Abstract
Cannabidiol (CBD) is a bioactive compound isolated from Cannabis plants that has garnered attention within the medical community due to its potent anti-inflammatory properties. To better understand how CBD limits excessive neuroinflammation we administered CBD via oral gavage (20 mg/kg) in a murine model of multiple sclerosis (MS) known as experimental autoimmune encephalomyelitis (EAE). Using single cell RNA sequencing (scRNA Seq) and array-based transcriptomics we were able to delineate how CBD limits excessive inflammation within the central nervous system (CNS) as well as within the intestinal lining in EAE. In-depth scRNA Seq analysis of CNS tissue demonstrated that CBD treatment resulted in a significant reduction in CXCL9, CXCL10 and IL-1β expression within the CNS, leading to inhibited infiltration of inflammatory macrophages. CBD inhibited IL-1β production independent of the classical cannabinoid receptors, CB1 and CB2. CBD treatment also led to induction of Myeloid-derived Suppressor Cells (MDSCs) both in the CNS and periphery. Interestingly, CBD treatment of EAE mice revealed significant suppression of inflammation in the gastrointestinal (GI) tract. The intestinal epithelial cells (IECs) of CBD treated mice demonstrated a transcriptional inhibition of a family of pyroptosis initiators that drive localized inflammation known as gasdermins (GSDMs). Further investigation into the GI tract via 16s sequencing of cecal and fecal contents demonstrated that oral administration of CBD resulted in no significant changes in the intestinal microbiota composition. These findings demonstrate the beneficial effect of CBD treatment on autoimmune neuroinflammation by ablating expression of pro-inflammatory chemoattractants, regulating inflammatory macrophage activity, promoting MDSC expansion, and limiting the systemic low-grade inflammation in the GI tract, culminating in the attenuation of EAE.
Keywords: Neuroinflammation, Multiple sclerosis, Cannabidiol, Experimental autoimmune encephalomyelitis, Cannabinoids
Introduction
MS is an incurable autoimmune disorder in which the host immune system inadvertently recognizes peptides deemed antigenic within the myelin sheath surrounding the axon terminals within the CNS (Goldenberg 2012). The immunity-dependent pathology of MS is characterized by flairs of neuroinflammation within the CNS that drives neurodegenerative lesions, which manifest in paralytic symptoms, discomfort, impaired cognitive function, and atrophy (Huang et al. 2017). Current immunosuppressant therapies aimed at slowing MS disease progression are often complicated by external factors that further worsen the quality of life for afflicted patients due to their exorbitant costs, lack of long-term efficacy, susceptibility to infections, and inadvertent toxicity (Hartung et al. 2015; Dargahi et al. 2017).
Some of the most consumed and postulated alternative therapeutics hoped to combat inflammatory disorders include the major cannabinoids isolated from Cannabis sativa, namely CBD and delta-9 tetrahydrocannabinol (THC) (Nagarkatti et al. 2009; Ahmed and Katz 2016). While clinical research pertaining to cannabinoids is still in its relative infancy, the treatment of animal models of inflammatory disorders with CBD and THC has yielded results that demonstrate their respective abilities to inhibit the excessive inflammatory processes that drive pathogenesis by promoting expansion anti-inflammatory regulatory immune cell subsets, and inhibiting pro-inflammatory effector immune cell activity (Nichols and Kaplan 2020; Becker et al. 2020a, b; Mohammed et al. 2020a, b; Rodríguez Mesa et al. 2021). Studies such as these have culminated into the adoption of cannabinoid-based treatments, such as Sativex®, consisting of a combination of THC and CBD from Cannabis plant, which has been shown to improve the quality of life in patients with MS without the associated psychoactive effects of THC due to the oromucosal consumption route yielding low plasma levels of soluble THC (Vermersch 2011). While THC exhibits psychotropic effects, CBD does not and thus, use of CBD alone to suppress inflammation looks clinically more promising. To that end, more research is needed to understand the mechanism of action of CBD to suppress inflammation.
To further understand the changes occurring in various inflammatory cells in the CNS of EAE mice treated with CBD, in the current study, we used single cell RNA sequencing (scRNA Seq) and array-based transcriptomics. Additionally, because of the crosstalk between the gut and the brain during autoimmune diseases such as MS/EAE (Dopkins et al. 2018), we also investigated immunological changes occurring in the peripheral immune system, including the mesenteric lymph nodes (MLN) and the gut.
The primary findings of our study demonstrate that CBD treatment regulates the pro-inflammatory phenotype of resident and infiltrating myeloid cells by inhibiting macrophage infiltration and inhibiting the production of soluble inflammatory mediators such as IL-1β, CXCL9 and CXCL10. Outside of the CNS, we saw an increase in anti-inflammatory processes that combat the chronic low-grade inflammation observed systemically in EAE mice. Specifically, within the GI tract, we observed a reduction in the expression of a family of pyroptosis initiators known as GSDMs in the intestinal epithelium, and a reduction of neutrophils present within the mesenteric lymph nodes. Within the spleen, we observed an expansion of the anti-inflammatory immune cells, MDSCs. Collectively, these data further suggest that CBD treatment yields potent inhibition of neuroinflammation resulting in alleviated paralytic symptoms, while limiting the chronic low-grade inflammation in the periphery, including the GI tract.
Materials and Methods
Reagents
The following reagents were used during the course of the experiments and were purchased as follows: CBD from Cayman Chemical (Ann Arbor, MI); A784168 (A78) from Tocris (Minneapolis, MN); red blood cell (RBC) lysis buffer and T007, from Millipore Sigma (Burlington, MA); Percoll from GE Healthcare Life Sciences (Pittsburgh, PA); Myelin oligodendrocyte glycoprotein peptide subunit 35–55 (MOG35–55) from PolyPeptide Laboratories (San Diego, CA); Pertussis Toxin (PTX) from List Biological Laboratories (Campbell, CA); Heat killed Mycobacterium tuberculosis (H37Ra) from Difco (Detroit, MI); β-mercaptoethanol, Freund’s Adjuvant, toluene based mounting medium and Tween-80, from Sigma-Aldrich (St. Louis, MO); Absolute ethanol from Fisher Scientific (Hampton, NH); RPMI 1640, fetal bovine serum (FBS), L- glutamine, phosphate buffered saline (PBS) and HEPES, from VWR (West Chester, PA); SSO Advanced SYBR Green Supermix, from Bio-Rad (Hercules, CA); DNA oligos, from Integrated DNA Technologies (Coralville, IA); Bovine serum albumin (BSA), 4’,6-Diamidino-2-Phenylindole (DAPI), Texas Red conjugated-phalloidin, pHrodo™ green dextran, and GeneChip™ WT Pico Kits, from Thermo Fisher (Waltham, MA); macrophage-colony stimulating factor (M-CSF), fluorophore conjugated antibodies, from Biolegend (San Diego, CA); Chromium Next GEM Single Cell 5’ Library Gel and Bead Kit v1.1, Chromium Single Cell 5’ Library Construction Kit, Chromium Next GEM Chip Single Cell Kit and Chromium i7 Multiplex Kit, from 10x Genomics (Pleasanton, CA); Illumina MiSeq reagents and NextSeq 500/550 v2.5 kits, from Illumina Inc (San Diego, CA); HRP-conjugated anti-biotin and biotinylated protein ladder, from Cell Signaling (Danvers, MA).
Mice
Six-week-old female wildtype (WT) C57BL/6 were purchased from Jackson laboratories (Bar Harbor, ME) and housed in the Animal Resource Facility (ARF) at University of South Carolina School of Medicine. Prior to the experiments, mice were cohoused in groups during a 2-week period to acclimate to the facilities as well as to establish a baseline microbiota. All C57BL/6CB1−/− (CB1KO), CB57BL/6CB2 −/− (CB2KO), and C57BL/6CB1−/−CB2−/− (CNRO) mice were bred in-house, as describe previously (Becker et al. 2020a). All experiments were conducted in accordance with National Institute of Health (NIH) guidelines. All protocols involving vertebrate animals were approved by the University of South Carolina Institutional Animal Care and Use Committee (IACUC). All samples, unless denoted otherwise, from EAE mice were collected on day 17 at the conclusion of studies.
EAE Induction and CBD Treatment
Chronic progressive EAE was induced in C57BL/6 mice according to previously published protocols (Singh et al. 2007; Constantinescu et al. 2011; Rouse et al. 2013; Al-Ghezi et al. 2019). Briefly, on day 0 mice were given 150 μg of MOG35–55 and 600 mg H37Ra suspended within an emulsion of sterile PBS and Freund’s adjuvant via 2 subcutaneous injections. This immunization was followed up by intra-peritoneal injections of PTX suspended within sterile PBS on day 0 (200ng) and day 2 (400ng). For CBD treatment, EAE mice were randomly divided into groups receiving either CBD or vehicle gavages daily. The CBD group received a 200μl oral gavage composed of 20 mg/kg of CBD suspended with 89.9% PBS, 10% ethanol and 0.1% Tween 80 daily, for 17 days. Control mice received vehicle treatment consisting of 200ul of 89.9% PBS, 10% ethanol and 0.1% Tween 80 via oral gavage daily. This dosage of CBD has been demonstrated to be effective in murine studies on inflammation was deemed to be a safe dose when converted to a human equivalent (Nair and Jacob 2016; Elliott et al. 2018; Millar et al. 2019). Mice were monitored, weighed, and scored for the onset of clinical symptoms associated with EAE severity daily.
EAE Scoring
The measurement for paralysis symptoms and EAE clinical scores were recorded as following according to previous studies (Dopkins et al. 2020): 0 = no symptoms, 1 = inability to curl the distal portion of the tail, 2 = complete tail atony/impaired movement, 3 = partial hind/fore limb paralysis, 4 = complete hind limb paralysis, 5 = tetraplegia/moribund state.
Flow Cytometry
Spleens, whole CNS, and mesenteric lymph nodes (MLNs) were isolated from mice for flow cytometry analysis of immune cells present. Whole spleens and MLNs were manually dissociated, incubated at room temp (RT) with RBC lysis buffer and filtered (70 μm) to produce single cell suspension. CNS tissue was manually dissociated and washed twice in 30% percoll to remove excess myelin. Single cell suspensions were tagged with fluorescently labeled monoclonal antibodies (mAbs) purchased from Biolegend (APC labeled anti-CD45, PE labeled anti-CD45, BV421 labeled anti-MHCII, AF647 labeled anti-CD11b, FITC labeled anti-CD11b, AF700 labeled anti-CX3CR1, FITC labeled anti-CD45, BV510 labeled anti-Ly6C, V450 labeled anti-Ly6G, PerCP Cy5 labeled anti-CD3, BV786 labeled anti-CD4, BV421 labeled anti-Foxp3, PE labeled anti-RORγT). Flow cytometry of fluorescent-labeled mAb tagged samples was conducted using a BD FACs Celesta flow cytometer. Analysis of FCS files was conducted using FlowJo software purchased from BD Biosciences (San Jose, CA).
Phylogenetic Characterization of Bacterial Species by 16s rDNA Sequencing
Microbiota analysis was conducted using 16s rDNA sequencing of the V3 and V4 region of rDNA isolated from cecal and stool contents of EAE mice belonging to vehicle and CBD-treated groups. Briefly, mice were cohoused for 2 weeks prior to EAE induction and randomly divided into groups that would receive either vehicle or CBD treatments daily. DNA was isolated from stool content collected on day 0 and cecal content collected on day 17 by using the QIAamp Stool Mini Kit according to manufacturer’s instructions. Sequencing of samples was performed using the Illumina MiSeq platform. OTU tables were generated from fastq. files derived from the sequencing run by the Nephele pipeline provided by the National Institute of Allergy and Infectious Diseases (Weber et al. 2018b; Bolyen et al. 2019). OTU tables were formatted and analyzed in R Studio using the phyloseq, ape and ggplot2 packages (Paradis et al. 2004; McMurdie and Holmes 2013; Wickham 2016; Gérikas Ribeiro et al. 2016).
Isolation of F4/80+ and CD326+ Cells
Positive selection using PE conjugated anti-F4/80 and PE conjugated anti-CD326 was used to isolate and enrich for myeloid cells and intestinal epithelial cells (IECs) respectively. Briefly, single cell suspensions from the CNS tissue and intestinal tract were Fc Receptor blocked, and then stained for their respective differentiation marker. EasySep™ PE selection cocktail followed by EasySep™ magnetic nanoparticles were added to the cell suspensions. The cell suspensions were brought up to an appropriate volume of 2.5mLs and placed within an EasySep™ magnet for retention. This step was repeated 4 times to ensure that primarily PE-tagged cells were retained. The remaining cell pellet was suspended in QIAzol™ lysis reagent for downstream RNA quantification.
Array Based Quantification of mRNA Production within Intestinal Epithelial Cells
Transcriptomic analysis was conducted on RNA isolated from IECs from VEH and CBD-treated EAE mice according to the GeneChip™ WT Pico Reagent Kit. Briefly, cDNA with a specified adaptor sequence was produced from whole mRNA content of CD326 positive cells. cDNA was purified prior to in-vitro transcription to produce labeled cRNA. Twenty ng of cRNA was used for production of ss-cDNA. After production, ss-cDNA was isolated, fragmented, labeled, and hybridized to a mouse-specific Clariom™ D assay. Hybridized Clariom™ D assay chips were washed in accordance with manufacturer’s recommended fluidics protocol FS450_0001 using GeneChip™ Fluidics Station 450. After completion of the fluidics protocol, the assay chips were analyzed using a GeneChip™ Scanner. Downstream transcriptomic analysis was conducted using Applied Biosystems™ Transcriptomic Analysis Console version 4.0.
Single Cell RNA Sequencing of CNS Tissue
scRNA Seq was conducted as described previously (Becker et al. 2020b), using the 10X Genomics Chromium Controller Instrument and Chromium single cell 5’ library & gel bead kit according to manufacturer’s instructions. Briefly, 3000 cells (>90% viability) were loaded onto the controller to generate single-cell gel bead emulsions. After cell-lysis, cDNA was generated via barcoded reverse transcription. cDNA was then amplified, fragmented, and indexed prior to being sequenced on the NextSeq 550. Cell Ranger version 3.1.0 (10x Genomics) was used to process raw sequencing data. Seurat Suite version 3.0 was used to perform downstream analysis of the Cell Ranger outputs(Butler et al. 2018; Stuart et al. 2019). The data files of reads from CBD-treated and VEH-treated EAE mice were integrated within Seurat using anchor and integration function. Scaling and principal component analysis (PCA) was conducted on integrated data. Clusters were identified following PCA analysis with a granularity resolution of 0.15.
Ex-Vivo Treatment of Bone Marrow Derived Macrophages
Bone marrow derived macrophages (BMMs) were generated from the bone marrow cells isolated from the tibia and femur of 6-week-old naïve female C57Bl/6 WT, CB1KO, CB2KO, and CNRO mice. Briefly, the femurs and tibias of each mouse were collected aseptically in a sterile hood. After collection, the bones were cut at the proximal and distal ends to produce a conduit of the long bone section that was then flushed with complete 5mL of DMEM/F12 using a 27-gauge needle. The resulting bone marrow was then physically dissociated via pipetting up and down before being washed in DMEM/F12 three times. Cells after isolation were cultured at 2×106 cells / mL in complete DMEM/F12 medium containing 10% FBS, 1% penicillin/streptomycin, 2mM L-glutamine and 1U/ml of M-CSF for 7 days as previously described (Miranda et al. 2018). After 7 days, the BMMs were activated with lipopolysaccharide (LPS) at a concentration of 100 ng/ml for 24 hours in DMEM/F12 supplemented with 10 μg/mL CBD for experimental treatment groups. In studies aimed at blocking PPARγ, cells were incubated with 25 μM T007, a concentration previously established to demonstrate the effects of PPARγ antagonism (Li et al. 2017). Cells were incubated with T007 for a period of 2 hours prior to incubation with CBD and LPS.
Real-Time Quantitative PCR for Validation of Gene Expression
Quantitative PCR (qPCR) was performed to determine the expression of genes of interest on cDNA synthesized from RNA isolated from whole tissue, bone marrow derived macrophages, and lymphocytes/myeloid cells. cDNA was synthesized according to manufacturer protocol for the generation of messenger RNA using the miScript II RT Kit supplied by Qiagen (Germantown, MD). qPCR was conducted using the SSO Advanced qPCR master mix according to manufacturer’s protocol supplied by Bio-Rad. Fold changes for mRNA expression were collected using the ΔΔCt method, where Ct is the threshold cycle to detect fluorescence. qPCR was used to detect the expression levels of IL-1β relative to GAPDH. A detailed list of primer sequences can be found in Table 1.
Table 1.
Primer sequences for qPCR
| Gene name | F Primer Sequence | R Primer Sequence |
|---|---|---|
|
| ||
| IL-iβ | TGGACCTTCCAGGATGAGGACA | GTTCATCTCGGAGCCTGTAGTG |
| GAPDH | TGATGGGTGTGAACCACGAG | CAGGGATGATGTTCTGGGCA |
| TNFα | CTGAACTTCGGGGTGATCGG | GGCTTGTCACTCGAATTTTGAGA |
| IL-6 | CCAAGAGGTGAGTGCTTCCC | CTGTTGTTCAGACTCTCTCCCT |
| IL-10 | GCTCTTACTGACTGGCATGAG | CGCAGCTCTAGGAGCATGTG |
| IL-13 | CCTGGCTCTTGCTTGCCTT | GGTCTTGTGTGATGTTGCTCA |
Fluorescent Microscopy
For conducting fluorescent microscopy, mice were euthanized with isoflurane and subsequently perfused with 10mL of heparinized PBS followed by 10mL of 10% formalin. Whole brains were isolated and embedded in paraffin blocks before being cut into 7 μm sections placed on glass slides. Slides were then deparaffinized by complete submersion into the following solutions in the listed order: Xylene for 6 minutes, 1:1 xylene and ethanol for 3 minutes, ethanol for 6 minutes, 95% ethanol for 3 minutes, 70% ethanol for 3 minutes, 50% ethanol for 3 minutes, and lastly held in tap water. Slides were then incubated with a blocking solution of filtered PBS with 1% BSA w/v for 10 minutes. Slides were then incubated with a primary FITC-conjugated antibody suspended in blocking solution at a concentration of 2.5 μg/mL for 1 hour in the dark. Slides were then washed three times for 1 minute in PBS to remove excess antibody. Slides were then incubated with Texas Red-conjugated phalloidin antibodies at a concentration of 165nM in blocking solution for 20 minutes in the dark. Slides were then washed three times for 1 minute in PBS to remove excess phalloidin. The slides were then incubated with DAPI dye diluted to a final concentration of 200nM in PBS for 5 minutes in the dark. After incubation, the slides were washed 3 times for 1 minute in PBS to remove excess DAPI. The slides were then mounted using a toluene based medium and sealed prior to being visualized on LAS X version 3.4.2.18368 software on a Leica DM 2500 instrument.
Endocytosis Assay
Endocytosis was measured via the uptake of pHrodo™ green dextran by macrophages isolated from the peritoneal cavity of naïve WT mice. Briefly, the mononuclear cells isolated from the peritoneal wash of naïve WT mice was plated in complete DMEM/F12 at 1 million cells/mL in the presence of DMSO, CBD (10μg/mL), and/or T007 (25μM) for 2 hours at 37 degrees Celsius. Cells were pelleted, washed in sterile PBS, and Fc blocked in sterile PBS for 15 minutes at room temperature. Cells were then pelleted and resuspended in a solution containing 20μg/mL pHrodo™ green dextran, BV605 conjugated anti-CD45, AF647 conjugated anti-CD11b, and PE conjugated anti-F4/80 for 10 minutes at room temperature. Cells were then pelleted, washed in PBS, and ran on the FACSCelesta flow machine. Endocytosis activity was measured as the mean fluorescent intensity of FITC on the gated population of CD11b+CD45+F4/80+ cells.
Statistical Analysis
All data are presented as the mean ± standard error of the mean. The number of biological replicates per experiment are identified in the figure legends. All statistical analysis was conducted by using unpaired T tests to compare 2 groups differing by one variable. Statistical analysis of experiments with multiple variables was conducted using a 2-way ANOVA tests to determine significant interactions. Post hoc analysis to determine the degree of significance was conducted using Bonferroni’s test. Degree of significance was demonstrated using the following key: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Results
CBD Ameliorates EAE Symptomology
Previous studies have shown that CBD and a variety of other phytocannabinoids possess potent anti-inflammatory properties that are capable of ameliorating symptoms in neuroinflammatory disorders (Corey-Bloom et al. 2012; Elliott et al. 2018; Maroon and Bost 2018; Al-Ghezi et al. 2019; Yang et al. 2019). To better understand the processes by which treatment with CBD regulates anti-inflammatory neuroprotection during encephalitis, we first confirmed that oral administration of CBD at a concentration of 20 mg/kg results in a significant amelioration of paralysis-associated EAE symptoms (Fig. 1A–B). Concurrent with the reduction in paralysis symptoms observed in the CBD treated group was a resistance to neuroinflammation-associated atrophy observed from day 11 onward, however for the time course of these experiments there were no significant changes in weight observed over time between groups (Fig. 1C–D).
Fig. 1.

CBD ameliorates EAE symptomology. (A) Clinical scores, outlined in the Materials and Methods section, over time of EAE mice receiving either vehicle (n=15) or CBD treatment (n=15) by oral gavage at a dose of 20 mg/kg body weight. (B) Composite score of paralysis symptoms per mouse. (C) Change in body weight represented as a percentage of starting body weight. (D) Body weight over time
CBD Treatment Results in an Anti-Inflammatory Shift in the Transcriptional Profile of Infiltrating Macrophages and Resident Microglia within the CNS
To better define how CBD accomplishes neuroprotective effects that combat autoimmune encephalomyelitis, scRNA Seq was conducted on a single cell suspension isolated from whole CNS tissue on day 17. Overlaying tSNE plots demonstrated transcriptional diversity between EAE mice treated with CBD or the Vehicle (Fig. 2A). Clustering of sequenced cells was performed using Seurat package, which identified 8 unique clusters (Butler et al. 2018) (Fig. 2B). CD11b expression signified that the identified clusters 0–1 and 3–5 belonged to a myeloid lineage (Fig. 2C). Ly6G expression further signified cluster 1 to be either PMN MDSCs or neutrophils dependent on the relative expression of Ly6C2 per cell (Fig. 2D). Ly6c2 (Fig. 2E) and CCR2 (Fig. 2F) expression further identified clusters 0 as being infiltrating inflammatory macrophages. CXCL9 (Fig. 2G), CXCL10 (Fig. 2H), and IL-1β (Fig. 2I) expression revealed a distinct pro-inflammatory cytokine expression amongst the resident and infiltrating myeloid cells of the CNS of vehicle-treated mice when compared to CBD-treated group. Gsdmd expression revealed a decrease in the expression of the inflammatory pyroptosis-initiator within the infiltrating myeloid cells of cluster 0, 4 and 5 in the CBD treated group when compared to vehicle (Fig. 2J). CD40 expression signified that clusters 0, 3 and 4 within the vehicle group, shown to be Ly6C and CD11b+, was used to identify cells capable of antigen presentation, while cluster 7 within the treatment group was the only cluster participating in antigen presentation (Fig. 2K). Expression of STAT1 amongst the clusters demonstrated that CBD treatment globally inhibited STAT1 activity amongst all cell types present in the CNS (Fig. 2L), while demonstrating modest changes in STAT2 (Fig. 2M) and STAT3 (Fig. 2N). Collectively, these data demonstrated that CBD treatment limits macrophage infiltration and activity within the CNS of EAE mice, potentially through inhibited transcription of STAT1 and a variety of soluble mediators such as CXCL9, CXCL10, and IL-1β. The suppressed expression of these pro-inflammatory soluble mediators in the CBD group correlated with the observed attenuation of pro-inflammatory macrophage infiltration as signified by cluster 0, when compared to the vehicle group.
Fig. 2.
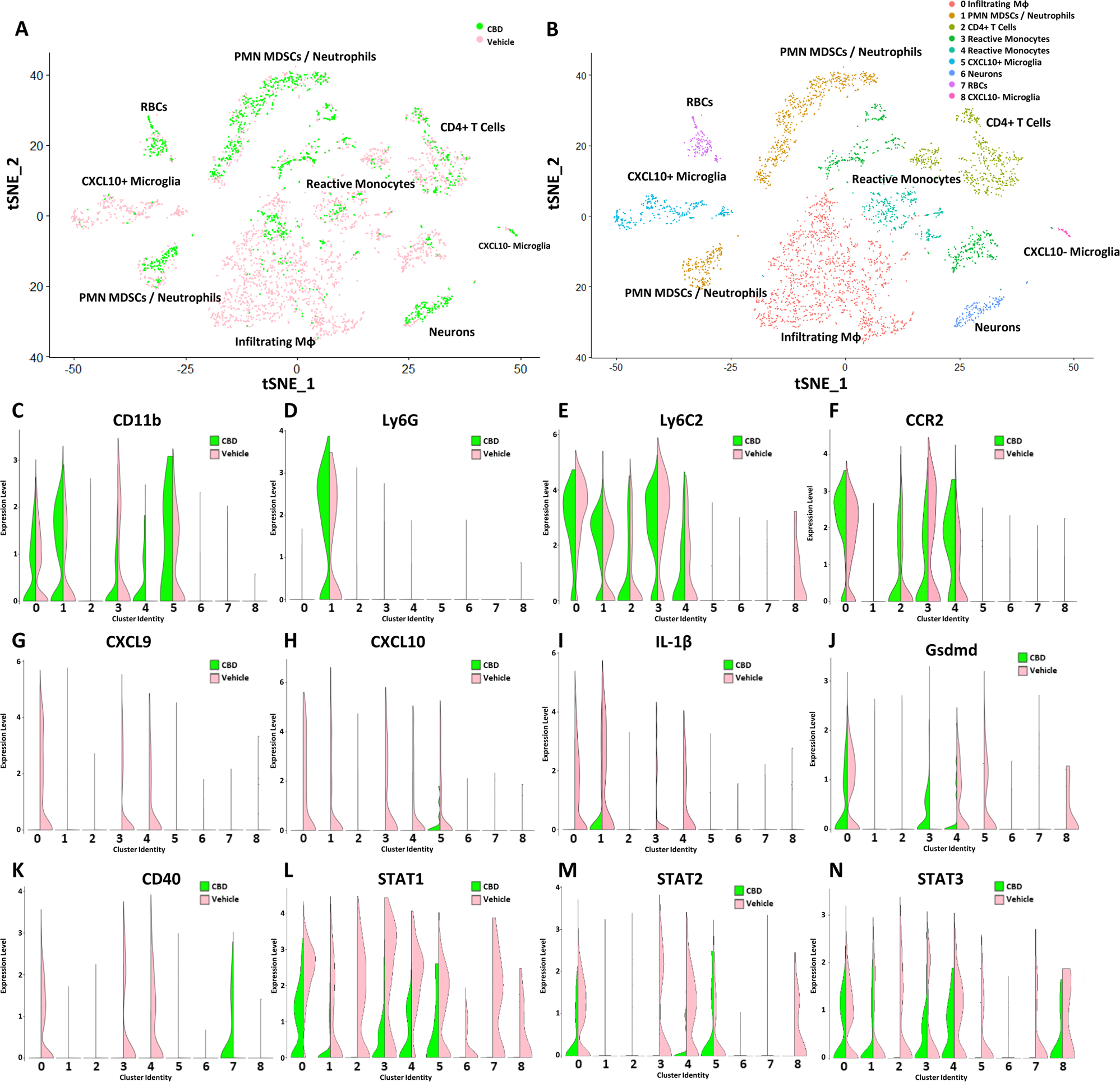
CBD Treatment Results in an Anti-Inflammatory Shift in the Transcriptional Profile of Infiltrating Macrophages and Resident Microglia within the CNS. (A) tSNE mapping of scRNA-seq reads from single cell suspensions of whole CNS tissue from vehicle (pink) and CBD (green) treated mice. Vehicle (3,011 Cells – 88,022,395 Reads – 29,233 Mean Reads per Cell) Treatment (1492 Cells – 103,253,015 Reads – 69,204 Mean Reads per Cell). (B) Clusters identified by Seurat based on a resolution of 0.15. Violin plots demonstrating relative expression levels of CD11b (C), Ly6G (D), Ly6C (E), CCR2 (F), CXCL9 (G), CXCL10 (H), IL-1β (I), GSDMD (J), CD40 (K), STAT1 (L), STAT2 (M), and STAT3 (N) amongst clusters identified by Seurat analysis of scRNA sequencing
CBD Treatment Inhibits Macrophage Derived Production of IL-1β Under Inflammatory Conditions
To better define how CBD limits macrophage-mediated inflammation, we further investigated the effects of CBD on IL-1β production by inflammatory macrophages. First, we quantified the expression of IL-1β within F4/80+ cells from the mononuclear cell fraction of CNS tissue from EAE mice on day 17. Quantified gene expression revealed that the F4/80+ cells from the CNS of CBD-treated EAE mice demonstrated a significant reduction in IL-1β expression when compared to vehicle- treated EAE mice (Fig. 3A). To test if CBD directly inhibits the expression of IL-1β by macrophages under inflammatory conditions, we cultured BMMs from naïve wild-type mice and stimulated them in the presence of LPS. IL-1β expression across these samples, as detected by qPCR, demonstrated that CBD treated BMMs under LPS stimulating conditions contained an 85-fold reduction in IL-1β expression (Fig. 3B). To ensure that this change in expression was represented by a change in IL-1β excretion, ELISA quantification of IL-1β present within supernatants from these cultures was conducted. These data revealed that LPS-stimulated samples contained high levels of soluble IL-1β, while all other samples, including the CBD-treated samples stimulated with LPS, had no detectable levels of IL-1β in the supernatant (Fig. 3C). Next, we used scatter plots to demonstrate which immunologically relevant markers are demonstrating the largest reduction in IL-1β expression in the scRNA Seq data from the CNS tissue of EAE mice treated with CBD. Amongst CD45+ cells present in the CNS of EAE mice treated with CBD (green) compared to vehicle (pink), there was a reduction of IL-1β expression (Fig. 3D), however there was a more drastic reduction in IL-1β expression in the CCR2+ cells infiltrating the CNS (Fig. 4E). Inasmuch as IL-1β has been demonstrated to be produced by as well as grant access to CCR2high infiltrates that drive neuroinflammation in EAE via a positive feedback loop, we used immunofluorescence to visualize CCR2 positive cells present within the cerebral cortex of vehicle- and CBD-treated mice (Paré et al. 2018, p. 2). Representative immunofluorescent images demonstrated that the CNS of vehicle treated EAE mice has sizable infiltration of CCR2+ cells that were not observed in the CNS of CBD treated EAE mice via microscopy (Fig. 3F).
Fig. 3.

CBD Treatment Inhibits Macrophage Derived Production of IL-1β Under Inflammatory Conditions. (A) Expression of IL-1β is significantly reduced within in F4/80+ cells selected from the CNS of CBD treated mice in comparison with vehicle treated (n=5). (B) Bone marrow macrophages treated with CBD and stimulated with LPS displayed a significant decrease in IL-1β expression when compared to macrophages treated with DMSO and stimulated with LPS. Bone marrow macrophages treated with CBD and stimulated LPS show no significant change in the expression of IL-1β when compared to bone marrow macrophages treated with CBD alone (n=5, 2-Way ANOVA with Bonferroni’s multiple comparisons test, F (1.000, 4.000) = 28.78. Vehicle + LPS vs CBD + LPS: p=0.0349). (C) Secreted IL-1β protein concentration in media collected from bone marrow macrophages treated with or without CBD under normal and LPS stimulated conditions (n=5, 2-Way ANOVA with Bonferroni’s multiple comparisons test, Vehicle + LPS vs CBD + LPS: p < 0001). (D) Scatter plot demonstrating co-expression of CD45 and IL-1β in CNS infiltrating cells as identified by scRNA Seq data. (E) Scatter plot demonstrating co-expression of CCR2 and IL-1β in CNS infiltrating cells as identified by scRNA Seq data. (F) Representative fluorescent microscopy images of CCR2 expression within 7 μm sections of CNS tissue isolated from vehicle treated (left) and CBD treated (right) EAE mice (Green = CCR2, Red = actin, Blue = cell nuclei)
Fig. 4.
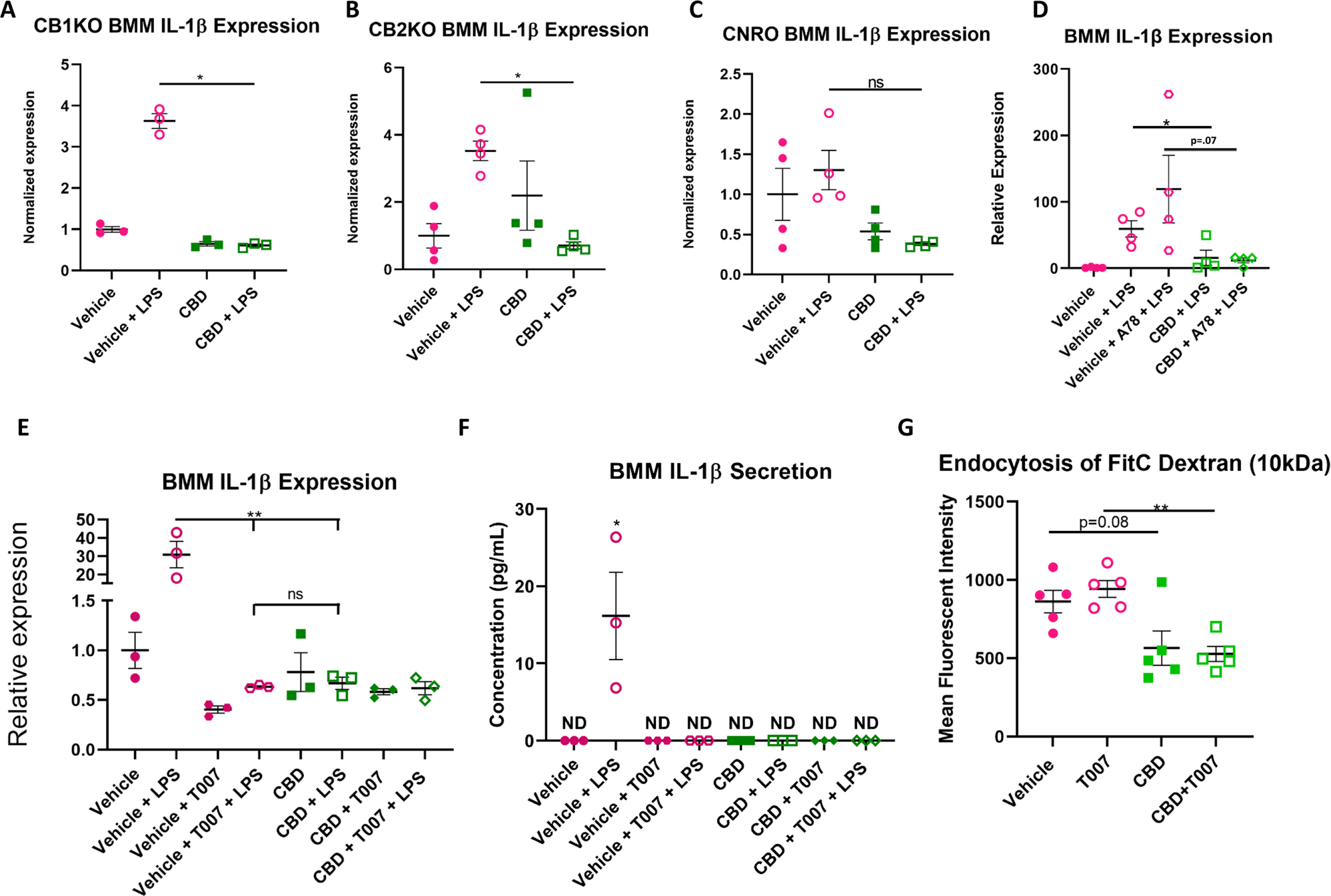
CBD Reduces IL-1β Expression in LPS-Stimulated BMMs Independent of Classical Cannabinoid Receptors. (A) Bone marrow macrophages isolated from CB1KO mice treated with CBD and stimulated with LPS displayed a significant decrease in IL-1β expression when compared to macrophages treated with DMSO and stimulated with LPS (n=3, 2-Way ANOVA with Bonferroni’s multiple comparisons test, F (1.000, 2.000) = 149.8; Post hoc: Vehicle + LPS vs CBD + LPS: p=0.0143). (B) Bone marrow macrophages isolated from CB2KO mice treated with CBD and stimulated with LPS displayed a significant decrease in IL-1β expression when compared to macrophages treated with DMSO and stimulated with LPS (n=4. 2-Way ANOVA with Bonferroni’s multiple comparisons test, F (1.000, 3.000) = 14.03; Post hoc: Vehicle + LPS vs CBD + LPS: p=0.016). (C) Bone marrow macrophages isolated from CNRO mice treated with CBD and stimulated with LPS displayed a significant decrease in IL-1β expression when compared to macrophages treated with DMSO and stimulated with LPS (n=4. 2-Way ANOVA with Bonferroni’s multiple comparisons test, F (1.000, 3.000) = 3.284; Post hoc: Vehicle + LPS vs CBD + LPS: p=0.22). (D) WT LPS stimulated BMMs treated with CBD display decreased expression of IL-1β independent of TRPV1 antagonism with A784168 (25nM) (n=4). (E) WT LPS stimulated BMDMs treated with PPARγ antagonist T007 (25μM) display decreased expression of IL-1β in a manner that is indistinguishable from CBD treatment. (n=3. 2-Way ANOVA with Bonferroni’s multiple comparisons test, F (3, 6) = 17.25; Post Hoc: Vehicle + LPS vs T007 + LPS p=0.0046, Vehicle + LPS vs CBD + LPS: p=0.0046). (F) WT LPS Stimulated BMDMs treated with PPARγ antagonist secrete no detectable levels of IL-1β (n=3. 2-Way ANOVA with Bonferroni’s multiple comparisons test, F (3, 6) = 7.356; Post hoc: Vehicle + LPS p=0.036). (G) CBD significantly inhibits endocytosis activity in peritoneal macrophages while T007 has no effect (n=5. 2-Way ANOVA with Bonferroni’s multiple comparisons test, F (1.000, 4.000) = 2.042; Post hoc Vehicle vs CBD p=0.0889; T007 vs CBD + T007 p=0.002)
CBD Reduces IL-1β Expression in LPS-Stimulated BMMs Independent of Classical Cannabinoid Receptors
To discern how CBD treatment was suppressing the production of the pro-inflammatory cytokine IL-1β, we investigated the likely receptors which were propagating this anti-inflammatory effect. Intuitively, we first investigated the potential roles of the classical cannabinoid receptors CB1 and CB2 by use of BMMs derived from CB1KO, CB2KO and CNRO donor mice. Analysis of gene expression demonstrated that LPS-stimulated macrophages derived from CB1KO (Fig. 4A) and CB2KO (Fig. 4B) mice demonstrated a significant reduction in IL-1β production in the presence of CBD, while LPS-stimulated macrophages isolated from CNRO mice demonstrated a trend suggesting CBD still inhibits IL-1β expression (Fig. 4C). These results demonstrated that CBD treatment of inflammatory macrophages results in a suppression of IL-1β production independent of the classical cannabinoid receptors. To study the potential of CBD enacting anti-inflammatory effects through other receptors, we investigated the potential role of the receptors TRPV1 and PPARγ that are known to enable some cannabinoid activities. First, we investigated the potential role of TRPV1 by incubating WT BMMs with the TRPV1 antagonist A78. Incubation of LPS activated BMMs with A78 yielded no changes between the CBD treated groups (Fig. 4D). Next, to explore the role of PPARγ, we treated these WT BMMs with the PPARγ antagonist T007 prior to incubation with CBD. Surprisingly, addition of PPARγ antagonist caused a significant reduction in IL-1β expression by BMM cells stimulated with LPS that resembled the levels of suppression caused by CBD (Fig. 4E). ELISA quantification of excreted IL-1β from these samples also demonstrated that T007 treatment caused significant inhibition like CBD (Fig. 4F). To ascertain if PPARγ antagonism inhibits other aspects of macrophage activity, we investigated the effects of T007 and CBD given in tandem on the endocytosis of pHrodo™ green dextran labeled beads by peritoneal macrophages isolated from naïve mice (Supplemental Figure 1). T007 treatment in vitro resulted in no changes in endocytosis activity, while CBD treatment significantly reduced endocytosis activity independent of PPARγ (Fig. 4G). These data suggested that other aspects of macrophage activity, such as endocytosis, that are reduced following CBD treatment occur entirely independent of PPARγ.
Oral Treatment with CBD Alleviates Inflammation via the Promotion of MDSCs and Limitation of Neutrophils in a Tissue Site-Specific Manner
Previous research has shown that CBD treatment in an inflamed state induces an expansion of immunosuppressive MDSCs (Elliott et al. 2018), however, their presence in the secondary lymphoid organs has not been previously defined. Thus, when we tested the spleens for monocytic MDSCs with the phenotype of CD45+CD11b+Ly6C+Ly6G− (gating shown in Supplemental Figure 2), we found that the percentage of this population was significantly increased in CBD group when compared to Vehicle controls at day 17 (Fig. 5A). Within the spleen, there were no further changes in the PMN MDSCs (Fig. 5B) or neutrophils at this timepoint (Fig. 5C). Representative flow plots demonstrating MDSC expansion in the splenic tissue of CBD treated EAE mice compared to Vehicle treated are displayed (Fig. 5D). Within the inflamed CNS of EAE mice, CBD treatment caused no significant changes in the monocytic MDSCs (Fig. 5E) or neutrophils (Fig. 5G) but did result in an increase in the PMN MDSCs having the phenotype CD45+CD11b+Ly6C+Ly6G+ at day 17 (Fig. 5F and Supplemental Figure 3). Representative flow plots demonstrating MDSC expansion in the CNS tissue of CBD treated EAE mice compared to Vehicle treated are displayed (Fig. 5H). To better define how CBD may be suppressing the immune response against MOG peripherally, and because EAE is also regulated by the intestinal microbiota and inflammation (Belkaid and Hand 2014; Miyake et al. 2015; Mestre et al. 2018; Boziki et al. 2020) we analyzed the cellular composition of the MLNs which drain the intestinal tract. Within the MLNs of EAE mice, CBD treatment resulted in no significant changes in the monocytic MDSCs (Fig. 5I) or PMN MDSCs (Fig. 5J) but did result in a decrease in the percentage of neutrophils at day 17 (Fig. 5K) possessing the phenotype of CD45+CD11b+Ly6C−Ly6G+ (Fig. 5L and Supplemental Figure 4). Collectively, these data demonstrate that CBD does increase MDSC subsets both in the periphery and in the CNS of EAE mice, while simultaneously limiting neutrophil abundance in the intestinal tract.
Fig. 5.

Oral Treatment with CBD Alleviates Inflammation via the Promotion of MDSCs and Limitation of Neutrophils in a Tissue Site-Specific Manner. (A–C) Percentage of MDSCs and Neutrophils of all CD45+CD11b+ cells within the spleen in vehicle (n=10) vs treated (n=10) mice. (D) Representative flow plots for Figure 5A–C displaying Ly6C expression vs Ly6G expression on the vertical and horizontal axes, respectively. (E–G) Percentage of MDSCs and Neutrophils of all CD45+CD11b+ cells within the CNS in vehicle (n=6) vs treated (n=6) mice. (H) Representative flow plots for Fig. 5E–G displaying Ly6C expression vs Ly6G expression on the vertical and horizontal axes, respectively. (I–K) Percentage of MDSCs and Neutrophils of all CD45+CD11b+ cells within the MLN in vehicle (n=10) vs treated (n=10) mice. (L) Representative flow plots for Figures 5I–K displaying Ly6C expression vs Ly6G expression on the vertical and horizontal axes, respectively. CD11b+CD45+Ly6C+LyG− cells are assumed as Monocytic MDSCs, CD11b+CD45+Ly6C+LyG+ cells are assumed as PMN MDSCs and CD11b+CD45+Ly6C−LyG+ cells are assumed as neutrophils
Oral Treatment with CBD Alleviates Intestinal Inflammation via Inhibition of GSDM Within the Intestinal Tract of EAE Mice
In order to better understand how CBD regulates intestinal immunity by suppressing neutrophil abundance in the MLN and define any potential effects on limiting autoimmune neuroinflammation, we analyzed transcriptomic changes observed in the IECs that encompass the barrier between the GI microbiota and host. Array based transcriptomic analysis of purified IECs isolated from the colonic epithelium at day 17 revealed significant changes in the expression of 390 coding genes as denoted by a fold change of >2 (Fig. 6A) and p < 0.05 (Fig. 6B) when compared between the vehicle and CBD-treated EAE mice. PCA analysis revealed that the transcriptomic profiles of the IECs displayed proximity between the CBD-treated samples that was functionally different from the vehicle-treated groups (Fig. 6C). Heat maps were utilized to visualize the significantly regulated genes between the IECs of the vehicle treated and CBD treated mice groups (Fig. 6D). The top 3 downregulated genes by fold change in the intestinal epithelium of CBD-treated mice, when compared to vehicle-treated mice, were all isoforms of the pyroptosis initiating gsdmc gene (Fig. 6E). Examination of H&E-stained sections of the proximal colon revealed that the CBD-treated EAE mice had fewer infiltrating cells in the underlying colonic tissue in comparison to the vehicle-treated EAE mice (Fig. 6F).
Fig. 6.
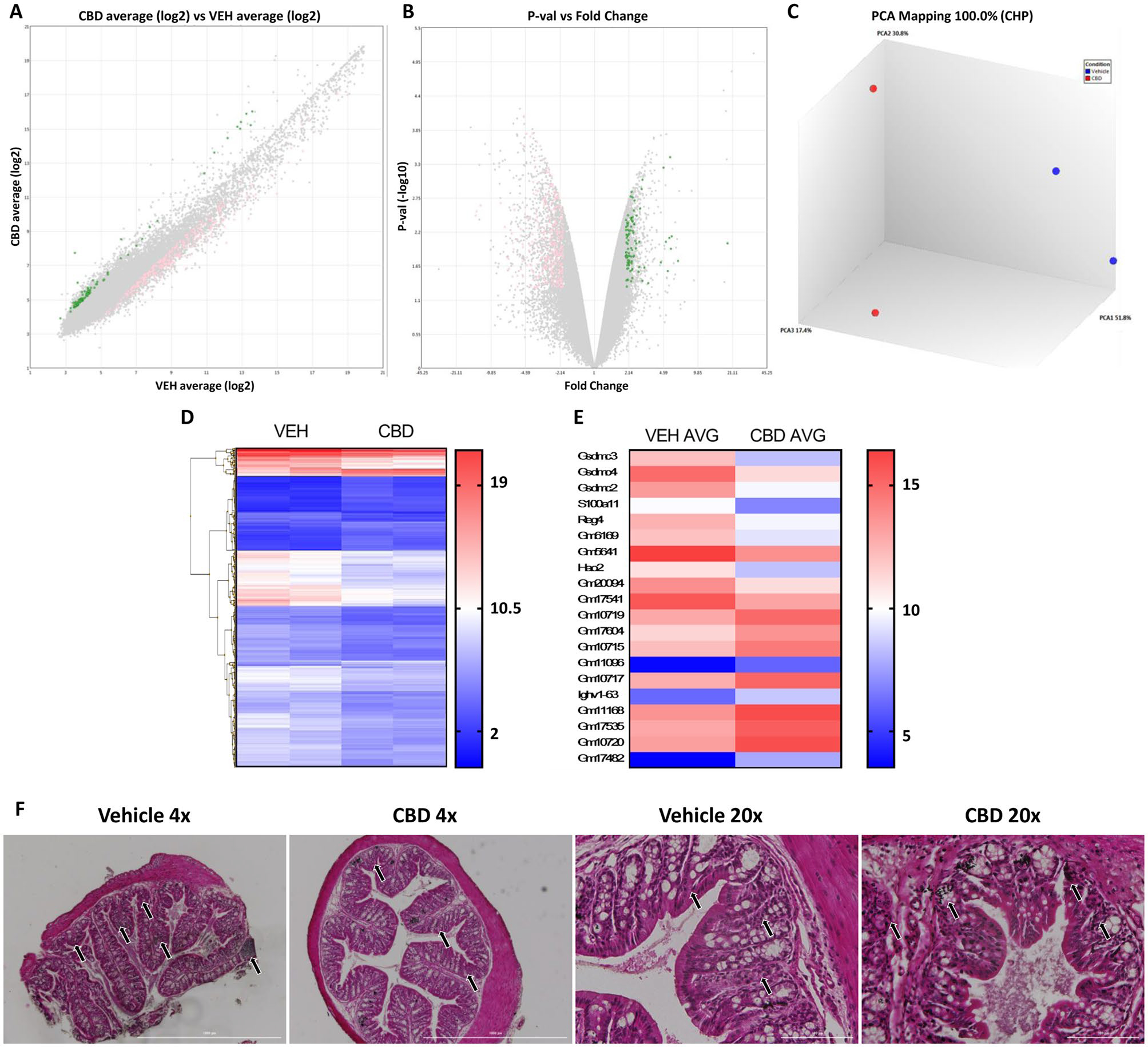
Oral Treatment with CBD Alleviates Intestinal Inflammation via Inhibition of GSDM Within the Intestinal Tract of EAE Mice. (A) log2 fold change between the mRNA content of IECs isolated from CBD vs VEH treated mice with coding genes significantly upregulated in the IECs of treatment mice (green) and vehicle mice (pink) highlighted (>=2 fold change; p < 0.05). (B) lP-val vs fold change between the mRNA content of IECs isolated from CBD vs VEH treated mice with coding genes significantly upregulated in the IECs of treatment mice (green) and vehicle mice (pink) highlighted (>=2 fold change; p < 0.05). (C) PCA Mapping of the transcriptome of vehicle treated (blue) vs CBD treated (red) IECs (n=2; 5 mice per sample). (D) Heat map of all 390 genes significantly regulated between vehicle and CBD treated groups(>=2 fold change; p < 0.05). (E) Heat map of the 10 significantly regulated genes with the highest and 10 significantly regulated genes with the lowest fold change value between vehicle and treated mice (>=2 fold change; p < 0.05). (F) Representative histology sections of the proximal colon of EAE mice at 4x magnification (left) and 20x magnification (right)
Effect of CBD on Gut Microbiota
Because the cross-talk between neuronal inflammation in EAE and gut microbiota is being increasingly appreciated as a major factor that regulates neuroinflammation severity (Zhu et al. 2020; Kadowaki and Quintana 2020), we tested if the oral administration of CBD had an impact on the composition of the gut microbiota in a murine model of experimental MS. 16s sequencing and subsequent analysis with Phyloseq software packages in R Studio demonstrated that oral administration of CBD at 20 mg/kg to EAE mice resulted in no significant changes to the cecal microbiota when compared to that of the vehicle-treated EAE mice. To confirm these results, 16s sequencing was performed on material isolated from fecal contents of previously cohoused mice on day 0 (n=10 per group) and on the cecal contents taken at time of sacrifice on day 17(n=10 per group). Metrics measuring alpha diversity, such as Chao1 index (Fig. 7A) and Shannon index (Fig. 7B), demonstrated no discernable changes in the microbiota composition across groups at either timepoint. Next, we studied beta diversity by use of PCA plots which demonstrate that the fecal content prior to disease induction, as well as the cecal content after sacrifice between vehicle-treated and CBD-treated EAE mice, showed no separation between the vehicle- and CBD-treated groups when analyzed at either timepoint (Fig. 7C). Further investigation into the specific reads as demonstrated by stacked bar charts illustrating the phyla (Fig. 7D), classes (Fig. 7E), orders (Fig. 7F), and families (Fig. 7G) identified by 16s sequencing confirmed that there were no significant changes in bacterial composition between the genera at either time point. To discern if there was any potential change in bacterial metabolism affecting host immunity following CBD treatment, we analyzed the concentration of the well-defined anti-inflammatory metabolites namely the short chain fatty acids (SCFAs) within the cecal contents via mass spectrometry (n=10 per group). These data revealed that there were no significant changes in any of the detectable SCFAs between vehicle vs CBD treated mice (Fig. 7H). These results collectively suggested that the anti-inflammatory effects of CBD on neuroinflammation and intestinal inflammation during EAE were not directly related to changes in the microbiota composition.
Fig. 7.

Effect of CBD on Gut Microbiota. (A) Alpha diversity of 16s sequencing reads as measured by Chao1 index for cecal contents from vehicle and CBD treated mice at sacrifice and stool contents at day 0 (n=10 per group). (B) Alpha diversity as measured by Shannon index for cecal contents from vehicle and CBD treated mice at sacrifice and stool contents at day 0 (n=10 per group). (C) PCA plot demonstrating beta diversity of 16s sequencing reads as measured by Chao1 index for cecal contents from vehicle and CBD treated mice at sacrifice and stool contents at day 0 (n=10 per group). (D) Stacked bar charts demonstrating average abundance of identified bacterial phyla from 16s sequencing reads for cecal contents from vehicle and CBD treated mice at sacrifice and stool contents at day 0 (n=10 per group). (E) Stacked bar charts demonstrating average abundance of identified bacterial classes from 16s sequencing reads for cecal contents from vehicle and CBD treated mice at sacrifice and stool contents at day 0 (n=10 per group). (F) Stacked bar charts demonstrating average abundance of identified bacterial orders from 16s sequencing reads for cecal contents from vehicle and CBD treated mice at sacrifice and stool contents at day 0 (n=10 per group). (G) Stacked bar charts demonstrating average abundance of identified bacterial families from 16s sequencing reads for cecal contents from vehicle and CBD treated mice at sacrifice and stool contents at day 0 (n=10 per group). (H) Concentration of detectable short chain fatty acids within the cecal contents of vehicle vs CBD treated EAE mice (n=10 per group)
Discussion
MS is a debilitating autoimmune disorder in which afflicted patients suffer chronic encephalomyelitis that manifests symptoms of atrophy, discomfort and paralysis (Reich et al. 2018). Current complications surrounding MS treatment include: complications arising from primary treatments, exorbitant treatment costs, lapses in medication, inefficient pharmaceuticals, and a complex etiology that makes early detection for proactive interventions associated with a beneficial prognosis incredibly difficult to accomplish (Waldman et al. 2014; Lublin et al. 2016; Paolicelli et al. 2020). MS pathology is exasperated by an inadvertent recognition of antigenic peptides within the myelin sheath by encephalitogenic T cells that directly and indirectly drive inflammatory degradation of CNS tissue (Aranami and Yamamura 2008; Fletcher et al. 2010). This antigen recognition triggers a variety of diverse inflammatory cascades that incorporate aspects of both adaptive and innate immunity; this underlying immunological diversity and variability that drives encephalomyelitis makes effective treatment of MS difficult to accomplish in the clinical setting. This multifactorial immunological diversity within encephalitic tissue is further contributed to by resident glia cells, recruited myeloid cells, and recruited lymphocytes all synergistically driving neuroinflammation (Fletcher et al. 2010; Ghasemi et al. 2017; Herz et al. 2017). Despite all the seemingly insurmountable barriers posed by such a convoluted and complex etiology driving disease progression, treatments that sway the balance of power in host immunity from a pro-inflammatory landscape towards an anti-inflammatory regulatory immune cell-dominated landscape, such as that associated with MDSC and Treg induced by cannabinoids, may provide novel therapeutic modalities (Dargahi et al. 2017).
The cannabinoid system has previously been established as an attractive candidate for the treatment of neurological and inflammatory disorders alike due to the safety of consumption, potent immunosuppressive potential, and low production costs of phytocannabinoids (Bellnier et al. 2018; Bruni et al. 2018). Our study demonstrates that treatment of EAE mice with the phytocannabinoids CBD results in potent anti-inflammatory effects within the CNS and GI tract of mice suffering from EAE, further validating the potential of CBD as a treatment of MS (Elliott et al. 2018; Nichols and Kaplan 2020). In the current study, we further defined the potential of CBD treatment on myeloid cell activity by use of scRNA seq on mononuclear cells isolated from the CNS tissue collected from EAE mice. Broadly, the results of this sequencing demonstrated that CBD reduces the abundance of infiltrating macrophages and inhibits the production of CXCL9, CXCL10 and IL-1β by macrophages, monocytes, and resident microglia. ScRNA seq further revealed that CBD treatment resulted in a dramatic reduction in the total number of infiltrating macrophages while promoting microglia to adopt a less inflammatory transcription profile with decreased CXCL10 or IL-1β expression. Despite this specific change in the transcription profile, flow cytometric analysis revealed that there were insignificant changes in the proportion and total number of activated microglia, as denoted by a CD11b+CD45highMHCII+CX3CR1+ phenotype (Supplemental Figure 5). Collectively, these results demonstrated that CBD limits macrophage infiltration into the CNS and inhibits the expression of IL-1β, CXCL9, and CXCL10 within the microglia and macrophage populations. This in-depth analysis technique was able to provide expansive results that demonstrated on a single-cell level what is occurring in the CNS when CBD is consumed orally during encephalitogenic conditions. Our novel insights into how CBD alleviates neuroinflammation in a cell-specific manner at the site of inflammation in a murine model of experimental MS gives an unprecedented and timely insight into the mechanisms by which cannabinoids regulate myeloid-derived immune cell function and subsequently suppress the production of soluble mediators that contribute to the pathogenic autoimmune neuroinflammation.
Next, we tested the direct effects of CBD on IL-1β production by inflammatory macrophages. We tested this in vitro by use of BMM cultures stimulated with LPS in the presence and absence of CBD. Our results demonstrated that supplementation of media with CBD resulted in ablated expression and excretion of IL-1β by BMMs stimulated with LPS, while having no noticeable effect on unstimulated BMMs, strengthening the hypothesis that CBD inhibits IL-1β production in activated macrophages. Because IL-1β driven neuroinflammation has previously been shown to depend on the recruitment of bone marrow derived CCR2+ infiltrates (Paré et al. 2018, p. 2), we used immunofluorescent imaging to test this possibility and found that CBD treatment resulted in the reduction of CCR2+ cells highlighted with a GFP- conjugated anti-CCR2 antibody. Collectively, these results demonstrated that CBD may attenuate IL-1β induction thereby preventing the recruitment of CCR2+ immune cells during EAE.
In the current study, in addition to the effect of CBD on inflammatory macrophages, we also tested the effect of CBD on MDSC abundance. MDSCs are an anti-inflammatory myeloid-derived cell subset that naturally migrate to site of inflammation in autoimmunity (Cripps and Gorham 2011). Studies focused on increasing the relative abundance of MDSCs have been beneficial in the treatment of autoimmune inflammation(Cripps and Gorham 2011). CBD treatment resulted in an induction of monocytic MDSCs and PMN MDSCs in the spleen and CNS, respectively. Monocytic MDSCs possess previously defined immunosuppressive functions, primarily by inhibiting effector T cell function through the excretion of nitric oxide, in inflammatory disorders that include EAE (Zhu et al. 2007; Crook and Liu 2014). PMN MDSCs however accomplish their immunosuppressive functions primarily via the inhibition of auto-antigen priming for presentation to encephalitogenic Th lymphocytes in EAE (Ioannou et al. 2012; Crook and Liu 2014). These results confirm previous studies which demonstrated that CBD treatment induces MDSCs in EAE (Elliott et al. 2018), while further defining the phenotype of MDSCs induced in a site-specific manner. The site specificity in MDSC accumulation observed in the treatment of EAE with CBD can likely be attributed to the discrepancy of soluble mediators capable of recruiting MDSC subsets to sites of inflammation. While certain mediators such as complement proteins can selectively promote PMN MDSC recruitment (Ostrand-Rosenberg and Sinha 2009), various chemokines are more selective in promoting the migration of monocytic MDSCs (Umansky et al. 2016). These data contribute to the literature established by previous studies which demonstrate the ability of CBD to directly induce anti-inflammatory MDSCs to alleviate inflammation-dependent symptomology and simultaneously promote immunotolerance in models of experimental autoimmunity (Hegde et al. 2011; Elliott et al. 2018).
While CBD is known not to directly activate CB1 or CB2 cannabinoid receptors, some studies have indicated that CBD may increase the levels of endocannabinoids such as anandamide (Leweke et al. 2012), thereby activating CB receptors. To test this possibility, we isolated BMMs from CB1KO mice, CB2KO mice, and CNRO mice and activated them with LPS in the presence of CBD. Our data shows that BMMs derived from classical cannabinoid receptor knockouts still demonstrate a reduction in IL-1β expression when stimulated LPS in the presence of CBD. These findings suggest that the inhibition of IL-1β expression by CBD occurs in a manner independent of the classical cannabinoid receptors CB1 and CB2.
Previous studies have shown that CBD may activate PPARγ, which in turn leads to suppression of inflammation (Varga et al. 2011; Wahli and Michalik 2012; O’Sullivan 2016). To determine if PPARγ binding is required for CBD to inhibit IL-1β production in macrophages, we inhibited PPARγ activation by supplementation of the cell culture media with T007 prior to treatment with CBD. In these experiments, addition of T007 alone to antagonize PPARγ in LPS stimulated cultures caused a significant decrease in IL-1β production and moreover, combination of CBD and PPARγ inhibitor failed to have an additive or inhibitory effect when compared to T007 or CBD alone. This discrepancy suggesting CBD may function other than an agonist of PPARγ in murine macrophages may be due to previously established complexities that underly function, such as PPARγ splicing variants present in macrophages or endogenously produced PPARγ ligands, such as prostaglandins, perpetuating LPS stimulated inflammatory processes (Fajas et al. 1997; Zhou et al. 2002; Grygiel-Górniak 2014; Zasłona et al. 2017). While these data showed that CBD acted like T007, the precise role of PPARγ in the regulation of LPS-mediated IL-1β production by CBD is also convoluted. While the primary role of PPARγ in regulating inflammatory processes has been shown to be dependent on agonistic ligands perpetuating anti-inflammatory effects via activation of this receptor, previous studies have demonstrated that suppression of PPARγ activity can also promote macrophages to develop an immunotolerant phenotype (Zizzo and Cohen 2015; Weber et al. 2018a). It has also been shown that PPARγ knockout macrophages, when stimulated with LPS, initially show a significant reduction in IL-1β production early on at 3 hours of culturing, followed by a significant increase in IL-1β expression at the 24 hour mark (Heming et al. 2018). Thus, deviation from normal PPARγ activity can lead to both a suppression and an enhancement in the expression of the pro-inflammatory cytokine IL-1β with the immune environment playing a major role. Additionally, we tested if other anti-inflammatory effects of CBD on macrophages, such as the suppression of endocytosis, can potentially be attributed to the inhibition of PPARγ. To test this, we measured endocytosis of pHrodo™ green dextran beads by CBD-treated peritoneal macrophages in the presence or absence of T007. Our results demonstrated that CBD significantly reduced the endocytosis activity of peritoneal macrophages, while T007 given at the given concentration capable of inhibiting IL-1β expression had no effect on suppressing endocytosis or reversing the effects of CBD on endocytosis, thereby showing that the inhibition of endocytosis activity by CBD was independent of PPARγ.
To further study how CBD exerts potent immunosuppression under encephalitic conditions when supplied orally, we investigated any changes in the GI tract by focusing on the draining MLNs of the GI tract and the IECs lining the colonic epithelium. Flow cytometry revealed that the MLNs of CBD treated EAE mice show a decrease in the proportions of neutrophils when compared to the MLNs from vehicle treated EAE mice, with no changes in the anti-inflammatory MDSCs being observed. To dissect what was occurring in the intestinal lining to potentially contribute to this reduction of neutrophils, we conducted transcriptome arrays that quantified the abundance of mRNA transcripts within the RNA content isolated from IECs. This array-based analysis demonstrated that the 3 most highly regulated genes between the vehicle- and CBD-treated mice (GSDMC2, GSDMC3, and GSDMC4) belong to a family of pyroptosis initiators known to instigate inflammatory processes in a variety of myeloid cells, and in neutrophils (Ding et al. 2016; Broz et al. 2019). To confirm that CBD treatment alleviated the low-grade inflammation observed within the GI tract of EAE mice, we next investigated the morphology of the colonic epithelium and underlying tissue with H&E histology. This histology demonstrated that the CBD treatment group contained fewer cellular infiltrates, demonstrating an alleviation of the state of intestinal inflammation that resembles low-grade neutrophil-driven cryptitis as observed in the underlying colonic tissue of vehicle treated mice (Greenson et al. 1997; Erben et al. 2014). Collectively, these results demonstrated that orally administered CBD alleviates the systemic low-grade intestinal inflammation that occurs in models of autoimmune neuroinflammation by inhibiting neutrophil activity and regulating the expression of GSDMC within the epithelium of the intestinal lining (Fournier and Parkos 2012; Nouri et al. 2014; Miyauchi et al. 2020). The relevance of intestinal inflammation following cannabinoid treatment in EAE has already been demonstrated, as the anti-inflammatory properties of cannabinoids are at least partially dependent on the control of the gut-brain axis by regulating intestinal immunity and regulating microbial dysbiosis (Mestre et al. 2018; Al-Ghezi et al. 2019).
Previous studies have shown that gut microbiota composition and associated metabolome regulates the severity of EAE and associated neuroinflammation (Miyake et al. 2015; Dopkins et al. 2018). To that end, we tested if oral administration of CBD would alter the gut microbiota to create an anti-inflammatory effect stemming from the gut microbiota. Our results demonstrated that the GI contents of mice that were treated with oral CBD administration during the entirety of the EAE model displayed no significant changes in the alpha diversity, beta diversity or phylogeny of the detected bacterial species within the fecal contents prior to EAE induction or within the cecal contents at day 17 post-induction of EAE. Collectively, these results demonstrated that there were no significant changes in the bacterial composition of the EAE mice treated with CBD down to the genus level (Supplemental Figure 6). Additionally, to detect if the reduction of neuroinflammation driven paralysis was potentially due to changes in the metabolomic profile of the GI microbiota we quantified the concentrations of SCFAs within the cecal contents of EAE mice, which also demonstrated no significant changes between the vehicle treated and CBD treated group. Collectively, these results suggested that oral CBD treatment during EAE induces an anti-inflammatory state in the CNS and the gut, but this occurs independent of inducing changes in the GI microbiota composition.
Collectively, the study demonstrates that CBD treatment in a murine model of experimental MS results in a significant amelioration of paralysis symptoms accredited to a reduction in inflammation within the CNS, as well as systemically within the secondary lymphoid organs and GI tract. Mechanistically, CBD may limit excessive neuroinflammation by suppressing the pathogenic infiltration of macrophages and inhibiting the expression of pro-inflammatory chemo-attractants such as CXCL9, CXCL10 and IL-1β. This specified regulation of excessive inflammation was identified in key tissues such as the CNS, lymph nodes, spleen, and intestinal lining. In addition to affecting the inflammatory macrophages, oral administration of CBD was also able to induce MDSCs.
While CBD has become a controversial therapeutic treatment for autoimmune disorders, we feel that studies like these are of the upmost importance to better demonstrate the benefits of cannabinoids in treating incurable autoimmune inflammation. CBD is a relatively cheap and readily available compound that can immediately be used to better the lives of individuals afflicted with debilitating autoimmune neuroinflammation who have exhausted all previously available therapeutic possibilities due to financial barriers and developed resistances to anti-inflammatory agents. We believe that studies such as these that solidify the benefits of cannabinoids is of the utmost importance when considering the treatment MS and other immunological disorders.
Supplementary Material
Acknowledgments
This work was supported in part by NIH grants P01AT003961, P20GM103641, R01ES030144, R01AI129788 and R01AI123947. The graphical abstract for this manuscript was created using Biorender.com
Footnotes
Declarations
Conflicts of Interest We the authors declare that the research was conducted in the absence of any potential commercial or financial relationships that can be construed as a potential conflict of interest.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s11481-021-10023-6.
Data Availability
The datasets presented in this study can be found in online repositories hosted via https://www.ncbi.nlm.nih.gov/ under the bioproject numbers PRJNA745189 (16s) and PRJNA (scRNA Seq).
References
- Ahmed W, Katz S (2016) Therapeutic Use of Cannabis in Inflammatory Bowel Disease. Gastroenterol Hepatol 12:668–679 [PMC free article] [PubMed] [Google Scholar]
- Al-Ghezi ZZ, Miranda K, Nagarkatti M, Nagarkatti PS (2019) Combination of Cannabinoids, Δ9-Tetrahydrocannabinol and Cannabidiol, Ameliorates Experimental Multiple Sclerosis by Suppressing Neuroinflammation Through Regulation of miRNA-Mediated Signaling Pathways. Front Immunol 10:1921. 10.3389/fimmu.2019.01921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranami T, Yamamura T (2008) Th17 Cells and Autoimmune Encephalomyelitis (EAE/MS). Allergol Int 57:115–120. 10.2332/allergolint.R-07-159 [DOI] [PubMed] [Google Scholar]
- Becker W, Alrafas HR, Busbee PB et al. (2020a) Cannabinoid Receptor Activation on Haematopoietic Cells and Enterocytes Protects against Colitis. J Crohns Colitis jjaa253. 10.1093/ecco-jcc/jjaa253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker W, Alrafas HR, Wilson K et al. (2020b) Activation of Cannabinoid Receptor 2 Prevents Colitis-Associated Colon Cancer through Myeloid Cell De-activation Upstream of IL-22 Production. iScience 23:101504. 10.1016/j.isci.2020.101504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Hand TW (2014) Role of the microbiota in immunity and inflammation. Cell 157:121–141. 10.1016/j.cell.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellnier T, Brown GW, Ortega TR (2018) Preliminary evaluation of the efficacy, safety, and costs associated with the treatment of chronic pain with medical cannabis. Ment Health Clin 8:110–115. 10.9740/mhc.2018.05.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolyen E, Rideout JR, Dillon MR et al. (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. 10.1038/s41587-019-0209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boziki MK, Kesidou E, Theotokis P et al. (2020) Microbiome in Multiple Sclerosis; Where Are We, What We Know and Do Not Know. Brain Sci. 10.3390/brainsci10040234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz P, Pelegrín P, Shao F (2019) The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 10.1038/s41577-019-0228-2 [DOI] [PubMed] [Google Scholar]
- Bruni N, Della Pepa C, Oliaro-Bosso S et al. (2018) Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Mol Basel Switz. 10.3390/molecules23102478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A, Hoffman P, Smibert P et al. (2018) Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol 36:411–420. 10.1038/nbt.4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu CS, Farooqi N, O’Brien K, Gran B (2011) Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol 164:1079–1106. 10.1111/j.1476-5381.2011.01302.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey-Bloom J, Wolfson T, Gamst A et al. (2012) Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ Can Med Assoc J J Assoc Medicale Can 184:1143–1150. 10.1503/cmaj.110837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps JG, Gorham JD (2011) MDSC in autoimmunity. Int Immunopharmacol 11:789–793. 10.1016/j.intimp.2011.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook KR, Liu P (2014) Role of myeloid-derived suppressor cells in autoimmune disease. World J Immunol 4:26–33. 10.5411/wji.v4.i1.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargahi N, Katsara M, Tselios T et al. (2017) Multiple Sclerosis: Immunopathology and Treatment Update. Brain Sci. 10.3390/brainsci7070078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Wang K, Liu W et al. (2016) Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535:111–116. 10.1038/nature18590 [DOI] [PubMed] [Google Scholar]
- Dopkins N, Becker W, Miranda K et al. (2020) Tryptamine Attenuates Experimental Multiple Sclerosis Through Activation of Aryl Hydrocarbon Receptor. Front Pharmacol 11:619265. 10.3389/fphar.2020.619265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopkins N, Nagarkatti PS, Nagarkatti M (2018) The role of gut microbiome and associated metabolome in the regulation of neuroinflammation in multiple sclerosis and its implications in attenuating chronic inflammation in other inflammatory and autoimmune disorders. Immunology 154:178–185. 10.1111/imm.12903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott DM, Singh N, Nagarkatti M, Nagarkatti PS (2018) Cannabidiol Attenuates Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis Through Induction of Myeloid-Derived Suppressor Cells. Front Immunol 9:1782. 10.3389/fimmu.2018.01782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erben U, Loddenkemper C, Doerfel K et al. (2014) A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol 7:4557–4576 [PMC free article] [PubMed] [Google Scholar]
- Fajas L, Auboeuf D, Raspé E et al. (1997) The Organization, Promoter Analysis, and Expression of the Human PPARγ Gene. J Biol Chem 272:18779–18789. 10.1074/jbc.272.30.18779 [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Lalor SJ, Sweeney CM et al. (2010) T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol 162:1–11. 10.1111/j.1365-2249.2010.04143.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier BM, Parkos CA (2012) The role of neutrophils during intestinal inflammation. Mucosal Immunol 5:354–366. 10.1038/mi.2012.24 [DOI] [PubMed] [Google Scholar]
- Gérikas Ribeiro C, Lopes dos Santos A, Marie D et al. (2016) Pico and nanoplankton abundance and carbon stocks along the Brazilian Bight. PeerJ 4:e2587. 10.7717/peerj.2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi N, Razavi S, Nikzad E (2017) Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J 19:1–10. 10.22074/cellj.2016.4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg MM (2012) Multiple sclerosis review. P T Peer-Rev J Formul Manag 37:175–184 [PMC free article] [PubMed] [Google Scholar]
- Greenson JK, Stern RA, Carpenter SL, Barnett JL (1997) The clinical significance of focal active colitis. Hum Pathol 28:729–733. 10.1016/s0046-8177(97)90183-0 [DOI] [PubMed] [Google Scholar]
- Grygiel-Górniak B (2014) Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications–a review. Nutr J 13:17. 10.1186/1475-2891-13-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung DM, Bourdette DN, Ahmed SM, Whitham RH (2015) The cost of multiple sclerosis drugs in the US and the pharmaceutical industry: Too big to fail? Neurology 84:2185–2192. 10.1212/WNL.0000000000001608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde VL, Nagarkatti PS, Nagarkatti M (2011) Role of myeloid-derived suppressor cells in amelioration of experimental autoimmune hepatitis following activation of TRPV1 receptors by cannabidiol. PloS One 6:e18281. 10.1371/journal.pone.0018281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heming M, Gran S, Jauch S-L et al. (2018) Peroxisome Proliferator-Activated Receptor-γ Modulates the Response of Macrophages to Lipopolysaccharide and Glucocorticoids. Front Immunol 9:893. 10.3389/fimmu.2018.00893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Filiano AJ, Smith A et al. (2017) Myeloid Cells in the Central Nervous System. Immunity 46:943–956. 10.1016/j.immuni.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W-J, Chen W-W, Zhang X (2017) Multiple sclerosis: Pathology, diagnosis and treatments. Exp Ther Med 13:3163–3166. 10.3892/etm.2017.4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou M, Alissafi T, Lazaridis I et al. (2012) Crucial Role of Granulocytic Myeloid-Derived Suppressor Cells in the Regulation of Central Nervous System Autoimmune Disease. J Immunol 188:1136–1146. 10.4049/jimmunol.1101816 [DOI] [PubMed] [Google Scholar]
- Kadowaki A, Quintana FJ (2020) The Gut-CNS Axis in Multiple Sclerosis. Trends Neurosci 43:622–634. 10.1016/j.tins.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leweke FM, Piomelli D, Pahlisch F et al. (2012) Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2:e94. 10.1038/tp.2012.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H-D, Chen X, Yang Y et al. (2017) Wogonin attenuates inflammation by activating PPAR-γ in alcoholic liver disease. Int Immunopharmacol 50:95–106. 10.1016/j.intimp.2017.06.013 [DOI] [PubMed] [Google Scholar]
- Lublin F, Miller DH, Freedman MS et al. (2016) Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Lond Engl 387:1075–1084. 10.1016/S0140-6736(15)01314-8 [DOI] [PubMed] [Google Scholar]
- Maroon J, Bost J (2018) Review of the neurological benefits of phytocannabinoids. Surg Neurol Int 9:91. 10.4103/sni.sni_45_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurdie PJ, Holmes S (2013) phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS One 8:e61217. 10.1371/journal.pone.0061217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre L, Carrillo-Salinas FJ, Mecha M et al. (2018) Gut microbiota, cannabinoid system and neuroimmune interactions: New perspectives in multiple sclerosis. Biochem Pharmacol 157:51–66. 10.1016/j.bcp.2018.08.037 [DOI] [PubMed] [Google Scholar]
- Millar SA, Stone NL, Bellman ZD et al. (2019) A systematic review of cannabidiol dosing in clinical populations. Br J Clin Pharmacol 85:1888–1900. 10.1111/bcp.14038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda K, Yang X, Bam M et al. (2018) MicroRNA-30 modulates metabolic inflammation by regulating Notch signaling in adipose tissue macrophages. Int J Obes 42:1140–1150. 10.1038/s41366-018-0114-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake S, Kim S, Suda W et al. (2015) Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PloS One 10:e0137429. 10.1371/journal.pone.0137429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi E, Kim S-W, Suda W et al. (2020) Gut microorganisms act together to exacerbate inflammation in spinal cords. Nature 585:102–106. 10.1038/s41586-020-2634-9 [DOI] [PubMed] [Google Scholar]
- Mohammed A, Alghetaa HK, Zhou J et al. (2020a) Protective effects of Δ9 -tetrahydrocannabinol against enterotoxin-induced acute respiratory distress syndrome are mediated by modulation of microbiota. Br J Pharmacol 177:5078–5095. 10.1111/bph.15226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A, Alghetaa H FK, Miranda K et al. (2020b) Δ9-Tetrahydrocannabinol Prevents Mortality from Acute Respiratory Distress Syndrome through the Induction of Apoptosis in Immune Cells, Leading to Cytokine Storm Suppression. Int J Mol Sci. 10.3390/ijms21176244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarkatti P, Pandey R, Rieder SA et al. (2009) Cannabinoids as novel anti-inflammatory drugs. Future Med Chem 1:1333–1349. 10.4155/fmc.09.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7:27–31. 10.4103/0976-0105.177703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols JM, Kaplan BLF (2020) Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid Res 5:12–31. 10.1089/can.2018.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouri M, Bredberg A, Weström B, Lavasani S (2014) Intestinal barrier dysfunction develops at the onset of experimental autoimmune encephalomyelitis, and can be induced by adoptive transfer of auto-reactive T cells. PloS One 9:e106335. 10.1371/journal.pone.0106335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S, Sinha P (2009) Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol Baltim Md 182:4499–4506. 10.4049/jimmunol.0802740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan SE (2016) An update on PPAR activation by cannabinoids. Br J Pharmacol 173:1899–1910. 10.1111/bph.13497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli D, Manni A, Iaffaldano A, Trojano M (2020) Efficacy and Safety of Oral Therapies for Relapsing-Remitting Multiple Sclerosis. CNS Drugs. 10.1007/s40263-019-00691-7 [DOI] [PubMed] [Google Scholar]
- Paradis E, Claude J, Strimmer K (2004) APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics 20:289–290. 10.1093/bioinformatics/btg412 [DOI] [PubMed] [Google Scholar]
- Paré A, Mailhot B, Lévesque SA et al. (2018) IL-1β enables CNS access to CCR2 hi monocytes and the generation of pathogenic cells through GM-CSF released by CNS endothelial cells. Proc Natl Acad Sci 115:E1194–E1203. 10.1073/pnas.1714948115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich DS, Lucchinetti CF, Calabresi PA (2018) Multiple Sclerosis. N Engl J Med 378:169–180. 10.1056/NEJMra1401483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez Mesa XM, Moreno Vergara AF, Contreras Bolaños LA et al. (2021) Therapeutic Prospects of Cannabinoids in the Immunomodulation of Prevalent Autoimmune Diseases. Cannabis Cannabinoid Res. 10.1089/can.2020.0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse M, Singh NP, Nagarkatti PS, Nagarkatti M (2013) Indoles mitigate the development of experimental autoimmune encephalomyelitis by induction of reciprocal differentiation of regulatory T cells and Th17 cells. Br J Pharmacol 169:1305–1321. 10.1111/bph.12205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, Hegde VL, Hofseth LJ et al. (2007) Resveratrol (trans-3,5,4’-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol Pharmacol 72:1508–1521. 10.1124/mol.107.038984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart T, Butler A, Hoffman P et al. (2019) Comprehensive Integration of Single-Cell Data. Cell 177:1888–1902.e21. 10.1016/j.cell.2019.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umansky V, Blattner C, Gebhardt C, Utikal J (2016) The Role of Myeloid-Derived Suppressor Cells (MDSC) in Cancer Progression. Vaccines 4:E36. 10.3390/vaccines4040036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga T, Czimmerer Z, Nagy L (2011) PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta BBA - Mol Basis Dis 1812:1007–1022. 10.1016/j.bbadis.2011.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermersch P (2011) Sativex ® (tetrahydrocannabinol + cannabidiol), an endocannabinoid system modulator: basic features and main clinical data. Expert Rev Neurother 11:15–19. 10.1586/ern.11.27 [DOI] [PubMed] [Google Scholar]
- Wahli W, Michalik L (2012) PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol Metab 23:351–363. 10.1016/j.tem.2012.05.001 [DOI] [PubMed] [Google Scholar]
- Waldman A, Ghezzi A, Bar-Or A et al. (2014) Multiple sclerosis in children: an update on clinical diagnosis, therapeutic strategies, and research. Lancet Neurol 13:936–948. 10.1016/S1474-4422(14)70093-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KJ, Sauer M, He L et al. (2018) PPARγ Deficiency Suppresses the Release of IL-1β and IL-1α in Macrophages via a Type 1 IFN–Dependent Mechanism. J Immunol 201:2054–2069. 10.4049/jimmunol.1800224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber N, Liou D, Dommer J et al. (2018) Nephele: a cloud platform for simplified, standardized and reproducible microbiome data analysis. Bioinformatics 34:1411–1413. 10.1093/bioinformatics/btx617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H (2016) ggplot2: elegant graphics for data analysis, 2nd edn. Springer, Cham [Google Scholar]
- Yang X, Bam M, Nagarkatti PS, Nagarkatti M (2019) Cannabidiol Regulates Gene Expression in Encephalitogenic T cells Using Histone Methylation and noncoding RNA during Experimental Autoimmune Encephalomyelitis. Sci Rep 9:15780. 10.1038/s41598-019-52362-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasłona Z, Pålsson-McDermott EM, Menon D et al. (2017) The Induction of Pro–IL-1β by Lipopolysaccharide Requires Endogenous Prostaglandin E 2 Production. J Immunol 198:3558–3564. 10.4049/jimmunol.1602072 [DOI] [PubMed] [Google Scholar]
- Zhou J, Wilson KM, Medh JD (2002) Genetic analysis of four novel peroxisome proliferator activated receptor-gamma splice variants in monkey macrophages. Biochem Biophys Res Commun 293:274–283. 10.1016/S0006-291X(02)00138-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Bando Y, Xiao S et al. (2007) CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol Baltim Md 179:5228–5237. 10.4049/jimmunol.179.8.5228 [DOI] [PubMed] [Google Scholar]
- Zhu S, Jiang Y, Xu K et al. (2020) The progress of gut microbiome research related to brain disorders. J Neuroinflammation 17:25. 10.1186/s12974-020-1705-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizzo G, Cohen PL (2015) The PPAR-γ antagonist GW9662 elicits differentiation of M2c-like cells and upregulation of the MerTK/Gas6 axis: a key role for PPAR-γ in human macrophage polarization. J Inflamm 12:36. 10.1186/s12950-015-0081-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories hosted via https://www.ncbi.nlm.nih.gov/ under the bioproject numbers PRJNA745189 (16s) and PRJNA (scRNA Seq).


