Abstract
Simian immunodeficiency virus (SIV) infection of macaques is remarkably similar to that of human immunodeficiency virus type 1 (HIV-1) in humans, and the SIV-macaque system is a good model for AIDS research. We have constructed an SIV proviral DNA clone that is deleted of 97 nucleotides (nt), i.e., construct SD, at positions (+322 to +418) immediately downstream of the primer binding site (PBS) of SIVmac239. When this construct was transfected into COS-7 cells, the resultant viral progeny were severely impaired with regard to their ability to replicate in C8166 cells. Further deletion analysis showed that a virus termed SD1, containing a deletion of 23 nt (+322 to +344), was able to replicate with wild-type kinetics, while viruses containing deletions of 21 nt (+398 to +418) (construct SD2) or 53 nt (+345 to +397) (construct SD3) displayed diminished capacity in this regard. Both the SD2 and SD3 viruses were also impaired with regard to ability to package viral RNA, while SD1 viruses were not. The SD and SD3 constructs did not revert to increased replication ability in C8166 cells over 6 months in culture. In contrast, long-term passage of the SD2 mutated virus resulted in a restoration of replication capacity, due to the appearance of four separate point mutations. Two of these substitutions were located in leader sequences of viral RNA within the PBS and the dimerization initiation site (DIS), while the other two were located within two distinct Gag proteins, i.e., CA and p6. The biological relevance of three of these point mutations was confirmed by site-directed mutagenesis studies that showed that SD2 viruses containing each of these substitutions had regained a significant degree of viral replication capacity. Thus, leader sequences downstream of the PBS, especially the U5-leader stem and the DIS stem-loop, are important for SIV replication and for packaging of the viral genome.
Simian immunodeficiency virus (SIV) and human immunodeficiency virus type 1 (HIV-1) belong to the primate lentivirus subfamily of retroviruses. They both possess at least six auxiliary genes and are considered complex retroviruses (7, 8). SIV can induce an AIDS-like disease in certain monkeys, such as rhesus macaques, and is an excellent animal model for the study of human HIV disease (15). The 5′ untranslated leader sequences of HIV possess a number of functional domains, including elements for transactivation of transcription, initiation of reverse transcription, packaging of viral RNA, and integration of the proviral genome (5, 6, 11, 12, 17, 31). A 54-nucleotide (nt) leader sequence in HIV-1, located downstream of the primer binding site (PBS) and upstream of the dimerization initiation site (DIS), has been shown to be involved in efficient HIV-1 gene expression and virus replication (16, 18, 20). SIVmac239 has 97-nt sequences in this region, which is therefore much longer than that of HIV-1 (29). The 5′ untranslated leader sequence of the SIV RNA genome has little sequence similarity with that of HIV-1, but similar secondary structures have been predicted (30). SIV also shows certain unique features in the leader sequence, such as an intron located in the 5′ R-U5 region and an internal ribosome entry site found in the SIV leader sequence but not in HIV-1 (26, 32). We conducted studies to determine the role of the region located downstream of the PBS and upstream of the DIS in SIV, an area that is not well understood.
Using mutational analysis, we show that SIV mutants containing a 97-nt deletion of these leader sequences is severely impaired with regard to both viral replication and packaging of viral RNA. A 23-nt sequence within the 5′ portion of this 97-nt region had only minor effects on viral genomic RNA packaging and SIV replication in C8166 cells. However, the remaining 74 nt within this region played a significant role in viral genomic RNA packaging and replication in the aforementioned cell line.
(Research performed by James B. Whitney was in partial fulfillment of the Ph.D. degree, Faculty of Graduate Studies and Research, McGill University, Montreal, Canada.)
MATERIALS AND METHODS
Construction of deletion mutations.
The two half-genome plasmids of SIVmac239, molecular clones p239SpSp5′ and p239SpE3′, were obtained through the AIDS Research and Reference Reagent Program (28). Nucleotide designations for SIVmac 239 are based on the published sequence; the transcription initiation site corresponds to +1. Table 1 shows the primers used in our experiments. To obtain the full-length clone, the 5′ cellular sequence was replaced with an EcoRI site, and the 3′ cellular sequence was replaced with a XhoI site by PCR-based methodology, using primers pSU3 and pSPBS and primers pSU5-1 and pSenf. A full-length clone was constructed by inserting the ligation product of the 5′ EcoRI-SphI fragment and the 3′ SphI-XhoI fragment into the EcoRI-XhoI site of a pSP73 vector. Deletion mutants were then constructed based on this full-length infectious clone, termed SIV-WT. We used PCR-based mutagenesis methods to generate deletions downstream of the PBS. Pfu polymerase was used to increase the reliability of the PCR. All constructs were confirmed by sequencing. Figure 1 presents a graphic description of the mutants generated in regard to both the sequence and the tertiary structure. Briefly, the region between the NarI and BamHI sites in SIV-WT was replaced by PCR fragments to generate mutant constructs (primers pSD and pSgag1 were used for SD deletion, and primers pSD1 and pSgag1 were used for SD1 deletion). For construction of SD2 and SD3 deletion, PCR fragments (pSD2 and pSgag1 for SD2, pSD3 and pSgag1 for SD3) were purified and were then used as a mega-primer paired with primer pSU5 to generate PCR fragments to replace the region between NarI and BamHI sites in SIV-WT.
TABLE 1.
Primer utilized in these experiments
| Name | Sequence | Locationa |
|---|---|---|
| pSU3 | 5′-GCGGAATTCTGGAAGGGATTTATTACAGTG-3′ | −518 to −498 |
| pSU5 | 5′-AAGCTAGTGTGTGTTCCCATCTC-3′ | +175 to +197 |
| pSgag1 | 5′-GCAACCCCAGTTGGATCCATCTCCTGT-3′ | +1348 to +1322 |
| PSD | 5′-TGGCGCCTGAACAGGGAC/GGTACCAGACGGCGTGAGG-3′ | +304 to +321/+419 to +437 |
| pSD1 | 5′-TGGCGCCTGAACAGGGAC/GAGTACGGCTGAGTGAAG-3′ | +304 to +321/+345 to +362 |
| pSD2 | 5′-GGAACCAACCACGACGGAGT/GGTACCAGACGGCGTGAGG-3′ | +378 to +397/+419 to +437 |
| pSD3 | 5′-GAAGGAGAGTGAGAGACTCCT/GCTCCTATAAAGGCGCGG-3′ | +324 to +344/+398 to +415 |
| PSPBS | 5′-CCTGTTCAGGCGCCAATCTG-3′ | +318 to +299 |
| pSPBS-1 | 5′-TGGCGCCTGAACAGGGAC-3′ | +304 to +321 |
| Sg | 5′-CTTCCCTGACAAGACGGAG-3′ | +567 to +549 |
| sg1 | 5′-GAAGCATGTAGTATGGGCAG-3′ | +627 to +646 |
| sg2 | 5′-GGCACTAATGGAGCTAAGACCG-3′ | +746 to +725 |
| PSenf | 5′-GGCTTGAGCTCACTCTCTTGTGAG-3′ | +8705 to +8728 |
| pSU5-1 | 5′-GGGCTCGAGTGCTAGGGATTTTCCTGC-3′ | +301 to +284 |
Location of the primer is relative to the transcription initiation site (+1).
FIG. 1.
Deletion mutations and RNA secondary structure. (A) Deletions are located between the arrows, and their positions are shown relative to the transcription initiation site. (B) Secondary structure of SIVmac239 leader RNA model was predicted by free-energy minimization (33, 34) and was adapted from published structures (1, 30). All hairpin motifs are named after their putative function or after similar elements encoded by HIV-1. The following sequence motifs are noted: the polyadenylation signal at position 153, the PBS at position 303, the DIS palindrome at position 419, and the Gag start codon at position 534. The splice donor and acceptor sites in the R-U5 region (positions 60 and 204) are marked by a dotted arrow, while the major splice donor site at position 466 is marked by a solid arrow. The positions of deletion constructs are shown above the structure.
Cells and preparation of virus stocks.
COS-7 cells were maintained in Dulbecco modified Eagle medium supplemented with 10% heat-inactivated fetal bovine serum. C8166 cells were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum. All media and sera were from GIBCO (Burlington, Ontario, Canada). Molecular constructs were purified using a Maxi Plasmid Kit (Qiagen, Inc. Mississauga, Ontario, Canada). COS-7 cells were transfected using these constructs with Lipofectamine-Plus reagent (GIBCO). Virus containing supernatant was harvested at 60 h after transfection and was clarified by centrifugation for 10 min at 4°C at 3,000 rpm in a Beckman GS-6R centrifuge. Viral stocks were stored in 0.5- or 1-ml aliquots at −70°C. The concentration of p27 antigen in these stocks was quantified using a Coulter SIV core antigen assay kit (Immunotech, Inc., Westbrook, Maine).
Virus replication in C8166 cells.
Viral stocks were thawed and treated with 100 U of DNase I in the presence of 10 mM MgCl2 at 37°C for 1 h to eliminate any residual contaminating plasmids from the transfection. Infection of C8166 cells was performed by incubating 106 cells at 37°C for 2 h with an amount of virus equivalent to 10 ng of p27 antigen. Infected cells were then washed twice with phosphate-buffered saline and incubated with fresh medium. Cells were split at a 1:3 ratio twice per week if they had grown to a sufficient level; otherwise the culture fluid was replaced with fresh medium. Supernatants were monitored for virus production by both reverse transcriptase (RT) assay and SIV core antigen capture assay (Immunotech).
Detection of viral DNA.
At various times postinfection, C8166 cells were collected and washed with phosphate-buffered saline. To ensure that no contaminating plasmid remained, fluid from the wash was routinely checked by PCR using SIV-specific primers. Cellular DNA was isolated using a QIAamp DNA Mini Kit (Qiagen). DNA samples were analyzed by PCR using primers the pSPBS-1 and Sg to amplify the deletion region between the PBS and the major splice donor site of SIV. PCR assays were performed with 0.1 to 1 μg of sample DNA, 50 mM Tris-HCl (pH 8.0), 50 mM KCl, 1.5 mM MgCl2, 2.5 U of Taq polymerase, 0.2 mM concentrations of deoxynucleoside triphosphates (dNTPs) 10 pmol of 32P-end-labeled reverse primer, and 20 pmol of unlabeled forward primer and then programmed as follows: 95°C at 3 min and 25 cycles at 94°C for 30 s, 55°C for 30 s, 72°C for 1 min, and 72°C for 10 min. Reactions were standardized by a simultaneous amplification of a 567-bp DNA fragment of human β-actin gene as an internal control. Products were separated through 5% native polyacrylamide gels. Products derived from PCR using unlabeled primers were separated in agarose gels and extracted using a Qiaex II Gel Extraction Kit (Qiagen). The purified DNA was used as template to confirm deletion mutations via sequencing.
Detection of viral proteins produced by transfected COS-7 cells.
Expression of viral proteins by transfected COS-7 cells was determined using a Coulter SIV core antigen assay and a Western blot. For the Western blot, nascent extracellular virions were precipitated by ultracentrifugation and used as protein samples. Western blotting was performed using SIVmac 251 antiserum according to a standard protocol (23).
Detection of RNA in virions by RT-PCR.
To study packaging of viral genomic RNA, viral RNA was isolated using the QIAamp viral RNA Mini Kit (Qiagen) from equivalent amounts of COS-7 cell-derived viral preparations based on levels of SIV p27 antigen. RNA samples were treated with RNase-free DNase I at 37°C for 30 min to eliminate possible DNA contamination. DNase I was then inactivated by incubation at 75°C for 10 min. The viral RNA samples were quantified by RT-PCR, using the Titan One Tube RT-PCR system (Boehringer Mannheim, Montreal, Quebec, Canada). The primer pairs sg1 and sg2 were used to amplify a 114-bp fragment representing full-length viral genome. The primer sg2 was radioactively labeled in order to visualize PCR products. Equivalent RNA samples, based on p27 antigen levels, were used as templates in an 18-cycle RT-PCR. The products were fractionated on 5% polyacrylamide gels and exposed to X-ray film. Relative amounts of products were quantified by molecular imaging (Bio-Rad Imaging). Levels of genomic packaging were calculated on the basis of four different reactions, with wild-type virus levels arbitrarily set at 1.0.
Site-directed mutagenesis.
For the introduction of point mutations into the SD2 genome, the fragment between the BamHI and SphI sites was subcloned into the pSP73 vector to generate a clone termed pSIV-BSp, and the fragment between the EcoRI and BamHI sites was subcloned into the pSP73 vector to generate the clone termed pSIV-EB-SD2. The QuikChange site-directed mutagensis kit (Stratagene, La Jolla, Calif.) was used to introduce the M2, CA1, and Mp6 point mutations into SD2 DNA by procedures that have been previously described (21) and utilizing the following primer pairs, i.e., M2-1 (5′-CCAACCACGACGGAGTGGTGCCAGACGGCGTGAGG-3′) and M2-2 (5′-CCTCACGCCGTCTGGCACCACTCCGTCGTGGTTGG-3′) for M2, CA1-1 (5′-GCTAACCCAGATTGCAGGCTAGTGCTGAAGGG-3′) and CA1-2 (5′-CCCTTCAGCACTAGCCTGCAATCTGGGTTAGC-3′) for CA1, and Mp6-1 (5′-GCCTTACAAGGAGGTGACAAAGGATTTGCTGCACCTC-3′) and Mp6-2 (5′-GAGGTGCAGCAAATCCTTTGTCACCTCCTTGTAAGGC-3′) for Mp6. The EcoRI-BamHI fragment was cloned back into the SD2 genome to generate the SD2-M2 clone; the BamHI-SphI fragment was cloned into the SD2 genome to generate both the SD2-CA1 and SD2-Mp6 clones. To generate the M1 mutation, the fragment which was produced by PCR using primers PBS-M1 (5′-TGGCGCCCGAACAGGGACTTG-3′) and pSgag1 (based on the SD2 template, see above) was inserted into the SD2 genome between the NarI and BamHI sites to yield the SD2-M1 clone. The presence of all point mutations was confirmed by direct sequencing.
RESULTS
Sequences downstream of the PBS are important for SIV replication in C8166 cells.
To investigate the role of leader sequences located downstream of the PBS in SIVmac239, we constructed deletion mutations in this region (Fig. 1). First, a 97-nt (positions +322 to +418) deletion was introduced into the region immediately downstream of the PBS, i.e., construct SD; this construct abolished both the putative U5-leader stem and the DIS stem-loop. Alternatively, three subdeletions within this 97-nt region were generated, termed SD1 (+322 to +344), SD2 (+398 to +418), and SD3 (+345 to +397), respectively. SD1 retains a stable U5-leader stem but is deleted of the small stem-loop within the U5-leader stem. SD2 is deleted of the left side half of the DIS stem-loop. Finally, SD3 retains the DIS stem-loop but is deleted of the U5-leader stem (Fig. 1).
To investigate the replicative potential of these constructs, the viral stock was thawed and treated with DNase I to eliminate any possible contaminating plasmids. Viruses containing 10 ng of p27 antigen were used to infect C8166 cells, and culture fluids were monitored for virus replication by RT assay and by SIV p27 antigen capture assay. Figure 2 shows that each of the SD, SD2, and SD3 deletion mutants were significantly impaired in their ability to replicate in C8166 cells, while wild-type virus and one of the deletion mutants (SD1) replicated efficiently, as determined by levels of RT activity in culture fluids. The data in Table 2 also show that the SD1 construct yielded levels of p27 antigen similar to those of wild-type virus, while the SD, SD2, and SD3 deletion constructs were severely impaired in this regard.
FIG. 2.
Growth curves of mutated viruses in C8166 cells. Equivalent amounts of virus from COS-7 transfected cells were used to infect C8166 cells based on levels of p27 antigen (10 ng/106 cells). Viral replication was monitored by RT assay of culture fluids. Mock infection denotes exposure of cells to heat-inactivated wild-type virus as a negative control.
TABLE 2.
Levels of p27 antigen expression in C8166 cellsa
| Viral construct | p27 concn (ng/ml) at day:
|
||||
|---|---|---|---|---|---|
| 7 | 10 | 14 | 17 | 28 | |
| Wild type | 27.75 | 126.8 | 211.8 | ND | ND |
| SD | 0.20 | 0.12 | 0.17 | 0.17 | 0.05 |
| SD1 | 29.05 | 125.0 | 179.2 | 151.4 | ND |
| SD2 | 0.22 | 0.25 | 0.18 | 0.32 | 1.7 |
| SD3 | 0.14 | 0.24 | 0.18 | ND | 0.06 |
C8166 cells were infected with various viral constructs (10 ng), and p27 levels in culture fluids were measured using an SIV p27 antigen capture assay. ND, not detected.
We also measured levels of proviral DNA in these studies by PCR. The sequencing of PCR products indicated that the deletions were retained, even after replication over several passages (results not shown). Figure 3A shows the PCR results of samples at 7 days postinfection, confirming that these deleted viruses were indeed able to infect C8166 cells but that the levels of proviral genomic DNA with regard to the SD, SD2, and SD3 viruses were diminished relative to the wild-type virus (Fig. 3B).
FIG. 3.
Detection of viral DNA. (A) Viruses derived from COS-7 cells were standardized on the basis of p27 and used to infect C8166 cells. Total cellular DNA was isolated from infected cells at 7 days after infection and subjected to PCR analysis with primers pSPBS-1 and sg, that specifically amplify an SIV cDNA fragment between the PBS and a site downstream of the DIS. The size of the PCR products vary based on the type of construct used and are 264 bp for wild-type virus (lane 1), 241 bp for the SD1 deletion virus (lane 2), 243 bp for the SD2 deletion virus (lane 3), 211 bp for the SD3 deletion virus (lane 4), and 167 bp for the SD construct (lane 5). Primers amplifying a 587-bp fragment of β-actin were used as an internal control. Mock infection was done by inoculation of cells with heat-inactivated viruses (lane 6). A DNA marker of a 100-bp ladder is also shown (lane 7). (B) The intensity of each band was quantified by molecular imaging, and the band intensity of viral DNA relative to cellular DNA for each sample is shown.
The deletion mutations affect the packaging of viral genomic RNA.
To investigate the potential mechanisms whereby virus replication was compromised, we determined the levels of virus production by transfected COS-7 cells. Levels of extracellular SIV p27 antigen were quantified using the SIV p27 antigen capture assay. The results show that similar amounts of p27 were produced in each case (Table 3). We next analyzed viral proteins by Western blotting, and the results also show that no significant differences were present with regard to viral protein production (Fig. 4).
TABLE 3.
Levels of p27 antigen expressed by transfected COS-7 cells
| Virus | p27 concn (ng/ml) |
|---|---|
| Wild-type | 46.8 ± 12.1 |
| SD | 46.1 ± 13.8 |
| SD1 | 46.3 ± 13.5 |
| SD2 | 43.1 ± 13.8 |
| SD3 | 42.4 ± 13.8 |
FIG. 4.
Western blot to detect viruses derived from COS-7 cells. Viruses were pelleted by ultracentrifugation at 60 h after transfection, and viral proteins were detected by using SIV-positive serum. The band indicating the p27 protein is indicated by the arrow.
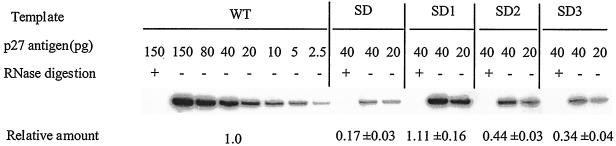
To determine the efficiency of packaging of the viral genome, RNA samples were isolated from equivalent amounts of SIV virus, based on p27 levels. A 114-bp fragment that represents the full-length, unspliced RNA genome was amplified and quantified by RT-PCR. The results of Fig. 5 show that the SD1 deletion had no effect on the encapsidation of viral RNA, while the SD, SD2, and SD3 constructs resulted in diminution of RNA packaging by about six-, two-, and threefold, respectively. Therefore, sequences in each of SD2 and SD3 are likely involved in the packaging of the viral genome, while those in SD1 are not.
FIG. 5.
Viral RNA packaging in wild-type and mutated viruses. Viral RNA was purified from virus stock derived from transfected COS-7 cells. Equivalent amounts of virus based on levels of p27 antigen were used as a template. Quantitative RT-PCR was performed to detect full-length viral RNA genome in an 18-cycle PCR reaction. Relative amounts of a 114-bp DNA product were quantified by molecular imaging, with wild-type levels arbitrarily set at 1.0. Reactions run with RNA template, digested by DNase-free RNase, served as a negative control for each sample to exclude any potential DNA contamination. Relative amounts of viral RNA that were packaged were determined on the basis of four different experiments.
Long-term culture results in reversion of SD2 viruses.
To investigate the possibility of reversion, we cultured the infected cells over longer periods and did not find any sign of reversion of the SD and SD3 constructs at over 6 months of passage. In contrast, modest amounts of RT activity in cultures infected by the SD2 viruses were present after 6 weeks. The supernatant fluids of the SD2 infection were then used to infect new C8166 cells, and viral culture fluids at peak levels of RT activity were again passaged onto new C8166 cells. After four passages (18 weeks), viral replication capacity was now similar to that of wild-type viruses (Fig. 6). Proviral DNA of these reverted viruses was detected by PCR, and the region from the 5′ long terminal repeat to the end of the gag gene was cloned. Six of these clones were sequenced, and the results showed that the original deletion had been retained in each case but that four additional point mutations were also present. These four point mutations were located within the PBS (termed M1), the putative DIS loop (termed M2), the capsid protein (termed CA1), and the p6 protein (termed Mp6) of the gag gene (Fig. 7). Each of these mutations is novel with the exception of M1, which has been observed in sequences of some wild-type viruses. The CA1 mutation involved a change of Lys-197 to Arg, while the Mp6 substitution results in a change from Glu-49 to Lys. Neither the M1 nor the M2 mutations involve amino acid substitutions, since both are located in noncoding areas of the viral genome. The M1 mutation (thymidine [T] to cytidine [C] at position 310) resulted in an alteration of the PBS, such that complementarity now existed with the 3′ end of tRNA3Lys instead of the original tRNA5Lys (31). The M2 substitution involved a change from adenosine (A) to guanosine (G) at position 423, which is located in the loop of the putative DIS stem-loop structure. RNA secondary structure analysis suggests that this point mutation cannot restore the destroyed DIS stem-loop structure in SD2 (data not shown).
FIG. 6.
Reversion of the SD2 mutant after long term culture in C8166 cells. (A) Growth curves of viruses in long-term culture. Equivalent amounts of virus from COS-7 transfected cells were used to infect C8166 cells based on levels of p27 antigen (10 ng/106 cells). Infected cells were cultured over protracted periods, and culture fluids were monitored by RT assay. Mock infection denotes exposure of cells to heat-inactivated wild-type virus as a negative control. (B) Growth curves of reverted SD2 viruses in C8166 cells. The SD2 virus at 42 days after the initial infection was passaged in fresh C8166 cells. Growth curves of the first and fourth passages of the SD2 viruses are shown.
FIG. 7.
Locations of the point mutations M1, M2, CA1, and Mp6 within the SIV genome, as indicated by asterisks. The substitutions observed are as follows: M1, T-+310 to C within the PBS; M2, A-+423 to G within the loop of the DIS; CA1, Lys-197 to Arg within CA; and Mp6, Glu-49 to Lys within p6. Letters in boldface indicate the original bases and amino acids, as well as the mutations. The PBS and the putative DIS are also indicated. Sequences that were deleted in SD2 are underlined.
In order to pursue the biological relevance of these various substitutions. we performed site-directed mutagenesis to introduce each of these four point mutations into the SD2 genome. The resultant DNA clones termed SD2-M1, SD2-M2, SD2-CA1, and SD2-Mp6 were then transfected into COS-7 cells, and the virus particles thereby recovered were assayed for viral replication capacity in C8166 cells. The results (Fig. 8) show that each of the constructs tested, except for SD2-M1, was able to replicate more efficiently than SD2 in the C8166 cell line, although not as efficiently as the wild-type virus. Thus, each of the M2, CA1, and Mp6 point mutations was able to partially compensate for the SD2 deletion, whereas the M1 substitution could not.
FIG. 8.
Growth curves of reverted viruses in C8166 cells. Equivalent amounts of virus from COS-7 transfected cells were used to infect C8166 cells based on levels of p27 antigen (10 ng/106 cells). Viral replication was monitored by RT assay of culture fluids. Mock infection denotes exposure of cells to heat-inactivated wild-type virus as a negative control.
DISCUSSION
Previous work has shown that leader sequences downstream of the PBS are important for HIV-1 gene expression and replication, but little about this subject is known with regard to SIV. In the present work, we have investigated this subject by constructing a series of mutated SIV clones containing deletions within a 97-nt region immediate downstream of the PBS. The results show that mutants containing deletions in the entire 97-nt region, as well as two subregions, were significantly impaired with respect to replication capacity in C8166 cells. A potential mechanism that may affect viral replication capacity in this context is that these sequences appear to be important for the packaging of the viral RNA genome. These results imply that both the U5-leader stem and the DIS stem-loop structures are important for SIV replication and for packaging of viral genomic RNA.
Packaging determinants have not been completely described for any lentivirus, but interactions of multiple regions that are distributed widely within the HIV-1 genome have been proposed (3). The encapsidation of the HIV-1 viral genome is dependent on cis-acting RNA elements located around the major splice donor site, and the core-packaging signal is composed of a series of stem-loops (2, 10). It was originally thought that RNA sequences downstream of the major splice donor site were responsible for the specific packaging of viral genomic RNA in a manner that would exclude the packaging of spliced viral RNA species in the case of HIV-1. However, it has been reported that sequences upstream of the splice donor are also important for efficient packaging of HIV-1 viral genomic RNA (1, 4, 21, 24, 28). Similar results for HIV-2 have also been reported, but it was suggested that sequences upstream of the major splice donor site were more important than those downstream for efficient encapsidation of HIV-2 RNA. Therefore, HIV-2 may use different mechanisms to select unspliced RNA for encapsidation (13, 14, 25, 28).
With regard to SIV RNA packaging determinants, only one study has reported that leader sequences upstream of the major splice donor site can be packaged into HIV-1 particles (30). Our results now show that sequences located downstream of the PBS and upstream of the major splice donor site, nt +345 to +418, are necessary for the efficient encapsidation of SIV genomic RNA, since deletions within this region have a detrimental effect on RNA packaging. This region includes half of the putative DIS and half of the putative U5-leader stem (1, 30). Therefore, these proposed structures likely serve a functional role in the encapsidation process. The fact that genomes with deletions of this entire region can still be packaged to some extent indicates that sequences in disparate regions may also play a role in the encapsidation of SIV genomic RNA.
Deletions in this region that result in impaired replication may not only affect RNA packaging. Comparable work with HIV-1 has indicated that sequences in this region also affect HIV-1 gene expression and may affect Gag polyprotein processing (16, 18–21). Although our results show that these deletions do not have any significant effect on SIV protein expression in transfected COS-7 cells, further work is required to characterize whether these deletions can affect proviral DNA synthesis and gene expression in permissive cell lines.
Reversions of deleted mutated viruses have also been observed in similar studies on HIV-1, and point mutations within four distinct Gag proteins were shown to contribute to the increased replication capacity of these viruses (22). Our results reveal that two of our SIV constructs, i.e., SD and SD3, did not revert to increased replication ability in C8166 cells over 6 months in culture. In contrast, long-term passage of the SD2 mutated virus in these cells did result in a restoration of replication capacity, due to the appearance of four point mutations: M1, M2, CA1, and Mp6. Interestingly, two of these mutations were located in leader sequences that flank the deletion site, i.e., M1 and M2, and only two of these mutations were located in Gag proteins, i.e., CA1 and Mp6. The M1 mutation was located within the PBS 87 nt upstream of the SD2 deletion, while the M2 substitution was identified in the loop of the DIS only 3 nt downstream of the SD2 deletion. These findings imply that there may be important differences between SIV and HIV-1 with regard to mechanism(s) of RNA packaging and in regard to the interactions between Gag proteins and leader sequences.
Site-directed mutagenesis studies have confirmed the biological relevance of each of these substitutions with the exception of M1. Since the M1 mutation has also been observed in infections with both wild-type and SD1 virus (data not shown), this substitution does not appear to be novel; rather, it may be a natural polymorphism involved in the binding of tRNA3Lys, which is used more efficiently by SIV than tRNA5Lys in human cells as a primer of reverse transcription (9). The M2 mutation was best able to rescue the SD2 deletion, but could not restore the putative DIS stem-loop structure. This implies that functions other than dimer formation may account for the partially restored replication capacity of SD2-M2 virus in C8166 cells. Further work is needed to determine how these point mutations are individually involved in the restoration of viral replication of the SD2 deletion virus; such studies are in progress and also involve analyses of the M2, CA1, and Mp6 mutations in various combinations. While M2 alone was not capable of restoring the DIS stem-loop, it remains possible that a combination of M2 with other mutations not yet discovered could do this, while simultaneously enabling viral replication to resume with wild-type kinetics.
ACKNOWLEDGMENTS
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health: SIVmac 251 antiserum, plasmids of p239SpSp5′ and p239SpE3′. We thank Chen Liang for helpful advice and Mervi Detorio and Maureen Oliveira for expert technical assistance.
This research was supported by grant R01 AI43878-01 from the National Institutes of Health.
REFERENCES
- 1.Berkhout B, van Wamel J L B. Role of the DIS hairpin in replication of human immunodeficiency virus type 1. J Virol. 1996;70:6723–6732. doi: 10.1128/jvi.70.10.6723-6732.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkowitz R D, Fisher J, Goff S P. RNA packaging. Curr Top Microbiol Immunol. 1996;214:177–218. doi: 10.1007/978-3-642-80145-7_6. [DOI] [PubMed] [Google Scholar]
- 3.Berkowitz R D, Hammarskjold M L, Helga-Maria C, Rekosh D, Goff S P. 5′ regions of HIV-1 RNAs are not sufficient for encapsidation: implications for the HIV-1 packaging signal. Virology. 1995;212:718–723. doi: 10.1006/viro.1995.1530. [DOI] [PubMed] [Google Scholar]
- 4.Clevel J L, Eckstein D A, Parslow T G. Genetic dissociation of the encapsidation and reverse transcription functions in the 5′R region of human immunodeficiency virus type 1. J Virol. 1999;73:101–109. doi: 10.1128/jvi.73.1.101-109.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cobrinik D, Aiyar A, Ge Z, Katzman M, Huang H, Leis J. Overlapping U5 sequence elements are required for efficient integration and initiation of reverse transcription. J Virol. 1991;62:3622–3630. doi: 10.1128/jvi.65.7.3864-3872.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cobrinik D, Soskey L, Leis J. A retroviral RNA secondary structure required for efficient initiation of reverse transcription. J Virol. 1988;62:3622–3630. doi: 10.1128/jvi.62.10.3622-3630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cullen B R. Human immunodeficiency virus as a prototypic complex retrovirus. J Virol. 1991;65:1053–1056. doi: 10.1128/jvi.65.3.1053-1056.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cullen B R. Mechanism of action of regulatory proteins of the primate immunodeficiency viruses. Microbiol Rev. 1992;56:375–394. doi: 10.1128/mr.56.3.375-394.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das A T, Klaver B, Berkhout B. Sequence variation of the human immunodeficiency virus primer-binding site suggests the use of an alternative tRNA Lys molecular in reverse transcription. J Gen Virol. 1997;78:837–840. doi: 10.1099/0022-1317-78-4-837. [DOI] [PubMed] [Google Scholar]
- 10.Harrison G P, Miele G, Hunter E, Lever A M L. Functional analysis of the core HIV-1 packaging signal in a permissive cell line. J Virol. 1998;72:5886–5896. doi: 10.1128/jvi.72.7.5886-5896.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu W S, Temin H M. Retroviral recombination and reverse transcription. Science. 1990;250:1227–1233. doi: 10.1126/science.1700865. [DOI] [PubMed] [Google Scholar]
- 12.Kao S Y, Calman A F, Luciew P A, Peterlin B M. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature. 1987;330:33–38. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- 13.Kaye J F, Lever A M L. Human immunodeficiency virus types 1 and 2 differ in the predominant mechanism used for selection of genomic ENA for encapsidation. J Virol. 1999;73:3023–3031. doi: 10.1128/jvi.73.4.3023-3031.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaye J F, Lever A M L. Nonreciprocal packaging of human immunodeficiency virus type 1 and type 2 RNA: a possible role for the p2 domain of Gag in RNA encapsidation. J Virol. 1998;72:5877–5885. doi: 10.1128/jvi.72.7.5877-5885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kestler H, Kodama T, Ringler D, Sehgal P, Daniel M D, King N, Desrosiers R. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990;248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 16.Lenz C, Scheid A, Schaal H. Exon 1 leader sequences downstream of U5 are important for efficient human immunodeficiency virus type 1 gene expression. J Virol. 1997;71:2757–2764. doi: 10.1128/jvi.71.4.2757-2764.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lever A M L, Gottlinger H, Haseltine W, Sodroski J. Identification of a sequence required for efficient packaging of human immunodeficiency virus type 1 RNA into virions. J Virol. 1989;63:4085–4087. doi: 10.1128/jvi.63.9.4085-4087.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Liang C, Quan Y, Chandok R, Laughrea M, Parniiak M A, Kleiman L, Wainberg M A. Identification of sequences downstream of the primer-binding site that is important for efficient replication of human immunodeficiency virus type 1. J Virol. 1997;71:6003–6010. doi: 10.1128/jvi.71.8.6003-6010.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang C, Li X, Quan Y, Langhrea M, Kleiman L, Hiscott J, Wainberg M A. Sequence elements downstream of human immunodeficiency virus type 1 long terminal repeat are required for efficient viral gene transcription. J Mol Biol. 1997;272:167–177. doi: 10.1006/jmbi.1997.1239. [DOI] [PubMed] [Google Scholar]
- 20.Liang C, Rong L, Cherry E, Kleiman L, Laughrea M, Wainberg M A. Deletion mutagenesis within the dimerization initiation site of human immunodeficiency virus type 1 results in delayed processing of the p2 peptide from precursor proteins. J Virol. 1999;73:6147–6151. doi: 10.1128/jvi.73.7.6147-6151.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang C, Rong L, Laughrea M, Kleiman L, Wainberg M A. Compensatory point mutations in the human immunodeficiency virus type 1 Gag region that are distal from deletion mutations in the dimerization initiation site can restore viral replication. J Virol. 1998;72:6629–6636. doi: 10.1128/jvi.72.8.6629-6636.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang C, Rong L, Quan Y, Laughrea M, Kleiman L, Wainberg M A. Mutations within four distinct Gag proteins are required to restore replication of human immunodeficiency virus type 1 after deletion mutagenesis within the dimerization initiation site. J Virol. 1999;73:7014–7020. doi: 10.1128/jvi.73.8.7014-7020.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 24.McBride M S, Panganiban A T. Position dependence of functional hairpins important for human immunodeficiency virus type 1 RNA encapsidation in vivo. J Virol. 1997;71:2050–2058. doi: 10.1128/jvi.71.3.2050-2058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCann E M, Lever A M L. Location of cis-acting signals important for RNA encapsidation in the leader sequence of human immunodeficiency virus type 2. J Virol. 1997;71:4133–4137. doi: 10.1128/jvi.71.5.4133-4137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohlmann T, Lopez-Lastra M, Darlix J L. An internal ribosome entry segment promotes translation of the simian immunodeficiency virus genomic RNA. J Biol Chem. 2000;275:11899–11906. doi: 10.1074/jbc.275.16.11899. [DOI] [PubMed] [Google Scholar]
- 27.Pailllart J-C, Berthoux L, Ottmann M, Darlix J-L, Marquet R, Ehresmann B, Ehresmann C. A dual role of the putative RNA dimerization initiation site of human immunodeficiency virus type 1 in genomic RNA packaging and proviral DNA synthesis. J Virol. 1996;70:8348–8354. doi: 10.1128/jvi.70.12.8348-8354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poeschla E, Gilbert J, Li X, Huang S, Ho A, Wong-Staal F. Identification of human immunodeficiency virus type 2 (HIV-2) encapsidation determinant and transduction of nondividing human cells by HIV-2 based lentivirus vectors. J Virol. 1998;72:6527–6536. doi: 10.1128/jvi.72.8.6527-6536.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Regier D A, Desrosiers R C. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res Hum Retrovir. 1990;6:1221–1231. doi: 10.1089/aid.1990.6.1221. [DOI] [PubMed] [Google Scholar]
- 30.Rizvi T A, Panganiban A T. Simian immunodeficiency virus RNA is efficiently encapsidated by human immunodeficiency virus type 1 particles. J Virol. 1993;67:2681–2688. doi: 10.1128/jvi.67.5.2681-2688.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steffy K, Wong-Staal F. Genetic regulation of human immunodeficiency virus. Microbiol Rev. 1991;55:173–205. doi: 10.1128/mr.55.2.193-205.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viglianti G A, Rubinstein E P, Graves K L. Role of the TAR RNA splicing in translational regulation of simian immunodeficiency virus from rhesus macaques. J Virol. 1992;66:4824–4833. doi: 10.1128/jvi.66.8.4824-4833.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuker M. On finding all suboptimal foldings of an RNA molecule. Science. 1989;244:48–52. doi: 10.1126/science.2468181. [DOI] [PubMed] [Google Scholar]
- 34.Zuker M, Mathews D H, Turner D H. Algorithms and thermodynamics for RNA secondary structure prediction: a practical guide in RNA biochemistry and biotechnology. In: Barciszewski J, Clark B F C, editors. NATO ASI series. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1999. pp. 11–43. [Google Scholar]