Abstract
Background
Fabry disease (FD) is an inherited lysosomal storage disorder, leading to multisystemic manifestations and causing significant morbidity and mortality.
Objective
The aim of this narrative review is to present the current and novel therapeutic strategies in FD, including symptomatic and specific treatment options.
Methods
A systematic literature search was conducted to identify relevant studies, including completed and ongoing randomized-controlled clinical trials (RCTs), prospective or retrospective cohort studies, case series and case reports that provided clinical data regarding FD treatment.
Results
A multidisciplinary symptomatic treatment is recommended for FD patients, personalized according to disease manifestations and their severity. During the last two decades, FD-specific treatments, including two enzyme-replacement-therapies (agalsidase alfa and agalsidase beta) and chaperone treatment with migalastat have been approved for use and allowed for symptoms’ stabilization or even disease burden reduction. More therapeutic agents are currently under investigation. Substrate reduction therapies, including lucerastat and venglustat, have shown promising results in RCTs and may be used either as monotherapy or as complementary therapy to established enzyme-replacement-therapies. More stable enzyme-replacement-therapy molecules that are associated with less adverse events and lower likelihood of neutralizing antibodies formation have also been developed. Ex-vivo and in-vivo gene therapy is being tested in animal models and pilot human clinical trials, with preliminary results showing a favorable safety and efficacy profile.
Conclusion
The therapeutic landscape in FD appears to be actively expanding with more treatment options expected to become available in the near future, allowing for a more personalized approach in FD patients.
Keywords: Fabry disease, enzyme replacement therapy, chaperone, gene therapy, rare neurological diseases, mutation
1. INTRODUCTION
Fabry disease (FD) is an inherited lysosomal storage disorder caused by mutations in the GLA gene (Xq21.3-q22), leading to the absence or deficiency of alpha-galactosidase A (α-Gal A) [1]. The markedly reduced activity of α-Gal A results in the diminished metabolism and the following accumulation of neutral glycosphingolipids and globotriaosylceramide (Gb3) in the plasma and cellular lysosomes of various tissues and organs [2], affecting the heart, kidneys, central and peripheral nervous system, skin and eyes [3]. More than 1,000 mutations in the GLA gene have been pathogenetically linked to FD manifestations [4]. The estimated prevalence of FD is calculated at approximately 1 in 3,000 individuals [4]. Among female carriers, the prevalence varies between 1:6,000 to 1:40,000 females [5]. Despite that FD may be observed in all ethnic, racial and demographic groups, significant variations in epidemiology exist and reported incidence ranges with different geographic distributions, as assessed by several newborn screening programs [6-11].
Unless treated, FD is associated with significant mortality and morbidity, with a 20- and 10-year reduction of life expectancy in males and females, respectively, versus the general population, mostly due to end-stage renal disease and life-threatening cardiovascular or cerebrovascular complications [4]. During the last two decades, there has been a significant change of scenery in FD management [12]. Symptomatic treatment has shifted to a more integrated therapeutic approach using FD-specific treatments, including enzyme replacement therapies (ERT) and chaperone treatment that have been approved for use and are currently available [13]. Current treatment options allow for FD stabilization or even disease burden reduction, while they improve quality of life [14]. Increased awareness is of utmost importance for a timely and accurate diagnosis of FD, since available FD treatments are capable of preventing complications and should be initiated before irreversible damage to target organs has developed [15].
Apart from accurate diagnosis and prompt treatment initiation, management by a multidisciplinary expert team and adopting a personalized approach according to the genotype and phenotype of each patient are key aspects to consider when developing a strategic treatment plan for FD patients [16]. In this narrative review, we sought to present the FD-specific treatment options that are currently available and provide an overview of developing treatments and potential future approaches.
2. METHODS
A systematic literature search was conducted to identify relevant studies published in MEDLINE, Scopus, Embase, Cochrane Library, EudraCT (European Union Drug Regulating Authorities Clinical Trials Database) and ClinicalTrials.gov. The combination of search strings applied to query all databases included the following keywords: “Fabry disease” and “enzyme replacement therapy”, “chaperone treatment”, “gene therapy”, “substrate reduction therapy”, or “mRNA-based therapy”. Database interrogation was performed by two independent researchers (LP, CZ), who additionally manually searched conference abstracts and reference lists of published articles to ensure the comprehensiveness of the bibliography. The last electronic search was performed on March 21st, 2022.
Completed and ongoing randomized-controlled clinical trials (RCTs), prospective or retrospective cohort studies, case series and case reports that provided clinical data regarding FD-specific treatments were evaluated, and relevant information was abstracted. Additionally, relevant editorials, commentaries and reviews that provided an overview of FD management were also assessed to ensure the comprehensiveness of bibliography.
This article is presented according to the Narrative Review Reporting Checklist, available as a Supplementary File [1 (118.3KB, pdf) ].
3. RESULTS
3.1. Clinical Characteristics
FD is a multiorgan disease affecting various systems and presenting with a variety of manifestations and a phenotypic spectrum of severity that mostly depends on the genotype, the residual activity of α-Gal A and the disease duration [2]. Female carriers may also present with milder phenotypes comprising atypical symptoms. An overview of FD-associated manifestations is presented in Table 1.
Table 1.
Manifestations associated with Fabry Disease, in order of descending frequency of involvement.
| Organ | Involvement % | Manifestations |
|---|---|---|
| Heart | 68% | Left ventricular hypertrophy |
| - | - | Right ventricular hypertrophy |
| - | - | Congestive heart failure |
| - | - | Arrhythmias |
| - | - | Myocardial ischemia |
| Kidney | 45% | Microalbuminuria |
| - | - | Proteinuria |
| - | - | Progressive renal failure |
| Peripheral Nervous System | 45% | Neuropathic pain |
| - | - | Acroparaesthesias |
| - | - | Acute pain crisis |
| - | - | Thermal sensation deficits |
| - | - | Dysautonomia |
| - | - | Perspiration disorders |
| - | - | Gastrointestinal dysmotility |
| - | - | Median nerve entrapment |
| - | - | Cramp-fasciculation syndrome |
| Eye | 38% | Cornea verticillata |
| - | - | Cataract |
| Central Nervous System |
34% | Ischemic stroke |
| - | - | Transient ischemic attack |
| - | - | Vascular dementia |
| - | - | Hemorrhagic stroke Vertebrobasilar dolichoectasia |
| Skin | 34% | Angiokeratomas |
| - | - | Lymphedema |
| - | - | Coarse facial features |
| Ear | 19% | Hearing loss |
| - | - | Vestibular disturbances |
| Miscellaneous | - | Chronic cough |
| - | - | Osteopenia |
| - | - | Anemia |
| - | - | Azoospermia |
| - | - | Hypothyroidism |
Cardiac involvement may be present in 40-70% of FD patients [18], with the most common manifestation being progressive diastolic dysfunction with concentric left ventricular hypertrophy [19]. Fibrosis of the posterior wall, septum thickness, right ventricular hypertrophy, arrhythmias, electrocardiographic abnormalities, and, less often, coronary artery disease may also be present in FD patients [20-24]. Congestive heart failure occurs with disease progression [25, 26]. Notably, a “cardiac variant” also exists as an atypical variant of FD, affecting predominately the heart, with minimal, if any, other organ manifestations [27-29].
Both male and female patients may exhibit renal manifestations during the FD course [30, 31]. Renal involvement is initially manifested by microalbuminuria and proteinuria, which are typically present during the second or third decade [32]. During the earlier stages of FD, renal dysfunction may be masked by glomerular hyperfiltration, but with disease progression, fibrosis, sclerosis, tubular atrophy, and, ultimately, kidney failure follow during the third to fifth decade of life [18]. Renal involvement is one of the most important causes of morbidity and mortality in FD. Characteristically, the 5-year survival rate has been calculated at 41% among patients with FD-associated nephropathy, compared to 68% in nephropathies of more common pathologies [33, 34].
Central and peripheral nervous systems are both involved during the course of FD [35]. Cerebrovascular disease is the hallmark of central system manifestations in FD patients and may include transient ischemic attacks, ischemic strokes of multiple underlying mechanisms, intracerebral hemorrhage, subarachnoid hemorrhage, and vascular dementia [36, 37]. Stroke may complicate both male (6.9%) and female (4.3%) FD patients at a median age of 39 and 45.7 years, respectively, as reported in the Fabry registry [38]. Nevertheless, while relative risk of stroke in FD patients is greatly increased, the absolute risk remains low [39] and a FD-targeted evaluation should be pursued mostly in the cases of recurrent cryptogenic strokes, when other, more common, causes of stroke have already been excluded [39, 40]. Regarding the peripheral nervous system, FD-associated manifestations are present at a much younger age. Characteristically, neuropathic pain, gastrointestinal dysmotility, hypοhidrosis and heat intolerance are the most commonly reported symptoms in FD during childhood [41]. Although not life-threatening, small-fiber peripheral neuropathy and autonomic nerve dysfunction clearly impact the health and quality of life of affected children [42, 43]. Over time, the positive neuropathic symptoms (i.e., burning pain, acroparaesthesias, pain crises) seem to ameliorate, probably due to reduction of axonal sensory hyperexcitability and central sensitization, while negative symptoms, such as numbness and hypoalgesia, become apparent during adulthood. Furthermore, during the later stages of FD, large nerve fibers might also be affected, resulting in diminished position and vibration sensation, as well as motor dysfunction [44, 45].
Other manifestations include dermatological, ophthalmological, and miscellaneous symptoms. A characteristic dermatological manifestation of FD is angiokeratoma corporis diffusum, which presents as small, red-to-purple, raised skin lesions, commonly localized in the “swimsuit area” (i.e., buttocks, groin, thighs, abdomen), as well as in the inner part of the lips or the genitals [46, 47]. The prevalence of angiokeratomas has been calculated at 83% in males and 80% in females with FD [48]. Other dermatological manifestations include lymphoedema [49] and coarse facial features [50, 51]. Cornea verticillata, reported in up to 70% of FD patients [52, 53], and posterior subcapsular cataract (i.e., the “Fabry cataract”) [54] are pathognomonic ocular symptoms in FD. Therefore, ophthalmological evaluation should be performed in suspected FD patients, even when no ocular symptoms are reported. Finally, miscellaneous manifestations may include hearing loss [55, 56], respiratory symptoms [57, 58], osteoporosis [59], anemia [60], hypothyroidism [61], azoospermia [62], or priapism [63].
3.2. Diagnosis
Prompt FD diagnosis requires increased awareness and clinical suspicion regarding the phenotypic spectrum of the disease. Measuring the activity of α-Gal A in plasma or in leukocytes is the initial laboratory test in suspected FD cases. Demonstration of α-Gal A reduction is a mainstay of diagnosis in male FD patients [64]. However, false-negative results are quite common in female patients [65]. Characteristically, female patients with late-onset FD may have residual α-Gal A function and Gb3 concentrations that are within the range observed in healthy individuals [66]. Thus, genotypic analysis is warranted for the diagnosis of female patients [67]. When a pathogenetic variant is identified during direct molecular analysis of the GLA gene, FD diagnosis can be confirmed. On the other hand, when novel mutations and mutations of unknown significance are present, molecular diagnosis of FD may be more challenging. Despite the fact that genetic testing should be performed for FD to be confirmed in both male and female patients, its results should be evaluated critically and in adjunction with the clinical signs of the disease and the other biomarkers as well [68].
As part of confirmatory testing, especially when a genetic variant of unknown significance is detected by GLA gene mutation analysis in male and female patients with FD suspicion, measurement of the deacylated form of Gb3 in the plasma or urine, called the lyso-Gb3, may be performed. Lyso-Gb3 evaluation has been proven an important biomarker for FD diagnosis and monitoring [69], and increased lyso-Gb3 values are considered very suggestive for FD [70]. Furthermore, in ambiguous cases, histopathological analysis of kidney, skin, or myocardial biopsies under electron microscopy may provide useful information [71, 72].
A proposed gender-specific diagnostic algorithm developed by the National Society of Genetic Counselors (United States) is presented in Table 2 [66, 73].
Table 2.
Proposed algorithm for Fabry Disease diagnosis.
| In Patients with Suspected FD* | |||
|---|---|---|---|
| Gender | Step 1 | Step 2 | Confirmatory Test (if needed)† |
| Males | Measurement of α-Gal A activity in plasma or leukocytes | GLA gene mutation analysis | Measurement of lyso-Gb3 in plasma or urine; biopsy (heart, kidney, skin) |
| Females | GLA gene mutation analysis | - | |
FD: Fabry Disease; α-Gal A: alpha-galactosidase A; lyso-Gb3: globotriaosysphingosine.
* Suspicion of FD could be indicated by one of the following:
• presence of FD-characteristic signs, such as angiokeratoma or cornea verticillata.
• known or suspicious family history.
• otherwise unexplained hypertrophic cardiomyopathy or left ventricular hypertrophy.
• otherwise unexplained proteinuria or renal failure.
• otherwise unexplained stroke in young patients.
• otherwise unexplained small-fiber neuropathy.
† Confirmatory testing may be needed in suspected male and female FD patients when a genetic variant of unknown significance is detected by GLA gene mutation analysis.
Apart from the initial diagnostic tests for FD, further ancillary testing should be performed with the aim of evaluating the involvement of each target organ. Peripheral nervous system involvement may be evaluated through electrophysiological studies, including sympathetic skin response, thermal quantitative sensory testing, sensory and motor nerve conduction studies and electromyography [74] and through skin biopsy for estimating intradermal nerve fiber density [75]. Central nervous system involvement may be assessed by brain MRI in several neuroradiological aspects [35], including strokes, leukoencephalopathy, cerebral microbleeds or pulvinar sign [76]. Renal manifestations may be assessed by serum biochemical assays, evaluating plasma urea, creatinine, age-corrected eGFR, microalbuminuria and proteinuria [31], and imaging techniques, such as renal ultrasound or MRI [77]. Finally, electrocardiography, echocardiography and cardiac MRI may be performed for the assessment of cardiac involvement in FD patients [20, 78]. Specialized ophthalmologists, dermatologists and otolaryngologists should also examine an FD patient as part of the initial evaluation and during follow-up.
3.3. Treatment
The mainstay of FD treatment lies upon two pillars: symptomatic management of FD-associated symptoms and FD-specific treatment.
3.4. Symptomatic Treatment
Symptomatic treatment requires a multidisciplinary approach that should be individualized according to the patient’s manifestations. In cases of cardiac involvement, antianginal and antihypertensive therapies are recommended, including administration of calcium antagonists, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, whereas b-blockers may aggravate potential arrhythmias, sinus bradycardia and atrioventricular block and should be avoided [79]. Amiodarone is another drug that should be avoided as a therapeutic option in FD-associated arrythmias, since it has been shown to interfere with lysosome function with the potential of inducing further decline of intracellular lysosomal enzyme levels [80]. Cardiac pacing and cardioverter-defibrillator implantation may be suggested in cases of documented clinically significant cardiac arrhythmias. Furthermore, when end-stage heart failure occurs, the patient may be evaluated and referred for heart transplantation [81]. Interestingly, although FD is not listed among the various indications for heart transplantation according to the International Society for Heart Lung Transplantation [82], several case reports highlight that this may be a viable therapeutic option [83-87] that could be offered in carefully selected patients [88].
FD-nephropathy, which initially manifests with microalbuminuria and proteinuria, may be managed by the use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers that limit urinary protein excretion and additionally control blood pressure [89-92]. As a treatment goal, protein excretion less than 500 mg/day is recommended for FD patients [93]. Sodium-glucose co-transporter-2 (SGLT2) inhibitors have also been shown to delay disease progression in patients with chronic kidney disease, irrespective of diabetes mellitus status [94]. Considering that they may also have an additional cardioprotective role in patients with heart failure [95], SGLT2 inhibitors might be used as another therapeutic agent for the symptomatic treatment of FD. When end-stage renal disease occurs, renal replacement therapy is required with either dialysis treatment or kidney transplantation [96-98]. Importantly, the risks of all-cause graft failure and all-cause mortality do not differ between FD patients compared to transplanted patients with other causes of end-stage renal disease [99]. Therefore, kidney transplant should be offered as an option in patients with FD, especially in the current era of ERT, which carries an additional protective role in terms of graft and patient survival [100].
Involvement of the peripheral nervous system may manifest with neuropathic pain, often accompanied by pain crises. Avoidance of certain stimuli that may aggravate the pain, such as temperature changes or physical stress, should be recommended. Pharmacological measures in cases of persistent acroparaesthesias or chronic neuropathic pain include carbamazepine, phenytoin, gabapentin, serotonin‐norepinephrine reuptake inhibitors and duloxetine [101-103]. Analgesic drugs, such as non-steroidal anti-inflammatory drugs or opioids, should be reserved for acute relief during pain crisis management [101].
Cerebrovascular complications in the setting of FD necessitate a targeted secondary stroke prevention treatment based on individual characteristics. In cases of ischemic strokes, antithrombotic therapy, with either antiplatelets or anticoagulants according to specific indications, coupled with adequate control of other risk factors (e.g., diabetes mellitus, arterial hypertension, hyperlipidemia, hyperhomocysteinemia), should be recommended as preventive measures [104]. In the acute setting, there are no specific guidelines regarding the administration of acute reperfusion therapies in ischemic stroke of FD patients who are otherwise eligible for receiving those treatments. However, no safety concerns have arisen in several case reports presenting FD patients with acute ischemic stroke treated with intravenous thrombolysis and/or mechanical thrombectomy [105-108].
Other FD-related symptoms should be treated as well since they may contribute to the significant disability of the patients. When gastrointestinal dysmotility is evident, metoclopramide and H-2 blockers may be used to alleviate delayed gastric emptying and dyspepsia symptoms. However, these pharmacological measures should be adjunctive, and adopting dietary changes such as increased fiber intake and eating more frequent and smaller meals should be encouraged [16]. Acute hearing loss may be treated by vasodilators and/or steroids [56], while chronic hearing loss may be managed by placing hearing aids or cochlear implants. Supportive therapy for vertigo and tinnitus, which can be quite disturbing for FD patients, should be offered using combinations of antiemetic and antivertigo agents [16]. Ocular manifestations can be managed by wearing polarized glasses to correct night vision and by applying artificial tears or ointment in cases of excessive eye dryness [16]. When angiokeratomas cause discomfort to the patients, laser or other cosmetic techniques may be applied locally to remove these lesions, although they are generally considered benign [16]. Symptomatic management using compression stockings may also be recommended in patients with lymphedema [16].
3.5. FD-Specific Treatment
3.5.1. Enzyme Replacement Therapy
Symptomatic treatment in FD is crucial fo patients; however, it has no effect on the pathophysiological history of the disease. On the other hand, an FD-specific treatment should have the potential to correct or improve the flaws in the corrupted metabolic path (Fig. 1), prevent rather than treat the various complications of FD, and finally alter the natural history course of the disease.
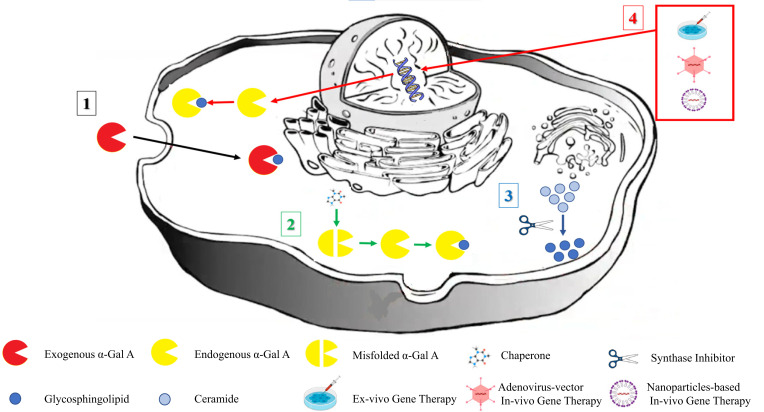
Fig. (1).
Metabolic paths targeted by Fabry Disease-specific treatments. Enzyme replacement therapy is intravenously administered, providing exogenous recombinant forms of the human α-Gal A enzyme embedded in cells via endocytosis, where they perform glycosphingolipid hydrolysis (metabolic path 1; black arrow). Corrupted or misfolded endogenous α-Gal A enzymes are repaired by chaperone treatment, allowing for sufficient glycosphingolipid hydrolysis (metabolic path 2; green arrows). Substrate reduction therapy acts by restricting the accumulation of the deficient α-Gal A byproduct (metabolic path 3; blue arrow). Ex- or in-vivo gene therapy delivers the therapeutic gene into the nuclear and endogenous α-Gal A enzyme, leading to glycosphingolipid hydrolysis (metabolic path 4; red arrows). α-Gal A: alpha-galactosidase A.
During the last two decades, a significant progression in FD treatment was noted with the approval of ERT. Currently, two forms of ERT are authorized for use: agalsidase alfa at a dose of 0.2 mg/kg biweekly and agalsidase beta at a dose of 1.0 mg/kg biweekly [109, 110]. Both agents are indicated for FD treatment in adults, adolescents and children aged 8 years and older [109, 110].
3.6. Agalsidase Alfa
Agalsidase alfa is a recombinant form of the human α-Gal A enzyme that is produced in a cultured human cell line after activating the relevant encoding gene [111]. Following the preliminary but encouraging results of a pilot study showing that agalsidase alfa administration was associated with reduced Gb3 levels in both the liver and the urine sediment of 11 FD patients [112], the first placebo-controlled RCT was designed and conducted in 18 adult males with FD investigating the safety and efficacy of intravenous treatment with agalsidase alfa in this population [113]. In this study, treatment with agalsidase alfa led to improvement of neuropathic pain and renal function as well as stabilization of cardiac involvement; it also confirmed the Gb3 reduction in plasma and urine sediments. Furthermore, it was shown that the treatment was well-tolerated, while the common mild infusion reactions could be controlled with antihistamines and/or low-dose corticosteroids. A second RCT confirmed the positive results of agalsidase alfa on cardiac function among treated patients, presenting significant regression of the hypertrophic cardiomyopathy and reduction in myocardial Gb3 deposits [114].
Safety and efficacy of agalsidase alfa treatment were confirmed in pediatric patients as well, with a long term follow-up of 6.5 years in an open-label setting [115]. Although adverse events were common, with the majority of them being infusion-related disorders, none of the patients had to discontinue treatment due to them. On the contrary, significant stabilization of the disease course was noted, with no progression in renal or cardiac manifestations and plasma and urinary Gb3 reduced.
Following the results of the RCTs, in 2001, the European Medicines Agency authorized agalsidase alfa for use in all patients with confirmed FD at a dose of 0.2 mg/kg bodyweight administered during a 40-minute intravenous infusion every two weeks. Since then, real-world data on the commercial use of agalsidase alfa have confirmed tolerability and efficacy in FD patients [116-118]. Importantly, no sex-related differences have been noted, with both sexes presenting a similar response to the treatment [119]. Nevertheless, it has been shown that the earlier treatment initiation, before irreversible damage has occurred, was associated with better clinical outcomes [120, 121] and better biochemical response, as shown by plasma lyso-Gb3 reduction [122]. Thus, it has been recommended by the European Fabry Working Group consensus that treatment with ERT should be initiated in all FD patients as soon as there present early clinical signs of kidney, heart or brain FD-related involvement, while it may be considered even in asymptomatic FD male patients aged ≥ 16 years old [123]. Although ERT initiation in pre-symptomatic FD is based on Class IIB recommendation according to the European Fabry Working Group consensus [123], such a proactive approach has been proven safe and effective in isolated asymptomatic male pediatric cases by reducing Gb3 accumulation [124]. Therefore, more recent consensus recommendations propose that asymptomatic male patients may initiate ERT, even earlier than the age of 7 years old, based on individualized criteria, such as the presence of a pathogenic GLA variant associated with the classic phenotype, an undetectable α-Gal A activity, high levels of lysoGb3 in plasma or positive family history of severe FD [125]. Currently, no data support ERT initiation in asymptomatic girls [125], mostly owing to the expected milder course of FD, if female carriers develop any symptoms.
Following the initial drug-producing processes based on a “roller-bottle” technique using bovine serum and animal-derived proteins, other agalsidase alfa regimens were manufactured following more sophisticated techniques with the aim of operational improvement, limiting the risk of drug supplies shortages. Agalsidase alfa produced using an animal component-free bioreactor process has been recently introduced [126] and presented a tolerable safety profile for both adult and pediatric patients [127, 128].
3.7. Agalsidase Beta
Another recombinant form of human α-Gal A enzyme, called agalsidase beta, is produced in mammalian Chinese hamster ovary (CHO) cell culture [129, 130]. Agalsidase beta is also administered intravenously, at a dose of 1 mg/kg bodyweight during a 180-minute infusion every two weeks. Approval of this treatment has been granted by both the European Medicines Agency and the Food and Drug Administration in 2001 and 2003, respectively. A phase 1/2 open-label dose-escalation study of replacement therapy with agalsidase beta was the first study that provided persuasive evidence that the treatment could lead to a substantial reduction of Gb3 deposits in both plasma and tissues (kidney, heart and skin) [131]. Later, during the year 1999, the first RCT was conducted and confirmed the efficacy of agalsidase beta in reducing Gb3 deposits, while providing further safety data, highlighting that agalsidase beta was only associated with transient mild-to-moderate infusion-associated reactions that could be prevented by reducing the infusion rate and/or administering premedication with antipyretics and antihistamines [132]. Apart from the biochemical improvement, a clear clinical benefit was demonstrated by another placebo-controlled RCT that proved a significantly reduced likelihood of experiencing any clinical event (renal, cardiac, or cerebrovascular event or death from any cause) in the active treatment arm [133]. Smaller studies were also confirmatory of the clinical improvement associated with agalsidase beta treatment, with significant recovery observed in cardiac [134-136] and renal function [137], while gastrointestinal [138, 139] and neuropathic symptoms [140] were also controlled. Importantly, overall quality of life was improved in both men and women with FD treated with agalsisade beta [141].
A significant shortage of agalsidase beta occurred globally in 2009 due to viral contamination in the manufacturing process. This led to the need for dose reduction or treatment shifting of the patients from agalsidase beta to agalsisade alfa [142]. Most of the observational studies reporting on patients who received a reduced dose of agalsidase beta or changed their treatment to agalsidase alfa demonstrated a similar effect of the treatments before and after the change of the treatment regimen, with no significant clinical events occurring after the shifting [143-145]. However, some of the FD-related symptoms, such as pain attacks, chronic pain, gastrointestinal pain, and diarrhea, as well as renal function, slightly deteriorated in the dose-reduction and switch groups [145].
So far, two RCTs have been designed with a head-to-head comparison of agalsidase alfa versus agalsidase beta [146,147]. None of the studies reported any significant differences regarding efficacy or safety outcomes. The two treatments seem comparable, and regimen selection should be based on individualized criteria [148]. Neutralizing antibody formation is reported in both ERT regimens, usually after 3-6 months of continuous treatment [149]; however, it occurs more commonly after treatment with agalsidase beta (83%) than agalsidase alfa (55%) [150]. Importantly, antibody formation may be related to increased Gb3 accumulation [151], while it may attenuate the favorable treatment effect of ERT [152]. Further studies addressing the prevention of neutralizing antibody formation, potentially with increased dosages of infused enzymes or immunomodulatory therapy, are warranted [153, 154].
3.8. Chaperone Therapy
The main disadvantages of ERT are the need for frequent intravenous infusions, the formation of neutralizing antibodies and the fact that they are large molecules that do not cross the blood-brain barrier. In order to deal with these drawbacks, chaperone treatment was introduced. Chemical chaperones are small molecules that facilitate the folding, maturation, binding or trafficking of the deficient, yet partially present, enzymes and have already been used in several lysosome storage disorders [155, 156]. In FD, the currently approved chaperone, the iminosugar 1-deoxygalactono-jirimycin (migalastat), which is a reversible, competitive inhibitor of α-Gal A, has the potential to stabilize the endogenous enzyme of FD patients and increase its enzymatic function by assisting the trafficking from the endoplasmic reticulum to the lysosomes [157-159]. Treatment with migalastat is indicated in patients with FD attributed to amenable mutations, aged ≥12 years [160]. The safety and efficacy of migalastat in children younger than 12 years old have not yet been established. It is an oral treatment, administered every other day at a dose of 123 mg.
Migalastat treatment was initially compared to placebo in the phase III FACETS trial [159]. In this RCT, when patients with amenable mutations were treated, migalastat was associated with stable renal function, reduced cardiac mass, and reduced the severity of gastrointestinal symptoms [159, 161]. Amenable mutations allow for the production of a-Gal A enzyme that retains some residual catalytic activity despite having abnormal protein folding. Although migalastat-amenable mutations account for 37% to 60% of all FD patients [162,163], the treatment effect of migalastat in these patients is comparable to ERT. In another phase III RCT, the ATTRACT trial, FD patients with amenable mutations were randomized to either continue RCT or shift to migalastat treatment [164]. Clinical outcomes, including renal function, left ventricular mass index, the composite of renal, cardiac or cerebrovascular events, and all-cause mortality, were similar in both treatment groups. Furthermore, Gb3 levels in plasma and adverse events did not differ between migalastat and ERT. Importantly, no safety issues emerged due to the switching from ERT to migalastat [165], which is a procedure that requires no special precautions [166].
A post-hoc analysis of the open-label extension phase of the FACETS and ATTACT trials has shown that the renal function of FD patients treated with migalastat for more than 2 years remained stable during long-term follow-up [167]. This treatment effect was independent of sex, previous FD-specific treatment (ERT-naïve or experienced patients) or disease phenotype [167]. Real-world data confirmed the positive effect of migalastat treatment in FD patients [168, 169]. Improvement of renal and cardiac function, coupled with increased a-Gal A activity and reduced Gb3 accumulation after chaperone therapy, led to the general concept that migalastat may be a feasible therapeutic option and a safe alternative to ERT in FD patients [168]. Furthermore, it should be noted that migalastat has the potential to cross the blood-brain barrier, and its efficacy on central nervous system manifestations remains to be elucidated in future trials [170, 171]. The patient perspective, including treatment adherence, quality of life and self-reported pain reduction, is the primary outcome of another ongoing, observational, investigator-initiated clinical trial [172]. Migalastat limitations include the strict indication in FD patients harboring amenable mutations and the contraindications in pregnant women and patients with eGFR (estimated glomerular filtration rate) <30ml/min.
3.9. Developing Treatments
3.9.1. Substrate Reduction Therapies
Substrate reduction therapies act by restricting the biosynthesis of the substrate that is accumulated due to the deficient α-Gal A [173]. The main advantage of these therapies is that they are active irrespective of the genetic mutation leading to the enzyme deficiency. Importantly, they may be administered as a monotherapy but also as a complementary therapy to ERT [173]. Lucerastat is a glucosylceramide synthase inhibitor that has the potential to reduce glycosphingolipids accumulated in FD [174]. An oral dose of 1000 mg twice daily has been shown to be well-tolerated in FD patients [175]. Caution and dose adjustments are needed in cases of renal impairment, since lucerastat is excreted in the urine [176]. In the first RCT testing of this treatment, the patients were randomized to receiving lucerastat in addition to standard of care treatment, including ERT (10 patients) versus ERT alone (4 patients) [177]. Plasma concentrations of Gb3, as well as its precursors, glucosylceramide and lactosylceramide, were significantly reduced in the lucerastat treatment arm. However, no clinical benefit regarding renal and cardiac function could be demonstrated, possibly due to the short duration of the study (12 weeks of treatment). Lucerastat treatment was well tolerated, with no drug-related safety issues emerging. A long-term, multicenter, but single-arm clinical trial addressing the safety and tolerability of lucerastat treatment in adult FD patients is currently ongoing [178]. Another clinical trial, the MODIFY trial, is a phase III RCT evaluating the efficacy and safety of lucerastat as a monotherapy in FD patients [179, 180]. Apart from plasma Gb3 reduction, MODIFY trial focuses on patient-reported clinical outcomes, including management of neuropathic pain and control of FD-related gastrointestinal symptoms [181]. This RCT has now been completed, and the results are awaited.
Venglustat is another small-molecule glucosylceramide synthase inhibitor that is being under consideration as a potential substrate reduction therapy, not only in FD, but in other diseases as well, including Gaucher disease type 3, Parkinson's disease associated with GBA mutations, GM2 gangliosidosis, and autosomal dominant polycystic kidney disease [182]. A phase II single-arm clinical trial tested venglustat as monotherapy in ERT-naïve adult FD patients [183]. This study showed that venglustat treatment, administered in a daily dose of 15mg orally, was associated with a significant reduction in skin capillary endothelial cell Gb3 deposits, plasma Gb3, plasma lyso-Gb3, and urine Gb3, while proteinuria and estimated glomerular filtration rate were also stabilized. No significant FD-related clinical events occurred during the 3-year treatment. Following these promising results, a phase III RCT, the CARAT trial, has been designed and is currently initiating recruitment, with the aim to investigate the effect of venglustat on the left ventricular mass index compared to standard of care treatment (i.e., ERT or migalastat) in adult FD patients, irrespective of their previous FD-specific treatment [184]. Other outcomes, such as the composite of FD-related clinical events, all-cause mortality, reduction of pain, and adverse events, will also be explored. Another placebo-controlled RCT, the PERIDOT trial, will randomize treatment-naïve FD patients and specifically assess the efficacy of venglustat in reducing neuropathic and abdominal pain [185]. After the completion of the 12-month treatment duration, the study will be followed by an open-label extension phase, when all patients will be treated with venglustat.
3.9.2. Other Enzyme Replacement Therapies
Following the wide implementation of ERT in FD patients and the 20-year experience of administering this treatment, the need for manufacturing more stable ERT molecules has emerged. The goal of the “new-era” ERT is to present longer half-lives and less adverse events, while ongoing treatment should not activate the development of neutralizing antibodies with the aim to maintain a high efficacy throughout time.
Pegunigalsidase alfa is a pegylated form of α-Gal A, manufactured in plant cell cultures, which is currently under investigation as an ERT agent in FD patients [186, 187]. In a phase 1/2 dose-ranging clinical trial, 16 FD patients received an intravenous infusion of pegunigalsidase alfa every other week for 1 year [188]. The prolonged enzyme stability, initially demonstrated in in-vitro studies [187], has also been confirmed in this study, showing sustained drug plasma concentrations and favorable pharmacodynamics throughout the study follow-up [188]. With regard to efficacy outcomes, Gb3 levels in kidney biopsies of patients receiving pegunigalsidase alfa were significantly reduced, while the renal function remained stable. Additionally, adverse events were mild or moderate, and the developing antidrug antibodies were diminished with ongoing treatment. Following these promising results indicating attenuated immune response, favorable safety profile and clinical stability in patients treated with pegunigalsidase alfa, several phase-3 clinical trials are currently ongoing (BALANCE study [189]) or have just been completed (BRIGHT study [190] and BRIDGE study [191]). Preliminary results during an interim analysis of the BRIDGE study have recently been published, showing that the transition of FD patients from agalsidase alfa to pegunigalsidase alfa at a dose of 1 mg/kg every 2 weeks was feasible and safe, without any serious adverse events [192]. Moreover, the renal function in patients on pegunigalsidase alfa seems to be stabilized [193] or at least deteriorate at a slower rate compared to agalsidase alfa [192].
Another agent of ERT can be generated using the moss Physcomitrella patens as a production host [194, 195]. The produced enzyme, called moss-aGal, has been shown to be successfully embedded in cells through the mannose receptors in in vitro and animal studies, disputing the previously established theory that mannose 6-phosphate receptor-mediated endocytosis and phosphorylated terminal mannose residues, which are present exclusively in enzymes produced via a mammalian cell based-method, are a prerequisite for ERT in FD [196]. In the first phase-1 clinical trial, six FD patients received a single intravenous injection of moss-aGal at a dose of 0.2 mg/kg and were followed for 28 days [197]. No serious adverse events emerged, antidrug immune response developing neutralizing antibodies did not activate, and importantly, Gb3 excretion was reduced throughout the study duration [197]. Further RCTs testing moss-aGal in FD patients are currently under preparation.
3.9.3. Gene Therapy
Gene therapy is a highly promising treatment strategy, which is expected to correct the enzyme deficiency by one single drug administration. The delivery of a therapeutic gene and the following endogenous cellular expression will guide the generation of the previously deficient enzyme, completely changing the natural history of FD [198]. Gene therapy may be applied by ex- or in-vivo delivery [198]. Ex-vivo delivery constitutes transplanting hematopoietic stem cells that have been previously harvested from the patient and undergone gene editing in order to express α-Gal A [13]. On the other hand, the in-vivo method demands the infusion of recombinant adeno-associated virus vectors that carry the gene and stimulate the patient’s cells to undergo gene editing themselves, leading to the missing protein expression [13].
Infusion of autologous CD34+-selected hematopoietic stem/progenitor cells engineered to express α-Gal A via lentivirus transduction was successfully performed in 5 FD patients in a pilot study addressing this ex-vivo gene therapy [199, 200]. Intriguingly, produced α-Gal A levels were measured near normal within the first week post-infusion in all the patients, Gb3 and lyso-Gb3 levels were reduced, and 3 of the patients managed to withdraw ERT [200]. Moreover, an immune reaction to the produced α-Gal A was minimal, while gene therapy was also found to ameliorate pre-existing immunity to foreign ERT. Another open-label, phase 1/2 study, called FAB-GT (NCT03454893), also evaluated the ex-vivo lentiviral gene therapy AVR-RD-01 in FD patients, with preliminary results showing a promising safety and efficacy profile as announced in the American Society of Gene & Cell Therapy annual meeting in 2020 [201]. However, the results that followed were discordant, questioning the efficacy of the AVR-RD-01 agent and leading to the early termination of the study and the deprioritizing of the Fabry disease program by the company [202].
In-vivo gene therapy is currently being tested in several phase 1/2 clinical trials. Different biological agents comprising adeno-associated virus vectors, such as the liver- and cardiac-directed 4D-310 [203], the liver-directed ST-920 [204], and the liver-directed FLT190 [205], are under investigation. Early data regarding 4D-310 treatment of 3 FD patients have been announced in the 18th Annual World Symposium annual meeting held in 2022, showing sustained near-normal α-Gal A activity, reduced Gb3 levels and an indication of benefits in the cardiac function [206]. An increase in the α-Gal A activity was noticed in the two patients that have already received FLT190 treatment during the MARVEL-1 study; however, mild myocarditis was noticed in both of them, and this potential safety concern will be further evaluated [207].
Apart from viral vectors, non-viral vectors using solid lipid nanoparticles are being studied in animal models. Non-viral vectors are considered less immunogenic compared to viral vectors, and their development is simpler and cheaper [208]. Recently, the administration of a solid lipid nanoparticle-based vector carrying DNA encoding α-Gal A in an animal model was shown to be associated with an increase of α-Gal A activity in plasma and different tissues without promoting liver toxicity [209]. Another potential application of nanoparticles is to deliver mRNA, which directly codes wild-type α-Gal A. The administration of such nanoparticle-formulated mRNA has led to significant Gb3 reduction in animal FD models [210, 211]. Future studies are warranted for the better characterization of nanoparticle-based gene therapy in FD.
All FD-specific treatment options that have been approved or are being under investigation are summarized in Table 3.
Table 3.
Overview of Fabry Disease-specific treatment options that have been approved or are currently under investigation.
| Treatment Options | ||
|---|---|---|
| Available | - | - |
| Enzyme Replacement Therapy | - | Agalsidase alfa (EMA) |
| - | Agalsidase beta (EMA, FDA) | |
| Chaperone Therapy | - | Migalastat (EMA, FDA) |
| Under Investigation | - | - |
| Substrate Reduction Therapy | - | Lucerastat |
| - | Venglustat | |
| Enzyme Replacement Therapy | - | Pegunigalsidase alfa |
| - | Moss-aGal | |
| Gene Therapy | Ex-vivo | Infusion of autologous hematopoietic stem cells engineered to express α-Gal A (ex-vivo lentiviral gene therapy AVR-RD-01) |
| In-vivo | Recombinant adeno-associated virus vectors (4D-310, ST-920, FLT190) carrying DNA encoding α-Gal A | |
| Solid lipid nanoparticle-based vector carrying DNA encoding α-Gal A | ||
| mRNA-based Therapy | - | Exogenous mRNA via lipid nanoparticles |
α-Gal A: alpha-galactosidase A; EMA: European Medicine Agency; FDA: U.S. Food and Drug Administration.
4. DISCUSSION
During the last two decades, FD-specific treatment has become available, comprising ERT and, most recently, chaperone treatment. The prompt administration of those therapies is associated with symptoms’ stabilization and significant change in FD prognosis, prolonging the patient survival and improving the quality of life. Although the long-term outcomes of available FD-specific treatments remain to be elucidated and may further be affected by several baseline patient characteristics (such as age at treatment initiation, male sex, classical phenotype) [212], a significant prolongation in the survival of more than 15 years has been shown by the Fabry Outcome Survey study [213]. However, the currently available therapeutic options suffer from several limitations. The need for frequent drug infusions, the occurrence of infusion-related adverse events, the development of neutralizing antibodies, and the inability to access the brain may limit the therapeutic benefit of ERT. These limitations were particularly challenging during the COVID-19 pandemic. Regarding chaperone treatment, the most important disadvantage is the strict selection of FD patients with an amenable-to-treatment GLA mutation to be eligible for migalastat treatment.
The scientific community took a step further in order to overcome those drawbacks. State-of-the-art therapies are under investigation with preliminary results looking quite promising. Human studies have been performed showing a favorable safety and efficacy profile for substrate reduction therapies and “new-era” ERT. Additionally, gene therapy has shown to be feasible in several pilot human studies, while more sophisticated methods of non-viral gene delivery are being investigated in animal models.
Nevertheless, the importance of symptomatic treatment in FD should not be overshadowed by the ongoing development of FD-specific agents. A multidisciplinary team should be involved in planning and executing a holistic approach for each patient with the aim of alleviating any FD-related signs and symptoms. In the end, patient survival and quality of life will be determined by the comprehensive management of the complications affecting each FD-targeted organ system.
CONCLUSION
FD used to be an untreatable disease causing significant morbidity and mortality. However, during the last two decades, different FD-specific treatments have been approved for use and are provided together with a multidisciplinary symptomatic treatment. Importantly, the therapeutic landscape in FD seems to be actively expanding, with more treatment options expected to become available in the near future, allowing for a more personalized approach to FD patients and, hopefully, achieving a definite management of the disease.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- FD
Fabry Disease
- RCTs
Randomized-controlled Clinical Trials
- α-Gal A
Alpha-galactosidase A
- Gb3
Globotriaosylceramide
- ERT
Enzyme Replacement Therapies
- SGLT2
Sodium-glucose co-transporter-2
- CHO
Chinese Hamster Ovary
- eGFR
Estimated Glomerular Filtration Rate
AUTHORS’ CONTRIBUTIONS
LP and GT contributed to the conception and study design. LP, PK, CZ, GP, EB, MP, VZ, DP, CV, and GT contributed to the acquisition and analysis of data. LP and GT contributed to drafting a significant portion of the manuscript or figures. PK, CZ, GP, EB, MP, VZ, DP, and CV contributed with critical comments during manuscript revision.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website along with the published article.
REFERENCES
- 1.Kint J.A. The enzyme defect in Fabry’s disease. Nature. 1970;227(5263):1173. doi: 10.1038/2271173b0. [DOI] [PubMed] [Google Scholar]
- 2.Germain D.P. Fabry disease. Orphanet J. Rare Dis. 2010;5(1):30. doi: 10.1186/1750-1172-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiffmann R. Fabry disease. Pharmacol. Ther. 2009;122(1):65–77. doi: 10.1016/j.pharmthera.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Abecasis G.R., Auton A., Brooks L.D., DePristo M.A., Durbin R.M., Handsaker R.E., Kang H.M., Marth G.T., McVean G.A. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491(7422):56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laney D.A., Fernhoff P.M. Diagnosis of Fabry disease via analysis of family history. J. Genet. Couns. 2008;17(1):79–83. doi: 10.1007/s10897-007-9128-x. [DOI] [PubMed] [Google Scholar]
- 6.Scott C.R., Elliott S., Buroker N., Thomas L.I., Keutzer J., Glass M., Gelb M.H., Turecek F. Identification of infants at risk for developing Fabry, Pompe, or mucopolysaccharidosis-I from newborn blood spots by tandem mass spectrometry. J. Pediatr. 2013;163(2):498–503. doi: 10.1016/j.jpeds.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue T., Hattori K., Ihara K., Ishii A., Nakamura K., Hirose S. Newborn screening for Fabry disease in Japan: Prevalence and genotypes of Fabry disease in a pilot study. J. Hum. Genet. 2013;58(8):548–552. doi: 10.1038/jhg.2013.48. [DOI] [PubMed] [Google Scholar]
- 8.Colon C., Ortolano S., Melcon-Crespo C., Alvarez J.V., Lopez-Suarez O.E., Couce M.L., Fernández-Lorenzo J.R. Newborn screening for Fabry disease in the north-west of Spain. Eur. J. Pediatr. 2017;176(8):1075–1081. doi: 10.1007/s00431-017-2950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mechtler T.P., Stary S., Metz T.F., De Jesús V.R., Greber-Platzer S., Pollak A., Herkner K.R., Streubel B., Kasper D.C. Neonatal screening for lysosomal storage disorders: Feasibility and incidence from a nationwide study in Austria. Lancet. 2012;379(9813):335–341. doi: 10.1016/S0140-6736(11)61266-X. [DOI] [PubMed] [Google Scholar]
- 10.Hwu W.L., Chien Y.H., Lee N.C., Chiang S.C., Dobrovolny R., Huang A.C., Yeh H.Y., Chao M.C., Lin S.J., Kitagawa T., Desnick R.J., Hsu L.W. Newborn screening for Fabry disease in Taiwan reveals a high incidence of the later-onset GLA mutation c.936+919G>A (IVS4+919G>A). Hum. Mutat. 2009;30(10):1397–1405. doi: 10.1002/humu.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spada M., Pagliardini S., Yasuda M., Tukel T., Thiagarajan G., Sakuraba H., Ponzone A., Desnick R.J. High incidence of later-onset fabry disease revealed by newborn screening. Am. J. Hum. Genet. 2006;79(1):31–40. doi: 10.1086/504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linhart A., Paleček T. Narrative review on Morbus Fabry: Diagnosis and management of cardiac manifestations. Cardiovasc. Diagn. Ther. 2021;11(2):650–660. doi: 10.21037/cdt-20-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felis A., Whitlow M., Kraus A., Warnock D.G., Wallace E. Current and investigational therapeutics for fabry disease. Kidney Int. Rep. 2019;5(4):407–413. doi: 10.1016/j.ekir.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenders M., Brand E. Fabry disease: The current treatment landscape. Drugs. 2021;81(6):635–645. doi: 10.1007/s40265-021-01486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carubbi F., Bonilauri L. Fabry disease: Raising awareness of the disease among physicians. Intern. Emerg. Med. 2012;7(S3) Suppl. 3:S227–S231. doi: 10.1007/s11739-012-0821-x. [DOI] [PubMed] [Google Scholar]
- 16.Ortiz A., Germain D.P., Desnick R.J., Politei J., Mauer M., Burlina A., Eng C., Hopkin R.J., Laney D., Linhart A., Waldek S., Wallace E., Weidemann F., Wilcox W.R. Fabry disease revisited: Management and treatment recommendations for adult patients. Mol. Genet. Metab. 2018;123(4):416–427. doi: 10.1016/j.ymgme.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Green B.N., Johnson C.D., Adams A. Writing narrative literature reviews for peer-reviewed journals: Secrets of the trade. J. Chiropr. Med. 2006;5(3):101–117. doi: 10.1016/S0899-3467(07)60142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiffmann R., Warnock D.G., Banikazemi M., Bultas J., Linthorst G.E., Packman S., Sorensen S.A., Wilcox W.R., Desnick R.J. Fabry disease: Progression of nephropathy, and prevalence of cardiac and cerebrovascular events before enzyme replacement therapy. Nephrol. Dial. Transplant. 2009;24(7):2102–2111. doi: 10.1093/ndt/gfp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Senechal M., Germain D.P. Fabry disease: A functional and anatomical study of cardiac manifestations in 20 hemizygous male patients. Clin. Genet. 2003;63(1):46–52. doi: 10.1034/j.1399-0004.2003.630107.x. [DOI] [PubMed] [Google Scholar]
- 20.Linhart A., Kampmann C., Zamorano J.L., Sunder-Plassmann G., Beck M., Mehta A., Elliott P.M. Cardiac manifestations of Anderson-Fabry disease: Results from the international Fabry outcome survey. Eur. Heart J. 2007;28(10):1228–1235. doi: 10.1093/eurheartj/ehm153. [DOI] [PubMed] [Google Scholar]
- 21.Linhart A., Germain D.P., Olivotto I., Akhtar M.M., Anastasakis A., Hughes D., Namdar M., Pieroni M., Hagège A., Cecchi F., Gimeno J.R., Limongelli G., Elliott P. An expert consensus document on the management of cardiovascular manifestations of Fabry disease. Eur. J. Heart Fail. 2020;22(7):1076–1096. doi: 10.1002/ejhf.1960. [DOI] [PubMed] [Google Scholar]
- 22.Anastasakis A., Papatheodorou E., Steriotis A.K. Fabry disease and cardiovascular involvement. Curr. Pharm. Des. 2013;19(33):5997–6008. doi: 10.2174/13816128113199990353. [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa H., Takano H., Shindo S., Takeda S., Funabashi N., Nakagawa K., Toyozaki T., Kuwabara Y., Komuro I. Images in cardiovascular medicine. Transition from left ventricular hypertrophy to massive fibrosis in the cardiac variant of Fabry disease. Circulation. 2006;113(16):e720–e721. doi: 10.1161/CIRCULATIONAHA.105.584292. [DOI] [PubMed] [Google Scholar]
- 24.Palecek T., Dostalova G., Kuchynka P., Karetova D., Bultas J., Elleder M., Linhart A. Right ventricular involvement in Fabry disease. J. Am. Soc. Echocardiogr. 2008;21(11):1265–1268. doi: 10.1016/j.echo.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Kampmann C., Linhart A., Baehner F., Palecek T., Wiethoff C.M., Miebach E., Whybra C., Gal A., Bultas J., Beck M. Onset and progression of the Anderson-Fabry disease related cardiomyopathy. Int. J. Cardiol. 2008;130(3):367–373. doi: 10.1016/j.ijcard.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Takenaka T., Teraguchi H., Yoshida A., Taguchi S., Ninomiya K., Umekita Y., Yoshida H., Horinouchi M., Tabata K., Yonezawa S., Yoshimitsu M., Higuchi K., Nakao S., Anan R., Minagoe S., Tei C. Terminal stage cardiac findings in patients with cardiac Fabry disease: An electrocardiographic, echocardiographic, and autopsy study. J. Cardiol. 2008;51(1):50–59. doi: 10.1016/j.jjcc.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa K., Sugamata K., Funamoto N., Abe T., Sato T., Nagashima K., Ohkawa S. Restricted accumulation of globotriaosylceramide in the hearts of atypical cases of Fabry’s disease. Hum. Pathol. 1990;21(10):1067–1073. doi: 10.1016/0046-8177(90)90258-7. [DOI] [PubMed] [Google Scholar]
- 28.Nakao S., Takenaka T., Maeda M., Kodama C., Tanaka A., Tahara M., Yoshida A., Kuriyama M., Hayashibe H., Sakuraba H., Tanaka H. An atypical variant of Fabry’s disease in men with left ventricular hypertrophy. N. Engl. J. Med. 1995;333(5):288–293. doi: 10.1056/NEJM199508033330504. [DOI] [PubMed] [Google Scholar]
- 29.Kubo T. Fabry disease and its cardiac involvement. J. Gen. Fam. Med. 2017;18(5):225–229. doi: 10.1002/jgf2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta A., Ricci R., Widmer U., Dehout F., Garcia de Lorenzo A., Kampmann C., Linhart A., Sunder-Plassmann G., Ries M., Beck M. Fabry disease defined: Baseline clinical manifestations of 366 patients in the Fabry Outcome Survey. Eur. J. Clin. Invest. 2004;34(3):236–242. doi: 10.1111/j.1365-2362.2004.01309.x. [DOI] [PubMed] [Google Scholar]
- 31.Wanner C., Oliveira J.P., Ortiz A., Mauer M., Germain D.P., Linthorst G.E., Serra A.L., Maródi L., Mignani R., Cianciaruso B., Vujkovac B., Lemay R., Beitner-Johnson D., Waldek S., Warnock D.G. Prognostic indicators of renal disease progression in adults with Fabry disease: Natural history data from the Fabry Registry. Clin. J. Am. Soc. Nephrol. 2010;5(12):2220–2228. doi: 10.2215/CJN.04340510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fervenza F.C., Torra R., Lager D.J. Fabry disease: An underrecognized cause of proteinuria. Kidney Int. 2008;73(10):1193–1199. doi: 10.1038/sj.ki.5002677. [DOI] [PubMed] [Google Scholar]
- 33.Tsakiris D., Simpson H.K., Jones E.H., Briggs J.D., Elinder C.G., Mendel S., Piccoli G., dos Santos J.P., Tognoni G., Vanrenterghem Y., Valderrabano F. Report on management of renale failure in Europe, XXVI, 1995. Rare diseases in renal replacement therapy in the ERA-EDTA Registry. Nephrol. Dial. Transplant. 1996;11(Suppl. 7):4–20. doi: 10.1093/ndt/11.supp7.4. [DOI] [PubMed] [Google Scholar]
- 34.Thadhani R., Wolf M., West M.L., Tonelli M., Ruthazer R., Pastores G.M., Obrador G.T. Patients with Fabry disease on dialysis in the United States. Kidney Int. 2002;61(1):249–255. doi: 10.1046/j.1523-1755.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- 35.Ranieri M., Bedini G., Parati E.A., Bersano A. Fabry disease: Recognition, diagnosis, and treatment of neurological features. Curr. Treat. Options Neurol. 2016;18(7):33. doi: 10.1007/s11940-016-0414-5. [DOI] [PubMed] [Google Scholar]
- 36.Mitsias P., Levine S.R. Cerebrovascular complications of Fabry’s disease. Ann. Neurol. 1996;40(1):8–17. doi: 10.1002/ana.410400105. [DOI] [PubMed] [Google Scholar]
- 37.Mendez M.F., Stanley T.M., Medel N.M., Li Z., Tedesco D.T. The vascular dementia of Fabry’s disease. Dement. Geriatr. Cogn. Disord. 1997;8(4):252–257. doi: 10.1159/000106640. [DOI] [PubMed] [Google Scholar]
- 38.Sims K., Politei J., Banikazemi M., Lee P. Stroke in Fabry disease frequently occurs before diagnosis and in the absence of other clinical events: Natural history data from the Fabry Registry. Stroke. 2009;40(3):788–794. doi: 10.1161/STROKEAHA.108.526293. [DOI] [PubMed] [Google Scholar]
- 39.Ortiz J.F., Parwani J., Millhouse P.W., Eissa-Garcés A., Hassen G., Cuenca V.D., Alzamora I.M., Khurana M., Herrera-Bucheli D., Altamimi A., Atoot A., Cueva W. Prevalence of Fabry disease in patients with cryptogenic strokes: A systematic review. Cureus. 2021;13(11):e19358. doi: 10.7759/cureus.19358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rolfs A., Böttcher T., Zschiesche M., Morris P., Winchester B., Bauer P., Walter U., Mix E., Löhr M., Harzer K., Strauss U., Pahnke J., Grossmann A., Benecke R. Prevalence of Fabry disease in patients with cryptogenic stroke: A prospective study. Lancet. 2005;366(9499):1794–1796. doi: 10.1016/S0140-6736(05)67635-0. [DOI] [PubMed] [Google Scholar]
- 41.Ellaway C. Paediatric Fabry disease. Transl. Pediatr. 2016;5(1):37–42. doi: 10.3978/j.issn.2224-4336.2015.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ries M., Gupta S., Moore D.F., Sachdev V., Quirk J.M., Murray G.J., Rosing D.R., Robinson C., Schaefer E., Gal A., Dambrosia J.M., Garman S.C., Brady R.O., Schiffmann R. Pediatric Fabry disease. Pediatrics. 2005;115(3):e344–e355. doi: 10.1542/peds.2004-1678. [DOI] [PubMed] [Google Scholar]
- 43.Ries M., Ramaswami U., Parini R., Lindblad B., Whybra C., Willers I., Gal A., Beck M. The early clinical phenotype of Fabry disease: A study on 35 European children and adolescents. Eur. J. Pediatr. 2003;162(11):767–772. doi: 10.1007/s00431-003-1299-3. [DOI] [PubMed] [Google Scholar]
- 44.Dütsch M., Marthol H., Stemper B., Brys M., Haendl T., Hilz M.J. Small fiber dysfunction predominates in Fabry neuropathy. J. Clin. Neurophysiol. 2002;19(6):575–586. doi: 10.1097/00004691-200212000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Luciano C.A., Russell J.W., Banerjee T.K., Quirk J.M., Scott L.J., Dambrosia J.M., Barton N.W., Schiffmann R. Physiological characterization of neuropathy in Fabry’s disease. Muscle Nerve. 2002;26(5):622–629. doi: 10.1002/mus.10236. [DOI] [PubMed] [Google Scholar]
- 46.Vadher P., Agarwal P., Mistry A., Gajjar K., Bansal N., Neazee S. Angiokeratoma corporis diffusum: An uncommon case with suspected anderson fabry disease. Indian Dermatol. Online J. 2020;11(2):212–215. doi: 10.4103/idoj.IDOJ_136_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Möhrenschlager M., Braun-Falco M., Ring J., Abeck D. Fabry disease: Recognition and management of cutaneous manifestations. Am. J. Clin. Dermatol. 2003;4(3):189–196. doi: 10.2165/00128071-200304030-00005. [DOI] [PubMed] [Google Scholar]
- 48.Larralde M., Boggio P., Amartino H., Chamoles N. Fabry disease: A study of 6 hemizygous men and 5 heterozygous women with emphasis on dermatologic manifestations. Arch. Dermatol. 2004;140(12):1440–1446. doi: 10.1001/archderm.140.12.1440. [DOI] [PubMed] [Google Scholar]
- 49.Anderson W. A case of angeio-keratoma. 1898;10(4):113-117. [Google Scholar]
- 50.Cox-Brinkman J., Vedder A., Hollak C., Richfield L., Mehta A., Orteu K., Wijburg F., Hammond P. Three-dimensional face shape in Fabry disease. Eur. J. Hum. Genet. 2007;15(5):535–542. doi: 10.1038/sj.ejhg.5201798. [DOI] [PubMed] [Google Scholar]
- 51.Ries M., Moore D.F., Robinson C.J., Tifft C.J., Rosenbaum K.N., Brady R.O., Schiffmann R. Krasnewich, D Quantitative dysmorphology assessment in Fabry disease. Genet. Med. 2006;8(2):96–101. doi: 10.1097/01.gim.0000200950.25118.dd. [DOI] [PubMed] [Google Scholar]
- 52.Sodi A., Ioannidis A.S., Mehta A., Davey C., Beck M., Pitz S. Ocular manifestations of Fabry’s disease: Data from the Fabry Outcome Survey. Br. J. Ophthalmol. 2007;91(2):210–214. doi: 10.1136/bjo.2006.100602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moiseev S.V., Ismailova D.S., Moiseev A.S., Bulanov N.M., Karovaikina E.A., Nosova N.R., Fomin V.V. Cornea verticillata in Fabry disease. Ter. Arkh. 2018;90(12):17–22. doi: 10.26442/00403660.2018.12.000003. [DOI] [PubMed] [Google Scholar]
- 54.Spaeth G.L., Frost P. Fabry’s disease. Its ocular manifestations. Arch. Ophthalmol. 1965;74(6):760–769. doi: 10.1001/archopht.1965.00970040762005. [DOI] [PubMed] [Google Scholar]
- 55.Conti G., Sergi B. Auditory and vestibular findings in Fabry disease: A study of hemizygous males and heterozygous females. Acta paediatrica. 2003;92(443):33–37. doi: 10.1111/j.1651-2227.2003.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 56.Germain D.P., Avan P., Chassaing A., Bonfils P. Patients affected with Fabry disease have an increased incidence of progressive hearing loss and sudden deafness: An investigation of twenty-two hemizygous male patients. BMC Med. Genet. 2002;3(1):10. doi: 10.1186/1471-2350-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Magage S., Lubanda J.C., Susa Z., Bultas J., Karetová D., Dobrovolný R., Hrebícek M., Germain D.P., Linhart A. Natural history of the respiratory involvement in Anderson-Fabry disease. J. Inherit. Metab. Dis. 2007;30(5):790–799. doi: 10.1007/s10545-007-0616-9. [DOI] [PubMed] [Google Scholar]
- 58.Rosenberg D.M., Ferrans V.J., Fulmer J.D., Line B.R., Barranger J.A., Brady R.O., Crystal R.G. Chronic airflow obstruction in Fabry’s disease. Am. J. Med. 1980;68(6):898–905. doi: 10.1016/0002-9343(80)90224-7. [DOI] [PubMed] [Google Scholar]
- 59.Mersebach H., Johansson J.O., Rasmussen A.K., Bengtsson B.A., Rosenberg K., Hasholt L., Sørensen S.A., Sørensen S.S., Feldt-Rasmussen U. Osteopenia: A common aspect of Fabry disease. Predictors of bone mineral density. Genet. Med. 2007;9(12):812–818. doi: 10.1097/gim.0b013e31815cb197. [DOI] [PubMed] [Google Scholar]
- 60.Kleinert J., Dehout F., Schwarting A., de Lorenzo A.G., Ricci R., Kampmann C., Beck M., Ramaswami U., Linhart A., Gal A., Houge G., Widmer U., Mehta A., Sunder-Plassmann G. Anemia is a new complication in Fabry disease: Data from the fabry outcome survey. Kidney Int. 2005;67(5):1955–1960. doi: 10.1111/j.1523-1755.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- 61.Hauser A.C., Gessl A., Lorenz M., Voigtländer T., Födinger M., Sunder-Plassmann G. High prevalence of subclinical hypothyroidism in patients with Anderson-Fabry disease. J. Inherit. Metab. Dis. 2005;28(5):715–722. doi: 10.1007/s10545-005-0003-3. [DOI] [PubMed] [Google Scholar]
- 62.Papaxanthos-Roche A., Deminière C., Bauduer F., Hocké C., Mayer G., Lacombe D. Azoospermia as a new feature of Fabry disease. Fertil. Steril. 2007;88(1):212.e15–212.e18. doi: 10.1016/j.fertnstert.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 63.Foda M.M., Mahmood K., Rasuli P., Dunlap H., Kiruluta G., Schillinger J.F. High-flow priapism associated with Fabry’s disease in a child: A case report and review of the literature. Urology. 1996;48(6):949–952. doi: 10.1016/S0090-4295(96)00320-2. [DOI] [PubMed] [Google Scholar]
- 64.Mayes J.S., Scheerer J.B., Sifers R.N., Donaldson M.L. Differential assay for lysosomal alpha-galactosidases in human tissues and its application to Fabry’s disease. Clin. Chim. Acta. 1981;112(2):247–251. doi: 10.1016/0009-8981(81)90384-3. [DOI] [PubMed] [Google Scholar]
- 65.Linthorst G.E., Vedder A.C., Aerts J.M., Hollak C.E. Screening for Fabry disease using whole blood spots fails to identify one-third of female carriers. Clinica Chimica Acta. 2005;353(1-2):201–203. doi: 10.1016/j.cccn.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 66.Stiles A.R., Zhang H., Dai J., McCaw P., Beasley J., Rehder C., Koeberl D.D., McDonald M., Bali D.S., Young S.P. A comprehensive testing algorithm for the diagnosis of Fabry disease in males and females. Mol. Genet. Metab. 2020;130(3):209–214. doi: 10.1016/j.ymgme.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 67.Germain D.P., Benistan K., Angelova L. X-linked inheritance and its implication in the diagnosis and management of female patients in Fabry disease. Rev. Med. Interne. 2010;31(Suppl. 2):S209–S213. doi: 10.1016/S0248-8663(10)70013-8. [DOI] [PubMed] [Google Scholar]
- 68.Vardarli I., Rischpler C., Herrmann K., Weidemann F. Diagnosis and screening of patients with Fabry disease. Ther. Clin. Risk Manag. 2020;16:551–558. doi: 10.2147/TCRM.S247814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nowak A., Mechtler T.P., Desnick R.J., Kasper D.C. Plasma LysoGb3: A useful biomarker for the diagnosis and treatment of Fabry disease heterozygotes. Mol. Genet. Metab. 2017;120(1-2):57–61. doi: 10.1016/j.ymgme.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 70.Smid B.E., van der Tol L., Biegstraaten M., Linthorst G.E., Hollak C.E., Poorthuis B.J. Plasma globotriaosylsphingosine in relation to phenotypes of Fabry disease. J. Med. Genet. 2015;52(4):262–268. doi: 10.1136/jmedgenet-2014-102872. [DOI] [PubMed] [Google Scholar]
- 71.Tschöpe C., Dominguez F., Canaan-Kühl S., Blaschke D., Kühl U., Pieske B., Haverkamp W. Endomyocardial biopsy in Anderson-Fabry disease: The key in uncertain cases. Int. J. Cardiol. 2015;190:284–286. doi: 10.1016/j.ijcard.2015.04.130. [DOI] [PubMed] [Google Scholar]
- 72.Navarro C., Teijeira S., Dominguez C., Fernandez J.M., Rivas E., Fachal C., Barrera S., Rodriguez C., Iranzo P. Fabry disease: An ultrastructural comparative study of skin in hemizygous and heterozygous patients. Acta Neuropathol. 2006;111(2):178–185. doi: 10.1007/s00401-005-0026-8. [DOI] [PubMed] [Google Scholar]
- 73.Laney D.A., Bennett R.L., Clarke V., Fox A., Hopkin R.J., Johnson J., O’Rourke E., Sims K., Walter G. Fabry disease practice guidelines: Recommendations of the National Society of Genetic Counselors. J. Genet. Couns. 2013;22(5):555–564. doi: 10.1007/s10897-013-9613-3. [DOI] [PubMed] [Google Scholar]
- 74.Gomes I., Nora D.B., Becker J., Ehlers J.A., Schwartz I.V., Giugliani R., Ashton-Prolla P., Jardim L. Nerve conduction studies, electromyography and sympathetic skin response in Fabry’s disease. J. Neurol. Sci. 2003;214(1-2):21–25. doi: 10.1016/S0022-510X(03)00172-2. [DOI] [PubMed] [Google Scholar]
- 75.Üçeyler N., He L., Schönfeld D., Kahn A.K., Reiners K., Hilz M.J., Breunig F., Sommer C. Small fibers in Fabry disease: Baseline and follow-up data under enzyme replacement therapy. J. Peripher. Nerv. Syst. 2011;16(4):304–314. doi: 10.1111/j.1529-8027.2011.00365.x. [DOI] [PubMed] [Google Scholar]
- 76.Cocozza S., Russo C., Pontillo G., Pisani A., Brunetti A. Neuroimaging in Fabry disease: Current knowledge and future directions. Insights Imaging. 2018;9(6):1077–1088. doi: 10.1007/s13244-018-0664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Glass R.B., Astrin K.H., Norton K.I., Parsons R., Eng C.M., Banikazemi M., Desnick R.J. Fabry disease: Renal sonographic and magnetic resonance imaging findings in affected males and carrier females with the classic and cardiac variant phenotypes. J. Comput. Assist. Tomogr. 2004;28(2):158–168. doi: 10.1097/00004728-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 78.Imbriaco M., Nappi C., Ponsiglione A., Pisani A., Dell’Aversana S., Nicolai E., Spinelli L., Aiello M., Diomiaiuti C.T., Riccio E., Esposito R., Galderisi M., Losi M., Greiser A., Chow K., Cuocolo A. Hybrid positron emission tomography-magnetic resonance imaging for assessing different stages of cardiac impairment in patients with Anderson-Fabry disease: AFFINITY study group. Eur. Heart J. Cardiovasc. Imaging. 2019;20(9):1004–1011. doi: 10.1093/ehjci/jez039. [DOI] [PubMed] [Google Scholar]
- 79.Linhart A., Elliott P.M. The heart in Anderson-Fabry disease and other lysosomal storage disorders. Heart. 2007;93(4):528–535. doi: 10.1136/hrt.2005.063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ikeda K., Hirayama M., Hirota Y., Asa E., Seki J., Tanaka Y. Drug-induced phospholipidosis is caused by blockade of mannose 6-phosphate receptor-mediated targeting of lysosomal enzymes. Biochem. Biophys. Res. Commun. 2008;377(1):268–274. doi: 10.1016/j.bbrc.2008.09.121. [DOI] [PubMed] [Google Scholar]
- 81.Cantor W.J., Daly P., Iwanochko M., Clarke J.T., Cusimano R.J., Butany J. Cardiac transplantation for Fabry’s disease. Can. J. Cardiol. 1998;14(1):81–84. [PubMed] [Google Scholar]
- 82.Mehra M.R., Canter C.E., Hannan M.M., Semigran M.J., Uber P.A., Baran D.A., Danziger-Isakov L., Kirklin J.K., Kirk R., Kushwaha S.S., Lund L.H., Potena L., Ross H.J., Taylor D.O., Verschuuren E.A.M., Zuckermann A. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J. Heart Lung Transplant. 2016;35(1):1–23. doi: 10.1016/j.healun.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 83.Karras A., De Lentdecker P., Delahousse M., Debauchez M., Tricot L., Pastural M., Bruneval P., Zemoura L., Duong Van Huyen J.P., Lidove O. Combined heart and kidney transplantation in a patient with Fabry disease in the enzyme replacement therapy era. Am. J. Transplant. 2008;8(6):1345–1348. doi: 10.1111/j.1600-6143.2008.02245.x. [DOI] [PubMed] [Google Scholar]
- 84.Rajagopalan N., Dennis D.R., O’Connor W. Successful combined heart and kidney transplantation in patient with Fabry’s disease: A case report. Transplant. Proc. 2019;51(9):3171–3173. doi: 10.1016/j.transproceed.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 85.Tran Ba S.N., Lidove O., Dorent R., Debauchez M., Nataf P., Delahousse M., Karras A., Azeroual L., De Lentdecker P., Chauveheid M.P., Sené T., Ziza J.M. Combined heart and kidney transplantation in Fabry’s disease: Long-term outcomes in two patients. Rev. Med. Interne. 2017;38(2):137–142. doi: 10.1016/j.revmed.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 86.Verocai F., Clarke J.T., Iwanochko R.M. Case report: Long-term outcome post-heart transplantation in a woman with Fabry’s disease. J. Inherit. Metab. Dis. 2010;33(S3) Suppl. 3:S385–S387. doi: 10.1007/s10545-010-9194-3. [DOI] [PubMed] [Google Scholar]
- 87.Di Nora C., Livi U. Heart transplantation in cardiac storage diseases: Data on Fabry disease and cardiac amyloidosis. Curr. Opin. Organ Transplant. 2020;25(3):211–217. doi: 10.1097/MOT.0000000000000756. [DOI] [PubMed] [Google Scholar]
- 88.Azevedo O., Cordeiro F., Gago M.F., Miltenberger-Miltenyi G., Ferreira C., Sousa N., Cunha D. Fabry disease and the heart: A comprehensive review. Int. J. Mol. Sci. 2021;22(9):4434. doi: 10.3390/ijms22094434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Warnock D.G., Thomas C.P., Vujkovac B., Campbell R.C., Charrow J., Laney D.A., Jackson L.L., Wilcox W.R., Wanner C. Antiproteinuric therapy and Fabry nephropathy: Factors associated with preserved kidney function during agalsidase-beta therapy. J. Med. Genet. 2015;52(12):860–866. doi: 10.1136/jmedgenet-2015-103471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wanner C., Breunig F. Fabry nephropathy and the case for adjunctive renal therapy. J. Am. Soc. Nephrol. 2007;18(9):2426–2428. doi: 10.1681/ASN.2007070783. [DOI] [PubMed] [Google Scholar]
- 91.Tahir H., Jackson L.L., Warnock D.G. Antiproteinuric Therapy and Fabry Nephropathy: Sustained Reduction of Proteinuria in Patients Receiving Enzyme Replacement Therapy with Agalsidase-β. 2007;18(9):2609-2617. doi: 10.1681/ASN.2006121400. [DOI] [PubMed] [Google Scholar]
- 92.Jain G., Warnock D.G. Blood pressure, proteinuria and nephropathy in Fabry disease. Nephron Clin. Pract. 2011;118(1):c43–c48. doi: 10.1159/000320903. [DOI] [PubMed] [Google Scholar]
- 93.Waldek S., Feriozzi S. Fabry nephropathy: A review - how can we optimize the management of Fabry nephropathy? BMC Nephrol. 2014;15(1):72–72. doi: 10.1186/1471-2369-15-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mende C.W. Chronic kidney disease and SGLT2 inhibitors: A review of the evolving treatment landscape. Adv. Ther. 2022;39(1):148–164. doi: 10.1007/s12325-021-01994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hundertmark M.J., Agbaje O.F., Coleman R., George J.T., Grempler R., Holman R.R., Lamlum H., Lee J., Milton J.E., Niessen H.G., Rider O., Rodgers C.T., Valkovič L., Wicks E., Mahmod M., Neubauer S. Design and rationale of the EMPA-VISION trial: Investigating the metabolic effects of empagliflozin in patients with heart failure. ESC Heart Fail. 2021;8(4):2580–2590. doi: 10.1002/ehf2.13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mignani R., Feriozzi S., Schaefer R.M., Breunig F., Oliveira J.P., Ruggenenti P., Sunder-Plassmann G. Dialysis and transplantation in Fabry disease: Indications for enzyme replacement therapy. Clin. J. Am. Soc. Nephrol. 2010;5(2):379–385. doi: 10.2215/CJN.05570809. [DOI] [PubMed] [Google Scholar]
- 97.Cybulla M., Kurschat C., West M., Nicholls K., Torras J., Sunder-Plassmann G., Feriozzi S. Kidney transplantation and enzyme replacement therapy in patients with Fabry disease. J. Nephrol. 2013;26(4):645–651. doi: 10.5301/jn.5000214. [DOI] [PubMed] [Google Scholar]
- 98.Shah T., Gill J., Malhotra N., Takemoto S.K., Bunnapradist S. Kidney transplant outcomes in patients with Fabry disease. Transplantation. 2009;87(2):280–285. doi: 10.1097/TP.0b013e318191a842. [DOI] [PubMed] [Google Scholar]
- 99.Suarez M.L.G., Thongprayoon C., Hansrivijit P., Medaura J., Vaitla P., Mao M.A., Bathini T., Boonpheng B., Kanduri S.R., Kovvuru K., Basu A., Cheungpasitporn W. Outcomes of kidney transplantation in Fabry disease: A meta-analysis. Diseases. 2020;9(1):2. doi: 10.3390/diseases9010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Capelli I., Aiello V., Gasperoni L., Comai G., Corradetti V., Ravaioli M., Biagini E., Graziano C., La Manna G. Kidney transplant in Fabry disease: A revision of the literature. Medicina (Kaunas) 2020;56(6):284. doi: 10.3390/medicina56060284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Politei J.M., Bouhassira D., Germain D.P., Goizet C., Guerrero-Sola A., Hilz M.J., Hutton E.J., Karaa A., Liguori R., Üçeyler N., Zeltzer L.K., Burlina A. Pain in Fabry disease: Practical recommendations for diagnosis and treatment. CNS Neurosci. Ther. 2016;22(7):568–576. doi: 10.1111/cns.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sommer C., Uçeyler N., Duning T., Arning K., Baron R., Brand E., Canaan-Kühl S., Hilz M., Naleschinski D., Wanner C., Weidemann F. Pain therapy for Fabry’s disease. Internist (Berl.) 2013;54(1):121–122, 124-130. doi: 10.1007/s00108-012-3204-5. [DOI] [PubMed] [Google Scholar]
- 103.Schuller Y., Linthorst G.E., Hollak C.E.M., Van Schaik I.N., Biegstraaten M. Pain management strategies for neuropathic pain in Fabry disease--a systematic review. BMC Neurol. 2016;16(1):25–25. doi: 10.1186/s12883-016-0549-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eng C.M., Germain D.P., Banikazemi M., Warnock D.G., Wanner C., Hopkin R.J., Bultas J., Lee P., Sims K., Brodie S.E., Pastores G.M., Strotmann J.M. Wilcox, WR Fabry disease: Guidelines for the evaluation and management of multi-organ system involvement. Genet. Med. 2006;8(9):539–548. doi: 10.1097/01.gim.0000237866.70357.c6. [DOI] [PubMed] [Google Scholar]
- 105.Theodorou A., Palaiodimou L., Kokotis P., Papadopoulou M., Fradelos S., Voudouri A., Zompola C., Magoufis G., Arvaniti C., Bonakis A., Tsivgoulis G. Teaching NeuroImages: An uncommon cause of carotid artery dissection: Fabry disease. Neurology. 2020;95(19):e2711–e2713. doi: 10.1212/WNL.0000000000010650. [DOI] [PubMed] [Google Scholar]
- 106.Kargiotis O., Psychogios K., Safouris A., Kalyvas P., Magoufis G., Stamboulis E., Tsivgoulis G. Intravenous thrombolysis for acute ischemic stroke in Fabry disease. Neurologist. 2019;24(5):146–149. doi: 10.1097/NRL.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 107.Zenone T., Chan V. Young woman with recurrent ischemic strokes diagnosed as Fabry disease: Lessons learned from a case report. Clin. Neurol. Neurosurg. 2011;113(7):586–588. doi: 10.1016/j.clineuro.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 108.Saarinen J.T., Sillanpää N., Kantola I. A male Fabry disease patient treated with intravenous thrombolysis for acute ischemic stroke. J. Clin. Neurosci. 2015;22(2):423–425. doi: 10.1016/j.jocn.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 109. Replagal (agalsidase alfa). European Public Assessment Report (EPAR). Available from: http://www.emea.europa.eu/humandocs/Humans/EPAR/replagal/replagal.htm (Accessed on Mar 22, 2022).
- 110. Fabrazyme (agalsidase beta); European Public Assessment Report (EPAR). Available from: http://www.emea.europa.eu/humandocs/Humans/EPAR/fabrazyme/fabrazyme.htm (Accessed on Mar 22, 2022).
- 111.Pastores G.M. Agalsidase alfa (Replagal) in the treatment of Anderson-Fabry disease. Biologics. 2007;1(3):291–300. [PMC free article] [PubMed] [Google Scholar]
- 112.Schiffmann R., Murray G.J., Treco D., Daniel P., Sellos-Moura M., Myers M., Quirk J.M., Zirzow G.C., Borowski M., Loveday K., Anderson T., Gillespie F., Oliver K.L., Jeffries N.O., Doo E., Liang T.J., Kreps C., Gunter K., Frei K., Crutchfield K., Selden R.F., Brady R.O. Infusion of alpha-galactosidase A reduces tissue globotriaosylceramide storage in patients with Fabry disease. Proc. Natl. Acad. Sci. USA. 2000;97(1):365–370. doi: 10.1073/pnas.97.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schiffmann R., Kopp J.B., Austin H.A., III, Sabnis S., Moore D.F., Weibel T., Balow J.E., Brady R.O. Enzyme replacement therapy in Fabry disease: A randomized controlled trial. JAMA. 2001;285(21):2743–2749. doi: 10.1001/jama.285.21.2743. [DOI] [PubMed] [Google Scholar]
- 114.Hughes D.A., Elliott P.M., Shah J., Zuckerman J., Coghlan G., Brookes J., Mehta A.B. Effects of enzyme replacement therapy on the cardiomyopathy of Anderson-Fabry disease: A randomised, double-blind, placebo-controlled clinical trial of agalsidase alfa. Heart. 2008;94(2):153–158. doi: 10.1136/hrt.2006.104026. [DOI] [PubMed] [Google Scholar]
- 115.Schiffmann R., Pastores G.M., Lien Y-H.H., Castaneda V., Chang P., Martin R., Wijatyk A. Agalsidase alfa in pediatric patients with Fabry disease: A 6.5-year open-label follow-up study. Orphanet J. Rare Dis. 2014;9(1):169–169. doi: 10.1186/s13023-014-0169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schiffmann R., Ries M., Timmons M., Flaherty J.T., Brady R.O. Long-term therapy with agalsidase alfa for Fabry disease: Safety and effects on renal function in a home infusion setting. Nephrol. Dial. Transplant. 2006;21(2):345–354. doi: 10.1093/ndt/gfi152. [DOI] [PubMed] [Google Scholar]
- 117.Feriozzi S., Schwarting A., Sunder-Plassmann G., West M., Cybulla M. Agalsidase alfa slows the decline in renal function in patients with Fabry disease. Am. J. Nephrol. 2009;29(5):353–361. doi: 10.1159/000168482. [DOI] [PubMed] [Google Scholar]
- 118.Feriozzi S., Torras J., Cybulla M., Nicholls K., Sunder-Plassmann G., West M., Investigators F.O.S. The effectiveness of long-term agalsidase alfa therapy in the treatment of Fabry nephropathy. Clin. J. Am. Soc. Nephrol. 2012;7(1):60–69. doi: 10.2215/CJN.03130411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hughes D.A., Barba Romero M.Á., Hollak C.E., Giugliani R., Deegan P.B. Response of women with Fabry disease to enzyme replacement therapy: Comparison with men, using data from FOS--the Fabry Outcome Survey. Mol. Genet. Metab. 2011;103(3):207–214. doi: 10.1016/j.ymgme.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 120.Arends M., Biegstraaten M., Hughes D.A., Mehta A., Elliott P.M., Oder D., Watkinson O.T., Vaz F.M., van Kuilenburg A.B.P., Wanner C., Hollak C.E.M. Retrospective study of long-term outcomes of enzyme replacement therapy in Fabry disease: Analysis of prognostic factors. PLoS One. 2017;12(8):e0182379. doi: 10.1371/journal.pone.0182379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Weidemann F., Niemann M., Breunig F., Herrmann S., Beer M., Störk S., Voelker W., Ertl G., Wanner C., Strotmann J. Long-term effects of enzyme replacement therapy on fabry cardiomyopathy: Evidence for a better outcome with early treatment. Circulation. 2009;119(4):524–529. doi: 10.1161/CIRCULATIONAHA.108.794529. [DOI] [PubMed] [Google Scholar]
- 122.Arends M., Wijburg F.A., Wanner C., Vaz F.M., van Kuilenburg A.B.P., Hughes D.A., Biegstraaten M., Mehta A., Hollak C.E.M., Langeveld M. Favourable effect of early versus late start of enzyme replacement therapy on plasma globotriaosylsphingosine levels in men with classical Fabry disease. Mol. Genet. Metab. 2017;121(2):157–161. doi: 10.1016/j.ymgme.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 123.Biegstraaten M., Arngrímsson R., Barbey F., Boks L., Cecchi F., Deegan P.B., Feldt-Rasmussen U., Geberhiwot T., Germain D.P., Hendriksz C., Hughes D.A., Kantola I., Karabul N., Lavery C., Linthorst G.E., Mehta A., van de Mheen E., Oliveira J.P., Parini R., Ramaswami U., Rudnicki M., Serra A., Sommer C., Sunder-Plassmann G., Svarstad E., Sweeb A., Terryn W., Tylki-Szymanska A., Tøndel C., Vujkovac B., Weidemann F., Wijburg F.A., Woolfson P., Hollak C.E.M. Recommendations for initiation and cessation of enzyme replacement therapy in patients with Fabry disease: The European Fabry Working Group consensus document. Orphanet J. Rare Dis. 2015;10(1):36–36. doi: 10.1186/s13023-015-0253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kritzer A., Siddharth A., Leestma K., Bodamer O. Early initiation of enzyme replacement therapy in classical Fabry disease normalizes biomarkers in clinically asymptomatic pediatric patients. Mol. Genet. Metab. Rep. 2019;21:100530–100530. doi: 10.1016/j.ymgmr.2019.100530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Germain D.P., Fouilhoux A., Decramer S., Tardieu M., Pillet P., Fila M., Rivera S., Deschênes G., Lacombe D. Consensus recommendations for diagnosis, management and treatment of Fabry disease in paediatric patients. Clin. Genet. 2019;96(2):107–117. doi: 10.1111/cge.13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. ClinicalTrials.gov. A phase II comparability study between Replagal ® produced from agalsidase alfa manufactured by 2 different processes in adult male patients with fabry disease. NCT: NCT01304277, Available from: https://clinicaltrials.gov/ct2/show/study/NCT01304277 Accessed on Mar 22, 2022.
- 127.Khan A., Sirrs S.M., Bichet D.G., Morel C.F., Tocoian A., Lan L., West M.L. The safety of agalsidase alfa enzyme replacement therapy in Canadian patients with fabry disease following implementation of a bioreactor process. Drugs R D. 2021;21(4):385–397. doi: 10.1007/s40268-021-00361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goker-Alpan O., Longo N., McDonald M., Shankar S.P., Schiffmann R., Chang P., Shen Y., Pano A. An open-label clinical trial of agalsidase alfa enzyme replacement therapy in children with Fabry disease who are naïve to enzyme replacement therapy. Drug Des. Devel. Ther. 2016;10:1771–1781. doi: 10.2147/DDDT.S102761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Keating G.M., Simpson D. Agalsidase Beta: A review of its use in the management of Fabry disease. Drugs. 2007;67(3):435–455. doi: 10.2165/00003495-200767030-00007. [DOI] [PubMed] [Google Scholar]
- 130.Matsuura F., Ohta M., Ioannou Y.A., Desnick R.J. Human alpha-galactosidase A: Characterization of the N-linked oligosaccharides on the intracellular and secreted glycoforms overexpressed by Chinese hamster ovary cells. Glycobiology. 1998;8(4):329–339. doi: 10.1093/glycob/8.4.329. [DOI] [PubMed] [Google Scholar]
- 131.Eng C.M., Banikazemi M., Gordon R.E., Goldman M., Phelps R., Kim L., Gass A., Winston J., Dikman S., Fallon J.T., Brodie S., Stacy C.B., Mehta D., Parsons R., Norton K., O’Callaghan M., Desnick R.J. A phase 1/2 clinical trial of enzyme replacement in fabry disease: Pharmacokinetic, substrate clearance, and safety studies. Am. J. Hum. Genet. 2001;68(3):711–722. doi: 10.1086/318809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Eng C.M., Guffon N., Wilcox W.R., Germain D.P., Lee P., Waldek S., Caplan L., Linthorst G.E., Desnick R.J. Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry’s disease. N. Engl. J. Med. 2001;345(1):9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- 133.Banikazemi M., Bultas J., Waldek S., Wilcox W.R., Whitley C.B., McDonald M., Finkel R., Packman S., Bichet D.G., Warnock D.G., Desnick R.J. Agalsidase-beta therapy for advanced Fabry disease: A randomized trial. Ann. Intern. Med. 2007;146(2):77–86. doi: 10.7326/0003-4819-146-2-200701160-00148. [DOI] [PubMed] [Google Scholar]
- 134.Waldek S. PR interval and the response to enzyme-replacement therapy for Fabry’s disease. N. Engl. J. Med. 2003;348(12):1186–1187. doi: 10.1056/NEJM200303203481224. [DOI] [PubMed] [Google Scholar]
- 135.Weidemann F., Breunig F., Beer M., Sandstede J., Turschner O., Voelker W., Ertl G., Knoll A., Wanner C., Strotmann J.M. Improvement of cardiac function during enzyme replacement therapy in patients with Fabry disease: A prospective strain rate imaging study. Circulation. 2003;108(11):1299–1301. doi: 10.1161/01.CIR.0000091253.71282.04. [DOI] [PubMed] [Google Scholar]
- 136.Spinelli L., Pisani A., Sabbatini M., Petretta M., Andreucci M.V., Procaccini D., Lo Surdo N., Federico S., Cianciaruso B. Enzyme replacement therapy with agalsidase beta improves cardiac involvement in Fabry’s disease. Clin. Genet. 2004;66(2):158–165. doi: 10.1111/j.1399-0004.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- 137.Wilcox W.R., Banikazemi M., Guffon N., Waldek S., Lee P., Linthorst G.E., Desnick R.J., Germain D.P. Long-term safety and efficacy of enzyme replacement therapy for Fabry disease. Am. J. Hum. Genet. 2004;75(1):65–74. doi: 10.1086/422366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Banikazemi M., Ullman T., Desnick R.J. Gastrointestinal manifestations of Fabry disease: Clinical response to enzyme replacement therapy. Mol. Genet. Metab. 2005;85(4):255–259. doi: 10.1016/j.ymgme.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 139.Breunig F., Weidemann F., Strotmann J., Knoll A., Wanner C. Clinical benefit of enzyme replacement therapy in Fabry disease. Kidney Int. 2006;69(7):1216–1221. doi: 10.1038/sj.ki.5000208. [DOI] [PubMed] [Google Scholar]
- 140.Guffon N., Fouilhoux A. Clinical benefit in Fabry patients given enzyme replacement therapy--a case series. J. Inherit. Metab. Dis. 2004;27(2):221–227. doi: 10.1023/B:BOLI.0000028726.11177.8b. [DOI] [PubMed] [Google Scholar]
- 141.Watt T., Burlina A.P., Cazzorla C., Schönfeld D., Banikazemi M., Hopkin R.J., Martins A.M., Sims K., Beitner-Johnson D., O’Brien F., Feldt-Rasmussen U. Agalsidase beta treatment is associated with improved quality of life in patients with Fabry disease: Findings from the Fabry Registry. Genet. Med. 2010;12(11):703–712. doi: 10.1097/GIM.0b013e3181f13a4a. [DOI] [PubMed] [Google Scholar]
- 142.Pisani A., Riccio E., Sabbatini M. Agalsidase alfa and agalsidase beta in the treatment of Fabry disease: Does the dose really matter? Genet. Med. 2015;17(1):21–23. doi: 10.1038/gim.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tsuboi K., Yamamoto H. Clinical observation of patients with Fabry disease after switching from agalsidase beta (Fabrazyme) to agalsidase alfa (Replagal). Genet. Med. 2012;14(9):779–786. doi: 10.1038/gim.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pisani A., Spinelli L., Visciano B., Capuano I., Sabbatini M., Riccio E., Messalli G., Imbriaco M. Effects of switching from agalsidase Beta to agalsidase alfa in 10 patients with anderson-fabry disease. JIMD Rep. 2013;9:41–48. doi: 10.1007/8904_2012_177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Weidemann F., Krämer J., Duning T., Lenders M., Canaan-Kühl S., Krebs A., Guerrero González H., Sommer C., Üçeyler N., Niemann M., Störk S., Schelleckes M., Reiermann S., Stypmann J., Brand S.M., Wanner C., Brand E. Patients with Fabry disease after enzyme replacement therapy dose reduction versus treatment switch. J. Am. Soc. Nephrol. 2014;25(4):837–849. doi: 10.1681/ASN.2013060585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vedder A.C., Linthorst G.E., Houge G., Groener J.E., Ormel E.E., Bouma B.J., Aerts J.M., Hirth A., Hollak C.E. Treatment of Fabry disease: Outcome of a comparative trial with agalsidase alfa or beta at a dose of 0.2 mg/kg. PLoS One. 2007;2(7):e598. doi: 10.1371/journal.pone.0000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sirrs S.M., Bichet D.G., Casey R., Clarke J.T., Lemoine K., Doucette S., West M.L. Outcomes of patients treated through the Canadian Fabry disease initiative. Mol. Genet. Metab. 2014;111(4):499–506. doi: 10.1016/j.ymgme.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 148.El Dib R., Gomaa H., Carvalho R.P., Camargo S.E., Bazan R., Barretti P., Barreto F.C. Enzyme replacement therapy for Anderson-Fabry disease. Cochrane Database Syst. Rev. 2016;7(7):CD006663–CD006663. doi: 10.1002/14651858.CD006663.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lenders M., Brand E. Effects of enzyme replacement therapy and antidrug antibodies in patients with Fabry disease. J. Am. Soc. Nephrol. 2018;29(9):2265–2278. doi: 10.1681/ASN.2018030329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Arends M., Biegstraaten M., Wanner C., Sirrs S., Mehta A., Elliott P.M., Oder D., Watkinson O.T., Bichet D.G., Khan A., Iwanochko M., Vaz F.M., van Kuilenburg A.B.P., West M.L., Hughes D.A., Hollak C.E.M. Agalsidase alfa versus agalsidase beta for the treatment of Fabry disease: An international cohort study. J. Med. Genet. 2018;55(5):351–358. doi: 10.1136/jmedgenet-2017-104863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rombach S.M., Aerts J.M., Poorthuis B.J., Groener J.E., Donker-Koopman W., Hendriks E., Mirzaian M., Kuiper S., Wijburg F.A., Hollak C.E., Linthorst G.E. Long-term effect of antibodies against infused alpha-galactosidase A in Fabry disease on plasma and urinary (lyso)Gb3 reduction and treatment outcome. PLoS One. 2012;7(10):e47805. doi: 10.1371/journal.pone.0047805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lenders M., Stypmann J., Duning T., Schmitz B., Brand S.M., Brand E. Serum-mediated inhibition of enzyme replacement therapy in fabry disease. J. Am. Soc. Nephrol. 2016;27(1):256–264. doi: 10.1681/ASN.2014121226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lenders M., Brand E. Mechanisms of neutralizing anti-drug antibody formation and clinical relevance on therapeutic efficacy of enzyme replacement therapies in fabry disease. Drugs. 2021;81(17):1969–1981. doi: 10.1007/s40265-021-01621-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lenders M., Oder D., Nowak A., Canaan-Kühl S., Arash-Kaps L., Drechsler C., Schmitz B., Nordbeck P., Hennermann J.B., Kampmann C., Reuter S., Brand S.M., Wanner C., Brand E. Impact of immunosuppressive therapy on therapy-neutralizing antibodies in transplanted patients with Fabry disease. J. Intern. Med. 2017;282(3):241–253. doi: 10.1111/joim.12647. [DOI] [PubMed] [Google Scholar]
- 155.Bernier V., Lagacé M., Bichet D.G., Bouvier M. Pharmacological chaperones: Potential treatment for conformational diseases. Trends Endocrinol. Metab. 2004;15(5):222–228. doi: 10.1016/j.tem.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 156.Parenti G., Andria G., Valenzano K.J. Pharmacological chaperone therapy: Preclinical development, clinical translation, and prospects for the treatment of lysosomal storage disorders. Mol. Ther. 2015;23(7):1138–1148. doi: 10.1038/mt.2015.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Benjamin E.R., Flanagan J.J., Schilling A., Chang H.H., Agarwal L., Katz E., Wu X., Pine C., Wustman B., Desnick R.J., Lockhart D.J., Valenzano K.J. The pharmacological chaperone 1-deoxygalactonojirimycin increases alpha-galactosidase A levels in Fabry patient cell lines. J. Inherit. Metab. Dis. 2009;32(3):424–440. doi: 10.1007/s10545-009-1077-0. [DOI] [PubMed] [Google Scholar]
- 158.Germain D.P., Fan J.Q. Pharmacological chaperone therapy by active-site-specific chaperones in Fabry disease: In vitro and preclinical studies. Int. J. Clin. Pharmacol. Ther. 2009;47(Suppl. 1):S111–S117. [PubMed] [Google Scholar]
- 159.Germain D.P., Hughes D.A., Nicholls K., Bichet D.G., Giugliani R., Wilcox W.R., Feliciani C., Shankar S.P., Ezgu F., Amartino H., Bratkovic D., Feldt-Rasmussen U., Nedd K., Sharaf El Din U., Lourenco C.M., Banikazemi M., Charrow J., Dasouki M., Finegold D., Giraldo P., Goker-Alpan O., Longo N., Scott C.R., Torra R., Tuffaha A., Jovanovic A., Waldek S., Packman S., Ludington E., Viereck C., Kirk J., Yu J., Benjamin E.R., Johnson F., Lockhart D.J., Skuban N., Castelli J., Barth J., Barlow C., Schiffmann R. Treatment of Fabry’s disease with the pharmacologic chaperone migalastat. N. Engl. J. Med. 2016;375(6):545–555. doi: 10.1056/NEJMoa1510198. [DOI] [PubMed] [Google Scholar]
- 160. Galafold (migalastat). European Public Assessment Report (EPAR). Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/galafold.
- 161.Schiffmann R., Bichet D.G., Jovanovic A., Hughes D.A., Giugliani R., Feldt-Rasmussen U., Shankar S.P., Barisoni L., Colvin R.B., Jennette J.C., Holdbrook F., Mulberg A., Castelli J.P., Skuban N., Barth J.A., Nicholls K. Migalastat improves diarrhea in patients with Fabry disease: Clinical-biomarker correlations from the phase 3 FACETS trial. Orphanet J. Rare Dis. 2018;13(1):68–68. doi: 10.1186/s13023-018-0813-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu X., Katz E., Della V.M.C., Mascioli K., Flanagan J.J., Castelli J.P., Schiffmann R., Boudes P., Lockhart D.J., Valenzano K.J., Benjamin E.R. A pharmacogenetic approach to identify mutant forms of α-galactosidase A that respond to a pharmacological chaperone for Fabry disease. Hum. Mutat. 2011;32(8):965–977. doi: 10.1002/humu.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Benjamin E.R., Della V.M.C., Wu X., Katz E., Pruthi F., Bond S., Bronfin B., Williams H., Yu J., Bichet D.G., Germain D.P., Giugliani R., Hughes D., Schiffmann R., Wilcox W.R., Desnick R.J., Kirk J., Barth J., Barlow C., Valenzano K.J., Castelli J., Lockhart D.J. The validation of pharmacogenetics for the identification of Fabry patients to be treated with migalastat. Genet. Med. 2017;19(4):430–438. doi: 10.1038/gim.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hughes D.A., Nicholls K., Shankar S.P., Sunder-Plassmann G., Koeller D., Nedd K., Vockley G., Hamazaki T., Lachmann R., Ohashi T., Olivotto I., Sakai N., Deegan P., Dimmock D., Eyskens F., Germain D.P., Goker-Alpan O., Hachulla E., Jovanovic A., Lourenco C.M., Narita I., Thomas M., Wilcox W.R., Bichet D.G., Schiffmann R., Ludington E., Viereck C., Kirk J., Yu J., Johnson F., Boudes P., Benjamin E.R., Lockhart D.J., Barlow C., Skuban N., Castelli J.P., Barth J., Feldt-Rasmussen U. Oral pharmacological chaperone migalastat compared with enzyme replacement therapy in Fabry disease: 18-month results from the randomised phase III ATTRACT study. J. Med. Genet. 2017;54(4):288–296. doi: 10.1136/jmedgenet-2016-104178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hughes D.A., Nicholls K., Sunder-Plassmann G., Jovanovic A., Feldt-Rasmussen U., Schiffmann R., Giugliani R., Jain V., Viereck C., Castelli J.P., Skuban N., Barth J.A., Bichet D.G. Safety of switching to Migalastat from enzyme replacement therapy in Fabry disease: Experience from the Phase 3 ATTRACT study. Am. J. Med. Genet. A. 2019;179(6):1069–1073. doi: 10.1002/ajmg.a.61105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chimenti C., Nencini P., Pieruzzi F., Feriozzi S., Mignani R., Pieroni M., Pisani A. The GALA project: Practical recommendations for the use of migalastat in clinical practice on the basis of a structured survey among Italian experts. Orphanet J. Rare Dis. 2020;15(1):86. doi: 10.1186/s13023-020-1318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bichet D.G., Torra R., Wallace E., Hughes D., Giugliani R., Skuban N., Krusinska E., Feldt-Rasmussen U., Schiffmann R., Nicholls K. Long-term follow-up of renal function in patients treated with migalastat for Fabry disease. Mol. Genet. Metab. Rep. 2021;28:100786–100786. doi: 10.1016/j.ymgmr.2021.100786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Müntze J., Gensler D., Maniuc O., Liu D., Cairns T., Oder D., Hu K., Lorenz K., Frantz S., Wanner C., Nordbeck P. Oral chaperone therapy migalastat for treating Fabry disease: Enzymatic response and serum biomarker changes after 1 year. Clin. Pharmacol. Ther. 2019;105(5):1224–1233. doi: 10.1002/cpt.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lenders M., Nordbeck P., Kurschat C., Karabul N., Kaufeld J., Hennermann J.B., Patten M., Cybulla M., Müntze J., Üçeyler N., Liu D., Das A.M., Sommer C., Pogoda C., Reiermann S., Duning T., Gaedeke J., Stumpfe K., Blaschke D., Brand S.M., Mann W.A., Kampmann C., Muschol N., Canaan-Kühl S., Brand E. Treatment of Fabry’s disease with migalastat: Outcome from a prospective observational multicenter study (FAMOUS). Clin. Pharmacol. Ther. 2020;108(2):326–337. doi: 10.1002/cpt.1832. [DOI] [PubMed] [Google Scholar]
- 170. Clinicaltrials.Gov. German observational multicenter study of patients with fabry disease under chaperone therapy with migalastat- HCl. NCT: NCT03135197, Available from: https://clinicaltrials.gov/ct2/show/NCT03135197 Accessed on Mar 22, 2022.
- 171. Clinicaltrials.Gov. French Prospective, Observational Cohort Study of Patients With Fabry Disease Treated With Migalastat. Nct: NCT04602364, Available from: https://Clinicaltrials.Gov/Ct2/Show/NCT04602364 Accessed on Mar 22, 2022.
- 172. Clinicaltrials.Gov. MigALastat Therapy Adherence Among FABRY Patients: A Prospective Multicentral Observational Study (MALTA-FABRY). NCT: NCT03683966, Available from: https://Clinicaltrials.Gov/Ct2/Show/NCT03683966 Accessed on Mar 22, 2022.
- 173.Coutinho M.F., Santos J.I., Alves S. Less is more: Substrate reduction therapy for lysosomal storage disorders. Int. J. Mol. Sci. 2016;17(7):1065. doi: 10.3390/ijms17071065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Welford R.W.D., Mühlemann A., Garzotti M., Rickert V., Groenen P.M.A., Morand O., Üçeyler N., Probst M.R. Glucosylceramide synthase inhibition with lucerastat lowers globotriaosylceramide and lysosome staining in cultured fibroblasts from Fabry patients with different mutation types. Hum. Mol. Genet. 2018;27(19):3392–3403. doi: 10.1093/hmg/ddy248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Guérard N., Morand O., Dingemanse J. Lucerastat, an iminosugar with potential as substrate reduction therapy for glycolipid storage disorders: Safety, tolerability, and pharmacokinetics in healthy subjects. Orphanet J. Rare Dis. 2017;12(1):9. doi: 10.1186/s13023-017-0565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Guérard N., Zwingelstein C., Dingemanse J. Lucerastat, an iminosugar for substrate reduction therapy: Pharmacokinetics, tolerability, and safety in subjects with mild, moderate, and severe renal function impairment. J. Clin. Pharmacol. 2017;57(11):1425–1431. doi: 10.1002/jcph.944. [DOI] [PubMed] [Google Scholar]
- 177.Guérard N., Oder D., Nordbeck P., Zwingelstein C., Morand O., Welford R.W.D., Dingemanse J., Wanner C. Lucerastat, an iminosugar for substrate reduction therapy: Tolerability, pharmacodynamics, and pharmacokinetics in patients with fabry disease on enzyme replacement. Clin. Pharmacol. Ther. 2018;103(4):703–711. doi: 10.1002/cpt.790. [DOI] [PubMed] [Google Scholar]
- 178. Clinicaltrials.Gov. A Study to Evaluate the Long-term Safety and Tolerability of Lucerastat in Adult Subjects With Fabry Disease. NCT: NCT03737214, Available from: https://Clinicaltrials.Gov/Ct2/Show/NCT03737214 Accessed on Mar 22, 2022.
- 179. Clinicaltrials.Gov. Efficacy and Safety of Lucerastat Oral Monotherapy in Adult Subjects With Fabry Disease (MODIFY). NCT: NCT03425539, Available from: https://Clinicaltrials.Gov/Ct2/Show/NCT03425539 Accessed on Mar 22, 2022.
- 180.Frey A., Trokan L., Vogler M., Hughes D., Gimona A. Measures to mitigate disruption due to the COVID-19 pandemic of the MODIFY phase 3 pivotal trial in patients with Fabry disease. Mol. Genet. Metab. 2021;132(2):S39–S40. doi: 10.1016/j.ymgme.2020.12.080. [DOI] [Google Scholar]
- 181.Morand O., Johnson J., Walter J., Atkinson L., Kline G., Frey A., Politei J., Schiffmann R. Symptoms and quality of life in patients with Fabry disease: Results from an international patient survey. Adv. Ther. 2019;36(10):2866–2880. doi: 10.1007/s12325-019-01061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Peterschmitt M.J., Crawford N.P.S., Gaemers S.J.M., Ji A.J., Sharma J., Pham T.T. Pharmacokinetics, pharmacodynamics, safety, and tolerability of oral venglustat in healthy volunteers. Clin. Pharmacol. Drug Dev. 2021;10(1):86–98. doi: 10.1002/cpdd.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Clinicaltrials.Gov. Evaluate the Safety, Pharmacodynamics, Pharmacokinetics, and Exploratory Efficacy of GZ/SAR402671 in Treatment-naïve Adult Male Patients With Fabry Disease. NCT: NCT02228460, Available from: https://Clinicaltrials.Gov/Ct2/Show/NCT02228460 Accessed on Mar 22, 2022.
- 184. Clinicaltrials.Gov. A study to evaluate the effect of venglustat tablets on left ventricular mass index in male and female adult participants with fabry disease (CARAT). NCT: NCT05280548, Available from: https://Clinicaltrials.Gov/Ct2/Show/NCT05280548 Accessed on Mar 22, 2022.
- 185. Clinicaltrials.Gov. A study to evaluate the effect of venglustat tablets on neuropathic and abdominal pain in male and female adult participants with fabry disease (PERIDOT). NCT: NCT05206773, Available from: https://Clinicaltrials.Gov/Ct2/Show/NCT05206773 Accessed on Mar 22, 2022.
- 186.Tekoah Y., Shulman A., Kizhner T., Ruderfer I., Fux L., Nataf Y., Bartfeld D., Ariel T., Gingis-Velitski S., Hanania U., Shaaltiel Y. Large-scale production of pharmaceutical proteins in plant cell culture-the Protalix experience. Plant Biotechnol. J. 2015;13(8):1199–1208. doi: 10.1111/pbi.12428. [DOI] [PubMed] [Google Scholar]
- 187.Kizhner T., Azulay Y., Hainrichson M., Tekoah Y., Arvatz G., Shulman A., Ruderfer I., Aviezer D., Shaaltiel Y. Characterization of a chemically modified plant cell culture expressed human α-Galactosidase-A enzyme for treatment of Fabry disease. Mol. Genet. Metab. 2015;114(2):259–267. doi: 10.1016/j.ymgme.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 188.Schiffmann R., Goker-Alpan O., Holida M., Giraldo P., Barisoni L., Colvin R.B., Jennette C.J., Maegawa G., Boyadjiev S.A., Gonzalez D., Nicholls K., Tuffaha A., Atta M.G., Rup B., Charney M.R., Paz A., Szlaifer M., Alon S., Brill-Almon E., Chertkoff R., Hughes D. Pegunigalsidase alfa, a novel PEGylated enzyme replacement therapy for Fabry disease, provides sustained plasma concentrations and favorable pharmacodynamics: A 1-year Phase 1/2 clinical trial. J. Inherit. Metab. Dis. 2019;42(3):534–544. doi: 10.1002/jimd.12080. [DOI] [PubMed] [Google Scholar]
- 189. Clinicaltrials.Gov. Study of the Safety and Efficacy of PRX-102 Compared to Agalsidase Beta on Renal Function (BALANCE). NCT: NCT02795676, Available from: https://Clinicaltrials.Gov/Ct2/Show/Study/NCT02795676 Accessed on Mar 22, 2022.
- 190. Clinicaltrials.Gov. Study of the safety, efficacy, & PK of pegunigalsidase Alfa (PRX-102) 2 mg/kg IV administered every 4 weeks in Fabry disease patients (BRIGHT). NCT: NCT03180840, Available from: https://Clinicaltrials.Gov/Ct2/Show/Study/NCT03180840 Accessed on Mar 22, 2022.
- 191. Clinicaltrials.Gov. Safety and efficacy of PRX 102 in patients with fabry disease currently treated with REPLAGAL® (Agalsidase alfa). NCT: NCT03018730, Available from: https://Clinicaltrials.Gov/Ct2/Show/Study/NCT03018730 Accessed on Mar 22, 2022.
- 192.Linhart A., Dostalova G., Nicholls K., West M., Tøndel C., Jovanovic A., Giraldo P., Vujkovac B., Hiwot T., Brill-Almon E., Alon S., Szlaifer M., Chertkoff R., Hughes D. Switching from agalsidase alfa to pegunigalsidase alfa for treating Fabry disease: One year of treatment data from BRIDGE, a phase III open label study. Mol. Genet. Metab. 2020;129(2):S98–S99. doi: 10.1016/j.ymgme.2019.11.249. [DOI] [Google Scholar]
- 193.Dostálová G., Hulkova H., Linhart A. Anderson-Fabry disease: No histological signs of pathological accumulation in arterial and venous endothelium during pegunigalsidase alfa therapy. Kardiol. Pol. 2021;79(12):1385–1386. doi: 10.33963/KP.a2021.0139. [DOI] [PubMed] [Google Scholar]
- 194.Niederkrüger H., Dabrowska-Schlepp P., Schaaf A. Suspension culture of plant cells under phototrophic conditions. In: Industrial Scale Suspension Culture of Living Cells, 2014. p. pp. 259-292. [DOI]
- 195.Reski R., Parsons J., Decker E.L. Moss-made pharmaceuticals: From bench to bedside. Plant Biotechnol. J. 2015;13(8):1191–1198. doi: 10.1111/pbi.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shen J.S., Busch A., Day T.S., Meng X.L., Yu C.I., Dabrowska-Schlepp P., Fode B., Niederkrüger H., Forni S., Chen S., Schiffmann R., Frischmuth T., Schaaf A. Mannose receptor-mediated delivery of moss-made α-galactosidase A efficiently corrects enzyme deficiency in Fabry mice. J. Inherit. Metab. Dis. 2016;39(2):293–303. doi: 10.1007/s10545-015-9886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hennermann J.B., Arash-Kaps L., Fekete G., Schaaf A., Busch A., Frischmuth T. Pharmacokinetics, pharmacodynamics, and safety of moss-agalactosidase A in patients with Fabry disease. J. Inherit. Metab. Dis. 2019;42(3):527–533. doi: 10.1002/jimd.12052. [DOI] [PubMed] [Google Scholar]
- 198.Domm J.M., Wootton S.K., Medin J.A., West M.L. Gene therapy for Fabry disease: Progress, challenges, and outlooks on gene-editing. Mol. Genet. Metab. 2021;134(1-2):117–131. doi: 10.1016/j.ymgme.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 199.Medin J.A., Khan A., Huang J., Barber D., Anthony Rupar C., Auray-Blais C., Fraser G., Fowler D.H., Keating A., West M.L., Foley R. FACTs Fabry gene therapy clinical trial: Two-year data. Mol. Genet. Metab. 2019;126(2):S99. doi: 10.1016/j.ymgme.2018.12.248. [DOI] [Google Scholar]
- 200.Khan A., Barber D.L., Huang J., Rupar C.A., Rip J.W., Auray-Blais C., Boutin M., O’Hoski P., Gargulak K., McKillop W.M., Fraser G., Wasim S., LeMoine K., Jelinski S., Chaudhry A., Prokopishyn N., Morel C.F., Couban S., Duggan P.R., Fowler D.H., Keating A., West M.L., Foley R., Medin J.A. Lentivirus-mediated gene therapy for Fabry disease. Nat. Commun. 2021;12(1):21321–21371. doi: 10.1038/s41467-021-21371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Fabry Disease news. Clinical trial data support AVR-RD-01 gene therapy for fabry disease. Available from: https://fabrydiseasenews.com/2020/06/16/clinical-trial-data-support-avr-rd-01-gene-therapy-for-fabry-disease/ Accessed on Mar 22, 2022.
- 202. Biopharma Dive. Avrobio stops work on rare disease gene therapy after unexpected study results. Available from: https://www.biopharmadive.com/news/avrobio-fabry-discontinue-gene-therapy-data/616594/ Accessed on May 21, 2022.
- 203. Clinicaltrials.Gov. An open-label, phase 1/2 trial of gene therapy 4D-310 in adult males with fabry disease. NCT: NCT04519749, Available from: https://Clinicaltrials.Gov/Ct2/Show/Study/ NCT04519749 Accessed on Mar 22, 2022.
- 204. Clinicaltrials.Gov. Dose-ranging study of ST-920, an AAV2/6 human alpha galactosidase a gene therapy in subjects with fabry disease. NCT: NCT04046224, Available from: https://Clinicaltrials.Gov/Ct2/Show/Study/NCT04046224 Accessed on Mar 22, 2022.
- 205. Clinicaltrials.Gov. A Fabry Disease Gene Therapy Study (MARVEL1). NCT: NCT04040049, Available from: https://Clinicaltrials.Gov/Ct2/Show/Study/NCT04040049 Accessed on Mar 22, 2022.
- 206. Fabrydiseasenews. Experimental gene therapy may be safe and effective, early data shows. Available from: https://fabry diseasenews.com/2022/03/11/4dmts-4d-310-gene-therapy-safe-effective-interim-data Accessed on Mar 22, 2022.
- 207. Fabrydiseasenews. Gal A activity nears normal in 2nd man given FLT190 gene therapy. Available from: https://fabrydiseasenews.com/2021/11/19/gal-a-activity-nears-normal-in-2nd-man-given-flt190-gene-therapy Accessed on Mar 22, 2022.
- 208.Del Pozo-Rodríguez A., Solinís M., Rodríguez-Gascón A. Applications of lipid nanoparticles in gene therapy. Euro. J. Pharmaceut. Biopharma. 2016;109:184–193. doi: 10.1016/j.ejpb.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 209.Rodríguez-Castejón J., Alarcia-Lacalle A., Gómez-Aguado I., Vicente-Pascual M., Solinís Aspiazu M.Á., Del Pozo-Rodríguez A., Rodríguez-Gascón A. α-Galactosidase A Augmentation by non-viral gene therapy: Evaluation in fabry disease mice. Pharmaceutics. 2021;13(6):771. doi: 10.3390/pharmaceutics13060771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.DeRosa F., Smith L., Shen Y., Huang Y., Pan J., Xie H., Yahalom B., Heartlein M.W. Improved efficacy in a fabry disease model using a systemic mRNA liver depot system as compared to enzyme replacement therapy. Mol. Ther. 2019;27(4):878–889. doi: 10.1016/j.ymthe.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhu X., Yin L., Theisen M., Zhuo J., Siddiqui S., Levy B., Presnyak V., Frassetto A., Milton J., Salerno T., Benenato K.E., Milano J., Lynn A., Sabnis S., Burke K., Besin G., Lukacs C.M., Guey L.T., Finn P.F., Martini P.G.V. Systemic mRNA therapy for the treatment of fabry disease: Preclinical studies in wild-type mice, fabry mouse model, and wild-type non-human primates. Am. J. Hum. Genet. 2019;104(4):625–637. doi: 10.1016/j.ajhg.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hopkin R.J., Cabrera G., Charrow J., Lemay R., Martins A.M., Mauer M., Ortiz A., Patel M.R., Sims K., Waldek S., Warnock D.G., Wilcox W.R. Risk factors for severe clinical events in male and female patients with Fabry disease treated with agalsidase beta enzyme replacement therapy: Data from the Fabry Registry. Mol. Genet. Metab. 2016;119(1-2):151–159. doi: 10.1016/j.ymgme.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 213.Beck M., Hughes D., Kampmann C., Larroque S., Mehta A., Pintos-Morell G., Ramaswami U., West M., Wijatyk A., Giugliani R. Long-term effectiveness of agalsidase alfa enzyme replacement in Fabry disease: A Fabry Outcome Survey analysis. Mol. Genet. Metab. Rep. 2015;3:21–27. doi: 10.1016/j.ymgmr.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s website along with the published article.