Abstract
Brain disorders are a prevalent and rapidly growing problem in the medical field as they adversely affect the quality of life of a human. With an increase in life expectancy, it has been reported that diseases like Alzheimer’s, Parkinson’s, stroke and brain tumors, along with neuropsychological disorders, are also being reported at an alarmingly high rate. Despite various therapeutic methods for treating brain disorders, drug delivery to the brain has been challenging because of a very complex Blood Brain Barrier, which precludes most drugs from entering the brain in effective concentrations. Nano-carrier-based drug delivery systems have been reported widely by researchers to overcome this barrier layer. These systems due to their small size, offer numerous advantages; however, their short residence time in the body owing to opsonization hinders their success in vivo. This review article focuses on the various aspects of modifying the surfaces of these nano-carriers with polymers, surfactants, protein, antibodies, cell-penetrating peptides, integrin binding peptides and glycoproteins such as transferrin & lactoferrin leading to enhanced residence time, desirable characteristics such as the ability to cross the blood-brain barrier (BBB), increased bioavailability in regions of the brain and targeted drug delivery.
Keywords: Blood brain barrier, CNS disorders, nanoparticles, surface modification, mucoadhesive, antibodies, transferrin, lactoferrin
1. INTRODUCTION
Central Nervous System disorders are one of the leading disorders affecting people of all age groups with increasing concern in the ageing population and have a serious impact on society due to the increased number of suffering people and its death rate. It immensely affects the quality of human life and leads to struggle in maintaining a normal lifestyle for them [1-3]. The Central Nervous System disorders include neurological disorders such as stroke, multiple sclerosis, migraine, brain tumor, Alzheimer’s disease (AD) or Parkinson’s disease (PD) [4]. Certain neuropsychiatry diseases include depression, ADHD (Attention Deficit Hyperactivity Disorder), epilepsy and dementia [2]. The cause of these neurological disorders could be genetic or environmental. Neurodegeneration is one of the causes of diseases like Parkinson’s and Alzheimer’s [5], whereas disturbances in neurotransmitters such as dopamine, norepinephrine and serotonin cause neuropsychiatric diseases such as depression, anxiety-related disorders and bipolar disorder [6]. The high prevalence of CNS disorders has led to the need and development of effective therapeutics for neurological diseases [7]. One of the major obstacles to treating CNS diseases is the presence of a barrier layer, Blood Brain Barrier (BBB), as most drugs fail to permeate this barrier and therefore do not reach the brain in effective concentrations. BBB only allows small lipophilic molecules to permeate and restricts the entry of large and hydrophilic molecules (>500 KDa) [8]. The blood-brain barrier is a complex structure of astrocytes, pericytes and endothelial cells. Tight junctions are present between endothelial cells to restrict the entry of large hydrophilic molecules and certain microorganisms [9]. Astrocytes and pericytes play an important role in maintaining endothelial cell barrier homeostasis as they help stabilize the basement membrane and communicate with brain cells [10].
Increasing challenges for drug delivery to the brain has led to the development of several drug delivery systems, amongst which nanoparticles-based drug delivery system has acquired high attention for treating various CNS disorders. Due to the diversity in nanomaterials, nano-based medicines have been proven to be promising next-generation therapeutics for advanced drug delivery [11]. The use of polymeric nanoparticles developed with synthetic, biodegradable polymers like poly (lactic acid) (PLA), poly (caprolactone) (PCL) and poly(lactic-co-glycolic acid) (PLGA) and natural polymers such as albumin, collagen, gelatin, fibroin, chitosan, and alginate are commonly reported in the literature [12]. Various lipid-based nano-formulations like nanostructured lipid carriers, liposomes and nanoemulsions are also being experimented with both in vitro and in-vivo. Nanoparticle delivery systems provide many desirable applications compared to conventional delivery and dosage forms. It enhances the bioavailability, exhibits high encapsulation of the drug, prevents the drug from mucociliary clearance, BBB crossing, and enzymatic degradation and facilitates the controlled release and good stability while storage [13, 14].
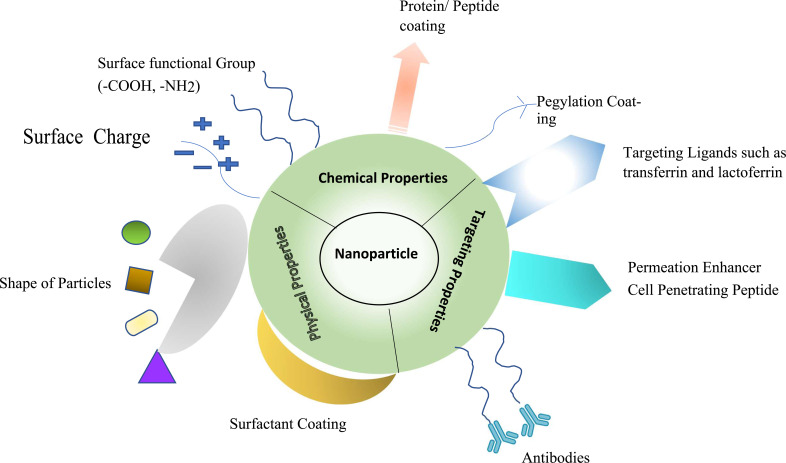
Despite many benefits of drug-loaded nano-carriers, the body often recognizes nano-carriers as foreign particles and invades them by opsonization, resulting in a very low circulation half-life in the body. Another limitation of degradation/ denaturation of macromolecules within the solid matrix is due to enzymes or the natural scavenging mechanism of the body, leading to challenges in the clinical implications of nanoformulations. To overcome the limitations of opsonization in-vivo, low stability, degradation, and surface modification are reported to improve the in vivo performance by camouflaging the nanocarrier; hence, the opsonins cannot identify the nano-carrier as a foreign body anymore, leading to long circulation half-lives in-vivo [15]. Surface modification is a concept where the surfaces of nano-carriers can be effectively and precisely designed by modifying their surface with appropriate moieties, which could provide a steric hindrance to enzymatic attack or provide for mucoadhesion for better permeation or a moiety/ligand which can attach to a specific receptor hence reducing the peripheral circulation etc. It can be said that the shape, size, surface charge, surface nature (hydrophobic/hydrophilic) and the material of the nanoparticles impact their interaction with the biological environment. Thus, an appropriate approach to surface engineering of nanoparticles with desirable characteristics can be chosen, such as changing shape, adding surface charge, adding surface functionality group, proteins or peptides and antibodies, which either results into increases mucus adhesivity/penetrance or targeting a receptor (diseased organ) [16, 17]. Most of the literature reports in vitro and in-vivo studies to evaluate the therapeutic effects of surface-modified nanoparticles. However, clinical trials are required to ascertain efficacy to assess effective drug delivery.
This review focuses on work based on surface modification of nanoparticles for delivery in the brain. Specific targeting approaches by the protein, antibody and ligands conjugation, like cell-penetrating peptides, transferrin and other related family members like lactoferrin etc., over the surface of nanoparticles have been discussed.
2. VARIOUS APPROACHES FOR SURFACE MODIFICATION OF NANO-CARRIERS
The nanoparticle-based delivery system is designed to achieve effective drug delivery to various target sites, and a crucial role is played by the size of nanoparticles in the delivery system and the surface modification or design of the particles. It is generally reported to be below 1000 nm, much less than the maximum body cell size (~100 µm); thus, it can permeate well through the epithelial layers [18]. Surface modification is a method of modifying the surface of nanoparticles by altering the surface charge and shape or by providing a coating of polymer, surfactant, peptides or antibodies conjugation and ligand anchoring onto the surface of nanoparticles for efficient drug delivery [18]. In recent years, work based on surface modification of nanoparticles for delivery in the brain has increased substantially as the modified nanoparticles can overcome the problems faced by conventional nanocarrier use. In another study by Xiaoling et al. 2006, the advantage of modified nanoparticles was observed over unmodified nanoparticles. Conjugation was done using wheat germ agglutinin lectins to the surface of poly (ethylene glycol)-poly (lactic acid) (PEG-PLA) nanoparticles. As a result, the nanoparticles gave negligible nasal ciliotoxicity, and the uptake of a fluorescent marker-coumarin carried by Wheat germ agglutinin conjugated nanoparticles was about 2 times higher in brain tissue than that of coumarin incorporated in the rats with unmodified nanoparticles [19]. In another study by Huang et al. 2013, the difference between surface-modified and unmodified nanoparticles was observed as Angiopep-conjugated NPs showed higher cellular uptake and gene expression in brain cells than unmodified nanoparticles. The pharmacodynamic results in data showed that rats in the group treated with modified nanoparticles showed improved locomotor activity and recovery of dopaminergic neurons compared to rats in other groups [20].
Several studies have reported that surface modification of nanoparticles for drug delivery to the brain has huge importance in drug delivery. This can be used to maintain the integrity of the loaded drug, protein, peptides, gene or other payloads. Surface modification leads to higher cellular uptake, absorption, and site-specific delivery in required concentrations, reducing the severe side effects associated with desired drug [18]. Although BBB poses a serious challenge to brain-targeted drug delivery system, limiting the clinical translation of various nanoparticulate formulations, surface-modified nanoparticles accomplish the goal of crossing the BBB as surface-modified nanoparticles can help in opening of tight junctions present in BBB or by transcytosis, or by retention at the BBB’s site per se and increasing the absorption along the concentration gradient across the endothelial layer of cells [21]. It can also control the release of drugs for a longer duration of time [18]. Surface modification of nanoparticles also facilitates the transport of poorly soluble drugs and hydrophobic molecules to the brain.
Surface modification of nanoparticles can be done through various approaches. Their physiochemical properties can be altered to get better in-vivo results to obtain the best efficiency out of the nanoparticulate system. Properties such as surface charge, size, stiffness and various other properties can be controlled and manipulated per requirement [17].
Mucoadhesion is one approach to prolong the delivery system's retention at the absorption site. Certain Polymeric nanoparticles, because of mucoadhesive properties, can adhere to the nasal epithelium and attain intimate contact with mucus and, hence, can potentially cross the BBB [22]. For easy absorption and effective delivery of nanoparticles via the intranasal route, the surface can be modified by exploiting adhesive or penetrating properties for mucus. Other approaches to improve drug stability, drug release, drug bioavailability, reduced toxicity and enhanced drug targeting efficiency (DTE) were developed by fabricating them with ligand conjugation such as protein/peptides and/or antibodies.
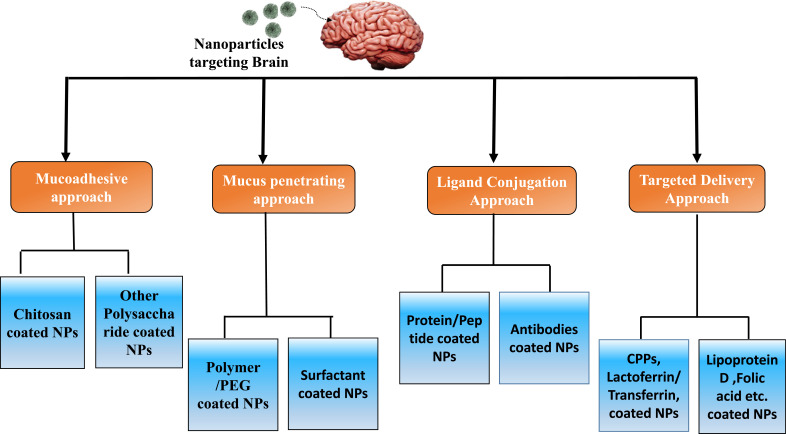
Further, nanoparticles can establish another level of modification to achieve specific targeted delivery, which involves using certain biomolecules as cell-penetrating peptides (CPPs), which are short, positively charged sequences and possess the ability to cross biological membranes such as BBB. Hence, CPPs have been known to mediate targeted drug delivery in the brain. Another approach is targeting nanoparticles to the nasal epithelium, including lectin-modified and cell-penetrating peptide (CPP) as surface Ligands specifically designed for targeted delivery [22]. Advanced approaches to enhance the targeting effect include ligands that can potentially bind the specific cell receptor, such as Lactoferrin which belongs to a transferrin family; it binds efficiently to the receptors expressed on the surface of neural cells [22]. Figs. (1 and 2) represents various types of approaches to surface modification.
Fig. (1).
Diagrammatic representation of various approaches to developing surface-modified nanoparticles.
Fig. (2).
Flow chart describing various approaches for the development of surface-coated nanoparticles.
Similar approaches have been developed for targeted delivery to the brain, like using glycoproteins such as transferrins, their family member and integrin targeting ligands conjugation. These molecules have been proven to show specific targeted delivery in the brain as receptors of these molecules rise in number in the person suffering through neurological disorder; hence nanoparticles attached with such molecules can easily target the affected region of the brain leading to reduced non-specific binding leading to lesser extrapyramidal side effects.
3. NANOPARTICLES DEVELOPED THROUGH THE MUCOADHESIVE APPROACH
Mucoadhesion is the state in which two materials adhere to each other through interfacial forces for prolonged periods. It is also called bio-adhesion when one of the materials is biological [23]. In terms of nanotherapeutics delivery, mucoadhesion is advantageous for drug delivery as it involves the attachment of the drug-loaded carrier to the biological membrane. The complex phenomenon of mucoadhesion includes various stages like wetting particles, their adsorption and the interpenetration of the polymer chains across the mucosal membrane. The particle adsorption mechanism for the nasal route leads to some brain therapeutics' uptake [23]. In order to get adhesive properties towards the mucosal surface, generally, mucoadhesive polymers are added to the formulation.
The nasal route has the mucociliary component present in the nasal epithelium, which protects the respiratory system from harmful contaminants, microorganisms and unwanted matter which can enter through this route, but this mucociliary clearance also acts as a strong physiological barrier to restrict the entry of drug and thus impacting its permeation and efficiency [22]. The nasal epithelium comprises goblet cells and a submucosal gland responsible for the secretion of mucus lining. Its major components are 90-95% water, 2% glycoproteins, 1% salts, 1% albumin, immunoglobulins, lysozyme and lactoferrin, and some cellular debris and products like DNA lipids and bacteria [24, 25]. Due to certain properties of glycoproteins, such as their molecular weight, hydration and chemical bonding, the nasal secretion has a high viscosity and elasticity and also, the presence of high levels of sialic acid and sulfate accumulations give a negative charge to the polymer chains which forms rigid networks [26]. The intranasal route can potentially transport active agents to the brain as it bypasses BBB. However, limitations like mucociliary clearance lead to a short residence time of nose-to-brain-delivered formulations, thus decreasing the amount of nasal drug uptake. Low volume of nasal cavity leads to fewer doses being administered, poor adhesion to olfactory sites etc., often limits its potential to be translated into clinical products. Mucociliary clearance can be regulated by using specific and desirable components for the formulation as viscosity enhancers and/or mucoadhesive polymers [24]. Mucoadhesive polymers can form bonds with mucus, mix with mucin fibers, and disorder the bonds like disulfide bridges, hydrophobic interactions, hydrogen, and attractive electrostatic forces [27]. Polymers can be used for surface modification to use their mucoadhesive properties as their surface area to volume ratio forms an alliance for more stable and durable interactions with the mucus. Apart from this, small-sized nanoparticles, less than 500 nm, can easily bond within the intertwined mucin fibers. Moreover, the positive charge and hydrophobicity may enhance the interaction of nanoparticles with the negatively charged and hydrophobic mucin networks through the hydrophobic and electrostatic interactive forces [22]. As per the ideal property of mucoadhesive polymer, it must swell up in the biological environment at the absorption site and interact with mucus and its components for appropriate adhesion. Various polymers such as natural, semisynthetic and synthetic have been used extensively for intranasal delivery of drugs to the brain [24]. Some of them, such as chitosan, gelatin, alginate, cellulose derivatives and low molecular weight pectins, have shown improved nasal drug delivery with increased residence time in the nasal region [22].
3.1. Chitosan-Based Nanoparticles
Chitosan is a linear polysaccharide derived from chitin obtained from a hard outer shell of shellfish such as crab, shrimp and lobster. It has emerged as a considerable and important drug delivery vehicle because of its biodegradability, biocompatibility, polycationic and mucoadhesive nature, and ease of physical and chemical alteration [28]. Chitosan-based nanoparticles are prepared by the ionic gelation method by combining chitosan with Tri Polyphosphate (TPP) for cross-linkage. A cross linkage lattice is formed between the two polymers of opposite charge as chitosan is cationic and TPP is anionic [29]. Chitosan has been reported to enhance epithelial permeability by opening tight junctions within epithelial cells [30]. Chitosan-based nanoparticles can be further modified by adding a thiol group, enhancing their mucoadhesiveness and permeation properties without impacting their biodegradability [31].
Further modification led to the development of thiolated chitosan, which was used to enhance the mucoadhesive property as it gets involved in the chemical bond formation, named di-sulphide bond between the thiol group of chitosan and glycoprotein present in the mucus. In a study in 2015 by Singh et al., thiolated chitosan nanoparticles were prepared by encapsulating selegiline HCl (antidepressant drug) of size 215 ± 34.71 nm, with entrapment efficiency of 70% ± 2.71% and zeta potential + 17.06 mV. According to the author, thiolated chitosan nanoparticles were more consequential than unmodified traditional nanoparticles in-vivo; rats showed improved behavioral assessment and higher drug concentration in the brain. The maximum amount of drug in the brain was 8.2 mg/mL with thiolated chitosan nanoparticles, while the concentration of selegiline solution in the brain was lower, and for unmodified chitosan nanoparticle, the highest concentration in the brain was around 4 mg/mL [31]. Further, chitosan was used as a surface modifier in certain other formulations made up of other materials. For example, Chlorpromazine hydrochloride encapsulated chitosan grafted PLGA nanoparticles for treating schizophrenia were developed by self-assembly-nanoprecipitation method with dextran sulphate to form a hydrophobic complex. Mucoadhesion studies were performed on sheep nasal mucosa, and nanoparticles have shown mucoadhesion of 87 ± 8.9% and were sufficiently adhered to mucosa [32].
An intranasal delivery for the brain was reported in a study by Niyaz Ahmad in 2017 through the chitosan-coated PLGA nanoparticles for treating Parkinson's disease. Rasagiline-loaded Chitosan-coated PLGA nanoparticles were formulated by double emulsification-solvent evaporation technique and were characterized. The average particle size, polydispersity index, and encapsulation efficiency (EE%) were found to be 122.38 ± 3.64 nm, 0.212 ± 0.009, and 75.83 ± 3.76%, respectively. The mucoadhesive potential of the formulation was tested by performing high-performance liquid chromatography–mass spectroscopy, which resulted in favor of chitosan-coated PLGA nanoparticles as they showed higher mucoadhesive potential compared to unmodified nanoformulations. In-vivo results of chitosan-coated PLGA nanoparticles in the brain and plasma of Wistar rats showed a high (p < 0.005) AUC in 24 hrs and increased Cmax of intranasally treated rats than an intravenously treated group of rats [33].
In a recent study in 2019, chitosan coating was used to improve the efficacy of human serum albumin nanoparticles for nose-to-brain drug delivery. Particles exhibited a size of 261± 18 nm with a zeta potential of +45 ±1 mV. Developed nanoparticles showed good stability as they possessed the same physical characteristics upon storage for a long period of time. Permeability studies were also performed across hCMEC/D3 monolayer, showing that chitosan-coated nanoparticles have higher permeability values. Similarly, uptake studies were performed in the presence of inhibitors such as sodium azide, chlorpromazine and indomethacin, which resulted in reduced uptake of both the formulation but comparatively higher uptake was observed in chitosan-coated nanoparticles than uncoated nanoparticles [34].
An efficient nose-to-brain delivery was observed through an enhanced mucoadhesive approach by developing chitosan-coated PLGA nanoparticles encapsulating ropinirole hydrochloride by Theodora et al. 2020. Particles were developed via the nanoprecipitation method, which resulted in spherical-shaped nanoparticles with a mean size of 468.0 nm. Ropinirole hydrochloride-loaded chitosan-coated PLGA nanoparticles gave a complete release of the ropinirole hydrochloride within 24 hrs in a simulated nasal electrolyte solution (SNES). Permeation studies were performed using sheep nasal mucosa, and it was observed that drug permeation was 3.22-fold higher from chitosan-coated PLGA nanoparticles compared to PLGA nanoparticles. In in-vitro studies on cell lines (Raw 264.7), neither chitosan-coated PLGA nanoparticles nor PLGA nanoparticles loaded with ropinirole induced hemolysis in whole blood or lead to the production of reactive oxygen species (ROS) in cells [35].
Saini et al., in 2021, used the applications of chitosan for the management of neurological disease, i.e., Alzheimer’s. Chitosan-coated solid lipid nanoparticles were prepared to enclose Ferulic acid, a natural bioactive compound known for its neuroprotective potential. Particles exhibited a size of 185 nm, a zeta potential of 12.4 mV and an entrapment efficiency (EE) of 51.2%. Ex vivo mucoadhesive experiments were performed, and it was observed that mucoadhesive strength was increased from 6.88 to 8.55 N. Permeation studies also showed that ferulic acid permeation was higher from chitosan-coated solid lipid nanoparticles (i.e., 35.49%) than with uncoated solid lipid nanoparticles and in-vivo studies performed in rats showed a decline in cognitive behavior (i.e., 25.35%) [36].
In another recent study by Aline et al. 2022, simvastatin-loaded chitosan-coated lipid-core nanocapsules were developed and delivered intranasally to treat glioblastoma. Nanocapsules were developed of optimum size in the nanometric range (200 nm) with PDI of 0.2, positive zeta potential with 100% encapsulation efficiency. In-vitro cytotoxicity was performed; as a result, chitosan-coated nanocapsules loaded with simvastatin showed higher toxicity than simvastatin solution in the U-138 MG glioblastoma cell line from a human. Animal studies were performed in rats through which an enhanced amount of the drug was observed when delivered through chitosan-coated nanocapsules intranasally (2.4-fold times than simvastatin) when compared with free simvastatin. Furthermore, simvastatin-loaded chitosan-coated lipid-core nanocapsules lead to a noticeable decrease in tumor growth [37].
3.2. Other Polysaccharide Based Nanoparticles
Certain natural polysaccharides like Delonix regiagum were isolated from seeds of D. regia, which belongs to the family Fabaceae, are reported to be used as a mucoadhesive polymer and in a study by Devkar et al., they were used for intranasal delivery of ondansetron hydrochloride. The nanostructured lipid carriers were prepared by high-pressure homogenization technique. Mucoadhesive properties were evaluated by calculating binding efficiency, which turned out to be (72%). In-vivo data presented high brain targeting efficiency (DTE) of 506% and direct transport percentage (DTP) of 97% in rats, although the study lacked some clarity because of a lack of non-mucoadhesive solution control [38].
Alginate nanoparticles loaded with venlafaxine (antidepressant) were produced by ionotropic gelation with calcium ions and a polycation, low-molecular-weight chitosan glutamate. Ex vivo permeation studies were performed across porcine nasal mucosa for 24 h, which showed that the permeation was twice for venlafaxine-loaded nanoparticles compared to venlafaxine solution. The DTE and DTP were 426% and 76%, respectively, in in-vivo studies in albino Wistar rats. These results proved that mucoadhesive properties were enhanced if particles' surfaces were modified with alginate, and thus, they showed better permeation across nasal mucosa [39].
In a study by Chandrakantsing V. Pardeshi in 2018, flaxseed oil-based neuronanoemulsions for nose-to-brain delivery modified by mucoadhesive polymer N, N, N'trimethyl chitosan loaded with ropinirole-dextran sulfate for management of Parkinson’s disease was developed. Ropinirole hydrochloride is a nonergoline dopamine agonist that binds specifically to the striatal and nigral D2-receptors complexed with dextran sulfate to form an aropinirole hydrochloride nanoplex. Particles obtained ranged between 32.39 to 99.00 nm with zeta potential -28.5 to -38.5 mV. In in-vivo pharmacokinetics studies, the concentration of ROPI from mucoadhesive neuro nanoemulsions formulation delivered intranasally was higher in the brain of swiss albino mice model compared to aqueous ropinirole hydrochloride-plain drug suspension. Also, a significant increase in Cmax was observed in the brain for mNNE formulation (22.43 μg/mL) compared to ropinirole hydrochloride plain drug suspension (1.67 μg/mL). An approximately 13-fold increase in Cmax for mucoadhesive neuro nanoemulsion formulation to ROPI-plain drug suspension delivered intranasally suggests improved CNS bioavailability [40].
Mohammad et al. in 2020 developed nanoparticles loaded with drugs such as doxorubicin, rhodamine B, and angiotensin II, which were modified by butylglyceryl polysaccharides (chitosan, guar gum, and pullulan). It was identified that nanoformulations developed using pullulan were more stable than chitosan and had increased membrane permeability across brain endothelial cells (bEnd3). Apart from these results, enhanced cellular uptake and cytoplasmic localisation of NPs were confirmed using confocal microscopy and flow cytometry [41].
4. NANOPARTICLES DEVELOPED THROUGH MUCUS PENETRATION APPROACH
Mucus is a dense network of mucin fibers composed of disulfide bonds and hydrophobic interactions forming a porous membrane. It acts as a strong semi-permeable membrane and allows the permeation of selective molecules through its pores, either on size, known as size filtering or interaction with the mucus components, known as interaction filtering. However, mucus pore size is different according to the area it is present in the body. Filtration based on size allows particles smaller than the pore size of mucin fibers, while interaction filtration depends upon how strongly nanoparticles interact with mucus; particles that show less interaction with mucin fibres can easily permeate through mucus [22]. Thus, even after effective mucoadhesive properties of nanoparticles, there could be chances of these nanoparticles being unable to permeate the mucus membrane and reach the brain region. Thus, this led to the usage of some small nanocarriers so that they could pass through mucin pores and coated nanocarriers with a polymer like PEG, surfactant, co-polymer surfactant or protein, which can increase the diffusivity of nanoparticles across mucin networks. Surface modification with these molecules can enhance better uptake in nasal epithelium and effective trans-mucosal drug delivery. Properties like hydrophobicity and surface charge of nanoparticles highly impact the penetration of nanoparticles into mucus; for example, to develop mucus-penetrating nanoparticles, there should be reduced hydrophobicity and electrostatic interaction which will decrease the formation of adhesive bonds between mucus and nanoparticles [42]. Hydrophilic PEG polymers have been commonly used to modify the surface of inorganic NPs to overcome this problem. This property of PEG can be used similarly for other hydrophobic nature molecules and helps enhance the stability and prolong the circulation time in biological fluids [17].
4.1. Polymer Coated Nanoparticles/PEGylated Nanoparticles
Polymeric nanoparticles represent an effective nanocarrier to show mucus penetration abilities and cross the BBB [18]. Specific interactions with biological targets can be accomplished by polymeric coating, and macromolecular drugs can also be loaded [15]. One of the approaches of polymer coating with PEG (polyethylene glycol), also known as PEGylation, is useful for effective brain delivery as it decreases interaction towards mucin and makes them more permeable [22]. In some of the earlier research, PEG was generally used to increase mucus adhesion due to its hydrophilicity and uncharged character, but as per a study done by Wang et al. in 2008, it was concluded that low molecular weight and high-density PEG coating exhibits reduced interactions of nanoparticles towards mucus. This probably happens because the molecular weight of PEG is very less to show adhesion when polymer chains interpenetrate; while PEG is hydrophilic when used at high density, it is sufficient to shield the hydrophobic core effectively [42].
A PEGylation is an effective tool for targeted brain delivery, which can use various influx transport methods present over the cerebral endothelium, such as carrier-mediated transport, receptor-mediated endocytosis and adsorptive-mediated endocytosis [43]. Nose-to-brain delivery of an antipsychotic drug was performed in another study in 2014 using the PEG-based formulation functionalized with Solanum tuberosum lectin. Haloperidol, an antipsychotic drug, was loaded with polyethylene glycol–block-poly-(D,L)-lactic-co-glycolic acid nanoparticles, lectin-functionalized for the effective drug delivery via intranasal administration. Before this study, previous studies were performed by encapsulating the drug into poly (D,L)-lactic- co-glycolic acid (PLGA) for intranasal delivery of antipsychotic drugs. PLGA-based formulations resulted in aggregation of particles and removal through nasal clearance mechanism and thus unable to deliver a drug into the brain in effective concentration, whereas, in this study, the presence of PEG improves therapeutics efficiency by reducing aggregation of particles, as well as Solanum tuberosum lectin conjugation leads to effective delivery as intranasally administered Solanum tuberosum lectin -functionalized nanoparticles increased haloperidol concentrations in the brain tissue of rats by 1.5-3-fold more than non-Solanum tuberosum lectin functionalized particles [44].
Liposomes have been widely used as nanocarriers for drug delivery in the brain because of their property of fusion with the cell membrane and higher cell uptake. Liposomes are composed of an aqueous core surrounded by a lipid bilayer and are amphipathic and thus can load both hydrophobic and hydrophilic drugs [45]. Conventional liposomes face drawbacks due to their rapid removal through the reticuloendothelial system (RES) from blood, leading to accumulation in the liver and spleen [45]. The following studies modified liposomes with PEG polymer to provide stability and prolonged circulation time. This study was done to safely improve drug delivery by formulating glutathione PEGylated liposomes. In-vivo pharmacokinetics and distributional studies in rats were also performed using the auto-quenched fluorescent tracer, carboxy-fluorescein, when delivered intraperitoneally and intravenously. As a result, no significant difference was observed in the release of carboxy-fluorescein from PEG and glutathione PEGylated liposomes in 24 hr at 37°C. Some amount of release of carboxy-fluorescein in culture medium was observed for both the formulations giving values 0.5 ± 0.1% (glutathione PEGylatedand 0.4 ±0.1% (PEGylated) of total liposomal carboxy-fluorescein concentration after 24 h. Later, a microdialysis study was performed to identify the exact amount of free fluorescent tracer inside the brain and to determine the precise quantification of carboxy-fluorescein level passing through BBB. In the microdialysis experiment, 4-fold higher (p<0.001) fluorescent tracers were found in the brain after intravenous administration of glutathione PEGylated liposomes compared with PEG control liposomes, this was majorly observed due to the beneficial effect of glutathione coating on PEG liposomes [46].
Recently, PEG coating has been explored for developing modified dendrimers resulting in Pegylated dendrimers. Dendrimers are nanocarriers composed of highly branched, tree-like globular structures and believed to be efficient in drug delivery to the brain [47]. Some studies have been performed using PEGylating dendrimers resulting in effective results. For instance, in a study performed by Parashar et al. 2018, the development of Angiopep-2 PEGylated Poly propyleneimine dendrimers was done for targeted delivery of paclitaxel to treat brain cancer. In the following study, the researcher firstly developed the PEGylated Poly propyleneimine dendrimers and then did the surface modification with the help of PEG and later also conjugated with Angiopep-2 for further enhancing the delivery across the BBB. The developed dendrimers were loaded with paclitaxel. Characterization resulted in 47±0.20 nm mean size of dendrimers with drug loading % of 57.42±0.8%. Cell cytotoxicity assay was also performed on C6 glioma cells; cell viability of paclitaxel-loaded dendrimers was reported to be around 75% at lower concentrations (<0.01 μg/ml), although it decreases with an increase in concentration. The researchers concluded that Angiopep-2 conjugated PEGylated Poly propyleneimine dendrimers were one of the promising nanocarriers of paclitaxel drug for targeted delivery to the brain [48].
In an interesting study by Santos et al. in 2018, an effort was made to develop and deliver cationic poly-(amido amine) dendrimers with a functionalized coating of PEG loaded with Rhodamine B isothiocyanate for the treatment of brain ischemia. PEGylation has remarkably enhanced the circulation time and decreased the toxicity impacts of drug-loaded nanocarriers. In the in-vitro cytotoxicity test, PEGylated cationic poly-(amido amine) dendrimers were not toxic to the brain endothelioma cell line (bEnd.3) cells, on which test was performed at the lowest concentration of 0.01 mg/mL. At the same time, similar results were not obtained for unmodified cationic poly-(amido amine) dendrimers. Also, as an interesting fact, the PEG-modified dendrimers reduced blood clotting, which is one of the beneficial factors for maintaining stroke conditions [49].
Elsewedy et al., in 2020, developed poly (D,L)-lactic- co-glycolic acid (PLGA) nanoparticles loaded with brucine for treating cancer. The particles were further modified with the help of polyethylene glycol (PEG) to increase the brucine action on the target site. Nanoparticles were characterized by physiochemical parameters and various other studies. The size observed was in the range between 94 ± 3.05 to 253 ± 8.7 nm with a zeta potential of 1.09 ± 0.15 to 3.71 ± 0.44 mV. Entrapment efficiency was obtained between 37.5 ± 1.8% and 77 ± 1.3% with a drug release value of >99.1% at 168 h. A decrease in tumor growth rate was observed in tumor-bearing mice by applying PEG PLGA nanoparticles more than by using PLGA nanoparticles of brucine solution [50].
In 2021 a study by Bin et al. was conducted to synthesize the H-MnO2-PEG hollow nanoparticles modified with polyethylene glycol to cure ischemic stroke. Prepared modified nanoparticles were further verified by observing their protective effect in an ischemic model of SD rats. As a result, it was observed that the induced nanoparticles decreased cerebral infraction area and recovered cognitive behavior in the stroke mice model. Studies predicted the inhibition in the expression of reactive oxygen species (ROS), inflammatory factors and declination in cell apoptosis [51].
Another recent study was performed by Kanawat et al. 2022 wherein with the advent of the surface modification method, drug delivery for paclitaxel to the brain was enhanced and improved the survival of the glioblastoma multiforme murine model. Kanawat et al. developed the paclitaxel-loaded PLGA-PEG nanoparticles and modified them with two different surface modifiers, i.e., poly(amidoamine) (PAMAM) and poly(ethylenimine) (PEI), separately. The developed surface-modified nanoparticles were analyzed for their size, surface charge, in-vitro permeability and in-vivo biodistribution studies. Results were comparable as nanoparticles coated with PAMAM or PEI showed higher brain accumulation than unmodified nanoparticles. Also, according to this report, orthotopic human GBM xenografts studies were done, and results revealed that PAMAM-modified nanoparticles encapsulating paclitaxel were efficacious and enhanced survival and were equally safe as paclitaxel solution [52].
4.2. Surfactant Coated Nanoparticles
Surfactant coating of nanoparticles was first introduced by Troster and coworkers, who also studied the distribution of surface-coated nanoparticles through in-vivo experiments. In rats, the body distribution of surfactant-coated and non-coated poly(methyl methacrylate) nanoparticles of 131 ± 30 nm was obtained. Although there was no direct correlation between the distribution pattern and the contact angles of the surfactant solutions on poly(methyl methacrylate), it was observed that all surfactants were less accumulated in the liver and uptake in other parts of the body was enhanced. Specifically, poloxamer 338 and poloxamine 908were effective in decreasing the liver uptake to a value of less than 30% of the dose [53, 54]. Studies have used surfactant as a coating for surface modification, hypothesizing it to possess mucus-penetrating properties. Various surfactants and co-surfactants have been widely used and proven successful for neural drug delivery, reflecting surface properties and bio-distribution. Some include poloxamers, i.e., 184, 188, 338, 407 and 908, and polysorbate 20, 60, and 80. The development of a surfactant modified by Poloxamer 80-coated poly (butyl cyano acrylate) leads to the confirmation of effective crossing of the BBB by receptor-mediated endocytosis and good therapeutic values when compared to intravenous administration [18]. In a study, nanovesicular spanlastics were prepared to encapsulate risperidone to increase its bioavailability and accomplish effective delivery via the intranasal route [55]. Risperidone is a drug used to treat various CNS disorders such as schizophrenia, bipolar disorder and, in some cases, autism [56]. Spanlastics were formulated with span 60 (5 mg/mL) and polyvinyl alcohol (30 mg/mL) and were reported for their elasticity according to the study. It helped the drug permeate through the mucosal membrane, making them mucus penetrating nanocarrier. The particle size obtained was around 300 nm, under the nanometric range, with an average PDI value (of 0.44). Encapsulation efficiency was between 54.78% and 74.29%. In ex-vivo permeation studies, the drug flux was remarkably higher for the optimized formula i.e. 184.52 μg/h/cm2 as compared to the plain drug solution i.e. 85.56 μg/h/cm2 with p-value <0.0001 [55].
In some of the recent studies, surfactant coating has been used and elaborated for its significance. Polysorbate 80 coated chitosan nanoparticles were prepared in one of the studies by Ray et al., where ropinirole hydrochloride was encapsulated for targeted delivery into the brain for the treatment of Parkinson’s disease. The results obtained were improved and satisfactory for the polysorbate 80 coated ropinirole hydrochloride loaded chitosan nanoparticles as the particles were 201-233 nm in range with a zeta potential of -19.60 mV, negatively charged particles are reported to show more circulation period in blood [57]. In vitro drug release study, polysorbate 80 coated ropinirole hydrochloride loaded chitosan nanoparticles showed a sustained release of ropinirole hydrochloride compared to uncoated chitosan nanoparticles and plain drug solution. The stability was confirmed when no change was observed in particle size and PDI values of nanoparticles even after a period of three months of storage. In-vivo biodistribution studies were done in Wistar rats, which showed higher ropinirole hydrochloride concentrations in the brain (4780.0 ± 98.6 ng/ml), with fewer accumulations of ropinirole hydrochloride in the liver 2370.0 ± 45.8 ng/ml, spleen 1598.0 ± 79.3 ng/ml and kidney 2160.0 ± 78.6 ng/ml, for polysorbate 80 coated ropinirole hydrochloride loaded chitosan nanoparticles after 1 hr of intake [57].
Another recent study by Soudi et al. in 2019 explored surface modification in which they tried to study the protective effects of surface-modified berberine-loaded chitosan nanoparticles for neurodegenerative disorders. The particles were developed through the ionic gelation method, and the surface of nanoparticles was modified with Tween 80, polyethylene glycol 4000, and miltefosine and delivered to rats induced by lipopolysaccharide to generate neurodegenerative changes in them. The BBR-loaded NPs had a size > 190 nm, good enough for delivery across BBB [30], and significantly high zeta potential symbolizes good colloidal stability along with high Entrapment Efficiency (EE%) (> 98%). In animal studies, LPS treatment in rats has relatively elevated the AChE activity leading to the degradation of acetylcholine at a faster rate. Berberine is known and used to control AChE activity, thereby showing neuroprotective activity, but as a drawback, over suppression may lead to a collection of acetylcholine at the postsynaptic cleft. Thus, nano-encapsulating berberine has moderately controlled anti-AChE activity. Moreover, using Tween 80, PEG and miltefosine enhanced this effect as Tween 80 maintained the anti-AChE suppression, and PEG and miltefosine coatings moderated AChE inhibition and brought its level to normal [58].
Polysorbate 80 coated albumin nanoparticles were synthesized by Barnabas et al. 2020. By targeted delivery, nanoparticles were developed to increase antiepileptic drug efficacy (levetiracetam). Levetiracetam drug concentration was observed to be increased in the brain significantly when delivered via levetiracetam-loaded polysorbate 80-coated nanoparticles. A markable difference in levetiracetam concentration was detected in male Wistar rats’ brains between the administration of levetiracetam loaded polysorbate 80 nanoparticles and free drug, i.e.18.54 ± 2.38 μg/gm and 5.28 ± 1.79 and respectively. Thus, levetiracetam-loaded polysorbate 80 coated nanoparticles increased the drug concentration about 3.5 times compared to the free drug [59].
Another recent study by Yusuf et al. in 2021 delivered thymoquinone to treat Alzheimer’s disease. Thymoquinone is a potential therapeutic phytocompound known for its neuroprotective and antioxidative properties but has low bioavailability due to its lipophilic nature. In this study, thymoquinone-loaded PLGA nanoparticles were prepared with the surface coating of polysorbate 80 to achieve targeted delivery and increase the bioavailability of thymoquinone. As a result, particles were 226.2 nm along with ζ = −45.6 mV. Nanoparticles were evaluated by Super-Oxide Dismutase (SOD) biochemical assay and by behavioural test in Alzheimer’s induced mice model. Administration of thymoquinone-loaded PLGA P80 coated nanoparticles increased the functioning of SOD (8.33 ± 2.61 units/mg) after treating mice for 28 days with thymoquinone-loaded PLGA P80 nanoparticles. In-vivo studies showed that thymoquinone release in the brain was higher as AUC (0–600mins) was 4.5 times higher when delivered through thymoquinone-loaded PLGA P80 coated nanoparticles in comparison to free thymoquinone. AD-induced mice also improved behavior and cognitive function [60].
5. NANOPARTICLES DEVELOPED THROUGH LIGAND CONJUGATION APPROACH- PROTEIN/PEP-TIDE & ANTIBODIES CONJUGATED NPS
Proteins are natural molecules with unique properties and functional abilities in biological substances [61]. Protein-based nanoparticles have several advantages, including reduced toxicity, improved drug release and increased bioavailability, providing better formulations and drug delivery into the brain. There is a large number of nanomaterials obtained from proteins such as albumin and gelatin [61]. It has been observed that when nanoparticles enter rich fluids, they form the protein corona around themselves, ultimately destabilizing the particles. But protein-coating of nanoparticles has been seen as helpful in attaining stability of nanocarriers in biological fluids as proteins are macromolecules with a charged group on them, providing electrostatic stability and colloidal stability in physiological fluids [62]. Some recent investigations have been mentioned to better understand polymer-coated nanoparticles.
In a study by Shamarekh et al. 2020, Tacrine-loaded protamine-coated PLGA nanoparticles were developed for intranasal delivery and evaluated through in-vitro and in-vivo studies for the treatment of Alzheimer’s disease. PLGA nanoparticles were prepared using the nanoprecipitation technique, and the surface was modified using protamine sulphate to get better targeting via the nose to the brain. Particles were of size 70.55-237.67 nm, small in range, with a low polydispersity index between 0.075-0.224. The in-vitro drug release studies showed sustained release manner with the highest release of 43.65% in 120 hrs. The in-vivo study on rats revealed good brain targeting efficiency for protamine sulphate-coated nanoparticles compared touncoated nanoparticles when delivered intranasally and demonstrated much higher brain absolute bioavailability (265.24 ± 62.99%) of the drug tacrine [63].
In a study by Lu et al. Fas, ligand antibody conjugated PEG-lipid nanoparticles were prepared for targeted delivery in brain ischaemia. 3-n-Butylphthalide (NBP) loaded PEG nanoparticles were prepared by solvent diffusion method. Amino-terminated polyethylene glycol monostearate was used for polyethylene glycol monostearate to generate antibody-modified NPs. Particles were obtained of size, Polydispersity Index, Entrapment efficiency and drug loading values as 60.97 ± 7.95 nm, 0.329 ± 0.06, 93.05 ± 0.44 and 15.51 ± 0.07, respectively. Fluorescent tracing of the mice brain showed that 3-n-Butylphthalide through Fas ligand antibody conjugated PEG nanoparticles were efficiently delivered to the region of the ischaemic brain [64].
In another research by Loureiroa et al., for the treatment of Alzheimer’s disease, anti-transferrin receptor monoclonal antibody (OX26) and anti-Aß(DE2B4) antibodies were used for better cellular uptake of PLGA nanoparticles. Antibodies were used to overcome the drawback of low BBB permeability resulting in less uptake of drug-peptide iAß5 into the affected brain region. As a result of the experiment, the uptake of antibody-conjugated nanoparticles loaded with peptide iAß5 and controlled delivery was significantly increased compared to the unconjugated (without monoclonal antibody) nanoparticles [65].
In a recent study, Monge et al. (2020) functionalized PLGA nanoparticles prepared by coating them with monoclonal anti-transferrin receptor antibody (8D3 mAb). The main objective of this study was to identify the protein that binds to polymeric nanoparticles and binds to the target site of BBB. Firstly, 8D3 mAb coated PLGA nanoemulsion was prepared by phase inversion composition (PIC) method encapsulation thiazolidinedione drug followed by the development of nanoparticles from nanoemulsion templating and functionalized with 8D3 mAb with the help of carbodiimide coupling reaction. The particle size obtained for the 8D3 functionalized thiazolidinedione PLGA-coated nanoparticle was 65 ± 1.4 nm. For protein corona formation, nanoparticles were incubated in 5 and 25% fetal bovine serum at 37°C for 24 or 48 hours. Protein-bound to nanoparticles were eluted and isolated using sodium dodecyl sodium (SDS) buffer elution protocol. It was identified that 68 proteins interacted with all the developed nanoparticles, out of which 7 proteins were specifically bound to 8D3 functionalized thiazolidinedione PLGA-coated nanoparticles. Albumin and apolipoprotein E (apoE) were attached with plain PLGA nanoparticles and 8D3 functionalized thiazolidinedione PLGA-coated nanoparticles. Studies reported that Afamin helps in molecular transport, such as transporting specific molecules to the cerebrovascular endothelial cells model [66].
6. NANOPARTICLES DEVELOPED BASED ON A TARGETED DELIVERY APPROACH
A promising strategy to target the central nervous system with an active drug is targeting ligands such as cell-penetrating peptides (CPPs), transferrins, lactoferrins etc. CPPs can show targeted transport as they can show selective permeability across the plasma membrane and translocate across the cells. They have been used to deliver a large variety of cargo, including small molecules, DNAs, antibodies and nanocarriers [67]. Furthermore, the use of strategies like transferrin and lactoferrin conjugation has been investigated by many researchers and scientists. The transferrin and lactoferrin receptors are abundant among the various potential receptors at the BBB, ensuring sufficient drug delivery to the brain [68, 69]. In the following section of the review, we provide a full account of the use of CPPs targeting BBB, transferrin, lactoferrin targeting TfR (Transferrin receptors) & LfR (Lactoferrin receptors) respectively and integrin binding peptides along with studies performed using them.
6.1. Cell Penetrating Peptide Nanoparticles
Cell-penetrating peptides (CPPs) are also called protein transduction domains (PTDs) which are arginine (Arg) rich basic peptide sequences. These are the short positively charged sequence of around 5-30 amino acids, which can easily penetrate biological membranes like the intestinal wall, BBB and skin [70]. Cell-penetrating peptides (CPP) have been reported to mediate the delivery of macromolecules through various biological barriers and can deliver many cargos for drug translocation through nose-to-brain transport such as protein molecules, nucleic acids and nanocarriers as CPP have the advantage of small size and can easily pass across the plasma membrane by various endocytosis pathway [71] and also being positively charged these are more attractive to negatively charge BBB [72]. These cell-penetrating peptides were discovered in 1988, HIV transactivator of transcription (Tat) protein, which was the first known sequence to translocate cell membranes and get inside the cell [73].
These properties of CPPs make them suitable drug delivery vehicles for the nose-to-brain targeted delivery of nanocarriers. For example, in a study by Gartziandia et al. 2017, polymeric-poly (D,L-lactide-co-glycolide) (PLGA) nanoparticles and nanostructured lipid carrier (NLC) were prepared using the cell-penetrating peptide (CPPs) moieties, i.e., Tat and Penetration (Pen), characterized for physiochemical properties and their in-vitro olfactory cell monolayers assessment to validate whether they can transport the olfactory cell monolayers and reach the brain. As a result, 0.7% of PLGA nanoparticles were able to cross the olfactory cell monolayers, rather than 8% and 22% of NLC and chitosan-coated NLC were transported across monolayers, respectively. While interestingly, the addition and use of CPPs to NLC surface increased their transport even more, resulting in 46% transported NPs. Hence it could be concluded that CPP-modified chitosan nanostructure lipid carrier represents a promising brain vehicle for nose-to-brain drug delivery [74].
In a similar study, CPP-modified liposomes were prepared and studied; their in-vitro and in-vivo assessments were also performed for transport analysis. Cellular uptake of peptides was evaluated in the presence of sodiumazide (metabolic inhibitor) and various other inhibitors named colchicines, Oxophenylarsine, and sucrose at 37oC. Compared to the controls, when cells were treated with inhibitors - colchicines, sucrose and Oxophenylarsine, showed a significant decrease in the uptake of TAT(peptide terminal Cysteine) Cys-AYGRKKRRQRRR)) liposome by 28.43, 37.10% and 17.35, respectively. As a result of biodistribution studies in miceliposome modified with the S-TAT (Cys-AYGGQQGGQGGG) was obtained in lesser quantity in the brain fractions, while the TAT-liposomes gave significantly higher levels compared to `other two liposomes [75].
In the latest study by Nai et al. 2021, functionalized thermosensitive liposomes with macrophage membrane, cyclic Arg-Gly-Asp peptide, and cell-penetrating peptide were fabricated for the successful delivery of small interfering RNA (siRNA)for targeting the tumor. Nai et al. combined the thermotherapy with the targeting approach by using thermosensitive liposomes with the macrophage membrane, cyclic Arg-Gly-Asp peptide, and cell-penetrating. As a result, it was identified that there was minimal uptake by macrophages and increased HepG2 cells, leading to apoptosis and the highest inhibition of Bcl-2 protein and Bcl-2 mRNA. It was also observed that the distribution of siRNA was much higher in tumors, whereas the least amount of siRNA was found in any other organ in mice. Hence, it was concluded that functionalized thermosensitive liposomes lead to a higher accumulation of siRNA in the targeted region, i.e., tumor, than in the reticuloendothelial system, which improves the therapeutic potential of siRNA and can be a promising candidate for tumor therapy [76].
Another study has been recently conducted by Arora et al. (2022) for brain-targeting BDNF (Brain-derived neurotrophic factor) gene therapy to cure Alzheimer’s disease pathology in a mouse model (transgenic). It has been observed that BDNF gene therapy with viral vectors successfully achieved the proliferation of neurons, increased synaptic protein expression, and attenuated amyloidogenic processes. Therefore, to minimize virus-associated safety concerns and invasiveness, scientists have developed a lipid-based nanoparticle targeting the brain for the delivery of BDNF safely and efficiently. Liposomes were developed and surface functionalized via brain-targeting ligands such as mannose and cell-penetrating peptides (rabies virus-derived peptide or penetratin). Formulated liposomes were tested for efficacy and safety in transgenic APP/PS1 AD mice in both early (6 months) and advanced stage (9 months). Results demonstrated that the modified liposomes have increased the expression of BDNF by 2-fold and have reduced the toxicity (>40%) of amyloid-beta peptides in both 6- and 9-months old APP/PS1 mice brains in comparison to same-aged untreated controls [77].
6.2. Transferrin/Lactoferrin Modified Nanoparticles
Specific targeted delivery can be achieved through various other approaches by modifying nanoparticles with potential ligands, which can act as effective alternatives to cell-penetrating peptides [22]. Targeted delivery is crucial for drug delivery in the brain as BBB is a major obstacle that prevents the entry of most large molecules and nanomedicines [78]. Hence, strategies to bind ligands to nutrient receptors at the BBB membrane site are explored to withstand this problem. Some of the most potential targets of various efficient targeting ligands have been discovered, amongst which the transferrin, lactoferrin, integrin-targeting ligand, etc. [22]. Numerous receptors are present for transferring, and lactoferrin at the BBB membrane site ensures that these are the most common targets to ensure effective drug delivery to the brain [78].
Targeting the transferrin receptor is one of the advantageous approaches for drug delivery in the brain. Transferrin is a monomeric 78kDa glycoprotein; its concentrations ranges between 25 to 50 μM (2–4 μg/ml) in human blood plasma [78]. It is closely related to the glycoproteins family, including lactoferrin, melanoTf and ovoTf. Physiologically, the iron in the circulation is mostly bound to transferrin [79-81]. Transferrin receptor (TfR) is abundantly expressed by brain capillary endothelial cells through which receptor-mediated transport (RMT) occurs across the BBB to deliver the iron in the brain [82, 83]. Receptor-mediated transport is one of the finest transport mechanisms for the active and targeted delivery of therapeutics crossing BBB with specific ligands attached as this method bypasses the multi-drug resistant and P-glycoprotein efflux transporters present in the BBB and cancerous cells [84, 85]. Thereafter, the high expression of TfR in brain endothelial cells and brain tumor cells increases the affinity of transferring towards the brain region compared to other organs and parts of the body, leading to targeted delivery. Because of such attractive targeting characteristics of transferrin, it is widely used for functionalizing nanoparticles for selective binding towards BBB endothelium and generating the RMT of nanoparticles passing the BBB [86]. For example, in a study by Ghadiri et al. in 2017, magnetic dextran-spermine nanoparticles loaded with capecitabine drug were prepared through the ionic gelation method and later conjugated by transferrin (Tf) as a targeting entity. Tf conjugated nanoparticles were characterized and analyzed, and their cytotoxicity test on U87MG (human glioblastoma) cells was performed. Biodistribution studies were also done to observe the iron concentration in the brain and other female organs of female mice to estimate targeted delivery. The particles generated of size varying between 74-110 nm with 0.16-0.23 polydispersity index and zeta potential of +17- 36 mV. Entrapment efficiency and loading of capecitabine obtained were 11-22% and 6-10%, respectively. As a result of the cytotoxicity test, it was observed that Tf conjugated capecitabine-loaded NPs gave an increase in the cytotoxic effects of dextran-spermine nanoparticles on U87MG cell lines with cell viability ranging between 20-30% at a concentration of 0.25, 0.5, 0.75 and 1 mg/ml at 72 hr duration of incubation. Biodistribution studies demonstrated a substantial increase of iron concentrations in the brain after 1 and 7 days post-injection. Dextran-spermine nanoparticles and transferrin-conjugated dextran-spermine nanoparticles were more concentrated in the brain region with increased time (p<0.05). Thus, it can be remarked that the presence of Tf on the dextran-spermine nanoparticles surface increased the biodistribution of the nanoparticles into the brain through the Tf receptor-mediated transcytosis mechanism [87].
Another study was conducted by Han et al. to develop antitumor therapeutics with the help of a conjugated nanocarrier. Transferrin-modified nanostructure lipid carriers for codelivery of DNA and doxorubicin were developed. Nanostructures were evaluated for particle size, zeta potential, encapsulation efficiency, in-vitro cellular cytotoxicity, and in-vivo anticancer therapy. Particle size obtained 198 nm with a zeta potential of +19 mV with an encapsulation efficiency of 86.7 ± 2.7%. Cell viability of transferring modified NLC was more than 80% compared with the control. In-vivo results demonstrated that tumor growth was remarkably inhibited by the transferrin-NLC formulation (P< 0.05), and it was observed that after 15 days of administration into mice of this formulation, tumor weight in mice was reduced by 66% compared with control [88].
In recent years, transferrin has greatly focused on treating brain glioma and various central nervous disorders. For instance, in a recent study by Lopalco et al. in 2018, transferring functionalized liposomes were prepared and loaded with Dopamine HCl to treat Parkinson’s disease. The physical characteristics of the formulated liposomes were estimated, and their endothelial permeability across an in vitro model of the blood-brain barrier was determined via human cerebral microvascular endothelial cells (hCMEC/D3). Through characterization procedures, particles resulted in a size of 180 nm with a polydispersity of 0.2, a surface charge equal to +7.5 mV, and an encapsulation efficiency of 35%. As a result, for permeability tests in hCMEC/D3 cell line, the value obtained for transferrin modified Dopamine HCL loaded liposomes was 4.97 ± 0.41 × 10-3 cm/min, while the value registered for unfunctionalized liposomes was 0.92 ± 0.24 × 10-3 cm/min, indicating an increase of about five-fold [89].
Pinheiro et al. (2020) developed quercetin-loaded lipid nanoparticles functionalized with transferrin to ease the delivery of nanoparticles across the BBB and enhance the activity of quercetin to show neuroprotective effects. Nanoparticles were formulated and characterized by particle size, zeta potential and TEM (Transmission Electron Microscopy) analysis. Entrapment efficiency was calculated along with in-vitro cytotoxicity and permeability studies across hCMEC/ D3 cell monolayers. Particles were found in a range of 200 nm with a zeta potential of -30 mV, and TEM results demonstrated that all particles were spherical. The entrapment efficiency of quercetin was reported to be around 80-90%. Cytotoxicity tests showed that cells were viable even at a higher concentration of 30 μM, and nanoparticles did not show cytotoxicity. Permeability studies across hCMEC/D3 cell monolayers showed quercetin-loaded lipid nanoparticles permeate more across the blood-brain barrier, while amyloid-beta studies revealed transferrin functionalized lipid nanoparticles can inhibit fibril formation [90].
Another study by Bruna et al. in 2020 was performed in which they developed liposomes encapsulating plasmid DNA (pDNA) with the coating of two ligands, i.e., CPP(PFVYLI or R9F2) and transferrin. The objective was to overcome the limitations of liposomes in drug delivery and to enhance the therapeutic effect in the brain. The administration of pDNA through dual modified liposomes with CPP(PFVYLI or R9F2) and transferrin resulted in significantly higher (p<0.05) in-vitro transfection efficiency in comparison to single modified nanoparticles. CPP(PFVYLI or R9F2) and transferrin functionalized liposomes showed the ability to cross across in-vitro BBB and also reached the brain and accumulated in higher amounts (6.6%) as compared to R9F2-liposomes (2.5%). Therefore, transferrin can be used to functionalize nanocarriers for efficient delivery and permeability across BBB [91].
A recent study was conducted by Ramalho et al. (2022) by using transferrin as a targeting molecule for the delivery of Asiatic acid to treat glioblastoma cells. PLGA nanoparticles were optimized and developed by encapsulating Asiatic acid and were modified with transferrin to attain the targeted delivery in glioblastoma cells. Particles were characterized for physiochemical properties and were found to be smaller than 200 nm with low PDI and negative zeta potential. Nanoparticles exhibited higher encapsulation efficiency and resulted in Asiatic acid's slow release for up to 20 days. In-vitro cell studies with transferrin-modified nanoparticles showed equal anti-tumor activity as the natural compound, and transferrin has also led to the increased uptake of nanoparticles in GBM cells [92].
Similarly, the use of lactoferrin has also been reported for the surface modification of nanoparticles for targeted delivery of drugs. Lactoferrin is a naturally occurring iron-binding glycoprotein belonging to the transferrin family, showing numerous beneficiary characteristics in terms of safety and biocompatibility. Various studies have been reported for their uses in modifying nanoparticles, emulsions and hydrogels to encapsulate and safely deliver the drug and bioactive compounds [93]. For this reason, lactoferrin has also been numerously used to modify nanoparticles. In a study by Liu et al. in 2013, lactoferrin conjugated PEG-PCL nanoparticle was developed through a maleimide-thiol reaction for the lactoferrin conjugation with incorporated coumarin-6 for the treatment of Alzheimer’s diseases. Particles were analyzed with various parameters and resulted in desirable characteristics such as particle size of 88.4 ± 7.8 nm, polydispersity index of 0.22 and zeta potential of -23.56 ± 0.96 mV. The pharmacokinetics data was evaluated to establish the enhanced effect of lactoferrin-modified accumulation inside the rat brain, and the data obtained was in favor of the modified nanoparticles as the AUC0-8h (pg h/ml or pg h/g) after intranasal administration of the formulations was higher (1180.10, 3179.18, 2679.98, 4484.86, 4212.75 and 4143.74) than AUC0-8h obtained for unmodified formulation (966.49, 1238.44, 993.90, 1901.42, 1663.84 and 1854.70) for blood; OB, olfactory bulb; OT, olfactory tract; CR, cerebrum with hippocampus removed; CL, cerebellum; HI, hippocampus organs. Hence, lactoferrin nanoparticles may become an effective nose-to-brain drug delivery carrier for drugs, peptides and proteins [69]. However, solid lipid nanoparticles have been reported to deliver a high amount of drug into the brain but show extrapyramidal side effects due to their non-specificity [94].
In a study, lactoferrin-modified Solid lipid nanoparticles were developed and loaded with docetaxel for delivery in the brain. Lactoferrin conjugation was proven to show selective drug delivery towards the brain. Cellular uptake studies of SLN and lactoferrin-modified Solid lipid nanoparticles were performed, and values obtained were 7.49±0.33 and 20.9± 5.44%, respectively. Distribution studies in the brain of female Swiss albino mice were also performed, resulting in the area under curves (AUCs) of docetaxel in the brain were obtained to be 10.09±2.61 and 22.88±3.33 mg h/g for SLN and lactoferrin-modified Solid lipid nanoparticles [95].
In another latest study in 2020, the construction of 7,8-dihydroxyflavone (7,8-DHF) loaded zein/lactoferrin nanoparticles was done as 7,8-DHF is a tyrosine kinase B (TrkB) receptor agonist which can mimic the action of brain-derived neurotrophic factor (BDNF) to protect neurological diseases such as Parkinson disease, Alzheimer's disease, depression etc. Particles were fabricated and analyzed, and results demonstrated mean particle size of zein/LF nanoparticles was around 74 nm with turbidity (<0.300) and polydispersity index (<0.200) values. Chemical stability of Zein/lactoferrin loaded with 7,8-dihydroxyflavone nanoparticles was established, and as data obtained, Zein/lactoferrin-7,8-dihydroxyflavone slowed down the degradation speed in comparison to free 7,8-DHF and zein-DHF. After 15 days of storage at 25oC in light, free 7,8-DHF was completely degraded, but zein/LF nanoparticles showed the stability of 7,8-DHF was significantly higher, and the retention percentage of, Zein/lactoferrin-7,8-dihydroxyflavone was maximum, i.e., 27.40% retention. The bioaccessibility of free 7, 8-DHF and zein/LF-DHF was also determined, and the values were 18.06% and 31.85%, respectively [96].
One more interesting study was performed by John D. Hoekman and his group with an approach of using an integrin-targeting ligand. They fabricated Arg-Gly-Asp (RGD) liposomes attached with acylated integrin-binding peptides (palmitoyl-Gly-Arg-Gly-Asp-Ser) to deliver analgesic opioid fentanyl. An RGD-expressing liposome increased binding and permeability to the nasal epithelial cells so that fentanyl residence time and absorption increased. In-vivo experiments were conducted where rats treated with fentanyl in RGD liposome displayed better analgesic effect when compared with the free drug (AUC effect = 1387.1% vs. 760.1% MPE*min), whereas round about 20% reduced plasma drug exposure was exhibited (AUC0-120 = 208.2 vs. 284.8 ng min/mL) [97].
Some recent studies have also been performed to develop surface-modified nanocarriers using lactoferrin. Zhang et al.(2021) developed doxorubicin hydrochloride-loaded hyaluronic acid nanogels with surface functionalized Lactoferrin/phenylboronic acid for targeting glioma. They formulated doxorubicin-loaded phenylboronic acid nanogels and further coated the surface of nanogels with the help of lactoferrin. The size of lactoferrin and phenylboronic acid-coated doxorubicin-loaded nanogels was obtained in 193.9 ± 3.7 nm with PDI of 0.125 ± 0.007 and zeta potential of 21.63 ± 1.14 mV. In various cytological studies, lactoferrin/ phenylboronic acid-coated doxorubicin-loaded nanogels have shown better cytotoxicity, improved cellular uptake and higher brain permeability compared to doxorubicin solution. The pharmacokinetic studies were also performed, which reported that area under the curve (AUC) of different formulations (doxorubicin-loaded phenylboronic acid nanogels, lactoferrin-coated doxorubicin loaded phenylboronic acid nanogels and doxorubicin-loaded nanogels)increased by 8.12, 4.32 and 4.20 and times as compared to that of doxorubicin solution, respectively [98].
Another study was also performed by Kim et al. in 2021, developed orally absorbable gold nanoparticles conjugated with lactoferrin for the treatment of glioblastoma multiforme because of its easy absorption mechanism in the intestine, BBB and glioblastoma cells as its receptors are highly expressed in these areas. Primarily formed coated gold nanoparticles with glutathione and polyethylene glycol (PEG) for more stability and circulation and coated them with the help of lactoferrin to form lactoferrin-coated PEG gold nanoparticles. Pharmacokinetics studies were done in glioma model of mice, and it was exhibited that with the oral administration of lactoferrin-coated PEG gold nanoparticles into the orthotopic GBM-containing mice, 11-fold higher concentrations of gold nanoparticles were determined in the blood and GBM in the brain as compared with unmodified nanoparticles [99].
Teixeira et al. (2022) formulated and characterized the lactoferrin functionalized lipid nanoparticles for treating a neurodegenerative disorder Amyotrophic lateral sclerosis (ALS) through the targeted delivery of riluzole to the brain to facilitate its transport across the BBB. Nanostructured Lipid carriers (lipid nanoparticles) were characterized for their physiochemical properties such as size, polydispersity index and zeta potential and were also analyzed for their encapsulation efficiency, stability studies, morphology, cell viability and in-vitro drug release percentage. As a result, nanoparticles attained a size range of 180-200 nm with a polydispersity index of less than 0.3 and encapsulation efficiency of around 94-98%. It was found that nanoparticles showed stability for at least 3 months. In a cell viability assay, lipid nanoparticles did not affect the viability of NSC-34 and hCMEC/D3 cells at a high drug concentration (10 μM); hence are biocompatible and safe to be used for drug delivery in the brain [100].
Certain other targeting ligands have been used to develop functionalized nanoparticles such as folate, lipoprotein, bacterial outer membrane etc. Donepzil-loaded lipoprotein-based polymeric nanoparticles were developed to improve the efficacy of donepezil in treating Alzheimer's disease. Apolipoprotein (ApoE3) facilitates drug bioavailability and reduces doses in Wistar rats [101]. Lecithin-coated lipophilic nanoparticles embedded with ApoE3 have been formulated, revealing ApoE-dependent penetration of the BBB via transcytosis, preventing lysosomal degradation [102]. In another study, folic acid was used for conjugation to enhance drug penetration across the BBB in rats using a targeted delivery approach. SPIONS were developed by surface conjugation with folic acid [103]. Biomimetic nanoparticles functionalized with lipopolysaccharide-free bacterial outer membranes have been developed to achieve brain-targeted delivery. The biomimetic nanoparticle provides the drugs with prolonged circulation, high biocompatibility and intracranial interstitial [104].
Table 1 summarizes some recent development of surface-modified nanoparticles for drug delivery to the brain.
Table 1.
Summary of various recently developed surface-modified nanocarriers for drug delivery to the brain.
|
Type of
Nanocarrier |
Surface | Core (Drug/Gene) |
Clinical
Application |
Size (nm) | Zeta Potential (mV) | Key Properties | Refs. |
|---|---|---|---|---|---|---|---|
| PLA Nanoparticles | Lectin and PEG | Wheat germ agglutinin | Brain drug delivery | 85-90 | - | Negligible nasal ciliatoxicity, higher uptake in rats’ brains. | [19] |
| Angiopep conjugated nanoparticles | Angiopep and PEG | Dendrigraft poly-L-lysine (DGL) | Neuroprotective Effect | 119±12 | 8.2±0.7 | Higher cellular uptake and gene expression in brain cells improved locomotory activity in rats. |
[20] |
| PLGA Nanoparticles | Chitosan | Chlorpromazine hydrochloride | Schizophrenia | 463.9 12 | + 21.0 2.0 | Increased mucoadhesive particles in sheep nasal mucosa. | [32] |
| Human serum albumin nanoparticles | Chitosan | sulforhodamine B sodium salt | Neuroprotective Effect | 261⌖8 | +45⌖1 | Higher cellular uptake and increased permeation in rabbit nasal mucosa. |
[34] |
| Solid Lipid Nanoparticle | Chitosan | Ferulic acid | Alzheimer’s disease | 185 | +12.4 | Higher permeation and neuroprotective effect in rats. | [36] |
| Nanostructured lipid Carriers | Delonix regia gum | Ondansetron hydrochloride | Brain drug delivery | 92.28-135 | -11.5 to -36.2 | higher drug targeting efficiency and direct transport percentage observed in rats. | [38] |
| Nanoparticles | Alginate | Venlafaxine | Depression | 173.7 ± 2.5 | 37.40 ± 1.74 | Increased permeation across nasal mucosa, sustained drug release, improved locomotory in albino Wistar rats. | [39] |
| Nanoemulsion | N,N,N'trimethyl chitosan | Ropinirole hydrochloride |
Parkinson's Disease | 32.39 to 99.00 | -28.5 to -38.5 | Higher uptake in the brain of swiss albino mice improved CNS bioavailability | [40] |
| Nanoparticles | Butylglyceryl polysaccharides | Doxorubicin, rhodamine B, angiotensin II |
Brain disorders | - | - | Increased biological membrane permeability and cellular uptake | [41] |
| PLGA Nanoparticles | Solanum tuberosum lectin and PEG | Haloperidol | Schizophrenia | <150nm | -11 to -16 | Increases the efficacy of particle transport across the nasal epithelium and increases the concentration in the brain of rats. | [44] |
| Liposomes | Glutathione and PEG | - | Brain drug delivery | 108 | - | Stability and prolonged circulation time in rats | [46] |
| Poly propyleneimine dendrimers | Angiopep-2 and PEG | Paclitaxel | Brain cancer | 47±0.20 nm | - | Targeted delivery to the brain | [48] |
| Poly-(amido amine) dendrimers | PEG | Rhodamine B isothiocyanate |
Brain ischemia | 24.2 nm ± 16.2 nm | 11.4 ± 1.69 | Increased bioavailability in the neuron, diffusion of the dendrimers through the brain tissue of mice. |
[49] |
| PLGA Nanoparticles | PEG | Brucine | Cancer | 94 ± 3.05 to 253 ± 8.7 nm | 1.09 ± 0.15 to 3.71 ± 0.44 mV | Decrease in tumor growth in tumor-bearing mice. | [50] |
| Solid silica Nanoparticles |
PEG | MnO2 (H-MnO2) | Stroke | - | - | Protective effect on ischemic stroke mice model | [51] |
| Nanovesicularspanlastics | Span 60 and polyvinyl alcohol | Risperidone | CNS Disorders | 300 nm | -46.7 ± 2.19 | Showed elasticity to permeate through mucosal membrane, significantly higher concentration in swiss albino mice. | [55] |
| Chitosan Nanoparticles |
Polysorbate 80 | Ropinirole hydrochloride |
CNS Disorders | 201-233 | -19.6 | Sustained release, stability of particles and higher concentration of drug in the brain of Wistar rats. | [57] |
| Chitosan nanoparticles |
Tween 80, polyethylene glycol 4000, and miltefosine | Berberine | Neuroprotective Effect | > 190 | 36.3 ± 1.44 | Showed neuroprotective and hepatoprotective effects in rats. | [58] |
| Albumin nanoparticles |
Polysorbate 80 | Levetiracetam | Epilepsy | 153.7 ± 44.8 nm | - 10.8 | Increased drug concentration in male Wistar rats. | [59] |
| PLGA nanoparticles | Polysorbate 80 | Thymoquinone | Alzheimer’s disease | 226.2 nm | −45.6 mV | Improvement in behavior and cognitive effect in mice model. | [60] |
| PLGA nanoparticles | Protamine | Tacrine | Alzheimer’s disease | 196.43 ± 0.55 | 22.53 ± 0.32 | Sustained release manner and good brain targeting efficiency and brain absolute bioavailability in rats’ model. | [63] |
| PEG-lipid nanoparticles |
Fas ligand antibody | 3-n-Butylphthalide (NBP) | Brain ischaemia | 60.97 ±7.95nm | - | Effectively delivered to the ipsilateral region of the ischaemic brain, significantly reduced dosages observed in rats. |
[64] |
| PLGA nanoparticles | Anti-transferrin receptor monoclonal antibody (OX26) and anti-Aβ (DE2B4) | Peptide iAβ5 | Alzheimeŕs disease | 163 ± 3, 166 ± 2 | −10.1 ± 0.4, −13 ± 1 | Substantial increase in uptake of immune nanoparticles with a controlled delivery of the peptide iA5 | [65] |
| PLGA nanoparticles | Monoclonal anti-transferrin receptor antibody (8D3 mAb). |
Thiazolidinedione | Brain drug delivery | 65± 1.4 | -22.10 | Selective interaction with BBB | [66] |
| Liposomes | Arg-Gly-Asp peptide | Small interfering RNA (siRNA) | Tumor | - | - | Increased distribution of siRNA in tumors and improved therapeutic efficiency in mice. | [76] |
| Liposomes | Transferrin | Dopamine HCL | Parkinson’s disease | 180 nm | +7.5 | Higher permeability and increased concentration of dopamine |
[89] |
| Solid Lipid Nanoparticles |
Lactoferrin | Docetaxel | Brain Cancer | 121.0 ± 5.65 | -21.5 +1.2 | Increased the targeting potential for brain tumors of swiss albino mice. | [95] |
| Lipophilic Nanoparticles |
Apolipoprotein E3 | Model Drug | Brain Drug delivery | 103.3 ± 5.5 to 115.7 ± 1.6 | −53.0 ± 2.0 to −49.1 ± 8.5 | Higher penetration of drug across BBB, apolipoprotein mediated transcytosis. Enhanced pharmacokinetics in Wistar rats. | [101] |
| Nanoparticles | Folic Acid | Temozolomide | Glioblastoma | 58.61 | -29.85 ± 0.47 | Enhanced anti-cancer activity and improved drug targeting in rat brain | [103] |
| Nanocubic vesicles | Poloxamer 188 or 407 | Olanzapine | Antipsychotic disorders | 363–645 nm | - | Increased drug targeting efficiency and bioavailability in rats. |
[105] |
| Human serum albumin-based Nanoparticles |
Apolipoprotein E | - | Neuroprotective Effect | 197.8±4.8 | −42.5±6.3 | The active endocytotic uptake mechanism | [106] |
| Liposomes | Cell-penetrating Peptides | Doxorubicin | Glioblastoma | 95 | - | Increased cellular uptake and reduced cell viability | [107] |
| Nanoparticles | Lactoferrin | Dopamine | Parkinson’s Disease | 175.3 ± 9.6 | -15.7 ± 0.86 | Increased dopamine delivery to the brain via the intranasal route. |
[108] |
CONCLUSION & FUTURE PROSPECTS
Due to BBB, central nervous system disorders are one of the major challenges to treat pharmacologically. Therefore, there is a need to develop novel drug delivery strategies. Hence, surface-modification of nanoparticles are gaining attention and emerging to be a useful approach to overcome the drawbacks faced by conventional nanoparticles not only for neurodegenerative disorders but also for another life-threatening disease. Through surface modification, delivery of poor water-soluble molecules and macromolecular have been made possible without affecting the integrity of loaded drug molecules. Surface modification leads to the generation of desirable characteristics to the nanoparticles, which ease the delivery process, such as crossing BBB in case of brain drug delivery, resulting in more biocompatibility, enhanced drug release and targeted delivery. Various surface modification methods result in mucoadhesive NPs, mucus penetrating NPs, protein conjugated, and antibody-conjugated nanoparticles. Other approaches have been applied for targeted delivery, such as cell-penetrating peptides (CPPs), transferrin, lactoferrin and integrin-targeting ligands. CPPs being small and positively charged, are quite favorable for transportation across BBB. Whereas, transferrin and lactoferrin possess unique physiological and biological characteristics, which implies that these can be used in conjugation with the developed nanoparticles for more effective and targeted delivery, especially in the brain region of the affected individual where transferrin and lactoferrin receptors are expressed abundantly. Hence, targeted drug delivery systems could be considered the most promising strategy to deliver across BBB and the brain.
Despite the huge potential of nanocarriers and surface-modified nanoformulations on drug delivery and treatment of diseases, there are several challenges like in vivo stability, biocompatibility, scale-up of formulations etc. which restrict the clinical translation and commercialization of these nanocarriers and this aspect needs to be addressed and resolved.
Some of the basic concerns that need to be addressed are given to signify the superiority of the developed nanomedicines and facilitate the data necessary for further development of a therapeutic product:
1) The material used to develop particles must be biodegradable, biocompatible and safe to use so that it does not harm the brain and other organs, eliminates with time and provides safe, targeted delivery.
2) A uniform method for preparation must be used to generate more homogenous nanoparticles, and also it should be validated and up-scalable fabrication.
3) Generally, particles with smaller sizes of 100-400 nm are more likely to enter the CNS and create less cytotoxicity. Therefore, try to develop particles within this range.
4) To carry out in-vivo experiments, organ perfusion must be performed before dissection to avoid blood contamination from analytical quantitation.
5) The in-vivo studies must be analyzed and evaluated carefully to determine all the factors influencing the in-vivo behavior and stability of delivered nanoparticles, which is an important factor for developing brain-targeted drug delivery systems.
6) The targeting efficiency must be checked before clinical trials, and necessary improvements must be made.
ACKNOWLEDGEMENTS
The authors would like to acknowledge JaypeeInstitute of Information Technology, Noida, U.P, India.
LIST OF ABBREVIATIONS
- AD
Alzheimer’s Disease
- ALS
Amyotrophic Lateral Sclerosis
- BBB
Blood Brain Barrier
- CPPs
Cell-Penetrating Peptides
- DTE
Drug Targeting Efficiency
- DTP
Direct Transport Percentage
- EE
Entrapment Efficiency
- NLC
Nanostructured Lipid Carrier
- PD
Parkinson’s Disease
- PLGA
Poly(lactic-co-glycolic acid)
- RES
Reticuloendothelial System
- RMT
Receptor-mediated Transport
- ROS
Reactive Oxygen Species
- SDS
Sodium Dodecyl Sodium
- SNES
Simulated Nasal Electrolyte Solution
- TPP
Tri Polyphosphate
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Barnabas W. Drug targeting strategies into the brain for treating neurological diseases. J. Neurosci. Methods. 2019;311:133–146. doi: 10.1016/j.jneumeth.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Masserini M. Nanoparticles for brain drug delivery. ISRN Biochem. 2013;2013:238428. doi: 10.1155/2013/238428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Y., Wei L., Wang H. Progress and perspectives on nanoplatforms for drug delivery to the brain. J. Drug Deliv. Sci. Technol. 2020;57:101636. doi: 10.1016/j.jddst.2020.101636. [DOI] [Google Scholar]
- 4.Tam V.H., Sosa C., Liu R., Yao N., Priestley R.D. Nanomedicine as a non-invasive strategy for drug delivery across the blood brain barrier. Int. J. Pharm. 2016;515(1-2):331–342. doi: 10.1016/j.ijpharm.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 5.Di Luca M., Nutt D., Oertel W., Boyer P., Jaarsma J., Destrebecq F., Esposito G., Quoidbach V. Towards earlier diagnosis and treatment of disorders of the brain. Bull. World Health Organ. 2018;96(5):298–298A. doi: 10.2471/BLT.17.206599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong G.F., Qin N., Sun L.W. Development and evaluation of Desvenlafaxine loaded PLGA-chitosan nanoparticles for brain delivery. Saudi Pharm. J. 2017;25(6):844–851. doi: 10.1016/j.jsps.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang A.E. Clinical trials of disease-modifying therapies for neurodegenerative diseases: The challenges and the future. Nat. Med. 2010;16(11):1223–1226. doi: 10.1038/nm.2220. [DOI] [PubMed] [Google Scholar]
- 8.Katare Y.K., Piazza J.E., Bhandari J., Daya R.P., Akilan K., Simpson M.J., Hoare T., Mishra R.K. Intranasal delivery of antipsychotic drugs. Schizophr. Res. 2017;184:2–13. doi: 10.1016/j.schres.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 9.Dimitrijevic I., Pantic I. Application of nanoparticles in psychophysiology and psychiatry research. Mater. Sci. 2014;38:1–6. [Google Scholar]
- 10.Zlokovic B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011;12(12):723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J., Ahn S.I., Kim Y. Nanotherapeutics engineered to cross the blood-brain barrier for advanced drug delivery to the central nervous system. J. Ind. Eng. Chem. 2019;73:8–18. doi: 10.1016/j.jiec.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pande V.V. Studies on the characteristics of zaltoprofen loaded gelatin nanoparticles by nanoprecipitation. Inventi Rapid: NDDS. 2015. p. 1-7.
- 13.Mahmoudi Saber M. Strategies for surface modification of gelatin-based nanoparticles. Colloids Surf. B Biointerfaces. 2019;183:110407. doi: 10.1016/j.colsurfb.2019.110407. [DOI] [PubMed] [Google Scholar]
- 14.Pelaz B., Alexiou C., Alvarez-Puebla R.A., Alves F., Andrews A.M., Ashraf S., Balogh L.P., Ballerini L., Bestetti A., Brendel C., Bosi S., Carril M., Chan W.C.W., Chen C., Chen X., Chen X., Cheng Z., Cui D., Du J., Dullin C., Escudero A., Feliu N., Gao M., George M., Gogotsi Y., Grünweller A., Gu Z., Halas N.J., Hampp N., Hartmann R.K., Hersam M.C., Hunziker P., Jian J., Jiang X., Jungebluth P., Kadhiresan P., Kataoka K., Khademhosseini A., Kopeček J., Kotov N.A., Krug H.F., Lee D.S., Lehr C.M., Leong K.W., Liang X.J., Ling Lim M., Liz-Marzán L.M., Ma X., Macchiarini P., Meng H., Möhwald H., Mulvaney P., Nel A.E., Nie S., Nordlander P., Okano T., Oliveira J., Park T.H., Penner R.M., Prato M., Puntes V., Rotello V.M., Samarakoon A., Schaak R.E., Shen Y., Sjöqvist S., Skirtach A.G., Soliman M.G., Stevens M.M., Sung H.W., Tang B.Z., Tietze R., Udugama B.N., VanEpps J.S., Weil T., Weiss P.S., Willner I., Wu Y., Yang L., Yue Z., Zhang Q., Zhang Q., Zhang X.E., Zhao Y., Zhou X., Parak W.J. Diverse applications of nanomedicine. ACS Nano. 2017;11(3):2313–2381. doi: 10.1021/acsnano.6b06040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felipe A. Surface-Modified nanoparticles to improve drug delivery. In: Dekker Encyclopedia of Nanoscience and nanotechnology, 3rd ed. 2014. p. pp. 1-7.
- 16.Mout R., Moyano D.F., Rana S., Rotello V.M. Surface functionalization of nanoparticles for nanomedicine. Chem. Soc. Rev. 2012;41(7):2539–2544. doi: 10.1039/c2cs15294k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen Z., Nieh M.P., Li Y. Decorating nanoparticle surface for targeted drug delivery: Opportunities and challenges. Polymers (Basel) 2016;8(3):83. doi: 10.3390/polym8030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh D., Kapahi H., Rashid M., Prakash A., Majeed A.B., Mishra N. Recent prospective of surface engineered nanoparticles in the management of neurodegenerative disorders. Artif. Cells Nanomed. Biotechnol. 2016;44(3):780–791. doi: 10.3109/21691401.2015.1029622. [DOI] [PubMed] [Google Scholar]
- 19.Gao X., Tao W., Lu W., Zhang Q., Zhang Y., Jiang X., Fu S. Lectin-conjugated PEG–PLA nanoparticles: Preparation and brain delivery after intranasal administration. Biomaterials. 2006;27(18):3482–3490. doi: 10.1016/j.biomaterials.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 20.Huang R., Ma H., Guo Y., Liu S., Kuang Y., Shao K., Li J., Liu Y., Han L., Huang S., An S., Ye L., Lou J., Jiang C. Angiopep-conjugated nanoparticles for targeted long-term gene therapy of Parkinson’s disease. Pharm. Res. 2013;30(10):2549–2559. doi: 10.1007/s11095-013-1005-8. [DOI] [PubMed] [Google Scholar]
- 21.Barbu E., Molnàr É., Tsibouklis J., Górecki D.C. The potential for nanoparticle-based drug delivery to the brain: Overcoming the blood–brain barrier. Expert Opin. Drug Deliv. 2009;6(6):553–565. doi: 10.1517/17425240902939143. [DOI] [PubMed] [Google Scholar]
- 22.Sonvico F., Clementino A., Buttini F., Colombo G., Pescina S., Stanisçuaski G.S., Raffin P.A., Nicoli S. Surface-modified nanocarriers for nose-to-brain delivery: From bioadhesion to targeting. Pharmaceutics. 2018;10(1):34. doi: 10.3390/pharmaceutics10010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sosnik A. das Neves, J.; Sarmento, B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: A review. Prog. Polym. Sci. 2014;39(12):2030–2075. doi: 10.1016/j.progpolymsci.2014.07.010. [DOI] [Google Scholar]
- 24.Ugwoke M., Agu R., Verbeke N., Kinget R. Nasal mucoadhesive drug delivery: Background, applications, trends and future perspectives. Adv. Drug Deliv. Rev. 2005;57(11):1640–1665. doi: 10.1016/j.addr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Pardeshi C.V., Kulkarni A.D., Sonawane R.O., Belgamwar V.S., Chaudhari P.J., Surana S.J. Mucoadhesive nanoparticles: A roadmap to encounter the challenge of rapid nasal mucociliary clearance. Indian J. Pharm. Edu. Res. 2019;53(2s):s17–s27. doi: 10.5530/ijper.53.2s.45. [DOI] [Google Scholar]
- 26.Lai S.K., Wang Y.Y., Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009;61(2):158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sosnik A., Neves J., Sarmento B. Mucoadhesive polymers in the design of nano-drug delivery system for administration by non-parenteral routes: A review. Prog. Polym. Sci. 2014;39(12):2030–2075. doi: 10.1016/j.progpolymsci.2014.07.010. [DOI] [Google Scholar]
- 28.Lee D., Powers K., Baney R. Physicochemical properties and blood compatibility of acylated chitosan nanoparticles. Carbohydr. Polym. 2004;58(4):371–377. doi: 10.1016/j.carbpol.2004.06.033. [DOI] [Google Scholar]
- 29.Wang X., Chi N., Tang X. Preparation of estradiol chitosan nanoparticles for improving nasal absorptionand brain targeting. Eur. J. Pharm. Biopharm. 2008;70(3):735–740. doi: 10.1016/j.ejpb.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Casettari L., Illum L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release. 2014;190:189–200. doi: 10.1016/j.jconrel.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Singh D., Rashid M., Hallan S.S., Mehra N.K., Prakash A., Mishra N. Pharmacological evaluation of nasal delivery of selegiline hydrochloride-loaded thiolated chitosan nanoparticles for the treatment of depression. Artif. Cells Nanomed. Biotechnol. 2016;44(3):865–877. doi: 10.3109/21691401.2014.998824. [DOI] [PubMed] [Google Scholar]
- 32.Chalikwar S.S., Mene B.S., Pardeshi C.V., Belgamwar V.S., Surana S. Self-assembled, chitosan grafted PLGA nanoparticles for intranasal delivery: Design, development and ex vivo characterization. Polym. Plast. Technol. Eng. 2013;52(4):368–380. doi: 10.1080/03602559.2012.751999. [DOI] [Google Scholar]
- 33.Ahmad N. Rasagiline-encapsulated chitosan-coated PLGA nanoparticles targeted to the brain in the treatment of Parkinson’s disease. J. Liq. Chromatogr. Relat. Technol. 2017;40(13):677–690. doi: 10.1080/10826076.2017.1343735. [DOI] [Google Scholar]
- 34.Piazzini V., Landucci E., D’Ambrosio M., Tiozzo Fasiolo L., Cinci L., Colombo G., Pellegrini-Giampietro D.E., Bilia A.R., Luceri C., Bergonzi M.C. Chitosan coated human serum albumin nanoparticles: A promising strategy for nose-to-brain drug delivery. Int. J. Biol. Macromol. 2019;129:267–280. doi: 10.1016/j.ijbiomac.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Chatzitaki A.T., Jesus S., Karavasili C., Andreadis D., Fatouros D.G., Borges O. Chitosan-coated PLGA nanoparticles for the nasal delivery of ropinirole hydrochloride: In vitro and ex vivo evaluation of efficacy and safety. Int. J. Pharm. 2020;589:119776. doi: 10.1016/j.ijpharm.2020.119776. [DOI] [PubMed] [Google Scholar]
- 36.Saini S., Sharma T., Jain A., Kaur H., Katare O.P., Singh B. Systematically designed chitosan-coated solid lipid nanoparticles of ferulic acid for effective management of Alzheimer’s disease: A preclinical evidence. Colloids Surf. B Biointerfaces. 2021;205:111838. doi: 10.1016/j.colsurfb.2021.111838. [DOI] [PubMed] [Google Scholar]
- 37.Bruinsmann F.A., de Cristo Soares A.A., de Fraga Dias A., Lopes S.L.F., Visioli F., Raffin P.A., Figueiró F., Sonvico F., Stanisçuaski G.S. Nose-to-brain delivery of simvastatin mediated by chitosan-coated lipid-core nanocapsules allows for the treatment of glioblastoma in vivo. Int. J. Pharm. 2022;616:121563. doi: 10.1016/j.ijpharm.2022.121563. [DOI] [PubMed] [Google Scholar]
- 38.Devkar T.B., Tekade A.R., Khandelwal K.R. Surface engineered nanostructured lipid carriers for efficient nose to brain delivery of ondansetron HCl using Delonix regia gum as a natural mucoadhesive polymer. Colloids Surf. B Biointerfaces. 2014;122:143–150. doi: 10.1016/j.colsurfb.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 39.Haque S., Md S., Sahni J.K., Ali J., Baboota S. Development and evaluation of brain targeted intranasal alginate nanoparticles for treatment of depression. J. Psychiatr. Res. 2014;48(1):1–12. doi: 10.1016/j.jpsychires.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 40.Pardeshi C.V., Belgamwar V.S. N. N,N-trimethyl chitosan modified flaxseed oil based mucoadhesive neuronanoemulsions for direct nose to brain drug delivery. Int. J. Biol. Macromol. 2018;120(Pt B):2560-2571. doi: 10.1016/j.ijbiomac.2018.09.032. [DOI] [PubMed] [Google Scholar]
- 41.Bostanudin M.F., Lalatsa A., Górecki D.C., Barbu E. Engineering butylglyceryl-modified polysaccharides towards nanomedicines for brain drug delivery. Carbohydr. Polym. 2020;236:116060. doi: 10.1016/j.carbpol.2020.116060. [DOI] [PubMed] [Google Scholar]
- 42.Liu M., Zhang J., Shan W., Huang Y. Developments of mucus penetrating nanoparticles. Asian J. Pharm. Sci. 2015;10(4):275–282. doi: 10.1016/j.ajps.2014.12.007. [DOI] [Google Scholar]
- 43.Gajbhiye K.R., Pawar A., Mahadik K.R., Gajbhiye V. PEGylated nanocarriers: A promising tool for targeted delivery to the brain. Colloids Surf. B Biointerfaces. 2020;187:110770. doi: 10.1016/j.colsurfb.2019.110770. [DOI] [PubMed] [Google Scholar]
- 44.Piazza J., Hoare T., Molinaro L., Terpstra K., Bhandari J., Selvaganapathy P.R., Gupta B., Mishra R.K. Haloperidol-loaded intranasally administered lectin functionalized poly(ethylene glycol)–block-poly(d,l)-lactic-co-glycolic acid (PEG–PLGA) nanoparticles for the treatment of schizophrenia. Eur. J. Pharm. Biopharm. 2014;87(1):30–39. doi: 10.1016/j.ejpb.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Pinzón-Daza M., Campia I., Kopecka J., Garzón R., Ghigo D., Rigant C. Nanoparticle- and liposome-carried drugs: New strategies for active targeting and drug delivery across blood-brain barrier. Curr. Drug Metab. 2013;14(6):625–640. doi: 10.2174/1389200211314060001. [DOI] [PubMed] [Google Scholar]
- 46.Rip J., Chen L., Hartman R., van den Heuvel A., Reijerkerk A., van Kregten J., van der Boom B., Appeldoorn C., de Boer M., Maussang D., de Lange E.C.M., Gaillard P.J. Glutathione PEGylated liposomes: Pharmacokinetics and delivery of cargo across the blood–brain barrier in rats. J. Drug Target. 2014;22(5):460–467. doi: 10.3109/1061186X.2014.888070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tiwari A., Kesharwani P., Gajbhiye V., Jain N.K. Synthesis and characterization of dendro-PLGA nanoconjugate for protein stabilization. Colloids Surf. B Biointerfaces. 2015;134:279–286. doi: 10.1016/j.colsurfb.2015.06.064. [DOI] [PubMed] [Google Scholar]
- 48.Parashar A.K., Jain N.K., Gupta A.K. Synthesis and characterization of Agiopep-2 anchored PEGylated poly propyleneimine dendrimers for targeted drug delivery to glioblastoma multiforme. J. Drug Deliv. Ther. 2018;8(6-A):74–79. [Google Scholar]
- 49.Santos S.D., Xavier M., Leite D.M., Moreira D.A., Custódio B., Torrado M., Castro R., Leiro V., Rodrigues J., Tomás H., Pêgo A.P. PAMAM dendrimers: Blood-brain barrier transport and neuronal uptake after focal brain ischemia. J. Control. Release. 2018;291:65–79. doi: 10.1016/j.jconrel.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Elsewedy H.S., Dhubiab B.E.A., Mahdy M.A., Elnahas H.M. Development, optimization, and evaluation of PEGylated brucine-loaded PLGA nanoparticles. Drug Deliv. 2020;27(1):1134–1146. doi: 10.1080/10717544.2020.1797237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang S.B., Li X.L., Li K., Zhang X.X., Yuan M., Guo Y.S., Bi X. The colossal role of H-MnO2-PEG in ischemic stroke. Nanomedicine. 2021;33:102362. doi: 10.1016/j.nano.2021.102362. [DOI] [PubMed] [Google Scholar]
- 52.Wiwatchaitawee W., Ebeid K., Quarterman J.C., Naguib Y., Ali Y., Oliva C., Griguer C., Salem A.K. Surface modification of nanoparticles enhances drug delivery to the brain and improves survival in a glioblastoma multiforme murine model. Bioconjugate Chem; 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tröster S.D., Kreuter J. Contact angles of surfactants with a potential to alter the body distribution of colloidal drug carriers on poly (methyl methacrylate) surfaces. Int. J. Pharm. 1988;45(1-2):91–100. doi: 10.1016/0378-5173(88)90037-3. [DOI] [Google Scholar]
- 54.Tröster S.D., Müller U., Kreuter J. Modification of the body distribution of poly(methyl methacrylate) nanoparticles in rats by coating with surfactants. Int. J. Pharm. 1990;61(1-2):85–100. doi: 10.1016/0378-5173(90)90047-8. [DOI] [Google Scholar]
- 55.Abdelrahman F.E., Elsayed I., Gad M.K., Elshafeey A.H., Mohamed M.I. Response surface optimization, ex vivo and in vivo investigation of nasal spanlastics for bioavailability enhancement and brain targeting of risperidone. Int. J. Pharm. 2017;530(1-2):1–11. doi: 10.1016/j.ijpharm.2017.07.050. [DOI] [PubMed] [Google Scholar]
- 56.Grant S., Fitton A. Risperidone. Drugs. 1994;48(2):253–273. doi: 10.2165/00003495-199448020-00009. [DOI] [PubMed] [Google Scholar]
- 57.Ray S., Sinha P., Laha B., Maiti S., Bhattacharyya U.K., Nayak A.K. Polysorbate 80 coated crosslinked chitosan nanoparticles of ropinirole hydrochloride for brain targeting. J. Drug Deliv. Sci. Technol. 2018;48:21–29. doi: 10.1016/j.jddst.2018.08.016. [DOI] [Google Scholar]
- 58.Soudi S.A., Nounou M.I., Sheweita S.A., Ghareeb D.A., Younis L.K., El-Khordagui L.K. Protective effect of surface-modified berberine nanoparticles against LPS-induced neurodegenerative changes: A preclinical study. Drug Deliv. Transl. Res. 2019;9(5):906–919. doi: 10.1007/s13346-019-00626-1. [DOI] [PubMed] [Google Scholar]
- 59.Wilson B., Selvam J., Mukundan G.K., Premakumari K.B., Jenita J.L. Albumin nanoparticles coated with polysorbate 80 for the targeted delivery of antiepileptic drug levetiracetam into the brain. Drug Deliv. Transl. Res. 2020;10(6):1853–1861. doi: 10.1007/s13346-020-00831-3. [DOI] [PubMed] [Google Scholar]
- 60.Yusuf M., Khan M., Alrobaian M.M., Alghamdi S.A., Warsi M.H., Sultana S., Khan R.A. Brain targeted Polysorbate-80 coated PLGA thymoquinone nanoparticles for the treatment of Alzheimer’s disease, with biomechanistic insights. J. Drug Deliv. Sci. Technol. 2021;61:102214. doi: 10.1016/j.jddst.2020.102214. [DOI] [Google Scholar]
- 61.Verma D., Gulati N., Kaul S., Mukherjee S., Nagaich U. Protein based nanostructures for drug delivery. J. Pharm. (Cairo) 2018;2018:9285854. doi: 10.1155/2018/9285854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guerrini L., Alvarez-Puebla R., Pazos-Perez N. Surface modifications of nanoparticles for stability in biological fluids. Materials (Basel) 2018;11(7):1154. doi: 10.3390/ma11071154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.ShamarekhHeba. K.S.; Gad, H.A.; Soliman, M.A.; Sammour, O.A. Development and evaluation of protamine-coated PLGA nanoparticles for nose-tobrain delivery of tacrine: In vitro and in vivo assessment. J. Drug Deliv. Sci. Technol. 2020;57:101724. doi: 10.1016/j.jddst.2020.101724. [DOI] [Google Scholar]
- 64.Lu Y.M., Huang J.Y., Wang H., Lou K.F., Liao M.H., Hong L.J., Tao R., Ahmed M., Shan C.L., Wang X.L., Fukunaga K., Du Y.Z., Han F. Targeted therapy of brain ischaemia using Fas ligand antibody conjugated PEG-lipid nanoparticles. Biomaterials. 2014;35(1):530–537. doi: 10.1016/j.biomaterials.2013.09.093. [DOI] [PubMed] [Google Scholar]
- 65.Joana A. Cellular uptake of PLGA nanoparticles targeted with anti-amyloid andanti-transferrin receptor antibodies for Alzheimer’s disease treatment. Colloids Surf. B Biointerfaces. 2016;145:8–13. doi: 10.1016/j.colsurfb.2016.04.041. [DOI] [PubMed] [Google Scholar]
- 66.Monge M., Fornaguera C., Quero C., Dols-Perez A., Calderó G., Grijalvo S., García-Celma M.J., Rodríguez-Abreu C., Solans C. Functionalized PLGA nanoparticles prepared by nano-emulsion templating interact selectively with proteins involved in the transport through the blood-brain barrier. Eur. J. Pharm. Biopharm. 2020;156:155–164. doi: 10.1016/j.ejpb.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Lin T., Liu E., He H., Shin M.C., Moon C., Yang V.C., Huang Y. Nose-to-brain delivery of macromolecules mediated by cell-penetrating peptides. Acta Pharm. Sin. B. 2016;6(4):352–358. doi: 10.1016/j.apsb.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sonali P.A., Agrawal P., Singh R.P., Rajesh C.V., Singh S., Vijayakumar M.R., Pandey B.L., Muthu M.S. Transferrin receptor-targeted vitamin E TPGS micelles for brain cancer therapy: preparation, characterization and brain distribution in rats. Drug Deliv. 2016;23(5):1788–1798. doi: 10.3109/10717544.2015.1094681. [DOI] [PubMed] [Google Scholar]
- 69.Liu Z., Jiang M., Kang T., Miao D., Gu G., Song Q., Yao L., Hu Q., Tu Y., Pang Z., Chen H., Jiang X., Gao X., Chen J. Lactoferrin-modified PEG-co-PCL nanoparticles for enhanced brain delivery of NAP peptide following intranasal administration. Biomaterials. 2013;34(15):3870–3881. doi: 10.1016/j.biomaterials.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 70.Derakhshankhah H., Jafari S. Cell penetrating peptides: A concise review with emphasis on biomedical applications. Biomed. Pharmacother. 2018;108:1090–1096. doi: 10.1016/j.biopha.2018.09.097. [DOI] [PubMed] [Google Scholar]
- 71.Xie J., Bi Y., Zhang H., Dong S., Teng L., Lee R.J., Yang Z. Cell-penetrating peptides in diagnosis and treatment of human diseases: From preclinical research to clinical application. Front. Pharmacol. 2020;11:697. doi: 10.3389/fphar.2020.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm. Sin. B. 2016;6(4):268–286. doi: 10.1016/j.apsb.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fonseca S.B., Pereira M.P., Kelley S.O. Recent advances in the use of cell-penetrating peptides for medical and biological applications. Adv. Drug Deliv. Rev. 2009;61(11):953–964. doi: 10.1016/j.addr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 74.Gartziandia O., Egusquiaguirre S.P., Bianco J., Pedraz J.L., Igartua M., Hernandez R.M., Préat V., Beloqui A. Nanoparticle transport across in vitro olfactory cell monolayers. Int. J. Pharm. 2016;499(1-2):81–89. doi: 10.1016/j.ijpharm.2015.12.046. [DOI] [PubMed] [Google Scholar]
- 75.Qin Y., Zhang Q., Chen H., Yuan W., Kuai R., Xie F., Zhang L., Wang X., Zhang Z., Liu J., He Q. Comparison of four different peptides to enhance accumulation of liposomes into the brain. J. Drug Target. 2012;20(3):235–245. doi: 10.3109/1061186X.2011.639022. [DOI] [PubMed] [Google Scholar]
- 76.Nai J., Zhang J., Li J., Li H., Yang Y., Yang M., Wang Y., Gong W., Li Z., Li L., Gao C. Macrophage membrane- and cRGD-functionalized thermosensitive liposomes combined with CPP to realize precise siRNA delivery into tumor cells. Mol. Ther. Nucleic Acids. 2021;27:349–362. doi: 10.1016/j.omtn.2021.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arora S., Kanekiyo T., Singh J. Functionalized nanoparticles for brain targeted BDNF gene therapy to rescue Alzheimer’s disease pathology in transgenic mouse model. Int. J. Biol. Macromol. 2022;208:901–911. doi: 10.1016/j.ijbiomac.2022.03.203. [DOI] [PubMed] [Google Scholar]
- 78.Johnsen K.B., Burkhart A., Thomsen L.B., Andresen T.L., Moos T. Targeting the transferrin receptor for brain drug delivery. Prog. Neurobiol. 2019;181:101665. doi: 10.1016/j.pneurobio.2019.101665. [DOI] [PubMed] [Google Scholar]
- 79.Huebers H.A., Finch C.A. The physiology of transferrin and transferrin receptors. Physiol. Rev. 1987;67(2):520–582. doi: 10.1152/physrev.1987.67.2.520. [DOI] [PubMed] [Google Scholar]
- 80.Moos T., Morgan E.H. Transferrin and transferrin receptor function in brain barrier systems. Cell. Mol. Neurobiol. 2000;20(1):77–95. doi: 10.1023/A:1006948027674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morgan E.H. Studies on the mechanism of iron release from transferrin. Biochim. Biophys. Acta Protein Struct. 1979;580(2):312–326. doi: 10.1016/0005-2795(79)90144-2. [DOI] [PubMed] [Google Scholar]
- 82.Li H., Qian Z.M. Transferrin/transferrin receptor-mediated drug delivery. Med. Res. Rev. 2002;22(3):225–250. doi: 10.1002/med.10008. [DOI] [PubMed] [Google Scholar]
- 83.Visser C.C., Stevanović S., Heleen Voorwinden L., Gaillard P.J., Crommelin D.J.A., Danhof M., de Boer A.G. Validation of the transferrin receptor for drug targeting to brain capillary endothelial cells in vitro. J. Drug Target. 2004;12(3):145–150. doi: 10.1080/10611860410001701706. [DOI] [PubMed] [Google Scholar]
- 84.Sahoo S.K., Labhasetwar V. Enhanced antiproliferative activity of transferrin-conjugated paclitaxel-loaded nanoparticles is mediated via sustained intracellular drug retention. Mol. Pharm. 2005;2(5):373–383. doi: 10.1021/mp050032z. [DOI] [PubMed] [Google Scholar]
- 85.Jones A.R., Shusta E.V. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm. Res. 2007;24(9):1759–1771. doi: 10.1007/s11095-007-9379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Das M., Wang C., Bedi R., Mohapatra S.S., Mohapatra S. Magnetic micelles for DNA delivery to rat brains after mild traumatic brain injury. Nanomedicine. 2014;10(7):1539–1548. doi: 10.1016/j.nano.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ghadiri M., Vasheghani-Farahani E., Atyabi F., Kobarfard F., Mohamadyar-Toupkanlou F., Hosseinkhani H. Transferrin-conjugated magnetic dextran-spermine nanoparticles for targeted drug transport across blood-brain barrier. J. Biomed. Mater. Res. A. 2017;105(10):2851–2864. doi: 10.1002/jbm.a.36145. [DOI] [PubMed] [Google Scholar]
- 88.Han Y., Zhang Y., Li D., Chen Y., Sun J., Kong F. Transferrin-modified nanostructured lipid carriers as multifunctional nanomedicine for codelivery of DNA and doxorubicin. Int. J. Nanomedicine. 2014;9:4107–4116. doi: 10.2147/IJN.S67770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lopalco A., Cutrignelli A., Denora N., Lopedota A., Franco M., Laquintana V. Transferrin functionalized liposomes loading dopamine HCl: Development and permeability studies across an in vitro model of human blood–brain barrier. Nanomaterials (Basel) 2018;8(3):178. doi: 10.3390/nano8030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pinheiro R.G.R., Granja A., Loureiro J.A., Pereira M.C., Pinheiro M., Neves A.R., Reis S. Quercetin lipid nanoparticles functionalized with transferrin for Alzheimer’s disease. Eur. J. Pharm. Sci. 2020;148:105314. doi: 10.1016/j.ejps.2020.105314. [DOI] [PubMed] [Google Scholar]
- 91.dos Santos Rodrigues B., Kanekiyo T., Singh J. In vitro and in vivo characterization of CPP and transferrin modified liposomes encapsulating pDNA. Nanomedicine. 2020;28:102225. doi: 10.1016/j.nano.2020.102225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ramalho M.J., Bravo M., Loureiro J.A., Lima J., Pereira M.C. Transferrin-modified nanoparticles for targeted delivery of Asiatic acid to glioblastoma cells. Life Sci. 2022;296:120435. doi: 10.1016/j.lfs.2022.120435. [DOI] [PubMed] [Google Scholar]
- 93.Liu F., Zhang S., Li J., McClements D.J., Liu X. Recent development of lactoferrin-based vehicles for the delivery of bioactive compounds: Complexes, emulsions, and nanoparticles. Trends Food Sci. Technol. 2018;79:67–77. doi: 10.1016/j.tifs.2018.06.013. [DOI] [Google Scholar]
- 94.Allen T.M., Cullis P.R. Drug delivery systems: Entering the mainstream. Science. 2004;303(5665):1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 95.Singh I., Swami R., Pooja D., Jeengar M.K., Khan W., Sistla R. Lactoferrin bioconjugated solid lipid nanoparticles: A new drug delivery system for potential brain targeting. J. Drug Target. 2016;24(3):212–223. doi: 10.3109/1061186X.2015.1068320. [DOI] [PubMed] [Google Scholar]
- 96.Chen Y., Zhao Z., Xia G., Xue F., Chen C., Zhang Y. Fabrication and characterization of zein/lactoferrin composite nanoparticles for encapsulating 7,8-dihydroxyflavone: Enhancement of stability, water solubility and bioaccessibility. Int. J. Biol. Macromol. 2020;146:179–192. doi: 10.1016/j.ijbiomac.2019.12.251. [DOI] [PubMed] [Google Scholar]
- 97.Hoekman J.D., Srivastava P., Ho R.J.Y. Aerosol-stable peptide-coated liposome nanoparticles: A proof-of-concept study with opioid fentanyl in enhancing analgesic effects and reducing plasma drug exposure. J. Pharm. Sci. 2014;103(8):2231–2239. doi: 10.1002/jps.24022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang M., Asghar S., Tian C., Hu Z., Ping Q., Chen Z., Shao F., Xiao Y. Lactoferrin/phenylboronic acid-functionalized hyaluronic acid nanogels loading doxorubicin hydrochloride for targeting glioma. Carbohydr. Polym. 2021;253:117194. doi: 10.1016/j.carbpol.2020.117194. [DOI] [PubMed] [Google Scholar]
- 99.Kim H.S., Lee S.J., Lee D.Y. Milk protein-shelled gold nanoparticles with gastrointestinally active absorption for aurotherapy to brain tumor. Bioact. Mater. 2022;8:35–48. doi: 10.1016/j.bioactmat.2021.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Teixeira M.I., Lopes C.M., Gonçalves H., Catita J., Silva A.M., Rodrigues F., Amaral M.H., Costa P.C. Formulation, characterization, and cytotoxicity evaluation of lactoferrin functionalized lipid nanoparticles for riluzole delivery to the brain. Pharmaceutics. 2022;14(1):185. doi: 10.3390/pharmaceutics14010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krishna K.V., Wadhwa G., Alexander A., Kanojia N., Saha R.N., Kukreti R., Singhvi G., Dubey S.K. Design and biological evaluation of lipoprotein-based donepezil nanocarrier for enhanced brain uptake through oral delivery. ACS Chem. Neurosci. 2019;10(9):4124–4135. doi: 10.1021/acschemneuro.9b00343. [DOI] [PubMed] [Google Scholar]
- 102.Wünsch A., Mulac D., Langer K. Lipoprotein imitating nanoparticles: Lecithin coating binds ApoE and mediates non-lysosomal uptake leading to transcytosis over the blood-brain barrier. Int. J. Pharm. 2020;589:119821. doi: 10.1016/j.ijpharm.2020.119821. [DOI] [PubMed] [Google Scholar]
- 103.Afzalipour R., Khoei S., Khoee S., Shirvalilou S., Jamali Raoufi N., Motevalian M., Karimi M.R. Dual-targeting temozolomide loaded in folate-conjugated magnetic triblock copolymer nanoparticles to improve the therapeutic efficiency of rat brain gliomas. ACS Biomater. Sci. Eng. 2019;5(11):6000–6011. doi: 10.1021/acsbiomaterials.9b00856. [DOI] [PubMed] [Google Scholar]
- 104.Chen H., Zhou M., Zeng Y., Miao T., Luo H., Tong Y., Zhao M., Mu R., Gu J., Yang S., Han L. Biomimetic lipopolysaccharide‐free bacterial outer membrane‐functionalized nanoparticles for brain‐targeted drug delivery. Adv. Sci. (Weinh.) 2022;9(16):2105854. doi: 10.1002/advs.202105854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Salama H.A., Mahmoud A.A., Kamel A.O., Abdel Hady M., Awad G.A.S. Phospholipid based colloidal poloxamer–nanocubic vesicles for brain targeting via the nasal route. Colloids Surf. B Biointerfaces. 2012;100:146–154. doi: 10.1016/j.colsurfb.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 106.Wagner S., Zensi A., Wien S.L., Tschickardt S.E., Maier W., Vogel T., Worek F., Pietrzik C.U., Kreuter J., von Briesen H. Uptake mechanism of ApoE-modified nanoparticles on brain capillary endothelial cells as a blood-brain barrier model. PLoS One. 2012;7(3):e32568. doi: 10.1371/journal.pone.0032568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yuan B., Zhao Y., Dong S., Sun Y., Hao F., Xie J., Teng L., Lee R.J., Fu Y., Bi Y. Cell-penetrating peptide-coated liposomes for drug delivery across the blood–brain barrier. Anticancer Res. 2019;39(1):237–243. doi: 10.21873/anticanres.13103. [DOI] [PubMed] [Google Scholar]
- 108.Tang S., Wang A., Yan X., Chu L., Yang X., Song Y., Sun K., Yu X., Liu R., Wu Z., Xue P. Brain-targeted intranasal delivery of dopamine with borneol and lactoferrin co-modified nanoparticles for treating Parkinson’s disease. Drug Deliv. 2019;26(1):700–707. doi: 10.1080/10717544.2019.1636420. [DOI] [PMC free article] [PubMed] [Google Scholar]