Abstract
Background
Claudin6(CLDN6) is a tight junction protein of claudin-tetraspanin family and is of the earliest molecules expressed in embryonic epithelium. CLDN6 is frequently aberrantly expressed in testicular germ-cell tumors(GCT). ASP1650 is a chimeric-mouse/human-IgG1 antibody directed against CLDN6.
Methods
Two-part, open-label, phase-II trial investigating ASP1650 in patients with relapsed/refractory GCT and no curable options. Part1 was a safety lead-in to establish the recommended-phase-II-dose(RP2D). Part2 was a phase-II study designed to evaluate the antitumor effects of ASP1650. CLDN6 expression was centrally assessed on archival tumor tissue using immunohistochemistry. The primary objectives were to establish the RP2D(safety lead-in) and the antitumor activity(phase-II) of ASP1650.
Results
Nineteen male patients were enrolled: 6 patients in 1000mg/m2 safety lead-in group, and 13 in 1500mg/m2 group. Median age 37.2 years(range,20–58). Histology was non-seminoma in 17/19 patients. Median number of previous chemotherapy regimens was 3. Thirteen patients had prior high-dose chemotherapy. No dose-limiting toxicity events were reported at any study drug dose. A RP2D of 1500mg/m2 every 2weeks was established. No partial or complete responses were observed. The study was stopped at the end of Simon Stage-I due to lack of efficacy. 15/16 subjects with available tissue had CLDN6 positive staining. The mean percent membrane staining was 71.6% and the mean membrane H score was 152.6(SD 76).
Conclusion
ASP1650 did not appear to have clinically meaningful single-agent activity in relapsed/refractory GCT. CLDN6 expression seems ubiquitous in all elements of GCT and is worthy of investigation as a diagnostic biomarker and therapeutic target.
Keywords: Testicular cancer, Germ cell tumor, ASP1650
INTRODUCTION
Germ-cell tumors (GCT) are remarkably chemosensitive and patients diagnosed with metastatic disease have up to an 80% cure rate with first-line cisplatin-based combination chemotherapy.1 Patients who relapse after initial chemotherapy can still be cured with salvage therapy including conventional dose chemotherapy or high-dose chemotherapy plus peripheral-blood stem cell transplant (PBSCT).2–6 Salvage surgery is reserved for a subset of patients who relapse with anatomically confined disease.7 Despite remarkable advances in curative systemic combination therapy, there remains a subset of 15–20% of patients with refractory GCT who are incurable with the current therapeutic options. Novel therapeutic approaches are needed for these patients.
Claudin 6 (CLDN6) is a tight junction protein of the claudin tetraspanin family and is one of the earliest molecules expressed in embryonic epithelium.8 Preclinical studies have indicated that, in normal tissue, CLDN6 expression is largely confined to embryonic and fetal cells.9–11 CLDN6 is ectopically expressed in several human malignancies including ovarian, testicular, uterine, and lung cancer.12–14 It was reported that immunohistochemical staining showed >90% (n=97/104) of testicular cancer tissue samples tested positive for CLDN6 expression.15 Of 104 testicular GCT tissue samples tested, 54 were pure seminoma, 39 were non-seminoma, and 11 were not otherwise specified. Most testicular GCT tissue samples had strong intensity CLDN6 staining (≥2+).15
ASP1650 (also known as IMAB027) is a monoclonal antibody targeted against CLDN6 that can induce antibody-dependent cell-mediated and complement-dependent cytotoxicity in testicular GCT cell lines expressing human CLDN6.16 ASP1650 demonstrated significant antitumor effects in preclinical models of testicular GCT.16
Targeting CLDN6 on testicular GCT with ASP1650 is a novel approach for salvage in patients with relapsed/refractory GCT. In a multicenter single-arm open-label phase II trial, we evaluated the efficacy and safety of ASP1650 in patients with relapsed/refractory GCT and no further curative treatment options.
PATIENTS AND METHODS
Patients
Eligible patients had histologically confirmed metastatic GCT (seminoma or non-seminoma histology) and progressed after first-line cisplatin-based chemotherapy as well as at-least one salvage regimen: standard-dose chemotherapy or high-dose chemotherapy plus peripheral-blood stem-cell transplant. Patients with late relapse defined as progression after 2 years of cisplatin-based combination chemotherapy were eligible. Patients with late relapse did not require a salvage chemotherapy regimen to enroll. An Eastern Cooperative Oncology Group (ECOG) performance status score of ≤2 was required. Patients had measurable metastatic disease according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 and/or elevation of tumors markers (alpha-fetoprotein [AFP] and/or β-human chorionic gonadotropin [hCG]).17 If a rising tumor marker was the only evidence of progressive disease, 2 consecutive rising values at least one week apart were required. Patients with treated or asymptomatic brain metastases were eligible. All patients were required to provide archived tumor tissue from a biopsy or the original primary tumor for CLDN6 expression evaluation. Full eligibility criteria are listed in the trial protocol, available online with the full text of this article.
Study Design
This two-part, open-label study was designed to be conducted in up to 46 male patients with relapsed/refractory GCT. Part 1 was a safety lead-in to establish the recommended phase 2 dose (RP2D) of ASP1650. Part 2 was a phase II study designed to evaluate the antitumor effects of ASP1650 (Figure 1).
Fig 1:
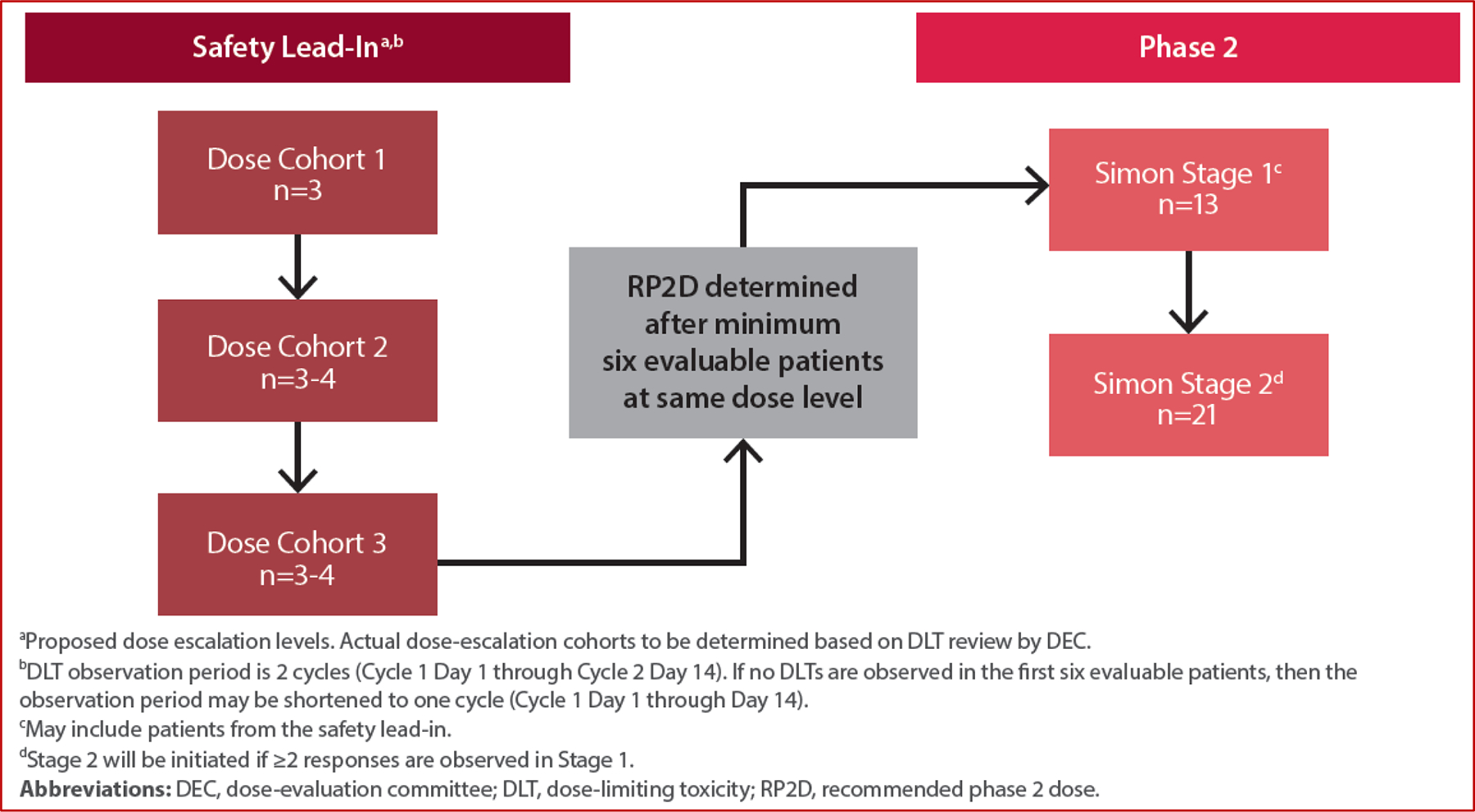
Study Design
In the safety lead-in, an initial ASP1650 dose level was to be evaluated in 3 patients; if tolerated, a new cohort of 3 patients would begin at the next dose level per the Bayesian Optimal Interval Design. ASP1650 was administered intravenously in successive cycles every 2 weeks for a maximum of 12 cycles. A dose-evaluation committee (DEC) assessed tolerability and determined the maximum tolerated dose (MTD) and RP2D. Nine to 18 patients were planned for enrollment in the safety lead-in. Evaluable patients were defined as patients who experience a dose-limiting toxicity (DLT), or in the absence of DLT, receive at least two doses of ASP1650. Dose-limiting toxicities were assessed during the safety lead-in between Cycle 1 Day 1 through Cycle 2 Day 14. If no DLTs were observed in the first 6 evaluable subjects, the DLT observation period could be reduced to Cycle 1 Day 1 through Cycle 1 Day 14 (one cycle). DLT definitions are available in the study protocol (online).
Once the RP2D was established, up to 34 patients could be enrolled in phase II portion with a primary endpoint of confirmed overall response rate (ORR) defined as the proportion of subjects who have a best overall response of confirmed complete response or confirmed partial response, as assessed by modified RECIST v1.1. Phase II utilized a Simon’s 2-stage design to allow for early termination. During stage I, 13 patients, including patients receiving the RP2D during the safety lead-in, were to be evaluated for response. If ≤1 response was observed among these 13 patients, then the study would be terminated, otherwise an additional 21 patients would be enrolled into stage II. The investigational treatment would be considered promising if at least 6 of the 34 patients achieved a response.
Evaluation of Response and Toxicity
Patients were assessed on day 1 of each 2-week cycle. Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. Tumor imaging with CT scan of the chest, abdomen, and pelvis was performed at baseline followed by every 6 weeks for the initial 24 weeks. Subsequently, imaging was spaced to every 12 weeks until discontinuation of study drug. Tumor markers including AFP and hCG were measured at day 1 of every cycle and during the post-treatment follow-up period. Sampling for pharmacokinetics and immunogenicity was performed pre-dose on Day 1 of Cycles 1–12 and post-dose on Day 1 of Cycles 1 and 3. The full assessment schedule is provided in the trial protocol. CLDN6 expression was assessed in formalin-fixed archived tumor samples at central laboratory.
Endpoints and Statistical Analysis
The primary objectives of the study were to establish the RP2D (safety lead-in) and the antitumor activity (phase II) of ASP1650 in patients with relapsed/refractory GCT. Primary endpoints were DLT assessment by the Dose Evaluation Committee (DEC) [safety lead-in] and confirmed ORR as assessed by modified RECIST v1.1 response criteria, incorporating serum tumor biomarker response criteria (AFP and hCG) [phase II]. Secondary endpoints included determination of safety/tolerability and pharmacokinetic profiles of ASP1650, clinical benefit rate, duration of response (DoR), progression-free survival (PFS) based on modified RECIST v1.1, and percent change in AFP and hCG. The null hypothesis was that the true ORR is 10% and the alternative hypothesis was that the true ORR is 25%. The type I error rate was 10% yielding a power of 80%. Confirmed ORR by dose level, as assessed by modified RECIST v1.1, was calculated and its 90% confidence interval (CI) constructed using the Clopper-Pearson method. A futility analysis was conducted 24 weeks after the first study drug dose was administered for the 13th patient in stage I of phase II portion. If ≤1 confirmed response by modified RECIST v1.1 was observed, the trial would close early. Time-to-event analyses (eg, PFS; DoR) were estimated using Kaplan-Meier methodology. Noncompartmental analysis was performed to calculate the pharmacokinetic parameters of ASP1650.
RESULTS
Patient and Disease Characteristics
Nineteen male patients were enrolled between March 2019 and October 2020: 6 patients in the 1000 mg/m2 safety lead-in group, 6 patients in the 1500 mg/m2 safety lead-in group, and 7 subjects in the 1500 mg/m2 phase II group. Patient characteristics are provided in Table 1. Median age was 37 years (range, 20 to 58 years). Primary tumor site was testis in 16 patients (84%), retroperitoneum in 2 patients (11%), and mediastinum in 1 patient (5%). Histology was non-seminoma in 17 (89%) and seminoma in 2 patients (11%). Twelve patients (63%) had ≥3 prior lines of systemic therapy. Thirteen patients (68%) received prior high-dose chemotherapy plus autologous stem-cell transplant as a previous line of therapy. Four patients (21%) had late relapse defined as disease that relapsed >2 years after cisplatin-based combination chemotherapy.
Table 1.
Patient and Disease Characteristics
| Characteristic | No. of Patients (%) | ||
|---|---|---|---|
| ASP1650 1,000mg/m2 (N=6) | ASP1650 1,500mg/m2 (N=13) | Total (N=19) | |
| Median age (range) | 41 (23–52) | 36 (20–58) | 37 (20–58) |
| Location of primary tumor | |||
| • Testis | 5 | 11 | 16 |
| • Retroperitoneum | 1 | 1 | 2 |
| • Mediastinum | 0 | 1 | 1 |
| Tumor histology | |||
| • Seminoma | 0 | 2 | 2 |
| • Non-seminoma | 6 | 11 | 17 |
| Metastatic site(s) | |||
| • Retroperitoneum | 2 | 5 | 7 |
| • Pulmonary | 4 | 6 | 10 |
| • NPVM | 3 | 8 | 11 |
| –Liver metastasis | 1 | 6 | 7 |
| –Brain metastasis* | 2 | 1 | 3 |
| –Bone metastasis* | 0 | 1 | 1 |
| No. of previous chemotherapy regimens | |||
| • 1 | 1 | 2 | 3 |
| • 2 | 1 | 3 | 4 |
| • 3 | 1 | 5 | 6 |
| • >3 | 3 | 3 | 6 |
| Late Relapse (> 2 years) | 1 | 3 | 4 |
| Median Serum AFP ng/mL (range) | 85 (2–5,155) | 190 (2–50,000) | 167 (2–50,000) |
| Median Serum hCG mIu/mL (range) | 2,780 (1–176,486) | 1 (1–6,203) | 1 (1–176,486) |
| ECOG performance status | |||
| • 0 | 4 | 9 | 13 |
| • 1 | 2 | 3 | 5 |
| • 2 | 0 | 1 | 1 |
Abbreviations: NPVM, non-pulmonary visceral metastasis; AFP, alpha fetoprotein; HCG, human chorionic gonadotropin; IU, international unit; ECOG, Eastern Cooperative Oncology Group;
Brain/bone imaging was not mandatory
Safety Lead-in
All 19 patients enrolled received at least one dose of ASP1650 IV per study protocol. Overall, the mean (standard deviation) duration of exposure per subject was 38.4 (44.5) days; the median duration of exposure was 18 days (range, 1–186 days).
A total of 6 patients were enrolled in the safety lead-in 1000mg/m2 dose cohort and 6 patients in the 1500mg/m2 safety lead-in cohort. No subjects had a DLT during the study and the RP2D of 1500 mg/m2 every 2 weeks was established.
Efficacy
None of the enrolled patients had a partial or complete response and the overall response rate (ORR) as assessed by modified RECIST v1.1 was 0 (95% CI, 0.0–14.6%). Three patients, 1 in the 1000 mg/m2 safety lead-in group and 2 in the 1500 mg/m2 group, had stable disease. This included 1 patient in the 1500mg/m2 cohort who had stable disease for >3months (166 days). The remaining patients had progressive disease as the best response. The clinical benefit rate was 5.3% (95% CI, 0.3–22.6%). None of the patients on study had confirmed and continued tumor marker decline while on treatment with ASP1650. Median PFS at the RP2D was 1.18 months (95% CI, 0.92–1.48). At most recent follow-up, all patients had disease progression and 8 patients died of disease progression. Per study protocol, at least 2 objective responses on the RP2D cohort were required to continue enrolling on the second stage of the study; therefore, the trial was terminated at the completion of the first stage. Figures 2 and 3 illustrate best overall response by RECIST v1.1 criteria and swimmer plot of study events, respectively. Figure 2 includes patients who had measurable radiographic disease at baseline. Patients enrolled based on rising tumor markers only are not included in this figure.
Fig 2:
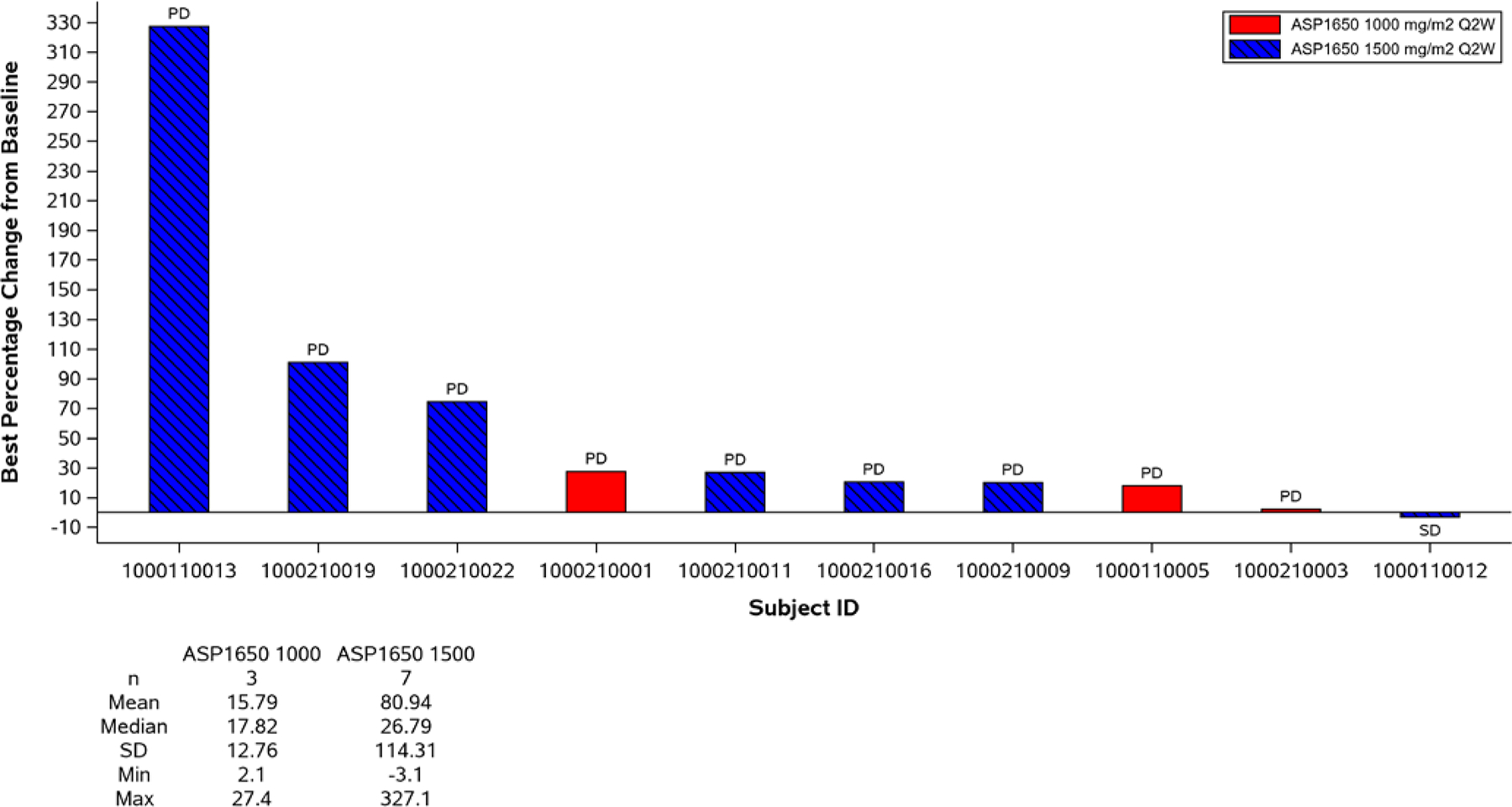
Waterfall Plot of Best Percentage Change from Baseline in Size of Target Lesions in Subjects with Both Baseline and Post-Baseline Measurements with Tumor Marker (AFP and hCG) integration
*Note: Subjects not included progressed clinically or with rising tumor markers and therefore did not have post-baseline imaging measurements
*Note: Only subjects with measurable radiographic disease at enrollment are included. Subjects enrolled based on rising tumor markers only are not included. This includes 2 subjects who had stable disease by tumor marker criteria but no measurable disease and therefore not included in this plot.
Fig 3:
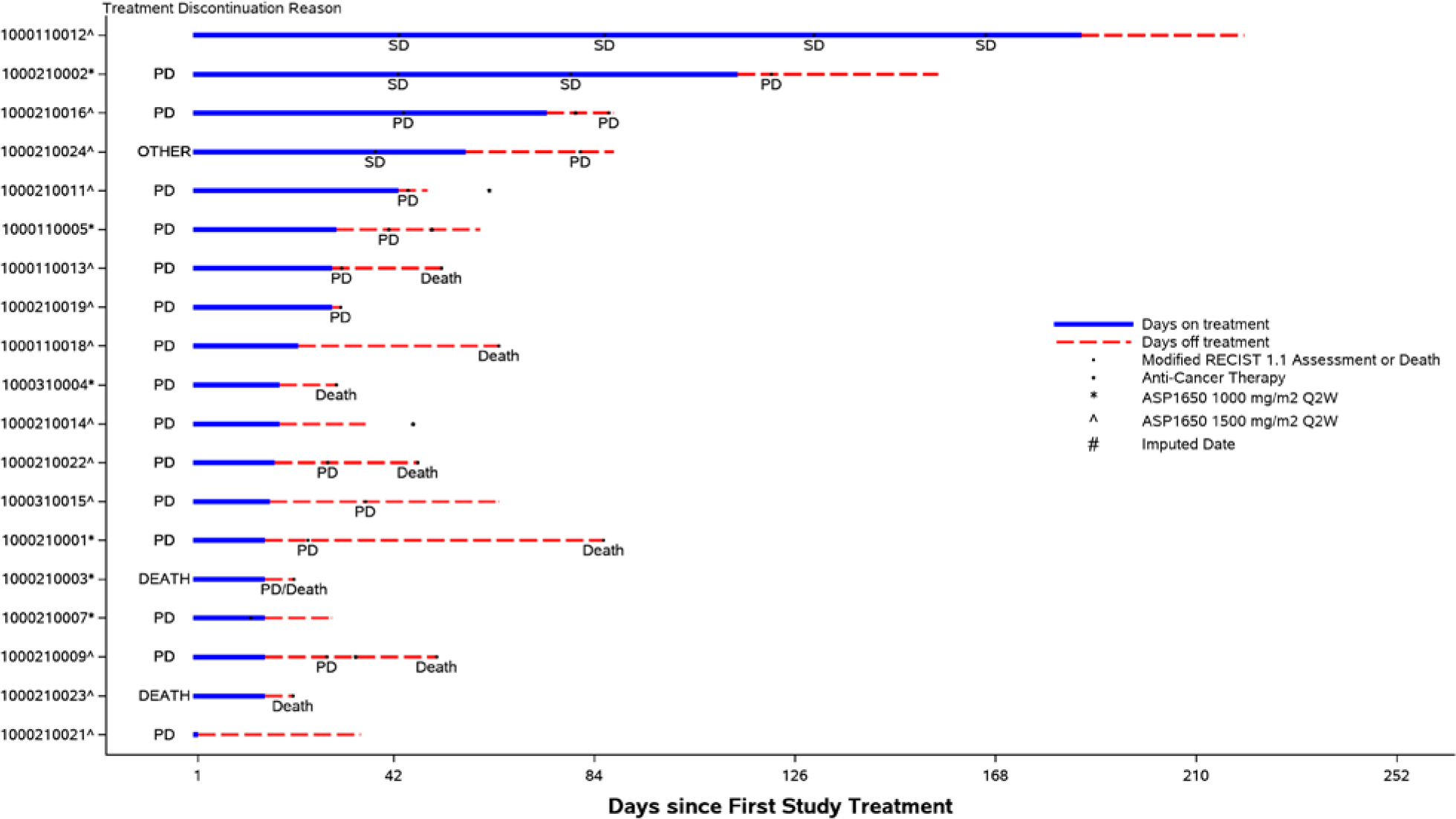
Swimmer Plot of Study Events
Pharmacokinetics and Biomarker Analysis
ASP1650 exposure was approximately dose-proportional in the 1000 to 1500 mg/m2 dosing range. The mean half-life of ASP1650 was approximately 134 to 148 hours (i.e. ~ 6 days). Pharmacokinetic data after multiple dose administrations were limited. ASP1650 Ctrough reached steady state during cycle 3 to cycle 5 in most subjects with multiple dose data. A total of 8 out of 19 patients enrolled in the study had a sample collected after receiving at least 1 dose of ASP1650 for immunogenicity analysis. None of the 8 patients had positive anti-drug antibody detected.
A total of 16 out of 19 patients enrolled in the study had archival tissue samples available for biomarker analysis. Fifteen out of 16 (93.8%) subjects had CLDN6 positive cells (any positivity; 1+/2+/3+). The mean (SD) percent membrane staining positive cells (any positivity; 1+/2+/3+) was 71.6% (31.1). The mean (SD) Membrane H Score was 152.6 (76.0).
Safety
Table 2 summarizes adverse events reported regardless of attributions. In general, ASP1650 had a favorable safety profile. Seven patients (36.8%) had a drug-related treatment-emergent adverse event and 1 patient (5.3%) had a drug-related serious treatment-emergent adverse event. There were no drug-related adverse events that led to discontinuation of study treatment or death. No DLTs were observed on this trial.
Table 2.
Most Frequent Treatment Emergent Adverse Events
| Toxicity | Patients, N (%) | |||
|---|---|---|---|---|
| ASP1650 1,000mg/m2 (N=6) | ASP1650 1,500mg/m2 (N=13) | Total (N=19) | Grade≥3 | |
| Anemia | 3 (50) | 3 (23.1) | 6 (31.6) | 6 (31.6) |
| Abdominal Pain | 1 (16.7) | 2 (15.4) | 3 (15.8) | 2 (10.5) |
| Nausea | 2 (33.3) | 1 (7.7) | 3 (15.8) | 1 (5.2) |
| Vomiting | 3 (50%) | 0 | 3 (15.8) | 1 (5.2) |
| Decreased appetite | 2 (33.3) | 1 (7.7) | 3 (15.8) | 1 (5.2) |
| Anxiety | 2 (33.3) | 1 (7.7) | 3 (15.8) | 0 |
| Diarrhea | 0 | 2 (15.4) | 2 (10.5) | 0 |
| Fatigue | 2 (33.3) | 0 | 2 (10.5) | 0 |
| Peripheral edema | 1 (16.7) | 1 (7.7) | 2 (10.5) | 0 |
| Increased serum creatinine | 1 (16.7) | 1 (7.7) | 2 (10.5) | 0 |
| Arthralgia | 0 | 2 (15.4) | 2 (10.5) | 0 |
| Insomnia | 1 (16.7) | 1 (7.7) | 2 (10.5) | 0 |
| Dyspnea | 1 (16.7) | 1 (7.7) | 2 (10.5) | 0 |
| Rash | 0 | 2 (15.4) | 2 (10.5) | 0 |
DISCUSSION
Multimodal treatment including risk-adapted cisplatin-based combination therapy +/− surgical resection of residual disease will cure ~80% of patients with metastatic GCT.18 Patients who relapse after first-line therapy can still achieve cures with high-dose chemotherapy plus PBSCT, standard-dose salvage chemotherapy, or salvage surgery. There remains a subset of patients who have chemo-refractory disease and are deemed incurable with current treatment options. Understanding the mechanism(s) of resistance to treatment is warranted. Studies with molecularly targeted therapies such as imatinib, thalidomide, and trastuzumab have yielded negative results.19–22 Phase II trials with sunitinib and pazopanib showed signals of brief activity.23,24
Claudin 6 (CLDN6) is a tight junction protein with high expression frequency in GCT samples. Preclinical data indicates >90% (n=97/104) of testicular cancer tissue samples with various histological components tested positive for CLDN6 including seminoma, embryonal carcinoma, yolk sac tumor, choriocarcinoma, and teratoma.15 Most GCT tissue samples had strong intensity CLDN6 staining (≥2+).15
In this phase II clinical trial, we investigated the safety and efficacy of ASP1650, a monoclonal antibody targeted against CLDN6, in patients with relapsed refractory GCT. No DLTs were observed and the RP2D of 1500mg/m2 was established. ASP1650 had a favorable toxicity profile in this patient population and there were no drug-related adverse events leading to treatment discontinuation. No confirmed radiographic responses were observed. Most patients had rising tumor markers indicating continued progression on treatment. Among 16 available archival tissues samples, 93.8% of subjects had CLDN6+ expression. The mean (SD) Membrane H Score was 152.6 (76.0). This is consistent with previous reports where >90% of testicular cancer tissue samples with various histologic components tested positive for CLDN6.15
Although this phase II trial investigated a novel approach for salvage in refractory GCT, there are limitations to report. The small cohort of patients limits generalizability. Immunohistochemistry staining for CLDN6 was performed on archival tumor tissues which may not represent the current status of CLDN6 expression or the tumor microenvironment at the time of treatment with ASP1650.
It is noteworthy that 15 of 16 patients with evaluable tumor samples on this trial had positive CLDN6 expression on archival tissue. This includes patients with various histological subtypes of GCT. This data reinforces that CLDN6 is highly expressed on tissue from patients with GCT and could serve as a potential future diagnostic or therapeutic target. Whether the expression of CLDN6 changes over time and post-chemotherapy warrants further investigation. A change in CLDN6 expression over time and/or the mechanism of action of ASP1650, might be reasons for the lack of clinical activity observed in this trial. If CLDN6 expression is found to be retained in tumor tissue obtained at time of relapsed disease, it would be worthwhile investigating other means of targeting this tight junction protein with agents having a different mechanism of action.
In conclusion, this is the first reported clinical trial evaluating targeting CLDN6 with a monoclonal antibody, ASP1650, in patients with relapsed refractory GCT. Single agent ASP1650 was safe but did not demonstrate clinical benefit in this cohort of patients despite high expression rates of CLDN6 from archival tissue in various histological subtypes of GCT.
ACKNOWLEDGEMENTS:
The authors thank all patients and their families, investigators, and research teams who participated in this study. This study was funded by Astellas.
Footnotes
Conflict of interest statement: will be disclosed online
Ethics Approval and Consent to Participate:
The study was approved by the institutional review board (IRB) of each participating institution (Indiana University, University of Pennsylvania, Memorial Sloan Kettering)
Presented in part at Genitourinary Symposium, American Society of Clinical Oncology, San Francisco, February 2020
Clinical trial information:NCT03760081
Availability of Data and Materials:
If further data is requested, please contact Nabil Adra, MD (nadra@iu.edu)
REFERENCES
- 1.Hanna NH, Einhorn LH: Testicular cancer--discoveries and updates. N Engl J Med 371:2005–16, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Einhorn LH, Williams SD, Chamness A, et al. : High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumors. N Engl J Med 357:340–8, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Feldman DR, Sheinfeld J, Bajorin DF, et al. : TI-CE high-dose chemotherapy for patients with previously treated germ cell tumors: results and prognostic factor analysis. J Clin Oncol 28:1706–13, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kondagunta GV, Bacik J, Donadio A, et al. : Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ cell tumors. J Clin Oncol 23:6549–55, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Loehrer PJ Sr., Einhorn LH, Williams SD: VP-16 plus ifosfamide plus cisplatin as salvage therapy in refractory germ cell cancer. J Clin Oncol 4:528–36, 1986 [DOI] [PubMed] [Google Scholar]
- 6.Loehrer PJ Sr., Gonin R, Nichols CR, et al. : Vinblastine plus ifosfamide plus cisplatin as initial salvage therapy in recurrent germ cell tumor. J Clin Oncol 16:2500–4, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Murphy BR, Breeden ES, Donohue JP, et al. : Surgical salvage of chemorefractory germ cell tumors. J Clin Oncol 11:324–9, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Günzel D, Fromm M: Claudins and other tight junction proteins. Comprehensive Physiology, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Hashizume A, Ueno T, Furuse M, et al. : Expression patterns of claudin family of tight junction membrane proteins in developing mouse submandibular gland. Developmental dynamics: an official publication of the American Association of Anatomists 231:425–431, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Hewitt KJ, Agarwal R, Morin PJ: The claudin gene family: expression in normal and neoplastic tissues. BMC cancer 6:186, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turksen K, Troy TC: Claudin-6: A novel tight junction molecule is developmentally regulated in mouse embryonic epithelium. Developmental dynamics: an official publication of the American Association of Anatomists 222:292–300, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Micke P, Mattsson JSM, Edlund K, et al. : Aberrantly activated claudin 6 and 18.2 as potential therapy targets in non-small-cell lung cancer. International journal of cancer 135:2206–2214, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Ushiku T, Shinozaki-Ushiku A, Maeda D, et al. : Distinct expression pattern of claudin-6, a primitive phenotypic tight junction molecule, in germ cell tumours and visceral carcinomas. Histopathology 61:1043–1056, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Jin X, Lin D, et al. : Clinicopathologic significance of claudin-6, occludin, and matrix metalloproteinases− 2 expression in ovarian carcinoma. Diagnostic pathology 8:1–8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Türeci Ö, Wagner M, Paret C, et al. : Claudin 6 is a carcinoembryonic antigen with cancer stem cell marker features, AACR, 2018 [Google Scholar]
- 16.Türeci Ö, Kreuzberg M, Walter K, et al. : The anti-claudin 6 antibody, IMAB027, induces antibody-dependent cellular and complement-dependent cytotoxicity in claudin 6-expressing cancer cells, AACR, 2018 [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–47, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Ko JJ, Bernard B, Tran B, et al. : Conditional Survival of Patients With Metastatic Testicular Germ Cell Tumors Treated With First-Line Curative Therapy. J Clin Oncol 34:714–20, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Kollmannsberger C, Pressler H, Mayer F, et al. : Cisplatin-refractory, HER2/neu-expressing germ-cell cancer: induction of remission by the monoclonal antibody Trastuzumab. Ann Oncol 10:1393–4, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Rick O, Braun T, Siegert W, et al. : Activity of thalidomide in patients with platinum-refractory germ-cell tumours. Eur J Cancer 42:1775–9, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Feldman DR, Turkula S, Ginsberg MS, et al. : Phase II trial of sunitinib in patients with relapsed or refractory germ cell tumors. Invest New Drugs 28:523–8, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Einhorn LH, Brames MJ, Heinrich MC, et al. : Phase II study of imatinib mesylate in chemotherapy refractory germ cell tumors expressing KIT. Am J Clin Oncol 29:12–3, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Feldman DR, Turkula S, Ginsberg MS, et al. : Phase II trial of sunitinib in patients with relapsed or refractory germ cell tumors. Investigational new drugs 28:523–528, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Necchi A, Vullo SL, Giannatempo P, et al. : Pazopanib in advanced germ cell tumors after chemotherapy failure: results of the open-label, single-arm, phase 2 Pazotest trial. Annals of Oncology 28:1346–1351, 2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
If further data is requested, please contact Nabil Adra, MD (nadra@iu.edu)


