Abstract
Mycophenolate mofetil has an important role as immunosuppressive agent in solid organ transplant recipients. Exposure to the active mycophenolic acid (MPA) can be monitored using therapeutic drug monitoring. We present three cases in which MPA exposure severely decreased after oral antibiotic coadministration. By diminishing gut bacteria β‐glucuronidase activity, oral antibiotics can prevent deglucuronidation of the inactive MPA‐7‐O‐glucuronide metabolite to MPA and thereby possibly prevent its enterohepatic recirculation. This pharmacokinetic interaction could result in rejection, which makes it clinically relevant in solid organ transplant recipients, especially when therapeutic drug monitoring frequency is low. Routine screening for this interaction, preferably supported by clinical decision support systems, and pragmatic close monitoring of the MPA exposure in cases is advised.
Keywords: drug interactions, enterohepatic circulation, mycophenolic acid, organ transplantation, patient safety
MPA exposure can be severely decreased after oral antibiotic coadministration. By diminishing gut bacteria β‐glucuronidase activity, oral antibiotics can prevent deglucuronidation of the inactive MPA‐7‐O‐glucuronide metabolite to MPA and thereby possibly prevent its enterohepatic recirculation. This pharmacokinetic interaction could result in rejection, which makes it clinically relevant in solid organ transplant recipients, especially when therapeutic drug monitoring frequency is low.
Abbreviations
- AUC
area under the concentration–time curve
- Cmax
maximum concentration
- MMF
mycophenolate mofetil
- MPA
mycophenolic acid
- MPAG
MPA‐7‐O‐glucuronide
- TDM
therapeutic drug monitoring
- UGT
uridine diphosphate‐glucuronosyltransferase
1. INTRODUCTION
Mycophenolate mofetil (MMF) is the backbone of immunosuppression in solid organ transplantation patients to reduce the risk of rejection. 1 MMF interferes with de novo synthesis of purine nucleotides in B‐ and T‐lymphocytes by reversibly inhibiting inosine monophosphate dehydrogenase. This results in decreased lymphocyte proliferation and decreased antibody production. 2 , 3
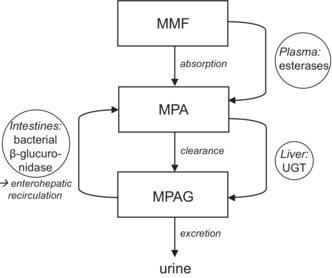
Upon ingestion, the prodrug MMF is rapidly hydrolyzed to the active metabolite mycophenolic acid (MPA; Figure 1). 4 MPA is metabolized by uridine diphosphate‐glucuronosyltransferase (UGT) isoenzymes into the inactive MPA‐7‐O‐glucuronide (MPAG). 5 MPAG is excreted via the bile to the intestines and deglucuronidated into MPA again by β‐glucuronidase enzymes present in intestinal bacteria. MPA is then reabsorbed into the circulation. 4 This enterohepatic recirculation of MPA comprises up to 60% of the exposure. 4
FIGURE 1.
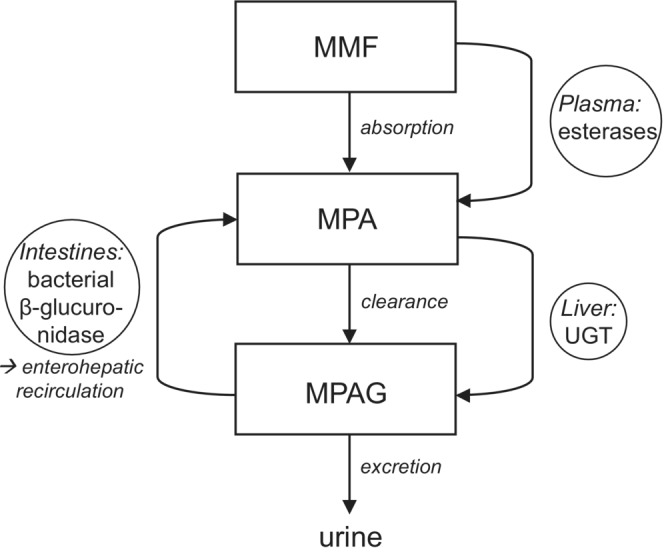
Pharmacokinetics of mycophenolate mofetil (MMF). MMF, mycophenolate mofetil; MPA, mycophenolic acid; MPAG, MPA‐7‐O‐glucuronide; UGT, uridine diphosphate glucuronosyltransferases.
MPA and its metabolites exhibit large interindividual pharmacokinetic variability. A relationship between MPA concentrations and allograft rejection has been documented, which makes therapeutic drug monitoring (TDM) of MPA an important tool to prevent inadequate drug concentrations that increase the risk of organ rejection or MPA toxicity. 6 , 7 , 8 The most adequate measurement of MPA exposure is a 12‐h area under the concentration–time curve (AUC[0–12 h], reference: 30–60 mg*h/L). 9 The MPA Cmax is observed 0–6 h after a single dose of MMF orally. A second peak after 6 h represents reabsorption of MPA through enterohepatic recirculation. 4 This method is sometimes used in clinical setting to address MPA exposure, however, steady‐state MPA trough concentrations are more frequently assessed as a surrogate parameter for exposure.
Emerging evidence suggests disturbance of the MPA exposure during concomitant use of other drugs, for example, antibiotics. 10 , 11 Furthermore, the summary of product characteristics of MMF‐originator CellCept® specifically mentions ciprofloxacin, amoxicillin/clavulanic acid, norfloxacin/metronidazole, and trimethoprim/sulfamethoxazole for this interaction. 12 Nevertheless, routine screening for this interaction is currently not routine clinical practice. We discuss three patients with decreased MPA concentrations during concomitant oral antibiotics.
2. CASE #1
A 53‐year‐old male patient received a liver transplant because of end‐stage liver disease caused by primary sclerosing cholangitis. He started on tacrolimus, MMF, and prednisolone orally. Because of adequate tacrolimus concentrations, MMF was discontinued. For an intra‐abdominal infection, intravenous vancomycin and ciprofloxacin were administered. Because of neurological side effects of tacrolimus, the tacrolimus dose was reduced and MMF was reintroduced. The first plasma concentration of MPA (day 4; Figure 2A) was 1.48 mg/L (reference: 1–3 mg/L according to local protocol). 9 However, on day 8, the MPA concentration was very low and remained low (0.19–0.25 mg/L) after increasing the MMF dose to 1000 mg t.i.d. Concomitant albumin concentrations were normal (36–42 g/L, reference: 35–50 g/L). As on day 6, ciprofloxacin was switched to oral, an oral ciprofloxacin‐induced disturbance of MPA concentrations was suspected. On day 20, all antibiotics were discontinued and on day 22, the MPA concentration was above therapeutic range: 3.47 mg/L. The MMF dose was then reduced to 1000 mg b.i.d. The kidney function was mildly decreased in this period of time (eGFR 50–70 mL/min), which was slightly better than the week before (eGFR 40–50 mL/min), but decreased compared to the week after this period of time (eGFR 70–80 mL/min).
FIGURE 2.
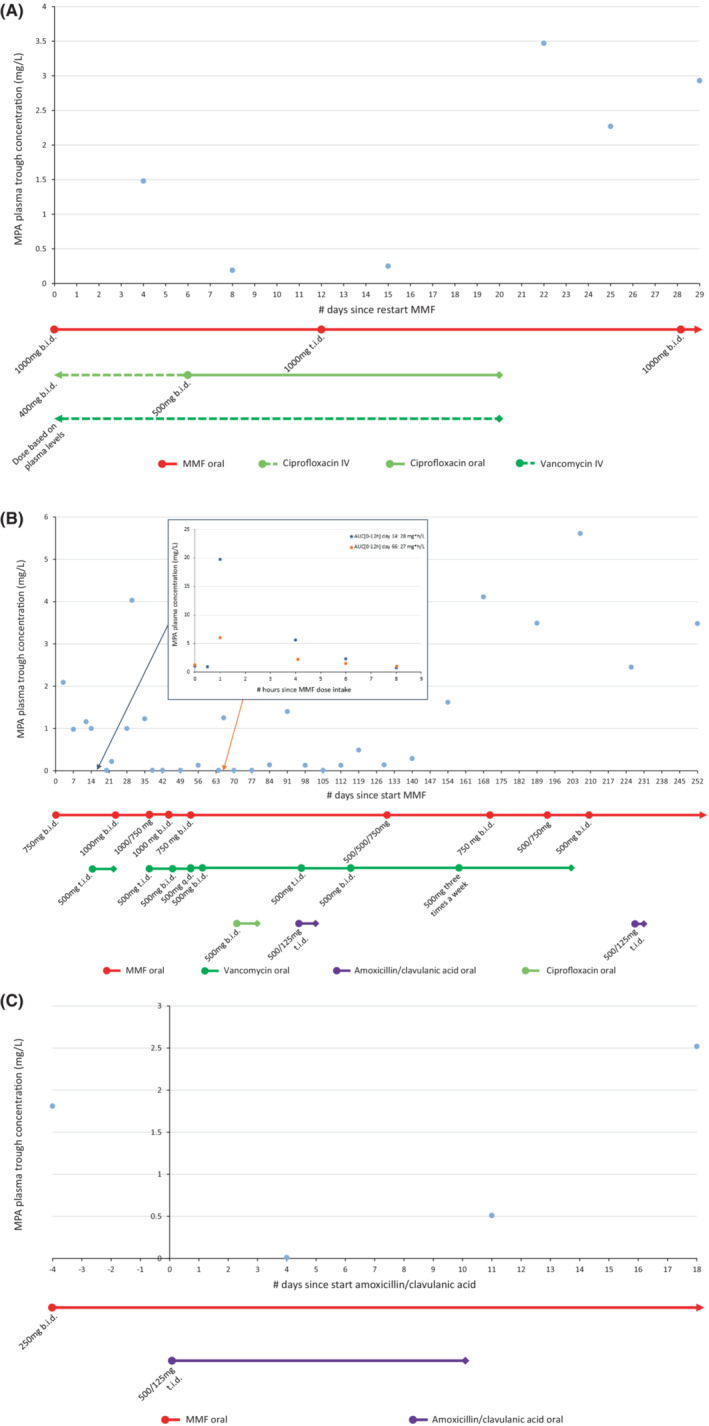
Plasma MPA concentrations and co‐medication over time. Lines connect individual measurements but do not themselves reflect measured values. (A). Case 1; (B) Case 2; and (C) Case 3. AUC[0–12 h] reference 30–60 mg*h/L. 9 AUC, area under the curve; MPA, mycophenolic acid; MMF, mycophenolate mofetil; b.i.d., twice daily; q.d., once daily; t.i.d., three times a day; IV, intravenous.
3. CASE #2
A 13‐year‐old boy was admitted to the hospital for an elective living related kidney transplantation because of congenital uropathy. He received immunosuppression with tacrolimus, MMF and prednisolone orally. When the patient developed diarrhea, proven due to intestinal Clostridium difficile by fecal PCR, oral vancomycin was started for 10 days. Soon thereafter, MPA plasma concentrations decreased to undetectable concentrations (Figure 2B). After increasing the MMF dose to 1000 mg b.i.d. and stopping vancomycin, MPA concentrations increased again to above therapeutic range. Unfortunately, due to a persistent Clostridium infection, the patient received prolonged vancomycin therapy and short courses of ciprofloxacin and amoxicillin/clavulanic acid. On several occasions, subtherapeutic MPA concentrations and AUC[0–12 h]s were measured for which the MMF dose was adjusted to doses ranging from 1000 to 2500 mg per day, taking into account vancomycin dose changes. Upon lowering the dose and eventually stopping vancomycin as the diarrhea improved, MPA concentrations increased quickly to (above) therapeutic range and MMF was reduced to 500 mg b.i.d. The kidney function was stably mildly decreased in this period of time (eGFR 58–74 mL/min), while the albumin concentration was in the normal range (32–49 g/L).
4. CASE #3
A 13‐year‐old girl visited the emergency room with a fever, abdominal pain, and a suspected urinary tract infection, 1 year and 9 months since her second renal transplant. She was on immunosuppression with tacrolimus and MMF with adequate exposure (AUC[0–12 h] 51 mg*h/L). While awaiting urine cultures, amoxicillin/clavulanic acid 500/125 mg t.i.d. was administered for 10 days (Figure 2C). On day 4, she reported at the outpatient clinic to feel much better although now having diarrhea. The MPA concentration was undetectable. The diarrhea was a suspected side effect of amoxicillin/clavulanic acid, but not likely to be solely responsible for the undetectable MPA plasma concentration. Seven days after completing the antibiotic course, the MPA concentration was in range: 2.52 mg/L (reference: >1.9 mg/L according to local protocol). 9 The kidney function was stably mildly decreased in this period of time (eGFR 52–62 mL/min). No albumin concentration was measured.
All three patients were Caucasian. These patients were all medication adherent—both anamnestically and proven by administration registration and/or TDM. None of the patients were co‐treated with ciclosporin, which is known to inhibit the MPA enterohepatic recirculation, or any other nonantibiotic medication that interacts with MMF or its metabolites. 12
5. DISCUSSION AND CONCLUSION
We describe three patients with a significant reduction in MPA exposure after starting oral antibiotics, which increased again after lowering the dose or discontinuation of the antibiotics.
Our findings are in line with the few available small cohorts and case series. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 It has been shown that many oral antibiotics can cause an interaction with decreasing MMF concentrations, including rifampicin, norfloxacin/metronidazole, selective bowel decontamination (mycostatin/tobramycin/cefuroxime), ciprofloxacin, and amoxicillin/clavulanic acid. 10 , 11 , 13 , 14 , 15 , 16 , 17 However, the summary of product characteristics of MMF‐originator CellCept® specifically mentions only ciprofloxacin, amoxicillin/clavulanic acid, norfloxacin/metronidazole, and trimethoprim/sulfamethoxazole for such an interaction. 12 The coadministered oral ciprofloxacin and amoxicillin/clavulanic acid in case 2 may thus have had an additive effect on the MPA concentrations, but it seems unlikely that this fully explains the prolonged decreased trough concentrations and AUCs in this case. To the best of our knowledge, we add to the existing literature by being the first to report a similar effect for oral vancomycin.
The exact mechanism underlying the interaction is still unclear. As the AUC[6–12 h] and MPA trough concentration are affected predominantly, without a significant effect on AUC[0–6 h], Cmax or tmax, the previously suggested mechanism of the interaction is interference with the enterohepatic recirculation. 15 A recent study in hematopoietic cell transplantation patients showed that the MPA trough concentrations, MPA AUC[4–8 h], and acyl‐glucuronide metabolite (acylMPAG) AUC[4–8 h]/AUC[0–8 h] ratio and also the abundance of Bacteroides species were greater in patients with a higher MPA enterohepatic recirculation compared to patients with low MPA enterohepatic recirculation. 18 This suggests that Bacteroides species and the enterohepatic recirculation indeed play an important role in the MPA pharmacokinetics and that antibiotics affecting this system may influence MPA exposure. We observed not only a rapid decline of MPA trough concentrations after start of antibiotic treatment, but also rapid recovery after cessation of antibiotics. This suggests that the deglucuronidating activity of the gut flora and accordingly enterohepatic circulation can be reconstituted. 16 This is corroborated by previous literature, which showed profound and rapid effects of antibiotics, with dysbiosis occurring within three to 4 days after start of ciprofloxacin in human and recovering—although to an altered state—1 week after discontinuation. 19 , 20 As shown in an in vitro experiment, the reduction of MPA exposure might not solely depend on eradication of β‐glucuronidase‐producing bacteria, but also on direct noncompetitive inhibition of intestinal β‐glucuronidase activity. 21 This is also illustrated by case 1, in which MPA trough concentrations recovered very quickly after withdrawal of ciprofloxacin. A possible enterohepatic circulation interfering effect therefore seems antibiotic‐specific rather than a group effect, as inhibition of in vitro β‐glucuronidase was observed for ciprofloxacin and enoxacin but not for levofloxacin and ofloxacin. 21
Recently, more evidence is appearing regarding the influence of immunosuppressants on the gut microbiome. Tacrolimus and prednisolone are associated with pro‐inflammatory dysbiosis, and alterations in the intestinal barrier and MMF is associated with pro‐inflammatory dysbiosis and increased endotoxemia. 22 In mice, it is shown that MMF was responsible for an increase in Clostridia and Bacteroides spp. β‐glucuronidase is expressed by some Bacteroides and as a consequence MMF stimulates the activity of gut β‐glucuronidase in the cecum and the colon. 23 Furthermore, in these mice, it was shown that addition of vancomycin was responsible for a decrease in Bacteroides, β‐glucuronidase activity, and free MPA in mice stool. 23 This is an interesting finding, as Bacteroides are a genus of Gram‐negative bacteria and vancomycin only affects Gram‐positive bacteria such as enterococci and staphylococci. Nevertheless, antibiotics against Gram‐negative bacteria might be suspect for having a significant impact on the MPA blood concentrations in transplant recipients, but other antibiotics may have a similar effect through an alternative or indirect mechanism. This warrants further research.
Because the enterohepatic recirculation may account for up to 60% of the MPA AUC[0–12 h] and bacterial infections are common in patients using immunosuppressants such as MMF, interference with the enterohepatic recirculation by antibiotics may have a significant impact on MPA exposure and result in potentially ineffective immunosuppression. 4 In a clinical setting, TDM of MPA is performed regularly. However, outpatient prescribers less familiar with transplant patients, may start antibiotics for various indications. Unfortunately, this interaction is not regularly monitored and many physicians and (community) pharmacists' may not be aware of this effect of oral antibiotics on MMF. Furthermore, most of these interactions are not included in clinical decision support systems, which makes routine identification and management of these interactions difficult. Without digital monitoring for the MMF–antibiotics interaction, medication reconciliation is essential for prescribers to be informed about the current (antibiotic) drug use by their patients. Altered exposure to MPA can be detected using TDM. However, trough concentrations were mostly measured in our cases according to local routine clinical practice, are not strongly associated with the exposure, for which the AUC[0–12 h] (full or even with limited sampling strategies) is a better measure. 9 MPA trough concentrations also exhibit more intra‐individual variability than the AUC[0–12 h]. 9 In addition, it is important to take into account that the enterohepatic recirculation predominantly influences the AUC[6–12 h]. For this reason too, trough concentrations may not adequately represent changes in overall MPA exposure as a result of the interaction. Also, one should bear in mind that the effect on the MPA plasma concentrations may reduce again with continued antibiotic use and usually diminishes within days after antibiotic discontinuation. 12 Although a preemptive dose increase is not supported by the literature so far, close monitoring of the MPA exposure, ideally with AUC[0–12 h], and graft function during and shortly after antibiotic use is necessary. A pragmatic approach would be to measure the MPA trough concentration 3–4 days after the start of a >1 week course of an interfering antibiotic, adjust the dose accordingly, and repeat this about a week after the antibiotic course.
Although more prospective research is needed into which antibiotics are involved in this interaction and through which exact mechanism, we recommend caution in transplant recipients on MMF with co‐prescriptions for oral antibiotics to prevent organ rejection. Furthermore, we suggest routine screening for the combination of MMF and oral antibiotics interfering with the enterohepatic recirculation, preferably using clinical decision support systems.
NOMENCLATURE OF TARGETS AND LIGANDS
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), 24 and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019). 25
AUTHOR CONTRIBUTIONS
Midas B. Mulder and Brenda C. M. de Winter conceived of the presented idea. Mirjam Simoons and Kishan A. T. Naipal collected the data. Kishan A. T. Naipal drafted a preliminary version of the article, which Mirjam Simoons supplemented, revised, and finalized with input from all authors. Kishan A. T. Naipal, Huib de Jong, Caroline M. den Hoed, Brenda C. M. de Winter, and Midas B. Mulder reviewed and commented on the article.
ETHICS APPROVAL STATEMENT
Not applicable.
PATIENT CONSENT STATEMENT
Not applicable.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Not applicable.
CLINICAL TRIAL REGISTRATION
Not applicable.
ACKNOWLEDGMENTS
Not applicable.
Simoons M, Naipal KAT, de Jong H, den Hoed CM, de Winter BCM, Mulder MB. Oral antibiotics lower mycophenolate mofetil drug exposure, possibly by interfering with the enterohepatic recirculation: A case series. Pharmacol Res Perspect. 2023;11:e01103. doi: 10.1002/prp2.1103
DATA AVAILABILITY STATEMENT
Data from the cases are available upon request.
REFERENCES
- 1. van Gelder T, Hesselink DA. Mycophenolate revisited. Transpl Int. 2015;28(5):508‐515. [DOI] [PubMed] [Google Scholar]
- 2. Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47(2–3):85‐118. [DOI] [PubMed] [Google Scholar]
- 3. McMurray RW, Harisdangkul V. Mycophenolate mofetil: selective T cell inhibition. Am J Med Sci. 2002;323(4):194‐196. [DOI] [PubMed] [Google Scholar]
- 4. Bullingham RE, Nicholls AJ, Kamm BR. Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet. 1998;34(6):429‐455. [DOI] [PubMed] [Google Scholar]
- 5. Picard N, Ratanasavanh D, Premaud A, Le Meur Y, Marquet P. Identification of the UDP‐glucuronosyltransferase isoforms involved in mycophenolic acid phase II metabolism. Drug Metab Dispos. 2005;33(1):139‐146. [DOI] [PubMed] [Google Scholar]
- 6. Yamani MH, Starling RC, Goormastic M, et al. The impact of routine mycophenolate mofetil drug monitoring on the treatment of cardiac allograft rejection. Transplantation. 2000;69(11):2326‐2330. [DOI] [PubMed] [Google Scholar]
- 7. van Gelder T, Hilbrands LB, Vanrenterghem Y, et al. A randomized double‐blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation. 1999;68(2):261‐266. [DOI] [PubMed] [Google Scholar]
- 8. Rhu J, Lee KW, Park H, Park JB, Kim SJ, Choi GS. Clinical implication of mycophenolic acid trough concentration monitoring in kidney transplant patients on a tacrolimus triple maintenance regimen: a single‐center experience. Ann Transplant. 2017;22:707‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuypers DR, Le Meur Y, Cantarovich M, et al. Consensus report on therapeutic drug monitoring of mycophenolic acid in solid organ transplantation. Clin J Am Soc Nephrol. 2010;5(2):341‐358. [DOI] [PubMed] [Google Scholar]
- 10. Bhagat V, Pandit RA, Ambapurkar S, Sengar M, Kulkarni AP. Drug interactions between antimicrobial and immunosuppressive agents in solid organ transplant recipients. Indian J Crit Care Med. 2021;25(1):67‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benjanuwattra J, Pruksakorn D, Koonrungsesomboon N. Mycophenolic acid and its pharmacokinetic drug‐drug interactions in humans: review of the evidence and clinical implications. J Clin Pharmacol. 2020;60(3):295‐311. [DOI] [PubMed] [Google Scholar]
- 12. European Medicines Agency . CellCept Summary of Product Characteristics. 2009. accessed 24 January 2022. https://www.ema.europa.eu/en/documents/product‐information/cellcept‐epar‐product‐information_en.pdf
- 13. Naesens M, Kuypers DR, Streit F, et al. Rifampin induces alterations in mycophenolic acid glucuronidation and elimination: implications for drug exposure in renal allograft recipients. Clin Pharmacol Ther. 2006;80(5):509‐521. [DOI] [PubMed] [Google Scholar]
- 14. Naderer OJ, Dupuis RE, Heinzen EL, Wiwattanawongsa K, Johnson MW, Smith PC. The influence of norfloxacin and metronidazole on the disposition of mycophenolate mofetil. J Clin Pharmacol. 2005;45(2):219‐226. [DOI] [PubMed] [Google Scholar]
- 15. Schmidt LE, Rasmussen A, Norrelykke MR, Poulsen HE, Hansen BA. The effect of selective bowel decontamination on the pharmacokinetics of mycophenolate mofetil in liver transplant recipients. Liver Transpl. 2001;7(8):739‐742. [DOI] [PubMed] [Google Scholar]
- 16. Borrows R, Chusney G, Loucaidou M, et al. The magnitude and time course of changes in mycophenolic acid 12‐hour predose levels during antibiotic therapy in mycophenolate mofetil‐based renal transplantation. Ther Drug Monit. 2007;29(1):122‐126. [DOI] [PubMed] [Google Scholar]
- 17. Ratna P, Mathew BS, Annapandian VM, et al. Pharmacokinetic drug interaction of mycophenolate with co‐amoxiclav in renal transplant patients. Transplantation. 2011;91(6):e36‐e38. [DOI] [PubMed] [Google Scholar]
- 18. Saqr A, Carlson B, Staley C, et al. Reduced enterohepatic recirculation of Mycophenolate and lower blood concentrations are associated with the stool bacterial microbiome after hematopoietic cell transplantation. Transplant Cell Ther. 2022;28(7):372.e1‐372.e9. [DOI] [PubMed] [Google Scholar]
- 19. Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4554‐4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patangia DV, Anthony Ryan C, Dempsey E, Paul Ross R, Stanton C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiology. 2022;11(1):e1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kodawara T, Masuda S, Yano Y, Matsubara K, Nakamura T, Masada M. Inhibitory effect of ciprofloxacin on beta‐glucuronidase‐mediated deconjugation of mycophenolic acid glucuronide. Biopharm Drug Dispos. 2014;35(5):275‐283. [DOI] [PubMed] [Google Scholar]
- 22. Gabarre P, Loens C, Tamzali Y, Barrou B, Jaisser F, Tourret J. Immunosuppressive therapy after solid organ transplantation and the gut microbiota: bidirectional interactions with clinical consequences. Am J Transplant. 2022;22(4):1014‐1030. [DOI] [PubMed] [Google Scholar]
- 23. Taylor MR, Flannigan KL, Rahim H, et al. Vancomycin relieves mycophenolate mofetil‐induced gastrointestinal toxicity by eliminating gut bacterial beta‐glucuronidase activity. Sci Adv. 2019;5(8):eaax2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 2018;46(D1):D1091‐D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alexander SPH, Kelly E, Mathie A, et al. THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: introduction and other protein targets. Br J Pharmacol. 2019;176(Suppl 1):S1‐S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from the cases are available upon request.


