Abstract
Alcohol-related poisoning is the foremost cause of death resulting from excessive acute alcohol consumption. Respiratory failure is crucial to the pathophysiology of fatal alcohol poisoning. Alcohol increases accumulation of extracellular adenosine. Adenosine suppresses breathing. The goal of this investigation was to test the hypothesis that adenosine signaling contributes to alcohol-induced respiratory suppression. In the first experiment, the breathing of mice was monitored following an injection of the non-selective adenosine receptor antagonist caffeine (40 mg/kg), alcohol (5 g/kg), or alcohol and caffeine combined. Caffeine reduced alcohol-induced respiratory suppression suggesting that adenosine contributes to the effects of alcohol on breathing. The second experiment utilized the same experimental design, but with the blood brain barrier impermeant non-selective adenosine receptor antagonist 8-sulfophenyltheophylline (8-SPT, 60 mg/kg) instead of caffeine. 8-SPT did not reduce alcohol-induced respiratory suppression suggesting that adenosine is contributing to alcohol-induced respiratory suppression in the central nervous system. The third and fourth experiments used the same experimental design as the first, but with the selective A1 receptor antagonist DPCPX (1 mg/kg) and the selective A2A receptor antagonist istradefylline (3.3 mg/kg). Istradefylline, but not DPCPX, reduced alcohol-induced respiratory suppression indicating an A2A receptor mediated effect. In the fifth experiment, alcohol-induced respiratory suppression was evaluated in Adk+/− mice which have impaired adenosine metabolism. Alcohol-induced respiratory suppression was exacerbated in Adk+/− mice. These findings indicate that adenosinergic signaling contributes to alcohol-induced respiratory suppression. Improving our understanding of how alcohol affects breathing may lead to better treatment strategies and better outcomes for patients with severe alcohol poisoning.
Keywords: Adenosine, Alcohol, Breathing, Caffeine, Respiratory suppression
1. Introduction
Alcohol consumption contributes to more than 5% of global mortality (WHO, 2019). This problem is potentially becoming more serious given the increase in alcohol consumption and alcohol-related deaths during the COVID-19 pandemic (White et al., 2022). In the United States, alcohol-related poisonings are the primary cause of death attributable to the acute effects of alcohol with an incidence approximately twice that of alcohol-related motor vehicle accidents (CDC, 2022b).
Respiratory arrest is crucial to the pathophysiology of fatal alcohol poisoning (Lignian et al., 1983; Adinoff et al., 1988; Vale, 2007; Wang et al., 2019). Alcohol suppresses breathing under normal conditions (Wilson and Saukkonen, 2004; Langhan, 2013) and in response to hypoxia and hypercapnia (Sahn et al., 1975; Michiels et al., 1983; McCauley et al., 1988). Identifying the mechanisms of alcohol-induced respiratory suppression may lead to better treatment strategies for alcohol poisoning. Several hypotheses for alcohol-induced respiratory suppression have been put forward:
Acute alcohol consumption increases endogenous opioid signaling potentially via an increase in corticotrophin-releasing hormone (Gianoulakis, 2001; Lam et al., 2008). Opioid receptor activation suppresses breathing (Santiago and Edelman, 1985; Bachmutsky et al., 2020). Opioid receptor antagonism attenuates the effect of alcohol on respiratory responsiveness to hypoxia and hypercapnia in humans (Michiels et al., 1983). These observations have led to the hypothesis that endogenous opioid signaling contributes to alcohol-induced respiratory suppression (Michiels et al., 1983); however, this hypothesis is somewhat undermined by regional differences in alcohol-induced opioid release. Breathing is primarily controlled by brainstem structures (Smith et al., 1991; Del Negro et al., 2018). Alcohol at high doses increases opioid signaling in the forebrain, but not the brainstem (Lam et al., 2008; Jarjour et al., 2009). Furthermore, opioid receptor antagonism does not counteract alcohol-induced respiratory arrest in animal models (Lignian et al., 1983). These observations do not support a purely opioid based interpretation of alcohol-induced respiratory suppression (Lam et al., 2008; Jarjour et al., 2009).
Acute alcohol exposure increases GABAergic signaling via both presynaptic and postsynaptic mechanisms (Nestoros, 1980; Roberto et al., 2004; Weiner and Valenzuela, 2006). GABAergic signaling contributes to respiratory rhythmogenesis (Bonham, 1995), but excessive GABA receptor activation suppresses breathing (Hedner et al., 1981; Yamada et al., 1981). GABA receptor antagonism counteracts the respiratory effects of alcohol and barbiturate coadministration (Ren et al., 2012). Similarly, facilitation of glutamatergic signaling via AMPA receptors counteracts the respiratory effects of alcohol (Xiao et al., 2020). A case report indicates that GABA receptor antagonism may alleviate alcohol-induced respiratory suppression (Linowiecki et al., 1992); however, this effect has never been empirically investigated.
Alcohol affects the central nervous system through a variety of disparate mechanisms beyond endogenous opioids and GABA. Among those, alcohol causes extracellular adenosine levels to rise by two distinct mechanisms: (1) Alcohol is metabolized into acetaldehyde, and then acetate that, along with ATP, produces acetyl-CoA and AMP which is then converted into adenosine (Carmichael et al., 1991; Pardo et al., 2013). (2) Alcohol impairs adenosine reuptake through equilibrative nucleoside transporter 1 (Nagy et al., 1990). Adenosine is an inhibitory modulator of neuronal activity (Dunwiddie and Masino, 2001). Excessive adenosinergic signaling suppresses breathing under normal conditions (Eldridge et al., 1984, 1985; Lagercrantz et al., 1984) and in response to hypercapnia (Falquetto et al., 2018). Increases in extracellular adenosine are associated with the respiratory dysregulation seen in traumatic brain injury (Lusardi et al., 2020) and sudden unexpected death in epilepsy (Shen et al., 2010; Ashraf et al., 2021). Experimental manipulations in animal models have implicated increased adenosinergic signaling in the effect of alcohol on vigilance state (Dar et al., 1983; El Yacoubi, 2003; Fang et al., 2017), motor control (Dar, 1990; Connole et al., 2004), memory (Spinetta et al., 2008; Lopez-Cruz et al., 2016), portal vein blood flow to the liver (Orrego et al., 1988), and anxiety (Prediger et al., 2004). The goal of this investigation was to test the hypothesis that adenosine signaling contributes to the effects of alcohol on breathing. To test this hypothesis we quantified alcohol-induced respiratory suppression under conditions of: (1) systemic non-selective adenosine receptor antagonism; (2) peripheral non-selective adenosine receptor antagonism; (3) systemic A1 and (4) A2A selective adenosine receptor antagonism; and (5) transgenic downregulation of adenosine metabolism. Clarification of the mechanistic underpinnings of alcohol-induced respiratory suppression may lead to the development of more effective treatment strategies and better outcomes for patients with severe acute alcohol poisoning.
2. Materials and methods
Ethical approval
All procedures and protocols used in this study were approved by the Rutgers University Institutional Animal Care and Use Committee in accordance with the guidelines set by the Association for Assessment and Accreditation of Laboratory Animal Care and the National Research Council. Care was taken to use the minimum number of animals possible and to minimize their pain and distress.
2.1. Experimental design
2.1.1. Experiment 1. determine whether systemic non-selective adenosine receptor antagonism reduces vulnerability to alcohol-induced respiratory suppression
Adult, male, C57BL6/J mice were injected with either caffeine (40 mg/kg, i.p.), alcohol (5 g/kg, i.p.), or a combination of alcohol (5 g/kg, i. p.) and caffeine (40 mg/kg, i.p.; n = 8–9 per group). The breathing of mice was monitored for 30 min before and 3 h after the injection. Each animal received one experimental trial of each condition in a counterbalanced order to avoid sequence effects. We predicted that animals treated with the non-selective adenosine receptor antagonist caffeine would undergo less severe alcohol-induced respiratory suppression than animals treated with alcohol alone, our data was consistent with this prediction.
2.1.2. Experiment 2. determine whether peripheral non-selective adenosine receptor antagonism reduces vulnerability to alcohol-induced respiratory suppression
Adult, male, C57BL6/J mice were injected with either 8-(p-sulfo-phenyl)theophylline (8-SPT; 60 mg/kg, i.p.), alcohol (5 g/kg, i.p.), or a combination of alcohol (5 g/kg, i.p.) and 8-SPT (60 mg/kg, i.p.; n = 8–9 per group). 8-SPT is a non-selective adenosine receptor antagonist which, unlike caffeine, is unable to cross the blood brain barrier. The breathing of mice was monitored for 30 min before and 3 h after the injection. Each animal received one experimental trial of each condition in a counterbalanced order to avoid sequence effects. We predicted that animals peripherally treated with the non-selective adenosine receptor antagonist 8-SPT would undergo the same degree of alcohol-induced respiratory suppression as animals treated with alcohol alone, our data was consistent with this prediction.
2.1.3. Experiment 3. determine whether systemic A1 receptor antagonism reduces vulnerability to alcohol-induced respiratory suppression
Adult, male, C57BL6/J mice were injected with either Dipropylcyclopentylxanthine (DPCPX; 1 mg/kg, i.p.), alcohol (5 g/kg, i.p.), or a combination of alcohol (5 g/kg, i.p.) and DPCPX (1 mg/kg, i.p.; n = 9 per group). The breathing of mice was monitored for 30 min before and 3 h after the injection. Each animal received one experimental trial of each condition in a counterbalanced order to avoid sequence effects. We predicted that animals treated with the A1 receptor antagonist DPCPX would undergo the same degree of alcohol-induced respiratory suppression as animals treated with alcohol alone, our data was consistent with this prediction.
2.1.4. Experiment 4. determine whether systemic A2A receptor antagonism reduces vulnerability to alcohol-induced respiratory suppression
Adult, male, C57BL6/J mice were injected with either istradefylline (3.3 mg/kg, i.p.), alcohol (5 g/kg, i.p.), or a combination of alcohol (5 g/kg, i.p.) and istradefylline (3.3 mg/kg, i.p.; n = 8–9 per group). The breathing of mice was monitored for 30 min before and 3 h after the injection. Each animal received one experimental trial of each condition in a counterbalanced order to avoid sequence effects. We predicted that animals treated with the A2A receptor antagonist istradefylline would undergo less severe alcohol-induced respiratory suppression than animals treated with alcohol alone, our data was consistent with this prediction.
2.1.5. Experiment 5. determine whether transgenic downregulation of adenosine metabolism increases vulnerability to alcohol-induced respiratory suppression
Adk+/− mice are heterozygous for a genetic knockout of the adenosine kinase (Adk) gene which encodes the major metabolic (adenosine removing) enzyme ADK (Boison et al., 2002). Adult, male and female, Adk+/+ and Adk+/− mice were injected with alcohol (5 g/kg, i.p.; n = 8–9 per group). The breathing of mice was monitored for 30 min before and 3 h after the injection. We predicted that animals with impaired metabolic adenosine clearance (Adk+/−) would experience more severe alcohol-induced respiratory suppression than animals with normal adenosine clearance (Adk+/+), our data was consistent with this prediction.
2.2. Animals and drug injections
The C57BL/6J mice used in this study were acquired from Jackson Labs at 7–8 weeks old (000664; Bar Harbor, ME). The Adk+/− mice used in this study were produced, genotyped, and maintained as previously described (Boison et al., 2002). All mice were housed under normal 12:12 LD conditions (lights on at 0600, lights off at 1800). Mice had ad libitum access to food (PicoLab 5058; LabDiet, St. Louis, MO) and water. Following the end of experimentation, animals used in this study were euthanized by CO2 exposure followed by cervical dislocation.
All injections were given intraperitoneally. Injections of ethyl alcohol (Decon Labs; King of Prussia, PA), caffeine (Sigma-Aldrich; St. Louis, MO) and 8-SPT (United States Biological; Salem, MA) were given with 0.9% saline (Hospira; Lake Forest, IL) as a vehicle. Injections of DPCPX (Tocris Bioscience; Bristol, UK) and istradefylline (Sigma-Aldrich) were given with 20% dimethyl sulfoxide (DMSO; VWR International, Radnor, PA) and 80% saline as a vehicle. Alcohol injections were prepared at 20% v/v. An alcohol dose of 5 g/kg was selected with the expectation that it would generate substantive respiratory disruption without approaching the LD50 (Dinh and Gailis, 1979). Non-alcohol drug injections were prepared to have a volume of ~0.15 mL. In alcohol only, caffeine only, 8-SPT only, DPCPX only, and istradefylline only experimental trials, a saline or 20% DMSO injection of appropriate volume was also given so that animals in all three conditions in these experiments received approximately the same volume of injected fluid. Experimental trials were conducted at least 72 h apart to allow the animals to fully recover.
2.3. Whole body plethysmography
Mice were acclimated to the plethysmography chamber during two separate sessions of at least 30 min prior to experimentation. During experimental trials, animals were removed from their home cage, placed in the plethysmography chamber (Data Sciences International, St. Paul, MN) and breathing was recorded for 1 h. Data from the first 30 min of the initial recording period was not analyzed, data from the second 30 min was analyzed as pre-injection baseline. Following the baseline recording period, mice were removed from the plethysmography chamber, injected with alcohol and/or an adenosinergic drug, and returned to the chamber. Room air was drawn through the plethysmography chamber at 0.5 L/min. A barometric flow-through method (Drorbaugh and Fenn, 1955) was used to determine respiratory frequency, tidal volume, and minute ventilation. Minute ventilation was used to determine degree of respiratory suppression as this parameter most directly relates to the capacity of the animal to prevent blood gas derangement and the adverse health outcomes that might result; however, the other parameters have been included as they can provide crucial information regarding the cause of differences in overall ventilation. Stable, artifact free segments of breathing were identified and analyzed using a ‘Drorbaugh & Fenn Reduced Rejection’ algorithm with a 2 s log interval (FinePointe, Data Sciences International; Drorbaugh and Fenn, 1955).
2.4. Statistical analyses
Statistical analyses were conducted using GraphPad Prism 9 (GraphPad Software Inc.) or Microsoft Excel (Microsoft Corp.). Two-way ANOVAs on baseline-normalized time series data were used to evaluate differences between conditions. Unless otherwise specified, data are expressed as mean ± standard error. Group sizes were determined using power calculations based on the effect sizes seen in pilot data in the lab. Significance threshold was set at p < 0.05 for all comparisons.
3. Results
3.1. Systemic non-selective adenosine receptor antagonism reduces alcohol-induced respiratory suppression
To determine the effect of systemic non-selective adenosine receptor antagonism on alcohol-induced respiratory suppression, breathing was monitored in mice before and after an injection of caffeine, alcohol, or a combination of alcohol and caffeine.
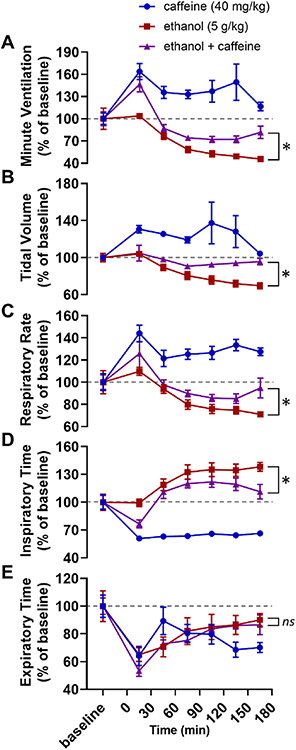
Two-way ANOVAs were used to compare baseline-normalized respiratory parameters between alcohol alone and caffeine with alcohol treatment conditions. Alcohol with caffeine experimental trials were significantly different than alcohol alone in minute ventilation (F1,111 = 34.98, p < 0.001; Fig. 1A), tidal volume (F1,112 = 31.43, p < 0.001.; Fig. 1B), respiratory rate (F1,112 = 8.04, p = 0.005; Fig. 1C), and inspiratory time (F1,112 = 15.93, p < 0.001; Fig. 1D), but not expiratory time (F1,112 = 0.83, p = 0.365; Fig. 1E).
Fig. 1. Caffeine reduces alcohol-induced respiratory suppression.
Time series graphs depicting (A) minute ventilation, (B) tidal volume, (C) respiratory rate, (D) inspiratory time, and (E) expiratory time before and after injections of caffeine alone (40 mg/kg, blue circles), alcohol alone (5 g/kg, red squares), and alcohol with caffeine (5 g/kg alcohol and 40 mg/kg caffeine, purple triangles). *, p < 0.05; ns, p ≥ 0.05; two-way ANOVA. Data has been normalized to baseline and depicted as mean with SEM. Baseline is defined as the 30 min prior to drug administration. Grey dotted line, baseline level.
3.2. Peripheral non-selective adenosine receptor antagonism does not reduce alcohol-induced respiratory suppression
To determine whether the effects of caffeine observed in Experiment 1 were due to adenosine receptor antagonism in the central nervous system or in the periphery, breathing was monitored in mice before and after an injection of 8-SPT, alcohol, or a combination of alcohol and 8-SPT. 8-SPT cannot cross the blood brain barrier; thus, its effects on adenosine receptors are limited to the periphery.
Two-way ANOVAs were used to compare baseline-normalized respiratory parameters between alcohol alone and 8-SPT with alcohol treatment conditions. There were no significant differences between alcohol alone and alcohol with caffeine in minute ventilation (F1,105 = 0.04, p = 0.843; Fig. 2A), tidal volume (F1,105 = 3.68, p = 0.058; Fig. 2B), respiratory rate (F1,105 = 3.15, p = 0.079; Fig. 2C) or expiratory time (F1,105 = 0.39, p = 0.533; Fig. 2D), but there was a difference in inspiratory time (F1,105 = 6.18, p = 0.015; Fig. 2C).
Fig. 2. 8-SPT does not reduce alcohol-induced respiratory suppression.
Time series graphs depicting (A) minute ventilation, (B) tidal volume, (C) respiratory rate, (D) inspiratory time, and (E) expiratory time before and after injections of 8-SPT alone (60 mg/kg, blue circles), alcohol alone (5 g/kg, red squares), and alcohol with 8-SPT (5 g/kg alcohol and 60 mg/kg 8-SPT, purple triangles). *, p < 0.05; ns, p ≥ 0.05; two-way ANOVA. Data has been normalized to baseline and depicted as mean with SEM. Baseline is defined as the 30 min prior to drug administration. Grey dotted line, baseline level.
3.3. Systemic A1 receptor antagonism does not reduce alcohol-induced respiratory suppression
To determine whether the effects of adenosine receptor antagonism observed in Experiment 1 were mediated by the A1 receptor, breathing was monitored in mice before and after an injection of DPCPX, alcohol, or a combination of alcohol and DPCPX. Because DPCPX is selective to the A1 receptor, it should only counteract adenosinergic effects which are mediated by the A1 receptor.
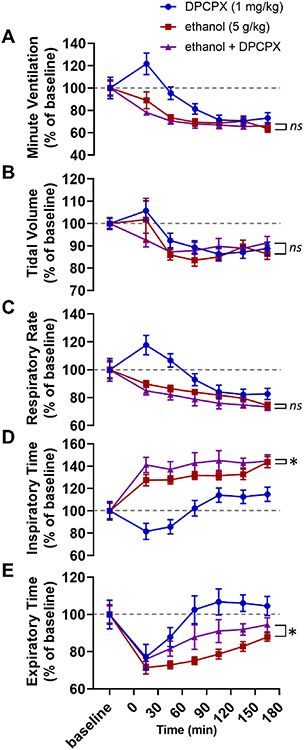
Two-way ANOVAs were used to compare baseline-normalized respiratory parameters between alcohol alone and DPCPX with alcohol treatment conditions. Alcohol with DPCPX experimental trials were not significantly different than alcohol alone in minute ventilation (F1,112 = 1.54, p = 0.217; Fig. 3A), tidal volume (F1,112 = 0.16, p = 0.696; Fig. 3B), or respiratory rate (F1,112 = 3.68, p = 0.058; Fig. 3C). Alcohol with DPCPX experimental trials were significantly different than alcohol alone in inspiratory time (F1,112 = 6.54, p = 0.012; Fig. 3D) and expiratory time (F1,112 = 13.33, p < 0.001; Fig. 3E).
Fig. 3. DPCPX does not reduce alcohol-induced respiratory suppression.
Time series graphs depicting (A) minute ventilation, (B) tidal volume, (C) respiratory rate, (D) inspiratory time, and (E) expiratory time before and after injections of DPCPX alone (1 mg/kg, blue circles), alcohol alone (5 g/kg, red squares), and alcohol with DPCPX (5 g/kg alcohol and 1 mg/kg DPCPX, purple triangles). *, p < 0.05; ns, p ≥ 0.05; two-way ANOVA. Data has been normalized to baseline and depicted as mean with SEM. Baseline is defined as the 30 min prior to drug administration. Grey dotted line, baseline level.
3.4. Systemic A2A receptor antagonism reduces alcohol-induced respiratory suppression
To determine whether the effects of adenosine receptor antagonism observed in Experiment 1 were mediated by the A2A receptor, breathing was monitored in mice before and after an injection of istradefylline, alcohol, or a combination of alcohol and istradefylline. Because istradefylline is selective to the A2A receptor, it should only counteract adenosinergic effects which are mediated by the A2A receptor.
Two-way ANOVAs were used to compare baseline-normalized respiratory parameters between alcohol alone and istradefylline with alcohol treatment conditions. Alcohol with istradefylline experimental trials were different than alcohol alone in minute ventilation (F1,105 = 30.11, p < 0.001; Fig. 4A), tidal volume (F1,105 = 19.26, p < 0.001; Fig. 4B), respiratory rate (F1,105 = 29.71, p < 0.001; Fig. 4C), inspiratory time (F1,105 = 36.12, p < 0.001; Fig. 4D), and expiratory time (F1,105 = 4.13, p = 0.045; Fig. 4E).
Fig. 4. Istradefylline reduces alcohol-induced respiratory suppression.
Time series graphs depicting (A) minute ventilation, (B) tidal volume, (C) respiratory rate, (D) inspiratory time, and (E) expiratory time before and after injections of istradefylline alone (3.3 mg/kg, blue circles), alcohol alone (5 g/kg, red squares), and alcohol with istradefylline (5 g/kg alcohol and 3.3 mg/kg istradefylline, purple triangles). *, p < 0.05; ns, p ≥ 0.05; two-way ANOVA. Data has been normalized to baseline and depicted as mean with SEM. Baseline is defined as the 30 min prior to drug administration. Grey dotted line, baseline level.
3.5. Transgenic downregulation of adenosine metabolism increases alcohol-induced respiratory suppression
Adenosine kinase is the primary enzyme responsible for the metabolic clearance of adenosine (Boison and Jarvis, 2021). To determine whether transgenic downregulation of adenosine kinase would exacerbate alcohol-induced respiratory suppression, breathing was monitored in mice with impaired adenosine metabolism (Adk+/−) and their phenotypically normal littermates (Adk+/+) before and after an injection of alcohol.
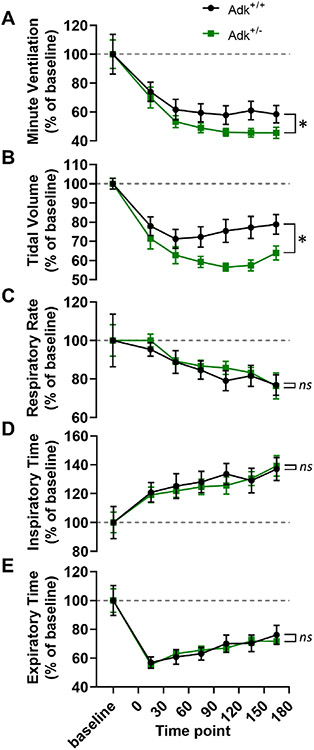
Two-way ANOVAs were used to compare baseline-normalized respiratory parameters between Adk+/− and Adk+/+ mice. Breathing following alcohol treatment was different between Adk+/− and Adk+/+ mice in minute ventilation (F1,105 = 6.28, p = 0.014; Fig. 5A) and tidal volume (F1,105 = 25.30, p < 0.001; Fig. 5B), but not in respiratory rate (F1,105 = 0.47, p = 0.494; Fig. 5C), inspiratory time (F1,105 = 0.23, p = 0.634; Fig. 5D), or expiratory time (F1,104 = 0.02, p = 0.884; Fig. 5E).
Fig. 5. Adk+/− mice are more vulnerable to alcohol-induced respiratory suppression.
Time series graphs depicting (A) minute ventilation, (B) tidal volume, (C) respiratory rate, (D) inspiratory time, and (E) expiratory time before and after alcohol injection (5 g/kg) in adenosine kinase deficient (Adk+/−) and phenotypically wildtype (Adk+/+) mice. *, p < 0.05; ns, p ≥ 0.05; two-way ANOVA. Data has been normalized to baseline and depicted as mean with SEM. Baseline is defined as the 30 min prior to drug administration. Grey dotted line, baseline level.
4. Discussion
Alcohol generally, and acute alcohol poisoning specifically, are serious contributors to premature mortality (WHO, 2019; CDC, 2022b). The primary cause of mortality in acute alcohol poisoning is typically attributed to respiratory arrest (Lignian et al., 1983; Wang et al., 2019). The mechanistic antecedents of this respiratory dysfunction are not well understood. Given that (1) alcohol exposure is known to increase extracellular adenosine levels (Nagy et al., 1990), (2) excessive adenosine receptor activation suppresses breathing (Eldridge et al., 1984, 1985; Lagercrantz et al., 1984), and (3) adenosine has been implicated in other effects of alcohol (Dar, 1990; Prediger et al., 2004; Fang et al., 2017; Lusardi et al., 2020) we hypothesized that adenosine signaling contributes to the effect of alcohol on breathing. We tested this hypothesis, and further clarified adenosinergic contributions to alcohol-induced respiratory suppression, over the course of five experiments.
In the first experiment, a high dose of the non-selective adenosine receptor antagonist caffeine was administered with alcohol. We hypothesized that the effects of alcohol on breathing were mediated by adenosine. We expected that non-selective adenosine receptor blockade via caffeine would alleviate alcohol-induced respiratory suppression. Indeed, we observed that caffeine attenuated alcohol-induced reduction in ventilation suggesting that adenosine contributes to alcohol-induced respiratory suppression. (Fig. 1A). This effect was mediated by reduced suppression of both tidal volume (Fig. 1B) and, to a lesser extent, respiratory rate (Fig. 1C).
A critical detail in the interpretation of the data from the caffeine experiment (Exp. 1) is that breathing increased in response to caffeine alone (Fig. 1A). As a result, it is not possible to differentiate between (a) the action of caffeine on a mechanistic level (i.e. caffeine blocking adenosine receptors thereby counteracting the increased extracellular adenosine levels caused by alcohol) and (b) the action of caffeine as a stimulant which increases respiration (i.e. alcohol decreases breathing, caffeine increases breathing, giving caffeine with alcohol might increase breathing relative to alcohol alone without necessarily interacting with the underlying mechanism). Regardless, this limitation of the caffeine experiment was not present in the 8-SPT experiment (Exp. 2, Fig. 2A), the DPCPX experiment (Exp. 3, Fig. 3A), the istradefylline experiment (Exp. 4, Fig. 4A), or our transgenic approach (Exp. 5, Fig. 5A; the Adk+/− and Adk+/+ mice had no differences in baseline breathing).
Metabolism and breathing are intimately linked processes (Wasserman, 1994) and it seems plausible that a drug that would increase breathing (e.g. caffeine) would increase metabolism in a way that would accelerate the clearance of alcohol from the blood. This increased alcohol metabolism due to caffeine might alleviate respiratory suppression without any direct involvement of adenosine signaling. The blood alcohol content of the mice used in this study was not measured as it would have necessitated removing the animal from the plethysmograph thereby disrupting the respiratory recording; however, this confound is unlikely given that it has been previously demonstrated that caffeine does not alter alcohol metabolism in humans (Azcona et al., 1995) or rodents (Kunin et al., 2000; Ferreira et al., 2004; Fritz et al., 2014). There is evidence to suggest that alcohol impairs caffeine metabolism (Mitchell et al., 1983), but this does not pose the same inter-pretational challenges for our results as the inverse.
In the second experiment, we evaluated whether the effects of adenosine receptor blockade observed in the first experiment were centrally or peripherally mediated. To test this, we peripherally administered the blood brain barrier impermeant non-selective adenosine receptor antagonist 8-SPT with alcohol. We hypothesized that the adenosinergic contributions to alcohol-induced respiratory suppression were occurring in the central nervous system (the rationale for this will be discussed in more detail below). As a result, we expected that peripheral non-selective adenosine receptor blockade via 8-SPT would not alleviate alcohol-induced ventilatory suppression. As predicted, 8-SPT did not reduce alcohol-induced respiratory suppression.
Alcohol increases adenosine signaling and affects breathing in both the brain and the periphery; however, its effects on breathing are regionally dichotomous. In the central nervous system adenosine and its analogs suppress breathing (Eldridge et al., 1984; Lagercrantz et al., 1984) and this suppression is counteracted by adenosine receptor antagonism (Eldridge et al., 1985). By contrast, in the periphery, adenosine analogs (Monteiro and Ribeiro, 1987) and inhibitors of adenosine metabolism (Monteiro and Ribeiro, 1989) stimulate breathing by increasing carotid body chemosensitivity. We hypothesized that adenosinergic contributions to alcohol-induced respiratory suppression are occurring in the central nervous system because if increased adenosine signaling in the periphery was the primary driving factor, it seems likely that alcohol would increase respiration, but the inverse is true (Wilson and Saukkonen, 2004; Langhan, 2013). In fact, increased adenosine signaling in the periphery might be having a beneficial effect on breathing which is outweighed by the detrimental effect in the central nervous system.
In the third and fourth experiments, we sought to determine the receptor responsible for adenosinergic contributions to alcohol-induced respiratory suppression. The results of Experiment 2 suggested that the effect of adenosine receptor antagonism on alcohol-induced respiratory suppression was occurring in the central nervous system. As a result, we focused on the A1 and A2A receptors which are expressed in the brain (Dixon et al., 1996; Sheth et al., 2014). We hypothesized that the effects of alcohol on breathing were mediated by the A2A receptor in light of its role in mediating other effects of alcohol, such as its hypnotic effects (El Yacoubi, 2003; Fang et al., 2017); however, given the effects of A1 receptor activation on breathing and chemoreception (Herlenius et al., 1997; Gettys et al., 2013; Falquetto et al., 2018) A1 receptor mediation of alcohol-induced respiratory suppression would not have been overly surprising. We expected that the A2A receptor antagonist istradefylline, but not the A1 receptor antagonist DPCPX, would reduce alcohol-induced respiratory suppression. In accordance with our expectations, A2A receptor antagonism, but not A1 receptor antagonism, reduced alcohol-induced ventilatory suppression (Figs. 3A and 4A). Like caffeine, the effect of istradefylline on ventilation was mediated by alleviated suppression of both tidal volume (Fig. 4B) and, to a lesser extent, respiratory rate (Fig. 4C).
In the fifth experiment, the effect of alcohol on breathing was evaluated in Adk+/− mice which have an ~50% reduction in adenosine kinase (Boison et al., 2002). Adenosine kinase is the enzyme primarily responsible for adenosine metabolism (Boison and Jarvis, 2021). We hypothesized that adenosine kinase has a beneficial effect in the context of alcohol intoxication by facilitating the metabolic clearance of adenosine. As a result, we expected that Adk+/− mice would undergo more severe alcohol-induced respiratory suppression. In accordance with our hypothesis, we found that alcohol-induced ventilatory suppression was exacerbated in Adk+/− mice (Fig. 5A). As with the adenosine receptor antagonists, the effect on ventilation was primarily mediated by tidal volume (Fig. 5B) rather than respiratory rate (Fig. 5C).
The data collected using Adk+/− mice does not suffer from the limitations of a pharmacological approach, but is subject to the caveats inherent to all constitutive transgenic manipulations. Reduced adenosine kinase expression might have resulted in compensatory changes during development that could have unpredictable second-order effects on physiology. Furthermore, this is an indirect manipulation of adenosine signaling that targets the enzymatic clearance of adenosine, not adenosine itself. Fortunately, the pharmacological and transgenic experimental approaches used in this investigation have nonoverlapping limitations and provide convergent evidence that adenosine contributes to alcohol-induced respiratory suppression.
The causal relationships between elevated adenosine signaling, the hypnotic effects of alcohol, and the respiratory suppression caused by alcohol are difficult to parse. Increased A2A receptor activation contributes to the effect of alcohol on sleep (El Yacoubi, 2003; Fang et al., 2017), our data indicate that the same may be true of breathing. We hypothesize that increased A2A receptor activation following alcohol exposure precipitates respiratory suppression and hypersomnolence by the same mechanism; however, on the basis of our results, we cannot rule out the possibility that alcohol increases A2A receptor activation which causes hypersomnolence or frank sedation which then causes the respiratory effects. By the same count, previous studies indicating that adenosine contributes to other effects of alcohol exposure are not able to rule out the possibility that this is mediated by a change in breathing. In terms of correcting the respiratory suppression induced by alcohol in a clinical context, this distinction may not matter.
Currently, respiratory dysfunction consequent to severe alcohol poisoning is treated with intubation, artificial ventilation, supplemental oxygen and, in the most serious cases, hemodialysis (Adinoff et al., 1988; Vacca and Correllus, 2013; Atassi et al., 2018; Sauter et al., 2020). Mechanical ventilation is prone to serious side effects such as lung injury (Slutsky, 1999) and pneumonia (Chastre and Fagon, 2002). Pneumonia is of particular concern for individuals who are being treated for alcohol poisoning as alcohol use disorder nearly quadruples the risk of pneumonia becoming fatal (Lujan et al., 2010). Patient outcomes might be improved if an adenosine-based intervention could be used with, or instead of, existing approaches. Our results suggest that an intervention targeting adenosine signaling, perhaps with a high dose of caffeine, may counteract alcohol-induced respiratory suppression.
The United States Department of Health and Human Services, Centers for Disease Control and Prevention, and Federal Trade Commission caution against the concurrent consumption of caffeine and alcohol (FTC, 2010; DHHS, 2020; CDC, 2022a). Though our data suggest that caffeine counteracts alcohol-induced respiratory suppression, this investigation should not be viewed as casting doubt on the aforementioned guidance. The dangers of consuming caffeine and alcohol in large quantities are behaviorally mediated and occur prior to the point where alcohol might seriously affect breathing (Ferre and O’Brien, 2011; Emond et al., 2014). Specifically, by counteracting the sedative/hypnotic effects of alcohol, caffeine facilitates the consumption of additional alcohol potentially bringing the person closer to serious alcohol poisoning (Ferre and O’Brien, 2011). Furthermore, the dose of caffeine in this study was chosen as a tool to interrogate the mechanisms of alcohol-induced respiratory suppression, not to be analogous to recreational caffeine consumption. In an 80 kg human, an equivalent dose of caffeine (40 mg/kg) would be 3.2 g of caffeine or about 17 doses of coffee (McCusker et al., 2003). With that said, a lower dose might also have a greater effect. It has been observed in mice that caffeine at 25 mg/kg causes the righting reflex to be restored more quicky following alcohol exposure, but this effect is substantially reduced at 50 mg/kg and entirely absent at 100 mg/kg (El Yacoubi, 2003).
Future investigations should: (1) Evaluate the effect of concurrent reductions of adenosine, GABA, and endogenous opioid signaling on alcohol-induced respiratory suppression. (2) Determine whether the increase in adenosine receptor expression caused by chronic caffeine exposure increases vulnerability to alcohol-induced respiratory suppression (Fredholm, 1995). (3) Investigate whether adenosine signaling contributes to the dangerous interactions between alcohol and opiates (Hill et al., 2016) or benzodiazepines (Tanaka, 2002). (5) Lastly, given that traumatic brain injury elicits a surge in extracellular adenosine (Bell et al., 1998), it might be beneficial to explore the role of adenosine in the increased mortality seen in highly intoxicated traumatic brain injury patients with prior alcohol exposure (Tien et al., 2006).
Acknowledgements
This work was supported by National Institutes of Health (DB, NS065957; DB, NS103740; BP, NS117792), and the Rutgers INSPIRE (IRACDA New Jersey/New York for Science Partnerships in Research & Education) Postdoctoral Program (BP).
Abbreviations:
- ADK
adenosine kinase
- GABA
γ-Aminobutyrie acid
Footnotes
Declaration of competing interest
The authors have no conflict of interest to declare.
CRediT authorship contribution statement
Benton S. Purnell: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Sydney Thompson: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Tenise Bowman: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Jayant Bhasin: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Steven George: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Brian Rust: Data curation, Formal analysis and Writing – review & editing. Madhuvika Murugan: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Denise Fedele: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Detlev Boison: Data curation, Formal analysis, Writing – original draft, Writing – review & editing.
Data availability
Data will be made available on request.
References
- Adinoff B, Bone GH, Linnoila M, 1988. Acute ethanol poisoning and the ethanol withdrawal syndrome. Med Toxicol Adverse Drug Exp 3, 172–196. 10.1007/BF03259881. [DOI] [PubMed] [Google Scholar]
- Ashraf O, Huynh T, Purnell BS, Murugan M, Fedele DE, Chitravanshi V, Boison D, 2021. Suppression of phrenic nerve activity as a potential predictor of imminent sudden unexpected death in epilepsy (SUDEP). Neuropharmacology 184, 108405. 10.1016/j.neuropharm.2020.108405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi WA, Noghnogh AA, Hariman R, Jayanthi S, Cheung SF, Kjellstrand CM, Ing TS, 2018. Hemodialysis as a treatment of severe ethanol poisoning. Int. J. Artif. Organs 22, 18–20. 10.1177/039139889902200105. [DOI] [PubMed] [Google Scholar]
- Azcona O, Barbanoj MJ, Torrent J, Jane F, 1995. Evaluation of the central effects of alcohol and caffeine interaction. Br. J. Clin. Pharmacol 40, 393–400. 10.1111/j.1365-2125.1995.tb04562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmutsky I, Wei XP, Kish E, Yackle K, 2020. Opioids depress breathing through two small brainstem sites. Elife 9. 10.7554/eLife.52694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MJ, Kochanek PM, Carcillo JA, Mi Z, Schiding JK, Wisniewski SR, Clark RS, Dixon CE, Marion DW, Jackson E, 1998. Interstitial adenosine, inosine, and hypoxanthine are increased after experimental traumatic brain injury in the rat. J. Neurotrauma 15, 163–170. 10.1089/neu.1998.15.163. [DOI] [PubMed] [Google Scholar]
- Boison D, Jarvis MF, 2021. Adenosine kinase: a key regulator of purinergic physiology. Biochem. Pharmacol 187, 114321 10.1016/j.bcp.2020.114321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Scheurer L, Zumsteg V, Rulicke T, Litynski P, Fowler B, Brandner S, Mohler H, 2002. Neonatal hepatic steatosis by disruption of the adenosine kinase gene. Proc. Natl. Acad. Sci. U. S. A 99, 6985–6990. 10.1073/pnas.092642899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham AC, 1995. Neurotransmitters in the CNS control of breathing. Respir. Physiol 101, 219–230. 10.1016/0034-5687(95)00045-f. [DOI] [PubMed] [Google Scholar]
- Carmichael FJ, Israel Y, Crawford M, Minhas K, Saldivia V, Sandrin S, Campisi P, Orrego H, 1991. Central-nervous-system effects of acetate - contribution to the central effects of ethanol. J. Pharmacol. Exp. Therapeut 259, 403–408. [PubMed] [Google Scholar]
- CDC, Centers for Disease Control and Prevention, 2022a. Alcohol and Caffeine Facts Sheet. https://www.cdc.gov/alcohol/fact-sheets/caffeine-and-alcohol.htm#:~:text=When%20alcohol%20is%20mixed%20with,risk%20of%20alcohol%2Dattributable%20harms.
- CDC, Centers for Disease Control and Prevention, 2022b. Alcohol and Public Health: Alcohol-Related Disease Impact (ARDI). https://nccd.cdc.gov/DPH_ARDI/Default/Report.aspx?T=AAM&P=612EF325-9B55-442B-AE0C-789B06E3A8D5&R=C877B524-834A-47D5-964D-158FE519C894&M=DB4DAAC0-C9B3-4F92-91A5-A5781DA85B68&F=&D=,.
- Chastre J, Fagon JY, 2002. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med 165, 867–903. 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- Connole L, Harkin A, Maginn M, 2004. Adenosine A1 receptor blockade mimics caffeine’s attenuation of ethanol-induced motor incoordination. Basic Clin. Pharmacol. Toxicol 95, 299–304. 10.1111/j.1742-7843.2004.pto950509.x. [DOI] [PubMed] [Google Scholar]
- Dar MS, 1990. Central adenosinergic system involvement in ethanol-induced motor incoordination in mice. J. Pharmacol. Exp. Therapeut 255, 1202–1209. [PubMed] [Google Scholar]
- Dar MS, Mustafa SJ, Wooles WR, 1983. Possible role of adenosine in the CNS effects of ethanol. Life Sci. 33, 1363–1374. 10.1016/0024-3205(83)90819-6. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Funk GD, Feldman JL, 2018. Breathing matters. Nat. Rev. Neurosci 19, 351–367. 10.1038/s41583-018-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhhs UDoHaHS., 2020. Dietary Guidelines for Americans, pp. 2020–2025.
- Dinh TKH, Gailis L, 1979. Effect of body temperature on acute ethanol toxicity. Life Sci. 25, 547–551. 10.1016/0024-3205(79)90569-1. [DOI] [PubMed] [Google Scholar]
- Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC, 1996. Tissue distribution of adenosine receptor mRNAs in the rat. Br. J. Pharmacol 118, 1461–1468. 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drorbaugh JE, Fenn WO, 1955. A barometric method for measuring ventilation in newborn infants. Pediatrics 16, 81–87. [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA, 2001. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci 24, 31–55. 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, 2003. Caffeine reduces hypnotic effects of alcohol through adenosine A2A receptor blockade. Neuropharmacology 45, 977–985. 10.1016/s0028-3908(03)00254-5. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Kiley JP, 1984. Respiratory effects of a long-acting analog of adenosine. Brain Res. 301, 273–280. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Kiley JP, 1985. Antagonism by theophylline of respiratory inhibition induced by adenosine. J. Appl. Physiol 59, 1428–1433. 10.1152/jappl.1985.59.5.1428, 1985. [DOI] [PubMed] [Google Scholar]
- Emond JA, Gilbert-Diamond D, Tanski SE, Sargent JD, 2014. Energy drink consumption and the risk of alcohol use disorder among a national sample of adolescents and young adults. J. Pediatr 165, 1194–1200. 10.1016/j.jpeds.2014.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falquetto B, Oliveira LM, Takakura AC, Mulkey DK, Moreira TS, 2018. Inhibition of the hypercapnic ventilatory response by adenosine in the retrotrapezoid nucleus in awake rats. Neuropharmacology 138, 47–56. 10.1016/j.neuropharm.2018.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang T, Dong H, Xu XH, Yuan XS, Chen ZK, Chen JF, Qu WM, Huang ZL, 2017. Adenosine A2A receptor mediates hypnotic effects of ethanol in mice. Sci. Rep 7, 12678 10.1038/s41598-017-12689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, O’Brien MC, 2011. Alcohol and caffeine: the perfect storm. J. Caffeine Res 1, 153–162. 10.1089/jcr.2011.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SE, Hartmann Quadros IM, Trindade AA, Takahashi S, Koyama RG, Souza-Formigoni ML, 2004. Can energy drinks reduce the depressor effect of ethanol? An experimental study in mice. Physiol. Behav 82, 841–847. 10.1016/j.physbeh.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, 1995. Adenosine, adenosine receptors and the actions of caffeine. Pharmacol. Toxicol 76, 93–101. 10.1111/j.1600-0773.1995.tboo111.x. [DOI] [PubMed] [Google Scholar]
- Fritz BM, Companion M, Boehm SL, 2014. Wired," yet intoxicated: modeling binge caffeine and alcohol co-consumption in the mouse. Alcohol Clin. Exp. Res 38, 2269–2278. 10.1111/acer.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ftc FTC, 2010. FTC Sends Warning Letters to Marketers of Caffeinated Alcohol Drinks. www.ftc.gov. [Google Scholar]
- Gettys GC, Liu F, Kimlin E, Baghdoyan HA, Lydic R, 2013. Adenosine A(1) receptors in mouse pontine reticular formation depress breathing, increase anesthesia recovery time, and decrease acetylcholine release. Anesthesiology 118, 327–336. 10.1097/ALN.0b013e31827d413e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C, 2001. Influence of the endogenous opioid system on high alcohol consumption and genetic predisposition to alcoholism. J. Psychiatry Neurosci 26, 304–318. [PMC free article] [PubMed] [Google Scholar]
- Hedner J, Hedner T, Jonason J, Lundberg D, 1981. GABA-ergic mechanisms in central respiratory control in the anesthetized rat. Naunyn-Schmiedeberg’s Arch. Pharmacol 317, 315–320. 10.1007/BF00501312. [DOI] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H, Yamamoto Y, 1997. Adenosine modulates inspiratory neurons and the respiratory pattern in the brainstem of neonatal rats. Pediatr. Res 42, 46–53. 10.1203/00006450-199707000-00008. [DOI] [PubMed] [Google Scholar]
- Hill R, Lyndon A, Withey S, Roberts J, Kershaw Y, MacLachlan J, Lingford-Hughes A, Kelly E, Bailey C, Hickman M, Henderson G, 2016. Ethanol reversal of tolerance to the respiratory depressant effects of morphine. Neuropsychopharmacology 41, 762–773. 10.1038/npp.2015.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarjour S, Bai L, Gianoulakis C, 2009. Effect of acute ethanol administration on the release of opioid peptides from the midbrain including the ventral tegmental area. Alcohol Clin. Exp. Res 33, 1033–1043. 10.1111/j.1530-0277.2009.00924.x. [DOI] [PubMed] [Google Scholar]
- Kunin D, Gaskin S, Rogan F, Smith BR, Amit Z, 2000. Caffeine promotes ethanol drinking in rats. Alcohol 21, 271–277. 10.1016/s0741-8329(00)00101-4. [DOI] [PubMed] [Google Scholar]
- Lagercrantz H, Yamamoto Y, Fredholm BB, Prabhakar NR, von Euler C, 1984. Adenosine analogues depress ventilation in rabbit neonates. Theophylline stimulation of respiration via adenosine receptors? Pediatr. Res 18, 387–390. 10.1203/00006450-198404000-00018. [DOI] [PubMed] [Google Scholar]
- Lam MP, Marinelli PW, Bai L, Gianoulakis C, 2008. Effects of acute ethanol on opioid peptide release in the central amygdala: an in vivo microdialysis study. Psychopharmacology (Berl) 201, 261–271. 10.1007/s00213-008-1267-8. [DOI] [PubMed] [Google Scholar]
- Langhan ML, 2013. Acute alcohol intoxication in adolescents: frequency of respiratory depression. J. Emerg. Med 44, 1063–1069. 10.1016/j.jemermed.2012.11.041. [DOI] [PubMed] [Google Scholar]
- Lignian H, Fontaine J, Askenasi R, 1983. Naloxone and alcohol intoxication in the dog. Hum. Toxicol 2, 221–225. 10.1177/096032718300200208. [DOI] [PubMed] [Google Scholar]
- Linowiecki K, Paloucek F, Donnelly A, Leikin JB, 1992. Reversal of ethanol-induced respiratory depression by flumazenil. Vet. Hum. Toxicol 34, 417–419. [PubMed] [Google Scholar]
- Lopez-Cruz L, San-Miguel N, Bayarri P, Baqi Y, Muller CE, Salamone JD, Correa M, 2016. Ethanol and caffeine effects on social interaction and recognition in mice: involvement of adenosine A2A and A1 receptors. Front. Behav. Neurosci 10, 206. 10.3389/fnbeh.2016.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan M, Gallego M, Belmonte Y, Fontanals D, Valles J, Lisboa T, Rello J, 2010. Influence of pneumococcal serotype group on outcome in adults with bacteraemic pneumonia. Eur. Respir. J 36, 1073–1079. 10.1183/09031936.00176309. [DOI] [PubMed] [Google Scholar]
- Lusardi TA, Lytle NK, Gebril HM, Boison D, 2020. Effects of preinjury and postinjury exposure to caffeine in a rat model of traumatic brain injury. J Caffeine Adenosine Res 10, 12–24. 10.1089/caff.2019.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley VB, Grunstein RR, Sullivan CE, 1988. Ethanol-induced depression of hypoxic drive and reversal by naloxone–a sex difference. Am. Rev. Respir. Dis 137, 1406–1410. 10.1164/ajrccm/137.6.1406. [DOI] [PubMed] [Google Scholar]
- McCusker RR, Goldberger BA, Cone EJ, 2003. Caffeine content of specialty coffees. J. Anal. Toxicol 27, 520–522. 10.1093/jat/27.7.520. [DOI] [PubMed] [Google Scholar]
- Michiels TM, Light RW, Mahutte CK, 1983. Effect of ethanol and naloxone on control of ventilation and load perception. J. Appl. Physiol. Respir. Environ. Exerc. Physiol 55, 929–934. 10.1152/jappl.1983.55.3.929. [DOI] [PubMed] [Google Scholar]
- Mitchell MC, Hoyumpa AM, Schenker S, Johnson RF, Nichols S, Patwardhan RV, 1983. Inhibition of caffeine elimination by short-term ethanol administration. J. Lab. Clin. Med 101, 826–834. 10.5555/uri:pii:0022214383901270. [DOI] [PubMed] [Google Scholar]
- Monteiro EC, Ribeiro JA, 1987. Ventilatory effects of adenosine mediated by carotid body chemoreceptors in the rat. Naunyn-Schmiedeberg’s Arch. Pharmacol 335, 143–148. 10.1007/BF00177715. [DOI] [PubMed] [Google Scholar]
- Monteiro EC, Ribeiro JA, 1989. Adenosine-deaminase and adenosine uptake inhibitions facilitate ventilation in rats. N-S Arch Pharmacol 340, 230–238. [DOI] [PubMed] [Google Scholar]
- Nagy LE, Diamond I, Casso DJ, Franklin C, Gordon AS, 1990. Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. J. Biol. Chem 265, 1946–1951. [PubMed] [Google Scholar]
- Nestoros JN, 1980. Ethanol specifically potentiates GABA-mediated neurotransmission in feline cerebral cortex. Science 209, 708–710. 10.1126/science.7394531. [DOI] [PubMed] [Google Scholar]
- Orrego H, Carmichael FJ, Saldivia V, Giles HG, Sandrin S, Israel Y, 1988. Ethanol-induced increase in portal blood flow: role of adenosine. Am. J. Physiol 254, G495–G501. 10.1152/ajpgi.1988.254.4.G495. [DOI] [PubMed] [Google Scholar]
- Pardo M, Betz AJ, San Miguel N, Lopez-Cruz L, Salamone JD, Correa M, 2013. Acetate as an active metabolite of ethanol: studies of locomotion, loss of righting reflex, and anxiety in rodents. Front. Behav. Neurosci 7, 81. 10.3389/fnbeh.2013.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prediger RD, Batista LC, Takahashi RN, 2004. Adenosine A1 receptors modulate the anxiolytic-like effect of ethanol in the elevated plus-maze in mice. Eur. J. Pharmacol 499, 147–154. 10.1016/j.ejphar.2004.07.106. [DOI] [PubMed] [Google Scholar]
- Ren J, Ding X, Greer JJ, 2012. Respiratory depression in rats induced by alcohol and barbiturate and rescue by ampakine CX717. J. Appl. Physiol 113, 1004–1011. 10.1152/japplphysiol.00752.2012, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR, 2004. Increased GABA release in the central amygdala of ethanol-dependent rats. J. Neurosci 24, 10159–10166. 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahn SA, Lakshminarayan S, Pierson DJ, Weil JV, 1975. Effect of ethanol on the ventilatory responses to oxygen and carbon dioxide in man. Clin. Sci. Mol. Med 49, 33–38. 10.1042/cs0490033. [DOI] [PubMed] [Google Scholar]
- Santiago TV, Edelman NH, 1985. Opioids and breathing. J. Appl. Physiol 59, 1675–1685. 10.1152/jappl.1985.59.6.1675, 1985. [DOI] [PubMed] [Google Scholar]
- Sauter TC, Ronz K, Hirschi T, Lehmann B, Hutt C, Exadaktylos AK, Muller M, 2020. Intubation in acute alcohol intoxications at the emergency department. Scand J Trauma Resus 28. 10.1186/s13049-020-0707-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HY, Li T, Boison D, 2010. A novel mouse model for sudden unexpected death in epilepsy (SUDEP): role of impaired adenosine clearance. Epilepsia 51, 465–468. 10.1111/j.1528-1167.2009.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth S, Brito R, Mukherjea D, Rybak LP, Ramkumar V, 2014. Adenosine receptors: expression, function and regulation. Int. J. Mol. Sci 15, 2024–2052. 10.3390/ijms15022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky AS, 1999. Lung injury caused by mechanical ventilation. Chest 116, 9S–15S. 10.1378/chest.116.suppl_1.9sa. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL, 1991. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254, 726–729. 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinetta MJ, Woodlee MT, Feinberg LM, Stroud C, Schallert K, Cormack LK, Schallert T, 2008. Alcohol-induced retrograde memory impairment in rats: prevention by caffeine. Psychopharmacology (Berl) 201, 361–371. 10.1007/s00213-008-1294-5. [DOI] [PubMed] [Google Scholar]
- Tanaka E, 2002. Toxicological interactions between alcohol and benzodiazepines. J. Toxicol. Clin. Toxicol 40, 69–75. 10.1081/clt-120002887. [DOI] [PubMed] [Google Scholar]
- Tien HC, Tremblay LN, Rizoli SB, Gelberg J, Chughtai T, Tikuisis P, Shek P, Brenneman FD, 2006. Association between alcohol and mortality in patients with severe traumatic head injury. Arch. Surg 141, 1185–1191. 10.1001/archsurg.141.12.1185.; discussion 1192. [DOI] [PubMed] [Google Scholar]
- Vacca VM Jr., Correllus DF 3rd, 2013. Alcohol poisoning. Nursing 43, 14–16. 10.1097/01.NURSE.0000427975.97543.d5. [DOI] [PubMed] [Google Scholar]
- Vale A, 2007. Ethanol. Medicine 35, 615–616. 10.1016/j.mpmed.2007.08.015. [DOI] [Google Scholar]
- Wang H, Xu H, Li W, Li B, Shi Q, Ma K, Xiao B, Chen L, 2019. Forensic appraisal of death due to acute alcohol poisoning: three case reports and a literature review. Forensic Sci Res 5, 341–347. 10.1080/20961790.2019.1572259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman K, 1994. Coupling of external to cellular respiration during exercise: the wisdom of the body revisited. Am. J. Physiol 266, E519–E539. 10.1152/ajpendo.1994.266.4.E519. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF, 2006. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol. Ther 111, 533–554. 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- White AM, Castle IP, Powell PA, Hingson RW, Koob GF, 2022. Alcohol-related deaths during the COVID-19 pandemic. JAMA. 10.1001/jama.2022.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2019. Global Status Report on Alcohol and Health 2018. World Health Organization. [Google Scholar]
- Wilson KC, Saukkonen JJ, 2004. Acute respiratory failure from abused substances. J. Intensive Care Med 19, 183–193. 10.1177/0885066604263918. [DOI] [PubMed] [Google Scholar]
- Xiao D, Xie F, Xu X, Zhou X, 2020. The impact and mechanism of ampakine CX1739 on protection against respiratory depression in rats. Future Med. Chem 12, 2093–2104. 10.4155/fmc-2020-0256. [DOI] [PubMed] [Google Scholar]
- Yamada KA, Hamosh P, Gillis RA, 1981. Respiratory depression produced by activation of GABA receptors in hindbrain of cat. J. Appl. Physiol. Respir. Environ. Exerc. Physiol 51, 1278–1286. 10.1152/jappl.1981.51.5.1278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.