Abstract
Over 98% of cervical cancers (CC) are caused by regular infections with "high risk" genotype of the human papilloma virus (HPV). However, this is not always the causative factor. Therefore, production of HPV vaccinations represents a significant chance to minimize the risk of CC. Phase III studies for a number of preventative HPV vaccines based on L1-virus-like particle (VLPs) have just been completed and the preliminary results are very convincing. However, there are a lot of practical concerns that need to be resolved before the use of these vaccinations. These vaccines were challenged with obvious queries such as protection time, subject receiving vaccines, time of vaccination, and how to include them into ongoing screening programs. Although these vaccines were 90% effective at preventing HPV infection as these offered only modest advantages for the removal of pre-existing infections. New advancements in the creation of therapeutic vaccinations have been explored for further improvement and post-vaccination surveillance. Therapeutic vaccines attempted to boost cell-mediated immunities and these are detrimental to the infected cell as opposed to neutralizing antibodies (different from prophylactic vaccines).
Keywords: Cervical cancers (CC), Human papillomavirus (HPV), Vaccines to control CC, Clinical trials
Introduction
Cervical cancer (CC) is the deadliest and life-threatening malignant health issue (fourth most common cancer) among women globally. With an expected 570,000 cases and 311,000 deaths in 2018, CC is the second most frequent malignancy in women living in developing countries (World Health Organization, WHO). In developing and less developed countries, estimated death reached to about 85% of 311,000 in 2018 [1]. As per the current report, 84% of HPV-related cancer lesions are CC which is transmitted sexually and causes CC [2]. Despite virus, few bacteria have been known to cause cancer and these are named as oncogenic or carcinogenic bacteria. These bacteria are exemplified as Salmonella typhi (gall bladder cancer), Streptococcus bovis (colorectal cancer), Chlamydia pneuminae (lungs cancer), Mycoplasma (various cancers such as prostate cancer), H. pylori (stomach cancer). Cervical cancer and bacterial vaginosis (BV) are linked together. However, the pathogenesis of BV in cervical cancer is complicated to understand and conclude a single causative bacteria as its pathogenesis involve various bacteria (G. vaginalis, Atopobium vagina, Bacterioides, Mycoplasma, Fusobacterium, Peptostreptococci, Mobiluncus spp, Ureaplasma urealyticum) [3]. The genus sneathia is related to various cancers such as cervical, vaginal, oral, and gastrointestinal types of cancer. The genus includes two species such as S. amnii, and S. sanguinegens commonly observed in oral, intestinal, vaginal, and cervical area. In a large cohort study (736 women), about 43% of sample specimens contained Sneathia [4].
Only the epithelium's basal cells are infected with HPV. Unchecked cell proliferation and mutations cause HPV lesions, which eventually progress to cancer. It is the second main cause of women getting cancer worldwide, especially in developing nations. Interestingly, epidemiological studies in the past suggested a link between the emergence of cervical cancer cases and the metabolic syndrome (MetS). In contrast to endometrial cancer, there is a dearth of solid data that might support the association between MetS and cervical cancer. On the other hand, a number of epidemiological studies shakily connect MetS presence with increased risk of cervical cancer [5]. HPV infection is virtually always a factor in the development of cervical cancer. Over 200 distinct HPV strains have been found that infect epithelial cells [6], and about 40 of them show a preference for mucosal tissues. Depending on their ability to cause cancer, they are further categorized into low-risk and high-risk HPV (lr-HPV and hr-HPV, respectively) [7]. While hr-HPV types are linked to cervical intraepithelial neoplasia (CIN) and CC, lr-HPV types are linked to the growth of anogenital warts. Around 70% of CC cases worldwide are caused by hr-HPV, primarily HPV-16 and HPV-18 [8, 9]. Although HPV infection alone is insufficient to cause CC, the emergence of persistent infection is a critical element in the development of cervical lesions and the course of the disease. Immune responses control infection, avoiding cervical lesion formation and its development into cancer in the majority of HPV-infected women [10]. As a result, only a small percentage of infected women are unable to control their infection and go on to develop CC. The progression of CIN to CC or its regression may be influenced by additional factors, according to this fact. The complex cervical milieu contains immune cells as well as a unique microbiome that controls regional immune responses [11–13]. Based on the types of bacteria present, the cervicovaginal microbiota was determined using 16S rRNA high-throughput sequencing (16S-HTS) data [14]. They are referred to as community state types (CSTs). Dysbiosis is a condition characterized by an unbalanced cervicovaginal microbiota composition with high variety and low Lactobacillus abundance, similar to CST IV. Dysbiosis can cause some women to experience symptoms such abnormal vaginal discharge, irritation, odor, and itching. In these cases, bacterial vaginosis is diagnosed [15]. Despite the fact that some women have symptoms, the majority of women are asymptomatic. However, women are more likely to contract HIV, HPV, and other infections when they are both symptomatic and asymptomatic [16]. There are various preventive strategies which are classified as primary, secondary, tertiary and palliative care. HPV based vaccines and chemotherapy-based treatment (and diagnosis) of cancerous lesions, are under primary and secondary prevention whereas diagnosis and treatment of invasive CC are tertiary prevention method. Moreover, palliative care involves a comprehensive CC control strategy. Preventing HPV-related disease and death successfully require both screening for pre-cancerous and cancerous lesions, and primary immunization [17]. Many nations have approved the use of three preventative vaccines. These are categorized as (a) quadrivalent-HPV vaccine, (b) bivalent-HPV vaccine, and (c) nonavalent-HPV vaccines. These three vaccinations demonstrate safety and efficacy in randomized studies and post marketing surveillance preventing 70%–90% of HPV-related malignancies. The HPV6 and HPV11 vaccines are nonavalent and quadrivalent to provide protection against anogenital warts. The first dose of the HPV vaccines was administered to about 118 million women globally [18]. These vaccines are substantially efficient and effective to prevent HPV infections and neoplastic illnesses. As these are preventive in nature, these have limited therapeutic benefits and no effect on treating pre-existing infections. Additionally, it appears that the impact of vaccination may not lower the incidence of cancer due to the lengthy period of time needed for the development of pre-cancerous lesions [19]. HPV16 and HPV18 are two the most common HPV-caused cancer-causing viral strains causing about 70% of CC and these are the focus of the two vaccines presently under evaluation. As discussed before, these three prophylactic vaccines are available in the market (a quadrivalent HPV vaccine, a bivalent HPV vaccine, and a novel nonavalent HPV vaccine). In India, Merck and GlaxoSmithKline legally marketed Gardasil™ (quadrivalent, type 6,11, 16, and 18) for 9–45 ages and Cervarix™ (bivalent vaccine), respectively to control CC. Gardasil™-9 can protect against HPV type 6, 11, 16,18, 31, 33, 45, 52, and 58. For developing both vaccines, a recombinant DNA technology was used and created non-infectious VLPs containing HPV-L1 protein. Effectiveness against CIN-2/3 and adenocarcinoma in-situ (AIS) using HPV based vaccines has been employed as key end points in clinical trials. The cross-protection against HPV strains (not included in the relevant vaccine) has also been studied for both vaccines. Notably, these vaccines are not effective and protective against previous infection as diagnosed in serotype diagnosis with HPV virus [20]. The main financial advantage of HPV vaccines in industrialized nations would be the cost savings from a decrease in the annual number of abnormal cervical smears found by screening that required further investigation. The main effect on developing nations would be a decrease in the actual number of CC cases and the overall cost. However, the cost of producing and delivering the current HPV VLP vaccines must be weighed against these advantages. In response to an HPV vaccine, the body produces antibodies that attach to the virus and stop it from infecting cells in subsequent contacts with the virus. The basis for the current HPV vaccines is the formation of virus-like particles (VLPs) from HPV surface elements. VLPs are not contagious since the virus's DNA is missing from them. In contrast, they resemble the natural virus and antibodies to the VLPs also work against the natural virus. According to research, the VLPs are strongly immunogenic to produce substantial antibodies, effective and safe to control CC.
Preventive measures and treatments
The dysplastic process takes times about 1–2 decades to mature and gives systematic screening programmed time to find and treat pre-invasive disease. According to estimates, cervical screening stops about 5000 deaths in the UK each year [21]. In fact, the increased incidence of HPV infection due to altered sexual behavior patterns may be an underestimation [21]. In a pilot trials study, the United Kingdom (UK) adopted a liquid-based cytology method for screening as per the National Institute for Health and Clinical Excellence (NICE) advice. Similarly, the ARTISTIC trial (a randomized trial of HPV-Testing in primary CC Screening) of Manchester, adopted a new HPV DNA testing method by hybrid capture and it is currently considered as a primary screening test [22]. To detect high grade cervical intraepithelial neoplasia (CIN), HPV-testing seems to be highly sensitive but less specific than cytology [23]. As it can identify numerous transitory illnesses, it lacks specificity. A potential screening paradigm includes HPV-testing as a main screening test and cytological triage for those who found as positive for HPV. It has been discovered that repeat testing after a 12-month break is just as beneficial as early colposcopy for women with normal or borderline cytology who have tested positive for HPV (Cuzick et al. 2003) [23]. This might increase the diagnosis and detection of high-grade CIN while reducing the cases of women who are recommended for colposcopy. Currently, screening aimed to find CIN and treat high grade illness. In contrast, 25% of CIN II advances to late-stage dysplasia within five years, and around one-third of CIN III progresses to late stage cancer. This might be easy to correlate with the ˃ 70% of mild dysplasia (including CIN I) being cured spontaneously. Therefore, treatment is not always necessary when CIN-1 is discovered. It is important to manage treatment strategy and diagnosis follow up. It was said that until therapy is necessary, cytological and colposcopic follow up should be accomplished (NHSCSP 2004). It might be possible that the follow up process may be restricted to high-risk HPV type CC women only. There is a chance of recurrence and this method of treatment does not always completely remove HPV infection from the upper genital tract. Pathologically, routine histological examination and cytological assessment of even high-risk HPV can be used to monitor any residual disease that develops during follow-up. Physical removal has already failed. Therefore, an ant treatment strategy capable of targeting the underlying HPV strain could be certainly preferable and ideal. A method of treatment for successful therapeutic immunization could be considered as preferable and acceptable to control pre-malignant and malignant CC whereas prevention through prophylactic vaccination could potentially eliminate the development of CC.
HPV vaccines development pipeline
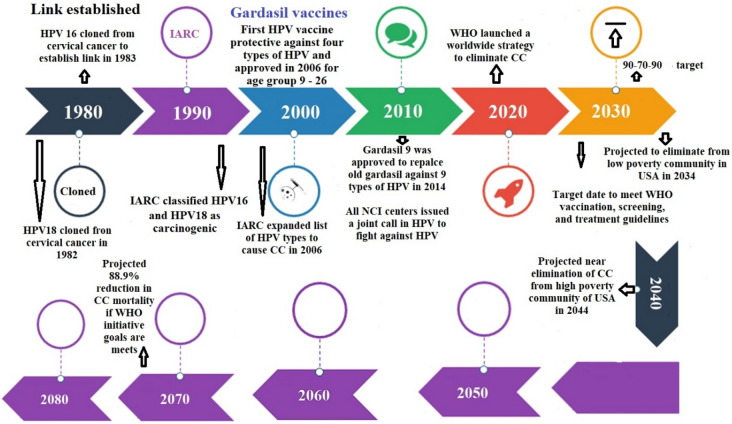
Historically, vaccination has been the least expensive and the most efficient method of preventing infectious disease. There is therefore a profound volume of research on the topic of HPV vaccines and CC [24–26]. In this article, we focused on some of the most therapeutically pertinent findings. According to the estimates, CC mortality would decline by 88.9% by 2070 and 98.6% by 2120 if all three WHO targets for eliminating the disease by 2030 were met. Without the screening and treatment targets, achieving the 90% immunization goal would reduce mortality by 61.7% by 2070 and by 89.5% by 2120 (Fig. 1).
Fig. 1.
Timelines of HPV vaccines: The HPV vaccine was first developed by the University of Queensland in Australia by Professors Ian Frazer and Jian Zhou. In 1990, Frazer and Zhou began to synthesize particles that mimicked HPV, from which the vaccine would later be made. These particles are called “virus-like particles” (VLPs), and are small particles that contain proteins from the outer layer of the HPV virus. In 1991, Frazer and Zhou’s findings were first presented to the scientific community. After seven years of design and testing, the first human trials for the vaccine, named Gardasil, were completed. Since then, two further vaccines have been approved: a bivalent vaccine called Cervarix approved in 2007 that prevents two HPV types (HPV16 and 18) and a nonavalent vaccine called Gardasil 9 in 2014 that protects against nine HPV types (HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58). As of October 2019, 100 countries worldwide vaccinate against HPV as part of their regular vaccine schedule. Currently there are six licensed HPV vaccines: three bivalent, two quadrivalent, and one nonavalent vaccine. Those that have been prequalified are being marketed in countries throughout the world (“Created with BioRender.com”)
Evidence for achievability
Is it possible to eliminate CC to the WHO threshold? A worldwide study team used comparative modelling to estimate how long it would take to eradicate CC in the United States in order to respond to this question. According to a simulation model, the WHO incidence criterion of less than four cases per 100,000 women-years may reach by 2038 assuming the status quo assumptions for immunization and screening. In a different scenario, it assumes screening, scaling up to 90%, and predicts the eradication of CC by 2028. Interestingly, a third scenario with the assumption of 90% vaccination coverage predicted CC elimination at about the same time as the status quo scenario. So, this suggests that the most effective intervention and the fastest way for the United States to achieve the goal of CC elimination is to scale up screening and treatment, especially focusing on the under-screened and undertreated [27]. There is evidence from multiple countries to support a reduction in HPV infection after the vaccine is rolled out into public health practice. One study comparing HPV prevalence among Australian women aged 18–24 pre and post-vaccination found a large reduction in the four types of HPV (Gardasil protected). The prevalence was 22.7% (n = 88) pre-vaccination in the years 2005–2007 whereas the post-vaccination prevalence dropped to 7.3% (n = 688) from 2010 to 2012 and to 1.5% (n = 200) in 2015 [28]. What is the evidence that the vaccine will work? While the HPV vaccine is the only one part of the three-legged stool, when it is added to screening and treatment. It reduces infection in the population. In sequence over time, this reduction in infection is followed by a reduction in genital warts, reduction in cervical intraepithelial neoplasia (CIN), and a reduction in CC. There is evidence from multiple countries to support a reduction in HPV infection after the vaccine is rolled out into public health practice. In the United States, even with a relatively low vaccine dissemination, there are significant reductions in genital wart incidence, pointing to the potential benefits in public health. Flagg and Torrone observed decreases in the prevalence of genital warts in young women likely to be affected by HPV several years after licensure of the HPV vaccine [29]. Similar to the incidence pattern for genital warts, CIN 2 incidence has declined in the United States despite the low dissemination of vaccine. To evaluate the impact of HPV vaccination on the reduction of cervical pre-cancer, McClung et al. examined archived specimens from women aged 18–39 with CIN 2 + . They found that between the years 2008 and 2014, the proportion of CIN 2 + cases decreased from 51.0% to 47.3% [30]. Finally, there is evidence that oral HPV infection is also declining as a consequence of vaccination. One study in the United Kingdom examined the effect of HPV vaccination on the prevalence of oropharyngeal HPV-16 infection among girls and young adult women and compared infection levels with those in unvaccinated young males of similar age [31]. They found that the UK female-only vaccination program was associated with a significantly lower oral HPV-16 prevalence among vaccinated females compared to unvaccinated females [31].
HPV pathogenesis
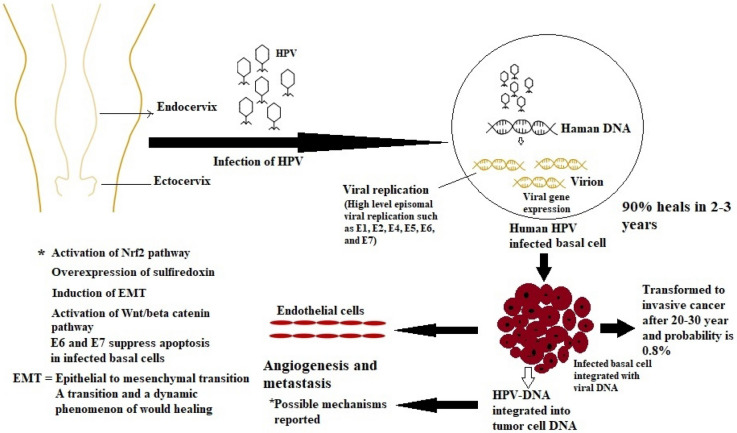
Mucosal or epidermal epithelial cells are infected by HPVs. The host's immune system eliminates the majority of infections. However, benign cervical lesions were caused by chronic HPV infections that the immune system was unable to eradicate. Three stages are frequently used to categorize cervical intraepithelial neoplasia (CIN-1, CIN-2, and CIN-3). The low-grade lesion stage known as CIN-1 is seen in many HPV infections. Within few months, the immune system eradicates almost 80% of CIN1 instances. The development of CIN2/3 as high-grade CIN lesions from an untreated HPV infection, can lead to aggressive CC [32, 33]. The main route by which HPVs infect the multi-layered stratified epithelium of the ectocervix and enter the basal lamina is a micro-wound [34, 35]. The early promoters can be turned on in the infected basal-layer cells early in the HPV life cycle to start the E1-viral helicase expression, which interacts with E2. The viral episomes are then amplified and quickly reproduced [36]. The virus genomes maintain a small amount of intact genomes replication at this point [37]. Daughter cells of infected basal cells gradually move to the top layers while also differentiating into distinct types of epithelium. Cell division then expedites bulk viral genome amplification, inducing delayed gene expressions, virosome assembly, and in vivo release. (Fig. 2).
Fig. 2.
Pathogenesis of HPV infection: Most probably HPV accesses basal cells, which rest on the basal membrane, supported by the dermis, through micro-abrasions in cervical epithelium. HPV infection of these cells leads to the activation of a cascade of viral gene expression that results in the production of approximately 20 to 100 extrachromosomal copies of viral DNA per cell. This average copy number is stably maintained in undifferentiated basal cells throughout the course of the infection. The most important players involved in the malignant transformation of HPV-related lesions are E6 and E7 proteins. They have the ability to interfere with cell proliferation and differentiation (“Created with BioRender.com”)
HPV vaccine history
The HPV L1 main capsid protein is expressed via recombinant DNA technology in Saccharomyces cerevisiae (yeast), which self-assembles to create shells (empty) resembling viruses, known as virus-like particles (VLPs). The VLPs lack genetic material but have the same exterior L1-protein. These VLPs are used in the vaccine as an antigen to trigger powerful immune responses that are protective in the event of exposure. Antibodies to the L1-protein will bind the HPV to stop releasing the genetic materials [38].
Vaccination as the best form of treatment strategy
Currently, the only ways to avoid genital HPV infections are lifetime mutual monogamy and abstinence. The use of physical barrier techniques (condom and contraceptive) is not the effective and conclusive proof to provide protection against HPV infections. Second, the virus has no symptoms other than genital warts [39, 40]. Even in developed nations, the susceptible female population has not adhered to routine screening by periodic Pap smears, and in developing nations like India, it is challenging to implement routine screening on a wide scale.
The HPV vaccines to control CC
The primary mechanism of vaccine-induced protection is the induction of antibodies. In the event of HPV contact, the vaccination attaches to the HPV viruses and stops to infect further epithelial tissues. The amount of antibody generated during natural infection is typically not enough to stop a reinfection. There isn't secondary lymphoid tissue in the infected cervical region, which would have produced antibodies and neutralized the virus before absorption (if it did contain a lot of memory B cells) [41]. Additionally, large and extended levels of natural antibodies generated by vaccines are necessary to maintain the protective immune responses during sexually active life. Therefore, a perfect HPV vaccination should boost the immune system's defenses and protect against all high-risk HPV types as well as those forms that are probably or maybe carcinogenic. The HPV vaccines available today are based on VLPs, which lack viral DNA and are made from recombinant HPV capsid proteins. Different forms of type-specific nAbs can be induced by VLP vaccination, and these nAbs can bind to native VPs and neutralize the virus by inhibiting cellular uptake (epithelial cells).
Prophylactic HPV vaccines
As we discussed before, there are three approved commercial vaccines to control CC for prophylactic therapy. The yeast strain was used to express these proteins and manufactured the 4vHPV and 9vHPV vaccines. These proteins were further conjugated with aluminum hydroxy phosphate sulfate (AAHS) adjuvant to get an amorphous construct. The 4vHPV vaccine was recommended to prevent from genital wart, dysplasia lesion, precancerous lesion, and CC for both sexes aged between 9 to 26 years [42]. On the other hand, the 9vHPV vaccine protects from five more HPV strains in both sexes aged between 9 to 45 years and is effective to protect from other cancers such as vulvar, CC, vaginal, and anal cancers [43]. The production of the 2vHPV, which originally got permission in 2007, uses a baculovirus expression vector system and an adjuvant called AS04 (aluminum hydroxide and 3-deacylated monophosphorylate lipid A) [44]. AIS, CC, and CIN may be prevented by HPV vaccination [45, 46]. These three preventative vaccinations use VLPs that were put together using L1's recombinant expression. Recombinant L1 protein has been demonstrated to self-assemble into VLPs that resemble natural virions morphologically and lack the oncogenic viral genome [47]. By inducing high and long-lasting antibodies titers that bind to the native virion and destroy the virus, HPV vaccinations can prevent HPV infection and render people immunized to subsequent viral challenges.
Prophylactic vaccine schedules and strategies
A three-dose vaccination schedule was first approved for the preventive HPV. Recent research has demonstrated that the 2 or even 1 dosage of the 2vHPV vaccination was significantly more effective than the 3-doses strategy [48]. Girls aged from 9 to 14 years who received two doses of the 2vHPV vaccine at months 0 and 6 showed superior HPV-16/18 antibody responses compared to those who received two doses of the 4vHPV vaccine at months 0 and six or three doses of the 4vHPV vaccine at months 0, 2, and 6, in an observer-blind study [49]. The 2-doses vaccination schedule appeared to be the most cost-effective assuming the vaccines could provide protection for more than 20 years [50]. The WHO has suggested that individuals between the ages of 9 and 15 receiving two doses of the HPV vaccine at least six months apart, the gap between the first and second doses should be between 6 and 15 months. A third-dose regimen is necessary for people older than 14 or immunocompromised patient regardless of age who have their two doses less than five months apart [51].
Safety concern to receive prophylactic HPV vaccination
A rough estimate of ˃ 200 million doses of the preventive HPV vaccines had been administered globally [52]. Several evidences support the vaccine's safety. However, a number of safety concerns, have surfaced including discomfort, swelling in the arm at the shot site, redness, slight fever, fatigue, nausea, headache, and muscle, and joint pain. The Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD), and the Clinical Immunization Safety Assessment (CISA) Project are three systems used by the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) to monitor the safety aspects of vaccines. Prior to the FDA granting the license in 2014, seven studies investigated the 9vHPV vaccine's safety. According to these pre-licensure trials, the 9vHPV vaccination is equally safe as the 4vHPV vaccine. Additionally, according to statistics from the 4vHPV vaccine reports, 92% of them were not considered significant [53]. Serious adverse reactions are extremely rare after receiving an HPV vaccine, and the causes still remained unknown. While the immunizations do not appear to increase the likelihood of miscarriage, the fetal adverse events following HPV vaccination primarily involve spontaneous miscarriage. There are no risks of unfavorable pregnancy outcomes when receiving the HPV vaccine during pregnancy, post-pregnancy, or right after conception [54, 55]. Although, it is not advised for pregnant women to obtain the HPV vaccine. Lactating women can get the vaccine. Additionally, the safety profile of the 9vHPV vaccination was supported by preclinical investigation on rat models wherein the nonavalent HPV vaccine had no negative impact on reproductive performance, fertility, and any signs of developmental toxicity.
The new version of HPV vaccines (second generation HPV)
The aforementioned vaccines (three) are now approved and these are developed by yeast or insect cell expression systems, making their manufacturing procedures complicated and this is the main cause of the high cost. The manufacture of VLP-based vaccines has been improved via the efforts of many professionals, with notable success. In preclinical or clinical studies, HPV L1 VLP-based vaccines made in methylotrophic yeast species such as Hansenula Polymorpha, P. pastoris), S. cerevisiae, and S. cerevisiae. (Table 1) [56].
Table 1.
Various stages of clinical trials on HPV vaccines
| Trial name | Phase/disease condition | Vaccine type | Number |
|---|---|---|---|
| Immunogenicity and safety of Havrix co-administered with other vaccines (DTP and haemophilus b vaccine) in children (18 months). Neutralization dose: 1440 EL.U. for age 19 years and older | Phase III (completed)/hepatitis A. Not protective in all vaccine recipients. Adverse reaction at the injection site | VLP (prevention) | NCT00197236 |
| Gardasil® vaccine (quadrivalent HPV composed of type 6, 11, 16, and 18 L1 VLP). To investigate efficacy against external genital warts (condyloma acuminata), penile, PIN, perianal or perineal cancer, and genital infections in young men due to HPV. Aluminum hydroxyphosphate sulfate adjuvant at 25 µg/0.5 ml IM (amorphous) | Phase III (completed)/prevention against papillomavirus. Mid-adult women (16 to 23 years). Possible adverse reactions (redness, irritation and swelling) at the injection site | VLP (prevention) at three dose times (0, 3 and 6 months) | NCT00090220 |
| pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine three doses at weeks 0, 4 and 8 in absence of disease or 8, 15, and 19 for patients undergoing colposcopy. Three doses as low (0.003 mg), medium (1–3 mg) and high (3 mg per dose) Primary outcomes: safety, toxicity and efficacy | Phase I/II (completed)/prevention against cervical cancer in patients of papillomavirus-16 positive grade 2/3 cervical intraepithelial neoplasia and precancerous condition | Recombinant DNA | NCT00121173 |
| SGN-00101 vaccine to treat HPV infected patient with CC using three doses at week 1, 4, and 8 subcutaneously in ˃ 18 years old | Phase II (completed)/treatment of cervical cancer due to HPV | HPV Prevention | NCT00091130 |
| HPV16 E7 peptide-pulsed autologous DCs was used in immunotherapy to control recurrent CC using DCs pulsed with human papillomavirus-type 16 E7 antigen | Phase I (unknown) / recurrent CC in patients with age 18–75 years | HPV16 E7 prevention | NCT00155766 |
| Combination of PD-1 Monoclonal Antibody and HPV Vaccine in Patients with Cervical Cancer | Phase II, Cervical Cancer | Quadrivalent HPV vaccine | NCT04096911 |
| Vaccine To Prevent Cervical Intraepithelial Neoplasia or Cervical Cancer in Younger Healthy Participants | Cervical Cancer, Phase III | Human papillomavirus 16/18 L1 virus-like particle/AS04 vaccine | NCT00128661 |
| Immunogenicity Study of Recombinant Human Papillomavirus Vaccine(6,11,16,18,31,33,45,52,58 Type)(E.Coli) | Phase II, Cervical Cancer, | HPV Vaccine,270 μg/1.0 ml | NCT03935204 |
| An Immuno-bridging Study of a Nonavalent HPV Vaccine (E.Coli) in Healthy Population Aged 9–17 vs Aged 18–26 Years Old | Phase III, Cervical Cancer | 3 doses of the Recombinant Human Papillomavirus Nonavalent (Types 6,11,16,18,31,33,45,52,58)Vaccine(E.Coli) | NCT05056402 |
| Immunogenicity of a Recombinant Human Papillomavirus Nonavalent (Types 6,11,16,18,31,33,45,52,58)Vaccine(E.Coli) Versus Gardasil®9 in Healthy Females 18–26 Years of Age | Phase III, Cervical Cancer | Recombinant Human Papillomavirus Nonavalent (Types 6,11,16,18,31,33,45,52,58)Vaccine(E.Coli) | NCT04782895 |
| Alternate Dosing Schedules Study for HPV Vaccine | Phase IV, Cervical Cancer | Received HPV vaccine firs | NCT00862810 |
| Dose-Ranging Study of Recombinant Human Papillomavirus Virus 16/18 Bivalent Vaccine | Phase 2, Cervical Intraepithelial Neoplasia, Cervical Cancer | Hepatitis B vaccine | NCT01356823 |
| Safety and Immunogenicity Study of Human Papilloma Virus Vaccine in Women Aged 9 to 30 and Men Aged 9 to 17 | Phase I, Cervical Cancer | Tetravalent recombinant human papilloma virus vaccine (6,11,16,18 type) (Hansenula polymorpha) | NCT02888418 |
| Efficacy, Immunogenicity and Safty Study of Recombinant Human Papillomavirus Vaccine (6,11,16,18,31,33,45,52,58 Type) (E. coli) | Phase III, Cervical Intraepithelial Neoplasia, Cervical Cancer | Nonavalent HPV vaccine, Bivalent HPV vaccine | NCT04537156 |
| A Controlled Trial to Assess the Immunogenicity of a Proposed Paediatric Dosing Schedule of Human Papillomavirus Vaccine | Phase III, Cervical Cancer | HPV (Human Papillomavirus) Vaccine | NCT00501137 |
| Broad Spectrum HPV Vaccine Dose Ranging Study (V502-001) | Cervical Cancer, Phase II | Octavalent HPV Vaccine—dose formulation 1 | NCT00260039 |
| Vaccine Therapy in Preventing HPV in HIV-Positive Women in India | Phase I, Cervical Cancer | Quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine | NCT00667563 |
| Vaccine Therapy in Treating Patients with Recurrent or Persistent Cervical Cancer | Cervical Cancer, Phase I | Human papillomavirus 16 E7 peptide | NCT00003977 |
| Study to Test the Safety of HPV Vaccine in Women (V501-011) (COMPLETED) | Phase III, Cervical Cancer | V501, Gardasil, Quadrivalent Human Papillomavirus (Types 6, 11, 16, 18) Recombinant Vaccine | NCT00517309 |
| Protecting Young Special Risk Females from Cervical Cancer Through Human Papilloma Virus (HPV) Vaccination | Phase III, Cervical Cancer | Licensed quadrivalent HPV vaccine, Gardasil | NCT00964210 |
| Long-term Follow-up of Broad Spectrum Human Papillomavirus (HPV) Vaccine Study in Women (V503-021) | Cervical Cancer | GARDASIL vaccine | NCT02653118 |
| Single Dose of Cervarix Vaccine in Girls or Three Doses of Gardasil Vaccine in Women for the Prevention of Human Papillomavirus Infection, the PRIMAVERA-ESCUDDO Trial | Human Papillomavirus-Related Cervical Carcinoma, Phase III | Quadrivalent Human Papillomavirus (types 6, 11, 16, 18) Recombinant Vaccine | NCT03728881 |
| Cervical Intraepithelial Neoplasm (CIN)-Warts Efficacy Trial in Women (Gardasil)(V501-013) (COMPLETED) | Cervical Cancer, Phase III | Human Papillomavirus (HPV) 16 Monovalent | NCT00092521 |
| Efficacy and Immunogenicity Study of Recombinant Human Papillomavirus Bivalent(Type 16/18) Vaccine | Cervical Intraepithelial, Neoplasia Cervical Cancer, Phase III | HPV Vaccine, HEV vaccine | NCT01735006 |
| Immunogenicity AND Safety Study of the 11 Valent Recombinant Human Papillomavirus Vaccine (Hansenulapolymorpha) | HPV-Related Cervical Carcinoma, Phase II | Biological/Vaccine: 11-valent recombinant human papilloma virus vaccine (Hansenula polymorpha) | NCT04436133 |
| Vaccine Therapy in Preventing Cervical Cancer in Patients with Cervical Intraepithelial Neoplasia | Cervical Cancer, Phase II | pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine | NCT00121173 |
| Comparing One or Two Doses of the Human Papillomavirus Vaccine for the Prevention of Human Papillomavirus Infection, ESCUDDO Study | Human Papillomavirus Infection, Phase IV | Diphtheria Toxoid/Tetanus Toxoid/Acellular Pertussis Vaccine Adsorbed | NCT03180034 |
| A Randomized, Blinded, Placebo-controlled Phase I Clinical Trial Evaluating the Safety and Preliminary Immunogenicity of a 11-valent Recombinant Human Papillomavirus Vaccine (Hansenulapolymorpha) in Chinese Women Aged 9–45 Years | HPV-Related Cervical Carcinoma, Phase I | 11-valent recombinant human papilloma virus vaccine (Hansenula polymorpha) | NCT04083196 |
| A Vaccine (PDS0101) and Chemoradiation for the Treatment of Stage IB3-IVA Cervical Cancer, the IMMUNOCERV Trial | IB3 Cervical Cancer FIGO 2018, Phase II | Liposomal HPV-16 E6/E7 Multipeptide Vaccine PDS0101 | NCT04580771 |
| Vvax001 Cancer Vaccine in (Pre) Malignant Cervical Lesions | Cervical Cancer, Phase I | Vvax001 therapeutic cancer vaccine | NCT03141463 |
| Lot Consistency Clinical Trial of of Recombinant HPV Bivalent Vaccine in 9 to14 Years Old Healthy Female | Cervical Intraepithelial, Neoplasia Cervical Cancer, Phase IV | Recombinant Human Papillomavirus Bivalent (Types 16, 18) Vaccine (Escherichia coli) / Cecolin® | NCT05426148 |
| Cervical Intraepithelial Neoplasm (CIN) in Women (Gardasil) (V501-015) | Cervical Cancer, Phase III | Gardasil, human papillomavirus (type 6, 11, 16, 18) recombinant vaccine | NCT00092534 |
| The Durability of Protection and Immuno-persistence Study of a Recombinant HPV 16/18 Bivalent Vaccine in Female | Cervical Intraepithelial, Neoplasia Cervical Cancer | Recombinant Human Papillomavirus Bivalent (Types 16, 18) Vaccine (Escherichia coli) | NCT05045755 |
| Immuno-persistence Study of a Recombinant Human Papillomavirus 16/18 Bivalent Vaccine in Preadolescent Girls | Cervical Intraepithelial Neoplasia Cervical Cancer | 3 doses of HPV 16/18 bivalent vaccine | NCT03206255 |
| Vaccine Therapy in Treating Patients with Persistent or Recurrent Cervical Cancer | Cervical Adenocarcinoma, Phase II | Attenuated Live Listeria Encoding HPV 16 E7 Vaccine ADXS11-001 | NCT01266460 |
| A Single Centre Study to Evaluate the Safety and Immunogenicity of the Human Papillomavirus Vaccine (GSK-580299) in Chinese Females | Infections, Papillomavirus, Phase I | HPV -16/18 L1 VLP AS04 vaccine) | NCT00549900 |
| Evaluation of Immunogenicity and Safety of Human Papillomavirus (HPV) Vaccine Co-administered With Another Vaccine in Healthy Female Subjects | Infections, Papillomavirus, Phase III | HPV Vaccine (GSK580299) Cervarix TM | NCT00652938 |
| A Phase III Trial to Evaluate the Efficacy, Immunogenicity and Safety Profile of Recombinant Nonavalent (Types 6/11/16/18/31/33/45/52/58) Human Papillomavirus (HPV) Vaccine (Escherichia Coli) | Human Papillomavirus Infection, Phase III | Recombinant Nonavalent (types 6/11/16/18/31/33/45/52/58) Human Papillomavirus (HPV) Vaccine (Escherichia coli) | NCT05668572 |
| Immunobridging Study of 9-valent Human Papillomavirus Recombinant Vaccine in Chinese Females Aged 9 to 19 Years | HPV Infections, Cervical Cancer, Phase III | 9-valent HPV vaccine | NCT04895020 |
| Evaluate the Immunogenicity and Safety of 4-valent and 9-valent HPV Recombinant Vaccine in Chinese Healthy Females | Cervical Cancer, Phase III | 4-valent HPV Vaccine, 9-valent HPV Vaccine | NCT04425291 |
| Evaluate the Efficacy, Immunogenicity and Safety of 9-valent HPV Recombinant Vaccine in Chinese Healthy Females | Cervical Cancer, Phase III | 9-valent Human Papillomavirus (Types 6, 11, 16, 18,31,33,45,52 and 58) Recombinant Vaccine (Hansenula Polymorpha) | NCT04422366 |
| Evaluate the Immunogenicity and Safety of 9-valent HPV Recombinant Vaccine in Chinese Healthy Females | HPV Infections Cervical Cancer, Phase III | Experimental: Experimental: 9-valent Human Papillomavirus (Types 6, 11, 16, 18, 31, 33, 45, 52, 58) | NCT05372016 |
| A Phase III Study to Evaluate the Efficacy, Immunogenicity, Safety of Quadrivalent HPV Recombinant Vaccine in Chinese Healthy Females | Cervical Cancer, Phase III | Quadrivalent Human Papillomavirus (Types 6, 11, 16, and 18) Recombinant Vaccine (Hansenula Polymorpha) | NCT05584332 |
| DNA Plasmid-encoding Interleukin-12/HPV DNA Plasmids Therapeutic Vaccine INO-3112 and Durvalumab in Treating Patients with Recurrent or Metastatic Human Papillomavirus Associated Cancers | Human Papillomavirus-16 Positive, Phase II | DNA Plasmid-encoding Interleukin-12/HPV DNA Plasmids Therapeutic Vaccine MEDI0457 | NCT03439085 |
| Efficacy, Immunogenicity and Safety of GSK Biologicals' HPV GSK 580,299 Vaccine in Healthy Chinese Female Subjects | Infections, Papillomavirus, Phase III | HPV GSK 580,299 vaccine | NCT00779766 |
| Evaluate the Safety of Recombinant Human Papillomavirus Bivalent (Types 16 and 18) Vaccine (Yeast) in Healthy Females | Human Papillomavirus, Phase I | HPV 16/18 vaccine, 0,5 ml | NCT01548118 |
| Safety and Immunogenicity Study of V503 (GARDASIL™9, 9vHPV Vaccine) Administered to 9- to 26-Year-Old Females and Males in Vietnam (V503-017) | Papillomavirus Infections, Phase III | 9vHPV vaccine | NCT03546842 |
| Immunogenicity of GlaxoSmithKline Biological's Human Papillomavirus (HPV) Vaccine (580,299) Versus Merck's Gardasil® in Healthy Females 18–45 Years of Age | Infections, Papillomavirus, Phase III | GSK Biologicals HPV 16/18 vaccine 580,299 (CervarixTM) | NCT00423046 |
| Evaluation of Safety and Immunogenicity of a Human Papillomavirus (HPV) Vaccine in Human Immunodeficiency Virus (HIV) Infected Females | Infections, Papillomavirus, Phase IV | GSK Biologicals' HPV vaccine 580,299, Merck's Human Papillomavirus Quadrivalent (Types 6, 11, 16, and 18) Vaccine (Gardasil) | NCT01031069 |
| Safety Study of GSK Biologicals' Human Papillomavirus Vaccine in 580,299/008 Subjects from Canada or the US | Infections, Papillomavirus, Phase III | Biological: GSK Biological's HPV vaccine GSK580299 (Cervarix™) | NCT00799825 |
| Safety Study of GSK Biologicals' Human Papillomavirus Vaccine in 580,299/008 Subjects from Brazil, Taiwan or Thailand | Infections, Papillomavirus, Phase III | GSK580299, GSK Biological's HPV vaccine | NCT00849381 |
| A Study to Evaluate the Immune Response and Safety of GSK Biologicals' HPV-16/18 L1 VLP AS04 Vaccine/Cervarix TM Vaccine in Healthy Females Aged 15–25 Years | Infections, Papillomavirus, Phase III | HPV-16/18 VLP/AS04 vaccine (Cervarix TM) | NCT00485732 |
| Human Papilloma Virus (HPV) Vaccine Trial in Young Adolescent Women with GlaxoSmithKline Biologicals' (GSK Bio) HPV-16/18 Vaccine | Cervical Intraepithelial Neoplasia, Phase III | GSK Biologicals' HPV-16/18 Vaccine (Cervarix™) | NCT00316706 |
| A Study to Evaluate the Immunogenicity and Safety of HPV Vaccine in Healthy Female Participants Aged 9–26 Years in China | Human Papillomavirus Infection, Phase III | Recombinant Nonavalent (types 6/11/16/18/31/33/45/52/58) Human Papillomavirus (HPV) Vaccine (Escherichia coli) | NCT05662020 |
| HPV DNA Vaccine Via Electroporation for HPV16 Positive Cervical Neoplasia | Human Papillomavirus Type 16, Phase I | NCT04131413 |
DTP Diphtheria, tetanus, and pertussis, CIN cervical intraepithelial neoplasia; DCs dendritic cells, DNA deoxyribonucleic acid, PIN Penile intraepithelial neoplasia, VLP Virus like particles
Therapeutically used HPV vaccination
The prime goal of preventative HPV vaccinations is to stimulate humoral-immunity against the target proteins (late proteins L1 and L2), which will induce antibodies production and antigens neutralization. Therapeutic vaccinations, as opposed to preventative ones, are primarily intended to treat precancerous lesions and chronic HPV infections. In HPV infection, the majority of CC, and precancerous lesions, the early proteins E6 and E7 are consistently produced (but not in normal tissues). Notably, these early proteins (E6 and E7) are the most preferred target proteins to develop antigen-specific immunotherapy and treatment therapy of HPV infections, and related disorders. Their continuous expression is crucial to induce and maintain the malignant phenotype of cancer cells. Various therapeutic vaccines to control CC using the same viral strains have been reported to target these early proteins (E6, E7), late proteins (L1 and L2), and virus antigens over the last two decades. These vaccines included nucleic acid, live vector, and acid/protein/peptide based approaches. The following Table 2 lists the characteristics of vaccination platform technologies and the development of the several therapeutic HPV vaccines available (Table 2). For a number of reasons, developing prophylactic vaccines are considerably more difficult than developing preventive vaccinations. E6 and E7 oncoproteins are expressed in high grade CIN lesions at a low level only.
Table 2.
Summary of few HPV vaccines published after clinical trials for therapeutic use
| Vectors | Antigens | Major results | Clinical phases | Refs. |
|---|---|---|---|---|
| Viral vector | TA-HPV, Xenova E6-E7 fusion protein, | Five patients were used to develop specific novel HPV T- cell responses post-vaccination. However, no clinical responses were obtained (phase I/II) | Phase I/II | [82–84] |
| Fusion protein PD-E7 | 5/7 CTL responses were observed (phase I/II) | Phase I/II | ||
| Davidson et al. 2003 | Eight women had partial clinical responses whereas 13 women had HPV specific immune responses post- vaccination (phase II) | Phase II | ||
| No correlation between response and pre-vaccination local immune obtained | ||||
| Peptide | 1. HPV 16 E7 peptides | 1. T helper responses in 4 patients, No specific CTL responses | Phase I/II | [85, 86] |
| 2. E7 Peptide + IFA | 2. 10/16 CTL responses | Phase I | ||
| Protein | Protein/Iscomatrix adjuvant | Most developed antibody responses | Phase I | [24, 87] |
| TA-CIN- L2, E6, E7 Fusion protein, Xenova | 2. TA-CIN specific IgG antibody and T-cell proliferative responses in majority | Phase I | ||
| Prime boost | TA-HPV + TA-CIN, Xenova | To study immunogenicity of heterogeneous prime boost human HPV oncogene vaccine in anogenital intraepithelial neoplasia (AGIN) | Phase I/II | [88] |
| 29 women with high grade AGIN at three doses were administered (IM) | ||||
| No established relationship between induced systemic immunity and outcomes | ||||
| DNA | Plasmid DNA responsible to encode multiple HLA-A2 epitopes of HPV-E2-16 protein | To assess safety and efficacy of novel ZYC101a to control high grade cervical intraepithelial neoplasia | Phase I/II | [89] |
| 127 out of 161 subjects were evaluated after three doses (100 or 200 µg) (IM) | ||||
| Exhibited well tolerance in women younger than 25 years | ||||
| DC | DCs pulsed with recombinant HPV16E7 & HPV18E7 | 15 women stage IV to control CC | Phase I/II | [90] |
Live vector-based vaccines
Recombinant technology has been used to tailor vaccines to control CC. These are viral and bacterial vector-based vaccines used to transport HPV antigen within host cells, and elicit immune response against HPV infection. These are of two subgroups of live vector-based vaccinations. Additionally, these are capable of promoting antigen presentation via both MHC-I and MHC-II class pathways which offered significant level of immunogenic responses. The development of natural antibodies against pre-existing bacteria and viruses may restrict the use of these vectors in recurrent therapy. Another barrier to the use of live vector vaccinations is the probable immuno-dominance of the vectors rather than the HPV antigen [57, 58].
Bacterial vector vaccines
An ideal vaccination vector should stimulate the infected host's innate and adaptive immune systems strongly. In order to defend the host from pathogen, attenuated bacteria can activate immunogenic responses (cellular and humoral immune). Peptidoglycans, flagellin, lipoteichoic acid, and lipopolysaccharides are examples of conserved molecular patterns that are identified by pattern recognition receptors (PRRs) and trigger innate immune responses [59]. It is efficient to create novel vaccines by delivering heterologous antigens through the bacterial vector. The bacterial vector executes several benefits over other vaccines such as (a) bacterial vectors to deliver heterologous antigen for reducing the challenges of targeting antigen purification and (b) making large-scale manufacture and inoculation relatively simple and affordable. The stimulation of adaptive and innate immune responses to antigens (pathogens) could boost attenuated bacteria to execute specific immune responses against target antigen [60]. Attenuated Lm is currently being employed for vaccine development, treatment and prevention of HPV infection, and disorders associated with it. A live attenuated Lm-based immunotherapy called ADXS11-001 (ADXS-HPV) secretes the antigen-adjuvant fusion protein Lm-LLO-E7 that targets HPV-related cancers [61].
Viral vector vaccines
Viral vectors like adenoviruses (Ad), alphaviruses, adeno-associated viruses, and vaccinia viruses have been extensively investigated due to their high infection effectiveness, high antigen expression, and natural tendency for transduction of genetic material into the host for virus replications. Notably, undesired viral genes are swapped out for foreign genes responsible to encode immunogenic protein expression from pathogens. Then, recombinant viral vectors are allowed to transduce the target cell and express the encoded antigens. In order to prevent HPV16 and HPV18-related diseases, Khan et al. [62] produced two unique replication deficient vaccines called “replication-deficient Ad26 and Ad35 vector vaccines” employing fusion proteins of HPV-E6, E26, and E7. The created vaccine vector formed a viable therapeutic active vaccine immunotherapy (active), as evidenced by their significant therapeutic efficacy in the TC-1 mouse model. In a recent clinical phase-III experiment including 180 male and 1176 female patients, Rosales et al. assessed an MVA-viral vector targeting HPV16-E2 to cure intraepithelial lesion linked to the HPV infections. Direct injections of MVA-E2 virus particles were made into the urethra, uterus, vulva, and anus. All of the male patients and 89.3% of the female patients who received MVA-E2 treatment saw the lesions completely disappear, and no adverse symptoms were noticed. In this study, a control vaccine was not used in the trial. Therefore, the actual effectiveness of the vaccine is still unknown and questionable [63].
Nucleic acid/protein/peptide-based vaccines
DNA-based vaccines
For both human and animal illnesses, DNA-based vaccinations have emerged as a secure substitute for traditional live and inactivated vaccines [64]. The DNA vaccine GX-188E was created in 2014 by Kim et al. [65], expressed the HPV16/18-E6 and E7 antigens as well as the Fms-like tyrosine kinase-3 ligand (Flt3L), to provoke DCs (dendritic cells). To improve immunization, they employed electroporation. The first most effective therapeutic DNA vaccine to date has demonstrated efficacy against CIN2/3 is VGX3100. A phase III trial is currently evaluating VGX-3100's effectiveness, safety, and tolerability in women with CIN2/3 that has been histologically confirmed to be HPV 16/18-positive (NCT03185013).
RNA-based vaccines
The foundation of RNA-based vaccines is the RNA-replicon system developed from non-pathogenic ssRNA (single stranded RNA) viruses with negative or positive polarity [66]. Various RNA viruses, including picornaviruses, measles, lentiviruses, alphaviruses, flaviviruses, rhabdoviruses, and retroviruses, have been modified to serve as vectors. These vectors can expression the targeted protein to control diseases by triggering immune responses [67]. Variety of studies have shown that self-replicating RNA-viral vectors can effectively deliver recombinant heterologous antigens and promote their expression at high levels due to their high autonomous RNA replication capability. They can also induce strong immune responses and protect against challenges from infectious agents and tumor cells. However, RNA-based vaccines are constrained by their poor durability and inability to disseminate between cells [68]. Few clinical trials for RNA-based vaccinations against illnesses linked to HPV have been conducted.
Protein and Peptide-based vaccines
The advantages of these vaccines are safety, stability, storage, and ease of manufacturing [69]. Low immunogenicity and the required MHC-specific drugs matching the patient's HLA haplotypes are major drawbacks [70]. Particular epitope (short) peptides and synthetic long peptides (SLPs) are two categories for peptide-based vaccinations. Prior to injection, the short peptides must be pre-HLA typed as these are limited to the patient's unique HLA types [71]. Seven women with high-grade CIN and HPV16-positive, underwent testing for short peptide-based therapeutic HPV vaccines (CIGB-228) adjuvant with very tiny size proteoliposomes (VSSP). Five individuals experienced lesion regression and HPV eradication as a result of the vaccine-induced IFN-associated T-cell response [72]. SLPs, as opposed to short peptides, need to be digested and presented by APCs, but patient selection with a specific MHC profile is not necessary [73]. PepCan (NCT01653249), a peptide-based vaccine made up of four synthetic HPV16 E6 peptides and a novel adjuvant called Candin, was tested on 24 patients in the dose-escalation phase I clinical research.
Cellular HPV vaccines
Dendritic cell-based vaccines
In physiology and anatomy, it is well known that there are several antigen presenting cells responsible to execute immunological reactions and responses against certain pathogens and external antigens. Among them dendritic cells (DCs) are the excellent target for immunotherapy. These cells have been identified as the most potent cells in in-vivo condition, mediating and generating both innate and adaptive immunological responses. DCs exhibit significant quantities of either co-stimulatory or co-inhibitory molecules and have a strong ability to gather and prepare antigens for presentation to T lymphocytes [74]. Additionally, DCs can act as organic adjuvants to boost the effectiveness of antigen-specific immunotherapy for the treatment of cancer [75]. The two types of DC-based vaccinations are categorized as (a) transduced with DNA or viral vectors responsible to encode foreign antigens and (b) pulsed with HPV-specific protein (or peptide) antigens [76]. Both mature and immature cells can be used to make DCs. It was found that immature types of DCs lack the full T-cell co-stimulatory ability whereas mature DCs were used in the majority of experiments [77]. Thirty-two patients with advanced cervical malignancies were treated with the PIDC pulsed with HPV16 E6 or E7 peptide by Rahma et al., [78].
Adaptive T-cell therapy
The process of adaptive T-cell treatment (ACT) entails the isolation, in vitro growth, and re-infusion of T-cells with the desired specificity and phenotype. With the use of ACT, T cells can be selected and handled appropriately in vitro for immunotherapy, which results in tenfold more antigen-specific T cells than existing vaccine regimens used alone [79]. Nine individuals who had been diagnosed with metastatic CC were investigated for ACT by Stevanovic et al. [80]. Selected HPV-targeted tumor infiltrating T-cells (HPV-TILs) were administered to patients again. Three individuals had objective tumor responses at 22 and 15 months after therapy. There were two full responses in those patients. Recently, Doran et al. treated 12 patients with metastatic HPV-associated epithelial malignancies using autologous genetically modified T cells expressing a T-cell receptor specific for HPV16 E6 [81].
Prophylactic and therapeutic vaccines to control CC
The two vaccines designed to prevent CC are composed of empty VLPs generated by expression systems for recombinant capsid antigen L1 [91, 92]. VLPs do not contain viral DNA and these are non-infectious. Recombinant L1 VLPs strongly resemble authentic papilloma virions in terms of structure and immunogenicity [93]. Recent clinical studies with L1 VLP-based vaccines have primarily revolved around the prevention of cancer associated with the female genital tract, including CC. L1 VLP vaccines may also prevent additional HPV-associated cancers. Although prophylactic vaccines against high-risk HPV types have been highly effective. Unresolved issues may shape the future of CC vaccines. Current vaccines must contain intact L1 VLPs to promote the generation of strong, type-restricted, and neutralizing IgG antibody responses. The minimum antibody titer needed for protection in human is unknown. Though, the importance of antibody responses to prophylactic VLP vaccination has been suggested by studies in preclinical models. A denatured L1 VLP does not induce protection in the cotton tail rabbit papillomavirus or canine oral papillomavirus (COPV) challenge models, suggesting the importance of L1 conformation-dependent antibody responses [94]. Increased COPV L1-specific antibody titers were associated with protection from papillomas, suggesting a certain level of humoral immunity associated with protection against papilloma viruses [95]. Prophylactic VLP-based vaccines do not eliminate pre-existing persistent infections [96]. However, therapeutic vaccination could have an immediate impact in reducing the incidence of CC. L1 and L2 late capsid proteins are not expressed in the basal cells that harbor infection in precancerous tissues or cancerous tissues, [97] suggesting that they are not good targets for therapeutic vaccines [98]. Conversely, E6 and E7 proteins are very promising target proteins for therapeutic vaccines, as these are the only viral proteins constitutively expressed in CC cells and are required to maintain the disease phenotype [99]. Table 3 gives brief description on prophylactic and therapeutic vaccines intended for CC [100]. Although prophylactic HPV vaccines are available in the market whereas HPV remained prevalent globally due to the unevenness of vaccination. Therefore, there is a great demand for effective treatment of established HPV infection, and therapeutic HPV vaccines are expected to become an effective method to treat HPV. In an attempt to eliminate existing HPV infection or lesions, therapeutic vaccines mainly trigger robust cytotoxic T lymphocyte (CTL) responses against antigens that can be continuously expressed in abnormal cells, thereby enhancing the antitumor effect.
Table 3.
Novel prophylactic and therapeutic vaccine strategies for cervical cancer
| Approach | Types | Advantage(s) | Limitation (s) |
|---|---|---|---|
| Peptide | Prophylactic and therapeutic | Safe; easily synthesized and purified | Only contains selected epitopes, therefore can be less effective for a diverse population; small size may reduce immunogenicity |
| Recombinant protein | Prophylactic (L2) and therapeutic | Can induce strong antibodies against L1 or L2 and weak cellular immune responses | Cost, storage, and purification |
| Dendritic cell delivery of novel vaccines | Therapeutic | Direct targeting of vaccine to professional antigen presenting cells | Each immunization is labor intensive and can only be performed in a limited number of facilities |
| DNA | Prophylactic and therapeutic | Inexpensive; easy storage | Has had limited immunogenicity in humans and has the potential to transform cells |
| Recombinant vaccinia virus | Therapeutic | Induces strong and protective cellular immune responses | Could cause disease in immunocompromised individuals |
| Recombinant adenovirus | Prophylactic and therapeutic | Induces strong and protective cellular immune response | Previously existing natural immunity can limit efficacy |
Challenges with HPV vaccinations
CC poses a very critical public health challenge to Sub-Saharan African (SSA) countries, where it is the major cause of female cancer deaths [101]. However, implementing HPV vaccination at this region has been met with stiff challenges ranging from sociocultural belief, logistical difficulties, and lack of proper financing. Notably, the most developed countries have a high uptake of the HPV vaccine, countries like the United States still have many challenges associated with the low uptake of the HPV vaccine [102]. This challenges ranges from misconceptions about HPV and the HPV vaccine and socio-economic status. Myriads of challenges hampering the implementation of HPV vaccination also abounds in the Middle East and North Africa, where there are no organized CC screening systems in place [103]. Thus, it results in CC cases being diagnosed at advanced stages of the diseases. Other factors hampering the implementation of HPV vaccination include cultural and religious sensitivities, weak health systems and infrastructures, political instability, financial constraints, and limited public health funding amid competing priorities. In Europe, challenges with HPV vaccination uptake were associated with concerns about the long-term effectiveness and possible side effects of the vaccine [104]. Despite these myriads of issues and challenges, studies have shown that education can be used as a tool towards influencing the acceptance of the HPV vaccine. In addition, the provision of the right information to clients by healthcare workers would go a long way in helping to improv the acceptance of HPV vaccination [105]. Massive Public Health education on issues relating to CC and its causal relationship with HPV is needed towards the successful implementation of the HPV Vaccination programs among all age groups. Governments needs to invest more into this primary preventive initiative, and donor agencies and supporting partners all need to forge a formidable front at combating the menace of this deadly disease especially in Low and Middle-income countries. International efforts at mitigating the impact of this disease through the Global Alliance for Vaccine and Immunization (GAVI Alliance) are also strongly encouraged. Large pharmaceutical companies producing these vaccines could also be supported towards subsidizing the price of these vaccines to making them more affordable to poorer countries. The involvement of the men folks as active partners in the advocacy for HPV vaccination initiatives is also strongly advocated [106].
Current landscape and future perspectives
Phase III worldwide studies are being carried out by significant pharmaceutical companies, who are close to receiving regulatory approval for their preventative vaccinations. The first generation of preventive vaccinations is almost finished and there is still room for an enhanced second generation of vaccines to addresses some of the first generation's flaws (production and distribution costs). Various implementation-related concerns, such as (a) who should receive the vaccines, (b) at what time and age, (c) what about the length of protection, and (d) how to coordinate them with present screening and various pathogen related immunization programs. These concerns have yet to be answered for the vaccines entered in the final phases of development. There is little expectation that any current therapeutic vaccine will have a significant impact on public-health in future. However, there are several therapeutically potential vaccines to use various delivery systems (tested or under ongoing clinical trials). A pan-oncogenic vaccination with both therapeutic and preventative potential would be ideal. The WHO Strategic Advisory Group of Experts on Immunization (SAGE) met from 4–7 April, 2022 to review the mounting scientific evidence showing single-dose schedules are just as effective as two- or three-dose regimens. The Human Papillomavirus (HPV) vaccine offers reliable protection against HPV and it is comparable to 2-dose schedules, as per SAGE's evaluation. Increasing the number of girls who can receive the potentially life-saving vaccine, might be game changer in terms of disease prevention. CC, often referred to as the "silent killer," is a disease of unequal access. The new SAGE recommendation is supported by worries over the poor HPV vaccine access into the immunization programs and low population covered in less developed and poor countries. CC is almost entirely preventable. CC, the fourth most frequent type of cancer in women worldwide with 90% of these women living in low- and middle-income countries, is caused by sexually transmitted HPV in more than 95% of cases [107]. CC vaccine produced in India (Serum Institute of India) “Ceravac” is an in-house manufactured vaccine for CC brought on by the human papillomavirus (HPV), will cost between Rs 200 and 400 per dosage and it may be available within few months. Depending upon age, the vaccination is administered in a two- or three-dose course. Currently, there are two foreign-produced HPV vaccinations on the private market: Cervarix by Glaxo Smithkline and Gardasil by Merck. Serum India's arrival is anticipated to lower prices for HPV vaccines. 483.5 million Indian women over the age of 15 are at risk for developing CC. According to current figures, 123,907 women are given a CC diagnosis annually, and 77,348 of them passed away from the condition. In India, CC is the second most common malignancy in women. CC should be included in the National Immunization Mission, according to the standing technical subcommittee of the National Technical Advisory Group on Immunization (NTAGI) (NIM) [108]. Even while the progress being made on HPV vaccines, it is encouraging. Women who had HPV infection before vaccine administration or post vaccination, should continue identification and routine screening [109]. Additional study is required to determine the duration of protection brought on by these vaccinations, the necessity of booster shots, and the impact on occurrence and prevalence of the HPV types covered by the vaccines. Additional information regarding various HPV vaccine types for various populations, safety and pregnancy outcomes, immunogenicity of concurrent administration with other vaccines, and other information are needed to be considered. After the current common varieties of HPV, further research must be performed to investigate the effectiveness in patients (both sexes) with age ˃ 26 years, the function of regular HPV vaccination in men to prevent the genital warts using additional, and rarer HPV types. Future research is needed to determine CC screening methods, safe sexual behavior, and more economic analysis. The target group for vaccination will be prepubescent females between the ages of 9 and 10 because vaccines will be effective as a preventative before viral exposure. However, this will cause cultural and societal problems. Epidemiological research on the logistics, long-term effectiveness, and economics of widespread HPV-vaccination (women) are urgently needed in nations like India. Various vaccine delivery systems such as different routes of immunization and physical/chemical delivery methods have been used in cancer therapy with the goal to induce immunity against tumor-associated antigens. Two basic delivery approaches including physical delivery to achieve higher levels of antigen production and formulation with microparticles to target antigen-presenting cells (APCs) have demonstrated to be effective in animal models. New developments in vaccine delivery systems will improve the efficiency of clinical trials in the near future. Among them, nanoparticles (NPs) such as dendrimers, polymeric NPs, metallic NPs, magnetic NPs and quantum dots have emerged as effective vaccine adjuvants for infectious diseases and cancer therapy [110].
Conclusion
CC is the most deadly, life threatening, and lethal cancer in women globally and a serious concern for health care personnel particularly in poor or less developed countries. These counties have poor access to drugs and vaccines. Comparing vaccines with chemotherapy, immunotherapy using specific vaccines at the right dose is relatively beneficial and patient friendly. Various approaches have been used to achieve simplicity, scalability, translation to industrial production, effectiveness and economic burden. Despite several benefits of these vaccines, there were still limitations and gaps for further investigation and research at the preclinical and clinical stage. Luckily, the developed prophylactic HPV-vaccines successfully saved lives by treating HPV infected patients in the last ten years after HPV vaccine introduction. It was estimated that about 95% of those who received the vaccine demonstrated high safety and efficacy in preventing persistent infections of precancerous lesions. The widespread use is constrained by a number of restrictions, including the limited HPV types for prevention and expensive manufacturing. Preclinical or clinical trials for a variety of HPV L2-based and second-generation preventive vaccinations are currently being conducted. Additionally, the approved HPV-vaccines are unable to execute effectively in patients already infected with HPV and develop the accompanying cancers. Therefore, therapeutic HPV-vaccines are urgently needed to minimize the incidence of CC in these infected patients. Research on the immune evasion mechanism and the cancer microenvironment is still largely undefined. In our opinion, the development of successful vaccines in the future will be facilitated by initiatives to improve vaccine design, including the use of effective adjuvants, as more knowledge about the immune mechanisms against HPV infection becomes available. Although many of the novel vaccines described in this review are reported to be immunogenic in humans, it may be necessary to continue developing novel adjuvants to establish clinical efficacy.
Acknowledgements
Thankful to BIT, Meerut for helping in the drafting of this manuscript.
Funding
None.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Human Papillomavirus (HPV) and Cervical Cancer, https://www.who.int/en/ news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer [accessed on Sep 02, 2022].
- 2.Human papillomavirus vaccines WHO position paper, May 2017- recommendations. Vaccine. 2017;35:5753–5755. doi: 10.1016/j.vaccine.2017.05.069. [DOI] [PubMed] [Google Scholar]
- 3.Biswala BM, Singh KKB, Ismail MB, Jalal MIBA, Safruddin EISBE. Current concept of bacterial vaginosis in cervical cancer. J Clin Gynecol Obstet. 2014;3(1):1–7. [Google Scholar]
- 4.Harwich MD, Serrano MG, Fettweis JM, Alves JM, Reimers MA, Buck GA, Jefferson KK. Genomic sequence analysis and characterization of Sneathia amnii sp. nov. BMC Genomics. 2012;13(8):S4. doi: 10.1186/1471-2164-13-s8-s4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashique S, Upadhyay A, Kumar N, Chauhan S, Mishra N. Metabolic syndromes responsible for cervical cancer and advancement of nanocarriers for efficient targeted drug delivery-A review. Adv Cancer Biol-Metast. 2022; 100041.
- 6.Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci. 2006;110:525–541. doi: 10.1042/CS20050369. [DOI] [PubMed] [Google Scholar]
- 7.De Villiers EM, Fauquet C, Broker TR. Bernard HU. Zur Hausen H. Classification of papillomaviruses. Virol. 2004; 324: 17–27 [DOI] [PubMed]
- 8.Clifford GM, Smith JS, Aguado T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer. 2003;89:101–105. doi: 10.1038/sj.bjc.6601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braaten P, Laufer MR. Human papillomavirus (HPV), HPV-related disease, and the HPV vaccine. Rev Obstet Gynecol. 2008;1:2–10. [PMC free article] [PubMed] [Google Scholar]
- 10.Shulzhenko N, Lyng H, Sanson GF, Morgun A. Mé nage à trois: An evolutionary interplay between human papillomavirus, a tumor, and a woman. Trends Microbiol. 2014;22:345–353. doi: 10.1016/j.tim.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Anahtar MN, Byrne EH, Doherty KE, Bowman BA, Yamamoto HS, Soumillon M, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity. 2015;42:965–976. doi: 10.1016/j.immuni.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gosmann C, Anahtar MN, Handley SA, Farcasanu M, Abu-Ali G, Bowman BA, et al. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity. 2017;46:29–37. doi: 10.1016/j.immuni.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Audirac-Chalifour A, Torres-Poveda K, Bahena-Román M, Téllez-Sosa J, Martínez-Barnetche J, Cortina-Ceballos B, et al. Cervical microbiome and cytokine profile at various stages of cervical cancer: a pilot study. PLoS ONE. 2016;11:e0153274. doi: 10.1371/journal.pone.0153274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci. 2011;108:4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White BA, Creedon DJ, Nelson KE, Wilson BA. The vaginal microbiome in health and disease. Trends Endocrinol Metab. 2011;22:389–393. doi: 10.1016/j.tem.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van de Wijgert JHHM, Jespers V. The global health impact of vaginal dysbiosis. Res Microbiol. 2017;168:859–864. doi: 10.1016/j.resmic.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Harper DM, DeMars LR. HPV vaccines - a review of the first decade. Gynecol Oncol. 2017;146:196–204. doi: 10.1016/j.ygyno.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Bruni L, Diaz M, Barrionuevo-Rosas L, Herrero R, Bray F, Bosch FX, et al. Castellsague, global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;4:e453–463. doi: 10.1016/S2214-109X(16)30099-7. [DOI] [PubMed] [Google Scholar]
- 19.Hancock G, Hellner K, Dorrell L. Therapeutic HPV vaccines, best practice & research. Clin Obstet Gynaecol. 2018;47:59–72. doi: 10.1016/j.bpobgyn.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Singhal T. Indian Academy of Pediatrics Committee on Immunisation (IAPCOI) - consensus recommendations on immunization 2008. Indian Pediatr. 2008;45:635–648. [PubMed] [Google Scholar]
- 21.Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet. 2004;364:249–256. doi: 10.1016/S0140-6736(04)16674-9. [DOI] [PubMed] [Google Scholar]
- 22.Kitchener, H., M. Almonte, and P. Wheeler. The ARTISTIC trial of HPV testing in primary cervical screening: methods and baseline data. In 22nd Internation Papillomavirus Conference. 2005; 5
- 23.Cuzick J, Szarewski A, Cubie H. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet. 2003;362:1871–1876. doi: 10.1016/S0140-6736(03)14955-0. [DOI] [PubMed] [Google Scholar]
- 24.Frazer IH. Prevention of cervical cancer through papillomavirus vaccination. Nat Rev Immunol. 2004;4:46–54. doi: 10.1038/nri1260. [DOI] [PubMed] [Google Scholar]
- 25.Kadish AS, Einstein MH. Vaccine strategies for human papillomavirus-associated cancers. Current Opinion Oncol. 2005;17(5):456–461. doi: 10.1097/01.cco.0000174038.92526.29. [DOI] [PubMed] [Google Scholar]
- 26.Stern PL. Immune control of human papillomavirus (HPV) associated anogenital disease and potential for vaccination. J Clin Virol. 2005;32:72–81. doi: 10.1016/j.jcv.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Burger EA, Smith MA, Killen J, Sy S, Simms KT, Canfell K, et al. Projected time to elimination of cervical cancer in the USA: a comparative modelling study. Lancet Public Health. 2020;5(4):213–222. doi: 10.1016/S2468-2667(20)30006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Machalek DA, Garland SM, Brotherton JML, Bateson D, McNamee K, Stewart M, et al. Very low prevalence of vaccine human papillomavirus types among 18- to 35-year old Australian women 9 years following implementation of vaccination. J Infect Dis. 2018;217:1590–1600. doi: 10.1093/infdis/jiy075. [DOI] [PubMed] [Google Scholar]
- 29.Flagg EW, Torrone EA. Declines in anogenital warts among age groups most likely to be impacted by human papillomavirus vaccination, United States, 2006–2014. Am J Public Health. 2018;108:112–119. doi: 10.2105/AJPH.2017.304119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClung NM, Gargano JW, Bennett NM, Niccolai LM, Abdullah N, Griffn MR, et al. Trends in human papillomavirus vaccine types 16 and 18 in cervical precancers, 2008–2014. Cancer Epidemiol Biomark Prev. 2019;3:602–609. doi: 10.1158/1055-9965.EPI-18-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehanna H, Bryant TS, Babrah J, Louie K, Bryant JL, Spruce RJ, et al. Human papillomavirus (HPV) vaccine effectiveness and potential herd immunity for reducing oncogenic oropharyngeal HPV-16 prevalence in the United Kingdom: a cross-sectional study. Clin Infect Dis. 2019;8:1296–1302. doi: 10.1093/cid/ciy1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graham SV. The human papillomavirus replication cycle, and its links to cancer progression: a comprehensive review. Clin Sci. 2017;17:2201–2221. doi: 10.1042/CS20160786. [DOI] [PubMed] [Google Scholar]
- 33.Doorbar J, Egawa N, Griffin H, Kranjec C, Murakami I. Human papillomavirus molecular biology and disease association. Rev Med Virol. 2015;25:2–23. doi: 10.1002/rmv.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doorbar J, Quint W, Banks L. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30:55–70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 35.Albert E, Laimins L. Regulation of the human papillomavirus life cycle by DNA damage repair pathways and epigenetic factors. Viruses. 2020;7:744. doi: 10.3390/v12070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mac M, Moody CA. Epigenetic regulation of the human papillomavirus life cycle. Pathogens. 2020;6:744. doi: 10.3390/pathogens9060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burley M, Roberts S, Parish JL. Epigenetic regulation of human papillomavirus transcription in the productive virus life cycle. Semin Immunopathol. 2020;2:159–171. doi: 10.1007/s00281-019-00773-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang CM. Human Papillomavirus and vaccination. Mayo Clin Proc. 2008;83:701–707. doi: 10.1016/S0025-6196(11)60898-7. [DOI] [PubMed] [Google Scholar]
- 39.Winer RL, Hughes JP, Feng Q, O’Reilly S, Kiviat NB, Holmes KK, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354:2645–2654. doi: 10.1056/NEJMoa053284. [DOI] [PubMed] [Google Scholar]
- 40.Ferenczy A, Franco E. Cervical-cancer screening beyond the year 2000. Lancet Oncol. 2001;2:27–32. doi: 10.1016/S1470-2045(00)00192-3. [DOI] [PubMed] [Google Scholar]
- 41.Schwarz TF, Kocken M, Petaja T, Einstein TM, Spaczynski M, Louwers JA, et al. Correlation between levels of human papillomavirus (HPV)-16 and 18 antibodies in serum and cervicovaginal secretions in girls and women vaccinated with the HPV-16/18 AS04-adjuvanted vaccine. Hum Vaccine. 2010;6:1054–1061. doi: 10.4161/hv.6.12.13399. [DOI] [PubMed] [Google Scholar]
- 42.Gardasil, https://www.fda.gov/vaccines-blood-biologics/vaccines/gardasil [accessed on Sep 02, 2022].
- 43.Gardasil, 9 https://www.fda.gov/vaccines-blood-biologics/vaccines/gardasil-9 [accessed on Sep 02, 2022].
- 44.Crosbie EJ, Kitchener HC. Cervarix–a bivalent L1 virus-like particle vaccine for prevention of human papillomavirus type 16- and 18-associated cervical cancer. Expert Opin Biol Ther. 2007;7:391–396. doi: 10.1517/14712598.7.3.391. [DOI] [PubMed] [Google Scholar]
- 45.Szarewski A. HPV vaccine: cervarix. Expert Opin Biol Ther. 2010;10:477–487. doi: 10.1517/14712591003601944. [DOI] [PubMed] [Google Scholar]
- 46.Cervarix, https://www.fda.gov/vaccines-blood-biologics/vaccines/cervarix [accessed on Sep 02, 2022]
- 47.Buck CB, Day PM, Trus BL. The papillomavirus major capsid protein L1. Virol. 2013;445:169–174. doi: 10.1016/j.virol.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, et al. C.V.T.V. Group, Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Nat Cancer Inst. 2011; 103: 1444–1451. [DOI] [PMC free article] [PubMed]
- 49.Leung TF, Liu AP, Lim FS, Thollot F, Oh HML, Lee BW, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and 4vHPV vaccine administered according to two- or three-dose schedules in girls aged 9–14years: results to month 36 from a randomized trial. Vaccine. 2018;36:98–106. doi: 10.1016/j.vaccine.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 50.Jit M, Brisson M, Laprise JF, Choi YH. Comparison of two dose and three dose human papillomavirus vaccine schedules: cost effectiveness analysis based on transmission model. BMJ. 2015;350:7584. doi: 10.1136/bmj.g7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organization Electronic address, human papillomavirus vaccines: WHO position paper, May 2017-recommendations. Vaccine. 2017;35:5753–5755. doi: 10.1016/j.vaccine.2017.05.069. [DOI] [PubMed] [Google Scholar]
- 52.Garland SM, Kjaer SK, Munoz N, Block SL, Brown DR, DiNubile MJ, et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 Years of real-world experience. Clin Infect Dis Off Publ Infect Dis Soc Am. 2016;63:519–527. doi: 10.1093/cid/ciw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Human Papillomavirus (HPV) Vaccine Safety, https://www.cdc.gov/ vaccinesafety/vaccines/hpv-vaccine.html.
- 54.Bonde U, Joergensen JS, Lamont RF, Mogensen O. Is HPV vaccination in pregnancy safe? Hum Vaccines Immunother. 2016;12:1960–1964. doi: 10.1080/21645515.2016.1160178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kharbanda EO, Vazquez-Benitez G, Lipkind HS, Sheth SS, Zhu J, Naleway AL, et al. Risk of spontaneous abortion after inadvertent human papillomavirus vaccination in pregnancy. Obstet Gynecol. 2018;132:35–44. doi: 10.1097/AOG.0000000000002694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.https://www.clinicaltrials.gov/ct2/results?cond=Cervical+cancer%2C+vaccines&term=HPV&cntry=&state=&city=&dist [accessed on Sep 02, 2022]
- 57.Medina E, Guzman CA. Use of live bacterial vaccine vectors for antigen delivery: potential and limitations. Vaccine. 2001;19:1573–1580. doi: 10.1016/S0264-410X(00)00354-6. [DOI] [PubMed] [Google Scholar]
- 58.Saxena M, Van TT, Baird FJ, Coloe PJ, Smooker PM. Pre-existing immunity against vaccine vectors–friend or foe? Microbiology (Read) 2013;159:1–11. doi: 10.1099/mic.0.049601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dietrich G, Spreng S, Favre D, Viret JF, Guzman CA. Live attenuated bacteria as vectors to deliver plasmid DNA vaccines. Curr Opin Mol Ther. 2003;5:10–19. [PubMed] [Google Scholar]
- 60.Schoen C, Loeffler DI, Frentzen A, Pilgrim S, Goebel W, Stritzker J. Listeria monocytogenes as novel carrier system for the development of live vaccines. Int J Med Microbiol. 2008;298:45–58. doi: 10.1016/j.ijmm.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 61.Wallecha A, French C, Petit R, Singh R, Amin A, Rothman J. Lm-LLO-based immunotherapies and HPV-associated disease. J Oncol. 2012; 542851. [DOI] [PMC free article] [PubMed]
- 62.Khan S, Oosterhuis K, Wunderlich K, Bunnik EM, Bhaggoe M, Boedhoe S, et al. Development of a replication-deficient adenoviral vector-based vaccine candidate for the interception of HPV16- and HPV18-induced infections and disease. Int J Cancer. 2017;141:393–404. doi: 10.1002/ijc.30679. [DOI] [PubMed] [Google Scholar]
- 63.Rosales R, Lopez-Contreras M, Rosales C, Magallanes-Molina JR, GonzalezVergara R, Arroyo-Cazarez JM, et al. Villarreal, regression of human papillomavirus intraepithelial lesions is induced by MVA E2 therapeutic vaccine. Hum Gene Ther. 2014;25:1035–1049. doi: 10.1089/hum.2014.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Delany I, Rappuoli R, De Gregorio E. Vaccines for the 21st century. EMBO Mol Med. 2014;6:708–720. doi: 10.1002/emmm.201403876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim TJ, Jin HT, Hur SY, Yang HG, Seo YB, Hong SR, et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat Commun. 2014;5:5317. doi: 10.1038/ncomms6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Afrough B, Dowall S, Hewson R. Emerging viruses and current strategies for vaccine intervention. Clin Exp Immunol. 2019;196:157–166. doi: 10.1111/cei.13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lundstrom K. RNA viruses as tools in gene therapy and vaccine development. Genes. 2019;3:189. doi: 10.3390/genes10030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Varnavski AN, Young PR, Khromykh AA. Stable high-level expression of heterologous genes in vitro and in vivo by noncytopathic DNA-based Kunjin virus replicon vectors. J Virol. 2000;74:4394–4403. doi: 10.1128/JVI.74.9.4394-4403.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hung CF, Wu TC, Monie A, Roden R. Antigen-specific immunotherapy of cervical and ovarian cancer. Immunol Rev. 2008;222:43–69. doi: 10.1111/j.1600-065X.2008.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paris R, Bejrachandra S, Thongcharoen P, Nitayaphan S, Pitisuttithum P, Sambor A, et al. HLA class II restriction of HIV-1 clade-specific neutralizing antibody responses in ethnic Thai recipients of the RV144 prime-boost vaccine combination of ALVAC-HIV and AIDSVAX((R)) B/E. Vaccine. 2012;30:832–836. doi: 10.1016/j.vaccine.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 71.Vici P, Pizzuti L, Mariani L, Zampa G, Santini D, Di Lauro L, et al. Targeting immune response with therapeutic vaccines in premalignant lesions and cervical cancer: hope or reality from clinical studies. Expert Rev Vaccines. 2016;15:1327–2133. doi: 10.1080/14760584.2016.1176533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Solares AM, Baladron I, Ramos T, Valenzuela C, Borbon Z, Fanjull S, et al. Safety and immunogenicity of a human papillomavirus peptide vaccine (CIGB-228) in women with high-grade cervical intraepithelial neoplasia: first-in-human, proof-of-concept trial. ISRN Obstet Gynecol 2011; 292951. [DOI] [PMC free article] [PubMed]
- 73.Sercarz EE, Maverakis E. MHC-guided processing: binding of large antigen fragments. Nat Rev Immunol. 2003;3:621–629. doi: 10.1038/nri1149. [DOI] [PubMed] [Google Scholar]
- 74.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 75.Santin AD, Bellone S, Roman JJ, Burnett A, Cannon MJ, Pecorelli S. Therapeutic vaccines for cervical cancer: dendritic cell-based immunotherapy. Curr Pharmaceut Des. 2005;11:3485–3500. doi: 10.2174/138161205774414565. [DOI] [PubMed] [Google Scholar]
- 76.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 78.Rahma OE, Herrin VE, Ibrahim RA, Toubaji A, Bernstein S, Dakheel O, et al. Pre-immature dendritic cells (PIDC) pulsed with HPV16 E6 or E7 peptide are capable of eliciting specific immune response in patients with advanced cervical cancer. J Transl Med. 2014;12:353. doi: 10.1186/s12967-014-0353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zsiros E, Tsuji T, Odunsi T. Adoptive T-cell therapy is a promising salvage approach for advanced or recurrent metastatic cervical cancer. J Clin Oncol. 2015;33:1521–1522. doi: 10.1200/JCO.2014.60.6566. [DOI] [PubMed] [Google Scholar]
- 80.Stevanovic S, Draper LM, Langhan MM, Campbell TE, Kwong ML, Wunderlich JR, et al. Hinrichs, Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol. 2015;33:1543–1550. doi: 10.1200/JCO.2014.58.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Doran SL, Stevanovic S, Adhikary S, Gartner JJ, Jia L, Kwong MLM, et al. Hinrichs, Tcell receptor gene therapy for human papillomavirus-associated epithelial cancers: a first-in-human, phase I/II study. J Clin Oncol. 2019;37:2759–2768. doi: 10.1200/JCO.18.02424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith KL, Tristram A, Gallagher KM. Epitope specificity and longevity of a vaccine-induced human T cell response against HPV18. Int Immunol. 2005;17:167–176. doi: 10.1093/intimm/dxh197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hallez S, Simon P, Maudoux F. Phase I/II trial of immunogenicity of a human papillomavirus (HPV) type 16 E7 proteinbased vaccine in women with oncogenic HPV-positive cervical intraepithelial neoplasia. Cancer Immunol Immunother. 2004;53:642–650. doi: 10.1007/s00262-004-0501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davidson EJ, Boswell CM, Sehr P. Immunological and clinical responses in women with vulval intraepithelial neoplasia vaccinated with a vaccinia virus encoding human papillomavirus 16/18 oncoproteins. Cancer Res. 2003;63:6032–6041. [PubMed] [Google Scholar]
- 85.Ressing ME, van Driel WJ, Brandt RM. Detection of T helper responses, but not of human papillomavirus-specific cytotoxic T lymphocyte responses, after peptide vaccination of patients with cervical carcinoma. J Immunother. 2000;23:255–266. doi: 10.1097/00002371-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 86.Muderspach L, Wilczynski S, Roman L. A phase I trial of a human papillomavirus (HPV) peptide vaccine for women with highgrade cervical and vulvar intraepithelial neoplasia who are HPV 16 positive. Clin Cancer Res. 2000;6:3406–3416. [PubMed] [Google Scholar]
- 87.de Jong A, O’Neill T, Khan AY. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine. 2000;20:3456–3464. doi: 10.1016/S0264-410X(02)00350-X. [DOI] [PubMed] [Google Scholar]
- 88.Smyth LJ, Van Poelgeest MI, Davidson EJ. Immunological responses in women with human papillomavirus type 16 (HPV-16)- associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. Clin Cancer Res. 2004;10:2954–2961. doi: 10.1158/1078-0432.CCR-03-0703. [DOI] [PubMed] [Google Scholar]
- 89.Garcia F, Petry KU, Muderspach L. ZYC101a for treatment of high grade intraepithelial neoplasia. A randomised controlled trial. Obstet Gynecol. 2004;103:317–326. doi: 10.1097/01.AOG.0000110246.93627.17. [DOI] [PubMed] [Google Scholar]
- 90.Ferrara A, Nonn M, Sehr P. Dendritic cell-based tumor vaccine for cervcal cancer II: results of a clinical pilot study in 15 individual patients. J Cancer Res Clin Oncol. 2003;129:521–530. doi: 10.1007/s00432-003-0463-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomized control trial. Lancet. 2006;9518:1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 92.Bryan JT. Developing an HPV vaccine to prevent cervical cancer and genital warts. Vaccine. 2007;16:3001–3006. doi: 10.1016/j.vaccine.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 93.Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/ aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24:5937–5949. doi: 10.1016/j.vaccine.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 94.Suzich JA, Ghim SJ, Palmer-Hill FJ, White WI, Tamura JK, Bell JA, et al. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci USA. 1995;25:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, et al. Immunization with virus like particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;6:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.FUTURE, II. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007; 19:1915–27. [DOI] [PubMed]
- 97.Brinkman JA, Caffrey AS, Muderspach LI, Roman LD, Kast WM. The impact of anti HPV vaccination on cervical cancer incidence and HPV induced cervical lesions: consequences for clinical management. Europ J Gynecol Oncol. 2005;2:129–142. [PubMed] [Google Scholar]
- 98.Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, et al. Effect of human papillomavirus 16/18 L1 virus-like particle vaccine among young women with pre-existing infection: a randomized trial. JAMA. 2007;7:743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 99.Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;4:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huh WK, Roden RB. The future of vaccines for cervical cancer. Gynecol Oncol. 2008;2:S48–56. doi: 10.1016/j.ygyno.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Black E, Richmond R. Prevention of cervical cancer in Subsaharan Africa: the advantage and challenges of HPV vaccination. Vaccines. 2000;3:61. doi: 10.3390/vaccines6030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Strohl AE, Mendoza G, Ghant MS. Barriers to prevention: knowledge of HPV, cervical cancer, and HPV vaccination among African American women. Am J Obstet Gynecol. 2015;1:65.e5. doi: 10.1016/j.ajog.2014.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McGhee E, Harper AUH, Baker M. Elimination of cancer health disparities through the acceleration of HPV vaccines and vaccination: a simplified version of the president’s cancer panel report on HPV vaccinations. J Vaccines Vaccin. 2017;8(3):361. doi: 10.4172/2157-7560.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baghi HB, Yousefi B, Oskouee MA. HPV vaccinations: a Middle Eastern and North African Dilemma. Lancet Infect Dis. 2017;1:18–19. doi: 10.1016/S1473-3099(16)30553-9. [DOI] [PubMed] [Google Scholar]
- 105.Korfage IJ, Essink-Bot ML, Daamen R. Women show mixed intentions regarding the uptake of HPV vaccinations in pre-adolescent: a questionnaire study. Eur J Cancer. 2008;9:1186–1192. doi: 10.1016/j.ejca.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 106.Lee KN, Chang KHJ, Cho SS. Attitudes regarding HPV vaccinations of children among mothers with adolescent daughters in Korea. J Korean Med Sci. 2017;32(1):130–134. doi: 10.3346/jkms.2017.32.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.https://www.who.int/news/item/11-04-2022-one-dose-human-papillomavirus-(hpv)-vaccine-offers-solid-protection-against-cervical-cancer [accessed on Sep 02, 2022]
- 108.https://www.business-standard.com/article/current-affairs/home-made-serum-institute-s-hpv-vaccine-to-cost-between-rs-200-400-per-dose-122090100955_1.html [accessed on Sep 02, 2022].
- 109.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the advisory committee on immunization practices (ACIP) MMWR Recomm Rep. 2007;56:1–24. [PubMed] [Google Scholar]
- 110.Ashique S, Sandhu NK, Chawla V, Chawla PA. Targeted drug delivery: trends and perspectives. Current Drug Deliv. 2021;10:1435–1455. doi: 10.2174/1567201818666210609161301. [DOI] [PubMed] [Google Scholar]