Abstract
Background
Individuals with paraplegia and coexisting trunk and postural control deficits rely on their upper extremities for function, which increases the risk of shoulder pain. A multifactorial etiology of shoulder pain includes “impingement” of the supraspinatus, infraspinatus, long head of the biceps tendons, and/or subacromial bursa resulting from anatomic abnormalities, intratendinous degeneration, and altered scapulothoracic kinematics and muscle activation. Targeting serratus anterior (SA) and lower trapezius (LT) activation during exercise, as part of a comprehensive plan, minimizes impingement risk by maintaining optimal shoulder alignment and kinematics during functional activities. To prevent excessive scapular upward translation, minimizing upper trapezius (UT) to SA and LT activation is also important.
Objectives
To determine which exercises (1) maximally activate SA and minimize UT:SA ratio and (2) maximally activate LT and minimize UT:LT ratio.
Methods
Kinematic and muscle activation data were captured from 10 individuals with paraplegia during four exercises: “T,” scaption (sitting), dynamic hug, and SA punch (supine). Means and ratios were normalized by percent maximum voluntary isometric contraction (MVIC) for each muscle. One-way repeated measures analysis of variance determined significant differences in muscle activation between exercises.
Results
Exercises were rank ordered: (1) maximum SA activation: SA punch, scaption, dynamic hug, “T”; (2) maximum LT activation: “T,” scaption, dynamic hug, SA punch; 3) minimum UT:SA ratio: SA punch, dynamic hug, scaption, “T”; and (4) minimum UT:LT ratio: SA punch, dynamic hug, “T,” scaption. Exercise elicited statistically significant changes in percent MVIC and ratios. Post hoc analyses revealed multiple significant differences between exercises (p < .05).
Conclusion
SA punch produced the greatest SA activation and lowest ratios. Dynamic hug also produced optimal ratios, suggesting supine exercises minimize UT activation more effectively. To isolate SA activation, individuals with impaired trunk control may want to initiate strengthening exercises in supine. Participants maximally activated the LT, but they were not able to minimize UT while upright.
Keywords: exercise, paraplegia, serratus anterior, surface electromyography, trapezius
Individuals with paraplegia rely on their upper extremities for essential activities of daily living, including reaching overhead from a seated position, bed mobility, wheelchair propulsion, and transferring. The reliance on the upper extremities is hindered by musculoskeletal shoulder pain, a common secondary complaint following spinal cord injury (SCI).1 In individuals with paraplegia, prevalence of shoulder pain ranges up to 66%.2–6 Per Alm et al.,4 92% of individuals with paraplegia reported no shoulder pain before becoming a wheelchair user, whereas 67% reported a history of shoulder pain since becoming a wheelchair user. Shoulder pain is commonly attributed to chronic rotator cuff impingement syndromes and tears.2,3 Akbar et al.7 reported a 10-fold higher risk of rotator cuff rupture in individuals with long-term paraplegia than in age-matched controls.
Musculoskeletal pain is the most common type of pain following SCI8 and frequently affects the shoulder joint.9 Although risk factors associated with musculoskeletal shoulder pain have been studied in SCI, there is a dearth of SCI literature regarding the specific biomechanical etiology of this pain. Therefore, using best evidence as informed by non-SCI literature, a multifactorial etiology of shoulder pain from “impingement” includes anatomic abnormalities, posterior capsule or pectoralis minor tightness, and altered scapulothoracic kinematics and muscle activation. In non-weightbearing reaching activities, expected scapulothoracic joint motion during humerothoracic elevation in healthy shoulders includes upward rotation, posterior tilt, and, although there is variability between studies and planes of motion, external rotation.10,11 Simultaneously, the humerus laterally rotates between 20 and 120 degrees of elevation, increasing subacromial space and decreasing impingement risk.10 Prior investigations demonstrated that individuals with subacromial impingement syndrome have altered kinematics, including decreased scapular posterior tilt,12–16 external rotation,12,17–19 and upward rotation,12,14–15,20 and increased scapular elevation13 during arm elevation tasks. In weightbearing weight-relief raises, bed mobility, and transfer activities, individuals with SCI must also rely on reverse actions to lift the trunk and pelvis relative to a fixed scapula and humerus. Controlled motion during these newly acquired skills can be particularly challenging when performed by individuals with balance deficits.
Muscle activation guides glenohumeral and scapular kinematics. The serratus anterior (SA), referred to as the “prime mover” of the scapula, contributes to all components of optimal scapular motion (posterior tilt, external and upward rotation) with respect to the thorax during non-weightbearing arm elevation.21 The lower trapezius (LT) is also responsible for external and upward rotation, although not posterior tilt. Collectively, the SA, LT, and upper trapezius (UT) position the glenoid to maximize glenohumeral elevation. However, without balanced activation of the rotator cuff and other scapulothoracic musculature, the UT can contribute to unwanted scapular upward translation with an excessive “shoulder-shrug” during humeral elevation, as demonstrated in individuals with impingement or rotator cuff damage.22 Although variable in the literature, a reduction in SA,12–13,15,23 LT,23,24 and/or excessive UT12,23,25 activation has been observed in individuals with shoulder pain and symptoms of impingement. Muscles also work synergistically and can be depicted as ratios (UT:SA, UT:LT) with the goal to minimize excessive UT activation with respect to SA and LT. For example, Michener et al.26 reported that individuals with subacromial impingement syndrome had a higher UT:LT ratio during arm elevation. In weightbearing weight-relief raises, bed mobility, and transfer activities, the thoracohumeral depressor muscles, including the latissimus dorsi and pectoralis major, are also responsible for elevating the trunk and pelvis, protecting the rotator cuff from impingement. Although not the focus of this article, deficient thoracohumeral depressor muscles have been linked to impingement and resultant shoulder pain in this population.
Addressing the causes of shoulder pain is complicated and requires a comprehensive approach. Targeted exercise is an important element of this multifaceted rehabilitation program along with seating and postural improvements, glenohumeral exercises, and activity selection and performance. Home exercise programs varying from a scapula-focused exercise program27 to hypertrophy and endurance exercises28 to a high-dose scapular stabilizer and rotator cuff strengthening program29 have resulted in shoulder pain reduction up to 30% as measured by the Wheelchair User’s Shoulder Pain Index (WUSPI).
Although previous SCI interventions have reduced shoulder pain, there is room for improvement as pain is not consistently eliminated.27–29 Instead of muscle-specific strengthening purposefully guiding optimal shoulder kinematics, many investigations rely on global strengthening.28–30 Additionally, there is an absence of biomechanical analysis to verify that specific musculature is effectively targeted during selected exercises in individuals with paraplegia.28–30 Alternatively, exercises can be selected that target specific musculature, either by decreasing UT and/or increasing SA or LT activation. These muscles can be represented collectively as ratios, UT:SA and UT:LT. Recall that individuals with paraplegia, seated at a wheelchair level, by necessity overuse their upper extremities for essential functional activities. Overuse occurs during non-weightbearing reaching overhead from the seated position and during weightbearing to lift the body to and from various surfaces including the bed, wheelchair, commode, and car. Targeted strengthening of scapular muscles has the potential to mimic muscle activation required during daily functional activities. For example, by targeting the scapular stabilizers, scapulothoracic rhythm can be optimized for reaching into a cupboard, for example, and a stable base can be created for humerus movement in both non-weightbearing and dynamic weightbearing activities.
Exploring non-SCI literature and prioritizing the “prime mover,” we investigated exercises that emphasize SA activation and provide the lowest UT:SA ratio. Examples of exercises that produced high SA activation included standard push-up plus (123% maximum voluntary isometric contraction [MVIC]),31 dynamic hug (109% MVIC [greatest peak]),32 loaded scaption (55.2% MVIC),33 and unilateral shoulder press (62% MVIC).34 Examples of exercises with low UT:SA ratio (<0.2) included standard push-up plus (<0.2),31 bilateral scapular protraction (0.13),34 and supine press (0.11).34 Informed by non-SCI literature, four common exercises were selected that were hypothesized to maximize SA activation and minimize UT:SA ratio, and they could be easily performed in rehabilitation and/or home settings by individuals with paraplegia. Additionally, exercises were selected to eliminate confounding factors as necessitated in a surface electromyography (EMG) study comparing exercises (i.e., length of muscle, type of muscle contraction) and were physically possible in a group with paraplegia (i.e., standing or pushup on toes not required).
In summary, a potential primary cause of shoulder pain in individuals with paraplegia is rotator cuff impingement due to altered muscle activation and kinematics. Although exercise interventions focused on muscle groups have had some success, there is potential for targeted muscle strengthening interventions, as a component of a comprehensive program, to increase effectiveness. However, there is a lack of investigations into exercises that optimize SA while minimizing UT muscle activation. The primary aim of this study was to determine which exercises maximally activate the SA and minimize the UT:SA ratio in individuals with paraplegia. The secondary aim was to determine which exercises maximally activate the LT and minimize the UT:LT ratio. It was hypothesized that SA punch would produce the greatest SA activation with a minimal UT:SA ratio.
Methods
Design and participants
A convenience sample of 10 individuals with paraplegia (52.6 ± 7.6 years; range, 39–62), nine SCI and one hereditary/familial spastic paraplegia, who were primary wheelchair users participated in this cross-sectional observational design study (Table 1). Participants self-reported their activity level with an average of 17.7 ± 6.5 transfers/day. Based on pilot data, a sample size of at least 10 participants was calculated with a power analysis for a within-factors repeated-measures analysis of variance (RM-ANOVA) to detect differences in SA, LT, and UT muscle activation across exercises while achieving a moderate effect size of at least 0.4 with an alpha set at 0.05 and 80% power. A 0.4 effect size is a conservative estimate because it allows between-exercise differences of 10% to 15% to be statistically identified with variables that are twice that magnitude. Additionally, with a repeated-measures design, each participant is compared with themselves, which decreases variance in the error term. Inclusion criteria included at least 1-year post SCI from congenital conditions or trauma, vascular, or orthopedic origin resulting in American Spinal Injury Association (ASIA) International Standards for the Neurological Classification of Spinal Cord Injury (ISNCSCI)35 classification at or distal to the second thoracic neurological level of injury. Although enrollment was not specifically restricted based on ASIA Impairment Scale (AIS),35 participants were required to use a manual wheelchair for primary mobility. To rule out shoulder pain that potentially interferes with exercise performance or muscle activation, exclusion criteria included positive clinical tests (painful arc, Hawkins-Kennedy, Neer),36–39 shoulder pain greater than 3 out of 10 during one-repetition maximum (1RM) testing, or a score greater than 10/150 on the WUSPI. Participants signed university-approved human subject informed consent documents prior to participation.
Table 1.
Participant characteristics
| Etiology of paraplegia | Age, years | Sex | Time since injury, years | Level of injury | AIS |
|---|---|---|---|---|---|
| Traumatic SCI | NS | Female | 24 | T4/5 | AIS B |
| Traumatic SCI | NS | Male | 21 | T6/7 | AIS C |
| Traumatic SCI | NS | Male | 2 | T10/12 | AIS A |
| Traumatic SCI | NS | Male | 5 | T4 | AIS C |
| Traumatic SCI | NS | Male | 3 | T10/11 | AIS C |
| Traumatic SCI | NS | Male | 22 | T10 | AIS A |
| Traumatic SCI | NS | Male | 10 | T4 | AIS A |
| Traumatic SCI | NS | Male | 24 | T10/12 | AIS A |
| Traumatic SCI | NS | Male | 26 | T7/8 | AIS A |
| Hereditary spastic paraplegia | NS | Male | NA | NA | NA |
| Mean (total sample traumatic SCI/total sample paraplegia) | 53.6/53.1 | NA | 13.9/NA | NA | NA |
| SD (total sample traumatic SCI/total sample paraplegia) | 8.3/7.9 | NA | 9.8/NA | NA | NA |
Note: AIS = American Spinal Injury Association Impairment Scale; N/A = not applicable; NS = not specified (to ensure de-identification of participants); SCI = spinal cord injury.
Instrumentation
Three-dimensional kinematic data of the right humerus relative to the thorax were captured by the 11-Camera Vicon Motion Capture System (Oxford, UK) at a 100 Hz sampling rate. Right shoulder surface EMG data were captured by 16-channel Delsys Trigno Wireless EMG System (Natick, Massachusetts) using Trigno Avanti sensors (27 × 37 × 13 mm) with interelectrode spacing of 10 mm. The sensors have a noise level of <0.75 μV, a common mode rejection ratio of less than −80 dB at 60 Hz, and a bandwidth of 20 to 450 Hz. The EMGworks oscilloscope (Delsys Inc., Natick, MA) was used to verify raw signals, and Vicon Nexus software (Oxford Metrics plc, Yarnton, UK) was used to acquire data.
Procedures
Participants attended a single session lasting 1 to 2 hours and were overseen by the same licensed physical therapist. During the musculoskeletal examination, demographic and medical data were collected from each participant (i.e., sex, age, height, weight, and activity level). Activity level was self-reported by the participant describing transfers performed within a typical day. The investigator tallied each transfer, scoring both to and from each surface as a single transfer. All participants were tested in their custom wheelchair.
Three-dimensional kinematics
To identify the concentric phase of each exercise, motion capture markers were applied to determine the joint axis and track three-dimensional motion of the right humerus relative to the thorax. Markers were applied with double-sided adhesive to the following locations: seventh cervical and sixth thoracic spinous processes, midway between the medial border of both the right and left scapula and spine, midway and inferior to the left scapular spine, superior aspect of the right acromion, lateral mid portion of right upper arm, superior and lateral to right lateral epicondyle, manubrium, center of radius, and radial and ulnar styloid processes (Figure 1).
Figure 1.
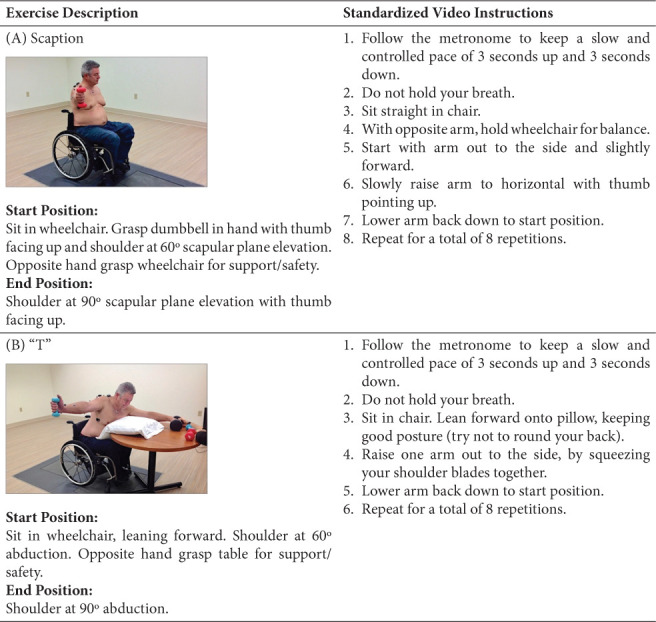
Exercises. (A) scaption; (B) “T”; (C) dynamic hug; (D) serratus anterior punch.
Figure 1.

(cont.)
EMG electrode placement
After skin preparation with an alcohol swab, surface electrodes were attached parallel to muscle fibers on the right upper extremity: SA (level of scapular inferior angle, anterior to latissimus dorsi), UT (halfway between the seventh cervical spinous process and acromion process), LT (55-degree oblique angle, 5 cm down from the scapular spine, next to scapular medial border), and middle deltoid (lateral aspect upper arm, 3 cm below the acromion) (Figure 1).
Normalization of EMG data
To compare EMG data across participants and exercises, muscle activation during each exercise was normalized to the maximum voluntary isometric contraction (MVIC) for each muscle. Standardized muscle testing positions were used.40 Each muscle’s resting level was recorded to identify baseline background activity. Data were then recorded for two repetitions of manually resisted MVICs for each muscle. Mean EMG value of the middle 3 seconds of two trials was used to normalize EMG data for each muscle. During the concentric phase of each exercise, data for each muscle were reported as percent (%) MVIC.
Exercise training and resistance determination
After the musculoskeletal examination, participants were shown standardized instructional exercise videos. Participants practiced with a 1-lb weight to ensure correct form without risk of fatigue. Similar to other SCI investigations,41–43 the Mayhew Regression44 was utilized to safely predict the 1RM for each exercise in a potentially vulnerable population. For each exercise, weight was increased incrementally until the participant was able to complete greater than three but fewer than eight repetitions with a dumbbell. Completed weight (Wt) and number of repetitions (Reps) were input into the Mayhew Regression: [1RM = Wt / (0.533 + 0.419 × e−0.055 × Reps)].
Exercise trials
Participants completed eight repetitions per exercise: “T” and scaption in sitting and dynamic hug and SA punch in supine (Figure 1). Exercises were tested using dumbbells at 60% 1RM in random order and paced by a metronome. To lessen fatigue potential, a minimum of 5 minutes of rest was allocated between exercises. Exercises were performed unilaterally with the exception of dynamic hug, which was performed bilaterally to accomplish the movement pattern and to keep the participant balanced (Figure 1). Simultaneous kinematic and muscle activation data were captured during the exercises.
Data reduction and statistical analysis
Using C-Motion Visual3D software, muscle activation data were normalized as %MVIC as described earlier. Mean SA, LT, and UT muscle activation during the concentric phase of each exercise were calculated; from these means, UT:SA and UT:LT ratios were calculated for each participant. Means, standard deviations, and confidence intervals of SA, LT, and UT muscle activation and UT:SA and UT:LT ratios across all participants were then calculated. One-way RM-ANOVA was conducted using PASW software version 27.0 (SPSS, Inc., Chicago, IL) to determine statistically significant differences in muscle activation (%SA, %LT, or %UT) or ratios (UT:SA or UT:LT) between the four exercises. Data were assessed for outliers and normal distribution. If assumption of sphericity was violated as demonstrated by Mauchly’s test of sphericity, Greenhouse-Geisser correction was used.
Results
Using Greenhouse-Geisser correction when indicated, exercise intervention elicited statistically significant changes in UT muscle activation [F(2.264, 0.657) = 31.004, p < .0005, partial eta squared (η2) = 0.775], SA muscle activation [F(3,27) = 36.924, p < .0005, partial η2 = 0.804], LT muscle activation [F(1.914, 17.228) = 20.727, p < .0005, partial η2 = 0.697], UT:SA ratio [F(1.010, 9.086) = 12.074, p < .007], and UT:LT ratio [F(3, 27) = 12.588, p < .0005, partial η2 = .583]. Bonferroni post hoc analysis revealed multiple significant differences between exercises (p ≤ .05) (Tables 2 and 3). For each muscle, exercises are rank ordered from most to least optimal muscle activation (Table 2). Similar to prior investigations, each exercise is also categorized by activation level: low (0%–20% MVIC), moderate (21%–40% MVIC), high (41%–60% MVIC), and very high (>60% MVIC) (Table 2).45 Exercises are also rank ordered from most to least optimal UT:SA and UT:LT ratios (Table 3).
Table 2.
Descriptive statistics for serratus anterior, lower trapezius, and upper trapezius muscle activity level represented as maximum voluntary isometric contraction (% MVIC) and associated 95% confidence intervals (95% CI)
| Exercise | Activation level category | Mean(% MVIC) | SD (% MVIC) | Lower boundary 95% CI (% MVIC) | Upper boundary 95% CI (% MVIC) | Post hoc analysis |
|---|---|---|---|---|---|---|
| Serratus anterior | ||||||
|
| ||||||
| SA punch | Very high | 73.7 | ± 19.2 | 59.9 | 87.4 | > T, DH |
| Scaption | Very high | 62.1 | ± 19.9 | 48.2 | 76.3 | > T, DH |
| Dynamic hug | Moderate | 32.4 | ± 8.8 | 26.1 | 38.6 | < S, > T, < SAP |
| “T” | Low | 16.5 | ± 12.0 | 8.0 | 25.1 | < S, DH, SAP |
|
| ||||||
| Lower trapezius | ||||||
|
| ||||||
| “T” | Very high | 69.3 | ± 38.9 | 41.5 | 97.1 | > DH, SAP |
| Scaption | Moderate | 49.1 | ± 30.4 | 27.3 | 70.8 | > DH, SAP |
| Dynamic hug | Low | 19.4 | ± 22.1 | 3.6 | 3.5 | < S, T |
| SA punch | Low | 14.6 | ± 13.6 | 4.9 | 2.4 | < S, T |
|
| ||||||
| Upper trapezius | ||||||
|
| ||||||
| SA punch | Low | 4.9 | ± 5.0 | 1.3 | 8.4 | < S, T |
| Dynamic hug | Low | 5.6 | ± 5.1 | 2.0 | 9.3 | < S, T |
| Scaption | High | 50.6 | ± 21.4 | 35.3 | 65.9 | > DH, SAP |
| “T” | High | 54.9 | ± 29.9 | 33.5 | 76.3 | > DH, SAP |
Note: Bonferroni post hoc analysis revealed multiple significant differences between exercises (p < .05). Differences between exercises are identified by the following abbreviations: scaption (S), T (T), dynamic hug (DH), SA punch (SAP). Exercises are displayed in rank order for each muscle from most to least optimal muscle activation. Each exercise is categorized by activation level from low (0%–20% MVIC), moderate (21%–40% MVIC), high (41%–60% MVIC), and very high (>60% MVIC).
Table 3.
UT:SA and UT:LT ratios and associated 95% confidence intervals (95% CI)
| Exercise | Mean | SD | Lower boundary 95% CI | Upper boundary 95% CI | Post hoc analysis |
|---|---|---|---|---|---|
| UT:SA | |||||
|
| |||||
| SA punch | 0.06 | ± 0.05 | 0.03 | 0.10 | < S, T |
| Dynamic hug | 0.20 | ± 0.20 | 0.06 | 0.35 | < S, T |
| Cutoff mean values < 0.6 | |||||
| Scaption | 0.87 | ± 0.38 | 0.60 | 1.14 | > DH, SAP |
| T | 5.46 | ± 4.77 | 2.05 | 8.88 | > DH, SAP |
|
| |||||
| UT:LT | |||||
|
| |||||
| SA punch | 0.38 | ± 0.24 | 0.21 | 0.55 | < S, T |
| Dynamic hug | 0.53 | ± 0.61 | 0.10 | 0.97 | < S |
|
| |||||
| Cutoff mean values < 0.6 | |||||
|
| |||||
| T | 0.86 | ± 0.33 | 0.62 | 1.10 | > SAP |
| Scaption | 1.28 | ± 0.62 | 0.83 | 1.73 | > DH, SAP |
Note: Bonferroni post hoc analysis revealed multiple significant differences between exercises (p < .05). Differences between exercises are identified by the following abbreviations: scaption (S), T (T), dynamic hug (DH), SA punch (SAP). A cutoff is displayed with mean values < 0.6 considered optimal. Exercises are displayed in rank order from smallest to largest ratio with arrows pointing toward the most optimal ratios. UT:LT = upper trapezius to lower trapezius ratio; UT:SA = upper trapezius to serratus anterior ratio.
Discussion
Although prior interventions offer some shoulder pain relief with global strengthening exercises, individuals with paraplegia must continue using their upper extremities for functional independence without the luxury of resting painful joints. A current guide to address shoulder pain includes scapular stabilizer strengthening, as a component of a comprehensive program, alongside strengthening of the thoracohumeral depressor muscles, stretching to maintain shoulder joint flexibility, and activity modification.46 Despite an abundance of evidence in non-SCI literature supporting exercises that target specific scapulothoracic musculature, we cannot assume individuals with paraplegia will respond similarly. Individuals with paraplegia and strength deficits involving the trunk often perform exercises while stabilizing with their upper extremities to maintain their balance and in positions of poor postural alignment. To our knowledge, this is the first study to examine targeted muscle activation across commonly prescribed upper extremity exercises selected specifically for individuals with paraplegia.
Our hypothesis was supported that SA punch produced the greatest SA activation and most optimal UT:SA ratio. Although seated scaption produced comparable levels of SA activation to SA punch (>60% MVIC; Table 2 and Figure 2), it resulted in a higher UT:SA ratio (.87; Table 3). Only dynamic hug, a supine exercise, was able to maintain a comparably low UT:SA ratio (0.2; Table 2). Conversely, LT was activated significantly more in sitting (“T” and scaption) versus either supine exercise (Figure 2). Sitting exercises also had highest UT activation, resulting in UT:LT at 0.86 and 1.28, respectively. These results in a population with impaired trunk control are consistent with non-SCI investigations that noted increased UT activity when upper extremity exercises are performed in a vertical trunk position.47
Figure 2.
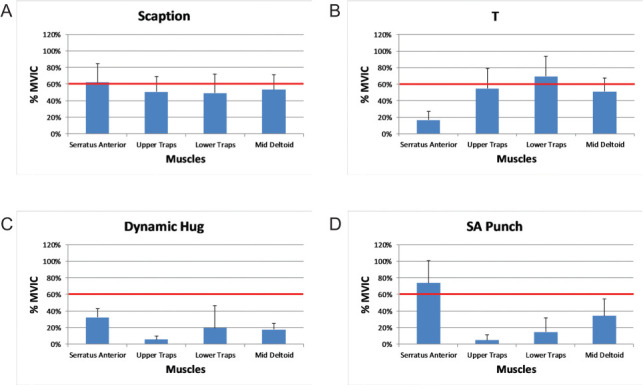
Percent muscle activation across exercises. (A) Scaption; (B) “T”; (C) dynamic hug; (D) serratus anterior (SA) punch. % MVIC = percent maximum voluntary isometric contraction.
Three out of four exercises in this investigation achieved a comparably low UT:SA ratio (Table 3) as compared with standard pushup plus31 and all phases of weighted scapular plane elevation in a non-SCI control group (0.92–1.27).47 Our findings for UT:SA ratios during supine exercises were slightly lower (<0.2) than Huang et al.48 reported for the concentric phases of weighted forward flexion (0.67), knee pushup plus (0.45), and side lying external rotation (1.02). Supine UT:LT ratios were slightly higher than those for UT:SA (Table 3), which was not surprising considering the extremely low LT activation in supine (Table 2). Our recommendations are consistent with prior recommendations of ratios of less than 0.6 being considered optimal.49,50
Importantly, our investigation included commonly performed exercises in positions feasible for those with paraplegia and explored options from supine to sitting (leaning anteriorly with opposite hand or forearm supported on table) to sitting (upright). These findings support a logical progression of exercises transitioning from supine with more trunk support to a more challenging, yet functional upright position. Investigations using surface EMG performed in the non-SCI population frequently use positions like pushups in various prone (i.e., standard,31,32 knee,31,32 or elbow pushup plus31) or standing (i.e., wall pushup plus,31 towel wall slide33) that are not always feasible for individuals with paraplegia to perform. Similar to the pushup exercises utilized in the non-SCI population, the supine exercises (SA punch and dynamic hug) emphasize protraction, which is a primary function of the SA. Undoubtedly, active scapular upward rotation during humeral elevation would necessitate more coordinated, balanced activation from all muscles in the force couple, SA, LT, and UT, thus supporting the progression from supine to more challenging sitting exercises.
Limitations
The complex nature of shoulder pain undisputedly requires a complex solution, including but not limited to targeted muscle activation, hand positioning during functional activities, and seated posture. Although attention to altered scapulothoracic kinematics and muscle activation is considered an important element of a comprehensive rehabilitation program, it is unclear whether these alterations are a primary cause of or consequence of shoulder pain.51 Despite consistent improvements in pain and disability with therapeutic interventions,51 the most essential elements in prevention and treatment of subacromial impingement pain are unknown. In individuals with paraplegia, who perform frequent overhead reaching from a wheelchair level, the important role of the upper trapezius, assisting with scapular upward rotation and suspending the shoulder girdle, must be acknowledged. The goal should not be to eliminate upper trapezius functioning during exercise prescription but rather to balance its functioning alongside the other muscles within the force couple. Although limited to two muscles at a time, muscle ratios are one way to appreciate balanced muscle contributions. Despite the symbiotic relationship, glenohumeral musculature was not included in this investigation. There is a fair amount of evidence on incidence, prevalence, and risk factors of shoulder pain after SCI. However, there is a dearth of SCI literature on biomechanical or neuropathic origin of shoulder pain, which necessitates reliance on non-SCI literature as best evidence. Biomechanical origin of shoulder pain (muscle activation or kinematics) may differ in individuals with paraplegia. Additionally, our interventions focused on the mechanisms of impingement during non-weightbearing reaching activities such as reaching overhead while in bed or from a wheelchair. Our interventions did not target the weightbearing mechanisms of impingement in SCI, including the role of thoracohumeral depressors during critical functional tasks, such as lifting the trunk and pelvis during bed mobility, weight relief, and transfers. Likewise, our interventions did not focus on the mechanisms of impingement during non-weightbearing static activities such as the role of pectoralis minor flexibility or glenohumeral musculature strength in achieving upright posture and shoulder alignment in seated positions. Our sample included individuals with paraplegia without shoulder pain. It is feasible that participants with musculoskeletal shoulder pain may have different muscle activation than those without pain. Additionally, we had a heterogenous population with paraplegia by including one individual with hereditary spastic paraplegia, who could theoretically respond differently from those with traumatic SCI. However, data were consistent without notable outliers. It was beyond the scope of this study to determine if various etiologies of paraplegia or levels of injury including tetraplegia factor into results.
Controlling for potential confounding factors (length, type of muscle contraction, and velocity) allowed us to more confidently interpret surface EMG as an indirect measurement of muscle force. Specifically, exercises were selected where arm elevation remained between 60 and 90 degrees, only concentric phases were analyzed, and a metronome ensured consistent pacing. We were not able to compare and contrast other potentially beneficial exercises including those in prone positions in which we could not control for confounding factors. For instance, prone “Is” and “Ys” would surpass the 60 to 90 degree elevation requirement. Despite all four exercises ensuring humeral elevation between 60 and 90 degrees, the two supine exercises (dynamic hug and SA punch) produce predominantly scapular protraction, without necessitating the same degree of active upward rotation as the sitting exercises (“T” and scaption). Therefore, one should expect more isolation of the SA during supine exercises, with minimal UT or LT activation. As noted, we used surface EMG for all muscles, including SA, which can be challenging in participants with higher body mass index. Mean (SD) for body mass index for our population (27.0 ± 6.0) is considered outside the normal range (24.9) recommended by the Centers for Disease Control and Prevention.52 To verify electrode placement, an oscilloscope was used during initial MVIC testing.
Clinical implications
Addressing potential biomechanical origin of musculoskeletal shoulder pain following paraplegia, a comprehensive rehabilitation program should include targeted muscle activation. Findings from this investigation demonstrated that SA punch produced the greatest SA activation and lowest UT:SA and UT:LT ratios. Dynamic hug also produced optimal UT:SA and UT:LT ratios, suggesting that supine exercises are better at minimizing excessive UT activation. Individuals with more impaired trunk control may initially perform strengthening exercises in supine to more selectively isolate muscle activation. However, to maximally activate the LT, an upright position may be indicated. Upright positioning may be preferred for some wheelchair users to limit transfers or to improve access in public gyms, for example, or to simulate functional positions. Biofeedback may provide valuable insight with isolating muscle activation with a vertical, unsupported trunk.
Acknowledgments
The authors acknowledge Dr. Jonathan Riek, Scott Andler, Damond Archung, Krista Cooley, Josie Andler, Brianna Hill, and Grant Levermore for their assistance with this project.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
REFERENCES
- 1.Mulroy S, Hatchett P, Eberly V, Haubert L, Conners S, Requejo P. Shoulder strength and physical activity predictors of shoulder pain in people with paraplegia from spinal injury: Prospective cohort study. Phys Ther . 2015;95(7):1027–1038. doi: 10.2522/ptj.20130606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kentar Y, Zastrow R, Bradley H. Prevalence of upper extremity pain in a population of people with paraplegia. Spinal Cord . 2018;56:695–703. doi: 10.1038/s41393-018-0062-6. [DOI] [PubMed] [Google Scholar]
- 3.Samuelsson KAM, Tropp H, Gerdle B. Shoulder pain and its consequences in paraplegic spinal cord-injured, wheelchair users. Spinal Cord . 2004;42:41–46. doi: 10.1038/sj.sc.3101490. [DOI] [PubMed] [Google Scholar]
- 4.Alm M, Saraste H, Norrbrink C. Shoulder pain in persons with thoracic spinal cord injury: Prevalence and characteristics. J Rehabil Med . 2008;40(4):277–283. doi: 10.2340/16501977-0173. [DOI] [PubMed] [Google Scholar]
- 5.Azadvari M, Razavi S, Tavasol T, Rakhshan A. Prevalence of shoulder pain in spinal cord injury patients referring to the Brain and Spinal Cord Injury Research Center of Tehran University of Medical Sciences. Arch Neurosci . 2020;7(1):e96150. [Google Scholar]
- 6.Bossuyt F, Arnet U, Brinkhof M et al. Shoulder pain in the Swiss spinal cord injury community: Prevalence and associated factors. Disabil Rehabil . 2018;40(7):798–805. doi: 10.1080/09638288.2016.1276974. [DOI] [PubMed] [Google Scholar]
- 7.Akbar M, Balean G, Brunner M et al. Prevalence of rotator cuff tear in paraplegic patients compared with controls. J Bone Joint Surg Am . 2010;92(1):23–30. doi: 10.2106/JBJS.H.01373. [DOI] [PubMed] [Google Scholar]
- 8.Muller R, Brinkhof MWG, Arnet U et al. Prevalence and associated factors of pain in the Swiss spinal cord injury population. Spinal Cord . 2017;55:346–354. doi: 10.1038/sc.2016.157. [DOI] [PubMed] [Google Scholar]
- 9.Barbetta DC, Lopes ACG, Chagas FNMR et al. Predictors of musculoskeletal pain in the upper extremities of individuals with spinal cord injury. Spinal Cord . 2016;54:145–149. doi: 10.1038/sc.2015.126. [DOI] [PubMed] [Google Scholar]
- 10.Ludewig PM, Phadke V, Braman J, Hassett D, Cieminski C, Laprade R. Motion of the shoulder complex during multiplanar humeral elevation. J Bone Joint Surg Am . 2009;91-A(2):378–389. doi: 10.2106/JBJS.G.01483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McClure PW, Michener LA, Sennett BJ, Karduna AR. Direct 3 dimensional measurement of scapular kinematics during dynamic movements in vivo. J Shoulder Elbow Surg . 2001;10:269–277. doi: 10.1067/mse.2001.112954. [DOI] [PubMed] [Google Scholar]
- 12.Ludewig PM, Cook TM. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys Ther . 2000;80(3):276–291. [PubMed] [Google Scholar]
- 13.Lin J, Hsieh S-C, Cheng W-C, Chen WC, Lai Y. Adaptive patterns of movement during arm elevation test in patients with shoulder impingement syndrome. J Orthop Res . 2011;29(5):653–657. doi: 10.1002/jor.21300. [DOI] [PubMed] [Google Scholar]
- 14.Endo K, Ikata T, Katoh S, Takeda Y. Radiographic assessment of scapular rotational tilt in chronic shoulder impingement syndrome. J Orthop Sci . 2001;6:3–10. doi: 10.1007/s007760170017. [DOI] [PubMed] [Google Scholar]
- 15.Lin JJ, Hanten WP, Olson SL, et al. Functional activity characteristics of individuals with shoulder dysfunctions. J Electromyogr Kinesiol . 2005;15(6):576–586. doi: 10.1016/j.jelekin.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Lukasiewicz AC, McClure P, Michener L, Pratt N, Sennett B. Comparison of 3-dimensional scapular position and orientation between subjects with and without impingement. J Orthop Sports Phys Ther . 1999;29:574–583. doi: 10.2519/jospt.1999.29.10.574. [DOI] [PubMed] [Google Scholar]
- 17.Timmons MK, Thigpen CA, Seitz AL, Karduna AR, Arnold BL, Michener LA. Scapular kinematics and subacromial-impingement syndrome: A meta-analysis. J Sport Rehabil . 2012;21:354–370. doi: 10.1123/jsr.21.4.354. [DOI] [PubMed] [Google Scholar]
- 18.Lopes AD, Timmons MK, Grover M, Ciconelli RM, Michener LA. Visual scapular dyskinesis: Kinematics and muscle activity alternations in patients with subacromial impingement syndrome. Arch Phys Med Rehabil . 2015;96:298–306. doi: 10.1016/j.apmr.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 19.Hebert LJ, Moffet H, McFadyen BJ, Dionne CE. Scapular behavior in shoulder impingement syndrome. Arch Phys Med Rehabil . 2002;83:60–69. doi: 10.1053/apmr.2002.27471. [DOI] [PubMed] [Google Scholar]
- 20.Su KP, Johnson MP, Gracely EJ, Karduna AR. Scapular rotation in swimmers with and without impingement syndrome: Practice effects. Med Sci Sports Exerc . 2004;36:1117–1123. doi: 10.1249/01.mss.0000131955.55786.1a. [DOI] [PubMed] [Google Scholar]
- 21.Dvir Z, Berme N. The shoulder complex in elevation of the arm: A mechanism approach. J Biomech. 1978;11:219–225. doi: 10.1016/0021-9290(78)90047-7. [DOI] [PubMed] [Google Scholar]
- 22.Sgroi T, Cilenti M. Rotator cuff repair: Post-operative rehabilitation concepts. Curr Rev Musculoskeletal Med. 2018;11(1):86–91. doi: 10.1007/s12178-018-9462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Struyf F, Cagnie B, Cools A et al. Scapulothoracic muscle activity and recruitment timing in patients with shoulder impingement symptoms and glenohumeral instability. J Electromyogr Kinesiol. 2014;24(2):277–84. doi: 10.1016/j.jelekin.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Cools AM, Dedercq GA, Majleu NN, Witrouw EE. Trapezius activity and intramuscular balance during isokinetic exercise in overhead athletes with impingement symptoms. Scand J Med Sci Sports . 2007;17:25–33. doi: 10.1111/j.1600-0838.2006.00570.x. [DOI] [PubMed] [Google Scholar]
- 25.Shinozaki N, Sano H, Omi et al. Differences in muscle activities during shoulder elevation in patients with symptomatic and asymptomatic rotator cuff tears: Analysis by positron emission tomography. J Shoulder Elbow Surg. 2014;23:e61–e67. doi: 10.1016/j.jse.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Michener LA, Sharma S, Cools AM, Timmons MK. Relative scapular muscle activity ratios are altered in subacromial pain syndrome. J Shoulder Elbow Surg . 2016;25(11):1861–1867. doi: 10.1016/j.jse.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Nawoczenski D, Ritter-Soronen J, Wilson C, Howe B, Ludewig P. Clinical trial of exercise for shoulder pain in chronic spinal injury. Phys Ther . 2006;86(12):1604–1618. doi: 10.2522/ptj.20060001. [DOI] [PubMed] [Google Scholar]
- 28.Mulroy SJ, Thompson S, Kemp B, Hattchett PP, Newsam CJ. Strengthening and optimal movements for painful shoulders (STOMPS) in chronic spinal cord injury: A randomized control trial. J Am Phys Ther Assoc . 2011;91:305–324. doi: 10.2522/ptj.20100182. [DOI] [PubMed] [Google Scholar]
- 29.Van Straaten M, Cloud BA, Morrow MM, Ludewig PM, Zhao KD. Effectiveness of home exercise on pain, function, and strength of manual wheelchair users with spinal cord injury: A high-dose shoulder program with telerehabilitation. Arch Phys Med Rehabil . 2014;95(10):1810–1817.e2. doi: 10.1016/j.apmr.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curtis KA, Tyner TM, Zachary L et al. Effect of a standard exercise protocol on shoulder pain in long-term wheelchair users. Spinal Cord . 1999;37:421–429. doi: 10.1038/sj.sc.3100860. [DOI] [PubMed] [Google Scholar]
- 31.Ludewig PM, Hoff M, Osowski E, Meschke S, Rundquist P. Relative balance of serratus anterior and upper trapezius muscle activity during push-up exercises. Am J Sports Med . 2004;32(2):484–493. doi: 10.1177/0363546503258911. [DOI] [PubMed] [Google Scholar]
- 32.Decker M, Hintermeister R, Faber K, Hawkins R. Serratus anterior muscle activity during selected rehabilitation exercises. Am J Sports Med . 1999;27(6):784–791. doi: 10.1177/03635465990270061601. [DOI] [PubMed] [Google Scholar]
- 33.Castelein B, Cagnie B, Parlevliet T, Cools A. Superficial and deep scapulothoracic muscle electromyographic activity during elevation exercises in the scapular plane. J Orthop Sports Phys Ther . 2016;46(3):184–193. doi: 10.2519/jospt.2016.5927. [DOI] [PubMed] [Google Scholar]
- 34.Ekstrom R, Donatelli R, Soderberg G. Surface electromyographic analysis of exercises for the trapezius and serratus anterior muscles. J Orthop Sports Phys Ther . 2003;33(5):247–58. doi: 10.2519/jospt.2003.33.5.247. [DOI] [PubMed] [Google Scholar]
- 35. International Standards for Neurological Classification of SCI (ISNCSCI) worksheet . Richmond, VA: The American Spinal Injury Association; 2021. Retrieved from https://asia-spinalinjury.org/international-standards-neurological-classification-sci-isncsci-worksheet/ Accessed January 21, 2020. [Google Scholar]
- 36.Neer CS. Impingement lesions. Clin Orthop Rel Res . 1983;173:70–77. [PubMed] [Google Scholar]
- 37.Calis M, Akgun K, Birtane M et al. Diagnostic values of clinical diagnostic tests in subacromial impingement syndrome. Ann Rheum Dis . 2000;59:44–47. doi: 10.1136/ard.59.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawkins RJ, Hobeika PE. Impingement syndrome in the athletic shoulder. Clin Sports Med . 1983;2:391–405. [PubMed] [Google Scholar]
- 39.Leroux JL, Thomas E, Bonnel F, Blotman F. Diagnostic value of clinical tests for shoulder impingement syndrome. Revue du Rhumatisme (English ed) 1995;62:423–428. [PubMed] [Google Scholar]
- 40.Kendall FP, et al. Muscles Testing and Function with Posture and Pain . Baltimore, MD: Williams & Wilkins; 1993. [Google Scholar]
- 41.Riek LM, Tome J, Ludewig PM, Nawoczenski DA. Improving shoulder kinematics in individuals with paraplegia: Comparison across circuit resistance training exercises and modifications in hand position. Phys Ther . 2016;96(7):1006–1017. doi: 10.2522/ptj.20140602. [DOI] [PubMed] [Google Scholar]
- 42.Nash MS, Jacobs PL, Woods JM et al. A comparison of 2 circuit exercise training techniques for eliciting matched metabolic responses in persons with paraplegia. Arch Phys Med Rehabil . 2002;83:201–209. doi: 10.1053/apmr.2002.28011. [DOI] [PubMed] [Google Scholar]
- 43.Nash MS, Van de Ven I, Van Elk N et al. Effects of circuit resistance training on fitness attributes and upper extremity pain in middle aged men with paraplegia. Arch Phys Med Rehabil . 2007;88:70–75. doi: 10.1016/j.apmr.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Mayhew JL, Ball TE, Arnold MD, Bowen JC. Relative muscular endurance performance as a predictor of bench press strength in college men and women. J Appl Sport Sci Res . 1992;6(4):200–206. [Google Scholar]
- 45.Edwards PK, Ebert JR, Littlewood C, Ackland T, Wang A. A systematic review of electromyography studies in normal shoulders to inform postoperative rehabilitation following rotator cuff repair. J Orthop Sports Phys Ther . 2017;47(12):931–944. doi: 10.2519/jospt.2017.7271. [DOI] [PubMed] [Google Scholar]
- 46.Mulroy SJ, Hafdahl L, Dyson-Hudson T. A primary care provider’s guide to shoulder pain after spinal cord injury. Top Spinal Cord Inj Rehabil . 2020;26(3):186–196. doi: 10.46292/sci2603-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uhl T, Muir T, Lawson L. Electromyographical assessment of passive, active assistive, and active shoulder rehabilitation exercises. PM R . 2010;2(2):132–141. doi: 10.1016/j.pmrj.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Huang H, Lin J, Guo YL, Wang W, Chen Y. EMG biofeedback effectiveness to alter muscle activity pattern and scapular kinematics in subjects with and without shoulder impingement. J Electromyogr Kinesiol . 2013;23(1):267–274. doi: 10.1016/j.jelekin.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Kibler WB, Sciascia A, Wilkes T. Scapular dyskinesis and its relation to shoulder injury. J Am Acad Orthop Surg . 2012;20(6):364–372. doi: 10.5435/JAAOS-20-06-364. [DOI] [PubMed] [Google Scholar]
- 50.Phadke V, Ludewig PM. Study of the scapular muscle latency and deactivation time in people with and without shoulder impingement. J Electromyogr Kinesiol . 2013;23(2):469–475. doi: 10.1016/j.jelekin.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Takeno K, Glaviano NR, Norte GE, Ingersoll CD. Therapeutic interventions for scapular kinematics and disability in patients with subacromial impingement: A systematic review. J Athletic Train . 2019;54(3):283–295. doi: 10.4085/1062-6050-309-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Assessing Your Weight . Centers for Disease Control and Prevention website. Reviewed April 6, 2022. Accessed April 18, 2022. https://www.cdc.gov/healthyweight/assessing/bmi/index.html.


