Abstract
Background
Improving the quality of life of scoliosis patients with appropriate preventive measures is critical. This study aimed to investigate the relationships among bone mass, Cobb angle, and complete blood count (CBC) parameters in patients with scoliosis.
Material/Methods
This joint study was conducted by the pediatric department and orthopedics clinics, which used the medical records of patients aged 10–18 years between 2018 and 2022. Patients were divided into 3 groups according to the Cobb angle. Patient blood count levels from medical records and bone mineral density (BMD) Z scores (g/cm2) were compared among groups. Notably, BMD Z scores were calculated using a (BMD) dataset from local Turkish children after adjusting for height and age.
Results
A total of 184 individuals (120 females, 64 males) were included in the study. There were statistically significant differences among the groups in platelet-to-lymphocyte ratio (PLR). Significant differences in DXA Z scores among groups were found. There was a significantly strong and positive correlation between DXA Z scores and all CBC parameters in patients with severe scoliosis.
Conclusions
This study found that CBC parameters can predict BMD in adolescents. Furthermore, the association between vitamin D deficiency and low BMD may contribute to the follow-up of body adaptation in patients with scoliosis receiving conservative treatment.
Keywords: Bone Density, Blood Cell Count, Scoliosis
Background
Bone mineral density (BMD) has been increasingly investigated since the 1980s after 2 reports showed that children with adolescent idiopathic scoliosis (AIS) have lower bone mineral density than their peers [1–3]. Various studies have suggested an association between osteoporosis and scoliosis [2–7]. Notably, some studies have reported a significant association between scoliosis and osteopenia [2,6,8–15], and some studies have reported that scoliosis can even develop in adulthood [13]. Patients with osteopenia and AIS have been reported to have twice the risk of curve progression than those with normal BMD [16]. Because osteopenia poses a risk of curve progression and is thought to cause worsening scoliosis, it has been proposed as a potential prognostic factor [16,17].
Clonal hematopoiesis is a somatic mutation caused by various changes in the hematopoietic stem cells [18,19]. Any chronic inflammation in the body causes a suppression of erythropoiesis in the bone marrow and alters red blood cell heterogeneity [20]. Moreover, the inflammation directly or indirectly affects BMD and fracture risk [21].
Osteoimmunology has shown that the immune system and inflammatory factors play important roles in the development of osteoporosis [22]. Several studies of immune diseases have reported that monocyte-to-lymphocyte ratio (MLR) has diagnostic value and can reflect systemic inflammation and the severity of immune system damage [23]. Therefore, MLR has been recognized as a marker of inflammation [23]. Moreover, elevated MLR has been reported to be associated with osteoporosis and bone diseases [24,25].
Notably, neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), which are novel markers of systemic inflammatory response [26,27], have also been found to be associated with low BMD [28,29].
Appropriate preventive measures are critical in preventing the development of scoliosis and improving the quality of life of patients [30,31]. The present study aimed to investigate the associations among bone mass, Cobb angle, and complete blood count (CBC) parameters in patients with scoliosis.
Material and Methods
In this retrospective study we examined prospectively collected data of 184 AIS patients (all Turkish, 120 females, 64 males), conducted by the pediatric department and orthopedics clinics, which used the medical records of patients aged 10–18 years between 2018 and 2022. This study received approval from the Local Ethics Committee (approval date, 08.24.2022; registration number, 2022/09).
Patients who applied to our hospital with posture disorder, back pain, humpback, or vitamin D deficiency were examined. After evaluating the patients, those with a Cobb angle less than 10 degrees were enrolled in the control group. Patients who had not received treatment before and whose initial Cobb angle was >10° when they first applied were enrolled in the patient group.
All children were healthy and had good nutritional status; moreover, they had normal thyroid, parathyroid, liver, and kidney functions. Notably, patients were excluded if they had psychiatric conditions; had undergone spinal surgery; had spinal cord infections that could affect the accuracy of dual-energy X-ray absorptiometry (DXA) measurements; were taking medications known to affect bone metabolism; had chronic conditions affecting the impaired bone metabolism; sustained from skeletal dysplasia or congenital connective tissue abnormalities; or had a history of other musculoskeletal, neurological, neuromuscular, or endocrine disorders or pathological fractures.
Cobb angles of patients with AIS were confirmed using clinical examinations as well as erect and bending radiographs.
Evaluation of Spinal Curvature
The Cobb angle between the most inclined vertebrae was calculated by a single experienced orthopedist using a standard full-spine posteroanterior radiograph [32]. Specifically, scoliosis was defined as the presence of a curvature of ≥10°. Moreover, AIS was confirmed using erect and bending radiographs. Other causes of scoliosis were excluded by examination and investigations at the pediatric endocrinology clinic.
The 2016 Scientific Society on Scoliosis Orthopaedic and Rehabilitation Treatment guidelines define mild and moderate scoliosis as a Cobb angle of <20° and 21–35°, respectively [33,34]. In this study, patients were divided into 3 groups according to the Cobb angle: less than 10 degrees (control group), 10–20 degrees, and over 20 degrees.
Data Collection
Demographic data of the patients were extracted from electronic medical records, and based on these data, calcium (CA), phosphorus (P), 25-hydroxyvitamin D (25-OH-D3), alkaline phosphatase (ALP), parathyroid hormone (PTH), NLR, MLR, and PLR were calculated.
Complete blood counts were obtained using an automated hematology analyzer (Abbott, Cell-Dyn Ruby, IL, USA). Moreover, biochemical parameters were measured using a clinical chemistry analyzer (Abbott, c16000, IL, USA). In addition, hormone parameters were measured using chemiluminescence immunoassay (Abbott, i2000sr, IL, USA). MLR, NLR, and PLR were calculated using monocyte count/lymphocyte count, neutrophil count/lymphocyte count, and platelet count/lymphocyte count, respectively.
Bone Mineral Density Measurements
The T score indicates how much a person’s bone mass differs from the bone mass of an average healthy 30-year-old adult. In contrast, the Z score refers to standard deviations based on a comparison of a child’s BMD to the mean BMD of a standard population of the same sex and age [35]. When calculating age- and sex-adjusted Z scores, DXA measures the density of different bones in the body; however, the lumbar spine (L1–L4) and femoral neck are most commonly used [36]. In the present study, Z scores were obtained for the spine using BMD (g/cm2) from DXA scans. BMD Z scores were calculated using the BMD dataset of local Turkish children after adjustment for height and age [37,38]. All DXA procedures were performed using Lunar Prodigy (General Electric, GE Healthcare, Lunar DPX, NT+150301, USA) at a single institution.
Statistical Analysis
Descriptive data are presented as number (percentages) or median and interquartile ranges (IQR: 25th–75th percentiles), according to data distribution. The normality of continuous variables was assessed with the Shapiro-Wilk test and histogram plots. Kruskal-Wallis and post hoc Dunn’s tests were used for the comparison of continuous variables among groups. Pearson correlation analysis was conducted to assess the relationship between DXA Z scores and hemogram parameters. The analyses were performed using SPSS 26.0 for Windows (SPSS, Inc., Chicago, Illinois, USA) and R 4.2.1 statistical software. The results were considered to be significant at a level of P<0.05.
Results
A total of 184 individuals (120 females, 64 males) were included in the study. The median age of participants was 14 (IQR: 12–16) years. The comparison of hemogram parameters among the control group with no scoliosis (n=96), patients with mild scoliosis (n=72), and patients with moderate scoliosis (n=16) is presented in Table 1. There were statistically significant differences among the groups in terms of PLR (P=0.022). Post hoc comparisons revealed that the median PLR was significantly lower in patients with moderate scoliosis (median: 80.4, IQR: 76.3–194.4) compared to the patients with mild scoliosis (median: 104.2, IQR: 94.1–125.6) (P=0.024) and no scoliosis (median: 107.9, IQR: 89.3–125.5) (P=0.026).
Table 1.
The comparison of hemogram parameters across scoliosis groups.
| Hemogram parameters | COBB angle degrees | p-value | ||
|---|---|---|---|---|
| <10 (n=96) | 10–20 (n=72) | 21–35 (n=16) | ||
| NLR | 1.4 (0.9–1.9) | 1.4 (1–2.4) | 1.4 (1.1–2.4) | 0.493 |
| MLR | 0.2 (0.2–0.3) | 0.2 (0.2–0.3) | 0.2 (0.1–0.3) | 0.629 |
| PLR | 107.9 (89.3–125.5)a | 104.2 (94.1–125.6)b | 80.4 (76.3–194.4)ab | 0.022 |
Values are expressed as median (25th–75th percentile). p-values are calculated using Kruskal Wallis test
Same superscript letters indicate statistically significant difference between the two groups based on the Dunn’s test. Bold p-values indicate statistical significance at α=0.05.
NLR – neutrophil-to-lymphocyte ratio; MLR – monocyte-to-lymphocyte ratio; PLR – platelet-to-lymphocyte ratio.
The distribution of DXA Z scores among groups are displayed in Figure 1. Significant differences in terms of DXA Z scores among groups were found (P<0.001) – patients with moderate scoliosis had significantly lower DXA Z scores (median: −0.5, IQR: −1.38-0.06) compared to the patients with mild scoliosis (median: 1.12, IQR: 0.11–1.80) (P<0.001) and the control group (median: 1.02, IQR: −0.24–1.54) (P<0.001).
Figure 1.
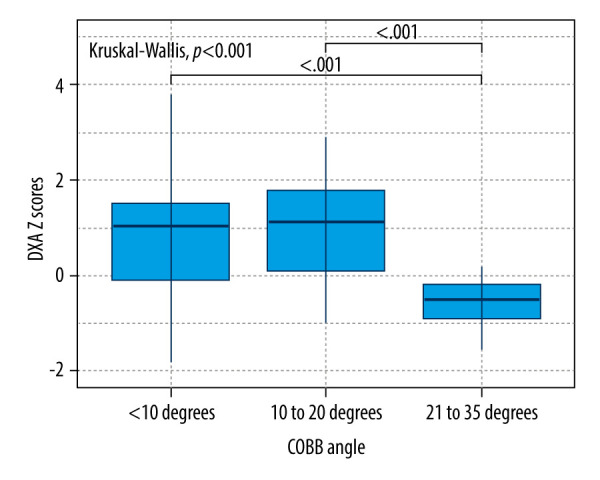
Comparison of DXA Z scores across scoliosis groups.
Correlations between DXA Z scores and hemogram parameters were evaluated separately for groups (Table 2). There was no significant correlation between DXA Z score and hemogram parameters in the control group. In the patients with mild scoliosis, a significant weak positive correlation was found between DXA Z Score and PLR (0.324, P=0.005). There was a strong and significant positive correlation between DXA Z scores and all hemogram parameters in patients with moderate scoliosis (NLR: r=0.814, P<0.001; MLR: r=0.544, P=0.029; PLR: r=0.714, P=0.002).
Table 2.
Correlation analysis between DXA Z scores and hemogram parameters according to severity of scoliosis.
| Hemogram parameters | COBB angle degrees | |||||
|---|---|---|---|---|---|---|
| <10 | 10–20 | 21–35 | ||||
| r | p | r | p | r | p | |
| NLR | 0.039 | 0.709 | −0.040 | 0.736 | 0.814 | <0.001 |
| MLR | 0.021 | 0.837 | −0.090 | 0.453 | 0.544 | 0.029 |
| PLR | 0.097 | 0.346 | 0.324 | 0.005 | 0.714 | 0.002 |
r: Pearson’ correlation coefficient. Bold p-values indicate statistical significance at α=0.05. NLR – neutrophil-to-lymphocyte ratio; MLR – monocyte-to-lymphocyte ratio; PLR – platelet-to-lymphocyte ratio.
Other serum blood parameters were compared among groups in Table 3. Significant differences were found in 25-OH-D3 (P=0.023), ALP (P<0.001), and PTH (P=0.017) among groups. The patients with mild scoliosis had significantly lower 25-OH-D3 (median: 10.9, IQR: 7.0–17.7) compared to the control group (median: 16.7, IQR: 7.5–22.7) (P=0.021). For ALP, all pairwise comparisons were statistically significant. The median ALP was highest in the control group (median: 194.5, IQR: 108.5–269.3), followed by patients with mild scoliosis (median: 125.5, IQR: 95.0–176.0) and moderate scoliosis (median: 90.0, IQR: 76.3–100.8). ALP was significantly higher in the control group compared to the mild (P=0.011) and moderate (P<0.001) groups, and it was significantly higher in the mild group compared to the moderate group (P=0.005). PTH was significantly lower in the moderate scoliosis group (median: 38.5, IQR: 34.7–65.3) compared to the mild scoliosis group (medians: 63.7, IQR: 37.5–93.0) (P=0.032).
Table 3.
Comparison of serum blood parameters across scoliosis groups.
| Serum blood parameters | COBB angle degree | p | ||
|---|---|---|---|---|
| <10 (n=96) | 10–20 (n=72) | 21–35 (n=16) | ||
| 25-OH-D3 (ng/mL) | 16.7 (7.5–22.7)a | 10.9 (7.0–17.7)a | 15.9 (7.4–21.7) | 0.023 |
| CA(mg/dL) | 9.5 (9.2–9.7) | 9.5 (9.4–10) | 9.8 (9.2–10) | 0.089 |
| P(mg/dL) | 4.3 (3.6–5) | 4.1 (3.7–4.5) | 4.3 (4–4.5) | 0.123 |
| ALP(IU/L) | 194.5 (108.5–269.3)ab | 125.5 (95.0–176.0)ac | 90.0 (76.3–100.8)bc | <0.001 |
| PTH(pg/mL) | 41.5 (36.4–77.9) | 63.7 (37.5–93)a | 38.5 (34.7–65.3)a | 0.017 |
Values are expressed as median (25th–75th percentile). p-values are calculated using Kruskal Wallis test
Same superscript letters indicate statistically significant difference between the two groups based on the post-hoc Dunn’s test. Bold p-values indicate statistical significance at α=0.05.
CA – calcium; P – phosphorus, 25-OH-D3 – 25-hydroxyvitamin D, ALP – alkaline phosphatise; PTH – parathyroid hormone.
Discussion
Increased load on a bone triggers remodeling, whereas decreased load causes the bone to become less dense and weaker. This indicates the shift of homeostatic mechanisms toward an anabolic or catabolic state [39]. Adaptive changes in bone caused by repeated loading are recognized by Wolff’s law [40–42]. The biomechanical characteristics of bone include both material and structural aspects [43–45]. In particular, BMD is a critical mechanical property of bone [11,12,46]. Chronic inflammation is known to be associated with low BMD, osteoporosis, and fractures [47]. Notably, leukocyte subset tests are commonly used to identify inflammatory diseases [48]; however, recent studies have shown that NLR, MLR, and PLR are better indicators of inflammation than are leukocyte subsets [49]. The present study reported that PLR was significantly lower in patients with moderate scoliosis than in patients with mild scoliosis and patients without scoliosis group. Compared to patients with mild scoliosis and individuals in the control group, patients with moderate scoliosis had significantly lower DXA Z scores. In patients with mild scoliosis, DXA Z scores had a significant weak positive correlation with PLR, whereas in patients with moderate scoliosis, DXA Z scores had a significantly strong and positive correlation with all CBC parameters. Other serum biochemistry parameters (25-hydroxyvitamin D3, alkaline phosphatase, and parathyroid hormone) were significantly different across the study groups.
Gao et al reported a significant association between osteoporosis and MLR in adult patients [25]. Notably, the results of our study support those of the relevant literature, and we found that MLR had a statistically significant correlation with BMD in children with AIS. In contrast, MLR did not correlate significantly with Cobb angle.
Tang et al [50] found that PLR had predictive value in postmenopausal women aged >50 years. Our study found that PLR is significantly correlated with both increased Cobb angles and BMD in children with AIS.
In a study involving adult patients with osteopenia and osteoporosis, Öztürk et al [28] found a correlation between NLR and BMD and reported that multivariate analysis showed NLR as an independent predictor of osteoporosis. Moreover, our study found a positive correlation between BMD and NLR in patients with moderate scoliosis, but there was no significant correlation between scoliotic curvature and NLR. This finding supports the consideration of NLR and MLR as independent predictors of BMD.
Dede et al did not find a statistically significant correlation between bone mineral density and the development of scoliosis in a bipedal rat model. They suggested that osteopenia alone may not be a major factor in the development of coronal deformity, but it may increase its frequency and severity, but not significantly [3]. Therefore, they argued that there is a BMD threshold between osteoporosis and idiopathic scoliosis [3]. Cheng et al reported that children with idiopathic scoliosis have lower bone mineral density, but the density does not correlate with the type or severity of the curves [3,6].
Nishida et al found a correlation between the low BMD and the severity of scoliosis in the AIS patients they operated on [51]. Sadat-Ali et al reported that scoliosis causes low bone mass and that it depends on the degree of the Cobb angle [52]. Moreover, they noted that patients with curves of >30° had significantly lower bone mass. Notably, our study observed significantly lower BMD in patients with curves of >20°, but BMD was not lower in patients with mild curves.
Lee et al [53] argued that patients with AIS have low calcium intake, which contributes to the development of scoliosis. Further, Cheung et al [54] reported that low bone mass may be caused by abnormally rapid growth during adolescence, which – with low calcium intake – causes abnormal mineralization. Notably, a report by Hung et al [17] sheds light on this important aspect of the curve shape. They identified osteopenia as an important risk factor for the course of curves in patients with AIS. In addition, they suggested that the BMD Z score should be added to other predictive factors of curve shape. Caan et al [55] found vitamin D deficiency in patients with idiopathic scoliosis, but calcium levels were normal in all pateints. Some other studies have discovered a possible association between vitamin D levels and scoliosis, independent of calcium [56,57]. In contrast, Balioğlu et al reported that vitamin D levels were lower in patients with scoliosis; however, they found a positive correlation between vitamin D and calcium levels [58]. Our study found no significant difference between the 2 groups in terms of calcium levels; notably, vitamin D levels were below the normal range in all study groups, whereas calcium levels were in the normal range. However, vitamin D levels were significantly lower in the scoliosis group than in the control group.
Study Limitations
This study has some limitations. First, this was a single-center study and included a small number of patients. Longitudinal and molecular biology studies may be required to obtain clearer results. Second, BMD measured using DXA can be confusing because bone growth is three-dimensional and uneven [35,59]. Third, bone microarchitecture, particularly that of trabecular bone, is another critical mechanical quality of bone; however, our study performed a comprehensive and systemic study of BMD but failed to assess trabecular bone microarchitecture. In addition, although physical activity has an effect on bone health, the fact that patients were not classified according to their physical activities is another limitation of our study [60]. Nonetheless, this study used a heterogeneous patient population and attempted to identify the factors that best correlated with low BMD and high Cobb angle.
Conclusions
To the best of our knowledge, this is the first study to provide evidence of an association of PLR with the Cobb angle in patients with AIS. Moreover, this study reported that CBC parameters play a predictive role for BMD in adolescent. Furthermore, the association between vitamin D deficiency and low BMD may contribute to the follow-up of body adaptation in patients with scoliosis receiving conservative treatment.
Footnotes
Conflict of interest: None declared
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding authors.
Ethics Committee Approval
This study received approval from the Ethics Committee of Health Sciences University Trabzon Kanuni Training and Research Hospital (approval date, 08.24.2022; registration number, 2022/09). We obtained the informed consent in writing from all patients before the study.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
References
- 1.Bartal E, Gage JR. Idiopathic juvenile osteoporosis and scoliosis. J Pediatr Orthop. 1982;2(3):295–98. doi: 10.1097/01241398-198208000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Burner WL, 3rd, Badger VM, Sherman FC. Osteoporosis and acquired back deformities. J Pediatr Orthop. 1982;2(4):383–85. doi: 10.1097/01241398-198210000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Dede O, Akel I, Demirkiran G, et al. Is decreased bone mineral density associated with development of scoliosis? A bipedal osteopenic rat model. Scoliosis. 2011;6(1):24. doi: 10.1186/1748-7161-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Healey JH, Lane JM. Structural scoliosis in osteoporotic women. Clin Orthop Relat Res. 1985;(195):216–23. [PubMed] [Google Scholar]
- 5.Thevenon A, Pollez B, Cantegrit F, et al. Relationship between kyphosis, scoliosis, and osteoporosis in the elderly population. Spine (Phila Pa 1976) 1987;12(8):744–45. doi: 10.1097/00007632-198710000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Cheng JC, Guo X. Osteopenia in adolescent idiopathic scoliosis. A primary problem or secondary to the spinal deformity? Spine (Phila Pa 1976) 1997;22(15):1716–21. doi: 10.1097/00007632-199708010-00006. [DOI] [PubMed] [Google Scholar]
- 7.Pappou IP, Girardi FP, Sandhu HS, et al. Discordantly high spinal bone mineral density values in patients with adult lumbar scoliosis. Spine (Phila Pa 1976) 2006;31(14):1614–20. doi: 10.1097/01.brs.0000222030.32171.5f. [DOI] [PubMed] [Google Scholar]
- 8.Cook SD, Harding AF, Morgan EL, et al. Trabecular bone mineral density in idiopathic scoliosis. J Pediatr Orthop. 1987;7(2):168–74. doi: 10.1097/01241398-198703000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Zhang J, Wang Y, et al. Abnormal lacuno-canalicular network and negative correlation between serum osteocalcin and Cobb angle indicate abnormal osteocyte function in adolescent idiopathic scoliosis. FASEB J. 2019;33(12):13882–92. doi: 10.1096/fj.201901227R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diarbakerli E, Savvides P, Wihlborg A, et al. Bone health in adolescents with idiopathic scoliosis. Bone Joint J. 2020;102-B(2):268–72. doi: 10.1302/0301-620X.102B2.BJJ-2019-1016.R1. [DOI] [PubMed] [Google Scholar]
- 11.Ito M, Ohki M, Hayashi K, et al. Trabecular texture analysis of CT images in the relationship with spinal fracture. Radiology. 1995;194(1):55–59. doi: 10.1148/radiology.194.1.7997582. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Y, Zhao J, White DL, Genant HK. Micro CT and micro MR imaging of 3D architecture of animal skeleton. J Musculoskelet Neuronal Interact. 2000;1(1):45–51. [PubMed] [Google Scholar]
- 13.Cheng JC, Qin L, Cheung CS, et al. Generalized low areal and volumetric bone mineral density in adolescent idiopathic scoliosis. J Bone Miner Res. 2000;15(8):1587–95. doi: 10.1359/jbmr.2000.15.8.1587. [DOI] [PubMed] [Google Scholar]
- 14.Roach JW. Adolescent idiopathic scoliosis. Orthop Clin North Am. 1999;30(3):353–65. vii–viii. doi: 10.1016/s0030-5898(05)70092-4. [DOI] [PubMed] [Google Scholar]
- 15.Zhu F, Qiu Y, Yeung HY, et al. Trabecular bone micro-architecture and bone mineral density in adolescent idiopathic and congenital scoliosis. Orthop Surg. 2009;1(1):78–83. doi: 10.1111/j.1757-7861.2008.00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yip BHK, Yu FWP, Wang Z, et al. Prognostic value of bone mineral density on curve progression: A longitudinal cohort study of 513 girls with adolescent idiopathic scoliosis. Sci Rep. 2016;6:39220. doi: 10.1038/srep39220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hung VWY, Qin L, Cheung CSK, et al. Osteopenia: A new prognostic factor of curve progression in adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2005;87(12):2709–16. doi: 10.2106/JBJS.D.02782. [DOI] [PubMed] [Google Scholar]
- 18.Steensma DP. Clinical implications of clonal hematopoiesis. Mayo Clin Proc. 2018;93(8):1122–30. doi: 10.1016/j.mayocp.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–98. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lippi G, Turcato G, Cervellin G, Sanchis-Gomar F. Red blood cell distribution width in heart failure: A narrative review. World J Cardiol. 2018;10(2):6–14. doi: 10.4330/wjc.v10.i2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandra A, Park SS, Pignolo RJ. Potential role of senescence in radiation-induced damage of the aged skeleton. Bone. 2019;120:423–31. doi: 10.1016/j.bone.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Limmer A, Wirtz DC. Osteoimmunology: Influence of the immune system on bone regeneration and consumption. Z Orthop Unfall. 2017;155(3):273–80. doi: 10.1055/s-0043-100100. [DOI] [PubMed] [Google Scholar]
- 23.Huang Y, Deng W, Zheng S, et al. Relationship between monocytes to lymphocytes ratio and axial spondyloarthritis. Int Immunopharmacol. 2018;57:43–46. doi: 10.1016/j.intimp.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Yapar A, Ulucaköy C, Sezgin EA, et al. Diagnostic role of neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio in patients with enchondroma and low-grade chondrosarcoma. Jt Dis Relat Surg. 2020;31(2):286–90. doi: 10.5606/ehc.2020.73629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao K, Zhu W, Liu W, et al. The predictive role of monocyte-to-lymphocyte ratio in osteoporosis patient. Medicine (Baltimore) 2019;98(34):e16793. doi: 10.1097/MD.0000000000016793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gary T, Pichler M, Belaj K, et al. Platelet-to-lymphocyte ratio: A novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. PLoS One. 2013;8(7):e67688. doi: 10.1371/journal.pone.0067688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grau AJ, Boddy AW, Dukovic DA, et al. Leukocyte count as an independent predictor of recurrent ischemic events. Stroke. 2004;35(5):1147–52. doi: 10.1161/01.STR.0000124122.71702.64. [DOI] [PubMed] [Google Scholar]
- 28.Öztürk ZA, Yesil Y, Kuyumcu ME, et al. Inverse relationship between neutrophil lymphocyte ratio (NLR) and bone mineral density (BMD) in elderly people. Arch Gerontol Geriatr. 2013;57(1):81–85. doi: 10.1016/j.archger.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Koseoglu SB. Bone loss & platelet-to-lymphocyte ratio. Biomark Med. 2017;11(1):5–10. doi: 10.2217/bmm-2016-0188. [DOI] [PubMed] [Google Scholar]
- 30.Tomé-Bermejo F, Piñera AR, Alvarez L. Osteoporosis and the management of spinal degenerative disease (II) Arch bone Jt Surg. 2017;5(6):363–74. [PMC free article] [PubMed] [Google Scholar]
- 31.McCoy S, Tundo F, Chidambaram S, Baaj AA. Clinical considerations for spinal surgery in the osteoporotic patient: A comprehensive review. Clin Neurol Neurosurg. 2019;180:40–47. doi: 10.1016/j.clineuro.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Engelke K, Stampa B, Timm W, et al. Short-term in vivo precision of BMD and parameters of trabecular architecture at the distal forearm and tibia. Osteoporos Int. 2012;23(8):2151–58. doi: 10.1007/s00198-011-1829-1. [DOI] [PubMed] [Google Scholar]
- 33.Negrini S, Donzelli S, Aulisa AG, et al. 2016 SOSORT guidelines: Orthopaedic and rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis Spinal Disord. 2018;13:3. doi: 10.1186/s13013-017-0145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horng M-H, Kuok C-P, Fu M-J, et al. Cobb angle measurement of spine from X-ray images using convolutional neural network. Comput Math Methods Med. 2019;2019:6357171. doi: 10.1155/2019/6357171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harindhanavudhi T, Petryk A, Jones R, et al. Lumbar spine bone mineral density Z-score discrepancies by dual X-ray absorptiometry do not predict vertebral fractures in children. J Investig Med. 2018;66(6):980–85. doi: 10.1136/jim-2018-000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Syed Z, Khan A. Bone densitometry: Applications and limitations. J Obstet Gynaecol Can. 2002;24(6):476–84. doi: 10.1016/s1701-2163(16)31095-7. [DOI] [PubMed] [Google Scholar]
- 37.Goksen D, Darcan S, Coker M, Kose T. Bone mineral density of healthy Turkish children and adolescents. J Clin Densitom. 2006;9(1):84–90. doi: 10.1016/j.jocd.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Demir K, Konakci E, Ozkaya G, et al. New features for child metrics: further growth references and blood pressure calculations. J Clin Res Pediatr Endocrinol. 2020;12(2):125–29. doi: 10.4274/jcrpe.galenos.2019.2019.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Zou D, Sun Z, et al. Hounsfield unit for assessing vertebral bone quality and asymmetrical vertebral degeneration in degenerative lumbar scoliosis. Spine (Phila Pa 1976) 2020;45(22):1559–66. doi: 10.1097/BRS.0000000000003639. [DOI] [PubMed] [Google Scholar]
- 40.Gorissen BMC, Wolschrijn CF, van Vilsteren AAM, et al. Trabecular bone of precocials at birth; Are they prepared to run for the wolf(f)? J Morphol. 2016;277(7):948–56. doi: 10.1002/jmor.20548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson KA. Wolff’s law continues to inspire orthopaedic research. Vet Comp Orthop Traumatol. 2014;27(1):V–VI. doi: 10.3415/VCOT-13-12-0142. [DOI] [PubMed] [Google Scholar]
- 42.Teichtahl AJ, Wluka AE, Wijethilake P, et al. Wolff’s law in action: A mechanism for early knee osteoarthritis. Arthritis Res Ther. 2015;17(1):207. doi: 10.1186/s13075-015-0738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lill CA, Fluegel AK, Schneider E. Sheep model for fracture treatment in osteoporotic bone: A pilot study about different induction regimens. J Orthop Trauma. 2000;14(8):556–59. doi: 10.1097/00005131-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Parfitt AM, Drezner MK, Glorieux FH, et al. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2(6):595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 45.Siu WS, Qin L, Cheung WH, Leung KS. A study of trabecular bones in ovariectomized goats with micro-computed tomography and peripheral quantitative computed tomography. Bone. 2004;35(1):21–26. doi: 10.1016/j.bone.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 46.Kapadia RD, Stroup GB, Badger AM, et al. Applications of micro-CT and MR microscopy to study pre-clinical models of osteoporosis and osteoarthritis. Technol Heal Care. 1998;6(5–6):361–72. [PubMed] [Google Scholar]
- 47.Lencel P, Magne D. Inflammaging: The driving force in osteoporosis? Med Hypotheses. 2011;76(3):317–21. doi: 10.1016/j.mehy.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 48.Weitzmann MN, Ofotokun I. Physiological and pathophysiological bone turnover – role of the immune system. Nat Rev Endocrinol. 2016;12(9):518–32. doi: 10.1038/nrendo.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bozan N, Alpaycı M, Aslan M, et al. Mean platelet volume, red cell distribution width, platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios in patients with ankylosing spondylitis and their relationships with high-frequency hearing thresholds. Eur Arch Otorhinolaryngol. 2016;273(11):3663–72. doi: 10.1007/s00405-016-3980-y. [DOI] [PubMed] [Google Scholar]
- 50.Tang Y, Peng B, Liu J, et al. Systemic immune-inflammation index and bone mineral density in postmenopausal women: A cross-sectional study of the national health and nutrition examination survey (NHANES) 2007–2018. Front Immunol. 2022;13:975400. doi: 10.3389/fimmu.2022.975400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishida M, Yagi M, Suzuki S, et al. Persistent low bone mineral density in adolescent idiopathic scoliosis: A longitudinal study. J Orthop Sci. 2022. [Online ahead of print] [DOI] [PubMed]
- 52.Sadat-Ali M, Al-Othman A, Bubshait D, Al-Dakheel D. Does scoliosis causes low bone mass? A comparative study between siblings. Eur Spine J. 2008;17(7):944–47. doi: 10.1007/s00586-008-0671-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee WTK, Cheung CSK, Tse YK, et al. Generalized low bone mass of girls with adolescent idiopathic scoliosis is related to inadequate calcium intake and weight bearing physical activity in peripubertal period. Osteoporos Int. 2005;16(9):1024–35. doi: 10.1007/s00198-004-1792-1. [DOI] [PubMed] [Google Scholar]
- 54.Cheung CSK, Lee WTK, Tse YK, et al. Generalized osteopenia in adolescent idiopathic scoliosis--association with abnormal pubertal growth, bone turnover, and calcium intake? Spine (Phila Pa 1976) 2006;31(3):330–38. doi: 10.1097/01.brs.0000197410.92525.10. [DOI] [PubMed] [Google Scholar]
- 55.Caţan L, Cerbu S, Amaricai E, et al. Assessment of static plantar pressure, stabilometry, vitamin D and bone mineral density in female adolescents with moderate idiopathic scoliosis. Int J Environ Res Public Health. 2020;17(6):2167. doi: 10.3390/ijerph17062167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Batista R, Martins DE, Hayashi LF, et al. Association between vitamin D serum levels and adolescent idiopathic scoliosis. Scoliosis. 2014;9(Suppl 1):O45. [Google Scholar]
- 57.Ng S-Y, Bettany-Saltikov J, Cheung IYK, Chan KKY. The role of vitamin D in the pathogenesis of adolescent idiopathic scoliosis. Asian Spine J. 2018;12(6):1127–45. doi: 10.31616/asj.2018.12.6.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balioglu MB, Aydin C, Kargin D, et al. Vitamin-D measurement in patients with adolescent idiopathic scoliosis. J Pediatr Orthop B. 2017;26(1):48–52. doi: 10.1097/BPB.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 59.Ryan PJ, Blake GM, Herd R, Parker J, Fogelman I. Distribution of bone mineral density in the lumbar spine in health and osteoporosis. Osteoporos. 1994;4(2):67–71. doi: 10.1007/BF01623225. [DOI] [PubMed] [Google Scholar]
- 60.Lau RW, Cheuk KY, Ng BK, et al. Effects of a home-based exercise intervention (E-Fit) on bone density, muscle function, and quality of life in girls with adolescent idiopathic scoliosis (AIS): A pilot randomized controlled trial. Int J Environ Res Public Health. 2021;18(20):10899. doi: 10.3390/ijerph182010899. [DOI] [PMC free article] [PubMed] [Google Scholar]


