Abstract
Despite therapeutic advancements, the prognosis of locally advanced non-small cell lung cancer (LANSCLC), which has invaded multiple lobes or the other lung and intrapulmonary lymph nodes, remains poor. The emergence of immunotherapy with immune checkpoint blockade (ICB) is transforming cancer treatment. However, only a fraction of lung cancer patients benefit from ICB. Significant clinical evidence suggests that the proinflammatory tumor microenvironment (TME) and programmed death-ligand 1 (PD-L1) expression correlate positively with response to the PD-1/PD-L1 blockade. We report here a liposomal nanoparticle loaded with cyclic dinucleotide and aerosolized (AeroNP-CDN) for inhalation delivery to deep-seated lung tumors and target CDN to activate stimulators of interferon (IFN) genes in macrophages and dendritic cells (DCs). Using a mouse model that recapitulates the clinical LANSCLC, we show that AeroNP-CDN efficiently mitigates the immunosuppressive TME by reprogramming tumor-associated macrophage from the M2 to M1 phenotype, activating DCs for effective tumor antigen presentation and increasing tumor-infiltrating CD8+ T cells for adaptive anticancer immunity. Intriguingly, activation of interferons by AeroNP-CDN also led to increased PD-L1 expression in lung tumors, which, however, set a stage for response to anti-PD-L1 treatment. Indeed, anti-PD-L1 antibody-mediated blockade of IFNs-induced immune inhibitory PD-1/PD-L1 signaling further prolonged the survival of the LANSCLC-bearing mice. Importantly, AeroNP-CDN alone or combination immunotherapy was safe without local or systemic immunotoxicity. In conclusion, this study demonstrates a potential nano-immunotherapy strategy for LANSCLC, and mechanistic insights into the evolution of adaptive immune resistance provide a rational combination immunotherapy to overcome it.
Keywords: locally advanced non-small cell lung cancer (LANSCLC), nanoparticle immunotherapeutic, aerosol inhalation, stimulator of interferon genes, programmed death 1/programmed death-ligand 1 (PD-1/PD-L1) blockade
Graphical Abstract
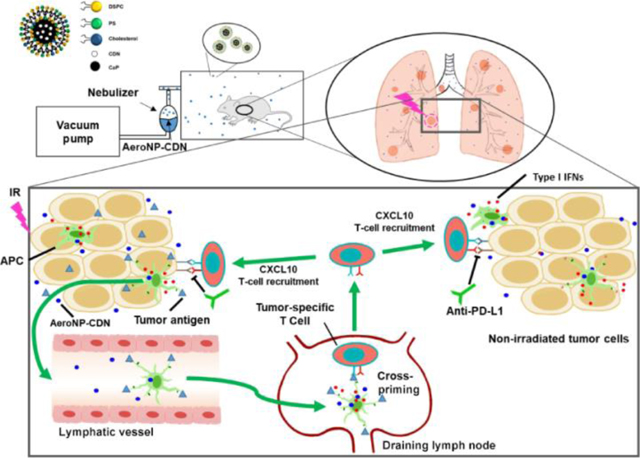
Inhalation of aerosolized nanoparticles loaded with cyclic dinucleotide (AeroNP-CDN) synergizes with irradiation and anti-programmed death-ligand 1 (PD-L1) immunotherapy for locally advanced non-small cell lung cancer (LANSCLC).
1. Introduction
Lung cancer is the second most common cancer in the United States, and non-small cell lung cancer (NSCLC) comprises the majority of cases [1, 2]. Locally advanced NSCLC (LANSCLC) with tumor cells invading lymph nodes and other lobes or the other lung, but without distant metastasis, represents about one third of the total NSCLC at diagnosis [3]. Often, patients at this stage are inoperable, and chemo-radiotherapy is the standard of care treatment. However, the prognosis for these patients is poor, with five-year survival rate of approximately 20% [4]. Immunotherapy, in particular, immunotherapeutic antibodies (Abs) against the immune checkpoint molecules cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed death 1 (PD-1) and its ligand, programmed death-ligand 1 (PD-L1), have shown impressive clinical efficacy in several solid tumors including NSCLC [5–7]. However, only a fraction of NSCLC patients benefit from the immune checkpoint blockade (ICB) therapy.
Clinical data suggest that tumor PD-L1 expression and tumor-infiltrating CD8+ lymphocytes (TILs) are biomarkers predictive of ICB responsiveness, and the inflamed NSCLC tumors with more TILs and the PD-L1-positive status generally show better response to anti-PD-1/PD-L1 antibodies [8]. Thus, immunomodulation of the tumor microenvironment (TME) to convert the immunosuppressive tumors into proinflammatory tumors likely benefits ICB immunotherapy in NSCLC. Much effort has been made to develop new immunomodulators as well as non-conventional pharmacological strategies in order to maximize the immune responses in tumors while reducing the off-target immunotoxicity to normal tissues. One of the potent immunostimulants, stimulators of interferon genes (STING) agonist, has recently been identified to play an important role in induction of antitumor immunity through its activation of STING signaling and type I interferons (IFNs) production [9–11]. While intratumoral injection of STING agonist, cyclic dinucleotide (CDN), has been investigated in preclinical studies, and is being evaluated in clinical trials for solid tumors, this approach is largely limited to those accessible tumors. Attempts are being made to develop nanoparticle or microparticle-loaded immunostimulants to facilitate extended in situ application [12–18] or systemic delivery to visceral tumors and metastases [19–28]. However, efficient delivery and possibly repeated applications of these immunostimulants to deep-seated tumors while avoiding systemic immunotoxicity remain technically challenging.
Previous studies have shown that activation of STING signaling and type I IFNs production within tumor–resident antigen-presenting cells (APCs) is necessary for induction of adaptive CD8+ T cell anticancer immunity [10, 29–31], whereas non-targeted free CDN may induce T cell apoptosis [32–34] or increase the resistance of tumor cells to ICB [35, 36]. Recently, we have developed a liposomal nanoplatform to efficiently load CDN and target CDN to macrophage and dendritic cells [37]. We have also established a nebulizer system to aerosolize the liposomal CDN (AeroNP-CDN) for inhalation delivery to deep-seated lung tumors. To further explore its utility, we established an orthotopic NSCLC mouse model that developed multifocal lung tumors in both lungs, which recapitulates the clinical LANSCLC. We tested the combination of AeroNP-CDN and irradiation (IR) in the LANSCLC mice. Although the combination treatment led to durable survival gains, a large fraction of the mice relapsed. We then examined the molecular and immunological response to the treatment and detected increased levels of PD-L1 in tumor tissues, which resulted plausibly from IFN-γ-mediated feedback inhibition [35, 38]. While the increased PD-L1 could attribute to the evolvement of T cell exhaustion and consequently treatment resistance, it may also provide an opportunity for a rational combination with anti-PD-1/PD-L1 ICB to reinvigorate the exhausted T cells. We thus investigated our hypothesis that AeroNP-CDN-induced proinflammatory TME and upregulation of PD-L1 would set the stage for response to anti-PD-L1 immunotherapy against lung tumors in the LANSCLC mouse model.
2. Results and discussion
2.1. Uptake of NP-CDN by macrophages and dendritic cells (DCs) and upregulation of type I IFN genes
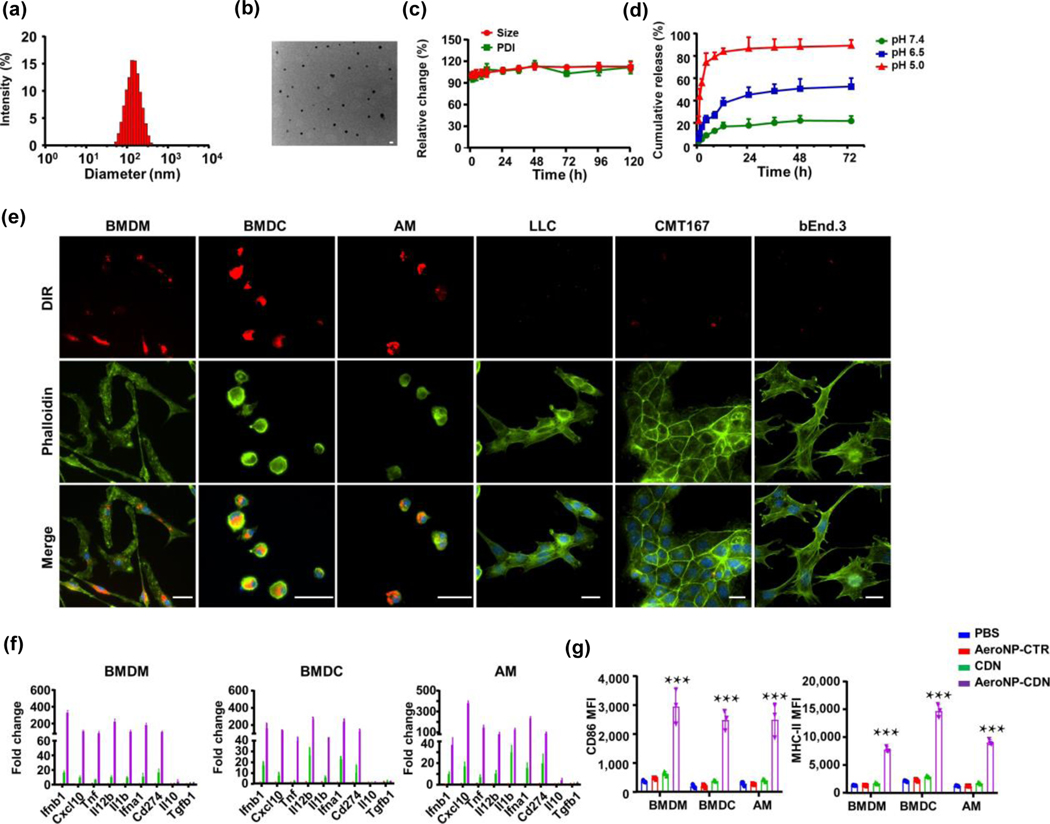
The CDN-calcium phosphate (CaP)-loaded, phosphatidylserine (PS)-coated NP-CDN has an average diameter of ~ 120 nm (Figs. 1(a) and 1(b)) and a negative surface charge of −40 mV. High-performance liquid chromatography (HPLC) analysis indicated high encapsulation efficiency of cGAMP (74.6% ± 5.1%) and high payload of cGAMP (32.5 ± 1.7 μg/mg lipid). Both the size and polydispersity index (PDI) of NP-CDN showed little change in phosphate buffered saline (PBS) with 10% fetal bovine serum (FBS) over a time course of 120 h (Fig. 1(c)). NP-CDN was stable at a physiological pH (7.4), but released CDN over time at 6.5 or 5.0 (Fig. 1(d)), indicating an acidic pH-responsive dissociation of the CDN-CaP complex. In vitro incubation of NP-DiR with APCs including bone marrow-derived macrophage (BMDM) and dendritic cell (BMDC), and alveolar macrophage (AM), or NSCLC Lewis lung carcinoma cells stably transfected with luciferase (LLC-Luc) and CMT-167 cells or vascular endothelial cells, bEnd.3 clearly showed preferential uptake of the nanoparticles by APCs over the other cell types (Fig. 1(e)). It is well known that membrane-exposed PS serves as an “eat me” signal for phagocytes via their surface PS receptors [39, 40]. After ingestion by APCs, real-time quantitative polymerase chain reaction (qPCR) measurements of mRNA levels of inflammation-related cytokines/chemokines in APCs revealed that NP-CDN (100 nM CDN) induced marked upregulation of type I IFNs and other inflammatory response genes such as Il-12b and Cxcl10 but minimal change in anti-inflammatory Il-10 and Tgfb1 (Fig. 1(f)). NP-CDN also promoted activation and maturation of APCs by overexpressing surface markers of CD86 and major histocompatibility complex (MHC) class II (Fig. 1(g)). By contrast, free CDN at 100 nM led to a smaller increase in the genes of inflammatory cytokines/chemokines and expression of CD86 and MHC-II (Figs. 1(f) and 1(g)). Structurally, CDN contains two phosphodiester bonds that restrict its penetration through the plasma membrane [11], which likely accounts for its inferior activity to NP-CDN.
Figure 1.
Characterization of NP-CDN and in vitro uptake studies. (a) Size distribution assessed by dynamic light scattering (DLS). (b) A representative transmission electron microscopy (TEM) image of NP-CDN, scale bar = 100 nm. (c) and (d) Stability of NP-CDN at pH 7.4 in 10% FBS (37 °C), but pH-dependent release of CDN. (e) In vitro uptake study by a 30 min-incubation of NP-DiR with APCs including BMDM, BMDC, and AM or NSCLC LLC and CMT167 cells or normal mouse vascular endothelial cells, bEnd.3. Immunofluorescence microscopy observed intracellular localization of NP-CDN in APCs. Scale bar = 25 μm. (f) Real-time qPCR measurements of mRNA levels in BMDM, BMDC, and AM after 4 h incubation with NP-CTR (red), free CDN (green), or NP-CDN (purple; 100 nM). NP-CTR: NP-2′ 5′ -GpAp as a control of NP-CDN. (g) NP-CDN induced increased expression of surface markers, CD86 and MHC-II on APCs. n = 3 biologically independent experiments. ***p < 0.001.
2.2. AeroNP-CDN targets CDN to macrophages and DCs in individual tumors of both lungs
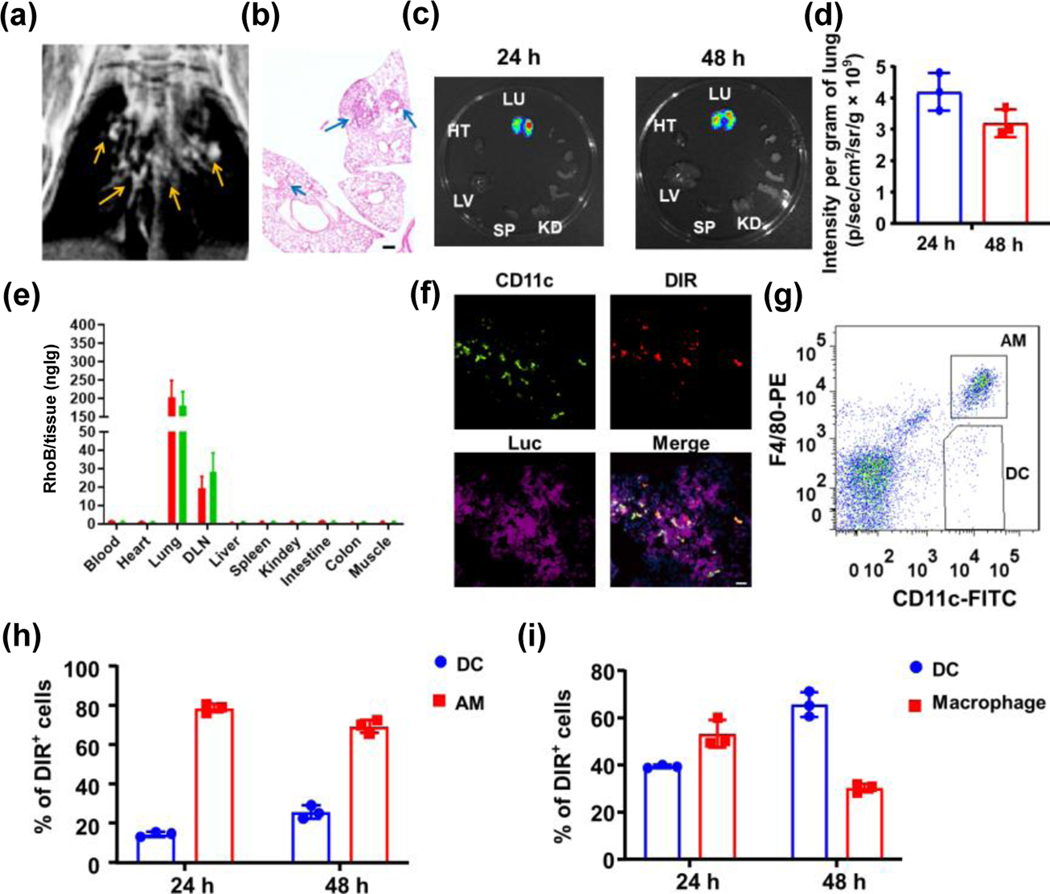
An orthotopic LANSCLC model that develops multiple lesions in both lungs has been established in this study, as confirmed by magnetic resonance imaging (MRI) and histology (Figs. 2(a) and 2(b)). The LANSCLC mice were placed in a chamber connected with a nebulizer system to expose to AeroNP-DiR. The nebulizer generates aerosols with a mass mean aerodynamic diameter (MMAD) of ~ 1.4 μm and geometric standard deviation (GSD) of 1.25, which falls into the size range optimal for deep lung deposition [37, 41]. Ex vivo IVIS optical imaging revealed exclusive accumulation of AeroNP-DiRs in both lungs at 24 h, which sustained at 48 h (Figs. 2(c) and 2(d)). HPLC analysis of major organs and tissues confirmed that the AeroNPs confined in lungs and tumor draining lymph nodes (DLNs) with minimal amount detected in blood or other organs (Fig. 2(e)). Immunohistochemical co-staining of Luc+ tumor cells detected intratumoral AeroNP-DiRs, where many of them were co-localizing with CD11c+ APCs (Fig. 2(f)). To determine which subsets of APCs were capturing AeroNPs, we conducted flow cytometry analysis and found more AeroNPs in AMs than DCs in tumor-bearing lung tissues (Figs. 2(g) and 2(h)). AeroNPs were also detected in DCs and CD11b+ macrophages at DLNs, and found more in DCs than macrophages at 48 h (Fig. 2(i)). We have previously demonstrated that we can control the dose of AeroNPs delivered to lung tissues by changing inhalation doses or durations [37]. Under the current protocol with a 28 min-inhalation of AeroNP-CDN (37 μM CDN) in 5 mL PBS, we estimated 0.1 μg CDN deposited in lungs of a mouse, which was less than one-hundredth of the dose of free CDN used for intratumoral injection in several studies [42, 43]. Taken together, our data demonstrate that AeroNP-CDN inhalation enables effective delivery of CDN to macrophages and DCs in individual lung tumors and their draining lymph nodes in both lungs.
Figure 2.
AeroNP targets macrophages and DCs in individual lung tumors. (a) and (b) An orthotopic LANSCLC mouse model bearing multiple lung tumors (arrows in an MRI and in hematoxylin and eosin (H&E) stained sections) in both lungs mimics the clinical LANSCLC. Scale bar = 200 μm. (c) and (d) Ex vivo IVIS imaging of major organs from LLC-Luc tumors-bearing mice detected DiR signals exclusively from both lungs 24 and 48 h post AeroNP-DiR. LU: Lung, HT: heart, LV: liver, SP: spleen, KD: kidney. (e) In addition to lung tissues, HPLC detected high concentrations of NPs in tumor draining lymph nodes at 24 h (red) and 48 h (green). (f) Co-staining of CD11c+ APCs and Luc+ tumor cells in tumor-bearing lung tissue sections revealed intratumoral NP-DiRs, many of which co-localized with CD11c+ APCs. Scale bar = 20 μm. (g) Flow cytometry gating for the subsets of APCs, CD11c+ DCs and CD11c+F4/80+ AMs and (h) their differential uptake of AeroNP-DiRs in tumor-bearing lung tissues. (i) AeroNP-DiRs were also detected in CD11c+ DCs and CD11b+F4/80+ macrophages in DLNs. n = 3 biologically independent experiments.
2.3. AeroNP-CDN remodels the immune landscape in individual lung tumors and enhances radiation therapy
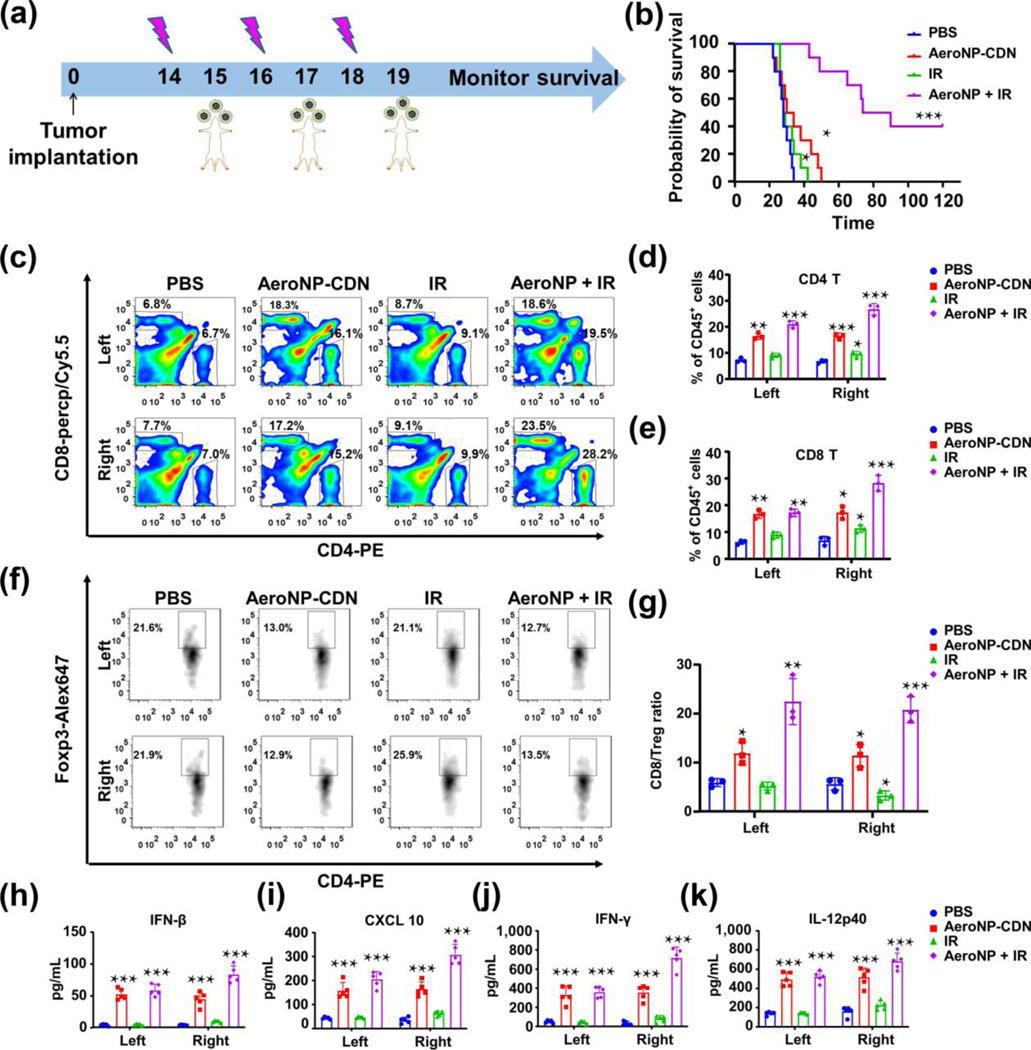
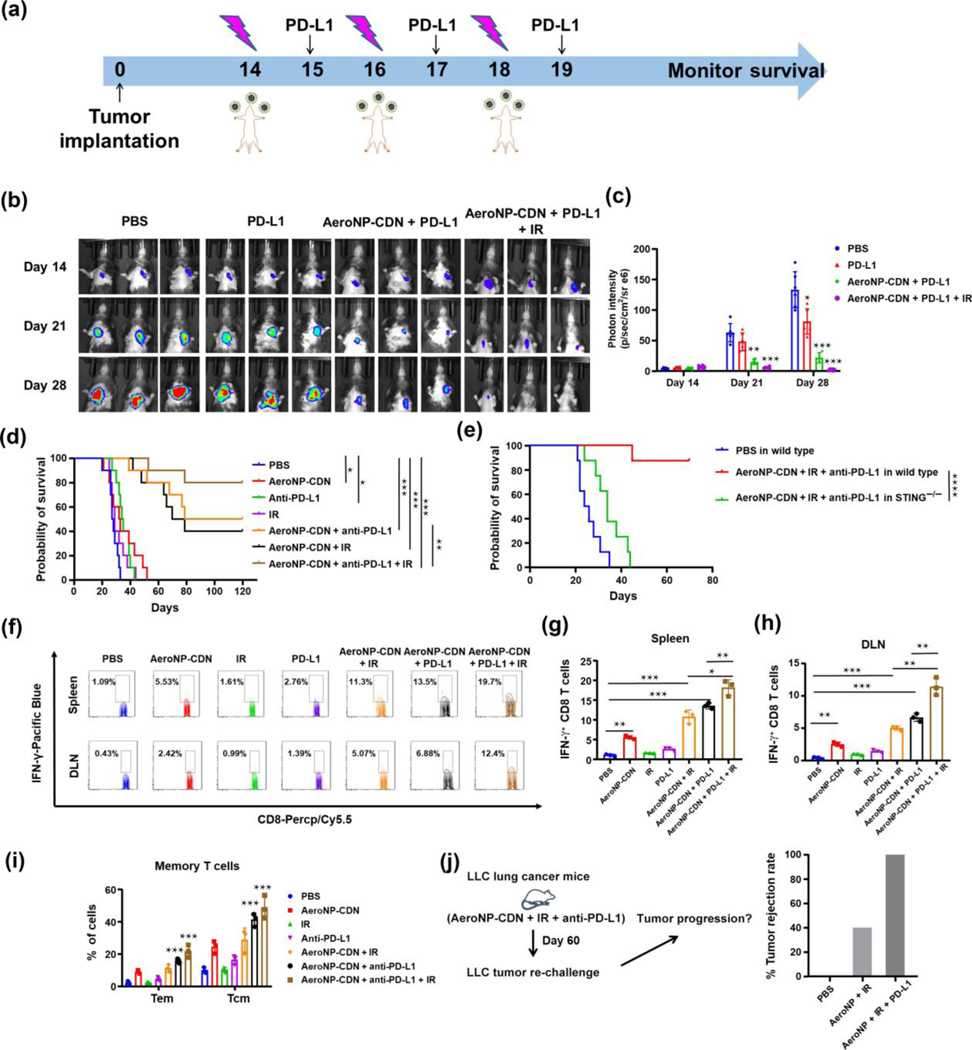
Radiotherapy is a standard of care treatment for lung cancer. Previous studies by others and us have shown that STING agonist in combination with focal IR elicits robust antitumor immune responses in multiple cancer models [37, 42]. Here, we investigated the therapeutic efficacy of AeroNP-CDN in combination with IR in the LANSCLC mouse model. After imaging confirmation of lung tumor development on day 14, a region bearing lung tumors in the right lung was irradiated with 6 Gy/every other day for 3 doses, while AeroNP-CDN was inhaled (a 28 min-inhalation of AeroNP-CDN (37 μM CDN) in 5 mL PBS) a day after each IR (Fig. 3(a)). AeroNP-CDN + IR significantly prolonged survival of the mice, 40% of which survived for at least 120 days (Fig. 3(b)). By contrast, focal IR alone showed minimal survival benefit (Fig. 3(b)). While radiation is known to cause immunogenic cell death by releasing tumor antigens (TAs), it rarely induces local or systemic immune response against non-irradiated tumors [44–46].
Figure 3.
AeroNP-CDN enhances radiation treatment. (a) Treatment of LLC-bearing mice with AeroNP-CDN, focal IR (to a tumor-bearing region in the right lung) or both (n = 6–8/group) was initiated on day 14 after confirming development of lung tumors. (b) Therapeutic efficacy was evaluated by Kaplan–Meier survival assay. (c)–(e) Flow cytometry detected significantly increased numbers of tumor-infiltrating CD4+ and CD8+ T cells in the left and right lung 48 h after the first dose of AeroNP-CDN alone or combined with IR (c). Quantitative data of CD4+ T cells and CD8+ T cells were presented in (d) and (e), respectively. (f) Regulatory T cells (Treg; Foxp3+CD4+) were quantified and (g) ratios of CD8+ T/Treg were plotted. (h)–(k) ELISA measurements of IFN-β, IFN-γ, IL-12p40, and CXCL10 in tumor-bearing lung tissues from the left and right lung. n = 3 biologically independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001.
To interrogate the treatment-induced immune responses, we analyzed the tumor-bearing lung tissues from both lungs by flow cytometry (Fig. S1 in the Electronic Supplementary Material (ESM)). Our data showed that AeroNP-CDN alone or in combination with IR induced increased expression of CD86 and MHC-II in both AMs and CD11c+CD103+ DCs (Fig. S2 in the ESM). Notably, CD103+/CD8α+ DCs have been indicated as the most competent APCs for cross-priming CD8+ T cells in mice [47, 48]. AeroNP-CDN alone or the combination treatment also led to a significant increase in tumor-infiltrating CD4+ and CD8+ T cells in both lungs (Figs. 3(c)–3(e)). Whereas IR induced some increase in regulatory T cells (Treg; Foxp3+CD4+) in the irradiated right lung, AeroNP-CDN decreased the number of Treg, and consequently a significantly increased ratio of CD8+ T/Tregs in both lungs (Figs. 3(f) and 3(g)). A high ratio of CD8+ T/Tregs has been indicated in clinic to correlate with favorable response to immunotherapy in NSCLC and other cancer types [49, 50]. In line with the flow cytometry results, enzyme-linked immunosorbent assay (ELISA) measurements of cytokines/chemokines in tumor tissues showed significant increase in type I IFN, IFN-β, and other pro-inflammatory IL-12p40, IFNγ, and CXCL10 (Figs. 3(h)–3(k)). CXCL10 is an important chemokine responsible for recruitment of T cells [48], which likely contributed to the increased number of TILs in not only the irradiated but also the non-irradiated lung. Collectively, our data indicate that AeroNP-CDN promotes the proinflammatory TME, activates both the innate APCs and adaptive cytotoxic T cells, and synergizes with focal irradiation against the LANSCLC.
2.4. AeroNP-CDN upregulates PD-L1 and blockade of PD-L1 enhances antitumor immune responses
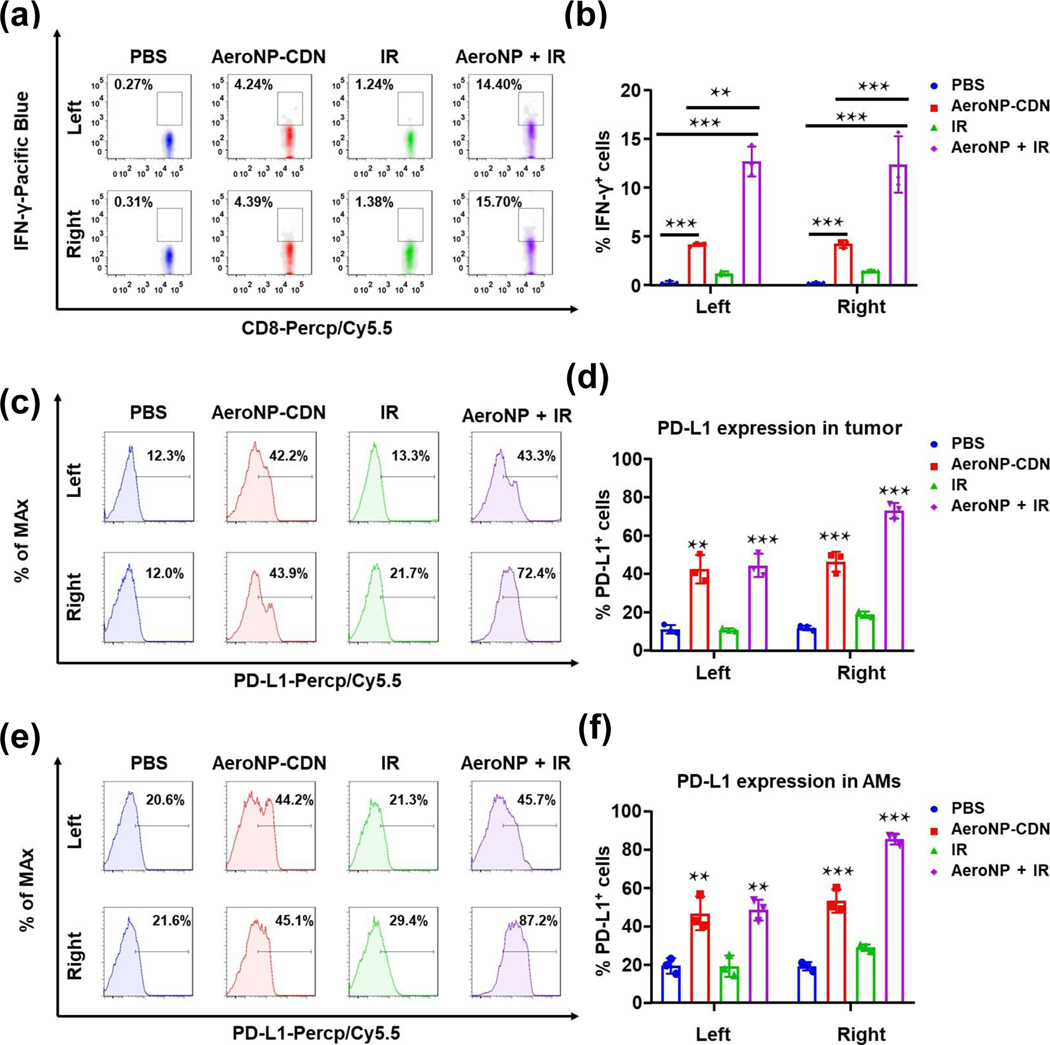
To further explore its therapeutic potential for enhanced anticancer efficacy, we looked into the immunological responses to AeroNP-CDN. AeroNP-CDN stimulates APCs to produce type I IFNs and IL-12 (Figs. 3(h) and 3(k)), which can activate T cells to secret IFN-γ and exert their antitumor cytotoxic effects [10, 35]. Indeed, our flow cytometry data showed that AeroNP-CDN significantly increased frequency of IFN-γ-secreting CD8+ T cells, which was further enhanced with the combination of IR (Figs. 4(a) and 4(b)). However, it has been recognized that certain proinflammatory cytokines, in particular, IFNγ, induce PD-L1 expression as a feedback inhibitory mechanism that limits local immunity [35, 51]. We thus investigated if AeroNP-CDN could induce PD-L1 expression in tumor tissues. As expected, AeroNP-CDN alone or in combination with IR induced a drastic increase in tumor cells expressing PD-L1 in both lungs, compared to the control PBS treatment (Figs. 4(c) and 4(d)). Besides tumor cells, there was significantly increased PD-L1 expression on CD11c+F4/80+ AM (Figs. 4(e) and 4(f)). PD-L1 on stromal cells such as macrophages also contributes to T cell exhaustion via the PD-1/PD-L1 axis [52].
Figure 4.
AeroNP-CDN activates effector CD8+ T cells while upregulating PD-L1. (a) and (b) AeroNP-CDN alone or in combination with focal IR activated CD8+ T cells to secret IFN-γ. (c)–(f) Upregulation of PD-L1 was detected on the surface of tumor cells ((c) and (d)) and AMs ((e) and (f)) in both lungs. n = 3 biologically independent experiments. **p < 0.01; ***p < 0.001.
Increased expression of PD-L1 in tumor tissues could attribute to evolvement of T cell exhaustion and a sequel to treatment resistance, as observed in Fig. (3). However, we reasoned that a combination with PD-L1 blockade would augment antitumor immunity of AeroNP-CDN by counteracting the overexpressed PD-L1. Indeed, therapeutic efficacy of anti-PD-L1 Abs combined with AeroNP-CDN was found comparable to that of IR plus AeroNP-CDN, and the triple combination treatment achieved long-term survival in 80% of the mice in the wild type but not STING−/− mice bearing LLC-Luc lung cancer (Figs. 5(a)–5(e)). Similar to IR, PD-L1 blockade alone showed a little survival benefit. To interrogate if the combination treatment could induce systemic antitumor immune responses, we examined both DLNs and spleens of the treated mice and found that there was indeed a significant increase in IFN-γ+ CD8+ T cells in both tissues in response to AeroNP-CDN in combination with anti-PD-L1 Ab and/or IR (Figs. 5(f)–5(h)). We also observed marked increase in the number of memory CD8+ T cells including both CD44+CD62L+ central memory and CD44+CD62L− effector memory T cells in spleens of the mice treated with the combination immunotherapy with/without IR (Fig. 5(i)). Moreover, the long-term surviving mice from the triple combination treatment group resisted secondary tumor challenge (Fig. 5(j)), further supporting that the treatment triggers systemic antitumor memory. Together, combination immunotherapy with blockade of PD-L1, which was upregulated by AeroNP-CDN, represents a rational strategy.
Figure 5.
Anti-PD-L1 Ab enhances antitumor activity of AeroNP-CDN. (a) LLC-Luc-bearing mice were treated with IR (morning) and AeroNP-CDN (afternoon), and followed by anti-PD-L1 Ab on next day. (b)–(d) Treatment efficacy was monitored by IVIS imaging ((b) and (c)) and Kaplan–Meier survival assay (d). (e) Kaplan–Meier survival curves of indicated treatment in wild type mice or STING knockout mice. n = 8/treatment. (f)–(h) Spleens and DLNs from the mice with indicated treatment were analyzed by flow cytometry for IFN-γ+ activated CD8+ effector T cells. (i) Memory CD8+ T cells of both CD44+CD62L+ central memory T cells (Tcm) and CD44+CD62L− effector memory T cells (Tem) detected in spleens on day 26. (j) Mice treated with AeroNP-CDN + IR or AeroNP-CDN + IR + anti-PD-L1 Ab were re-challenged 80 days later with LLC-Luc cells. Naive mice challenged at the same time served as positive controls. Data showed the percent of mice rejecting LLC-Luc tumor re-challenge in each group. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
2.5. AeroNP-CDN in combination with ICB is safe
We have examined the safety of inhalation of AeroNP-CDN. Our initial studies showed that AeroNP-CDN was well-tolerated by unanesthetized mice, and the same inhalation dose as used in this study with/without focal irradiation did not cause any short or long-term adverse events in healthy or lung metastasis mice [37]. Immunotherapy with anti-PD-1/PD-L1 and anti-CTLA4 Abs is frequently associated with immunopathology. We thus investigated if the combination immunotherapy of AeroNP-CDN with anti-PD-L1 Ab may induce immunotoxicity locally to lungs or systemically to distant organs. Histopathological examinations of tissues from lung, kidney, heart, and liver revealed no morphological difference between the control PBS, AeroNP-CDN alone, and the combination treatment (Fig. 6(a)). There was no significant change in liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) or serum levels of various cytokines (Figs. 6(b) and 6(c) and Fig. S3 in the ESM). Liposome is biocompatible and widely used as a drug carrier [53–55]. Altogether, the low dose of AeroNP-CDN, the inhalation approach, and APC-targeted delivery led to negligible amounts of CDN entering other types of lung cells or the blood circulation (Fig. 2(c)–2(g)), which contributed to its excellent safety.
Figure 6.
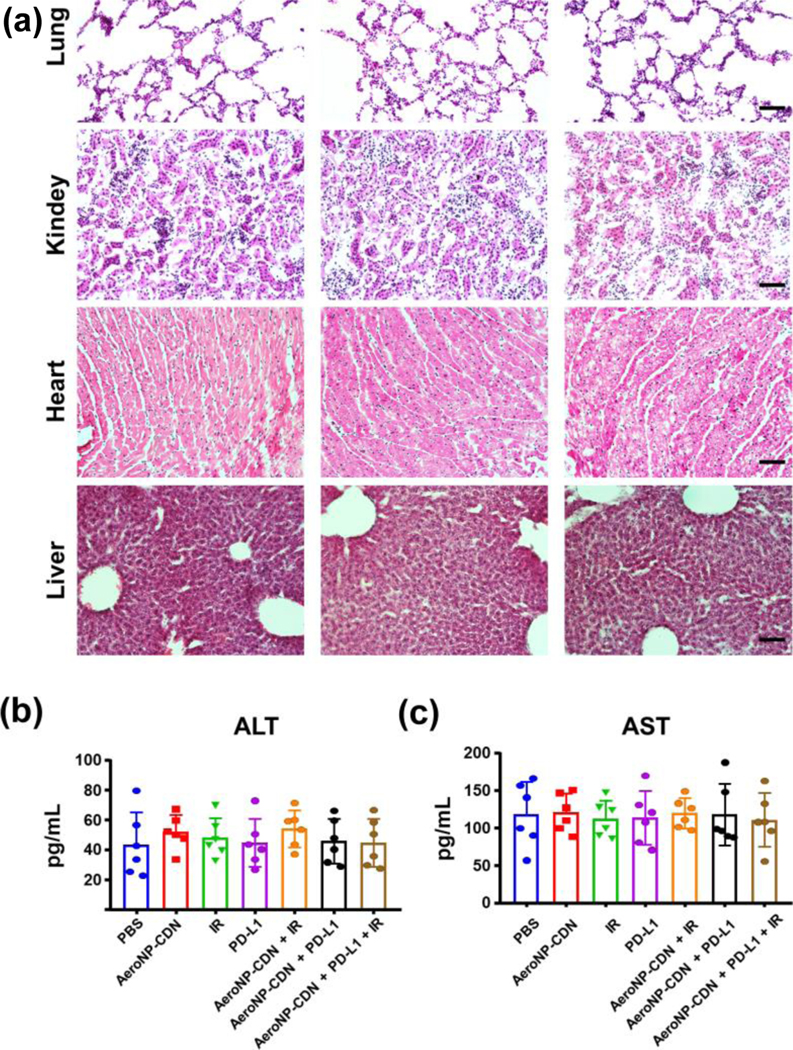
Immunotherapy of AeroNP-CDN with anti-PD-L1 Ab is safe. (a) H&E staining of major organs from healthy mice with indicated treatment on day 26 showed no morphological change in these organs between the PBS control and AeroNP-CDN alone or in combination with anti-PD-L1 Ab. (b) and (c) Serum levels of AST and ALT were measured in the lung cancer mice with indicated treatment (n = 6).
3. Conclusion
In summary, we have demonstrated an inhalable nanoparticle system that enables delivery of immunotherapeutics to deep-seated lung tumors for targeted activation of APCs-mediated antitumor immune responses. We show that AeroNP-CDN transforms the immune landscape in lung tumors and their draining lymph nodes in both lungs in a mouse model mimicking the locally advanced NSCLC. Therapeutically, AeroNP-CDN synergizes with focal irradiation to engage both the innate and adaptive antitumor immunity. Furthermore, combination immunotherapy with anti-PD-L1 Abs, which counteract IFN-γ signaling-induced overexpression of PD-L1 in tumor tissues, elicits durable systemic antitumor immune responses, conferring long-term survival of the LANSCLC mice. Because it is non-invasive, accessible to multiple lung lesions/lobes at the same time and feasible for repeated uses without immunotoxicity, this nanoparticle system may have the translational potential for treating lung cancer.
4. Methods
4.1. Materials
1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC, 18:0 PC), cholesterol, brain PS (L-α-phosphatidylserine), and Rhod-b (18:1 Liss Rhod PE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl)) were purchased from Avanti Polar Lipids. CDN (2′3′-cGAMP, cyclic [G(2′,5′)pA(3′,5′)p]) and its control 2′ 5′-GpAp were purchased from InvivoGen. Anti-PD-L1 (10F.9G2) was purchased from BioXcell.
4.2. Preparation and characterization of AeroNP-CDN
The liposomal CDN was prepared in two steps using the water-in-oil reverse microemulsion method, as detailed in our previous study [37]. In brief, 150 μL CaCl2 (2.5 M in ddH2O, Ph = 7.0) was added to 5 mL mixed oil phase 1 (cyclohexane:igepal co-520 = 80:20) and stirred to form a well-dispersed micro-emulsion phase. Oil phase 2 was prepared by adding 50 μL lipid mixture (20 mM, PS:DSPC:cholesterol = 5:4:1) to 5 mL mixed oil phase (cyclohexane:igepal co-520 = 80:20). A similar micro-emulsion containing sodium phosphate was prepared by adding 150 μL Na2HPO4 (25 mM in ddH2O, pH = 9.0) to 5 mL mixed oil phase 2. The CaP core with the single lipid coating was formed by mixing oil phase 1 and oil phase 2. After washing with ethanol three times, the collected CaP cores were dispersed in 1 mL chloroform and centrifuged at 2,000g for 5 min. The supernatants were further mixed with 70 μL of the lipid mixture, followed by chloroform evaporation under reduced pressure to form a lipid film. NP-CaP nanoparticles with bilayer lipid coating were formed by adding 1 mL PBS and rehydrating under water bath sonication for 5 min and sonic probe at 20 W for 2 min at 70 °C. The resulting nanoparticles were further filtered with 0.45 μm membrane to remove the free lipid aggregates and stored at 4 °C. To load CDN, half of the desired content CDN was mixed with each of the CaCl2 and Na2HPO4 solutions. For fluorescently labeling NPs, 18:1 Liss Rhod PE (Rhod-b) was added to the second lipid mixture with a molar ratio of 1%. DiR was further used for labeling the NP (NP-DiR) by adding DiR directly to the second lipid mixture at a molar ratio of 5%. The size, size distribution, and zeta potential of AeroNP-CDN in aqueous solution were measured by a Malvern Zetasizer Nano ZS90. TEM measurements were performed on an FEI Tecnai Bio Twin transmission electron microscope. To determine the CDN loading efficiency and release kinetics, HPLC analyses were conducted using an Agilent 1100 HPLC system.
4.3. Cells
NSCLC cells including luciferase-expressing Lewis lung carcinoma cells (LLC-Luc, provided by Dr. Yong Lu, Wake Forest) and CMT-167 (ATCC), and mouse vascular endothelial cells, bEnd.3 (ATCC) were maintained in Dulbecco’s modified Eagle medium (DMEM), supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. BMDMs were generated and cultured in complete α-MEM containing 10% heat-inactivated FBS, penicillin/streptomycin, and 50 ng/mL macrophage colony-stimulating factor (M-CSF). BMDCs were induced and cultured in RPMI-1640 containing 10% heat-inactivated FBS, penicillin/streptomycin, 20 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF), 5 ng/mL interleukin 4 (IL-4), and 1× 2-mercaptoethanol. AMs from bronchoalveolar lavage (BAL) were cultured in DMEM with 10% heat-inactivated FBS, 2-mercaptoethanol (1×), and penicillin/streptomycin. All the cells were maintained in a humidified atmosphere containing 5% CO2 at 37 °C.
4.4. Cellular uptake of PS-coated nanoparticles in vitro
Briefly, 6,000 BMDM, BMDC, AM, LLC-Luc, CMT-167, or bEnd.3 cells were seeded in 24 well plates with poly-lysine coated coverslips for 24 h before adding NP-DiR. After 0.5 h, cells were then washed twice with cold PBS and fixed with 4% formaldehyde for 15 min. After 1% Triton X-100 treatment for another 15 min, the cells were co-stained with Alexa Fluor 488 phalloidin (Thermo Fisher) for 30 min and then with 4’,6-diamidino-2-phenylindole (DAPI) for 5 min. The cellular uptake of NP-DiR was observed by fluorescence microscope.
4.5. Real-time qPCR of type I IFN and other inflammatory genes
BMDM, BMDC, or AM cells (2 × 105) were seeded into 35-mm culture dishes and cultured for 24 h. The cells were then incubated at 37 °C with free CDN (100 nM) or AeroNP-CDN (100 nM CDN) suspended in complete culture medium for 4 h. All cellular RNA was collected using TRIzol reagent (Thermo Fisher), of which 1 μg of total RNA was transcribed into cDNA using High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher) and real-time qPCR was performed using PowerUp SYBR Green Master Mix (Thermo Fisher) with primers listed (Table S1 in the ESM). mRNA levels were normalized against the housekeeping gene GAPDH.
4.6. Flow cytometry
Flow cytometry was performed on a BD Canto II flow cytometer and analyzed using FlowJo software (BD Biosciences). CD3-FITC (17A2), CD4-PE (GK1.5), CD8α-PerCP-Cyanine5.5 (53–6.7), CD45-APC-Cyanine7 (30-F11), CD45-Pacific Blue (30-F11), CD11b-FITC (M1/70), CD11b-PE-Cyanine7 (M1/70), F4/80-PE (BM8), CD11c-FITC (N418), PD-L1-PerCP-Cyanine5.5 (10F.9G2), PD-L1-PE (10F.9G2), PD-L1-APC (10F.9G2), CD86-APC-Cyanine7 (GL-1), MHC-II-APC (M5/114.15.2), CD103-Pacificl Blue (2E7), CD44-APC (IM7), CD62L-PE-Cyanine7 (MEL-14), IFN-γ-Brilliant Violet 510 (XMG1.2), and CD16/CD32 (93) were purchased from Biolegend (San Diego, CA). FOXP-3-Alexa Fluor 647(MF-14) and intracellular staining kit for Foxp-3 were from eBioscience (Thermo Fisher Scientific, Waltham, MA). For intracellular staining of IFN-γ, fresh isolated cells were treated with phorbol 12-myristate 13-acetate/ionomycin cocktail according to the manufacturer’s specification (BioLegend). Doublets and debris of dead cells were excluded before various gating strategies were applied. Gates and quadrants were set based on isotype control staining, and the mean fluorescence intensity (MFI) values were calculated by minus the MFI of isotype control antibodies.
4.7. ELISA assay
Cytokines were analyzed with ELISA Kit from BioLegend by following the manufacturer’s instructions. After adding the horseradish peroxidase (HRP) substrates, optical densities were determined at a wavelength of 450 nm in an ELISA plate reader (Bio-Rad).
4.8. Liver enzyme assay
Serum aspartate transaminase activity (Enzychrom Aspartate Transaminase Assay kit, BioAssay Systems, Haymard, CA) and alanine transaminase activity (Enzychrom Alanine Transaminase Assay kit, BioAssay Systems) were performed following manufacturer’s instructions.
4.9. Establishment of an orthotopic NSCLC mouse model
All animal experiments complied with all relevant ethical regulations for animal testing and research and were performed with approved of the Institutional Animal Care and Use Committee at the Wake Forest University School of Medicine. Anesthetized C57BL/6 mice (6–8 weeks, female:male at 1:1; Charles River Laboratories, Wilmington, MA) or STING−/− mice (The Jackson Laboratory, Bar Harbor, MA) were instilled intratracheally with LLC-Luc cells (2 × 106) in 50 μL PBS buffer with 0.1 M ethylene diamine tetraacetic acid (EDTA) [56]. The Intubation Illumination System (Braintree Scientific, Braintree, MA) with fiber-optic light was used for guiding an intratracheal catheter insertion. By day 14, multifocal primary lung carcinomas in both sides of lungs were confirmed by MRI and bioluminescence imaging (BLI). Development of LLC-Luc lung cancer was monitored by longitudinal BLI. In vivo studies of pharmacokinetics, immunological effects and treatment were subsequently conducted on the mice, as described below in details.
4.10. Aerosolized PS-coated nanoparticles inhalation
Aerosolized nanoparticles were generated by a custom-built nebulizing system. A clear plastic box with a wire-netting floor was used for inhalation treatment. Aerosol was generated via a medical-grade nebulizer (MEDNEB Compressor Nebulizer, REF MQ5600, Drive DeVilbiss Healthcare, NY) attached by medical tubing to the animal chamber. Aerosol was discharged from an opening in the opposite end of the cage. For in vivo biodistribution study, DiR- or Rhod-b-labeled PS-coated NP-CaP (DiR 700 μg; Rhod-b 100 μg) in 5 mL PBS was loaded into the nebulizer. For in vivo treatment in LLC lung cancer mouse model, AeroNP-CDN (37 μM CDN) in 5 mL PBS was nebulized and delivered to unanesthetized mice via aerosol inhalation.
4.11. In vivo biodistribution and quantification
C57BL/6 mice bearing multifocal LLC-Luc lung tumors were placed in the chamber to inhale NP-DiR for 28 min. The animals were sacrificed at 24 and 48 h (n = 3/time) and major organs were dissected and ex vivo imaging was conducted using an IVIS® Lumina system (Caliper Life Sciences). The signal intensity was analyzed using Living Image® 3.1 software. Immediately after ex vivo imaging, the tumors-bearing lung tissues were preserved and sectioned for immunofluorescence microscopy. Cryosections (6 μm thick) were co-stained with anti-mouse CD11c-FITC (1:200; BioLegend, N418) and anti-luciferase (1:500; Sigma-Aldrich, L0159) followed by cy3-anti-rabbit secondary antibody (1:800; Jackson Immuno) and observed using a fluorescence microscope. DiR signals were recorded and merged with the CD11c image and the luciferase-stained image of the same field. DiR+ cells in lung tissues and DLNs were also assessed by fluorescence-activated cell sorting (FACS). To quantify tissue concentrations of PS-coated nanoparticles, the LLC-Luc lung cancer mice were sacrificed at 24 and 48 h after inhaling NP-Rhod-b (n = 3/time). Major organs and blood were collected for HPLC analyses.
4.12. Focused irradiation
Animals were anesthetized with inhaled 3% isoflurane and positioned with the lung 50 cm below the aperture of an X-RAD 320 orthovoltage irradiator (Precision X-ray). Fractionated irradiation (6 Gy × 3 daily fractions) was delivered at a rate of 176 cGy/min (300 kVp voltage and 10 mA current) through a custom fabricated lipowitz alloy shield. The semi-circular aperture in the lipowitz alloy shield with a radius of 5 mm was placed~ 3.5 cm above the animal. This shield yields a semi-circular field of approximately 1.08 cm in diameter, allowing radiation beam to a specified area of the right lung with tumors while minimizing the radiation dose to important mediastinum and normal tissues. To ensure good reproducibility, the light-field was marked on the animal for the second and third irradiation.
4.13. MRI
MRI was conducted blinded on a 7T Bruker BioSpec small animal scanner (Bruker Biospin, Rheinstetten, Germany). Mice were anesthetized with isoflurane (3% induction, 1.5% maintenance). Respiration was monitored with a respiratory bulb under the chest and a SHARPII animal monitoring system was used for respiratory gating. Anatomical T2-weighted imaging was conducted using a RARE sequence with repetition time/echo time (TR/TE) = 1,600/23 ms, echo train length (ETL): 8, number of signal averages/acquisitions (NSA): 8, matrix size: 128 × 128, and scan time: 3 min 53 s.
4.14. In vivo experimental treatment
For the LLC-Luc-bearing mice, multifocal lung lesions in both lungs were visualized by BLI and MRI before treatment on day 14. The mice (n = 8/group) were then randomly grouped and treated as follows: (i) PBS (inhalation), (ii) inhalation of AeroNP-CDN, (iii) anti-PD-L1 (10F.9G2; 100 μg/mouse × 3, i.p.), (iv) IR (6 Gy × 3, to the tumor-bearing right lung), (v) IR (6 Gy × 3) + AeroNP-CDN, (vi) AeroNP-CDN + anti-PD-L1, and (vii) IR + AeroNP-CDN + anti-PD-L1. Orthotopic lung cancer burden was monitored longitudinally by BLI. Data were quantified with the Living Imaging software by using absolute photon counts (photons/s/cm2/Sr) in a region of interest (ROI), manually drawn to outline the BLI signal of the chest. Survival of the mice was followed for up to 120 days. For tumor re-challenge study, surviving mice from the combination treatment group (80 days) were injected subcutaneously with LLC-Luc cells (2 × 106). Additional group of mice without previous treatment were also injected and observed for tumor growth.
4.15. Histology
H&E staining was performed on cryosections (10 μm) of different tissues including normal heart, lung, liver, and spleen, as well as lung cancer bearing lungs.
4.16. Statistical analysis
Statistical analysis was performed using Microsoft Excel and Prism 7.0 (GraphPad). No priori sample size calculation was performed. No inclusion and exclusion of data were conducted. Treatment groups were not blinding to investigators. Confounders were not controlled during animal study. Data were presented as mean ± SD. Statistical significance was determined by Student’s t-test. All t-tests were one-tailed and unpaired and were considered statistically significant if p < 0.05. The survival assay was analyzed using a log-rank test and considered statistically significant if p < 0.05.
Supplementary Material
Acknowledgements
We thank Dr. Yong Lu for providing the LLC-Luc cell line, and Drs. J. Daniel Bourland and Ravi Singh for technical and collegial support. The research is supported in part by NIH/NCI 1R01CA264102-01 (D. Z.) and Wake Forest Comprehensive Cancer Center P30 CA01219740. A. A. H. is supported by funding from the Department of Veteran’s Affairs (No. 2I01BX002559-07) and from the National Institutes of Health (No. 1R01CA244212-01A1).
Footnotes
Electronic Supplementary Material:
Supplemental material (a list of primers for real-time PCR; flow cytometry gating strategy; flow data of DC maturation markers; ELISA of serum cytokines) is available in the online version of this article at https://doi.org/10.1007/s12274-022-5205-6.
References
- [1].American Cancer Society. What is non-small cell lung cancer? [Online]. 2019. https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/what-is-non-small-cell-lung-cancer.html (accessed May 30, 2022)
- [2].National Cancer Institute, SEER. Cancer stat facts: Lung and bronchus cancer [Online]. 2019. https://seer.cancer.gov/statfacts/html/lungb.html (accessed May 30, 2022)
- [3].Postmus PE; Kerr KM; Oudkerk M; Senan S; Waller DA; Vansteenkiste J; Escriu C; Peters S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol 2017, 28, IV1–IV21. [DOI] [PubMed] [Google Scholar]
- [4].Aupérin A; Le Péchoux C; Rolland E; Curran WJ; Furuse K; Fournel P; Belderbos J; Clamon G; Ulutin HC; Paulus R. et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J. Clin. Oncol 2010, 28, 2181–2190. [DOI] [PubMed] [Google Scholar]
- [5].Barlesi F; Vansteenkiste J; Spigel D; Ishii H; Garassino M; de Marinis F; Özgüroğlu M; Szczesna A; Polychronis A; Uslu R. et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): An open-label, randomised, phase 3 study. Lancet Oncol. 2018, 19, 1468–1479. [DOI] [PubMed] [Google Scholar]
- [6].Brahmer JR; Tykodi SS; Chow LQM; Hwu WJ; Topalian SL; Hwu P; Drake CG; Camacho LH; Kauh J; Odunsi K. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med 2012, 366, 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brahmer J; Reckamp KL; Baas P; Crinò L; Eberhardt WEE; Poddubskaya E; Antonia S; Pluzanski A; Vokes EE; Holgado E. et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med 2015, 373, 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yu YF; Zeng DQ; Ou QY; Liu SB; Li AL; Chen YJ;Lin DG; Gao QL; Zhou HY; Liao WJ et al. Association of survival and immune-related biomarkers with immunotherapy in patients with non-small cell lung cancer: A meta-analysis and individual patient-level analysis. JAMA Netw. Open 2019, 2, e196879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Barber GN STING: Infection, inflammation and cancer. Nat. Rev. Immunol 2015, 15, 760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Woo SR; Fuertes MB; Corrales L; Spranger S; Furdyna MJ; Leung MYK; Duggan R; Wang Y; Barber GN; Fitzgerald KA et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 2014, 41, 830–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sun L; Wu J; Du F; Chen X; Chen ZJ Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu Y; Wang LL; Song QQ; Ali M; Crowe WN; Kucera GL; Hawkins GA; Soker S; Thomas KW; Miller LD et al. Intrapleural nano-immunotherapy promotes innate and adaptive immune responses to enhance anti-PD-L1 therapy for malignant pleural effusion. Nat. Nanotechnol 2022, 17, 206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Park CG; Hartl CA; Schmid D; Carmona EM; Kim HJ; Goldberg MS Extended release of perioperative immunotherapy prevents tumor recurrence and eliminates metastases. Sci. Transl. Med 2018, 10, eaar1916. [DOI] [PubMed] [Google Scholar]
- [14].Chao Y; Xu LG; Liang C; Feng LZ; Xu J; Dong ZL; Tian LL; Yi X; Yang K; Liu Z. Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses. Nat. Biomed. Eng 2018, 2, 611–621. [DOI] [PubMed] [Google Scholar]
- [15].Lee D; Huntoon K; Wang YF; Jiang W; Kim BYS Harnessing innate immunity using biomaterials for cancer immunotherapy. Adv. Mater 2021, 33, 2007576. [DOI] [PubMed] [Google Scholar]
- [16].Jin QT; Liu Z; Chen Q. Controlled release of immunotherapeutics for enhanced cancer immunotherapy after local delivery. J. Control. Release 2021, 329, 882–893. [DOI] [PubMed] [Google Scholar]
- [17].Chen Q; Wang C; Zhang XD; Chen GJ; Hu QY; Li HJ; Wang JQ; Wen D; Zhang YQ; Lu YF et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol 2019, 14, 89–97. [DOI] [PubMed] [Google Scholar]
- [18].Zhang BD; Wu JJ; Li WH; Hu HG; Zhao L; He PY; Zhao YF; Li YM STING and TLR7/8 agonists-based nanovaccines for synergistic antitumor immune activation. Nano Res. 2022, 15, 6328–6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cheng N; Watkins-Schulz R; Junkins RD; David CN; Johnson BM; Montgomery SA; Peine KJ; Darr DB; Yuan H; McKinnon KP et al. A nanoparticle-incorporated STING activator enhances antitumor immunity in PD-L1-insensitive models of triple-negative breast cancer. JCI Insight 2018, 3, e120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Garland KM; Sheehy TL; Wilson JT Chemical and biomolecular strategies for STING pathway activation in cancer immunotherapy. Chem. Rev 2022, 122, 5977–6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liang JJ; Wang HF; Ding WX; Huang JX; Zhou XF; Wang HY; Dong X; Li GY; Chen EG; Zhou F. et al. Nanoparticle-enhanced chemo-immunotherapy to trigger robust antitumor immunity. Sci. Adv 2020, 6, eabc3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang X; Wilhelm J; Li W; Li SX; Wang ZH; Huang G; Wang J; Tang HL; Khorsandi S; Sun ZC et al. Polycarbonate-based ultra-pH sensitive nanoparticles improve therapeutic window. Nat. Commun 2020, 11, 5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bennett ZT; Li SX; Sumer BD; Gao JM Polyvalent design in the cGAS-STING pathway. Semin. Immunol 2021, 56, 101580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sun XQ; Zhang Y; Li JQ; Park KS; Han K; Zhou XW; Xu Y; Nam J; Xu J; Shi XY et al. Amplifying STING activation by cyclic dinucleotide-manganese particles for local and systemic cancer metalloimmunotherapy. Nat. Nanotechnol 2021, 16, 1260–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Qiu XY; Qu Y; Guo BB; Zheng H; Meng FH; Zhong ZY Micellar paclitaxel boosts ICD and chemo-immunotherapy of metastatic triple negative breast cancer. J. Control. Release 2022, 341, 498–510. [DOI] [PubMed] [Google Scholar]
- [26].Lu ZD; Chen YF; Shen S; Xu CF; Wang J. Co-delivery of phagocytosis checkpoint silencer and stimulator of interferon genes agonist for synergetic cancer immunotherapy. ACS Appl. Mater. Interfaces 2021, 13, 29424–29438. [DOI] [PubMed] [Google Scholar]
- [27].Chen YP; Xu L; Tang TW; Chen CH; Zheng QH; Liu TP; Mou CY; Wu CH; Wu SH STING activator c-di-GMP-loaded mesoporous silica nanoparticles enhance immunotherapy against breast cancer. ACS Appl. Mater. Interfaces 2020, 12, 56741–56752. [DOI] [PubMed] [Google Scholar]
- [28].Nakamura T; Sato T; Endo R; Sasaki S; Takahashi N; Sato Y; Hyodo M; Hayakawa Y; Harashima H. STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation. J. Immunother. Cancer 2021, 9, e002852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fuertes MB; Kacha AK; Kline J; Woo SR; Kranz DM; Murphy KM; Gajewski TF Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8α+ dendritic cells. J. Exp. Med 2011, 208, 2005–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Diamond MS; Kinder M; Matsushita H; Mashayekhi M; Dunn GP; Archambault JM; Lee H; Arthur CD; White JM; Kalinke U. et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med 2011, 208, 1989–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Benci JL; Johnson LR; Choa R; Xu YM; Qiu JY; Zhou ZL; Xu BH; Ye D; Nathanson KL; June CH et al. Opposing functions of interferon coordinate adaptive and innate immune responses to cancer immune checkpoint blockade. Cell 2019, 178, 933–948.E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gulen MF; Koch U; Haag SM; Schuler F; Apetoh L; Villunger A; Radtke F; Ablasser A. Signalling strength determines proapoptotic functions of STING. Nat. Commun 2017, 8, 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cerboni S; Jeremiah N; Gentili M; Gehrmann U; Conrad C; Stolzenberg MC; Picard C; Neven B; Fischer A; Amigorena S. et al. Intrinsic antiproliferative activity of the innate sensor STING in T lymphocytes. J. Exp. Med 2017, 214, 1769–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Larkin B; Ilyukha V; Sorokin M; Buzdin A; Vannier E; Poltorak A. Cutting edge: Activation of STING in T cells induces type I IFN responses and cell death. J. Immunol 2017, 199, 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Benci JL; Xu BH; Qiu Y; Wu TJ; Dada H; Twyman-Saint Victor C; Cucolo L; Lee DSM; Pauken KE; Huang AC et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell 2016, 167, 1540–1554.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen JZ; Cao YH; Markelc B; Kaeppler J; Vermeer JAF; Muschel RJ Type I IFN protects cancer cells from CD8+ T cell-mediated cytotoxicity after radiation. J. Clin. Invest 2019, 129, 4224–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Liu Y; Crowe WN; Wang LL; Lu Y; Petty WJ; Habib AA; Zhao DW An inhalable nanoparticulate STING agonist synergizes with radiotherapy to confer long-term control of lung metastases. Nat. Commun 2019, 10, 5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gong K; Guo G; Panchani N; Bender ME; Gerber DE; Minna JD; Fattah F; Gao BN; Peyton M; Kernstine K. et al. EGFR inhibition triggers an adaptive response by co-opting antiviral signaling pathways in lung cancer. Nat. Cancer 2020, 1, 394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Freeman GJ; Casasnovas JM; Umetsu DT; DeKruyff RH TIM genes: A family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol. Rev 2010, 235, 172–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhao DW; Stafford JH; Zhou HL; Thorpe PE Near-infrared optical imaging of exposed phosphatidylserine in a mouse glioma model. Transl. Oncol 2011, 4, 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Knight V; Koshkina NV; Waldrep JC; Giovanella BC; Gilbert BE Anticancer effect of 9-nitrocamptothecin liposome aerosol on human cancer xenografts in nude mice. Cancer Chemother. Pharmacol 1999, 44, 177–186. [DOI] [PubMed] [Google Scholar]
- [42].Deng LF; Liang H; Xu M; Yang XM; Burnette B; Arina A; Li XD; Mauceri H; Beckett M; Darga T. et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 2014, 41, 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Baird JR; Friedman D; Cottam B; Dubensky TW Jr.; Kanne DB; Bambina S; Bahjat K; Crittenden MR; Gough MJ Radiotherapy combined with novel STING-targeting oligonucleotides results in regression of established tumors. Cancer Res. 2016, 76, 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Reits EA; Hodge JW; Herberts CA; Groothuis TA; Chakraborty M; Wansley EK; Camphausen K; Luiten RM; de Ru AH; Neijssen J. et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med 2006, 203, 1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ko EC; Raben D; Formenti SC The integration of radiotherapy with immunotherapy for the treatment of non-small cell lung cancer. Clin. Cancer Res 2018, 24, 5792–5806. [DOI] [PubMed] [Google Scholar]
- [46].Wang J; Li ZM; Wang ZT; Yu YH; Li D; Li BS; Ding JX Nanomaterials for combinational radio-immuno oncotherapy. Adv. Funct. Mater 2020, 30, 1910676. [Google Scholar]
- [47].den Haan JMM; Lehar SM; Bevan MJ CD8+ but not Cd8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med 2000, 192, 1685–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Spranger S; Dai D; Horton B; Gajewski TF Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell 2017, 31, 711–723.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sato E; Olson SH; Ahn J; Bundy B; Nishikawa H; Qian F; Jungbluth AA; Frosina D; Gnjatic S; Ambrosone C. et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ganesan AP; Johansson M; Ruffell B; Yagui-Beltrán A; Lau J; Jablons DM; Coussens LM Tumor-infiltrating regulatory T cells inhibit endogenous cytotoxic T cell responses to lung adenocarcinoma. J. Immunol 2013, 191, 2009–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Greenwald RJ; Freeman GJ; Sharpe AH The B7 family revisited. Annu. Rev. Immunol 2005, 23, 515–548. [DOI] [PubMed] [Google Scholar]
- [52].Li HY; McSharry M; Bullock B; Nguyen TT; Kwak J; Poczobutt JM; Sippel TR; Heasley LE; Weiser-Evans MC; Clambey ET et al. The tumor microenvironment regulates sensitivity of murine lung tumors to PD-1/PD-L1 antibody blockade. Cancer Immunol. Res 2017, 5, 767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhang L; Zhang ZW; Mason RP; Sarkaria JN; Zhao DW Convertible MRI contrast: Sensing the delivery and release of anti-glioma nano-drugs. Sci. Rep 2015, 5, 9874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhang L; Zhou HL; Belzile O; Thorpe P; Zhao DW Phosphatidylserine-targeted bimodal liposomal nanoparticles for in vivo imaging of breast cancer in mice. J. Control. Release 2014, 183, 114–123. [DOI] [PubMed] [Google Scholar]
- [55].Torchilin VP Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov 2005, 4, 145–160. [DOI] [PubMed] [Google Scholar]
- [56].Sato T; Shimosato T; Ueda A; Ishigatsubo Y; Klinman DM Intrapulmonary delivery of CpG microparticles eliminates lung tumors. Mol. Cancer Ther 2015, 14, 2198–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.