Abstract
Objectives:
Geriatric depression is difficult to treat and frequently accompanied by treatment resistance, suicidal ideations and polypharmacy. New adjunctive mind-body treatment strategies can improve clinical outcomes in geriatric depression and reduce risk for side-effects of pharmacological treatments.
Methods:
We conducted a 3-month randomized controlled trial to assess the efficacy and tolerability of combining Tai Chi Chih (TCC) or Health Education and Wellness training (HEW) with the stable standard antidepressant treatment on mood and cognitive functioning in depressed older adults (NCT02460666). Primary outcome was change in depression as assessed by the Hamilton Rating Scale for Depression (HAM-D) post-treatment. Remission was defined as HAM-D ≤ 6; naturalistic follow-up continued for 6 months. We also assessed psychological resilience, health-related quality of life and cognition.
Results:
Of the 178 randomized participants, 125 completed the 3-month assessment and 117 completed the 6-month assessment. Dropout and tolerability did not differ between groups. Remission rate within TCC was 35.5% and 33.3%, compared to 27.0% and 45.8% in HEW, at 3 and 6 months respectively (x2(1) = 1.0, p = 0.3; x2(1) = 1.9, p =0.2). Both groups improved significantly on the HAM-D at 3 and 6 months. TCC demonstrated a greater improvement in general health compared to HEW.
Conclusions:
Both TCC and HEW combined with a standard antidepressant treatment improved symptoms of depression in older adults. While TCC was superior to HEW in improving general health, we did not find group differences in improvement in mood and cognition.
Late-life depression (LLD) occurs in 5% –15% of community-dwelling elderly and is associated with high rates of relapse, morbidity, mortality, and suicide.1–3 Over 60% of the depressed elderly fail to achieve symptomatic remission and functional recovery with pharmacotherapy,4,5 and LLD is often accompanied by unremitting cognitive dysfunction and poor health function.6,7 Finding new treatments that enhance resilience and improve outcomes is an important healthcare imperative.8,9
Mind-body therapies, including Tai Chi and Qi Gong have been consistently reported to reduce negative emotions and improve psychological and physical wellbeing.10–15 In a recent meta-analysis, Tai Chi (TC) interventions had beneficial effects for various populations on a range of psychological well-being measures, including depression, anxiety, and general stress management.16 In mechanistic studies, TC has demonstrated decreased sympathetic output, improved viral immunity and vaccine response, and health functioning in older adults, indicating that the mechanisms by which TC can help improve symptoms of depression and overall mental health include rebalancing autonomic nervous system (ANS) and immune/inflammatory function.17–19 Emerging evidence from brain imaging studies have also revealed the neuroplastic potential of TC practices to increase gray matter volumes and to enhance functional connectivity in younger and older adults.20,21
In our pilot study,13 Tai Chi Chih (TCC), a brief manualized version of Tai Chi practice, improved treatment response to the antidepressant, escitalopram, compared to the active control, health education and wellness training (HEW) in older depressed adults. As compared to depressed elderly randomized to escitalopram and HEW, those receiving escitalopram and TCC exhibited greater rates of remission, greater clinical, health and cognitive improvement and reduced markers of inflammation.7,22 In other studies of Tai Chi Chih in non-depressed older adults,23 TCC improved sleep quality, decreased sympathetic sympathetic activity as indexed by pre-ejection period24; and decreased the levels of proinflammatory peripheral cytokines.25 To-date, there are no published neuroimaging studies of TCC.
In this paper, we report the results of a 3-month randomized single-blind controlled trial (RCT) of TCC versus HEW in older depressed adults stable on standard antidepressant therapies for at least 4 months, but still reporting clinically significant depressive symptoms. HEW was used as an active control condition to account for non-specific attention and social support effects. The primary outcome was depression severity at post-treatment (3 months), and at naturalistic follow-up (6 months). Secondary outcomes included self-reported depressive symptoms, health-related quality of life and psychological resilience. We also assessed domains of cognition frequently impaired in LLD: memory (delayed recall),26 attention/executive function27, and language at 3 and 6 months.28,29 Based on our pilot data, we hypothesized that depression and cognition would improve more with TCC compared to HEW at 3 and 6 months.
METHODS
Participants
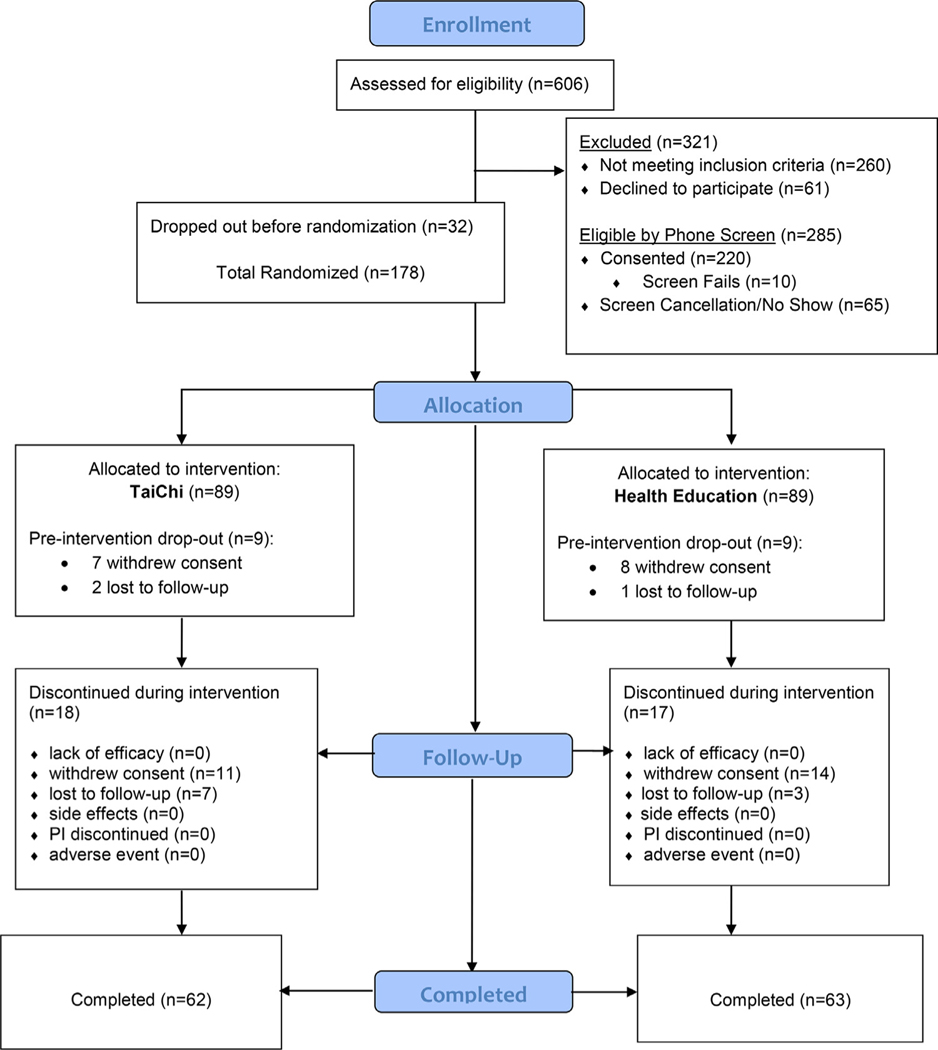
The UCLA Institutional Review Board approved all study procedures (NCT02460666). Participants were recruited from the UCLA Neuropsychiatric Hospital inpatient and outpatient service and from community advertising between September 2016 and January 2020. A total of 606 individuals were assessed via phone screening, yielding 285 participants eligible for in-person diagnostic interview. Of these, 220 participants completed the in-person screen. One hundred seventy-eight met all inclusion criteria, and were randomized to receive either TCC (n = 89) or HEW (n = 89) (see CONSORT diagram; Fig. 1). Participants provided written informed consent prior to study enrollment.
FIGURE 1.
Consort diagram.
Inclusion and Exclusion Criteria
The Structured Clinical Interview for DSM-IVR/DSM-5 (SCID) was administered by HL, SAN, and PW to diagnose major depressive disorder (MDD) and rule out other diagnoses. Inclusion criteria were: 1) presence of MDD according to DSM-IVR/DSM-5 criteria; 2) score of ≥15 on the 24-item Hamilton Rating Scale for Depression (HAM-D)30; 3) absence of dementia (see “Screening for Dementia” below); and 4) age≥60 years. Exclusion criteria were: 1) lifetime history of any psychiatric disorder except MDD, comorbid anxiety, or insomnia; 2) recent and/or current unstable medical or neurological disorders; or 3) diagnosis of moderate or severe neurocognitive impairment. Participants were stable on antidepressant therapies for at least 4 months before starting the trial. All participants were TCC naïve and did not have any other ongoing mind-body practices. They were asked not to initiate any new mind-body classes for the duration of the study.
Screening for Dementia
The evaluation for dementia included: 1) An interview by a study psychiatrist who also administered the Clinical Dementia Rating Scale (CDR)31 to identify physical and cognitive limitations; 2) a standard battery of hematologic studies, blood chemistries, liver and thyroid function tests, B12 and folate levels, and RPR test; 3) neurological examination (Unified Parkinson’s Disease Rating Scale); 4) neuropsychological examination (detailed below); and 5) psychiatric evaluation (SCID-DSM-IVR/5). In addition, individuals with an established dementia diagnosis or who scored ≤ 24 on the Mini-Mental State Examination (MMSE)32,33 were considered to have dementia and were excluded.
Diagnosis of Mild Cognitive Impairment
Participants were diagnosed with mild cognitive impairment (MCI) using established guidelines.34 MCI was defined as: 1) a stage between normal cognition and dementia (CDR score of 0.531); 2) patient-reported decline in cognition; 3) objective impairment on neurocognitive testing; 4) no significant functional impairment. Objective impairment on neurocognitive testing was defined as scoring one standard deviation (SD) below age- and education-specific norms on at least 2 memory tests (California Verbal Learning Test-II (CVLT-II) Long-Delay Free Recall35 and Rey–Osterrieth Complex Figure Test (ROCFT) 30-minute Delayed Recall36).
Randomization
Eligible participants were randomized in a 1:1 ratio to TCC or HEW using a computer-generated random assignment scheme. A block randomization strategy (with randomly selected blocks of length 4 and 6) was used to maintain balance throughout the trial.
Blinding Procedures
We described treatment groups to potential participants as “exercise and wellness education,” in order to minimize potential “placebo effects” and generate equal expectations for benefit. Each group had the option of attending the opposite intervention classes after six months of follow up. All subjects were blind to the differences in the interventions and that symptomatic, functional, and cognitive improvement were the outcomes of interest. Assessment was performed by trained behavioral raters “blind” to the treatment assignment, as were the statistician, the PI, and the database staff.
Interventions
Classes (TCC or HEW) were held in person for 60 minutes per week for 12 weeks and were taught by 2 different TCC instructors and 6 different HEW instructors. Groups of 6 −8 participants were formed for each intervention. The last recruited cohort was switched to a virtual administration following COVID-19 quarantine order on March 17, 2020 and received the 6 remaining classes virtually along with virtual assessments through November of 2020. Homework assignments were equal in both groups and asked for at least 20 minutes of either TCC (accompanied by the training CD), or computer searches on the topics of wellness at home. Regular supervisions with the therapists and inter-rater reliability sessions with behavioral and cognitive raters assured compliance with the study protocol. Biweekly follow ups were conducted for the first three months of the study to document changes in depression severity, adherence to practice, and adverse events. In addition, monthly follow-ups were conducted at 4, 5 and 6 months.
Tai Chi Chih (TCC)
Patients were informed that TCC constitutes a health management intervention, which incorporates meditation and physical activity to promote a sense of well-being and control over negative symptoms associated with depression. The standard detailed protocol for TCC is adapted from “Tai-Chi-Chih! Joy Through Movement”37 and has been used in several studies by our research group and others. Each class allowed 10 minutes of warm-up (e.g., stretching, breathing) and 5 minutes of cool down. Participants were instructed to practice at home for at least 20 minutes per day using handouts. Validity of the intervention was maintained via certification requirements for the TCC trainers and weekly supervisions by HL. The first cohort was co-taught by the two trainers to calibrate the teaching techniques.
Health education and wellness training (HEW)
This condition served as an active control for non-specific treatment elements such as attention and group support that could pose rival explanations for the effectiveness of TCC. Participants were informed that this intervention is designed to help reduce the severity of depressive symptoms. The trained study staff implemented the HEW protocol using a manual of educational information and learning objectives and patient activities to promote integration of material. Validity of the intervention was maintained via monthly supervisions by LME. This novel use of a non-exercise control intervention, which matches the exercise intervention in duration, frequency and social contact, represents an important methodological advance.38 Participants were instructed to practice at home in computer searches addressing the health topics discussed in the session for 20 minutes per day, which was discussed at the next class. Adherence to homework was monitored at each visit and each subsequent class.
Safety and Physical Health Assessments
All participants received an initial baseline medical assessment including a complete physical examination with neurological and neuropsychiatric examinations, electrocardiogram, and laboratory testing. Medical comorbidity was quantified using the clinician-rated Cumulative Illness Rating Scale for Geriatrics (CIRS-G39). Cardiovascular risk (CVRF) was assessed via the Stroke Risk Factor Prediction Chart from the Framingham Study.40 Anxiety symptoms were assessed via the 14-item clinician-rated Hamilton Anxiety Rating Scale (HAM-A).41
Outcome Measures (Baseline, 3 months and 6 months)
Depression
The primary outcome measure was change on the HAM-D at post-treatment (3 months).30 Remission of depressive symptoms was defined as a HAM-D score less than or equal to 6. Inter-rater reliability sessions were performed at the beginning of the study and every 6 months thereafter and yielded r of 0.9 on HAMD among all clinical raters (HL, LME, SAN, and PW). The Geriatric Depression Scale (GDS), a self-report measure of depression in older adults, was used as a secondary measure of depressive symptoms.
Resilience and Health-related Quality of Life
Because lower quality of life and resilience are common correlates of LLD, these 2 constructs were examined as secondary outcome measures. Psychological resilience was assessed via the 25-item Connor-Davidson Resilience Scale (CD-RISC).42 The Medical Outcomes Study 36-Item Short Form Health Survey (SF-36) was used to measure health-related quality of life.43
Cognition
Participants completed a comprehensive neuropsychological test battery to assess the following three cognitive domains frequently impaired in LLD: 1) Delayed recall (CVLT-II [Long-Delay Free Recall],35 ROCFT [30-minute Delayed Recall]36); 2) Attention/Executive function (Trail Making Test A and B44; Stroop Interference [Golden version]45); 3) Language (Controlled Oral Word Association test [FAS]28,29; Animal Fluency46; and Boston Naming Test48). We first z-transformed the raw scores for each test and averaged the z-scores within each domain to produce domain z-scores (transforming z-scores as necessary so that higher z-scores represent higher performance for all measures).
Statistical analysis
Data were entered at the time of collection and analyzed after completion of the trial. All data were inspected for outliers, homogeneity of variance and other assumptions to ensure their appropriateness for parametric statistical tests. Treatment groups were compared using t-tests (continuous variables) or chi-squared tests (categorical variables) on all demographic and clinical measures at baseline. Intent-to-treat analyses were used for all outcomes. The proportion of participants who achieved remission (HAM-D≤6) was analyzed using a chi-squared test. Continuous outcomes were analyzed using a mixed effects general linear model, as implemented in SAS PROC MIXED, including treatment group, time, and the interaction between time and treatment group. Age, sex, and education (only for cognitive outcomes) were used as covariates. Although recruitment was completed in February of 2020, some of the follow up assessments were performed remotely due to COVID-19 lockdown, we included a covariate to indicate whether the assessment took place pre-COVID versus post-COVID. Significance of the interaction between time and treatment group was used to assess whether the groups differed in changes in outcome measures. Post-hoc analyses determined the significance of specific pair-wise group differences and within-group changes. We present test scores and statistics as well as effect sizes (Cohen’s d) for group differences. Given the novel nature of the study, we present complete results of all analyses conducted on the secondary outcome measures and set the level of significance at p <0.05, two-tailed, without accounting for multiple comparisons. As such, results for secondary outcome measures should be interpreted with caution.
We also examined predictors of remission using logistic regression models. Predictors included demographic (age, sex, race, education), treatment (TCC versus HEW), cognitive (MMSE, and cognitive domain scores), and clinical (age of onset, number of depressive episodes, physical health [CIRS-G, CVRF], baseline depression [HAM-D, GDS], quality of life [SF-36], and resilience [CD-RISC]) variables. First, a series of logistic regression models were estimated with remission as the dependent and each of the above predictors as the independent variable. Predictors significant at a level of p <0.1 from these preliminary analyses as well as those identified using a stepwise selection method were then examined in a multivariable logistic regression. Inferences are made only from this final model, with significance set at p <0.05. The predictive ability of the final model was quantified with calculated area under the curve (AUC).
RESULTS
Sample Characteristics
Baseline demographic and clinical characteristics of the randomized sample (n = 178) by treatment group are summarized in Table 1. Average age of participants at baseline was 69.3 (SD = 6.6) years, the mean depression severity was 19.1 (SD = 3.9) on the HAM-D, and the average MMSE score was 28.8 (SD = 1.2). At baseline, treatment groups did not differ significantly in age, sex ratio, education, race, comorbidities variables, MMSE, HAM-D, GDS, CD-RISC, CIRS and CVRF scores. Nine participants (5.1% of the sample) met criteria for MCI at baseline (6 TCC and 3 HEW). Medication and therapy use of participants did not differ between groups at baseline (see Supplementary Table 1).
TABLE 1.
Comparison of Baseline Demographic and Clinical Characteristics
| Variable | TCCa (n = 89) | HEWa (n = 89) | Statistic |
|---|---|---|---|
|
| |||
| Demographics | |||
| Age | 69.2 (6.9) | 69.4 (6.2) | t(176) = 0.3, p =0.8 |
| Women (%) | 62 (69.7%) | 67 (75.3%) | χ2(1) = 0.7, p = 0.4 |
| Education | 16.0 (2.0) | 15.5(2.1) | t(176)=−1.6, p =0.1 |
| Race | Fisher’s exact p = 0.8 | ||
| Caucasian | 73 (82.0%) | 74 (83.2%) | |
| African American | 5 (5.6%) | 8 (9.0%) | |
| Asian | 5 (5.6%) | 3 (3.4%) | |
| Hispanic | 3 (3.4%) | 3 (3.4%) | |
| Other | 3 (3.4%) | 1 (1.1%) | |
| Chronic Depression (> 24 mos.) | 58 (65.9%) | 67 (75.3 %) | χ2(1)= 1.9, p = 0.2 |
| Age of Onset | 37.6 (21.0) | 37.6 (20.9) | t(174) = 0.0, p =1.0 |
| Illness duration | 31.7 (22.4) | 31.9 (21.1) | t(174) = 0.1, p =1.0 |
| Number of Episodes | 6.3(12.0) | 5.6(12.3) | t(163) = 0.4, p =0.7 |
| Number of Medications | 6.0 (3.7) | 6.0 (4.4) | t(174) = 0.0, p = 1.0 |
| MMSE | 28.8 (1.3) | 28.9(1.1) | t(176) = 0.9, p =0.4 |
| Mild Cognitive Impairment | 6 (6.7%) | 3 (3.4%) | Fisher’s exact p = 0.5 |
| Clinical Scores | |||
| HAM-D | 19.0 (4.0) | 19.3 (3.9) | t(176) = 0.6, p =0.5 |
| GDS | 16.8 (7.9) | 15.8(7.0) | t(176) =−0.9, p =0.4 |
| HAM-A | 10.8 (4.2) | 10.9 (5.1) | t(176) = 0.2, p =0.8 |
| CD-RISC | 60.0(15.1) | 60.4(15.3) | t(176) = 0.2, p =0.9 |
| CIRS-G | 6.2 (3.7) | 6.8 (4.2) | t(176)= 1.0, p =0.3 |
| CVRF | 10.0 (4.7) | 10.4 (4.4) | t(176)=0.7, p=.5 |
| Cognitive Domain Z-Scores | |||
| Delayed recall | −0.04(1.08) | 0.04 (0.92) | t(175) = 0.6, p =0.6 |
| Attention/Executive function | 0.08(1.02) | −0.08(0.98) | t(175) = 1.0, p =0.3 |
| Language | −0.08 (0.95) | 0.08(1.05) | t(175) = 1.1, p =0.3 |
TCC : Tai Chi Chih; HEW : Health Education and Wellness
Sixty-two TCC and 63 HEW participants completed the 3-month treatment trial; 57 TCC and 60 HEW participants completed the post-treatment assessment at 6 months (Fig. 1). Dropout rates did not significantly differ between the 2 arms (3-months: 27 (30.3%) TCC and 26 (29.2%) HEW, χ2(1)= 0.0, p = 0.9; 6-months: 32 (36.0%) TCC and 30 (33.7%) HEW, χ2(1)=0.1, p = 0.8). Tolerability and number of side effects also did not differ: 4 TCC participants reported muscle soreness and/or aches; 2 HEW participants reported increased tension/inner unrest and 1 HEW participant reported failing memory. Class attendance for the 2 arms were comparable: TCC group had a mean attendance percentage of 82.3 (SD = 24.5) compared to 79.2 (SD = 22.9) for HEW. Further, 88.7% of TCC and 81.0% of HEW participants attended at least 9 of the 12 sessions offered (χ2(1) = 1.5, p = 0.2). Both groups reported similar levels of homework (mean number of homework days per week, TCC: 4.1 (SD = 2.1); HEW: 3.7 (2.9), t(123) = 0.89, p = 0.4).
Changes in outcome measures
Changes in all outcome measures at 3 months and 6 months from baseline for the two study arms as well as between-group and within-group statistics are presented in Table 2 and estimated effect sizes (Cohen’s d) with associated 95% confidence intervals are presented in Table 3.
TABLE 2.
Changes in Outcome Measures at 3- and 6-Months
| Measure | 3-mo change | ||||
|---|---|---|---|---|---|
|
| |||||
| TCCa (N = 62) | HEWa (N = 63) | Between-Group Statistics | |||
|
|
|
||||
| Estimate (SD) | Within-Group Statistics | Estimate (SD) | Within-Group Statistics | ||
|
| |||||
| Clinical | |||||
| HAM-D | −9.27 (5.29) | t(174) = −14.2, p <0.0001 | −9.40 (5.35) | t(174) =−12.3, p <0.0001 | F(6,174) = 1.2, p =0.3 |
| GDS | −4.90 (5.36) | t(174) =−6.7, p <0.0001 | −3.77 (6.62) | t(174) =−5.1, p <0.0001 | F(6,174) = 0.5, p =0.8 |
| CD-RISC | 4.71 (13.25) | t(174) = 3.4, p = 0.0008 | 3.66 (9.75) | t(174) = 2.7, p = 0.008 | F(1,174) = 0.3, p =0.6 |
| SF-36 General Health | 2.71 (11.35) | t(174) = 2.1, p =0.04 | 0.32(9.31) | t(174) = 0.2, p =0.8 | F(1,174) = 1.8, p =0.2 |
| Cognitive Domains | |||||
| Delayed Recall | −0.15 (0.57) | t(173) =−1.7, p =0.08 | −0.002 (0.61) | t(173) = −0.2, p =0.9 | F(1,173) = 1.8, p =0.2 |
| Attention/Executive Function | −0.04 (0.61) | t(173) = −0.3, p =0.8 | 0.03 (0.53) | t(173) = 0.7, p =0.5 | F(1,173) = 0.5, p =0.5 |
| Language | 0.03 (0.40) | t(172) = 0.5, p =0.6 | −0.12 (0.46) | t(172) =−1.9, p =0.06 | F(1,172) = 2.9, p =0.09 |
|
| |||||
| Measure | 6-mo change | ||||
|
| |||||
| TCCa (N = 57) | HEWa (N = 59) | Between-Group Statistics | |||
|
|
|
||||
| Estimate (SD) | Within-Group Statistics | Estimate (SD) | Within-Group Statistics | ||
|
| |||||
|
| |||||
| Clinical | |||||
| HAM-D | −10.00 (5.99) | t(174)=−13.7, p <0.0001 | −10.46 (5.63) | t(174) =−14.3, p <0.0001 | F(9,174) = 0.8, p =0.6 |
| GDS | −5.31 (5.71) | t(174) =−6.3, p <0.0001 | −3.40(7.27) | t(174) =−4.3, p <0.0001 | F(9,174) = 1.0, p =0.4 |
| CD-RISC | 5.86(12.65) | t(174)=3.8, p = 0.0002 | 5.09(11.76) | t(174)=3.2, p = 0.002 | F(2,174) = 0.1, p =0.9 |
| SF-36 General Health | 5.31 (12.12) | t(174)=3.1,p = 0.003 | 0.61 (12.06) | t(174)=0.3, p =0.8 | F(2,174) = 2.1, p =0.1 |
| Cognitive Domains | |||||
| Delayed Recall | −0.20(0.83) | t(173)=−1.8, p =0.08 | −0.08 (0.62) | t(173)=−0.6, p =0.5 | F(2,173) = 0.9, p =0.4 |
| Attention/Executive Function | −0.08 (0.50) | t(173)=1.0, p =0.3 | 0.07(0.51) | t(173) = 1.3, p =0.2 | F(2,173)= 1.3, p =0.3 |
| Language | 0.04 (0.42) | t(172)=0.6, p = 0.6 | −0.12(0.46) | t(172)=−1.8, p =0.08 | F(2,172) = 1.9, p =0.2 |
TCC = Tai Chi Chih; HEW = Health Education and Wellness
TABLE 3.
Effect Size Estimates for Changes in Outcome Measures at 3- and 6-Months
| 3-Mo Change | 6-Mo Change | |||
|---|---|---|---|---|
|
|
|
|||
| Measure | Effect Sizea | 95% CI | Effect Sizea | 95% CI |
|
| ||||
| Clinical | ||||
| HAM-D | −0.02 | (−0.38, 0.33) | −0.08 | (−0.45, 0.29) |
| GDS | 0.19 | (−0.17, 0.55) | 0.29 | (−0.09, 0.67) |
| CD-RISC | −0.09 | (−0.45, 0.27) | −0.06 | (−0.44, 0.31) |
| SF-36 General Health | −0.23 | (−0.59, 0.13) | −0.29 | (−0.76,−0.01) |
| Cognitive Domains | ||||
| Delayed recall | 0.26 | (−0.11, 0.63) | 0.17 | (−0.21, 0.55) |
| Attention/Executive function | 0.13 | (−0.24, 0.49) | 0.29 | (−0.09, 0.68) |
| Language | −0.35 | (−0.72, 0.02) | −0.35 | (−0.73, 0.03) |
Effect sizes are Cohen’s d estimates. For HAM-D and GDS, where a higher score represents worse symptoms, a positive value indicates a better treatment effect of TCC versus HEW; for CD-RISC, SF-36 and cognitive domains where a higher score represents better resilience/performance, a negative value indicates a better treatment effect of TCC versus HEW.
Primary outcome, HAM-D
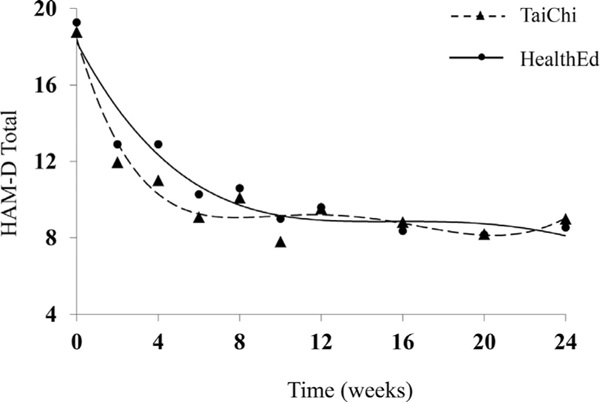
The two groups did not differ in their HAM-D changes (3-month: F(6,174)= 1.2, p = 0.3; 6-month: F (9,174) = 0.8, p = 0.6) and both groups demonstrated significant improvement in HAM-D at 3 months (TCC: mean change=−9.27 (5.29), t(174) =−14.2, p <0.0001; HEW: −9.40 (5.35), t(174) = −12.3, p <0.0001) and 6 months (TCC: −10.00 (5.99), t(174) = −13.7, p <0.0001; HEW: −10.46 (5.63), t(174)=−13.7, p <0.0001). Changes in HAM-D over time are depicted graphically in Figure 2.
FIGURE 2.
Change in depression severity over six month follow up.
Twenty-two TCC participants (35.5% of 62 completers) met remission criteria (HAM-D≤6) at 3 months, and seventeen HEW participants (27.0% of 63 completers) remitted. This difference was not statistically significant (χ2(1)= 1.0, p = 0.3). At 6 months, nineteen TCC participants (33.3% of 57 completers) and 27 HEW participants (45.8% of 59 completers) continued in remission, also not statistically significant (χ2(1) = 1.9, p = 0.2).
Secondary Outcomes
Cognition
There were no significant between-group differences in cognitive domain z-score changes (Delayed recall, Attention/Executive function, and Language) at 3 months or 6 months (Table 2). Further, none of the within-group changes reached significance.
GDS
Both groups demonstrated significant improvement in GDS (TCC: 3-month change = −4.90 (5.36), t (174) = −6.7, p < 0.0001; 6-month: −5.31 (5.71), t (174) = −6.3, p <0.0001; HEW, 3-month: −3.77 (6.62), t (174) = −5.1, p < 0.0001; 6-month: −3.40 (7.27), t (174) = −4.3, p < 0.0001). The 0 groups did not differ in their changes (3-month: F(6,174)=0.5, p = 0.8; 6-month: F(9,174) = 1.0, p = 0.4).
Resilience
Both groups improved in CD-RISC (TCC: 3-month change=4.71 (13.25), t(174) = 3.4, p =0.0008; 6-month: 5.86 (12.65), t(174) = 3.8, p =0.0002; HEW, 3-month: 3.66 (9.75), t(174) = 2.7, p =0.008; 6-month: 5.09 (1.76), t(174) = 3.2, p =0.002). The two groups did not differ in their changes (3-month: F(1,174) = 0.3, p =0.6; 6-month: F(2,174) = 0.1, p =0.9).
Health-related quality of life
TCC improved in SF-36 General Health (TCC, 3-month change = 2.71 (11.35), t(174)=2.1, p =0.04; 6-month 5.31 (12.12), t(174) = 3.1, p =0.003) while HEW did not (HEW, 3-month: 0.32 (9.31), t(174) =0.2, p =0.8; 6-month: 0.61 (12.16), t(174) = 0.3, p = 0.8). The 2 groups did not differ in their changes (3-month: F (1,174) = 1.8, p =0.2; 6-month: F(2,174) = 2.1, p =0.1). No other changes in SF-36 scales showed group differences: both groups showed improvement in Social, Energy, Wellbeing, and Role Emotional scales and neither group showed significant change in Role Physical, Pain, and Physical functioning scales.
Predictors of remission
Higher resilience scores (Odds Ratio (OR) = 1.39, 95% CI [1.04, 1.86], p =0.03) as well as lower levels of HAM-D (OR = 0.14 [0.03, 0.56], p =0.006) at baseline were significantly associated with treatment remission (AUC = 0.74) at 3 months. At 6 months, in addition to higher CD-RISC (OR = 1.76 [1.29, 2.39], p =0.0003) and lower HAM-D (OR = 0.24 [0.06, 0.95], p =0.04), male sex (OR = 3.07 [1.13, 8.29], p =0.03) was also a significant predictor of remission (AUC = 0.78).
DISCUSSION
We found that the addition of either TCC or Health Education to the standard antidepressants improved symptoms of depressed mood, and psychological resilience, and did not significantly change cognition. We did not find any group differences in dropout, attendance, or safety. Greater resilience and lower depression at baseline predicted remission at 3- and 6-months, and in addition, male sex predicted remission at 6-month follow-up.13 Our results uniquely address mood, resilience, health-related quality of life, and cognition in older adults with major depression, a population that is rarely examined by research studies. In addition, we used an active attention control and added these behavioral interventions to the heterogeneous antidepressant treatment ranging from combination of antidepressant and other pharmacological treatments. Both interventions were fully matched by group time exposure, homework and the amount of attention and follow up by the study staff. It is possible that the lack of significant difference in improvement in TCC versus HEW is because social support, behavioral activation and other non-specific factors play a significant role in improvement in late-life depression. All subjects also were receiving a standard treatment for depression, either pharmacological or psychotherapeutic, that added to the overall antidepressant response.
Our present results do not support our previous pilot findings of greater improvement in depression and cognition in the TCC group compared to HEW when we used a standardized antidepressant, escitalopram, for all study participants and where the study was of a longer 16 week duration.13 Thus, the difference in findings between the two studies might be due to the heterogeneity of antidepressant therapies in the current study or differences in the duration of the interventions. However, the strength of this current design reflects the real-life circumstances of mind-body therapies being added to a variety of antidepressant treatments. Our finding of a greater improvement in SF-36 general health with TCC compared to HEW is consistent with our prior findings of improvement in physical and mental health.
Our report differs from some reports of beneficial effect of Tai Chi on cognitive function in non-depressed older adults.48,49 A meta-analysis of outcomes related to executive function in cognitively healthy adults indicated a large effect size when Tai Chi participants were compared with non-intervention controls and a moderate effect size when compared with exercise controls.48 Outcomes related to global cognitive function in cognitively impaired adults also showed smaller but statistically significant effects when Tai Chi was compared with nonintervention controls and other active interventions. Notably, the effect sizes were lower and similar to ours (ES = 0.3) with the use of an active control. A similar large-scale meta-analysis,50 demonstrated benefits of mind-body practices including tai chi in improving global cognition and promoting cognitive flexibility, working memory, verbal fluency, and learning in cognitively intact or impaired non-depressed older adults. It is possible that mind-body practices including Tai Chi, are more effective in improving cognition in those without major depression, whereas our sample was depressed with preserved cognition, with only 5% of participants diagnosed with MCI. Presence of depression may attenuate the effects of mind-body interventions, whether performed alone or in combination with standard therapies.
We also found that greater psychological resilience at baseline predicted remission in depressive symptoms. This replicates our previous finding that participants with greater resilience were more likely to experience improvement or remission from depression with antidepressant treatment, regardless of the antidepressant medications to which they were randomized.51 It is also interesting to note that male sex was a significant predictor of remission, when baseline depression and resilience were in the model. While the distribution of men and women was comparable between the 2 intervention arms, 23.4% of HEW women remitted compared to 34.1% of TCC women, whereas the proportion of men remitting was similar in both groups (HEW: 37.5%; TCC: 38.9%). It is thus possible that women were more responsive to TCC than the control condition, consistent with previous findings that women may have more favorable responses than men to mindfulness training.52
Limitations
Several limitations of the current study should be noted. First, we used a convenience sample of outpatients with moderate MDD. Our sample was relatively demographically homogenous, with the majority being Caucasian and well-educated. Relatively few met criteria for MCI, and participants with psychiatric comorbidity, suicidality, or moderate-to-severe neurocognitive impairment were excluded. As such, our results may not generalize to patients with acute medical illness, severe depression, more severe cognitive impairment, or more demographically diverse samples. Second, standard antidepressant treatment varied greatly among patients and added heterogeneity to observed outcomes. Third, the duration of our follow-up was rather short for detection of cognitive improvement, and a longer follow-up duration over 12 months may have detected group differences. Fourth, both arms had a roughly 30% drop-out at 3-month follow-up, which although higher than the less than 20% drop-out desired in the CONSORT statement for RCTs, is very reasonable in this moderate MDD outpatient sample. Finally, we note that due to the novel nature of the study and out of concern that multiplicity adjustment might obscure possibly important findings, we did not correct for multiple comparisons in our analyses. As such, there is a distinct possibility of Type 1 error, and analyses of secondary outcomes should be interpreted with caution.
CONCLUSIONS
Despite these limitations, our study is the first randomized controlled RCT to address the efficacy and safety of TCC in combination with standard antidepressant therapies to enhance clinical and cognitive outcomes in geriatric depression. Our trial used an active control group to rigorously assess behavioral interventions for late-life mental disorders. The primary hypothesis of greater improvement in mood and cognition with TCC compared to HEW was not confirmed. Our results suggest that both the combinations are safe and may improve symptoms of depression, and psychological resilience in this difficult-to-treat population. TCC may be particularly useful in those depressed older adults who prefer using mind-body therapies. The use of active control may have limited our ability to detect group differences over a short follow up.
To shed light on the underlying mechanisms of the heterogeneity of treatment response, we are currently investigating differences between subjects via multimodal neuroimaging as well as methylome and transcriptome mapping. Future studies with longer follow-up period should address cognitive benefits of TCC in older adults with depression, as well as examine baseline predictors of treatment response with Tai Chi, to identify patient subgroups who may preferentially benefit from mind-body therapies in addition to patient preference. Given that the remission rate in the Tai Chi dropped at 6-month follow-up, it would also be important to examine the effects of a longer-term Tai Chi intervention in order to sustain effects, as has been attempted with mindfulness based cognitive therapy,53 and as we have done using yoga-based interventions in older adults with mild-cognitive impairment.54,55
Supplementary Material
Highlights.
Geriatric depression is difficult to treat and frequently accompanied by treatment resistance, suicidal ideations and polypharmacy. New adjunctive mind-body treatment strategies can improve clinical outcomes in geriatric depression and reduce risk for side-effects of pharmacological treatments.
We report the results of a 3-month randomized controlled trial to assess the efficacy and tolerability of combining Tai Chi Chih (TCC) or Health Education and Wellness training (HEW) with the stable standard antidepressant treatment on mood and cognitive functioning in depressed older adults with 6 month follow up.
The combination of TCC or HEW with a standard antidepressant treatment was equally effective for depression and cognition in older adults. TCC was superior to HEW in improving general health.
Acknowledgments
This work was supported by NIH grants R01AT008383 (NCT02460666), AT009198, and the National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881. The authors thank the Semel Institute Biostatistics Core (SIStat) for database management and support.
Footnotes
All other authors report no financial relationships with commercial interests.
SUPPLEMENTARY MATERIALS
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jagp.2021.07.008.
REFERENCES
- 1.Alexopoulos GS: Mechanisms and treatment of late-life depression. Transl Psychiatry 2019; 9:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butters MA, Whyte EM, Nebes RD, et al. : The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry 2004; 61:587–595 [DOI] [PubMed] [Google Scholar]
- 3.Turvey CL, Conwell Y, Jones MP, et al. : Risk factors for late-life suicide: a prospective, community-based study. Am J Geriatr Psychiatry 2002; 10:398–406 [PubMed] [Google Scholar]
- 4.Charney DS, Nemeroff CB, Lewis L, et al. : National Depressive and Manic-Depressive Association consensus statement on the use of placebo in clinical trials of mood disorders. Arch Gen Psychiatry 2002; 59:262–270 [DOI] [PubMed] [Google Scholar]
- 5.Thase ME: Achieving remission and managing relapse in depression. J Clin Psychiatry 2003; 64(Suppl 18)):3–7 [PubMed] [Google Scholar]
- 6.Alexopoulos GS: Depression in the elderly. Lancet 2005; 365:1961–1970 [DOI] [PubMed] [Google Scholar]
- 7.Cho KL: Effect of Tai Chi on depressive symptoms amongst Chinese older patients with major depression: the role of social support. Med Sport Sci 2008; 52:146–154 [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine of National Academics: Retooling for an Aging America. Reducing suicide: A national imperative. Washington, D.C.: National Academic Press, 2008 [Google Scholar]
- 9.Lavretsky H, Irwin MR,: Resilience and aging. Aging Health 2007; 3:309–323 [Google Scholar]
- 10.Abbott R, Lavretsky H: Tai Chi and Qigong for the treatment and prevention of mental disorders. Psychiatr Clin North Am 2013; 36:109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibanez GE, Fennie K, Larkey L, et al. : A tai chi/qigong intervention for older adults living with HIV: a study protocol of an exploratory clinical trial. Trials 2020; 21:804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laird KT, Paholpak P, Roman M, et al. : Mind-body therapies for late-life mental and cognitive health. Curr Psychiatry Rep 2018; 20:2. [DOI] [PubMed] [Google Scholar]
- 13.Lavretsky H, Alstein LL, Olmstead RE, et al. : Complementary use of tai chi chih augments escitalopram treatment of geriatric depression: a randomized controlled trial. Am J Geriatr Psychiatry 2011; 19:839–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solloway MR, Taylor SL, Shekelle PG, et al. : An evidence map of the effect of Tai Chi on health outcomes. Syst Rev 2016; 5:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C, Bannuru R, Ramel J, et al. : Tai Chi on psychological well-being: systematic review and meta-analysis. BMC Complement Altern Med 2010; 10:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang F, Lee EK, Wu T, et al. : The effects of tai chi on depression, anxiety, and psychological well-being: a systematic review and meta-analysis. Int J Behav Med 2014; 21:605–617 [DOI] [PubMed] [Google Scholar]
- 17.Irwin M, Pike J, Oxman M: Shingles immunity and health functioning in the Elderly: Tai Chi Chih as a behavioral treatment. Evid Based Complement Alternat Med 2004; 1:223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irwin MR, Olmstead R, Oxman MN: Augmenting immune responses to varicella zoster virus in older adults: a randomized, controlled trial of Tai Chi. J Am Geriatr Soc 2007; 55:511–517 [DOI] [PubMed] [Google Scholar]
- 19.Irwin MR, Pike JL, Cole JC, et al. : Effects of a behavioral intervention, Tai Chi Chih, on varicella-zoster virus specific immunity and health functioning in older adults. Psychosom Med 2003; 65:824–830 [DOI] [PubMed] [Google Scholar]
- 20.Cui L, Yin H, Lyu S, et al. : Tai Chi Chuan vs general aerobic exercise in brain plasticity: a multimodal MRI study. Sci Rep 2019; 9:17264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Tao J, Liu W, et al. : Different modulation effects of Tai Chi Chuan and Baduanjin on resting-state functional connectivity of the default mode network in older adults. Soc Cogn Affect Neurosci 2019; 14:217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenberger NI, Inagaki TK, Mashal NM, et al. : Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun 2010; 24(4):558–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irwin MR, Olmstead R, Motivala SJ: Improving sleep quality in older adults with moderate sleep complaints: a randomized controlled trial of Tai Chi Chih. Sleep 2008; 31:1001–1008 [PMC free article] [PubMed] [Google Scholar]
- 24.Motivala SJ, Sollers J, Thayer J, et al. : Tai Chi Chih acutely decreases sympathetic nervous system activity in older adults. J Gerontol A Biol Sci Med Sci 2006; 61:1177–1180 [DOI] [PubMed] [Google Scholar]
- 25.Irwin MR, Olmstead R: Mitigating cellular inflammation in older adults: a randomized controlled trial of Tai Chi Chih. Am J Geriatr Psychiatry 2012; 20:764–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elderkin-Thompson V, Kumar A, Bilker WB, et al. : Neuropsychological deficits among patients with late-onset minor and major depression. Arch Clin Neuropsychol 2003; 18:529–549 [DOI] [PubMed] [Google Scholar]
- 27.Lockwood KA, Alexopoulos GS, van Gorp WG: Executive dysfunction in geriatric depression. Am J Psychiatry 2002; 159:1119–1126 [DOI] [PubMed] [Google Scholar]
- 28.Reitan RM, Wolfson D: The halstead-reitan neuropsychological test battery and REHABIT: a model for integrating evaluation and remediation of cognitive impairment. Cogn Rehabil 1988; 6:10–17 [Google Scholar]
- 29.Benton A, Hamsher K, Varney RN, et al. : Contribution to Neuropsychological Assessment: A Clinical Manual. New York, NY: Oxford University Press, 1983 [Google Scholar]
- 30.Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes CP, Berg L, Danziger W, et al. : A new clinical scale for the staging of dementia. Br J psychiatry 1982; 140:566–572 [DOI] [PubMed] [Google Scholar]
- 32.Folstein MF, Folstein SE, McHugh PR: “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198 [DOI] [PubMed] [Google Scholar]
- 33.Folstein MF, Anthony JC, Parhad I, et al. : The meaning of cognitive impairment in the Elderly. JAm Geriatr Soc 1985; 33:228–235 [DOI] [PubMed] [Google Scholar]
- 34.Petersen RC: Mild cognitive impairment as a diagnostic entity. J Int Medicine 2004; 256:183–194 [DOI] [PubMed] [Google Scholar]
- 35.Delis DC: California Verbal Learning Test: Adult version Manual. San Anotnio, TX: Psychological Corporation, 2000 [Google Scholar]
- 36.Meyers JE, Meyers KR: Rey complex figure test under four different administration procedures. Clin Neuropsychol 1995; 9:63–67 [Google Scholar]
- 37.Stone JF: Tai-Chi-Chih! Joy Through Movement. Boston, MA: Good Karma Publishing, 1996 [Google Scholar]
- 38.Lawlor DA, Hopker SW: The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ 2001; 322:763–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller MD, Paradis CF, Houck PR, et al. : Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res 1992; 41:237–248 [DOI] [PubMed] [Google Scholar]
- 40.Wolf PA, D’Agostino RB, Belanger AJ, et al. : Probability of stroke: a risk profile from the Framingham Study. Stroke 1991; 22:312–318 [DOI] [PubMed] [Google Scholar]
- 41.Hamilton M: The assessment of anxiety states by rating. Br J Med Psychol 1959; 32:50–55 [DOI] [PubMed] [Google Scholar]
- 42.Connor KM, Davidson JRT: Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety 2003; 18:76–82 [DOI] [PubMed] [Google Scholar]
- 43.Ware JEJ, Kosinski M, Keller SD: SF-36 Physical and Mental Health Summary Scores: A User’s Manual. Boston, MA: The Health Institute, New England Medical Center, 1994 [Google Scholar]
- 44.Arnett JA, Labovitz SS: Effect of physical layout in performance of the Trail Making Test. Psychol Assess 1995; 7(2):220–221 [Google Scholar]
- 45.Golden CJ, Marsella AJ, Golden EE: Cognitive relationships of resistance to interference. J Consult Clin Psychol 1975; 43:432. [DOI] [PubMed] [Google Scholar]
- 46.Tombaugh TN, Kozak J, Rees L: Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 1999; 14:167–177 [PubMed] [Google Scholar]
- 47.Kaplan E, Goodglass H, Weintraub S: Boston Naming Test. 2nd Edition (BNT-2). Pro-Ed, 8700 Shoal Creek Blvd, Austin, TX. [Google Scholar]
- 48.Wayne PM, Walsh JN, Taylor-Piliae RE, et al. : Effect of tai chi on cognitive performance in older adults: systematic review and meta-analysis. J Am Geriatr Soc 2014; 62:25–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Su Q, Guo H, et al. : Long-term Tai Chi training is related to depressive symptoms among Tai Chi practitioners. J Affect Disord 2014; 169:36–39 [DOI] [PubMed] [Google Scholar]
- 50.Wu C, Yi Q, Zheng X, et al. : Effects of mind-body exercises on cognitive function in older adults: a meta-analysis. J Am Geriatr Soc 2019; 67:749–758 [DOI] [PubMed] [Google Scholar]
- 51.Laird KT, Lavretsky H, St Cyr N, et al. : Resilience predicts remission in antidepressant treatment of geriatric depression. Int J Geriatr Psychiatry 2018; 33:1596–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rojiani R, Santoyo JF, Rahrig H, et al. : Women benefit more than men in response to college-based meditation training. Front Psychol 2017; 8:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dikaios E, Escobar S, Nassim M, et al. : Continuation Sessions of Mindfulness-Based Cognitive Therapy (MBCT-C) vs. treatment as usual in late-life depression and anxiety: an open-label extension study. Int J Geriatr Psychiatry 2020; 35:1228–1232 [DOI] [PubMed] [Google Scholar]
- 54.Eyre HA, Siddarth P, Acevedo B, et al. : A randomized controlled trial of Kundalini yoga in mild cognitive impairment. Int Psychogeriatr 2017; 29:557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eyre HA, Acevedo B, Yang H, et al. : Changes in neural connectivity and memory following a yoga intervention for older adults: a pilot study. J Alzheimers Dis 2016; 52:673–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.