Abstract
Background:
Recent record-breaking hot temperatures in Alaska have raised concerns about the potential human health implications of heat exposure among this unacclimated population.
Objectives:
We estimated cardiorespiratory morbidity associated with days above summer (June–August) heat index (HI, apparent temperature) thresholds in three major population centers (Anchorage, Fairbanks, and the Matanuska-Susitna Valley) for the years 2015–2019.
Methods:
We implemented time-stratified case–crossover analyses of emergency department (ED) visits for International Classification of Diseases, 10th Revision codes indicative of heat illness and major cardiorespiratory diagnostic codes using data from the Alaska Health Facilities Data Reporting Program. Using conditional logistic regression models, we tested maximum hourly HI temperature thresholds between 21.1°C (70°F) and 30°C (86°F) for a single day, 2 consecutive days, and the absolute number of previous consecutive days above the threshold, adjusting for the daily average concentration of particulate matter .
Results:
There were increased odds of ED visits for heat illness above a HI threshold as low as 21.1°C (70°F) [; 95% confidence interval (CI): 4.05, 47.29], and this increased risk continued for up to 4 d (; 95% CI: 1.15, 5.10). Asthma and pneumonia were the only respiratory outcomes positively associated with the HI: ED visits for both were highest the day after a heat event (Asthma: ; 95% CI: 1.00, 1.39; Pneumonia: ; 95% CI: 1.06, 1.84). There was a decreased odds of bronchitis-related ED visits when the HI was above thresholds of 21.1–28°C (70–82°F) across all lag days. We found stronger effects for ischemia and myocardial infarction (MI) than for respiratory outcomes. Multiple days of warm weather were associated with an increased risk of health impacts. For each additional preceding day above a HI of 22°C (72°F), the odds of ED visits related to ischemia increased 6% (95% CI: 1%, 12%); for each additional preceding day above a HI of 21.1°C (70°F), the odds of ED visits related to MI increased 7% (95% CI: 1%, 14%).
Discussion:
This study demonstrates the importance of planning for extreme heat events and developing local guidance for heat warnings, even in areas with historically mild summertime climates. https://doi.org/10.1289/EHP11363
Introduction
Morbidity and mortality from exposure to extreme heat represent a growing yet preventable public health concern in the United States.1–4 Excessive temperatures have been associated with a number of adverse health outcomes, including acute heat illness,5 diabetes,6 acute renal failure,7 suicide and mental health-related hospital admissions,8 and all-cause mortality9,10 in adults and children.11 In addition, the epidemiological evidence from multiple countries on the impact of extreme heat on cardiovascular and respiratory diseases is robust.12–16 Further, the social, natural, and built environment as well as individual socioeconomic and demographic characteristics are important effect modifiers of the relationships between heat and adverse health outcomes.17,18
Early notification of forecasted heat events is intended to mitigate the health impact of heat waves. These warnings are often paired with recommendations to avoid heat exposure and seek health care when needed.19,20 To issue heat-related public health notifications, most cities in the United States use the National Weather Service (NWS) excessive heat alerts (e.g., watch, advisory, warning) that are based on forecasts of the 24- to 48-h heat index (HI), a measure of “how hot it really feels” when humidity is considered alongside air temperature.21 For example, the NWS issues a heat advisory when the HI is forecast to exceed 38°C (100°F) in northern states or 41°C (105°F) in southern states with nighttime lows higher than 24°C (75°F) for 1–2 d.22,23 Although the NWS provides this national guidance, it encourages weather forecast offices (WFOs) to develop locally relevant thresholds and criteria for heat warning systems in their areas.22 Of the 122 WFOs in the United States, about half (predominantly those in the western and southern states) have developed their own revised policy with local criteria for issuing heat products.22 In line with recommendations to develop heat warning systems based on environmental triggers of human health end points,19,24 several epidemiological studies have used local weather data and health records to identify heat exposure metrics that are associated with adverse health outcomes in their region.25–29
Although historically, diagnoses related to heat-related illness in Alaska seemed unlikely given the typically mild summer conditions, recent unprecedented hot temperatures in the state have raised concerns about the potential human health implications of temperature anomalies among this unacclimated population.30,31 For example, in 2019, a heat wave brought record-breaking temperatures to much of the state; Anchorage reached 32°C (90°F) for the first time and experienced 6 consecutive days with temperatures above 27°C (80°F).32 Exacerbating the issue, smoke from wildfires throughout the state created unhealthy air quality conditions that have been associated with increases in emergency department visits for cardiorespiratory conditions in Alaska’s primary population centers.33 Ongoing advisories during the summer to avoid hazardous outdoor air quality encouraged people to stay indoors, but Alaska’s public health agencies worried that the dearth of air conditioners and well-ventilated homes in the state would exacerbate the risk of heat illness, given the lack of locally relevant guidelines for dangerous heat exposure (W. Kays, personal communication).
To identify appropriate thresholds for issuing heat alerts in Alaska, we evaluated associations between the daily maximum heat index (testing thresholds between 21.1°C (70°F) and 30°C (86°F) and cardiorespiratory emergency department visits in three major population centers between 2015 and 2019 for lags up to 5 d. In addition, we assessed the impacts of prolonged heat events using two different methods for defining “heat waves,” and we evaluated differential impacts of heat on unique demographic groups through stratified analyses by age, sex, race, and geography.
Methods
Study Area
The study areas represent three geographically and climatologically distinct regions in Alaska: Anchorage, communities in the Matanuska-Susitna Valley, and Fairbanks. These are three major population centers, representing 65% of Alaska’s population. The populations of these boroughs (county equivalents) are , 110,000, and 95,000, respectively. The Matanuska-Susitna valley is located 35 mi north of Anchorage, and Fairbanks is located in the interior region of Alaska, more than 350 mi north of Anchorage and the Matanuska-Susitna Valley. Anchorage and the Matanuska-Susitna Valley have a mild, coastal, subarctic climate with average maximum summer temperatures historically (1991–2020) ranging from 18.1°C to 18.9°C (64.5–66°F)34 and average summer relative humidity of 77% and 75%, respectively. Fairbanks has a continental, subarctic climate with an average maximum summer temperature of 21.3°C (70.3°F).34
Exposure Characterization
Weather data were compiled from the National Oceanic and Atmospheric Administration (NOAA) Automated Surface Observing System (ASOS) monitoring network, using previously published methods.33 Temperature and relative humidity data were accessed from an ASOS monitor located nearest to the population center in each study area. The hourly HI was calculated from paired temperature and humidity measurements, using methods implemented by the NWS.35,36 Specifically, the calculation was done using a multiple regression equation with adjustments specified by the NWS within relevant ranges of temperature and humidity.37,38 The equation used was:
where T is temperature in degrees Fahrenheit and RH is percent relative humidity. After calculating the HI for every hour, the maximum value for each day was used to assign the daily HI for each study area, which was the primary exposure metric. For days during which fewer than 18 hourly temperature and relative humidity measurements were available from the nearest ASOS monitor, data from the second or third closest monitor were used. If 2 or fewer consecutive days had missing data, daily HI was imputed by taking the average of the first leading and lagging days around the missing period.
Data for fine particulate matter (PM) with aerodynamic diameter () were obtained from ground monitoring sensors maintained by the Alaska Department of Environmental Conservation (DEC) for U.S. Environmental Protection Agency (U.S. EPA) regulatory monitoring. The DEC monitoring sites that were located within the study areas had one, two, or three monitors that were DEC-designated as primary, secondary, or tertiary, respectively. Summarized daily data from primary monitors were selected preferentially. Daily data were excluded if fewer than 75% of hourly measurements were taken or if the recorded concentration was implausible (e.g., ). During the study period, there were two active DEC monitoring sites in Anchorage. The assigned daily concentration was the average of the measurements from these two sites. One or 2 consecutive days with missing data were imputed in the same way as temperature and relative humidity.
Health Outcomes
Hospitalization records were obtained from the Alaska Health Facilities Data Reporting Program (HFDR). Beginning in 2015, all private, municipal, state, federal, and Alaskan Native hospitals are required to report to the program. Alaska’s two military hospitals are encouraged to participate. Records were requested based on International Classification of Diseases, 9th Revision and 10th Revision, Clinical Modification codes indicative of cardiorespiratory health outcomes and heat illness (ICD-9-CM: 992; ICD-10-CM: T67). Cardiorespiratory health outcomes that were modeled individually included: asthma (493; J45), chronic obstructive pulmonary disease (COPD) (490–492, 494, 496; J40–J44, J47), pneumonia (480–486; J12–J18), acute bronchitis (466; J20–J22), arrhythmia (427; I46–I49), cerebrovascular disease (430–438; I60–I63, I65–I69, G45, I23), heart failure (428; I50), ischemic heart disease (410–414; I20–I22, I24–I25), and myocardial infarction (410; I21–I22). The primary diagnosis code was used as the outcome of interest.
HFDR data were filtered to only include emergency department (ED) encounters during the summer months (June–August) of 2015–2019. Other visit types were excluded from the data set to isolate acute cardiorespiratory- or heat illness–related incidents that could have been acutely associated with the changing daily HI. Demographic information included in the HFDR data set also permitted stratified analyses by age, sex, and race.
HI Threshold Analysis
The associations between the daily HI and cardiorespiratory and heat illness ED visits in the study areas were evaluated using a time-stratified case–crossover study design.39,40 Each ED encounter that occurred within one of the study areas during the summer season (June–August) in 2015–2019 was considered a case. For each case, referent days were selected as the same day of the week for the duration of the summer season of the same year.
Single-day conditional logistic regression models were used to identify HI thresholds at which the likelihood of cardiorespiratory or heat illness ED visits increased. For each health outcome, daily HI cutoffs ranging from 21.1°C to 30°C (70°F to 86°F) at 2°F intervals were evaluated as potential thresholds by including daily HI in the model as an indicator variable for above vs. below the cutoff. These cutoffs were selected based on the range of the mean to the maximum daily HI during the 2015–2019 summer months in our three study regions. The first whole number that was above the mean for all study locations was 70°F, and consistent 2°F thresholds ensured that results were easily interpretable and could be readily applied toward the development of regional heat warnings. Using this approach, up to nine conditional logistic regression models were constructed for each health outcome of interest. This approach was also repeated using daily HI lagged up to 5 d. Previous studies in this population observed an increased risk of ED visits for cardiorespiratory outcomes for up to 5 d after environmental exposure events.33 Daily concentration was included in all models as a time-varying confounder and was lagged in the same way as daily HI.
Previous studies of exposure to extreme heat have found differential risk across demographic groups, potentially related to dissimilarities in susceptibility to cardiovascular aging or social factors that may affect access to care, such as living alone.41,42 To understand how different populations may experience the potential health effects of heat, stratified analyses were also conducted by age, race, and sex. Age was categorized into three groups: , 15–65, and y of age. Race was stratified into two groups: Alaska Native people and non-Alaska Native people. We also explored potential geographic differences by stratifying the single-day models by study area.
HI-Based Heat Wave Analysis
To understand the potential effects of prolonged elevated heat, or heat waves, on cardiorespiratory- and heat illness–related ED visits, two different approaches were used. In the first approach, an indicator variable was created for which a value of “1” was assigned if both the same-day HI and the HI on the previous day were above a given cutoff, and a value of “0” was assigned if the same-day HI and/or the HI on the previous day were below the given cutoff. As with the threshold analysis, the cutoffs that were tested ranged from 21°C to 30°C (70°F to 86°F) at 2°F intervals. Here, same-day daily concentration was included in the model as a potential confounder. This model is referred to as the “acute heat wave” analysis.
The second approach that was employed to understand the effect of heat waves vs. transient (i.e., single-day) elevated heat built on the threshold analysis described previously by incorporating an additional variable to capture the effect of multiple consecutive warm days. In addition to the indicator variable used in the threshold analysis (“1” signified a same-day HI above a relevant cutoff, and “0” signified a same-day HI below the cutoff), these models also included a linear variable for the total number of previous consecutive days that were above the same cutoff (counting until the HI drops below the cutoff). For example, when the recent daily HIs were 27.8°C, 27.2°C, 26.1°C, 27.2°C, 27.8°C, and 25°C (82°F, 81°F, 79°F, 81°F, 82°F, and 77°F) on lag days 1, 2, 3, 4, 5, and 6, respectively, and the cutoff was 25.6°C (78°F), then the number of previous consecutive days above the cutoff was 5 (lag days 1–5). If the cutoff being tested was 26.7°C (80°F), then there were only 2 d in a row above this threshold (lag days 1 and 2), and then no further days are counted because of the drop in the HI to 26.1°C (79°F) on lag day 3. Again, same-day daily concentration was included in all models. This approach is referred to as the “ongoing heat wave” analysis. Similar to the single-day threshold analysis, stratified models were built by age, race, and sex for both the acute and ongoing heat wave approaches.
Interaction between Elevated HI and
We also assessed potential multiplicative effects between daily concentration and elevated HI on ED visits. As with the threshold analysis, single-day conditional logistic regression models were used to estimate the relationship between elevated HI and cardiorespiratory or heat illness ED visits. These models also included an interaction term between daily concentration and an indicator variable for a day with a HI above vs. below the previously described thresholds. Likelihood-ratio tests were conducted to test the goodness of fit between the threshold models with and without the interaction term. For models in which the interaction term was significant, ORs were estimated for HI above vs. below the relevant threshold when the daily concentration on the same lag day was 5, 10, 20, or .
Human subject approval was provided by the University of Alaska-Anchorage institutional review board (Protocol no. 1596177). All data analyses were conducted using R (version 3.6.1; R Development Core Team).
Results
HI, Heat Waves, and
The average daily HI during the summer months (June–August) of 2015–2019 in Anchorage, Fairbanks, and the Matanuska-Susitna Valley was 19.8°C, 20.9°C, and 19.5°C (67.6°F, 69.7°F, and 67.1°F), respectively. Anchorage and the Matanuska-Susitna Valley tended to have a similar HI across summer days (Pearson correlation ). Fairbanks is climatologically distinct from the other two study areas, and the HI in Fairbanks was weakly correlated with the HI in both Anchorage (Pearson correlation ) and the Matanuska-Susitna Valley (Pearson correlation ). During the study period, Fairbanks reached the highest HI of the three study areas, with of days during the study period () reaching a maximum HI above 82°F (Table 1). In general, the distribution of the HI on summer days in Fairbanks (range: 6.7–30.3°C, 44.1–86.5°F) was more dispersed than it was in Anchorage (range: 12.1–29.9°C, 53.8–85.9°F) or the Matanuska-Susitna Valley (range: 10.9–30.1°C, 51.6–86.1°F). Furthermore, Fairbanks generally had lower daily relative humidity [; ] than Anchorage (; ) or the Matanuska-Susitna Valley (; ). Maximum daily temperature, however, was generally higher in Fairbanks (, 71.0°F, , 8.0°F) than it was in Anchorage (, 68.7°F, , 6.6°F) or the Matanuska-Susitna (, 68.1°F, , 6.4°F).
Table 1.
Distribution of elevated HI days and emergency department visit data for same-day exposure used in single-day threshold analysis in Anchorage, Fairbanks, and the Matanuska-Susitna Valley, Alaska, 2015–2019.
| Total | HI threshold | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 21.1°C (70°F) | 22.2°C (72°F) | 23.3°C (74°F) | 24.4°C (76°F) | 25.6°C (78°F) | 26.7°C (80°F) | 27.8°C (82°F) | 28.9°C (84°F) | 30°C (86°F) | ||
| Number of days above each HI threshold by study area | ||||||||||
| Anchorage [ (%)] | 460 (100) | 163 (35.4) | 123 (26.7) | 91 (19.8) | 65 (14.1) | 42 (9.1) | 19 (4.1) | 9 (2.0) | 2 (0.4) | 0 (0.0) |
| Fairbanks North Star [ (%)] | 460 (100) | 227 (49.3) | 182 (39.6) | 151 (32.8) | 110 (23.9) | 80 (17.4) | 54 (11.7) | 24 (5.2) | 9 (2.0) | 3 (0.7) |
| Matanuska-Susitna [ (%)] | 460 (100) | 140 (30.4) | 105 (22.8) | 79 (17.2) | 53 (11.5) | 26 (5.7) | 15 (3.3) | 8 (1.7) | 5 (1.1) | 1 (0.2) |
| Number of ED visits on elevated HI days by health outcome | ||||||||||
| Asthma [ (%)] | 2,911 (100) | 1,111 (38.2) | 866 (29.7) | 668 (22.9) | 490 (16.8) | 340 (11.7) | 194 (6.7) | 101 (3.5) | 31 (1.1) | 3 (0.1) |
| COPD [ (%)] | 2,158 (100) | 777 (36.0) | 600 (27.8) | 455 (21.1) | 327 (15.2) | 212 (9.8) | 95 (4.4) | 38 (1.8) | 13 (0.6) | 3 (0.1) |
| Pneumonia [ (%)] | 2,081 (100) | 718 (34.5) | 555 (26.7) | 411 (19.8) | 291 (14.0) | 205 (9.9) | 118 (5.7) | 56 (2.7) | 20 (1.0) | 5 (0.2) |
| Bronchitis [ (%)] | 1,972 (100) | 648 (32.9) | 509 (25.8) | 372 (18.9) | 251 (12.7) | 154 (7.8) | 94 (4.8) | 38 (1.9) | 17 (0.9) | 6 (0.3) |
| Arrhythmia [ (%)] | 1,617 (100) | 592 (36.6) | 449 (27.8) | 344 (21.3) | 233 (14.4) | 152 (9.4) | 73 (4.5) | 33 (2.0) | 10 (0.6) | 2 (0.1) |
| Cerebrovascular [ (%)] | 434 (100) | 159 (36.6) | 114 (26.3) | 97 (22.4) | 70 (16.1) | 47 (10.8) | 27 (6.2) | 15 (3.5) | 7 (1.6) | 2 (0.5) |
| Ischemic [ (%)] | 200 (100) | 76 (38.0) | 63 (31.5) | 51 (25.5) | 36 (18.0) | 25 (12.5) | 14 (7.0) | 7 (3.5) | 1 (0.5) | 1 (0.5) |
| Myocardial infarction [ (%)] | 81 (100) | 33 (40.7) | 26 (32.1) | 23 (28.4) | 17 (21.0) | 10 (12.3) | 5 (6.2) | 1 (1.2) | 0 (0.0) | 0 (0.0) |
| Heart failure [ (%)] | 441 (100) | 152 (34.5) | 113 (25.6) | 91 (20.6) | 67 (15.2) | 42 (9.5) | 23 (5.2) | 9 (2.0) | 4 (0.9) | 1 (0.2) |
| Heat illness [ (%)] | 35 (100) | 31 (88.6) | 29 (82.9) | 28 (80.0) | 27 (77.1) | 23 (65.7) | 22 (62.9) | 13 (37.1) | 8 (22.9) | 0 (0.0) |
Note: COPD, chronic obstructive pulmonary disease; HI, heat index.
To understand prolonged elevated heat on cardiorespiratory outcomes among the study population, two different exposure metrics were used, as previously described. Using the acute heat wave approach at a HI threshold of 28.9°C (84°F), there were only four acute heat wave days (i.e., a day during which the HI exceeded 28.9°C (84°F), as did the HI on the previous day) identified in Fairbanks and two identified in Matanuska-Susitna Valley; no 28.9°C (84°F) acute heat wave days occurred in Anchorage during the study period (Table 2). More than 50% of heat illness–related ED visits occurred on a 25.6°C (78°F) acute heat wave day. Per the ongoing heat wave method, in which the exposure metric was the number of previous consecutive days with an elevated HI, prolonged heat wave events at lower HI thresholds may have lasted up to several weeks. For example, the longest 21.1°C (70°F) ongoing heat wave occurred on 20 July 2019 in Fairbanks when the HI on the previous 39 d in a row all exceeded 21.1°C (70°F). In Anchorage and the Matanuska-Susitna Valley, the longest 21.1°C (70°F) ongoing heat waves were 18 and 21 d long, respectively. Although Fairbanks tended to have hotter and more frequent heat wave events than Anchorage and the Matanuska-Susitna Valley, the longest 27.8°C (82°F) ongoing heat wave occurred in the Matanuska-Susitna Valley (4–9 July 2019); this heat wave event accounted for 6 of the 8 d during the entire study period that had a HI above 27.8°C (82°F) in this study area.
Table 2.
Distribution of acute heatwaves and ED visit data used in acute heat wave analysis ( consecutive elevated HI days) in Anchorage, Fairbanks, and the Matanuska-Susitna Valley, Alaska, 2015–2019.
| Total | HI threshold | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 21.1°C (70°F) | 22.2°C (72°F) | 23.3°C (74°F) | 24.4°C (76°F) | 25.6°C (78°F) | 26.7°C (80°F) | 27.8°C (82°F) | 28.9°C (84°F) | 30°C (86°F) | ||
| Number of acute heat wave days above each HI threshold by study area | ||||||||||
| Anchorage [ (%)] | 460 (100) | 126 (27.4) | 94 (20.4) | 67 (14.6) | 44 (9.6) | 24 (5.2) | 14 (3.0) | 4 (0.9) | 0 (0.0) | 0 (0.0) |
| Fairbanks North Star [ (%)] | 460 (100) | 189 (41.1) | 145 (31.5) | 115 (25.0) | 78 (17.0) | 58 (12.6) | 36 (7.8) | 13 (2.8) | 4 (0.9) | 0 (0.0) |
| Matanuska-Susitna [ (%)] | 460 (100) | 110 (23.9) | 78 (17.0) | 57 (12.4) | 33 (7.2) | 17 (3.7) | 11 (2.4) | 6 (1.3) | 2 (0.4) | 0 (0.0) |
| Number of ED visits on acute heat wave days by health outcome | ||||||||||
| Asthma [ (%)] | 2,911 (100) | 899 (30.9) | 687 (23.6) | 516 (17.7) | 357 (12.3) | 232 (8.0) | 146 (5.0) | 58 (2.0) | 9 (0.3) | 0 (0.0) |
| COPD [ (%)] | 2,158 (100) | 620 (28.7) | 462 (21.4) | 335 (15.5) | 215 (10.0) | 113 (5.2) | 58 (2.7) | 18 (0.8) | 5 (0.2) | 0 (0.0) |
| Pneumonia [ (%)] | 2,081 (100) | 558 (26.8) | 407 (19.6) | 302 (14.5) | 202 (9.7) | 137 (6.6) | 82 (3.9) | 34 (1.6) | 4 (0.2) | 0 (0.0) |
| Bronchitis [ (%)] | 1,972 (100) | 521 (26.4) | 388 (19.7) | 273 (13.8) | 166 (8.4) | 98 (5.0) | 63 (3.2) | 17 (0.9) | 7 (0.4) | 0 (0.0) |
| Arrhythmia [ (%)] | 1,617 (100) | 483 (29.9) | 351 (21.7) | 256 (15.8) | 159 (9.8) | 94 (5.8) | 44 (2.7) | 16 (1.0) | 4 (0.2) | 0 (0.0) |
| Cerebrovascular [ (%)] | 434 (100) | 129 (29.7) | 96 (22.1) | 73 (16.8) | 51 (11.8) | 35 (8.1) | 19 (4.4) | 7 (1.6) | 1 (0.2) | 0 (0.0) |
| Ischemic [ (%)] | 200 (100) | 65 (32.5) | 54 (27.0) | 44 (22.0) | 28 (14.0) | 14 (7.0) | 8 (4.0) | 4 (2.0) | 0 (0.0) | 0 (0.0) |
| Myocardial infarction [ (%)] | 81 (100) | 32 (39.5) | 24 (29.6) | 18 (22.2) | 14 (17.3) | 4 (4.9) | 2 (2.5) | 1 (1.2) | 0 (0.0) | 0 (0.0) |
| Heart failure [ (%)] | 441 (100) | 120 (27.2) | 85 (19.3) | 64 (14.5) | 43 (9.8) | 26 (5.9) | 15 (3.4) | 6 (1.4) | 2 (0.5) | 0 (0.0) |
| Heat illness [ (%)] | 35 (100) | 30 (85.7) | 27 (77.1) | 25 (71.4) | 24 (68.6) | 19 (54.3) | 15 (40.0) | 7 (20.0) | 2 (5.7) | 0 (0.0) |
Note: In this analysis, an acute heat wave day was one when the HI exceeded the indicated threshold, as did the previous day (i.e., an acute heat wave occurred if there were at least two elevated HI days in a row.) COPD, chronic obstructive pulmonary disease; ED, emergency department; HI, heat index.
Because of the unique geographies of these three study areas and the impact of nearby wildfires, average daily concentrations covered a wide range of values during the study period. As with HI, daily concentrations in Anchorage and the Matanuska-Susitna Valley were more highly correlated with each other (Pearson correlation ) than they were with Fairbanks (Pearson correlation for the Matanuska-Susitna and 0.23 for Anchorage). The mean daily concentrations during the study period were () in Anchorage, () in Fairbanks, and () in the Matanuska-Susitna Valley. There were 43, 19, and 35 d during the study period that were potentially impacted by wildfire smoke (i.e., a smoke plume was within of the study area and the daily concentration exceeded 1 SD above the long-term monthly mean) in Anchorage, Fairbanks, and the Matanuska-Susitna Valley, respectively. On these days, concentrations reached levels as high as 72.5, 211.6, and in Anchorage, Fairbanks, and the Matanuska-Susitna Valley, respectively.
Health Outcomes
Nearly 12,000 ED visits related to cardiorespiratory health outcomes or heat illness were captured within the three study areas during the summer months of 2015–2019 (Table 1). The vast majority of these were related to respiratory outcomes (76.5%, ). With the exception of pneumonia, ED visits related to respiratory outcomes were more common among females than males; for all cardiovascular health outcomes, however, ED visits were more common among males (Table 3). Less than 1% () of ED visits were among children y of age for all health outcomes except asthma, pneumonia, bronchitis, and heat illness. The percentage of ED visits due to respiratory outcomes that were made by Alaska Native people ranged from 22.1% () for COPD to 31.6% () for asthma. Alaska Native people accounted for 13.7% (), 14.3% (), and 17.5% () of arrhythmia-, cerebrovascular-, and ischemia-related ED visits, respectively, although only 7%–8.2% of the Anchorage, Matanuska-Susitna Valley, and Fairbanks populations identified as American Indian or Alaska Native, alone in the most recent U.S. census.
Table 3.
Summary of ED visits that occurred during the summer months (June–August) of 2015–2019 in Anchorage, Fairbanks, and the Matanuska-Susitna Valley, Alaska, stratified by demographic variables.
| Reason for ED visit | Total ED visits during study period | Sex | Age | Racea | |||||
|---|---|---|---|---|---|---|---|---|---|
| Female (%) | Male (%) | y (%) | 15–65 y (%) | y (%) | Alaska Native (%) | Non-Alaska Native (%) | Unknown | ||
| Asthma [ (%)] | 2,911 (100) | 1,651 (56.7) | 1,260 (43.3) | 523 (18.0) | 2,217 (76.2) | 171 (5.9) | 920 (32.5) | 1,907 (67.5) | 84 |
| COPD [ (%)] | 2,158 (100) | 1,138 (52.7) | 1,020 (47.3) | 12 (0.6) | 1,293 (59.9) | 853 (39.5) | 477 (22.7) | 1,627 (77.3) | 54 |
| Pneumonia [ (%)] | 2,081 (100) | 999 (48.0) | 1,082 (52.0) | 330 (15.9) | 1,231 (59.2) | 520 (25.0) | 625 (31.3) | 1,372 (68.7) | 84 |
| Bronchitis [ (%)] | 1,972 (100) | 1,130 (57.3) | 842 (42.7) | 402 (20.3) | 1,294 (65.7) | 275 (13.9) | 597 (31.5) | 1,301 (68.5) | 74 |
| Arrhythmia [ (%)] | 1,617 (100) | 676 (41.8) | 941 (58.2) | 14 (0.9) | 879 (54.4) | 724 (44.8) | 222 (14.4) | 1,325 (85.6) | 70 |
| Cerebrovascular [ (%)] | 434 (100) | 207 (47.7) | 227 (52.3) | 0 (0) | 211 (48.6) | 223 (51.4) | 62 (15.0) | 350 (85.0) | 22 |
| Ischemia [ (%)] | 200 (100) | 62 (31.0) | 138 (69.0) | 1 (0.5) | 100 (50.0) | 99 (49.5) | 35 (18.3) | 156 (81.7) | 9 |
| Myocardial infarction [ (%)] | 81 (100) | 21 (25.9) | 61 (74.1) | 0 (0) | 53 (65.4) | 28 (34.6) | 20 (26.7) | 55 (73.3) | 6 |
| Heart failure [ (%)] | 441 (100) | 162 (36.7) | 279 (63.3) | 1 (0.2) | 241 (54.8) | 199 (45.1) | 135 (32.1) | 286 (67.9) | 20 |
| Heat illness [ (%)] | 35 (100) | 14 (40.0) | 21 (60.0) | 6 (17.1) | 27 (77.1) | 2 (5.7) | 10 (30.3) | 23 (69.7) | 2 |
Note: COPD, chronic obstructive pulmonary disease; ED, emergency department.
Hospitalizations with “unknown” race were excluded from stratified analyses by race.
HI Thresholds
Below we highlight HI thresholds at which the estimated ORs for a given health outcome were statistically significantly elevated (i.e., the 95% CI did not include the null), especially when the effect remained significantly elevated for higher HI cutoffs, and note where nonsignificant or negative associations were observed. We present results by health outcome. Complete results of the primary threshold analysis for all health outcomes are reported in Tables 4–9. The results of stratified analyses (by age group, race, and sex) are reported if significant differences were observed across groups. Similarly, we only present the geographically stratified results for asthma because it was the most notable and consistent effect in this set of analyses. Complete results of stratified threshold analyses can be found in the supplementary materials.
Table 4.
ORs and 95% CIs for ED visits for same-day HI above vs. below the threshold from single-day lag models in Anchorage, Fairbanks, and the Matanuska-Susitna Valley, Alaska, 2015–2019.
| Reason for ED visit | Number of cases | 21.1°C (70°F) HI threshold | 22.2°C (72°F) HI threshold | 23.3°C (74°F) HI threshold | 24.4°C (76°F) HI threshold | 25.6°C (78°F) HI threshold | 26.7°C (80°F) HI threshold | 27.8°C (82°F) HI threshold | 28.9°C (84°F) HI threshold | 30°C (86°F) HI threshold |
|---|---|---|---|---|---|---|---|---|---|---|
| Heat illness | 35 | 13.84 (4.05, 47.29)a | 12.25 (4.22, 35.58)a | 12.21 (4.55, 32.75)a | 16.84 (6.67, 42.53)a | 16.72 (6.80, 41.12)a | 48.64 (14.70, 160.94)a | 22.21 (7.51, 65.67)a | 11.57 (4.09, 32.73)a | — |
| Asthma | 2,911 | 1.00 (0.92, 1.09) | 1.01 (0.93, 1.10) | 1.00 (0.91, 1.10) | 1.01 (0.90, 1.12) | 1.02 (0.90, 1.16) | 1.07 (0.91, 1.27) | 1.09 (0.87, 1.37) | 0.98 (0.66, 1.45) | 1.00 (0.30, 3.28) |
| COPD | 2,158 | 0.94 (0.86, 1.04) | 0.98 (0.88, 1.08) | 0.97 (0.86, 1.08) | 0.97 (0.86, 1.11) | 1.00 (0.85, 1.17) | 0.83 (0.66, 1.04) | 0.69 (0.49, 0.98)a | 0.78 (0.44, 1.39) | 0.98 (0.30, 3.17) |
| Pneumonia | 2,081 | 0.93 (0.84, 1.02) | 0.96 (0.86, 1.06) | 0.92 (0.82, 1.04) | 0.95 (0.83, 1.08) | 1.06 (0.91, 1.25) | 1.24 (1.01, 1.53)a | 1.23 (0.92, 1.65) | 1.38 (0.86, 2.21) | 1.63 (0.64, 4.15) |
| Bronchitis | 1,972 | 0.76 (0.69, 0.85)a | 0.82 (0.73, 0.91)a | 0.80 (0.70, 0.90)a | 0.75 (0.65, 0.87)a | 0.72 (0.60, 0.87)a | 0.82 (0.65, 1.04) | 0.72 (0.51, 1.02) | 1.08 (0.65, 1.80) | 1.51 (0.65, 3.53) |
| Arrhythmia | 1,617 | 0.96 (0.86, 1.07) | 0.96 (0.85, 1.08) | 0.98 (0.86, 1.11) | 0.91 (0.78, 1.06) | 0.90 (0.75, 1.08) | 0.79 (0.61, 1.03) | 0.77 (0.53, 1.12) | 0.71 (0.37, 1.36) | 0.81 (0.19, 3.38) |
| Cerebrovascular | 434 | 0.95 (0.77, 1.19) | 0.89 (0.70, 1.12) | 1.03 (0.80, 1.33) | 1.10 (0.83, 1.47) | 1.09 (0.78, 1.54) | 1.10 (0.69, 1.73) | 1.43 (0.81, 2.53) | 1.73 (0.77, 3.89) | 2.08 (0.46, 9.28) |
| Ischemia | 200 | 1.14 (0.83, 1.57) | 1.28 (0.92, 1.78) | 1.35 (0.95, 1.92) | 1.37 (0.92, 2.04) | 1.45 (0.91, 2.31) | 1.87 (0.99, 3.53) | 1.94 (0.83, 4.55) | 0.57 (0.07, 4.37)b | 6.37 (0.57, 71.32)b |
| Myocardial infarction | 81 | 1.53 (0.93, 2.49) | 1.60 (0.95, 2.67) | 1.91 (1.12, 3.29)a | 2.12 (1.17, 3.84)a | 1.96 (0.94, 4.08) | 2.14 (0.74, 6.19) | 0.63 (0.07, 6.10)b | — | — |
| Heart failure | 441 | 0.82 (0.66, 1.01) | 0.79 (0.62, 1.00)a | 0.85 (0.66, 1.10) | 0.91 (0.68, 1.22) | 0.84 (0.59, 1.21) | 0.86 (0.53, 1.38) | 0.66 (0.31, 1.39) | 1.25 (0.43, 3.58) | 1.19 (0.15, 9.43)b |
Note: Conditional logistic regression models adjusted for daily concentration were used to estimate ORs. —, Model did not converge due to limited sample size; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ED, emergency department; HI, heat index; OR, odds ratio.
95% CI does not include the null.
Only one ED visit related to the indicated health outcome occurred on an exposed day (i.e., a day during which the HI exceeded the indicated threshold) during the study period.
Table 9.
ORs and 95% CIs for ED visits for HI on lag day 5 above vs. below the threshold from single-day lag models in Anchorage, Fairbanks, and the Matanuska-Susitna Valley, Alaska, 2015–2019.
| Reason for ED visit | Number of cases | 21.1°C (70°F) HI threshold | 22.2°C (72°F) HI threshold | 23.3°C (74°F) HI threshold | 24.4°C (76°C) HI threshold | 25.6°C (78°F) HI threshold | 26.7°C (80°F) HI threshold | 27.8°C (82°F) HI threshold | 28.9°C (84°F) HI threshold | 30°C (86°F) HI threshold |
|---|---|---|---|---|---|---|---|---|---|---|
| Heat illness | 35 | 2.03 (0.98, 4.21) | 1.65 (0.82, 3.32) | 2.13 (1.04, 4.35)a | 2.49 (1.19, 5.22)a | 2.69 (1.18, 6.10)a | 4.21 (1.64, 10.83)a | 5.09 (1.69, 15.28)a | 1.86 (0.38, 9.00) | — |
| Asthma | 2,911 | 0.92 (0.85, 1.00) | 0.90 (0.82, 0.98)a | 0.94 (0.86, 1.04) | 0.94 (0.84, 1.05) | 0.99 (0.87, 1.13) | 0.97 (0.81, 1.16) | 1.04 (0.81, 1.33) | 0.53 (0.30, 0.94)a | — |
| COPD | 2,158 | 0.90 (0.82, 0.99)a | 0.86 (0.78, 0.96)a | 0.86 (0.77, 0.97)a | 0.81 (0.71, 0.93)a | 0.77 (0.65, 0.91)a | 0.81 (0.64, 1.01) | 0.68 (0.49, 0.96)a | 0.68 (0.38, 1.23) | 1.96 (0.82, 4.68) |
| Pneumonia | 2,081 | 0.93 (0.84, 1.02) | 0.89 (0.80, 0.99)a | 0.88 (0.78, 1.00)a | 0.88 (0.76, 1.01) | 0.88 (0.75, 1.05) | 0.69 (0.54, 0.89)a | 0.81 (0.58, 1.13) | 0.82 (0.46, 1.46) | 0.76 (0.18, 3.17) |
| Bronchitis | 1,972 | 0.80 (0.72, 0.88)a | 0.80 (0.71, 0.89)a | 0.79 (0.70, 0.89)a | 0.79 (0.68, 0.92)a | 0.74 (0.62, 0.89)a | 0.72 (0.55, 0.92)a | 0.92 (0.66, 1.29) | 1.10 (0.63, 1.92) | 1.33 (0.47, 3.73) |
| Arrhythmia | 1,617 | 0.92 (0.82, 1.03) | 0.90 (0.80, 1.01) | 0.93 (0.81, 1.06) | 0.95 (0.82, 1.10) | 1.03 (0.87, 1.23) | 1.24 (1.00, 1.55) | 1.07 (0.77, 1.48) | 0.93 (0.54, 1.58) | 0.66 (0.16, 2.75) |
| Cerebrovascular | 434 | 1.10 (0.89, 1.36) | 1.13 (0.90, 1.40) | 1.08 (0.85, 1.38) | 1.17 (0.89, 1.53) | 1.02 (0.73, 1.43) | 0.94 (0.60, 1.48) | 1.41 (0.80, 2.48) | 1.05 (0.37, 2.93) | 1.29 (0.17, 10.12)b |
| Ischemia | 200 | 1.22 (0.89, 1.67) | 1.21 (0.87, 1.68) | 1.16 (0.80, 1.67) | 1.31 (0.88, 1.97) | 1.16 (0.71, 1.90) | 1.37 (0.77, 2.44) | 0.67 (0.23, 1.94) | 1.09 (0.30, 3.91) | — |
| Myocardial infarction | 81 | 1.77 (1.08, 2.93)a | 2.06 (1.23, 3.43)a | 2.32 (1.34, 3.99)a | 2.40 (1.31, 4.41)a | 1.99 (0.92, 4.31) | 1.96 (0.74, 5.21) | 0.55 (0.07, 4.30)b | 1.05 (0.13, 8.49)b | — |
| Heart failure | 441 | 1.04 (0.84, 1.28) | 0.98 (0.79, 1.23) | 0.90 (0.69, 1.16) | 0.65 (0.47, 0.90)a | 0.77 (0.53, 1.11) | 0.83 (0.51, 1.36) | 0.69 (0.31, 1.55) | 0.62 (0.15, 2.68) | — |
Note: Conditional logistic regression models adjusted for daily concentration were used to estimate ORs. —, no data; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ED emergency department; HI, heat index; OR, odds ratio.
95% CI does not include the null.
Only one ED visit related to the indicated health outcome occurred 5 d after an exposure (i.e., a day during which the HI exceeded the indicated threshold) during the study period.
Table 6.
ORs and 95% CIs for ED visits for HI on lag day 2 above vs. below the threshold from single-day lag models in Anchorage, Fairbanks, and the Matanuska-Susitna Valley, Alaska, 2015–2019.
| Reason for ED visit | Number of cases | 21.1°C (70°F) HI threshold | 22.2°C (72°F) HI threshold | 23.3°C (74°F) HI threshold | 24.4°C (76°F) HI threshold | 25.6°C (78°F) HI threshold | 26.7°C (80°F) HI threshold | 27.8°C (82°F) HI threshold | 28.9°C (84°F) HI threshold | 30°C (86°F) HI threshold |
|---|---|---|---|---|---|---|---|---|---|---|
| Heat illness | 35 | 6.31 (2.38, 16.68)a | 4.27 (1.94, 9.39)a | 3.77 (1.83, 7.78)a | 4.93 (2.39, 10.18)a | 6.80 (3.17, 14.56)a | 5.69 (2.23, 14.55)a | 2.86 (0.70, 11.77) | 9.61 (1.26, 73.47)a | — |
| Asthma | 2,911 | 0.98 (0.90, 1.06) | 1.02 (0.94, 1.12) | 0.98 (0.89, 1.08) | 1.05 (0.95, 1.17) | 1.04 (0.91, 1.18) | 1.10 (0.93, 1.30) | 1.10 (0.87, 1.39) | 0.91 (0.60, 1.39) | 0.74 (0.23, 2.40) |
| COPD | 2,158 | 0.87 (0.79, 0.96)a | 0.89 (0.81, 0.99)a | 0.87 (0.78, 0.98)a | 0.85 (0.74, 0.97)a | 0.79 (0.67, 0.94)a | 0.76 (0.60, 0.96)a | 0.82 (0.59, 1.15) | 0.73 (0.40, 1.31) | 0.64 (0.15, 2.67) |
| Pneumonia | 2,081 | 0.91 (0.82, 1.00) | 0.89 (0.80, 0.99)a | 0.90 (0.80, 1.01) | 1.00 (0.87, 1.14) | 1.15 (0.98, 1.34) | 1.28 (1.04, 1.57)a | 1.25 (0.93, 1.67) | 0.96 (0.56, 1.64) | 1.06 (0.38, 2.95) |
| Bronchitis | 1,972 | 0.78 (0.71, 0.87)a | 0.84 (0.76, 0.94)a | 0.78 (0.68, 0.88)a | 0.79 (0.69, 0.92)a | 0.81 (0.68, 0.96)a | 0.86 (0.68, 1.10) | 0.78 (0.55, 1.11) | 0.54 (0.28, 1.07) | 0.91 (0.33, 2.50) |
| Arrhythmia | 1,617 | 1.05 (0.94, 1.17) | 1.01 (0.90, 1.14) | 0.98 (0.86, 1.12) | 0.96 (0.83, 1.12) | 0.95 (0.79, 1.14) | 0.87 (0.67, 1.12) | 0.86 (0.60, 1.22) | 0.80 (0.43, 1.48) | 1.05 (0.32, 3.41) |
| Cerebrovascular | 434 | 1.01 (0.82, 1.26) | 0.97 (0.78, 1.22) | 1.05 (0.82, 1.34) | 1.02 (0.77, 1.35) | 0.92 (0.65, 1.32) | 0.80 (0.49, 1.32) | 0.50 (0.22, 1.16) | 0.80 (0.24, 2.58) | 0.79 (0.10, 6.01)b |
| Ischemia | 200 | 1.41 (1.04, 1.92)a | 1.48 (1.08, 2.03)a | 1.29 (0.91, 1.83) | 1.22 (0.82, 1.82) | 1.03 (0.62, 1.69) | 1.08 (0.55, 2.10) | 0.94 (0.36, 2.50) | 0.64 (0.08, 4.85)b | — |
| Myocardial infarction | 81 | 2.00 (1.24, 3.23)a | 2.13 (1.29, 3.49)a | 2.11 (1.24, 3.61)a | 1.50 (0.79, 2.85) | 1.23 (0.53, 2.86) | 1.62 (0.51, 5.07) | 1.37 (0.29, 6.37) | — | — |
| Heart failure | 441 | 0.84 (0.68, 1.04) | 0.85 (0.68, 1.07) | 0.83 (0.64, 1.06) | 0.90 (0.68, 1.20) | 0.96 (0.68, 1.36) | 0.76 (0.46, 1.28) | 0.92 (0.47, 1.81) | 0.29 (0.04, 2.14)b | 1.28 (0.16, 10.09)b |
Note: Conditional logistic regression models adjusted for daily concentration were used to estimate ORs. —, no data; CI; confidence interval; COPD, chronic obstructive pulmonary disease; ED, emergency department; HI, heat index; OR, odds ratio.
95% CI does not include the null.
Only one ED visit related to the indicated health outcome occurred 2 d after an exposure (i.e., a day during which the HI exceeded the indicated threshold) during the study period.
Table 7.
ORs and 95% CI for ED visits for HI on lag day 3 above vs. below the threshold from single-day lag models in Anchorage, Fairbanks, and the Matanuska-Susitna Valley, Alaska, 2015–2019.
| Reason for ED visit | Number of cases | 21.1°C (70°F) HI threshold | 22.2°C (72°F) HI threshold | 23.3°C (74°F) HI threshold | 24.4°C (76°F) HI threshold | 25.6°C (78°F) HI threshold | 26.7°C (80°F) HI threshold | 27.8°C (82°F) HI threshold | 28.9°C (84°F) HI threshold | 30°C (86°F) HI threshold |
|---|---|---|---|---|---|---|---|---|---|---|
| Heat illness | 35 | 3.16 (1.44, 6.93)a | 3.46 (1.63, 7.35)a | 3.55 (1.69, 7.45)a | 1.94 (0.90, 4.20) | 2.33 (0.96, 5.63) | 1.05 (0.28, 3.88) | 1.05 (0.22, 4.97) | 2.40 (0.28, 20.62)b | — |
| Asthma | 2,911 | 0.95 (0.87, 1.03) | 0.95 (0.87, 1.03) | 0.96 (0.87, 1.06) | 0.98 (0.88, 1.10) | 0.99 (0.86, 1.12) | 1.01 (0.85, 1.20) | 0.93 (0.72, 1.20) | 0.87 (0.56, 1.34) | 0.44 (0.11, 1.81) |
| COPD | 2,158 | 0.88 (0.80, 0.97)a | 0.92 (0.83, 1.02) | 0.83 (0.74, 0.93)a | 0.83 (0.73, 0.96)a | 0.83 (0.70, 0.98)a | 0.76 (0.60, 0.97)a | 0.70 (0.49, 0.99)a | 0.62 (0.34, 1.15) | 0.74 (0.18, 3.10) |
| Pneumonia | 2,081 | 0.92 (0.83, 1.01) | 0.90 (0.81, 1.00) | 0.91 (0.81, 1.03) | 0.92 (0.80, 1.06) | 0.93 (0.79, 1.10) | 1.05 (0.84, 1.31) | 1.23 (0.92, 1.64) | 1.36 (0.87, 2.12) | 0.29 (0.04, 2.12)b |
| Bronchitis | 1,972 | 0.77 (0.69, 0.85)a | 0.75 (0.67, 0.84)a | 0.76 (0.67, 0.87)a | 0.72 (0.62, 0.83)a | 0.76 (0.64, 0.91)a | 0.78 (0.61, 1.00) | 0.79 (0.55, 1.14) | 0.71 (0.37, 1.36) | 0.66 (0.16, 2.75) |
| Arrhythmia | 1,617 | 0.93 (0.83, 1.04) | 0.89 (0.79, 1.00) | 0.88 (0.77, 1.00) | 0.88 (0.75, 1.02) | 0.88 (0.73, 1.06) | 1.03 (0.82, 1.30) | 1.09 (0.79, 1.50) | 0.92 (0.53, 1.58) | 0.52 (0.13, 2.16) |
| Cerebrovascular | 434 | 0.93 (0.75, 1.15) | 0.97 (0.77, 1.22) | 0.89 (0.69, 1.16) | 0.93 (0.69, 1.25) | 0.77 (0.53, 1.13) | 0.79 (0.48, 1.29) | 0.51 (0.22, 1.20) | — | — |
| Ischemia | 200 | 1.18 (0.86, 1.61) | 1.08 (0.78, 1.51) | 1.03 (0.71, 1.48) | 1.21 (0.81, 1.80) | 1.22 (0.76, 1.96) | 1.26 (0.70, 2.28) | 1.48 (0.66, 3.31) | 2.26 (0.84, 6.06) | — |
| Myocardial infarction | 81 | 1.35 (0.81, 2.24) | 1.47 (0.87, 2.48) | 1.70 (0.97, 2.98) | 1.82 (0.97, 3.39) | 1.51 (0.67, 3.42) | 2.32 (0.87, 6.20) | 3.27 (0.92, 11.59) | 2.62 (0.62, 11.04) | — |
| Heart failure | 441 | 0.86 (0.70, 1.07) | 0.87 (0.69, 1.09) | 0.92 (0.72, 1.19) | 0.92 (0.69, 1.23) | 0.85 (0.59, 1.22) | 0.73 (0.44, 1.22) | 1.25 (0.68, 2.29) | 1.56 (0.65, 3.73) | 1.10 (0.14, 8.60)b |
Note: Conditional logistic regression models adjusted for daily concentration were used to estimate ORs. —, Model did not converge due to limited sample size; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ED, emergency department; HI, heat index; OR, odds ratio.
95% CI does not include the null.
Only one ED visit related to the indicated health outcome occurred 3 d after an exposure (i.e., a day during which the HI exceeded the indicated threshold) during the study period.
Table 8.
ORs and 95% CIs for ED visits for HI on lag day 4 above vs. below the threshold from single-day lag models in Anchorage, Fairbanks, and the Matanuska-Susitna Valley, Alaska, 2015–2019.
| Reason for ED visit | Number of cases | 21.1°C (70°F) HI threshold | 22.2°C (72°F) HI threshold | 23.3°C (74°F) HI threshold | 24.4°C (76°F) HI threshold | 25.6°C (78°F) HI threshold | 26.7°C (80°F) HI threshold | 27.8°C (82°F) HI threshold | 28.9°C (84°F) HI threshold | 30°C (86°F) HI threshold |
|---|---|---|---|---|---|---|---|---|---|---|
| Heat illness | 35 | 2.43 (1.15, 5.10)a | 1.51 (0.74, 3.05) | 1.89 (0.91, 3.92) | 1.32 (0.59, 2.94) | 1.63 (0.65, 4.10) | 2.56 (0.98, 6.68) | 3.36 (1.04, 10.90)a | 5.33 (1.28, 22.18)a | — |
| Asthma | 2,911 | 0.89 (0.82, 0.97)a | 0.90 (0.82, 0.98)a | 0.92 (0.84, 1.02) | 0.93 (0.83, 1.04) | 1.03 (0.90, 1.17) | 1.08 (0.91, 1.28) | 1.04 (0.81, 1.33) | 0.80 (0.50, 1.28) | 0.27 (0.04, 1.97)b |
| COPD | 2,158 | 0.88 (0.80, 0.97)a | 0.88 (0.79, 0.98)a | 0.89 (0.80, 1.00) | 0.91 (0.80, 1.04) | 0.82 (0.69, 0.96)a | 0.84 (0.67, 1.06) | 0.79 (0.56, 1.10) | 0.86 (0.51, 1.48) | 0.61 (0.15, 2.53) |
| Pneumonia | 2,081 | 0.91 (0.82, 1.01) | 0.95 (0.85, 1.05) | 0.88 (0.78, 1.00)a | 0.87 (0.76, 1.00) | 0.88 (0.74, 1.04) | 0.91 (0.72, 1.14) | 0.94 (0.68, 1.29) | 0.97 (0.57, 1.65) | 0.67 (0.16, 2.76) |
| Bronchitis | 1,972 | 0.73 (0.66, 0.81)a | 0.74 (0.66, 0.83)a | 0.76 (0.67, 0.87)a | 0.76 (0.66, 0.89)a | 0.75 (0.63, 0.90)a | 0.75 (0.58, 0.96)a | 0.60 (0.40, 0.89)a | 0.55 (0.26, 1.18) | 0.63 (0.15, 2.61) |
| Arrhythmia | 1,617 | 0.91 (0.82, 1.02) | 0.92 (0.81, 1.03) | 0.88 (0.77, 1.00) | 0.93 (0.80, 1.09) | 0.91 (0.76, 1.10) | 1.08 (0.86, 1.37) | 1.23 (0.90, 1.68) | 1.31 (0.78, 2.19) | 1.04 (0.32, 3.39) |
| Cerebrovascular | 434 | 0.97 (0.79, 1.21) | 0.91 (0.73, 1.15) | 0.96 (0.75, 1.23) | 0.87 (0.65, 1.17) | 0.83 (0.58, 1.19) | 0.77 (0.48, 1.25) | 0.64 (0.31, 1.35) | 0.94 (0.33, 2.63) | — |
| Ischemia | 200 | 0.94 (0.69, 1.30) | 1.13 (0.81, 1.57) | 1.28 (0.90, 1.83) | 1.35 (0.91, 2.00) | 1.22 (0.76, 1.97) | 1.24 (0.69, 2.21) | 1.37 (0.63, 2.98) | 0.41 (0.06, 3.08)b | — |
| Myocardial infarction | 81 | 1.26 (0.75, 2.10) | 1.49 (0.87, 2.53) | 2.00 (1.14, 3.51)a | 3.06 (1.69, 5.55)a | 2.22 (1.09, 4.53)a | 2.04 (0.84, 4.96) | 2.64 (0.80, 8.77) | — | — |
| Heart failure | 441 | 1.02 (0.82, 1.25) | 0.87 (0.69, 1.09) | 0.91 (0.70, 1.17) | 0.71 (0.52, 0.97) | 0.82 (0.56, 1.18) | 1.04 (0.67, 1.62) | 0.60 (0.29, 1.25) | 0.77 (0.24, 2.51) | 1.48 (0.34, 6.50) |
Note: Conditional logistic regression models adjusted for daily concentration were used to estimate ORs. —, no data; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ED, emergency department; HI, heat index, OR, odds ratio.
95% CI does not include the null.
Only one ED visit related to the indicated health outcome occurred 4 d after an exposure (i.e., a day during which the HI exceeded the indicated threshold) during the study period.
A visual summary of threshold results provides a quick reference for Alaska’s public health practitioners and clinicians (Figure 1). These spider diagrams show significant ORs only for each health outcome at each same-day HI threshold; colored wedges represent the stratified ORs for individual demographic groups. The bold red line on each spider diagram is the null (). For example, at a HI threshold of 21.1°C (70°F), the value of the wedges is for bronchitis, COPD, pneumonia, and heart failure, indicating a negative association (decreased risk) between same-day HI above 21.1°C (70°F) and ED visits related to these outcomes. In contrast, for myocardial infarction (MI), the wedge for y olds (light blue) at the same temperature is outside the red line; so, the OR is for this demographic group at a threshold of 21.1°C (70°F). Similarly, for heat illness, the wedges for 15–65 y olds (blue), Alaska Native people (light orange), non-Alaska Native people (dark orange), both males (light green) and females (dark green), and the overall population (pink) are outside the red line, indicating an OR , or an increased odds of ED visits for heat illness among these demographic groups on days when the HI exceeds 21.1°C (70°F). Blocks with no spider diagram indicate no significant associations between the HI and that health outcome at that temperature threshold.
Figure 1.
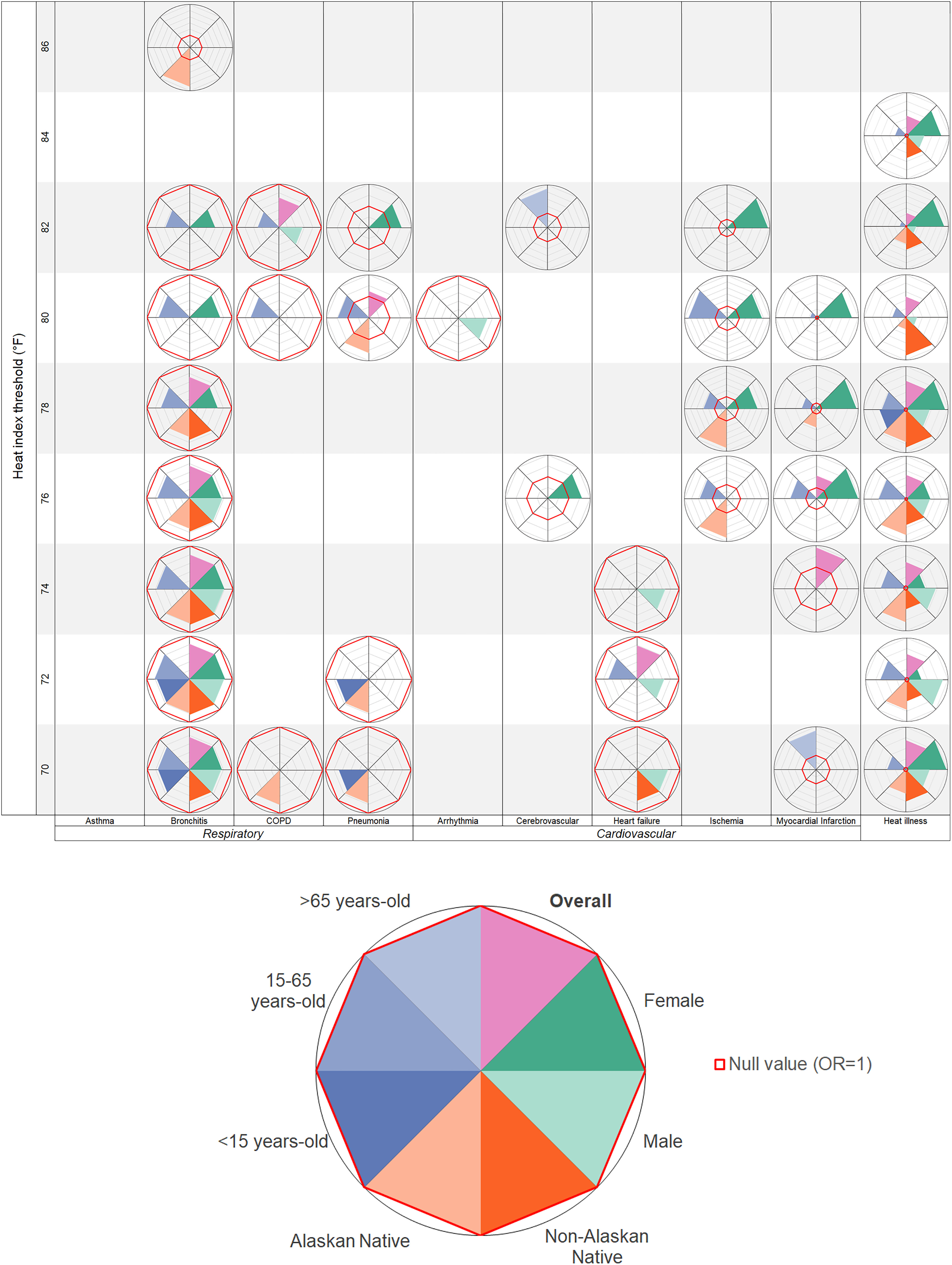
Summary of significant odds ratios for cardiorespiratory-related emergency department visits on days above vs. below the heat index (HI) threshold by demographic group in Anchorage, Fairbanks, and the Matanuska-Susitna Valley, Alaska, 2015–2019. Note: Complete results of stratified threshold analyses can be found in the supplementary materials (Tables S1a–l, Tables S2a–l, Tables S3a–r).
Spider diagrams of each health outcome for lag days 0–5 are available in the supplemental material. Again, these additional figures are meant to be a quick reference for public health practitioners or clinicians who are focused on a specific outcome and want to learn at which temperature thresholds and lag days specific demographic groups may be at increased risk. For example, from the asthma figure (Figures S1 and S2), it is easy to see that adults y of age are particularly vulnerable, generally 1–3 d after a day with a HI above 23.3°C (74°F). As the HI exceeds 27.8°C (82°F), females also have a higher risk of ED visits for asthma.
Heat illness.
We observed a substantially increased risk of ED visits for heat illness on days above a HI threshold as low as 21.1°C (70°F). The effect of elevated heat on the odds of heat illness–related ED visits persisted up to 5 d later when the HI was above 23.3°C (74°F) (Tables 4–9). Although there were still significant effects of elevated HI after 5 d, we observed that they were attenuated. The OR of an ED visit related to heat illness on a day above vs. below 23.3°C (74°F) was 12.21 (95% CI: 4.55, 32.75); after 5 d, the OR was only 2.13 (95% CI: 1.04, 4.35). The effect of the HI on heat illness-related ED visits was only observed among Alaska Native people for 1 d after an elevated HI, but among non-Alaska Native people, the increased risk persisted for 5 d (Tables S2a–l). There were too few cases to estimate risk of heat illness–related ED visits among people y of age. Among people between 15–65 y of age, there was an increased risk of heat illness–related ED visits on days with a HI above 21.1°C (70°F) that lasted for up to 3 d (Tables S3a–r). In contrast, the most pronounced effects for children y of age were on the day of or the day after a HI above thresholds of 24.4–26.7°C (76–80°F).
Respiratory: asthma.
Although there were no significant associations between same-day elevated HI and asthma-related ED visits, there was a significant increase in asthma-related ED visits the day after a HI of at least 26.7°C (80°F) (lag day 1 ; 95% CI: 1.00, 1.39). When stratified by sex, this lag day 1 threshold was observed only among females (Tables S1a–l). At later lag days, several negative associations between elevated HI and asthma-related ED visits were observed among the overall study populations [21.1–22.2°C (70–72°F) for lag day 4; 22.2°C (72°F) and 28.9°C (84°F) for lag day 5]. Similar negative associations were observed among females on lag days 4 and 5. When stratified by study area, these negative associations were observed in Anchorage and the Matanuska-Susitna Valley, but the odds of asthma-related ED visits were elevated in Fairbanks through lag day 5 [for 24.4°C (76°F) on lag day 5: ; 95% CI: 1.09, 1.74]. By age, the association between HI and asthma-related ED visits was strongest among individuals (Tables S3a–r). We observed an increased likelihood of asthma-related ED visits among this group 2 d after a HI above 74°F and the day following a HI above 25.6°C (78°F). For children , no significantly elevated ORs were estimated for asthma-related ED visits at any HI thresholds on any lag days. In fact, we observed a decrease in asthma-related ED visits among children 3–5 d after the HI was above thresholds of 21.1–23.3°C (70–74°F).
Respiratory: COPD.
Across all lag days, associations between the HI and COPD-related ED visits were generally negative, and on lag days 0 and 1 they were mostly nonsignificant (Tables 4–9). When stratified by sex, significantly negative associations were experienced consistently and exclusively by males, but not by females (Tables S1a–l). In general, significant negative associations between HI and COPD-related ED visits were more commonly observed among non-Alaska Native people, especially for lag days 2–5, than among Alaska Native people for whom few negative ORs were estimated for lag days 0–2 (Tables S2a–l).
Respiratory: pneumonia.
Within the total study population, there was an increase in pneumonia-related ED visits when the same-day HI was above 26.7°C (80°F); this positive association lasted for 2 d after the elevated HI (Tables 4–9). Although there were no significant associations detected on lag day 3, the risk of pneumonia-related ED visits significantly decreased on lag days 4 and 5 at lower HI thresholds. Significantly elevated odds of pneumonia-related ED visits were observed among females on days with a HI above 26.7°C (80°F), 1 and 2 d after a HI above 26.7°C (80°F), and 3 d after a HI above 27.8°C (82°F). These elevated odds for pneumonia-related ED visits, however, were not observed among males (Tables S1a–l). In contrast, a significantly decreased risk was observed among males 5 d after an elevated HI, but not among females. When stratified by age, adverse pneumonia-related outcomes associated with an elevated HI above 27.8°C (82°F) were only observed among individuals 15–65 y old (Tables S3a–r).
Respiratory: bronchitis.
We observed decreased odds of bronchitis-related ED visits when the HI was above thresholds of 21.1–27.8°C (70–82°F) across all lag days (Tables 4–9). Only nonsignificant ORs were estimated at higher thresholds. Stratified analyses showed that these negative associations were more consistent among non-Alaska Native people and 15–65 y olds (Tables S2a–l, S3a–r).
Cardiovascular: arrhythmia.
No significant ORs were estimated for the association between elevated HI and arrhythmia-related ED visits among the total study population (Tables 4–9). Among people of age, however, a consistent negative association was observed 3 d after an elevated HI above thresholds of 21.1–24.4°C (70–76°F) (Tables S3a–r). As with the overall study population, estimated ORs for the relationship between elevated HI and arrhythmia-related ED visits were overwhelmingly nonsignificant among all other stratified groups.
Cardiovascular: cerebrovascular.
No significant associations were detected in the analyses of cerebrovascular-related ED visits in the full study population (Tables 4–9). Among people , there was a significantly increased odds of cerebrovascular-related ED visits on days when the HI was above 27.8°C (82°F) and 1 d after an elevated HI above 25.6°C (78°F) (Tables S3a–r). Associations between elevated HI and cerebrovascular-related ED visits were primarily nonsignificant among all other stratified groups.
Cardiovascular: ischemia.
Sporadic positive associations between HI above various thresholds and ischemia-related ED visits were found on lag days 1 and 2, but there were no consistent patterns for the overall study population, and most estimated ORs were nonsignificant (Tables 4–9). Among females, however, there was a consistent increased odds of ischemia-related ED visits for same-day elevated HI above a threshold of at least 25.6°C (78°F), 1 d after a HI of at least 24.4°C (76°F), 3 d after a HI of at least 27.8°C (82°F), and 4 and 5 d after a HI of at least 23.3°C (74°F). None of these thresholds were apparent for males, among whom only sporadic positive associations were estimated for low HI thresholds on lag day 2 (Tables S1a–l). There were consistent associations between elevated HI (above thresholds of 23.3–25.6°C (74–78°F) and an increase in ischemia-related ED visits among Alaska Native people, especially on lag days 0 and 1, that were not observed among non-Alaska Native people (Tables S2a–l). When stratified by age, the associations between an increase in ischemia-related ED visits and the HI were most consistent among people 15–65 y of age (Tables S3a–r).
Cardiovascular: myocardial infarction.
Positive associations between HI and ED visits related to MI were observed on almost all lag days at HI thresholds of 21.1–25.6°C (70–78°F) among the overall study population (Tables 4–9). The increased odds of an MI-related ED visit related to HI were much stronger among females than males (Tables S1a–l). Although increased risk of MI was found among all age groups y of age, we observed that people y of age had an increase in same-day MI-related ED visits at lower HI thresholds [21.1°C (70°F) in comparison with 24.4–25.6°C (76–78°F) for 15–65 y olds], but the effect among people y of age was also more transient (Tables S3a–r).
Cardiovascular: congestive heart failure.
Only sporadic negative associations were found between an elevated HI and heart failure–related ED visits. When stratified by sex, there were no significant ORs estimated for an association between elevated HI and heart failure–related ED visits among females (Tables S1a–l). However, there was a decreased risk for lower HI thresholds [21.1°C–25.6°C (70°F–78°F)] at most lag days among males. A similar pattern was observed for Alaska Native people, among whom no associations were significant, and non-Alaska Native people, among whom there were several negative associations between HI elevated above low-temperature thresholds and heart failure–related ED visits.
HI-Based Heat Wave Effects
Below we describe the results of both heat wave analyses to understand the effects of prolonged elevated HI on heat illness– and cardiorespiratory-related ED visits. We highlight key differences between the acute and ongoing heat wave models and important differences across stratified groups. We present results by health outcome. Complete results of the acute heat wave analysis for all health outcomes are reported in Table 10. It is important to note that there were no instances of 2 consecutive days that had a maximum HI above the 86°F (i.e., a heat wave day as defined by the acute heat wave analysis approach). The complete results of the ongoing heat wave analysis for all health outcomes are reported in Table 11. The complete results of stratified heat wave analyses can be found in the supplementary materials.
Table 10.
ORs and 95% CIs for ED visits on heat wave days vs. non–heat wave days in Anchorage, Fairbanks, and the Matanuska-Susitna Valley, Alaska, 2015–2019.
| Reason for ED visit | Number of cases | 21.1°C (70°F) HI threshold | 22.2°C (72°F) HI threshold | 23.3°C (74°F) HI threshold | 24.4°C (76°F) HI threshold | 25.6°C (78°F) HI threshold | 26.7°C (80°F) HI threshold | 27.8°C (82°F) HI threshold | 28.9°C (84°F) HI threshold | 30°C (86°F) HI threshold |
|---|---|---|---|---|---|---|---|---|---|---|
| Heat illness | 35 | 15.26 (4.58, 50.80)a | 10.10 (4.05, 25.24)a | 10.92 (4.67, 25.54)a | 21.02 (8.22, 53.75)a | 21.49 (8.26, 55.92)a | 27.71 (9.65, 79.59)a | 12.35 (3.95, 38.66)a | 11.95 (1.68, 84.81)a | — |
| Asthma | 2,911 | 1.03 (0.95, 1.13) | 1.03 (0.94, 1.13) | 1.04 (0.94, 1.16) | 1.03 (0.91, 1.17) | 1.05 (0.90, 1.23) | 1.04 (0.85, 1.26) | 1.20 (0.89, 1.62) | 0.71 (0.33, 1.55) | — |
| COPD | 2,158 | 0.97 (0.88, 1.08) | 0.97 (0.86, 1.08) | 0.96 (0.84, 1.09) | 0.96 (0.82, 1.12) | 0.82 (0.66, 1.01) | 0.68 (0.51, 0.91)a | 0.60 (0.37, 0.97)a | 1.23 (0.48, 3.13) | — |
| Pneumonia | 2,081 | 0.91 (0.82, 1.01) | 0.89 (0.79, 1.00) | 0.91 (0.80, 1.04) | 0.97 (0.83, 1.14) | 1.20 (0.98, 1.45) | 1.20 (0.93, 1.54) | 1.37 (0.95, 1.98) | 1.11 (0.39, 3.10) | — |
| Bronchitis | 1,972 | 0.81 (0.72, 0.90)a | 0.82 (0.72, 0.92)a | 0.79 (0.68, 0.91)a | 0.74 (0.62, 0.88)a | 0.73 (0.58, 0.93)a | 0.74 (0.56, 0.99)a | 0.60 (0.36, 0.99)a | 1.35 (0.61, 2.99) | — |
| Arrhythmia | 1,617 | 1.01 (0.90, 1.13) | 1.00 (0.88, 1.14) | 0.98 (0.84, 1.13) | 0.91 (0.76, 1.09) | 0.93 (0.73, 1.17) | 0.71 (0.51, 0.99)a | 0.75 (0.45, 1.27) | 1.11 (0.39, 3.12) | — |
| Cerebrovascular | 434 | 1.01 (0.80, 1.27) | 1.04 (0.80, 1.33) | 1.08 (0.81, 1.44) | 1.17 (0.84, 1.63) | 1.41 (0.95, 2.09) | 1.13 (0.66, 1.93) | 1.22 (0.54, 2.74) | 0.80 (0.10, 6.21)b | — |
| Ischemia | 200 | 1.31 (0.94, 1.83) | 1.48 (1.04, 2.10)a | 1.69 (1.16, 2.48)a | 1.73 (1.11, 2.71)a | 1.51 (0.82, 2.77) | 1.76 (0.77, 4.00) | 1.98 (0.64, 6.14) | — | — |
| Myocardial infarction | 81 | 2.18 (1.32, 3.60)a | 2.11 (1.22, 3.66)a | 2.24 (1.22, 4.11)a | 2.85 (1.45, 5.60)a | 1.66 (0.52, 5.27) | 1.43 (0.26, 7.74) | 1.09 (0.10, 11.59)b | — | — |
| Heart failure | 441 | 0.79 (0.63, 1.00)a | 0.77 (0.59, 1.00) | 0.81 (0.60, 1.09) | 0.87 (0.61, 1.24) | 0.81 (0.51, 1.30) | 0.74 (0.41, 1.34) | 0.94 (0.38, 2.35) | 1.87 (0.39, 8.90) | — |
Note: For the acute heat wave analysis, the results of which are reported in this table, a heat wave day was defined as a day on which the observed HI was above the indicated threshold as was the HI on the previous day. Days with a HI below the threshold and/or those that were preceded by a daily HI below the threshold were considered non–heat wave days. Conditional logistic regression models adjusted for daily concentration were used to estimate ORs. —, no data; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ED, emergency department; HI, heat index; OR, odds ratio.
95% CI does not include the null.
Only one ED visit related to the indicated health outcome occurred on an exposed day (i.e., an acute heat wave day as defined above) during the study period.
Table 11.
ORs and 95% CIs for one additional previous day on which the observed HI was above the indicated threshold in Anchorage, Fairbanks, and the Matanuska-Susitna Valley, Alaska, 2015–2019.
| Reason for ED visit | Number of cases | 21.1°C (70°F) HI threshold | 22.2°C (72°F) HI threshold | 23.3°C (74°F) HI threshold | 24.4°C (76°F) HI threshold | 25.6°C (78°F) HI threshold | 26.7°C (80°F) HI threshold | 27.8°C (82°F) HI threshold | 28.9°C (84°F) HI threshold | 30°C (86°F) HI threshold |
|---|---|---|---|---|---|---|---|---|---|---|
| Heat illness | 35 | 1.05 (0.98, 1.11) | 1.07 (0.98, 1.17) | 1.08 (0.98, 1.20) | 1.20 (0.95, 1.51) | 1.18 (0.91, 1.53) | 1.31 (0.89, 1.92) | 1.17 (0.39, 3.54) | 3.56 (1.06, 11.92)a | — |
| Asthma | 2,911 | 1.01 (0.99, 1.02) | 1.01 (0.99, 1.02) | 1.01 (0.99, 1.03) | 1.00 (0.97, 1.03) | 1.01 (0.97, 1.05) | 1.04 (0.98, 1.10) | 1.04 (0.92, 1.17) | 1.04 (0.77, 1.41) | 0.74 (0.27, 2.04) |
| COPD | 2,158 | 0.99 (0.98, 1.01) | 0.98 (0.97, 1.00) | 0.98 (0.96, 1.00) | 0.97 (0.93, 1.01) | 0.93 (0.88, 0.99)a | 0.89 (0.81, 0.97)a | 0.88 (0.74, 1.05) | 0.88 (0.60, 1.30) | 1.24 (0.44, 3.48) |
| Pneumonia | 2,081 | 1.00 (0.99, 1.01) | 0.99 (0.97, 1.01) | 1.00 (0.97, 1.02) | 1.01 (0.97, 1.05) | 1.03 (0.97, 1.08) | 1.05 (0.98, 1.13) | 1.10 (0.96, 1.25) | 1.25 (0.93, 1.68) | 0.78 (0.19, 3.27) |
| Bronchitis | 1,972 | 0.99 (0.97, 1.00) | 0.97 (0.95, 0.99)a | 0.96 (0.93, 0.98)a | 0.94 (0.90, 0.99)a | 0.95 (0.89, 1.01) | 0.91 (0.83, 1.00)a | 0.92 (0.78, 1.09) | 0.95 (0.66, 1.35) | 0.89 (0.32, 2.46) |
| Arrhythmia | 1,617 | 1.00 (0.99, 1.02) | 1.01 (0.99, 1.03) | 1.00 (0.97, 1.02) | 1.00 (0.96, 1.05) | 1.02 (0.97, 1.08) | 1.00 (0.93, 1.09) | 1.03 (0.88, 1.20) | 0.98 (0.64, 1.50) | 2.30 (0.79, 6.71) |
| Cerebrovascular | 434 | 0.98 (0.95, 1.01) | 0.98 (0.94, 1.03) | 0.99 (0.94, 1.04) | 0.99 (0.91, 1.09) | 0.98 (0.88, 1.10) | 0.96 (0.81, 1.14) | 0.73 (0.48, 1.11) | 0.20 (0.03, 1.31) | — |
| Ischemia | 200 | 1.04 (1.00, 1.08) | 1.06 (1.01, 1.12)a | 1.05 (0.98, 1.11) | 1.03 (0.92, 1.16) | 1.09 (0.94, 1.25) | 1.15 (0.98, 1.36) | 1.12 (0.79, 1.60) | 1.93 (0.92, 4.07) | 4.89 (0.95, 25.32) |
| Myocardial | 81 | 1.07 (1.01, 1.14)a | 1.08 (1.00, 1.18) | 1.09 (0.98, 1.20) | 0.98 (0.81, 1.17) | 1.03 (0.83, 1.28) | 1.11 (0.88, 1.41) | 1.15 (0.70, 1.90) | 1.17 (0.25, 5.59) | — |
| Heart failure | 441 | 1.00 (0.97, 1.03) | 1.01 (0.96, 1.05) | 1.00 (0.95, 1.05) | 0.97 (0.88, 1.07) | 1.01 (0.89, 1.14) | 0.97 (0.82, 1.15) | 0.99 (0.72, 1.37) | 0.45 (0.12, 1.64) | 1.89 (0.41, 8.57) |
Note: For the ongoing heat wave analysis, the results of which are reported in this table, the effect of prolonged elevated HI was estimated via a linear variable in the model that represented the number of previous consecutive days on which the observed HI was above the relevant threshold. An indicator variable was also included to control for same-day elevated HI. As with the threshold analysis, a value of “1” indicated that the same-day HI was above the relevant threshold, and a value of “0” indicated that the same-day HI was below the relevant threshold. Conditional logistic regression models adjusted for daily concentration were used to estimate ORs. —, no data; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HI, heat index; OR, odds ratio.
95% CI does not include the null.
Heat illness.
In the acute heat wave analysis, the odds of an ED visit related to heat illness occurring on a heat wave day (i.e., a day with a HI above the tested threshold that was immediately preceded by another day with an elevated HI) was significantly higher than non–heat wave days at all HI thresholds tested (Table 10). Using the ongoing heat wave approach, we observed that the odds of heat illness-related ED visits increased significantly by 3.56 times per additional previous day with a HI above 28.9°C (84°F) (Table 11). When stratified by sex, the effect of prolonged heat was more apparent among females than males. From the ongoing heat wave analysis, it was observed that prolonged elevated HI above thresholds as low as 24.4–26.7°C (76–80°F) significantly increased the risk of heat illness–related ED visits among females, with the odds of a heat illness–related ED visit increasing 2.56 times (95% CI: 1.11, 5.89) for each additional consecutive day above 26.7°C (Table S5a). Among males, no significantly increased odds of heat illness–related ED visits were observed for additional previous days with a HI above any of the thresholds tested. Similarly, when stratified by race, the effects of prolonged heat on heat illness–related ED visits were observed only among non-Alaska Native people, but not Alaska Native people. Among non-Alaska Native people, additional previous elevated HI days were significantly associated with increased odds of heat illness-related ED visits for almost all thresholds tested [22.2°C, 24.4°C, 26.7°C, and 28.9°C (72°F, 76°F, 80°F, and 84°F) Table S5d].
Respiratory: asthma.
No significant associations were found between prolonged elevated HI above any thresholds and asthma-related ED visits among the total population, or when stratified by sex or race. When stratified by age, however, there was some evidence of a positive association between prolonged elevated HI and asthma-related ED visits among people y of age in the ongoing heat wave analysis. Among this age group, the odds of an asthma-related ED visit increased by 19% (95% CI: 5, 36%) and 33% (95% CI: 13, 57%) for each additional day with a HI above 25.6°C and 26.7°C (78°F and 80°F), respectively (Table S5g). For the groups of age and 15–65 y of age, there were no significant associations between prolonged elevated HI and asthma-related ED visits in either the acute or the ongoing heat wave analysis.
Respiratory: COPD.
The effect of prolonged elevated HI on COPD-related ED visits was generally a reduction in odds that was significant at HI thresholds of 26.7°C (80°F) and 27.8°C (82°C) in the acute heat wave analysis and 25.6°C (78°F) and 26.7°C (80°F) in the ongoing heat wave analysis. When stratified by age, there is some evidence that the negative associations with a heat wave as evaluated using the acute heat wave approach were more consistent among people 15–65 y of age than those among people y of age (Tables S4e–g). However, there were no significant associations among the group 15–65 y of age using the ongoing heat wave approach.
Respiratory: pneumonia.
No significant associations between prolonged elevated HI and pneumonia-related ED visits were observed among the total study population using either the ongoing or the acute heat wave approach (Tables 10 and 11). When stratified by age, however, a significant heat wave effect was estimated for the group 15–65 y of age on acute heat wave days above HI thresholds of 25.6°C (78°F) (Tables S4e–g). When stratified by sex, it became apparent that among this study population females are more susceptible to the negative impacts of prolonged heat on pneumonia-related ED visits than males. Although no significant associations were observed among males, significantly higher odds of pneumonia-related ED visits were observed on acute heat wave days above HI thresholds of 25.6–27.8°C (78–82°F) among females using the acute heat wave approach [at 27.8°C (82°F) threshold, ; 95% CI: 1.12, 3.00] (Tables S4a–b). Similarly, additional previous elevated HI days above a threshold of 26.7°C (80°F) were significantly associated with increased risk of pneumonia-related ED visits among females using the ongoing heat wave approach (Table S5a). For each additional previous day with a HI above 26.7°C (80°F), the odds of pneumonia-related ED visit among females increased by 13% (95% CI: 4%, 24%). There were a few significant negative associations among Alaska Native people at lower HI thresholds using the acute heat wave approach that were not present among non-Alaska Native people (Table S4c–d).
Respiratory: bronchitis.
Negative associations were observed between prolonged elevated HI and bronchitis-related ED visits in both the acute and ongoing heat wave models (Tables 10 and 11). When stratified by age, acute heat wave effects were only observed among people 15–65 y of age, and negative associations were estimated using the ongoing heat wave analysis for both age groups y of age (Tables S4e–g). There were obvious differences in the effect of both acute and ongoing heat wave events on bronchitis-related ED visits when stratified by race (Tables S4c–d, S5c–d). A significant reduction in odds was identified among non-Alaska Native people for additional previous elevated HI days for almost all thresholds tested 21.1–28.9°C (70–84°F) and for acute heat wave events for several HI thresholds 22.2–26.7°C (72–80°F). However, there was only one significant effect of acute heat wave events on bronchitis-related ED visits among Alaska Native people, and no significant effects of ongoing heat wave events.
Cardiovascular: arrhythmia.
Among the entire study population, one isolated negative association was identified between elevated HI on arrhythmia-related ED visits in the acute heat wave analysis; the remainder of estimated ORs were not significant (Table 10). When stratified by age, only one sporadic significant finding was identified: an OR of 3.67 (95% CI: 1.01, 13.37) was estimated for arrhythmia-related ED visits for each additional day with an elevated HI above a 30°C (86°F) among people 15–65 y of age. No significant findings were observed among Alaska Native or non-Alaska Native people when stratified by race.
Cardiovascular: cerebrovascular.
No significant associations between elevated HI and cerebrovascular-related ED visits were observed in any of the total or stratified heat wave models.
Cardiovascular: ischemia.
From both the acute and ongoing heat wave analyses, there is some evidence of a positive association between prolonged elevated HI and risk of ischemia-related ED visits among our study population. Significant elevated ORs were estimated for acute heat wave days in comparison with non–heat wave days for HI thresholds of 22.2–24.4°C (72–76°F) (Table 10) and for additional previous elevated HI days above a threshold of 22.2°C (72°F) (Table 11). Strong positive associations between ischemia-related ED visits and prolonged elevated HI were observed among individuals y of age at thresholds of 28.9–30°C (84–86°F) using the ongoing heat wave analysis; the odds of an ischemia-related ED visits increased by 2.66 times (95% CI: 1.06, 6.67) for each additional day with a HI above 28.9°C (84°F) for this age group (Table S5g). Among Alaska Native people, there were significant adverse effects associated with both acute and ongoing heat wave events. The odds of an ischemia-related ED visit among this group was 4.16 times higher (95% CI: 1.79, 9.69) on an acute heat wave day above a HI of 24.4°C (76°F) than a non–heat wave day (Table S4c). The negative impact of prolonged elevated HI on the risk of ischemia-related ED visits was consistent and strong among females, but no significant ORs were estimated for males using either of the heat wave analyses (Tables S4a–b, S5a–b). For example, the odds of ED visits for ischemia among females increased by 32% (95% CI: 5%, 65%) for each additional previous day with a HI above 26.7°C (80°F).
Cardiovascular: myocardial infarction.
Acute heat wave events were significantly associated with increased odds of MI-related ED visits among the total study population at HI thresholds of 21.1–24.4°C (70–76°F) (Table 10). Positive associations were also observed for ongoing heat wave events with the odds of MI-related ED visits increasing by 7% (95% CI: 1%, 14%) for each additional consecutive day with a HI above 70°F (Table 11). However, the estimated increase in odds of MI-related ED visits related to ongoing heat wave events among the entire study population were not significant at higher thresholds. When stratified by sex, the adverse effects of acute heat wave events on the odds of MI-related ED visits were predominantly observed among females (Tables S4a–b). Statistically significant elevated ORs were estimated for MI-related ED visits among females on heat wave days vs. non–heat wave days at HI thresholds of 22.2–25.6°C (72–78°F); a significantly elevated OR among males was only estimated for an acute heat wave at the 21.1°C (70°F) threshold.
Cardiovascular: congestive heart failure.
Only one significant negative association between prolonged elevated HI and heart failure–related ED visits was observed among the entire study population using the acute heat wave analysis [for 21.1°C (70°F) threshold: ; 95% CI: 0.62, 1.00] (Table 5). Among individuals 15–65 y of age, the odds of an ED visit for heart failure was 7.43 (95% CI: 1.01, 54.77) times higher on a heat wave day above an HI of 28.9°C (84°F) in comparison with a non–heat wave day (Table S4f). There were also sporadic positive associations among this age group using the ongoing heat wave analysis. When stratified by sex, several significant negative associations between acute heat wave events and heart failure–related ED visits emerged among males [for HI thresholds of 21.1–22.2°C (70–72°F)] that were not observed among females. The associations between prolonged elevated HI and heart failure–related ED visits were primarily nonsignificant among non-Alaska Native people and Alaska Native people when stratified by race.
Table 5.
ORs and 95% CIs for ED visits for HI on lag day 1 above vs. below the threshold from single-day lag models in Anchorage, Fairbanks, and the Matanuska-Susitna Valley, Alaska, 2015–2019.
| Reason for ED visit | Number of cases | 21.1°C (70°F) HI threshold | 22.2°C (72°F) HI threshold | 23.3°C (74°F) HI threshold | 24.4°C (76°F) HI threshold | 25.6°C (78°F) HI threshold | 26.7°C (80°F) HI threshold | 27.8°C (82°F) HI threshold | 28.9°C (84°F) HI threshold | 30°C (86°F) HI threshold |
|---|---|---|---|---|---|---|---|---|---|---|
| Heat illness | 35 | 13.63 (3.99, 46.50)a | 8.14 (3.26, 20.36)a | 7.83 (3.44, 17.80)a | 13.22 (5.70, 30.68)a | 12.91 (5.66, 29.43)a | 18.38 (7.47, 45.19)a | 10.60 (3.60, 31.20)a | 18.00 (3.01, 107.72)a | — |
| Asthma | 2,911 | 1.00 (0.92, 1.08) | 1.06 (0.97, 1.16) | 1.05 (0.95, 1.15) | 1.05 (0.94, 1.17) | 1.10 (0.97, 1.24) | 1.18 (1.00, 1.39)a | 1.23 (0.99, 1.53) | 1.10 (0.75, 1.62) | 0.79 (0.28, 2.18) |
| COPD | 2,158 | 0.93 (0.85, 1.03) | 0.94 (0.85, 1.04) | 0.94 (0.84, 1.05) | 0.96 (0.84, 1.09) | 0.85 (0.72, 1.00) | 0.68 (0.53, 0.87)a | 0.72 (0.51, 1.00)a | 0.77 (0.44, 1.36) | 1.26 (0.45, 3.55) |
| Pneumonia | 2,081 | 0.90 (0.82, 1.00)a | 0.90 (0.81, 1.00) | 0.91 (0.81, 1.02) | 0.97 (0.84, 1.10) | 1.09 (0.93, 1.28) | 1.24 (1.01, 1.53)a | 1.40 (1.06, 1.84)a | 1.48 (0.96, 2.28) | 0.75 (0.18, 3.15) |
| Bronchitis | 1,972 | 0.78 (0.71, 0.87)a | 0.82 (0.73, 0.91)a | 0.80 (0.71, 0.91)a | 0.76 (0.66, 0.88)a | 0.79 (0.67, 0.95)a | 0.79 (0.62, 1.01) | 0.81 (0.58, 1.13) | 0.99 (0.60, 1.63) | 0.92 (0.33, 2.54) |
| Arrhythmia | 1,617 | 1.02 (0.91, 1.14) | 1.02 (0.91, 1.15) | 0.98 (0.86, 1.11) | 0.94 (0.81, 1.09) | 1.07 (0.89, 1.27) | 0.88 (0.68, 1.14) | 0.84 (0.58, 1.20) | 1.10 (0.61, 1.96) | 2.32 (0.80, 6.77) |
| Cerebrovascular | 434 | 0.96 (0.77, 1.19) | 0.90 (0.71, 1.13) | 0.90 (0.70, 1.17) | 1.07 (0.80, 1.42) | 1.18 (0.85, 1.65) | 1.05 (0.66, 1.67) | 0.68 (0.32, 1.44) | 0.21 (0.03, 1.50)b | — |
| Ischemia | 200 | 1.23 (0.90, 1.69) | 1.36 (0.98, 1.89) | 1.51 (1.07, 2.13)a | 1.40 (0.93, 2.09) | 1.22 (0.74, 2.01) | 1.54 (0.82, 2.87) | 1.53 (0.65, 3.59) | 3.26 (1.06, 10.02)a | 4.74 (0.92, 24.44) |
| Myocardial infarction | 81 | 1.81 (1.12, 2.93)a | 1.90 (1.13, 3.17)a | 2.07 (1.20, 3.56)a | 1.86 (1.00, 3.46)a | 1.03 (0.41, 2.58) | 1.59 (0.48, 5.30) | 1.78 (0.45, 7.10) | 1.43 (0.17, 11.90)b | — |
| Heart failure | 441 | 0.82 (0.66, 1.02) | 0.82 (0.65, 1.03) | 0.88 (0.69, 1.14) | 0.85 (0.64, 1.14) | 1.00 (0.71, 1.41) | 0.83 (0.51, 1.37) | 1.08 (0.55, 2.10) | 0.44 (0.10, 1.95) | 1.99 (0.44, 9.07) |
Note: Conditional logistic regression models adjusted for daily concentration were used to estimate ORs. —, no data; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HI, heat index; OR, odds ratio.
95% CI does not include the null.
Only one ED visit related to the indicated health outcome occurred 1 d after an exposure (i.e., a day during which the HI exceeded the indicated threshold) during the study period.
Interaction between Elevated HI and
The interaction term between elevated HI and significantly contributed to model fit for several respiratory outcomes, but few interaction effects were significant in models for cardiovascular outcomes (two for heart failure–, two for ischemia-related, and one for MI-related ED visits). Table 12 compares ORs from interaction models with ORs from the relevant model of the primary analysis. We have only included estimates from models in which the interaction term significantly contributed to the fit.
Table 12.
ORs and 95% CIs for ED visits associated with a maximum daily HI above vs. below the indicated threshold on the indicated lag day for models in which an interaction term between and elevated HI was significant in Anchorage, Fairbanks, and the Matanuska-Susitna Valley, Alaska, 2015–2019.
| Reason for ED visit | Lag day | HI threshold | Original modela | Interaction modelb | |||
|---|---|---|---|---|---|---|---|
| Asthma | 1 | 84 | 1.10 (0.75, 1.62) | 1.59 (0.98, 2.60) | 1.50 (0.95, 2.37) | 1.34 (0.90, 2.00) | 1.13 (0.77, 1.64) |
| Asthma | 2 | 84 | 0.91 (0.60, 1.39) | 1.31 (0.79, 2.18) | 1.24 (0.77, 2.00) | 1.12 (0.73, 1.73) | 0.96 (0.63, 1.44) |
| Asthma | 5 | 70 | 0.92 (0.85, 1.00) | 0.93 (0.86, 1.01) | 0.90 (0.82, 0.98)c | 0.83 (0.74, 0.94)c | 0.74 (0.60, 0.92)c |
| Asthma | 5 | 72 | 0.90 (0.82, 0.98)c | 0.91 (0.84, 1.00) | 0.88 (0.81, 0.96)c | 0.82 (0.72, 0.93)c | 0.74 (0.59, 0.91)c |
| Asthma | 5 | 74 | 0.94 (0.86, 1.04) | 0.96 (0.87, 1.07) | 0.93 (0.84, 1.02) | 0.86 (0.75, 0.97)c | 0.76 (0.61, 0.94)c |
| Bronchitis | 2 | 82 | 0.78 (0.55, 1.11) | 0.64 (0.42, 0.96)c | 0.67 (0.46, 1.00)c | 0.75 (0.52, 1.09) | 0.89 (0.61, 1.31) |
| Bronchitis | 2 | 86 | 0.91 (0.33, 2.50) | — | — | — | — |
| Bronchitis | 5 | 82 | 0.92 (0.66, 1.29) | 0.77 (0.52, 1.14) | 0.81 (0.56, 1.17)c | 0.89 (0.62, 1.27) | 1.03 (0.71, 1.50) |
| Bronchitis | 5 | 84 | 1.10 (0.63, 1.92) | 0.77 (0.39, 1.52) | 0.82 (0.43, 1.58)c | 0.93 (0.50, 1.72) | 1.12 (0.62, 2.01) |
| COPD | 2 | 80 | 0.76 (0.60, 0.96)c | 0.68 (0.53, 0.89)c | 0.72 (0.56, 0.92)c | 0.80 (0.62, 1.03) | 0.93 (0.68, 1.28) |
| COPD | 3 | 74 | 0.83 (0.74, 0.93)c | 0.81 (0.72, 0.92)c | 0.86 (0.76, 0.97)c | 0.96 (0.80, 1.16) | 1.13 (0.82, 1.57) |
| COPD | 3 | 76 | 0.83 (0.73, 0.96)c | 0.81 (0.70, 0.93)c | 0.86 (0.74, 0.99)c | 0.96 (0.79, 1.16) | 1.14 (0.82, 1.58) |
| COPD | 3 | 80 | 0.76 (0.60, 0.97)c | 0.69 (0.53, 0.89)c | 0.72 (0.56, 0.93)c | 0.80 (0.62, 1.02) | 0.92 (0.68, 1.26) |
| COPD | 3 | 84 | 0.62 (0.34, 1.15) | 0.41 (0.18, 0.90)c | 0.43 (0.20, 0.93)c | 0.48 (0.23, 1.00)c | 0.57 (0.29, 1.13) |
| COPD | 5 | 70 | 0.90 (0.82, 0.99)c | 0.91 (0.83, 1.00) | 0.87 (0.78, 0.96)c | 0.78 (0.67, 0.91)c | 0.67 (0.52, 0.88)c |
| COPD | 5 | 72 | 0.86 (0.78, 0.96)c | 0.88 (0.79, 0.97)c | 0.83 (0.75, 0.93)c | 0.75 (0.65, 0.88)c | 0.65 (0.50, 0.84)c |
| COPD | 5 | 74 | 0.86 (0.77, 0.97)c | 0.88 (0.79, 0.99)c | 0.84 (0.75, 0.94)c | 0.76 (0.65, 0.89)c | 0.65 (0.50, 0.85)c |
| COPD | 5 | 76 | 0.81 (0.71, 0.93)c | 0.84 (0.73, 0.96)c | 0.80 (0.70, 0.92)c | 0.73 (0.62, 0.86)c | 0.64 (0.49, 0.83)c |
| COPD | 5 | 78 | 0.77 (0.65, 0.91)c | 0.81 (0.68, 0.96)c | 0.77 (0.65, 0.91)c | 0.69 (0.58, 0.83)c | 0.59 (0.45, 0.78)c |
| Heart failure | 2 | 84 | 0.29 (0.04, 2.14) | — | — | — | — |
| Heart failure | 3 | 86 | 1.10 (0.14, 8.60) | — | — | — | — |
| Heat illness | 3 | 82 | 1.05 (0.22, 4.97) | 32.77 (0.72, 1,497.60) | 4.10 (0.73, 23.05) | 0.06 (0.00, 23.79) | — |
| Ischemic | 2 | 82 | 0.94 (0.36, 2.50) | 3.21 (0.85, 12.16) | 1.95 (0.69, 5.53) | 0.72 (0.21, 2.50) | 0.16 (0.01, 2.21) |
| Ischemic | 5 | 80 | 1.37 (0.77, 2.44) | 1.03 (0.54, 1.98) | 1.25 (0.66, 2.36) | 1.81 (0.84, 3.91) | 3.18 (0.97, 10.49) |
| Myocardial infarction | 0 | 70 | 1.53 (0.93, 2.49) | 1.76 (0.97, 3.19) | 3.00 (0.98, 9.18) | 8.69 (0.76, 99.82) | 42.84 (0.47, 3,881.40) |
| Pneumonia | 1 | 70 | 0.82 (0.66, 1.02) | 0.89 (0.81, 0.98)c | 0.94 (0.85, 1.05) | 1.06 (0.89, 1.26) | 1.26 (0.93, 1.71) |
| Pneumonia | 1 | 72 | 0.82 (0.65, 1.03) | 0.88 (0.79, 0.98)c | 0.94 (0.84, 1.05) | 1.06 (0.89, 1.25) | 1.26 (0.94, 1.71) |
| Pneumonia | 1 | 74 | 0.88 (0.69, 1.14) | 0.88 (0.78, 1.00)c | 0.94 (0.83, 1.06) | 1.05 (0.89, 1.25) | 1.26 (0.93, 1.69) |
| Pneumonia | 1 | 76 | 0.85 (0.64, 1.14) | 0.93 (0.81, 1.07) | 0.98 (0.85, 1.12) | 1.08 (0.90, 1.29) | 1.24 (0.93, 1.67) |
| Pneumonia | 1 | 80 | 0.83 (0.51, 1.37) | 1.11 (0.88, 1.39) | 1.18 (0.95, 1.46) | 1.33 (1.07, 1.66)c | 1.60 (1.18, 2.15)c |
Note: Conditional logistic regression models were used to estimate ORs. —, Model did not converge due to limited sample size; the model is nonetheless listed to indicate that the interaction term was significant for the relevant outcome, threshold, and lag day; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ED, emergency department; HI, heat index; OR, odds ratio; , particulate matter with aerodynamic diameter .
Reported ORs from the original model can be interpreted as the odds of an ED visit when the HI on the given lag day is above vs. below the given threshold, while controlling for daily mean concentration.
Reported ORs from the model that included an interaction between the indicator variable for HI above vs. below the threshold and daily mean concentration can be interpreted as the odds of an ED visit when the HI on the given lag day is above vs. below the given threshold and the concentration on the given lag day is 5, 10, 20, or .
95% CI does not include the null.
There was some evidence that amplified the negative association between elevated HI and asthma-related ED visits 5 d after an elevated HI. When controlling for , it was 0.90 (95% CI: 0.82, 0.98) times as likely that an asthma-related ED visit occurred 5 d after a HI above 22.2°C (72°F) than below 22.2°C (72°F). When an interaction term was included in the same model, decreasing ORs of 0.88 (95% CI: 0.81, 0.96), 0.82 (95% CI: 0.72, 0.93), and 0.74 (95% CI: 0.59, 0.91) were estimated at increasing concentrations of 10, 20, and , respectively. In contrast, high concentrations (from 20–) increased the ORs of pneumonia-related ED visits for the day after a HI above vs. below 26.7°C (80°F) to reach a significantly positive association.
Discussion
Climate scientists predict that northern climates will experience the most dramatic effects of climate change.43 These effects require public health agencies to be prepared to protect populations who have previously had little experience with heat and where high-temperature days would be considered temperate in other places. In Alaska, we found heterogeneity in heat illness and cardiorespiratory health effects across temperature thresholds, by outcome, by lag, and by sociodemographic factors. Recently, people living in major population centers in Alaska experienced between 15 and 54 d with a during the five summers during the period 2015–2019. Although more infrequent, days with a occurred across all three study regions, highlighting the need for heat preparedness efforts in these northern communities.
Our results demonstrate an increased risk of several cardiorespiratory health outcomes on summer days above a HI threshold as low as 21.1°C (70°F). In particular, our study found strong associations between the HI and ED visits for heat illness for up to 4 d after the HI exceeded 21.1°C (70°F) and 5 d after the HI exceeded 23.3°C (74°F). Asthma and pneumonia were the only respiratory outcomes with notable positive associations with elevated HI. We found stronger effects for cardiovascular outcomes, including ischemia and MI. Other studies of emergency department admissions associated with elevated temperatures have found mixed results regarding cardiorespiratory outcomes. In California, positive associations were found between same-day apparent temperature and ED visits for cardiovascular events (including ischemic heart disease, acute MI, and cardiac dysrhythmias).7 In contrast to the respiratory outcomes results in our study, Basu et al. found positive associations with pneumonia and bronchitis and negative associations with asthma.7 In North Carolina, an assessment of three heat waves found statistically significant increases in the number of ED visits for ischemic heart disease, but not for other cause-specific cardiovascular outcomes, and increases in the number of pneumonia/influenza and COPD visits.44 In Georgia, an evaluation of hospitalization risk associated with a change in maximum temperature between 27°C and 32°C (80.6°F and 89.6°F) (25th and 75th percentiles in the region) showed predominantly negative associations with cardiovascular outcomes (hypertension, congestive heart failure) and an increase in risk of asthma/wheeze.45 Biologically, heat can trigger a physiological chain of events stemming from the dilation of blood vessels to redirect blood to the skin to dissipate heat, leaving inadequate blood flow to other organs, resulting in cell damage and decreased vital functions.46 Regarding one of the respiratory outcomes assessed in this study, the most common cause of bacterial pneumonia worldwide is Streptococcus pneumoniae,47 which thrives at temperatures between 30°C and 35°C (86°F and 95°F). Ambient conditions approaching this range may increase the likelihood of bacterial growth and infection.
In the present study, multiple consecutive days of warm weather were associated with an increased risk of health impacts. For each additional preceding day above a HI of 22.2°C (72°F), the odds of ED visits for ischemia-related visits increased 6%, and for each additional preceding day above a HI of 21.1°C (70°F) the odds of MI visits increased 7%. In addition, the odds of ischemia- and MI-related ED visits were significantly higher following 2 consecutive days above HI thresholds of 21.1–23.3°C (70–74°F). There is no standard definition for a heat wave, complicating cross-study comparisons. However, other studies have used an approach similar to that of the present study in modeling the effect of both a single day and multiple days of extreme heat to facilitate investigations of heat wave effects. For example, in a study of cardiorespiratory hospital admissions in Finland, there were no significant associations between daily temperature and any health outcomes on single lag days 0–5, but the researchers found significant positive associations for pneumonia, all respiratory diseases, COPD, and MI in age-stratified analyses of the effect of at least 3 or 4 d of consecutive extreme temperatures.48 In contrast, a study on temperature and mortality from 400 communities in 18 countries found that heat wave effects on mortality were affected by temperature and that the increased risk of mortality lasted 3–4 d after a warm day; however, additional days of extreme heat did not confer additional risk across most regions.49 The authors controlled for lagged effects in their heat wave model, which they suggested may explain why other studies that modeled these effects separately (including the present study) may have found evidence of compounding risk from consecutive warm days.
This study also provided evidence of lagged impacts on the risk of heat illness and cardiorespiratory ED visits over a period of 5 d after elevated temperatures occurred in the study region. Although the risk of ED visits for heat illness was highest on the day when the HI was elevated and was attenuated at longer lags, some of the cardiorespiratory outcomes we assessed either had consistent or increasing risk 1–5 d after a heat event. In particular, there was no evidence of increased odds of asthma-related ED visits on the same day that the HI was elevated, but significantly higher odds were evident on the following day. In addition, although the odds of MI-related ED visits doubled on days when the HI was above 23.3°C or 24.4°C (74°F or 76°F), the risk tripled 4 d after these heat thresholds were exceeded. This finding is in contrast to those of a number of other studies that have found the strongest associations at early lag periods with weakening effects at later lags. Among the Medicare population in the United States, the strongest associations between all-cause respiratory hospitalizations and heat were on the same day as the exposure and then decreased when lags of up to 2 d were assessed.16 A study of indoor heat and respiratory, circulatory, and renal mortality and hospitalizations in census block groups in Houston, Texas, found similar attenuated effects after 2 d.50 In a multicity study in Brazil,51 found positive associations between heat and cardiovascular hospitalization on the first day of exposure followed by a negative association on subsequent exposure days. They attributed this pattern to either displacement of hospitalizations that would likely have occurred at a more regular interval had there been no heat event or to high rates of mortality subsequent to heat exposure. Most epidemiology studies involving heat have assessed a narrow time frame (lags of 0–7 d after a heat event). Consequently, the differences in hospitalization patterns found in these studies are fairly minor; however, in contrast with observations in other regions where hospitalization risk increased in conjunction with high temperatures and then decreased, in our study we observed that risk for some cardiovascular outcomes peaked 5 d after a warm day, which may have implications for ED staff, medical facilities, and public health officials.
Among demographic subgroups, females had an increased odds of asthma-, pneumonia-, ischemia-, and MI-related events during days with an elevated HI. This increase was in contrast to that of the male population, for which there was little to no change in odds of ED visits related to these health outcomes in association with elevated HI. There is recognition of sex-related differences in cardiovascular aging52 that may affect susceptibility to elevated heat. Others have noted that behavioral factors linked to heat exposure (e.g., outdoor work, outdoor activities) may affect the likelihood of heat-related ED visits by sex.42
On days with an elevated HI, the Alaska Native population was more likely to visit the ED for ischemia in comparison with the non-Alaska Native population; however, we observed an increased odds of MI-related ED visits among the non-Alaska Native population 2–5 d after HI was above thresholds of 21.1–24.4°C (70–76°F) that was not observed among the Alaska Native population. In addition, although the risk of heat illness among the Alaska Native population increased for a day after a heat event, the risk for the non-Alaska Native population remained elevated for 5 d. In this study, there is no clear disparity in heat-related ED visits in urban Alaska for Alaska Native vs. non-Alaska Native residents; however, other social, cultural, or neighborhood factors may influence heat sensitivity in Alaska’s communities. Future heat–health assessments could consider other race strata, socioeconomic characteristics (e.g., income, education), behavior (e.g., social isolation), or neighborhood features (e.g., poverty, property ownership, greenspace).53
In comparison with other age groups, people y of age were more prone to asthma- and cerebrovascular-related events associated with elevated HI. This age group also experienced increased odds of ED visits related to MI up to 1 d after an elevated HI. Older adults have been previously highlighted as particularly sensitive to heat because of decreasing physiological function, a high rate of preexisting conditions, and social factors such as living alone or being homebound.41,54,55 Some studies of heat-related hospitalizations have found elevated cardiovascular admissions in the elder population,56,57 whereas others have not found an age effect.58–60 In an assessment of heat and respiratory hospitalizations among people y of age, Anderson et al.16 observed a 4.3% (95% CI: 3.8%, 4.8%) increase in respiratory hospitalizations for each 10°F increase, with similar results after adjusting for exposure to ozone and PM.
In our analysis, people 15–65 y of age experienced increased risk of heat illness at HI thresholds as low as 21.1°C (70°F), whereas the risk for children y of age did not increase until a HI threshold of 25.6°C (78°F) was reached. Children are generally considered more vulnerable to extreme heat than adults because of a less-developed thermoregulatory response, higher metabolism, less ability to protect themselves, and more time spent outdoors participating in vigorous activities than adults leading to a higher likelihood of exposure.11 Because the ability to seek health care is substantially different for adults and children, we hesitate to draw conclusions regarding potential age-dependent effects of heat from our threshold analysis of ED visits. More important, it is critical to ensure that the development of local heat mitigation and adaptation policies consider this younger age group.
Because this is the first study to assess heat-related morbidity in Alaska, we consider similar studies in other regions to contextualize our findings on temperature thresholds. In an assessment across the contiguous United States, Vaidyanathan et al.26 found that peak heat-attributable hospitalizations occurred when the HI was between 27.2°C (81°F) and 29.4°C (85°F) in the northwestern and western regions, between 86°F and 90°F in the central and east north central (Minnesota, Iowa, Wisconsin, Michigan) regions, between 32.8°C (91°F) and 35°C (95°F) in the northeast and west north central (Montana, Wyoming, North Dakota, South Dakota, Nebraska) regions, between 35°C (95°F) and 37.8°C (100°F) in the southeast, and over 37.8°C (100°F) in the south and southwest regions. Extending this dose–response relationship to the milder climate of Alaska, we would expect to see health impacts below a HI of 26.7°C (80°F), as have been observed in studies in other high-latitude regions. Based on the current threshold for heat warnings in Sweden, several studies have reported increased mortality when temperatures reach 26.1–26.7°C (79–80°F) but have not tested lower temperature thresholds.61–63 During the 2010 heat wave in Russia, 3 consecutive days of average daily temperatures of 23.6°C (74.5°F) in Moscow were associated with increased mortality, after controlling for the effect of wildfire smoke in the area.64
Geographic differences in temperature thresholds associated with increased health risk may be because of acclimatization to the local climate. The role of acclimatization on decreasing sensitivity to extreme heat has been explored in a number of ways. Assessments of heat-related health impacts over the course of a summer season have found higher risk of mortality during the first heat wave of the year9,65 or early in the season in comparison with subsequent temperature anomalies.66 Multisite time-series studies that compared heat effects between communities have found a higher risk of heat-related mortality in cities with mild summers and less access to air conditioning.56,67 A study assessing long-term trends in heat-related mortality in the United States found that, overall, the number of deaths per year between 1987 and 2005 declined, suggesting that residents are adapting to incremental changes in heat, although the authors emphasized that substantial health risks remain because of increasing heat caused by climate change.59 In addition, within specific cohorts, epidemiological studies have found higher rates of heat illness among soldiers or athletes from cooler regions in comparison with those from warmer areas,68,69 suggesting that being unacclimatized to hot weather is a risk factor for heat intolerance. A notable finding in the current study is that we found an increased risk of heat-related morbidity among Alaska residents at HI thresholds as low as 21.1°C (70°F). Given that this is the average maximum summer temperature in Fairbanks and above average in Anchorage,34 it is likely that many Alaskans are not physically adapted to extreme heat above this threshold. In addition, others have shown that high nighttime temperatures confer additional risk after a warm day.70 With abundant sunlight during the summer, high temperatures in Alaska can last into the evening, preventing people from getting a reprieve from the extreme heat. However, over the course of heat waves during our study period, there was some evidence of acute acclimatization. Although there were still significant effects of elevated HI after 5 d, these effects were attenuated. The OR of an ED visit related to heat illness on a day above vs. below 24.4°C (76°F) was 15.54 (95% CI: 5.70, 42.34); after 5 d, the OR was only 2.33 (95% CI: 1.13, 4.81). Several scenarios could explain this finding: Either the population became more acclimated to the heat or engaged in mitigation behaviors (e.g., obtained fans, air conditioning, or other modes of relief), or the most heat-sensitive people visited the ED early in the heat wave.
Finally, we found that increasing concentrations of decreased the odds of asthma-related visits and increased the odds of pneumonia-related ED visits on days of elevated heat or shortly thereafter. For people with chronic asthma, increasingly poor air quality may serve as a visual or physical indicator to stay inside, which may help them avoid heat exposure. In contrast, at warm temperatures that are hospitable for the bacteria that causes pneumonia, poor air quality may exacerbate inflammation in the lungs, increasing the likelihood of respiratory infection.71 For additional information on the independent effect of on cardiorespiratory ED visits in Alaska, see Hahn et al.33
These results can be used to create local heat response plans and develop guidance for issuing heat alerts in Alaska. For example, if heat indices above 21.1°C (70°F) are forecast, recommendations for strategies to keep cool (e.g., stay hydrated, visit air-conditioned places, wear light-colored clothes) could be shared with the general public in multiple languages,72–74 and hospitals could be notified that they may see an increase in ED visits for heat illness for up to 5 d after the heat event. In fact, heat alerts may actually increase hospitalizations if these public notices help people recognize symptoms of heat illness and access needed medical care.75 If forecasts predict several consecutive days above 24.4°C (76°F), alerts could include additional information regarding potential cardiovascular symptoms in addition to heat illness. Risk messages targeted to different demographic groups could include specific information about the most pertinent health risks (e.g., people y of age are at high risk of asthma exacerbations or MI).76 Other community-level public health strategies for extreme heat include identifying and designating buildings with air conditioning as public cooling centers, activating a heat hotline that people can call for advice and resources, providing water in public spaces, organizing public transportation to cooling centers, or developing guidelines for rescheduling outdoor events.72,77–79 Public health departments can also work with utility companies or planning departments on initiatives such as home energy assistance and weatherization programs80 or adding to and enhancing tree canopy and greenspace in urban areas.81–83
This study has a number of limitations that should be considered. The temperature information used to calculate the HI, the primary exposure in this study, was from one monitoring station in each study location. A number of factors affect individuals’ true heat exposure, including where they live in the city, their housing, and the amount of time they spend outside. Therefore, there may be misclassification of the exposure related to the health outcomes assessed in this study. Similarly, because some weather data were missing, we imputed the temperature for 1 d in the Matanuska-Susitna Valley and the PM data for 2 d in Anchorage, 9 d in Fairbanks, and 30 d in Matanuska-Susitna Valley. The major factor causing daily swings in summertime PM concentration in these regions is wildfire smoke. Among the days that were imputed, only 1 d during June 2015 in Fairbanks was potentially impacted by wildfire smoke (i.e., a plume was within of the study area and PM exceeds 1 SD above the long-term monthly mean). Although this approach may have contributed to exposure misclassification, we expect that the impact was minimal.
Although a strength of this study is that we controlled for , we did not control for ozone. A recent assessment of the role of ozone in heat-related mortality studies concluded that ozone is a causal intermediate in the pathway between heat and mortality.84 Based on this causal framework, our study results should be interpreted as the total effects of temperature on heat-related morbidity, including the potential impact caused by increased ozone on warm days. Although study data were sampled from Alaska’s primary population centers, small sample sizes may have limited our ability to detect significant associations between elevated HI and cardiorespiratory- or heat illness-related ED visits among some demographic groups at high temperature thresholds that occur infrequently in Alaska. For example, of the 1,021 case days and 12,403 control days for COPD-related ED visits among males, only 1 case day and 22 control days fell above a same-day HI threshold of 30°C (86°F). In addition, some large effect estimates identified in our study were driven by relatively few ED visits (e.g., a total of 35 ED visits for heat illness in our study area between 2015 and 2019). Although these estimates are statistically significant, it is important to note the magnitude of ED visits driving the effect estimates when interpreting the potential public health burden and planning for clinical resources to prepare for heat events in Alaska. Despite the low number of heat illness events in this data set, ED visits likely only capture the most severe cases, particularly in a region where symptoms of heat illness may be unfamiliar. It is likely that the actual incidence of adverse health effects related to extreme heat was higher than reported here because people were navigating illness on their own or through their regular medical provider.
This study is the first to assess the health impacts of extreme temperature in Alaska, and we used a HI threshold approach to facilitate the practical application of our results to public health preparedness in the state. Projections of extreme heat days in the contiguous United States show that by the middle of the century (2036–2065), the annual number of days with heat indices exceeding 37.8°C (100°F) and 40.6°C (105°F) are projected to double and triple in comparison with a 1971–2000 baseline, with portions of Texas and Florida projected to experience 100–150 d per year with a .85 Future projections for Alaska show that the number of days per year with a high temperature above 25°C (77°F) is expected to increase from the historical baseline (1981–2010) of 1.5 d per year to 29.7 d per year by the end of the century.86 It is notable that these warm temperatures have historically been limited to interior Alaska, but even near-term projections (2011–2040) show that these extreme temperatures will expand across the state.86 Our results suggest that Alaskans may be at increased risk of heat illness and cardiorespiratory health effects at HI thresholds as low as 21.1°C (70°F). Preparedness efforts could focus on collaboration between meteorological agencies and local health authorities to develop a heat–health warning system87 and associated public outreach materials. Local governments can also focus on “win–win” strategies, such as weatherization programs that simultaneously lower energy use, lower utility costs, and retrofit homes and buildings to accommodate extreme temperatures. Given the future frequency of extreme heat events, the imperative to assess and plan for this situation, even in regions with historically mild climates, should not be underestimated.
Supplementary Material
Acknowledgments
The authors thank the Alaska Department of Health and Social Services Health Analytics and Vital Records for their assistance in accessing the Health Facilities Data Reporting Program data. This assistance is a valuable service for public health research in Alaska.
All code for this project is available on GitHub (https://github.com/grelber13/Alaska_heat_2022).
References
- 1.Taylor EV, Vaidyanathan A, Flanders WD, Murphy M, Spencer M, Noe RS. 2018. Differences in heat-related mortality by citizenship status: United States, 2005–2014. Am J Public Health 108(S2):S131–S136, PMID: , 10.2105/AJPH.2017.304006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaidyanathan A, Malilay J, Schramm P, Saha S. 2020. Heat-related deaths—United States, 2004–2018. MMWR Morb Mortal Wkly Rep 69(24):729–734, PMID: , 10.15585/MMWR.MM6924A1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarofim MC, Saha S, Hawkins MD, Mills DM, Hess J, Horton R, et al. 2016. Ch. 2: Temperature-Related Death and Illness. In: The Impacts of Climate Change on Human Health in the United States: A Scientific Assessment. Washington, DC: U.S. Global Change Research Program, 43–68. 10.7930/J0MG7MDX. [DOI] [Google Scholar]
- 4.Jones B, O’Neill BC, McDaniel L, McGinnis S, Mearns LO, Tebaldi C. 2015. Future population exposure to US heat extremes. Nat Clim Chang 5(7):652–655, 10.1038/nclimate2631. [DOI] [Google Scholar]
- 5.Hess JJ, Saha S, Luber G. 2014. Summertime acute heat illness in U.S. emergency departments from 2006 through 2010: analysis of a nationally representative sample. Environ Health Perspect 122(11):1209–1215, PMID: , 10.1289/ehp.1306796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherbakov T, Malig B, Guirguis K, Gershunov A, Basu R. 2018. Ambient temperature and added heat wave effects on hospitalizations in California from 1999 to 2009. Environ Res 160:83–90, PMID: , 10.1016/j.envres.2017.08.052. [DOI] [PubMed] [Google Scholar]
- 7.Basu R, Pearson D, Malig B, Broadwin R, Green R. 2012. The effect of high ambient temperature on emergency room visits. Epidemiology 23(6):813–820, PMID: , 10.1097/EDE.0b013e31826b7f97. [DOI] [PubMed] [Google Scholar]
- 8.Thompson R, Hornigold R, Page L, Waite T. 2018. Associations between high ambient temperatures and heat waves with mental health outcomes: a systematic review. Public Health 161:171–191, PMID: , 10.1016/j.puhe.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Anderson BG, Bell ML. 2011. Heat waves in the United States: mortality risk during heat waves and effect modification by heat wave characteristics in 43 U.S. communities. Environ Health Perspect 119(2):210–218, PMID: , 10.1289/ehp.1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovats RS, Hajat S. 2008. Heat stress and public health: a critical review. Annu Rev Public Health 29:41–55, PMID: , 10.1146/annurev.publhealth.29.020907.090843. [DOI] [PubMed] [Google Scholar]
- 11.Xu Z, Sheffield PE, Su H, Wang X, Bi Y, Tong S. 2014. The impact of heat waves on children’s health: a systematic review. Int J Biometeorol 58(2):239–247, PMID: , 10.1007/s00484-013-0655-x. [DOI] [PubMed] [Google Scholar]
- 12.Bunker A, Wildenhain J, Vandenbergh A, Henschke N, Rocklöv J, Hajat S, et al. 2016. Effects of air temperature on climate-sensitive mortality and morbidity outcomes in the elderly: a systematic review and meta-analysis of epidemiological evidence. EBioMedicine 6:258–268, PMID: , 10.1016/j.ebiom.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green H, Bailey J, Schwarz L, Vanos J, Ebi K, Benmarhnia T. 2019. Impact of heat on mortality and morbidity in low and middle income countries: a review of the epidemiological evidence and considerations for future research. Environ Res 171:80–91, PMID: , 10.1016/j.envres.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Phung D, Thai PK, Guo Y, Morawska L, Rutherford S, Chu C. 2016. Ambient temperature and risk of cardiovascular hospitalization: an updated systematic review and meta-analysis. Sci Total Environ 550:1084–1102, PMID: , 10.1016/j.scitotenv.2016.01.154. [DOI] [PubMed] [Google Scholar]
- 15.Gronlund CJ, Zanobetti A, Wellenius GA, Schwartz JD, O’Neill MS. 2016. Vulnerability to renal, heat and respiratory hospitalizations during extreme heat among U.S. elderly. Clim Change 136(3):631–645, PMID: , 10.1007/s10584-016-1638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson GB, Dominici F, Wang Y, McCormack MC, Bell ML, Peng RD. 2013. Heat-related emergency hospitalizations for respiratory diseases in the Medicare population. Am J Respir Crit Care Med 187(10):1098–1103, PMID: , 10.1164/rccm.201211-1969OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Son JY, Liu JC, Bell ML. 2019. Temperature-related mortality: a systematic review and investigation of effect modifiers. Environ Res Lett 14(7):073004, 10.1088/1748-9326/ab1cdb. [DOI] [Google Scholar]
- 18.Benmarhnia T, Deguen S, Kaufman JS, Smargiassi A. 2015. Review article: vulnerability to heat-related mortality. Epidemiology 26(6):781–793, PMID: , 10.1097/EDE.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 19.Hess JJ, Ebi KL. 2016. Iterative management of heat early warning systems in a changing climate. Ann NY Acad Sci 1382(1):21–30, PMID: , 10.1111/nyas.13258. [DOI] [PubMed] [Google Scholar]
- 20.Lowe D, Ebi KL, Forsberg B. 2011. Heatwave early warning systems and adaptation advice to reduce human health consequences of heatwaves. Int J Environ Res Public Health 8(12):4623–4648, 10.3390/ijerph8124623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NOAA National Weather Service. Excessive Heat Conditions. Published 2021. https://www.weather.gov/phi/heatcond [accessed 13 December 2021].
- 22.Hawkins MD, Brown V, Ferrell J. 2017. Assessment of NOAA National Weather Service methods to warn for extreme heat events. Weather Clim Soc 9(1):5–13, 10.1175/WCAS-D-15-0037.1. [DOI] [Google Scholar]
- 23.NWS (National Weather Service). 2019. National Weather Service Instruction 10-515: WFO Non-Precipitation Weather Products Specification. https://www.nws.noaa.gov/directives/sym/pd01005015curr.pdf [accessed 13 December 2021].
- 24.McGregor G, Bessemoulin P, Ebi K, Menne B. 2015. Heatwaves and Health: Guidance on Warning-System Development. Geneva, Switzerland: World Meteorological Organization. https://public.wmo.int/en/resources/library/heatwaves-and-health-guidance-warning-system-development [accessed 13 December 2021]. [Google Scholar]
- 25.Metzger KB, Ito K, Matte TD. 2010. Summer heat and mortality in New York City: how hot is too hot? Environ Health Perspect 118(1):80–86, PMID: , 10.1289/ehp.0900906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaidyanathan A, Saha S, Vicedo-Cabrera AM, Gasparrini A, Abdurehman N, Jordan R, et al. 2019. Assessment of extreme heat and hospitalizations to inform early warning systems. Proc Natl Acad Sci USA 116(12):5420–5427, PMID: , 10.1073/pnas.1806393116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Issa MA, Chebana F, Masselot P, Campagna C, Lavigne É, Gosselin P, et al. 2021. A heat-health watch and warning system with extended season and evolving thresholds. BMC Public Health 21(1):1–13, 10.1186/s12889-021-10982-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang K, Rood RB, Michailidis G, Oswald EM, Schwartz JD, Zanobetti A, et al. 2012. Comparing exposure metrics for classifying ‘dangerous heat’ in heat wave and health warning systems. Environ Int 46:23–29, PMID: , 10.1016/j.envint.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McElroy S, Schwarz L, Green H, Corcos I, Guirguis K, Gershunov A, et al. 2020. Defining heat waves and extreme heat events using Sub-regional meteorological data to maximize benefits of early warning systems to population health. Sci Total Environ 721:137678, PMID: , 10.1016/j.scitotenv.2020.137678. [DOI] [PubMed] [Google Scholar]
- 30.Burke J. 2016. Heat stroke in Alaska’s Arctic. Anchorage Daily News. 27 September 2016. https://www.adn.com/arctic/article/heat-stroke-alaskas-arctic/2011/07/21/ [accessed 13 December 2021].
- 31.Berwyn B. Alaska chokes on wildfires as heat waves dry out the Arctic. Inside Climate News. 11 July 2019. https://insideclimatenews.org/news/11072019/arctic-wildfires-alaska-climate-change-heat-wave-2019-university-funding/ [accessed 13 December 2021].
- 32.di Liberto T. 2019. High temperatures smash all-time records in Alaska in early July 2019. https://www.climate.gov/news-features/event-tracker/high-temperatures-smash-all-time-records-alaska-early-july-2019 [accessed 5 December 2021].
- 33.Hahn MB, Kuiper G, O’Dell K, Fischer EV, Magzamen S. 2021. Wildfire smoke is associated with an increased risk of cardiorespiratory emergency department visits in Alaska. Geohealth 5(5):e2020GH000349, PMID: , 10.1029/2020GH000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alaska Climate Research Center. 2021. Temperature Normals. https://akclimate.org/data/air-temperature-normals/ [accessed 5 December 2021].
- 35.NOAA (National Oceanic and Atmospheric Administration) NWS Weather Prediction Center. 2014. The Heat Index Equation. https://www.wpc.ncep.noaa.gov/html/heatindex_equation.shtml [accessed 5 December 2021].
- 36.Rothfusz L. 1990. The Heat Index “Equation” (or, More Than You Ever Wanted to Know About Heat Index). https://wonder.cdc.gov/wonder/help/Climate/ta_htindx.PDF [accessed 5 December 2021].
- 37.Steadman R. 1979. The assessment of sultriness. Part II: effects of wind, extra radiation and barometric pressure on apparent temperature. J Appl Meteor 18(7):874–885, . [DOI] [Google Scholar]
- 38.Steadman R. 1979. The assessment of sultriness. Part I: a temperature-humidity index based on human physiology and clothing science. J Appl Meteor 18(7):861–873, . [DOI] [Google Scholar]
- 39.Janes H, Sheppard L, Lumley T. 2005. Case-crossover analyses of air pollution exposure data: referent selection strategies and their implications for bias. Epidemiology 16(6):717–726, PMID: , 10.1097/01.ede.0000181315.18836.9d. [DOI] [PubMed] [Google Scholar]
- 40.Janes H, Sheppard L, Lumley T. 2005. Overlap bias in the case-crossover design, with application to air pollution exposures. Stat Med 24(2):285–300, PMID: , 10.1002/sim.1889. [DOI] [PubMed] [Google Scholar]
- 41.Stapleton JM, Larose J, Simpson C, Flouris AD, Sigal RJ, Kenny GP. 2014. Do older adults experience greater thermal strain during heat waves? Appl Physiol Nutr Metab 39(3):292–298, PMID: , 10.1139/apnm-2013-0317. [DOI] [PubMed] [Google Scholar]
- 42.Jung J, Uejio CK, Adeyeye TE, Kintziger KW, Duclos C, Reid K, et al. 2021. Using social security number to identify sub-populations vulnerable to the health impacts from extreme heat in Florida, U.S. Environ Res 202:111738, PMID: , 10.1016/j.envres.2021.111738. [DOI] [PubMed] [Google Scholar]
- 43.Markon CJ, Gray ST, Berman M, Eerkes-Medrano L, Hennessy T, Huntington P, et al. 2018. Chapter 26: Alaska. In: Impacts, Risks, and Adaptation in the United States: The Fourth National Climate Assessment, Vol. II. Washington, DC: U.S. Global Change Research Program. 10.7930/NCA4.2018.CH26. [DOI] [Google Scholar]
- 44.Fuhrmann CM, Sugg MM, Konrad CE, Waller A. 2016. Impact of extreme heat events on emergency department visits in North Carolina (2007–2011). J Community Health 41(1):146–156, PMID: , 10.1007/s10900-015-0080-7. [DOI] [PubMed] [Google Scholar]
- 45.Winquist A, Grundstein A, Chang HH, Hess J, Sarnat SE. 2016. Warm season temperatures and emergency department visits in Atlanta, Georgia. Environ Res 147:314–323, PMID: , 10.1016/j.envres.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mora C, Counsell CWW, Bielecki CR, Louis L. 2017. Twenty-seven ways a heat wave can kill you: deadly heat in the era of climate change. Circ Cardiovasc Qual Outcomes 10(11):e004233, PMID: , 10.1161/CIRCOUTCOMES.117.004233. [DOI] [PubMed] [Google Scholar]
- 47.Pahal P, Rajasurya V, Sharma S. 2022. Typical Bacterial Pneumonia. Treasure Island, FL: StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK534295/ [accessed 4 August 2022]. [PubMed] [Google Scholar]
- 48.Sohail H, Lanki T, Kollanus V, Tiittanen P, Schneider A. 2020. Heat, heatwaves and cardiorespiratory hospital admissions in Helsinki, Finland. Int J Environ Res Public Health 17(21):7892, PMID: , 10.3390/ijerph17217892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo Y, Gasparrini A, Armstrong BG, Tawatsupa B, Tobias A, Lavigne E, et al. 2017. Heat wave and mortality: a multicountry, multicommunity study. Environ Health Perspect 125(8):087006, 10.1289/EHP1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Lenick CR, Baniassadi A, Michael R, Monaghan A, Boehnert J, Yu X, et al. 2020. A case-crossover analysis of indoor heat exposure on mortality and hospitalizations among the elderly in Houston, Texas. Environ Health Perspect 128(12):127007–127017, PMID: , 10.1289/EHP6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao Q, Li S, Coelho MSZS, Saldiva PHN, Hu K, Arblaster JM, et al. 2019. Geographic, demographic, and temporal variations in the association between heat exposure and hospitalization in Brazil: a nationwide study between 2000 and 2015. Environ Health Perspect 127(1):017001, PMID: , 10.1289/EHP3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parker BA, Kalasky MJ, Proctor DN. 2010. Evidence for sex differences in cardiovascular aging and adaptive responses to physical activity. Eur J Appl Physiol 110(2):235–246, PMID: , 10.1007/s00421-010-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yardley J, Sigal RJ, Kenny GP. 2011. Heat health planning: the importance of social and community factors. Glob Environ Change 21(2):670–679, 10.1016/j.gloenvcha.2010.11.010. [DOI] [Google Scholar]
- 54.Bobb JF, Obermeyer Z, Wang Y, Dominici F. 2014. Cause-specific risk of hospital admission related to extreme heat in older adults. JAMA 312(24):2659–2667, PMID: , 10.1001/jama.2014.15715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meade RD, Akerman AP, Notley SR, McGinn R, Poirier P, Gosselin P, et al. 2020. Physiological factors characterizing heat-vulnerable older adults: a narrative review. Environ Int 144:105909, PMID: , 10.1016/j.envint.2020.105909. [DOI] [PubMed] [Google Scholar]
- 56.Anderson BG, Bell ML. 2009. Weather-Related mortality: how heat, cold, and heat waves affect mortality in the United States. Epidemiology 20(2):205–213, PMID: , 10.1097/EDE.0b013e318190ee08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin S, Luo M, Walker RJ, Liu X, Hwang SA, Chinery R. 2009. Extreme high temperatures and hospital admissions for respiratory and cardiovascular diseases. Epidemiology 20(5):738–746, PMID: , 10.1097/EDE.0b013e3181ad5522. [DOI] [PubMed] [Google Scholar]
- 58.Gronlund CJ, Zanobetti A, Schwartz JD, Wellenius GA, O’Neill MS. 2014. Heat, heat waves, and hospital admissions among the elderly in the United States, 1992–2006. Environ Health Perspect 122(11):1187–1192, PMID: , 10.1289/ehp.1206132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bobb JF, Peng RD, Bell ML, Dominici F. 2014. Heat-related mortality and adaptation to heat in the United States. Environ Health Perspect 122(8):811–816, PMID: , 10.1289/ehp.1307392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Michelozzi P, Accetta G, De Sario M, D’Ippoliti D, Marino C, Baccini M, et al. 2009. High temperature and hospitalizations for cardiovascular and respiratory causes in 12 European cities. Am J Respir Crit Care Med 179(5):383–389, PMID: , 10.1164/rccm.200802-217OC. [DOI] [PubMed] [Google Scholar]
- 61.Oudin Åström D, Åström C, Forsberg B, Vicedo-Cabrera AM, Gasparrini A, Oudin A, et al. 2020. Heat wave–related mortality in Sweden: a case-crossover study investigating effect modification by neighbourhood deprivation. Scand J Public Health 48(4):428–435, PMID: , 10.1177/1403494818801615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Åström C, Ebi KL, Langner J, Forsberg B. 2014. Developing a heatwave early warning system for Sweden: evaluating sensitivity of different epidemiological modelling approaches to forecast temperatures. Int J Environ Res Public Health 12(1):254–267, PMID: , 10.3390/ijerph120100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rocklov J, Barnett AG, Woodward A. 2012. On the estimation of heat-intensity and heat-duration effects in time series models of temperature-related mortality in Stockholm, Sweden. Environ Health 11(1):1–12, 10.1186/1476-069X-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shaposhnikov D, Revich B, Bellander T, Bedada GB, Bottai M, Kharkova T, et al. 2014. Mortality related to air pollution with the Moscow heat wave and wildfire of 2010. Epidemiology 25(3):359–364. PMID: , 10.1097/EDE.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liss A, Wu R, Chui KKH, Naumova EN. 2017. Heat-related hospitalizations in older adults: an amplified effect of the first seasonal heatwave. Sci Rep 7(1):39581, PMID: , 10.1038/srep39581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee M, Nordio F, Zanobetti A, Kinney P, Vautard R, Schwartz J. 2014. Acclimatization across space and time in the effects of temperature on mortality: a time-series analysis. Environ Health 13(1):89, PMID: , 10.1186/1476-069X-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Medina-Ramón M, Schwartz J. 2007. Temperature, temperature extremes, and mortality: a study of acclimatisation and effect modification in 50 US cities. Occup Environ Med 64(12):827–833, PMID: , 10.1136/oem.2007.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carter R, Cheuvront SN, Williams JO, Kolka MA, Stephenson LA, Sawka MN, et al. 2005. Epidemiology of hospitalizations and deaths from heat illness in soldiers. Med Sci Sports Exerc 37(8):1338–1344, PMID: , 10.1249/01.mss.0000174895.19639.ed. [DOI] [PubMed] [Google Scholar]
- 69.Poore S, Grundstein A, Cooper E, Shannon J. 2020. Regional differences in exertional heat illness rates among Georgia USA high school football players. Int J Biometeorol 64(4):643–650, PMID: , 10.1007/s00484-019-01853-4. [DOI] [PubMed] [Google Scholar]
- 70.Murage P, Hajat S, Kovats RS. 2017. Effect of night-time temperatures on cause and age-specific mortality in London. Environ Epidemiol 1(2):e005, PMID: , 10.1097/EE9.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yee J, Cho YA, Yoo HJ, Yun H, Gwak HS. 2021. Short-term exposure to air pollution and hospital admission for pneumonia: a systematic review and meta-analysis. Environ Health 20(1):6, PMID: , 10.1186/s12940-020-00687-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Minnesota Department of Health. 2012. Minnesota Extreme Heat Toolkit. https://www.health.state.mn.us/communities/environment/climate/docs/mnextremeheattoolkit.pdf [accessed 6 February 2022].
- 73.San Francisco Department of Public Health. 2014. Multilingual “Stay Safe in the Heat” Resources. https://sfclimatehealth.org/wp-content/uploads/2018/12/DPH_Heat_Waves_Multilingual.pdf [accessed 6 February 2022].
- 74.Health Canada. 2011. Communicating the Health Risks of Extreme Heat Events: Toolkit for Public Health and Emergency Management Officials. https://ghhin.org/wp-content/uploads/heat-chaleur-eng.pdf [accessed 6 February 2022].
- 75.Weinberger KR, Wu X, Sun S, Spangler KR, Nori-Sarma A, Schwartz J, et al. 2021. Heat warnings, mortality, and hospital admissions among older adults in the United States. Environ Int 157:106834, PMID: , 10.1016/j.envint.2021.106834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arizona Department of Health Services. 2022. Extreme Weather & Public Health. https://www.azdhs.gov/preparedness/epidemiology-disease-control/extreme-weather/heat-safety/index.php#heat-home [accessed 6 February 2022].
- 77.Maricopa County Public Health. 2022. Staying Safe in the Extreme Heat. https://www.maricopa.gov/1871/Extreme-Heat [accessed 6 February 2022].
- 78.City of Vancouver. 2022. Staying safe in hot weather. https://vancouver.ca/people-programs/hot-weather.aspx [accessed 6 February 2022].
- 79.NYC (New York City) Parks. 2022. Cool It! NYC. https://www.nycgovparks.org/about/health-and-safety-guide/cool-it-nyc [accessed 6 February 2022].
- 80.CALIHEAP. 2022. California Low Income Home Energy Assistance Program (LIHEAP). https://www.caliheapapply.com/ [accessed 6 February 2022].
- 81.Venter ZS, Krog NH, Barton DN. 2020. Linking green infrastructure to urban heat and human health risk mitigation in Oslo, Norway. Sci Total Environ 709:136193, PMID: , 10.1016/j.scitotenv.2019.136193. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Y, Murray AT, Turner BL. 2017. Optimizing green space locations to reduce daytime and nighttime urban heat island effects in Phoenix, Arizona. Landsc Urban Plan 165:162–171, 10.1016/j.landurbplan.2017.04.009. [DOI] [Google Scholar]
- 83.Doick KJ, Peace A, Hutchings TR. 2014. The role of one large greenspace in mitigating London’s nocturnal urban heat island. Sci Total Environ 493:662–671, PMID: , 10.1016/j.scitotenv.2014.06.048. [DOI] [PubMed] [Google Scholar]
- 84.Reid CE, Snowden JM, Kontgis C, Tager IB. 2012. The role of ambient ozone in epidemiologic studies of heat-related mortality. Environ Health Perspect 120(12):1627–1630, PMID: , 10.1289/ehp.1205251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dahl K, Licker R, Abatzoglou JT, Declet-Barreto J. 2019. Increased frequency of and population exposure to extreme heat index days in the United States during the 21st century. Environ Res Commun 1(7):075002, 10.1088/2515-7620/ab27cf. [DOI] [Google Scholar]
- 86.Lader R, Walsh JE, Bhatt US, Bieniek PA. 2017. Projections of twenty-first-century climate extremes for Alaska via dynamical downscaling and quantile mapping. J Appl Meteorol Climatol 56(9):2393–2409, 10.1175/JAMC-D-16-0415.1. [DOI] [Google Scholar]
- 87.Casanueva A, Burgstall A, Kotlarski S, Messeri A, Morabito M, Flouris AD, et al. 2019. Overview of existing heat-health warning systems in Europe. Int J Environ Res Public Health 16(15):2657, PMID: , 10.3390/ijerph16152657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


