Abstract
Monostiliferous nemerteans in the genus Tetrastemma Ehrenberg, 1828 are generally characterized as having four eyes, and they occur worldwide, from the intertidal zone to the deep-sea bottom. Recent extensive sampling of Tetrastemma has explored the high species diversity, including many undescribed forms, but phylogenic analysis has revealed non-monophyly of the genus. We herein describe three new species of the genus (T.albumsp. nov., T.personasp. nov., and T.shohoensesp. nov.) from northwestern Pacific waters based on specimens collected by dredging or by use of a remotely operated vehicle at depths of 116–455 m. Since anatomical and histological characters traditionally used in systematics of the genus are sometimes interspecifically uniform, a histology-free approach is applied for the species descriptions in this study. To confirm the generic affiliation of the new species, a molecular phylogenetic analysis based on partial sequences of cytochrome c oxidase subunit I, 16S rRNA, 18S rRNA, 28S rRNA, and histone H3 genes was performed. Our result shows that all three new species are nested in a subclade formed by species from the North Pacific and American Atlantic, inferring that geographic distribution does not reflect the cladogenesis of Tetrastemma. Furthermore, two Tetrastemma species with a cylindrical stylet basis, T.freyaeChernyshev et al., 2020 from off the coast of India and Hawaii and T.shohoensesp. nov. from Shoho Seamount, Japan, constitute a clade in the resulting tree.
Keywords: Deep sea, Eumonostilifera, Japan, marine invertebrate, Monostilifera, Nemertea, Pacific, Tetrastemmatidae
Introduction
A histology-free description with DNA barcoding has been progressively introduced to nemertean systematics in the past decade (e.g., Kajihara 2015; Gonzalez-Cueto et al. 2017; Simpson et al. 2017; Kajihara et al. 2018, 2022; Chernyshev et al. 2020; Hookabe et al. 2021a, b; Leiva et al. 2021; Abato et al. 2022). This approach has been applied to two cases, one of which is a description of species with internal characters interspecifically differentiated and observable without histology (e.g., number of proboscis branches in Gorgonorhynchus Dakin & Fordham, 1931 [Kajihara 2015; Hookabe et al. 2021a)]. In the other case, especially when internal morphology is uniform between most species in a genus, a species description has been performed solely based on characters examined in-vivo (shape of head, body coloration and markings, number of eyes, blood color, and stylet apparatus) [e.g., Baseodiscus Diesing, 1850 (Kajihara et al. 2022) and Ototyphlonemertes Diesing, 1863 (Kajihara et al. 2018)]. Recent descriptions of species in the genus Tetrastemma Ehrenberg, 1828, fitting the latter case, have been performed based on characters of living specimens without histological observations (Chernyshev et al. 2020; Hookabe et al. 2021b; Abato et al. 2022).
Tetrastemma is a species-rich genus in Monostilifera (Kajihara 2021), currently encompassing about 110 species from tropical to polar areas (Chernyshev et al. 2021). As the generic name suggests–a composite of the Latin feminine “tetra” (= four) + “stemma” (= simple eyes)–members in the genus are generally characterized by four eyes, but this feature is also found in other genera. Several species in Tetrastemma were described based on internal morphology; however, the internal characters were inferred to be almost homogenous within the genus by taxonomic reappraisal based on molecular phylogeny (Chernyshev et al. 2021). Recent examples of a histology-free approach based on characteristics studied in-vivo and molecular data are descriptions of T.freyaeChernyshev et al., 2020, T.cupido Hookabe, Kohtsuka & Kajihara, 2021, and T.parallelos Abato, Yoshida & Kajihara, 2022.
Here, we establish three new species based on specimens collected in 2019–2021 from the lower sublittoral to upper bathyal zones of Sagami Bay and the Nishi-Shichito Ridge. The descriptions are histology-free, based on characters of living specimens examined with a light microscope. To test phylogenetic relationships with the congeners, we performed molecular phylogenetic reconstruction using partial sequences of the 16S rRNA (16S), cytochrome c oxidase subunit I (COI), 18S rRNA (18S), 28S rRNA (28S), and histone H3 genes (H3).
Materials and methods
Specimens were collected in 2019–2021 by use of a biological dredge in Sagami Bay (116–200 m) or a remotely operated vehicle (ROV) on Shoho Seamount of the Nishi-Shichito Ridge (455 m), northwestern Pacific Ocean. External morphology of the living specimens was documented on the vessel or in the laboratory with a Nikon D5600 digital SLR camera equipped with an AF-S DX Micro-NIKKOR 40mm f/2.8G macro lens (Nikon, Japan). A single specimen collected from Shoho Seamount was further observed under a compound light microscope by preparing a squeezed specimen with a cover slip and a glass slide. Specimens were anaesthetized with a few drops of bitterns Tenpi Nigari (Amashio, Japan); after the worms were relaxed, the posterior tips were preserved in 99% ethanol for DNA extraction and the rest of the body was fixed in Bouin’s fluid for 24–48 hours and later transferred to 70% ethanol. Type specimens have been deposited in the National Museum of Nature and Science, Tsukuba (NSMT), Japan.
DNA extraction, PCR amplification, and sequencing followed Hookabe et al. (2022). DNA sequences determined in the present study have been deposited in DDBJ/EMBL/GenBank (Table 1).
Table 1.
List of species included in the phylogenetic analysis and DDBJ/EMBL/GenBank accession numbers for each gene. Country names of each species sampling location are abbreviated as follows: CA = Canada, JP = Japan, RU = Russia, USA = United States of America, and VE = Venezuela.
*Erroneously registered in GenBank under the taxon name Quasitetrastemmanigrifrons.
To elucidate phylogenetic positions of specimens examined, we performed phylogenetic analyses based on the maximum-likelihood (ML) method. The newly obtained sequences from four Tetrastemma species were aligned using MAFFT v. 7 (Katoh and Standley 2013) employing L-INS-i strategy with sequences of other species in the genus, most of which were recently determined by Chernyshev et al. (2021). Ambiguous nucleotide sites in the dataset were removed with Gblocks v. 0.91b (Castresana 2000) using a less stringent option, resulting in 380-bp 16S, 626-bp COI, 1738-bp 18S, 505-bp 28S, and 329-bp H3. The ML analyses were performed with RAxML-NG (Kozlov et al. 2019), for which the best-fit partition scheme and substitution model were selected using PartitionFinder v. 2.1.1 (Lanfear et al. 2017). Nodal support values were derived from 1000 bootstrap pseudoreplicates.
Result
Systematics
Genus Tetrastemma Ehrenberg, 1828
. Tetrastemma album sp. nov.
2E514ABC-244C-55E4-AFBD-77128ECF605E
https://zoobank.org/F73378DB-B867-4ABA-A2D7-18CF781D10A7
Fig. 2A–C [New Japanese name: misaki-oshiroi-himomushi]
Figure 2.
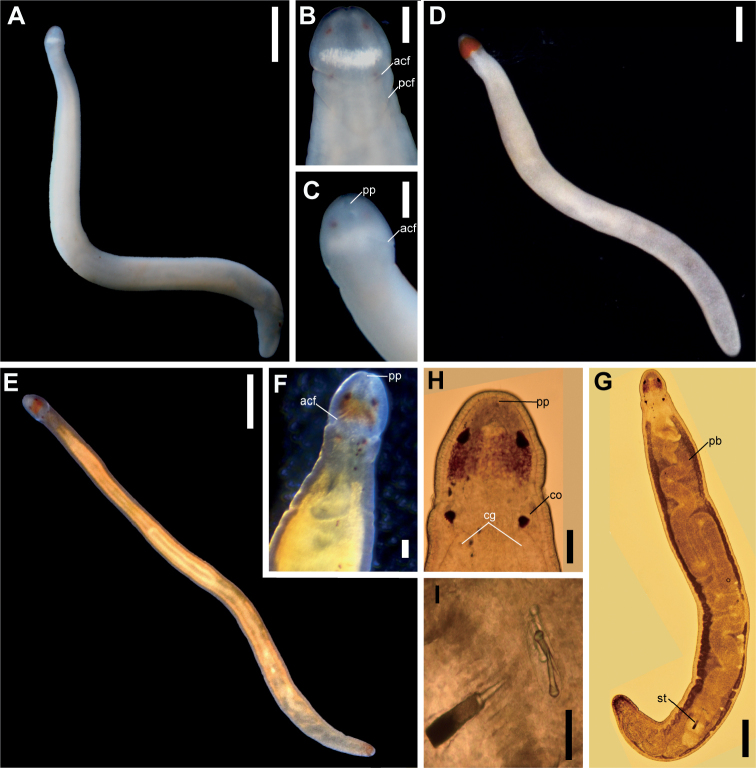
Holotype specimens of new Tetrastemma species; photographs were taken in life by NH A–CT.album sp. nov. A complete body, dorsal view B head, dorsal view C head, ventral view DT.persona sp. nov., complete body, dorsal view E–IT.shohoense sp. nov. E complete body F head, ventral view G squeezed specimen under a cover slip, complete body, dorsal view H head, dorsal view I stylet apparatus. Abbreviations: acf, anterior cephalic furrow; pcf, posterior cephalic furrow, cg, cerebral ganglia; co, cerebral organ; pb, proboscis; pp, proboscis pore. Scale bars: 2 mm (A); 500 μm (B, C, G); 1 mm (D, E); 100 μm (F, H); 50 μm (I).
Etymology.
The species name is derived from the Latin album (white), referring to pure white body of the new species. The Japanese name is named after the white powder foundation traditionally used by Maiko, Geisha, Kabuki actors in Japan.
Material examined.
Holotype: NMST-NE-H-06, unsectioned complete specimen except for the posterior tip, fixed in Bouin’s fluid and later preserved in 70% ethanol, posterior tip preserved in 99% ethanol, collected on March 12, 2021 by NH, biological dredge (R/V Rinkai-maru) at depths of 144–200 m, off Jogshima (35°07.41'N, 139°34.11'E–35°07.32'N, 139°33.572'E), Miura, Kanagawa, Japan, NW Pacific.
Description.
Head spatulate to rounded in profile (Fig. 2A–C), demarcated by posterior cephalic furrows from body (Fig. 2A). Before anesthetization, body of a live specimen 17 mm long and 1.0–1.2 mm wide. Body uniformly pale colored, without longitudinal or transverse stripe markings (Fig. 2A). Pure white transverse cephalic patch present between anterior and posterior pairs of eyes (Fig. 2B). Head not wider than maximum body width (Fig. 2A–C). A pair of cephalic furrows present; anterior pair not meeting mid-dorsally and ventrally curving anteriorly but not reaching to proboscis pore; posterior pair V-shaped and barely meeting mid-dorsally (Fig. 2B) and running transversely on ventral surface (Fig. 2C). Cerebral ganglia and blood not red and probably uncolored. Internal organs (proboscis, foregut, and intestine) visible as pale regions. Four reddish brown eyes regular in size (Fig. 2B).
Type locality and distribution.
The species is only known from the type locality, Sagami Bay, Kanagawa Prefecture, Japan, at depths of 144–200 m (Fig. 1).
Figure 1.
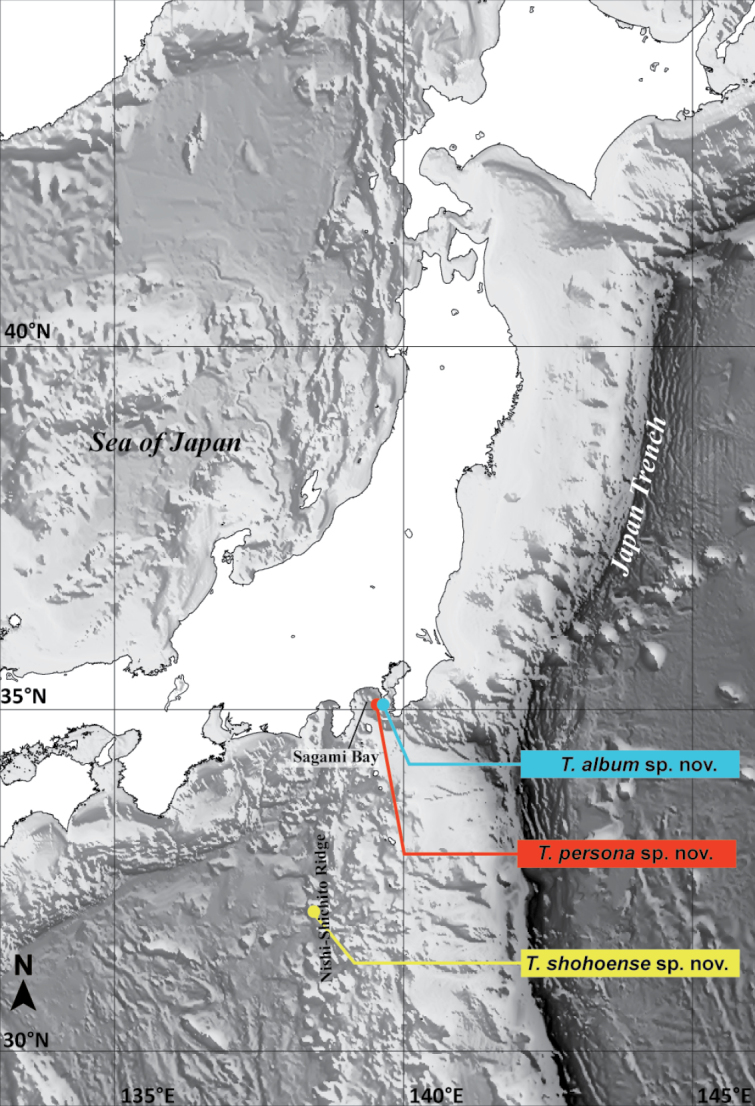
Collection sites of the specimens examined in the present study.
Remarks.
Having a pure white cephalic patch on a uniformly pale body, T.album sp. nov. differs from all the described species. Tetrastemmacoronatum (Quatrefages, 1846), T.diadema Hubrecht, 1879, T.olgarumChernyshev 1998, and T.pseudocoronatumChernyshev 1998 have white cephalic patches but are distinguished from T.album sp. nov. in possessing a light brown to dark transverse band on the head. Tetrastemmaalbomaculatum Chernyshev, 2016 also possesses a white cephalic patch but differs from the new species in having a pale-ochre body dorsally spotted with small white dots (Chernyshev 2016).
. Tetrastemma persona sp. nov.
220BA17D-A947-5A01-96EB-33B4D973ABA5
https://zoobank.org/E3E48065-E551-477D-9041-89DEE011DEB0
Fig. 2D [New Japanese name: misaki-kamen-himomushi]
Etymology.
The species name is derived from the Latin persōna (mask), referring to a broad cephalic patch of the new species masking eyes and internal organs in head region. The Japanese name “kamen” means a mask in Japanese.
Material examined.
Holotype: NMST-NE-H-07, unsectioned complete specimen except for the posterior tip, fixed in Bouin’s fluid and later preserved in 70% ethanol, posterior tip preserved in 99% ethanol, collected on July 31 2020 by NH, biological dredge (R/V Rinkai-maru) at depths of 116–211 m, off Jogshima (35°08.32'N, 139°32.857'E–35°08.40'N, 139°32.504'E), Miura, Kanagawa, Japan, NW Pacific. Paratype: NMST-NE-P-08, unsectioned complete specimen fixed in Bouin’s fluid and later preserved in 70% ethanol, collected on the same date and locality as the holotype.
Description.
Head slightly narrower than middle part of body and weakly demarcated from trunk (Fig. 2D). Before anesthetization, body of a live specimen 7.0–10 mm long and 0.8–1.0 mm wide. Body uniformly pale to yellow colored without longitudinal or transverse stripe markings (Fig. 2D). Vermilion-red cephalic patch spade-shaped (Fig. 2D), covering both anterior and posterior pairs of eyes (Fig. 2D) but not posteriorly reaching to anterior pair of cephalic furrows; eyes regular in sizes. A posterior pair of cephalic furrows not well distinguished probably due to the small body size. Cerebral ganglia and blood not red and probably uncolored. Internal organs (proboscis, foregut, and intestine) not well visible through body wall. Rhynchocoel visible as whitish region through body wall, extending about 1/2–2/3 of the body length.
Type locality and distribution.
The species is only known from the type locality, Sagami Bay, Kanagawa Prefecture, Japan, at depths of 116–211 m (Fig. 1, Table 1).
Remarks.
Tetrastemmapersona sp. nov. has atypically short rhynchocoel in the genus and most resembles T.roseocephalum (Yamaoka, 1947) and T.yamaokai Iwata, 1954 in having a pale body without any markings and a red cephalic patch. Pattern variation of a cephalic patch (shield shape or horse-shoe shape) was reported in both T.roseocephalum and T.yamaokai; referring to the original description of T.yamaokai, the name may be a junior synonym of T.roseocephalum, as suggested by Kajihara (2007). The external morphology of T.persona sp. nov. is similar to a form with a shield-shaped cephalic patch of T.roseocephalum (Iwata 1954).
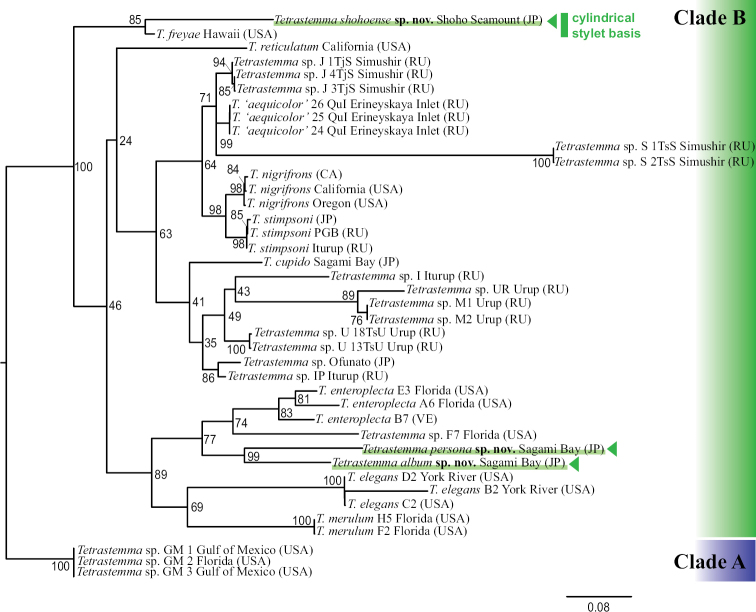
The subtle difference in the shape of cephalic patch between T.persona sp. nov. (spade-shaped) and T.roseocephalum (shield-shaped) was supported by our molecular analysis. The new species did not constitute a clade with T.roseocephalum but with T.album sp. nov. (Fig. 3); T.roseocephalum belongs to Clade C of Chernyshev et al. (2021).
Figure 3.
A maximum-likelihood (ML) tree based on concatenated sequences of two mitochondrial [16S rRNA (16S; 380 bp) and cytochrome c oxidase subunit I (COI; 626 bp)] and three nuclear gene markers [18S rRNA (18S; 1738 bp), 28S rRNA (28S; 505 bp), and histone H3 (H3; 329 bp)]. Numbers near each node are support values generated by a separate partitioned ML bootstrap analysis with 1000 replicates. Country names of each species sampling location are abbreviated as follows: CA = Canada, JP = Japan, RU = Russia, USA = United States of America, and VE = Venezuela.
An uncorrected genetic distance based on 657 bp of COI was 16% between T.album sp. nov. and T.persona sp. nov., comparable with interspecific values observed among Monostilifera (e.g., Sundberg et al. 2016; Hookabe et al. 2022).
. Tetrastemma shohoense sp. nov.
E64BFBDD-40A5-5912-9F6C-3659A7D127E0
https://zoobank.org/9D1CD900-900F-4114-8853-16C480FDD75D
Fig. 2E–I [New Japanese name: shoho-kakubari-himomushi]
Etymology.
The species is named after the type locality, Shoho Seamount of the Nishi-Shichito Ridge, Japan.
Material examined.
Holotype: NMST-Nem-H-05, unsectioned complete specimen except for the posterior tip, fixed in Bouin’s fluid, posterior tip preserved in 99% ethanol, collected on November 29 2020 by NH, by use of ROVKM-ROV (dive #123) during KM20-10C cruise of R/V Kaimei, at a depth of 455 m, near the summit of Shoho Seamount of the Nishi-Shichito Ridge (32°19.39'N, 138°44.48'E), Japan, NW Pacific.
Description.
Head spatulate in profile (Fig. 2E–H), not well demarcated from body by anterior cephalic furrows (Fig. 2E). Before anesthetization, body of a live specimen 5.5 mm long and 0.3 mm wide. Background body color generally white, tinged with bright yellow to orange, and almost transparent (Fig. 2E). Head with a red rectangle cephalic patch without extending behind a posterior pair of eyes (Fig. 2E, H). Anterior pair of cephalic furrows present (Fig. 2F) but posterior one not well distinguished. Cerebral ganglia and blood uncolored (Fig. 2G, H). Alimentary canals visible as bright yellow organs through body wall (Fig. 2E). Proboscis pale, extending about 3/4 of the body length (Fig. 2E). Four brown eyes present; anterior pair slightly larger than posterior ones (Fig. 2H).
Stylet basis cylindrical, 55.0 μm in length and 25.0 μm in maximum width; central stylet smooth, 47.0 μm in length; (stylet length) / (basis length) ratio 0.85 (Fig. 2I). Two accessory stylet pouches present, each containing two stylets (Fig. 2I).
Type locality and distribution.
The species is only known from the type locality, Shoho Seamount of the Nishi-Shichito Ridge, Japan, at a depth of 455 m (Fig. 1), among the sandy sediments on rocky substrates.
Remarks.
Having a dark cephalic patch and cylindrical stylet basis and lacking a longitudinal line on the dorsal surface of the body, T.shohoense sp. nov. resembles T.freyaeChernyshev et al., 2020 originally described based on Hawaiian and Indian specimens. The new species is differentiated from T.freyae in the color of the cephalic patch as well as the non-flared posterior margin of the cylindrical stylet basis.
A genetic distance based on COI between T.shohoense sp. nov. and T.freyae (specimens from Hawaii (MT247877) and India (MT247878) was 12.6%; the value is comparable with interspecific values observed among Monostilifera (e.g., Sundberg et al. 2016; Hookabe et al. 2022).
Molecular phylogeny
The sequence data set for molecular phylogenetic analyses in the present study is primarily based on Chernyshev et al. (2021). Since we confirmed that our new species are nested in Tetrastemma Clade B of Chernyshev et al. (2021), we used three species in Clade A (Tetrastemma sp. GM1 Gulf of Mexico, Tetrastemma sp. GM2 USA FL, and Tetrastemma sp. GM3 Gulf of Mexico) as outgroup taxa (Fig. 3). Clade B was subdivided into two clades with a full support value, one of which was a clade formed by T.freyae and T.shohoense sp. nov. The two species are characterized by having a cylindrical stylet basis in the proboscis. In the other subclade in Clade B, T.album sp. nov. and T.persona sp. nov. were included (Fig. 3). A clade constituted by newly described species, T.album sp. nov. and T.persona sp. nov., from Sagami Bay (Japan) with 99% of BS, was nested in the American Atlantic clade formed by T.elegans (Girard, 1852) (Virginia), T.enteroplecta (Corrêa, 1954) (Florida and Venezuela), T.merulum (Corrêa, 1954) (Florida), and Tetrastemma sp. F7 (Florida). The clade formed by T.album sp. nov. and T.persona sp. nov. was sister-related to a clade formed by T.enteroplecta (Florida and Venezuela) and Tetrastemma sp. F7 (Florida) with 77% of BS (Fig. 3).
Discussion
Three species herein described (T.album sp. nov., T.persona sp. nov., and T.shohoense sp. nov.) fell within a clade referred to as Tetrastemma Clade B of Chernyshev et al. (2021) (Fig. 3). One of the findings from the tree is that two species with cylindrical stylet basis, T.freyae and T.shohoense sp. nov., formed a clade regardless of the differences in habitat and collection depths of these two species; T.freyae was described based on specimens collected from live corals and mussel beds at depths shallower than 3 m in Hawaii and India (Chernyshev et al. 2020), while T.shohoense sp. nov. was found from sandy sediments in bathyal zone in Japan. A cylindrical stylet basis is likely to be acquired independently at least twice in Clade B (T.freyae and T.shohoense sp. nov.) and Clade C (T.albomaculatum and T.parallelos).
The other thing we can see on the phylogenetic tree is that T.album sp. nov. and T.persona sp. nov. are nested in a clade formed by several American Atlantic species, T.enteroplecta, T.elegans, T.merulum, and Tetrastemma sp. F7 (Chernyshev et al. 2021) (Fig. 3). A previous molecular analysis has inferred that Tetrastema clade B is subdivided into geographically distinct structures: North Pacific and American Atlantic subclades (Chernyshev et al. 2021). To obtain a more accurate picture of Tetrastemma phylogeny and speciation, again, further sampling of taxa, without bias toward shallow-water species, is needed for future phylogenetic analyses.
Supplementary Material
Acknowledgements
We thank Masanori Okanishi (Hiroshima Shudo University), Mamoru Sekifuji, and Michiyo Kawabata (MMBS) for their support in collecting specimens in Sagami Bay. NH thanks Toru Miura (MMBS) for providing NH with facilities for studying at MMBS. We are grateful to Katsunori Fujikura and Tetsuji Maki (JAMSTEC), the captain, the crew, the ROV operation team, and all the other participants in the research project Development of Biodiversity Monitoring Methods for the Management of Deep-sea Marine Protected Areas during KM20-10C cruise. NH also thanks Hiroshi Kajihara (Hokkaido University) for kindly sharing the relevant taxonomic literature with NH. This research was performed by the Environment Research and Technology Development Fund (JPMEERF20S20700) of the Environmental Restoration and Conservation Agency Provided by the Ministry of Environment of Japan and financially supported by JSPS KAKENHI (No. 21J14807 for NH) from Japan Society for the Promotion of Science. Finally, we thank the handling editor, Jon Norenburg and the three referees, Alexei V. Chernyshev, Hiroshi Kajihara, and Christina I. Ellison for the careful and insightful review of our manuscript.
Citation
Hookabe N, Kohtsuka H, Fujiwara Y, Tsuchida S, Ueshima R (2023) Three new species in Tetrastemma Ehrenberg, 1828 (Nemertea, Monostilifera) from sublittoral to upper bathyal zones of the northwestern Pacific. ZooKeys 1146: 135–146. https://doi.org/10.3897/zookeys.1146.95004
Funding Statement
Environmental Restoration and Conservation Agency of Japan
References
- Abato J, Yoshida R, Kajihara H. (2022) Histology-free description and phylogenetics of Tetrastemmaparallelos sp. nov. (Nemertea: Eumonostilifera) from Japan. Journal of Natural History 56(29–32): 1265–1277. 10.1080/00222933.2022.2118642 [DOI] [Google Scholar]
- Castresana J. (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution 17(4): 540–552. 10.1093/oxfordjournals.molbev.a026334 [DOI] [PubMed] [Google Scholar]
- Chernyshev AV. (1998) Nemerteans of the genus Tetrastemma (Enopla, Monostilifera) from the Far East seas of Russia. Zoologicheskii Zhurnal [Зоологический журнал] 77: 995–1002. [Google Scholar]
- Chernyshev AV. (2016) Nemerteans of the coastal waters of Vietnam. In: Adrianov AV, Lutaenko KA. (Eds) Biodiversity of the Western Part of the South China Sea.Dalnauka, Vladivostok, 279–314.
- Chernyshev AV, Polyakova NE, Vignesh MS, Jain RP, Sanjeevi P, Norenburg JL, Rajesh RP. (2020) A histology-free description of a new species of the genus Tetrastemma (Nemertea: Hoplonemertea: Monostilifera) from Hawaii and India. Zootaxa 4808(2): 379–383. 10.11646/zootaxa.4808.2.10 [DOI] [PubMed] [Google Scholar]
- Chernyshev AV, Polyakova NE, Norenburg JL, Kajihara H. (2021) A molecular phylogeny of Tetrastemma and its allies (Nemertea, Monostilifera). Zoologica Scripta 50(6): 824–836. 10.1111/zsc.12511 [DOI] [Google Scholar]
- Corrêa DD. (1954) Nemertinos do litoral Brasileiro. Boletim da Faculdade de Filosofia, Ciências e Letras, Universidade de São Paulo 19: 1–90. 10.11606/issn.2526-3382.bffclzoologia.1954.120084 [DOI] [Google Scholar]
- Dakin WJ, Fordham MGC. (1931) A new and peculiar marine nemertean from the Australian coast. Nature 128(3236): e796. 10.1038/128796b0 [DOI]
- de Quatrefages A. (1846) Étude sur les types inférieurs de l’embrachement des annelés. Annales des Sciences Naturelles, Zoologie, Série 3(6): 173–303. [Google Scholar]
- Diesing CM. (1850) Systema Helminthum (Vol. I). W Braumuller, Vindobonae.
- Diesing KM. (1863) Nachträge zur Revision der Turbellarien. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften. Mathematisch-Naturwissenschaftliche Classe 46: 173–188. [Google Scholar]
- Ehrenberg CG. (1828–1831) Phytozoa turbellaria Africana et Asiatica in Phytozoorum Tabula IV et V delineata. In: Hemprich FG, Ehrenberg CG. (Eds) Symbolae Physicae, seu Icones et Descriptiones Corporum Naturalium Novorum aut Minus Cognitorum Quae ex Itineribus per Libyam, Aegyptium, Nubiam, Dongalam, Syriam, Arabiam et Habessiniam, pars zoologica II, animalia evertebrata exclusis insectis.Officina Academica, Berlin, 53–67. [pls IV, V] 10.5962/bhl.title.107403 [DOI]
- Girard C. (1852) Descriptions of two new genera and two species of Nemertes. Proceedings of the Boston Society of Natural History 4: 185–186. [Google Scholar]
- Gonzalez-Cueto J, Castro LR, Quiroga S. (2017) Nipponnemertesincainca sp. n. Adoption of the new taxonomic proposal for nemerteans (Nemertea, Cratenemertidae). ZooKeys 693: 1–15. 10.3897/zookeys.693.12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hookabe N, Xu CM, Tsuyuki A, Jimi N, Sun SC, Kajihara H. (2021a) A new nemertean with a branched proboscis, Gorgonorhynchuscitrinus sp. nov. (Nemertea: Pilidiophora), with molecular systematics of the genus. Invertebrate Systematics 35: 350–359. 10.1071/IS20057 [DOI] [Google Scholar]
- Hookabe N, Kohtsuka H, Kajihara H. (2021b) A histology-free description of Tetrastemmacupido sp. nov. (Nemertea: Eumonostilfera) from Sagami Bay, Japan. Marine Biology Research 17(5–6): 467–474. 10.1080/17451000.2021.1979236 [DOI] [Google Scholar]
- Hookabe N, Kajihara H, Chernyshev AV, Jimi N, Hasegawa N, Kohtsuka H, Okanishi M, Tani K, Fujiwara Y, Tsuchida S, Ueshima R. (2022) Molecular phylogeny of the genus Nipponnemertes (Nemertea: Monostilifera: Cratenemertidae) and descriptions of 10 new species, with notes on small body size in a newly discovered clade. Frontiers in Marine Science 9: e906383. 10.3389/fmars.2022.906383 [DOI]
- Hubrecht AAW. (1879) The genera of European nemerteans critically revised, with description of several new species. Note from the Leyden Museum 1: 193–232. [Google Scholar]
- Iwata F. (1954) The fauna of Akkeshi Bay. XX. Nemertini in Hokkaido (revised report). Journal of the Faculty of Science, Hokkaido University. Series 6, Zoology 12: 1–39.
- Kajihara H. (2007) A taxonomic catalogue of Japanese nemerteans (phylum Nemertea). Zoological Science 24(4): 287–326. 10.2108/zsj.24.287 [DOI] [PubMed] [Google Scholar]
- Kajihara H. (2015) A histology-free description of the branched-proboscis ribbonworm Gorgonorhynchusalbocinctus sp. nov. (Nemertea: Heteronemertea). Publications of the Seto Marine Biological Laboratory 43: 92–102. 10.5134/199852 [DOI] [Google Scholar]
- Kajihara H. (2021) Higher classification of the Monostilifera (Nemertea: Hoplonemertea). Zootaxa 4920(2): 151–199. 10.11646/zootaxa.4920.2.1 [DOI] [PubMed] [Google Scholar]
- Kajihara H, Tamura K, Tomioka S. (2018) Histology-free descriptions for seven species of interstitial ribbon worms in the genus Ototyphlonemertes (Nemertea: Monostilifera) from Vietnam. Species Diversity 23(1): 13–37. 10.12782/specdiv.23.13 [DOI] [Google Scholar]
- Kajihara H, Abukawa S, Chernyshev AV. (2022) Exploring the basal topology of the heteronemertean tree of life: establishment of a new family, along with turbotaxonomy of Valenciniidae (Nemertea: Pilidiophora: Heteronemertea). Zoological Journal of the Linnean Society 196(1): 503–548. 10.1093/zoolinnean/zlac015 [DOI] [Google Scholar]
- Katoh K, Standley DM. (2013) MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution 30(4): 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov AM, Darriba D, Flouri T, Morel B, Stamatakis A. (2019) RAxML-NG: A fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 35(21): 4453–4455. 10.1093/bioinformatics/btz305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. (2017) PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution 34: 772–773. 10.1093/molbev/msw260 [DOI] [PubMed] [Google Scholar]
- Leiva NV, Ñacari L, Baeza JA, González MT. (2021) First report of an egg-predator nemertean worm in crabs from the south-eastern Pacific coast: Carcinonemertescamanchaco sp. nov. Scientific Reports 11(1): 1–13. 10.1038/s41598-021-98650-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson LA, Ambrosio LJ, Baeza JA. (2017) A new species of Carcinonemertes, Carcinonemertesconanobrieni sp. nov. (Nemertea: Carcinonemertidae), an egg predator of the Caribbean spiny lobster, Panulirusargus. PLoS ONE 12(5): e0177021. 10.1371/journal.pone.0177021 [DOI] [PMC free article] [PubMed]
- Sundberg P, Kvist S, Strand M. (2016) Evaluating the utility of single-locus DNA barcoding for the identification of ribbon worms (phylum Nemertea). PLoS ONE 11(5): e0155541. 10.1371/journal.pone.0155541 [DOI] [PMC free article] [PubMed]
- Yamaoka T. (1947) Prostomaroseocephalum Yamaoka, as submitted by S. Okuda. In: Uchida S (Ed.) Revised Edition Illustrated Encyclopedia of the Fauna of Japan (Exclusive of Insects). Hokuryukan, Tokyo, 1469 pp. [In Japanese] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.