Abstract
Macrophages are suspected to play a major role in human immunodeficiency virus (HIV) infection pathogenesis, not only by their contribution to virus dissemination and persistence in the host but also through the dysregulation of immune functions. The production of NO, a highly reactive free radical, is thought to act as an important component of the host immune response in several viral infections. The aim of this study was to evaluate the effects of HIV type 1 (HIV-1) Ba-L replication on inducible nitric oxide synthase (iNOS) mRNA expression in primary cultures of human monocyte-derived macrophages (MDM) and then examine the effects of NO production on the level of HIV-1 replication. Significant induction of the iNOS gene was observed in cultured MDM concomitantly with the peak of virus replication. However, this induction was not accompanied by a measurable production of NO, suggesting a weak synthesis of NO. Surprisingly, exposure to low concentrations of a NO-generating compound (sodium nitroprusside) and l-arginine, the natural substrate of iNOS, results in a significant increase in HIV replication. Accordingly, reduction of l-arginine bioavailability after addition of arginase to the medium significantly reduced HIV replication. The specific involvement of NO was further demonstrated by a dose-dependent inhibition of viral replication that was observed in infected macrophages exposed to NG-monomethyl l-arginine and NG-nitro-l-arginine methyl ester (l-NAME), two inhibitors of the iNOS. Moreover, an excess of l-arginine reversed the addition of L-NAME, confirming that an arginine-dependent mechanism is involved. Finally, inhibitory effects of hemoglobin which can trap free NO in culture supernatants and in biological fluids in vivo confirmed that endogenously produced NO could interfere with HIV replication in human macrophages.
The macrophage represents one of the major target cell for human immunodeficiency virus (HIV) infection and is likely to play a major role in persistence and tissue dissemination of this virus (35, 39). Macrophage immune functions are also altered by HIV type 1 (HIV-1) infection: (i) synthesis of inflammatory cytokines is dysregulated (8, 29, 94); (ii) in vitro-infected monocyte-derived macrophages (MDM) have decreased ability to act as accessory cells for T-lymphocyte proliferation (28); and (iii) the production of free radicals, such as hydrogen peroxide (H2O2), superoxide anion (O2−), and hydroxyl radicals (HO · ) (13, 74, 84), is impaired and may therefore facilitate the development of opportunistic intracellular pathogens. Among free radicals, NO is of particular interest. This molecule is generated by nitric oxide synthase (NOS) from l-arginine and rapidly reacts in vivo with oxygen to form nitrite and nitrate, its two stable end products (72, 76, 90). Three distinct isoforms of NOS have been described. Two are constitutive and mainly found in endothelial and neuronal cells (32, 53); the third, inducible NOS (iNOS), was originally described in murine macrophage (95). NO mediates numerous physiological functions and is known to be implicated in several immunological disorders. Besides its participation to the relaxation of blood vessels and glutamate-induced neurotoxicity (11, 12, 49), the production of NO represents an important component of the host immune response against viral infections (20, 52, 67, 69) including retroviruses (Friend leukemia virus) (2). Antiviral effects occur through its microbiostatic and microbicidal activity, and probably also through its proinflammatory and immunoregulatory properties (33, 50, 71).
In some cases, the production of NO during infectious diseases may also be deleterious. This may be particularly true in HIV infection, where NO may contribute to AIDS pathogenesis: significant increases in nitrite and nitrate (the two stable end products of NO) concentrations were evidenced in peripheral blood mononuclear cells (PBMC), polymorphonuclear leukocytes, and sera of patients with AIDS, especially in individuals with neurological disorders and pulmonary disease caused by intracellular opportunistic pathogens (30, 92; D. Torre, G. Ferrario, G. Bonetta, C. Zeroli, M. Giola, and G. P. Fioli, Abstr. 10th Int. Conf. AIDS, Int. Conf. STD, abstr. PAO114, 1996). HIV-related neurological disorders could in part be attributed to excessive production of NO. Indeed, high concentrations of NO could be obtained in vitro (i) after direct interactions between viral components and neuronal cells, since gp120-induced injury in primary neuronal cultures involves NO (23, 24, 26), and (ii) after HIV-1 infection of macrophages infiltrating the brain tissue (55). Direct evidence for the presence and distribution of iNOS has been reported in human pulmonary tissue (54, 82) and in the central nervous system of HIV-infected patients, especially in areas of acute and chronic inflammation (1). The production of NO by human monocytes/macrophages could result from the induction of iNOS expression by the proinflammatory cytokines (21, 65, 77, 78, 85). Synthesis of these cytokines is induced in vivo and in vitro in response to HIV-1 infection (42, 89) and may directly regulate iNOS expression (15, 83). However, we cannot exclude that HIV-1 can also directly interact with iNOS expression in monocytes/macrophages. Indeed, viral regulatory proteins such as Tat may directly enhance the transcription of the iNOS.
Interestingly, Groeneveld et al. have shown that levels of nitrate in the serum are positively correlated with plasma and cell-associated virus loads, suggesting that HIV could induce NO synthesis in vivo (46). In the simian immunodeficiency virus/macaque model, significantly increased concentrations of NO2− and NO3− were measured in plasma during primary infection, coincident with viremia peaks, and in the absence of opportunistic infections (10).
The objectives of this study were to (i) evaluate the effects of HIV-1 infection on iNOS mRNA expression and NO production in cultured human MDM, (ii) assess whether the endogenous NO release interfere with HIV replication in cultured MDM, and (iii) study the mechanisms of NO regulation in response to MDM infection with primary HIV-1 isolates.
MATERIALS AND METHODS
Isolation and characterization of monocytes.
Fresh human PBMC were obtained from healthy HIV-1-seronegative donors after centrifugation of heparinized venous blood over Ficoll-Hypaque gradients. Monocytes were isolated from PBMC by centrifugal elutriation (Beckman J2-21/ME centrifuge, JE-5 rotor; Beckman Instruments, Gagny, France) as previously described by Figdor et al. (31). Purified monocytes were cultivated at the concentration of 2 × 105 cells/ml in 48-well microtiter plates and progressively allowed to differentiate into macrophages for 7 days in a 5% CO2 atmosphere at 37°C. The culture medium was constituted of RPMI 1640 medium (Boehringer Mannheim, Mannheim, Germany) supplemented with 10% heat-inactivated fetal calf serum (Boehringer Mannheim), 2 mM glutamine (Boehringer Mannheim), and 0.2 μM antibiotics (penicillin-streptomycin-neomycin [Life Technologies, Inc., Berlin, Germany]). Immunophenotyping of the monocyte fraction was performed by standard fluorescence-activated cell sorter analysis as previously described (10), using a FACScan Plus cytofluorometer and LYSIS II software (Becton Dickinson, Mountain View, Calif.). Cellular purity was greater than 97%; these cells were negative for expression of CD3 (CD3 Leu4, immunoglobulin G1 [IgG1]; Becton Dickinson); and CD19 (CD19-fluorescein isothiocyanate [FITC], IgG1; Immunotech, Marseille, France) but positive for CD14 (CD14-FITC, IgG1; Becton Dickinson) and CD64 (CD64-FITC, IgG1; Medarex, West Lebanon, N.H.) expression (81.6 and 88.5%, respectively).
Infection of MDM and detection of virus replication.
MDM were infected with macrophagetropic HIV-1 reference strain Ba-L, a generous gift from A.-M. Aubertin (Strasbourg, France). This strain, initially obtained from a primary culture of postmortem lung tissue from an infant who died from AIDS (35, 36), replicates well in human macrophage cultures. HIV-1 Ba-L was grown to high titers in phytohemagglutinin-stimulated human cord blood mononuclear cells. The cell-free supernatant was clarified at 10,000 × g for 5 min and ultracentrifuged at 360,000 × g (Beckman TL100; Beckman Instruments) for 10 min. The viral pellet was resuspended in phosphate-buffered saline. Virus stock was titered on cord blood lymphocytes in a 96-well microplate assay as measured by endpoint dilution. The 50% tissue culture infectious dose (TCID50) was determined by the Karber's formula (47). The virus stock used was endotoxin free, as assessed by the limulus amebocyte lysate assay (Sigma, St. Louis, Mo.). MDM were infected with HIV-1 Ba-L at 105 TCID50/106 cells. Twenty-four hours after onset of HIV-1 infection, MDM were washed with phosphate-buffered saline (Boehringer Mannheim) to remove excess virus. MDM were then treated or not with various reagents at the desired concentration. Medium and reagents were replaced every 3 or 4 days. Cells were maintained in culture for 4 weeks after infection. Culture supernatants were kept frozen at −20°C before HIV replication measurement. For each tested compound, morphology and viability of culture MDM were evaluated by microscope examination and trypan blue exclusion dye.
HIV replication was assessed by reverse transcriptase (RT) activity measurement in the culture supernatants. RT activity was determined as previously described by Rey et al. (86). Briefly, the culture supernatants were ultracentrifuged for 5 min at 360,000 × g (Beckman TL100), and viral pellets were lysed in 20 μl of NTE (10 mM NaCl, 10 mM Tris [pH 7.8], 1 mM EDTA) containing 0.1% Triton X-100. Ten microliters of viral lysate was added to a reaction mixture containing 5 mM MgCl2, 1 mM dithiothreitol, 2.5 mg of poly(rA)-oligo(dT) per ml as a template-primer, and [methyl-3H]TTP (4 pmol/ml; TRK 354, 1.1 TBq/mmol; Amersham Life Science, Buckinghamshire, England). The mixture was incubated for 1 h at 37°C, placed on nitrocellulose filters, and extensively washed. Filters were dried for 20 min at 80°C. RT activity was quantified by measuring the level of incorporated [3H]TTP (results are expressed as counts per minute per hour per milliliter). It is of note that the RT activity measured in supernatants of HIV-infected MDM followed similar kinetics as reported previously (19, 64, 66).
Two types of representation were used to characterize HIV replication in human MDM: (i) kinetic curves of HIV-1 replication indicating the mean of three independent culture wells and (ii) the sum of the RT activities, for an individual well, of each time point of medium exchange (every 2 or 3 days). Three wells were monitored simultaneously; results are presented as the mean for the three wells ± standard deviation (SD).
Statistical analysis was performed using the nonparametric Mann-Whitney U test (Statview 4.5; Abacus Concepts, Berkeley, Calif.). Differences between treated and untreated HIV-infected cultures were considered significant if P was <0.05.
RNA extraction.
At different culture time points, infected and noninfected MDM were scraped off and lysed in guanidinium isothiocyanate solution. Total cellular RNA was extracted as described by Chomczynski and Sacchi (18) by a phenol-chloroform method, precipitated in the presence of isopropanol at −20°C overnight, and then washed twice in 75% ethanol. Total RNA extracted was resuspended in sterilized distilled water. The RNA concentration was determined by the absorbancy at 260 nm.
Quantification of iNOS mRNA expression by RT-PCR.
RT-PCR was performed using 106 freshly isolated MDM for the quantification of mRNA expression. Total RNA was subjected to first-strand cDNA synthesis for 1 h at 42°C in a 30-μl reaction volume containing 0.25 M Tris-HCl (pH 8.3), 0.375 M KCl, 15 mM MgCl2, 30 U of recombinant RNase inhibitor (Clontech, Palo Alto, Calif.), 30 μM each deoxynucleoside triphosphate, 0.3 μg of oligo(dT)12–18 (Sigma), and 150 U of Moloney murine leukemia virus RT (GIBCO-BRL, Grand Island, N.Y.). After completion of first-strand synthesis, the reaction mixture was diluted to 160 μl. Five microliters of this dilution was used for each PCR. The PCR mixture (in a volume of 50 μl) contained a 10 μM each deoxynucleoside triphosphate, 100 ng of each specific primer, buffer as supplied by manufacturer, and 0.5 U of Taq polymerase (ATGC Biotechnologie, Noisy le Grand, France). Sequences of primers specific for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (9, 17, 93) and iNOS (85) were as follows: iNOS-5′, 5′-TCCGAGGCAAACAGCACATTCA; iNOS-3′, 5′-GGGTTGGGGGTGTGGTGATG; GAPDH-5′, 5′-ACCACCATGGAGAAGGCTGG; and GAPDH-3′, 5′-CTAAGTGTAGCCCAGGATGC. All primers crossed introns to avoid amplification of potentially contaminant genomic DNA. However, the absence of DNA contaminants was controlled by DNase treatment before RT-PCR amplification. PCR was performed in an Omnigene thermocycler (Céra-labo, Aubervilliers, France). The cycle program for GAPDH amplification included denaturing at 94°C for 45 s, annealing at 60°C for 2 min, and extension at 72°C for 1 min, for a total of 32 cycles. The iNOS cycle program included denaturing at 94°C for 45 s, annealing at 60°C for 45 s, and extension at 72°C for 2 min, for a total of 40 cycles. The optimal number of PCR cycles was determined by using a variable number of cycles to identify a linear range of amplification for each transcript. Eight-microliter aliquots of amplification mixtures were electrophoresed on a 1.5% agarose gel, and PCR products were detected by ethidium bromide staining. The intensity of the signal was quantified using NIH Image 1.52 software (developed by Wayne Rasband, National Institutes of Health, Bethesda, Md.). Results were expressed, as previously published (16, 17), as the ratio of the signal obtained for each tested mRNA to the signal obtained for GAPDH mRNA.
Nucleotide sequence determination.
PCR specificity was confirmed by determination of nucleotide sequence. PCR products were purified with a USB US 70995 reagent pack (Amersham Life Science, Cleveland, Ohio), using exonuclease I and shrimp alkaline phosphatase, before sequencing with a Dye Terminator Cycle Sequencing kit (Perkin-Elmer). The product was then loaded onto a 6% polyacrylamide gel in an automated laser fluorescent DNA sequencer (ABI model 377; Perkin-Elmer). Direct cycle sequencing was done with Taq DNA polymerase and antisense iNOS primers. DNA sequences were aligned and analyzed using the Sequed program (Applied Biosystems). We confirmed that mRNA encoding the iNOS had been amplified and that PCR products had no significant similarity to human neuronal constitutive NOS and endothelial constitutive NOS (ncNOS and ecNOS, respectively).
NO colorimetric assay.
At various time points of culture, supernatants were harvested and NO accumulation was assessed by a colorimetric assay using the Griess reaction (56). Nitrite (NO2−) measurement was used as an indicator of NO production (the RPMI 1640 medium was free of nitrite, according to the manufacturer's data) and biological fluids. Briefly, 100 μl of culture supernatant was added to 100 μl of Griess reagent, made of a 1/1 mixture of 1% (wt/vol) sulfanilamide and 0.5% (wt/vol) N-(1-naphthyl)ethylenediamine dihydrochloride (Sigma) in 30% acetic acid, in each well of a 96-well plate. Reactions were performed in triplicate at room temperature for 10 min. Chromophore absorbancy was then measured at 550 nm in a microplate reader (Bio-TEK Instruments model EL 311; OSI, Paris, France). Nitrite concentration was evaluated by comparison with a sodium nitrite or nitrate standard curve (Sigma). The lower limit of the method for nitrite or nitrate concentration determination is 250 nM.
Materials and reagents.
l-Arginine was obtained from Sigma. Sodium nitrite, sodium nitroprusside (SNP), potassium ferricyanide (KPC), S-nitroso-N-acetylpenicillamine (SNAP), and N-acetylpenicillamine were purchased from Sigma. The iNOS inhibitors NG-monomethyl l-arginine (l-NMMA) and NG-nitro-l-arginine methyl ester (l-NAME) were obtained from Cayman Chemical Company (Ann Arbor, Mich.). NG-monomethyl d-arginine (d-NMMA) was provided by Cayman, and d-arginine was from Sigma. Arginase and bovine hemoglobin (Hb) were obtained from ICN Pharmaceuticals (Orsay, France).
RESULTS
Effect of HIV-1 Ba-L replication on iNOS mRNA transcription and NO2− production in human MDM cultures.
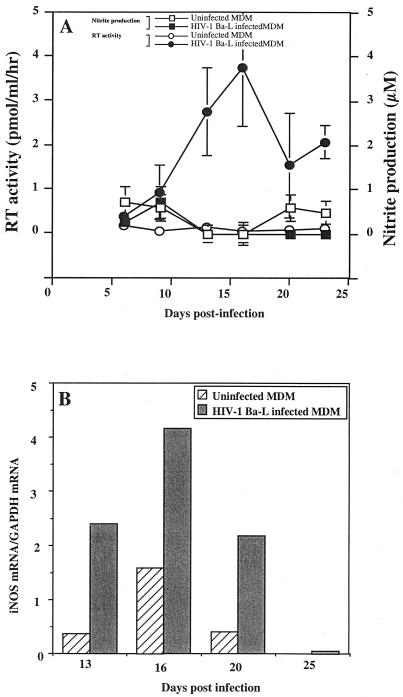
Human monocytes obtained from healthy HIV-seronegative donors by centrifugal elutriation were allowed to differentiate into MDM, without granulocyte-macrophage colony-stimulating factor stimulation, for 7 days before infection with HIV-1 Ba-L. The RT activity measured in culture supernatants peaked between days 15 and 21 (Fig. 1). As observed in Fig. 1, a significant induction of iNOS gene expression occurred at the time of viral replication peak (Fig. 1B). However, this was not associated with the production of detectable amounts of nitrites in culture supernatants (Fig. 1A).
FIG. 1.
Effects of HIV-1 Ba-L replication on iNOS mRNA transcription and NO2− production in human MDM cultures. (A) ●, level of HIV replication determined by the measure of RT activity in culture supernatants (mean of three independent culture wells ± SD); ○, RT activity in uninfected controls (mean of three independent culture wells ± SD); ■, production of nitrites in culture supernatants of infected macrophages (mean of three independent culture wells ± SD); □, nitrite production in uninfected control cultures (mean of three independent culture wells ± SD). (B) Expression of iNOS mRNA in the same culture of infected, or uninfected macrophages, determined as the ratio of the signal obtained for tested mRNA to the signal obtained for GAPDH mRNA (mean of two independent culture wells). Bars represent the mean of two independent measures.
Effects of NO-generating compounds on HIV-1 replication in MDM.
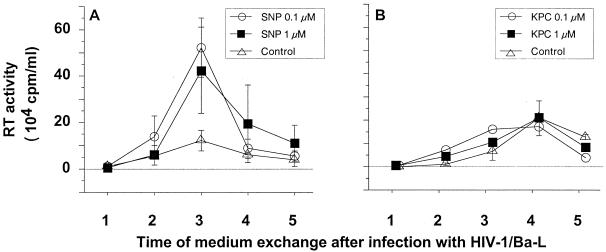
As reported by Bukrinsky et al. (14, 15), low expression of iNOS may lead to low production of nitrites remaining undetectable by the Griess assay, the sensitivity of which is approximately 250 nM. We therefore designed an experimental approach to determine whether low levels of NO released in culture supernatants could modulate HIV replication in MDM. HIV-1 Ba-L-infected MDM were treated with low concentrations of NO donors such as SNP, which is a classically used NO-generating compound (6, 37, 61, 70, 88). Doses of SNP that we used were reported by others to be efficient in human cell cultures (27, 60, 70) and appeared to be suitable for the in vivo situation, consistent with the levels of NO detected in plasma of asymptomatic seropositive patients (5, 62, 92). We verified that in our model, NO2− was generated from SNP in a dose-dependent manner (data not shown). A sequential and time-dependent release of nitrite was consistently obtained at the dose of 10 μM. Therefore, 24 h after HIV infection, MDM were treated with SNP at doses ranging from 0.1 to 10 μM. Concentration in the culture medium was maintained constant throughout the postinfection period. As control, HIV-1 Ba-L-infected MDM were treated with identical concentrations of KPC, a compound that is very similar in chemical structure to SNP but does not generate free NO in culture supernatants.
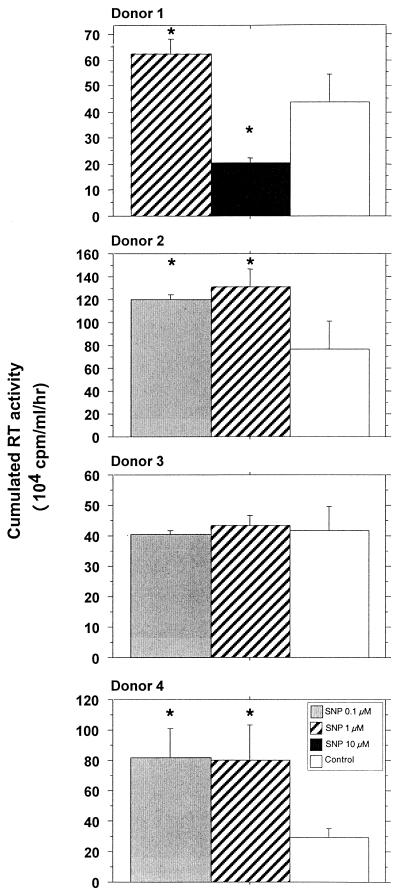
Unexpectedly, treatment at lower SNP concentrations, 0.1 and 1 μM, resulted in a significant increase of viral replication in MDM cultures (Fig. 2A). This was confirmed in cultures of MDM obtained from three out of the four tested donors (Fig. 3). At a higher concentration of SNP, 10 μM, HIV-1 replication in MDM was inhibited (Fig. 3) as previously reported (68). NO generated by SNP in culture medium is naturally and rapidly reduced in nitrite (NO2−). We verified that NO2− was not, by itself, responsible for the increased replication of HIV-1 by treating MDM with NaNO2 with doses ranging between 0.1 and 10 μM (data not shown).
FIG. 2.
Effects of NO-generating compounds on HIV-1 replication in MDM. Purified monocytes from PBMC were cultured for 7 days in 24-well microtiter plates and then infected with HIV-1 Ba-L. Culture supernatant was completed removed every 2 or 3 days and replaced by fresh culture medium. HIV replication in macrophages was determined by the mean level of RT (±SD) activity in culture supernatants of three independent wells. The x axis indicates the time points of medium exchange. (A) Replication of HIV in infected macrophages treated or not with SNP; (B) effect of the KPC control on HIV replication in macrophages.
FIG. 3.
Effects of the NO-generating compound SNP on HIV replication in macrophages obtained from four different donors. HIV replication is represented by the sum, at the end of the culture of an individual well, of the RT activities of each time point of medium exchange (every 2 or 3 days). Bars indicate the mean (±SD) of RT activity of three independent wells. Statistical analysis was performed using the nonparametric Mann-Whitney U test. ∗, significant difference between treated and untreated HIV-infected cultures (P < 0.05).
l-Arginine causes a dose-dependent enhancement of HIV-1 replication in human macrophages.
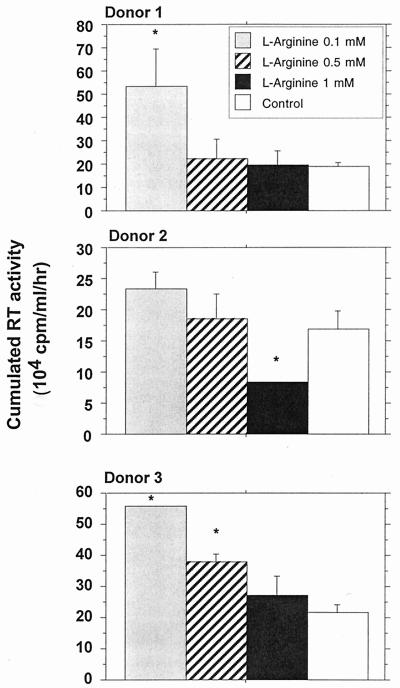
l-Arginine is the natural substrate of iNOS. In this experiment, MDM were cultured in the presence of different doses of l-arginine, ranging from 0.1 to 1 mM, previously reported by Belenky et al. (7) to modulate NO synthesis in cultured macrophages. These doses did not appear to affect MDM viability (data not shown). Low concentrations of l-arginine (0.1 and 0.5 nM) appeared to enhance replication of HIV-1 Ba-L in MDM; this was statistically significant for two different donors tested. Addition of 1 mM l-arginine had no marked effect on viral replication (Fig. 4).
FIG. 4.
Effects of l-arginine on HIV replication in macrophages obtained from three different donors. HIV replication is represented by the sum, at the end of the culture of an individual well, of the RT activities of each time point of medium exchange (every 2 or 3 days). Bars indicate the mean (±SD) of RT activity of three independent wells. Statistical analysis was performed using the nonparametric Mann-Whitney U test. ∗, significant difference between treated and untreated HIV-infected cultures (P < 0.05).
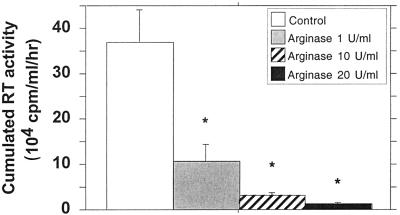
As a reciprocal control, arginase was used to deplete l-arginine in the culture medium. This enzyme has potential implication in AIDS pathogenesis since abnormal concentrations could be identified in patient fluids. A dose-dependent inhibition of HIV replication was observed in the MDM cultures maintained in the presence of arginase (1 to 20 U/ml) (Fig. 5) with no significant decrease of cellular viability. At the dose of 10 U/ml, arginase significantly reduces the replication of HIV (P < 0.05).
FIG. 5.
Effects of arginase on HIV replication in macrophages. HIV replication is represented by the sum, at the end of the culture of an individual well, of the RT activities of each time point of medium exchange (every 2 or 3 days). Bars indicate the mean (±SD) of RT activity of three independent wells. Statistical analysis was performed using the nonparametric Mann-Whitney U test. ∗, significant difference between treated and untreated HIV-infected cultures (P < 0.05).
Effect of iNOS inhibitors on HIV-1 replication in human macrophages.
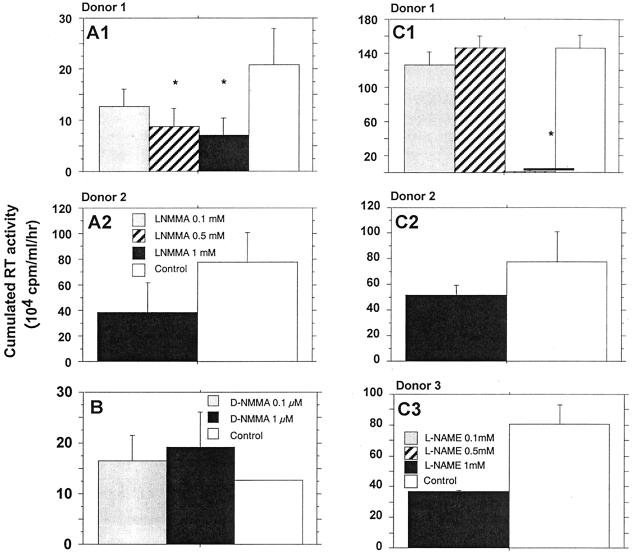
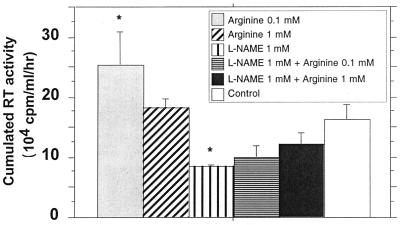
Two specific competitive inhibitors of iNOS, l-NMMA and l-NAME, represent powerful tools to confirm that endogenous production of NO modulates viral replication (25, 41, 43, 51). Twenty-four hours after HIV infection, MDM were treated with one of the two inhibitors at doses ranging from 0.1 to 1 mM. These components were maintained at constant concentrations in the culture medium throughout the postinfection period. l-NMMA significantly reduced, in a dose-dependent manner, the replication of HIV-1 Ba-L (Fig. 6A). Moreover, treatment of infected MDM with equal molar concentrations of d-NMMA, an inactive enantiomer of l-NMMA, had no significant effect on HIV-1 replication (Fig. 6B). Similarly, 1 mM l-NAME substantially reduced the level of HIV-1 Ba-L in MDM of three out of the four tested donors (Fig. 6C and D). The addition of l-arginine reversed the effects of l-NAME in a dose-dependent manner (Fig. 7), confirming that an arginine-dependent mechanism is involved in the modulation of HIV replication in MDM.
FIG. 6.
Effects of iNOS inhibitors on HIV-1 replication in human macrophages obtained from different donors. (A) Effect of the inhibitor l-NMMA; (B) effect of d-NMMA, used as inactive control; (C) effect of the inhibitor l-NAME. HIV replication is represented by the sum, at the end of the culture of an individual well, of the RT activities of each time point of medium exchange (every 2 or 3 days). Bars indicate the mean (±SD) of RT activity of three independent wells. Statistical analysis was performed using the nonparametric Mann-Whitney U test. ∗, significant difference between treated and untreated HIV-infected cultures (P < 0.05).
FIG. 7.
Reversion of the effect of the inhibitor l-NAME on HIV-1 replication in human macrophages by the addition of arginine. HIV replication is represented by the sum, at the end of the culture of an individual well, of the RT activities of each time point of medium exchange (every 2 or 3 days). Bars indicate the mean (±SD) of RT activity of three independent wells. Statistical analysis was performed using the nonparametric Mann-Whitney U test. ∗, significant differences between treated and untreated HIV-infected cultures (P < 0.05).
Effects of a NO scavenger, Hb, on HIV-1 replication in MDM.
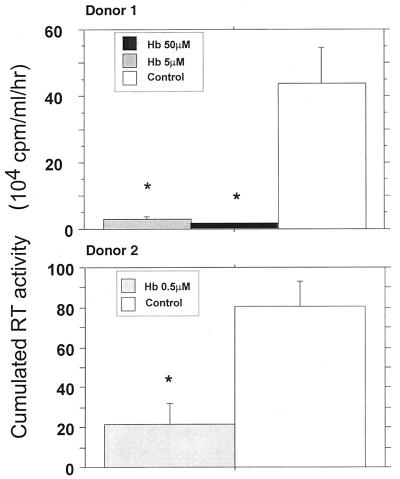
NO is a potent local messenger molecule capable of rapid migration from cell to cell, exerting its effect in both autocrine and paracrine manners (72). The paracrine mode is the major mechanism of NO activity (57). The biological activity of NO could be abolished in vivo by Hb, which oxidizes NO to nitrate (48). Zinetti et al. have reported that monocyte hyperactivation by lipopolysaccharide could be affected by Hb and l-NMMA, through the modulation of the NO-dependent release of tumor necrosis factor alpha (TNF-α) (97). Addition of Hb in culture supernatants mainly affects extracellular NO activity (91) by trapping NO produced in culture medium (40). We observed that 0.5 μM Hb is able to significantly decrease the viral replication in HIV-1 Ba-L-infected MDM (Fig. 8). At that concentration, no toxic effects of Hb on cultured MDM have been observed. This confirms the data reported by others (14, 70). In our culture system, Hb concentrations above 200 μM are needed to affect MDM viability (data not shown).
FIG. 8.
Effects of the NO scavenger Hb on HIV-1 replication in MDM in macrophages obtained from two different donors. HIV replication is represented by the sum, at the end of the culture of an individual well, of the RT activities of each time point of medium exchange (every 2 or 3 days). Bars indicate the mean (±SD) of RT activity of three independent wells. Statistical analysis was performed using the nonparametric Mann-Whitney U test. ∗, significant differences between treated and untreated HIV-infected cultures (P < 0.05).
DISCUSSION
The aim of this study was to determine the relationships between the production of NO and modulation of HIV-1 replication in human macrophages. We first observed that iNOS gene expression is induced in primary human macrophage cultures during infection in vitro with the macrophagetropic HIV-1 Ba-L. Conflicting reports have been published regarding the expression of an iNOS gene in human macrophages; nevertheless, the increase that we observed confirms the results found by Bukrinsky et al. in HIV-infected cultures (15). Despite a significant induction of iNOS expression, we did not succeed, as others have previously reported (15), in detecting any nitrite production in culture supernatants. This observation is nevertheless in agreement with results of Padgett and Pruett (79), who also detected no nitrite production after activation of human macrophages with lipopolysaccharide, gamma interferon, phorbol myristate acetate, or opsonized zymosan. However, we cannot exclude that this discrepancy may also be attributable in part to the low sensitivity of the Griess reaction, which is estimated to be approximately 250 nM (15, 73, 83).
In vivo, there is convincing evidence that human macrophages may synthesize detectable NO during HIV infection. In plasma, the nitrite and nitrate concentrations correlate with levels of neopterin, a marker of activation of mononuclear phagocytes (34). Increased production of NO was evidenced in PBMC of AIDS patients, in particular in individuals with opportunistic infections. Asymptomatic seropositive patients exhibited low production of NO (<1 μM).
The detection of iNOS expression that we observed, without any substantial accumulation of nitrite, suggested that NO could be released at low levels after HIV-1 infection. We therefore investigated the direct effects of low concentrations of NO on HIV-1 replication in MDM. The release of this unstable free radical in culture was obtained by using SNP, an exogenous NO donor. Interestingly, treatment of MDM with low doses of SNP enhanced viral replication, indicating that NO may interfere with viral replication mechanisms. This interaction could be directly mediated by NO but may also result from indirect mechanisms related, for instance, to macrophage activation (14, 15, 60). Indeed, very low doses of NO (<1 μM) could enhance soluble guanylate cyclase and GTPase activities, two markers of macrophage activation.
The observation that the modulation of NO synthesis by infected macrophages, using l-arginine, iNOS inhibitors, Hb, and arginase, modulates in the same direction the replication of HIV in MDM argues for a pivotal role of NO in the induction of this phenomenon. The specific involvement of the iNOS pathway in our experimental model was further demonstrated by the dose-dependent inhibition of viral replication in the presence of specific NOS inhibitors.
In summary, the effects of l-NMMA, l-NAME, and arginase on the infected macrophage cultures show that NO can influence the viral replication through an inducible l-arginine-dependent pathway, which is in accordance with previous report (14). While it is possible that human macrophages may be stimulated to produce reactive nitrogen intermediate by cytokines and/or pathogens, our results confirm that the specific l-arginine-dependent mechanism could be also modulated in turn, by HIV replication in human macrophage cultures as described elsewhere (25, 38). This is potentially important, since it contrasts sharply with the well-established antimicrobial and antiviral properties of NO. The unusual low production of NO by HIV-infected human monocytes could probably explain the lack of antiviral activity. However, these low concentrations could be sufficient to affect the biology of MDM, resulting in enhanced HIV-1 replication.
The molecular mechanisms involved in the induction of NO production in HIV-infected MDM remain unclear. We can postulate that iNOS mRNA expression may result from direct interactions between virus and resident macrophages. Pietraforte et al. (83) reported that recombinant HIV envelope glycoprotein gp120 stimulates a very low production of NO by human MDM. Enhanced replication of HIV may also involve the activation of NF-κB, which is a cellular component regulating HIV replication and also the expression of several cytokines (4, 75). Indeed, NO induces the production of TNF-α, which may in turn activate viral replication in MDM (63, 64). Previous findings concerning the modulation of NF-κB activation by NO are controversial. An early study indicates that chemical NO donors (SNP and SNAP) are able to activate NF-κB in human peripheral blood mononuclear cells. Lander et al. (61) have reported that production of nitric oxide radicals activates the NF-κB transcription factor in doses within the range of those used in our experiments (68, 80). In our experiments, partial inhibition of HIV needs 10- to 100-times-higher concentrations of SNP to decrease activation of NF-κB. However, such high NO concentrations do not reflect the production of NO observed in vivo in human fluids (5, 30, 92, 96; Torre et al., Abstr. 10th Int. Conf. AIDS, 1996).
Primary targets of reactive nitrogen oxide species may be different in cells submitted to low (<1 μM) or steady-state concentrations of NO. An explanation for these conflicting results might be related to the different fluxes of NO used in these experiments. Indeed, Lander et al. found that micromolar or submicromolar concentrations of pharmacological sources of NO were sufficient to activate NF-κB via an enhancement of GTPase activity (58–60). In other reports, inhibitory concentrations of NO donors were frequently 100 times higher than micromolar amounts shown to activate NF-κB per se (68, 81). Therefore, it may be that low amounts of NO would activate NF-κB, whereas high fluxes of NO would be inhibitory. This hypothesis is reinforced by biphasic effects of NO on GTPase activity, which is inhibited by high concentrations of NO (58).
In the same way, the tendency to decrease HIV-1 replication that was observed with higher concentrations of l-arginine (1 mM) could be due to a negative feedback exerted by NO on NOS activity as previously described (3, 45, 87).
AIDS is associated with activation of the immune system. Correlations of nitrite and nitrate with the immune activation markers (sTNFR 55 and TNFR 75) and neopterin in HIV-1-infected patients (96) suggest that endogenous cytokines, like TNF-α, could activate inflammatory cells (44). This additional priming could be sufficient to amplify the induction of iNOS and increase NO production by the infected macrophage (10). The increased production of cytokines and NO may in turn contribute to the immunopathogenesis of HIV disease both by enhancing HIV replication and by direct effects on target tissues, such as the brain and lung.
The impact of NO production on HIV-1 infection is still difficult to predict. Our results suggest that NO, in vivo, may favor virus replication in MDM rather than exert an efficient antiviral activity. Nevertheless, considerable controversy remains regarding the ability of human macrophages to generate biologically significant amounts of NO, and it is not clearly established whether an elevated nitrite level in serum or tissues of HIV-infected patients is a cause, effect, or epiphenomenon of HIV-1 infection. However, Torre et al. have shown that HIV-1 stimulates NO production by human macrophages and that the NO concentration is increased in the sera of patients with AIDS, especially in those with neurological disorders and pulmonary disease caused by intracellular opportunistic pathogens (Torre et al., Abstr. 10th Int. Conf. AIDS, 1996). Moreover, Groeneveld et al. have demonstrated that serum nitrate in such patients correlates positively with viral load, strongly suggesting that our in vitro observations may be relevant for in vivo situations and thus should be considered with special attention for the design of new therapeutic strategies (46). In addition, the significant increased concentrations of NO2− and NO3− that we previously observed in plasma in the macaque model during primary simian immunodeficiency virus infection (10) seems to be closely related to active virus production. Peaks of NO3− in plasma and p27 antigenemia were detected simultaneously in the absence of any opportunistic infections, suggesting that NO production may therefore contribute to virus-induced pathogenesis as early as the first days following infection.
ACKNOWLEDGMENTS
We thank L. Minghetti for helpful discussions and for critical reading of the manuscript. We acknowledge the Centre de Cytaphérèse de l'Hôpital Saint-Louis (Paris, France) for technical assistance. We thank Dominique Marcé for technical support.
This work was supported by the Agence Nationale de Recherches sur le SIDA (ANRS; Paris, France), Centre de Recherches du Service de Santé des Armées (CRSSA; La Tronche, France), Commissariat à l'Energie Atomique (CEA; Fontenay aux Roses, France), and Institut Paris-Sud sur les Cytokines (IPSC) and Tous ensemble contre le SIDA (SIDACTION) (Paris, France).
REFERENCES
- 1.Adamson D C, Wildemann B, Sasaki M, Glass J D, McArthur J C, Christov V I, Dawson T D, Dawson V L. Immunologic NO synthase: elevation in severe AIDS dementia and induction by HIV-1 gp41. Science. 1996;274:1917–1921. doi: 10.1126/science.274.5294.1917. [DOI] [PubMed] [Google Scholar]
- 2.Akarid K, Sinet M, Desforges B, Gougerot-Pocidalo M A. Inhibitory effect of nitric oxide on the replication of a murine retrovirus in vitro and in vivo. J Virol. 1995;69:7001–7005. doi: 10.1128/jvi.69.11.7001-7005.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assreuy J, Cunha F Q, Liew F Y, Moncada S. Feedback inhibition of nitric oxide synthase activity by nitric oxide. Br J Pharmacol. 1993;108:833–837. doi: 10.1111/j.1476-5381.1993.tb12886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachelerie F, Alcami J, Arenzana-Seisdedos F, Virelizier J L. HIV enhancer activity perpetuated by NFκB induction on infection of monocytes. Nature. 1991;350:709–712. doi: 10.1038/350709a0. [DOI] [PubMed] [Google Scholar]
- 5.Baldeweg T, Sooranna S, Das I, Catalan J, Gazzard B. Serum nitrite concentration suggests a role for nitric oxide in AIDS. AIDS. 1996;10:451–452. doi: 10.1097/00002030-199604000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Bates J N, Baker M T, Guerra R J, Harrison D G. Nitric oxide generation from nitroprusside by vascular tissue. Evidence that reduction of the nitroprusside anion and cyanide loss are required. Biochem Pharmacol. 1991;42:S157. doi: 10.1016/0006-2952(91)90406-u. [DOI] [PubMed] [Google Scholar]
- 7.Belenky S N, Robbins R A, Rubinstein I. Nitric oxide synthase inhibitors attenuate human monocyte chemotaxis in vitro. J Leukoc Biol. 1993;49:380–389. doi: 10.1002/jlb.53.5.498. [DOI] [PubMed] [Google Scholar]
- 8.Bender B L, Davidson B L, Kline R, Brown C, Quinn T C. Role of the mononuclear phagocyte system in the immunopathogenesis of human immunodeficiency virus infection and the acquired immunodeficiency syndrome. Rev Infect Dis. 1988;10:1142–1154. doi: 10.1093/clinids/10.6.1142. [DOI] [PubMed] [Google Scholar]
- 9.Benveniste O, Vaslin B, Le Grand R, Chéret A, Matheux F, Théodoro F, Granage M P, Dormont D. Comparative interleukin (IL)-2/interferon (IFN)-γ and IL-4/IL-10 responses during acute infection of macaques inoculated with attenuated nef-truncated or pathogenic SIVmac251 virus. Proc Natl Acad Sci USA. 1996;93:3658–3663. doi: 10.1073/pnas.93.8.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blond D, Chéret A, Raoul H, Le Grand R, Caufour P, Théodoro F, Dormont D. Nitric oxide synthesis during acute SIVmac251 infection of macaques. Res Virol. 1998;149:75–86. doi: 10.1016/s0923-2516(98)80083-6. [DOI] [PubMed] [Google Scholar]
- 11.Bredt D S, Glatt C E, Hwang P M, Fotuhi M, Dawson T M, Snyder S H. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of mammalian CNS together with diaphorase. Neuron. 1991;7:615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- 12.Bredt D S, Hwang P M, Snyder S H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- 13.Buhl R. Imbalance between oxidants and antioxidants in the lungs of HIV-seropositive individuals. Chem Biol Interact. 1994;91:147–158. doi: 10.1016/0009-2797(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 14.Bukrinsky M, Schmidtmayerova H, Zybarth G, Dubrovsky L, Sherry B, Enikolopov G. A critical of nitric oxide in human immunodeficiency virus type 1-induced hyperresponsiveness of cultured monocytes. Mol Med. 1996;2:460–468. [PMC free article] [PubMed] [Google Scholar]
- 15.Bukrinsky M I, Nottet H S L M, Schmidtmayerova H, Dubrovsky L, Flanagan C R, Mullins M E, Lipton S A, Gendelman H E. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV-associated neurological disease. J Exp Med. 1995;181:735–745. doi: 10.1084/jem.181.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chéret A, Caufour P, Le Grand R, Théodoro F, Boussin F, Vaslin B, Dormont D. Macrophage inflammatory protein-1α mRNA expression in mononuclear cells from different tissues during acute simian immunodeficiency virus strain mac251 infection of macaques. AIDS. 1997;11:257–258. [PubMed] [Google Scholar]
- 17.Chéret A, Le Grand R, Caufour P, Dereudre-Bosquet N, Matheux F, Neildez O, Théodoro F, Maestrali N, Benveniste O, Vaslin B, Dormont D. Cytokines mRNA expression in mononuclear cells from different tissues during acute SIVmac251 infection of macaques. AIDS Res Hum Retroviruses. 1996;12:1263–1272. doi: 10.1089/aid.1996.12.1263. [DOI] [PubMed] [Google Scholar]
- 18.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Ann Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 19.Clayette P, Dereuddre-Bosquet N, Martin M, Fretier P, Dormont D. Effects of RP55778, a tumor necrosis factor alpha synthesis inhibitor, on antiviral activity of dideoxynucleosides. Antimicrob Agents Chemother. 1997;41:875–877. doi: 10.1128/aac.41.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Croen K D. Evidence for antiviral effect of nitric oxide. Inhibition of herpes simplex virus type 1 replication. J Clin Investig. 1993;91:2446–2452. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curran R D, Billiar T R, Stuehr D J, Ochoa J B, Harbrecht B G, Flint S G, Simmons R L. Multiple cytokines are required to induce hepatocyte nitric oxide production and inhibit total protein synthesis. Ann Surg. 1990;212:462–471. doi: 10.1097/00000658-199010000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Currie G A. Activated macrophages kill tumour cells by releasing arginase. Nature. 1978;273:758–759. doi: 10.1038/273758a0. [DOI] [PubMed] [Google Scholar]
- 23.Dawson V L, Dawson T M, Bartley D A, Uhl G R, Snyder S H. Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. J Neurosci. 1993;13:2651–2661. doi: 10.1523/JNEUROSCI.13-06-02651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawson V L, Dawson T M, London E D, Bredt D S, Snyder S H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci USA. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denis M. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell Immunol. 1991;132:150–157. doi: 10.1016/0008-8749(91)90014-3. [DOI] [PubMed] [Google Scholar]
- 26.Dreyer E B, Kaiser K, Offerman J T, Lipton S A. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990;248:364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- 27.Efron D T, Kirk S J, Regan M C, Wasserkrug H L, Barbul A. Nitric oxide generation from l-arginine is required for optimal human peripheral blood lymphocyte DNA synthesis. Surgery. 1991;110:327–334. [PubMed] [Google Scholar]
- 28.Ennen J, Seipp I, Norley S G, Kurth R. Decreased accessory cell function of macrophages after infection with human immunodeficiency virus type 1 in vitro. Eur J Immunol. 1990;20:2451–2456. doi: 10.1002/eji.1830201114. [DOI] [PubMed] [Google Scholar]
- 29.Estevez M E, Ballart I J, Diez R A, Planes N, Scaglione C, Sen L. Early defect of phagocytic cell function in subjects at risk for acquired immunodeficiency syndrome. Scand J Immunol. 1986;24:215–222. doi: 10.1111/j.1365-3083.1986.tb02088.x. [DOI] [PubMed] [Google Scholar]
- 30.Evans T G, Rasmussen K, Wiebke G, Hibbs J B. Nitric oxide synthesis in patients with advanced HIV infection. Clin Exp Immunol. 1994;97:83–86. doi: 10.1111/j.1365-2249.1994.tb06583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Figdor C G, Leemans J M M, Bont W S, Vries J E. Theory and practice of centrifugal elutriation (CE). Factors influencing the separation of human blood cells. Cell Biophys. 1983;5:105–118. doi: 10.1007/BF02796137. [DOI] [PubMed] [Google Scholar]
- 32.Forstermann U, Gorsky L D, Pollock J S, Ishii K, Schmidt H H H W, Heller M, Murad F. Hormone-induced biosynthesis of endothelium-derived relaxing factor/nitric-like material in NIE-115 neuroblastoma cells requires calcium and calmodulin. Mol Pharmacol. 1990;38:7–15. [PubMed] [Google Scholar]
- 33.Fu Y, Blankehorn E P. Nitric oxide-induced antimitogenic effects in high and low responder rat strains. J Immunol. 1992;148:2217–2223. [PubMed] [Google Scholar]
- 34.Fuchs D, Hausen A, Reibnegger G, Werner E R, Dierich P, Watcher H. Neopterin as a marker for activated cell-mediated-immunity: application in HIV infection. Immunol Today. 1988;369:150–155. doi: 10.1016/0167-5699(88)91203-0. [DOI] [PubMed] [Google Scholar]
- 35.Gartner S, Markovits P, Markovitz D M, Kaplan M H, Gallo R C, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 36.Gartner S, Markovitz D M, Markovitz R F, Betts P, Popovic M. Virus isolation from and identification of HTLV-III/LAV-producing cells in brain tissue from a patient with AIDS. JAMA. 1988;256:2365–2371. [PubMed] [Google Scholar]
- 37.Gartwright J E, Whitley G, Johnstone A P. Endothelial cell adhesion molecule expression and lymphocyte adhesion to endothelial cells: effect of nitric oxide. Exp Cell Res. 1997;235:431–434. doi: 10.1006/excr.1997.3723. [DOI] [PubMed] [Google Scholar]
- 38.Gazzinelli R T, Oswald I P, Hieny S, James S L, Sher A. The microbicidal activity of interferon-gamma-treated macrophages against Trypanosoma cruzi involves an l-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-beta. Eur J Immunol. 1992;22:2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- 39.Gendelman H E, Orenstein J M, Baca L M, Weiser B, Burger H, Kalter D C, Meltzer M S. The macrophage in the persistence and pathogenous of HIV infection. AIDS. 1989;3:475–495. doi: 10.1097/00002030-198908000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Goretski J, Hollocher T C. Trapping of nitric oxide produced during dentrification by extracellular hemoglobin. J Biol Chem. 1988;263:2316–2323. [PubMed] [Google Scholar]
- 41.Granger D L, Hibbs J B J, Perfect J R, Durack D I. Specific amino acid (l-arginine) requirement for the microbiostatic activity of murine macrophages. J Clin Investig. 1991;81:1129–1136. doi: 10.1172/JCI113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graziosi C, Gantt K R, Vaccarezza M, Demarest J F, Daucher M, Saag M S, Shaw G M, Quinn T C, Cohen O J, Welbon C C, Pantaleo G, Fauci A S. Kinetics of cytokine expression during primary human immunodeficiency virus type 1 infection. Proc Natl Acad Sci USA. 1996;93:4386–4391. doi: 10.1073/pnas.93.9.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green S J, Crawford R M, Hockmeyer J T, Meltzer M S, Nacy C A. Leishmania major amastigotes initiate the l-arginine-dependent killing mechanism in IFN-γ stimulated macrophages by induction of tumor necrosis factor-α. J Immunol. 1990;145:4290–4297. [PubMed] [Google Scholar]
- 44.Grimaldi L M E, Martino G V, Franciotta D M, Brustia R, Castagna A, Pristera R, Lazzarin A. Elevated alpha-tumor necrosis factor levels in spinal fluid from HIV-1-infected patients with central nervous system involvement. Ann Neurol. 1991;29:21–25. doi: 10.1002/ana.410290106. [DOI] [PubMed] [Google Scholar]
- 45.Griscavage J M, Rogers N E, Sherman M P, Ignarro L J. Inducible nitric oxide synthase from a rat alveolar macrophage cell line inhibited by nitric oxide. J Immunol. 1993;151:6329–6337. [PubMed] [Google Scholar]
- 46.Groeneveld P H, Kroon F P, Nibbering P H, Bruisten S M, van Swieten P, van Furth R. Increased production of nitric oxide correlates with viral load and activation of mononuclear phagocytes in HIV-infected patients. Scand J Infect Dis. 1996;28:341–345. doi: 10.3109/00365549609037916. [DOI] [PubMed] [Google Scholar]
- 47.Harada S, Koyanagi Y, Yamamoto N. Infection of HTLV-IIIB/LAV in HTLV-I-carrying cells MT2 and MT4 and application in a plate assay. Science. 1985;229:563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- 48.Haussmann N, Werringloer J. Nitric oxide and nitrite formation during degradation of N-nitrosoamines. Naunyn Schmiedbergs Arch Pharmakol. 1985;329:R21. [Google Scholar]
- 49.Ignarro L J, Burns R E, Buga G M, Wood K S. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987;61:866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- 50.Isobe K, Nakashima I. Feedback suppression of staphylococcal enterotoxin-stimulated T-lymphocyte proliferation by macrophages through inductive nitric oxide synthesis. Infect Immun. 1992;60:4832–4837. doi: 10.1128/iai.60.11.4832-4837.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.James S L, Glaven J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J Immunol. 1989;143:4208–4212. [PubMed] [Google Scholar]
- 52.Karupiah G, Xie Q, Buller R M L, Nathan C, Duarte C, MacMicking J D. Inhibition of viral replication by interferon-gamma induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 53.Knowles R G, Palacios M, Palmer R M J, Moncada S. Formation of nitric oxide from l-arginine in the central nervous system: a transduction mechanism for stimulation of guanylate cyclase. Proc Natl Acad Sci USA. 1989;86:5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kobzick L, Bredt D S, Lowenstein C J, Drazen J, Gaston B, Sugarbaker D, Stamler J S. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol. 1993;9:371–377. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- 55.Koening S, Gendelman H E, Orenstein J M, Dal-Canto M C, Pezeshkpour G H, Yungbluth M, Janotta F, Aksamit A, Martin M A, Fauci A S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 56.Kolb J P, Paul-Eugene N, Damais C, Yamaoka K, Drapier J C, Dugas B. Interleukin-4 stimulates cGMP production by IFN-γ-activated human monocytes. J Biol Chem. 1994;269:9811–9816. [PubMed] [Google Scholar]
- 57.Lancaster J R. Stimulation of the diffusion and reaction of endogenously produced nitric oxide. Proc Natl Acad Sci USA. 1994;91:8137–8141. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lander H M, Hajjar D P, Hempstead B L, Mirza U A, Chait B T, Campbell S, Quilliam L A. A molecular redox switch on p21(ras)-structural basis for the nitric oxide-p21(ras) interaction. J Biol Chem. 1997;272:4323–4326. doi: 10.1074/jbc.272.7.4323. [DOI] [PubMed] [Google Scholar]
- 59.Lander H M, Ogiste J S, Pearce S F A, Levi R, Novogrodsky A. Nitric oxide-stimulated guanine nucleotide exchange on p21ras. J Biol Chem. 1995;270:7017–7020. doi: 10.1074/jbc.270.13.7017. [DOI] [PubMed] [Google Scholar]
- 60.Lander H M, Sehajpal P, Levine D M, Novogrodsky A. Activation of human peripheral blood mononuclear cells by nitric oxide-generating compounds. J Immunol. 1993;150:1509–1516. [PubMed] [Google Scholar]
- 61.Lander H M, Sehajpal P K, Novogrosky A. Nitric oxide signaling: a possible role for G proteins. J Immunol. 1993;151:7182–7187. [PubMed] [Google Scholar]
- 62.Lane T, Buchmeier M J, Wtry D D, Fox S. Expression of inflammatory cytokines and inducible nitric oxide synthase in brains of SIV-infected rhesus monkeys: applications to HIV-induced central nervous system disease. Mol Med. 1996;2:27–37. [PMC free article] [PubMed] [Google Scholar]
- 63.Le Naour R, Clayette P, Henin Y, Mabondzo A, Raoul H, Bousseau A, Dormont D. Infection of human macrophages with endogenous tumor necrosis factor-α (TNF-α)-independent human immunodeficiency virus type 1 isolate is unresponsive to the TNF-α synthesis inhibitor RP55778. J Gen Virol. 1994;75:1379–1388. doi: 10.1099/0022-1317-75-6-1379. [DOI] [PubMed] [Google Scholar]
- 64.Le Naour R, Raoul H, Mabondzo A, Henin Y, Bousseau A, Dormont D. Treatment of human monocyte-derived macrophages with a TNF-α synthesis inhibitor prior to HIV-1 infection: consequences on cytokine production and viral replication. Res Virol. 1994;145:199–207. doi: 10.1016/s0923-2516(07)80023-9. [DOI] [PubMed] [Google Scholar]
- 65.Lorsbach R B, Murphy W J, Lowenstein C J, Snyder S H, Russel S W. Expression of the nitric oxide synthase gene in mouse macrophages activated for tumor cell killing. J Biol Chem. 1993;268:1908–1913. [PubMed] [Google Scholar]
- 66.Mabondzo A, Boussin F, Raoul H, Le Naour R, Gras G, Vaslin B, Bartholeyns J, Romet-Lemonne J-L, Dormont D. Antibody-dependent cellular cytotoxicity and neutralization of human immunodeficiency virus type 1 by high affinity cross-linking of gp41 to human macrophage Fc IgG receptor using bispecific antibody. J Gen Virol. 1994;75:1451–1456. doi: 10.1099/0022-1317-75-6-1451. [DOI] [PubMed] [Google Scholar]
- 67.Mannick J B. The antiviral role of nitric oxide. Res Immunol. 1995;146:693–697. doi: 10.1016/0923-2494(96)84920-0. [DOI] [PubMed] [Google Scholar]
- 68.Matthews J R, Botting C H, Panico M, Morris H R, Hay R T. Inhibition of NF-κB DNA binding by nitric oxide. Nucleic Acids Res. 1996;14:2236–2242. doi: 10.1093/nar/24.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Melkova Z, Esteban M. Inhibition of vaccinia virus DNA replication by inducible expression of nitric oxide synthase. J Immunol. 1995;155:5711–5718. [PubMed] [Google Scholar]
- 70.Merryman P F, Clancy R M, He X Y, Abramson S B. Modulation of human T cell responses by nitric oxide and its derivative, S-nitrosoglutathione. Arthritis Rheum. 1993;36:1414–1422. doi: 10.1002/art.1780361014. [DOI] [PubMed] [Google Scholar]
- 71.Mills C D. Molecular basis of suppressor macrophages: arginine metabolism via the nitric oxide synthetase pathway. J Immunol. 1991;146:2719–2723. [PubMed] [Google Scholar]
- 72.Moncada S, Palmer R M, Higgs E A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 73.Mordvintcev P, Mulsch A, Busse R, Vanin A. On-line detection of nitric oxide formation in liquid aqueous phase by electron paramagnetic resonance spectroscopy. Anal Biochem. 1991;199:142–146. doi: 10.1016/0003-2697(91)90282-x. [DOI] [PubMed] [Google Scholar]
- 74.Murray H W. Survival of intracellular pathogens within human mononuclear phagocytes. Semin Hematol. 1988;25:101–111. [PubMed] [Google Scholar]
- 75.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 76.Nathan C. Nitric oxide a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 77.Nussler A, DiSilvio M, Billiar T, Hoffman R, Geller D, Selby R, Madariaga J, Simmons R L. Stimulation of the nitric oxide synthase pathway in human hepatocytes by cytokines and endotoxin. J Exp Med. 1992;176:261–264. doi: 10.1084/jem.176.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nussler A K, Geller D A, Sweetland M A, DiSilvio M, Billiar T R, Madriaga J B, Simmons R L, Lancaster J R. Induction of nitric oxide synthesis and its reactions in cultured human hepatocytes and stimulated with cytokines plus LPS. Biochem Biophys Res Commun. 1993;194:826–835. doi: 10.1006/bbrc.1993.1896. [DOI] [PubMed] [Google Scholar]
- 79.Padgett E L, Pruett S B. Evaluation of nitrite production by human monocyte-derived macrophages. Biochem Biophys Res Commun. 1992;186:775–781. doi: 10.1016/0006-291x(92)90813-z. [DOI] [PubMed] [Google Scholar]
- 80.Peng H B, Libby P, Liao J K. Induction and stabilization of IκBα by nitric oxide mediates inhibition of NF-κB. J Biol Chem. 1995;270:14214–14219. doi: 10.1074/jbc.270.23.14214. [DOI] [PubMed] [Google Scholar]
- 81.Peng H B, Rajavaschisth T B, Libby P, Liao J K. Nitric oxide inhibits macrophage-colony stimulating factor gene transcription in vascular endothelial cells. J Biol Chem. 1995;270:17050–17055. doi: 10.1074/jbc.270.28.17050. [DOI] [PubMed] [Google Scholar]
- 82.Persoons J H A, Schornagel K, Tilders F F H, de Vente J, Berkenbosch F, Kraal G. Alveolar macrophages autoregulate IL-1 and IL-6 production by endogenous nitric oxide. Am J Respir Cell Mol Biol. 1996;14:272–278. doi: 10.1165/ajrcmb.14.3.8845178. [DOI] [PubMed] [Google Scholar]
- 83.Pietraforte D, Tritarelli E, Testa U, Minetti M. Gp 120 HIV envelope glycoprotein increases the production of nitric oxide in human monocyte-derived macrophages. J Leukoc Biol. 1994;55:175–182. doi: 10.1002/jlb.55.2.175. [DOI] [PubMed] [Google Scholar]
- 84.Raoul H, Le Naour R, Blond D, Dormont D. HIV-1 infection of human macrophages induces an up-regulation of manganese superoxide dismutase gene that may protect cells from death. AIDS Res Hum Retroviruses. 1998;14:427–434. doi: 10.1089/aid.1998.14.427. [DOI] [PubMed] [Google Scholar]
- 85.Reiling N, Ulmer A J, Duchrow M, Ernst M, Flad H D, Hauschildt S. Nitric oxide synthase: mRNA expression of different isoforms in human monocytes/macrophages. Eur J Immunol. 1994;24:1941–1944. doi: 10.1002/eji.1830240836. [DOI] [PubMed] [Google Scholar]
- 86.Rey M, Dormont D, Barré-Sinoussi F, Montagnier L, Chermann J C. Characterization of the RNA-dependent DNA polymerase of a new human lymphotropic retrovirus (lymphadenopathy-associated virus) Biochem Biophys Res Commun. 1984;121:126–133. doi: 10.1016/0006-291x(84)90696-x. [DOI] [PubMed] [Google Scholar]
- 87.Sheffler L A, Wink D A, Melillo G, Cox G W. Exogenous nitric oxide regulate IFN-γ plus lipopolysaccharide-induced nitric oxide synthase expression in mouse macrophages. J Immunol. 1995;155:886–894. [PubMed] [Google Scholar]
- 88.Shoker A S, Yang H, Muramit M A, Jamil H, Al-Ghoul A, Okasha K. Analysis of the in vitro of exogenous nitric oxide on human lymphocytes. Mol Cell Biochem. 1997;171:75–83. doi: 10.1023/a:1006815430622. [DOI] [PubMed] [Google Scholar]
- 89.Sinicco A, Biglino A, Sciandra M, Forno B, Pollono A M, Raiteri R, Gioannini P. Cytokine network and acute primary HIV-1 infection. AIDS. 1993;7:1167–1172. doi: 10.1097/00002030-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 90.Stamler J S, Singel D J, Loscalzo J. Biochemestry of nitric oxide and its redox-activated forms. Science. 1992;258:1896–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 91.Stuehr D J, Nathan C F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989;169:1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Torre D, Ferrario G, Bonetta G, Speranza F, Zeroli C. Production of nitric oxide from peripheral blood mononuclear cells and polymorphonuclear leukocytes of patients with HIV-1 infection. AIDS. 1995;1995:979–980. doi: 10.1097/00002030-199508000-00027. [DOI] [PubMed] [Google Scholar]
- 93.Villinger F, Hunt D, Mayne A, Vuchetich M, Findley H, Ansari A A. Qualitative and quantitative studies of cytokines synthesized and secreted by non-human primate peripheral blood mononuclear cells. Cytokine. 1993;5:469–479. doi: 10.1016/1043-4666(93)90038-7. [DOI] [PubMed] [Google Scholar]
- 94.Wright S C, Jewett C U, Mitsuyasu R, Bonavida B. Spontaneous cytotoxicity and tumor necrosis factor production by peripheral blood monocytes from AIDS patients. J Immunol. 1988;141:99. [PubMed] [Google Scholar]
- 95.Xie Q W, Cho H J, Calaycay J, Mumford R A, Swiderek K M, Lee T D, Ding A, Troso T, Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- 96.Zangerle R, Fuchs D, Reibnegger G, Werner-Felmayer G, Gallati H, Wachter H, Werner E R. Serum nitrite plus nitrate in infection with human immunodeficiency virus type-1. Immunobiology. 1995;193:59–70. doi: 10.1016/S0171-2985(11)80155-5. [DOI] [PubMed] [Google Scholar]
- 97.Zinetti M, Fantuzzi G, Delgado R, Di Santo E, Chezzi P, Fratelli M. Endogenous nitric oxide production by monocytic cells regulates LPS-induced TNF production. Eur Cytokine Netw. 1995;6:45–48. [PubMed] [Google Scholar]