Abstract
A systematic review was conducted to investigate the relationship between aminotransferases and the severity of dengue infection, which is a prevalent and significant infection in tropical and subtropical regions. Aminotransferases are enzymes that are often elevated in dengue due to the liver’s physiological and immunological response to the infection. This review focused on analyzing various studies that examined the correlation between aminotransferase levels and the severity of dengue. Extensive literature searches were performed using (“dengue*” OR “dengue fever*” OR “dengue haemorrhagic fever*” OR “dengue shock syndrome*”) AND (“alanine aminotransferase*” OR “aspartate aminotransferase*”) on PubMed. The selected articles were thoroughly reviewed, encompassing epidemiology, pathogenesis, and clinical manifestations of dengue. The consistent findings across the studies indicated that aminotransferases can serve as predictive markers for dengue severity. Therefore, early assessment of liver enzyme levels is crucial in dengue cases, and elevated levels should be closely monitored to prevent adverse outcomes.
Keywords: dengue severity, dengue hemorrhagic fever (dhf), dengue fever/complications, aminotransferases, dengue
Introduction and background
Caused by four different dengue virus serotypes which are the members of the flavivirus family, dengue hemorrhagic fever is spread by the Aedes mosquito. Globally, and particularly in Southeast Asian nations, dengue fever is a severe public health hazard. Because of the wide range of organ involvement caused by dengue outbreaks, they are far more likely to cause morbidity and fatality [1]. Nearly 3,500 million people living in the tropics and subtropics are susceptible to this infection, with cases sometimes reaching up to 100 million reported yearly [2]. Most dengue infections are asymptomatic. The spectrum of symptomatic dengue fever includes fever without warning signs, fever with warning signs, and severe dengue. Traditional dengue fever develops in three stages, namely, the febrile phase, followed by the critical phase, and then the recovery phase. Although dengue is a self-limiting viral disease, a proportionate number of patients develop life-threatening complications as a result of this disease [3]. In dengue fever, hepatic impairment is frequently observed [4]. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) blood levels rise as a result of reactive hepatitis or the virus’s direct hepatocyte injury. Hepatic dysfunction shown as a rise in serum aminotransferase levels is associated with increased bleeding episodes, lower platelet count, shock, respiratory distress, and renal failure [4,5]. Therefore, the rise of serum aminotransferase levels is crucial in determining the severity of dengue fever. This study aims to determine the severity of dengue illness in relation to aminotransferases.
Review
Methodology and results
Search Strategy
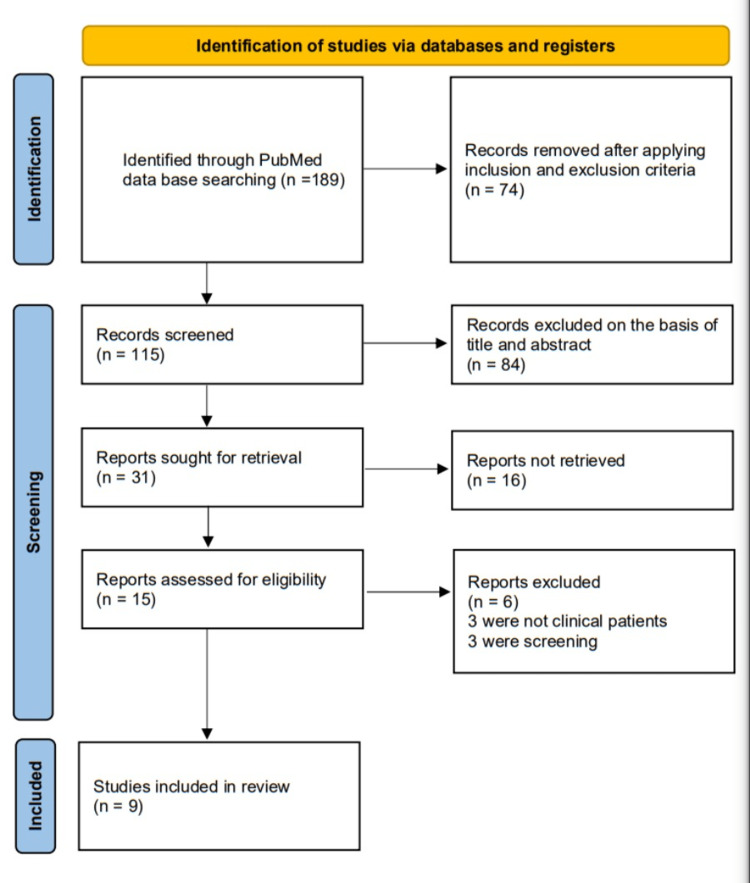
The search strategy and selection of studies for this systematic review are described in Figure 1 using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 flow diagram [6]. We conducted an extensive search through PubMed using the following: (“dengue*” OR “dengue fever*” OR “dengue haemorrhagic fever*” OR “dengue shock syndrome*”) AND (“alanine aminotransferase*” OR “aspartate aminotransferase*”).
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 flow diagram.
Inclusion and Exclusion Criteria
The inclusion criteria for this review consist of studies published after the year 2000, specifically focusing on original research conducted on adult symptomatic human subjects. The studies should be written in English to ensure accessibility and comprehension. On the other hand, the exclusion criteria involve studies published on or before the year 2000 and studies conducted in languages other than English and on asymptomatic individuals. Additionally, letters to editors, literature reviews, and systematic and meta-analyses were excluded. Furthermore, studies involving animal subjects and those focused on children were also excluded.
Data Extraction and Analysis
The identified papers underwent a selection process based on predetermined inclusion and exclusion criteria. Titles and abstracts of potentially relevant articles were retrieved for further evaluation. After reading the full texts of the retrieved articles, those that met the inclusion criteria were chosen. To assess the risk of bias and the applicability of the selected studies, the Prediction Model Risk of Bias Assessment Tool (PROBAST) was employed [7]. The included publications were evaluated with regard to participant age, outcome measures, categorization of dengue severity, observation period, method of model construction, and variables considered. We gathered information on the study’s location, type, and year of publication, as well as the number of participants (Table 1). After a thorough analysis of the study, a column was set aside for comments on the study.
Table 1. Study findings.
DF: dengue fever; DHF: dengue hemorrhagic fever; DSS: dengue shock syndrome; AST: aspartate aminotransferase; ALT: alanine aminotransferase
| Authors | Country | Study | Year of publication | Sample size | Comment |
| Trung et al. [8] | Singapore | Prospective observational study | 2010 | 644 | AST and ALT levels were considerably more significant in individuals who had shock than those who did not during the crucial time, according to the link between transaminase levels and indicators of illness severity (P < 0.01 by the Mann-Whitney test) |
| Souza et al. [9] | Brazil | Retrospective observational study | 2004 | 1585 | These patients frequently had reactive hepatitis and liver damage as a result of the dengue virus infection and showed high AST and ALT |
| Parkash et al. [10] | Pakistan | Cohort comparative study | 2010 | 699 patients categorized into DF/DHF/DSS | According to the study findings, severe hepatitis is a good predictor of dengue illness severity and is a bad prognostic indicator of outcome |
| Lee et al. [11] | Singapore | Retrospective observational study | 2012 | 690 patients categorized into DF/DHF | Despite the fact that aminotransferase levels increased along with the severity of the dengue, increased aminotransferase values failed to distinguish DF from DHF or mild and severe dengue |
| Saha et al. [12] | India | Retrospective study | 2013 | 1,226 patients categorized into DF/DHF | The research has shown that there are several cases of hepatic dysfunctions that resemble acute viral hepatitis |
| Rao et al. [13] | India | Prospective study | 2020 | 106 | The study demonstrated that serum AST and ALT levels and ferritin could serve as better biomarkers for determining the severity of the disease, and repeated estimation of these indicators should be taken into consideration |
| Md Sani et al. [14] | Malaysia | Retrospective cohort study | 2017 | 365 patients | The composite index, AST2/ALT can be employed as a marker for recognizing severe dengue, with two options for cut-off values: 402 and 653 |
| Huy al. [15] | Vietnam | Multicentric cross-sectional study | 2022 | 326 patients categorized as DF/DHF | Platelet count or serum albumin can be used as prognostic indications during the first three days of sickness. Prognostic indications should be AST, ALT, albumin, or total bilirubin from the fourth to the sixth day of clinical symptoms |
| Srisuphanunt et al. [16] | Thailand | Retrospective study | 2022 | 302 | The study suggested that patients with dengue infection could have their disease severity predicted using aminotransferases in combination with other parameters |
Discussion
Epidemiology and History
Dengue virus, an arbovirus, belongs to the Flaviviridae family and flavivirus genus. Four different dengue virus serotypes (DENV 1-4) have been identified and are transmitted by the Aedes mosquito [17,18]. It is the most prevalent arboviral disease globally, with tropical and subtropical areas bearing the brunt of its impact [19]. During the Caribbean exanthema epidemic accompanying arthralgia in 1827-1828, the name Dengue was first used in English-language medical literature, the Spanish equivalent of the Swahili term Ki denga Pepo, which means an unexpected cramp-like spasm brought on by an evil spirit. Since 1780, Dengue has been referred to as the Break bone fever.
Around 33% of the earth’s population is at risk of dengue infection [19]. Tropics and subtropics are at an increased risk compared to other regions. In some nations, the prevalence of infection follows a predictable pattern, low temperatures reduce the survival of adult mosquitoes, thus impacting transmission rates. Rain and temperature change the mosquito’s reproduction and feeding rhythm, which impacts the density of the vector population [20]. Dengue fever, which is spread by mosquitoes, was the second most important tropical infectious disease in 1998, after malaria, with an estimated 100 million illnesses, 500,000 cases of dengue hemorrhagic fever, and 25,000 fatalities annually. In the past 20 years, dengue has expanded to new geographical locations. Reasons for this expansion to new geographic areas are mainly attributed to changes in public health infrastructure and demographics over the past 30 years [21].
Pathophysiology
The four distinct dengue virus serotypes comprise a phylogenetic group and vary from one another in terms of nucleotide sequence [22]. The dengue virus genome consists of seven non-structural (NS) proteins, including NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5, as well as three structural protein genes that encode nucleocapsid of the core protein (C), a membrane-associated protein (M), and an envelope protein (E). The host immune system and the NS1 protein interact, which causes a T-cell response. Individuals with this infection have detectable amounts of the NS1 protein in their serum, which is a diagnostic indicator [23]. Although the precise pathogenesis of dengue fever is unclear, the clinical events can be distinguished by early infection, propagation of the virus, immunological response, and subsequent viral clearance [24]. Additionally, essential to the pathophysiology of dengue fever is the host immunological response. Most of the explanations for the signs and symptoms explained above center around one of the following three concepts: antibody-dependent enhancement (ADE), vasculopathy, cytokine storm, and coagulopathy [25].
Dendritic cells initially engulf the dengue virus, and after digesting it, they present the T cells with an antigen. The host immune system explicitly targets the E protein, precursor membrane protein (Pre-M), and NS1 as the three main proteins of the dengue virus. E protein-specific antibodies prevent binding to cell receptors and neutralize the infection. NS1-specific antibodies can drive complement-mediated lysis of infected cells but are ineffective in neutralizing the infection. Pre-M-specific antibodies can promote ADE but only exhibit partial binding to matured virions and have insufficient neutralization [26,27].
Neutralizing and non-neutralizing antibodies are created due to the invasion of the dengue virus. The neutralizing antibodies can offer a defense against a particular viral serotype. Non-neutralizing antibodies bind to an infecting virus; however, they cannot neutralize it. Instead, they facilitate the pathogen’s entry into the cell [28]. Within the cell, viral replication begins and raises blood virus titers. ADE of infection is the term used to describe this phenomenon. Dengue-specific CD4+ and CD8+ T cells release interferon-gamma (IFN-gamma), tumor necrosis factor-alpha (TNF-alpha), and lymphotoxin, which cause a cytokine storm and, eventually, further deterioration of the illness. Immunoglobulin receptor expression is also increased by IFN-gamma, which increases the antibody-dependent intensification of the infection [28].
Vasculopathy and coagulopathy are the two pathways of pathogenesis that support the clinical manifestation of dengue. Most symptomatic patients recover after a brief illness; a small percentage of patients have more severe sickness, which often presents as a vasculopathy with hemorrhagic diathesis and plasma leakage. The infection causes a small modification in the fiber matrix properties of the endothelium by temporarily disrupting the function of the endothelial glycocalyx layer. As autoantibodies, anti-NS1 antibodies interact with platelets and uninfected endothelial cells to cause intracellular signaling that alters capillary permeability. Plasma leakage leading to ascites, hemoconcentration, and effusions in pleural and pericardial spaces are brought on by a diffuse increase in capillary permeability. The severity of vasculopathy is correlated with the degree of organ involvement and shock. Typically, it shows up between the third and seventh day of the disease [25,26]. Raised activated partial thromboplastin time and low fibrinogen levels are generally regular observations. Thrombocytopenia as an outcome of coagulation defect is associated with worse features. Coagulation disorder is also exacerbated by releasing heparan sulfate or chondroitin sulfate from the glycocalyx, two molecules with structural similarities to heparin that can perform its anticoagulant action [27].
The immune enhancement and virus virulence hypothesis has been proposed to explain why severe disease occurs. Dengue shock syndrome is more frequent in people exposed to secondary dengue infection caused by a different dengue virus serotype. ADE therapy can explain this phenomenon. Complexes of prior non-neutralizing antibodies develop inside the viral particles. During secondary dengue virus infections, they boost their absorption and replication in the body’s macrophage system. T cells, mast cells, and monocytes create more cytokines when infected with dengue. INF-gamma, TNF-alpha, interleukin (IL)-2, and IL-6 are at their peak levels during the first three days of sickness, while an increase in IL-4, IL-5, and IL-10 is seen after three days [29].
Clinical Manifestations
Dengue with or without warning signs and severe dengue are included in the 2009 WHO classification changes. Dengue without warning signs includes fever, two skin rashes, bodily aches, nausea, vomiting, or retro-orbital pain. Dengue fever with warning signs includes fever with any two of the skin rash, bodily pains, nausea, or vomiting, and any one of the warning signs, which include abdominal tenderness, frequent vomiting, signs of fluid retention, such as effusions and ascites, mucosal bleeding, restlessness, lethargy, rise in hematocrit (20%) with a sharp decline in thrombocyte count (50,000/mm3), and liver enlargement >2 cm. The following three features characterize severe dengue: (1) Severe plasma leakage that results in oliguria, shock, or a delay in capillary filling and/or fluid build-up in the serosa leading to breathing difficulties. (2) Extreme bleeding symptoms. (3) Significant organ involvement: more than 1,000 units of AST or ALT, hepatomegaly and liver failure, consciousness impairment, and myocardial malfunction [30,31].
The symptoms of dengue fever, an acute febrile sickness, include muscle, bone, and joint pains; headaches; leukopenia; and rash. Severe fever, hemorrhage, frequently accompanied by hepatomegaly, and, in severe cases, circulatory failure are the main clinical symptoms of dengue hemorrhagic fever. Dengue hemorrhagic fever can occasionally lead to the potentially fatal dengue shock syndrome, which has features similar to anaphylaxis [32,33].
Dengue fever follows a course of illness consisting of three phases, namely, the febrile phase, the critical phase, and the recovery phase. During the febrile phase, which lasts around three to five days, the symptoms resemble those of other febrile diseases, such as body aches, headaches, joint pain, and loss of appetite. A notable indicator during this phase is a gradual reduction in the white blood cell count, which raises concerns about dengue. The critical phase is characterized by an abnormal increase in capillary permeability, leading to plasma leakage and a rise in hematocrit levels. This phase lasts from 24 to 48 hours and is accompanied by progressive leukopenia and a rapid decline in platelet counts. Warning signs often precede shock, and impairment of the organ systems, metabolic acidosis, and disseminated intravascular coagulation can be seen. The recovery phase begins after the critical phase, during which extravascular fluid is gradually absorbed. The patient’s general condition and hemodynamic status improve, and white blood cell counts return to normal once platelet counts have normalized. Avoiding excessive fluid management during this phase is essential to prevent complications such as pulmonary edema or congestive cardiac failure [34,35].
Prevention and Control
No specific medication or vaccination is available today to protect against the dengue virus. Therefore, control of the vector is a critical factor in controlling infection, including environmental, biological, and chemical control, apart from personal protective measures. Subjective measures include protective clothing, insect repellents, mosquito nets, and curtains treated with pesticides. Environmental measures include adequate provision of drinking water, proper roofing of overhead tanks, and an underground drainage system. Gambia and Peorilia reticulate, long-lived fish usage come under biological control. Chemical control uses malathion-based space sprayers and 1% temephos granules. Additionally, insect growth regulators can be used [36].
Lately, dengue vaccinations have gained much focus, with research in this area yielding promising results. However, the difficulty of performing long-term trials to evaluate vaccination efficacy and safety to eliminate the danger of vaccine-induced dengue hemorrhagic fever/dengue shock syndrome, particularly in children, has complicated efforts to create safe and effective vaccines to prevent dengue infections. At least seven vaccines have been tested in various stages of clinical trials, but just three (Dengvaxia, TV003, and TAK-003) have shown promising outcomes [37].
Dengue Fever and Aminotransferases
Hepatic injury is common in dengue infection, ranging from mild elevations in hepatic transaminases to severe acute liver failure (ALF) with fatal outcomes. While viral hepatitis and drugs are the leading causes of ALF, infectious diseases such as dengue are also recognized as etiological factors [38]. Various manifestations characterize liver injury in dengue. Hepatocytes and Kupffer cells are the primary targets of the dengue virus. The initial step in the viral infection process involves viral attachment to receptors on the host cell surface, with the E protein playing a crucial role in this attachment. It has been observed that the binding of dengue viruses to hepatocytes facilitates the subsequent binding of viral particles. Following viral attachment, the internalization of the virus occurs through direct fusion or endocytosis [38].
The pathogenesis of hepatic injury in dengue is primarily believed to be T-cell-mediated. The interaction between antibodies, the endothelium, and the concurrent cytokine storm is crucial. CD4+ cytotoxic T cells have been identified as responsible for liver dysfunction in dengue fever, involving mechanisms such as bystander lysis [39]. The pathogenesis of liver injury in dengue involves the virus’s direct effect on hepatocytes and immune-mediated hepatitis. During acute dengue attack, a varying range of liver involvement can be observed, such as apoptosis of liver cells induced by the dengue virus, hypoxic damage from altered perfusion, oxidative stress, or immunological injury. Elevated serum aminotransferase levels are commonly seen in hepatic involvement in dengue fever. Such patients are at a higher risk of bleeding. Acute respiratory distress syndrome, cholecystitis, shock, renal failure, and hepatic dysfunction play a significant role in bleeding [39]. Gagnon et al. [40] demonstrated that CD4+ T cells contribute to the destruction of hepatocytes in dengue fever. This is mediated by two pathways, namely, perforin and granzymes released by activated CD4+ T cells, and the interaction between Fas and Fas ligands on target and T cells, respectively [41,42].
The findings from various studies indicate that elevated liver enzymes, specifically AST and ALT, are commonly observed in patients with dengue fever. These studies have demonstrated significant liver dysfunction in over 50% of individuals diagnosed with dengue fever [43,44]. In addition, liver dysfunction rates are high in Asian populations ranging from 30% to 90% [45]. These studies also observed that increased AST and ALT were associated with severe bleeding manifestations. Additionally, it should be noted that vomiting from the first day of illness could indicate possible hepatic dysfunction. Early-onset vomiting may be a clinical sign that warrants attention to the liver function of dengue fever patients [8]. The mean values of AST and ALT have been observed to be higher in severe dengue than in dengue fever without warning signs, suggesting a correlation between increased transaminase levels and disease severity. AST is derived from various sources, including the liver, heart, striated muscle, and erythrocytes, whereas ALT is primarily hepatic. Non-hepatic tissue damage caused by the dengue virus can contribute to higher AST levels than ALT, indicating that AST elevations may not solely reflect liver involvement [46,47].
A negative correlation between liver enzyme levels and platelet count indicated a potential relationship between liver dysfunction and thrombocytopenia. In addition, several studies significantly associated bleeding tendencies with elevated liver enzymes [11,48]. Hepatomegaly was found in some patients, and elevated AST levels were detected in all cases of hepatomegaly. However, there was no significant association between elevated liver enzymes and free fluid in the abdomen. Ultrasound findings in dengue fever often include liver enlargement, thickening of the gallbladder, and third-space fluid build-up [49]. Moreover, it should be noted that there were no significant gender or age differences in terms of transaminase elevation levels in the studies [10,50]. In addition, jaundice, a known hepatic alteration in dengue infection, was reported in only a minority of patients [51].
Conclusions
Hepatic dysfunction in dengue fever can result from direct viral damage to the liver cells or reactive hepatitis. The elevation of liver enzymes typically occurs between the third and fifth day of fever onset. Patients with elevated liver enzymes during the febrile phase are at a higher risk of developing complications and require careful management during the critical phase. The study’s findings indicate a strong association between increased levels of liver enzymes, specifically AST and ALT, and the severity of dengue fever. Furthermore, the research suggests that patients with elevated liver enzymes are more likely to exhibit bleeding tendencies, hepatomegaly (enlarged liver), and the presence of fluid in the abdomen, providing additional support for the conclusion. Therefore, early evaluation of liver enzyme levels is crucial in all cases of dengue fever, and patients with elevated levels should receive close monitoring to prevent unfavorable outcomes.
Acknowledgments
We thank The Almighty for his mercy on us.
The authors have declared that no competing interests exist.
References
- 1.Dengue virus and the host innate immune response. Uno N, Ross TM. Emerg Microbes Infect. 2018;7:167. doi: 10.1038/s41426-018-0168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The global economic burden of dengue: a systematic analysis. Shepard DS, Undurraga EA, Halasa YA, Stanaway JD. Lancet Infect Dis. 2016;16:935–941. doi: 10.1016/S1473-3099(16)00146-8. [DOI] [PubMed] [Google Scholar]
- 3.Management of dengue: an updated review. Tayal A, Kabra SK, Lodha R. Indian J Pediatr. 2023;90:168–177. doi: 10.1007/s12098-022-04394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liver involvement in dengue viral infections. Dissanayake HA, Seneviratne SL. Rev Med Virol. 2018;28 doi: 10.1002/rmv.1971. [DOI] [PubMed] [Google Scholar]
- 5.Evaluation of aminotransferase abnormality in dengue patients: a meta analysis. Wang XJ, Wei HX, Jiang SC, He C, Xu XJ, Peng HJ. Acta Trop. 2016;156:130–136. doi: 10.1016/j.actatropica.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 6.The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Liberati A, Altman DG, Tetzlaff J, et al. J Clin Epidemiol. 2009;62:0–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 7.PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Wolff RF, Moons KG, Riley RD, et al. Ann Intern Med. 2019;170:51–58. doi: 10.7326/M18-1376. [DOI] [PubMed] [Google Scholar]
- 8.Liver involvement associated with dengue infection in adults in Vietnam. Trung DT, Thao le TT, Hien TT, et al. Am J Trop Med Hyg. 2010;83:774–780. doi: 10.4269/ajtmh.2010.10-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aminotransferase changes and acute hepatitis in patients with dengue fever: analysis of 1,585 cases. Souza LJ, Alves JG, Nogueira RM, et al. Braz J Infect Dis. 2004;8:156–163. doi: 10.1590/s1413-86702004000200006. [DOI] [PubMed] [Google Scholar]
- 10.Severity of acute hepatitis and its outcome in patients with dengue fever in a tertiary care hospital Karachi, Pakistan (South Asia) Parkash O, Almas A, Jafri SM, Hamid S, Akhtar J, Alishah H. BMC Gastroenterol. 2010;10:43. doi: 10.1186/1471-230X-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical relevance and discriminatory value of elevated liver aminotransferase levels for dengue severity. Lee LK, Gan VC, Lee VJ, Tan AS, Leo YS, Lye DC. PLoS Negl Trop Dis. 2012;6:0. doi: 10.1371/journal.pntd.0001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spectrum of hepatic dysfunction in 2012 dengue epidemic in Kolkata, West Bengal. Saha AK, Maitra S, Hazra SCh. Indian J Gastroenterol. 2013;32:400–403. doi: 10.1007/s12664-013-0382-6. [DOI] [PubMed] [Google Scholar]
- 13.Correlation of clinical severity and laboratory parameters with various serotypes in dengue virus: a hospital-based study. Rao P, Basavaprabhu A, Shenoy S, Dsouza NV, Sridevi Hanaganahalli B, Kulkarni V. Int J Microbiol. 2020;2020:6658445. doi: 10.1155/2020/6658445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evaluation of creatine kinase and liver enzymes in identification of severe dengue. Md Sani SS, Han WH, Bujang MA, Ding HJ, Ng KL, Amir Shariffuddin MA. BMC Infect Dis. 2017;17:505. doi: 10.1186/s12879-017-2601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prognostic indicators associated with progresses of severe dengue. Huy BV, Toàn NV. PLoS One. 2022;17:0. doi: 10.1371/journal.pone.0262096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prognostic indicators for the early prediction of severe dengue infection: a retrospective study in a University Hospital in Thailand. Srisuphanunt M, Puttaruk P, Kooltheat N, Katzenmeier G, Wilairatana P. Trop Med Infect Dis. 2022;7:162. doi: 10.3390/tropicalmed7080162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dengue virus: epidemiology, biology, and disease aetiology. Roy SK, Bhattacharjee S. Can J Microbiol. 2021;67:687–702. doi: 10.1139/cjm-2020-0572. [DOI] [PubMed] [Google Scholar]
- 18.The origin, emergence and evolutionary genetics of dengue virus. Holmes EC, Twiddy SS. Infect Genet Evol. 2003;3:19–28. doi: 10.1016/s1567-1348(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 19.Dengue: a growing problem with new interventions. Wong JM, Adams LE, Durbin AP, et al. Pediatrics. 2022;149:0. doi: 10.1542/peds.2021-055522. [DOI] [PubMed] [Google Scholar]
- 20.Dengue. Guzman MG, Harris E. Lancet. 2015;385:453–465. doi: 10.1016/S0140-6736(14)60572-9. [DOI] [PubMed] [Google Scholar]
- 21.Dengue and dengue hemorrhagic fever. Gubler DJ. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flavivirus structure and membrane fusion. Heinz FX, Allison SL. Adv Virus Res. 2003;59:63–97. doi: 10.1016/s0065-3527(03)59003-0. [DOI] [PubMed] [Google Scholar]
- 23.Dengue: a continuing global threat. Guzman MG, Halstead SB, Artsob H, et al. Nat Rev Microbiol. 2010;8:0–16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dengue: an update. Guzmán MG, Kourí G. Lancet Infect Dis. 2002;2:33–42. doi: 10.1016/s1473-3099(01)00171-2. [DOI] [PubMed] [Google Scholar]
- 25.Flaviviridae. Westaway EG, Brinton MA, Gaidamovich SYa, et al. Intervirology. 1985;24:183–192. doi: 10.1159/000149642. [DOI] [PubMed] [Google Scholar]
- 26.The mannose receptor mediates dengue virus infection of macrophages. Miller JL, de Wet BJ, Martinez-Pomares L, Radcliffe CM, Dwek RA, Rudd PM, Gordon S. PLoS Pathog. 2008;4:0. doi: 10.1371/journal.ppat.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Chen Y, Maguire T, Hileman RE, Fromm JR, Esko JD, Linhardt RJ, Marks RM. Nat Med. 1997;3:866–871. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 28.Dengue virus pathogenesis: an integrated view. Martina BE, Koraka P, Osterhaus AD. Clin Microbiol Rev. 2009;22:564–581. doi: 10.1128/CMR.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dengue virus infection: a tale of viral exploitations and host responses. Nanaware N, Banerjee A, Mullick Bagchi S, Bagchi P, Mukherjee A. Viruses. 2021;13:1967. doi: 10.3390/v13101967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emergence of co-infection of COVID-19 and dengue: a serious public health threat. Saddique A, Rana MS, Alam MM, et al. J Infect. 2020;81:0–8. doi: 10.1016/j.jinf.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meta-analysis of dengue severity during infection by different dengue virus serotypes in primary and secondary infections. Soo KM, Khalid B, Ching SM, Chee HY. PLoS One. 2016;11:0. doi: 10.1371/journal.pone.0154760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dengue hemorrhagic fever - a systemic literature review of current perspectives on pathogenesis, prevention and control. Wang WH, Urbina AN, Chang MR, Assavalapsakul W, Lu PL, Chen YH, Wang SF. J Microbiol Immunol Infect. 2020;53:963–978. doi: 10.1016/j.jmii.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Dengue and dengue haemorrhagic fever: Indian perspective. Chaturvedi UC, Nagar R. J Biosci. 2008;33:429–441. doi: 10.1007/s12038-008-0062-3. [DOI] [PubMed] [Google Scholar]
- 34.Dengue infection: global importance, immunopathology and management. Kularatne SA, Dalugama C. Clin Med (Lond) 2022;22:9–13. doi: 10.7861/clinmed.2021-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clinical and prognostic relevance of sST2 in adults with dengue-associated cardiac impairment and severe dengue. Teo A, Chia PY, Ramireddi GK, Khoo SK, Yeo TW. PLoS Negl Trop Dis. 2022;16:0. doi: 10.1371/journal.pntd.0010864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prevention and control of dengue-the light at the end of the tunnel. Pang T, Mak TK, Gubler DJ. Lancet Infect Dis. 2017;17:0–87. doi: 10.1016/S1473-3099(16)30471-6. [DOI] [PubMed] [Google Scholar]
- 37.Dengue vaccines: an update. Torres-Flores JM, Reyes-Sandoval A, Salazar MI. BioDrugs. 2022;36:325–336. doi: 10.1007/s40259-022-00531-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pathogenesis of liver involvement during dengue viral infections. Seneviratne SL, Malavige GN, de Silva HJ. Trans R Soc Trop Med Hyg. 2006;100:608–614. doi: 10.1016/j.trstmh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Cytotoxic T lymphocytes in dengue virus infection. Kurane I, Ennis FA. Curr Top Microbiol Immunol. 1994;189:93–108. doi: 10.1007/978-3-642-78530-6_6. [DOI] [PubMed] [Google Scholar]
- 40.Bystander target cell lysis and cytokine production by dengue virus-specific human CD4(+) cytotoxic T-lymphocyte clones. Gagnon SJ, Ennis FA, Rothman AL. J Virol. 1999;73:3623–3629. doi: 10.1128/jvi.73.5.3623-3629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evolution and taxonomy of positive-strand RNA viruses: implications of comparative analysis of amino acid sequences. Koonin EV, Dolja VV. Crit Rev Biochem Mol Biol. 1993;28:375–430. doi: 10.3109/10409239309078440. [DOI] [PubMed] [Google Scholar]
- 42.Lymphocyte activation and hepatic cellular infiltration in immunocompetent mice infected by dengue virus. Chen HC, Lai SY, Sung JM, et al. J Med Virol. 2004;73:419–431. doi: 10.1002/jmv.20108. [DOI] [PubMed] [Google Scholar]
- 43.The impact of dengue on liver function as evaluated by aminotransferase levels. de Souza LJ, Nogueira RM, Soares LC, et al. Braz J Infect Dis. 2007;11:407–410. doi: 10.1590/s1413-86702007000400007. [DOI] [PubMed] [Google Scholar]
- 44.Liver biochemical tests and dengue fever. Kuo CH, Tai DI, Chang-Chien CS, Lan CK, Chiou SS, Liaw YF. Am J Trop Med Hyg. 1992;47:265–270. doi: 10.4269/ajtmh.1992.47.265. [DOI] [PubMed] [Google Scholar]
- 45.Liver histopathology and biological correlates in five cases of fatal dengue fever in Vietnamese children. Huerre MR, Lan NT, Marianneau P, et al. Virchows Arch. 2001;438:107–115. doi: 10.1007/s004280000329. [DOI] [PubMed] [Google Scholar]
- 46.Diagnostic and prognostic value of the AST/ALT ratio in patients with sepsis and septic shock. Schupp T, Weidner K, Rusnak J, et al. Scand J Gastroenterol. 2023;58:392–402. doi: 10.1080/00365521.2022.2131331. [DOI] [PubMed] [Google Scholar]
- 47.Liver function tests: defining what's normal. Roderick P. BMJ. 2004;328:987. doi: 10.1136/bmj.38055.451968.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clinical profile of dengue infection at a teaching hospital in North India. Karoli R, Fatima J, Siddiqi Z, Kazmi KI, Sultania AR. J Infect Dev Ctries. 2012;6:551–554. doi: 10.3855/jidc.2010. [DOI] [PubMed] [Google Scholar]
- 49.Fulminant hepatic failure due to dengue. Sedhain A, Adhikari S, Regmi S, Chaudhari SK, Shah M, Shrestha B. Kathmandu Univ Med J (KUMJ) 2011;9:73–75. doi: 10.3126/kumj.v9i2.6293. [DOI] [PubMed] [Google Scholar]
- 50.Risk predictors of progression to severe disease during the febrile phase of dengue: a systematic review and meta-analysis. Sangkaew S, Ming D, Boonyasiri A, et al. Lancet Infect Dis. 2021;21:1014–1026. doi: 10.1016/S1473-3099(20)30601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The infective causes of hepatitis and jaundice amongst hospitalised patients in Vientiane, Laos. Syhavong B, Rasachack B, Smythe L, et al. Trans R Soc Trop Med Hyg. 2010;104:475–483. doi: 10.1016/j.trstmh.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]