Summary
Neurological complications that occur in SARS-CoV-2 infection, such as olfactory dysfunction, brain inflammation, malaise, and depressive symptoms, are thought to contribute to long COVID. However, in autopsies of patients who have died from COVID-19, there is normally no direct evidence that central nervous system damage is due to proliferation of SARS-CoV-2. For this reason, many aspects of the pathogenesis mechanisms of such symptoms remain unknown. Expressing SARS-CoV-2 S1 protein in the nasal cavity of mice was associated with increased apoptosis of the olfactory system and decreased intracerebral acetylcholine production. The decrease in acetylcholine production was associated with brain inflammation, malaise, depressive clinical signs, and decreased expression of the cytokine degrading factor ZFP36. Administering the cholinesterase inhibitor donepezil to the mice improved brain inflammation, malaise and depressive clinical signs. These findings could contribute to the elucidation of the pathogenesis mechanisms of neurological complications associated with COVID-19 and long COVID.
Subject areas: Neuroscience, Behavioral neuroscience, Virology
Graphical abstract
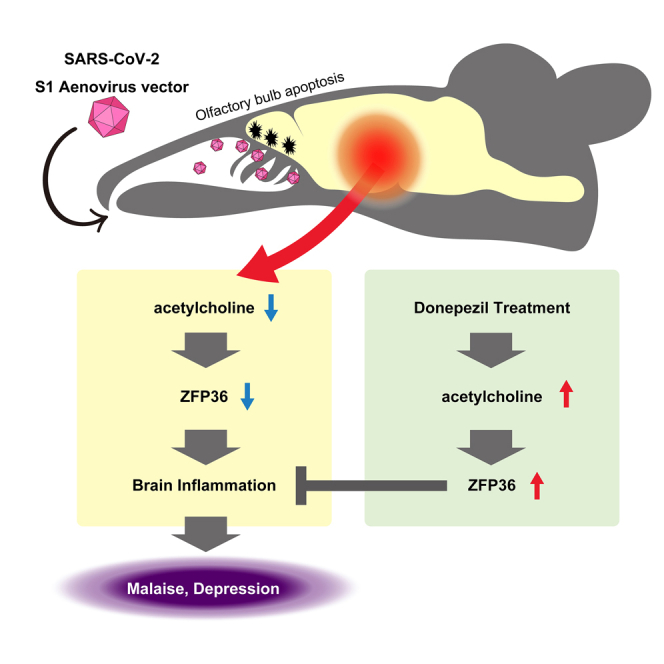
Highlights
-
•
Mice expressing S1 in the nasal cavity showed decreased acetylcholine production
-
•
In the brain, ZFP36 was decreased and inflammatory cytokine expression was enhanced
-
•
The mice exhibited malaise and depressive clinical signs
-
•
Donepezil normalized inflammatory cytokine production and mitigated depression
Neuroscience; Behavioral neuroscience; Virology
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative virus of coronavirus disease 2019 (COVID-19). Mainly affecting the lungs, it causes pneumonia and acute respiratory distress syndrome (ARDS). Also affecting the kidneys, brain, heart, liver, and other organs, it can cause multi-organ failure.1 Research on the etiology of SARS-CoV-2 has uncovered many neurological complications but many aspects of their pathogenesis mechanisms remain unknown.2,3 Highly prevalent neurological complications are those due to olfactory system damage and brain inflammation, such as olfactory dysfunction, fatigue, and depressive symptoms.4,5 If they persist, long COVID6,7,8 may develop. Recently, long COVID has been listed in the ICD-10 classification as post-COVID-19 condition. The WHO defines post-COVID-19 condition “as the continuation or development of new symptoms 3 months after the initial SARS-CoV-2 infection, with these symptoms lasting for at least 2 months with no other explanation”. Regarding symptoms, the WHO adds: “While common symptoms of long COVID can include fatigue, shortness of breath, and cognitive dysfunction over 200 different symptoms have been reported that can have an impact on everyday functioning”.9 Although several treatments have been established for COVID-19 and more are under development,10,11,12,13,14,15,16 there is still no treatment for long COVID.9,16,17,18
Tissue damage in virus infection is normally due to proliferation of the virus in the tissue. In the autopsy results for patients who died from COVID-19, however, despite the presence of tissue degeneration in the brain, there was no evidence of CNS damage directly due to SARS-CoV-2 proliferation.3,19,20,21,22 Also, since it has recently been reported that SARS-CoV-2 infection is not required for the onset of cognitive impairment in long COVID23 and that there are few histopathologic changes24 in patients with COVID-19 brain-related symptoms despite a substantial viral burden in their brains, many aspects of how SARS-CoV-2 causes such symptoms remain unknown. However, the point in common of these studies is that they suggest that factors other than the proliferation of the virus in the brain is at work.
Olfactory system cells are the first point of attack of SARS-CoV-2 and the plentiful expression of ACE2 and TMPRSS2, which is required for invasion of the virus,25,26 makes it possible for SARS-CoV-2 to invade them. Olfactory bulb damage has indeed been reported in COVID-19 patients.27,28,29
Furthermore, since it was reported that surgically induced disruption of olfactory bulb function by olfactory bulbectomy (OBX) resulted in brain inflammation,30,31,32 we considered that olfactory bulb damage due to SARS-CoV-2 was related in some way to the onset of brain inflammation.
It has been reported that the olfactory system cell damage, in particular that in the olfactory bulb, results from an increase in intracellular calcium in olfactory epithelial cells.33 In previous research, we demonstrated that production of the human herpesvirus-6 (HHV-6) protein SITH-1, which has calcium elevating activity in olfactory cells, induced apoptosis in the olfactory bulb and caused depression.34 Since, SARS-CoV-2 spike protein is also known to elevate calcium in cells,35 we created a mouse model expressing spike protein in the nasal cavity.
Results
Intracellular calcium elevating activity of S1 protein
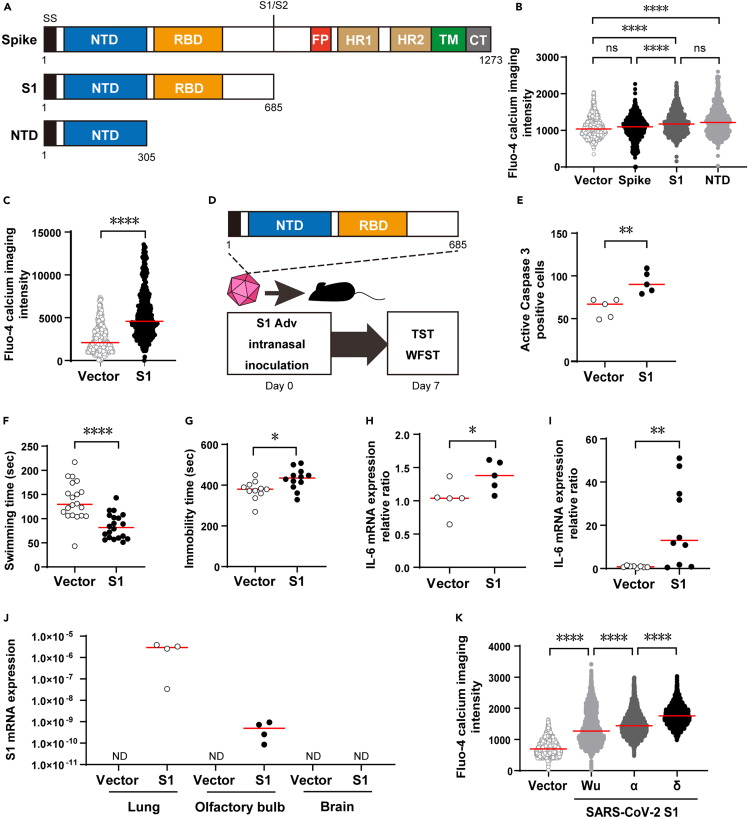
When spike protein invades cells, it is cleaved into S1 and S2 subunits. Using mouse cells (3T3), we found that the N-terminal domain (NTD) was responsible for calcium elevating activity on S1 protein (Figures 1A and 1B). S1 protein also elevated intracellular calcium in human cells (A549) (Figure 1C).
Figure 1.
Analysis of spike protein function and creation of COVID-19 encephalopathy model using S1 protein
(A) Structures of genes used in expression experiments. SS, signal sequence; S1/S2, S1/S2 protease cleavage site; FP, fusion peptide; HR1, heptad repeat 1; R2, heptad repeat 2; TM, transmembrane domain; CT, cytoplasmic tail.
(B) Comparison of intracellular calcium concentration elevation among vector control, spike protein, S1 protein and NTD in mouse 3T3 cells. Calcium concentration was measured by fluorescence of Fluo-4. Vector control, n = 750; spike protein, n = 750; S1 protein, n = 746; NTD, n = 740; Kruskal-Wallis test followed by Dunn’s post hoc test; median values; ∗∗∗∗, p < 0.0001.
(C) Measurement of intracellular calcium concentration in human A549 cells. Calcium concentration was measured by fluorescence of Fluo-4. Vector control, n = 444; S1 Adv, n = 477; Mann-Whitney U-test; median values; ∗∗∗∗, p < 0.0001.
(D) S1 mouse creation and evaluation.
(E) Measurement of apoptosis induction in S1 mouse olfactory bulb tissue based on caspase 3 positive cell count (vector control, n = 5; S1 mouse, n = 5; Mann-Whitney U-test; median values; ∗∗, p < 0.01).
(F) Reduced swimming time in S1 mouse WFST; i.e., increase in fatigue (vector control, n = 20; S1 mouse, n = 20; Mann-Whitney U-test; median values; ∗∗∗∗, p < 0.0001).
(G) Prolonged immobility time in S1 mouse TST, i.e., increase in depressive clinical signs (vector control, n = 11; S1 mouse, n = 12; Mann-Whitney U-test; median values; ∗, p < 0.05).
(H) Enhanced IL-6 expression in S1 mouse brain tissue (vector control, n = 5; S1 mouse, n = 5; Mann-Whitney U-test; median values; ∗, p < 0.05).
(I) Enhanced IL-6 expression in S1 mouse lung tissue (vector control, n = 10; S1 mouse, n = 10; Mann-Whitney U-test; median values; ∗∗, p < 0.01).
(J) S1 mRNA expression in lung, olfactory bulb and brain tissue (Ratios of S1 to 18s ribosomal RNA are shown.) (vector control, n = 4 each; S1 mouse, n = 4 each median values).
(K) Comparison of intracellular calcium concentration elevation among Wu strain, α strain, and δ strain in mouse 3T3 cells. Calcium concentration was measured by fluorescence of Fluo-4. Vector control, n = 3562; Wu strain, n = 2711; α strain, n = 2837; δ strain, n = 2339; Kruskal-Wallis test followed by Dunn’s post hoc test; median values; ∗∗∗∗, p < 0.0001.
Therefore, in order to investigate the effect of S1 protein’s intracellular calcium elevating activity on the olfactory bulb in vivo, we induced expression of S1 protein in the mouse olfactory cavity. In brief, we prepared a non-proliferative adenovirus vector (S1 Adv) expressing the S1 protein of the original Wuhan strain (Wu strain) and then created the S1 mouse through inoculation of S1 Adv into the nasal cavity. One week after inoculation, we conducted behavioral experiments and observed gene expression (Figure 1D).
S1 expressing mouse manifests olfactory bulb damage, malaise, depression, and brain inflammation
Apoptosis of the olfactory bulb was enhanced in the model mouse expressing S1 protein in the nasal cavity (S1 mouse) as expected (Figure 1E). Since it was reported that surgically induced disruption of olfactory bulb function by olfactory bulbectomy (OBX) resulted in depressive-like clinical signs36 and brain inflammation,31 we conducted depression- and malaise-related behavioral tests, and brain inflammation-related investigations. Owing to a decrease in swimming time in the weight-loaded forced swim test (WFST),37 we determined that fatigue had increased in the S1 mouse (Figure 1F). In addition, there was an increase in immobility time for the S1 mouse in the tail suspension test (TST)38 (Figure 1G), which we interpreted as presence of depressive clinical signs.
In addition, using histologic samples of the whole brain without the olfactory bulb, we observed enhanced expression of inflammatory cytokines (IL-6, TNFα) and a chemokine (CCL-2) with an inflammation promoting function (Figures 1H, S1A, and S1B) suggesting that inflammation had occurred in the brain.
We also observed enhanced expression of IL-6 in the lungs (Figure 1I). In the lungs, a correlation of inflammatory cytokine production with S1 mRNA expression (Figures S1C and S1D) suggested that inflammation in the lungs was due to the inflammation-inducing action of S1 protein.39 In contrast, since S1 mRNA expression was not detected in the brain, this suggested that inflammation in the brain was due to an indirect effect (Figure 1J).
Since the results up to this point had suggested an association of S1 protein’s calcium increasing activity and pathogenicity, we measured this for S1 protein from the Wu strain, α strain, and δ strain. The strength of intracellular calcium concentration elevation activity in cells expressing the different strains was in the order: Wu < α < δ (Figure 1K). This was consistent with the order for the severity and mortality rates of these strains: Wu < α < δ.40,41
Impairment of acetylcholine’s anti-inflammatory effect was associated with brain inflammation in S1 mouse
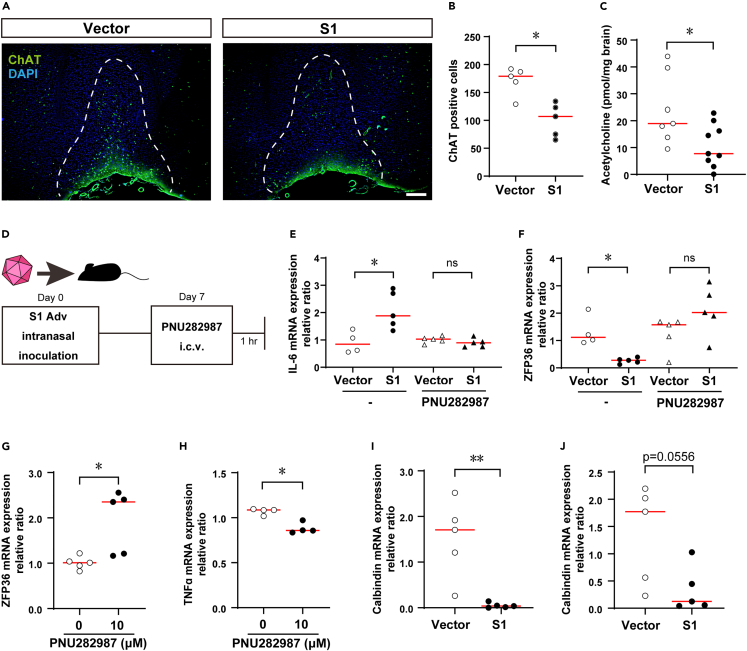
To elucidate a mechanism for the effect of olfactory bulb damage on the brain and the body overall, we focused on neurotransmitters in neurons connecting to the olfactory bulb. There was a decrease in cells positive for the acetylcholine synthesizing enzyme choline acetyltransferase (ChAT) in the medial septal (MS) and diagonal band of Broca (DBB) (Figures 2A and 2B) and the acetylcholine level in the brain overall was also decreased (Figure 2C). We therefore examined a relationship between brain inflammation in the S1 mouse and the anti-inflammatory response in which acetylcholine plays a role called cholinergic anti-inflammatory pathway (CAP).42 CAP was discovered as a mechanism for suppressing inflammation in peripheral tissues via autonomic nerve fibers. It is also known to have a function for suppressing inflammation in the brain.43,44 Since an α7 nicotinic acetylcholine receptor (α7nAchR) agonist was found to activate CAP,44 one week after inoculation with S1 Adv, we administered the α7nAchR agonist PNU282987 intracerebroventricularly (i.c.v.) and after an hour, measured inflammatory cytokine expression levels in the brain (Figure 2D). This resulted in normalizing the enhanced inflammatory cytokine expression in the S1 mouse (Figure 2E). These observations suggest that brain inflammation in the S1 mouse resulted from CAP disruption.
Figure 2.
Disruption of cholinergic anti-inflammatory pathway (CAP) in S1 mouse brain
(A) Decreased ChAT positive cells in S1 mouse MS and DBB (green, ChAT; blue, DAPI; scale bar, 200 μm).
(B) Decreased ChAT positive cells in S1 mouse MS and DBB (vector control, n = 5 S1 mouse, n = 5; Mann-Whitney U-test; median values; ∗, p < 0.05).
(C) Intracerebral acetylcholine (vector control, n = 8; S1 mouse, n = 9; Mann-Whitney U-test; median values; ∗, p < 0.05).
(D) Adv inoculation, PNU282987 administration, brain harvest schedule.
(E) Enhanced IL-6 expression in S1 mouse brain tissue and effect of PNU282987 (no treatment; vector control, n = 4 S1 mouse, n = 5; Mann-Whitney U-test; median values; ∗, p < 0.05. PNU282987; vector control, n = 5 S1 mouse, n = 5; Mann-Whitney U-test; median values; ns, not significant.).
(F) Decreased ZFP36 expression in S1 mouse brain tissue and effect of PNU282987 (no treatment; vector control, n = 4 S1 mouse, n = 5; Mann-Whitney U-test; median values; ∗, p < 0.05. PNU282987; vector control, n = 5 S1 mouse, n = 5; Mann-Whitney U-test; median values; ns, not significant.).
(G) ZFP36 mRNA expression in U373 cells (LPS stimulation, n = 5; LPS stimulation+PNU282987 treatment, n = 5; Mann-Whitney U-test; median values; ∗, p < 0.05).
(H) TNFα mRNA expression in U373 cells (LPS Stimulation, n = 5; LPS stimulation+PNU282987 treatment, n = 5; Mann-Whitney U-test; median values; ∗, p < 0.05).
(I) Expression of calbindin in olfactory bulb tissue of S1 mice additionally administered LPS (vector control, n = 5 S1 mouse, n = 5; Mann-Whitney U-test; median values; ∗∗, p < 0.01).
(J) Expression of calbindin in brain tissue of S1 mouse additionally administered LPS (vector control, n = 4 S1 mouse, n = 5; Mann-Whitney U-test; median values).
It has been known for some time that nerves in the CAP suppress inflammation,42,45 but inflammation suppressing factors were unknown. In the S1 mouse, the expression of the mRNA binding protein ZFP36,46 which has an inflammatory cytokine mRNA degrading function, was reduced when inflammation was present in the brain and was increased by PNU282987 (Figure 2F). In contrast, with another RNA binding protein having an inflammatory cytokine mRNA degrading function similar to that of ZFP36, expression was not decreased, and not induced by PNU282987 in the S1 mouse brain (Figure S2). Also, administering PNU282987 to the human astrocyte cell line U37347 expressing α7nAchR enhanced ZFP36 expression and suppressed TNFα expression (Figures 2G and 2H). These findings suggest that ZFP36 functions as an inflammation-suppressing factor in the CAP.
In order to investigate the influence of systemic inflammation in brain inflammation, we administered lipopolysaccharide (LPS) intraperitoneally to the S1 mouse to enhance systemic inflammation and investigated expression of neural differentiation markers in the olfactory bulb and brain. As a result, the expression of calbindin,48,49 a GABAergic neuron marker, was markedly decreased. (Figures 2I and 2J). As no changes were observed in other neuron differentiation markers (Figures S3A and S3B), this observation was considered specific to GABAergic neurons.
Acetylcholine esterase inhibitor donepezil improves brain symptoms
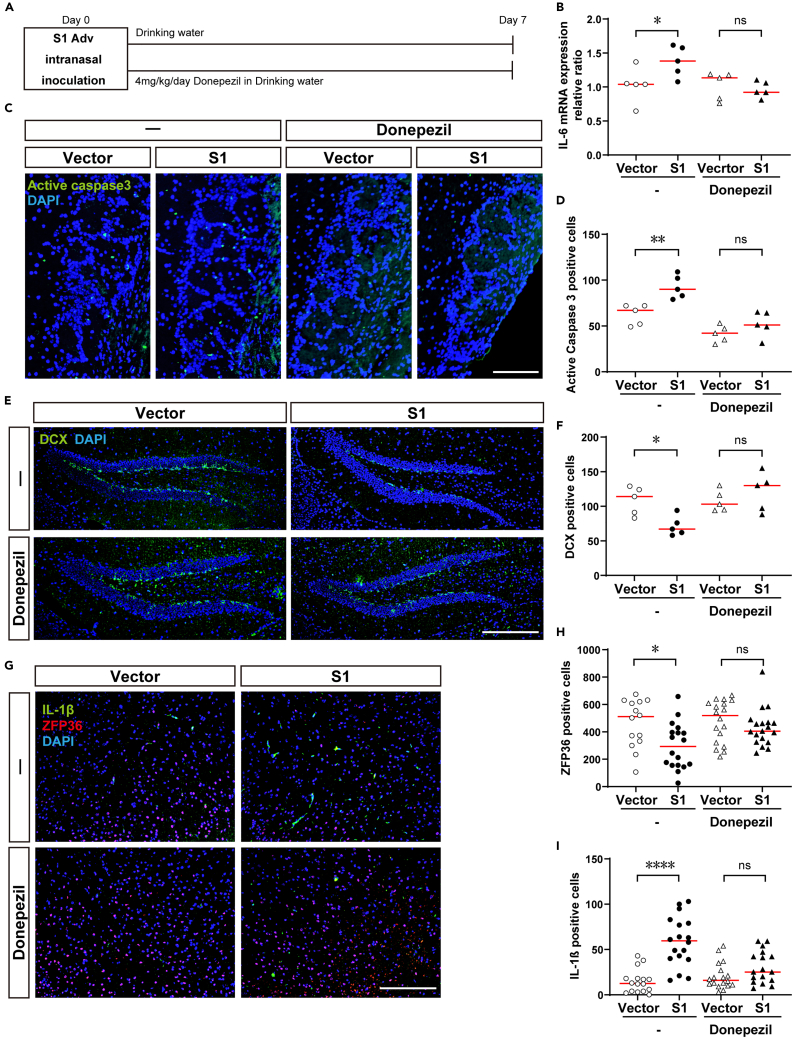
The acetylcholine esterase inhibitor donepezil is a central cholinergic agent used clinically for the treatment of dementia.50 To investigate the possibility of its repurposing for the treatment of neurological complications in COVID-19, we investigated symptom improvement due to donepezil in the S1 mouse.
We administered donepezil at the usual dose for animal experiments (4 mg/kg/day) from the day of S1 Adv inoculation daily in drinking water (Figure 3A). Administration of donepezil resulted in normalizing inflammatory cytokines (IL-6, TNFα) that had been enhanced by S1 protein (Figures 3B and S4A). Since IL-6 and TNFα genes are targets of ZFP36,46,51 this finding suggests that donepezil brought about a recovery in CAP. However, administration of donepezil did not mitigate the enhanced inflammatory cytokine production in the lungs (Figures S4B–S4D).
Figure 3.
Mitigation of olfactory and brain dysfunction in S1 mouse due to donepezil
(A) Adv inoculation, donepezil administration, brain harvest schedules.
(B) Enhanced IL-6 expression in S1 mouse brain tissue and improvement due to donepezil (no treatment; vector control, n = 4, S1 mouse, n = 5; Mann-Whitney U-test; median values; ∗, p < 0.05. Donepezil; vector control, n = 5, S1 mouse, n = 5; Mann-Whitney U-test; median values; ns, not significant).
(C) Increased apoptosis cells in olfactory bulb tissue of S1 mouse and improvement due to donepezil (green, Active caspase 3; blue, DAPI; scale bar, 100 μm).
(D) Increased caspase 3 positive apoptotic cells in S1 mouse olfactory bulb tissue and improvement due to donepezil (no treatment; vector control, n = 5, S1 mouse, n = 5; Mann-Whitney U-test; median values; ∗∗, p < 0.01. Donepezil; vector control, n = 5 S1 mouse, n = 5; Mann-Whitney U-test; median values; ns, not significant).
(E) Decreased DCX positive cells in S1 mouse hippocampus tissue and improvement due to donepezil (green, DCX; blue, DAPI; scale bar, 200 μm).
(F) Decreased DCX positive cells in S1 mouse hippocampus tissue and improvement due to donepezil (no treatment; vector control, n = 5, S1 mouse, n = 5; Mann-Whitney U-test; median values; ∗, p < 0.05. Donepezil; vector control, n = 5 S1 mouse, n = 5; Mann-Whitney U-test; median values; ns, not significant.).
(G) Increased IL-1β positive cells in S1 mouse amygdala tissue and improvement due to donepezil (green, IL-1β; red, ZFP36; blue, DAPI; scale bar, 200 μm).
(H) Decreased ZFP36 positive cells in S1 mouse amygdala tissue and improvement due to donepezil (no treatment; vector control, n = 14, S1 mouse, n = 18; Mann-Whitney U-test; median values; ∗, p < 0.05. Donepezil; vector control, n = 18 S1 mouse, n = 19; Mann-Whitney U-test; median values; ns, not significant.).
(I) Increased IL-1β positive cells in S1 mouse amygdala tissue and improvement due to donepezil (no treatment; vector control, n = 16, S1 mouse, n = 18; Mann-Whitney U-test; median values; ∗∗∗∗, p < 0.0001. Donepezil; vector control, n = 18 S1 mouse, n = 17; Mann-Whitney U-test; median values; ns, not significant.).
Regarding pathological changes in the brain, donepezil mitigated the enhanced apoptosis in the olfactory bulb of the S1 mouse (Figures 3C and 3D). There was also improvement due to donepezil with regard to doublecortin (DCX) positive cells, which indicate hippocampal neurogenesis and had been decreased in the S1 mouse (Figures 3E and 3F). A decrease in hippocampal neurogenesis has been reported to be associated with depressive symptoms52 and memory impairment,53 so we considered it likely that donepezil would improve depressive symptoms and memory impairment in COVID-19. However, donepezil did not reverse a decrease in ChAT positive cells in the MS and DBB (Figures S4E and S4F).
Using anti-ZFP 36 antibody and anti-IL-1β antibody available for use in immunohistochemistry, we performed histologic staining of sections of brain tissue sections innervated with cholinergic nerves from the MS and DBB. For the amygdala, we obtained distinct staining images of decreased ZFP36 production and enhanced IL-1β production due to administration of S1. These phenomena were canceled by administering donepezil (Figures 3G–3I).
Treatment of neurological complications in COVID-19 with donepezil
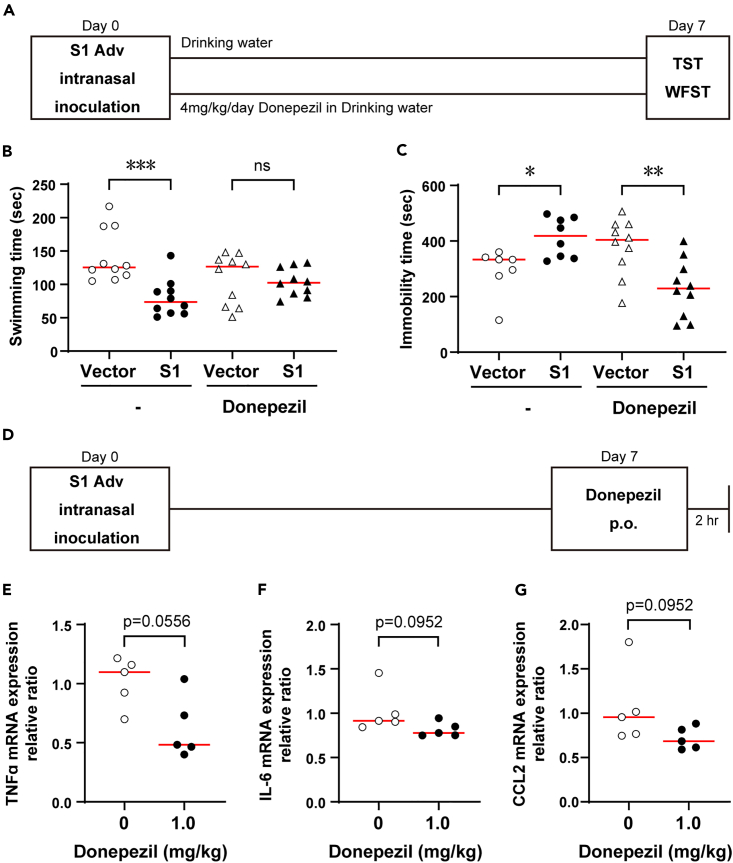
With the objective of using donepezil clinically to treat neurological complications in COVID-19 in mind, we examined the drug’s effects on clinical signs, as well as the dose that would be effective.
We administered donepezil at the usual dose for animal experiments (4 mg/kg/day) daily in drinking water from the day of S1 Adv inoculation (Figure 4A). The administration of donepezil mitigated the increased fatigue determined in the weigh-loaded swimming test and depressive clinical signs determined from the TST (Figures 4B and 4C).
Figure 4.
Therapeutic effects of donepezil with respect to COVID-19
(A) Adv inoculation, donepezil administration, behavior experiment schedules.
(B) Decreased S1 mouse swimming time in WFST, i.e., increase in fatigue, and therapeutic effect of donepezil (no treatment; vector control, n = 10, S1 mouse, n = 10; Mann-Whitney U-test; median values; ∗∗∗, p < 0.001. Donepezil; vector control, n = 10 S1 mouse, n = 10; Mann-Whitney U-test; median values; ns, not significant).
(C) Increased immobility time in S1 mouse TST and therapeutic effect of donepezil (no treatment; vector control, n = 7, S1 mouse, n = 8; Mann-Whitney U-test; median values; ∗, p < 0.05. Donepezil; vector control, n = 10 S1 mouse, n = 10; Mann-Whitney U-test; median values; ns, not significant).
(D) Adv inoculation, donepezil administration, organ harvest schedules.
(E) Inhibitory effect of donepezil on TNFα expression in S1 mouse brain tissue (0 mg/kg, n = 5; 1 mg/kg, n = 5; Mann-Whitney U-test; median values).
(F) Inhibitory effect of donepezil on IL-6 expression in S1 mouse brain tissue (0 mg/kg, n = 5; 1 mg/kg, n = 5; Mann-Whitney U-test; median values).
(G) Inhibitory effect of donepezil on CCL2 expression in S1 mouse brain tissue (0 mg/kg, n = 5; 1 mg/kg, n = 5; Mann-Whitney U-test; median values).
Next, we investigated whether the normal dose of donepezil used for dementia would be effective. We converted the normally administered dose for dementia patients (5 mg/day) for mice (1 mg/kg/day) and administered a single dose orally. Administration was one week after inoculation of S1 Adv and measurements were made 2 h later (Figure 4D). A tendency toward mitigation of the enhanced expression of inflammatory cytokines (IL-6, TNFα) and a chemokine (CCL-2) was observed in the S1 mouse brain (Figures 4E–4G). This result indicates the possibility of donepezil being effective against brain inflammation in COVID-19 at the normal dose.
Discussion
SARS-CoV-2 S1 protein has calcium elevating activity. By inducing its expression in the mouse nasal cavity, we created an S1 mouse exhibiting neurological complications, such as olfactory bulb damage, brain inflammation, fatigue, and depressive clinical signs. This was consistent with brain inflammation induced by olfactory bulb damage that was reported for OBX, as well as induction of depressive clinical signs due to expression in olfactory epithelium of HHV-6 SITH-1 protein, which also has calcium elevating activity. In addition, it has been reported that olfactory system cell damage due to calcium elevation is due to microangiopathy33 and this is consistent with autopsy results for COVID-19 patients.54
In addition, a lack of S1 mRNA expression in the brain led us to conclude that we had reproduced a state of encephalopathy similar to that reported for COVID-19, where the inflammation is not due to viral proliferation in the brain but caused indirectly.
The finding that strength of calcium elevating activity for different strains was in the order Wu < α < δ is of great interest because this is consistent with the order of their severity and mortality rates.40,41 Regarding the calcium-related pathogenicity of spike protein and SARS-CoV-2, an association with syncytia formation has been found.35 The mechanism indicated in the present study may also make consistency between strength of calcium enhancing activity and pathogenicity easier to explain.
In the brain of the S1 mouse, we observed a decrease in cells positive for ChAT in the MS and DBB, as well as a decrease in the amount of acetylcholine in the brain and CAP disruption.42,45 We considered neurological complications had developed in the S1 mouse because inflammation in the brain caused by inflammatory cytokines produced in the lungs could not be suppressed due to CAP disruption. Since it was reported that COVID-19 symptoms were mitigated by nicotine administered through smoking, much attention has been given to a relationship between COVID-19 and CAP.55,56 Decreased expression of acetylcholine receptors in the peripheral blood of COVID-19 patients has also been reported.57
Since it was reported that surgically induced disruption of olfactory bulb function by OBX resulted in a decrease in ChAT positive cells in the MS,58 the decrease in ChAT positive cells and disruption of the CAP in the S1 mouse could be due to olfactory bulb dysfunction. Thus, severe damage to the olfactory bulb due to SARS-CoV-2 infecting the olfactory system27,54 could be the main cause of CAP disruption in COVID-19. However, the olfactory bulb damage in the S1 mouse was thought to be due to the calcium elevating activity of S1 protein.
Our results thus far suggest that, in the S1 mouse, inflammatory cytokines produced in the lungs due to S1 protein expression induce inflammation in the brain because anti-inflammatory effects are diminished due to CAP impairment. Systemic inflammation due to proliferation of the virus throughout the body in COVID-19 could be stronger than that in observed in the S1 mouse. Therefore, we enhanced systemic inflammation through intraperitoneal administration of lipopolysaccharide (LPS), which resulted in markedly reduced expression of the GABAergic marker calbindin59 in the olfactory bulb and the brain. The olfactory bulb has many GABAergic neurons,60 and it has been reported that GABAergic dysfunction is the cause of cognitive difficulties in long COVID.61,62 Thus, our finding may help explain the pathogenesis mechanism of olfactory disturbance and brain fog in long COVID.
To mitigate the pathological state in the S1 mouse, we administered donepezil for inhibition of choline esterase, the enzyme that breaks down acetylcholine. This resulted in reversing the inflammation, reduced hippocampal neurogenesis and apoptosis in the olfactory bulb, and the fatigue and depressive clinical signs associated with them also disappeared.
In the histologic examination of the S1 mouse brain, decreased ZFP36 production, enhanced inflammatory cytokine (IL-1β) production as well as their mitigation by donepezil was more distinct in the amygdala than other parts. This suggests that the amygdala was the most strongly affected by enhancement of inflammation due to CAP disruption in the S1 mouse. It has been reported that the changes in gene expression,63 metabolic function, and64 vol65 indeed occur in the amygdala of COVID-19 patients. In addition, damage to the amygdala has been found to be associated with long COVID64,66,67 Therefore, the information that we obtained regarding inflammation in the amygdala of the S1 mouse and its mitigation by donepezil may be useful in research on long COVID.
As donepezil readily passes through the blood-brain barrier (BBB) and has a brain to blood drug concentration ratio of 4 or above,68 we considered that these improvements were due to the action of donepezil acting in the brain.
On the other hand, the decrease in ChAT positive cells in the MS and DBB was not reversed by donepezil. This finding again suggests that a decrease in ChAT positive cells is positioned upstream of a series of pathological states and is considered to provide a basis for an increase in acetylcholine due to donepezil having a therapeutic effect on the overall clinical signs in the S1 mouse.
Our findings indicate that administering donepezil according to the same administration and dosage directions as for the treatment of dementia would suppress brain inflammation. This suggests that within the guaranteed safety range, donepezil could be applied for the treatment of brain inflammation and neurological complications in COVID-19 and that drug repositioning would allow it to be used in clinical practice at an early date. Therefore, its usefulness needs to be confirmed in clinical trials. As many aspects of their pathogenesis mechanisms are unknown and effective treatments have yet to be established, the results of the present study should not only contribute to the elucidation of pathologies, but also have high clinical value.
The present study also suggests the importance of promoting ZFP36 production via α7nAchRs in suppressing brain inflammation due to COVID-19. With this in mind, through the development of α7nAchR agonists, it may be possible to create more effective drugs for the treatment of brain inflammation in COVID-19 and prevention of long COVID. Also, by discovering an association of CAP and ZFP36 in the brain, we partly elucidated the molecular mechanism of CAP and can expect this to provide clues for elucidating the mechanism of encephalopathy onset due to various causes including viruses and autoimmunity, as well as the development of treatments for them.
Limitations of the study
The effects of donepezil in the present research were obtained in an animal study. Therefore, in order to use it in the treatment of COVID-19 sequelae, its effects on patients will need to be confirmed through clinical trials or other methods.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-active Caspase 3 | Abcam | Cat# ab13847; RRID: AB_443014 |
| Rabbit polyclonal anti-Doublecortin | Abcam | Cat# ab18723; RRID: AB_732011 |
| Rabbit polyclonal anti- Choline Acetyltransferase | Abcam | Cat# ab178850; RRID: AB_2721842 |
| Goat anti-Rabbit IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | ThermoFisher | Cat# A-11034; RRID: AB_2576217 |
| Bacterial and virus strains | ||
| Vector-Ad | TaKaRa Bio | Cat# 6170 |
| S1-Ad | This paper | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Donepezil Hydrochloride | FUJIFILM Wako Chemicals | Cat# 041-32323 |
| PNU 282987 | FUJIFILM Wako Chemicals | Cat# 167-26363 |
| Lipopolysaccharides from Escherichia coli O111:B4 | Merk | Cat# L2630-100 MG |
| Image-iT FX Signal Enhancer | Invitrogen | Cat# I36933 |
| Can Get Signal Immunostain Solution A | TOYOBO | Cat# NKB-501 |
| ProLong Diamond Antifade Mountant with DAPI | ThermoFisher | Cat# P36962 |
| Premix Ex Taq (Perfect Real Time) | TaKaRa Bio | Cat# RR039A |
| Critical commercial assays | ||
| Adenovirus Dual Expression Kit | TaKaRa Bio | Cat# 6170 |
| ProFection Mammalian Transfection System | Promega | Cat# E1200 |
| Calcium Kit II – Fluo 4 | Dojindo | Cat# CS32 |
| Adeno-X Virus Purification kit | Takara Bio | Cat# 631532 |
| Adeno-X Rapid Titer kit | Takara Bio | Cat# 632250 |
| RNeasy Mini QIAcube Kit | QIAGEN | Cat# 74116 |
| PrimeScript RT Reagent Kit | Takara Bio | Cat# RR037A |
| Choline/Acetylcholine Assay Kit | Abcam | Cat# ab65345 |
| Deposited data | ||
| Original Data | Mendeley Data | https://doi.org/10.17632/ch52rydwjh.1 |
| Experimental models: Cell lines | ||
| Mouse: 3T3 (NIH/3T3) | RIKEN BRC | RCB2767 |
| Human: A549 | RIKEN BRC | RCB0098 |
| Human: U373 (U-373 MG) | ATCC | HBT-17 |
| Experimental models: Organisms/strains | ||
| C57BL/6NCrSlc | Japan SLC,Inc. | N/A |
| Oligonucleotides | ||
| SARS-CoV-2_S1 qPCR F: CTGACGGAGAGCAATAAGAAGTTTC R: CCAGGATTTCCAGTGTCTGAGG P: FAM- CCATTCCAGCAGTTCGGCAG AGACATTG -TAMRA |
This paper | N/A |
| Mm_IL-6 qPCR | ThermoFisher | Mm00446190_m1 |
| Mm_IL-1β qPCR | ThermoFisher | Mm00434228_m1 |
| Mm_TNFα qPCR | ThermoFisher | Mm00443258_m1 |
| Mm_CCL2 qPCR | ThermoFisher | Mm00441242_m1 |
| Mm_Zfp36 qPCR | ThermoFisher | Mm00457144_m1 |
| Mm_Calbindin qPCR | ThermoFisher | Mm00486647_m1 |
| Mm_GFAP qPCR | ThermoFisher | Mm01253033_m1 |
| Mm_Nestin qPCR | ThermoFisher | Mm00450205_m1 |
| Mm_IL-17a qPCR | ThermoFisher | Mm00439618_m1 |
| Mm_Sox2 qPCR | ThermoFisher | Mm03053810_s1 |
| Mm_Mcm2 qPCR | ThermoFisher | Mm00484815_m1 |
| Mm_DCX qPCR | ThermoFisher | Mm00438400_m1 |
| Mm_Calretinin qPCR | ThermoFisher | Mm00801461_m1 |
| Mm_HuR qPCR | ThermoFisher | Mm00516011_m1 |
| Mm_Roquin qPCR | ThermoFisher | Mm01284492_m1 |
| Mm_KHSRP qPCR | ThermoFisher | Mm01232838_g1 |
| Mm_Regnase-1 qPCR | ThermoFisher | Mm00462535_g1 |
| Mm_AUF1 qPCR | ThermoFisher | Mm01201314_m1 |
| 18s rRNA | ThermoFisher | Hs99999901_s1 |
| Recombinant DNA | ||
| pFlag-CMV-5b | Sigma Aldrich | Cat# E3762 |
| SARS-CoV-2 S1 (Wu)/pFlag-CMV-5b | This paper | N/A |
| SARS-CoV-2 S1 (α)/pFlag-CMV-5b | This paper | N/A |
| SARS-CoV-2 S1 (δ)/pFlag-CMV-5b | This paper | N/A |
| SARS-CoV-2 NTD/pFlag-CMV-5b | This paper | N/A |
| SARS-CoV-2 Spike/pFlag-CMV-5b | This paper | N/A |
| Software and algorithms | ||
| ImageJ | NIH | https://imagej.nih.gov/ij/ |
| Sequence Detection Software version 1.4 | Thermo Fisher | N/A |
| Prism 8 | GraphPad | N/A |
| BellCurve for Excel | Social Survey Research Information Co., Ltd | https://bellcurve.jp/ex/ |
| TailSuspScan | CleverSys Inc | N/A |
| TopScan | CleverSys Inc | N/A |
| ArrayScan XTI instrument | Thermo Fisher | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Kazuhiro Kondo (kkondo@jikei.ac.jp).
Materials availability
All unique reagents generated in this study are available from the lead contact with a completed Materials Transfer Agreement.
Experimental model and study participant details
Animals
Male 8-week-old C57BL/6NCrSlc mice obtained from Sankyo laboratories were used for all experiments. All mice were housed under standard conditions (12-h light–dark cycle [lights-on at 8:00 a.m.] at 24 ± 1°C) with food and water provided ad libitum. All animal experiments were performed in accordance with animal experiment regulations and approved by the Animal Care and Use Committee of the Jikei University School of Medicine.
Cell lines
The mouse fibroblast-like cell line 3T3, human lung epithelial cell line A549, human astrocytoma cell lines U373 and human embryonic kidney cell line HEK293A were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS. These cells were incubated at 37°C in a humidified 5% CO2 atmosphere. Sex and age at sampling for the cells were as follows: 3T3, male embryo; A549, male 58Y; U373, male 75Y; HEK293A, female fetus.
Method details
Production of recombinant adenovirus vectors
The recombinant adenovirus was produced using an Adenovirus Dual Expression Kit (Takara Bio) in accordance with the manufacturer’s protocol. The SARS-CoV-2 genes were cloned into an adenovirus cosmid vector (pAxCAwtit2) using standard methods (SARS-CoV-2 genes/pAxCAwtit2). HEK293A cells were transfected with the SARS-CoV-2 genes/pAxCAwtit2 cosmid and a cosmid that did not contain the target gene (pAxcwit2). The recombinant adenovirus was prepared in HEK293A cells and purified with an Adeno-X Virus Purification kit (Takara Bio). The purified virus titer was determined using an Adeno-X Rapid Titer kit (Takara Bio).
Intracellular calcium assay
3T3 cells or A549 cells were cultured on a 96-well plate and transient overexpression of each protein - S1 (Wu, α and δ) - was achieved for 24 h using ProFection Mammalian Transfection System (Promega) or adenovirus infection. Subsequently, cells were induced to take up Fluo 4-AM using Calcium Kit II-Fluo 4 (Dojindo) and then the fluorescence intensity in individual cells was measured using an ArrayScan XTI instrument (Thermo Fisher).
Nasal inoculation of adenovirus vectors
For the nasal inoculation of adenovirus vectors, 8-week-old male C57BL/6 mice were anesthetized with isoflurane. Recombinant adenoviruses were diluted in sterile water (not in isotonic buffer). A drop (25 μL) of S1-Ad or Vector-Ad solution containing the virus at a titer of 1 × 109 infectious units (ifu)/mL was placed at the entrance of the nasal cavity of the mouse. The solution entered the cavity through spontaneous respiration.
Drugs
The cholinesterase inhibitor donepezil (FUJIFILM Wako) was administered via the drinking water (40 mg/L, 4 mg/kg/day) from the day of intranasal inoculation. For oral administration, donepezil was diluted in saline and administered to mice by feeding tube at 0, 1 and 2 mg/kg. The α7nAChR agonist PNU282987 (FUJIFILM Wako) was diluted in saline and administered intracerebroventricularly at 400 nmol/mouse. Lipopolysaccharide (LPS) from Escherichia coli O111:B4 (Merck) was diluted in saline and administered intraperitoneally to S1 mice at 5 mg/kg. The brain and olfactory bulb were removed 15 and 30 min after administration, respectively, and used for gene expression analysis. For all oral, intracerebroventricular and intraperitoneal administrations, control mice received the solvent saline.
Animal behavior tests
Seven days after the inoculation of S1-Ad or Vector-Ad, a tail suspension test (TST) and a weight-loaded forced swim test (WFST) were performed to assess depression- and fatigue-like behavior. In the TST, the period of immobility during 10 min was analyzed by TailSuspScan software (CleverSys Inc). In the WFST, a weight equivalent to 10% of body weight was attached to the root of the mouse tail, the time taken for the mouse’s nose to be below the surface of the water for 10 s was measured by TopScan software (CleverSys Inc). At 24 h, after these behavior tests, the mice were euthanized for real-time PCR and immunohistochemical staining.
Real-time PCR
Total RNA was purified from animal tissues using the RNeasy Mini QIAcube Kit (Qiagen). cDNA was synthesized from total RNA with a PrimeScript RT Reagent Kit (Takara Bio). mRNA amounts were quantified with Premix Ex Taq (Perfect Real Time) (Takara Bio), and the Applied Biosystems 7300 Real-Time PCR system (Thermo Fisher). The thermal profile was 95°C for 30 s, followed by 45 cycles of 95°C for 5 s and 60°C for 31 s. Data analysis was performed with Sequence Detection Software version 1.4 (Thermo Fisher). Mouse IL-6, mouse IL-1β, mouse TNFα, mouse CCL-2, mouse ZFP36, mouse Calbindin, mouse GFAP, mouse Nestin, mouse Sox2, mouse Mcm2, mouse DCX, mouse Calretinin, mouse HuR, mouse Roquin, mouse KHSRP, mouse Regnase-1, mouse AUF1, human 18S and SARS-CoV-2 S1 were measured using the primers and probes described in the key resources table.
Immunohistochemistry
For the staining of tissues, mice were sacrificed and transcardially perfused with saline, followed by 10% neutral buffered formalin (pH 7.4). Formalin-fixed tissues were then embedded in paraffin and sectioned. For immunofluorescence staining, paraffin-embedded sections were deparaffinized, and blocking was performed in Image-iT FX Signal Enhancer (Life Technologies) for 30 min. Primary antibodies were obtained from Abcam (active caspase-3, doublecortin and Choline Acetyltransferase). Secondary antibodies were obtained from Thermo (Alexa Fluor 488 Goat Anti-rabbit IgG (H + L)). The antibodies were diluted with Can Get Signal Immunostain Solution A (TOYOBO). After the samples had been mounted on a slide with a cover glass, they were observed under a Keyence BZ-9000 fluorescence microscope.
Measurement of brain acetylcholine levels
Seven days after the inoculation of S1-Ad or Vector-Ad, mice were sacrificed and their brains immediately harvested. The amount of acetylcholine in the brain was measured by the Choline/Acetylcholine Assay Kit (abcam). Fluorescence was measured by TriStar LB941 (Berthold).
Induction of ZFP36 in cultured cells
U373 cells were cultured on a 6-well plate and stimulated with 10 μM PNU282987. After culturing U373 cells in the presence of 10 μg/mL LPS (Merck) for 48 h, they were stimulated with 10 μM PNU282987.Cells were recovered 2h after stimulation followed by RNA purification and cDNA synthesis by the methods mentioned above and then expression of anti-inflammatory factors was analyzed by RT-qPCR. Human TNFα, Human ZFP36 and human 18S were measured using the primers and probes described in the key resources table.
Quantification and statistical analysis
The Shapiro-Wilk normality test was performed to assess the normality of distributions. To compare two different groups, the Mann-Whitney U-test was used as the nonparametric test. To compare multiple groups, the Kruskal-Wallis test, and then Dunn’s post hoc test, were used as nonparametric tests. Red horizontal lines are medians. p < 0.05 was considered significant. Spearman’s rank correlation coefficients were used to determine correlations between variables. Statistical analyses were performed with Prism 8 (GraphPad) and BellCurve for Excel (Social Survey Research Information Co., Ltd.).
Acknowledgments
We thank Mr. Alexander Cox for editorial assistance with the manuscript. This research was supported by Japan Agency for Medical Research and Development (AMED) under Grant Number JP20fk0108541 and JP 21fk0108486.
Author contributions
N.O. and K.K. contributed to study design. N.O., K.S., A.I., N.K., and K.K. contributed to data collections or interpretation. K.K. coordinated all experiments.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: May 24, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.106954.
Supplemental information
Data and code availability
-
•
The data included in this manuscript have been deposited at Mendeley Data and publicly available as of the date of publication. The DOI is listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ariño H., Heartshorne R., Michael B.D., Nicholson T.R., Vincent A., Pollak T.A., Vogrig A. Neuroimmune disorders in COVID-19. J. Neurol. 2022 doi: 10.1007/s00415-022-11050-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon T. Neurological infection with SARS-CoV-2 - the story so far. Nat. Rev. Neurol. 2021;17:65–66. doi: 10.1038/s41582-020-00453-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guasp M., Muñoz-Sánchez G., Martínez-Hernández E., Santana D., Carbayo Á., Naranjo L., Bolós U., Framil M., Saiz A., Balasa M., et al. CSF biomarkers in COVID-19 associated encephalopathy and encephalitis predict long-term outcome. Front. Immunol. 2022;13:866153. doi: 10.3389/fimmu.2022.866153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vakili K., Fathi M., Hajiesmaeili M., Salari M., Saluja D., Tafakhori A., Sayehmiri F., Rezaei-Tavirani M. Neurological symptoms, comorbidities, and complications of COVID-19: a literature review and meta-analysis of observational studies. Eur. Neurol. 2021;84:307–324. doi: 10.1159/000516258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceban F., Ling S., Lui L.M.W., Lee Y., Gill H., Teopiz K.M., Rodrigues N.B., Subramaniapillai M., Di Vincenzo J.D., Cao B., et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: a systematic review and meta-analysis. Brain Behav. Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schou T.M., Joca S., Wegener G., Bay-Richter C. Psychiatric and neuropsychiatric sequelae of COVID-19 - a systematic review. Brain Behav. Immun. 2021;97:328–348. doi: 10.1016/j.bbi.2021.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibrahim E.A.A., Isam Farah Hassan R., Abbasher Hussien Mohamed Ahmed K., Taha Salah E., Eltahier Abdalla Omer M., Haroun M.S. Neurological manifestations of COVID-19: a potential gate to the determinants of a poor prognosis. Brain Behav. 2022;12:e2587. doi: 10.1002/brb3.2587. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V., WHO Clinical Case Definition Working Group on Post-COVID-19 Condition A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022;22:e102–e107. doi: 10.1016/s1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaur I., Behl T., Sehgal A., Singh S., Sharma N., Subramanian V., Fuloria S., Fuloria N.K., Sekar M., Dailah H.G., et al. A motley of possible therapies of the COVID-19: reminiscing the origin of the pandemic. Environ. Sci. Pollut. Res. Int. 2022;29:67685–67703. doi: 10.1007/s11356-022-22345-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behl T., Shah S., Kaur I., Yadav S., Kanwar R., Seth S., Wig N., Sharma K.K., Yadav H.N. Role of ACE 2 and vitamin D: the two players in global fight against COVID-19 pandemic. Ann. Natl. Acad. Med. Sci. 2021;57:186–196. [Google Scholar]
- 12.Dal-Ré R., Becker S.L., Bottieau E., Holm S. Availability of oral antivirals against SARS-CoV-2 infection and the requirement for an ethical prescribing approach. Lancet Infect. Dis. 2022;22:e231–e238. doi: 10.1016/s1473-3099(22)00119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards A.M., Baric R.S., Saphire E.O., Ulmer J.B. Stopping pandemics before they start: lessons learned from SARS-CoV-2. Science. 2022;375:1133–1139. doi: 10.1126/science.abn1900. [DOI] [PubMed] [Google Scholar]
- 14.Behl T., Kaur I., Aleya L., Sehgal A., Singh S., Sharma N., Bhatia S., Al-Harrasi A., Bungau S. CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target. Sci. Total Environ. 2022;808:152072. doi: 10.1016/j.scitotenv.2021.152072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philips R.L., Wang Y., Cheon H., Kanno Y., Gadina M., Sartorelli V., Horvath C.M., Darnell J.E., Jr., Stark G.R., O'Shea J.J. The JAK-STAT pathway at 30: much learned, much more to do. Cell. 2022;185:3857–3876. doi: 10.1016/j.cell.2022.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merad M., Blish C.A., Sallusto F., Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375:1122–1127. doi: 10.1126/science.abm8108. [DOI] [PubMed] [Google Scholar]
- 17.Borah P., Deb P.K., Chandrasekaran B., Goyal M., Bansal M., Hussain S., Shinu P., Venugopala K.N., Al-Shar'i N.A., Deka S., Singh V. Neurological consequences of SARS-CoV-2 infection and concurrence of treatment-induced neuropsychiatric adverse events in COVID-19 patients: navigating the uncharted. Front. Mol. Biosci. 2021;8:627723. doi: 10.3389/fmolb.2021.627723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorman E.A., O'Kane C.M., McAuley D.F. Acute respiratory distress syndrome in adults: diagnosis, outcomes, long-term sequelae, and management. Lancet. 2022;400:1157–1170. doi: 10.1016/s0140-6736(22)01439-8. [DOI] [PubMed] [Google Scholar]
- 19.Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., Mushumba H., Fitzek A., Allweiss L., Dandri M., et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/s1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang A.C., Kern F., Losada P.M., Agam M.R., Maat C.A., Schmartz G.P., Fehlmann T., Stein J.A., Schaum N., Lee D.P., et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature. 2021;595:565–571. doi: 10.1038/s41586-021-03710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukerji S.S., Solomon I.H. What can we learn from brain autopsies in COVID-19? Neurosci. Lett. 2021;742:135528. doi: 10.1016/j.neulet.2020.135528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McQuaid C., Brady M., Deane R. SARS-CoV-2: is there neuroinvasion? Fluids Barriers CNS. 2021;18:32. doi: 10.1186/s12987-021-00267-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-Castañeda A., Lu P., Geraghty A.C., Song E., Lee M.H., Wood J., O'Dea M.R., Dutton S., Shamardani K., Nwangwu K., et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell. 2022;185:2452–2468. doi: 10.1016/j.cell.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein S.R., Ramelli S.C., Grazioli A., Chung J.Y., Singh M., Yinda C.K., Winkler C.W., Sun J., Dickey J.M., Ylaya K., et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022;612:758–763. doi: 10.1038/s41586-022-05542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klingenstein M., Klingenstein S., Neckel P.H., Mack A.F., Wagner A.P., Kleger A., Liebau S., Milazzo A. Evidence of SARS-CoV2 entry protein ACE2 in the human nose and olfactory bulb. Cells Tissues Organs. 2020;209:155–164. doi: 10.1159/000513040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brann D.H., Tsukahara T., Weinreb C., Lipovsek M., Van den Berge K., Gong B., Chance R., Macaulay I.C., Chou H.J., Fletcher R.B., et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020;6:eabc5801. doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kandemirli S.G., Altundag A., Yildirim D., Tekcan Sanli D.E., Saatci O. Olfactory bulb MRI and paranasal sinus CT findings in persistent COVID-19 anosmia. Acad. Radiol. 2021;28:28–35. doi: 10.1016/j.acra.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altunisik E., Baykan A.H., Sahin S., Aydin E., Erturk S.M. Quantitative analysis of the olfactory system in COVID-19: an MR imaging study. AJNR. Am. J. Neuroradiol. 2021;42:2207–2214. doi: 10.3174/ajnr.A7278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan C.J.W., Tan B.K.J., Tan X.Y., Liu H.T., Teo C.B., See A., Xu S., Toh S.T., Kheok S.W., Charn T.C., Teo N.W.Y. Neuroradiological basis of COVID-19 olfactory dysfunction: a systematic review and meta-analysis. Laryngoscope. 2022;132:1260–1274. doi: 10.1002/lary.30078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rinwa P., Kumar A. Quercetin suppress microglial neuroinflammatory response and induce antidepressent-like effect in olfactory bulbectomized rats. Neuroscience. 2013;255:86–98. doi: 10.1016/j.neuroscience.2013.09.044. [DOI] [PubMed] [Google Scholar]
- 31.Almeida R.F.d., Ganzella M., Machado D.G., Loureiro S.O., Leffa D., Quincozes-Santos A., Pettenuzzo L.F., Duarte M.M., Duarte T., Souza D.O. Olfactory bulbectomy in mice triggers transient and long-lasting behavioral impairments and biochemical hippocampal disturbances. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017;76:1–11. doi: 10.1016/j.pnpbp.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Bansal Y., Singh R., Saroj P., Sodhi R.K., Kuhad A. Naringenin protects against oxido-inflammatory aberrations and altered tryptophan metabolism in olfactory bulbectomized-mice model of depression. Toxicol. Appl. Pharmacol. 2018;355:257–268. doi: 10.1016/j.taap.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Thyssen A., Hirnet D., Wolburg H., Schmalzing G., Deitmer J.W., Lohr C. Ectopic vesicular neurotransmitter release along sensory axons mediates neurovascular coupling via glial calcium signaling. Proc. Natl. Acad. Sci. USA. 2010;107:15258–15263. doi: 10.1073/pnas.1003501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi N., Oka N., Takahashi M., Shimada K., Ishii A., Tatebayashi Y., Shigeta M., Yanagisawa H., Kondo K. Human herpesvirus 6B greatly increases risk of depression by activating hypothalamic-pituitary -adrenal Axis during latent phase of infection. iScience. 2020;23:101187. doi: 10.1016/j.isci.2020.101187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braga L., Ali H., Secco I., Chiavacci E., Neves G., Goldhill D., Penn R., Jimenez-Guardeño J.M., Ortega-Prieto A.M., Bussani R., et al. Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia. Nature. 2021;594:88–93. doi: 10.1038/s41586-021-03491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramaker M.J., Dulawa S.C. Identifying fast-onset antidepressants using rodent models. Mol. Psychiatr. 2017;22:656–665. doi: 10.1038/mp.2017.36. [DOI] [PubMed] [Google Scholar]
- 37.Su K.Y., Yu C.Y., Chen Y.W., Huang Y.T., Chen C.T., Wu H.F., Chen Y.L.S. Rutin, a flavonoid and principal component of saussurea involucrata, attenuates physical fatigue in a forced swimming mouse model. Int. J. Med. Sci. 2014;11:528–537. doi: 10.7150/ijms.8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Can A., Dao D.T., Terrillion C.E., Piantadosi S.C., Bhat S., Gould T.D. The tail suspension test. J. Vis. Exp. 2011:e3769. doi: 10.3791/3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schroeder J.T., Bieneman A.P. The S1 subunit of the SARS-CoV-2 spike protein activates human monocytes to produce cytokines linked to COVID-19: relevance to galectin-3. Front. Immunol. 2022;13:831763. doi: 10.3389/fimmu.2022.831763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisman D.N., Tuite A.R. Evaluation of the relative virulence of novel SARS-CoV-2 variants: a retrospective cohort study in Ontario, Canada. CMAJ (Can. Med. Assoc. J.) 2021;193 doi: 10.1503/cmaj.211248. E1619–e1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venkatraja B., Srilakshminarayana G., Krishna Kumar B. The dominance of severe acute respiratory syndrome coronavirus 2 B.1.617 and its sublineages and associations with mortality during the COVID-19 pandemic in India between 2020 and 2021. Am. J. Trop. Med. Hyg. 2021;106:142–149. doi: 10.4269/ajtmh.21-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tracey K.J. Reflex control of immunity. Nat. Rev. Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoover D.B. Cholinergic modulation of the immune system presents new approaches for treating inflammation. Pharmacol. Ther. 2017;179:1–16. doi: 10.1016/j.pharmthera.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piovesana R., Salazar Intriago M.S., Dini L., Tata A.M. Cholinergic modulation of neuroinflammation: focus on α7 nicotinic receptor. Int. J. Mol. Sci. 2021;22:4912. doi: 10.3390/ijms22094912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reardon C. Neuro-immune interactions in the cholinergic anti-inflammatory reflex. Immunol. Lett. 2016;178:92–96. doi: 10.1016/j.imlet.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Makita S., Takatori H., Nakajima H. Post-transcriptional regulation of immune responses and inflammatory diseases by RNA-binding ZFP36 family proteins. Front. Immunol. 2021;12:711633. doi: 10.3389/fimmu.2021.711633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalashnyk O., Lykhmus O., Oliinyk O., Komisarenko S., Skok M. α7 Nicotinic acetylcholine receptor-specific antibody stimulates interleukin-6 production in human astrocytes through p38-dependent pathway. Int. Immunopharm. 2014;23:475–479. doi: 10.1016/j.intimp.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 48.Kuwajima T., Nishimura I., Yoshikawa K. Necdin promotes GABAergic neuron differentiation in cooperation with Dlx homeodomain proteins. J. Neurosci. 2006;26:5383–5392. doi: 10.1523/jneurosci.1262-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalinowski D., Bogus-Nowakowska K., Kozłowska A., Równiak M. Expression of calbindin, a marker of gamma-aminobutyric acid neurons, is reduced in the amygdala of oestrogen receptor β-deficient female mice. J. Clin. Med. 2022;11:1760. doi: 10.3390/jcm11071760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Birks J.S., Harvey R.J. Donepezil for dementia due to Alzheimer's disease. Cochrane Database Syst. Rev. 2018;6:Cd001190. doi: 10.1002/14651858.CD001190.pub3. [DOI] [PubMed] [Google Scholar]
- 51.Ricciardi L., Col J.D., Casolari P., Memoli D., Conti V., Vatrella A., Vonakis B.M., Papi A., Caramori G., Stellato C. Differential expression of RNA-binding proteins in bronchial epithelium of stable COPD patients. Int. J. Chronic Obstr. Pulm. Dis. 2018;13:3173–3190. doi: 10.2147/copd.S166284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahar I., Bambico F.R., Mechawar N., Nobrega J.N. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci. Biobehav. Rev. 2014;38:173–192. doi: 10.1016/j.neubiorev.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 53.Gonçalves J.T., Schafer S.T., Gage F.H. Adult neurogenesis in the Hippocampus: from stem cells to behavior. Cell. 2016;167:897–914. doi: 10.1016/j.cell.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 54.Ho C.Y., Salimian M., Hegert J., O'Brien J., Choi S.G., Ames H., Morris M., Papadimitriou J.C., Mininni J., Niehaus P., et al. Postmortem assessment of olfactory tissue degeneration and microvasculopathy in patients with COVID-19. JAMA Neurol. 2022;79:544–553. doi: 10.1001/jamaneurol.2022.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonzalez-Rubio J., Navarro-Lopez C., Lopez-Najera E., Lopez-Najera A., Jimenez-Diaz L., Navarro-Lopez J.D., Najera A. Cytokine release syndrome (CRS) and nicotine in COVID-19 patients: trying to calm the storm. Front. Immunol. 2020;11:1359. doi: 10.3389/fimmu.2020.01359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mehranfard D., Speth R.C. Cholinergic anti-inflammatory pathway and COVID-19. Bioimpacts. 2022;12:171–174. doi: 10.34172/bi.2022.23980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Courties A., Boussier J., Hadjadj J., Yatim N., Barnabei L., Péré H., Veyer D., Kernéis S., Carlier N., Pène F., et al. Regulation of the acetylcholine/α7nAChR anti-inflammatory pathway in COVID-19 patients. Sci. Rep. 2021;11:11886. doi: 10.1038/s41598-021-91417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han F., Shioda N., Moriguchi S., Qin Z.H., Fukunaga K. The vanadium (IV) compound rescues septo-hippocampal cholinergic neurons from neurodegeneration in olfactory bulbectomized mice. Neuroscience. 2008;151:671–679. doi: 10.1016/j.neuroscience.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 59.Ferrer I. Neurons and their dendrites in frontotemporal dementia. Dement. Geriatr. Cogn. Disord. 1999;10(Suppl 1):55–60. doi: 10.1159/000051214. [DOI] [PubMed] [Google Scholar]
- 60.Fujiwara N., Cave J.W. Partial conservation between mice and humans in olfactory bulb interneuron transcription factor codes. Front. Neurosci. 2016;10:337. doi: 10.3389/fnins.2016.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Versace V., Sebastianelli L., Ferrazzoli D., Romanello R., Ortelli P., Saltuari L., D'Acunto A., Porrazzini F., Ajello V., Oliviero A., et al. Intracortical GABAergic dysfunction in patients with fatigue and dysexecutive syndrome after COVID-19. Clin. Neurophysiol. 2021;132:1138–1143. doi: 10.1016/j.clinph.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ortelli P., Ferrazzoli D., Sebastianelli L., Maestri R., Dezi S., Spampinato D., Saltuari L., Alibardi A., Engl M., Kofler M., et al. Altered motor cortex physiology and dysexecutive syndrome in patients with fatigue and cognitive difficulties after mild COVID-19. Eur. J. Neurol. 2022;29:1652–1662. doi: 10.1111/ene.15278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piras I.S., Huentelman M.J., Walker J.E., Arce R., Glass M.J., Vargas D., Sue L.I., Intorcia A.J., Nelson C.M., Suszczewicz K.E., et al. Olfactory bulb and amygdala gene expression changes in subjects dying with COVID-19. medRxiv. 2021 doi: 10.1101/2021.09.12.21263291. Preprint at. [DOI] [Google Scholar]
- 64.Guedj E., Million M., Dudouet P., Tissot-Dupont H., Bregeon F., Cammilleri S., Raoult D. (18 F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Eur. J. Nucl. Med. Mol. Imag. 2021;48:592–595. doi: 10.1007/s00259-020-04973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Campabadal A., Oltra J., Junqué C., Guillen N., Botí M.Á., Sala-Llonch R., Monté-Rubio G.C., Lledó G., Bargalló N., Rami L., et al. Structural brain changes in post-acute COVID-19 patients with persistent olfactory dysfunction. Ann. Clin. Transl. Neurol. 2022;10:195–203. doi: 10.1002/acn3.51710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Besteher B., Machnik M., Troll M., Toepffer A., Zerekidze A., Rocktäschel T., Heller C., Kikinis Z., Brodoehl S., Finke K., et al. Larger gray matter volumes in neuropsychiatric long-COVID syndrome. Psychiatr. Res. 2022;317:114836. doi: 10.1016/j.psychres.2022.114836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martini A.L., Carli G., Kiferle L., Piersanti P., Palumbo P., Morbelli S., Calcagni M.L., Perani D., Sestini S. Time-dependent recovery of brain hypometabolism in neuro-COVID-19 patients. Eur. J. Nucl. Med. Mol. Imag. 2022;50:90–102. doi: 10.1007/s00259-022-05942-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shin C.Y., Kim H.S., Cha K.H., Won D.H., Lee J.Y., Jang S.W., Sohn U.D. The effects of donepezil, an acetylcholinesterase inhibitor, on impaired learning and memory in rodents. Biomol. Ther. (Seoul) 2018;26:274–281. doi: 10.4062/biomolther.2017.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The data included in this manuscript have been deposited at Mendeley Data and publicly available as of the date of publication. The DOI is listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.