Abstract
New psychoactive substances (NPS) have been rapidly emerged as legal alternatives to controlled drugs, which raised severe public health issue. The detection and monitoring of its intake by complete metabolic profiling is an urgent and vital task. Untargeted metabolomics approach has been applied for several NPS metabolites studies. Although the number of such works is relatively limited but with a rapidly growing need. The present study aimed to propose a procedure that includes liquid chromatography high-resolution mass spectrometry (LC-HRMS) analysis and a signal selection software, MetaboFinder, programed as a web tool. The comprehensive metabolites profile of one kind of NPS, 4-methoxy-α-pyrrolidinovalerophenone (4-MeO-α-PVP), was studied by using this workflow. In this study, two different concentrations of 4-MeO-α-PVP along with as blank sample were incubated with human liver S9 fraction for the conversion to their metabolites and followed by LC-MS analysis. After retention time alignment and feature identification, 4640 features were obtained and submitted to statistical analysis for signal selection by using MetaboFinder. A total of 50 features were considered as 4-MeO-α-PVP metabolite candidates showing significant changes (p < 0.00001 and fold change >2) between the two investigated groups. Targeted LC-MS/MS analysis was conducted focusing on these significantly expressed features. Assisted by chemical formula determination according to high mass accuracy and in silico MS2 fragmentation prediction, 19 chemical structure identifications were achieved. Among which, 8 metabolites have been reported derived from 4-MeO-α-PVP in a previous literature while 11 novel 4-MeO-α-PVP metabolites were identified by using our strategy. Further in vivo animal experiment confirmed that 18 compounds were 4-MeO-α-PVP metabolites, which demonstrated the feasibility of our strategy for screening the 4-MeO-α-PVP metabolites. We anticipate that this procedure may support and facilitate traditional metabolism studies and potentially being applied for routine NPS metabolites screening.
Keywords: 4-methoxy-α-pyrrolidinovalerophenone (4-MeO-α-PVP), LC-MS/MS, Metabolome, Metabolomics, New psychoactive substances
1. Introduction
Detection of small-molecule drug metabolism is always a high demand for monitoring drug overdosing or abuse [1]. The use of new psychoactive substances (NPS) continues to emerge in the drug market, which poses an urgent clinical/ forensic toxicology and public health issue due to the lack of their toxicodynamic and kinetic properties, particularly knowledge of their metabolism [2,3]. NPS include aminoindanes, synthetic cannabinoids, cathinones, ketamine, phenethylamines, tryptamines, piperazines, and other plant-based psychoactive substance [4]. A total of 1124 different NPS have been reported to the United Nations Office on Drugs and Crime’s Early Warning Advisory between 2009 and 2021, and the number is growing rapidly [5]. Safety data, carcinogenicity, and toxicology information of most NPS are very limited or even not available. It has been known that synthetic cannabinoids have more serious side-effects than cannabis [6]. Complete metabolic profiling of NPS are crucial for developing detection of intake and link adverse effects to the toxicants. For metabolism studies, since authentic human biosamples are usually not available and the controlled human studies are not practical owing to ethical reasons [2,7], metabolites can be generated through in vitro or in vivo models which mimic the endogenous enzymatic reaction [8,9].
Evolving techniques such as advanced nuclear magnetic resonance and high-resolution tandem mass spectrometry (HRMS/MS) have improved the capability of metabolites characterization [10,11]. HRMS/MS has great potential to become the gold standard in non-targeted screening [12,13], in which liquid chromatography coupled to mass spectrometry (LC-MS) provides on-line separation and accurate mass measurement to enable the elemental composition determination and confident identification. LC-MS analysis includes more than tens of thousands of features and data processing is one of the key steps to the success of metabolites profiling. One widely-used approach to assist characterizing metabolites includes in silico prediction. Based on the prior knowledge, theoretical m/z values of predicted metabolites can be calculated according to the formula of the target compound and phase I/II transformation. In silico prediction software, such as MetaSite [14], Meteor [15], StarDrop™, SyGMa [16], SOMP [17], and BioTransformer [18] were developed to provide information of sites of metabolism in the early discovery phase. However, the metabolizing enzyme database may not be complete in some software and the prediction was not always accurate for all target compounds [19]. Ellefsen et al. has conducted a suspect screening analysis and reported the metabolites of one kind of NPS, 4-methoxy-α-pyrrolidinovalerophenone (4-MeO-α-PVP), by using human hepatocyte incubation and high-resolution mass spectrometry. Among the 10 identified metabolites, only 3 metabolites matched the results from in silico prediction software that provided 14 candidates, which suggested that software didn’t readily predict and has limitations [20]. To this end, several studies have used untargeted metabolomics techniques for NPS metabolite identification [21–27], revealing that traditional metabolism studies of NPS can be supported and facilitated by metabolomics techniques for comprehensive quantitative analysis. Although the number of such works is relatively limited but this field is with a rapidly growing need. To facilitate this application, we aimed to propose an untargeted metabolic profiling workflow, including LC-MS based analysis coupled with a signal selection software programed as a web tool, that has potential to be routinely being applied for NPS metabolites screening. Besides, this approach was demonstrated by comprehensively characterizing the toxicometabolomics of 4-MeO-α-PVP by in vitro and in vivo experiments.
2. Materials and methods
2.1. Chemicals and reagents
4-MeO-α-PVP (purity ≥ 98%) was purchased from Cayman Chemicals (Ann Arbor, MI, USA). Pooled human liver S9 fraction (20 mg protein/mL, from 150 individual donors) was purchased from Corning ® UltraPoolTM (Woburn, MA, USA). NADPH Regenerating System (Solution A and Solution B) were purchased from Corning® GentestTM (Woburn, MA, USA). HPLC-grade acetonitrile (ACN, purity ≥99.9%) was purchased from J.T. Baker (Philipsburg, New Jersey, USA). The internal standard, N-(2-Hydroxybenzoyl)pyrrolidine (N2P), was obtained as powder (purity = 97%) from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Incubation of 4-MeO-α-PVP with human liver S9
The 4-MeO-α-PVP standard was firstly dissolved in the MeOH at 1 mg/mL and followed by being diluted 10 and 200 times the volume with sodium phosphate buffer (50 mM, pH 7.4) to constitute 100 ppm and 5 ppm of 4-MeO-α-PVP solution, respectively. The storage time of buffer-diluted standard solution was no longer than 1 h before enzyme incubation. Each reaction mixture containing 195 μL sodium phosphate buffer (50 mM, pH 7.4), 25 μL S9 fraction, and 2.5 μL Solution B was pre-incubated at 37 °C for approximately 3 min. The reaction was initiated with the addition of 12.5 μL Solution A and 15 μL of 100 ppm or 5 ppm 4-MeO-α-PVP solution to make 1 or 20 μM in the final mixture. Incubation with five replicates each were performed in a 37 °C water bath for 16 h. The final proportion of MeOH was less than 0.6% in the incubation mixture. Negative controls without 4-MeO-α-PVP were also prepared. Enzyme reactions were terminated by adding 250 μL cold ACN, stored at −20 °C for 30 min, and each sample was then centrifuged at 20,000×g for 20 min at 4 °C. Each vial containing 150 μL of the resulting supernatants was spiked with 0.02 μg/50 μL N2P and subsequently stored at −20 °C prior to analysis.
2.3. In vivo animal studies, urine collection, and sample preparation
Male Wistar rates with a 7-week-old weight of approximately 250 g were purchased from Bio-LASCO Taiwan Co. (Taipei, Taiwan). The material of feeding cages was polycarbonate. Breeding environments were maintained on a 12/12 h light/dark cycle (lights off at 20:00 h) at a constant temperature 20 °C and constant relative humidity 60%. Food and water were made available ad libitum. One group of male Wistar rats were administered orally with the 4-MeO-α-PVP (40 mg/kg body weight). The 4-MeOα-PVP samples were dissolved in 1 mL deionized water with the concentration corresponding to rat weight and fed to the rats by oral gavage. The used concentration of 4-MeO-α-PVP ranged from 7.4 to 11.8 mg/mL since the weight of the rat ranged from 185.3 to 295.7 g. The 4-MeO-α-PVP dissolved in water was homogenized by sonication before feeding. Another group administered the deionized water was used as a control group. Six rats were included in each group. After the drug administration, each rat was housed in individual metabolism cages for 24 h. The experimental procedures of the animal experiments were approved by the International Animal Care and Use Committee (NCKU IACUC number: 109360).
Urine samples were collected into glass conical flasks within 24 h and added with sodium azide (0.05% w/v). After collection, the urine samples were aliquoted and stored at −20 °C before analysis. According to the published procedures [28,29], aliquoted urines were centrifuged at 20,000 g at 4 °C for 20 min. To remove glucuronidation and focus on identifying phase I metabolites, the supernatant of the centrifuged urine (400 μL) was added with 40 μL β-glucuronidase (6 U) and incubated at 37 °C for 1.5 h. The pH was controlled at 6.0–6.5 as instructed by product document. The deconjugation reaction was terminated by adding 120 μL of 20% acetic acid. After centrifugation again at 20,000 g for 20 min, supernatants were diluted with 290 μL of deionized water contained internal standard N2P and submitted for ultra-high performance liquid chromatography (UHPLC)-HRMS analysis. For each prepared urine sample, the final dilution ratio was three and the concentration of internal standard was 0.05 μg/μL. Aliquot 5 μL of each prepared urine samples were mixed together as the QC sample for monitoring the instrument variation.
2.4. Liquid chromatography-tandem mass spectrometry (LC-MS) and targeted MS2 and MS3 analysis
The prepared samples were analyzed on a Waters ACQUITY UPLC system (Waters, Milford, MA) coupled with an Orbitrap Elite™ Hybrid Ion Trap-Orbitrap Mass Spectrometer system (Thermo Fisher Scientific, Bremen, Germany). Chromatographic separation was achieved on an ACQUITY UPLC BEH C18 Column (2.1 mm × 100 mm, 1.7 μm, Waters, Milford, MA) maintained at 40 °C. The mobile phase consisted of 2% (v/v) ACN with 0.1% (v/v) formic acid in deionized water (A) and 0.1% (v/v) formic acid in methanol (B) at a flow rate of 350 μL/ min with the following linear gradient: 0–2 min, 2% B; 2–16 min, 2–95% B; 16–17 min, 95% B; 17–18 min, 95-2% B; 18–20 min, 2% B.
The mass spectrometer contained a heated electrospray ionization source (HESI). The spray voltage was 3.2 kV, capillary temperature 360 °C, source heater temperature 350 °C, S-lens RF level 60%, sheath gas flow rate 30; auxiliary gas flow rate 15, and sweep gas flow 2 (manufacture’s units). Fullscan mass data were acquired in the range of m/z 100–650, with a resolution of 60,000 at m/z 200. AGC target was set at 1e6. As for the targeted MS2 analysis, parallel reaction monitoring (PRM) acquisition mode was performed to acquire MS2 spectra of all metabolite candidates. The LC condition was the same as mentioned above. The m/z values of the filtered metabolite candidates were set in the global list on the basis of the retention time. Higher energy collision dissociation (HCD)-MS2 was used to fragment the targeted ions within a 1-Da isolation window and ±4 min RT window from the RT of targeted metabolite candidates. Normalized collision energy (NCE) was set at 35%. AGC target was set at 5e4. For MS3 analysis, specific precursor ions were selected for CID fragmentation with NCE of 35% at MS2 and the fragment ions with the identical m/z value of in-source fragment candidates were further selected for HCD-MS3. Parameters of AGC value and resolution were identical to that for the MS2 method. Source fragmentation function was enabled and applied with additional collision energy of 0, 5, 20, and 40% for investigating in-source fragments.
2.5. Data processing
All the raw data files from UHPLC-MS analysis were converted to a peak list table, including m/z values, retention times (RTs), and signal abundances by using Progenesis QI software (version 2.0; Non-Linear Dynamics, a Waters company, Newcastle upon Tyne, United Kingdom) for feature detection, alignment, and quantification. Default parameters were used as follows: peak picking limits: automatic; maximum ion charge: 20; quantitation: relative quantitation; isotope number: 0). In order to remove noise and enable enough data points (≥10) to be collected for the quantification, custom filtering was then performed to extract feature signals based on the following parameters: (1)m/z charge state≤2, (2) peak width >0.05 min, (3) RT 0–20 min. To adjust differences among each sample, the abundance of individual peaks was subsequently normalized by spiked internals standard, N2P, before the metabolite candidate selection.
2.6. A web tool, MetaboFinder, for signal selection
Selected features were further submitted to an in-house developed software, MetaboFinder, for mining metabolite candidates. MetaboFinder, which can be access through https://cooperation.shinyapps.io/olsda/, was programed as a public web tool. Feature list including feature ID, m/z value, RT, and two group of sample abundance can be uploaded. Basic instruction was provided in Fig. S1 (https://www.jfda-online.com/cgi/viewcontent.cgi?filename=0&article=3447&context=journal&type=additional) while an example of input file can be download from the website. For this study, data obtained from different groups were processed according to setting m/z 0–1000, RT 0–20 min, and RT window 0–0.5 min. Raw abundance was normalized by N2P signal (feature ID: 38) by choosing the “Type of normalization” as “by internal standard”. After the abundance normalization, t-test and fold change calculation were performed in the software to determine the significantly changed features which was visualized by volcano plot and outputted as a list. Parameters used were set as below: XY-axis of volcano plot, fold change-p value of t test; Log base of fold change, 2. Differentially expressed features found in negative control samples were excluded. It is noted that some real metabolites may not have concentration-dependent difference between low and high concentration samples and will not be sieved out during peak selection process.
2.7. Chemical structure identification
The workflow of identifying metabolite chemical structure was illustrated in Fig. S2 (https://www.jfda-online.com/cgi/viewcontent.cgi?filename=0&article=3447&context=journal&type=additional). MS1 and MS2 spectra from targeted analysis with accurate masses (mass error <5 ppm) of each metabolite candidate were inputted to SIRIUS 4 software version 4.0.1 for generating tentative molecular formula by integrating high-resolution isotope pattern analysis and fragmentation trees [30]. The candidate formula with highest score was considered as the possible molecular formula. Chemical structure of each metabolite formula was inferred from that of parent compound, 4-MeO-α-PVP, based on biotransformation routes. For instance, if a mass difference between one molecular formula and parent compound equals to one hydroxyl group, this molecular formula was assigned with all possible hydroxylation structures. After compiling a tentative chemical structure list, the Mass Frontier™ software (version 7.0, Waters) was used to generate in silico MS2 fragment profile of each structure candidate. If the top 5 intense fragments in the experimental MS2 spectrum matched to the corresponding in silico predicted fragments with mass error <5 ppm, the inferred structure was considered as the putative chemical structure of the metabolite candidate. In addition to the Mass Frontier, alternative in silico MS/MS fragmentation tools such as CSI:FingerID [31] and MS-FINDER [32] can also be implemented in this step.
3. Results and discussion
3.1. Workflow for general identification of NPS metabolites
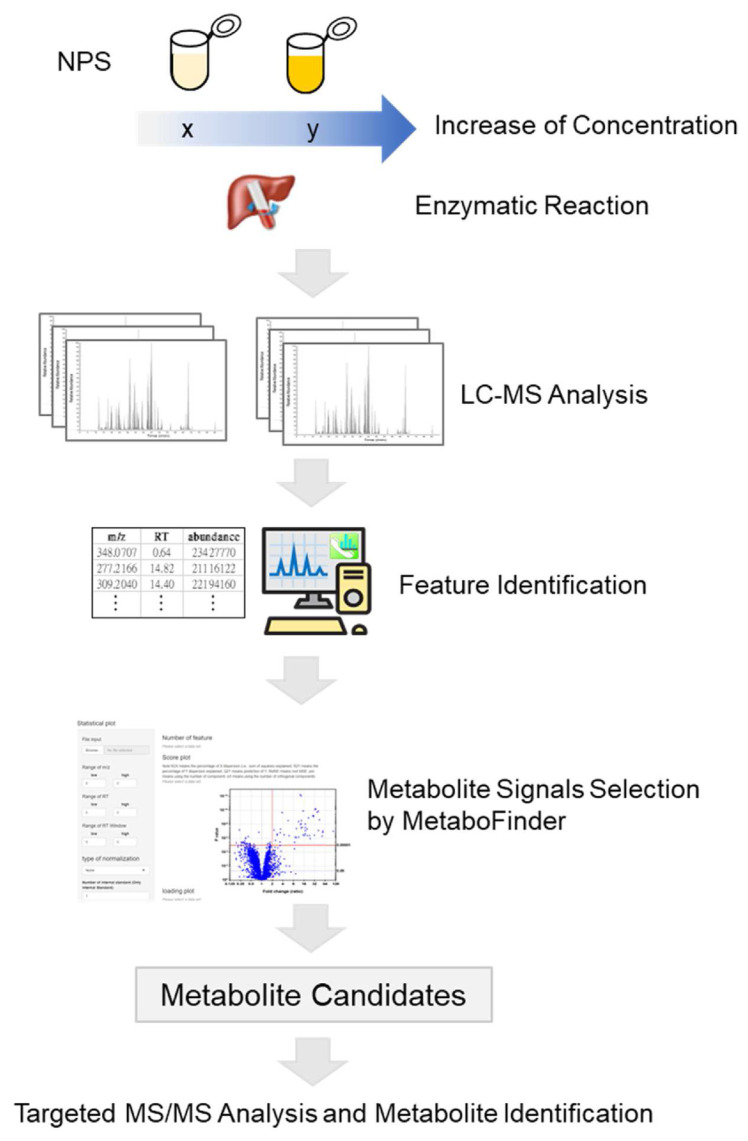
The experimental scheme employed in this study is depicted in Fig. 1. NPS with high or low concentration were both incubated with human liver S9 for the conversion from parent compounds into their metabolites. Samples were analyzed by LC-HRMS. The retention time alignment and feature identification of raw MS data from individual sample was performed. Generated features were further submitted to statistical analysis by using an in-house software, MetaboFinder. Significantly changed features were filtered out and submitted to targeted LC-MS/MS analysis for identification.
Fig. 1.
Analytical approach. One NPS, 4-MeO-α-PVP, with different concentration were incubated with human liver S9 followed by LC-MS analysis. Features in the raw LC-MS files were identified by Progenesis QI software. Matched features were further submitted to statistical analysis by using a web tool, MetaboFinder. Differentially expressed features were filtered out and identified by targeted LC-MS/MS analysis.
3.2. Selection of metabolite candidates
To demonstrate this strategy, 4-MeO-α-PVP, a substituted cathinone, was used as a NPS parent compound. Both 1 μM and 20 μM of 4-MeO-α-PVP along with a blank sample (each 5 replicates), spiked with N2P as an internal standard, were incubated with human liver S9 for 16 h and followed by LC-HRMS analysis. To reveal the data points, raw LC-MS data were processed by Progenesis QI to get 22081 features from all samples, and 4640 of which were sieved out based on custom filtering (listed in Table S1 (https://www.jfda-online.com/cgi/viewcontent.cgi?filename=1&article=3447&context=journal&type=additional)). Selected features were further submitted to an in-house developed software, MetaboFinder, for mining metabolite candidates (Fig. 2). As for 4-MeO-α-PVP, each 5 replicates of 4640 features from 1 μM to 20 μM conditions were processed by setting m/z 0–1000, RT 0–20 min, RT window 0–0.5 min and normalized by N2P signal (m/z 192.1024) abundance. After the abundance normalization, t-test p value and fold change of each normalized feature were calculated and illustrated as volcano plot (Fig. 3). As depicted in the volcano plot, 50 features, located in the upper-right panel, were considered as 4-MeO-α-PVP metabolite candidates for they show significant change (p < 0.00001 and fold change >2) between two different concentration of parent compound (listed in Table S2 (https://www.jfda-online.com/cgi/viewcontent.cgi?filename=1&article=3447&context=journal&type=additional)). The significance level (p < 0.00001) used in our study has been adjusted by dividing 0.05 with feature number (~5000) according to Bonferroni procedure [33,34]. Fold change greater than 2 was used based on reference [35]. It is noted that some real metabolites might not have concentration-dependent difference between low and high concentration samples and will not be sieved out during peak selection process.
Fig. 2.
MetaboFinder. An in-house constructed web tool (https://cooperation.shinyapps.io/olsda/) that can process the peak list from LC-MS analysis including m/z, RT, and intensity information. Significantly changed features can be selected out by statistical analysis, visualized by volcano plot, and outputted as a list.
Fig. 3.
Low vs high-dose treatments from in vitro experiment. Comparing the fold change and p-value of individual metabolites. Vertical dashed lines mark the two-fold change and horizontal lines mark the p-value cut-off of 0.00001. The upper right quadrants contained significant metabolites upregulated in comparison to low-dose treatment with a p-value<0.00001 and greater than two-fold change.
3.3. Targeted LC-MS/MS analysis and chemical structure elucidation for metabolite identifications
Targeted LC-MS/MS analysis were performed on the 50 selected potential metabolites. Although MS2 spectra were collected for the selected candidates, no spectral or structural database was available for most NPS metabolites, which hampered the metabolites identification. To facilitate the process, molecular formula for each feature was obtained based on measured m/z value and MS2 spectrum by using SIRIUS 4 and that with the highest score was accepted [30]. The potential chemical structures were manually calculated based on biotransformation rules for each formula, which was also inputted to Mass Frontier software for generating in silico MS2 fragments. A total of 26 metabolites (M01~M19, M0′, M02′A, M02′B, M03′, M04′, and M08′), as listed in Table 1, were identified. Take M02 as an example, M02 was identified from feature 17. Its extracted ion chromatogram (EIC) showed the retention time at 6.7 min and measured m/z value at 248.1651 (Fig. 4A). The molecular formula of M02 peak is proposed as C15H21NO2 by SIRIUS 4 software while chemical structure was manually calculated as 4-hydroxy-α-pyrrolidinovalerophenone (4-OH-α-PVP) based on biotransformation routes, which has the mass error of 2.45 ppm compared to the theoretical value. It was proposed that 4-OH-α-PVP derived from the O-demethylation of the 4-methoxy moiety in the aromatic ring of 4-MeO-α-PVP. As depicted in Fig. 4B, MS2 spectrum containing fragments at m/z 126.1279, 121.0285, 135.0442, and 177.0913 confirmed the identification. The mass errors of product ions between experimental data and Mass Frontier™ predication data were all below 3 ppm. The metabolite, 4-OH-α-PVP, has been reported as the dominant metabolite derived from incubation of 4-MeO-α-PVP with human liver microsomes and human hepatocyte [20].
Table 1.
Characteristics of the 26 identified 4-MeO-α-PVP potential metabolites.
| Metabolite ID | Measured m/z | RT (min) | Abundance ordera | Literatureb | In vivo Experiment | Fold change | t-test p-value | Chemical information | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Chemical formulac | Theoretical m/zd | Mass error (ppm) | Proposed structure | ||||||||
| M0 | 262.1807 | 8.63 | 4-MeO-α-PVP | ● | 33.9 | 5.87 × 10−11 | C16H23NO2 | 262.1802 | 2.05 |

|
|
| M01 | 246.1494 | 9.61 | 12 (1.1%) | ● | 17.4 | 8.17 × 10−8 | C15H19NO2 | 246.1488 | 2.52 |

|
|
| M02 | 248.1651 | 6.71 | 1 (100%) | M3 | ● | 12.7 | 1.06 × 10−8 | C15H21NO2 | 248.1652 | 2.45 |

|
| M03 | 250.1808 | 6.18 | 21 (0.5%) | M2 | ● | 16.4 | 3.87 × 10−7 | C15H23NO2 | 250.1802 | 2.50 |

|
| M04 | 260.1651 | 11.34 | 3 (4.0%) | M10 | ● | 13.1 | 8.58 × 10−13 | C16H21NO2 | 260.1645 | 2.22 |

|
| M05 | 262.1444 | 11.09 | 11 (1.3%) | ● | 17.5 | 3.11 × 10−8 | C15H19NO3 | 262.1438 | 2.16 |

|
|
| M06 | 264.1601 | 6.38 | 28 (0.3%) | ● | 11.2 | 6.76 × 10−7 | C15H21NO3 | 264.1594 | 2.58 |

|
|
| M07 | 264.1602 | 5.84 | 22 (0.5%) | ● | 8.9 | 6.83 × 10−7 | C15H21NO3 | 264.1594 | 2.91 |

|
|
| M08 | 264.1965 | 8.30 | 4 (3.8%) | M7 | 28.8 | 7.67 × 10−10 | C16H25NO2 | 264.1958 | 2.53 |

|
|
| M09 | 266.1754 | 6.58 | 15 (0.7%) | ● | 107.6 | 1.02 × 10−7 | C15H23NO3 | 266.1750 | 1.52 |

|
|
| M10 | 274.1443 | 9.79 | 48 (0.04%) | ● | 2.7 | 3.56 × 10−7 | C16H19NO3 | 274.1438 | 1.83 |

|
|
| M11 | 274.1444 | 10.12 | 19 (0.6%) | ● | 3.8 | 2.09 × 10−10 | C16H19NO3 | 274.1438 | 2.23 |

|
|
| M12 | 276.1599 | 8.63 | 5 (2.7%) | ● | 15.0 | 3.38 × 10−7 | C16H21NO3 | 276.1594 | 1.88 |

|
|
| M13 | 276.1600 | 12.35 | 6 (2.5%) | M11 | ● | 54.8 | 9.47 × 10−8 | C16H21NO3 | 276.1594 | 2.09 |

|
| M14 | 278.1757 | 6.56 | 45 (0.1%) | ● | 31.5 | 3.57 × 10−7 | C16H23NO3 | 278.1751 | 2.04 |

|
|
| M15 | 278.1757 | 8.38 | 8 (2.4%) | M4 | ● | 21.2 | 1.25 × 10−9 | C16H23NO3 | 278.1751 | 2.22 |

|
| M16 | 280.1550 | 6.56 | 13 (0.9%) | ● | 9.6 | 7.50 × 10−7 | C15H21NO4 | 280.1543 | 2.50 |

|
|
| M17 | 280.1913 | 8.61 | 10 (1.4%) | M5 | ● | 23.7 | 1.07 × 10−6 | C16H25NO3 | 280.1907 | 2.03 |

|
| M18 | 294.1706 | 8.63 | 2 (26.6%) | M6 | ● | 17.2 | 2.25 × 10−7 | C16H23NO4 | 294.1700 | 1.88 |

|
| M19 | 308.1498 | 10.96 | 14 (0.8%) | ● | 19.6 | 4.02 × 10−7 | C16H21NO5 | 308.1492 | 1.95 |

|
|
| In-source collision derived compounds | |||||||||||
| M0′ | 191.1071 | 8.63 | 7 (2.5%) | — | — | 15.0 | 1.25 × 10−10 | 191.1067* | 2.09 |

|
|
| M02′A | 126.1280 | 6.71 | 31 (0.2%) | — | — | 13.4 | 2.87 × 10−9 | C8H16N+ | 126.1278* | 1.59 |

|
| M02′B | 177.0914 | 6.71 | 26 (0.4%) | — | — | 5.6 | 3.01 × 10−9 | 177.0911* | 1.69 |

|
|
| M03′ | 232.1702 | 6.18 | 27 (0.3%) | — | — | 4.3 | 3.26 × 10−7 | C15H22NO+ | 232.1696* | 2.58 |

|
| M04′ | 175.1121 | 11.34 | 33 (0.2%) | — | — | 2.2 | 6.53 × 10−8 | C12H15O+ | 175.1118* | 1.71 |

|
| M08′ | 246.1859 | 8.30 | 9 (2.3%) | — | — | 29.9 | 7.23 × 10−10 | C16H24NO+ | 246.1853* | 2.44 |

|
Abundance ratio: abundance/max abundance (M02, m/z: 248.1651, RT: 6.71 min).
4-MeO-α-PVP metabolite identifier reported in the literature [20].
Calculated by SIRIUS 4 software using MS1 and MS2 spectra.
The theoretical m/z for all the ions are [M+H]+, except the ion denoted with a start means the ion is [M]+.
Fig. 4.
EIC and MS2 spectra of M02. (A) M02 were observed at 6.7 min with m/z value at 248.1651. (B) Fragments at m/z 121.0285, 126.1279, 135.0442, and 177.0913 confirmed the identification as 4-OH-α-PVP.
Among the 26 identified chemical structure, M0′, M02′A, M02′B, M03′, M04′, and M08′ were considered to be derived from their precursor ions, M0, M02~04, and M08, by in-source fragmentation, meeting the following three conditions: (1) the suspected in-source fragment coeluted with its precursor ion; (2) in-source fragmentation ion must exist in the MS2 spectrum of precursor ion; (3) MS2 spectrum of the in-source fragmentation ion was consistent with the MS3 spectrum from the precursor ion. Take M02 and M02′B as examples. M02 (m/z 248.1651, singly charged) and M02′B (m/z 177.0914, singly charged) both eluted at 6.7 min and were identified as 4-OH-α-PVP and 1-(4-hydroxyphenyl)-1-oxopentan-2-ylium, respectively (Fig. 5A and B). The structure of M02′B was a loss of pyrrolidine from M02. Besides, M02′B was observed in the MS2 spectrum of M02. The MS3 scan of m/z 177.0914 which was one of the product ion of M02 (Fig. 5C) showed similar pattern with product ion pattern from M02′B in full scan data (Fig. 5D). We further changed the collision energy of in-source fragmentation and found that as the collision energy increased, the abundance of M02′B that was produced from M02 increased (Fig. 5E). An in-source collision fragment identified is a proof that the precursor compound of which did present in the MS dataset. Here, a total of 19 metabolites were putatively identified as 4-MeO-α-PVP metabolites.
Fig. 5.
Metabolites derived from in-source fragmentation. (A) EIC of M02 (m/z 248.1651, singly charged) and (B) its in-source fragment, M02′B (m/z 177.0914, singly charged). (C) The MS3 scan of one product ion (m/z 177.0914) of M02. (D) Product ion pattern from M02′B. (E) Abundance of M02 and M02′B under different collision energy of in-source fragmentation.
Ten 4-MeO-α-PVP metabolites identified with full scan and MS2 spectra has been reported in Ellefsen et al.’s work [20]. It is noted that all the reported 4-MeO-α-PVP metabolites in Ellefsen et al.’s work was also found in our study, with the exception of two that yielded from O-demethylation and N-dealkylation of the pyrrolidine ring of M02 (C11H15NO2, m/z 194.1182) and aliphatic N-oxidation (C16H23NO3, m/z 278.1749). We speculated that this metabolite may be generated specifically from incubation with human hepatocytes. The metabolic pathway of 19 identified 4-MeO-α-PVP metabolites were illustrated in Fig. 6. Eight metabolites that has been reported in the previous literature were presented with solid-line arrow and 2 metabolites presented with dash line arrows indicated that it has been reported but was not found in our study. Eleven novel 4-MeO-α-PVP metabolites identified by using our strategy were shown with red arrow. For these newly identified targets, M01 and M07 derived from M02 through dehydrogenation and hydroxylation, respectively. M14 was the hydroxylated metabolite of parent compound and M06 was the O-demethylated form of M14. M13 was the results of carbonylation of the pyrrolidine ring, which is a common biotransformation for α-pyrrolididinophrnones [36]. We detected that it further converted to a O-demethylated form, M05. Another pyrrolidine ring opening and hydroxylated metabolite (M17) generated M09, M12, and M19 through O-demethylation, di-dehydrogenation, and di-hydroxylation reactions, respectively. M16 was the hydroxylated plus dehydrogenated form of M09. M10 was another hydroxylated metabolite of the parent compound which was converted to M11 through dehydrogenation reaction. The metabolites at m/z 194.1182 and 277.1672 were not found it our strategy, which may suggest that they might be specific metabolites from hepatocyte incubation.
Fig. 6.
Proposed metabolic pathway of 19 identified 4-MeO-α-PVP metabolites. Every metabolite is labeled by its metabolite identifier as listed in Table 1 and theoretical m/z value. Matabolites with black arrows were reported in the literature [20]; dotted arrows refer to metabolites that were reported in the literature but were not found in our study; red arrows represent newly identified metabolites of 4-MeO-α-PVP. Metabolic reactions were annotated on the arrows.
3.4. In vivo confirmation of identified metabolites
It is important to note that although incubation with enzymes reflects liver metabolism, additional processes were also observed in organism, such as extrahepatic metabolism and enterohepatic circulation existed. Since particular enzyme metabolism only represent one aspect of drug elimination, it is vital to confirm the identified metabolites in in vivo specimens. To highlight the capability of our strategy, the presence of the 19 metabolites derived from human liver S9 fraction incubation was confirmed in rat urine specimens. Six rats for each group were administered orally 0 or 40 mg/kg body weight of 4-MeO-α-PVP and their urines were collected after 24 h. After sample preparation, each sample was submitted to targeted MS2 analysis focused on the 19 identified metabolites. The presence of 18 metabolites were confirmed in the rat urine with both MS1 and MS2 analyses (as annotated in Table 1). All of them showed higher abundance in high dose compared to zero dose of intake (Fig. 7A). Take two newly discovered 4-MeO-α-PVP metabolites, M05 and M07, as examples. M05 contained characteristic fragments, m/z 86.0597, 98.0597, 107.0488, 140.1067, 159.0801, 177.0907, 244.1327 that derived from the structural information of parent compound, the carbonylation of the pyrrolindine, and O-demethylation of the 4-methoxy moiety (Fig. 7B). In Fig. 7C, characteristic fragments, m/z 70.0647, 88.0752, 107.0487, 121.0280, 142.1223, 177.0904, 246.1585 illustrated that M07 contained the structure of parent compound and the O-demethylation of the 4-methoxy moiety followed by introduction of the second hydroxyl group at the para-position. MS2 spectra that were obtained from the in vivo animal experiment were identical to that from the in vitro experiments. The EIC (from both high and zero doses) and MS2 spectra of the other 16 metabolites were provided in Supporting Fig. S3 (https://www.jfda-online.com/cgi/viewcontent.cgi?filename=0&article=3447&context=journal&type=additional). It is speculated that M08 was not detected in the rat urine may be due to its tendency to transform into M03. The presence of 18 metabolites obtained from in vitro experiment confirmed the results from in vitro experiment.
Fig. 7.
Confirmation of 18 metabolites in rat urine. (A) Heatmap of the log 10 abundance of parent drug and 18 metabolites from rats being administered orally with 40 or 0 mg/kg of 4-MeO-α-PVP. MS2 spectra of (B) M05 and (C) M07.
4. Conclusions
Lots of works need to be conducted to comprehensively identify metabolomics profiles for certain NPS. Here, we presented a workflow coupling LC-MS analysis, data processing by in-house statistical tool, and structure identification, to assist traditional metabolism studies, which may be applied for routine characterization of metabolites derived from NPS or other drugs. The MetaboFinder software was designed for the one-step drug metabolite candidate discovery. Users can do the m/z and retention time-based feature filtering, automatic data normalization, and univariate/multivariate statistical analysis all in this software, which may facilitate the metabolites profiling process. The feasibility of this workflow has been demonstrated for comprehensively characterizing 4-MeO-α-PVP metabolites. A total of 11 novel metabolites of 4-MeO-α-PVP were putatively characterized by in vitro and in vivo experiment. In general, there was a good agreement between them. A limitation of in vivo experiment conducted here was that it was based on an animal model. Confirmation in real samples from human consumers is required for further use of these metabolites. In principle, this procedure can be also applied to analyze metabolites of other NPS. We believe that aside from developing comprehensive knowledge to metabolomes, advances and ease of use in the analytical workflow can eliminate the loading and time for evaluation of NPS, which could facilitate the establishment of monitoring system for public health.
Supplementary Information
Acknowledgments
This work was supported by the National Science and Technology Council, Taiwan [grant number MOST109-2113-M-006-015, and MOST110-2113-M-006-014]. The authors gratefully acknowledge the use of ICP00401 and MS004000 equipment belonging to the Core Facility Center of National Cheng Kung University, and the National Taiwan University Consortia of Key Technologies, National Taiwan University Instrumentation Center, and the Metabolomics Core Facility, Scientific Instrument Center at Academia Sinica.
Funding Statement
This work was supported by the National Science and Technology Council, Taiwan [grant number MOST109-2113-M-006-015, and MOST110-2113-M-006-014].
Footnotes
Conflict of interest
The authors declare no competing financial interest.
References
- 1. Pizzolato TM, de Alda MJL, Barceló D. LC-based analysis of drugs of abuse and their metabolites in urine. Trends Anal Chem. 2007;26:609–24. [Google Scholar]
- 2. Richter LHJ, Maurer HH, Meyer MR. New psychoactive substances: studies on the metabolism of XLR-11, AB-PINACA, FUB-PB-22, 4-methoxy-α-PVP, 25-I-NBOMe, and meclonazepam using human liver preparations in comparison to primary human hepatocytes, and human urine. Toxicol Lett. 2017;280:142–50. doi: 10.1016/j.toxlet.2017.07.901. [DOI] [PubMed] [Google Scholar]
- 3. Richter LHJ, Maurer HH, Meyer MR. Metabolic fate of the new synthetic cannabinoid 7′N-5F-ADB in rat, human, and pooled human S9 studied by means of hyphenated high-resolution mass spectrometry. Drug Test Anal. 2019;11:305–17. doi: 10.1002/dta.2493. [DOI] [PubMed] [Google Scholar]
- 4. de Coning E, Stølsvik G. United Nations Office on drugs and Crime. Int J Mar Coast Law. 2013;28:189–204. [Google Scholar]
- 5.United Nations Office on Drugs Crime. World drug report 2021. United Nations: 2022. [Google Scholar]
- 6. Cooper ZD. Adverse effects of synthetic cannabinoids: management of acute toxicity and withdrawal. Curr Psychiatr Rep. 2016;18:52. doi: 10.1007/s11920-016-0694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meyer MR. New psychoactive substances: an overview on recent publications on their toxicodynamics and toxicokinetics. Arch Toxicol. 2016;90:2421–44. doi: 10.1007/s00204-016-1812-x. [DOI] [PubMed] [Google Scholar]
- 8.Meyer MR. Toxicokinetics of NPS: update 2017. In: Maurer HH, Brandt SD, editors. New psychoactive substances: pharmacology, clinical, forensic and analytical toxicology. Cham: Springer International Publishing; 2018. pp. 441–59. [DOI] [PubMed] [Google Scholar]
- 9.Wagmann L, Maurer HH. Bioanalytical methods for new psychoactive substances. In: Maurer HH, Brandt SD, editors. New psychoactive substances: pharmacology, clinical, forensic and analytical toxicology. Cham: Springer International Publishing; 2018. pp. 413–39. [DOI] [PubMed] [Google Scholar]
- 10. Dona AC, Kyriakides M, Scott F, Shephard EA, Varshavi D, Veselkov K, et al. A guide to the identification of metabolites in NMR-based metabonomics/metabolomics experiments. Comput Struct Biotechnol J. 2016;14:135–53. doi: 10.1016/j.csbj.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiao JF, Zhou B, Ressom HW. Metabolite identification and quantitation in LC-MS/MS-based metabolomics. Trends Anal Chem. 2012;32:1–14. doi: 10.1016/j.trac.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maurer HH, Meyer MR. High-resolution mass spectrometry in toxicology: current status and future perspectives. Arch Toxicol. 2016;90:2161–72. doi: 10.1007/s00204-016-1764-1. [DOI] [PubMed] [Google Scholar]
- 13. Meyer MR, Maurer HH. Review: LC coupled to low- and high-resolution mass spectrometry for new psychoactive substance screening in biological matrices–where do we stand today? Anal Chim Acta. 2016;927:13–20. doi: 10.1016/j.aca.2016.04.046. [DOI] [PubMed] [Google Scholar]
- 14. Cruciani G, Carosati E, De Boeck B, Ethirajulu K, Mackie C, Howe T, et al. MetaSite: understanding metabolism in human cytochromes from the perspective of the chemist. J Med Chem. 2005;48:6970–9. doi: 10.1021/jm050529c. [DOI] [PubMed] [Google Scholar]
- 15. Langowski J, Long A. Computer systems for the prediction of xenobiotic metabolism. Adv Drug Deliv Rev. 2002;54:407–15. doi: 10.1016/s0169-409x(02)00011-x. [DOI] [PubMed] [Google Scholar]
- 16. Ridder L, Wagener M. SyGMa: combining expert knowledge and empirical scoring in the prediction of metabolites. ChemMedChem: Chem Enab Drug Discov. 2008;3:821–32. doi: 10.1002/cmdc.200700312. [DOI] [PubMed] [Google Scholar]
- 17. Rudik A, Dmitriev A, Lagunin A, Filimonov D, Poroikov V. SOMP: web server for in silico prediction of sites of metabolism for drug-like compounds. Bioinformatics. 2015;31:2046–8. doi: 10.1093/bioinformatics/btv087. [DOI] [PubMed] [Google Scholar]
- 18. Djoumbou-Feunang Y, Fiamoncini J, Gil-de-la-Fuente A, Greiner R, Manach C, Wishart DS. BioTransformer: a comprehensive computational tool for small molecule metabolism prediction and metabolite identification. J Cheminf. 2019;11:1–25. doi: 10.1186/s13321-018-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diao X, Huestis MA. New synthetic cannabinoids metabolism and strategies to best identify optimal marker metabolites. Front Chem. 2019;7:109. doi: 10.3389/fchem.2019.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ellefsen KN, Wohlfarth A, Swortwood MJ, Diao X, Concheiro M, Huestis MA. 4-Methoxy-alpha-PVP: in silico prediction, metabolic stability, and metabolite identification by human hepatocyte incubation and high-resolution mass spectrometry. Forensic Toxicol. 2016;34:61–75. doi: 10.1007/s11419-015-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Manier SK, Keller A, Schäper J, Meyer MR. Untargeted metabolomics by high resolution mass spectrometry coupled to normal and reversed phase liquid chromatography as a tool to study the in vitro biotransformation of new psychoactive substances. Sci Rep. 2019;9:1–11. doi: 10.1038/s41598-019-39235-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mortele O, Vervliet P, Gys C, Degreef M, Cuykx M, Maudens K, et al. In vitro Phase I and Phase II metabolism of the new designer benzodiazepine cloniprazepam using liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. J Pharm Biomed Anal. 2018;153:158–67. doi: 10.1016/j.jpba.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 23. Vervliet P, Mortelé O, Gys C, Degreef M, Lanckmans K, Maudens K, et al. Suspect and non-target screening workflows to investigate the in vitro and in vivo metabolism of the synthetic cannabinoid 5Cl-THJ-018. Drug Test Anal. 2019;11:479–91. doi: 10.1002/dta.2508. [DOI] [PubMed] [Google Scholar]
- 24. Manier SK, Wagmann L, Flockerzi V, Meyer MR. Toxicometabolomics of the new psychoactive substances α-PBP and α-PEP studied in HepaRG cell incubates by means of untargeted metabolomics revealed unexpected amino acid adducts. Arch Toxicol. 2020;94:2047–59. doi: 10.1007/s00204-020-02742-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steuer AE, Kaelin D, Boxler MI, Eisenbeiss L, Holze F, Vizeli P, et al. Comparative untargeted metabolomics analysis of the psychostimulants 3,4-methylenedioxy-methamphetamine (MDMA), amphetamine, and the novel psychoactive substance mephedrone after controlled drug administration to humans. Metabolites. 2020:10. doi: 10.3390/metabo10080306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Montesano C, Vincenti F, Fanti F, Marti M, Bilel S, Togna AR, et al. Untargeted metabolic profiling of 4-fluorofuranylfentanyl and isobutyrylfentanyl in mouse hepatocytes and urine by means of LC-HRMS. Metabolites. 2021:11. doi: 10.3390/metabo11020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manier SK, Schwermer F, Wagmann L, Eckstein N, Meyer MR. Liquid chromatography-high-resolution mass spectrometry-based in vitro toxicometabolomics of the synthetic cathinones 4-MPD and 4-MEAP in pooled human liver microsomes. Metabolites. 2020;11:3. doi: 10.3390/metabo11010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Want EJ, Wilson ID, Gika H, Theodoridis G, Plumb RS, Shockcor J, et al. Global metabolic profiling procedures for urine using UPLC-MS. Nat Protoc. 2010;5:1005–18. doi: 10.1038/nprot.2010.50. [DOI] [PubMed] [Google Scholar]
- 29. Hsu J-F, Tien C-P, Shih C-L, Liao P-M, Wong HI, Liao P-C. Using a high-resolution mass spectrometry-based metabolomics strategy for comprehensively screening and identifying biomarkers of phthalate exposure: method development and application. Environ Int. 2019;128:261–70. doi: 10.1016/j.envint.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 30. Dührkop K, Fleischauer M, Ludwig M, Aksenov AA, Melnik AV, Meusel M, et al. SIRIUS 4: a rapid tool for turning tandem mass spectra into metabolite structure information. Nat Methods. 2019;16:299–302. doi: 10.1038/s41592-019-0344-8. [DOI] [PubMed] [Google Scholar]
- 31. Dührkop K, Shen H, Meusel M, Rousu J, Böcker S. Searching molecular structure databaseswith tandemmass spectra using CSI: FingerID. Proc Natl Acad Sci USA. 2015;112:12580–5. doi: 10.1073/pnas.1509788112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsugawa H, Kind T, Nakabayashi R, Yukihira D, Tanaka W, Cajka T, et al. Hydrogen rearrangement rules: computational MS/MS fragmentation and structure elucidation using MS-FINDER software. Anal Chem. 2016;88:7946–58. doi: 10.1021/acs.analchem.6b00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abdi H. Bonferroni and Šidák corrections for multiple comparisons. Encycl Measur Stat. 2007;3:103–7. [Google Scholar]
- 34.Portney LG, Watkins MP. Foundations of clinical research: applications to practice. Saddle River, NJ: Pearson/Prentice Hall Upper; 2009. [Google Scholar]
- 35. Kumar N, Hoque M, Sugimoto M. Robust volcano plot: identification of differential metabolites in the presence of outliers. BMC Bioinf. 2018;19:1–11. doi: 10.1186/s12859-018-2117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zaitsu K, Katagi M, Tsuchihashi H, Ishii A. Recently abused synthetic cathinones, α-pyrrolidinophenone derivatives: a review of their pharmacology, acute toxicity, and metabolism. Forensic Toxicol. 2014;32:1–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.