Abstract
There were few reports about antibiotic residues in egg-containing products. In the study, an effective method for the simultaneous determination of 24 sulfonamide antibiotics in two instant pastries based on a modified QuEChERS sample preparation technique coupled with ultra performance liquid chromatography-tandem mass spectrometry was developed. The results show that the average recoveries of the SAs at 5, 10, and 50 μg kg−1 levels were 67.6%–103.8%, with relative standard deviations (RSD) of 0.80–9.23%. The limit of detections (LODs) and limit of quantitations (LOQs) were 0.01–0.14 μg kg−1 and 0.02–0.45 μg kg−1, respectively. This method was suitable for analysis of 24 SAs in instant pastries.
Keywords: Instant pastries, QuEChERS, Sulfonamide antibiotics, Ultra performance liquid chromatography-tandem mass spectrometry
1. Introduction
According to market sales data, approximately 210,000 tons of antibiotics, including quinolones, sulfonamides, tetracyclines, and chloramphenicol, are estimated to be produced each year in China [1], making China the world’s largest producer and consumer of antibiotics. Sulfonamide antibiotics (SAs) are a general term for a class of antibiotics whose structure features a para-amino benzene sulfonamide moiety and were the earliest antibacterial drugs used for treatment [2]. In addition to their strong antibacterial activities [3], as well as their anti-cancer, anti-fungal, and anti-parasitic properties, SAs are also used to promote growth of animals [4]. The SAs most frequently applied in veterinary medicine are sulfadiazine (SDZ), sulfamerazine (SMR), sulfamonomethoxine (SMM), and sulfamethoxazole (SMX), etc [5]. Their mechanisms of action involve the inhibition of bacterial nucleoprotein synthesis by competitively inhibiting the synthesis of para-aminobenzoic acid, which is an intermediate in the synthesis of folic acid [6]. Many of these drugs are not fully metabolized in livestock and poultry and are excreted in their raw form from the animals through their feces [1]. When these excretions are prepared into organic fertilizers and used for crop cultivation, the presence of these antibiotics in the soil may enhance microbial resistance [7]. Moreover, since SAs are highly polar and soluble in water, they are easily transferred from the soil to surface and groundwater [8]. Unmetabolized antibiotics in animals may also migrate to foods derived from those animals, such as milk, eggs, and meat, resulting in antibiotic residues or metabolites in those foods [9], which poses a potential threat to human health. For example, antibiotic residues in the body can lead to antibiotic resistance, disruption of normal intestinal flora, and hypersensitivity reactions [10].
To ensure proper food safety and human health, many countries and regions have set maximum residue limit (MRL) standards for SAs in food. For example, the MRL in Brazil is 100 μg kg−1 for the sum of SAs in liver and other tissues [2], while the MRL for SAs in biological tissues and milk in the EU is also 100 μg kg−1 [11]. The Chinese national food safety standard GB 31650-2019 set the MRLs for SAs in foods (muscle, fat, liver, kidney, milk, etc.) at 25–100 μg kg−1 [12]. Given how low these limits are, it is very important to develop methods to measure the amounts of SA residues in agricultural products for monitoring the safety of agricultural products.
Several analytical methods have been developed to determine the composition of SAs in food and environmental samples. These methods include high-performance liquid chromatography (HPLC) [13], enzyme-linked immunosorbent assays (ELISAs) [14], capillary electrophoresis (CE) [15], HPLC coupled with tandem mass spectrometry (HPLC-MS/MS) [2,16,17], and ultra-high performance supercritical fluid chromatography (UHPSFC) [18], Among them, HPLC-MS/MS operating in multiple reaction monitoring (MRM) mode demonstrates significant advantages over the other methods, such as the ability to conduct high-throughput analysis, as well as selectivity and specificity; therefore, it has been widely applied veterinary applications to measure the levels of antibiotic residues. Sample treatment is a key step for ensuring the effective extraction and purification of different classes of drugs from animal tissue [19]. Compared to other pretreatment techniques, such as magnetic solid phase extraction (SPE) [20], solid-phase micro-extraction (SPME) [21], magnetic solid phase extraction (MSPE) [22], dispersive solid phase extraction (d-SPE) [23], pressurized liquid extraction (PLE) [24], and liquid–liquid micro-extraction (LLME) [25], the quick, easy, cheap, effective, rugged, and safe (QuEChERS) [3,26] sample preparation method enables higher recovery and accuracy as well as less exposure to harmful solvents. Petrarca et al. [3] developed a method that the determination of 10 SAs in complex infant formula matrices by QuEChERS, which showed good linearity in the concentration range of 5–120 μg kg−1 (R2 ≥ 0.991). Xu et al. [4] developed a method that surface-enhanced Raman spectroscopy combined with QuEChERS was used to determine sulfadiazine and sulfathiazole in swine urine. The method has a better precision with relative standard deviation (RSD) of 1.53%–5.18%. Abafe et al. [26] established a modified QuEChERS method for the simultaneous analysis of 10 SAs in animal and aquaculture fish tissues. The recoveries of analytes were generally satisfactory and typically fell between 80% and 113%.
In recent years, studies on SA residues have focused on animal products such as eggs and meat as well as environmental samples. In particular, there are many studies reporting on antibiotic residues in eggs, but there are few reports about antibiotic residues in egg-containing products (e.g. cakes and cookies), as antibiotic residues in raw materials could also be transferred to their finished products. In this study, we aimed to establish an ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method for the simultaneous determination of 24 SAs in cakes and pastries. Because of the complexity of pastry matrices, it was necessary to explore a very reasonable sample pretreatment technology to achieve the simultaneous determination of multiple SAs. For the QuEChERS method, although most of the standard operations remained unmodified in this study, the main steps of the QuEChERS method should be optimized to account for the variations in complexity between different samples. To ensure that the method could achieve high accuracy and sensitivity for different samples, we improved the QuEChERS method and optimized the UPLC-MS/MS detection conditions with satisfactory results.
2. Material and methods
2.1. Chemicals and reagents
Sulfaguanidine was obtained from INTERNATIONAL LABORATORY (New Jersey, USA), Sulfanilamide, Sulfisomidine, Trimethoprim, Sulfacetamide, Sulfathiazole, Sulfadiazine, Sulfapyridine, Sulfamerazine, Sulfamoxol, Sulfamethizole, Sulfamethazine, Sulfamethoxypyridazine, Sulfameter, Sulfamonomethoxine, Sulfachlorpyridazine, Sulfadoxine, Sulfamethoxazole, Sulfisoxazole, Sulfabenzamide, Sulfaquinoxaline, Sulfadimethoxine, Sulfaphenazole and Sulfanitran were obtained from Dr EhrenstorferGmbH (Augsburg, Germany). HPLC-grade acetonitrile (ACN) was obtained from Merck (Darm-stadt, Germany), methanol, acetone and ethyl acetate were purchased from Thermo Fisher (Waltham, USA). Hexane, formic acid and ammonium acetate were purchased from Dikma (Lake Forest, USA). Superior pure petroleum ether was purchased from Tianjin Kemiou Chemical Reagent (Tianjin, China). Sodium chloride (NaCl) and magnesium sulfate anhydrous were purchased from Tianjin Yongda Chemical Reagent (Tianjin, China) and were of analytical grade. Primary secondary amine (PSA) was supplied by Dikma (Lake Forest, USA). C18 was purchased from Welch (40–50 mm, Maryland, USA). Ultrapure water was prepared using a Millipore Milli-Q Gradient Water Purification System (Massachusetts, USA).
2.2. Standard solution preparation
Individual standard solutions of the 24 SAs of interest were prepared in ACN at a concentration of 1.0 mg mL−1. A working solution (100 μg mL−1) of each SA was prepared by diluting each standard solution with methanol 10-fold. The working solutions were then diluted furtherly with a 21:79 (v/v) mixture of acetonitrile and 0.1% formic acid solution to prepare solvent calibration standard solutions with different concentrations of 0.1, 0.2, 0.5, 1, 2, 5, 10, 20, 50, and 100 ng mL−1. The pastry samples (cakes and cookies) without the target SAs were extracted as described in extraction and purification (Section 2.5) to obtain a blank matrix solution. Matrix-matched calibration standard solutions were prepared in matrix solution containing different analytes contents. All standards solutions were stored at −20 °C in a bottle, and freshly prepared solvent or matrix standards were used before each analysis.
2.3. UPLC-MS/MS analysis conditions
A UPLC-MS/MS instrument (Exion-TRILPLE QUAD 5500, AB SCIEX, USA) was used to analyze the 24 target compounds. The LC unit was used to separate the target compounds using an Agilent ZORBAX XDB-C18 column (4.6 mm × 100 mm, 1.8 μm) held at 40 °C. The injection volume and flow rate were 3 μL and 0.3 mL min−1, respectively. The mobile phase was composed of 0.1% formic acid solution (A) and acetonitrile (B), and a linear-gradient elution program was set as follows: 0–7.5 min 21% B, 7.5–7.6 min 21–40% B, 7.6–11.0 min 40% B, 11.0–11.1 min 40–75% B, 11.1–15 min 75% B, 15.0–15.1 min 75–21% B and 15.1–18.0 min 21%. Ionization was carried out using an electrospray ionization (ESI) source in the positive mode, and mass spectrometric analysis was carried out in MRM mode. The parameters for MS/MS were set as follows: spray voltage, 5500 V; vaporizer temperature, 550 °C; nebulizing gas pressure, 55 psi; auxiliary gas pressure, 55 psi; and curtain gas pressure, 40 psi. Table 1 showed the optimized MRM data acquisitions.
Table 1.
Mass spectrometric parameters of 24 kinds of SAs.
| Compound | Retention time (min) | Precursor ion (m/z) | Declustering potential (V) | Product ion (m/z) | Collision energy (eV) |
|---|---|---|---|---|---|
| Sulfaguanidine | 3.32 | 215.2 | 58.1, 71.2 | 156.0a, 108.1 | 20.3, 30.9 |
| Sulfanilamide | 3.93 | 173.0 | 136.2, 106.9 | 93.0a, 108.1 | 30.2, 22.0 |
| Sulfisomidine | 3.90 | 279.2 | 81.8, 78.1 | 124.2a, 186.0 | 27.5, 23.0 |
| Trimethoprim | 4.48 | 291.2 | 77.0, 60.3 | 230.1a, 261.1 | 31.5, 34.8 |
| Sulfacetamide | 5.18 | 215.1 | 103.9, 116.0 | 92.0a, 156.1 | 29.0, 14.6 |
| Sulfathiazole | 5.47 | 256.1 | 83.8, 83.8 | 156.1a, 108.2 | 21.0, 31.8 |
| Sulfadiazine | 5.67 | 251.1 | 59.7, 48.9 | 156.2a, 108.0 | 22.0, 33.0 |
| Sulfapyridine | 6.04 | 250.1 | 73.3, 82.1 | 156.0a, 184.2 | 23.0, 23.9 |
| Sulfamerazine | 7.05 | 265.1 | 40.3, 61.1 | 156.1a, 172.0 | 23.1, 23.0 |
| Sulfamoxol | 7.20 | 268.2 | 88.0, 81.0 | 156.2a, 108.0 | 23.7, 33.4 |
| Sulfamethizole | 8.19 | 271.0 | 49.2, 45.1 | 156.0a, 108.2 | 20.8, 31.3 |
| Sulfamethoxypyridazine | 8.59 | 281.0 | 83.2, 77.9 | 156.2a, 92.1 | 24.0, 37.9 |
| Sulfamethazine | 8.81 | 279.2 | 85.1, 77.0 | 124.1a, 186.2 | 32.1, 24.1 |
| Sulfameter | 9.12 | 281.1 | 55.0, 61.1 | 156.1a, 108.2 | 24.9, 33.12 |
| Sulfamonomethoxine | 11.24 | 281.1 | 56.9, 18.1 | 156.2a, 108.2 | 24.2, 35.1 |
| Sulfachlorpyridazine | 12.19 | 285.1 | 82.0, 83.1 | 156.2a, 108.1 | 21.6, 34.0 |
| Sulfadoxine | 12.56 | 311.1 | 82.1, 73.1 | 156.0a, 108.1 | 24.1, 33.0 |
| Sulfamethoxazole | 12.64 | 254.1 | 106.9, 84.9 | 156.1a, 108.2 | 22.3, 31.8 |
| Sulfisoxazole | 13.02 | 268.0 | 87.7, 100.2 | 156.2a, 113.0 | 19.6, 21.8 |
| Sulfabenzamide | 13.99 | 277.1 | 72.9, 76.9 | 156.1a, 92.0 | 17.8, 38.8 |
| Sulfaquinoxaline | 14.16 | 301.1 | 38.0, 47.9 | 156.0a, 92.1 | 23.2, 39.7 |
| Sulfadimethoxine | 14.27 | 371.2 | 55.0, 39.8 | 156.2a, 108.1 | 27.4, 37.9 |
| Sulfaphenazole | 14.56 | 315.2 | 90.2, 93.5 | 156.2a, 108.2 | 27.7, 38.1 |
| Sulfanitran | 15.18 | 336.3 | 155.2, 153.8 | 156.2a, 294.0 | 17.1, 16.9 |
Quantifying ions.
2.4. Sample collection and preparation
Cake and cookie samples were purchased from local supermarkets in Shijiazhuang, Hebei Province, China. The cake sample was ground in a mortar and placed in a clean self-sealing bag. Then, it was stored in a refrigerator at 4 °C away from light until use. Blank samples were used for validation studies and matrix-matched standard calibrations for quantitation. The blank samples were spiked with 20 μg kg−1 standards solutions and left for 10 min before the extraction.
2.5. Extraction and purification
For the extraction of SAs, the ground pastry samples (1.00 g) were added to a 50 mL centrifuge tube, followed by 2 mL water, and the mixture was vortexed for 30 s. Then, 10 mL acetonitrile was added. After vortexing and ultrasonicating the mixture for 10 min, 1.5 g NaCl was added, and the mixture was vortexed again for 30 s. The mixture was then centrifuged at 8000 rpm for 5 min at 4 °C.
For purification of the SAs after extraction, the supernatant (7.5 mL) obtained from centrifugation was transferred to a 15 mL centrifuge tube containing 0.4 g C18 and 0.4 g MgSO4. Then, the mixture was vortexed for 30 s, left to stand for 2 min, and then centrifuged for 5 min at 8000 rpm. The super-natant (5 mL) was evaporated to dryness under a stream of nitrogen at 40 °C. The residue was dissolved in 1 mL of a 21:79 (v/v) mixture of acetonitrile and 0.1% formic acid solution. The mixture was vortexed for 30 s and passed through a 0.20 μm nylon filter membrane before UPLC-MS/MS analysis.
3. Results and discussion
3.1. Chromatographic conditions
Optimization of the chromatographic conditions, such as packing, the particle size, and the composition of the mobile phase, can increase the sensitivity and improve the peak shape of analytes. To obtain the optimal chromatographic conditions for the target compounds, we performed separations on three different-sized column sizes for comparison when acetonitrile was used as mobile phase B and 0.1% formic acid solution was used as mobile phase A. On the Zorbax Eclipse Plus C18 (2.1 × 100 mm, 1.8 μm), 15 SAs appeared in 3 min with poor separation (Fig. S1 (https://www.jfda-online.com/cgi/viewcontent.cgi?filename=5=article=3434=context=journal=type=additional)). In comparison, the 24 SAs were better separated, and the response abundance of sulfadimethoxine, sulfaquinoxaline and sulfaphenazole was significantly enhanced on the Eclipse XDB-C18 (4.6 × 50 mm, 1.8 μm) column (Fig. S2 (https://www.jfda-online.com/cgi/viewcontent.cgi?filename=5=article=3434=context=journal=type=additional)). Furtherly, the separation performance was investigated through increasing the length of the column. Compared to the other two columns, the Zorbax Eclipse XDB-C18 (4.6 × 100 mm, 1.8 μm) column demonstrated significantly better separation with sharp and symmetrical peak shapes, accompanying higher abundance (Fig. 1).
Fig. 1.
Chromatograms of 24 kinds of SAs. (50 μg·L−1). (1). Sulfaguanidine, (2). Sulfanilamide, (3). Sulfisomidine, (4). Trimethoprim, (5). Sulfacetamide, (6). Sulfathiazole, (7). Sulfadiazine, (8). Sulfapyridine, (9). Sulfamerazine, (10). Sulfamoxol, (11). Sulfamethizole, (12). Sulfamethoxypyridazine, (13). Sulfamethazine, (14). Sulfameter, (15). Sulfamonomethoxine, (16). Sulfachlorpyridazine, (17). Sulfadoxine, (18). Sulfamethoxazole, (19). Sulfisoxazole, (20). Sulfabenzamide, (21). Sulfaquinoxaline, (22). Sulfadimethoxine, (23). Sulfaphenazole, (24). Sulfanitran.
When developing the UPLC-MS/MS method, adjusting and optimizing the composition of the mobile phase is beneficial for the formation of ions and improving the peak shape [27]. Therefore, we assessed different mobile phases consisting of methanol or acetonitrile as component B and HPLC-grade water, 0.1% formic acid solution, or 0.1% formic acid solution (containing 5 mM ammonium acetate) as component A on the separation of the 24 SAs targets. When 0.1% formic acid solution was used as component A, the separation performance was compared using acetonitrile or methanol as component B. Compared to acetonitrile (Fig. 1), the response abundance of sulfanilamide was very low and the peak shape of trimethoprim was broad (Fig. S3 (https://www.jfda-online.com/cgi/viewcontent.cgi?filename=5=article=3434=context=journal=type=additional)) when methanol was component B. Using acetonitrile as component B, the response abundance of sulfanilamide and sulfanitran were low and sulfaguanidine appeared as split peak when water was used as component A (Fig. S4 (https://www.jfda-online.com/cgi/viewcontent.cgi?filename=5=article=3434=context=journal=type=additional)). After adding 0.1% formic acid in component A, the response abundance of sulfanilamide and sulfanitran became high and the split peak disappeared (Fig. 1). However, 5 mM ammonium acetate in 0.1% formic acid solution as component A made the response abundance of sulfanitran to disappear (Fig. S5 (https://www.jfda-online.com/cgi/viewcontent.cgi?filename=5=article=3434=context=journal=type=additional)). Therefore, 0.1% formic acid solution and acetonitrile were chosen as component A and component B, respectively. In addition, the gradient elution method shown in Section 2.3 resulted in a better separation in a shorter period of time than the other elution methods. Within 18 min, all 24 compounds were well-separated. The total ion flow chromatogram of all 24 SA standards is shown in Fig. 1.
3.2. Optimization of QuEChERS for analysis of the SAs in the cake samples
3.2.1. Optimization of the extraction
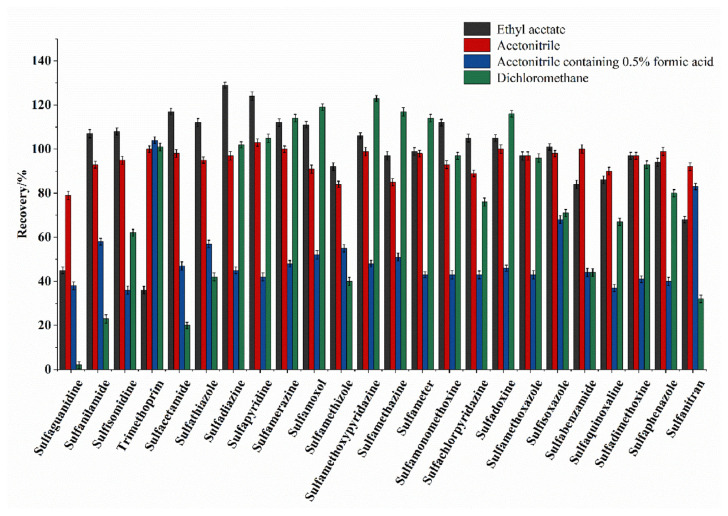
In this experiment, cakes were used as test samples to explore the effects of the extraction solvent, volume of the solvent, and type of purification agent on the recovery of the 24 spiked SAs from the cake samples. Since the protein and fat content of the cakes were high, they easily emulsified during the extraction, making it difficult to separate the target analytes from the other components in the sample, potentially affecting the reliability of the experiments [28]. Therefore, the selection of a suitable extraction solvent is very important for ensuring optimal analytical performance of the method. We compared the ability of different solvents, including acetonitrile, 0.5% formic acid in acetonitrile, ethyl acetate, petroleum ether, hexane, and dichloromethane to effectively extract the analytes.
First, the blank cake sample (1.00 g) was spiked with 20 μg kg−1 of each SA, and the extraction and purification procedures were performed as described in section 2.5 with the different extraction solvents, and the 0.4 g C18 + 0.4 g MgSO4 was used as the purifying sorbent. When either petroleum ether or hexane was used as the extraction solvent, more impurities were obtained after evaporation of the organic phase under nitrogen. The recoveries of most of the analytes were below 58% when acetonitrile containing 0.5% formic acid was used as the extraction solvent. The recovery of sulfaguanidine was only 2.2% when dichloromethane was used as the extraction solvent. The recoveries of trimethoprim and sulfaguanidine were 35.8% and 45.1% when ethyl acetate was used as the extraction solvent. Therefore, we determined that acetonitrile was the optimal extraction solvent because the recoveries of the 24 different SAs were highest (79.7–103.2%) (Fig. 2).
Fig. 2.
Recoveries of 24 kinds of SAs with different extractant.
Using acetonitrile as the extraction solvent, the effect of different volumes of acetonitrile on the recoveries of the SAs were compared. The recoveries of the 24 SAs were 74.1–118.2% when the volume of acetonitrile was 10 mL, which were collectively higher than the corresponding recoveries when 6 mL, 8 mL, 12 mL, and 14 mL of acetonitrile were used (Fig. 3). The decrease of recoveries may be due to the increase in impurities induced by increasing extractant volume [29]. Therefore, 10 mL of acetonitrile was finally chosen considering both the solvent dosage and recovery.
Fig. 3.
Recoveries of 24 kinds of SAs with different extractant volume.
3.2.2. Optimization of purification procedure
Interferents in the extraction solution can affect the chromatographic separation and the response values of the target, and they may contaminate the ion source; therefore, further purification of the extraction residues using adsorbents is required prior to LC-MS detection. Primary secondary amine (PSA), octadecyl silica (C18), and MgSO4 are often used as sorbents for purification. PSA is often used for the removal of fatty acids, sugars, organic acids, lipids, and some pigments, while C18 is effective for the removal of lipids. Moreover, MgSO4 is used as a desiccant [30].
In this study, different combinations and amounts of sorbents were investigated to determine which sorbent (or combination thereof) was optimal for removal of interfering substances from the extraction residues. After spiking the blank pastry samples with the 24 SAs (20 μg kg−1), they underwent extraction with acetonitrile, and the residues were purified with different sorbents as explained in section 2.5. For each sample, the recovery efficiencies of each of the 24 SAs using seven different sets of sorbents (PSA; C18; PSA + C18; PSA + MgSO4; C18 + MgSO4; MgSO4; C18 + PSA + MgSO4) were compared by purifying the sample extracts spiked with 20 μg kg−1 of the 24 target SAs.
The results are shown in Table 2. The PSA sorbent showed a strong adsorption capacity to sulfisomidine, sulfadoxine and sulfabenzamide, so the recoveries were low (6.7–16.4%). The other three sorbents without PSA were able to better purify the 24 SAs from the other component of each sample, enabling recoveries of each SA above 74.1%. Based on the results shown in Table 2, a mixture of 0.2 g C18 and 0.3 g MgSO4 was selected as optimal sorbent, with the corresponding recoveries of the 24 SAs between 79.4% and 97.9%.
Table 2.
Recoveries of 24 kinds of SAs with different purification sorbents (%).
| Compound | Unpurified | PSA | C18 | MgSO4 | PSA+C18 | PSA+MgSO4 | C18+MgSO4 | PSA+C18+MgSO4 |
|---|---|---|---|---|---|---|---|---|
| Sulfaguanidine | 52.4 | 90.0 | 94.8 | 74.1 | 143.5 | 170.2 | 79.4 | 29.5 |
| Sulfanilamide | 74.9 | 81.0 | 106.1 | 101.4 | 71.1 | 29.8 | 86.4 | 5.0 |
| Sulfisomidine | 70.2 | 16.4 | 103.5 | 94.5 | 44.6 | 1.9 | 83.4 | 2.2 |
| Trimethoprim | 97.0 | 107.6 | 112.3 | 97.9 | 95.0 | 90.2 | 96.5 | 89.7 |
| Sulfacetamide | 83.4 | 93.7 | 108.0 | 105.6 | 62.1 | 22.1 | 90.8 | 7.4 |
| Sulfathiazole | 68.1 | 84.9 | 104.0 | 106.9 | 46.1 | 19.4 | 84.6 | 1.5 |
| Sulfadiazine | 86.5 | 92.3 | 115.2 | 120.9 | 46.9 | 7.6 | 88.3 | 3.6 |
| Sulfapyridine | 80.1 | 65.7 | 110.5 | 122.1 | 61.6 | 10.1 | 85.7 | 3.5 |
| Sulfamerazine | 85.0 | 36.1 | 124.9 | 110.1 | 42.9 | 10.2 | 86.7 | 3.7 |
| Sulfamoxol | 70.5 | 48.0 | 113.8 | 106.5 | 69.9 | 8.6 | 83.8 | 2.8 |
| Sulfamethizole | 66.9 | 70.4 | 106.9 | 103.0 | 47.1 | 8.5 | 85.3 | 1.5 |
| Sulfamethoxypyridazine | 76.3 | 66.8 | 111.3 | 109.8 | 45.8 | 7.9 | 94.0 | 3.1 |
| Sulfamethazine | 73.4 | 55.0 | 113.0 | 110.6 | 50.8 | 8.7 | 92.9 | 3.9 |
| Sulfameter | 76.9 | 22.4 | 111.7 | 103.4 | 59.5 | 1.8 | 87.8 | 8.7 |
| Sulfamonomethoxine | 80.5 | 63.9 | 119.2 | 110.1 | 51.2 | 18.4 | 94.3 | 4.1 |
| Sulfachlorpyridazine | 77.4 | 46.0 | 111.0 | 100.7 | 57.2 | 6.7 | 88.8 | 5.4 |
| Sulfadoxine | 76.3 | 10.2 | 113.9 | 110.6 | 58.2 | 1.7 | 92.5 | 7.4 |
| Sulfamethoxazole | 76.4 | 22.2 | 114.9 | 102.4 | 57.1 | 2.4 | 92.8 | 8.3 |
| Sulfisoxazole | 75.2 | 90.8 | 106.5 | 102.0 | 107.4 | 124.3 | 79.5 | 49.3 |
| Sulfabenzamide | 74.0 | 6.7 | 104.3 | 95.9 | 57.7 | 1.4 | 87.1 | 11.4 |
| Sulfaquinoxaline | 74.8 | 30.4 | 105.7 | 96.2 | 55.5 | 7.2 | 95.7 | 6.1 |
| Sulfadimethoxine | 77.6 | 10.1 | 108.5 | 105.5 | 63.8 | 1.2 | 97.9 | 7.9 |
| Sulfaphenazole | 67.4 | 31.3 | 103.0 | 94.1 | 60.0 | 3.2 | 91.9 | 9.0 |
| Sulfanitran | 69.3 | 75.7 | 101.1 | 81.9 | 70.6 | 62.4 | 81.3 | 101.0 |
Recovery(%) = (Cspiked − Cmatrix)/Cmatrix standard ×100, Cspiked, Cmatrix and Cmatrix standard represent the concentrations of spiked, unspiked samples and matrix standard, respectively.
Since C18 and MgSO4 enabled the highest recoveries of the 24 SAs, the purification efficiencies of the 24 SAs using different amounts of C18 and MgSO4 were determined. Based on the results shown in Table 3, we determined that a mixture of 0.4 g C18 and 0.4 g MgSO4 was the most effective combination of sorbents for the purification of the cake samples, with recoveries of 91.0–110.5%.
Table 3.
Recoveries of 24 kinds of SAs with different purification sorbents dosages (%).
| Compound | C18 (with 0.3 g MgSO4) | MgSO4 (with 0.4 g C18) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 0.3 g | 0.4 g | 0.5 g | 0.3 g | 0.4 g | 0.5 g | |
| Sulfaguanidine | 63.8 | 84.2 | 77.3 | 81.4 | 91.0 | 83.4 |
| Sulfanilamide | 79.5 | 93.4 | 103.6 | 95.7 | 98.0 | 96.8 |
| Sulfisomidine | 79.3 | 95.7 | 86.9 | 81.3 | 104.6 | 99.7 |
| Trimethoprim | 78.6 | 95.2 | 87.3 | 83.7 | 103.7 | 100.6 |
| Sulfacetamide | 78.3 | 96.8 | 100.9 | 90.2 | 104.4 | 107.5 |
| Sulfathiazole | 84.7 | 91.2 | 96.7 | 94.3 | 96.2 | 99.0 |
| Sulfadiazine | 90.4 | 92.7 | 102.8 | 97.7 | 102.7 | 110.2 |
| Sulfapyridine | 90.4 | 98.2 | 98.2 | 100.3 | 101.7 | 102.0 |
| Sulfamerazine | 89.5 | 93.9 | 92.8 | 98.8 | 101.1 | 99.9 |
| Sulfamoxol | 83.5 | 85.2 | 103.9 | 96.1 | 103.2 | 96.6 |
| Sulfamethizole | 79.8 | 89.6 | 99.4 | 95.5 | 100.1 | 94.2 |
| Sulfamethoxypyridazine | 76.1 | 100.0 | 106.9 | 99.3 | 96.3 | 94.9 |
| Sulfamethazine | 93.4 | 93.3 | 104.5 | 105.2 | 109.2 | 99.7 |
| Sulfameter | 92.6 | 100.1 | 104.9 | 97.8 | 102.2 | 99.2 |
| Sulfamonomethoxine | 81.5 | 88.9 | 96.3 | 109.4 | 99.9 | 110.3 |
| Sulfachlorpyridazine | 79.3 | 93.2 | 121.9 | 97.9 | 101.9 | 96.8 |
| Sulfadoxine | 81.4 | 101.0 | 107.2 | 111.8 | 108.7 | 108.6 |
| Sulfamethoxazole | 85.1 | 92.2 | 105.9 | 103.5 | 110.0 | 103.3 |
| Sulfisoxazole | 86.9 | 90.1 | 103.8 | 98.9 | 99.5 | 103.7 |
| Sulfabenzamide | 80.3 | 89.6 | 96.2 | 97.5 | 98.1 | 95.7 |
| Sulfaquinoxaline | 84.1 | 93.6 | 92.2 | 97.2 | 104.0 | 92.8 |
| Sulfadimethoxine | 86.9 | 103.3 | 89.8 | 96.6 | 110.5 | 100.2 |
| Sulfaphenazole | 84.2 | 92.3 | 107.9 | 95.3 | 104.2 | 100.1 |
| Sulfanitran | 77.5 | 85.1 | 87.0 | 87.9 | 91.1 | 89.8 |
Recovery(%) = (Cspiked−Cmatrix)/Cmatrix standard ×100, Cspiked, Cmatrix and Cmatrix standard represent the concentrations of spiked, unspiked samples and matrix standard, respectively.
3.3. Method validation
3.3.1. Matrix effects
The presence of interferences in sample matrices can cause enhancements and suppressions in the analyte signals. This is considered to be an important source of error in the quantification of foods using LC-MS [31]. One of the methods for calculating the matrix effect (ME) entails comparing the slope of the solvent standard curve with the matrix standard curve [27], while another method entails comparing the peak area of an analyte at a representative concentration in a matrix extract to the corresponding peak area of the analyte in an organic solvent [26]. The ME can be calculated using the following equation: ME (%) = AX/AS × 00%, wherein AS is the slope of the solvent standard curve, and AX is the slope of the matrix-matched standard curve. A ME of 80–120% is considered small and can be ignored [27]. The experimental ME results of the 24 SAs in the cake and cookie samples are shown in Table 4. When the calculated ME was lower than 80% or higher than 120%, there were different degrees of inhibition or enhancement, which should be corrected with matrix-matched standard solutions.
Table 4.
Matrix effects of cakes and cookies (%).
| Compound | Cake | Cookie |
|---|---|---|
| Sulfaguanidine | 52.4 | 84.2 |
| Sulfanilamide | 93.4 | 90.6 |
| Sulfisomidine | 56.7 | 61.2 |
| Trimethoprim | 59.8 | 67.1 |
| Sulfacetamide | 50.4 | 66.0 |
| Sulfathiazole | 84.8 | 79.0 |
| Sulfadiazine | 68.5 | 68.1 |
| Sulfapyridine | 88.4 | 77.9 |
| Sulfamerazine | 82.5 | 81.8 |
| Sulfamoxol | 93.7 | 80.6 |
| Sulfamethizole | 99.7 | 87.7 |
| Sulfamethoxypyridazine | 95.9 | 80.1 |
| Sulfamethazine | 98.1 | 81.5 |
| Sulfameter | 93.4 | 80.0 |
| Sulfamonomethoxine | 92.4 | 89.8 |
| Sulfachlorpyridazine | 81.7 | 89.0 |
| Sulfadoxine | 92.2 | 93.9 |
| Sulfamethoxazole | 92.3 | 92.9 |
| Sulfisoxazole | 89.7 | 97.3 |
| Sulfabenzamide | 94.0 | 102.7 |
| Sulfaquinoxaline | 98.3 | 91.6 |
| Sulfadimethoxine | 96.0 | 83.6 |
| Sulfaphenazole | 87.5 | 46.5 |
| Sulfanitran | 89.9 | 51.2 |
Matrix effect (ME, %) = AX / AS ×100, AS and AX are the slope of the solvent standard curve and matrix-matched standard curve, respectively. Signal suppression: ME < 80%. Signal enhancement: ME > 120%. When ME is in the range of 80–120%, the matrix effect can be ignored.
3.3.2. Linearity, limit of detection (LOD), and limit of quantitation (LOQ)
Standard curves were generated by plotting the areas under the curves of the SAs against their concentrations (0.1, 0.2, 0.5, 1, 2, 5, 10, 20, 50, and 100 ng mL−1). Some of the SAs that exhibited matrix effects were calibrated using matrix standard curves. The correlation coefficients (R) were calculated after linear regression of the standard curves. Moreover, the LOD and LOQ were defined based on the three times and ten times standard deviation of the lowest concentration level response of standard curves and the slope obtained in the matrix-matched calibration curves, respectively [32]. The results of the standard curve experiments with the 24 SAs are shown in Table 5. The LODs and LOQs of all 24 SAs were 0.01–0.14 μg kg−1 and 0.03–0.45 μg kg−1, respectively. In addition, all standard curves were highly linear, with R ≥ 0.9990.
Table 5.
Linear range, linear equations, LODs and LOQs of 24 SAs in initial mobile phase.
| Compound | Calibration curve | r | Linearity Range (ng·mL−1) | LODa (μg·kg−1) | LOQb (μg·kg−1) |
|---|---|---|---|---|---|
| Sulfaguanidine | y = 98589.3x+22812.9 | 0.9998 | 0.2–100 | 0.05 | 0.17 |
| Sulfanilamide | y = 66188.1x+11505.3 | 0.9996 | 0.5–100 | 0.10 | 0.34 |
| Sulfisomidine | y = 472585.0x+490428.0 | 0.9990 | 0.1–100 | 0.01 | 0.02 |
| Trimethoprim | y = 215224.0x+64217.0 | 0.9998 | 0.1–100 | 0.01 | 0.03 |
| Sulfacetamide | y = 49776.6x+11408.8 | 0.9999 | 0.5–100 | 0.09 | 0.30 |
| Sulfathiazole | y = 136076.0x+6236.8 | 0.9996 | 0.2–100 | 0.03 | 0.12 |
| Sulfadiazine | y = 152251.0x+19593.8 | 0.9999 | 0.1–100 | 0.03 | 0.09 |
| Sulfapyridine | y = 214809.0x+162672.0 | 0.9995 | 0.1–100 | 0.01 | 0.03 |
| Sulfamerazine | y = 163287.0x+9159.3 | 0.9998 | 0.1–100 | 0.01 | 0.04 |
| Sulfamoxol | y = 160903.0x+15221.7 | 0.9999 | 0.1–100 | 0.02 | 0.06 |
| Sulfamethizole | y = 83717.3x+3512.5 | 0.9999 | 0.2–100 | 0.05 | 0.17 |
| Sulfamethoxypyridazine | y = 206949.0x+47387.3 | 0.9997 | 0.1–100 | 0.03 | 0.09 |
| Sulfamethazine | y = 161170.0x+108189.0 | 0.9992 | 0.5–100 | 0.14 | 0.45 |
| Sulfameter | y = 112411.0x+49259.3 | 0.9996 | 0.2–100 | 0.05 | 0.16 |
| Sulfamonomethoxine | y = 78473.4x+10125.3 | 0.9994 | 0.5–100 | 0.10 | 0.32 |
| Sulfachlorpyridazine | y = 99075.3x+2670.7 | 0.9998 | 0.1–100 | 0.02 | 0.07 |
| Sulfadoxine | y = 526485.0x+707291.0 | 0.9990 | 0.1–100 | 0.01 | 0.03 |
| Sulfamethoxazole | y = 159030.0x+18961.0 | 0.9998 | 0.1–100 | 0.02 | 0.07 |
| Sulfisoxazole | y = 137731.0x+13285.1 | 0.9999 | 0.1–100 | 0.02 | 0.06 |
| Sulfabenzamide | y = 249640.0x+135729.0 | 0.9997 | 0.1–100 | 0.01 | 0.04 |
| Sulfaquinoxaline | y = 112452.0x+37347.7 | 0.9999 | 0.1–100 | 0.02 | 0.06 |
| Sulfadimethoxine | y = 458534.0x+448431.0 | 0.9993 | 0.1–100 | 0.01 | 0.03 |
| Sulfaphenazole | y = 119221.0x+96506.0 | 0.9994 | 0.1–100 | 0.01 | 0.03 |
| Sulfanitran | y = 15751.8x+4390.2 | 0.9996 | 0.2–100 | 0.03 | 0.11 |
Limit of detection, LOD = 3 × standard deviation of the response at the lowest concentration / slope of the calibration curve.
Limit of quantitations, LOQ = 10 × standard deviation of the response at the lowest concentration / slope of the calibration curve.
3.3.3. Recovery and precision of standard addition
To evaluate the accuracy of using the recoveries, blank cake and cookie samples were spiked with 24 SAs at three different concentrations (5.0, 10.0, and 50.0 μg kg−1), and seven parallel samples were run at each concentration. The matrix calibration curves were used to calculate the recoveries and precisions of the SAs in the cake and cookie samples. The recoveries of the 24 SAs in cakes and cookies are shown in Tables 6 and 7, respectively. The average recoveries of the three spiked concentrations in the cakes were 79.6–103.3%, with relative standard deviations (RSD, n=7) of 0.80–6.48%. The mean recoveries of the three spiked concentrations in the cookies were 67.6–103.8%, with relative standard deviations (RSD, n = 7) of 1.02–9.23%. Based on these results, we determined that this method was advantageous for the rapid, accurate, and sensitive detection of 24 different SAs in the cakes and cookies.
Table 6.
The recoveries and RSDs of 24 kinds of SAs in cakes (n = 7).
| 50 μg·kg−1 | Spike level 5 μg·kg−1 |
Spike level 10 μg·kg−1 |
Spike level 50 μg·kg−1 |
|||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Recoverya(%) | RSDb(%) | Recoverya(%) | RSDb(%) | Recoverya(%) | RSDb(%) | |
| Sulfaguanidine | 79.9 | 4.01 | 81.4 | 3.82 | 79.9 | 3.88 |
| Sulfanilamide | 80.1 | 2.90 | 84.5 | 4.94 | 81.9 | 3.69 |
| Sulfisomidine | 79.8 | 1.73 | 92.7 | 3.54 | 98.9 | 2.46 |
| Trimethoprim | 80.4 | 4.64 | 90.4 | 3.97 | 80.9 | 4.57 |
| Sulfacetamide | 91.1 | 4.86 | 91.6 | 3.33 | 103.3 | 2.09 |
| Sulfathiazole | 80.1 | 1.93 | 82.5 | 4.26 | 81.7 | 1.75 |
| Sulfadiazine | 88.3 | 5.12 | 82.6 | 2.25 | 80.8 | 3.30 |
| Sulfapyridine | 83.0 | 3.56 | 91.3 | 5.78 | 84.7 | 2.38 |
| Sulfamerazine | 82.7 | 7.56 | 91.2 | 4.59 | 81.8 | 1.69 |
| Sulfamoxol | 79.6 | 1.27 | 79.6 | 4.95 | 81.1 | 3.49 |
| Sulfamethizole | 82.7 | 1.47 | 80.8 | 5.01 | 80.5 | 5.45 |
| Sulfamethoxypyridazine | 86.0 | 5.39 | 82.0 | 3.40 | 81.0 | 3.01 |
| Sulfamethazine | 82.0 | 5.12 | 83.0 | 2.55 | 84.9 | 2.25 |
| Sulfameter | 80.5 | 0.80 | 85.6 | 2.77 | 84.6 | 3.69 |
| Sulfamonomethoxine | 86.9 | 3.94 | 85.5 | 4.56 | 84.4 | 5.42 |
| Sulfachlorpyridazine | 83.9 | 5.30 | 80.0 | 4.08 | 89.8 | 2.84 |
| Sulfadoxine | 88.2 | 4.48 | 87.0 | 2.69 | 88.6 | 4.14 |
| Sulfamethoxazole | 90.9 | 2.42 | 89.7 | 2.74 | 85.2 | 3.23 |
| Sulfisoxazole | 87.7 | 4.41 | 89.6 | 2.26 | 90.1 | 3.84 |
| Sulfabenzamide | 79.6 | 3.04 | 83.8 | 2.47 | 84.6 | 3.87 |
| Sulfaquinoxaline | 82.6 | 3.98 | 80.3 | 2.56 | 86.9 | 3.82 |
| Sulfadimethoxine | 84.4 | 3.93 | 85.1 | 2.86 | 92.4 | 2.98 |
| Sulfaphenazole | 80.5 | 4.92 | 80.4 | 1.31 | 88.3 | 2.96 |
| Sulfanitran | 81.8 | 6.48 | 91.4 | 5.03 | 79.6 | 5.02 |
, Cspiked, i, Cmatrix and Cmatrix standard represent the concentrations of spiked, unspiked samples and matrix standard, respectively; n = 7.
Relative standard deviation
Table 7.
The recoveries and RSDs of 24 kinds of SAs in cookies (n = 7).
| Compound | Spike level | Spike level | Spike level | |||
|---|---|---|---|---|---|---|
| 5 μg·kg−1 | 10 μg·kg−1 | 50 μg·kg−1 | ||||
|
|
|
|
||||
| Recoverya(%) | RSDb(%) | Recoverya(%) | RSDb(%) | Recoverya(%) | RSDb(%) | |
| Sulfaguanidine | 67.8 | 6.81 | 70.8 | 6.58 | 73.0 | 3.78 |
| Sulfanilamide | 71.9 | 2.32 | 76.7 | 6.94 | 80.9 | 4.37 |
| Sulfisomidine | 73.5 | 1.02 | 81.6 | 5.26 | 89.9 | 4.06 |
| Trimethoprim | 79.9 | 3.87 | 80.2 | 1.37 | 81.9 | 1.44 |
| Sulfacetamide | 71.1 | 8.23 | 80.3 | 4.54 | 88.0 | 4.13 |
| Sulfathiazole | 69.6 | 2.57 | 80.8 | 4.99 | 89.6 | 3.67 |
| Sulfadiazine | 74.0 | 3.43 | 82.0 | 3.24 | 88.3 | 3.72 |
| Sulfapyridine | 75.8 | 4.66 | 78.5 | 4.45 | 88.5 | 3.12 |
| Sulfamerazine | 73.5 | 4.51 | 75.7 | 4.61 | 87.0 | 3.88 |
| Sulfamoxol | 69.5 | 4.25 | 76.8 | 3.91 | 82.5 | 2.44 |
| Sulfamethizole | 66.8 | 3.03 | 71.3 | 4.50 | 79.0 | 3.57 |
| Sulfamethoxypyridazine | 70.6 | 1.92 | 76.8 | 4.82 | 83.6 | 5.87 |
| Sulfamethazine | 74.7 | 3.30 | 76.8 | 4.81 | 91.0 | 4.26 |
| Sulfameter | 69.5 | 4.31 | 73.7 | 4.47 | 81.5 | 3.69 |
| Sulfamonomethoxine | 81.3 | 5.99 | 82.9 | 8.09 | 92.4 | 4.30 |
| Sulfachlorpyridazine | 74.2 | 2.60 | 80.9 | 6.55 | 88.0 | 3.86 |
| Sulfadoxine | 69.8 | 2.33 | 75.0 | 6.17 | 82.0 | 2.20 |
| Sulfamethoxazole | 81.1 | 3.01 | 84.4 | 4.49 | 96.6 | 4.38 |
| Sulfisoxazole | 75.5 | 3.02 | 79.5 | 4.51 | 88.8 | 3.04 |
| Sulfabenzamide | 70.7 | 5.66 | 77.4 | 8.33 | 82.4 | 4.10 |
| Sulfaquinoxaline | 74.0 | 4.99 | 82.3 | 5.15 | 90.7 | 3.00 |
| Sulfadimethoxine | 71.4 | 1.99 | 80.7 | 5.07 | 87.7 | 6.80 |
| Sulfaphenazole | 67.6 | 1.93 | 80.1 | 3.77 | 74.7 | 4.70 |
| Sulfanitran | 81.0 | 2.12 | 103.8 | 9.23 | 99.5 | 6.96 |
, Cspiked, i, C matrix and C matrix standard represent the concentrations of spiked, unspiked samples and matrix standard, respectively; n = 7.
Relative standard deviation.
3.4. Application to actual sample analysis
We applied this study in the identification and quantification of 24 SAs in 24 different cake and cookie samples that were obtained from local markets and supermarkets in Shijiazhuang and analyzed following preparation using the procedure previously mentioned. In one cake sample, trimethoprim (1.5 μg kg−1) and sulfamonomethoxine (5.0 μg kg−1) were observed, and the other 22 SAs were below the LODs. Moreover, 24 SAs in other 23 real samples were all lower than the LODs.
3.5. Method performance comparison
The QuEChERS-UPLC-MS/MS method developed for the determination of SAs was compared to other methods reported in the literature, the results of which are shown in Table 8. Among them, SPE and QuEChERS were the most used pretreatment methods for the detection of SAs. However, compared with SPE and MSPE [10,17,22], QuECh-ERS method could not only reduce the time and cost of the analysis but also reduce the number of steps and minimize the consumption of chemicals. In these comparative methods, the maximum number of SAs tested was 16 [20], and the samples are mostly pork, fish and milk [10,16,17,22]. The relevant detection of SAs in instant pastries were rarely reported. As shown in Table 8, the modified QuECh-ERS-UPLC-MS/MS method was developed for the determination of 24 SAs in instant pastries with satisfied recoveries and the lower LOQs, which showed high throughout, high sensitivity and accuracy with simple pretreatment procedure.
Table 8.
Performance comparisons for SAs determination with other reported analytical methods.
| Analytical method | Pretreatment method | Analytes | Sample | Sample amount | Total analytical time | Enrichment factor | Recovery (%) | LOQ | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| LC-MS/MS | On-line SPE | 15 sulfonamides | pork and fish samples | 2 g | 15.0 min | 25 | 78.3–99.3 | 0.25–5.00 μg·kg−1 | [10] |
| LC-MS/MS | QuEChERS | 13 sulfonamides | salmon | 3 g | 16.0 min | NO | 66–114 | 0.10–1.69 μg·kg−1 | [16] |
| LC-MS | SPE | 5 sulfonamides | meat products | 10 g | 21.0 min | 10 | 82.8–119.9 | 0.10–0.23 μg·kg−1 | [17] |
| LC-MS/MS | SPE | 16 sulfonamides | beeswax | 1 g | 13.0 min | 20 | 65.2–117.8 | 2–5 μg·kg−1 | [20] |
| LC-MS/MS | SPME | 10 sulfonamides | water | 1 mL | 5.5 min | 5 | 82.0–105.4 | 0.54–4.5 ng·L−1 | [21] |
| LC-MS/MS | MSPE | 8 sulfonamides | water, milk, pork and fish meat | 25 mL, 1 g | 5.5 min | 250, 1 | 80.13–108.6 | 0.70–3.50 ng·L−1, 1.10–3.75 μg·kg−1 | [22] |
| LC-MS/MS | QuEChERS | 24 sulfonamides | Cakes and cookies | 1 g | 18 min | 5 | 67.6–103.8 | 0.03–0.45 μg·kg−1 | This work |
4. Conclusion
Eggs are a high-quality and abundant source of dietary protein, vitamins, and minerals. Therefore, the detection of antibiotic residues in instant pastries is of great importance. In this study, a QuEChERS coupled with UPLC-MS/MS method was developed for the high-throughput determination of 24 different SAs in instant pastries. The QuEChERS method had the advantages of being quick, trivial, and inexpensive, and it enabled higher accuracies and recoveries for most of the antibiotics assessed compared to other methods. This method was successfully applied in the detection of antibiotics in cakes and cookies, offering the possibility to apply the method to other similar or even simpler samples for ensuring food quality and safety.
Supplementary Information
Acknowledgments
This work was supported by the Key Research and Development Program of Hebei Province, China (223777116D) and the Central Government Guides the Development of Local Science and Technology Project of Hebei Province (226Z7708G).
Funding Statement
This work was supported by the Key Research and Development Program of Hebei Province, China (223777116D) and the Central Government Guides the Development of Local Science and Technology Project of Hebei Province (226Z7708G).
Footnotes
Conflict of Interest
All authors declare that there are no conflict of interest.
References
- 1. Zhi S, Zhou J, Liu H, Wu H, Zhang Z, Ding Y, et al. Simultaneous extraction and determination of 45 veterinary antibiotics in swine manure by liquid chromatography-tandem mass spectrometry. J Chromatogr, B: Anal Technol Biomed Life Sci. 2020;1154:122286. doi: 10.1016/j.jchromb.2020.122286. [DOI] [PubMed] [Google Scholar]
- 2. Hoff R. Analysis of sulfonamide residues in bovine liver by liquid chromatography-tandem mass spectrometry without chemical extraction or clean-up procedures. Anal Biochem. 2020;611:114011. doi: 10.1016/j.ab.2020.114011. [DOI] [PubMed] [Google Scholar]
- 3. Petrarca MH, Braga PAC, Reyes FGR, Bragotto APA. Hydrophilic interaction liquid chromatography coupled to quadrupole time-of-flight mass spectrometry as a potential combination for the determination of sulfonamide residues in complex infant formula matrices. J Chromatogr A. 2020;1633:461606. doi: 10.1016/j.chroma.2020.461606. [DOI] [PubMed] [Google Scholar]
- 4. Xu L, Wu R, Geng X, Zhu X, Xiong Y, Chen T, et al. Rapid detection of sulfonamide antibiotics residues in swine urine by surface-enhanced Raman spectroscopy. Spectrochim Acta Mol Biomol Spectrosc. 2022;267:120570. doi: 10.1016/j.saa.2021.120570. [DOI] [PubMed] [Google Scholar]
- 5. Yang S, Ma S, Zhu K, Wang M, Li J, Arabi M, et al. Simultaneous enrichment/determination of six sulfonamides in animal husbandry products and environmental waters by pressure-assisted electrokinetic injection coupled with capillary zone electrophoresis. J Food Compos Anal. 2020;88:103462. [Google Scholar]
- 6. Chatzimitakos T, Samanidou V, Stalikas CD. Graphene-functionalized melamine sponges for microextraction of sulfonamides from food and environmental samples. J Chromatogr A. 2017;1522:1–8. doi: 10.1016/j.chroma.2017.09.043. [DOI] [PubMed] [Google Scholar]
- 7. Wang J, Xu J, Ji X, Wu H, Yang H, Zhang H, et al. Determination of veterinary drug/pesticide residues in livestock and poultry excrement using selective accelerated solvent extraction and magnetic material purification combined with ultra-high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2020;1617:460808. doi: 10.1016/j.chroma.2019.460808. [DOI] [PubMed] [Google Scholar]
- 8. Hong B, Yu S, Zhou M, Li J, Ding J, Niu Y. Development of a pH-paralleling approach of quantifying six-category pharmaceuticals in surface water using SPE-HPLC-MS/MS. Watersh Ecol Environ. 2021;3:1–16. [Google Scholar]
- 9. Chiesa LM, DeCastelli L, Nobile M, Martucci F, Mosconi G, Fontana M, et al. Analysis of antibiotic residues in raw bovine milk and their impact toward food safety and on milk starter cultures in cheese-making process. LWT (Lebensm-Wiss = Technol) 2020;131:109783. [Google Scholar]
- 10. Ma J, Fan S, Sun L, He L, Zhang Y, Li Q. Rapid analysis of fifteen sulfonamide residues in pork and fish samples by automated on-line solid phase extraction coupled to liquid chromatography-tandem mass spectrometry. Food Sci Hum Wellness. 2020;9:363–9. [Google Scholar]
- 11. Shirani M, Parandi E, Nodeh HR, Akbari-Adergani B, Shahdadi F. Development of a rapid efficient solid-phase microextraction: an overhead rotating flat surface sorbent based 3-D graphene oxide/lanthanum nanoparticles @ Ni foam for separation and determination of sulfonamides in animal-based food products. Food Chem. 2022;373:131421. doi: 10.1016/j.foodchem.2021.131421. [DOI] [PubMed] [Google Scholar]
- 12.GB/T 31650 National food safety standard-maximum residue limits for veterinary drugs in food. National standards of the People’s Republic of China; 2019. [Google Scholar]
- 13. Yao T, Du K. Simultaneous determination of sulfonamides in milk: in-situ magnetic ionic liquid dispersive liquid-liquid microextraction coupled with HPLC. Food Chem. 2020;331:127342. doi: 10.1016/j.foodchem.2020.127342. [DOI] [PubMed] [Google Scholar]
- 14. Galarini R, Diana F, Moretti S, Puppini B, Saluti G, Persic L. Development and validation of a new qualitative ELISA screening for multiresidue detection of sulfonamides in food and feed. Food Control. 2014;35:300–10. [Google Scholar]
- 15. Semail NF, Abdul Keyon AS, Saad B, Kamaruzaman S, Mohamad Zain NN, Lim V, et al. Simultaneous preconcentration and determination of sulfonamide antibiotics in milk and yoghurt by dynamic pH junction focusing coupled with capillary electrophoresis. Talanta. 2022;236:122833. doi: 10.1016/j.talanta.2021.122833. [DOI] [PubMed] [Google Scholar]
- 16. Castilla-Fernández D, Moreno-González D, Bouza M, Saez-Gómez A, Ballesteros E, García-Reyes JF, et al. Assessment of a specific sample cleanup for the multiresidue determination of veterinary drugs and pesticides in salmon using liquid chromatography/tandem mass spectrometry. Food Control. 2021;130:108311. [Google Scholar]
- 17. Shen R, Huang L, Liu R, Shuai Q. Determination of sulfonamides in meat by monolithic covalent organic frameworks based solid phase extraction coupled with high-performance liquid chromatography-mass spectrometric. J Chromatogr A. 2021;1655:462518. doi: 10.1016/j.chroma.2021.462518. [DOI] [PubMed] [Google Scholar]
- 18. Zhang Y, Zhou WE, Li SH, Ren ZQ, Li WQ, Zhou Y, et al. A simple, accurate, time-saving and green method for the determination of 15 sulfonamides and metabolites in serum samples by ultra-high performance supercritical fluid chromatography. J Chromatogr A. 2016;1432:132–9. doi: 10.1016/j.chroma.2015.12.075. [DOI] [PubMed] [Google Scholar]
- 19. Wang C, Li X, Yu F, Wang Y, Ye D, Hu X, et al. Multi-class analysis of veterinary drugs in eggs using dispersive-solid phase extraction and ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2021;334:127598. doi: 10.1016/j.foodchem.2020.127598. [DOI] [PubMed] [Google Scholar]
- 20. Mitrowska K, Antczak M. Determination of sulfonamides in beeswax by liquid chromatography coupled to tandem mass spectrometry. J Chromatogr, B: Anal Technol Biomed Life Sci. 2015;1006:179–86. doi: 10.1016/j.jchromb.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 21. Duan R, Sun L, Yang HY, Ma YR, Deng XY, Peng C, et al. Preparation of phenyl-boronic acid polymeric monolith by initiator-free ring-opening polymerization for microextraction of sulfonamides prior to their determination by ultra-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2020;1609:460510. doi: 10.1016/j.chroma.2019.460510. [DOI] [PubMed] [Google Scholar]
- 22. Zhao Y, Wu R, Yu H, Li J, Liu L, Wang S, et al. Magnetic solid-phase extraction of sulfonamide antibiotics in water and animal-derived food samples using core-shell magnetite and molybdenum disulfide nanocomposite adsorbent. J Chromatogr A. 2020;1610:460543. doi: 10.1016/j.chroma.2019.460543. [DOI] [PubMed] [Google Scholar]
- 23. Lu D, Liu C, Qin M, Deng J, Shi G, Zhou T. Functionalized ionic liquids-supported metal organic frameworks for dispersive solid phase extraction of sulfonamide antibiotics in water samples. Anal Chim Acta. 2020;1133:88–98. doi: 10.1016/j.aca.2020.07.074. [DOI] [PubMed] [Google Scholar]
- 24. Hoff RB, Pizzolato TM, Peralba M, Diaz-Cruz MS, Barcelo D. Determination of sulfonamide antibiotics and metabolites in liver, muscle and kidney samples by pressurized liquid extraction or ultrasound-assisted extraction followed by liquid chromatography-quadrupole linear ion trap-tandem mass spectrometry (HPLC-QqLIT-MS/MS) Talanta. 2015;134:768–78. doi: 10.1016/j.talanta.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 25. Shakirova F, Shishov A, Bulatov A. Automated liquid-liquid microextraction and determination of sulfonamides in urine samples based on Schiff bases formation in natural deep eutectic solvent media. Talanta. 2021;234:122660. doi: 10.1016/j.talanta.2021.122660. [DOI] [PubMed] [Google Scholar]
- 26. Abafe OA, Gatyeni P, Matika L. A multi-class multi-residue method for the analysis of polyether ionophores, tetracyclines and sulfonamides in multi-matrices of animal and aquaculture fish tissues by ultra-high performance liquid chromatography tandem mass spectrometry. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2020;37:438–50. doi: 10.1080/19440049.2019.1705399. [DOI] [PubMed] [Google Scholar]
- 27. Xu X, Xu X, Han M, Qiu S, Hou X. Development of a modified QuEChERS method based on magnetic multi-walled carbon nanotubes for the simultaneous determination of veterinary drugs, pesticides and mycotoxins in eggs by UPLC-MS/MS. Food Chem. 2019;276:419–26. doi: 10.1016/j.foodchem.2018.10.051. [DOI] [PubMed] [Google Scholar]
- 28. Yu YX, Zhang R, Liu H, Zhang Z, Shi X, Sun A, et al. Highly-selective complex matrices removal via a modified QuECh-ERS for determination of triazine herbicide residues and risk assessment in bivalves. Food Chem. 2021;347:129030. doi: 10.1016/j.foodchem.2021.129030. [DOI] [PubMed] [Google Scholar]
- 29. Hu J, Liang M, Xian Y, Chen R, Wang L, Hou X, et al. Development and validation of a multianalyte method for quantification of aflatoxins and bongkrekic acid in rice and noodle products using PRiME-UHPLC-MS/MS method. Food Chem. 2022;395:133598. doi: 10.1016/j.foodchem.2022.133598. [DOI] [PubMed] [Google Scholar]
- 30. Tian F, Qiao C, Luo J, Guo L, Pang T, Pang R, et al. Development and validation of a method for the analysis of five diamide insecticides in edible mushrooms using modified QuEChERS and HPLC-MS/MS. Food Chem. 2020;333:127468. doi: 10.1016/j.foodchem.2020.127468. [DOI] [PubMed] [Google Scholar]
- 31. Berset JD, Mermer S, Robel AE, Walton VM, Chien ML, Field JA. Direct residue analysis of systemic insecticides and some of their relevant metabolites in wines by liquid chromatography -mass spectrometry. J Chromatogr A. 2017;1506:45–54. doi: 10.1016/j.chroma.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 32. Izcara S, Casado N, Morante-Zarcero S, Perez-Quintanilla D, Sierra I. Miniaturized and modified QuEChERS method with mesostructured silica as clean-up sorbent for pyrrolizidine alkaloids determination in aromatic herbs. Food Chem. 2022;380:132189. doi: 10.1016/j.foodchem.2022.132189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.