ABSTRACT
The complications of endoscopic retrograde cholangiopancreatography (ERCP) are numerous and mainly intraluminal. We present a unique case of a patient who developed splenic hematoma after ERCP. A 41-year-old woman was hospitalized for evaluation of chronic abdominal pain, for which she underwent an ERCP. The next day, the patient developed hemorrhagic shock. She was found to have a large ruptured subcapsular splenic bleed. Splenic artery embolization was performed, and the patient was stabilized. In conclusion, a high index of suspicion should be kept when managing patients presenting with unstable vital signs and/or acute anemia after ERCP.
KEYWORDS: bile duct dilation, endoscopic retrograde cholangiopancreatography, pancreatic duct dilation, sphincterotomy, splenic subcapsular hematoma
INTRODUCTION
Endoscopic retrograde cholangiopancreatography (ERCP) is a minimally invasive diagnostic and therapeutic procedure, which is notably used in biliary and pancreatic diseases.1 Its complications include pancreatitis, hemorrhaging, perforation, infection, and cardiopulmonary disturbances. When post–ERCP-associated bleeding develops, it is usually intraluminal. Although rare, intraductal bleeding and hematomas, including hepatic, splenic, and intra-abdominal, can occur.2 Only few cases of splenic injury after ERCP were reported in the literature in the past 3 decades.3 In this report, we describe a unique case of a patient who developed a subcapsular splenic hematoma after ERCP, supported by objective radiologic evidence obtained before and after the procedure.
CASE REPORT
A 41-year-old woman with medical history of gallstone pancreatitis status post stent placement and cholecystectomy, opioid use disorder on naloxone, and an established diagnosis of sphincter of Oddi dysfunction presented to the hospital for evaluation of chronic abdominal pain.
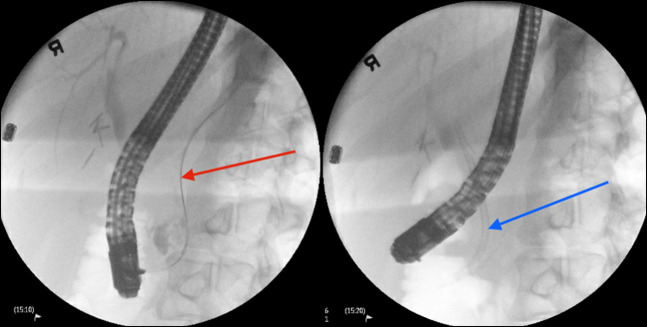
On admission, laboratory findings were unremarkable including lipase and liver function tests. An abdominal and pelvic computed tomography (CT) scan showed intrahepatic and extrahepatic bile ducts and pancreatic duct dilation with pneumobilia (Figure 1). The findings of magnetic resonance cholangiopancreatography, which was performed to rule out a tumor, were consistent with the results of the CT scan (Figure 2). ERCP showed the presence of a moderately dilated bile duct and ventral pancreatic duct in the head of the pancreas. The ventral pancreatic orifice appeared stenotic, and a 2-mm ventral pancreatic sphincterotomy was made with a monofilament traction sphinterotome using ERBE USA electrocautery. One 5-Fr by 5-cm plastic stent with 2 external flaps and 2 internal flaps was placed 4 cm into the ventral pancreatic duct. The biliary pancreatic junction contained a single localized stenosis of 2 mm in length, and a 4-mm biliary sphincterotomy was made with a monofilament traction sphincterotome using ERBE USA electrocautery. One 10-Fr by 5-cm plastic stent with a single external flap and a single internal flap was placed 4 cm into the common bile duct (Figure 3). Strictures appeared benign and were not biopsied. Ductal dilation was considered to be acquired because no specific causes of dilation were identified.
Figure 1.
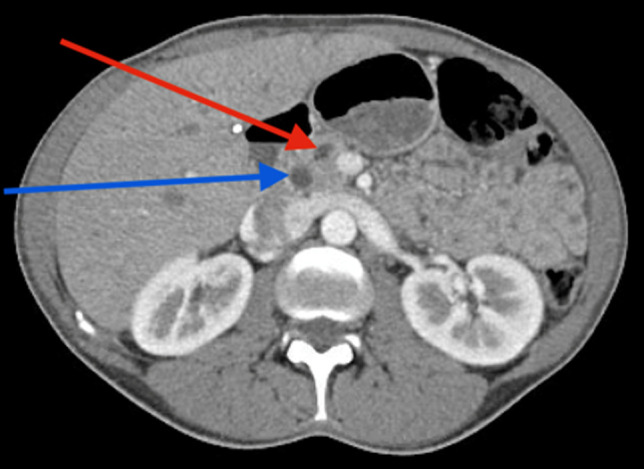
A late arterial phase CT scan showing common bile duct (blue arrow) and main pancreatic duct (red arrow) dilation. CT, computed tomography.
Figure 2.
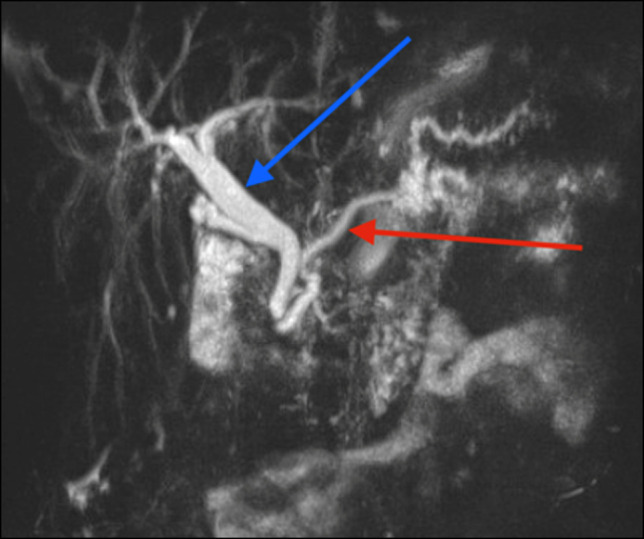
MRCP showing common bile duct (blue arrow) and main pancreatic duct (red arrow) dilation, without choledocholithiasis or mass effect. MRCP, magnetic resonance cholangiopancreatography.
Figure 3.
ERCP showing stent placement in the ventral pancreatic main duct (red arrow) and stent in the common bile duct (blue arrow) with presence of biliary tree dilation. ERCP, endoscopic retrograde cholangiopancreatography.
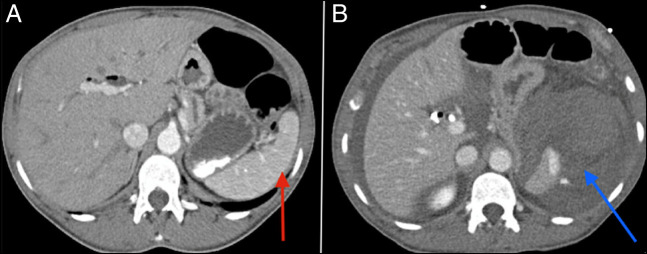
Twenty-four hours later, the patient complained of a severe sharp abdominal pain that was worse than usual. The vital signs were significant for a drop of the blood pressure from 101/62 to 75/39 mm Hg. Physical examination revealed the presence of severe abdominal tenderness and rigidity consistent with signs of peritonitis. Laboratory workup was unremarkable, except for a drop of hemoglobin level from 11.4 to 7.5 g/dL. Her vitals stabilized after she was transfused with 2 units of blood and was resuscitated with intravenous fluids. A CT angiography revealed the presence of a large ruptured subcapsular splenic hematoma with active bleeding and large-volume hemoperitoneum that were not present on the scan performed 1 day before the event (Figure 4). An emergent splenic artery embolization was performed by interventional radiology under general anesthesia. Intravenous meropenem was started and continued for 5 days after the procedure to prevent splenic abscess. She was recommended to follow the guidelines for patients with dysfunctional spleen, including being up to date with her vaccination record and starting prophylactic antibiotics as an outpatient.4 The patient eventually improved and was discharged from the hospital in stable condition. She was scheduled for follow-up with gastroenterology as an outpatient for further investigation of the origin of her chronic abdominal pain. The latter was unclear and could have been caused by multiple factors, including the presence of biliary and pancreatic strictures and sphincter of Oddi dysfunction as per gastroenterology.
Figure 4.
(A) A late arterial phase abdominal CT scan showing a normal spleen (red arrow). (B) A portal venous phase abdominal CT scan showing large perisplenic hematoma and hemoperitoneum with arterial extravasation of contrast consistent with active arterial bleeding (blue arrow). CT, computed tomography.
DISCUSSION
ERCP has become the mainstay of management in numerous pancreaticobiliary conditions. Accordingly, because the procedure has become widely used, uncommon adverse events are unraveling. Although splenic injury (SI) is a well-recognized complication of colonoscopy,5,6 ERCP-induced splenic injuries are rare with only a few cases reported in the literature.7,8 Among the reported complications, subcapsular hematoma, laceration, avulsion of splenic vessels, and direct tearing of the splenic capsule are the most frequent.8,9
The exact mechanism leading to SI after ERCP is still unclear. However, one of the postulated hypotheses seems to stand out. Excessive shear and traction forces seem to be the culprit. In fact, for optimal major papilla cannulation, the duodenoscope should be switched from the “long position” to the more preferred “short position.” This is performed by torqueing the scope to the right and slightly pulling it out of the patient's mouth, leading to paradoxical forward motion of the scope.3 This maneuver can cause bowing of the endoscope and torsion of the greater stomach curvature with possible traction on the short gastric vessels. The latter can lead to splenic capsular tears and vascular avulsions.10,11 Interestingly, in the case of our patient, the procedure was not deemed challenging or prolonged. Similar cases after uncomplicated ERCPs are reported in the literature.12,13 This raises questions about the possible under-recognition of excessive shearing during the procedure. Another possible hypothesis can be derived from the higher incidence of splenic injuries after therapeutic ERCPs in comparison with diagnostic ERCP as pointed out by Wang et al.14 Stenting (as it was the case with our patient) and other pancreatic ductal work could be associated with increased torque and traction maneuvering, increasing the risk of splenic injury.
Multiple predisposing risk factors have been proposed. First, conditions associated with limited spleen motility, such as chronic pancreatitis and adhesions from previous abdominal surgery, seem to be associated with an increased risk of SI. In fact, our patient has a history of cholecystectomy, which increases the risk of adhesions. Moreover, multiple reported cases of SI in patients with chronic pancreatitis were reported. It seems that calcification and fibrosis of the attaching ligaments significantly impairs splenic motility.8,15 Although this association is purely theoretical and observational, the potential danger of an unrecognized splenic hematoma could justify an increased level of suspicion in this population. Second, prolonged traction on attaching ligaments as seen in prolonged procedures, obstructing tumors, significant body habitus, and altered anatomy can increase the risk of SI after ERCP.3,16 Third, the use of anticoagulant or coagulopathy was also described as a risk factor for splenic hematoma.17
Although our patients developed hemorrhagic shock, the clinical features of subcapsular hematoma are nonspecific and can easily overlap with more common ERCP adverse events. Abdominal pain, hypotension, tachycardia, and acute anemia are most commonly described.18 However, patients do not always present with shock and acute abdomen, and the injury may be overlooked with delayed presentations and increased morbidity. Thus, a high index of suspicion should be kept, especially in patients with described risk factors. As with our patient, the definitive diagnosis requires imaging.
A unique feature in our case that differentiates it from the reports available in the literature is the fact that an abdominal CT scan performed immediately before the procedure and another one performed at the onset of symptoms the next day were both available. This confirms that the ERCP was the sole cause of the subcapsular hematoma formation. In previous case reports, splenectomy was performed as the mainstay of management. However, we believe that the approach could be tailored to the patient's hemodynamic status and managed as per blunt splenic trauma guidelines.19 For hemodynamically unstable patients, emergency splenectomy is the standard of care. Nonetheless, a less-invasive approach may be considered for stable patients. In our case, we elected to perform emergent splenic artery embolization because the patient was stabilized after blood transfusion. To note, this is the first reported case of post-ERCP subcapsular hematoma treated with splenic artery embolization.
Strategies to prevent ERCP-related splenic injuries include managing modifiable risk factors, such as coagulopathy. Cho et al20 suggest that the use of gentle epigastric hand pressure can help ease the long-bowing of the endoscope and alleviate mechanical stress on the spleen. We also highly recommend limiting shearing as much as possible during the procedure. Splenic hematoma after ERCP is a very unusual, albeit severe, complication. Thus, a high index of suspicion should be kept when managing patients presenting with unstable vital signs and/or acute anemia after the procedure.
DISCLOSURES
Author contributions: A. Boustany wrote the case presentation section, obtained consent, and is the article guarantor. J. Kassab wrote the discussion. N. Ramahi wrote the introduction. S. Onwuzo wrote the abstract and added the images with their description. P. Acar is a radiologist who chose the best CT sequences and revised image titles. I. Asaad is the senior author who supervised this study.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Contributor Information
Joseph Kassab, Email: kassabj@ccf.org.
Noor Ramahi, Email: ramahin3@ccf.org.
Somtochukwu Onwuzo, Email: onwuzos@ccf.org.
Imad Asaad, Email: imadasaad86@gmail.com.
REFERENCES
- 1.ElGeidie AA. Single-session minimally invasive management of common bile duct stones. World J Gastroenterol. 2014;20(41):15144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson MA, Fisher L, Jain R, et al. Complications of ERCP. Gastrointest Endosc. 2012;75(3):467–73. [DOI] [PubMed] [Google Scholar]
- 3.Lee R, Huelsen A, Saad N, Hodgkinson P, Hourigan LF. Splenic injury following endoscopic retrograde cholangiopancreatography: A case report and literature review. Case Rep Gastroenterol. 2017;11(1):241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies JM, Lewis MPN, Wimperis J, Rafi I, Ladhani S, Bolton‐Maggs PHB. Review of guidelines for the prevention and treatment of infection in patients with an absent or dysfunctional spleen: Prepared on behalf of the British Committee for Standards in Haematology by a Working Party of the Haemato‐Oncology Task Force. Br J Haematol. 2011;155(3):308–17. [DOI] [PubMed] [Google Scholar]
- 5.Sarhan M, Ramcharan A, Ponnapalli S. Splenic injury after elective colonoscopy. JSLS. 2009;13(4):616–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Shankar S, Rowe S. Splenic injury after colonoscopy: Case report and review of literature. Ochsner J, 2011;11(3):276–81. [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad S, Bragg D, Zaitoun AM, Lobo DN. A dangerous loop. Clin Case Rep. 2016;4(5):535–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaffney RR, Jain V, Moyer MT. Splenic injury and ERCP: A possible risk for patients with advanced chronic pancreatitis. Case Rep Gastroenterol. 2012;6(1):162–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis FW, Moloo N, Stiegmann GV, Goff JS. Splenic injury complicating therapeutic upper gastrointestinal endoscopy and ERCP. Gastrointest Endosc. 1991;37(6):632–3. [DOI] [PubMed] [Google Scholar]
- 10.Mallery JS, Baron TH, Dominitz JA, et al. Complications of ERCP. Gastrointest Endosc. 2003;57(6):633–8. [DOI] [PubMed] [Google Scholar]
- 11.Grammatopoulos A, Moschou M, Rigopoulou E, Katsoras G. Splenic injury complicating ERCP. Ann Gastroenterol. 2014;27(2):177–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Wu WC, Katon RM. Injury to the liver and spleen after diagnostic ERCP. Gastrointest Endosc. 1993;39(6):824–7. [DOI] [PubMed] [Google Scholar]
- 13.Chavalitdhamrong D, Donepudi S, Pu L, Draganov PV. Uncommon and rarely reported adverse events of endoscopic retrograde cholangiopancreatography. Dig Endosc. 2014;26(1):15–22. [DOI] [PubMed] [Google Scholar]
- 14.Wang K, Fang Y, Huang A, Gao M. Splenic laceration after endoscopic retrograde cholangiopancreatography: A literature review and our experience. Laparosc Endosc Robot Surg. 2020;3(3):80–4. [Google Scholar]
- 15.Kingsley DD, Schermer CR, Jamal MM. Rare complications of endoscopic retrograde cholangiopancreatography: Two case reports. JSLS. 2001;5(2):171–3. [PMC free article] [PubMed] [Google Scholar]
- 16.Furman G, Morgenstern L. Splenic injury and abscess complicating endoscopic retrograde cholangiopancreatography. Surg Endosc. 1993;7(4):343–4. [DOI] [PubMed] [Google Scholar]
- 17.Grammatopoulos A, Moschou M, Rigopoulou E, Katsoras G. Splenic injury complicating ERCP. Ann Gastroenterol. 2014;27(2):177–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Bajwa KS, Madabhushi AK, Jafri N, Shah SK, Felinski MM. Splenic hematoma as a rare complication of endoscopic retrograde cholangiopancreatography. J Clin Gastroenterol Treat. 2020;4:078. [Google Scholar]
- 19.Coccolini F, Montori G, Catena F, et al. Splenic trauma: WSES classification and guidelines for adult and pediatric patients. World J Emerg Surg. 2017;12(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho C, Yuen KK, Yuen C, Chong L, Chu RW. Case report: Splenic laceration after endoscopic retrograde cholangiopancreatography. Hong Kong Med J. 2008;14(2):145–7. [PubMed] [Google Scholar]