INTRODUCTION:
Cytomegalovirus (CMV) viral load detected by real-time polymerase chain reaction (PCR) in plasma or stool may facilitate detection of CMV colitis.
METHODS:
This prospective study enrolled 117 patients with clinically suspected CMV colitis. Patients presenting with gastrointestinal symptoms and having increased risk of CMV infection were eligible. All participants underwent colonoscopy with tissue biopsy. Five patients underwent colonoscopy twice because of clinical recurrence, resulting in a total of 122 colonoscopies. Stool CMV-PCR and plasma CMV-PCR were performed within 7 days before/after colonoscopy. Twenty asymptomatic volunteers also underwent the same protocol.
RESULTS:
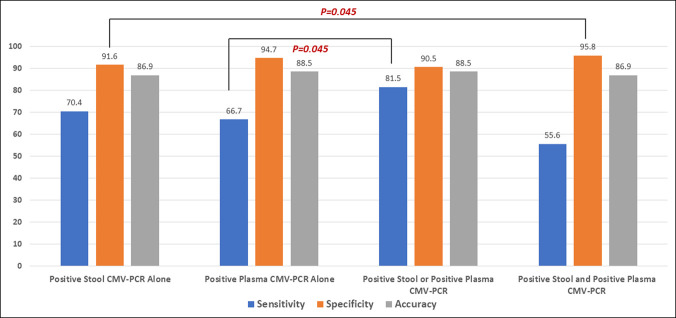
Twenty-seven (23.1%) of 122 colonoscopies yielded positive for CMV colitis. The sensitivity and specificity was 70.4% and 91.6% for stool CMV-PCR and 66.7% and 94.7% for plasma CMV-PCR, respectively. The sensitivity of either positive plasma or positive stool CMV-PCR was 81.5%, which is significantly higher than that of plasma CMV-PCR alone (P = 0.045). However, positive results from both tests yielded a specificity of 95.8%, which is significantly higher than that of stool CMV-PCR alone (P = 0.045). There was a good and significant correlation between stool CMV-PCR and plasma CMV-PCR (r = 0.71, P < 0.01), and both tests significantly correlated with the cytomegalic cell count (r = 0.62, P < 0.01 for stool and r = 0.64, P < 0.01 for plasma). There were no positive stool or plasma CMV-PCR assays among volunteers.
DISCUSSION:
The results of this study strongly suggest that the combination of stool CMV-PCR and plasma CMV-PCR can be used to confidently rule in (both positive) or rule out (both negative) a diagnosis of CMV colitis.
KEYWORDS: performance, real-time polymerase chain reaction assays, fecal and plasma specimens, diagnosing, cytomegalovirus colitis, CMV colitis
INTRODUCTION
Cytomegalovirus (CMV)-related gastrointestinal (GI) diseases are common and can involve various organs, including the oral cavity, esophagus, stomach, small intestine, and large intestine—the last of which is the most common site of CMV-GI infection (1–4). Two-thirds of patients with CMV-GI disease are immunocompromised (5–7). The risk factors for CMV-GI infection include HIV infection with low CD4 count (2,8,9), post–organ transplantation (10–14), and taking immunosuppressive agents, especially corticosteroids (6,7,15,16). Interestingly, CMV-GI disease is being increasingly reported in immunocompetent hosts. The important risk factors for CMV-GI disease in immunocompetent patients were reported to be old age and being critically ill with multiple comorbid illnesses (6,7,16–19). Inflammatory bowel disease (IBD) also increases the risk of developing CMV-GI disease, despite not receiving immunosuppressive agents (20–22).
Diagnosis of CMV colitis requires colonoscopy with tissue biopsy. The presence of viral inclusion on histologic specimens stained with hematoxylin and eosin (H&E) has high specificity, but low sensitivity for diagnosing CMV colitis, with reported sensitivities and specificities of 10%–87% and 92%–100%, respectively. Immunohistochemistry (IHC) stain increases the diagnostic sensitivity to 78%–93%. Therefore, the combination of H&E staining and IHC has been recommended as the gold standard for diagnosing CMV colitis (23–25). Tissue polymerase chain reaction (PCR) assay, which amplifies CMV DNA, has been reported to increase the diagnostic sensitivity to 92%–96.7% (23,26). However, because of its high sensitivity, colonic tissue PCR might detect latent CMV replication in colonic tissue in patients who do not have a clinically significant infection (27–29). Quantitative tissue PCR, which is able to select different cutoffs of tissue viral load, has been reported to increase the specificity while maintaining sensitivity (30). Furthermore, there is a good correlation between tissue CMV viral load and the density of CMV-infected cells (31). The European Crohn's and Colitis Organization guideline recommends that colonic tissue PCR can be used to diagnose CMV colitis in patients with IBD (32). However, the data to define the optimal cutoff of tissue viral load for diagnosis of CMV colitis are currently limited to a few studies (30,33).
Although colonoscopy with tissue sampling remains the mainstay method for diagnosing CMV colitis, colonoscopy may not be feasible in some situations because of the high risk of procedural complications, such as in patients with profound neutropenia, thrombocytopenia, or severe illnesses. Plasma CMV-PCR is a noninvasive test that detects CMV viremia, which could be associated with CMV-GI disease. However, the results of previous studies that investigated the use of plasma CMV-PCR for diagnosing CMV colitis are inconsistent (34,35). Moreover, there has been increasing interest in stool CMV-PCR in diagnosing CMV colitis. Stool CMV-PCR is a noninvasive test that can be either qualitative or quantitative. In a pilot study, Herfarth et al (36) reported a sensitivity and specificity of stool CMV-PCR for detecting CMV DNA of 83% and 93%, respectively, in 19 patients with IBD. Since then, only a few studies have reported the performance of stool CMV-PCR for diagnosing CMV colitis, and the results of those studies are inconsistent. The reported sensitivity ranges from 16.7% to 85%, and the specificity ranges from 71% to 96% (37–40). Furthermore, and importantly, the diagnostic performance of combining plasma and stool CMV-PCR, which may improve the diagnostic performance, has not been studied.
Accordingly, the aim of this study was to investigate the diagnostic performance of stool CMV-PCR, plasma CMV-PCR, and the combination of stool CMV-PCR and plasma CMV-PCR for diagnosing CMV colitis using tissue histopathology (H&E stain and IHC) as a standard reference in patients with clinical suspicion of CMV colitis. We also evaluated the correlation between the number of CMV-infected cells in colonic tissue and the CMV viral load in stool, plasma, and colonic tissue.
METHODS
Study design and participants
This prospective cohort study was conducted at Siriraj Hospital, Mahidol University, Bangkok, Thailand, from October 2020 to October 2021. Patients older than 18 years with clinical suspicion of CMV colitis were enrolled into the study group. Twenty asymptomatic volunteers were also enrolled—all of whom underwent the same study protocol. Clinical suspicion for CMV colitis was established by the presence of at least one risk factor from the list in section 1 below and presenting with at least one GI tract symptom from the list in section 2 below.
-
Risk factors for CMV colitis:
a. Immunocompromised status, including at least one of the following: HIV infection, solid organ or hematologic stem cell transplantation, active hematologic malignancy, or receiving immunosuppressive agents or chemotherapy at the time of enrollment.
b. Patients without obvious immunocompromised status, but having some risk factors for CMV colitis, including being critically ill (defined by the presence of organ failure or requiring inotropic agents); age older than 60 years; or having multiple comorbid illnesses, including diabetes mellitus (DM), atherosclerosis, or chronic kidney disease (6,7,16–19).
Presenting GI tract symptoms, including diarrhea, lower GI bleeding, bowel ileus, or pseudointestinal obstruction.
All participants underwent colonoscopy with tissue biopsy. Histopathologic analysis of the tissue biopsy was performed using both H&E and IHC stain. An experienced GI pathologist (N.A.) counted the number of infected cells in biopsy specimens with IHC. Two pieces of colonic tissue were sent for quantitative CMV-PCR (CMV R-GENE kit, limit of detection 450 copies/mL; bioMérieux SA, Marcy-l'Étoile, France). Patients who could not be proceeded to colonoscopy with tissue biopsy were excluded. Quantitative stool CMV-PCR (CMV R-GENE kit, limit of detection 450 copies/mL; bioMérieux SA) and quantitative plasma CMV-PCR (cobas CMV, limit of detection 150 copies/mL; Roche Diagnostics, Basel, Switzerland) were performed within 7 days before or after colonoscopy.
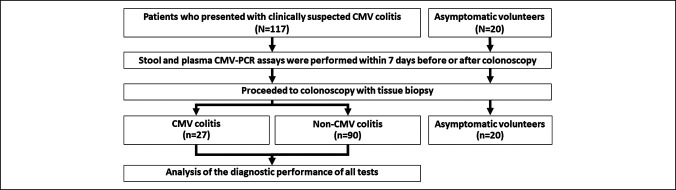
Patients diagnosed with CMV colitis based on detection of either cytomegalic cells on H&E stain or IHC-CMV cells were treated with ganciclovir for at least 2 weeks and then underwent follow-up colonoscopy with tissue biopsy to confirm complete treatment response. Stool specimens were also recollected and sent for CMV-PCR. The patients diagnosed with non-CMV colitis received treatment appropriate to their diagnosis. Twenty asymptomatic volunteers were enrolled into our asymptomatic control group, and all 20 of those subjects followed the same study protocol. A flow diagram describing subject enrollment, the study protocol, and the diagnostic outcome for both study patients and asymptomatic volunteers is shown in Figure 1.
Figure 1.
Flow diagram describing subject enrollment, the study protocol, and the diagnostic outcome for both study patients and asymptomatic volunteers. CMV, cytomegalovirus; CMV-PCR, cytomegalovirus polymerase chain reaction.
CMV-infected cell morphologic diagnosis and cell count methods
The H&E and CMV IHC study (clone CCH2+DDG9, DAKO, Denmark, 1:150 dilution) slides were retrieved from our archive and digitalized by whole slide scanner (Pannoramic 1,000, 3DHISTECH Ltd., Hungary) with ×40 objective lens (numerical aperture 0.95, pixel resolution 0.12 μm).
The digital H&E and CMV-IHC slides were reviewed (CaseViewer software, 3Dhistech) by pathologists. A CMV-positive cell is defined by the presence of IHC-positive intranuclear and/or intracytoplasmic inclusion. The CMV-positive cells and area of the biopsied tissue were counted and calculated by digital image analysis software (Quantcenter, 3DHISTECH) and recorded as the number of CMV-positive cells per mm2 (Figures 2a,b).
Figure 2.
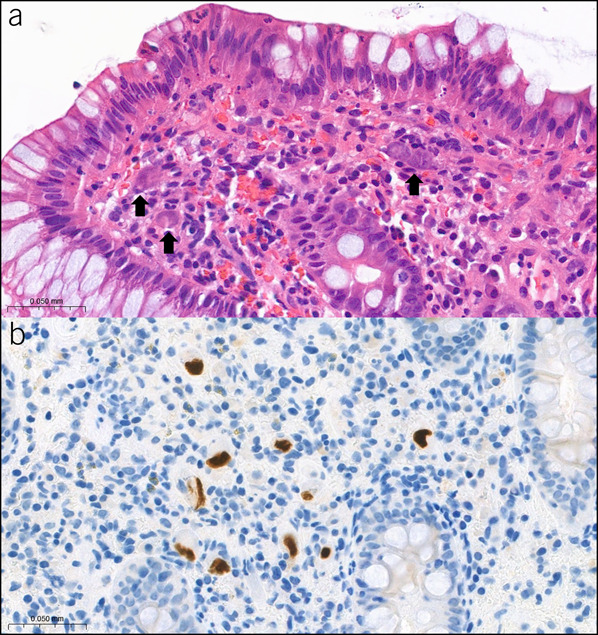
Images of H&E (×1,000) CMV-infected cells (arrow) reveal cytomegalic change with intranuclear and/or intracytoplasmic inclusions (a) and CMV IHC (×1,000) CMV-positive cells (b). CMV, cytomegalovirus; H&E, hematoxylin-eosin; IHC, immunohistochemistry.
Stool, plasma, and colonic tissue PCR technique
For stool CMV-PCR, CMV DNA from stool was extracted using a MagLEAD 12gC automated extraction platform (Precision System Science, Chiba, Japan). Briefly, the stool specimen was resuspended with phosphate-buffered saline at a concentration of 20% (g/mL) and then subjected to a freeze/thaw cycle 3 times. The supernatant was then collected and used for nucleic acid extraction. Quantitative CMV-PCR was performed using a CMV R-GENE kit (bioMérieux SA) according to the manufacturer's instructions.
For plasma CMV-PCR, 500 mL of plasma was placed into a Cobas AmpliPrep/Cobas TaqMan platform for nucleic acid extraction, amplification, and quantitation. Testing of plasma was performed using a Cobas AmpliPrep/Cobas TaqMan CMV assay (Roche Diagnostics) following the manufacturer's instructions.
For tissue CMV-PCR, the biopsied tissue was cut with scissors into small pieces and placed into a lysis buffer containing guanidine isothiocyanate. The tissue and buffer mixture was mixed thoroughly and incubated at room temperature for 20 minutes. The MagLEAD 12gC automated extraction platform (Precision System Science) was used to extract CMV DNA from 200 mL of lysed tissue. Extraction was performed according to the manufacturer's recommended protocol. Viral DNA was eluted with 100-mL buffer and used for real-time PCR assay. Quantitative CMV-PCR was performed using a CMV R-GENE kit (bioMérieux SA) according to the manufacturer's instructions. Ten milliliters of extracted DNA was added into the amplification mix, and the PCR was performed using a CFX96 Touch Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA).
Study outcomes
Primary outcomes.
The performance of stool CMV-PCR, plasma CMV-PCR, and their combinations was calculated using the detection of either cytomegalic cells on H&E stain or IHC-CMV cells as the reference standard for diagnosis of CMV colitis among 117 patients with clinically suspected CMV colitis. Correlation between cytomegalic cell count and quantitative tissue CMV-PCR, stool CMV-PCR, and plasma CMV-PCR was calculated. We also evaluated the performance of stool CMV-PCR in following up patients after treatment.
Secondary outcomes.
The performance of the diagnostic tests using tissue CMV-PCR as the reference standard was assessed. Subgroup analysis assessed the performance of the diagnostic tests in patients with IBD, patients with immunocompromised status, and patients without obvious immunocompromised status but with some risk factors for CMV colitis.
Statistical analysis
Descriptive statistics were used to summarize patient characteristics. Continuous variables are expressed as median and interquartile range (IQR) or mean ± SD, and categorical variables are presented as number of subjects and percentage. Standard 2-group comparison methods were used, including independent t test (for normally distributed data) or Wilcoxon rank-sum test (for non-normally distributed data) for continuous data, and χ2 test or Fisher exact test (depending on the size of the sample) for categorical data.
The diagnostic performance of stool CMV-PCR and plasma CMV-PCR is reported as the area under the receiver operating characteristic curve (AUC). The DeLong test was used to compare the AUCs of the stool and plasma CMV-PCR tests. After that, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated using the optimal cutoff value obtained from the Youden Index. The McNemar test was used to determine the statistical significance of differences among stool CMV-PCR, plasma CMV-PCR, and their combination. Spearman correlation coefficients (r) were used to determine the correlation between the number of infected cells in colonic tissue and stool CMV-PCR, plasma CMV-PCR, and colonic tissue CMV-PCR. All statistical analyses were performed using SAS version OnDemand for Academics (SAS Institute, Cary, NC). A 2-tailed P value of <0.05 was regarded as being statistically significant for all tests.
The protocol for this study was approved by the Siriraj Institutional Review Board on October 1, 2020 (COA no. Si 810/2020), and was in accordance with both the 1964 Declaration of Helsinki (and all of its subsequent amendments) and Good Clinical Practice guidelines. The study was registered at http://clinicaltrials.gov (NCT05522283). All study and volunteer participants provided signed informed consent before participation in the study.
RESULTS
Patient characteristics
A total of 117 patients with clinical suspicion of CMV colitis were included, and of those, 27 (23.1%) patients were definitively diagnosed with CMV colitis. The most common diagnoses in the non-CMV colitis group were ulcerative colitis (n = 21), Crohn's disease (n = 11), drug-induced colitis (n = 9), and acute hemorrhagic rectal ulcer syndrome or stercoral ulcers (n = 8). A comprehensive list of non-CMV colitis diagnoses is shown in Supplementary Digital Content (see Supplementary Table S1, http://links.lww.com/CTG/A917). Patient and asymptomatic volunteer baseline characteristics are shown in Table 1. The mean age of study patients was 53 years, and 48% were men. Sixty-seven of 117 (57.3%) patients were immunocompromised, 11 (9.4%) had HIV infection, and 43 (36.7%) were treated with corticosteroids. Seventy patients (59.8%) were hospitalized. Of those, 37 patients had GI symptoms at admission, and the other 33 patients were admitted because of other indications and developed GI symptoms during hospitalization.
Table 1.
Patient baseline characteristics of all study patients, compared between study patients with and without CMV colitis, and of asymptomatic volunteersa
| Characteristics | All study patients (N = 117) | CMV colitis (n = 27) | Non-CMV colitis (n = 90) | P | Asymptomatic volunteers (n = 20) |
| Age (yr) | 53.3 ± 18.4 | 55.8 ± 15.5 | 52.5 ± 19.2 | 0.41 | 52.10 ± 14.91 |
| Male sex | 56 (47.9%) | 13 (48.2%) | 43 (47.8%) | 0.97 | 9 (45.0%) |
| Underlying conditions | |||||
| Diabetes mellitus | 18 (15.4%) | 5 (18.5%) | 13 (14.4%) | 0.61 | 3 (15.0%) |
| Coronary artery disease | 10 (8.6%) | 5 (18.5%) | 5 (5.6%) | 0.03 | 3 (15.0%) |
| Cerebrovascular disease | 14 (12.0%) | 4 (14.8%) | 10 (11.1%) | 0.60 | 1 (5.0%) |
| Chronic kidney disease | 19 (16.2%) | 5 (18.5%) | 14 (15.6%) | 0.71 | 0 (0.0%) |
| HIV | 11 (9.4%) | 5 (18.5%) | 6 (6.7%) | 0.06 | 0 (0.0%) |
| CD4 count (cells/mm3) | 33.5 (18–293) | 33.5 (27–156) | 155.5 (8–495) | 1.00 | |
| Organ transplantation | 12 (10.3%) | 2 (7.4%) | 10 (11.1%) | 0.58 | 0 (0.0%) |
| Malignancy | 18 (15.4%) | 5 (18.5%) | 13 (14.4%) | 0.61 | 2 (10.0%) |
| Autoimmune disease | 10 (8.6%) | 2 (7.4%) | 8 (8.9%) | 0.81 | 1 (5.0%) |
| Inflammatory bowel disease | 39 (33.3%) | 6 (22.2%) | 33 (36.7%) | 0.16 | 0 (0.0%) |
| Active IBD | 27/39 (69.2%) | 4/6 (66.7%) | 23/33 (69.7%) | 1.00 | |
| Immunosuppressive drugs | 54 (46.1%) | ||||
| Corticosteroid | 43 (36.7%) | 7 (25.9%) | 36 (40.0%) | 0.18 | 0 (0.0%) |
| Prednisolone dose (mg/d) | 7.5 (5–15) | 30 (15–40) | 5 (5–15) | 0.003 | 0 (0.0%) |
| Other immunosuppressants | 44 (37.6%) | 10 (37.0%) | 34 (37.8%) | 0.94 | 0 (0.0%) |
| Chemotherapy | 11 (9.4%) | 4 (14.8%) | 7 (17.8%) | 0.27 | 1 (5.0%) |
| Immunocompromised status | 67 (57.3%) | 16 (59.3%) | 51 (56.7%) | 0.81 | 1 (5.0%) |
| Status | |||||
| Inpatient | 70 (59.8%) | 21 (77.8%) | 49 (54.4%) | 0.03 | 0 (0.0%) |
| GI symptoms at admission | 37/70 (52.8%) | 12/21 (57.1%) | 25/49 (51.0%) | 0.64 | |
| ICU | 15 (12.8%) | 7 (25.9%) | 8 (8.9%) | 0.02 | 0 (0.0%) |
| On ventilator | 19 (16.2%) | 6 (22.2%) | 13 (14.4%) | 0.33 | 0 (0.0%) |
| On inotropic drugs | 17 (14.5%) | 7 (25.9%) | 10 (11.1%) | 0.055 | 0 (0.0%) |
| Acute kidney injury | 24 (20.5%) | 11 (40.7%) | 13 (14.4%) | 0.003 | 0 (0.0%) |
| Clinical presentations | |||||
| Symptom duration (d) | 14 (2–60) | 15 (2–42) | 14 (2–60) | 0.54 | |
| Diarrhea | 81 (52.1%) | 14 (51.8%) | 47 (52.2%) | 0.97 | |
| Lower GI hemorrhage | 45 (38.5%) | 13 (48.1%) | 32 (35.6%) | 0.23 | |
| Abdominal pain | 19 (16.2%) | 1 (3.7%) | 18 (20.0%) | 0.07 | |
| Fever | 17 (14.5%) | 6 (22.2%) | 11 (12.2%) | 0.19 | |
| Laboratory findings | |||||
| Hemoglobin (mg/dL) | 9.99 ± 2.39 | 9.41 ± 2.31 | 10.16 ± 2.40 | 0.15 | |
| WBC (cells/uL) | 7,878 ± 4,450 | 8,456 ± 6,846 | 7,705 ± 3,462 | 0.59 | |
| Albumin (mg/dL) | 3.21 ± 0.87 | 2.77 ± 0.78 | 3.35 ± 0.86 | 0.002 | |
| Endoscopic findings | |||||
| Ulceration | 76 (65.0%) | 24 (88.9%) | 52 (57.8%) | 0.002 | |
| Irregular/geographic | 46 (39.7%) | 16 (59.3%) | 30 (33.7%) | 0.017 | |
| Deep ulcer | 18 (15.5%) | 7 (25.9%) | 11 (12.4%) | 0.09 | |
| Punch out lesion | 4 (3.5%) | 2 (7.4%) | 2 (2.2%) | 0.23 | |
| Inflamed mucosa | 65 (55.6%) | 18 (66.7%) | 47 (52.2%) | 0.18 | |
| Mucosal hemorrhage | 26 (22.2%) | 9 (33.3%) | 17 (18.9%) | 0.11 | |
| Location | |||||
| Ileocecum | 42 (36.2%) | 11 (40.7%) | 31 (34.8%) | 0.57 | |
| Ascending and/or transverse | 34 (29.3%) | 11 (40.7%) | 23 (25.8%) | 0.13 | |
| Descending and/or sigmoid | 50 (42.7%) | 15 (55.7%) | 35 (38.9%) | 0.12 | |
| Rectum | 60 (51.3%) | 18 (66.7%) | 42 (46.7%) | 0.06 |
Data presented as number and percentage, mean plus/minus SD, or median and interquartile range.
A P value < 0.05 indicates statistical significance which are highlighted in bold.
CD4, cluster of differentiation 4; CMV, cytomegalovirus; GI, gastrointestinal; IBD, inflammatory bowel disease; ICU, intensive care unit; WBC, white blood cells.
aAsymptomatic volunteers are the subjects who undergo colonoscopy for colorectal cancer screening without any gastrointestinal symptoms.
Study patient age and sex were not significantly different between the CMV and non-CMV groups. The proportion of patients with HIV infection tended to be higher in the CMV group, but the difference was not statistically significant. Furthermore, the dose of corticosteroids was significantly higher in the CMV colitis group (P = 0.003). The patients with CMV colitis were more seriously ill based on their higher rates of hospitalization, admission to the intensive care unit, acute kidney injury, and lower mean albumin levels. The common presentations in both groups were not different, including diarrhea and lower GI bleeding, with a median duration from symptom onset to hospital presentation of 14 days. Endoscopic findings revealed more ulcerative lesions in the CMV colitis group than in the non-CMV colitis group (88.9% vs 57.8%, respectively; P = 0.002). Among the 20 asymptomatic volunteers, the mean age was 52 years, and 45% were men. The mean age and sex distribution in the control group were not significantly different from the mean age and sex distribution in both of the study groups. All 20 volunteer subjects had normal colonoscopic findings.
Diagnostic performance of stool CMV-PCR, plasma CMV-PCR, and their combination using histopathologic diagnosis as the reference standard
Of the 117 study patients enrolled, 5 underwent colonoscopy twice because of recurrent symptoms, resulting in a total of 122 stool CMV-PCR tests, and 122 plasma CMV-PCR tests. Of those 122 colonoscopies, 27 colonoscopies yielded positive for CMV on histopathology. Table 2 shows the minimum (0th percentile), 25th percentile, median (50th percentile), 75th percentile, and maximum (100th percentile) values of each diagnostic test. As shown in Table 2, the median stool CMV viral load was 2,357 copies/mL (IQR: 0–6,626) in the CMV group, which was significantly higher than the viral load value of 0 copies/ml (IQR: 0–0) in the non-CMV group (P < 0.001). Similarly, the median plasma CMV viral load was significantly higher in the CMV group than in the non-CMV group (345 copies/mL [IQR: 0–25,900] vs 0 copies/mL (IQR: 0–0), respectively; P ≤ 0.001). As shown in Figure 3, the AUC for diagnosing CMV colitis was 0.818 (95% confidence interval [CI]: 0.724–0.911) for the stool CMV-PCR assay and 0.809 (95% CI: 0.715–0.902) for the plasma CMV-PCR assay. There was no significant difference between the stool CMV-PCR AUC and the plasma CMV-PCR AUC (P = 0.854). The identified optimal cutoff values to define a positive CMV finding was 450 copies/mL for the stool CMV-PCR assay and 161 copies/mL for the plasma CMV-PCR assay.
Table 2.
Range of quantitative data specific to cytomegalic cell counts per area of colonic tissue biopsy(cell/mm2) and copies per milliliter from tissue, stool, and plasma CMV-PCR compared among patients with CMV colitis, patients with non-CMV colitis, and asymptomatic volunteers
| Study group | Variables | Minimum (0th percentile) | 25th percentile | Median (50th percentile) | 75th percentile | Maximum (100th percentile) |
| CMV colitis | Cytomegalic cells, cells/mm2 | 1.46 | 4.57 | 6.26 | 7.97 | 11.82 |
| Tissue, copies/mL | 0 | 3,040 | 56,199 | 979,107 | 29,463,461 | |
| Stool, copies/mL | 0 | 0 | 2,357 | 6,626 | 425,317 | |
| Plasma, copies/mL | 0 | 0 | 345 | 25,900 | 283,000 | |
| Non-CMV colitis | Cytomegalic cells, cells/mm2 | 0 | 0 | 0 | 0 | 0 |
| Tissue, copies/mL | 0 | 0 | 0 | 0 | 1,992,358 | |
| Stool, copies/mL | 0 | 0 | 0 | 0 | 43,793 | |
| Plasma, copies/mL | 0 | 0 | 0 | 0 | 13,400 | |
| Asymptomatic volunteers | Cytomegalic cells, cells/mm2 | 0 | 0 | 0 | 0 | 0 |
| Tissue, copies/mL | 0 | 0 | 0 | 0 | 0 | |
| Stool, copies/mL | 0 | 0 | 0 | 0 | 0 | |
| Plasma, copies/mL | 0 | 0 | 0 | 0 | 0 |
CMV, cytomegalovirus; PCR, polymerase chain reaction.
Figure 3.
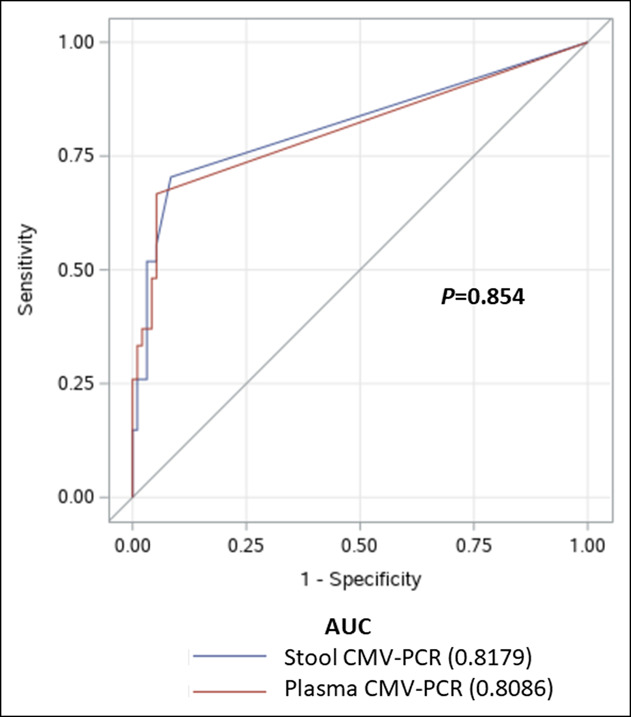
Receiver operating characteristic (ROC) curves to evaluate the sensitivity and specificity of the stool cytomegalovirus polymerase chain reaction (CMV-PCR) assay and the plasma CMV-PCR assay for diagnosing CMV colitis. AUC, area under the curve.
Using the immediately aforementioned cutoff values, 27 stool tests and 23 plasma tests were positive. As shown in Table 3 and Figure 4, stool CMV-PCR alone had a sensitivity and specificity of 70.4% and 91.6%, respectively, whereas the corresponding values for plasma CMV-PCR were 66.7% and 94.7%, respectively. When both tests were combined, positive results for both tests yielded a specificity of 95.8%, which is significantly higher than the 91.6% specificity of stool CMV-PCR alone (P = 0.045), and resulted in a PPV of 78.9% (95% CI: 60.6–97.3). Furthermore, the sensitivity of either positive plasma CMV-PCR or positive stool CMV-PCR was 81.5%, which was significantly higher than the 66.7% sensitivity of positive plasma CMV-PCR alone (P = 0.045), and resulted in a NPV of 94.5% (95% CI: 89.8–99.2). There were 12 inconsistent results between stool CMV-PCR and plasma CMV-PCR. Four of 8 (50%) positive stool CMV-PCR and negative plasma CMV-PCR tests were implicated in a final diagnosis of CMV colitis, and CMV colitis was diagnosed in 1 of 4 (25%) negative stool CMV-PCR and positive plasma CMV-PCR tests. There were no positive results for any stool CMV-PCR or plasma CMV-PCR tests among any of the 20 asymptomatic volunteer subjects.
Table 3.
Diagnostic performance of the 5 evaluated diagnostic modalities
| Testing modalities | Sensitivity (%) (95% CI) |
Specificity (%) (95% CI) |
PPV (%) (95% CI) |
NPV (%) (95% CI) |
| Stool CMV-PCR alone | 70.4% (53.1–87.6) | 91.6% (86.0–97.2) | 70.4% (53.1–87.6) | 91.6% (86.0–97.2) |
| Plasma CMV-PCR alone | 66.7% (48.9–84.4) | 94.7% (90.2–99.2) | 78.3% (61.4–95.1) | 90.9% (85.2–96.6) |
| Positive stool CMV-PCR and positive plasma CMV-PCR | 55.6% (36.8–74.3) | 95.8% (91.7-99.8)a | 78.9% (60.6-97.3)a | 88.3% (82.1–94.5) |
| Positive stool CMV-PCR or positive plasma CMV-PCR | 81.5% (66.8-96.1)a | 90.5% (84.6–96.4) | 71.0% (55.0–86.9) | 94.5% (89.8-99.2)a |
| Tissue PCR | 85.2% (71.8–98.6) | 83.2% (75.6–90.7) | 59.0% (43.5–74.4) | 95.2% (90.6–99.8) |
The highest value for that diagnostic performance parameter.
CI, confidence interval; CMV, cytomegalovirus; PCR, polymerase chain reaction; PPV, positive predictive value; NPV, negative predictive value.
Figure 4.
Diagnostic performance of a positive stool cytomegalovirus polymerase chain reaction (CMV-PCR) alone, a positive plasma CMV-PCR alone, a positive stool CMV-PCR or positive plasma CMV-PCR, and the combination of a positive stool CMV-PCR and a positive plasma CMV-PCR.
Correlation between cytomegalic cell count and quantitative tissue CMV-PCR, stool CMV-PCR, and plasma CMV-PCR
The correlations between cytomegalic cell count and quantitative data from tissue CMV-PCR, stool CMV-PCR, and plasma CMV-PCR are shown in Supplementary Digital Content (see Supplementary Table S2, http://links.lww.com/CTG/A917). The median cytomegalic cell count was 6.3 cells per mm2 of colonic tissue biopsy from patients diagnosed with CMV colitis. The median viral load from tissue, stool, and plasma CMV-PCR was 56,199 copies/mL, 2,357 copies/mL, and 345 copies/mL, respectively. There was a good and significant correlation between stool CMV-PCR and plasma CMV-PCR (r = 0.71, P < 0.0001). Furthermore, both of those tests significantly correlated with cytomegalic cell count (r = 0.62, P < 0.0001 for stool CMV-PCR, and r = 0.64, P < 0.01 for plasma CMV-PCR) and with tissue CMV-PCR (r = 0.68, P < 0.0001 for stool CMV-PCR and r = 0.68, P < 0.0001 for plasma CMV-PCR), as shown in Supplementary Digital Content (see Supplementary Table S2, http://links.lww.com/CTG/A917).
Follow-up stool CMV-PCR to evaluate for CMV colitis after treatment
Twenty-six of the 27 patients diagnosed with CMV colitis were treated with intravenous ganciclovir or oral valganciclovir. The median duration of treatment was 21 days. After treatment, 13 patients underwent follow-up colonoscopy, and stool CMV-PCR was performed in 8 of 13 patients. Among the 4 patients in that group who were initially diagnosed with CMV colitis and who had a positive stool CMV-PCR, all 4 of the follow-up stool tests were negative for CMV after treatment. The follow-up stool CMV-PCR remained negative in the other 4 patients with CMV colitis that had a negative stool CMV-PCR before treatment.
Diagnostic performance of stool CMV-PCR, plasma CMV-PCR, and their combination using tissue CMV-PCR as the reference standard
When using the tissue CMV-PCR as the reference standard, 40 patients had CMV colitis. The sensitivity and specificity of stool CMV-PCR were 60.0% and 96.3%, respectively, whereas the corresponding values of plasma CMV-PCR were 52.5% and 97.6%, respectively. The sensitivity and specificity of pathological diagnosis were 57.5% and 95.1%, respectively. No difference was observed among each diagnostic modality (P = 0.77 for stool CMV-PCR and plasma CMV-PCR, P = 0.80 for stool CMV-PCR and pathological diagnosis, P = 1.00 for serum CMV-PCR and pathological diagnosis).
Subgroup analysis in patients with IBD, patients with immunocompromised status, and patients without obvious immunocompromised status but with some risk factors for CMV colitis
Among 122 colonoscopies, 42 were performed in patients with IBD (6 CMV and 36 non-CMV), 36 were performed in immunocompromised patients (10 CMV and 26 non-CMV), and 44 were performed in immunocompetent patients (11 CMV and 33 non-CMV). Stool CMV-PCR had a sensitivity and specificity of 33.3% and 94.4% in patients with IBD, 70.0% and 84.6% in immunocompromised patients, and 90.9% and 93.9% in immunocompetent patients, respectively. In comparison, plasma CMV-PCR had a sensitivity of 0% for patients with IBD, a sensitivity and specificity of 90.0% and 84.6% for immunocompromised patients, and 81.8% and 96.9% for immunocompetent patients, respectively.
DISCUSSION
This prospective study reported the performance of stool CMV-PCR, plasma CMV-PCR, and their combinations in diagnosis of CMV colitis, the correlations among each diagnostic test, and the performance of stool CMV-PCR in following up after antiviral treatment. There have been few studies evaluating the performance of stool CMV-PCR in diagnosis of CMV colitis; however, the participants, the methods for definite diagnosis of CMV colitis, the PCR methods, and the results are varied as we summarized in Table 4 (36–43).
Table 4.
Summary of previous studies that investigated the diagnostic performance of stool CMV-PCR for diagnosing CMV colitis
| Study | Study design | Population, n | CMV colitis incidence | Reference standard | PCR method | Stool collection | Stool PCR Sn/Sp |
Plasma PCR Sn/Sp |
Tissue PCR Sn/Sp |
Cytomegalic cells |
| Michel et al, 1995 | Prospective | 36 ICM (17 AIDS and 19 post–stem cell transplant); 15 colonoscopies were performed in symptomatic patients |
6/15 (40%) | H&E or IHC | Taq DNA polymerase LOD 2 × 104 CMV genome equivalents per ml (In house analysis) |
Fresh stool 5 mL within 4 wk of colonoscopy | Positive 4/6 (66.7%) | Positive 6/15 (40%) | ||
| Harfarth et al, 2010 | Prospective pilot | 19 IBD (11 UC and 8 CD); (steroid 16/19, thiopurine/MTX 7/19, anti-TNF 4/19) |
6/19 (32%) | Tissue PCR | Primers and TaqMan probe LOD = 500 cp/mL (In house analysis) |
1.5 g of fresh stool | ✓ Sn 83% Sp 93% |
✓ 15 cases Sn 80% Sp 100% |
✓ Gold standard |
|
| Chan et al, 2014 | Retrospective | 18 ICU patients with CMV colitis (excluded: post-transplant, HIV) | Biopsy-proven CMV colitis = 8 Probable CMV colitis = 10 |
H&E or IHC Probable CMV: Symptomatic + blood CMV-PCR pos + colonic ulcer or stool CMV-PCR pos |
Primers and TaqMan probe (In house analysis) | Not mentioned (retrospective) | Positive 8/18 (44.4%), 2/3 biopsy-proven, 5/7 probable CMV | All positive | ||
| Ganzenmueller et al, 2014 | Retrospective | 66 ICM (42% SCT, 26% SOT, 5% HIV, and 14% IBD) | 12/66 (18%) | H&E or IHC tissue PCR >0.14 cp/cell | AB 7500 Cycler LOD = 500 cp/mL (In house analysis) |
Not mentioned (retrospective) | ✓ Sn 67% Sp 96% |
CMV Antigen Sn 83% |
✓ Gold standard |
Significant correlation between stool and tissue CMV-PCR |
| Sun et al, 2015 | Prospective | 56 GVHD biopsy-proven in post-HSCT | 7/58 (12%) | H&E or IHC | AB 7300 Cycler LOD = 1,000 cp/mL (QIAamp stool Mini Kit, Qiagen GmbH, German) |
Stool and blood PCR within 24 hr of colonoscopy | ✓ Sn 28.6% Sp 86.3% |
|||
| Prachasitthisak et al, 2017 | Prospective | 29 ICM (12 SOT, 4 SCT, 3 CMT, and 5 corticosteroid use) | 7/27 (26%) | H&E | LOD = 20 cp/mL (Abbott RealTime CMV assay, USA) | Not mentioned (prospective) | ✓ Sn 71% Sp 85% |
✓ Sn 100% Sp 85% |
||
| Zavrelova et al, 2018 | Retrospective | 45 SCT | 6/69 (8.7%) | H&E or IHC | LOD = 100 cp/mL (QIAamp DNA Mini Kit, Qiagen) | Not mentioned (retrospective) | ✓ Sn 16.7% Sp 76.2% |
✓ Sn 66.7% Sp 71.4% |
||
| Magdziak et al, 2020 | Prospective | 75 active UC (89 tests) (steroid 72%, immunosuppressant 40%, and biologic 10%) |
19/89 (21%) | H&E or IHC | Rotor Gene 6000/Rotor Gene Q apparatus LOD = 42.5 cp/mL (QIAamp Stool Mini Kit, Qiagen of Hilden, German) |
200 μL of supernatant from 215 to 220 mg of stool | ✓ Sn 84.7% Sp 71.4% |
✓ No correlation with stool PCR |
||
| This study, 2022 | Prospective | 117 patients (67 ICM and 50 ICP) (12 SOT, 11 HIV, 54 immunosuppressant, and 39 IBD) (122 tests) 20 asymptomatic volunteers |
27/122 (22%) | H&E or IHC | LOD 450 copies/mL (CMV R-gene kit, bioMérieux, France) | Stool and blood PCR within 7 d of colonoscopy | ✓ Sn 70.4% Sp 91.6% |
✓ Sn 66.7% Sp 94.7% |
✓ Sn 85.2% Sp 83.2% |
✓ Strong correlation with colonic biopsy, stool, and blood PCR |
AB, Applied Biosystems; CD, Crohn's disease; CMT, chemotherapy; CMV, cytomegalovirus; DNA, deoxyribonucleic acid; GVHD, graft-vs-host disease; H&E, hematoxylin and eosin stain; HSCT, hematopoietic stem cell transplantation; IBD, inflammatory bowel disease; ICM, immunocompromised; ICP, immunocompetent; ICU, intensive care unit; IHC, immunohistochemistry; LOD, limit of detection; MTX, methotrexate; PCR, polymerase chain reaction; SCT, stem cell transplant; Sn, sensitivity; SOT, solid organ transplant; Sp, specificity; TNF, tumor necrosis factor; UC, ulcerative colitis.
To best of our knowledge, our study is the first prospective study comprehensively collect and evaluate stool CMV-PCR, plasma CMV-PCR, tissue CMV-PCR, and CMV-infected cell count on histopathology from the same group of patients. All samples were collected within 7 days before or after colonoscopy. We included a broad variety of patients with risk factors for developing CMV colitis, including both immunocompromised and immunocompetent patients. We found an overall sensitivity and specificity of stool CMV-PCR for diagnosing CMV colitis of 70% and 91%, respectively, when using histopathologic findings as the reference standard, and our results are comparable with those from several previous reports (36–39). Most previous studies were conducted in immunocompromised patients. Micheal et al, Ganzenmeuller et al, and Prachasitthisak et al included various types of immunocompromised patients and reported sensitivity of 66%–71%, and specificity of 85%–96% (38,39,41). Two studies specifically included only patients with IBD. The reported sensitivity and specificity were 83%–84.7% and 71.4%–93%, respectively (36,37). Two studies specifically included only stem cell transplantation patients, and one of those studies required enrolled patients to have graft-vs-host disease. They reported the sensitivity and specificity of stool CMV-PCR to be 16.7%–28.6% and 76.2%–86.3%, respectively (40,43).
The diagnostic performance of plasma CMV-PCR for diagnosing CMV colitis in our study was 67% sensitivity and 95% specificity. A previous prospective study by Prachasitthisak et al (39) that included various types of immunocompromised patients reported the sensitivity and specificity of plasma CMV-PCR to be 100% and 85%, respectively. A prospective study by Harfarth et al (36) that included only patients with IBD reported a sensitivity of 80%, and a specificity of 100%, but plasma CMV-PCR was collected in only some patients because it was not in the study protocol. A retrospective study by Zavrelova et al (40) that included only stem cell transplant patients reported a sensitivity of 66.7%, and a specificity of 71.4%. Finally, a study by Chan et al (42) that included only intensive care unit patients reported the sensitivity of plasma CMV-PCR to be 100%.
Despite stool CMV-PCR alone and plasma CMV-PCR alone having been reported to have good performance for diagnosing CMV colitis, the reported sensitivity ranges from 60% to 70%, and the reported specificity ranges from 80% to 90%, as discussed above. The use of these 2 CMV-PCR tests in combination may improve the diagnosis of CMV colitis; however, combination use of these 2 tests in this clinical setting has not been previously studied. In this study, the combination of positive stool CMV-PCR and positive plasma CMV-PCR increased the specificity to 96% with a 79% PPV. Accordingly, when both tests are positive and colonoscopy is not feasible, the start of treatment for CMV colitis should be considered. When we evaluated this combination of CMV-PCR tests and only one or the other test was positive, we found the sensitivity to be 81% with a 94% NPV. As such, a negative result on both tests is strongly suggestive of no presence of CMV colitis.
To strengthen the reliability of the stool and plasma CMV-PCR tests, we analyzed their correlation to other parameters, particularly cytomegalic cell count from tissue histopathology. We found a significantly strong correlation among stool CMR-PCR, plasma CMV-PCR, cytomegalic cell count, and tissue CMV-PCR. By contrast, previous studies reported conflicting results. Ganzenmueller et al reported a significant association between stool CMV-PCR and tissue CMV-PCR, but Magdziac et al found no significant association between cytomegalic cell count and stool CMV-PCR (37,38). Furthermore, we performed follow-up stool CMV-PCR after treatment. We found that all previous positive results became negative after the treatment, which was consistent with the results of follow-up tissue histopathology.
Because tissue CMV-PCR was used as the standard diagnostic tool in some studies (36,38), we did another analysis using tissue CMV-PCR as the reference standard. The sensitivity of both stool and plasma CMV-PCR slightly decreased to 60% and 52.5%, respectively, whereas the specificities remained high, 96.3% for stool and 97.6% for plasma CMV-PCR.
We also did a subgroup analysis categorizing the patients according to the inclusion criteria. We found that the sensitivity of both stool and plasma CMV-PCR in patients with IBD was lower than previously reported (36,37). The difference in the PCR method might attribute to this variation (Table 4). Interestingly, we found that the sensitivity of stool CMV-PCR was higher than plasma CMV-PCR (33.3% vs 0%). This could be because CMV infection in patients with IBD was localized rather than systemic reactivation. The study by Ganzenmueller et al (30), which found that many patients with CMV intestinal disease showed no concomitant CMV antigenemia, suggesting a localized intestinal CMV replication, supported this hypothesis. By contrast, among immunocompromised patients, plasma CMV-PCR was more sensitive to stool CMV-PCR (90% vs 70%) in detecting CMV colitis. This result was consistent with the previous studies including only immunocompromised patients (39,40).
Strengths
This study has several important strengths. First, it is the largest study to investigate the performance of stool CMV-PCR for diagnosing CMV colitis. Second, our study is the first to report the results of stool CMV-PCR, plasma CMV-PCR, tissue CMV-PCR, and cytomegalic cell count from histopathology from the same cohort of patients. Having both stool CMV-PCR data and plasma CMV-PCR data allowed us to assess the performance of stool CMV-PCR, plasma CMV-PCR, and their combination, which our results strongly suggest will confer benefit in clinical practice. Furthermore, because all parameters were evaluated quantitatively, we are able to show strong correlations among stool CMV-PCR, plasma CMV-PCR, tissue CMV-PCR, and cytomegalic cell count from histology. Third, we repeated stool CMV-PCR after completing treatment to assess the correlation between stool CMV-PCR and histologic findings in the same patients at different time points. Fourth and last, we also enrolled 20 asymptomatic volunteers who were subjected to the same study protocol. Analysis of those 20 volunteers showed no presence of CMV in any volunteer for either the stool CMV-PCR or the plasma CMV-PCR. All these strengths support the value of the combined use of stool CMV-PCR and plasma CMV-PCR as a reliable alternative for diagnosing CMV colitis in clinical practice in scenarios where colonoscopy is, for some reason, contraindicated.
Limitations
This study has some mentionable limitations. First, this study was conducted in a single national tertiary care center that is routinely referred complex cases believed to be unmanageable at other levels of care. As such, the characteristics of patients in this study may not necessarily reflect the characteristics of patients in other care settings. Furthermore, the prevalence of CMV colitis in this study may be higher than in other settings. Second, the colonic tissue specimens were not obtained from the same anatomical location, and the number of biopsies was not equal among patients. However, the GI pathologist counted all cytomegalic cells in all tissue specimens, and we calculated the number of cytomegalic cells per mm2 of the biopsy piece. Third and last, we did not enroll a separate group of asymptomatic volunteers who were immunocompromised. This is potentially notable because a previous study reported the possible presence of CMV viremia in asymptomatic immunocompromised patients (44).
Conclusion
The results of this study strongly suggest that the combination of stool CMV-PCR and plasma CMV-PCR can be used to rule in (both tests positive) or rule out (both tests negative) a diagnosis of CMV colitis with a high degree of confidence. In addition to the advantage of these PCR tests being noninvasive, they can be used in hospitalized patients with critical illness, with multiple comorbidities, or with immunocompromised status—all of which render colonoscopy an unfeasible or less favorable diagnostic alternative. In patients with contradictory results between the 2 tests, colonoscopy with tissue biopsy should be performed to establish the diagnosis. Moreover, follow-up stool CMV-PCR may be performed after treatment to ensure treatment success.
CONFLICTS OF INTEREST
Guarantor of the article: Julajak Limsrivilai, MD, MSc.
Specific author contributions: Study design: J.L. and O.S. Data collection: O.S., J.L., P.P., N.S., N.H., A.P., and N.A. Data analysis: J.L. and O.S. Writing of the paper: O.S. and J.L. Giving intellectual comments: N.P., P.C., and M.C. All authors approved the manuscript.
Financial support: This study was funded by a grant from the Faculty of Medicine Siriraj Hospital, Mahidol University, Grant Number (IO) R016433007.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Diagnosis of cytomegalovirus colitis requires colonoscopy with tissue biopsy.
✓ Colonoscopy can cause serious complications, particularly in patients with severe illnesses.
WHAT IS NEW HERE
✓ The combined use of stool cytomegalovirus (CMV) polymerase chain reaction and plasma CMV polymerase chain reaction can be used to rule in (both tests positive) or rule out (both tests negative) a diagnosis of CMV colitis with a high degree of confidence.
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge the study patients and volunteer control subjects who generously agreed to participate in this study.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A917.
Contributor Information
Onuma Sattayalertyanyong, Email: onuma.gimed@gmail.com.
Phutthaphorn Phaophu, Email: phutthaphorn.p@gmail.com.
Nichcha Subdee, Email: nichcha.subdee@gmail.com.
Navin Horthongkham, Email: navmoo@gmail.com.
Ananya Pongpaibul, Email: ananya.pog@mahidol.edu.
Napat Angkathunyakul, Email: aonz_z@hotmail.com.
Methee Chayakulkeeree, Email: methee.cha@mahidol.ac.th.
Nonthalee Pausawasdi, Email: nonthaleep7@gmail.com.
Phunchai Charatcharoenwitthaya, Email: phunchai@yahoo.com.
References
- 1.Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med 1993;119(9):924–35. [DOI] [PubMed] [Google Scholar]
- 2.Francis ND, Boylston AW, Roberts AH, et al. Cytomegalovirus infection in gastrointestinal tracts of patients infected with HIV-1 or AIDS. J Clin Pathol 1989;42(10):1055–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nasa M, Sharma Z, Sud R, et al. Cytomegalovirus infection of gastrointestinal tract. Community Acquired Infect 2016;3(1):4–9. [Google Scholar]
- 4.O'Hara KM, Pontrelli G, Kunstel KL. An introduction to gastrointestinal tract CMV disease. JAAPA 2017;30(10):48–52. [DOI] [PubMed] [Google Scholar]
- 5.Patra S, Samal SC, Chacko A, et al. Cytomegalovirus infection of the human gastrointestinal tract. J Gastroenterol Hepatol 1999;14(10):973–6. [DOI] [PubMed] [Google Scholar]
- 6.Le P-H, Lin W-R, Kuo C-J, et al. Clinical characteristics of cytomegalovirus colitis: A 15-year experience from a tertiary reference center. Ther Clin Risk Manag 2017;13:1585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaemsupaphan T, Limsrivilai J, Thongdee C, et al. Patient characteristics, clinical manifestations, prognosis, and factors associated with gastrointestinal cytomegalovirus infection in immunocompetent patients. BMC Gastroenterol 2020;20(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salzberger B, Hartmann P, Hanses F, et al. Incidence and prognosis of CMV disease in HIV-infected patients before and after introduction of combination antiretroviral therapy. Infection 2005;33(5-6):345–9. [DOI] [PubMed] [Google Scholar]
- 9.Marques Jr O, Averbach M, Zanoni ECA, et al. Cytomegaloviral colitis in HIV positive patients: Endoscopic findings. Arquivos de Gastroenterologia 2007;44(4):315–9. [DOI] [PubMed] [Google Scholar]
- 10.Hammond SP, Martin ST, Roberts K, et al. Cytomegalovirus disease in lung transplantation: Impact of recipient seropositivity and duration of antiviral prophylaxis. Transpl Infect Dis 2013;15(2):163–70. [DOI] [PubMed] [Google Scholar]
- 11.Harvala H, Stewart C, Muller K, et al. High risk of cytomegalovirus infection following solid organ transplantation despite prophylactic therapy. J Med Virol 2013;85(5):893–8. [DOI] [PubMed] [Google Scholar]
- 12.Ramanan P, Razonable RR. Cytomegalovirus infections in solid organ transplantation: A review. Infect Chemother 2013;45(3):260–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotton CN, Kumar D, Caliendo AM, et al. The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2018;102(6):900–31. [DOI] [PubMed] [Google Scholar]
- 14.Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients-guidelines of the American society of transplantation infectious diseases community of practice. Clin Transplant 2019;33(9):e13512. [DOI] [PubMed] [Google Scholar]
- 15.Kwon S, Jung BK, Ko S-Y, et al. Comparison of quantitation of cytomegalovirus DNA by real-time PCR in whole blood with the cytomegalovirus antigenemia assay. Ann Lab Med 2015;35(1):99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko JH, Peck KR, Lee WJ, et al. Risk factors for cytomegalovirus gastrointestinal diseases in adult patients with cancer. Eur J Clin Microbiol Infect Dis 2014;33(10):1847–53. [DOI] [PubMed] [Google Scholar]
- 17.Ko JH, Peck KR, Lee WJ, et al. Clinical presentation and risk factors for cytomegalovirus colitis in immunocompetent adult patients. Clin Infect Dis 2015;60(6):e20–6. [DOI] [PubMed] [Google Scholar]
- 18.Galiatsatos P, Shrier I, Lamoureux E, et al. Meta-analysis of outcome of cytomegalovirus colitis in immunocompetent hosts. Dig Dis Sci 2005;50(4):609–16. [DOI] [PubMed] [Google Scholar]
- 19.Klauber E, Briski LE, Khatib R. Cytomegalovirus colitis in the immunocompetent host: An overview. Scand J Infect Dis 1998;30(6):559–64. [DOI] [PubMed] [Google Scholar]
- 20.Siegmund B. Cytomegalovirus infection associated with inflammatory bowel disease. Lancet Gastroenterol Hepatol 2017;2(5):369–76. [DOI] [PubMed] [Google Scholar]
- 21.Roblin X, Pillet S, Oussalah A, et al. Cytomegalovirus load in inflamed intestinal tissue is predictive of resistance to immunosuppressive therapy in ulcerative colitis. Am J Gastroenterol 2011;106(11):2001–8. [DOI] [PubMed] [Google Scholar]
- 22.Nakase H, Matsumura K, Yoshino T, et al. Systematic review: Cytomegalovirus infection in inflammatory bowel disease. J Gastroenterol 2008;43(10):735–40. [DOI] [PubMed] [Google Scholar]
- 23.Cotte L, Drouet E, Bissuel F, et al. Diagnostic value of amplification of human cytomegalovirus DNA from gastrointestinal biopsies from human immunodeficiency virus-infected patients. J Clin Microbiol 1993;31(8):2066–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garrido E, Carrera E, Manzano R, et al. Clinical significance of cytomegalovirus infection in patients with inflammatory bowel disease. World J Gastroenterol 2013;19(1):17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baniak N, Kanthan R. Cytomegalovirus colitis: An uncommon mimicker of common colitides. Arch Pathol Lab Med 2016;140(8):854–8. [DOI] [PubMed] [Google Scholar]
- 26.McCoy MH, Post K, Sen JD, et al. qPCR increases sensitivity to detect cytomegalovirus in formalin-fixed, paraffin-embedded tissue of gastrointestinal biopsies. Hum Pathol 2014;45(1):48–53. [DOI] [PubMed] [Google Scholar]
- 27.Beswick L, Ye B, van Langenberg DR. Toward an algorithm for the diagnosis and management of CMV in patients with colitis. Inflamm Bowel Dis 2016;22(12):2966–76. [DOI] [PubMed] [Google Scholar]
- 28.Matsuoka K, Iwao Y, Mori T, et al. Cytomegalovirus is frequently reactivated and disappears without antiviral agents in ulcerative colitis patients. Am J Gastroenterol 2007;102(2):331–7. [DOI] [PubMed] [Google Scholar]
- 29.Dimitroulia E, Spanakis N, Konstantinidou AE, et al. Frequent detection of cytomegalovirus in the intestine of patients with inflammatory bowel disease. Inflamm Bowel Dis 2006;12(9):879–84. [DOI] [PubMed] [Google Scholar]
- 30.Ganzenmueller T, Henke-Gendo C, Schlue J, et al. Quantification of cytomegalovirus DNA levels in intestinal biopsies as a diagnostic tool for CMV intestinal disease. J Clin Virol 2009;46(3):254–8. [DOI] [PubMed] [Google Scholar]
- 31.Zidar N, Ferkolj I, Tepes K, et al. Diagnosing cytomegalovirus in patients with inflammatory bowel disease--by immunohistochemistry or polymerase chain reaction?. Virchows Arch 2015;466(5):533–9. [DOI] [PubMed] [Google Scholar]
- 32.Kucharzik T, Ellul P, Greuter T, et al. ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis 2021;15(6):879–913. [DOI] [PubMed] [Google Scholar]
- 33.Mills AM, Guo FP, Copland AP, et al. A comparison of CMV detection in gastrointestinal mucosal biopsies using immunohistochemistry and PCR performed on formalin-fixed, paraffin-embedded tissue. Am J Surg Pathol 2013;37(7):995–1000. [DOI] [PubMed] [Google Scholar]
- 34.Mori T, Mori S, Kanda Y, et al. Clinical significance of cytomegalovirus (CMV) antigenemia in the prediction and diagnosis of CMV gastrointestinal disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transpl 2004;33(4):431–4. [DOI] [PubMed] [Google Scholar]
- 35.Nagata N, Kobayakawa M, Shimbo T, et al. Diagnostic value of antigenemia assay for cytomegalovirus gastrointestinal disease in immunocompromised patients. World J Gastroenterol 2011;17(9):1185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herfarth HH, Long MD, Rubinas TC, et al. Evaluation of a noninvasive method to detect cytomegalovirus (CMV)-DNA in stool samples of patients with inflammatory bowel disease (IBD): A pilot study. Dig Dis Sci 2010;55(4):1053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magdziak A, Szlak J, Mróz A, et al. A stool test in patients with active ulcerative colitis helps exclude cytomegalovirus disease. Scand J Gastroenterol 2020;55(6):664–70. [DOI] [PubMed] [Google Scholar]
- 38.Ganzenmueller T, Kluba J, Becker JU, et al. Detection of cytomegalovirus (CMV) by real-time PCR in fecal samples for the noninvasive diagnosis of CMV intestinal disease. J Clin Virol 2014;61(4):517–22. [DOI] [PubMed] [Google Scholar]
- 39.Prachasitthisak N, Tanpowpong P, Lertudomphonwanit C, et al. Short article: Stool cytomegalovirus polymerase chain reaction for the diagnosis of cytomegalovirus-related gastrointestinal disease. Eur J Gastroenterol Hepatol 2017;29(9):1059–63. [DOI] [PubMed] [Google Scholar]
- 40.Zavrelova A, Radocha J, Pliskova L, et al. Detection of cytomegalovirus DNA in fecal samples in the diagnosis of enterocolitis after allogeneic stem cell transplantation. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2018;162(3):227–31. [DOI] [PubMed] [Google Scholar]
- 41.Michel D, Marre E, Hampl W, et al. Intestinal cytomegalovirus disease in immunocompromised patients may be ruled out by search for cytomegalovirus DNA in stool samples. J Clin Microbiol 1995;33(11):3064–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan KS, Yang CC, Chen CM, et al. Cytomegalovirus colitis in intensive care unit patients: Difficulties in clinical diagnosis. J Crit Care 2014;29(3):474.e1-6. [DOI] [PubMed] [Google Scholar]
- 43.Sun YQ, Xu LP, Han TT, et al. Detection of human cytomegalovirus (CMV) DNA in feces has limited value in predicting CMV enteritis in patients with intestinal graft-versus-host disease after allogeneic stem cell transplantation. Transpl Infect Dis 2015;17(5):655–61. [DOI] [PubMed] [Google Scholar]
- 44.Spector SA, Wong R, Hsia K, et al. Plasma cytomegalovirus (CMV) DNA load predicts CMV disease and survival in AIDS patients. J Clin Invest 1998;101(2):497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.