Abstract
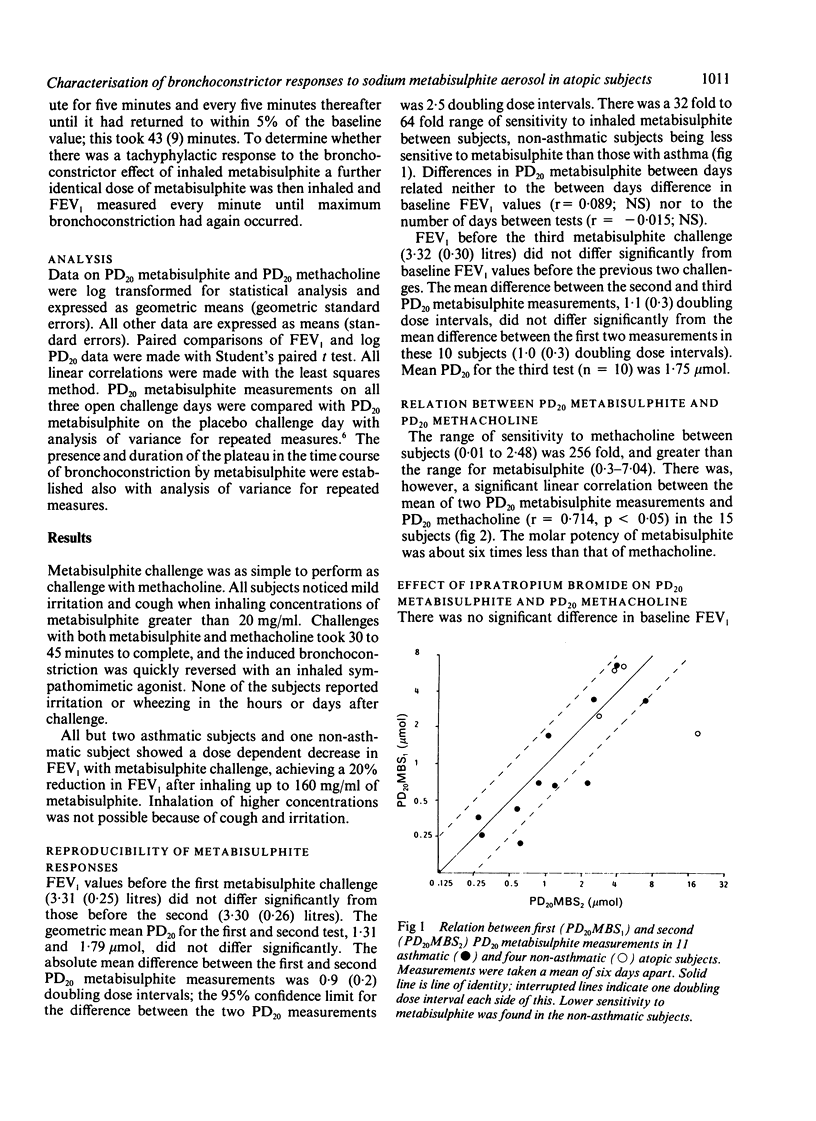
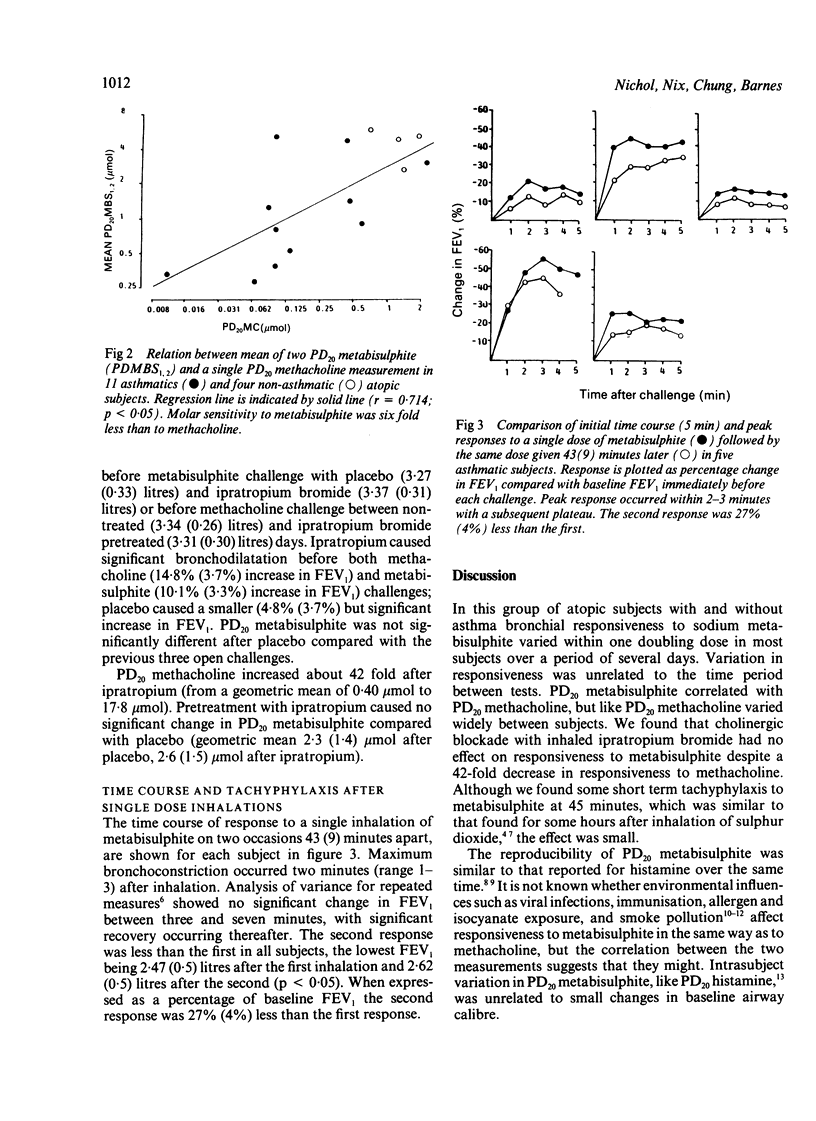
Inhalation of sodium metabisulphite is thought to induce bronchoconstriction by release of sulphur dioxide. We sought to establish the reproducibility of the airway response to inhaled sodium metabisulphite given in increasing doubling concentrations (0.3 to 160 mg/ml) to 13 asthmatic and five atopic non-asthmatic subjects and the contribution of cholinergic mechanisms to this response. In 15 of the 18 subjects bronchoconstriction was sufficient to allow calculation of the dose of metabisulphite causing a 20% reduction in the forced expiratory volume in one second (FEV1) from baseline values (PD20 metabisulphite). The 95% confidence limit for the difference between this and a second PD20 metabisulphite determined 2-14 days later was 2.5 doubling doses. The difference between repeat PD20 metabisulphite measurements was unrelated to the number of days between challenges or change in baseline FEV1. Ten subjects returned for a third study 3-120 days after the second challenge; variability in PD20 metabisulphite did not differ from that seen between the first and second challenges. PD20 methacholine was determined between the two metabisulphite challenges and found to correlate with PD20 metabisulphite (r = 0.71). Inhaled ipratropium bromide 200 micrograms given in a randomised, placebo controlled, crossover study to 10 subjects increased PD20 methacholine 42 fold but had no significant effect on the response to metabisulphite. A single inhalation of the PD20 metabisulphite in five subjects induced maximal bronchoconstriction 2-3 minutes after inhalation, with a plateau in FEV1 lasting a further four minutes before recovery. A further single inhalation of the same PD20 dose 43 minutes later produced a 27% (SEM 4%) smaller fall in FEV1 than the first inhalation. These results show that metabisulphite PD20 values measured over days and weeks show similar reproducibility to those reported for histamine inhalation and that PD20 metabisulphite correlates with methacholine responsiveness. Most of the bronchoconstriction is not inhibited by antimuscarinic agents; the underlying mechanisms require further investigation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker G. J., Collett P., Allen D. H. Bronchospasm induced by metabisulphite-containing foods and drugs. Med J Aust. 1981 Nov 28;2(11):614–617. doi: 10.5694/j.1326-5377.1981.tb113018.x. [DOI] [PubMed] [Google Scholar]
- Barnes P. J. Neural control of human airways in health and disease. Am Rev Respir Dis. 1986 Dec;134(6):1289–1314. doi: 10.1164/arrd.1986.134.5.1289. [DOI] [PubMed] [Google Scholar]
- Chung K. F., Snashall P. D. Effect of prior bronchoconstriction on the airway response to histamine in normal subjects. Thorax. 1984 Jan;39(1):40–45. doi: 10.1136/thx.39.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft D. W., Ruffin R. E., Dolovich J., Hargreave F. E. Allergen-induced increase in non-allergic bronchial reactivity. Clin Allergy. 1977 Nov;7(6):503–513. doi: 10.1111/j.1365-2222.1977.tb01481.x. [DOI] [PubMed] [Google Scholar]
- Dixon C. M., Barnes P. J. Bradykinin-induced bronchoconstriction: inhibition by nedocromil sodium and sodium cromoglycate. Br J Clin Pharmacol. 1989 Jun;27(6):831–836. doi: 10.1111/j.1365-2125.1989.tb03446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon C. M., Fuller R. W., Barnes P. J. Effect of nedocromil sodium on sulphur dioxide induced bronchoconstriction. Thorax. 1987 Jun;42(6):462–465. doi: 10.1136/thx.42.6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M., Jackson D. M., Richards I. M. The action of sodium cromoglycate on 'C' fibre endings in the dog lung. Br J Pharmacol. 1980 Sep;70(1):11–13. doi: 10.1111/j.1476-5381.1980.tb10898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Empey D. W., Laitinen L. A., Jacobs L., Gold W. M., Nadel J. A. Mechanisms of bronchial hyperreactivity in normal subjects after upper respiratory tract infection. Am Rev Respir Dis. 1976 Feb;113(2):131–139. doi: 10.1164/arrd.1976.113.2.131. [DOI] [PubMed] [Google Scholar]
- Fine J. M., Gordon T., Sheppard D. The roles of pH and ionic species in sulfur dioxide- and sulfite-induced bronchoconstriction. Am Rev Respir Dis. 1987 Nov;136(5):1122–1126. doi: 10.1164/ajrccm/136.5.1122. [DOI] [PubMed] [Google Scholar]
- Godard P. Chronic cough: bronchoscopy or pulmonary testing? Am Rev Respir Dis. 1983 Feb;127(2):257–257. doi: 10.1164/arrd.1983.127.2.257. [DOI] [PubMed] [Google Scholar]
- Koenig J. Q., Marshall S. G., van Belle G., McManus M. S., Bierman C. W., Shapiro G. G., Furukawa C. T., Pierson W. E. Therapeutic range cromolyn dose-response inhibition and complete obliteration of SO2-induced bronchoconstriction in atopic adolescents. J Allergy Clin Immunol. 1988 May;81(5 Pt 1):897–901. doi: 10.1016/0091-6749(88)90947-5. [DOI] [PubMed] [Google Scholar]
- Madsen F., Rathlou N. H., Frølund L., Svendsen U. G., Weeke B. Short and long term reproducibility of responsiveness to inhaled histamine: Rt compared to FEV1 as measurement of response to challenge. Eur J Respir Dis. 1985 Sep;67(3):193–203. [PubMed] [Google Scholar]
- NADEL J. A., SALEM H., TAMPLIN B., TOKIWA Y. MECHANISM OF BRONCHOCONSTRICTION DURING INHALATION OF SULFUR DIOXIDE. J Appl Physiol. 1965 Jan;20:164–167. doi: 10.1152/jappl.1965.20.1.164. [DOI] [PubMed] [Google Scholar]
- Pavlovic M., Holstein-Rathlou N. H., Madsen F., Svendsen U. G., Weeke B. Bronchial histamine challenge. A combined interrupter-dosimeter method compared with a standard method. Allergy. 1985 Nov;40(8):574–579. doi: 10.1111/j.1398-9995.1985.tb00885.x. [DOI] [PubMed] [Google Scholar]
- Schwartz H. J., Chester E. H. Bronchospastic responses to aerosolized metabisulfite in asthmatic subjects: potential mechanisms and clinical implications. J Allergy Clin Immunol. 1984 Oct;74(4 Pt 1):511–513. doi: 10.1016/0091-6749(84)90387-7. [DOI] [PubMed] [Google Scholar]
- Sheppard D., Epstein J., Bethel R. A., Nadel J. A., Boushey H. A. Tolerance to sulfur dioxide-induced bronchoconstriction in subjects with asthma. Environ Res. 1983 Apr;30(2):412–419. doi: 10.1016/0013-9351(83)90227-x. [DOI] [PubMed] [Google Scholar]
- Snashall P. D., Baldwin C. Mechanisms of sulphur dioxide induced bronchoconstriction in normal and asthmatic man. Thorax. 1982 Feb;37(2):118–123. doi: 10.1136/thx.37.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinacker A. Presynaptic effects of sodium bisulfite at the frog neuromuscular junction. J Neurosci Res. 1982;7(3):313–319. doi: 10.1002/jnr.490070309. [DOI] [PubMed] [Google Scholar]
- Steinacker A. Sulphonation of cholinergic receptor disulphide bond increases response to ACh. Nature. 1979 Mar 22;278(5702):358–360. doi: 10.1038/278358a0. [DOI] [PubMed] [Google Scholar]
- Stevenson D. D., Simon R. A. Sensitivity to ingested metabisulfites in asthmatic subjects. J Allergy Clin Immunol. 1981 Jul;68(1):26–32. doi: 10.1016/0091-6749(81)90119-6. [DOI] [PubMed] [Google Scholar]
- Tan W. C., Cripps E., Douglas N., Sudlow M. F. Protective effect of drugs on bronchoconstriction induced by sulphur dioxide. Thorax. 1982 Sep;37(9):671–675. doi: 10.1136/thx.37.9.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. T. Passive smoking and lung cancer. What is the risk? Am Rev Respir Dis. 1986 Jan;133(1):1–3. doi: 10.1164/arrd.1986.133.1.1. [DOI] [PubMed] [Google Scholar]


