Abstract
An 8-week feeding experiment was carried out to explore the effects of dietary n-3/n-6 polyunsaturated fatty acid (PUFA) ratio on growth performance, lipid metabolism, hepatic antioxidant status, and gut flora of spotted seabass (Lateolabrax maculatus). Six experimental diets were formulated to contain different levels of two purified oil sources including docosahexaenoic and eicosapentaenoic acids enriched oil (n-3) and linoleic acid-enriched oil (n-6) leading to n-3/n-6 PUFA ratios of 0.04, 0.35, 0.66, 1.35, 2.45 and 16.17. Each diet was fed to triplicate groups of juvenile L. maculatus (11.06 ± 0.20 g, 30 fish/tank). Final body weight (FBW), weight gain (WG), specific growth rates (SGR), protein efficiency ratio (PER) and feed utilization efficiency increased as n-3/n-6 PUFA ratio increased up to a certain level, and then decreased thereafter. Fish fed the diet with n-3/n-6 PUFA ratio of 0.66 exhibited the highest FBW, WG, SGR and PER and the lowest feed conversion ratio. Lower n-3/n-6 PUFA ratios induced up-regulated expression of lipid synthesis-related genes (fas, acc2 and srebp-1c) and down-regulated expression of lipolysis related genes (atgl, pparα, cpt-1 and aox). Higher expression of lipolysis-related genes (atgl, pparα and cpt-1) was recorded at moderate n-3/n-6 PUFA ratios (0.66 to 1.35). Moreover, inappropriate n-3/n-6 PUFA ratios triggered up-regulation of pro-inflammatory genes (il-6 and tnf-α) and down-regulation of anti-inflammatory genes (il-4 and il-10) in the intestine. The diet with n-3/n-6 PUFA ratio of 0.66 inhibited intestine inflammation, improved intestinal flora richness, increased the abundance of beneficial bacteria such as Lactobacillus, Alloprevotella and Ruminococcus, and reduced the abundance of harmful bacteria including Escherichia-Shigella and Enterococcus. In summary, it could be suggested that a dietary n-3/n-6 PUFA ratio of 0.66 can improve growth performance and feed utilization in L. maculatus, as is deemed to be mediated through regulation of lipid metabolism and intestinal flora.
Keywords: Lateolabrax maculatus, Growth performance, Lipid metabolism, Intestinal flora, n-3/n-6 PUFA ratio
1. Introduction
Fats play essential roles in vertebrates by serving as an energy source and participating in key metabolic processes (Watanabe, 1982; Zechner et al., 2012; Wang et al., 2022). Notably, it has been demonstrated that the fat requirement of animals is determined to a great extent by its fatty acids (Glencross, 2009). Most vertebrates lack de novo biosynthesis capacity for polyunsaturated fatty acids (PUFA) due to low relative desaturase and elongase activities (Sargent et al., 1999). Hence, PUFA, as crucial nutritional factors, should be exogenously supplied in the diet.
Recent nutrition studies have revealed that n-3 and n-6 PUFA are highly biologically active components of fats (Harris, 2018). Furthermore, it has been shown that a competitive relationship between n-3 and n-6 PUFA exists in common enzymatic systems in the metabolic processes (Contreas and Rapoport, 2002; Niu et al., 2004). Opposing physiological roles of n-3 and n-6 PUFA have been found in growth and development of organs, lipid metabolism and inflammatory response (Schmitz and Ecker, 2008). Metabolites of n-6 PUFA, such as prostaglandins, leukotrienes, and related compounds, are known to be ligands for the toll-like receptor 4 (TLR4) and inducers of inflammation (Alagawany et al., 2021). The critical contribution of the intestinal flora to the intestinal health and metabolic state of animals is widely recognized (Liu et al., 2021). Excessive intake of n-6 PUFA has been found to induce metabolic endotoxemia leading to dysbiosis of intestinal flora (Krajmalnik et al., 2012). Intriguingly, a previous study revealed that supplementation of n-3 PUFA to high n-6 PUFA diets improved intestinal flora disturbance and prevented organ damage (Ghosh et al., 2013). Also, n-3 PUFA displays a converse impact on TLR4 and inflammatory responses compared to n-6 PUFA (Mokkala et al., 2020).
Recent studies in mammals showed that an imbalance in dietary n-3/n-6 PUFA ratio is associated with the development of a spectrum of pathologies including metabolic disorders, cardiovascular diseases, and enteritis (Heerwagen et al., 2013; Husted and Bouzinova, 2016; Liu et al., 2013; Scorletti and Byrne, 2013). Moreover, dietary n-3/n-6 PUFA ratios ranging from 0.25 to 1 were shown to decrease the risk of breast, prostate, colon and renal cancers (Zarate et al., 2017). Similarly, the significance of n-3/n-6 PUFA ratio has also been emphasized in aquafeeds (Sargent et al., 1995). Previous reports have shown that an optimal n-3/n-6 PUFA ratio can improve growth performance, nutrient utilization, and health of aquatic animals (Glencross, 2009; Hundal et al., 2021a; Jin et al., 2019). However, the optimal dietary n-3/n-6 PUFA ratio varies considerably among species.
In aquafeeds, n-3 and n-6 PUFA are mainly provided by fish oil and vegetable oils, respectively (Marszalek and Lodish, 2005). Typically, fish oil is rich in n-3 PUFA including docosahexaenoic acid (C22:6n-3, DHA) and eicosapentaenoic acid (C20:5n-3, EPA), while most vegetable oils are rich in n-6 PUFA such as linoleic acid (18:2n-6, LA) (Jin et al., 2019; Torstensen et al., 2005). Due to decreasing supply of marine products globally, fish oil production has decreased considerably during the recent decades (Zuo et al., 2012b). Accordingly, substituting fish oil with vegetable oils in aquafeeds has become a common practice (Nasopoulou and Zabetakis, 2012; Turchini et al., 2009). However, the replacement of fish oil with vegetable oils corresponds with the considerable alteration of dietary n-3/n-6 PUFA ratio (Turchini et al., 2009). Furthermore, fish oil and vegetable oils produced from different raw materials often have varying n-3/n-6 ratios (Childs et al., 1990; Xue et al., 2006). Therefore, it is necessary to precisely determine the appropriate dietary n-3/n-6 PUFA ratio to provide a theoretical basis for the appropriate fish oil replacement level and dietary fat source.
Spotted seabass (Lateolabrax maculatus) is a euryhaline teleost fish classified in the genus Lateolabrax which used to be regarded as Japanese seabass (Lateolabrax japonicas) (Lu et al., 2020). Recently, this fish was proved to be a different species from L. japonicas according to morphological and molecular biological analyses (Chen et al., 2019; Liu et al., 2006). Owing to its high economic value, rapid growth and delicious flesh, spotted seabass production in China has been increasing continuously in recent years, having reached 195,000 tons in 2020 (Lu et al., 2020). Accordingly, numerous studies have been conducted to quantify its nutritional requirements such as protein (Cai et al., 2020), lipid (Xie et al., 2021; Xu et al., 2011), minerals (Song et al., 2017; Zhang et al., 2022), and amino acids (Cheng et al., 2021; Mai et al., 2006) requirements, and optimization of protein/energy ratio (Lu et al., 2020). However, to our knowledge, determination of the appropriate dietary n-3/n-6 PUFA ratio for spotted seabass is still needed. Traditionally, marine fish oil has been used as the main oil source for this species (Xue et al., 2006). Due to both economic and sustainability reasons, the use of vegetable oils and freshwater fish oils in aquafeeds is booming nowadays (Cai et al., 2020; Cheng et al., 2021). The revelation of an appropriate dietary n-3/n-6 PUFA ratio will help to obtain suitable combinations of different oils. Some previous studies on other fish species used different fish oil/vegetable oil ratios to formulate diets with varying n-3/n-6 ratios, which introduced additional variables (Zhu et al., 2017; Jin et al., 2019; Hundal et al., 2021a). In the present study, high purity n-3 and n-6 PUFA enriched oils were used to prepare semi-purified diets with varying n-3/n-6 PUFA ratios to determine the appropriate dietary n-3/n-6 PUFA ratio more precisely. Furthermore, for a better understanding of the physiological roles of n-3 and n-6 PUFA, diets with extremely low or high n-3/n-6 PUFA ratios were also prepared.
2. Materials and methods
2.1. Animal ethics statement
The animal study protocol of the present study was reviewed and approved by the Ethics Committee of Animal Research Institute Committee guidelines, Jimei University, China (No. 2019–32, Approval date: 5 March 2019).
2.2. Experimental diets
Six isonitrogenous (44.7% crude protein) and isolipidic (11% crude lipid) semi-purified diets were prepared. White fish meal, soy protein concentrate, and wheat gluten meal were used as the main protein sources. Two purified oils were used as the main lipid sources including a DHA + EPA-enriched oil as the source of n-3 PUFA, and an LA-enriched oil as the source of n-6 PUFA. The total amount of purified oils was fixed at 9% but with variable ratios to produce n-3/n-6 PUFA ratios of 0.04 (Diet 1), 0.35 (Diet 2), 0.66 (Diet 3), 1.35 (Diet 4), 2.45 (Diet 5) and 16.17 (Diet 6) (analyzed values). The formulation and proximate composition of the experimental diets are shown in Table 1, and fatty acid composition of diets is presented in Table 2. The diets were prepared as described in our previous study (Cai et al., 2020). Briefly, all coarse ingredients were finely pulverized and sifted through a 60-mesh sieve. All dry ingredients were thoroughly mixed, and after addition of the blend of purified oils, 30% (wt:wt) deionized water was added to make a dough. The resultant dough was made into a hard pelleted diet (diameter = 2 mm) diet using a cold-extruded pellet producer. The pellets were air-dried at room temperature, sealed in plastic bags, and stored at −20 °C until use.
Table 1.
Formulation and proximate composition of the experimental diets (% dry matter).
| Item | Diets (n-3/n-6 PUFA ratio) |
|||||
|---|---|---|---|---|---|---|
| Diet 1 (0.04) | Diet 2 (0.35) | Diet 3 (0.66) | Diet 4 (1.35) | Diet 5 (2.45) | Diet 6 (16.17) | |
| Ingredients | ||||||
| White fish meal | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 |
| Soy protein concentrate | 27.97 | 27.97 | 27.97 | 27.97 | 27.97 | 27.97 |
| Wheat gluten meal | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 |
| Corn starch | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 |
| DHA + EPA-enriched oil1 | 0.00 | 3.00 | 4.50 | 6.00 | 7.20 | 9.00 |
| LA-enriched oil2 | 9.00 | 6.00 | 4.50 | 3.00 | 1.80 | 0.00 |
| Microcrystalline cellulose | 4.68 | 4.68 | 4.68 | 4.68 | 4.68 | 4.68 |
| Vitamin C | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Premix3 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 |
| Choline chloride | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| CaH2PO4 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Proximate composition | ||||||
| Dry matter | 93.42 | 95.26 | 94.18 | 95.32 | 94.56 | 95.21 |
| Protein | 43.84 | 45.16 | 45.33 | 45.06 | 45.18 | 44.02 |
| Lipid | 10.30 | 10.65 | 10.22 | 10.39 | 10.44 | 10.19 |
| Ash | 9.75 | 9.77 | 9.65 | 9.89 | 9.86 | 9.64 |
PUFA = poly-unsaturated fatty acids; DHA = docosahexaenoic acid; EPA = eicosapentaenoic acid; LA = linoleic acid.
DHA + EPA-enriched oil: DHA content, 413.5 mg/g oil; EPA content, 424.3 mg/g oil.
LA-enriched oil: LA content, 853.2 mg/g oil.
The composition of premix was described in our previous study (Cai et al., 2020).
Table 2.
Fatty acid composition of the experimental diets (% total fatty acids).
| Fatty acids | Diets (n-3/n-6 PUFA ratio) |
|||||
|---|---|---|---|---|---|---|
| Diet 1 (0.04) | Diet 2 (0.35) | Diet 3 (0.66) | Diet 4 (1.35) | Diet 5 (2.45) | Diet 6 (16.17) | |
| C14:0 | 0.41 | 0.44 | 0.46 | 0.50 | 0.51 | 0.62 |
| C16:0 | 5.45 | 5.53 | 5.90 | 5.60 | 5.92 | 6.07 |
| C18:0 | 2.18 | 2.10 | 1.98 | 2.25 | 2.33 | 1.96 |
| C20:0 | 0.28 | 0.23 | 0.20 | 0.20 | 0.19 | 0.15 |
| ΣSFA | 8.32 | 8.30 | 8.54 | 8.54 | 8.95 | 8.81 |
| C16:1n-7 | 0.62 | 0.60 | 0.65 | 0.71 | 0.76 | 0.89 |
| C18:1n-9 | 7.77 | 7.40 | 7.13 | 6.90 | 6.63 | 7.10 |
| C20:1n-9 | 0.37 | 0.37 | 0.38 | 0.41 | 0.41 | 0.48 |
| ΣMUFA | 8.76 | 8.38 | 8.15 | 8.02 | 7.79 | 8.47 |
| C18:2n-6 | 72.30 | 55.85 | 47.34 | 32.87 | 22.77 | 4.27 |
| Σn-6 PUFA | 72.30 | 55.85 | 47.34 | 32.87 | 22.77 | 4.27 |
| C18:3n-3 | 0.47 | 0.51 | 0.52 | 0.56 | 0.56 | 0.77 |
| C20:5n-3 | 1.03 | 9.23 | 14.54 | 21.28 | 27.02 | 32.70 |
| C22:6n-3 | 1.12 | 10.06 | 16.08 | 22.52 | 28.23 | 35.53 |
| DHA/EPA ratio | 1.09 | 1.09 | 1.11 | 1.06 | 1.05 | 1.09 |
| Σn-3 PUFA | 2.62 | 19.79 | 31.14 | 44.36 | 55.81 | 69.01 |
| n-3/n-6 PUFA ratio | 0.04 | 0.35 | 0.66 | 1.35 | 2.45 | 16.17 |
PUFA = poly-unsaturated fatty acids; SFA = saturated fatty acids; MUFA = mono-unsaturated fatty acids; DHA = docosahexaenoic acid; EPA = eicosapentaenoic acid.
The trace amounts and not detectable fatty acids are not shown in the table.
2.3. Experimental fish and feeding trial
Spotted seabass juveniles used in the present study were purchased from a local fish breeding farm and were all from the same batch. The feeding trial was carried out at a local aquaculture facility (Zhangzhou, Fujian). Prior to the feeding trial, fish were stocked in a recirculating aquaculture system (RAS) and fed a commercial diet (crude protein: 44%, crude lipid: 10%; Fujian Yuehai Feeds Group Corp., Ltd) for environmental adaptation. After a month, a total of 540 healthy juveniles (initial weight 11.06 ± 0.20 g) were selected and randomly distributed into 18 tanks in a RAS at a density of 30 fish per tank. During the feeding trial, continuous flow of ultraviolet-disinfected freshwater and aeration were provided. The water temperature was maintained at 26.8 ± 0.5 °C, dissolved oxygen was 5.8 ± 0.2 mg/L, pH was maintained at 7.2 ± 0.2, and the photoperiod was controlled at 12:12 h (light:dark). The fish were hand-fed twice daily (08:00 and 17:30) with the test diets to apparent satiation for 8 weeks. After 30 min of each meal, uneaten feed was collected, dried, and weighed for subsequent calculation of feed intake.
2.4. Sampling
At termination of the feeding trial, fish were fasted for 24 h prior to sampling. All the fish in each tank were captured and euthanized for counting and weighing. A total of 14 fish per tank were randomly selected, of which 5 fish were stored at −20 °C for the analysis of body composition, and 9 fish per tank were used for drawing blood from the caudal vein using 1-mL sterile syringe. The blood samples were stored at 4 °C overnight and centrifuged (850 × g, 10 min) at 4 °C to separate the serum. The isolated serum was stored at −80 °C for subsequent analysis of biochemical parameters. After drawing blood, the dorsal muscle, liver, and intestine (9 fish per tank) were immediately removed and pooled, flash frozen in liquid nitrogen and then stored at −80 °C until further analysis. Moreover, a small piece of liver sample (approximately 5 mm3) from 3 fish per tank was isolated using a scalpel and fixed in Bouin's solution for histology examination.
2.5. Analytical methods
2.5.1. Proximate and fatty acid composition analyses
Moisture, crude protein, crude lipid, and ash contents in the experimental diets and whole-body of fish were determined according to the methods described in AOAC (2005). Moisture was analyzed by drying in an oven at 105 °C until a constant weight was reached. Crude protein (N × 6.25) was assayed by Dumas's combustion method. Crude lipid was detected through the Soxhlet extractor method. Ash was measured by the combustion method at 550 °C for 8 h. Fatty acid composition of the diets and dorsal muscle samples were determined by gas chromatography (GC-2010; Shimadzu, Kyoto, Japan) according to a previous study (Zuo et al., 2012a).
2.5.2. Liver antioxidant status
For determination of antioxidant status, liver samples (n = 3) were prepared as described by Lu et al. (2014). Superoxide dismutase (SOD) and catalase (CAT) activities, total antioxidant capacity (T-AOC) and malonaldehyde (MDA) concentration were detected in liver homogenate using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, China). The protein content of liver homogenate was determined by a BCA Protein Assay Kit (Beijing Solarbio Science & Technology Co., Ltd., China).
2.5.3. Liver histology
Histological examination of liver (n = 3) was performed according to our previous study (Lu et al., 2013). Briefly, liver samples were fixed in Bouin's solution for 24 h, then dehydrated by a graded series of ethanol solutions, cleared by xylene solution, and embedded in paraffin. Thereafter, 5-μm thick sections were cut and stained with hematoxylin and eosin solution (Nanjing Jiancheng Bioengineering Institute, China). The observation and photographs were carried out under a Leica DM5500B light microscope, and the representative images were selected for display.
2.5.4. Gene expression
Total RNA was isolated from 20 mg pooled liver samples (3 replicates per group) using a commercial kit (RC101, Vazyme Biotech Co., Ltd., Nanjing, China). After DNase treatment, the purity of hepatic RNA samples was determined spectrophotometrically (Nanodrop 2000, Thermo Fisher Scientific, USA), and the quality of RNA was tested using 1.5% agarose gel electrophoresis. The agarose was obtained from Lablead Inc. (Beijing, China). Then, 1 μg RNA was used for the reverse-transcription of cDNA using a commercial kit (R211, Vazyme Biotech Co., Ltd., Nanjing, China). Quantitative Real-time PCR (qRT-PCR) was used to determine the relative gene expression levels under an ABI QuantStudio 6 Flex qPCR system (Applied Biosystems, Foster City, CA, USA), using AceQ qPCR SYBR Green Master Mix (Vazyme Biotech Co., Ltd., Nanjing, China). The procedure was as follows: pre-denaturation at 95 °C for 5 min, followed by 40 circular reactions of 95 °C for 10 s, 60 °C for 30 s. The primers used in the present study were designed using an online tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) and synthesized by Genewiz Co. Ltd (Suzhou, China). The primer sequences are listed in Table S1. The relative gene expression levels were calculated by 2−ΔΔCt method, according to our previous study (Dong et al., 2022), and β-actin was used as house-keeping gene.
2.5.5. Intestinal flora
Six hindgut samples from each group were used for intestinal flora analysis. Total bacterial DNA was isolated from each sample using a commercial kit (Magen, Shanghai, China). After quantity and purity assessments, the total bacterial DNA samples were transferred to Gene Denovo Biotechnology Co. Ltd (Guangzhou, China) and used to perform 16S rDNA sequencing. Briefly, the 16S rDNA V3–V4 region was amplified by PCR with the forward and reverse primers 341 F (CCTACGGGNGGCWGCAG) and 806 R (GGACTACHVGGGTATCTAAT). Then, the high-throughput sequencing of purified products was carried out using the Illumina HiSeq 2500. Bioinformatic analysis was conducted using an online platform (http://www.omicsmart.com) provided by Gene Denovo Biotechnology Co. Ltd.
2.6. Statistical analysis
The differences among experimental groups were analyzed by one-way ANOVA and Tukey's post-hoc test using the SPSS 22.0 program. All data are presented as mean ± S.E.M. (standard error of the mean), and the difference was considered significant when P < 0.05.
3. Results
3.1. Growth performance, feed utilization, survival and body composition
In the present study, all the diets were accepted by the fish, and no mortality was recorded during the feeding trial. As shown in Table 3, final body weight (FBW), weight gain (WG), specific growth rate (SGR), and protein efficiency ratio (PER) increased as dietary n-3/n-6 PUFA ratio increased from 0.04 to 0.66, and decreased thereafter, while an opposite trend was observed for feed conversion ratio (FCR). The highest protein retention ratio (PRR) was obtained for fish fed Diet 3, which significantly differed from those of the groups fed Diets 1, 2, 5 and 6 (P < 0.05). Increasing the n-3/n-6 PUFA ratio resulted in a lower lipid retention ratio (LRR). The feeding rate (FR) remained unchanged among all groups. The abdominal fat percentage (AFP) consistently decreased with the increasing of dietary n-3/n-6 PUFA ratio, and the highest value was observed in the group fed Diet 1 (P < 0.05).
Table 3.
Effects of dietary n-3/n-6 PUFA ratio on growth performance and feed utilization in L. maculatus.
| Item | Diets (n-3/n-6 PUFA ratio) |
|||||
|---|---|---|---|---|---|---|
| Diet 1 (0.04) | Diet 2 (0.35) | Diet 3 (0.66) | Diet 4 (1.35) | Diet 5 (2.45) | Diet 6 (16.17) | |
| FBW1, g | 39.86 ± 1.30ab | 44.66 ± 1.10bc | 47.99 ± 0.41c | 40.94 ± 0.16ab | 39.77 ± 2.67ab | 34.63 ± 1.47a |
| WG2, % | 262.34 ± 11.85ab | 306.04 ± 10.01bc | 337.28 ± 4.28c | 272.15 ± 1.50ab | 261.56 ± 24.29ab | 214.81 ± 13.38a |
| SGR3, %/d | 2.30 ± 0.06ab | 2.50 ± 0.05bc | 2.63 ± 0.02c | 2.35 ± 0.01bc | 2.29 ± 0.12ab | 2.05 ± 0.08a |
| FCR4 | 1.40 ± 0.01cd | 1.31 ± 0.01ab | 1.25 ± 0.03a | 1.35 ± 0.02bc | 1.37 ± 0.01bcd | 1.44 ± 0.02d |
| PER5 | 1.62 ± 0.03a | 1.69 ± 0.01ab | 1.78 ± 0.04b | 1.64 ± 0.02ab | 1.62 ± 0.02a | 1.59 ± 0.03a |
| PRR6, % | 24.00 ± 0.42a | 23.48 ± 0.21a | 26.62 ± 0.56b | 25.22 ± 0.28ab | 23.96 ± 0.19a | 24.16 ± 0.33a |
| LRR7, % | 40.97 ± 0.81cd | 41.89 ± 0.34d | 38.05 ± 0.88bc | 41.24 ± 0.43cd | 35.14 ± 0.11b | 27.37 ± 0.91a |
| FR8, %/d | 4.26 ± 0.09 | 4.14 ± 0.05 | 4.00 ± 0.09 | 4.10 ± 0.04 | 4.13 ± 0.08 | 4.09 ± 0.02 |
| AFP9, % | 4.05 ± 0.19e | 3.34 ± 0.12d | 3.08 ± 0.03cd | 2.66 ± 0.11bc | 2.23 ± 0.11ab | 1.87 ± 0.05a |
PUFA = poly-unsaturated fatty acids.
Values in the same row with the same superscripts are not significantly different (P > 0.05).
Final body weight (g).
Weight gain (%) = (final body weight–initial body weight)/initial body weight × 100.
Specific growth rate (%/d) = (ln final body weight-ln initial body weight)/feeding days × 100.
Feed conversion ratio = dry feed intake/wet weight gain.
Protein efficiency ratio = wet weight gain/total protein intake.
Protein retention ratio (%) = (final body protein content – initial body protein content)/total protein intake × 100.
Lipid retention ratio (%) = (final body lipid content – initial body lipid content)/total lipid intake × 100.
Feeding rate (%/d) = dry feed intake/[(initial body weight + final body weight)/2]/feeding days × 100.
Abdominal fat percentage (%) = (individual abdominal fat weight/individual body weight) × 100.
Moreover, the results of whole-body composition analyses revealed no significant differences among groups (P > 0.05) (Table S2). The muscle fatty acid composition was obviously impacted by the dietary n-3/n-6 PUFA ratio (Table S3). The muscle PUFA composition reflected the PUFA profile of the diet, where increasing the dietary n-3/n-6 PUFA ratio led to increased muscle Ʃn-3 PUFA content and decreased Ʃn-6 PUFA content.
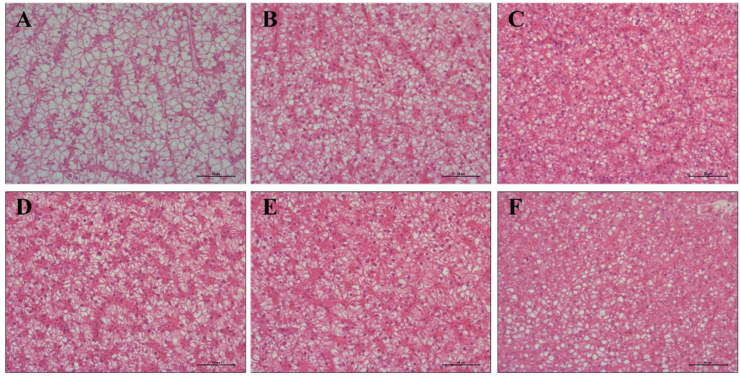
3.2. Liver histology
Representative histological images of fish liver are shown in Fig. 1. The liver of groups fed Diet 3, Diet 4 and Diet 5 had normal structure and hepatocytes arranged tightly, whereas the groups offered Diet 2 and Diet 6 showed some hepatocellular vacuolization. Furthermore, fish fed Diet 1 exhibited the most severe vacuolization and irregular arrangement of hepatocytes.
Fig. 1.
Liver section with H&E staining (magnification, 400 × , scale bar = 50 μm) of L. maculatus fed the experimental diets for 8 weeks (A: Diet 1, B: Diet 2, C: Diet 3, D: Diet 4, E: Diet 5, F: Diet 6).
3.3. Liver antioxidant status
CAT activity and T-AOC increased as dietary n-3/n-6 PUFA ratio increased from 0.04 to 0.66, and then decreased (Table 4). The lowest MDA concentration was achieved at the n-3/n-6 PUFA ratio of 0.66, which significantly differed from that of the other groups (P < 0.05). However, SOD activity did not change among groups.
Table 4.
Effects of dietary n-3/n-6 PUFA ratio on liver oxidative status in L. maculatus.
| Item | Diets (n-3/n-6 PUFA ratio) |
|||||
|---|---|---|---|---|---|---|
| Diet 1 (0.04) | Diet 2 (0.35) | Diet 3 (0.66) | Diet 4 (1.35) | Diet 5 (2.45) | Diet 6 (16.17) | |
| SOD, U/mgprot | 115.82 ± 7.58 | 119.22 ± 8.14 | 144.55 ± 1.77 | 122.35 ± 9.02 | 111.78 ± 3.48 | 129.26 ± 7.11 |
| CAT, U/mgprot | 438.45 ± 26.82ab | 579.27 ± 24.59b | 613.8 ± 48b | 555.3 ± 28.72b | 460.13 ± 8.99ab | 363.73 ± 60.34a |
| T-AOC, U/mgprot | 0.31 ± 0.01ab | 0.32 ± 0.02ab | 0.40 ± 0.02b | 0.28 ± 0.03a | 0.22 ± 0.01a | 0.21 ± 0.02a |
| MDA, mmol/mgprot | 1.66 ± 0.07cd | 1.31 ± 0.01b | 1.00 ± 0.07a | 1.42 ± 0.02bc | 1.36 ± 0.06b | 1.73 ± 0.10d |
PUFA = poly-unsaturated fatty acids; SOD = superoxide dismutase; CAT = catalase; T-AOC = total antioxidant capacity; MDA = malonaldehyde.
Values in the same row with the same superscripts are not significantly different (P > 0.05).
3.4. Relative expression of lipid metabolism-related genes
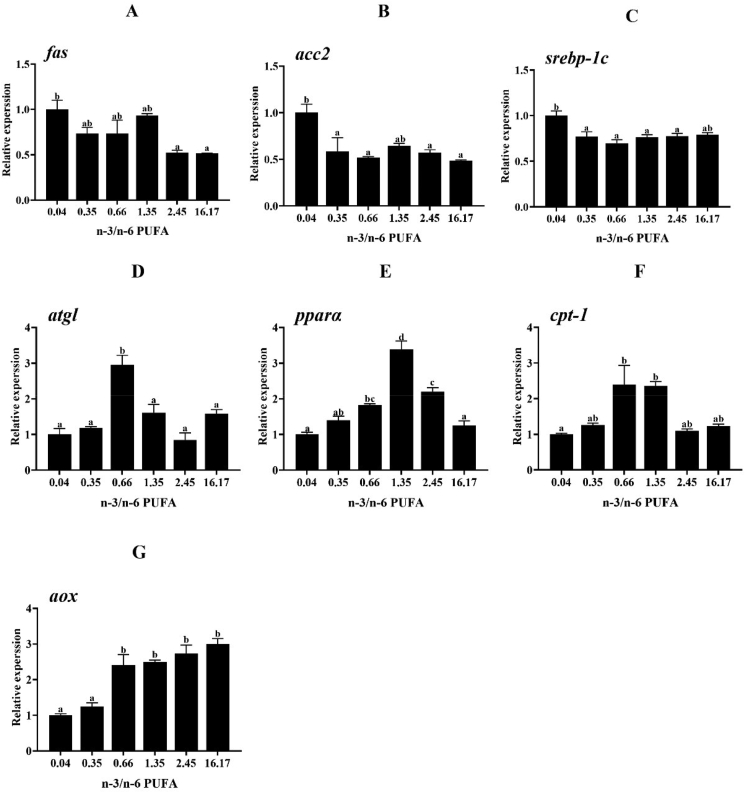
The relative expression of lipid metabolism-related genes in the liver is listed in Fig. 2. The fish fed Diet 1 exhibited the highest expression level of lipid synthesis-related genes such as fatty acid synthase (fas), acetyl CoA carboxylase 2 (acc2) and sterol regulatory element binding protein-1c (srebp-1c). Expression of the fas gene in fish fed Diets 5 and 6 was obviously lower than that of fish fed Diet 1 (P < 0.05). The fish fed Diets 2, 3, 5 and 6 exhibited a markedly lower expression level of the acc2 gene than the fish fed Diet 1 (P < 0.05). Also, the expression level of srebp-1c in the groups fed Diets 2, 3, 4 and 5 was significantly lower than fish fed Diet 1 (P < 0.05). A different pattern for the expression of lipolysis-associated genes such as adipose triglyceride lipase (atgl), peroxisome proliferator-activated receptor α (pparα), carnitine palmitoyl transferase-1 (cpt-1) and acyl-CoA oxidase (aox) was observed compared to the expression of lipid synthesis-related genes. Fish fed Diet 3 showed significantly higher expression of the atgl gene than the other groups (P < 0.05). Expression of pparα was enhanced concomitantly with increasing dietary n-3/n-6 PUFA ratio from 0.04 to 1.35, and then decreased at higher ratios. The expression of cpt-1 was up-regulated in fish fed Diet 3 and 4 in comparison to the fish fed Diet 1 (P < 0.05). Expression of aox was remarkably up-regulated at n-3/n-6 PUFA ratios of 0.66 to 16.17 (P < 0.05).
Fig. 2.
Relative expression of lipid metabolism-related genes in the liver of L. maculatus fed the experimental diets for 8 weeks (A: fas, B: acc2, C: srepb-1c, D: atgl, E: pparα, F: cpt-1, G: aox). Bars with the same superscripts are not significantly different (P > 0.05). Fas = fatty acid synthase; srebp-1c = sterol regulatory element binding protein-1c; acc2 = acetyl CoA carboxylase 2; pparα = peroxisome proliferator-activated receptor α; cpt-1 = carnitine palmitoyl transferase-1; aox = acyl-CoA oxidase; atgl = adipose triglyceride lipase.
3.5. Relative expression of inflammation-related genes
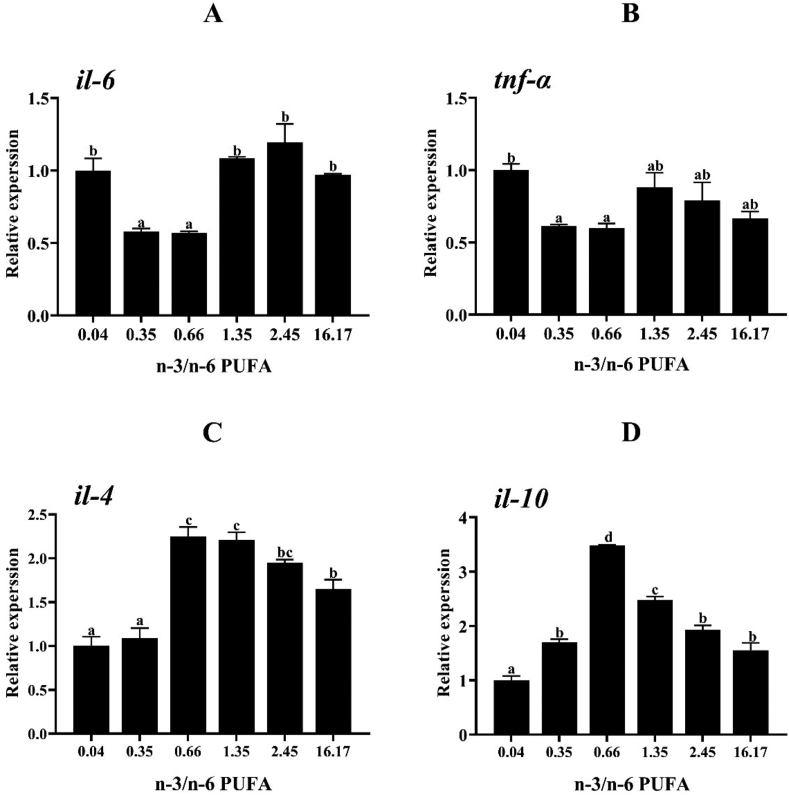
The relative expression of inflammation-related genes in the intestine is presented in Fig. 3. The expression level of interleukin-6 (il-6) in the groups fed Diets 2 and 3 was significantly lower than the other groups (P < 0.05). Also, expression of tumor necrosis factor-α (tnf-α) was down-regulated in groups fed Diets 2 and 3 compared to the fish fed Diet 1 (P < 0.05). The expression level of interleukin-4 (il-4) in groups offered Diets 3 and 4 was markedly higher than those fed Diets 1, 2 and 6 (P < 0.05). The expression level of interleukin-10 (il-10) was enhanced by increasing n-3/n-6 PUFA ratio up to 0.66, and then decreased (P < 0.05).
Fig. 3.
Relative expression of inflammation-related genes in the intestine of L. maculatus fed the experimental diets for 8 weeks (A: il-6, B: tnf-α, C: il-4, D: il-10). Bars with the same superscripts are not significantly different (P > 0.05). Il-4 = interleukin-4; il-6 = interleukin-6; il-10 = interleukin-10; tnf-α = tumor necrosis factor-α.
3.6. Intestinal flora analysis
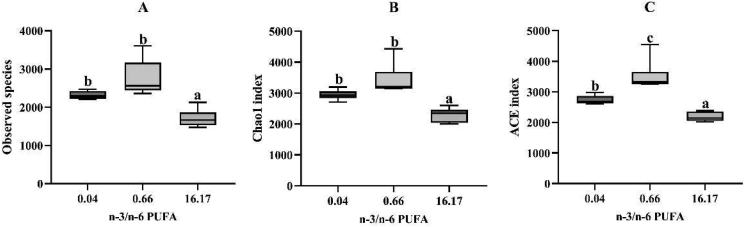
The intestinal flora of the groups fed Diets 1, 3 and 6 was analyzed through 16S rDNA sequencing. The fish fed the diet with the n-3/n-6 PUFA ratio of 16.17 exhibited the lowest observed species and Chao1 index values (P < 0.05) compared to the other two groups (Fig. 4A and B). The ACE index in fish fed the diet with the n-3/n-6 PUFA ratio of 0.66 was significantly higher than the other groups (P < 0.05) (Fig. 4C). The principal coordinates analysis (PCoA) was conducted to visualize the similarities and differences in the composition of the microbial communities (Fig. S1). The greater distance among the symbols indicates a higher difference among groups. It was obvious that the symbols from the same group were mostly clustered and dislocated from the other groups. Thus, there were clear differences in the intestinal microbiota composition among fish fed the diets with n-3/n-6 PUFA ratios of 0.04, 0.66 and 16.17.
Fig. 4.
The diversity of intestinal microbiota in L. maculatus fed the experimental diets for 8 weeks. (A) Observed species, (B) Chao1 index, (C) ACE index. Box plots with the same superscripts are not significantly different (P > 0.05).
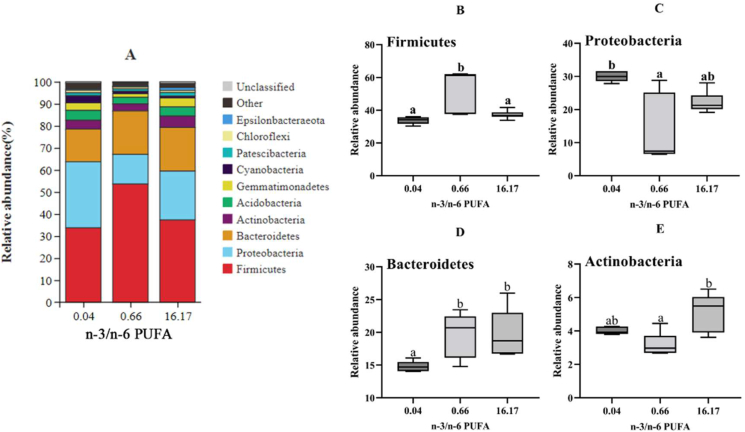
The intestinal microbiota was analyzed at the phylum and genus levels. As shown in Fig. 5A, Firmicutes, Proteobacteria, Bacteroidetes and Actinobacteria were the most predominant phyla in all groups, and their abundance showed obvious differences (Fig. 5B–E). The relative abundance of Firmicutes in the intestine of fish fed the diet with n-3/n-6 PUFA ratio of 0.66 was markedly higher compared to n-3/n-6 PUFA ratios of 0.04 and 16.17 (P < 0.05). Fish fed the diet with n-3/n-6 PUFA ratio of 0.04 had significantly higher abundance of Proteobacteria compared to n-3/n-6 PUFA ratio of 0.66 (P < 0.05). The diet with n-3/n-6 PUFA ratio of 0.04 resulted in a remarkably lower abundance of Bacteroidetes compared to the other diets (P < 0.05). Furthermore, the relative abundance of Actinobacteria in fish fed the diet with n-3/n-6 PUFA ratio of 16.17 was significantly higher compared to the n-3/n-6 PUFA ratio of 0.66 (P < 0.05).
Fig. 5.
The analysis of species differences in the intestinal microbiota of L. maculatus fed different diets for 8 weeks at the phylum level. (A) The proportion of microbial communities of the gut microbiota at the phylum level. The relative abundance of Firmicutes (B), Proteobacteria (C), Bacteroidetes (D), and Actinobacteria (E). Box plots with the same superscripts are not significantly different (P > 0.05).
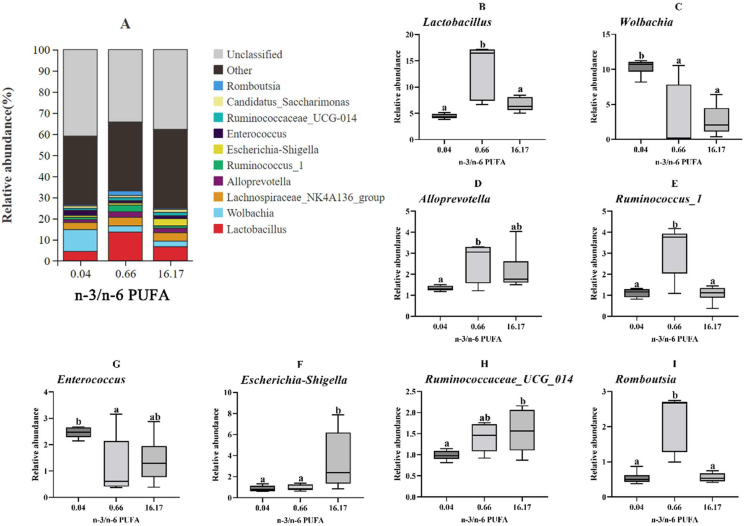
Lactobacillus, Wolbachia, Lachnospiraceae_NK4A136_group, Alloprevotella, Ruminococcus_1, Escherichia-Shigella, Enterococcus, Ruminococcaceae_UCG-014, Candidatus_Saccharimonas and Romboutsia were the dominant bacterial genera (Fig. 6A). The species with significant differences are shown in the box plots in Fig. 6B–I. The abundance of Lactobacillus, Ruminococcus_1 and Romboutsia in fish fed the diet with n-3/n-6 PUFA ratio of 0.66 was markedly higher compared to n-3/n-6 PUFA ratios of 0.04 and 16.17 (P < 0.05) (Fig. 6B, E and I). Dietary n-3/n-6 PUFA ratio of 0.04 resulted in a significantly higher abundance of Wolbachia compared to the other ratios (P < 0.05) (Fig. 6C). Furthermore, the abundance of Alloprevotella at a dietary n-3/n-6 PUFA ratio of 0.66 was remarkably higher than the ratio of 0.04 (P < 0.05) (Fig. 6D). The diet with n-3/n-6 PUFA ratio of 16.17 led to an increased abundance of Escherichia-Shigella compared to other groups (P < 0.05) (Fig. 6F). The abundance of Enterococcus in fish fed the diet with n-3/n-6 PUFA ratio of 0.04 was markedly higher than 0.66 (P < 0.05) (Fig. 6G). Moreover, the abundance of Ruminococcaceae_UCG-014 increased as dietary n-3/n-6 PUFA ratio increased (P < 0.05).
Fig. 6.
The analysis of species differences in the intestinal microbiota of L. maculatus fed different diets for 8 weeks at the genus level. (A) The proportion of microbial communities of the gut microbiota at the genus level. The relative abundance of Lactobacillus (B), Wolbachia (C), Alloprevotella (D), Ruminococcus_1 (E), Escherichia-Shigella (F), Enterococcus (G), Ruminococcaceae_UCG-014 (H) and Romboutsia (I). Box plots with the same superscripts are not significantly different (P > 0.05).
4. Discussion
The dietary n-3/n-6 PUFA ratio has been regarded as a potent factor for growth of fish (Glencross, 2009). In this study, FBW, WG and SGR of spotted seabass were impacted by the different dietary n-3/n-6 PUFA ratios. The maximum growth was achieved for fish maintained on the diet with n-3/n-6 PUFA ratio of 0.66 (Diet 3). Growth performance decreased at both low and high extreme dietary n-3/n-6 ratios, as agrees with studies on black seabream (Acanthopagrus schlegelii) (Jin et al., 2019), large yellow croaker (Larmichthys crocea) (Zuo et al., 2012a), European seabass (Dicentrarchus labrax) (Campos et al., 2019) and grouper (Epinephelus coioides) (He et al., 2021). In the current study, fish fed Diet 3 exhibited the lowest FCR and the highest PER, indicating an improvement in nutrient utilization. It is widely accepted that growth is associated with the deposition of nutrients, particularly protein, in fish (Dumas et al., 2007). As a result, the group fed Diet 3 showed the highest PRR among all experimental groups, implying that the growth promoting effect of optimal n-3/n-6 PUFA ratio could be explained by the facilitated protein retention. As carnivorous fish species including spotted seabass have limited capacity for carbohydrate utilization, dietary protein would be catabolized for energy production when lipid metabolism is disturbed. Thus, regulation of lipid metabolism can improve PER and PRR (Du et al., 2006; Li et al., 2012; Lu et al., 2020). Previous studies in mammals showed that low n-3/n-6 intake is a high-risk factor for metabolic disorders (Scorletti and Byrne, 2013), and that dietary supplementation of n-3 PUFA could inhibit excessive fat deposition (Spadaro et al., 2008). The results of our study showed the reduction of LRR and AFP by increasing dietary n-3/n-6 PUFA ratio, which might be the intrinsic reason for the low protein utilization and poor growth, as the deposition of fat is also an important contributor to the weight gain of fish (Du et al., 2006).
Numerous studies have reported the effect of dietary fatty acid profile on the lipid metabolism of fish (Bandarra et al., 2011; Dai et al., 2021; Hundal et al., 2021a, 2021b; Zhang et al., 2022; Zuo et al., 2012b). Muscle composition can reflect the internal causes of the difference in growth to a certain extent (Mommsen, 2001; Vélez et al., 2017). In this study, the fatty acid composition of muscle was responsive to dietary n-3/n-6 PUFA ratios, probably indicating that the dietary n-3/n-6 PUFA ratio may affect growth performance through regulating lipid metabolism. The liver is an essential organ for lipid metabolism (Zhou et al., 2019). In this study, we found that the extremely high and low dietary n-3/n-6 PUFA ratios induced liver damage as evidenced by the histological examination. As the center of lipid metabolism, the liver often displays a strong oxidant/antioxidant interplay (Mattioli et al., 2021). However, the dynamic balance of oxidant/antioxidant status is often interrupted by unsuitable nutritional conditions resulting in lipid metabolism dysfunction (Dong et al., 2022). In this study, fish fed Diet 3 exhibited the highest SOD and CAT activities and T-AOC and the lowest MDA content, while an opposite trend was true for the groups fed Diets 1 and 6. These results indicate that extreme n-3/n-6 PUFA ratios deteriorate the antioxidant capacity leading to promoted lipid peroxidation. It is widely accepted that PUFA serve as the key components of cellular membrane structure, and that the membrane n-3/n-6 ratio directly influences the membrane fluidity (Harris, 2018). As the major source of cellular active oxygen, mitochondrial redox homeostasis is largely dependent to the membrane fluidity (Schieber and Chandel, 2014). We speculate that the diet with n-3/n-6 PUFA ratio of 0.66 may improve redox homeostasis through improving the mitochondrial membrane fluidity.
The expression of lipid synthesis- and lipolysis-related genes were investigated in this study to further explore the alterations in lipid metabolism. ACC catalyzes the carboxylation of acetyl-CoA to malonyl-CoA, an important precursor of lipid synthesis. Malonyl-CoA is used to produce fatty acids catalyzed by fatty acid synthase (FAS) (Zhang et al., 2022). These two enzymes are key rate-limiting points of de novo synthesis of fatty acids (Dong et al., 2020). Transcription factor SREBP-1C is an important regulator of the expression of acc and fas, and some other genes involved in triglyceride (TAG) and cholesterol formation (Horton et al., 2002). Previous studies have demonstrated that the expression of srebp-1c could be affected by PUFA (Worgall et al., 1998). In the present study, fish fed Diet 1 had the highest expression level of acc2, fas and srebp-1c, indicating that a low dietary n-3/n-6 PUFA ratio enhances lipid synthesis, which agrees with previous reports in other fish species (He et al., 2021; Jin et al., 2019). ATGL is the rate-limiting enzyme for TAG hydrolysis (Yang et al., 2010). As the main product of TAG hydrolysis, fatty acids are further broken down by β-oxidation (Den Broeder et al., 2015). The β-oxidation of fatty acids with a carbon number ≥20 occurs in peroxisome, and other fatty acids are β-oxidated in mitochondria. The transfer of fatty acids into peroxisome or mitochondria is regulated by the key enzymes CPT-1 and AOX, respectively (Tocher, 2003). Moreover, the expression of cpt-1 and aox can be activated by the transcription factor PPARα (Pyper et al., 2010). In this study, the fish fed Diet 3 or/and Diet 4 showed high expression levels of atgl, pparα and cpt-1, indicating that inappropriate dietary n-3/n-6 PUFA ratios inhibit lipolysis, as is in parallel with previous studies (Ayala et al., 2014; Zhu et al., 2017). Increased expression of aox by increasing the n-3/n-6 PUFA ratio corresponded with enhanced AFP and serum TG and TC concentrations. These results indicate that spotted seabass may have a limited catabolic capacity of n-6 PUFA.
The intestine is a vital digestive organ which also plays key roles in the immune and metabolic functions in fish (Cai et al., 2020; Liu et al., 2021). Intestinal inflammation is a common phenomenon in cultured fish, and could be induced by unsuitable nutritional conditions (Liu et al., 2021). Fish suffering from intestinal inflammation often imply an inefficient utilization of nutrients and impaired health status (Venold et al., 2012; Wang et al., 2020). Several authors have found that inappropriate dietary fat level and fatty acid composition can activate inflammatory responses (Dai et al., 2021; Xie et al., 2021). In the current study, we determined the transcriptional levels of pro-inflammatory cytokines (IL-6 and TNF-α) and anti-inflammatory cytokines (IL-4 and IL-10). Fish fed the diet with the n-3/n-6 PUFA ratio of 0.66 (Diet 3) showed the lowest expression levels of il-6 and tnf-α and the highest expression levels of il-4 and il-10, indicating that a suitable dietary n-3/n-6 ratio can inhibit intestinal inflammation. The extreme n-3/n-6 PUFA ratios (Diet 1 or 6) triggered intestinal inflammation, as was evidenced by the up-regulated expression of il-6 and tnf-α, and down-regulated expression of il-4 and il-10. Furthermore, the fish fed Diet 1 displayed the highest expression levels of il-6 and tnf-α, and the lowest expression levels of il-4 and il-10. Interestingly, there are several reports indicating that excessive dietary n-6 PUFA can activate inflammation (Li et al., 2021; Tan et al., 2022), and that supplementation of n-3 PUFA can ameliorate the inflammatory response (Zhu et al., 2022), as are in agreement with the results of the current study.
Studies in mammalian models have demonstrated that the interactions between dietary fatty acid composition and intestinal flora play a significant role in regulation of intestinal inflammation (Ye et al., 2021). In the present study, the fish fed the diet with n-3/n-6 PUFA ratio of 0.66 and the extreme ratios (0.04 and 16.17) displayed large differences in intestinal inflammatory responses. Accordingly, we further analyzed the intestinal flora composition of these 3 groups. The results of the PCoA analysis showed that the intestinal flora composition of L. maculatus was clearly modified by dietary n-3/n-6 PUFA ratio. At the same time, the value of observed species, Chao1 index and ACE index indicated that at extreme dietary n-3/n-6 PUFA ratios (0.04 and 16.17), particularly at a high ratio (16.17), the richness of intestinal flora decreases. Recently, the link between intestinal flora and inflammation has been widely reported. Many members of the Lactobacillus species have anti-inflammatory activity (Jiang et al., 2018; Mohamadzadeh et al., 2011). Also, Alloprevotella and Ruminococcus play a role in anti-inflammatory processes through producing short-chain fatty acids (Clemente et al., 2018; Yang et al., 2019; Zhong et al., 2021). Moreover, the abundance of Escherichia-Shigella and Enterococcus has been shown to increase with the activation of the inflammatory response, thus serving as a biomarker of inflammation (Cattaneo et al., 2017; Grant et al., 2021). In the present study, the abundance of Lactobacillus, Alloprevotella and Ruminococcus increased at dietary n-3/n-6 PUFA ratio of 0.66 compared to the ratios of 0.04 and 16.17. Furthermore, the abundance of Escherichia-Shigella and Enterococcus increased at dietary n-3/n-6 PUFA ratios of 16.17 and 0.04, respectively. These results indicate that dietary n-3/n-6 PUFA ratio may affect intestinal inflammation through modulating the microbial flora. It has been well documented that EPA and DHA could directly promote the growth of beneficial bacteria or indirectly through increasing the production of short-chain fatty acids or short-chain fatty acid salts (Fu et al., 2021). In this study, at both low and high extremes of dietary n-3/n-6 PUFA ratio, the intestinal flora was negatively influenced indicating that n-6 PUFA is also an essential nutrient for probiotics. Accumulated evidence reveals that the intestinal flora is involved in the inflammatory response, digestive process and metabolic regulation of the host (Liu et al., 2019, 2021; Murakami et al., 2016). In the current study, the diet with the n-3/n-6 PUFA ratio of 0.66 enhanced the abundance of Gram-positive bacteria (Phylum Firmicutes) and reduced the relative abundance of Gram-negative bacteria (Phylum Proteobacteria). This phenomenon was seen as a positive modulation of the intestinal flora, which was associated with better growth performance and innate immunity (Liu et al., 2021).
In summary, results of the present study indicated that dietary n-3/n-6 PUFA ratio influences the growth performance of L. maculatus and that maximum growth was obtained at ratio of 0.66. Furthermore, an optimal n-3/n-6 PUFA ratio improved liver health and lipid metabolism. A sub-optimal n-3/n-6 PUFA ratio induced intestinal inflammation, which is speculated to be caused by the disturbance in intestinal flora. The diet with the n-3/n-6 PUFA ratio of 0.66 could inhibit intestinal inflammation through positively modulating the intestinal flora.
Author contributions
Yanzou Dong: conceptualization, data curation, writing-original draft preparation. Yu Wei: conceptualization, data curation. Ling Wang: methodology. Kai Song: methodology. Chunxiao Zhang: methodology, resources. Kangle Lu: conceptualization, supervision, writing-review & editing. Samad Rahimnejad: writing-review & editing.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (32072984), the Natural Science Foundation of Fujian Province (2020J01664), and China Agricultural Research System (CARS-47).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2023.04.005.
Appendix. Supplementary data
The following is the Supplementary data to this article:
References
- AOAC . 18th ed. Association of Official Anlytical Chemists International; Gaithersburg, MD: 2005. Official methods of analysis. [Google Scholar]
- Alagawany M., Elnesr S.S., Farag M.R., El-Sabrout K., Alqaisi O., Dawood M.A.O., Soomro H., Abdelnour S.A. Nutritional significance and health benefits of omega-3, -6 and -9 fatty acids in animals. Anim Biotechnol. 2021:1–13. doi: 10.1080/10495398.2020.1869562. [DOI] [PubMed] [Google Scholar]
- Ayala A., Munoz M.F., Argueelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014 doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandarra N.M., Rema P., Batista I., Pousão-Ferreira P., Valente L.M.P., Batista S.M.G., Ozório R.O.A. Effects of dietary n−3/n−6 ratio on lipid metabolism of gilthead seabream (Sparus aurata) Eur J Lipid Sci Technol. 2011;113:1332–1341. [Google Scholar]
- Cai L.S., Wang L., Song K., Lu K.L., Zhang C.X., Rahimnejad S. Evaluation of protein requirement of spotted seabass (Lateolabrax maculatus) under two temperatures, and the liver transcriptome response to thermal stress. Aquaculture. 2020;516 [Google Scholar]
- Campos I., Matos E., Maia M.R.G., Marques A., Valente L.M.P. Partial and total replacement of fish oil by poultry fat in diets for European seabass (Dicentrarchus labrax) juveniles: effects on nutrient utilization, growth performance, tissue composition and lipid metabolism. Aquaculture. 2019;502:107–120. [Google Scholar]
- Cattaneo A., Cattane N., Galluzzi S., Provasi S., Lopizzo N., Festari C., Ferrari C., Guerra U.P., Paghera B., Muscio C., Bianchetti A., Volta G.D., Turla M., Cotelli M.S., Gennuso M., Prelle A., Zanetti O., Lussignoli G., Mirabile D., Bellandi D., Gentile S., Belotti G., Villani D., Harach T., Bolmont T., Padovani A., Boccardi M., Frisoni G.B., Grp I.F. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- Chen B., Li Y., Peng W., Zhou Z., Shi Y., Pu F., Luo X., Chen L., Xu P. Chromosome-level assembly of the Chinese seabass (Lateolabrax maculatus) genome. Front Genet. 2019;10:275. doi: 10.3389/fgene.2019.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Li X., Wang L., Lu K., Song K., Ai Q., Mai K., Zhang C. Effects of dietary arginine levels on growth, immune function of physical barriers and serum parameters of spotted seabass (Lateolabrax maculatus) reared at different water temperatures. Aquaculture. 2021;541 [Google Scholar]
- Childs M.T., King I.B., Knopp R.H. Divergent lipoprotein responses to fish oils with various ratios of eicosapentaenoic acid and docosahexaenoic acid. Am J Clin Nutr. 1990;52:632–639. doi: 10.1093/ajcn/52.4.632. [DOI] [PubMed] [Google Scholar]
- Clemente J.C., Manasson J., Scher J.U. The role of the gut microbiome in systemic inflammatory disease. BMJ Br Med J (Clin Res Ed) 2018;360:j5145. doi: 10.1136/bmj.j5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreas M.A., Rapoport S.I. Recent studies on interactions between n-3 and n-6 polyunsaturated fatty acids in brain and other tissues. Curr Opin Lipidol. 2002;13:267–272. doi: 10.1097/00041433-200206000-00006. [DOI] [PubMed] [Google Scholar]
- Dai Y., Zhang L., Yan Z., Li Z., Fu M., Xue C., Wang J. A low proportion n-6/n-3 PUFA diet supplemented with Antarctic krill (Euphausia superba) oil protects against osteoarthritis by attenuating inflammation in ovariectomized mice. Food Funct. 2021;12:6766–6779. doi: 10.1039/d1fo00056j. [DOI] [PubMed] [Google Scholar]
- Den Broeder M.J., Kopylova V.A., Kamminga L.M., Legler J. Zebrafish as a model to study the role of peroxisome proliferating-activated receptors in adipogenesis and obesity. PPAR Res. 2015 doi: 10.1155/2015/358029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y.Z., Li L., Espe M., Lu K.L., Rahimnejad S. Hydroxytyrosol attenuates hepatic fat accumulation via activating mitochondrial biogenesis and autophagy through the AMPK pathway. J Agric Food Chem. 2020;68:9377–9386. doi: 10.1021/acs.jafc.0c03310. [DOI] [PubMed] [Google Scholar]
- Dong Y., Yu M., Wu Y., Xia T., Wang L., Song K., Zhang C., Lu K., Rahimnejad S. Hydroxytyrosol promotes the mitochondrial function through activating mitophagy. Antioxidants. 2022;11:5. doi: 10.3390/antiox11050893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z.Y., Clouet P., Zheng W.H., Degrace P., Tian L.X., Liu Y.J. Biochemical hepatic alterations and body lipid composition in the herbivorous grass carp (Ctenopharyngodon idella) fed high-fat diets. Br J Nutr. 2006;95:905–915. doi: 10.1079/bjn20061733. [DOI] [PubMed] [Google Scholar]
- Dumas A., Lange C.F.M., France J., Bureau D.P. Quantitative description of body composition and rates of nutrient deposition in rainbow trout (Oncorhynchus mykiss) Aquaculture. 2007;273:165–181. [Google Scholar]
- Fu Y., Wang Y., Gao H., Li D., Jiang R., Ge L., Tong C., Xu K. Associations among dietary omega-3 polyunsaturated fatty acids, the gut microbiota, and intestinal immunity. Mediat Inflamm. 2021 doi: 10.1155/2021/8879227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., DeCoffe D., Brown K., Rajendiran E., Estaki M., Dai C., Yip A., Gibson D.L. Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing sepsis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glencross B.D. Exploring the nutritional demand for essential fatty acids by aquaculture species. Rev Aquacult. 2009;1:71–124. [Google Scholar]
- Grant C.V., Loman B.R., Bailey M.T., Pyter L.M. Manipulations of the gut microbiome alter chemotherapy-induced inflammation and behavioral side effects in female mice. Brain Behav Immun. 2021;95:401–412. doi: 10.1016/j.bbi.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Qin Y., Wang Y., Li D., Chen W., Ye J. Effects of dietary replacement of fish oil with soybean oil on the growth performance, plasma components, fatty acid composition and lipid metabolism of groupers Epinephelus coioides. Aquacult Nutr. 2021;27:1494–1511. [Google Scholar]
- Heerwagen M.J.R., Stewart M.S., Houssaye B.A., Janssen R.C., Friedman J.E. Transgenic increase in N-3/N-6 fatty acid ratio reduces maternal obesity-associated inflammation and limits adverse developmental programming in mice. PLoS One. 2013;8:6. doi: 10.1371/journal.pone.0067791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris W.S. The Omega-6:Omega-3 ratio: a critical appraisal and possible successor. Prostag Leukotr Ess. 2018;132:34–40. doi: 10.1016/j.plefa.2018.03.003. [DOI] [PubMed] [Google Scholar]
- Horton J.D., Goldstein J.L., Brown M.S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundal B.K., Liland N.S., Rosenlund G., Bou M., Stubhaug I., Sissener N.H. Increasing dietary n-6 fatty acids while keeping n-3 fatty acids stable decreases EPA in polar lipids of farmed Atlantic salmon (Salmo salar) Br J Nutr. 2021;125:10–25. doi: 10.1017/S0007114520002494. [DOI] [PubMed] [Google Scholar]
- Hundal B.K., Liland N.S., Rosenlund G., Hoglund E., Araujo P., Stubhaug I., Sissener N.H. Increasing the dietary n-6/n-3 ratio alters the hepatic eicosanoid production after acute stress in Atlantic salmon (Salmo salar) Aquaculture. 2021;534 [Google Scholar]
- Husted K.S., Bouzinova E.V. The importance of n-6/n-3 fatty acids ratio in the major depressive disorder. Medicina. 2016;52:139–147. doi: 10.1016/j.medici.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Jiang X., Gu S., Liu D., Zhao L., Xia S., He X., Chen H., Ge J. Lactobacillus brevis 23017 relieves mercury toxicity in the colon by modulation of oxidative stress and inflammation through the interplay of MAPK and NF-kappaB signaling cascades. Front Microbiol. 2018;9:2425. doi: 10.3389/fmicb.2018.02425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M., Lu Y., Pan T., Zhu T., Yuan Y., Sun P., Zhou F., Ding X., Zhou Q. Effects of dietary n-3 LC-PUFA/n-6 C18 PUFA ratio on growth, feed utilization, fatty acid composition and lipid metabolism related gene expression in black seabream, Acanthopagrus schlegelii. Aquaculture. 2019;500:521–531. [Google Scholar]
- Krajmalnik R., Ilhan Z., Kang D., DiBaise J. Effects of gut microbes on nutrient absorption and energy regulation. Nutr Clin Pract. 2012;27:201–214. doi: 10.1177/0884533611436116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Chen Q., Li Q., Li J., Cui K., Zhang Y., Kong A., Zhang Y., Wan M., Mai K., Ai Q. Effects of high levels of dietary linseed oil on the growth performance, antioxidant capacity, hepatic lipid metabolism, and expression of inflammatory genes in large yellow croaker (Larimichthys crocea) Front Physiol. 2021;12 doi: 10.3389/fphys.2021.631850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Jiang Y., Liu W., Ge X. Protein-sparing effect of dietary lipid in practical diets for blunt snout bream (Megalobrama amblycephala) fingerlings: effects on digestive and metabolic responses. Fish Physiol Biochem. 2012;38:529–541. doi: 10.1007/s10695-011-9533-9. [DOI] [PubMed] [Google Scholar]
- Liu H.Q., Qiu Y., Mu Y., Zhang X.J., Liu L., Hou X.H., Zhang L., Xu X.N., Ji A.L., Cao R., Yang R.H., Wang F. A high ratio of dietary n-3/n-6 polyunsaturated fatty acids improves obesity-linked inflammation and insulin resistance through suppressing activation of TLR4 in SD rats. Nutr Res. 2013;33:849–858. doi: 10.1016/j.nutres.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Liu J.X., Gao T.X., Yokogawa K., Zhang Y.P. Differential population structuring and demographic history of two closely related fish species, Japanese sea bass (Lateolabrax japonicus) and spotted sea bass (Lateolabrax maculatus) in Northwestern Pacific. Mol Phylogenet Evol. 2006;39:799–811. doi: 10.1016/j.ympev.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Liu Z., Wang N., Ma Y., Wen D. Hydroxytyrosol improves obesity and insulin resistance by modulating gut microbiota in high-fat diet-induced obese mice. Front Microbiol. 2019;10:390. doi: 10.3389/fmicb.2019.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.Y., Yang H.L., Hu L.H., Yang W., Ai C.X., Sun Y.Z. Autochthonous probiotics alleviate the adverse effects of dietary histamine in juvenile grouper (Epinephelus coioides) Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.792718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K.L., Cai L.S., Wang L., Song K., Zhang C.X., Rahimnejad S. Effects of dietary protein/energy ratio and water temperature on growth performance, digestive enzymes activity and non-specific immune response of spotted seabass (Lateolabrax maculatus) Aquacult Nutr. 2020;26:2023–2031. [Google Scholar]
- Lu K., Xu W., Li J., Li X., Huang G., Liu W. Alterations of liver histology and blood biochemistry in blunt snout bream Megalobrama amblycephala fed high-fat diets. Fish Sci. 2013;79:661–671. [Google Scholar]
- Lu K.L., Xu W.N., Liu W.B., Wang L.N., Zhang C.N., Li X.F. Association of mitochondrial dysfunction with oxidative stress and immune suppression in blunt snout bream Megalobrama amblycephala fed a high-fat diet. J Aquat Anim Health. 2014;26:100–112. doi: 10.1080/08997659.2014.893460. [DOI] [PubMed] [Google Scholar]
- Mai K., Lu Z., Ai Q., Duan Q., Zhang C., Li H., Wan J., Liufu Z. Dietary lysine requirement of juvenile Japanese seabass, Lateolabrax japonicus. Aquaculture. 2006;258:535–542. [Google Scholar]
- Marszalek J.R., Lodish H.F. Docosahexaenoic acid, fatty acid–interacting proteins, and neuronal function: breastmilk and fish are good for you. Annu Rev Cell Dev Biol. 2005;21:633–657. doi: 10.1146/annurev.cellbio.21.122303.120624. [DOI] [PubMed] [Google Scholar]
- Mattioli S., Collodel G., Signorini C., Cotozzolo E., Noto D., Cerretani D., Micheli L., Fiaschi A.I., Brecchia G., Menchetti L., Moretti E., Oger C., De Felice C., Castellini C. Tissue antioxidant status and lipid peroxidation are related to dietary intake of n-3 polyunsaturated acids: a rabbit model. Antioxidants. 2021;10:681. doi: 10.3390/antiox10050681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamadzadeh M., Pfeiler E.A., Brown J.B., Zadeh M., Gramarossa M., Managlia E., Bere P., Sarraj B., Khan M.W., Pakanati K.C., Ansari M.J., O'Flaherty S., Barrett T., Klaenhammer T.R. Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid. P Natl Acad Sci USA. 2011;108:4623–4630. doi: 10.1073/pnas.1005066107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokkala K., Houttu N., Cansev T., Laitinen K. Interactions of dietary fat with the gut microbiota: evaluation of mechanisms and metabolic consequences. Clin Nutr. 2020;39:994–1018. doi: 10.1016/j.clnu.2019.05.003. [DOI] [PubMed] [Google Scholar]
- Mommsen T.P. Paradigms of growth in fish. Comp Biochem Physiol B. 2001;129:207–219. doi: 10.1016/s1096-4959(01)00312-8. [DOI] [PubMed] [Google Scholar]
- Murakami M., Tognini P., Liu Y., Eckel-Mahan K.L., Baldi P., Sassone-Corsi P. Gut microbiota directs PPARγ-driven reprogramming of the liver circadian clock by nutritional challenge. EMBO Rep. 2016;17:1292–1303. doi: 10.15252/embr.201642463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasopoulou C., Zabetakis I. Benefits of fish oil replacement by plant originated oils in compounded fish feeds. A review. Lebensm Wiss Technol. 2012;47:217–224. [Google Scholar]
- Niu S.L., Mitchell D.C., Lim S.Y., Wen Z.M., Kim H.Y., Salem N., Litman B.J. Reduced G protein-coupled signaling efficiency in retinal rod outer segments in response to n-3 fatty acid deficiency. J Biol Chem. 2004;279:31098–31104. doi: 10.1074/jbc.M404376200. [DOI] [PubMed] [Google Scholar]
- Pyper S.R., Viswakarma N., Yu S., Reddy J.K. PPARalpha: energy combustion, hypolipidemia, inflammation and cancer. Nucl Recept Signal. 2010;8 doi: 10.1621/nrs.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent J., Bell G., McEvoy L., Tocher D., Estevez A. Recent developments in the essential fatty acid nutrition of fish. Aquaculture. 1999;177:191–199. [Google Scholar]
- Sargent J.R., Bell J.G., Bell M.V., Henderson R.J., Tocher D.R. Requirement criteria for essential fatty acids. J Appl Ichthyol. 1995;11:183–198. [Google Scholar]
- Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz G., Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47:147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Scorletti E., Byrne C.D. Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annu Rev Nutr. 2013;33:231–248. doi: 10.1146/annurev-nutr-071812-161230. [DOI] [PubMed] [Google Scholar]
- Song J.Y., Zhang C.X., Wang L., Song K., Hu S.C., Zhang L. Effects of dietary calcium levels on growth and tissue mineralization in Japanese seabass, Lateolabrax japonicus. Aquacult Nutr. 2017;3:637–648. [Google Scholar]
- Spadaro L., Magliocco O., Spampinato D., Piro S., Oliveri C., Alagonan C., Papa G., Rabuazzo A.M., Purrello F. Effects of n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig Liver Dis. 2008;40:194–199. doi: 10.1016/j.dld.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Tan P., Ding Y., Li X., Dong X., Mai K., Ai Q. Nrf2 pathway in vegetable oil-induced inflammation of large yellow croaker (Larimichthys crocea) Fish Shellfish Immunol. 2022;127:778–787. doi: 10.1016/j.fsi.2022.05.046. [DOI] [PubMed] [Google Scholar]
- Tocher D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev Fish Sci. 2003;11:107–184. [Google Scholar]
- Torstensen B.E., Bell J.G., Rosenlund G., Henderson R.J., Graff I.E., Tocher D.R., Lie O., Sargent J.R. Tailoring of Atlantic salmon (Salmo salar L.) flesh lipid composition and sensory quality by replacing fish oil with a vegetable oil blend. J Agric Food Chem. 2005;53:10166–10178. doi: 10.1021/jf051308i. [DOI] [PubMed] [Google Scholar]
- Turchini G.M., Torstensen B.E., Ng W.K. Fish oil replacement in finfish nutrition. Rev Aquacult. 2009;1:10–57. [Google Scholar]
- Vélez E.J., Lutfi E., Azizi S., Perelló M., Salmerón C., Riera-Codina M., Ibarz A., Fernández-Borràs J., Blasco J., Capilla E., Navarro I., Gutiérrez J. Understanding fish muscle growth regulation to optimize aquaculture production. Aquaculture. 2017;467:28–40. [Google Scholar]
- Venold F.F., Penn M.H., Krogdahl Å., Overturf K. Severity of soybean meal induced distal intestinal inflammation, enterocyte proliferation rate, and fatty acid binding protein (Fabp2) level differ between strains of rainbow trout (Oncorhynchus mykiss) Aquaculture. 2012;364–365:281–292. [Google Scholar]
- Wang J., Liang D., Yang Q., Tan B., Dong X., Chi S., Liu H., Zhang S. The effect of partial replacement of fish meal by soy protein concentrate on growth performance, immune responses, gut morphology and intestinal inflammation for juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) Fish Shellfish Immunol. 2020;98:619–631. doi: 10.1016/j.fsi.2019.10.025. [DOI] [PubMed] [Google Scholar]
- Wang X., Xiao K., Jiang G.Z., Dai Y.J., Abasubong K., Wang M.M., Li X.F., Zhang D.D., Liu W.B. Dietary 18-carbon fatty acid unsaturation improves the muscle fiber development and meat quality of Megalobrama amblycephala. Aquacult Rep. 2022;24 [Google Scholar]
- Watanabe T. Lipid nutrition in fish. Comp Biochem Phys B. 1982;73:3–15. [Google Scholar]
- Worgall T.S., Sturley S.L., Seo T., Osborne T.F., Deckelbaum R.J. Polyunsaturated fatty acids decrease expression of promoters with sterol regulatory elements by decreasing levels of mature sterol regulatory element-binding protein. J Biol Chem. 1998;273:25537–25540. doi: 10.1074/jbc.273.40.25537. [DOI] [PubMed] [Google Scholar]
- Xie S., Lin Y., Wu T., Tian L., Liang J., Tan B. Dietary lipid levels affected growth performance, lipid accumulation, inflammatory response and apoptosis of Japanese seabass (Lateolabrax japonicus) Aquacult Nutr. 2021;27:807–816. [Google Scholar]
- Xu J.H., Qin J., Yan B.L., Zhu M., Luo G. Effects of dietary lipid levels on growth performance, feed utilization and fatty acid composition of juvenile Japanese seabass (Lateolabrax japonicus) reared in seawater. Aquacult Int. 2011;19:79–89. [Google Scholar]
- Xue M., Luo L., Wu X., Ren Z., Gao P., Yu Y., Pearl G. Effects of six alternative lipid sources on growth and tissue fatty acid composition in Japanese sea bass (Lateolabrax japonicus) Aquaculture. 2006;260:206–214. [Google Scholar]
- Yang F., Li J., Pang G., Ren F., Fang B. Effects of diethyl phosphate, a non-specific metabolite of organophosphorus pesticides, on serum lipid, hormones, inflammation, and gut microbiota. Molecules. 2019;24:10. doi: 10.3390/molecules24102003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Lu X., Lombès M., Rha G.B., Chi Y.I., Guerin T.M., Smart E.J., Liu J. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metabol. 2010;11:194–205. doi: 10.1016/j.cmet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z., Xu Y.J., Liu Y. Influences of dietary oils and fats, and the accompanied minor content of components on the gut microbiota and gut inflammation: a review. Trends Food Sci Technol. 2021;113:255–276. [Google Scholar]
- Zechner R., Zimmermann R., Eichmann T.O., Kohlwein S.D., Haemmerle G., Lass A., Madeo F. FAT signals - lipases and lipolysis in lipid metabolism and signaling. Cell Metabol. 2012;15:279–291. doi: 10.1016/j.cmet.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate R., Jaber-Vazdekis N., Tejera N., Perez J.A., Rodriguez C. Significance of long chain polyunsaturated fatty acids in human health. Clin Transl Med. 2017;6:25. doi: 10.1186/s40169-017-0153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zhang S., Lu K., Wang L., Song K., Li X., et al. Effects of dietary phosphorus level on growth, body composition, liver histology and lipid metabolism of spotted seabass (Lateolabrax maculatus) reared in freshwater. Aquaculture and Fisheries. 2022;8:528–537. [Google Scholar]
- Zhong Y.B., Kang Z.P., Wang M.X., Long J., Wang H.Y., Huang J.Q., Wei S.Y., Zhou W., Zhao H.M., Liu D.Y. Curcumin ameliorated dextran sulfate sodium-induced colitis via regulating the homeostasis of DCs and Treg and improving the composition of the gut microbiota. J Funct Foods. 2021;86 [Google Scholar]
- Zhou W., Rahimnejad S., Lu K., Wang L., Liu W. Effects of berberine on growth, liver histology, and expression of lipid-related genes in blunt snout bream (Megalobrama amblycephala) fed high-fat diets. Fish Physiol Biochem. 2019;45:83–91. doi: 10.1007/s10695-018-0536-7. [DOI] [PubMed] [Google Scholar]
- Zhu H., He A., Chen L., Qin J., Li E., Li Q., Wang H., Zhang T., Su X. Effects of dietary lipid level and n-3/n-6 fatty acid ratio on growth, fatty acid composition and lipid peroxidation in Russian sturgeon Acipenser gueldenstaedtii. Aquacult Nutr. 2017;23:879–890. [Google Scholar]
- Zhu S., Liu Q., Xiang X., Cui K., Zhao F., Mai K., et al. Docosahexaenoic acid ameliorates the toll-like receptor 22-triggered inflammation in fish by disrupting lipid raft formation. J Nutr. 2022;152:1991–2002. doi: 10.1093/jn/nxac125. [DOI] [PubMed] [Google Scholar]
- Zuo R., Ai Q., Mai K., Xu W., Wang J., Xu H., Liufu Z., Zhang Y. Effects of dietary docosahexaenoic to eicosapentaenoic acid ratio (DHA/EPA) on growth, nonspecific immunity, expression of some immune related genes and disease resistance of large yellow croaker (Larmichthys crocea) following natural infestation of parasites (Cryptocaryon irritans) Aquaculture. 2012;334:101–109. doi: 10.1016/j.fsi.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Zuo R., Ai Q., Mai K., Xu W., Wang J., Xu H., Liufu Z., Zhang Y. Effects of dietary n-3 highly unsaturated fatty acids on growth, nonspecific immunity, expression of some immune related genes and disease resistance of large yellow croaker (Larmichthys crocea) following natural infestation of parasites (Cryptocaryon irritans) Fish Shellfish Immunol. 2012;32:249–258. doi: 10.1016/j.fsi.2011.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.