Abstract
Endometrial tissue lines the inner cavity of the uterus and is under the cyclical control of estrogen and progesterone. It is a tissue that is composed of luminal and glandular epithelium, a stromal compartment, a vascular network, and a complex immune cell population. Mouse models have been a powerful tool to study the endometrium and have revealed critical mechanisms that control implantation, placentation, and cancer. The recent development of 3D endometrial organoid cultures presents a state-of-the-art model to dissect the signaling pathways that underlie endometrial biology. Establishing endometrial organoids from genetically engineered mouse models, analyzing their transcriptomes, and visualizing their morphology at a single-cell resolution are crucial tools for the study of endometrial diseases. This paper outlines the methods to establish 3D cultures of endometrial epithelium from mice and describes techniques to quantify gene expression and analyze the histology of the organoids. The goal is to provide a resource that can be used to establish, culture, and study the gene expression and morphological characteristics of endometrial epithelial organoids.
Keywords: endometrium, organoids, infertility, uterus, regeneration
SUMMARY:
This protocol describes the methodologies to establish mouse endometrial epithelial organoids for gene expression and histological analyses.
INTRODUCTION:
The endometrium, or inner lining mucosal tissue of the uterine cavity, is a unique and highly dynamic tissue that plays critical roles in a woman’s reproductive health. During the reproductive lifespan, the endometrium holds the potential to undergo hundreds of cycles of proliferation, differentiation, and breakdown, coordinated by the concerted action of the ovarian hormones, estrogen and progesterone. Studies of genetically engineered mice have uncovered basic biological mechanisms underpinning the endometrial response to hormones and control of embryo implantation, stromal cell decidualization, and pregnancy1. In vitro studies, however, have been limited due to difficulties in maintaining non-transformed primary mouse endometrial tissues in traditional 2D cell cultures2,3. Recent advances in the culture of endometrial tissues as 3D organ systems, or organoids, present a novel opportunity to investigate biological pathways that control endometrial cell regeneration and differentiation. Mouse and human endometrial organoid systems have been developed from pure endometrial epithelium encapsulated in various matrices4,5, while human endometrium has been cultured as scaffold-free epithelial/stromal co-cultures6,7, and more recently as collagen-encapsulated epithelial/stromal assembloids8. The growth and regenerative potential of epithelial organoid cultures is supported by a defined cocktail of growth factors and small molecule inhibitors that have been empirically determined to maximize the growth and regeneration of the organoids4,5,9. Furthermore, the ability to freeze and thaw endometrial organoids permits the long-term banking of endometrial organoids from mice and humans for future studies.
Genetically engineered mice have revealed the complex signaling pathways that control early pregnancy and decidualization and have been used as models of pregnancy loss, endometrial cancer, and endometriosis. These genetic studies have been largely achieved with cell-specific deletion of loxP flanked alleles (“floxed”) using cre recombinases that are specifically active in female reproductive tissues. These mouse models include the widely used progesterone receptor-cre10, which has strong recombinase activity in the endometrial epithelial and stromal tissues, lactoferrin i-cre, which induces endometrial epithelial recombination in adult mice11, or, Wnt7a-cre, which triggers epithelial-specific deletion in Müllerian-derived tissues12. Culturing endometrial tissues from genetically engineered mouse models as 3D organoids has provided an excellent opportunity to investigate endometrial biology and facilitate the identification of growth factors and signaling pathways that control endometrial cell renewal and differentiation13,14. Methods for the isolation and culture of mouse endometrial tissue are described in the literature and report the use of various enzymatic strategies for the isolation of uterine epithelium for subsequent culturing of endometrial epithelial organoids4. While previous literature provides a critical framework for endometrial epithelial organoid culture protocols4–6, this paper provides a clear, comprehensive method for generating, maintaining, processing, and analyzing these organoids. Standardization of these techniques is important for accelerating advancements in the field of women’s reproductive biology. Here, we report a detailed methodology for the enzymatic and mechanical purification of mouse endometrial epithelial tissue for the subsequent culture of endometrial organoids in a gel matrix scaffold. We also describe the methodologies for downstream histological and molecular analyses of the gel matrix-encapsulated mouse endometrial epithelial organoids.
PROTOCOL:
Mouse handling and experimental studies were performed under protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Baylor College of Medicine and guidelines established by the NIH Guide for the Care and Use of Laboratory Animals.
1. Isolation of uterine epithelium from mice using enzymatic and mechanical methods
NOTE: This section describes the steps required to establish, passage, freeze, and thaw epithelial endometrial organoids from mice using a gel matrix scaffold. Previous studies have determined that optimal cultures of mouse endometrial organoids are established from mice during the estrus phase4, which can be determined by cytological examination of a vaginal swab15. Adult female WT mice (6–8 weeks old, hybrid C57BL/6J and 129S5/SvEvBrd) were used for all experiments. Mice were humanely euthanized according to IACUC-approved guidelines using isoflurane sedation followed by cervical disarticulation. Once these mice are euthanized, the following steps should be followed. See Table of Materials and Table 1 for details related to materials and solutions used in this protocol.
Table 1:
Primer sequences and PCR conditions.
| Primers and PCR Conditions | ||
|---|---|---|
| Primer Sequence | Forward (5′-3′) | Reverse (5′-3′) |
| Lipocalin 2 (Lcn2) | GCAGGTGGTACGTTGTGGG | CTCTTGTAGCTCATAGATGGTGC |
| Lactoferrin (Ltf) | TGAGGCCCTTGGACTCTGT | ACCCACTTTTCTCATCTCGTTC |
| Progesterone (Pgr) | CCCACAGGAGTTTGTCAAGCTC | TAACTTCAGACATCATTTCCGG |
| Glyceraldehyde 3 phosphate dehydrogenase (Gapdh) | CAATGTGTCCGTCGTGGATCT | GCCTGCTTCACCACCTTCTT |
| PCR mixture contains: |
|---|
| 2X SYBR Green |
| 0.5μM Fwd Primer |
| 0.5μM Rev Primer |
| cDNA |
| RNAse/DNAse-Free H20 |
| Cycler Conditions: | |||
|---|---|---|---|
| Temperature | Time | Cycles | |
| Hold | 95 C | 10 min | 1 |
| Denature | 95 C | 15 sec | 40 |
| Extend | 60 C | 60 sec |
1.1. Allow the gel matrix to thaw on ice for approximately 1–2 h before use.
1.2. To dissect the mouse, use scissors to make a mid-line incision on the abdomen and gently peel back the skin to expose the underlying peritoneal layer. Use forceps to hold up the peritoneal layer and make lateral incisions with the scissors to expose the abdominal contents. Locate the uterine horns by gently moving aside the abdominal fat pads and dissect the uterine horns by first holding them at the cervical junction and use scissors to cut along the mesenteric fat. Following the dissection of the mouse uterus, thoroughly remove fat tissue from the uterine horn with small scissors.
NOTE: Maintain sterility during the cell isolation by sterilizing all surgery tools before use, spray the mouse abdomen with 70% ethanol, and perform all steps following dissection under a sterile tissue culture hood.
1.3. Cut each uterine horn into small fragments, each measuring approximately 4–5 mm.
1.4. Place all the uterine fragments from one uterus into one well of a 24-well plate containing 0.5 mL of 1% Trypsin. Allow the enzymatic solution to enter the uterine lumen, causing enzymatic separation of the endometrial epithelium from the underlying stroma.
1.5. Incubate the 24-well plate in a 37 °C humidified tissue culture incubator for approximately 1 h.
1.6. After the 1-h incubation, transfer the uterine fragments to a 35 mm tissue culture plate containing 1 mL of Dulbecco’s Phosphate-Buffered Solution (DPBS).
1.7. Under a dissection microscope, use fine forceps and a 1 mL pipette to mechanically separate the uterine epithelium from the uterine tube. While holding down one end of a uterine fragment with the forceps, gently run the pipette tip longitudinally across the fragment, squeezing the epithelium out of the other end of the uterine tube. Observe the separated epithelial sheets from the uterine fragment under the dissection microscope.
1.8. Use the 1 mL pipette to collect and gently transfer the epithelial sheets into a 1.5 mL tube.
1.9. Repeat the process for the remaining uterine fragments, transferring all the epithelial sheets to the same collection tube.
1.10. Pellet the dissociated epithelial sheets by centrifugation for 5 min at 375 × g.
1.11. Carefully remove the supernatant to avoid disturbing the cell pellet.
1.12. Resuspend the cell pellet in 0.5 mL of 2.5 mg/mL collagenase + 2 mg/mL DNase solution. Pipette up and down approximately 10x, or until a single-cell suspension is achieved.
1.13. Add 0.5 mL of DMEM/F12 + 10% fetal bovine serum (FBS) + antibiotics and centrifuge the cells for 5 min at 375 × g.
NOTE: For additional information on the broad-spectrum antibiotic used for cell culture, refer to the Table of Materials.
1.14. Carefully remove the supernatant and resuspend the cells in 1 mL of DMEM/F12 + 10% FBS + antibiotics. Centrifuge the cells for 5 min at 375 × g.
2. Processing of the stromal compartment
NOTE: This section outlines the protocols necessary for isolating the stromal compartment of the mouse endometrium. Given the increasing interest in epithelial/stromal co-culture experiments, it is important to be able to process the stromal cell populations in addition to the epithelial cells that will generate organoids.
2.1. Once all the epithelium has been enzymatically and mechanically separated from the uterine fragments, the remaining tube-like structures are the “stromal/myometrial” compartment. Collect this tissue in a 2.5 mg/mL collagenase + 2 mg/mL DNase in HBSS solution.
2.2. Incubate the stromal/myometrial sample on a 37 °C shaker for 15 min.
2.3. Following incubation, add 500 μL of DMEM/F12 + 10% FBS + antibiotics per mouse and filter the non-dissociated fragments through a 40 μm cell filter.
2.4. Pellet cells by centrifugation for 5 min at 375 × g.
2.5. Carefully remove the supernatant and resuspend the cell pellet in 1 mL of DMEM/F12 + 10% FBS + antibiotics. Add the mixture dropwise to 10 mL of DMEM/F12 + 10% FBS + antibiotics in a 10 cm cell culture plate. Incubate the plate at 37 °C in a humidified cell culture incubator.
2.6. To generate co-cultures of mouse endometrial epithelial and stromal cells, follow the methods described for human endometrial organoids using scaffold-free or collagen matrix systems6,8.
NOTE: While these techniques have not yet been published for mice, they can be adapted based on the published protocols with human endometrium.
3. Encapsulation of uterine epithelium into gel matrix to establish organoids
NOTE: Keep gel matrix on ice until it is ready to be used.
3.1. Remove the supernatant and resuspend the cell pellet in a volume of gel matrix that is 20x that of the cell pellet (i.e., if the cell pellet is 20 μL, resuspend the cells with 400 μL of gel matrix). Resuspend the pellet carefully to avoid introducing bubbles.
3.2. Allow the gel matrix/cell suspension to settle at room temperature for ~10 min.
3.3 Once the gel matrix/cell suspension becomes a semi-solid gel, use a P200 micropipette with a wide bore 200 μL tip to gently aspirate 25 μL of the gel matrix/cell suspension. Dispense three separate 25 μL domes per well of a 12-well plate and allow the gel matrix to cure for 15 min in a 37 °C humidified tissue culture incubator.
3.4. After the gel matrix has cured, add 750 μL of organoid medium to each well containing gel matrix domes. Incubate at 37 °C in a humidified tissue culture incubator.
NOTE: Organoid media formulation is noted in the Table of Materials. Organoids will typically form within 4 days of initial culture.
4. Gene expression analysis of endometrial organoids following treatment with estradiol
NOTE: This section describes the methods used to profile the gene expression of endometrial epithelial organoids using real-time qPCR following treatment with estradiol (E2). Because the endometrium is under the cyclical control of the ovarian hormone, E2, testing the responsiveness of the organoids to E2, is an important measure of physiological function. We have obtained high-quality RNA and generated sufficient mRNA to profile gene expression using qPCR and/or RNA-sequencing from our endometrial epithelial organoids. This section describes how to collect organoids and process them for downstream analysis of gene expression. The selected treatment medium reflects the one used to treat cultured endometrial cells. However, it should be noted that this treatment medium can be optimized accordingly, as done for treating of human endometrial 3D cultures with hormones 8,16,17.
4.1. Culture the endometrial organoids as described above.
4.2. Four days after seeding, remove the organoid medium and replace it with 750 μL of Starvation Medium. Incubate overnight.
4.3. The following morning, remove the Starvation Medium. Add 750 μL of Treatment Medium containing either vehicle or 10 nM E2. Incubate for 48 h.
4.4. Proceed with RNA isolation following the kit manufacturer’s protocol.
5. Histological analysis of endometrial organoids
NOTE: Imaging the morphological features of endometrial organoids is critical to evaluating the cellular effect of growth factors, genetic manipulations, or small molecule inhibitors. This section describes the techniques used to fix, process, and image endometrial epithelial organoids using histological stains and antibody immunofluorescent staining.
5.1. Prepare 1.5 mL microcentrifuge tubes containing 1 mL of 4% paraformaldehyde in 1x PBS. Place them on ice.
5.2. Aspirate the medium from the wells of the 12-well plate containing the organoids.
5.3. Using a 1 mL pipette tip with a cut tip, transfer 500 μL of 4% paraformaldehyde to each well and gently detach the gel matrix domes from the bottom of the plate.
5.4. Gently aspirate the entire gel matrix domes into the pipette tip and transfer to the 1.5 mL microcentrifuge tube.
5.5. Fix the organoids by placing them on a rotator at 4 °C overnight.
5.6. The following morning, centrifuging the tube at 600 × g for 5 min to pellet the organoids. Gently remove the 4% paraformaldehyde solution with a pipette and discard. Wash the organoids 2x with 70% ethanol.
5.7. After the last wash, remove all but 50–100 μL of ethanol from the tube. Set the tube aside.
5.8. Place a tube of specimen processing gel in a water bath and microwave for ~30 s to melt the gel. Ensure that the specimen processing gel does not boil over by monitoring the consistency of the gel.
NOTE: Once the specimen processing gel is melted, but not boiling hot, work quickly to encapsulate the organoids.
5.9. Transfer enough specimen processing gel to cover the entire surface of the mold or approximately 250 μL.
5.10. While the specimen processing gel is still molten, quickly transfer the 50 μL of 70% ethanol solution containing the organoids. Ensure that the organoids are sunken or pushed to the bottom portion of the mold.
5.11. Place the Histology mold on a bucket of ice and allow the specimen processing gel to cool and solidify.
5.12. Once the specimen processing gel is completely dry, carefully transfer the specimen processing gel square to a specimen bag, keeping track of the plane where the organoids are located.
5.13. Place the bag in a histology cassette and process using standard methods used for formalin fixation and paraffin embedding of tissues18.
5.14. Following fixation and embedding in paraffin, section into 5 μm sections using a microtome19. Proceed with standard H&E staining or immunostaining procedures as outlined below.
6. Hematoxylin & eosin staining
6.1. Deparaffinize the sections as follows: xylenes 2 x 10 min; 100% ethanol 2 x 3 min; 80% ethanol, 3 min; 60% ethanol, 3 min; dH2O 2 x 3 min; 1 min in hematoxylin; tap water (3 x 5 s); 1 min in eosin.
6.2. Dehydrate the sections as follows: 60% ethanol, 3 min; 80% ethanol, 3 min; 95% ethanol, 3 min; 100% ethanol 2 x 3 min; xylenes 2 x 15 min.
6.3. Mount using mounting medium.
7. Immunofluorescence staining
7.1. Deparaffinize the sections as described in step 6.1.
7.2. Perform antigen retrieval:
7.2.1. Submerge the slides in Antigen Retrieval Solution in a microwave-safe container.
7.2.2. Microwave at high heat for 20 min using 5 min intervals to ensure that the solution does not boil over.
7.3. After the 20 min antigen retrieval step is complete, allow slides to cool on ice for 40 min while still submerged in Antigen Retrieval Buffer.
7.4. Wash the slides with 1x TBST, 3 min
7.5. Block the slides by incubating in 3% BSA in TBST for 1 h at room temperature.
7.6. Primary antibody incubation
7.6.1. Dilute antibody in 3% BSA in TBST (1:50–1:1,000, depending on Ab)
7.6.2. Incubate overnight at 4 °C in a humidified chamber.
7.6.3. Wash 3 × 5 min with TBST.
7.7. Secondary antibody incubation
7.7.1. Dilute antibody in 3% BSA (in TBST) (1:250) or in 5% Normal Donkey Serum.
7.7.2. Incubate for 1 h at room temperature (RT) in the dark because antibody is conjugated to a fluorophore.
7.8. Nuclear stain.
7.8.1. Dilute 4′,6-diamidino-2-phenylindole (DAPI) 1:1,000 in TBST.
7.8.2. Incubate for 5 min at RT.
7.8.3. Wash 2 × 5 min with TBST.
7.9. Mount.
7.9.1. Use 1 drop of mounting medium to mount the coverslip.
7.9.2. Seal the coverslip using nail polish the following day.
REPRESENTATIVE RESULTS:
Phase contrast images of mouse endometrial organoids
We established organoids from WT mouse endometrial epithelium as described in the attached protocol (see diagram in Figure 1). Following enzymatic dissociation of the mouse endometrial epithelium, epithelial sheets were mechanically separated from the uterine stromal cells and further dissociated with collagenase to generate a single-cell suspension. If performed correctly, this method of epithelial and stromal cell separation yields samples with contamination of no more than 10–15% of the opposite cell type (see immunofluorescence images in Figure 2). The epithelial cell pellet was then resuspended in gel matrix, allowed to gel at room temperature, and plated into 25 μL domes on tissue culture plates. After the gel matrix domes solidified, they were overlaid with organoid medium and allowed to grow. We typically observed that endometrial epithelial cells assembled into organoids within 3–4 days, as pictured in Figure 3. The organoids were maintained in culture, with media changes occurring every 3 days, and passaging every 5–7 days.
Figure 1: Procedures used to establish endometrial organoids.
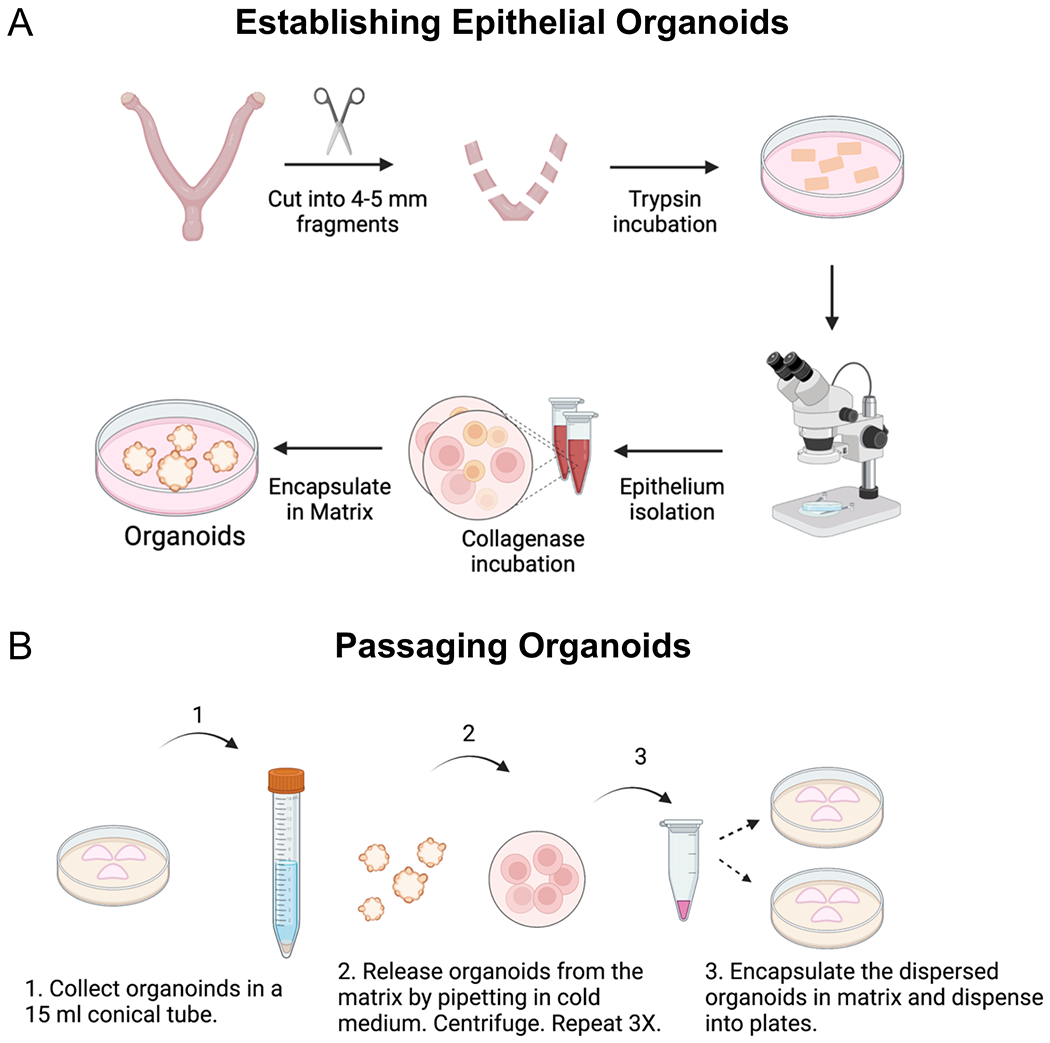
Diagram outlines the key steps that were followed to (A) establish and (B) passage epithelial organoids from the mouse endometrium. (A) Methods include the enzymatic and mechanical dissociation of endometrial epithelium from the mouse uterus, followed by single-cell dispersion and encapsulation of the epithelium into a gel matrix. To obtain the uterine tissues for enzymatic dissociation, the ovaries and oviducts are removed from the uterine horns with fine scissors, they are cut laterally into 2–3 mm fragments and then placed in the enzyme solution. After enzymatic incubation, the endometrial epithelium is mechanically separated from the underlying stroma under a microscope using forceps and a pipette to “squeeze” out the epithelium from the uterine tube. The epithelium is further digested into epithelial cells using a short incubation in collagenase, following by encapsulation into a gel matrix. (B) Passaging the endometrial organoids requires mechanical dissociation in cold medium and centrifugation to release the organoids from the matrix and to generate smaller organoid fragments. Once the organoids have been released from the matrix and mechanically dissociated into smaller fragments, they are encapsulated into matrix and replated.
Figure 2: Immunostaining of isolated endometrial epithelium and stromal cell populations.
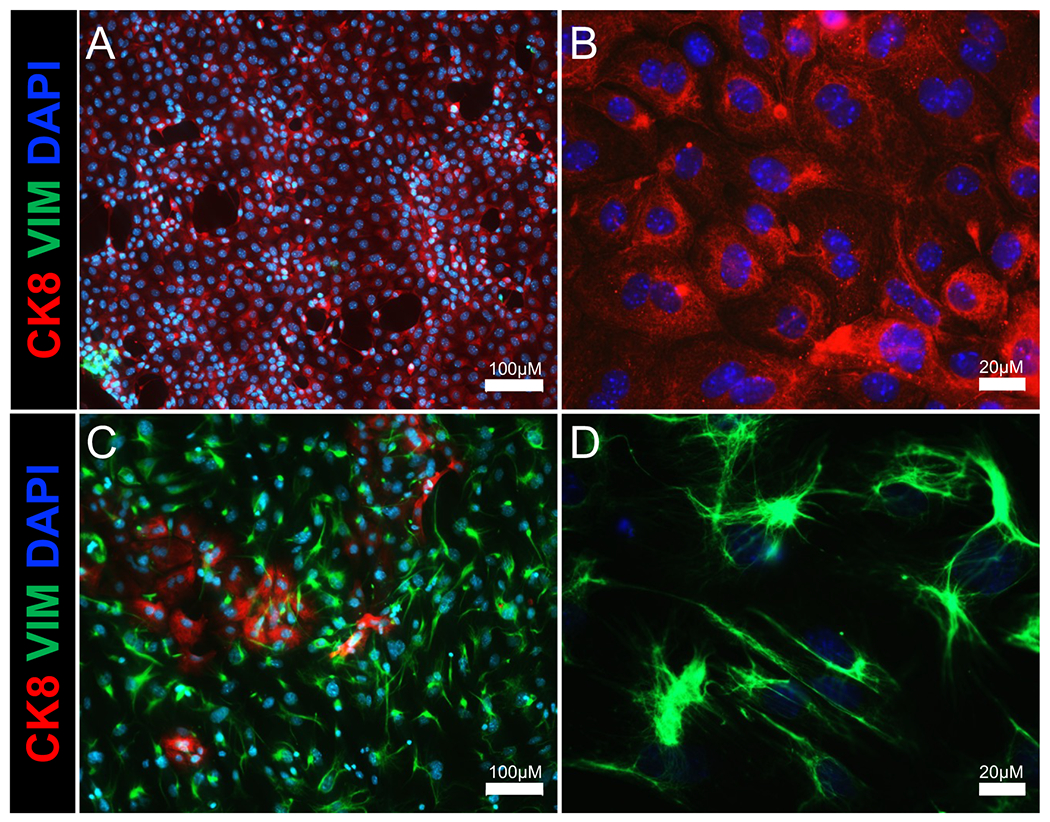
Immunofluorescence images show epithelial and stromal cell populations of the (A,B) epithelial cell and (C,D) stromal cell fractions following enzymatic and mechanical separation of the uterus. Cytokeratin 8 (an epithelial cell marker) is shown in red, vimentin (a stromal cell marker) is shown in green, and DAPI (a nuclear marker) is shown in blue. Scale bars = 100 μm (A,C), 20 μm (B,D). Abbreviations: CK8 = cytokeratin 8; VIM = vimentin; DAPI = 4′,6-diamidino-2-phenylindole.
Figure 3: Formation of endometrial organoids from WT mice over the course of 4 days.
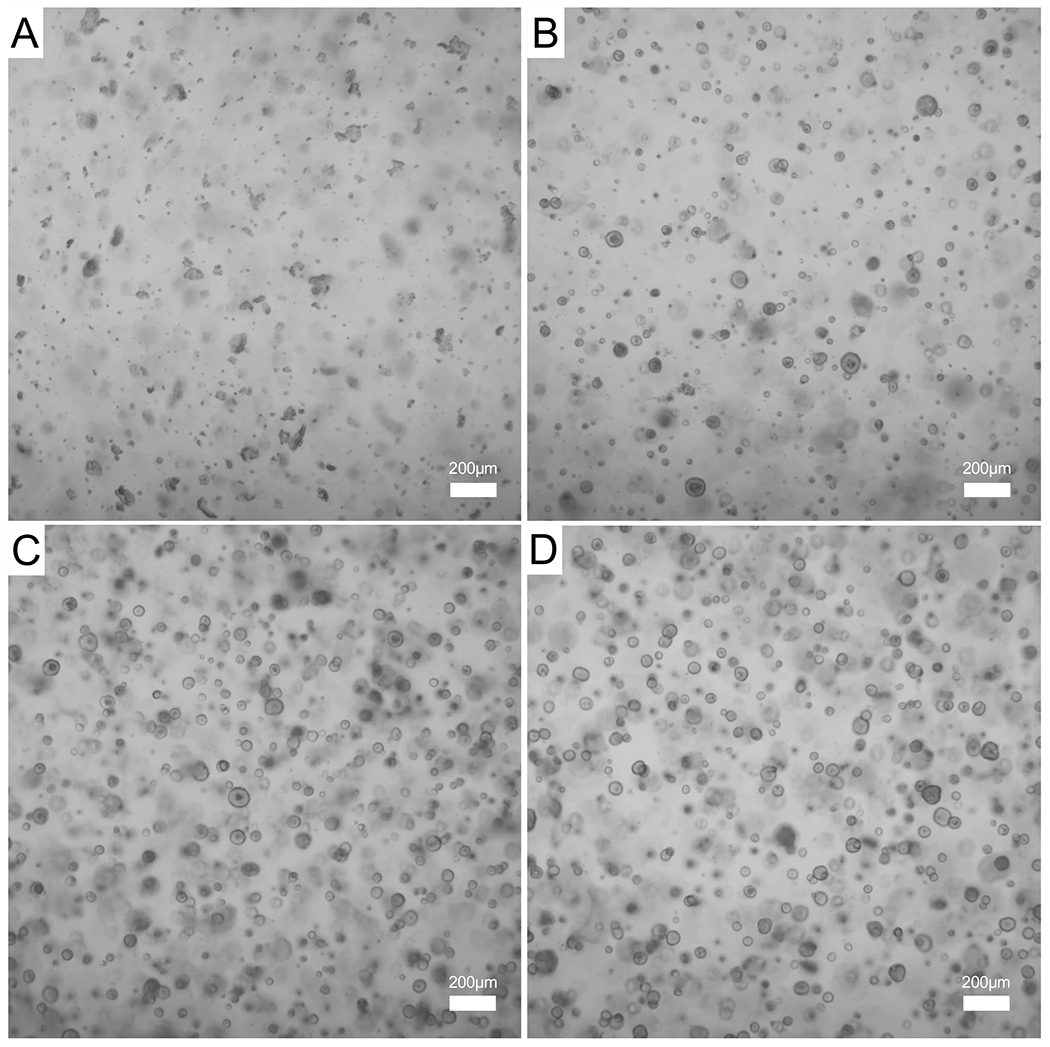
Endometrial epithelium was isolated from a WT mouse and used to generate organoids. (A) Phase contrast image of the epithelial cells encapsulated in the gel matrix immediately after digestion, day 0. (B,C) A few small organoids can be observed on day 1 (B) and day 2 (C) of culture. (D) Larger and mature epithelial organoids can be observed on day 3 of culture. Images were captured with a 5x objective; scale bars = 200 μm. Abbreviation: WT = wild type.
Imaging of mouse endometrial organoids using histological and immunofluorescent staining
To analyze the morphology of the mouse epithelial organoids, we adapted methods used for the encapsulation of cellular fine needle aspirates using specimen processing gel20. This allows for the preservation of the endometrial organoid morphology and encapsulation into a matrix that can be subjected to processing in preparation for paraffin embedding. Specimen processing gel is a modified agar that is widely used in clinical pathology laboratories for the analysis of fine needle aspirates and has been used to encapsulate vaginal organoids21,22. After the specimen processing gel/organoid mixture solidifies, it can be processed, stained, and imaged with techniques typically used to analyze tissues. In Figure 4A,B, we show that sectioned formalin-fixed paraffin-embedded endometrial organoids can be visualized by hematoxylin and eosin stains, which display the single layer of epithelium and hollow centers, or lumens, of the organoids. Certain organoids also contain secretions in the lumen, indicating that the organoids acquire the functional properties of glandular epithelial cells. We also describe the techniques to visualize the organoids using immunostaining (Figure 4C,D). Sectioned endometrial organoids were incubated with a Cytokeratin 8 primary antibody (TROMA-1) and a fluorophore-conjugated secondary antibody (secondary anti-rat-594). Nuclei were then visualized with DAPI, and the slides were imaged using a fluorescence microscope. We observed that all the cells in the organoids were Cytokeratin 8-positive and contained DAPI, indicating that the fixation, embedding, and processing of the endometrial organoids is compatible with antibody-based immunostaining.
Figure 4: Histological analysis of endometrial organoids.
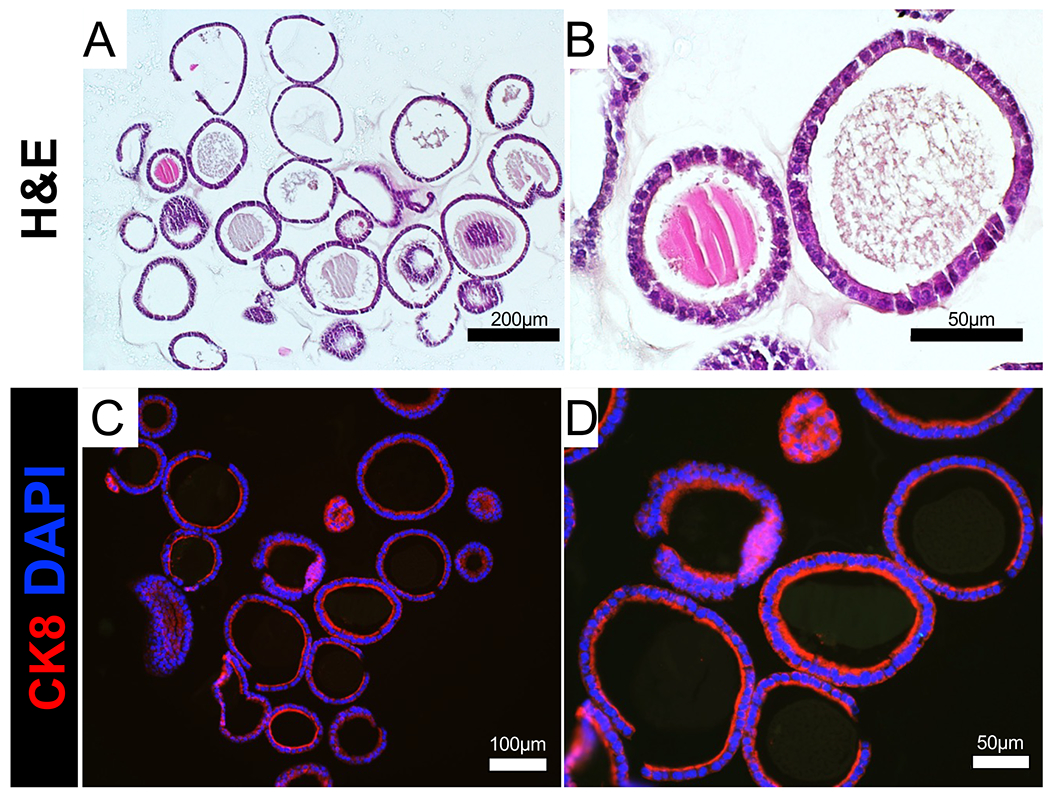
(A,B) FFPE endometrial epithelial organoids were sectioned and stained with H&E. (C,D) FFPE epithelial organoids were immunostained with the epithelial cell marker cytokeratin 8 (red). Cell nuclei were stained with DAPI (blue). Scale bars = 200 μm (A), 100 μm (C), 50 μm (B,D). Abbreviations: FFPE = Formalin-fixed paraffin embedded; H&E = hematoxylin & eosin; CK8 = cytokeratin 8; DAPI = 4′,6-diamidino-2-phenylindole.
RNA extraction and gene expression analysis
To determine the gene expression response of endometrial organoids, we allowed endometrial organoids from WT mice to grow for 4 days after the first passage. On the evening before treatment, the endometrial organoid medium was changed to starvation medium. The organoids were then treated in triplicate wells (each well containing three domes) with vehicle (ethanol, equal volume in solution as used for estradiol) or 10 nM estradiol (E2) for a total of 48 h. After 48 h, the medium was removed, and the organoids were processed for RNA extraction. Approximately 4 μg of RNA can be obtained from a total of two domes from each well, and 1 μg of RNA was used to reverse transcribe cDNA. Amplification of the genes encoding lipocalin 2 (Lcn2), lactoferrin (Ltf), and the progesterone receptor (Pgr) was performed using real-time quantitative PCR (Figure 5)23–25. As expected, we observed that the expression of Lcn2, Ltf, and Pgr increased in the epithelial organoids following stimulation with 10 nM E2 (Figure 5). Thus, these results show that endometrial epithelial organoids can be successfully used to measure the gene expression changes in response to E2.
Figure 5: Gene expression analysis of mouse endometrial organoids.
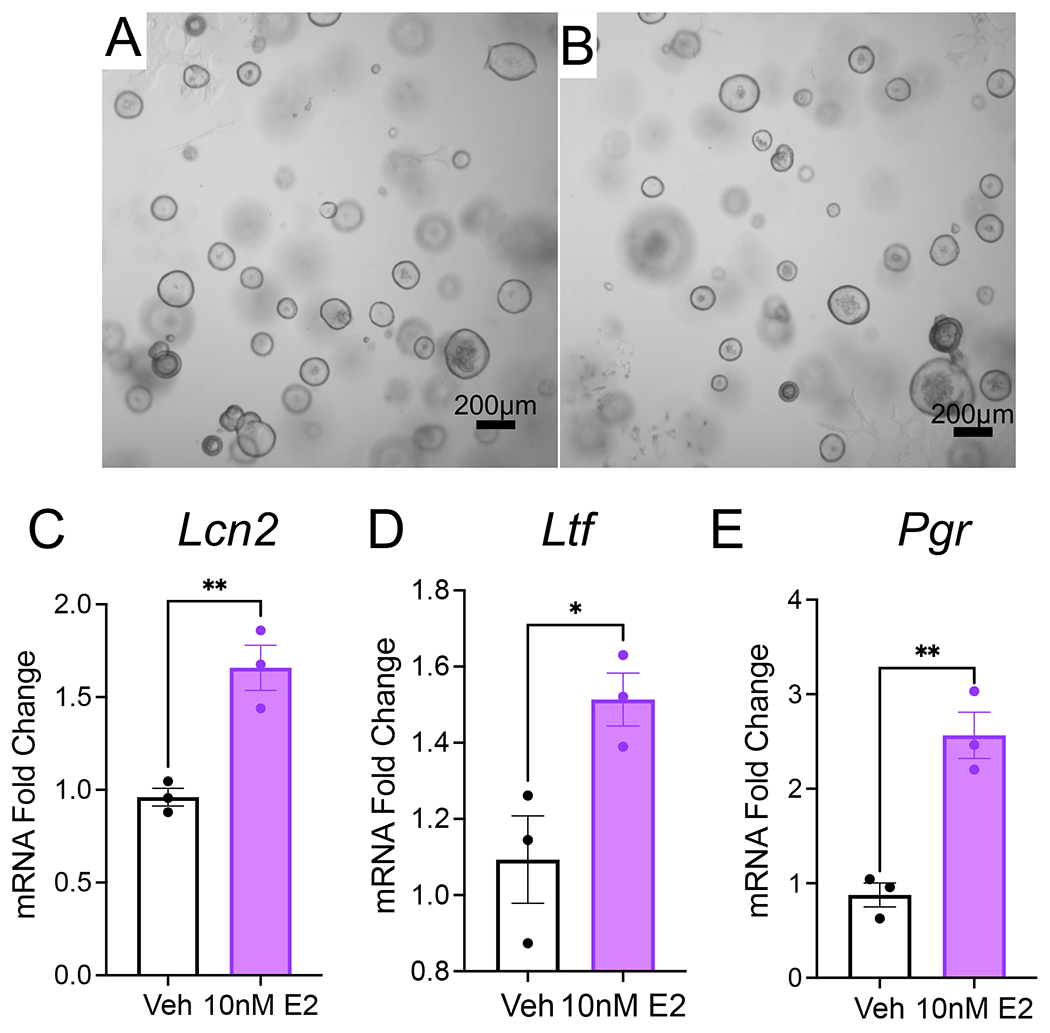
Mouse endometrial epithelial organoids from WT mice were cultured for 4 days in organoid growth medium, followed by treatment with vehicle or 10 nM E2 for 48 h in DMEM/F12 supplemented with 2% charcoal-stripped FBS. (A,B) Phase contrast images of the WT mouse organoids after treatment with vehicle (A) or 10 nM E2 (B). (C–E) Real time qPCR analysis of epithelial endometrial organoids treated with vehicle or 10 nM E2. Expression of E2-regulated genes, lipocalin 2 (Lnc2), lactoferrin (Ltf), and progesterone receptor (Pgr). Bars represent mean ± SEM, analyzed by a two-tailed t-test, *<0.033, **<0.002, ***<0.001. Repeated with organoids derived from n=3 WT mice per condition. Scale bars = 200 μm (A,B). Abbreviations: WT = wild type; E2 = estradiol; qPCR = quantitative PCR.
DISCUSSION:
We describe the methods to generate endometrial epithelial organoids from mouse endometrium and the protocols routinely used for their downstream analysis. Endometrial organoids are a powerful tool to study the mechanisms that control endometrial-related diseases, such as endometriosis, endometrial cancer, and implantation failure. Landmark studies published in 2017 reported the conditions to culture long-term and renewable cultures of endometrial organoids from mouse and human epithelium4,5. Although organoids have been widely used to study biological pathways in other systems, the study of endometrial tissue in vitro was limited by the absence of a suitable model to study the non-transformed primary epithelium in culture26. Endometrial organoids derived from human and mouse endometrium were successfully established using conditions typically used to culture organoids from other organ tissues, such as gut, kidney and lung, and by embedding them in an extracellular matrix scaffold composed of gel matrix4. Since these initial reports, the use of endometrial organoids to study the biology of the endometrium has increased significantly in the scientific community.
By adding minor modifications to a previously published method of epithelial isolation from the mouse uterus27,28, this protocol uniquely highlights key steps to isolate mouse uterine by directly placing the tissue into an enzyme solution, bypassing the need to transect the uterus, or using filtration. Using this method, we obtained epithelium with ~85–90% purity as determined by cytokeratin 8 immunostaining (Figure 2). This protocol also clearly outlines the details used to encapsulate the isolated epithelial cells into the growth matrix for generation, maintenance, and passaging of the organoids. Key limitations for the growth of these organoids include the use of the commercial matrix (i.e., Matrigel), which can result in batch-to-batch variations and contains growth factors that can affect organoid growth. Furthermore, while these organoids contain pure uterine epithelium, the use of additional endometrial cell types (i.e., immune, stromal) have been recently described in human organoid systems of the endometrium8,29. Other groups have shown the ability to isolate both luminal and glandular epithelial cells for organoid cultures from the mouse uterus14. Our images in Figure 4 show and our unpublished studies have identified glandular secretions (i.e., mucins) and expression of glandular-like cells (i.e., expressing FOXA230), indicating that the method presented here is effective for isolation of both glandular and luminal-epithelial cell populations from the mouse endometrium.
This approach uses tissues from a small animal model that is relatively common and available to most researchers; generating endometrial organoids from larger animal models, or humans, may pose ethical or technical challenges, which can be overcome by using induced pluripotent stem cell-based (iPSC) techniques. Such technologies have been previously described for endometrial tissues31, other tissues of the reproductive tract32 and have been effectively used to study endometrial/placental interactions in vitro33. Hence, using iPSCs as a source of endometrial stromal and epithelial cells of the endometrium from humans and other large-animal models poses a renewable and accessible source for generating endometrial organoids.
While the mouse is a widely used model for the study of implantation, there are key evolutional differences in the mechanism of implantation between mice and humans that should be noted. For example, while interstitial implantation is characterized by deep trophoblastic invasion through the luminal epithelium into the underlying stroma, the mechanism of the invasion process differs between mice and primates34. In mice, trophoblast invasion through the luminal epithelium involves apoptosis or entosis35,36, whereas trophoblasts migrate between luminal epithelium into the underlying stroma in primates34,35.
Providing endometrial organoids with the necessary growth factors to support self-assembly and long-term renewal is essential to obtain endometrial organoid cultures that recapitulate the native tissue. The complex media composition for the endometrial organoids indicates the multiple signaling pathways that contribute to epithelial cell characteristics and would arise from both paracrine and autocrine sources. The stromal compartment of the endometrium is a complex assembly of numerous cell types, including fibroblast-like stromal cells, endothelium, macrophages, and other specialized immune cells that are constantly changing in response to estrogen and progesterone37.
For example, the organoid culture medium contains factors that activate WNT/β-catenin signaling in the organoids, such as, WNT3a and R-Spondin. Studies in mice have demonstrated that WNT ligands are critical for uterine development and glandular function, therefore, activation of this signaling pathway is critical for organoid development and maintenance38,39. R-Spondin amplifies WNT signaling and is the ligand of the leucine-rich repeat-containing G-protein coupled receptor (LGR5)40. Endometrial organoid cultures also require inhibition of the transforming growth factor β (TGFβ) and bone morphogenetic protein (BMP) signaling pathways. For example, endometrial organoid culture medium contains the BMP inhibitor, Noggin, a secreted protein that binds to and inactivates BMP2, BMP4, and BMP741. The pharmacological inhibitor, A83-01, is also present in endometrial organoid medium. A83-01 is a small molecule inhibitor with high specificity to the receptors ALK4, ALK5, and ALK742. ALK4/5/7 are type 1 receptors of the TGFβ signaling family that bind to and transmit signals elicited by ligands such as TGFβ, activin, or nodal. BMP signals are critical for stem cell maintenance in tissues such as the gut43 and hair follicle44. Inhibition of TGFβ signaling contributes to the proliferative capacity and colony-formation efficiency of endometrial mesenchymal stem cells45 and this occurs by remodeling key regions in the chromatin46. Thus, modulation of these two key signaling pathways, BMP and TGFβ, is necessary to maintain organoid cultures with self-renewal capacity.
Although not displayed here, we found that long-term culture of the mouse organoids with the ALK4/5/7 inhibitor, A83-01, led to morphological changes in the epithelial organoids after 3–4 continous passages (unpublished). The morphological changes in the epithelial organoids were reminiscent of the “dense” epithelial organoids described by Boretto et al.,4, where the development of more secretory-like cells occurred due to decreased paracrine secretion of WNT ligands. Thus, it is likely that TGFβ and WNT signaling pathways intersect to control endometrial epithelial organoid regeneration and differentiation.
Recent studies have generated 3D endometrial epithelial/stromal co-culture systems from human tissues7,8,16. One method of co-culturing utilizes a scaffold-free system in which endometrial epithelial and stromal cells are combined and allowed to assemble into 3D structures in a scaffold-free well using a defined culture medium. These 3D endometrial epithelial/stromal organoids not only express receptors for estrogen, progesterone, and androgen, but also faithfully recapitulate the response to ovarian hormones, estrogen and progesterone. 6,7 In another co-culturing method, used in a study by Rawlings et al., endometrial epithelial organoids are combined with stromal cells in a collagen matrix and grown in a modified organoid medium8. These epithelial/stromal co-cultured organoids, or assembloids, organize into a suspended 3D-system where stromal cells surround epithelial gland-like structures.
The majority of endometrial organoids are cultured in growth-factor reduced gel matrix; however, the presence of active compounds in the matrix may exert undesired cellular responses within the organoids. To overcome this limitation, organoids have been established in gel matrix-free scaffolds, such as 3D printed agarose molds in which individual organoids are allowed to self-assemble6. Other groups have developed synthetic hydrogels to serve as 3D matrices for the culture of endometrial organoids47. These organoids assemble into 3D structures with apicobasal polarity and may also be combined with stromal cells of the endometrium to form complex organoids48. Since organoids can be successfully used for culturing non-transformed primary human and mouse endometrial tissues in ways that 2D cell cultures cannot, there is no doubt that endometrial organoids will advance the field of women’s reproductive health and be an incredible tool for studying reproductive pathologies such as recurrent pregnancy loss, endometriosis, and endometrial cancer16,49,50.
Supplementary Material
ACKNOWLEDGMENTS:
We thank Dr. Stephanie Pangas and Dr. Martin M. Matzuk (M.M.M) for critical reading and editing of our manuscript. Studies were supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development grants R00-HD096057 (D.M.), R01-HD105800 (D.M.), R01-HD032067 (M.M.M.) and R01-HD110038 (M.M.M.), and by NCI- P30 Cancer Center Support Grant (NCI-CA125123). Diana Monsivais, Ph.D. holds a Next Gen Pregnancy Award from the Burroughs Wellcome Fund.
Footnotes
A complete version of this article that includes the video component is available at http://dx.doi.org/10.3791/64448.
DISCLOSURES:
The authors have no conflicts of interest to disclose.
BIBLIOGRAPHY AND REFERENCES
- 1.Wang H & Dey SK Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 7 (3), 185–199, (2006). [DOI] [PubMed] [Google Scholar]
- 2.Hibaoui Y & Feki A Organoid Models of Human Endometrial Development and Disease. Front Cell Dev Biol. 8 84, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawlings TM, Makwana K, Tryfonos M & Lucas ES Organoids to model the endometrium: implantation and beyond. Reprod Fertil. 2 (3), R85–R101, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boretto M et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development. 144 (10), 1775–1786, (2017). [DOI] [PubMed] [Google Scholar]
- 5.Turco MY et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol. 19 (5), 568–577, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy AR, Wiwatpanit T, Lu Z, Davaadelger B & Kim JJ Generation of Multicellular Human Primary Endometrial Organoids. J Vis Exp. 10.3791/60384 (152), (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiwatpanit T et al. Scaffold-Free Endometrial Organoids Respond to Excess Androgens Associated With Polycystic Ovarian Syndrome. J Clin Endocrinol Metab. 105 (3), (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rawlings TM et al. Modelling the impact of decidual senescence on embryo implantation in human endometrial assembloids. Elife. 10, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lou L, Kong S, Sun Y, Zhang Z & Wang H Human Endometrial Organoids: Recent Research Progress and Potential Applications. Front Cell Dev Biol. 10 844623, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soyal SM et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 41 (2), 58–66, (2005). [DOI] [PubMed] [Google Scholar]
- 11.Daikoku T et al. Lactoferrin-iCre: a new mouse line to study uterine epithelial gene function. Endocrinology. 155 (7), 2718–2724, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR & Korach KS Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci U S A. 107 (45), 19272–19277, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seishima R et al. Neonatal Wnt-dependent Lgr5 positive stem cells are essential for uterine gland development. Nat Commun. 10 (1), 5378, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Syed SM et al. Endometrial Axin2(+) Cells Drive Epithelial Homeostasis, Regeneration, and Cancer following Oncogenic Transformation. Cell Stem Cell. 26 (1), 64–80 e13, (2020). [DOI] [PubMed] [Google Scholar]
- 15.Caligioni CS Assessing reproductive status/stages in mice. Curr Protoc Neurosci. Appendix 4 Appendix 4I, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzgerald HC, Schust DJ & Spencer TE In vitro models of the human endometrium: evolution and application for women’s health. Biol Reprod. 104 (2), 282–293, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hewitt SC et al. Progesterone Signaling in Endometrial Epithelial Organoids. Cells. 11 (11), (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadeghipour A & Babaheidarian P Making Formalin-Fixed, Paraffin Embedded Blocks. Methods Mol Biol. 1897 253–268, (2019). [DOI] [PubMed] [Google Scholar]
- 19.Qin C et al. The Cutting and Floating Method for Paraffin-embedded Tissue for Sectioning. J Vis Exp. 10.3791/58288 (139), (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rekhtman N et al. Novel Modification of HistoGel-Based Cell Block Preparation Method: Improved Sufficiency for Molecular Studies. Arch Pathol Lab Med. 142 (4), 529–535, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shidham VB CellBlockistry: Chemistry and art of cell-block making - A detailed review of various historical options with recent advances. Cytojournal. 16 12, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali A, Syed SM & Tanwar PS Protocol for In Vitro Establishment and Long-Term Culture of Mouse Vaginal Organoids. STAR Protoc. 1 (2), 100088, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurihara I et al. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 3 (6), e102, (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMaster MT, Teng CT, Dey SK & Andrews GK Lactoferrin in the mouse uterus: analyses of the preimplantation period and regulation by ovarian steroids. Mol Endocrinol. 6 (1), 101–111, (1992). [DOI] [PubMed] [Google Scholar]
- 25.Huang HL, Chu ST & Chen YH Ovarian steroids regulate 24p3 expression in mouse uterus during the natural estrous cycle and the preimplantation period. J Endocrinol. 162 (1), 11–19, (1999). [DOI] [PubMed] [Google Scholar]
- 26.Clevers H Modeling Development and Disease with Organoids. Cell. 165 (7), 1586–1597, (2016). [DOI] [PubMed] [Google Scholar]
- 27.Bigsby RM & Cunha GR Estrogen stimulation of deoxyribonucleic acid synthesis in uterine epithelial cells which lack estrogen receptors. Endocrinology. 119 (1), 390–396, (1986). [DOI] [PubMed] [Google Scholar]
- 28.Clementi C et al. Activin-like kinase 2 functions in peri-implantation uterine signaling in mice and humans. PLoS Genet. 9 (11), e1003863, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song Y et al. Endometriotic Organoids: A Novel In Vitro Model of Endometriotic Lesion Development. bioRxiv. 10.1101/2022.02.15.480583 2022.2002.2015.480583, (2022). [DOI] [Google Scholar]
- 30.Jeong JW et al. Foxa2 is essential for mouse endometrial gland development and fertility. Biol Reprod. 83 (3), 396–403, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyazaki K et al. Generation of Progesterone-Responsive Endometrial Stromal Fibroblasts from Human Induced Pluripotent Stem Cells: Role of the WNT/CTNNB1 Pathway. Stem Cell Reports. 11 (5), 1136–1155, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshimatsu S, Kisu I, Qian E & Noce T A New Horizon in Reproductive Research with Pluripotent Stem Cells: Successful In Vitro Gametogenesis in Rodents, Its Application to Large Animals, and Future In Vitro Reconstitution of Reproductive Organs Such as “Uteroid” and “Oviductoid”. Biology (Basel). 11 (7), (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheung VC et al. Pluripotent stem cell-derived endometrial stromal fibroblasts in a cyclic, hormone-responsive, coculture model of human decidua. Cell Rep. 35 (7), 109138, (2021). [DOI] [PubMed] [Google Scholar]
- 34.McGowen MR, Erez O, Romero R & Wildman DE The evolution of embryo implantation. Int J Dev Biol. 58 (2-4), 155–161, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carson DD et al. Embryo implantation. Dev Biol. 223 (2), 217–237, (2000). [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Sun X & Dey SK Entosis allows timely elimination of the luminal epithelial barrier for embryo implantation. Cell Rep. 11 (3), 358–365, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jain V, Chodankar RR, Maybin JA & Critchley HOD Uterine bleeding: how understanding endometrial physiology underpins menstrual health. Nat Rev Endocrinol. 10.1038/s41574-021-00629-4, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayashi K et al. Wnt genes in the mouse uterus: potential regulation of implantation. Biol Reprod. 80 (5), 989–1000, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunlap KA et al. Postnatal deletion of Wnt7a inhibits uterine gland morphogenesis and compromises adult fertility in mice. Biol Reprod. 85 (2), 386–396, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ter Steege EJ & Bakker ERM The role of R-spondin proteins in cancer biology. Oncogene. 40 (47), 6469–6478, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brazil DP, Church RH, Surae S, Godson C & Martin F BMP signalling: agony and antagony in the family. Trends Cell Biol. 25 (5), 249–264, (2015). [DOI] [PubMed] [Google Scholar]
- 42.Tojo M et al. The ALK-5 inhibitor A-83-01 inhibits Smad signaling and epithelial-to-mesenchymal transition by transforming growth factor-beta. Cancer Sci. 96 (11), 791–800, (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y & Que J BMP Signaling in Development, Stem Cells, and Diseases of the Gastrointestinal Tract. Annu Rev Physiol. 82 251–273, (2020). [DOI] [PubMed] [Google Scholar]
- 44.Plikus MV et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 451 (7176), 340–344, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gurung S, Werkmeister JA & Gargett CE Inhibition of Transforming Growth Factor-beta Receptor signaling promotes culture expansion of undifferentiated human Endometrial Mesenchymal Stem/stromal Cells. Sci Rep. 5 15042, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lucciola R et al. Impact of Sustained Transforming Growth Factor-beta Receptor Inhibition on Chromatin Accessibility and Gene Expression in Cultured Human Endometrial MSC. Front Cell Dev Biol. 8 567610, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernandez-Gordillo V et al. Fully synthetic matrices for in vitro culture of primary human intestinal enteroids and endometrial organoids. Biomaterials. 254 120125, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gnecco JS et al. Physiomimetic Models of Adenomyosis. Semin Reprod Med. 38 (2-03), 179–196, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nikolakopoulou K & Turco MY Investigation of infertility using endometrial organoids. Reproduction. 161 (5), R113–R127, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JJ Preparing for implantation. Elife. 10, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


