Abstract
The study aimed to evaluate the influence of solid-state fermentation on the nutritional value and enzymatic activity of rapeseed meal and its effects on the performance of broiler chickens and meat quality, including physicochemical properties (proximate analysis, pH, water holding capacity), antioxidant capabilities, dipeptide composition of the meat and sensory traits. Three dietary treatments were evaluated using broiler chickens: a control without incorporation of rapeseed meal; a second treatment with the incorporation of 3% unfermented rapeseed meal; and a third with the incorporation of 3% rapeseed meal fermented with Bacillus subtilis 67. The study showed that fermented compared to unfermented rapeseed meal was characterized by a significantly higher content of dry matter, crude ash, crude fat and metabolic energy (P < 0.05), and a significantly lower content of crude fiber and glucosinolates (P < 0.05). The B. subtilis 67 strain shows cellulolytic and xylulolytic activity. Fermented rapeseed meal has a positive effect on body weight of birds, daily gain, and European Production Efficiency Factor (P < 0.05). Both rapeseed meal treatments significantly reduced the pH of leg muscles and the water-holding capacity of breast muscles (P < 0.05). The fermented meal had a negative impact on some sensory parameters of poultry meat. There was no significant influence of fermented rapeseed meal on the composition of dipeptides in poultry meat and its antioxidant status.
Key words: rapeseed meal, meat quality, enzymatic activity, fermentation, Bacillus subtilis
INTRODUCTION
Soybean meal (SBM) is the most popular protein raw material used in the feed industry. However, due to the relatively high price of this feed material and the planned ban on the use of genetically modified organism (GMO) soybean in some countries, the need to use alternative local sources of protein in animal nutrition increases. A good alternative in some parts of the world is postextracted rapeseed meal (RSM), which is a by-product of pressing rapeseed oil. Rapeseed meal has enormous economic potential and can be used in the nutrition of all species of farm animals. However, the value of RSM is limited due to the low digestibility and the presence of antinutritional factors, causing a reduction in feed intake and poor feed utilization, as well as deterioration of production parameters such as laying or daily gains (Tie et al., 2020). Three important factors, nonstarch polysaccharides (NSP), glucosinolates, and phytic acid, found in RSM negatively impact broiler chicken nutrition. Nonstarch polysaccharides increase the viscosity of the chyme, which negatively affects the use of feed nutrients. Due to the increase in the viscosity of the feed content, the digestion and absorption of nutrients is limited. Methods are being sought to reduce the adverse effects of NSP in the digestive tract of poultry and other monogastric animals. Cellulose is an unbranched glucose polymer that can be hydrolyzed by cellulolytic enzymes produced by certain microorganisms, for example, from the genus Bacillus spp. Hemicellulose, on the other hand, is a polymer stored in seeds and is a structural component of cell walls. The most common fraction of hemicelluloses is xylan, which can contain acetyl, arabinosyl, and gluconosyl residues in its backbone (Magge and Kosaric, 1985; Filho et al., 1997). Glucosinolates negatively affect the health and productivity of birds, and phytic acid forms complexes with minerals, limiting their digestibility (Khajali and Slominski, 2012). There are also reports of a negative impact of rapeseed raw materials on meat quality (Salmon et al., 1984; Hopkins et al., 1995). The solid-state fermentation process using Bacillus subtilis bacteria can modify the chemical composition and biological properties of RSM, including the reduction of antinutritional factors (Olukomaiya et al., 2019; Zhang et al., 2020a,b,c), and improves the digestibility of nutrients (Grela et al., 2019; Szmigiel et al., 2021). It should be kept in mind that currently, the fermentation process increases the cost of rapeseed meal by about 2 times. Despite this, the meal treated in this way is characterized by a better nutritional value, and its biomass contains probiotic bacteria that have a positive effect on the health and performance of animals. Solid-state fermentation mimics the natural environment of most microorganisms, requires less energy for sterilization, is less susceptible to bacterial contamination, and allows for greater enzymatic productivity due to less susceptibility to substrate inhibition (Singhania et al., 2009). However, a small number of studies make it difficult to pinpoint all the potential benefits of fermented rapeseed meal (FRSM) in farm animal nutrition. However, based on the available literature, it can be hypothesized that the bacteria used in the RSM fermentation process will exhibit enzymatic activity, reducing the level of antinutritional factors in this feed material and positively affecting the performance of birds and the quality of meat obtained from them. The aim of this work was to evaluate the influence of solid-state fermentation on the nutritional value and enzymatic activity of rapeseed meal and its effects on the performance and meat quality of broiler chickens.
MATERIALS AND METHODS
Fermentation of RSM
Fermentation of RSM was carried out at InventionBio (Bydgoszcz, Poland) using a solid fermentation bioreactor with a capacity of 50 kg (serial number PM/2018/123, Pro-mill, Warsaw, Poland). The Bacillus subtilis 67 strain, isolated from Eisenia fetida earthworms, was used for this process. This strain was used because it is classified as generally recognized as safe (GRAS), so it does not pose a threat to animal and human health. This strain produces a wide range of metabolites, such as enzymes, biosurfactants, and biopolymers, and has probiotic properties. Bacterial inoculum with an optical density of OD600 = 0.1 (0.35 × 107 cfu/mL) was prepared by resuspending B. subtilis preculture in MIM1 (minimal medium) (sucrose 60 g/L (LabEmpire, Rzeszów, Poland), urea 2.3 g/L (LabEmpire, Rzeszów, Poland), MgSO4 0.5 g/L (LabEmpire, Rzeszów, Poland), Na2HPO4 8.4 g/L (Pol-Aura, Dywity, Poland), NaH2PO4 3.9 g/L (Pol-Aura, Dywity, Poland), FeSO4 1.2 mg/L (Pol-Aura, Dywity, Poland), CuSO4 1.6 mg/L (Pol-Aura, Dywity, Poland), MnSO4 5 mg/L (Pol-Aura, Dywity, Poland), pH = 7.0). The RSM was pasteurized for 8 min at 80°C (Laboratory Pasteurizer Armfield FT43, Merazet, Poznan, Poland), and then mixed with the prepared inoculum in a mass to volume (m/v) ratio of 1:1 to maintain 50% humidity. The fermentation was shaken at 20 rpm, with 50 L/min aeration for 24 h at 37°C. After 24 h, the RSM was immediately dried in a fluid dryer (Pro-mill, Warsaw, Poland). The B. subtilis count in the final product was 1.8 × 107 cfu/g.
Determination of the Enzymatic Activity of B. Subtilis Strain 67
The substrate for the xylanase reaction was a 1% xylan solution (SERVA Electrophoresis GmbH, Heidelberg, Germany). One gram of xylan was weighed and then ground in 5 mL of 1 M NaOH (Sigma-Aldrich, Saint Louis, MO) in a mortar until the powder was completely dissolved. Then 50 mL of distilled water (Merck, Darmstadt, Germany) was added and mixed thoroughly. The pH of the resulting solution was determined and adjusted to pH 5 with 1 M acetic acid (Pol-Aura, Dywity, Poland). Finally, the mixture volume was increased to 100 mL with 0.05 M acetate buffer (Pol-Aura, Dywity, Poland) and frozen at −20°C until analysis. The substrate for the cellulase reaction was a 1% sodium carboxymethylcellulose solution (Sigma-Aldrich, Saint Louis, MO). To prepare it, 1 g of sodium carboxymethylcellulose was dissolved in 80 mL of boiled, distilled water by slowly adding the substrate to the hot water while stirring constantly. After reconstitution of the powder, 10 mL of 0.5 M acetate buffer pH 4.8 (Pol-Aura, Dywity, Poland) was added, and then made up to 100 mL with distilled water. The substrate prepared in this way was also frozen at −20°C until the analysis. For the preparation of postculture supernatants, 10 g of dry postfermentation biomass was weighed, and 100 mL of sterile distilled water was added to it. The mixture was shaken at 180 rpm for 30 min at room temperature. The mixture was then set aside for 5 min for biomass sedimentation, then centrifuged (13,500 rpm, 5 min), and the centrifugation supernatant was collected. The clear supernatant was transferred to sterile Falcon tubes and immediately frozen at −20°C until analysis. To determine the enzymatic activity of the B. subtilis 67 strain, the Bernfeld method with the use of 3,5-dinitrosalicylic acid was used. The standard for determining xylulolytic activity was xylose (Sigma-Aldrich, Saint Louis, MO), and glucose for the cellulolytic activity assay (Sigma-Aldrich, Saint Louis, MO). The range of the standard curve was sugar concentrations from 125 µg/mL to 1,000 µg/mL. To prepare the standard curve, successive sugar concentrations in a volume of 200 µl were preincubated at 50°C for 5 min. The samples were then incubated at 50°C for 30 min. The reaction was stopped by adding 300 μL of 3,5-dinitrosalicylic acid (Pol-Aura, Dywity, Poland) and incubating the mixture for another 10 min at 100°C. After cooling to room temperature (21°C), the samples were diluted 5-fold. The absorbance was measured at 530 nm against a blank (200 μL of distilled water instead of sugar solution). Test and control samples were prepared to determine enzymatic activities.
Determination of the Nutritional Value of RSM Before and After Fermentation
The chemical composition of RSM was performed according to the Association of Official Analytical Chemists (AOAC) methodology, and included dry matter: Zalmed SML 32/250 dryer (AOAC, 930.15); crude ash: in a muffle furnace (AOAC, 942.05); nitrogen, by the Kjeldahl method (AOAC, 984.13): Kjeltec 2300 Foss Tecator (Hillerod, Denmark); crude protein conversion factor of 6.25 N; crude fat by ether extraction: Büchi Extraction System B-811 (AOAC, 920.39); crude fiber: Foss Fibertec 1020, Sweden (AOAC, 978.10). The gross energy (GE) content was determined using a calorimetric bomb. The metabolic energy (ME) content was expressed as the difference between the gross energy of the feed and the energy lost in feces. All necessary analyses were repeated 6 times. The content of glucosinolates in RSM was also determined before and after the fermentation process. The glucosinolates content was determined by high-pressure liquid chromatography (HPLC) method based on PN-ISO 10633-1: 2000, oil seed meal. Determination of glucosinolates content and PN-EN ISO 9167-1: 1999, rapeseed.
Ethical Statement
According to Polish law (Act of 15.01.2015 on the Protection of Animals Used for Scientific and Educational Purposes), ethical approval for this experiment was not required because the experiment was carried out under standard production conditions, therefore the birds were not exposed to excessive pain, suffering, and stress.
Experimental Design and Animal Treatment
The research was carried out at the Agricultural Experimental Station of Wroclaw University of Environmental and Life Sciences, Poland. Seven hundred twenty broiler chickens (Ross 308 line) were used in the experiment. Day-old male chickens were placed in 24 boxes, each 4 m2. The birds in the boxes were kept in a straw litter system. In each box, there was a bell-shaped drinker and a feeder. Birds were divided into 3 groups, each of them containing 8 replications (boxes) with 30 chickens (240 chickens per group). The C group was a negative control that did not receive the RSM in the feed mixture. The unfermented rapeseed meal (URSM) group was a positive control that received a feed mixture with a 3% addition of unfermented RSM. The FRSM group was the experimental group fed with a 3% addition of RSM fermented with the B. subtilis 67 strain. During the 35 d of the experiment, chickens were kept in accordance with the recommendations of the line producer (Aviagen). The temperature on the day of placing the chickens in the boxes was 32°C, it was lowered by 0.5°C daily until it reached 22°C on the 21st day of bird life. The relative humidity was maintained at 50 to 65%. During the first week of life, the light (L) was interrupted every 6 h for 10-min dark periods (D). Then the L:D ratio was maintained at 18:6. The exception was the last 3 d of the experiment, during which the L:D was 24:0.
Nutrition
The composition and nutritional value of feed mixtures are presented in Table 1.
Table 1.
The composition and nutritional value of feed mixtures used in the experiment.
| Composition of starter diet | |||
|---|---|---|---|
| Component | C (%) | URSM (%) | FRSM (%) |
| Wheat | 45.13 | 33.49 | 33.49 |
| Soybean meal (48% CP) | 32 | 31.12 | 31.12 |
| Maize (8% CP) | 15.77 | 25 | 25 |
| Unfermented rapeseed meal | 0 | 3 | 0 |
| Fermented rapeseed meal | 0 | 0 | 3 |
| Soybean oil | 2,92 | 3.29 | 3.29 |
| Phosphate 1-Ca | 0.99 | 1.01 | 1.01 |
| Chalk | 0,92 | 0.86 | 0.86 |
| Premix 0.5%* | 0.5 | 0.5 | 0.5 |
| DL-methionine 99% | 0.34 | 0.33 | 0.33 |
| Acidifier | 0.3 | 0.3 | 0.3 |
| HCL-lysine 78.5% | 0.26 | 0.26 | 0.26 |
| L-threonine 98.5% | 0.19 | 0.17 | 0.17 |
| Sodium bicarbonate | 0.17 | 0.16 | 0.16 |
| Sodium chloride | 0.16 | 0.16 | 0.16 |
| Lysine sulfate | 0.15 | 0.15 | 0.15 |
| Sodium sulfate | 0.12 | 0.12 | 0.12 |
| L-valine—96.5% | 0.08 | 0.06 | 0.06 |
| Nutritional value of starter diet | |||
| Nutrient | C | URSM | FRSM |
| Dry matter (%) | 90.25 | 90.1 | 90.07 |
| Crude ash (% d.m.) | 4.6 | 4.66 | 4.52 |
| Crude fat (% d.m.) | 4.64 | 4.99 | 5.03 |
| Crude protein (% d.m.) | 23.10 | 22.34 | 22.98 |
| Crude fiber (% d.m.) | 3.48 | 3.84 | 3.47 |
| Metabolic energy (kcal/kg d.m. | 2938.84 | 2946.95 | 2955.41 |
| Composition of grower I diet | |||
| Component | C (%) | URSM (%) | FRSM (%) |
| Wheat | 38.56 | 36.91 | 36.91 |
| Soybean meal (48% CP) | 29.69 | 27.94 | 27.94 |
| Maize (8% CP) | 25 | 25 | 25 |
| Unfermented rapeseed meal | 0 | 3 | 0 |
| Fermented rapeseed meal | 0 | 0 | 3 |
| Soybean oil | 3.3 | 3.76 | 3.76 |
| Phosphate 1-Ca | 0.73 | 0.72 | 0.72 |
| Chalk | 0.74 | 0.7 | 0.7 |
| Premix 0.5%* | 0.5 | 0.5 | 0.5 |
| DL-methionine 99% | 0.28 | 0.27 | 0.27 |
| Acidifier | 0.3 | 0.3 | 0.3 |
| HCL-lysine 78.5% | 0.14 | 0.14 | 0.14 |
| L-threonine 98.5% | 0.13 | 0.12 | 0.12 |
| Sodium bicarbonate | 0.15 | 0.15 | 0.15 |
| Sodium chloride | 0.16 | 0.16 | 0.16 |
| Lysine sulfate | 0.2 | 0.2 | 0.2 |
| Sodium sulfate | 0.1 | 0.1 | 0.1 |
| L-valine—96.5% | 0.02 | 0.02 | 0.02 |
| Nutritional value of grower I diet | |||
| Nutrient | C | URSM | FRSM |
| Dry matter (%) | 89.63 | 90.47 | 88.6 |
| Crude ash (% d.m.) | 4.26 | 4.12 | 4.15 |
| Crude fat (% d.m.) | 5.37 | 5.67 | 5.87 |
| Crude protein (% d.m.) | 21.16 | 20.79 | 21.03 |
| Crude fiber (% d.m.) | 3.47 | 4.09 | 2.99 |
| Metabolic energy (kcal/kg d.m. | 3016.09 | 3020.26 | 3028.93 |
| Composition of grower II diet | |||
| Component | C (%) | URSM (%) | FRSM (%) |
| Wheat | 37.73 | 35.65 | 35.65 |
| Soybean meal (48% CP) | 25.06 | 23.71 | 23.71 |
| Maize (8% CP) | 30 | 30 | 30 |
| Unfermented rapeseed meal | 0 | 3 | 0 |
| Fermented rapeseed meal | 0 | 0 | 3 |
| Soybean oil | 3.99 | 4.52 | 4.52 |
| Phosphate 1-Ca | 0.57 | 0.56 | 0.56 |
| Chalk | 0.6 | 0.56 | 0.56 |
| Premix 0.5%⁎⁎ | 0.5 | 0.5 | 0.5 |
| DL-methionine 99% | 0.27 | 0.26 | 0.26 |
| Acidifier | 0.3 | 0.3 | 0.3 |
| HCL-lysine 78.5% | 0.17 | 0.17 | 0.17 |
| L-threonine 98.5% | 0.14 | 0.13 | 0.13 |
| Sodium bicarbonate | 0.18 | 0.18 | 0.18 |
| Sodium chloride | 0.16 | 0.16 | 0.16 |
| Lysine sulfate | 0.2 | 0.2 | 0.2 |
| Sodium sulfate | 0.08 | 0.08 | 0.08 |
| L-valine—96.5% | 0.05 | 0.04 | 0.04 |
| Nutritional value of grower II diet | |||
| Nutrient | C | URSM | FRSM |
| Dry matter (%) | 90.09 | 89.99 | 89.82 |
| Crude ash (% d.m.) | 3.95 | 3.72 | 3.64 |
| Crude fat (% d.m.) | 5.3 | 5.46 | 6.01 |
| Crude protein (% d.m.) | 19.19 | 19.23 | 19.32 |
| Crude fiber (% d.m.) | 3.69 | 4.18 | 3.55 |
| Metabolic energy (kcal/kg d.m. | 3116.48 | 3119.34 | 3127.52 |
The composition of 1 kg of starter and grower 1 premixes: vitamin A—2,600,000 IU; vitamin D3—1,000,000 IU; vitamin E—15,000 mg; vitamin K3—800 mg; vitamin B1—800 mg; vitamin B2—2,000 mg; vitamin B6—1,200 mg; vitamin B12—8,000 µg; biotin—60,000 µm; niacinamide—12,000 mg; calcium D-pantothenate—3,000 mg; pantothenic acid—2,760 mg; folic acid—400 mg; choline chloride—90,000 mg; betaine—25,000 mg; Mg—3.240 g; Mn—24,000 mg; Fe—9,000 mg; Cu—4,000 mg; Zn—20,000 mg; Se—70 mg; I—300 mg; BHT—30 mg; propyl gallate—30 mg; clinoptilolite—3 mg; silicic acid—0.105 g; citric acid—0.015 g.
The composition of 1 kg of grower II premix: vitamin A—2,000,000 IU; vitamin D3—900,000 IU; vitamin E—12,000 mg; vitamin K3—600 mg; vitamin B1—500 mg; vitamin B2—1,600 mg; vitamin B6—1,000 mg; vitamin B12—7,000 µg; biotin—50,000 µm; niacinamide—10,000 mg; calcium D-pantothenate—2,600 mg; pantothenic acid—2,392 mg; folic acid—400 mg; choline chloride—75,000 mg; betaine—25,000 mg; Mg—5.230 g; Mn—20,000 mg; Fe—8,500 mg; Cu—4,000 mg; Zn—18,000 mg; Se—65 mg; I—280 mg; BHT—30 mg; propyl gallate—30 mg; clinoptilolite—3 mg; silicic acid—0.105 g; citric acid—0.015 g.
Growth Performance
The production parameters of the birds, such as body weight (BW), daily gains (DG), feed intake (FI), feed conversion ratio (FCR), mortality and European Production Efficiency Factor (EPEF) were determined. To determine the BW and DG, chickens were weighed at 1, 7, 14, 21, 28, and 35 d of life. The feed was weighed daily, and then the FI was determined. The cumulative FCR was calculated by dividing the total feed consumed per week by the body weight of the birds in the same week. Mortality was monitored throughout the experiment and was expressed as % of dead birds in each group. The EPEF was calculated from the formula:
At the end of the experiment, 8 birds from each group were randomly selected to collect the pectoral muscles and the leg muscles for further analysis. The euthanization process was performed according to the Directive 2010/63/EU of the European Parliament and Council of the European Union (2010). After evisceration, the pectoral and thigh muscles were collected for further analysis.
Meat Quality
The chemical composition of meat was performed according to AOAC (2005), and included crude ash: in a muffle furnace (AOAC, 942.05); nitrogen, by the Kjeldahl method (AOAC, 984.13): Kjeltec 2300 Foss Tecator (Hillerod, Denmark); crude protein as 6.25 N; crude fat by ether extraction: Büchi Extraction System B-811 (AOAC, 920.39). The analyses were done in 6 replicates.
The color of meat samples before and after cooking was measured using a colorimeter (Minolta spectrophotometer CM 3500d, Japan). The color reading includes lightness (L), redness (a*), and yellowness (b*). Before measurement, the equipment was standardized with a white color standard. The pH was measured by pH-meter (InoLab Mettler Toledo, AG 8603, Switzerland). The measurement was carried out before and after rigor. For the determination of water-holding capacity (WHC), the water content of a Whatman No. 1 filter paper was standardized prior to sample addition by drying it at 105°C to constant weight. The final weight of the sample was taken to calculate water content (ISO/DIS 1442 Meat and meat products—Determination of moisture content—Reference method). A 300 ± 5 mg portion of meat was placed on a glass plate with a Whatman No. 1 filter paper, covered with a 24 × 24 mm coverslip, and pressed with a 2 kg metal pad for 5 min. The WHC value is expressed as the percentage of water retained in the sample (despite weight loading) to the water content of the sample before pressing.
The determination of anserine, carnosine, and taurine was also performed. Samples were prepared as described by Aristoy and Toldrá (2004). Feed or sample tissue was finely ground, and 25 g of this was homogenized with 25 mL of redistilled water in a Polytron PT 2500 (3 × 15 s strokes) (Kinematica GmbH, Bern, Switzerland). The homogenate was centrifuged at 10,000 × g (Hettich 1720) for 20 min in the cold, and the supernatant was filtered through glass wool. The supernatant was deproteinized by taking 300 μL and adding 3 vol (900 μL) of methanol (HPLC grade source of chemical) the mixture was allowed to stand at 4°C for 15 min. The samples were centrifuged (12,000 × g) in an Eppendorf centrifuge (Hettich 1720) for 3 min, and the supernatant was directly analyzed for dipeptides content. The dipeptides were analyzed by liquid chromatography, HPLC, and OPA postcolumn derivatization as described in Henderson et al. (1999). The HPLC system used was the Agilent 1100 HPLC: G1312A Binary pump with G1315A Diode Array Detector (DAD), 6-mm or 10-mm flow cell and/or G1315A Fluorescence Detector (FLD). The diode array detector was used to determine the taurine and FLD detector to quantify the anserine and carnosine.
Following mincing, the samples were divided into 50 g portions. Each sample was homogenized with 50 mL of deionized water and centrifuged at 15,000 rpm at 4°C for 10 min (Beckman, J2-21). The supernatants were used for further determination of thiobarbituric reactive substances (TBARS) and selected antioxidant traces. Thiobarbituric acid assay (TBA) was estimated colorimetrically, and absorbance was measured at 532 nm using the UV-visible spectrophotometer model PharmaSpec 1700 (Shimadzu, Japan), using a method described by Batsoglou et al. (1994). The results were expressed as malonaldehyde (MDA) μmol per mL of the sample. Ferric reducing antioxidant power (FRAP) of the supernatant was evaluated by using 10 mM TPTZ and 20 mM ferric chloride solution in 0.3 M sodium acetate buffer (pH 3.6), at a ratio of 1:1:10 (Benzie and Strain, 1996). The formation of a colored TPTZ–Fe2+ complex was UV-visible at 595 nm. The results were expressed as Trolox Equivalents of Antioxidant Capacity (TEAC) and given in μmol/mL of supernatant. The 2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonate (ABTS) assay was carried out as per the method of (Cai et al., 2006). The ABTS radical cation (ABTS●+) solution was prepared by mixing 7 mM ABTS and 2.45 mM potassium persulfate and incubated in the dark at room temperature for 16 h. The ABTS●+ solution was then diluted with 80% (v/v) ethanol to obtain an absorbance of 0.700 ± 0.005 at 734 nm. ABTS●+ solution (3.9 mL) was added to 0.1 mL of the test sample (prediluted at a ratio of 1:50) and mixed vigorously. The reaction mixture was allowed to stand at 23°C for 6 min, and the absorbance was recorded at 734 nm immediately. A standard curve was obtained by using ascorbic acid in 80% ethanol. The results were expressed as ascorbic acid equivalents and given in μmol/mL of supernatant.
Sensory Traits
In order to determine the sensory parameters of meat, samples were prepared using the sous-vide method. The meat samples, packaged in cook-in plastic bags, were cooked in hot water at temperatures of 85°C or 95°C (hot water). All meat samples were heated until their geometric center temperature reached 72°C, which was monitored by a thermocouple. Then, a panel composed of 8 experts evaluated 3 sets of samples, scoring each sample for flavor, color, appearance, tenderness, and taste on a 5-point scale (1—dislike, 5—like).
Statistical Analysis
The results are presented as the mean and standard error of the mean (SEM). The normality, the data distribution relating to the content of nutrients and antinutritional factors, was assessed using the Shapiro-Wilk test. If the data distribution was normal, the paired Student t test was performed. If it was not normal, a Wilcoxon pairwise test was performed. In the case of bird performance, meat quality parameters (apart from pH) and meat sensory parameters, the normality of the data distribution also was assessed using the Shapiro-Wilk test. If the distribution was normal, a 1-way analysis of variance was performed where the differences between the groups were assessed using the Tukey test. If the distribution was not normal, the Kruskal-Wallis test was performed. For pH, a 2-way analysis of variance was performed, where the differences between the groups were assessed using the Bonferroni test. The differences were statistically significant when P < 0.05. All data were analyzed using the Statistica ver. 13.3.
RESULTS AND DISCUSSION
Enzymatic Activity of B. Subtilis 67 Strain
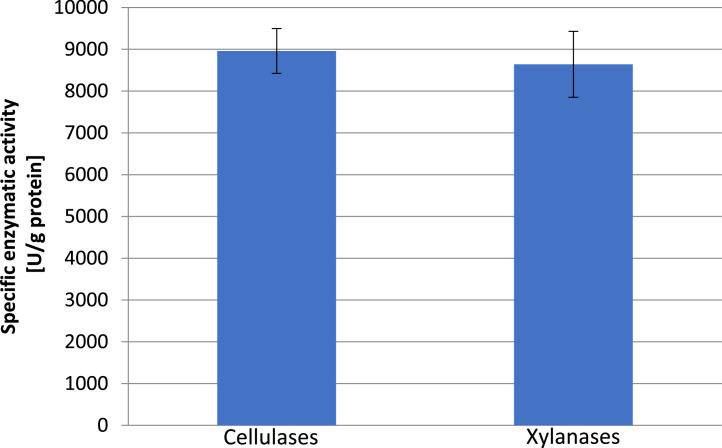
Enzymatic activity of B. subtilis 67 strain is shown in Figure 1.
Figure 1.
This figure shows the specific enzymatic activity of the B. subtilis 67 strain.
The obtained results show that the B. subtilis 67 strain is characterized by both cellulolytic and xylulolytic activity. During solid cultivation on rapeseed meal, this strain was able to produce approx. 9,000 U/g of cellulase protein and approx. 8,600 U/g of xylanase protein. This means that the use of this strain in the fermentation of rapeseed raw materials can help solve the problem of NSP. A similar value (0.72 U/mg) cellulase protein was obtained by Heck et al. (2002), simultaneously obtaining more than twice the amount of xylanases—1.75 U/mg of protein using the Bacillus sp. BL15. A much higher value, almost 300 U/mg, was obtained by Kalim and Ali (2016) using the environmental strain of B. subtilis. Regardless of the results obtained by other authors, it has been proven that the B. subtilis 67 strain produces relatively large amounts of cellulolytic and xylulolytic enzymes, and therefore it may reduce the negative effect of NSP on the digestibility of nutrients in the poultry digestive tract.
Nutritional Value of RSM After Fermentation
The fermentation process can change nutritional properties of RSM. Table 2 shows the nutritional value of RSM before and after the fermentation process.
Table 2.
Nutritional value and amino acid profile of RSM before and after solid-state fermentation.
| Nutrient | URSM | FRSM | SEM | P value |
|---|---|---|---|---|
| Dry matter (%) | 89.28a | 89.16b | 0.213 | 0.022 |
| Crude ash (% d.m.) | 7.315b | 7.410a | 0.0387 | 0.0089 |
| Crude fat (% d.m.) | 1.750b | 2.605a | 0.1297 | 0.0001 |
| Crude protein (% d.m.) | 38.60 | 38.55 | 0.184 | 0.917 |
| Crude fiber (% d.m.) | 12.54a | 11.20b | 0.203 | 0.001 |
| Metabolic energy (MJ/kg d.m.) | 9.637b | 10.30a | 0.1601 | 0.0001 |
| Glucosinolates (mmol/kg d.m.) | 15.51a | 9.84b | 0.021 | 0.027 |
| Amino acid profile | ||||
| Asp | 27,08 | 25,65 | - | - |
| Thr | 16,72 | 15,71 | - | - |
| Ser | 16,92 | 16,08 | - | - |
| Glu | 69,83 | 63,35 | - | - |
| Pro | 23,71 | 21,40 | - | - |
| Gly | 19,02 | 17,68 | - | - |
| Ala | 16,35 | 15,02 | - | - |
| Val | 17,40 | 16,03 | - | - |
| Ile | 11,48 | 11,07 | - | - |
| Leu | 27,91 | 25,95 | - | - |
| Tyr | 9,35 | 6,83 | - | - |
| Phe | 13,81 | 12,95 | - | - |
| His | 10,91 | 9,84 | - | - |
| Lys | 20,60 | 18,47 | - | - |
| Arg | 20,20 | 20,86 | - | - |
| Cys | 11,96 | 10,69 | - | - |
| Met | 13,21 | 12,12 | - | - |
Statistically significant differences on the level at P < 0.05; URSM, unfermented rapeseed meal; FRSM, fermented rapeseed meal; SEM, standard error of the mean.
It was shown that the FRSM was characterized by a significantly lower dry matter content compared to the URSM (P < 0.05). This was probably caused by the use of carbohydrates in RSM by bacteria in the fermentation process. These results are consistent with the results obtained by Xu et al. (2012), however, in their research, the difference in the level of dry matter was 3.5%. Different results were obtained by Hu et al. (2016), who recorded an almost 3% increase in the level of dry matter after the fermentation process. A mixture of B. subtilis, Candida albicans, and Enterococcus faecalis in 1:2:1 ratio, was used in these 2 studies, and the RSM was amended with wheat bran and corn flour (in the proportions 80, 10, and 10%). The FRSM contained significantly more crude ash, crude fat and metabolic energy (P < 0.05). These results may reflect the decrease in the dry matter content, not the actual increase in nutrient content, which is in line with the results obtained by Xu et al. (2012). The FRSM was also characterized by a significantly lower content of crude fiber (P < 0.05). This may be the result of the activity of cellulolytic and xylulolytic enzymes created by B. subtilis and the subsequent use of structural carbohydrates contained in RSM as an energy source by this bacteria. These results confirm the results obtained by Xu et al. (2012) and Hu et al. (2016). There were no statistically significant differences in the crude protein content. The glucosinolates content was significantly higher (P < 0.05) in the URSM treatment. These results were also obtained by Hu et al. (2016), who found a significant decrease (P < 0.01) of this factor and a decrease of isothiocyanates in FRSM. Similar results were obtained by Ashayerizadeh et al. (2018), reducing the level of glucosinolates in FRSM by almost 68%. Xu et al. (2012) showed a reduction in the content of isothiocyanates by almost 88%. Hao et al. (2020) also obtained a significant decrease in the level of glucosinolates after 1, 3, and 5 d of fermentation of RSM with B. subtilis and Actinomucor elegans. The results obtained during these studies, as well as results obtained by other authors, lead to the conclusion that the glucosinolates contained in RSM are degraded by enzymes produced by microorganisms in the fermentation process.
Performance
The production parameters of broiler chickens after 7, 14, 21, 28, and 35 d of the experiment are presented in Table 3.
Table 3.
Performance of broiler chickens.
| Parameter | C | URSM | FRSM | SEM | P value |
|---|---|---|---|---|---|
| D 7 | |||||
| BW (g) | 207.12 | 206.49 | 213.73 | 3.258 | 0.401 |
| DG (g) | 23.87 | 23.78 | 24.81 | 0.465 | 0.624 |
| FI (g) | 201.56 | 198.68 | 202.41 | 1.102 | 0.652 |
| FCR | 0.978 | 0.967 | 0.948 | 0.0116 | 0.707 |
| D 14 | |||||
| BW (g) | 523.08 | 528.57 | 535.95 | 4.431 | 0.513 |
| DG (g) | 45.13 | 46.01 | 46.02 | 0.576 | 0.784 |
| FI (g) | 549.19 | 548.48 | 548.65 | 0.592 | 0.888 |
| FCR | 1.051 | 1.038 | 1.025 | 0.0086 | 0.5326 |
| D 21 | |||||
| BW (g) | 1123.57b | 1134.13ab | 1171.31a | 8.339 | 0.041 |
| DG (g) | 85.78 | 86.51 | 90.76 | 1.018 | 0.093 |
| FI (g) | 1342.83 | 1342.36 | 1347.16 | 2.373 | 0.681 |
| FCR | 1.196 | 1.184 | 1.151 | 0.0083 | 0.0650 |
| D 28 | |||||
| BW (g) | 1885.54 | 1884.62 | 1933.04 | 13.735 | 0.268 |
| DG (g) | 108.85 | 107.21 | 108.81 | 1.636 | 0.903 |
| FI (g) | 2468.31 | 2478.43 | 2490.71 | 4.611 | 0.102 |
| FCR | 1.312 | 1.315 | 1.288 | 0.0092 | 0.4508 |
| D 35 | |||||
| BW (g) | 2508.48b | 2509.26b | 2604.57a | 17.338 | 0.0130 |
| DG (g) | 88.99 | 89.23 | 95.93 | 2.573 | 0.477 |
| FI (g) | 3561.82 | 3563.07 | 3652.85 | 18.936 | 0.073 |
| FCR | 1.420 | 1.421 | 1.402 | 0.0035 | 0.0537 |
| EPEF | 492b | 496b | 520a | 4.429 | 0.0199 |
Statistically significant differences on the level at P < 0.05; C, control group; URSM, group with addition 3% unfermented rapeseed meal; FRSM, group with addition 3% fermented rapeseed meal; BW, body weight; DG, daily gains; FI, feed intake; FCR, feed conversion ratio; EPEF, European Production Efficiency Factor; SEM, standard error of the mean.
After 7 and 14 d of the experiment, there were no significant differences in the assessed parameters. However, birds in the FRSM group had the highest numerically values of BW and the lowest numerically values of FCR. After 21 d of the experiment, the birds from the FRSM group had a significantly higher BW (P < 0.05) compared to the C group. There were no significant differences in these parameters after 28 d of the experiment. After 35 d, the birds in the FRSM group had a significantly higher BW (P < 0.05) compared to the C and URSM groups. Chickens in the FRSM group had a significantly greater EPEF (P < 0.05) compared to the other groups. The RSM subjected to the fermentation process positively influenced the production parameters of broiler chickens. This can be attributed to the content of antinutritional factors in RSM after fermentation being significantly lower. In addition, the feed mixtures containing FRSM had lower amounts of fiber, which improved the utilization of the feed nutrients. In addition, B. subtilis exhibits probiotic activity, which could also have a positive effect on the production parameters of birds. Similar results were obtained by Chiang et al. (2009), who showed that chickens receiving FRSM in the feed were characterized by significantly (P < 0.05) higher DG and significantly (P < 0.05) lower FCR compared to chickens receiving URSM. Xu et al. (2012) replaced SBM in the feed mixture with 0, 5, 10, or 15% FRSM demonstrating that SBM can be successfully replaced with up to 10% of FRSM with no negative impact on the performance of broiler chickens. Szmigiel et al. (2021) did not show the effect of RSM fermented with the use of B. subtilis 67 and 87Y strains on the broilers’ performance. However, in their research, FRSM was included in the feed mixture on the 21st day of life, and the birds were placed in metabolic cages at the same time, which could have influenced the results.
Proximate Composition of Meat
In general, leg muscles collected from FRSM-fed birds had a significantly (P < 0.05) higher protein content. This is probably due to the fact that the protein contained in FRSM was better absorbed and incorporated into the muscles (Table 4).
Table 4.
Proximate analysis.
| Parameter | Group of meat | C | URSM | FRSM | SEM | P value |
|---|---|---|---|---|---|---|
| Ash content (%) | Breast | 1.247 | 1.335 | 1.267 | 0.0203 | 0.1297 |
| Leg | 1.186 | 1.127 | 1.208 | 0.0226 | 0.1699 | |
| Protein content (%) | Breast | 22.75 | 22.98 | 22.72 | 0.0573 | 0.0757 |
| Leg | 19.04b | 19.01b | 19.68a | 0.1029 | 0.0166 | |
| Fat content (%) | Breast | 1.135 | 1.099 | 1.088 | 0.0275 | 0.5317 |
| Leg | 3.221 | 2.870 | 2.987 | 0.0621 | 0.0611 |
Statistically significant differences on the level at P < 0.05; C, control group; URSM, group with addition 3% unfermented rapeseed meal; FRSM, group with addition 3% fermented rapeseed meal; SEM, standard error of the mean.
Mikulski et al. (2012) reported that RSM does not cause changes in the composition of turkey breast muscles. On the other hand, Gopinger et al. (2014) argue that the content of dry matter and fat in the breast muscles of broiler chickens increases with higher levels of RSM in the feed mixture. Ashayerizadeh et al. (2018) showed that replacing some or all of SBM with RSM did not affect the fat content of broiler chicken breast muscles. Drażbo et al. (2019) did not show the effect of fermented rapeseed cake on the dry matter and fat content in turkey breast muscles. However, the muscles taken from the birds fed raw or fermented rapeseed cake had a significantly lower cholesterol content.
pH Values and WHC in Meat
Muscle pH is one of the most important factors influencing other quality parameters of meat, such as color, tenderness, taste, durability, and WHC (Nowakowicz-Dębek et al., 2021). Delaying the decrease in muscle pH leads to a reduction in the rate of protein denaturation, thus improving the muscle's ability to retain water (Berri et al., 2007).
The pH of the breast and leg muscles is shown in Table 5.
Table 5.
The pH of breast and leg muscles.
| Group | |||||
|---|---|---|---|---|---|
| pH | C | URSM | FRSM | SEM | P value |
| Breast | 5.701 | 5.619 | 5.669 | 0.0238 | 0.2985 |
| Leg | 5.721a | 5.574b | 5.625b | 0.0431 | 0.0013 |
| Time | |||||
| pH | 15 min | 24 h | SEM | P value | |
| Breast | 5.802a | 5.524b | 0.1390 | 0.0001 | |
| Leg | 5.687a | 5.592b | 0.0475 | 0.0040 | |
| Interaction | |||||
| Breast | C*15 min | 5.897 | |||
| C*24 h | 5.505 | ||||
| URSM*15 min | 5.708 | ||||
| URSM*24 h | 5.530 | ||||
| FRSM*15 min | 5.800 | ||||
| FRSM*24 h | 5.538 | ||||
| SEM | 0.0667 | ||||
| P value | 0.1339 | ||||
| Leg | C*15 min | 5.833a | |||
| C*24 h | 5.608b | ||||
| URSM*15 min | 5.557b | ||||
| URSM*24 h | 5.591b | ||||
| FRSM*15 min | 5.671ab | ||||
| FRSM*24 h | 5.578b | ||||
| SEM | 0.0417 | ||||
| P value | 0.0059 | ||||
Statistically significant differences on the level at P < 0.05; C, control group; URSM, group with addition 3% unfermented rapeseed meal; FRSM, group with addition 3% fermented rapeseed meal; SEM, standard error of the mean.
There was a statistically significant interaction in the case of leg muscles, where the pH was significantly higher (P < 0.05) in the muscles of the C group after 15 min compared to the muscles of the C group after 24 h, URSM after 15 min and 24 h, and FRSM after 24 h. In studies conducted by Ashayerizadeh et al. (2018), the pH of the breast and leg muscles after 45 min did not differ between the groups. After 24 h, the breast muscles taken from chickens fed with FRSM were significantly higher than the pH of the breast muscles collected from chickens fed with URSM and SBM. For leg muscles after 24 h, the highest pH value was observed in the foraging group, in which the SBM was replaced by 100% FRSM. The obtained results are difficult to explain because the relationship between FRSM and the improvement in meat pH is not well understood, and more research is needed to investigate the mechanisms of fermented feed action that are related to meat pH (Ashayerizadeh et al., 2018).
The WHC of protein is very important in meat processing, this characteristic can affect the juiciness, tenderness, and taste of the meat products (Mir et al., 2017). The consequence of poor WHC is low cook yields and often “dry (lack of juiciness)” meat, so these cooked qualities can also be used to indirectly measure WHC. Poor WHC results in high drip and purge loss from meat and meat products, which can represent a significant loss of weight from carcasses and cuts, and may affect the yield and quality of processed meats. The WHC was significantly higher (P < 0.05) in the breast muscles of the C (73.80%) group compared to the muscles of the URSM (65.84%) and FRSM (67.68%) groups.
This corresponds to the results relating to the pH of the breast muscles. In this case, the differences in pH were not statistically significant, but in the case of URSM and FRSM groups it was smaller. Other results were obtained by Ashayerizadeh et al. (2018), who found that the breast muscles of chickens fed with SBM replaced with 50% FRSM had a significantly higher WHC than the muscles collected from chickens fed with SBM replaced by URSM. In the case of leg muscles, WHC was also significantly higher in the group fed with FRSM. Drażbo et al. (2019) did not show that fermented rapeseed cake had a statistically significant effect on the WHC of turkey breast muscles.
Color Change
The color degradation of chicken meat was evaluated before and after cooking, and the result is shown in Table 6. The breast meat of chickens from the FRSM group was characterized by a significantly lower (P < 0.05) yellowness value (b*). The leg meat from the URSM and FRSM groups before cooking was characterized by a significantly higher (P < 0.05) b* value. After cooking, breast meat from the URSM and FRSM groups showed a significantly lower (P < 0.05) redness value (a*). In the case of the leg meat after cooking, the meat from the FRSM group was characterized by significantly lower (P <0.01) lightness (L*). On the other hand, leg meat from the URSM group was characterized by a significantly lower (P < 0.05) a* value. This result is not aligned with a previous study that identified an insignificant impact of FRSM on the breast and thigh muscles of broiler chickens (Ashayerizadeh et al., 2018). The color value in this study is higher than that in the previous study, which reported a range of L* between 48.1 - 59.5 and 47.6 - 50.1 for leg and breast muscles, respectively; b* at 1.3 - 4.4 (Ashayerizadeh et al., 2018; Biesek et al., 2020). This phenomenon might be due to the different levels of RSM addition. In a previous study, the addition of RSM was at 25, 50, and 100% (Ashayerizadeh et al., 2018; Biesek et al., 2020), while in this study it was 3%. It was mentioned that meat color is influenced by feed composition, including carotenoids and antioxidants, as well as its stability. Moreover, myoglobin content and pH are important factors in influencing the color of chicken meat (Mir et al., 2017). In other words, the color changes are influenced by protein availability in the meat.
Table 6.
Color properties of chicken meat before and after cooking process.
| Color | C | URSM | FRSM | SEM | P value | |
|---|---|---|---|---|---|---|
| Before cooking | ||||||
| Breast | L* | 63.68 | 63.54 | 63.81 | 0.422 | 0.972 |
| a* | 3.612 | 3.774 | 3.354 | 0.1257 | 0.4186 | |
| b* | 6.705a | 6.227a | 5.046b | 0.2452 | 0.0004 | |
| Legs | L* | 63.03 | 66.03 | 63.44 | 0.5817 | 0.061 |
| a* | 7.424 | 7.236 | 8.200 | 0.2604 | 0.2972 | |
| b* | 4.271b | 6.767a | 6.643a | 0.3663 | 0.0007 | |
| After cooking | ||||||
| Breast | L* | 93.43 | 93.26 | 93.98 | 0.240 | 0.479 |
| a* | 3.224a | 1.373b | 1.702b | 0.2444 | 0.0001 | |
| b* | 16.23 | 16.39 | 15.72 | 0.181 | 0.280 | |
| Legs | L* | 88.65a | 87.31a | 83.29b | 0.704 | 0.001 |
| a* | 3.416 | 2.732 | 3.732 | 0.1778 | 0.0501 | |
| b* | 16.37a | 15.58b | 17.20a | 0.316 | 0.008 | |
Statistically significant differences on the level at P < 0.05; C, control group; URSM, group with addition 3% unfermented rapeseed meal; FRSM, group with addition 3% fermented rapeseed meal; SEM, standard error of the mean; L*, lightness; a*, redness; b*, yellowness.
The Composition of Dipeptide on the Chicken Breast and Legs and Antioxidant Capacity of Chicken Meat
The composition of dipeptide in the chicken breast and legs and antioxidant capacity of chicken meat are presented in Table 7. In general, FRSM had no significant impact on the amount of anserine, and taurine. The carnosine content was significantly lower (P < 0.05) in the leg meat from the FRSM group. The amount of anserine in the current study is aligned with those in the previous reports, which observed a range of 1.7 to 6.1 mg/g (Kopeć et al., 2012; Jayasena et al., 2015). Compared to previous studies, the carnosine level of the breast in this study is lower, while its level in the legs is higher. It was reported that chicken breast had carnosine in a range of 1.3 to 1.8 mg/g tissue and 0.7 to 0.8 mg/g in breast and leg, respectively (Kopeć et al., 2012; Jayasena et al., 2015). In this study, the level of carnosine for breast and legs were 1.14 to 1.15 mg/g and 1.16 to 1.5 mg/g, respectively. The taurine content of the breast in the current study revealed a higher amount which was at a range of 0.7 to 0.8 mg/g, while the previous report identified an amount of 0.1 to 0.4 mg/g tissue (Huang et al., 2014). In contrast, the taurine level of legs in this study (0.6–0.8 mg/g) showed a lower level compared to the previous report, which observed a level of 1.6 to 3.1 mg/g (Huang et al., 2014). Different levels of the dipeptide, particularly anserine, might be strongly influenced by the feed treatments. The increase in the amount of dipeptide was observed due to the nutrient enrichment of animal feed (Kopeć et al., 2012; Huang et al., 2014; Jayasena et al., 2015). There were no significant changes in the antioxidant status of broiler chicken meat following the use of FRSM. The ABTS values are seen to be higher in the leg samples from the URSM and FRSM groups. Higher FRAP values are observed in leg samples from C and FRSM groups. The lowest TBA values are in the leg samples from the FRSM group and breast samples from the URSM group, indicating that the amount of malonaldehyde is very low, a product of lipid autoxidation in meat. The MDA concentration in tissue indirectly reflects lipid oxidation, which is the result of the attenuated antioxidant protection when reactive oxygen species increase (Bai et al., 2017). Thiobarbituric acid measured in the FRSM and URSM groups is much lower compared to other samples, indicating that these chickens were healthier and diet has contributed to the prevention of autoxidation of meat, especially here, the role of probiotics should be emphasized. In general, the highest values of ABTS and FRAP are measured in samples from URSM and FRSM, while the significantly lower values of TBA are observed in samples FRSM and URSM. The effect of fermentation on antioxidant activity in several plant-based materials, which are the major feed ingredients for broilers, has comprehensively been reviewed by (Hur et al., 2014).
Table 7.
The composition of dipeptide on the chicken breast and legs and antioxidant capacity of chicken meat.
| Parameter | C | URSM | FRSM | SEM | P value | |
|---|---|---|---|---|---|---|
| Anserine (mg/g) | Legs | 2.957 | 3.040 | 2.555 | 0.1702 | 0.4298 |
| Breast | 5.415 | 5.942 | 5.391 | 0.1693 | 0.3708 | |
| Carnosine (mg/g) | Legs | 1.508a | 1.329ab | 1.166b | 0.0604 | 0.0366 |
| Breast | 1.154 | 1.149 | 1.144 | 0.0866 | 0.9992 | |
| Taurine (mg/g) | Legs | 0.866 | 0.693 | 0.677 | 0.0480 | 0.2191 |
| Breast | 0.714 | 0.802 | 0.717 | 0.0554 | 0.8371 | |
| ABTS (μmol/L) | Legs | 130.5 | 153.3 | 171.6 | 8.629 | 0.144 |
| Breast | 121.2 | 140.4 | 101.2 | 11.85 | 0.491 | |
| FRAP (μmol/L) | Legs | 0.311 | 0.271 | 0.330 | 0.0185 | 0.3292 |
| Breast | 0.289 | 0.374 | 0.233 | 0.0293 | 0.1405 | |
| TBA (μmol/L) | Legs | 2.348 | 2.287 | 2.045 | 0.0750 | 0.2372 |
| Breast | 1.742 | 1.636 | 1.772 | 0.0427 | 0.4491 | |
Statistically significant differences on the level at P < 0.05; C, control group; URSM, group with addition 3% unfermented rapeseed meal; FRSM, group with addition 3% fermented rapeseed meal; SEM, standard error of the mean; ABTS, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid; FRAP, ferric reducing antioxidant power; TBA, thiobarbituric acid assay.
The production of amino acids, lactic acid and antioxidant vitamins during fermentation may also contribute to the increased antioxidant capacity of the fermented feed (Doblado et al., 2005). Indeed, the extent of enhancement of antioxidant activity with fermentation may vary depending on the starter (microorganism species), nature of substrates and fermentation conditions (pH, temperature, solvent, water content, duration of fermentation process and aerobic conditions). Also, carbohydrates in present foods may act as antioxidants. Some of the Maillard reaction products (glycated proteins) and complexes of oxidized lipids with proteins, such as moderate properties, also have such properties (Bartosz, 2013). It has been reported that polysaccharides from different resources extracted from plants and seaweed have strong antioxidant properties (free-radical scavenging, transition-metal binding) and can be explored as novel potential antioxidants (Bartosz, 2013). Samples of FRSM as an addition to their diet have rapeseed, and this meal is fermented with Bacillus subtilis 67, according to Hu et al. (2016). Fermentation could change the physical and nutritional characteristics of RSM. The results of the present study showed that the contents of crude protein, true protein, water-soluble protein, and peptides were increased after fermentation, and crude fiber, glucosinolates, ITC, tannin, and phytic acid were decreased.
Sensory Traits
The sensory evaluation of the meat of broiler chickens fed with feed with FRSM has not been carried out so far. The breast muscles of the chickens from the URSM groups had significantly better color (P < 0.05) compared to the breast muscles of the chickens from the C and FRSM groups (Table 8). Moreover, the breast muscles of the chickens from the URSM group had a better taste (P < 0.05) compared to the muscles from the FRSM group.
Table 8.
Sensory traits.
| Muscle | Parameter | C | URSM | FRSM | SEM | P value |
|---|---|---|---|---|---|---|
| Breast | Smell | 3.711 | 3.656 | 3.500 | 0.0668 | 0.3339 |
| Color | 3.867b | 4.250a | 3.726b | 0.0588 | 0.0009 | |
| Appearance | 3.891 | 4.140 | 3.835 | 0.0558 | 0.0793 | |
| Tenderness | 3.625 | 3.742 | 3.445 | 0.0675 | 0.1846 | |
| Flavor | 3.664ab | 3.750a | 3.391b | 0.0618 | 0.0413 | |
| Leg | Smell | 3.843 | 3.726 | 3.695 | 0.0662 | 0.6218 |
| Color | 3.992 | 3.921 | 3.882 | 0.0699 | 0.7636 | |
| Appearance | 3.914 | 3.804 | 3.781 | 0.0643 | 0.5339 | |
| Tenderness | 3.703 | 3.609 | 3.593 | 0.0669 | 0.7835 | |
| Flavor | 3.960 | 3.687 | 3.867 | 0.0591 | 0.1302 |
Statistically significant differences on the level at P < 0.05; C, control group; URSM, group with addition 3% unfermented rapeseed meal; FRSM, group with addition 3% fermented rapeseed meal; SEM, standard error of the mean.
There were no statistically significant differences in the remaining parameters assessed, however, according to consumers’ assessment, the breast muscles of the FRSM group were the worst. There were also no statistically significant differences in the assessed parameters for the muscles of the legs. For these muscles, the FRSM group was also rated the worst. In a study by McNeill et al. (2004), the consumers showed no differences in the meat of the birds from the control group and the birds fed with rapeseed meal (100 g/kg of feed). When the proportion of rapeseed meal was increased to 200 g/kg of feed, consumers identified the meat as “different,” but did not show a strong aversion to it.
CONCLUSIONS
B. subtilis 67 exhibits cellulolytic and xylulolytic activity. The use of this strain during the fermentation of rapeseed meal has a positive effect on its nutritional value. The addition of the FRSM to broiler chicken feed has a positive effect on their performance, especially BW and EPEF. The use of FRSM and URSM in the diet has a negative effect on the pH of the leg muscles and WHC of breast muscles. The leg muscles of chickens fed with FRSM have a higher protein content, but the FRSM diet showed a negative impact on the sensory traits of meat. The FRSM has no significant impact on dipeptide composition of chicken meat. Chickens fed with FRSM generated a higher FRAP antioxidant capability and lower ABTS. The ambiguous results show the need for further research on the fermentation process of vegetable protein sources, especially in the context of the planned ban on the use of GMO soybeans in some countries.
ACKNOWLEDGMENTS
The work was supported by the National Centre for Research and Development [POIR.01.02.00-00-0064/17]. The APC is co-financed by Wrocław University of Environmental and Life Sciences.
DISCLOSURES
All authors declare no competing financial interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2023.102742.
Appendix. Supplementary materials
REFERENCES
- Aristoy M.C., Toldrá F. Histidine dipeptides HPLC-based test for the detection of mammalian origin proteins in feeds for ruminants. Meat Sci. 2004;67:211–217. doi: 10.1016/j.meatsci.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Ashayerizadeh A., Dastar B., Shams Shargh M., Sadeghi Mahoonak A.R., Zerehdaran S. Effects of feeding fermented rapeseed meal on growth performance, gastrointestinal microflora population, blood metabolites, meat quality, and lipid metabolism in broiler chickens. Livest. Sci. 2018;216:183–190. [Google Scholar]
- Association of Official Analytical Chemists. 2005, Official Methods of Analysis of AOAC International, AOAC International. AOAC International, Gaithersburg, MD.
- Bai K., Huang Q., Zhang J., He J., Zhang L., Wang T. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult. Sci. 2017;96:74–82. doi: 10.3382/ps/pew246. [DOI] [PubMed] [Google Scholar]
- Bartosz G. CRC Press; Boca Raton, FL: 2013. Food Oxidants and Antioxidants: Chemical, Biological, and Functional Properties. [Google Scholar]
- Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Berri C., Le B.D.E., Debut M., Sante-Lhoutellier V., Baeza E., Gigaud V., Jego Y., Duclos M.J. Consequence of muscle hypertrophy on characteristics of pectoralis major muscle and breast meat quality of broiler chickens. J. Anim. Sci. 2007;85:2005–2011. doi: 10.2527/jas.2006-398. [DOI] [PubMed] [Google Scholar]
- Biesek J., Kuźniacka J., Banaszak M., Kaczmarek S., Adamski M., Rutkowski A., Zmudzińska A., Perz K., Hejdysz M. Growth performance and carcass quality in broiler chickens fed on legume seeds and rapeseed meal. Animals. 2020;10:1–16. doi: 10.3390/ani10050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsoglou N.A., Fletouris D.J., Papageorgiou G.E., Vassilopoulos V.N., Mantis A.J., Trakatellis A.G. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 1994;42:1931–1937. [Google Scholar]
- Cai Y.Z., Sun M., Xing J., Luo Q., Corke H. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006;78:2872–2888. doi: 10.1016/j.lfs.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Chiang G., Lu W.Q., Piao X.S., Hu J.K., Gong L.M., Thacker P.A. Effects of feeding solid-state fermented rapeseed meal on performance, nutrient digestibility, intestinal ecology and intestinal morphology of broiler chickens. Asian-Australas. J. Anim. Sci. 2009;23:263–271. [Google Scholar]
- Doblado R., Zielinski H., Piskula M., Kozlowska H., Muñoz R., Frías J., Vidal-Valverde C. Effect of processing on the antioxidant vitamins and antioxidant capacity of vigna sinensis var. Carilla. J. Agric. Food Chem. 2005;53:1215–1222. doi: 10.1021/jf0492971. [DOI] [PubMed] [Google Scholar]
- Drażbo A., Kozłowski K., Ognik K., Zaworska A., Jankowski J. The effect of raw and fermented rapeseed cake on growth performance, carcass traits, and breast meat quality in turkey. Poult. Sci. 2019;98:6161–6169. doi: 10.3382/ps/pez322. [DOI] [PubMed] [Google Scholar]
- European Parliament and Council of the European Union . Council of Europe; Strasbourg, France: 2010. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. [Google Scholar]
- Filho E.X.F., Ximenes F.A., Fonseca A.S., Ximenes E.A. Xylan-degrading enzyme production by solid-state cultures of aerobic fungi. Rev. Microbiol. 1997;28:22–28. [Google Scholar]
- Gopinger E., Xavier E.G., Elias M.C., Catalan A.A., Castro M.L., Nunes A.P., Roll V.F. The effect of different dietary levels of canola meal on growth performance, nutrient digestibility, and gut morphology of broiler chickens. Poult. Sci. 2014;93:1130–1136. doi: 10.3382/ps.2013-03426. [DOI] [PubMed] [Google Scholar]
- Grela E., Czech A., Kiesz M., Wlazło Ł., Nowakowicz-Dębek B. A fermented rapeseed meal additive: effects on production performance, nutrient digestibility, colostrum immunoglobulin content and microbial flora in sows. Anim. Nutr. 2019;5:373–379. doi: 10.1016/j.aninu.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y., Wang Z., Zou Y., He R., Ju X., Yuan J. Effect of static-state fermentation on volatile composition in rapeseed meal. Sci. Food Agric. 2020;100:2145–2152. doi: 10.1002/jsfa.10238. [DOI] [PubMed] [Google Scholar]
- Heck J.X., Hertz P.F., Ayub M.A. Cellulase and xylanase productions by isolated Amazon Bacillus strains using soybean industrial residue based solid-state cultivation. Braz. J. Microbiol. 2002;33:213–218. [Google Scholar]
- Henderson, J. W., R. D. Ricker, B. A. Bidlingmeyer, and C. Woodward. 1999. Rapid, accurate, sensitive and reproducible HPLC analysis of amino acids. Agilent Technical Note 5980_1193E.
- Hopkins D.L., Beattie A.S., Pirlot K.L. Meat quality, carcass fatness and growth of short scrotum lambs grazing either rape or irrigated perennial pasture. Aust. J. Exp. Agric. 1995;35:453–459. [Google Scholar]
- Hu Y., Wang Y., Li A., Wang Z., Zhang X., Yun T., Qiu L., Yin Y. Effects of fermented rapeseed meal on antioxidant functions, serum biochemical parameters and intestinal morphology in broilers. Food Agric. Immunol. 2016;27:182–193. [Google Scholar]
- Huang C.X., Wang B., Min Z., Yuan J. Dietary inclusion level and time effects of taurine on broiler performance, meat quality, oxidative status and muscle taurine content. Br. Poult. Sci. 2014;55:598–604. doi: 10.1080/00071668.2014.943692. [DOI] [PubMed] [Google Scholar]
- Hur S.J., Lee S.Y., Kim Y.C., Choi I., Kim G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014;160:346–356. doi: 10.1016/j.foodchem.2014.03.112. [DOI] [PubMed] [Google Scholar]
- Jayasena D.D., Jung S., Kim H.J., Yong H.I., Nam K.C., Jo C. Taste-active compound levels in Korean native chicken meat: the effects of bird age and the cooking process. Poult. Sci. 2015;94:1964–1972. doi: 10.3382/ps/pev154. [DOI] [PubMed] [Google Scholar]
- Kalim B., Ali N.M. Optimization of fermentation media and growth conditions for microbial xylanase production. 3 Biotech. 2016;122:1–7. doi: 10.1007/s13205-016-0445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khajali F., Slominski B.A. Factors that affect the nutritive value of canola meal for poultry. Poult. Sci. 2012;91:2564–2575. doi: 10.3382/ps.2012-02332. [DOI] [PubMed] [Google Scholar]
- Kopeć W., Jamroz D., Wiliczkiewicz A., Biazik E., Hikawczuk T., Skiba T., Pudło A., Orda J. Antioxidation status and histidine dipeptides content in broiler blood and muscles depending on protein sources in feed. Anim. Phys. Anim. Nutr. 2012;97:586–598. doi: 10.1111/j.1439-0396.2012.01303.x. [DOI] [PubMed] [Google Scholar]
- Magge R.J., Kosaric M. Bioconservation of hemicellulose. Adv. Biochem. Eng. Biotechnol. 1985;32:61. doi: 10.1007/BFb0009525. 93. [DOI] [PubMed] [Google Scholar]
- McNeill L., Bernard K., MacLeod M.G. Food intake, growth rate, food conversion and food choice in broilers fed on diets high in rapeseed meal and pea meal, with observations on sensory evaluation of the resulting poultry meat. Br. Poult. Sci. 2004;45:519–523. doi: 10.1080/00071660412331286235. [DOI] [PubMed] [Google Scholar]
- Mikulski D., Jankowski J., Zdunczyk Z., Juskiewicz J., Slominski B.A. The effect of different dietary levels of rapeseed meal on growth performance, carcass traits, and meat quality in turkeys. Poult. Sci. 2012;91:215–223. doi: 10.3382/ps.2011-01587. [DOI] [PubMed] [Google Scholar]
- Mir N.A., Rafiq A., Kumar F., Singh V., Shukla V. Determinants of broiler chicken meat quality and factors affecting them: a review. J. Food Sci. Technol. 2017;54:2997–3009. doi: 10.1007/s13197-017-2789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowicz-Dębek B., Wlazło Ł., Czech A., Kowalska D., Bielański P., Ryszkowska-Siwko M., Łukaszewicz M., Florek M. Effects of fermented rapeseed meal on gastrointestinal morphometry and meat quality of rabbits (Oryctolagus cuniculus) Livest. Sci. 2021;251 doi: 10.3390/ani11030716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olukomaiya O., Fernando C., Mereddy R.M., Li X., Sultanbawa Y. Solid-state fermented plant protein sources in the diets of broiler chickens: a review. Anim. Nutr. 2019;5:319–330. doi: 10.1016/j.aninu.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon R.E., Froehlich D., Butler G. Effect of canola meal, fish meal, and choline plus methionine on the sensory quality of broiler chickens. Poult. Sci. 1984;63:1994–1998. [Google Scholar]
- Singhania R.R., Patel A.K., Soccol C.R., Pandey A. Recent advances in solid-state fermentation. Biochem. Eng. J. 2009;44:13–18. [Google Scholar]
- Szmigiel I., Konkol D., Korczyński M., Łukaszewicz M., Krasowska A. Changes in the microbial composition of the cecum and histomorphometric analysis of its epithelium in broilers fed with feed mixture containing fermented rapeseed meal. Microorganisms. 2021;9:360. doi: 10.3390/microorganisms9020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie Y., Li L., Liu J., Liu C., Fu J., Xiao X., Wang G., Wang J. Two-step biological approach for treatment of rapeseed meal. J. Food Sci. 2020;85:340–348. doi: 10.1111/1750-3841.15011. [DOI] [PubMed] [Google Scholar]
- Xu F.Z., Zeng X.G., Ding X.L. Effects of replacing soybean meal with fermented rapeseed meal on performance, serum biochemical variables and intestinal morphology of broilers. Asian-Australas. J. Anim. Sci. 2012;25:1734–1741. doi: 10.5713/ajas.2012.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., He S., Liu H., Sun X., Ye Y., Cao X., Wu Z., Sun H. Effect of pH regulation on the components and functional properties of proteins isolated from cold-pressed rapeseed meal through alkaline extraction and acid precipitation. Food Chem. 2020;327 doi: 10.1016/j.foodchem.2020.126998. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang H., Zhang J., Lin B., Chen L., Wang Q., Li G., Deng J. Assessment of rapeseed meal as fish meal alternative in diets for juvenile Asian red-tailed catfish (Hemibagrus wyckioides) Aquacult. Rep. 2020;18:1–8. [Google Scholar]
- Zhang Z., Wen M., Chang Y. Degradation of glucosinolates in rapeseed meal by Lactobacillus delbrueckii and Bacillus subtilis. GOST. 2020;3:70–76. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.