Abstract
In ovo corticosterone (CORT) exposure reportedly reduces growth and alters body composition traits in meat-type chickens. However, the mechanisms governing alterations in growth and body composition remain unclear but could involve myogenic stem cell commitment, and/or the presence of yolk steroid hormones. This study investigated whether in ovo CORT exposure influenced yolk steroid hormone content, as well as embryonic myogenic development in meat-type chickens. Fertile eggs were randomly divided at embryonic day (ED) 11 and administered either a control (CON; 100 µL of 10 mM PBS) or CORT solution (100 µL of 10 mM PBS containing 1 µg CORT) into the chorioallantoic membrane. Yolk samples were collected at ED 0 and ED 5. At ED 15 and hatch, embryos were humanely killed, and yolk and breast muscle (BM) samples were collected. The relative abundance of 15 steroid hormones, along with total lipid content was measured in yolk samples collected at ED 0, ED 5, ED 15, and ED 21. Muscle fiber number, cross-sectional area, and fascicle area occupied by muscle fibers were measured in BM samples collected at hatch. Relative expression of MyoD, MyoG, Pax7, PPARγ, and CEBP/β, and the sex steroid receptors were measured in BM samples collected at hatch. The administration of CORT had a limited effect on yolk steroid hormones. In ovo CORT significantly reduced fascicle area occupied by muscle fibers and CEBP/β expression was increased in CORT exposed birds at hatch. In addition, the quantity of yolk lipid was significantly reduced in CORT-treated birds. In conclusion, in ovo exposure to CORT does not appear to influence early muscle development through yolk steroid hormones in embryonic meat-type chickens however, the results provide a comprehensive analysis of the composition of yolk steroid hormones in ovo at different developmental time points. The findings may suggest increased mesenchymal stem cell commitment to the adipogenic lineage during differentiation and requires further investigation.
Key words: poultry, androgens, yolk fat, developmental programming, body composition
INTRODUCTION
The phenotypic traits displayed by offspring as they transition into adulthood largely results from a complex interaction between genetic and maternal factors encountered during embryonic development (Groothuis et al., 2019; Peixoto et al., 2020a,b; Boulton et al., 2021). These maternal factors are a result of the environment encountered by a mother at the time of gestation, or in oviparous species, during egg formation. In avian species, maternally derived differences in offspring phenotypes are likely mediated through hormonal or nutritional alterations to the composition of the egg during formation (Henriksen et al., 2011b; Angove and Forder, 2020; Boulton et al., 2021). A range of factors can influence the egg composition, including but not limited to; environmental (Sheriff et al., 2017), nutritional status (Gatford et al., 2018), and stress (Gatford et al., 2018; Angove and Forder, 2020).
Maternal stress has been the focus of a number of studies in wild birds (Love et al., 2013; Berghänel et al., 2017; Sheriff et al., 2017) and commercial poultry (Henriksen et al., 2013; Bowling et al., 2018; Peixoto et al., 2020a,b; Boulton et al., 2021). The effects of maternal stress on subsequent progeny performance however have not been extensively investigated, especially in production species. Recent studies have highlighted the impact of both maternal and embryonic stress on growth and BW in commercial poultry. For example, increased feed restriction severity in meat-type chicken breeder hens was associated with elevated maternal plasma corticosterone (CORT) concentration and reduced male progeny BW (Bowling et al., 2018). An earlier study by Ahmed et al. (2014b) found that in ovo exposure to CORT reduced BW in commercial layer hens from 3 wk of age. Although these studies highlight the effect of maternal/in ovo stress on growth in commercial poultry, the mechanisms remains unclear. Recent findings have shown that in ovo exposure to CORT at embryonic day (ED) 11 decreased total lean mass, and enhanced total fat mass, in 35-day-old female meat-type chickens (Angove et al., 2021). These findings suggest alterations to adult body composition may be achieved through manipulations of the in ovo environment during critical developmental time points.
Muscle fiber development occurs embryonically in the chicken, including determination of muscle fiber number (Smith, 1963; Halevy, 2020). Muscle fibers are derived from the commitment of multipotent progenitor stem cells to embryonic myoblasts, which then proliferate and differentiate to form multinucleated myofibers (Herbst and Bhasin, 2004; Velleman, 2007). This period of progenitor cell commitment and embryonic myoblast differentiation may be more susceptible to alterations of the in ovo environment and therefore influence posthatch body composition via a reduction or increase in muscle fiber number. Posthatch muscle growth is due to hypertrophy, however myonuclei do not undergo cellular division and require external nuclei to facilitate hypertrophy mechanisms (Moss and Leblond, 1971). The predominant source of external nuclei is from a population of adult myoblast cells, termed satellite cells (SCs), which are detectable from ED 13 (Halevy et al., 2006). The number of SC peak around 2- to 3-days posthatch but continue to proliferate and differentiate until d 7 posthatch, after which they become quiescent until required to initiate muscle growth or repair (Halevy et al., 2006). This developmental period determines the quiescent SC population available for muscle hypertrophy therefore influencing the growth potential of the animal.
The state (i.e., proliferation, differentiation, quiescent) of SC is under tight myogenic regulation, primarily from the muscle-specific basic helix-loop-helix family of transcription factors, including myoblast determination protein 1 (MyoD) and myogenin (MyoG) (Olson, 1990). In chickens, MyoD is primarily expressed in proliferating SC with peak expression at hatch, whereas MyoG expression peaks at 3-days posthatch, coinciding with SC differentiation (Halevy et al., 2004). The paired box protein Pax-7 (Pax7) gene is also documented to be involved in SC mediated skeletal muscle development, with expression levels coinciding with the presence of quiescent SC's (Halevy et al., 2004). Thus, alterations to the expression of myogenic regulator genes as a result of alterations to the in ovo environment throughout development, as demonstrated by Stern et al. (2015), may also influence posthatch body composition traits.
Glucocorticoid hormones, including CORT, reportedly regulate the differentiation of mesenchymal stem cells to the various cell lineages (Salloum et al., 2013), and the proliferation and differentiation of SC's during their active phase (Salloum et al., 2013). Additionally, glucocorticoid exposure reportedly increases expression of the adipogenic regulator genes peroxisome proliferator-activated receptor gamma (PPARγ) and CCAAT/enhancer binding protein beta (CEBP/β), both of which promote adipogenic commitment of mesenchymal stem cells (Otto and Lane, 2005). Glucocorticoids also interact with various other endocrine growth regulators, including gestagen (Henriksen et al., 2011a), estrogen (Henriksen et al., 2011a), and androgen hormones (Brown and Spencer, 2013). Sex steroids also regulate skeletal muscle development (Li et al., 2020), where they promote increased myoblast number and myofiber size (Herbst and Bhasin, 2004; Li et al., 2020) and aid in the commitment of mesenchymal stem cells to the myogenic line (Herbst and Bhasin, 2004). However, for steroid hormones to influence embryonic and posthatch muscle development, embryos must first be exposed to these hormones (Groothuis and Schwabl, 2008; von Engelhardt et al., 2009), which by nature, are highly lipophilic (Moore and Johnston, 2008). How lipophilic hormones are transferred from the lipid-rich environment of the yolk, to the water-rich environment of the embryo remains unclear. Recent evidence suggests steroids are metabolized early during embryogenesis into inactive conjugates (Kumar et al., 2019; Vassallo et al., 2019). Whether these conjugates enable the transfer of steroids into the embryo, or act as a form of storage mechanism until the absorption of yolk lipids, which occurs primarily during the later stages embryogenesis (Yadgary et al., 2010), is not known.
The impact of early life stress on yolk hormone abundance and fat content during late embryonic development and the possible relationship with musculoskeletal development is also not well understood. This study aimed to investigate the effects of in ovo CORT administration on embryonic musculoskeletal development, steroid yolk hormone abundance, and total yolk lipid content in meat-type chickens. In ovo exposure to CORT was hypothesized to decrease the relative abundance of steroid hormones and lipid content within the yolk. It was further hypothesized that in ovo exposure to CORT would inhibit myogenic development, reducing muscle fiber number (MFN) at hatch and/or the expression of myogenic regulator genes, while increasing expression of adipogenic regulatory genes.
MATERIALS AND METHODS
In Ovo Treatment
Fertile Cobb 500 parent breeder eggs (n = 550) obtained from a commercial hatchery (Baiada Hatchery, Willaston, UK) were set and incubated in accordance with industry standards (see below). In ovo administration of treatments followed the methods described by Angove et al. (2021). Briefly, eggs were randomly allocated at ED 11 to control (CON) or corticosterone (CORT) treatment groups. Prior to treatment, eggs were candled and damaged or infertile eggs were excluded. A 23-gauge needle was used to puncture a hole in the egg. A 1 mL insulin syringe was then used to administer treatment solutions into the chorioallantoic membrane, via the air cell. CORT-treated eggs (n = 255) were injected with 100 µL of 10 mM phosphate buffer saline (PBS) solution containing 1 µg of CORT dissolved in absolute ethanol, while CON-treated eggs (n = 255) received 100 µL of 10 mM PBS solution containing the same concentration of absolute ethanol. The injection site was resealed using Selleys glass and eggs were immediately returned to the incubator. A subset of eggs (n = 20) were injected with a dye to verify administration of solutions into the chorioallantoic membrane, with an accuracy index of 90%. All injections were administered by a single individual only (with significant experience in this technique) to maximize uniformity.
Animals and Tissue Collection
Prior to incubation, eggs were left at room temperature (23°C) to acclimatize for 13.5 h then placed in an incubator under the following conditions: 38°C and 55% humidity from ED 0 to ED 18 and 36.7°C and 60% humidity from ED 18 until hatching (ED 21). CON and CORT treatments were evenly and randomly represented on each tray level. Eggs were rotated 90° every hour until ED 18 then placed into hatching trays. At ED 0, a random (across incubator level) sub sample of eggs (n = 20) were opened, measured for egg quality parameters and yolk samples obtained. Whole yolks were separated from egg whites, weighed, and homogenized. Homogenate aliquots were snap frozen in liquid nitrogen and stored at −20°C until further analysis.
At ED 5, a random (across incubator level) subsample of eggs (n = 20) was opened, and the egg contents emptied into a sterile petri dish. The embryo was removed from the egg contents and weighed, after which the embryo was decapitated, with embryonic head and body samples snap frozen in liquid nitrogen and stored at −80°C. The yolk sac membrane was weighed, snap frozen in liquid nitrogen, and stored at −80°C. The remaining egg contents were placed in 50 mL falcon tubes and centrifuged at 3,000 rpm for 10 min. After centrifugation, the yolk supernatant was transferred to a clean tube, snap frozen in liquid nitrogen, and stored at −80°C.
At ED 15, 42 birds per treatment (n = 84) were removed from their eggs (sampled evenly from all incubator tray levels) and humanely killed by decapitation. Total BW was recorded, after which the liver, yolk sac, left and right breast muscles (BM) were removed and weighed. Both left and right BM sliced in half on a horizontal plane. The bottom sections were stored in 3 mL of 10% buffered formalin, and top sections placed in cryogenic tubes. A yolk sample was collected from the yolk sac after its removal from the embryo. The gender of each bird was identified to ensure equal sex distribution within treatments (n = 21 samples/treatment/sex). All samples, other than those stored in 10% buffered formalin, were snap frozen in liquid nitrogen and stored at −80°C until further analysis.
An additional 42 birds per treatment (n = 84; n = 21/treatment/sex) were humanely killed at hatch (d 0) via cervical dislocation and total BW recorded. Liver, yolk sac, yolk, left, and right BM were removed, weighed and stored as described at ED 15, along with gender identification. Additionally, a blood sample was collected immediately after euthanasia via cardiac puncture.
Yolk Hormone Extraction
Yolk samples collected at ED 0 (n = 10), ED 5 (n = 10), ED 15 (n = 40; 10 per treatment/sex), and hatch (n = 40; 10 per treatment/sex) were thawed and homogenized in an MP BIO science Quick homogenizer (FastPrep-24 5G, Santa Ana, CA) using Lysing Matrix C (MP Bio Science, #116912050-CF, Solon, OH). Following homogenization, 200 mg of yolk was combined with 2 mL of molecular grade methanol (Sigma-Aldrich, #34860, Castle Hill, New South Wales, Australia). All samples were vortexed for 60 s, snap frozen in liquid nitrogen and stored at −20°C overnight to precipitate the lipid and protein contents. The next day, frozen samples were centrifuged at 2,000 rpm for 20 min, at 4°C. The ∼2 mL supernatant was transferred to a separate clean vial and stored at −20°C. An additional 2 mL of methanol was added to the original homogenized yolk sample and the overnight process repeated. Supernatants were combined into a single vial and stored at −20°C.
Solid phase extraction (SPE) was to separate the free and conjugated hormones from the methanol extracts. Briefly, 40 mL of MilliQ water was added to a 50 mL falcon tube, after which the 4 mL methanol extract was added. The methanol extract tube was rinsed with 6 mL of MilliQ then transferred to the 50 mL falcon tube ensuring all methanol sample was transferred and vortexed thoroughly. Samples were extracted using solid phase extraction cartridges (waters SEP-PAK plus tC18 environmental SPE cartridge #WAT036800) and a vacuum pump (Rocker 400, SKU 167400-11(22), Kaohsiung City, Taiwan). SPE cartridges were conditioned using 5 mL methanol, followed by 5 mL of MilliQ water, then samples were gradually loaded into 20 mL reservoirs attached to the SPE cartridge. The eluted solvent was discarded. After the samples were loaded onto the cartridge, 5 mL of 2% methanol in MilliQ water was added to the reservoir to wash the cartridge and remove possible interfering compounds still linked to the SPE phase. The cartridge was then dried for 5 min using the vacuum pump (10 mm Hg). The free hormones were eluted from the cartridge using 5 mL of diethyl ether (Chem-Supply, #EA012, Gillman, SA, Australia) and collected in a 20 mL glass test tube. Conjugated hormones were eluted from the cartridge using 5 mL of methanol, with the eluent collected in a separate glass test tube. Following eluent collection, all samples were dried in a nitrogen evaporator (Biotage, TurboVap LV #415000, Uppsala, Sweden), with a nitrogen flow rate of 3.5 mL/min at 30°C. Samples were reconstituted with methanol (100 uL) and dichloromethane (100 uL) and vortexed for 4 s. The reconstituted samples were transferred into a 4 mL Eppendorf tube and stored at 3°C for 10 min to allow precipitation of possible proteins still present. Samples were then centrifuged for 10 min at 14,000 rpm, the supernatant collected and transferred to a HPLC vial fitted with a 200 µL glass insert. Samples were stored at 4°C until further analysis.
Liquid Chromatography-Mass Spectroscopy/Mass Spectroscopy
Liquid chromatography-mass spectroscopy/mass spectroscopy (LC-MS/MS) analyses were performed at the Metabolomics Australia/Australian Wine Research Institute using an Agilent 1290 infinity II HPLC coupled to AB Sciex 4500 QQQ instrument. Data were acquired using Multiple Reaction Monitoring (MRM) in positive ionization mode for free hormones and negative ionization mode for conjugated hormones. Briefly, the liquid chromatography (LC) separation was performed on a Phenomenex Phenyl Hexyl column (2.1 × 150 mm, 3 µm) with mobile phase A (0.1% formic acid, 0.5% methanol MilliQ water) and mobile phase B (0.1% formic acid in acetonitrile). For both free and conjugated fractions, the flow rate was 0.4 mL/min. The linear gradient was as follows: 0 min: 0% B, 5 min: 20% B, 10 min: 30% B, 15 min: 50% B, 20 min: 70% B, 25 min: 95% B, 27.5 min: 10% B, 28 min: 90% B, 28.5 min: 10% B. For the free hormone fraction, column temperature was set at 40°C, source temperature was 450°C and the injection volume was 10 µL. The curtain gas, ion source gas 1 and ion source gas 2 were 10, 40, and 50 PSI respectively with an ion spray voltage of 5,500 V. For the conjugated fraction, column temperature was set at 40°C, source temperature was 500°C and the injection volume was 10 µL. The curtain gas, ion source gas 1, and ion source gas 2 were 35, 50, and 65 PSI respectively with an ion spray voltage of 5,500 V. Mass spectrometry parameters were optimized based on 3 standards, testosterone (Sigma-Aldrich, #86500, Castle Hill, New South Wales, Australia) CORT (Sigma-Aldrich, #27840, Castle Hill, New South Wales, Australia), and etiocholanolone glucuronide (Kinesis, D607, Redland Bay, Queensland, Australia).
For the free hormone fraction analysis, 15 metabolite compounds of sex steroid targets testosterone, progesterone, and estradiol, as well as corticosterone were targeted (Table 1). Two MRM transitions “precursor ion -> product ion” were recorded for each compound, except for estrone where only 1 MRM transition was monitored. The MRM transition of “precursor ion” to “quantifier ion” was used to record compound intensity. The MRM transition of “precursor ion” to “qualifier ion” was monitored to increase the confidence of the putative identification. For the analytical reference standards of testosterone, corticosterone, and etiocholanolone glucuronide, the mass spectrometer was optimized to achieve maximum sensitivity for the compounds characteristic MRM transitions. Where pure analytical standards were not available, MRM transitions monitored were determined based on previous studies described in the literature and mass spectroscopy/mass spectroscopy spectra present in online databases (m/z Cloud). Where differing isomers of separate compounds shared a similar formula and molecular structure (11β-hydroxy-progesterone/11-deoxycorticosterone), the same transitions were monitored and could not be separated. The compounds 3α androstanediol, 3β androstanediol, 5α dihydrotestosterone, 5β dihydrotestosterone, and androsterone could not be detected at any time point using the previously described methodology and were therefore excluded from the study.
Table 1.
Multiple reaction monitoring acquisition—scan segments for target compounds.
| Compounds | Precursor ion | Quantifier ion | Qualifier ion | Polarity |
|---|---|---|---|---|
| Estrone | 271 | 253 | / | Positive |
| 17β-Estradiol | 273 | 213 | 109 | Positive |
| Androstenedione | 287 | 97 | 109 | Positive |
| Testosterone | 289 | 97 | 107 | Positive |
| 5α-Dihydrotestosterone | 291 | 255 | 273 | Positive |
| 5β-Dihydrotestosterone | 291 | 255 | 273 | Positive |
| Androsterone | 291 | 255 | 273 | Positive |
| Etiocholanolone glucuronide | 291 | 255 | 273 | Positive |
| 3α-Androstanediol | 293 | 257 | 275 | Positive |
| 3β-Androstanediol | 293 | 257 | 275 | Positive |
| Progesterone | 315 | 109 | 97 | Positive |
| Pregnonelone | 317 | 109 | 97 | Positive |
| Corticosterone | 347 | 329 | 311 | Positive |
| 11β-Hydroxyprogesterone | 331 | 295 | 109 | Positive |
| 11β-Deoxycorticosterone | 331 | 295 | 109 | Positive |
Yolk Lipid Extraction
Yolk lipids were extracted following the methods of Yadgary et al. (2010). Briefly, ED 0 (n = 10), ED 5 (n = 10), ED 15 (n = 40 per treatment/sex), and hatch (n = 40 per treatment/sex) yolk samples were thawed and homogenized in an MP BIO science Quick homogenizer using Lysing Matrix C. Approximately 250 mg of homogenized yolk was combined with 5 mL of a 2:1 vol/vol chloroform-methanol solution in a new tube and left at room temperature for 30 min. Afterward, 2 mL of Milli Q water was added to each sample, vortexed, and incubated overnight at 4°C. The next morning, the 2.5 mL bottom layer was transferred into a clean, previously weighed test tube, then oven dried at 105°C. Tubes with remaining lipid content were weighed, after which yolk lipid content was calculated.
Muscle Fiber Development
Histological evaluations were performed on 10 birds per treatment/sex (n = 40 birds) at hatch, with both left and right BM samples evaluated for each individual bird (n = 80 samples in total). Tissue specimens were dehydrated in a graded series of ethanol then orientated to ensure transverse fiber sectioning and embedded in paraffin wax. Samples were sectioned at 5 µm using a microtome (Micron, #904030, Walldorf, Germany) and stained with hematoxylin and eosin. Sections were examined by light microscopy at ×40 magnification with 5 images taken from randomly selected locations within a sample. Images were analyzed for MFN, cross-sectional area (CSA) of the muscle fiber and percentage of fascicle area occupied by muscle fibers using the image analysis software ImageJ, with a minimum of 300 muscle fibers analyzed per individual left and right BM sample.
Isolation and Quantification of Total RNA From Chicken Breast Muscle
Total RNA from n = 32 (n = 8 treatment/sex) chicken BM samples was isolated using an Rneasy Plus Universal Mini Kit (Qiagen, Hilden, Germany) in accordance with the manufacturer's instruction. Briefly, 50 mg of chicken BM tissue (combined left and right) was homogenized in 900 uL of Qiazol reagent (Invitrogen, Carlsbad, CA) using the MP BIO QuickPrep tissue homogenizer, CoolPrep Adapter and lysing matrix D (MP Bio Science, #116913050-CF, Solon, OH) under the following conditions, speed: 6.0 m/s, time: 40 s, cycles: 2 (150-s interval). Homogenates were combined with 100 uL of gDNA eliminator solution and 180 uL of chloroform (Sigma-Aldrich, #C2432, Castle Hill, NSW, Australia), then vortexed. Samples were left to stand at room temperature for 3 min, then centrifuged at 10,000 rpm for 15 min at 4°C. The upper aqueous phase (300 µL) was combined with 300 µL of 70% ethanol, mixed, and transferred onto the Rneasy columns. The on-column wash steps were performed to manufacturer's specifications. Postwash, Rneasy columns were placed into new sleeves, and centrifuged for 1 min at 14,000 rpm to remove residual wash buffer, then placed into new 2 mL collection tubes along with 50 µL of Rnase-free water and centrifuged for 1 min at 10,000 rpm. A further 50 µL of Rnase-free water was added, followed by centrifugation at 10,000 rpm for 1 min. RNA integrity was analyzed using the RNA tape station (Agilent Technologies 2200, Santa Clara, CA), with all RNA integrity numbers (RIN) ≥8.0. Sample purity and concentration were determined using UV spectrophotometry (NanoDrop 2000, ThermoScientific, Waltham, MA). Pure RNA samples were stored at −80°C until further analysis.
cDNA Synthesis
RNA concentrations from chicken BM samples were normalized to 200 ng/µL using a liquid handling robotics system (EpMotion %075; Eppendorf, Hamburg, Germany). A high-capacity RNA-to-cDNA kit (#4387406, Applied Biosystems, Carlsbad, CA) was used to synthesize cDNA in accordance with the manufacture's specifications. Reactions were incubated for 60 min at 37°C, followed by a 5-min incubation period at 95°C to inactivate reverse transcriptase. Incubation steps were carried out using a thermomixer (Thermomixer C; Eppendorf, Hamburg, Germany). Reactions were then cooled to 4°C and placed on ice. Stock cDNA (1:4) dilutions were prepared in TE buffer (#12090-015, Applied Biosystems, Carlsbad, CA) and stored at −80°C until further analysis.
Real-Time Quantitative PCR
Primer sequences and references are presented in Table 2. A master mix was created before each assay, consisting of SYBR reagent 2× power (Applied Biosystems, #4367659, Warrington, UK) and both forward and reverse oligonucleotides. Stock cDNA (1:4) was diluted 5-fold to 1:20 with TE buffer (#12090-015, Carlsbad, CA) using the above-mentioned robotics system. Diluted (1:20) cDNA (6 µL) was combined with 19 µL of master mix, after which the cDNA/SYBR mixture (5 µL) was transferred in triplicate to a 384-well MicroAmp Plate (Applied Biosystems, Carlsbad, CA). A total of n = 32 cDNA preparations were examined along with a 5-point standard curve (1:8, 1:32, 1:128, 1:512, 1:2048 dilutions of 8 pooled cDNA samples and TE buffer blank). Quantitative PCR (qPCR) was performed using a real-time PCR Machine (Quant Studio 6 Flex, Applied Biosystems, Carlsbad, CA) under the following conditions: 95°C/10 min for 1 cycle, 95°C/15 s, 60°C/20 s and 72°C/40 s for 40 cycles and 95°C/15 s, 60°C/15 s and 95°C/15 s for melt curve data acquisition. Quant studio Real-time PCR software was used to process the data. Tata-binding protein (TBP), β-actin (ACTH), and glyceraldehyde-3-phoshate dehydrogenase (GAPDH) were chosen as reference genes. NormFinder (Andersen et al., 2004) was used to identify the optimal normalization gene, with TBP identified as most stable. The Pflaffl method (Pfaffl, 2001) for relative quantification and normalization to reference genes was used to determine the relative expression of Pax7, MyoD, MyoG, androgen receptor (AR), estrogen receptor alpha (ER-a), progesterone receptor (PR), PPARγ, and CEBP/β, see Table 2 for primers.
Table 2.
Primers used for real-time quantitative PCR.
| Target gene | Primer sequence (5′–3′) | Amplicon length (bp) | Reference | Accession number |
|---|---|---|---|---|
| Reference genes | ||||
| GAPDH | F: CAACCCCCAATGTCTCTGTT R: TCAGCAGCAGCCTTCACTAC |
94 | Gilani et al., 2018 | NM_204305 |
| TBP | F: GTCCACGGTGAATCTTGGTT R: GCGCAGTAGTACGTGGTTCTC |
128 | Gilani et al., 2018 | NM_205103 |
| β-actin | F: GAGAAATTGTGCGTGACATCA R: CCTGAACCTCTCATTGCCA |
152 | Liu et al., 2015 | NM_205518 |
| Steroid receptors | ||||
| AR | F: AAGAAGCTGGGCAGTCTGAAR: AGCAGGTTGGAGAAGGAGTC | 214 | Li et al., 2020 | NM_001040090 |
| ERα | F: TATTGATGATCGGCTTAGTCTGGCR: CGAGCAGCAGTAGCCAGTAGCA | 145 | Liu et al., 2015 | NM_205183.2 |
| PR | F: ATCATCGTTCTATTCACTGT R: CTCGTTCTCATCTCATCAA |
168 | Liu et al., 2015 | NM_205262.1 |
| Satellite cell development genes | ||||
| Pax7 | F: AGTTCGATTAGCCGTGTGCT R: CTCTTCAAAGGCAGGTCTGG |
185 | Li et al., 2020 | NM_205065 |
| MyoD | F: ATGTCCCATACTGCCTCCAG R: GTCTTGGAGCTTGGCTGAAC |
235 | Li et al., 2020 | NM_204214 |
| MyoG | F: GGCTTTGGAGGAGAAGGACT R: CAGAGTGCTGCGTTTCAGAG |
N.P | Cazzato et al., 2014 |
NM_204184 |
| Adipocyte differentiation genes | ||||
| PPARγ | F: CCACTGCAGGAACAGAACAA R: CTCCCGTGTCATGAATCCTT |
249 | Piestun et al., 2017 | NM_001001460.1 |
| CEBP/β | F: GCCGCCCGCCTTTAAA R: CCAAACAGTCCGCCTCGTAA |
N.P | Zhang et al., 2015 | NM_205253.2 |
N.P, not provided.
Statistical Analysis
The experimental design was a completely randomized 2 × 2 factorial design for the factors of sex and treatment (CON and CORT). Data were tested for normality using the Shapiro-Wilk test. Total MFN, CSA, and muscle fiber (% of fascicle area) data from both left and right BM sections were combined and analyzed as a whole. Yolk hormone relative abundance was analyzed for sex × treatment at ED15 and hatch only (sex and treatment were unknown and unapplied at ED0 and ED5) ED 15 17β-estradiol, ED 15 and hatch 11β-hydroxy-progesterone/11-deoxycorticosterone and hatch etiocholanolone relative abundance data were log transformed to meet the Shapiro-Wilk test requirements for normality. Normalized mRNA expression values were used in the analysis of gene expression data. Normally distributed data were processed using a full factorial univariate analysis with sex and treatment included as factors. Additionally, dissector was added as a random factor to ensure no dissector bias was present. A generalized linear model pairwise comparison was used to separate means between treatment groups where statistical significance was identified using the Fisher's least significant difference (LSD) adjustment for multiple comparisons. Data sets that did not meet the Shapiro-Wilk test requirements for normality were subjected to nonparametric Mann-Whitney U and Kruskal-Wallis tests. A nonparametric pairwise comparison was used to separate statistically significant means from nonparametric analyses. The Pearson's coefficient for normalized data and Spearman's coefficient for non-normalized data were utilized for correlation analyses. All data were processed through IBM, SPSS Statistics 25 (Armonk, NY), where a less than 5% (P < 0.05) probability was deemed statistically significant.
RESULTS
Embryonic Growth
A treatment × sex interaction was identified at ED 15 (P = 0.040), whereby BW was increased in CORT males compared to CORT females (Table 3); however, this effect was not detected at hatch (P = 0.850). There were no differences between sexes exposed to CORT or CON treatments.
Table 3.
Male (n = 42) and female (n = 42) body weight (BW, g), liver (% BW), and yolk Sac (% BW) at embryonic day (ED) 15 (A) and hatch (B) in meat-type chickens exposed to CON or CORT administered via injection into the chorioallantoic membrane at ED 11.
| ED 15 |
Hatch |
||||
|---|---|---|---|---|---|
| Body and organ weights | Treatment | Male | Female | Male | Female |
| BW (g) | CON | 16.24 ± 0.47ab | 16.91 ± 0.39ab | 49.68 ± 0.79 | 48.40 ± 0.69 |
| CORT | 17.15 ± 0.36a | 16.12 ± 0.38b | 50.20 ± 0.71 | 49.22 ± 0.98 | |
| P value | Treatment | P = 0.883 | P = 0.436 | ||
| Sex | P = 0.664 | P = 0.197 | |||
| Sex × treatment | P = 0.040 | P = 0.850 | |||
| Liver % BW | CON | 2.13 ± 0.06 | 2.06 ± 0.06 | 2.37 ± 0.06 | 2.54 ± 0.07 |
| CORT | 2.03 ± 0.5 | 2.06 ± 0.05 | 2.43 ± 0.07 | 2.41 ± 0.06 | |
| P value | Treatment | P = 0.446 | P = 0.671 | ||
| Sex | P = 0.729 | P = 0.288 | |||
| Sex × treatment | P = 0.386 | P = 0.149 | |||
| Yolk sac % BW | CON | 97.75 ± 6.68a | 88.83 ± 6.40a | 13.41 ± 0.57 | 13.28 ± 0.55 |
| CORT | 115.65 ± 6.28b | 109.87 ± 6.28b | 13.78 ± 0.55 | 13.71 ± 0.67 | |
| P value | Treatment | P = 0.002 | P = 0.498 | ||
| Sex | P = 0.221 | P = 0.876 | |||
| Sex × treatment | P = 0.793 | P = 0.962 | |||
Values are mean ± SEM.
Means with different superscripts are significantly different between sex and treatment factors at the corresponding age.
No differences in egg weight were recorded at ED 15 between treatments (P = 0.345) or sex (P = 0.992), nor was any interaction identified (P = 0.086). Yolk sac weight (% BW) at ED 15 was greater in CORT-treated birds (P = 0.002) (Table 3), irrespective of sex (P = 0.221). No sex by treatment interaction was identified for yolk sac weight at hatch (P = 0.793), nor did treatment (P = 0.498) or sex (P = 0.876) influence yolk sac weight (% BW) at hatch. A positive correlation was identified between yolk sac weight and hatch BW (r = 0.644, P < 0.001).
Liver % BW did not vary between CON and CORT treatments at both ED 15 (P = 0.446) and hatch (P = 0.671), or between sexes (ED 15: P = 0.729, hatch: P = 0.288) and no interaction between sex and treatment was observed at either time points (ED 15: P = 0.386, hatch: P = 0.149).
Yolk Hormone Relative Abundance
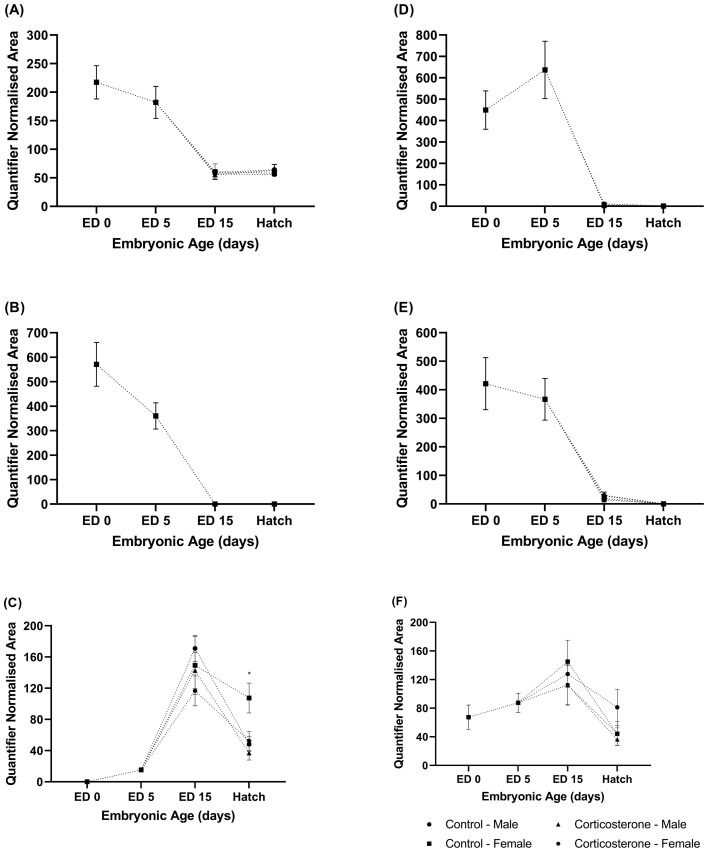
Testosterone, Androstenedione, Etiocholanolone Glucuronide. The relative abundance of testosterone (Figure 1A) and androstenedione (Figure 1B) within the yolk was highest at ED 0, with similar abundance levels detected at ED 5. At ED 15, the relative abundance of testosterone declined, and detection levels remained consistent between ED 15 and hatch. It should be noted that testosterone was detected in n = 28 of a possible 40 samples at ED 15, and n = 27 of a possible 40 samples at hatch. Androstenedione was undetected at ED 15 and detected in n = 1 sample at hatch. No sex by treatment interaction was identified for testosterone at ED 15 (P = 0.890), and no differences were identified for treatment (P = 0.467) or sex (P = 0.856). Furthermore, no 2-way interaction existed for treatment and sex at hatch (P = 0.833), and no difference in testosterone abundance was identified for treatment (P = 0.659) or sex (P = 0.111) at hatch. Androstenedione was undetectable in the yolk at ED 15 and was only detected in 1 sample at hatch and therefore excluded from analyses.
Figure 1.
The relative abundance of (A) testosterone, (B) androstenedione, (C) etiocholanolone, (D) progesterone, (E) pregnonelone, and (F) 11β-hydroxy-progesterone/11-deoxycorticosterone in the yolk of commercial male and female meat-type chickens at embryonic day (ED) 0 (n = 10), ED 5 (n = 10), ED 15 (n = 40), and hatch (n = 40). Embryos were exposed to a CON or CORT solution via the chorioallantoic membrane at ED 11. Values are mean ± SEM. Quantified normalized area refers to the integrals of the area under the chromatographic peak. * Indicates statistical (P < 0.05) significance.
Etiocholanolone glucuronide was undetected in the yolk at ED 0 and was detected in n = 7 samples at ED 5. Etiocholanolone glucuronide abundance peaked at ED 15, after which abundance levels tended to decline by hatch (Figure 1C). At ED 15, no sex by treatment interaction was identified (P = 0.931), nor were any differences present for treatment (P = 0.232) or sex (P = 0.346). At hatch (ED 21), a sex by treatment interaction was identified (P = 0.021), where yolk etiocholanolone glucuronide abundance was significantly increased in CON females, compared to CORT males.
Progesterone, Pregnonelone, and 11β-Hydroxy-progesterone/11-Deoxycorticosterone. The relative abundance of progesterone (Figure 1D) and pregnonelone (Figure 1E) within the yolk were highest at ED 5 and ED 0, respectively, after which levels declined at both ED 15 and hatch. Progesterone was detected in n = 34 samples at ED 15, and n = 16 samples at hatch and pregnonelone was detected in n = 24 of a possible 40 samples at ED 15, and n = 3 of 40 samples at hatch. Analyses for ED 15 did not identify any sex by treatment interactions for progesterone (P = 0.501) or pregnonelone (P = 0.984), or between treatment (progesterone: P = 0.740, pregnonelone: P = 0.928) or sex (progesterone: P = 0.176, pregnonelone: P = 0.925). Statistical analyses could not be performed for the factors of treatment and sex at the hatch time point due to an insufficient sample size.
11β-Hydroxy-progesterone/11-deoxycorticosterone relative abundance levels peaked at the ED 15, after which relative abundance levels appeared to decline up until hatch (Figure 1F). At ED 15, or sex by treatment interaction was identified (P = 0.997), or differences between sex (P = 0.979) or treatment (P = 0.200). Furthermore, no sex by treatment interaction was identified at hatch (P = 0.564), or sex (P = 0.783), or treatment (P = 0.142).
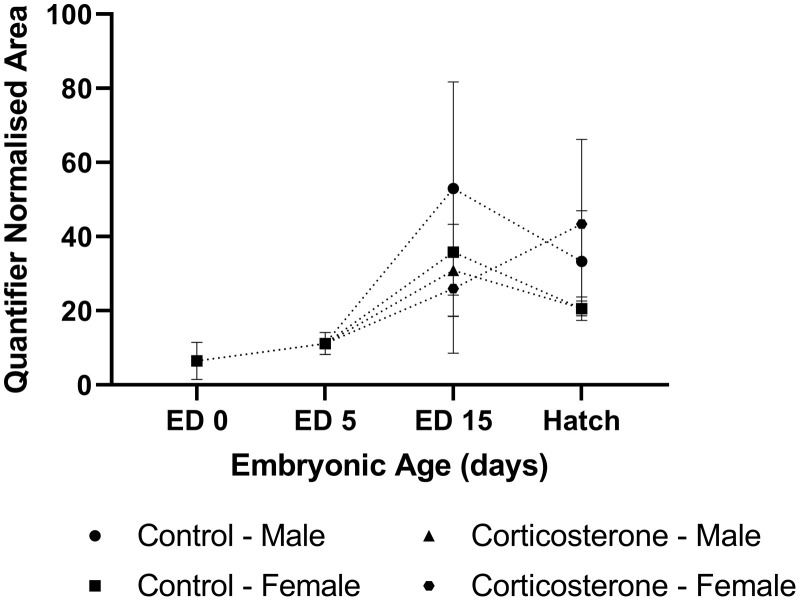
Corticosterone. The relative abundance of CORT within the yolk was detected in n = 2 samples at ED 0, and n = 6 samples at ED 5. The relative abundance of CORT peaked at ED 15; however significant variability existed between treatments (Figure 2). CORT relative abundance appeared to decline between ED 15 and hatch, although variability within treatment groups was again observed at hatch. No interaction of sex and treatment was identified at ED 15 (P = 0.766) and hatch (P = 0.941), nor was treatment (ED 15: P = 0.612, hatch: P = 0.790) or sex (ED 15: P = 0.351, hatch: P = 0.900) effects identified at either time points.
Figure 2.
The relative abundance of corticosterone in the yolk of commercial male and female meat-type chickens at embryonic day (ED) 0 (n = 10), ED 5 (n = 10), ED 15 (n = 40), and hatch (n = 40). Embryos were exposed to a CON or CORT solution via the chorioallantoic membrane at ED11. Values are mean ± SEM. Quantified normalized area refers to the integrals of the area under the chromatographic peak.
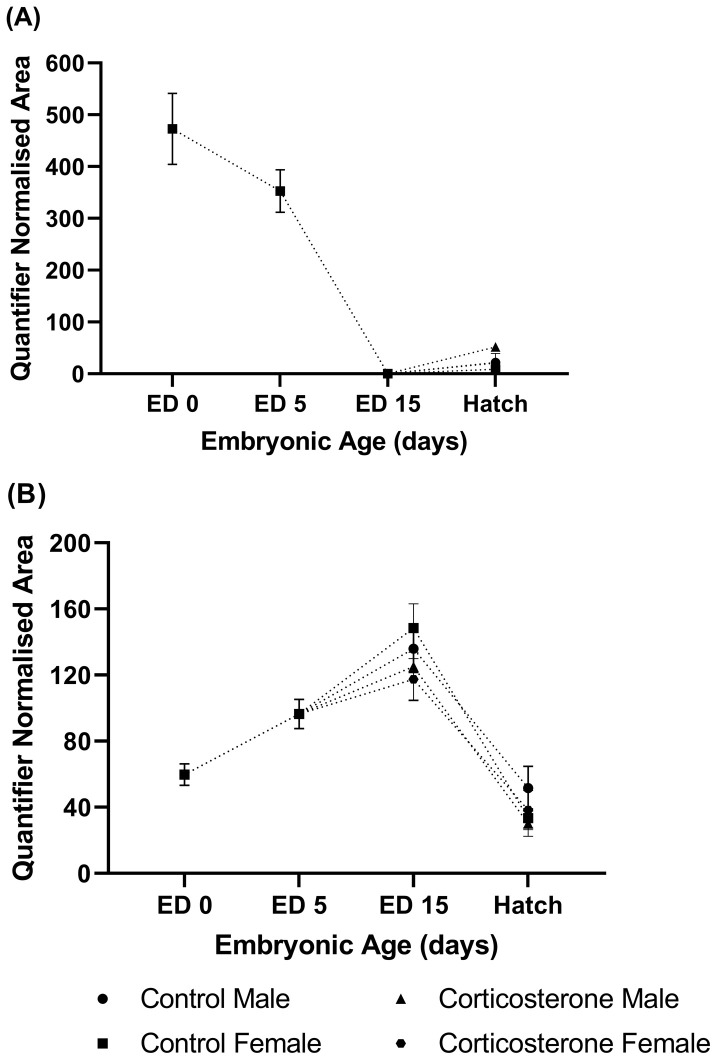
17β-Estradiol and Estrone. The relative abundance of estrone in the yolk was highest at ED 0 and declined throughout the course of embryonic develop (Figure 3A). Estrone was undetectable at ED 15 and detected in n = 8 samples at hatch and therefore could not be analyzed for treatment or sex effects.
Figure 3.
The relative abundance of (A) estrone, and (B) 17β-estradiol in the yolk of commercial male and female meat-type chickens at embryonic day (ED) 0 (n = 10), ED 5 (n = 10), ED 15 (n = 40) and hatch (n = 40). Embryos were exposed to a CON or CORT solution via the chorioallantoic membrane at ED 11. Values are mean ± SEM. Quantified normalized area refers to the integrals of the area under the chromatographic peak.
The relative abundance of 17β-Estradiol in yolk appeared to increase between ED 0 and ED 15, with abundance levels peaking at the ED 15 (Figure 3B). After ED 15, the relative abundance of 17β-estradiol declined, with 17β-estradiol abundance being lowest at the point of hatch. At ED 15, no sex by treatment interaction was identified (P = 0.411), nor were sex (P = 0.966), or treatment (P = 0.185) effects observed. Similarly at hatch, no sex by treatment interaction was observed (P = 0.494), along with no sex (P = 0.892) or treatment (P = 0.203) effects. The relative abundance of 17β-estradiol in the yolk positively correlated with the relative abundance of 11β-hydroxy-progesterone/11-deoxycorticosterone in the yolk at hatch (r = 0.929, P < 0.001).
Embryonic Musculoskeletal Development
MFN at hatch did not differ between CON (2.95 ± 0.07 × 104 fibers per mm2 fascicle area) and CORT (2.98 ± 0.07 × 104 fibers per mm2 fascicle area) treated birds (P = 0.774), or between sexes (P = 0.672) (male: 2.99 ± 0.07 × 104 fibers per mm2 fascicle area, Female: 2.95 ± 0.07 × 104 fibers per mm2 fascicle area). No interaction was identified between sex and treatment in relation to MFN. Average muscle fiber CSA followed a similar trend with no difference identified between CON (36.81 ± 0.75 µm2) and CORT (37.06 ± 0.79 µm2) treated birds (P = 0.826), or between male (36.52 ± 0.81 µm2) and female (37.27 ± 0.74 µm2) birds (P = 0.459). There was no 2-way interaction between treatment and sex (P = 0.527). A positive correlation was identified between CSA and yolk sac weight at hatch (r = 0.304, P = 0.051). Additionally, a negative correlation was present between MFN and CSA (r = −0.941, P < 0.001). The percentage of fascicle area occupied by muscle fibers was significantly greater in CON-treated birds (54.61 ± 0.31%) compared to CORT-treated birds (52.59 ± 0.28%) (P < 0.001). No sex differences were observed (P = 0.443), and no interaction for sex and treatment was identified (P = 0.269). A negative correlation was identified between MFN and the percentage of total fascicle area occupied by muscle fibers (r = −0.318, P = 0.040).
Yolk Fat
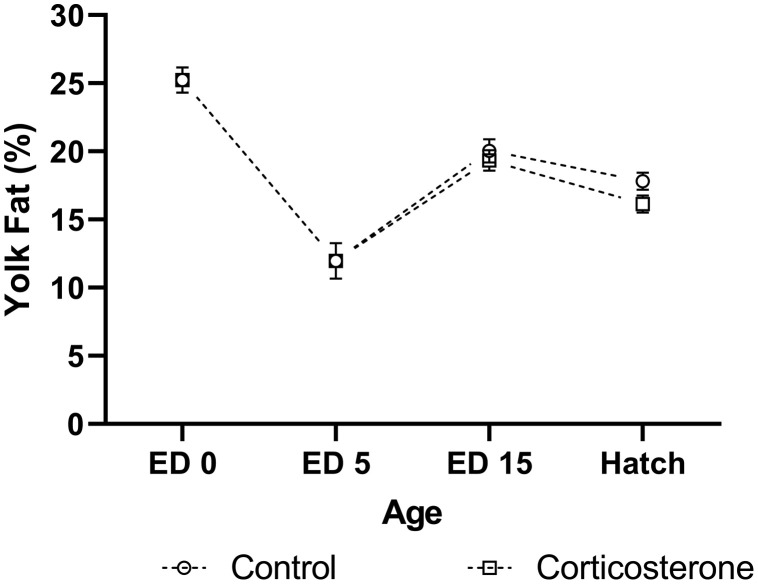
Baseline yolk fat percentage was 25.23 ± 0.92 at ED 0, and 11.96 ± 1.31 at ED 5 (Figure 4). At ED 15, yolk fat percentage did not differ between treatments (P = 0.479). The difference in yolk fat percentage between sexes approached significance at ED 15 (P = 0.058), with fat percentage increased in male birds. No 2-way interactions were identified at ED 15 (P = 0.566). At hatch, yolk fat percentage appeared to increase in CON exposed birds compared to CORT exposed birds (P = 0.062). No difference in yolk fat percentage was identified between sexes at hatch (P = 0.138), nor was a sex × treatment interaction present (P = 0.642). A negative correlation was identified between yolk fat content at hatch, and hatch BW (r = −0.364, P = 0.021).
Figure 4.
Percentage of fat content within the yolk of meat-type chickens at embryonic day (ED) 0 (n = 10), ED 5 (n = 10), ED 15 (n = 40), and hatch (n = 40). Embryos were exposed to a CON or CORT solution at embryonic ED 11 via the chorioallantoic membrane. Values are mean ± SEM.
Relative mRNA Expression
The relative expression of CEBP/β was increased in CORT birds (P = 0.040; Table 4), however no sex effect (P = 0.551) or significant interaction was identified (P = 0.186). No interaction between sex and in ovo treatment was observed for PPARγ (P = 0.491), however, relative expression tended to increase in CON females, compared to CORT females, and expression levels tended to increase in CORT males compared to CON males. A negative correlation was identified between PPARγ relative expression and CSA (r = −0.419, P = 0.019). Additionally, no significant differences were observed between treatments (P = 0.809) or sexes (P = 0.724).
Table 4.
Relative mRNA expression of the CEBP/β, MyoD, MyoG, Pax7, PPARγ, ER-α, AR, and PR gene in the breast muscle of chicken meat birds exposed to a CON or CORT solution at embryonic d 11.
| Treatment |
|||
|---|---|---|---|
| Gene | Sex | CON | CORT |
| PR | Male | 1.26 ± 0.17 | 1.03 ± 0.18 |
| Female | 1.07 ± 0.16 | 1.20 ± 0.27 | |
| AR | Male | 1.15 ± 0.10 | 1.13 ± 0.13 |
| Female | 1.07 ± 0.15 | 1.54 ± 0.23 | |
| ER-α | Male | 1.14 ± 0.14 | 1.06 ± 0.12 |
| Female | 1.17 ± 0.25 | 1.50 ± 0.19 | |
| MyoD | Male | 1.02 ± 0.05 | 0.96 ± 0.07 |
| Female | 1.03 ± 0.09 | 1.12 ± 0.07 | |
| MyoG | Male | 1.07 ± 0.11 | 1.17 ± 0.12 |
| Female | 1.04 ± 0.10 | 1.15 ± 0.15 | |
| Pax-7 | Male | 1.07 ± 0.04 | 1.02 ± 0.04 |
| Female | 1.02 ± 0.08 | 1.15 ± 0.07 | |
| PPARγ | Male | 0.82 ± 0.08 | 0.96 ± 0.18 |
| Female | 1.06 ± 0.14 | 0.79 ± 0.04 | |
| CEBP/β | Male | 1.25 ± 0.11a | 1.40 ± 0.18b |
| Female | 1.11 ± 0.19a | 1.76 ± 0.24b | |
Samples consist of pooled left and right breast muscle, which were obtained from birds at hatch (n = 32). Values are normalized average relative mRNA expression ± SEM.
Means with different superscripts are significantly different between treatments groups (CON/CORT).
The relative expression in chicken BM of AR, PR, and ER-α did not differ between in ovo treatment (P > 0.05), or sex (P > 0.05) at hatch. However, AR and ER-α relative mRNA expression was slightly elevated in CORT female birds, although not statistically significant. Additionally, no 2-way interactions for the factors of sex and in ovo treatment were identified (P > 0.05).
No in ovo treatment effects were identified for the relative expression of MyoD (P = 0.845), MyoG (P = 0.351), and Pax7 (P = 0.580). Additionally, sex did not affect the relative expression of MyoD (P = 0.278), MyoG (P = 0.835), and Pax7 (P = 0.505), nor were any interactions observed (MyoD: P = 0.333, MyoG: P = 0.967, Pax7: P = 0.141, Table 4).
DISCUSSION
The effects of maternal or in ovo stress in production poultry have not been investigated to the same extent as that of wild birds. However, such studies are relevant given that meat-type chicken breeder birds are exposed to various stressors throughout the production cycle (De Jong and Guemene, 2011). Work investigating the effects of embryonic stress in commercial poultry have identified altered growth rate (Bowling et al., 2018; Peixoto et al., 2020b), body weight (Gholami et al., 2017; Peixoto et al., 2020b), and more recently, body composition at market weight (Angove et al., 2021). The mechanisms regulating these phenotypic alterations in directly exposed progeny, or progeny hatched from exposed hens remains unknown, but are of importance to determine how the in ovo environment can influence progeny performance. The current study aimed to investigate whether in ovo exposure to CORT would alter embryonic muscle development, and whether this correlated with differences in yolk contents of steroid hormones and/or lipid content.
Exposure to CORT at ED 11 did not appear to influence embryonic muscle development with no differences in MFN or CSA detected. This is somewhat surprising, as exposure to high doses of glucocorticoids (10−6 M), such as CORT, is documented to inhibit the proliferation and differentiation of multipotent stem cells (Salloum et al., 2013), both of which are important determinants of MFN (Du et al., 2013). Additionally, increased glucocorticoid exposure tends to promote the commitment of multipotent stems cells to adipogenic (Campbell et al., 2011), osteogenic (Kerachian et al., 2009), and chondrogenic lineages (Salloum et al., 2013). Considering the present study utilized an “extreme” model of in ovo stress (Ahmed et al., 2014b), it was expected that exposure to CORT would reduce total MFN in CORT-treated birds due to the commitment of multipotent stem cells to nonmyogenic lineages (Salloum et al., 2013).
Evidence suggests multiple myogenic populations exist within developing muscles, with these populations termed, embryonic, fetal, and adult (Halevy et al., 2006). Embryonic myoblasts are most abundant in the chick at ED 5, and initiate the formation of the limb bud, which provides a source of mesoderm cells which eventually differentiate into myogenic cells and subsequent primary fibers (Stockdale, 1992). Additionally, fetal myoblasts appear most abundant in the chick between ED 8 and ED 12 and form secondary fibers which become dominant in growing muscles (Stockdale, 1992). Adult myoblasts, SC, first appear between ED 13 and ED 16, however don't peak until 2 to 3-days posthatch, after which they become quiescent until required for posthatch muscular repair and growth (Hartley et al., 1992). As the embryos in the present study were not exposed to CORT until ED 11, embryonic and fetal myoblast cell populations are likely to be set by this stage, and cell lineage redirection during the later stages of embryogenesis may therefore not be possible. This would in part also explain the finding that exposure to CORT did not alter the relative mRNA expression of any of the myogenic regulatory genes measured at hatch in this study. The possibility that muscle fiber CSA could differ between CON- and CORT-treated birds during the growth phase posthatch as result of muscular hypertrophy remains, as muscle fiber CSA is expected to increase posthatch in meat-type chickens (Halevy et al., 2006). The process of muscle hypertrophy, and a subsequent increase in fiber CSA, influenced by embryonic exposure to endogenous glucocorticoids has been previously documented (Baehr et al., 2011; Braun and Marks, 2015).
Despite CORT exposure having no apparent influence on MFN and fiber CSA, a significant difference was identified in the percentage of fascicle area occupied by muscle fibers, which was reduced in CORT-treated birds. The area may be represented by another tissue type, such as adipose tissue, as exposure to high CORT doses has been shown to redirect mesenchymal stem cells to the adipogenic line (Salloum et al., 2013). The intramuscular adipocyte numbers were not measured in the present study but have been identified in separate studies where intramuscular triglyceride content was increased in response to CORT (Campbell et al., 2011). The significant increase in CEBP/β relative mRNA expression in CORT-treated birds in the current study suggests a potential upregulation of adipogenic processes at the point of hatch. CEBP/β is documented to promote the expression of PPARγ, the primary regulator of adipocyte differentiation (Wang et al., 2017). PPARγ expression did not differ in the present study, which may suggest the onset of adipocyte differentiation at hatch, and thus PPARγ expression could be upregulated at a later time point, where PPARγ expression tends to increase with age (Sato et al., 2004). Additionally, differences in adipogenic activity could be irrespective of PPARγ regulation, as CEBP/β is documented to interact with CEBP/α, another primary regulator involved in adipogenesis (Wang et al., 2017). Whether CEBP/β expression is influenced by glucocorticoid exposure has not been extensively investigated in the chicken, although the results from this study suggest the potential for such an interaction in the BM of birds exposed to in ovo CORT. Furthermore, the phenotypic effects of in ovo CORT may not be a direct effect of CORT itself, but instead a result of CORT's documented ability to influence the synthesis and metabolism of separate endocrine factors (Tilbrook et al., 2000; Henriksen et al., 2011a; Natt et al., 2015).
In avians, the likely source of nonendogenous endocrine factors that influence early development is the yolk (Henriksen et al., 2011b; Angove and Forder, 2020). Interactions between maternal/in ovo stress, yolk hormones, and subsequent progeny phenotype have been previously identified (Henriksen et al., 2011a; Ahmed et al., 2014a; Bowling et al., 2018). Most studies have focused on interactions between glucocorticoids and sex steroids, such as the androgens, gestagens, and oestrogens (Rettenbacher et al., 2009; von Engelhardt et al., 2009; Henriksen et al., 2011a), due to the phenotypic differences identified in response to altered sex steroid concentrations in the yolk (Paitz and Bowden, 2008; Rettenbacher et al., 2009; Benowitz-Fredericks and Hodge, 2013; Giraudeau et al., 2017).
In the current study, exposure to CORT at ED 11 had minimal influence on the relative abundance of the various hormones in the yolk. The noted rapid reduction in testosterone and androstenedione abundance levels from ED 0 until ED 15 coincides with an increase in the relative abundance of etiocholanolone glucuronide in the yolk. Etiocholanolone glucuronide is the conjugate metabolite of the 5β-reductase metabolic pathway (Paitz et al., 2011; Kumar et al., 2019), and is thought to be the inactivation pathway of androgenic hormones (Balthazart et al., 1990). Although this study cannot conclude testosterone and androstenedione were metabolized to etiocholanolone glucuronide, previous work identified that androgens are rapidly metabolized to etiocholanolone glucuronide during the early stage of embryonic development in birds (Paitz et al., 2011; Kumar et al., 2019). Furthermore, our study suggests a potential sex difference in either absorption or clearance rates of etiocholanolone glucuronide from the yolk, as male birds had a faster decline in yolk compared to females and may explain why etiocholanolone glucuronide was significantly increased in CON female birds at hatch compared to other treatments. Although posthatch phenotypes were not assessed, differences in yolk absorption/metabolism of etiocholanolone glucuronide could explain the previously documented sex differences in progeny phenotypes as influenced by the maternal and/or in ovo environment (Haussmann et al., 2012; Chang et al., 2016; Bowling et al., 2018). Whether etiocholanolone glucuronide can induce phenotypic differences in progeny is unclear, although Campbell et al. (2020) identified no effect of embryonic exposure to free etiocholanolone in European Starlings. Embryos instead may metabolize androgens to etiocholanolone glucuronide to regulate their exposure to maternally sourced hormones (Paitz et al., 2011; Groothuis et al., 2019). Conversion to etiocholanolone glucuronide may also be a storage method, as glucuronides are less lipophilic, initiating uptake by the developing embryo (Paitz et al., 2011). Such absorption could initiate reconversion back to more potent androgens (Kumar et al., 2019), although no work has identified such a pathway exists within the avian embryo.
The estrogenic and gestagen hormones measured in the present study followed a similar metabolic pathway. The reduction in relative abundance levels of estrone throughout embryonic development coincided with an increase in the relative abundance of 17β-estradiol. Whether estrone is metabolized to 17β-estradiol within the yolk cannot be concluded from this study, although estrone and 17β-estradiol are interconvertible (Moghrabi et al., 1997). Previous work suggests 17β-estradiol is converted to estrone, followed by conjugation to estrone sulfonate and glucuronide (Paitz et al., 2020), a similar metabolic fate to the androgens. Further work is required to understand the metabolic fate of yolk oestrogens, as well as whether phenotypic variations eventuate in exposed progeny, or whether 17β-estradiol conjugates are converted back to active forms.
Reductions in the relative abundance of progesterone and pregnonelone coincided with an increase in abundance of 11-deoxycorticosterone/11β-hydroxyprogesterone, which again follows an inactivation metabolic pathway. Interestingly, the relative abundance of CORT in the yolk followed a similar pattern to 11-deoxycorticosterone/11β-hydroxyprogesterone, where both hormones act as precursors to CORT (Payne and Hales, 2004). CORT was almost undetectable in the yolk at ED 0 and ED 5, however was detected in all samples at ED 15 and hatch. Whether CORT within the yolk at ED 15 and hatch is synthesized from 11-deoxycorticosterone/11β-hydroxyprogesterone sourced from the metabolism of progesterone deposited in the yolk by the hen is not known. However, recent work suggests yolk progesterone is instead converted to the conjugate 5β-preganedione, a similar metabolic pathway to that of the androgen hormones (Paitz and Cagney, 2019). Alternatively, plasma CORT concentrations spike in the avian embryo at ED 15 and hatch (Jenkins and Porter, 2004; Angove and Forder, 2020). This coincides with the onset of a functional hypothalamic pituitary adrenal axis at ED 15 (Jenkins and Porter, 2004), and the stressors associated with the hatch process during late embryonic development (Jenkins and Porter, 2004). Whether the avian embryo is able to transfer hormones from the plasma to the yolk is not clear, although provides an alternative explanation for the presence of yolk CORT in the later stages of embryogenesis. In fact, it is still not clear how, and when, developing embryos are exposed to steroid hormones contained within the lipophilic environment of the yolk.
Steroid hormones are highly lipophilic and therefore unlikely to actively transfer from the yolk to the water-rich environment of the embryo. As mentioned, conversion of free hormones to metabolic conjugates may facilitate absorption of lipophilic hormones by the embryo (Paitz et al., 2011). Alternatively, conjugation of free hormones could provide a storage mechanism until the embryo begins to absorb yolk lipids. Yolk fat absorption is minimal through the first 2 wk of embryogenesis, and peaks in the final days of development (Yadgary et al., 2010). Thus, lipophilic steroid hormones may be absorbed during the period of peak fat utilization. Interestingly, our results suggest yolk fat content is reduced in CORT-treated birds at hatch, suggesting a greater fat absorption rate in these birds. To the best of our knowledge, no work has investigated whether early-life stress affects yolk fat absorption. A study by Mikec et al. (2006) did identify differences in yolk reabsorption rates in response to environmental stressors during the first 5-days posthatch, a potential reflection of bird energy requirements in response to early life stress. Energy requirements tend to increase during periods of stress (Duan et al., 2014), and so reductions in yolk fat could indicate a survival mechanism in response to early-life stress (Henriksen et al., 2011b). This is supported by the lack of BW variation at hatch between CON and CORT-treated birds. Similar results have been recorded in progeny exposed to CORT throughout embryonic development, with alterations in BW and body composition identified in adult birds (Ahmed et al., 2014b; Angove et al., 2021). Additionally, yolk sac weight was increased in CORT-treated birds at ED 15 and to a lesser extent, at hatch. This coupled with the reductions in yolk fat % in CORT-treated birds at ED 15 suggests that embryos exposed to CORT might prioritize fat absorption more so than CON birds through the later stages of development, again as a short-term survival mechanism. This is further supported by the negative correlation identified between yolk lipid content at hatch and hatch BW, as well as the positive correlation identified between hatch yolk sac weight (% BW) and hatch BW. The effects of altered yolk fat absorption during embryonic development are not well understood, but likely contribute to the posthatch phenotypes subsequently displayed in adult birds across various studies in response to alterations to the in ovo environment. This is because the period between ED 13- and 7-days posthatch, in which the majority of yolk fat is absorbed, coincides with the proliferation and differentiation of multipotent stem cells into adult myoblasts (Halevy et al., 2006). Yolk fat is potentially an energy source for this process, while also having potential to store and transport lipophilic hormones, exposing developing embryos to important endocrine factors during this developmental period.
CONCLUSIONS
The findings from this study suggest exposure to 1 µg of in 100 µL of PBS at ED 11 does not influence early musculoskeletal development in commercial chicken meat birds. Instead, early-life exposure to CORT may increase mesenchymal stem cells commitment to the adipogenic lineage during the process of differentiation. This is supported by the identified increase in mRNA expression of the CEBP/β gene in BM tissue at hatch and decrease in fascicle percent area occupied by muscle fibers. In addition, in ovo exposure to CORT reduced total yolk fat content in birds at hatch, with minimal influence on the relative abundance of the various steroid hormones measured in the yolk. To the best of our knowledge, this study is the first to identify differences in yolk fat content in response to the in ovo environment encountered. However, the effects of altered yolk fat absorption rates on subsequent performance traits, as well as yolk steroid hormone contents in chicken meat birds is not yet understood. An understanding of the mechanisms controlling posthatch phenotypes as a result of the in ovo environment would allow the chicken meat industry to implement subtle manipulations to production strategies in an effort to improve performance and carcass characteristics in commercial flocks.
ACKNOWLEDGMENTS
The authors would like to acknowledge the significant contributions made by Dr. Luca Nicolotti and Dr. Natoiya Lloyd from the Australian Wine and Research Institute in the generation of hormone relative abundance data.
Funding: This study was funded by the University of Adelaide.
Ethics Statement: Experimental protocols and the use of animals in this study were approved by the University of Adelaide Animal Ethics Committee (S-2020-034).
Data and Model Availability Statement: None of the data presented in this study were deposited in an official repository. The data generated from this study are available upon request.
Author Contributions: J. A. designed the experiment, performed the experiments, analyzed the data, performed the project administration, and developed the manuscript. R. F. and N.-L. W. designed the study, performed the experiments and assisted in data analysis and manuscript editing. R. B. and D. R. performed experiments and assisted in data analysis. All authors read and approved the final manuscript.
DISCLOSURES
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- Ahmed A.A., Ma W., Ni Y., Wang S., Zhao R. Corticosterone in ovo modifies aggressive behaviors and reproductive performances through alterations of the hypothalamic-pituitary-gonadal axis in the chicken. Anim. Reprod. Sci. 2014;146:193–201. doi: 10.1016/j.anireprosci.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Ahmed A.A., Ma W., Ni Y., Zhou Q., Zhao R. Embryonic exposure to corticosterone modifies aggressive behavior through alterations of the hypothalamic pituitary adrenal axis and the serotonergic system in the chicken. Horm. Behav. 2014;65:97–105. doi: 10.1016/j.yhbeh.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Andersen C.L., Jensen J.L., Ørntoft T.F. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- Angove J.L., Forder R.E.A. The avian maternal environment: exploring the physiological mechanisms driving progeny performance. Worlds Poult. Sci. J. 2020;76:100–118. [Google Scholar]
- Angove J.L., Willson N.-L., Cadogan D.J., Forder R.E. In ovo corticosterone administration alters body composition irrespective of arginine supplementation in 35-day-old female chicken meat birds. Anim. Prod. Sci. 2021;61:8–16. [Google Scholar]
- Baehr L.M., Furlow J.D., Bodine S.C. Muscle sparing in muscle RING finger 1 null mice: response to synthetic glucocorticoids. J. Physiol. 2011;589:4759–4776. doi: 10.1113/jphysiol.2011.212845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J., Schumacher M., Evrard L. Sex differences and steroid control of testosterone-metabolizing enzyme activity in the quail brain. J. Neuroendocrinol. 1990;2:675–683. doi: 10.1111/j.1365-2826.1990.tb00464.x. [DOI] [PubMed] [Google Scholar]
- Benowitz-Fredericks Z.M., Hodge M. Yolk androstenedione in domestic chicks (Gallus gallus domesticus): uptake and sex-dependent alteration of growth and behavior. Gen. Comp. Endocrinol. 2013;193:48–55. doi: 10.1016/j.ygcen.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Berghänel A., Heistermann M., Schülke O., Ostner J. Prenatal stress accelerates offspring growth to compensate for reduced maternal investment across mammals. Proc. Natl. Acad. Sci. 2017;114:10658–10666. doi: 10.1073/pnas.1707152114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton K., Wilson P.W., Bishop V.R., Perez J.H., Wilkinson T., Hogan K., Homer N.Z.M., Robert C., Smith J., Meddle S.L., Dunn I.C., Watson K. Parental methyl-enhanced diet and in ovo corticosterone affect first generation Japanese quail (Coturnix japonica) development, behaviour and stress response. Sci. Rep. 2021;11:21092. doi: 10.1038/s41598-021-99812-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling M., Forder R., Hughes R.J., Weaver S., Hynd P.I. Effect of restricted feed intake in broiler breeder hens on their stress levels and the growth and immunology of their offspring. Transl. Anim. Sci. 2018;2:263–271. doi: 10.1093/tas/txy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T.P., Marks D.L. The regulation of muscle mass by endogenous glucocorticoids. Front. Physiol. 2015;6:1–12. doi: 10.3389/fphys.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G.R., Spencer K.A. Steroid hormones, stress and the adolescent brain: a comparative perspective. Neuroscience. 2013;249:115–128. doi: 10.1016/j.neuroscience.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Campbell N.A., Angles R., Bowden R.M., Casto J.M., Paitz R.T. Characterizing the timing of yolk testosterone metabolism and the effects of etiocholanolone on development in avian eggs. J. Exp. Biol. 2020;223 doi: 10.1242/jeb.210427. [DOI] [PubMed] [Google Scholar]
- Campbell J.E., Peckett A.J., D'souza A.M., Hawke T.J., Riddell M.C. Adipogenic and lipolytic effects of chronic glucocorticoid exposure. Am. J. Physiol. Cell Physiol. 2011;300:198–209. doi: 10.1152/ajpcell.00045.2010. [DOI] [PubMed] [Google Scholar]
- Cazzato D., Assi E., Moscheni C., Brunelli S., De Palma C., Cervia D., Perrotta C., Clementi E. Nitric oxide drives embryonic myogenesis in chicken through the upregulation of myogenic differentiation factors. Exp. Cell Res. 2014;320:269–280. doi: 10.1016/j.yexcr.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Chang A., Halley J., Silva M. Can feeding the broiler breeder improve chick quality and offspring performance? Anim. Prod. Sci. 2016;56:1254–1262. [Google Scholar]
- De Jong I.C., Guemene D. Major welfare issues in broiler breeders. Worlds Poult. Sci. J. 2011;67:73–81. [Google Scholar]
- Du M., Huang Y., Das A., Yang Q., Duarte M., Dodson M., Zhu M. Manipulating mesenchymal progenitor cell differentiation to optimize performance and carcass value of beef cattle. J. Anim. Sci. 2013;91:1419–1427. doi: 10.2527/jas.2012-5670. [DOI] [PubMed] [Google Scholar]
- Duan Y., Fu W., Wang S., Ni Y., Zhao R. Effects of tonic immobility (TI) and corticosterone (CORT) on energy status and protein metabolism in pectoralis major muscle of broiler chickens. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2014;169:90–95. doi: 10.1016/j.cbpa.2013.12.019. [DOI] [PubMed] [Google Scholar]
- Gatford K.L., Roberts C.T., Kind K.L., Hynd P.I. Off to the right start: how pregnancy and early life can determine future animal health and production. Anim. Prod. Sci. 2018;58:459–475. [Google Scholar]
- Gholami M., Seidavi A., O'Shea C.J., Akter Y., Dadashbeiki M. Feeding regimen of breeder broiler hen influences growth performance of the broiler chickens. Livest. Sci. 2017;203:132–135. [Google Scholar]
- Gilani S., Howarth G.S., Nattrass G., Kitessa S.M., Barekatain R., Forder R.E.A., Tran C.D., Hughes R.J. Gene expression and morphological changes in the intestinal mucosa associated with increased permeability induced by short-term fasting in chickens. J. Anim. Physiol. Anim. Nutr. 2018;102:653–661. doi: 10.1111/jpn.12808. [DOI] [PubMed] [Google Scholar]
- Giraudeau M., Ziegler A.K., Pick J.L., Ducatez S., Canale C.I., Tschirren B. Interactive effects of yolk testosterone and carotenoid on prenatal growth and offspring physiology in a precocial bird. Behav. Ecol. 2017;28:31–38. [Google Scholar]
- Groothuis T.G., Hsu B.-Y., Kumar N., Tschirren B. Revisiting mechanisms and functions of prenatal hormone-mediated maternal effects using avian species as a model. Philos. Trans. R. Soc. B: Biol. Sci. 2019;374 doi: 10.1098/rstb.2018.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis T.G.G., Schwabl H. Hormone-mediated maternal effects in birds: mechanisms matter but what do we know of them? Philos. Trans. R. Soc. B: Biol. Sci. 2008;363:1647–1661. doi: 10.1098/rstb.2007.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O. Timing is everything—the high sensitivity of avian satellite cells to thermal conditions during embryonic and posthatch periods. Front. Physiol. 2020;11:235. doi: 10.3389/fphys.2020.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O., Piestun Y., Allouh M.Z., Rosser B.W., Rinkevich Y., Reshef R., Rozenboim I., Wleklinski-Lee M., Yablonka-Reuveni Z. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev. Dyn. 2004;231:489–502. doi: 10.1002/dvdy.20151. [DOI] [PubMed] [Google Scholar]
- Halevy O., Yahav S., Rozenboim I. Enhancement of meat production by environmental manipulations in embryo and young broilers. Worlds Poult. Sci. J. 2006;62:485–497. [Google Scholar]
- Hartley R.S., Bandman E., Yablonka-Reuveni Z. Skeletal muscle satellite cells appear during late chicken embryogenesis. Dev. Biol. 1992;153:206–216. doi: 10.1016/0012-1606(92)90106-q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann M.F., Longenecker A.S., Marchetto N.M., Juliano S.A., Bowden R.M. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc. R. Soc. B. 2012;279:1447–1456. doi: 10.1098/rspb.2011.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen R., Groothuis T.G., Rettenbacher S. Elevated plasma corticosterone decreases yolk testosterone and progesterone in chickens: linking maternal stress and hormone-mediated maternal effects. PLoS One. 2011;6:1–8. doi: 10.1371/journal.pone.0023824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen R., Rettenbacher S., Groothuis T.G.G. Prenatal stress in birds: pathways, effects, function and perspectives. Neurosci. Biobehav. Rev. 2011;35:1484–1501. doi: 10.1016/j.neubiorev.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Henriksen R., Rettenbacher S., Groothuis T.G.G. Maternal corticosterone elevation during egg formation in chickens (Gallus gallus domesticus) influences offspring traits, partly via prenatal undernutrition. Gen. Comp. Endocrinol. 2013;191:83–91. doi: 10.1016/j.ygcen.2013.05.028. [DOI] [PubMed] [Google Scholar]
- Herbst K.L., Bhasin S. Testosterone action on skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care. 2004;7:271–277. doi: 10.1097/00075197-200405000-00006. [DOI] [PubMed] [Google Scholar]
- Jenkins S.A., Porter T.E. Ontogeny of the hypothalamo-pituitary-adrenocortical axis in the chicken embryo: a review. Domest. Anim. Endocrinol. 2004;26:267–275. doi: 10.1016/j.domaniend.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Kerachian M.A., Séguin C., Harvey E.J. Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action. J. Steroid. Biochem. Mol. Biol. 2009;114:121–128. doi: 10.1016/j.jsbmb.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N., van Dam A., Permentier H., van Faassen M., Kema I., Gahr M., Groothuis T.G.G. Avian yolk androgens are metabolized rather than taken up by the embryo during the first days of incubation. J. Exp. Biol. 2019;222 doi: 10.1242/jeb.193961. [DOI] [PubMed] [Google Scholar]
- Li D., Wang Q., Shi K., Lu Y., Yu D., Shi X., Du W., Yu M. Testosterone promotes the proliferation of chicken embryonic myoblasts via androgen receptor mediated PI3K/Akt signaling pathway. Int. J. Mol. Sci. 2020;21:1152–1164. doi: 10.3390/ijms21031152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Li D., Gilbert E.R., Xiao Q., Zhao X., Wang Y., Yin H., Zhu Q. Effect of monochromatic light on expression of estrogen receptor (ER) and progesterone receptor (PR) in ovarian follicles of chicken. PLoS One. 2015;10 doi: 10.1371/journal.pone.0144102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love O.P., McGowan P.O., Sheriff M.J. Maternal adversity and ecological stressors in natural populations: the role of stress axis programming in individuals, with implications for populations and communities. Funct. Ecol. 2013;27:81–92. [Google Scholar]
- Mikec M., Biđin Z., Valentić A., Savić V., Zelenika T.A., Đurić R.R., Novak I.L., Baleńovic M. Influence of environmental and nutritional stressors on yolk sac utilization, development of chicken gastrointestinal system and its immune status. Worlds Poult. Sci. J. 2006;62:31–40. [Google Scholar]
- Moghrabi N., Head J.R., Andersson S. Cell type-specific expression of 17β-hydroxysteroid dehydrogenase type 2 in human placenta and fetal liver1. J. Clin. Endocr. 1997;82:3872–3878. doi: 10.1210/jcem.82.11.4391. [DOI] [PubMed] [Google Scholar]
- Moore M.C., Johnston G.I.H. Toward a dynamic model of deposition and utilization of yolk steroids. Integr. Comp. Biol. 2008;48:411–418. doi: 10.1093/icb/icn079. [DOI] [PubMed] [Google Scholar]
- Moss F.P., Leblond C.P. Satellite cells as the source of nuclei in muscles of growing rats. Anat. Rec. 1971;170:421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Natt D., Goerlich-Jansson V.C., Persson M., Hjelm J. Early stress causes sex-specific, life-long changes in behaviour, levels of gonadal hormones, and gene expression in chickens. PLoS One. 2015;10:1–15. doi: 10.1371/journal.pone.0125808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E.N. MyoD family: a paradigm for development? Genes Dev. 1990;4:1454–1461. doi: 10.1101/gad.4.9.1454. [DOI] [PubMed] [Google Scholar]
- Otto T.C., Lane M.D. Adipose development: from stem cell to adipocyte. Crit. Rev. Biochem. Mol. Biol. 2005;40:229–242. doi: 10.1080/10409230591008189. [DOI] [PubMed] [Google Scholar]
- Paitz R.T., Angles R., Cagney E. In ovo metabolism of estradiol to estrone sulfate in chicken eggs: implications for how yolk estradiol influences embryonic development. Gen. Comp. Endocr. 2020;287 doi: 10.1016/j.ygcen.2019.113320. [DOI] [PubMed] [Google Scholar]
- Paitz R.T., Bowden R.M. A proposed role of the sulfotransferase/sulfatase pathway in modulating yolk steroid effects. Integr. Comp. Biol. 2008;48:419–427. doi: 10.1093/icb/icn034. [DOI] [PubMed] [Google Scholar]
- Paitz R.T., Bowden R.M., Casto J.M. Embryonic modulation of maternal steroids in European starlings (Sturnus vulgaris) Proc. R. Soc. B. 2011;278:99–106. doi: 10.1098/rspb.2010.0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paitz R.T., Cagney E. In ovo metabolism of progesterone to 5β-pregnanedione in chicken eggs: implications for how yolk progesterone influences embryonic development. Gen. Comp. Endocr. 2019;282 doi: 10.1016/j.ygcen.2019.113221. [DOI] [PubMed] [Google Scholar]
- Payne A.H., Hales D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Peixoto M.R.L.V., Karrow N.A., Newman A., Widowski T.M. Effects of maternal stress on measures of anxiety and fearfulness in different strains of laying hens. Front. Vet. Sci. 2020;7:128. doi: 10.3389/fvets.2020.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto M.R.L.V., Karrow N.A., Widowski T.M. Effects of prenatal stress and genetics on embryonic survival and offspring growth of laying hens. Poult. Sci. 2020;99:1618–1627. doi: 10.1016/j.psj.2019.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29 doi: 10.1093/nar/29.9.e45. 45-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piestun Y., Patael T., Yahav S., Velleman S.G., Halevy O. Early posthatch thermal stress affects breast muscle development and satellite cell growth and characteristics in broilers. Poult. Sci. 2017;96:2877–2888. doi: 10.3382/ps/pex065. [DOI] [PubMed] [Google Scholar]
- Rettenbacher S., Möstl E., Groothuis T. Gestagens and glucocorticoids in chicken eggs. Gen. Comp. Endocrinol. 2009;164:125–129. doi: 10.1016/j.ygcen.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Salloum R.H., Rubin J.P., Marra K.G. The role of steroids in mesenchymal stem cell differentiation: molecular and clinical perspectives. Horm. Mol. Biol. Clin. Invest. 2013;14:3–14. doi: 10.1515/hmbci-2013-0016. [DOI] [PubMed] [Google Scholar]
- Sato K., Fukao K., Seki Y., Akiba Y. Expression of the chicken peroxisome proliferator-activated receptor-γ gene is influenced by aging, nutrition, and agonist administration1. Poult. Sci. 2004;83:1342–1347. doi: 10.1093/ps/83.8.1342. [DOI] [PubMed] [Google Scholar]
- Sheriff M.J., Bell A., Boonstra R., Dantzer B., Lavergne S.G., McGhee K.E., MacLeod K.J., Winandy L., Zimmer C., Love O.P. Integrating ecological and evolutionary context in the study of maternal stress. Integr. Comp. Biol. 2017;57:437–449. doi: 10.1093/icb/icx105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.H. Relation of body size to muscle cell size and number in the chicken. Poult. Sci. 1963;42:283–290. [Google Scholar]
- Stern R.A., Ashwell C.M., Dasarathy S., Mozdziak P.E. The effect of hyperammonemia on myostatin and myogenic regulatory factor gene expression in broiler embryos. Animal. 2015;9:992–999. doi: 10.1017/S1751731115000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockdale F.E. Myogenic cell lineages. Dev. Biol. 1992;154:284–298. doi: 10.1016/0012-1606(92)90068-r. [DOI] [PubMed] [Google Scholar]
- Tilbrook A.J., Turner A.I., Clarke I.J. Effects of stress on reproduction in non-rodent mammals: the role of glucocorticoids and sex differences. Rev. Reprod. 2000;5:105–113. doi: 10.1530/ror.0.0050105. [DOI] [PubMed] [Google Scholar]
- Vassallo B.G., Litwa H.P., Haussmann M.F., Paitz R.T. In ovo metabolism and yolk glucocorticoid concentration interact to influence embryonic glucocorticoid exposure patterns. Gen. Comp. Endocr. 2019;272:57–62. doi: 10.1016/j.ygcen.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velleman S. Muscle development in the embryo and hatchling. Poult. Sci. 2007;86:1050–1054. doi: 10.1093/ps/86.5.1050. [DOI] [PubMed] [Google Scholar]
- von Engelhardt N., Henriksen R., Groothuis T.G.G. Steroids in chicken egg yolk: metabolism and uptake during early embryonic development. Gen. Comp. Endocrinol. 2009;163:175–183. doi: 10.1016/j.ygcen.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Wang G., Kim W.K., Cline M.A., Gilbert E.R. Factors affecting adipose tissue development in chickens: a review. Poult. Sci. 2017;96:3687–3699. doi: 10.3382/ps/pex184. [DOI] [PubMed] [Google Scholar]
- Yadgary L., Cahaner A., Kedar O., Uni Z. Yolk sac nutrient composition and fat uptake in late-term embryos in eggs from young and old broiler breeder hens. Poult. Sci. 2010;89:2441–2452. doi: 10.3382/ps.2010-00681. [DOI] [PubMed] [Google Scholar]
- Zhang W., Bai S., Liu D., Cline M.A., Gilbert E.R. Neuropeptide Y promotes adipogenesis in chicken adipose cells in vitro. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015;181:62–70. doi: 10.1016/j.cbpa.2014.11.012. [DOI] [PubMed] [Google Scholar]