Abstract
O-linked GlcNAc (O-GlcNAc) is an emerging post-translation modification that couples metabolism with cellular signal transduction by crosstalk with phosphorylation and ubiquitination to orchestrate various biological processes. The mechanisms underlying the involvement of O-GlcNAc modifications in N6-methyladenosine (m6A) regulation are not fully characterized. Herein, we show that O-GlcNAc modifies the m6A mRNA reader YTH domain family 1 (YTHDF1) and fine-tunes its nuclear translocation by the exportin protein Crm1. First, we present evidence that YTHDF1 interacts with the sole O-GlcNAc transferase (OGT). Second, we verified Ser196/Ser197/Ser198 as the YTHDF1 O-GlcNAcylation sites, as described in numerous chemoproteomic studies. Then we constructed the O-GlcNAc-deficient YTHDF1-S196A/S197F/S198A (AFA) mutant, which significantly attenuated O-GlcNAc signals. Moreover, we revealed that YTHDF1 is a nucleocytoplasmic protein, whose nuclear export is mediated by Crm1. Furthermore, O-GlcNAcylation increases the cytosolic portion of YTHDF1 by enhancing binding with Crm1, thus upregulating downstream target (e.g. c-Myc) expression. Molecular dynamics simulations suggest that O-GlcNAcylation at S197 promotes the binding between the nuclear export signal motif and Crm1 through increasing hydrogen bonding. Mouse xenograft assays further demonstrate that YTHDF1-AFA mutants decreased the colon cancer mass and size via decreasing c-Myc expression. In sum, we found that YTHDF1 is a nucleocytoplasmic protein, whose cytosolic localization is dependent on O-GlcNAc modification. We propose that the OGT–YTHDF1–c-Myc axis underlies colorectal cancer tumorigenesis.
Keywords: Crm1, c-Myc, m6A, O-GlcNAc, YTHDF1
The N6-methyladenosine (m6A) modification is quite abundant on internal mRNAs, and its function and regulation have caught a wave of intense investigations (1, 2). Its numerous writers, erasers, and readers are under stringent control (3), and one of the readers is YTH domain family 1 (YTHDF1) (4), which promotes translation efficiency during arsenite recovery (5). YTHDF1 enhances translation in adult mouse dorsal root ganglions during injury recovery and augments axonal regeneration (6). YTHDF1 fuels translation upon neuronal stimuli, which is conducive to learning and memory (7). YTHDF1 also recognizes m6A-marked lysosomal protease mRNAs, thus mediating the decay of neoantigens and bolstering tumor suppressive immunotherapy (8). Recently, YTHDF1 and YTHDF3 are also found to promote stress granule formation, as m6A mRNAs are found to be enriched in stress granules (9).
The interconnection between m6A mRNA and cancer are being revealed (10, 11, 12), and m6A participates in many aspects of tumor biology: cancer stem cell, tumor cell proliferation, or oncogene expression. YTHDF1, in particular, has been found at the nexus of multiple tumorigenic pathways. YTHDF1 binds the m6A-modified mRNA of c-Myc, whose enhanced translation promotes glycolysis and cancer cell proliferation (13). In non–small cell lung cancer, YTHDF1 upregulates the translation efficiency of CDK2, CDK4, and cyclin D1, and YTHDF1 is also elevated in people who live at high altitudes, possibly through the hypoxia Keap1–Nrf2–AKR1C1 pathway (14). In gastric cancer, YTHDF1 enhances the expression of frizzled 7, a key Wnt receptor that hyperactivates the Wnt–β-catenin pathway (15). In ovarian cancer, YTHDF1 promotes the translation of eukaryotic translation initiation factor 3 subunit C (EIF3C), a component of the protein translation initiation factor EIF3 complex (16). In cervical cancer, YTHDF1 elevates the translation of hexokinase 2 via binding with its 3′-UTR, thus promoting the Warburg effect (17). All these findings suggest that YTHDF1 binds with its targets via m6A mRNA and is fundamental during human carcinogenesis.
Investigations show that some of the m6A regulators are subject to post-translational modifications. YTHDF2, another m6A reader that mediates mRNA decay (18), is subject to SUMOylation at K571 upon hypoxia stress (19). SUMOylation alters the binding affinity of YTHDF2 with m6A, thus deregulating the downstream target genes, leading to lung cancer progression (19). An m6A writer, methyltransferase-like 3 (METTL3), is modified by lactylation at its zinc-finger domain, changing its RNA capturing capacity and regulating immunosuppression of tumor-infiltrating myeloid cells (20). METTL3 is also acetylated, which regulates its localization and cancer metastasis (21).
The O-linked GlcNAc (O-GlcNAc) glycosylation occurs intracellularly (22, 23). Functioning as a rheostat to environmental stress or cellular nutrient status, O-GlcNAc monitors transcription, neural development, cell cycle, and stress response (22, 23). However, whether it plays a role in m6A regulation has remained enigmatic. Historically O-GlcNAc studies have been strenuous due to technical impediments. Recent years have witnessed the combined methodology of chemoenzymatic labeling, bioorthogonal conjugation, and electron-transfer dissociation mass spectrometry (MS), which have smoothened the way for biological investigations. Previously, an isotope-tagged cleavable linker together with chemoenzymatic labeling screen identified the O-GlcNAc sites of YTHDF1 to be S196 and S198 (24). A second enrichment strategy using Gal labeling followed by chemical oxidation points the YTHDF1 O-GlcNAcylation region as Ser196-198 (25). In another isotope-targeted glycoproteomic study in T cells, YTHDF1 O-GlcNAcylation occurs on several residues, including Ser196, Ser197, and Ser198 (26). In this manuscript, we first confirmed that YTHDF1 O-GlcNAcylation occurs on Ser196, Ser197, and Ser198. Then we found that YTHDF1 is a nucleocytoplasmic protein with exportin 1 (CRM1) mediating its cytoplasmic shuttling. We further presented evidence that O-GlcNAcylation promotes YTHDF1 cytosolic localization, thus enhancing downstream target expression, such as c-Myc. Our results were further correlated with The Cancer Genome Atlas analysis combined with mouse xenograft models. Our data highlight the significance of glycosylation in m6A regulation and tumorigenesis.
Results
YTHDF1 is O-GlcNAcylated at Ser196, Ser197, and Ser198
As YTHDF1 has been reproducibly identified in O-GlcNAc profiling screens (24, 27, 28), we first assessed the binding affinity between YTHDF1 and the sole O-GlcNAc writer-O-GlcNAc transferase (OGT). 293T cells were transfected with Flag-YTHDF1 and HA-OGT plasmids, and the two overproduced proteins coimmunoprecipitate (Fig. 1A). When the endogenous proteins were examined, YTHDF1 proteins were also present in the anti-OGT immunoprecipitates (Fig. 1B). Pull-down assays were then utilized to evaluate the physical association. 293T cells were transfected with HA-OGT, and the cell lysates were incubated with recombinant glutathione-S-transferase-YTHDF1 proteins, which pulled down overproduced OGT proteins (Fig. 1C). When pull-down assays were carried out between recombinant OGT and YTHDF1, again glutathione-S-transferase-YTHDF1 pulled down His-OGT (Fig. 1D), suggesting that OGT and YTHDF1 directly interact in vivo and in vitro.
Figure 1.
YTHDF1 is O-GlcNAcylated at Ser196 Ser197 Ser198.A, 293T cells were transfected with Flag-YTHDF1 and HA-OGT. The cell lysates were subject to immunoprecipitation and immunoblotting with the antibodies indicated. B, HeLa cell lysates were immunoprecipitated with anti-OGT antibodies and immunoblotted with the indicated antibodies. C, 293T cells were transfected with HA-OGT, and the cell lysates were incubated with recombinant GST-YTHDF1. D, recombinant His-OGT and GST-YTHDF1 proteins were incubated and subject to pull-down assays. E, cells were treated with the OGA inhibitor Thiamet-G(TMG) and glucose to enrich for O-GlcNAcylation as described previously (29). Then the cell lysates were immunoprecipitated with anti-YTHDF1 antibodies and immunoblotted with anti-O-GlcNAc RL2 antibodies. F, YTHDF1-S196A, S197F, S198A, -S196 (AFA) mutants were constructed, and the cells were transfected with HA-OGT together with SFB-YTHDF1-WT and SFB-YTHDF1-AFA mutants. The anti-Flag immunoprecipitates were immunoblotted with RL2 antibodies. ∗ indicates p < 0.05. GST, glutathione-S-transferase; O-GlcNAc, O-linked N-acetylglucosamine; OGT, O-GlcNAc transferase; TMG, Thiamet-G; YTHDF1, YTH domain family 1.
Then we assessed the O-GlcNAcylation of YTHDF1. 293T cells were enriched for O-GlcNAc by supplementing the media with glucose and Thiamet-G (TMG, the OGA inhibitor) (TMG + Glu) as previously described (29, 30). The endogenous YTHDF1 proteins were immunoprecipitated from the lysates, and RL2 antibodies detected a crisp band upon O-GlcNAc enrichment (Fig. 1E), suggesting that YTHDF1 is indeed O-GlcNAcylated. We then decided to mutate the three Ser, as several chemoproteomic studies have identified YTHDF1 O-GlcNAcylation sites to be Ser196-198 (24, 27, 28). Thus we generated a YTHDF1-S196AS197FS198A (AFA) mutant. When we transfected the WT and AFA mutant into cells, the AFA mutant significantly diminished YTHDF1 O-GlcNAcylation levels (Fig. 1F), suggesting that they are the main glycosylation sites.
Crm1 mediates the nuclear cytoplasmic shuttling of YTHDF1
To investigate the potential functions of YTHDF1 O-GlcNAcylation, we first employed an immunoprecipitation (IP)-MS analysis. Flag-YTHDF1 plasmids were transfected into cells, and the lysates were immunoprecipitated with anti-Flag antibodies. Interestingly, the MS results revealed many importins and exportins (Table S1). When we did literature research, YTHDF1 was among the binding partners of exportin 1 (Crm1) in a recent proteomic study (31). Therefore, we suspect that YTHDF1 might be a nuclear cytoplasmic protein and Crm1 might mediate the process.
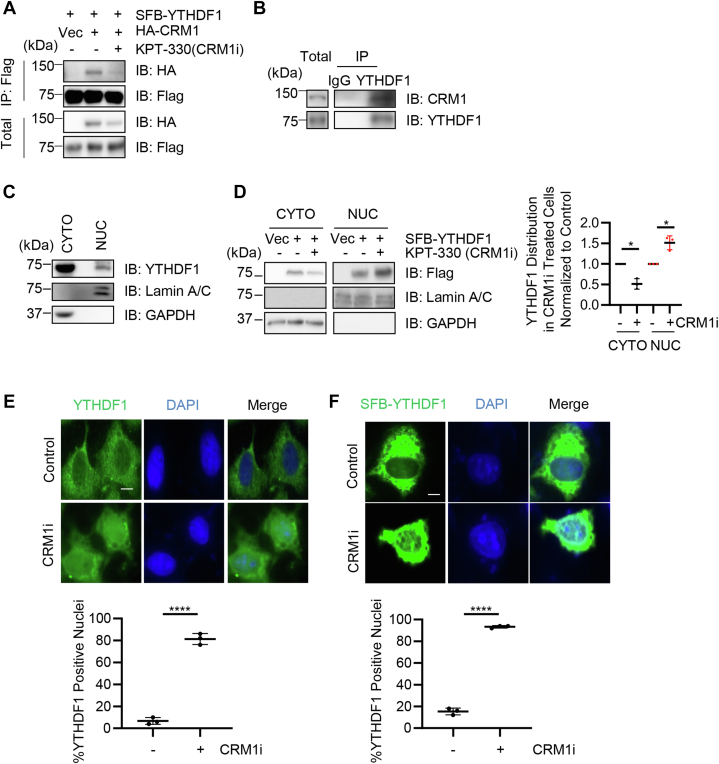
To test this possibility, we first assessed the association between YTHDF1 and Crm1. We also utilized KPT-330, a Crm1 inhibitor. Overexpressed YTHDF1 coimmunoprecipitates with Crm1, and KPT-3301 markedly reduced the interaction (Fig. 2A). Moreover, endogenous YTHDF1 interacts with Crm1 (Fig. 2B), suggesting that YTHDF1 could be a nuclear cytoplasmic protein. We then utilized the nuclear cytoplasmic fractionation assay, and fractionation results revealed a nuclear portion of YTHDF1 (Fig. 2C). We further adopted KPT-330 in the fractionation assay and found that it significantly enhanced the nuclear fraction of YTHDF1 (Fig. 2D). Furthermore, in immunofluorescence staining samples, both endogenous YTHDF1 and overproduced YTHDF1 manifested significant upregulation of nuclear staining signals (Fig. 2, E and F), suggesting that Crm1 could export YTHDF1 to the cytosol.
Figure 2.
Nuclear cytoplasmic shuttling of YTHDF1 is mediated by exportin 1 (Crm1).A, overproduced YTHDF1 interacts with Crm1. Cells were transfected with SFB-YTHDF1 and HA-CRM1, treated or untreated with KPT-330 (Crm1 inhibitor). B, endogenous YTHDF1 interacts with Crm1. Cell lysates were immunoprecipitated with anti-YTHDF1 antibodies. C, cell lysates were subject to nuclear cytoplasmic fractionation to indicate cytosolic (CYTO) and nuclear (NUC) portions. D, KPT-330 treatment increases nuclear YTHDF1. Cells were transfected with SFB-YTHDF1 and treated with or without KPT-330. E–F, indirect immunofluorescence demonstrated that KPT-330 treatment increases the nuclear localization of endogenous YTHDF1 (E) and overexpressed YTHDF1 (F). Scale bar represents 10 μM. ∗ indicates p < 0.05; ∗∗∗∗ indicates p < 0.0001. YTHDF1, YTH domain family 1.
YTHDF1 O-GlcNAcylation promotes interaction with Crm1
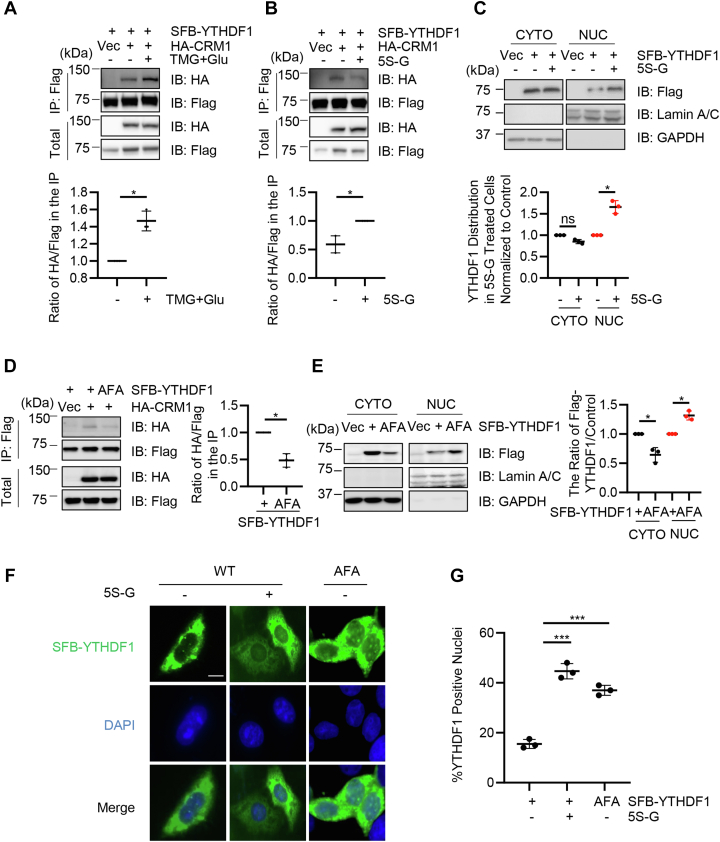
To determine if O-GlcNAcylation plays a role in Crm1-mediated YTHDF1 nuclear shuttling, we enriched for protein O-GlcNAcylation by TMG + Glu as previously described (29). We found that O-GlcNAc enrichment increased the binding between YTHDF1 and Crm1 (Fig. 3A). We also repressed O-GlcNAcylation using an OGT inhibitor, acetyl-5S-GlcNAc (5S-G) (32). 5S-G treatment significantly reduced the affinity between YTHDF1 and Crm1 (Fig. 3B). When 5S-G was included in the fractionation assay, nuclear YTHDF1 is upregulated notably (Fig. 3C), suggesting that O-GlcNAcylation increases the binding between YTHDF1 and Crm1.
Figure 3.
O-GlcNAcylation promotes the interaction between YTHDF1 and Crm1. A, cells were transfected with SFB-YTHDF1 and HA-CRM1 and enriched for O-GlcNAcylation by TMG plus glucose treatment (TMG + Glu) as previously described (29). O-GlcNAcylation enhanced the binding between YTHDF1 and Crm1. B, cells were transfected with SFB-YTHDF1 and HA-CRM1 and treated with the OGT inhibitor acetyl-5S-GlcNAc (5S-G). OGT inhibition downregulated the affinity between YTHDF1 and Crm1. C, cells were transfected with SFB-YTHDF1 and treated with 5S-G. Nuclear and cytoplasmic fractionation assays were carried out. OGT inhibition elevated nuclear YTHDF1. D, cells were transfected with HA-Crm1 together with SFB-YTHDF1-WT or SFB-YTHDF1-AFA plasmids. E, cells were transfected with SFB-YTHDF1-WT or SFB-YTHDF1-AFA mutants and subject to nuclear and cytoplasmic fractionation assays. F–G cells were transfected with SFB-YTHDF1-WT (treated or untreated with 5S-G) or SFB-YTHDF1-AFA. The cells were then stained with anti-Flag antibodies and DAPI. Scale bar represents 10 μM. ∗ indicates p < 0.05; ∗∗∗ indicates p < 0.001. DAPI, 4′,6-diamidino-2-phenylindole; O-GlcNAc, O-linked N-acetylglucosamine; OGT, O-GlcNAc transferase; TMG, Thiamet-G; YTHDF1, YTH domain family 1.
Then we directly measured the effect using the AFA mutant. YTHDF1-AFA displayed marked reduction in association with Crm1 (Fig. 3D). In the fractionation analysis, AFA again manifested a much higher portion in the nucleus (Fig. 3E). Lastly, we employed fluorescence microscopy to visualize whether O-GlcNAcylation affected YTHDF1 localization. As shown in Figure 3, F and G, both 5S-G treatment and the AFA mutant enhanced nuclear YTHDF1 staining, probably by blocking its nuclear export via Crm1. These assays suggest that YTHDF1 O-GlcNAcylation promotes the binding between YTHDF1 and Crm1 and the resultant nuclear export.
A potential NES lies in proximity to YTHDF1 O-GlcNAcylation sites
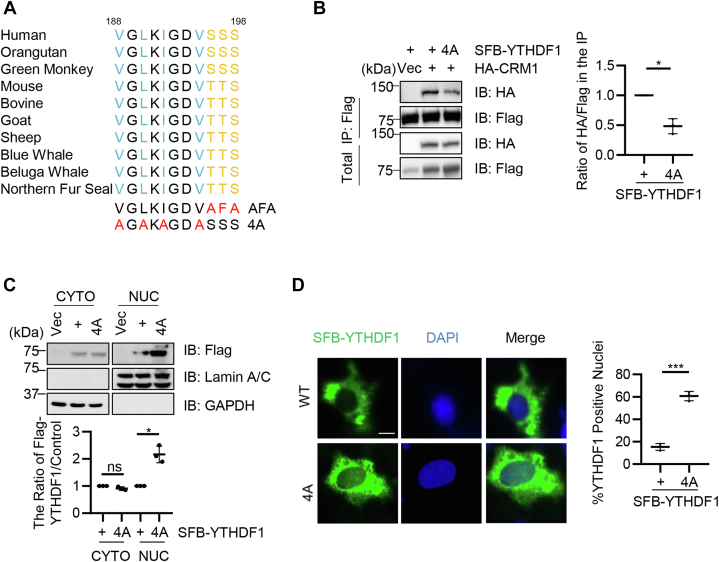
We were curious why O-GlcNAcylation has such a conspicuous effect on YTHDF1 localization and looked for potential nuclear export signals (NESs) surrounding the S196S197S198 region. As NES consists of the Φ1-X(2-3)-Φ2-X(2-3)-Φ3-X-Φ4 motif (Φ: hydrophobic amino acid) (33), we found a potential NES juxtaposing the 196-198 Ser cluster (Fig. 4A). We mutated the corresponding hydrophobic amino acid to Ala and generated 4A (Fig. 4A), as previously described for the NES of the cyclic GMP-AMP synthase (34). When we examined for YTHDF1-Crm1 association, the 4A mutant significantly downregulated binding with Crm1 (Fig. 4B). In the fractionation studies, 4A also elevated nuclear YTHDF1 localization (Fig. 4C). In the immunofluorescent staining experiments, 4A also had a more prominent nuclear localization pattern than the control (Fig. 4D). Combined, these data suggest that O-GlcNAcylation might boost the association of the neighboring NES with Crm1.
Figure 4.
There is a potential nuclear exportin signal in proximity to O-GlcNAcylation sites. A, sequence alignment of the YTHDF1-WT, O-GlcNAc-deficient AFA, and the NES-deficient 4A sequences. B, cells were transfected with HA-Crm1, together with YTHDF1-WT and YTHDF1-4A plasmids. C, cells were transfected with SFB-YTHDF1-WT or SFB-YTHDF1-4A plasmids and analyzed by nuclear cytoplasmic fractionation. D, cells were transfected with SFB-YTHDF1 or 4A and stained with anti-Flag antibodies and DAPI. Scale bar represents 10 μM. ∗ indicates p < 0.05; ∗∗∗ indicates p < 0.001, ns indicates nonspecific. DAPI, 4′,6-diamidino-2-phenylindole; NES, nuclear exportin signal; O-GlcNAc, O-linked N-acetylglucosamine; YTHDF1, YTH domain family 1.
MD simulations suggest that S197 O-GlcNAcylation increases the interaction between NES and Crm1 via hydrogen bonds
We then explored deeper as why O-GlcNAcylation increases binding with Crm1. Recently, a structural study focusing on the interface between NES and CRM1 found that many NESs might form hydrogen bonds with CRM1 (35); therefore, we wondered whether hydrophilic O-GlcNAc could enhance the interaction by increasing hydrogen bonding. And we utilized the molecular dynamics (MD) simulation approach and constructed the system. Since the AlphaFold Protein Structure Database cannot clearly predict the NES domain of YTHDF1 (pLDDT < 50) (36, 37), the ColabFold web server was used to build the initial structure of a short fragment (residues 182–210) including the NES domain (Fig. 5A) (38). The initial structure was further optimized for 300 ns with MD simulations (Fig. 5, B and C). The binding domain of CRM1 (residues 362–645, Fig. 5D) was cropped from the crystal structure of the PKI NES–CRM1–RanGTP nuclear export complex (PDB ID: 3NBY) (39). The Rosetta Docking protocol (version 3.12) was applied to build the YTHDF1 NES and CRM1 complex (40, 41, 42). The NES fragment was set as the input structure with 10 Å translation and 360º rotation. One hundred poses were created after the docking process (Fig. 5E) and only two obtained reasonable relative positions (with the NES domain close to the CRM1-binding domain) (Fig. 5F). After 500 ns of MD simulations, only one complex maintained a reasonable interaction (Fig. 5G). The last frame of this complex was chosen as the initial structure for further analysis.
Figure 5.
Molecular dynamics simulations suggest that S197 O-GlcNAcylation increases the interaction between NES and Crm1 via hydrogen bonds. A, initial structure of YTHDF1 fragment from ColabFold. The NES domain is colored in magenta, and serines that could be glycosylated are colored in yellow. B, RMSD of the backbone of YTHDF1 fragments during 300 ns of MD simulation. C, structure of YTHDF1 fragments after optimization. D, structure of CRM1. The NES-binding region cropped for docking is shown in cyan. E, docking results for CRM1 and the YTHDF1 fragment. F, reasonable poses (pose 37 in cyan and pose 97 in magenta) chosen from 100 poses. G, positions of YTHDF1 before and after MD simulations in poses 37 and 97. H, RMSDs of the backbone of CRM1 and YTHDF1 fragments in the nonglycosylated system (black for CRM1 and red for YTHDF1 fragments) and the glycosylated system (blue for CRM1 and magenta for YTHDF1 fragment) during 200 ns of MD simulations. I, binding energies between CRM1 and YTHDF1 fragments in the nonglycosylated and glycosylated systems. J, number of hydrogen bonds per frame in the nonglycosylated and glycosylated systems. K, detailed interaction between glycan and key residues in CRM1. The hydrogen bonds are shown in yellow dashed lines. MD, molecular dynamics; NES, nuclear exportin signal; O-GlcNAc, O-linked N-acetylglucosamine; YTHDF1, YTH domain family 1.
The RMSD values indicated that both systems can reach stable states in 200 ns (Fig. 5H). The trajectory of the last 100 ns was extracted for further analysis. The binding energy of glycosylated fragment to CRM1-binding domain was −118.04 ± 0.32 kcal/mol, which is lower than that of the unglycosylated fragment to the CRM1-binding domain (−116.21 ± 0.24 kcal/mol) (Fig. 5I). The number of hydrogen bonds between the fragment and CRM1 was increased when S197 was glycosylated (3.70 ± 0.06 in the glycosylated system versus 3.32 ± 0.04 in the nonglycosylated system, Fig. 5J) because the glycan at S197 frequently forms hydrogen bonds with H577 and D535 in CRM1 to pull the NES domain to the CRM1-binding domain (Fig. 5K). Taken together, MD simulations suggest that O-GlcNAc might increase hydrogen bonding between YTHDF1 and Crm1.
YTHDF1 O-GlcNAcylation promotes downstream target expression (e.g. c-Myc)
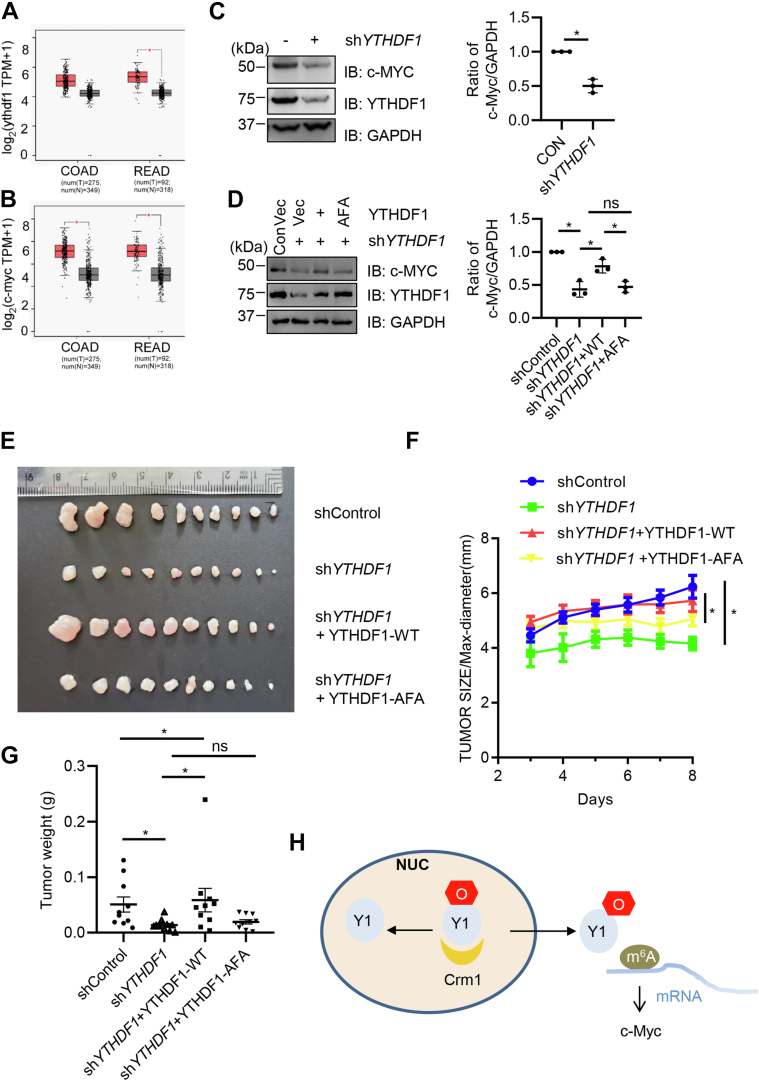
Recently, many YTHDF1-mediated m6A mRNA targets have been identified, such as the protein translation initiation factor EIF3 (16), the key Wnt receptor frizzled 7 (15), and c-Myc (13). We focused on c-Myc, as m6A-modified c-Myc mRNA has been demonstrated to recruit YTHDF1 (13). We reasoned that YTHDF1 O-GlcNAcylation would promote c-Myc expression as there is more cytosolic YTHDF1. We first carried out The Cancer Genome Atlas analysis and found that in colon adenocarcinoma and rectal adenocarcinoma samples, both YTHDF1 and c-Myc are overexpressed in the tumor samples (Fig. 6, A and B), indicative of a positive correlation between YTHDF1 and c-Myc in colorectal cancer. We therefore generated stable YTHDF1-knockdown SW620 cells using shYTHDF1, and c-Myc protein levels were attenuated upon YTHDF1 downregulation (Fig. 6C). When the knockdown cells were rescued with YTHDF1-WT or YTHDF1-AFA plasmids, c-Myc expression was comparable to the control in the YTHDF1-WT–rescued cells, but not in the YTHDF1-AFA–rescued cells (Fig. 6D). The stable SW620 cells were then utilized in mouse xenograft experiments, and the tumor size and weight were monitored (Fig. 6, E–G). As expected, the YTHDF1-WT–rescued cells produced much larger tumors than the AFA mutants, suggesting that YTHDF1 O-GlcNAcylation promotes colorectal cancer, probably via c-Myc.
Figure 6.
YTHDF1 O-GlcNAcylation promotes c-Myc expression in colorectal carcinoma.A and B, YTHDF1 and c-Myc mRNA levels in colon adenocarcinoma(COAD) and rectal adenocarcinoma (READ) samples from The Cancer Genome Atlas (TCGA) database. C, stable YTHDF1-knockdown SW620 cell lines were generated and examined for c-Myc expression. D, the cell lines in (C) were rescued with Flag-YTHDF1-WT or Flag-YTHDF1-AFA plasmids. Cellular lysates were immunoblotted with antibodies indicated. E–G, xenografts in nude mice. The stable SW620 cells were injected into nude mice. The tumors were imaged after 8 days. E shows the tumor images, F shows the tumor size, and G shows the tumor weight. ∗ indicates p < 0.05. H, a model illustrating the role of O-GlcNAc in YTHDF1 nuclear shuttling. O-GlcNAcylation of YTHDF1 at S196S197S198 will enhance the partnership between Crm1 and YTHDF1, thus promoting cytosol localization of YTHDF1 and translation of downstream target proteins, e.g., c-Myc. Such an OGT–YTHDF1–c-Myc pathway will enhance colorectal cancer. OGT, O-GlcNAc transferase; O-GlcNAc, O-linked N-acetylglucosamine; READ, rectal adenocarcinoma; TCGA, The Cancer Genome Atlas; YTHDF1, YTH domain family 1.
Discussion
In this work, we first confirmed that YTHDF1 O-GlcNAcylation occurs on Ser196/197/198, and then we identified that glycosylation promotes shuttling of YTHDF1 to the cytoplasm by CRM1. Consequently, cytosolic YTHDF1 will upregulate its downstream target expression (e.g., c-Myc), and then tumorigenesis will ensue.
Our work is in line with the observation that elevated O-GlcNAcylation correlates with different cancer types, such as breast cancer, prostate cancer, and bladder cancer (43). In colon cancer, both O-GlcNAc and OGT abundance increased in clinical patient samples (44). Here, we found that YTHDF1 O-GlcNAcylation boost the expression of c-Myc, at least in SW620 cells. In xenograft models, the O-GlcNAc–deficient YTHDF1-AFA mutants attenuated tumor progression, suggesting that OGT could regulate many more downstream substrates to promote cancer growth.
Of the many m6A readers, YTHDF1-3 has been considered as cytosolic proteins (45). We show here that YTHDF1 is partly localized to the nucleus, and we found a potential NES in YTHDF1. Incidentally, the NES neighbors the O-GlcNAcylation sites, suggesting that O-GlcNAcylation might promote the interaction between NES and Crm1. MD simulations suggest that the hydrophilic O-GlcNAcylation might increase the binding between NES and Crm1 through hydrogen bonding.
A great many investigations have shown that O-GlcNAcylation alters protein localization, such as pyruvate kinase M2 (PKM2) (46) and serine/arginine-rich protein kinase 2 (SRPK2) (47). PKM2 O-GlcNAcylation at Thr405/Ser406 promotes ERK-dependent phosphorylation of PKM2 at Ser37, which is required for PKM2 nuclear translocation (46, 48). And PKM2-T405A/S406A attenuates the interaction with importin α5 (46). SRPK2 is O-GlcNAcylated at Ser490/Thr492/Thr498, which is close to a nuclear localization signal (47); this nuclear localization signal mediates SRPK2 nuclear localization by importin α (47). Indeed, a general mechanism has been proposed that at least some O-GlcNAcylated proteins are imported to the nucleus by importin α (47). Our work here suggests that maybe in some other cases, O-GlcNAcylation might shuttle the O-GlcNAcylated proteins to the cytoplasm by exportin.
The intertwined relationship between RNA and glycosylation is just emerging. Recently, a “glycoRNA”concept has been coined as small noncoding RNAs are found to be decorated with sialylated glycans (49). As far as m6A is concerned, many readers have been identified in O-GlcNAc chemoproteomic profiling works (25, 26, 27, 28, 50), including YTHDF1, YTHDF3, and YTHDC1. Upon hepatitis B virus infection, YTHDF2 O-GlcNAcylation is found to be increased, which enhances its protein stability (51). In a recent investigation from our group (https://doi.org/10.1101/2022.09.03.506498), we found that YTHDC1 O-GlcNAcylation is induced upon DNA damage and takes part in homologous recombination by enhancing binding with m6A mRNA. Here we show that YTHDF1 O-GlcNAcylation mediates its localization by promoting binding with exportin. We envision that O-GlcNAcylation is likely to be found in many more aspects of RNA metabolism.
Experimental procedures
Cell culture, antibodies, and plasmids
Cells were purchased from ATCC. OGT plasmids and antibodies were described before (52). Antibodies are as follows: YTHDF1 (Proteintech, #17479-1-AP), c-Myc (Abcam, Ab32072), Lamin A/C (CST, 2032S). YTHDF1 shRNA sequence (TRCN0000286871) was as follows:
5′-CCGGCCCGAAAGAGTTTGAGTGGAACTCGAGTTCCACTCAAACTCTTTC GGGTTTTTG-3’
IP and IB assays
IP and immunoblotting (IB) experiments were performed as described before (53). Nuclear and cytoplasmic fractionation assays were carried out as before (54). The following primary antibodies were used for IB: anti-β-actin (1:10,000), anti-HA (1:1000), and anti-FLAG M2 (Sigma) (1:1000), anti-Myc (1:1000), anti-YTHDF1 (1:1000), and Lamin A/C (1:1000). Peroxidase-conjugated secondary antibodies were from Jackson ImmunoResearch. Blotted proteins were visualized using the ECL detection system (Amersham). Signals were detected by a LAS-4000 and quantitatively analyzed by densitometry using the Multi Gauge software (Fujifilm). All Western blots were repeated at least three times.
Cell culture treatment
Chemical utilization was as follows: TMG (OGA inhibitor) at 5 μM for 24 h; acetyl-5S-GlcNAc (5S-G) (OGT inhibitor) at 100 μM (prepared at 50 mM in dimethyl sulfoxide) for 24 h; KPT-300 (Crm1 inhibitor) at 5 μM for 24 h.
Indirect immunofluorescence
Indirect immunofluorescence staining was performed as described before (53). Dilutions of primary antibodies were 1:500 for mouse anti-YTHDF1 and 1:1000 for anti-Flag antibodies. Cell nuclei were stained with 4′,6-diamidino-2-phenylindole.
MD simulations
The O-glycan (β-N-Acetyl-D-Glucosamine) at S197 of the YTHDF1 fragment was built using the Glycan Reader & Modeler module (55). The role of O-glycosylation in the YTHDF1 fragment interacting with CRM1 was investigated via MD simulations with the GROMACS (version 2021.2) software package (56, 57). Two systems (unglycosylated fragment and O-GlcNAcylated fragment at S197 in complex with CRM1-binding domain, respectively) were neutralized and solvated by 150 mM KCl and TIP3P water molecules. The systems were minimized and equilibrated using default equilibration inputs from the CHARMM-GUI webserver (58) with the CHARMM36m force field (59, 60). In brief, the systems were equilibrated in the isothermal-isobaric (NPT) ensemble for 200 ns. The pressure was set at 1 atm maintained by the Parrinello-Rahman barostat (61), and the temperature was maintained at 310.15 K with the Nosé–Hoover thermostat (62). Periodic boundary conditions were applied throughout the simulations. The SHAKE algorithm was used to constrain all bonds with hydrogen atoms (63). The particle-mesh Ewald summation method was applied to treat long-range electrostatic interactions (64).
Analysis of MD trajectory data was performed through MDAnalysis (65). The binding energy (enthalpy) and per-residue energy contributions were calculated by the molecular mechanics/Poisson-Boltzmann (generalized-Born) surface area method with the gmx_MMPBSA tool (66, 67). Interactions between the YTHDF1 fragment and the CRM1-binding domain were displayed by PyMol (68).
Mouse xenograft
A total of 1 X 106 control, YTHDF1 shRNA, YTHDF1 shRNA; YTHDF1-WT or YTHDF1 shRNA;YTHDF1-AFA stable SW620 cells were resuspended in Matrigel (Corning) and then injected into the flanks of nude mice (4–6 weeks old). Tumor volumes were measured from day 3 to 9 after injection. At 9 days after the injection, tumors were dissected. The mice were obtained from the Animal Research and Resource Center, Yunnan University (Certification NO. SCXK[Dian]K2021-0001). All animal work procedures were approved by the Animal Care Committee of the Yunnan University (Kunming, China).
Data availability
All data are contained within the manuscript.
Supporting information
This article contains supporting information.
Conflicts of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We thank Dr Xing Chen (Peking Univ.) for reagents and Dr Qing Chang for providing facility support at the Protein Preparation and Characterization Platform of Tsinghua University Technology Center for Protein Research. Calculations were performed on the Tianhe Supercomputer at the National Supercomputer Center in Tianjin.
Author contributions
Jie L., M. A., L. S., Y. Z., Y. W., Y. Y., Y. L., W. M., M. L., Y. D., and R. Z. investigation; M.-Q. D., Y.-G. Y., and X. W. resources; M.-Q. D., Y.-G. Y., X. W., J. S., and Jing L. supervision; X. W., J. S., and Jing L. conceptualization; X. W., J. S., and Jing L. project administration; J. S. data curation; Jing L. funding acquisition.
Funding and additional information
This work was supported by the National Natural Science Foundation of China (NSFC) fund (32271285 and 31872720) and the R & D Program of Beijing Municipal Education Commission (KZ202210028043) to Jing L., NSFC (82273460) fund and the Yunnan Applied Basic Research Projects (202101AV070002 and 2019FY003030) to J. S., Beijing National Laboratory for Molecular Sciences (BNLMS202108) and the Chinese Academy of Sciences Pioneer Hundred Talents Program to X. W.
Reviewed by members of the JBC Editorial Board. Edited by Robert Haltiwanger
Contributor Information
Xiaohui Wang, Email: xiaohui.wang@ciac.ac.cn.
Jianwei Sun, Email: jwsun@ynu.edu.cn.
Jing Li, Email: jing_li@mail.cnu.edu.cn.
Supporting information
References
- 1.Zhao B.S., Roundtree I.A., He C. Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol. 2017;18:31–42. doi: 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adhikari S., Xiao W., Zhao Y.L., Yang Y.G. m(6)A: signaling for mRNA splicing. RNA Biol. 2016;13:756–759. doi: 10.1080/15476286.2016.1201628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei J., He C. Chromatin and transcriptional regulation by reversible RNA methylation. Curr. Opin. Cell Biol. 2021;70:109–115. doi: 10.1016/j.ceb.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi H., Wei J., He C. Where, when, and how: context-dependent functions of RNA methylation writers, readers, and erasers. Mol. Cell. 2019;74:640–650. doi: 10.1016/j.molcel.2019.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X., Zhao B.S., Roundtree I.A., Lu Z., Han D., Ma H., et al. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weng Y.L., Wang X., An R., Cassin J., Vissers C., Liu Y., et al. Epitranscriptomic m(6)A regulation of axon regeneration in the adult mammalian nervous system. Neuron. 2018;97:313–325.e316. doi: 10.1016/j.neuron.2017.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi H., Zhang X., Weng Y.L., Lu Z., Liu Y., Lu Z., et al. m(6)A facilitates hippocampus-dependent learning and memory through YTHDF1. Nature. 2018;563:249–253. doi: 10.1038/s41586-018-0666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han D., Liu J., Chen C., Dong L., Liu Y., Chang R., et al. Anti-tumour immunity controlled through mRNA m(6)A methylation and YTHDF1 in dendritic cells. Nature. 2019;566:270–274. doi: 10.1038/s41586-019-0916-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu Y., Zhuang X. m(6)A-binding YTHDF proteins promote stress granule formation. Nat. Chem. Biol. 2020;16:955–963. doi: 10.1038/s41589-020-0524-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han X., Wang M., Zhao Y.L., Yang Y., Yang Y.G. RNA methylations in human cancers. Semin. Cancer Biol. 2021;75:97–115. doi: 10.1016/j.semcancer.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 11.He P.C., He C. m(6) A RNA methylation: from mechanisms to therapeutic potential. EMBO J. 2021;40 doi: 10.15252/embj.2020105977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J., Harada B.T., He C. Regulation of gene expression by N(6)-methyladenosine in cancer. Trends Cell Biol. 2019;29:487–499. doi: 10.1016/j.tcb.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X., Shao F., Guo D., Wang W., Wang J., Zhu R., et al. WNT/beta-catenin-suppressed FTO expression increases m(6)A of c-Myc mRNA to promote tumor cell glycolysis and tumorigenesis. Cell Death Dis. 2021;12:462. doi: 10.1038/s41419-021-03739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Y., Fan S., Wu M., Zuo Z., Li X., Jiang L., et al. YTHDF1 links hypoxia adaptation and non-small cell lung cancer progression. Nat. Commun. 2019;10:4892. doi: 10.1038/s41467-019-12801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pi J., Wang W., Ji M., Wang X., Wei X., Jin J., et al. YTHDF1 promotes gastric carcinogenesis by controlling translation of FZD7. Cancer Res. 2021;81:2651–2665. doi: 10.1158/0008-5472.CAN-20-0066. [DOI] [PubMed] [Google Scholar]
- 16.Liu T., Wei Q., Jin J., Luo Q., Liu Y., Yang Y., et al. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucl. Acids Res. 2020;48:3816–3831. doi: 10.1093/nar/gkaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q., Guo X., Li L., Gao Z., Su X., Ji M., et al. N(6)-methyladenosine METTL3 promotes cervical cancer tumorigenesis and Warburg effect through YTHDF1/HK2 modification. Cell Death Dis. 2020;11:911. doi: 10.1038/s41419-020-03071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X., Lu Z., Gomez A., Hon G.C., Yue Y., Han D., et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou G., Zhao X., Li L., Yang Q., Liu X., Huang C., et al. SUMOylation of YTHDF2 promotes mRNA degradation and cancer progression by increasing its binding affinity with m6A-modified mRNAs. Nucl. Acids Res. 2021;49:2859–2877. doi: 10.1093/nar/gkab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong J., He J., Zhu J., Pan J., Liao W., Ye H., et al. Lactylation-driven METTL3-mediated RNA m(6)A modification promotes immunosuppression of tumor-infiltrating myeloid cells. Mol. Cell. 2022;82:1660–1677.e1610. doi: 10.1016/j.molcel.2022.02.033. [DOI] [PubMed] [Google Scholar]
- 21.Li Y., He X., Lu X., Gong Z., Li Q., Zhang L., et al. METTL3 acetylation impedes cancer metastasis via fine-tuning its nuclear and cytosolic functions. Nat. Commun. 2022;13:6350. doi: 10.1038/s41467-022-34209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart G.W., Slawson C., Ramirez-Correa G., Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X., Qian K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2017;18:452–465. doi: 10.1038/nrm.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin K., Zhu Y., Qin W., Gao J., Shao X., Wang Y.L., et al. Quantitative profiling of protein O-GlcNAcylation sites by an isotope-tagged cleavable linker. ACS Chem. Biol. 2018;13:1983–1989. doi: 10.1021/acschembio.8b00414. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y., Qin H., Yue X., Zhou J., Liu L., Nie Y., et al. Highly efficient enrichment of O-GlcNAc glycopeptides based on chemical oxidation and reversible hydrazide chemistry. Anal. Chem. 2021;93:16618–16627. doi: 10.1021/acs.analchem.1c04031. [DOI] [PubMed] [Google Scholar]
- 26.Woo C.M., Lund P.J., Huang A.C., Davis M.M., Bertozzi C.R., Pitteri S.J. Mapping and quantification of over 2000 O-linked glycopeptides in activated human T cells with isotope-targeted glycoproteomics (Isotag) Mol. Cell Proteomics. 2018;17:764–775. doi: 10.1074/mcp.RA117.000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J., Li Z., Duan X., Qin K., Dang L., Sun S., et al. An isotope-coded photocleavable probe for quantitative profiling of protein O-GlcNAcylation. ACS Chem. Biol. 2019;14:4–10. doi: 10.1021/acschembio.8b01052. [DOI] [PubMed] [Google Scholar]
- 28.Huo B., Zhang W., Zhao X., Dong H., Yu Y., Wang J., et al. A triarylphosphine-trimethylpiperidine reagent for the one-step derivatization and enrichment of protein post-translational modifications and identification by mass spectrometry. Chem. Commun. (Camb) 2018;54:13790–13793. doi: 10.1039/c8cc08416e. [DOI] [PubMed] [Google Scholar]
- 29.Ramakrishnan P., Clark P.M., Mason D.E., Peters E.C., Hsieh-Wilson L.C., Baltimore D. Activation of the transcriptional function of the NF-kappaB protein c-Rel by O-GlcNAc glycosylation. Sci. Signal. 2013;6:ra75. doi: 10.1126/scisignal.2004097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian J., Geng Q., Ding Y., Liao J., Dong M.Q., Xu X., et al. O-GlcNAcylation antagonizes phosphorylation of CDH1 (CDC20 homologue 1) J. Biol. Chem. 2016;291:12136–12144. doi: 10.1074/jbc.M116.717850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirli K., Karaca S., Dehne H.J., Samwer M., Pan K.T., Lenz C., et al. A deep proteomics perspective on CRM1-mediated nuclear export and nucleocytoplasmic partitioning. Elife. 2015;4 doi: 10.7554/eLife.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gloster T.M., Zandberg W.F., Heinonen J.E., Shen D.L., Deng L., Vocadlo D.J. Hijacking a biosynthetic pathway yields a glycosyltransferase inhibitor within cells. Nat. Chem. Biol. 2011;7:174–181. doi: 10.1038/nchembio.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim Y.H., Han M.E., Oh S.O. The molecular mechanism for nuclear transport and its application. Anat. Cell Biol. 2017;50:77–85. doi: 10.5115/acb.2017.50.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun H., Huang Y., Mei S., Xu F., Liu X., Zhao F., et al. A nuclear export signal is required for cGAS to sense cytosolic DNA. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2020.108586. [DOI] [PubMed] [Google Scholar]
- 35.Fung H.Y., Fu S.C., Chook Y.M. Nuclear export receptor CRM1 recognizes diverse conformations in nuclear export signals. Elife. 2017;6 doi: 10.7554/eLife.23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varadi M., Anyango S., Deshpande M., Nair S., Natassia C., Yordanova G., et al. AlphaFold protein structure database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucl. Acids Res. 2022;50:D439–D444. doi: 10.1093/nar/gkab1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mirdita M., Schutze K., Moriwaki Y., Heo L., Ovchinnikov S., Steinegger M. ColabFold: making protein folding accessible to all. Nat. Met. 2022;19:679. doi: 10.1038/s41592-022-01488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guttler T., Madl T., Neumann P., Deichsel D., Corsini L., Monecke T., et al. NES consensus redefined by structures of PKI-type and Rev-type nuclear export signals bound to CRM1. Nat. Struct. Mol. Biol. 2010;17:1367–1376. doi: 10.1038/nsmb.1931. [DOI] [PubMed] [Google Scholar]
- 40.Gray J.J., Moughon S., Wang C., Schueler-Furman O., Kuhlman B., Rohl C.A., et al. Protein-protein docking with simultaneous optimization of rigid-body displacement and side-chain conformations. J. Mol. Biol. 2003;331:281–299. doi: 10.1016/s0022-2836(03)00670-3. [DOI] [PubMed] [Google Scholar]
- 41.Wang C., Schueler-Furman O., Baker D. Improved side-chain modeling for protein-protein docking. Protein Sci. 2005;14:1328–1339. doi: 10.1110/ps.041222905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaudhury S., Berrondo M., Weitzner B.D., Muthu P., Bergman H., Gray J.J. Benchmarking and analysis of protein docking performance in rosetta v3.2. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Queiroz R.M., Carvalho E., Dias W.B. O-GlcNAcylation: the sweet side of the cancer. Front. Oncol. 2014;4:132. doi: 10.3389/fonc.2014.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mi W., Gu Y., Han C., Liu H., Fan Q., Zhang X., et al. O-GlcNAcylation is a novel regulator of lung and colon cancer malignancy. Biochim. Biophys. Acta. 2011;1812:514–519. doi: 10.1016/j.bbadis.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 45.Liao S., Sun H., Xu C. YTH domain: a family of N(6)-methyladenosine (m(6)A) readers. Genomics Proteomics Bioinform. 2018;16:99–107. doi: 10.1016/j.gpb.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y., Liu J., Jin X., Zhang D., Li D., Hao F., et al. O-GlcNAcylation destabilizes the active tetrameric PKM2 to promote the Warburg effect. Proc. Natl. Acad. Sci. U. S. A. 2017;114:13732–13737. doi: 10.1073/pnas.1704145115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan W., Jiang P., Zhang W., Hu Z., Lin S., Chen L., et al. Posttranscriptional regulation of de novo lipogenesis by glucose-induced O-GlcNAcylation. Mol. Cell. 2021;81:1890–1904.e1897. doi: 10.1016/j.molcel.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 48.Yang W., Zheng Y., Xia Y., Ji H., Chen X., Guo F., et al. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat. Cell Biol. 2012;14:1295–1304. doi: 10.1038/ncb2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flynn R.A., Pedram K., Malaker S.A., Batista P.J., Smith B.A.H., Johnson A.G., et al. Small RNAs are modified with N-glycans and displayed on the surface of living cells. Cell. 2021;184:3109–3124.e3122. doi: 10.1016/j.cell.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qin W., Qin K., Fan X., Peng L., Hong W., Zhu Y., et al. Artificial cysteine S-glycosylation induced by per-O-acetylated unnatural monosaccharides during metabolic glycan labeling. Angew. Chem. Int. Ed. Engl. 2018;57:1817–1820. doi: 10.1002/anie.201711710. [DOI] [PubMed] [Google Scholar]
- 51.Yang Y., Yan Y., Yin J., Tang N., Wang K., Huang L., et al. O-GlcNAcylation of YTHDF2 promotes HBV-related hepatocellular carcinoma progression in an N(6)-methyladenosine-dependent manner. Signal. Transduct. Target Ther. 2023;8:63. doi: 10.1038/s41392-023-01316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Z., Li X., Nai S., Geng Q., Liao J., Xu X., et al. Checkpoint kinase 1-induced phosphorylation of O-linked beta-N-acetylglucosamine transferase regulates the intermediate filament network during cytokinesis. J. Biol. Chem. 2017;292:19548–19555. doi: 10.1074/jbc.M117.811646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J., Wang J., Hou W., Jing Z., Tian C., Han Y., et al. Phosphorylation of Ataxin-10 by polo-like kinase 1 is required for cytokinesis. Cell Cycle. 2011;10:2946–2958. doi: 10.4161/cc.10.17.15922. [DOI] [PubMed] [Google Scholar]
- 54.Cao X., Li C., Xiao S., Tang Y., Huang J., Zhao S., et al. Acetylation promotes TyrRS nuclear translocation to prevent oxidative damage. Proc. Natl. Acad. Sci. U. S. A. 2017;114:687–692. doi: 10.1073/pnas.1608488114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jo S., Song K.C., Desaire H., MacKerell A.D., Jr., Im W. Glycan reader: automated sugar identification and simulation preparation for carbohydrates and glycoproteins. J. Comput. Chem. 2011;32:3135–3141. doi: 10.1002/jcc.21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Der Spoel D., Lindahl E., Hess B., Groenhof G., Mark A.E., Berendsen H.J. Gromacs: fast, flexible, and free. J. Comput. Chem. 2005;26:1701–1718. doi: 10.1002/jcc.20291. [DOI] [PubMed] [Google Scholar]
- 57.Abraham M.J., Murtola T., Schulz R., Páll S., Smith J.C., Hess B., et al. Gromacs: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1-2:19–25. [Google Scholar]
- 58.Jo S., Kim T., Iyer V.G., Im W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 2008;29:1859–1865. doi: 10.1002/jcc.20945. [DOI] [PubMed] [Google Scholar]
- 59.Best R.B., Zhu X., Shim J., Lopes P.E., Mittal J., Feig M., et al. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone phi, psi and side-chain chi(1) and chi(2) dihedral angles. J. Chem. Theor. Comput. 2012;8:3257–3273. doi: 10.1021/ct300400x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang J., Rauscher S., Nawrocki G., Ran T., Feig M., de Groot B.L., et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Met. 2017;14:71–73. doi: 10.1038/nmeth.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parrinello M., Rahman A. Polymorphic transitions in single-crystals - a new molecular-dynamics method. J. Appl. Phys. 1981;52:7182–7190. [Google Scholar]
- 62.Evans D.J., Holian B.L. The nose-hoover thermostat. J. Chem. Phys. 1985;83:4069–4074. [Google Scholar]
- 63.Blum C.A., MJB Roli A., Sampels M. Springer-Verlag; Berlin Heidelberg: 2008. Hybrid Metaheuristics, an Emerging Approach to Optimization. [Google Scholar]
- 64.Darden T., York D., Pedersen L. Particle mesh Ewald: AnN⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993;98:10089–10092. [Google Scholar]
- 65.Michaud-Agrawal N., Denning E.J., Woolf T.B., Beckstein O. MDAnalysis: a toolkit for the analysis of molecular dynamics simulations. J. Comput. Chem. 2011;32:2319–2327. doi: 10.1002/jcc.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valdes-Tresanco M.S., Valdes-Tresanco M.E., Valiente P.A., Moreno E. gmx_MMPBSA: a new tool to perform end-state free energy calculations with GROMACS. J. Chem. Theor. Comput. 2021;17:6281–6291. doi: 10.1021/acs.jctc.1c00645. [DOI] [PubMed] [Google Scholar]
- 67.Massova I., Kollman P.A. Combined molecular mechanical and continuum solvent approach (MM-PBSA/GBSA) to predict ligand binding. Perspect. Drug Discov. 2000;18:113–135. [Google Scholar]
- 68.PyMol . The PyMOL Molecular Graphics System. Schrödinger, LLC; New York, NY: 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the manuscript.