Abstract
Nanocarriers have therapeutic potential to facilitate drug delivery, including biological agents, small-molecule drugs, and nucleic acids. However, their efficiency is limited by several factors; among which, endosomal/lysosomal degradation after endocytosis is the most important. This review summarizes advanced strategies for overcoming endosomal/lysosomal barriers to efficient nanodrug delivery based on the perspective of cellular uptake and intracellular transport mechanisms. These strategies include promoting endosomal/lysosomal escape, using non-endocytic methods of delivery to directly cross the cell membrane to evade endosomes/lysosomes and making a detour pathway to evade endosomes/lysosomes. On the basis of the findings of this review, we proposed several promising strategies for overcoming endosomal/lysosomal barriers through the smarter and more efficient design of nanodrug delivery systems for future clinical applications.
Introduction
Owing to their unique physicochemical, biological, optical, electrical, and catalytic activities, nanodrugs can overcome the pharmacokinetic limitations associated with traditional pharmaceutical agents [1,2]. An intravenously delivered nanodrug typically has to pass through 5 consecutive processes [3]: circulation in the blood compartments, accumulation into the target area, subsequent penetration deeply into the tissue, cellular uptake by cells, and intracellular release of drug from endosome or lysosome. Therefore, researchers have designed nanocarriers with different assembly modes to overcome multiple intracellular obstacles during delivery [4]. Because most drug delivery systems pass through the endosome–lysosome pathway after cellular uptake, the ability of endosomal/lysosomal escape is one of the crucial factors affecting delivery efficiency [5]. Most intracellular transport pathways include internalization into an endocytic vesicle, fusion with the early endosome (EE), maturation into a late endosome (LE), and accumulation in the lysosome [6]. Significantly, compared with the physiological pH of 7.4, the pH gradually decreases during maturation of the endosome (EE pH ∼ 6.5, LE pH ∼ 6.0, and lysosome has a lower pH ∼ 5.0) [7]. Lysosomes contain various degradative enzymes (such as nucleases and phosphatases), and failure to escape rapidly from lysosomes usually results in entrapment and potential degradation, leading to the unsuccessful delivery of therapeutic drugs [8,9].
Overcoming the endosomal/lysosomal barrier is a crucial step in the successful delivery of nanodrugs for the treatment of various diseases, including cancer, neurodegenerative disorders, and infectious diseases. For example, the blood–brain barrier (BBB) can prevent nanodrugs from reaching the brain, making it challenging to treat brain glioma and neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease [10]. Most nanocarriers undergo lysosomal degradation after they are internalized by endothelial cells via endocytosis, failing to penetrate the BBB via transcytosis. Strategies to overcome the endosomal/lysosomal barrier are helpful to deliver therapeutic agents across BBB. Similarly, strategies such as pH-sensitive or fusogenic nanoparticles can help release drugs from the endosomal/lysosomal compartments of infected cells, improving their efficacy to treat hepatitis B and C viruses.
To achieve rapid drug release into the cytoplasm, various strategies have been reported in research articles and reviews. These strategies are mainly related to various biological mechanisms [11–16], including the proton sponge effect, membrane instability, membrane fusion, and membrane disruption. However, most previous reviews have primarily focused on strategies that promote lysosomal escape, thus limiting the findings, instead of summarizing more strategies from the perspective of cellular and intracellular transport mechanisms. For example, strategies that do not involve entry into endosomes or lysosomes have not been considered in some reviews [17]. To this end, this review highlights the following 3 strategies for overcoming lysosomal barriers based on the perspective of cellular uptake and intracellular transport mechanisms: (a) promoting endosomal/lysosomal escape, (b) crossing the cell membrane without entering endosomes or lysosomes, and (c) making a detour pathway to evade lysosomes and avoid degradation. These strategies may provide novel and more comprehensive design ideas for overcoming endosomal/lysosomal barriers to efficient nanodrug delivery.
Cellular Uptake and Intracellular Transport Mechanisms
An in-depth understanding of the mechanisms of cellular and intracellular transport is necessary to resolve the problem of endosomal/lysosomal degradation. Although studies on intracellular transport and lysosomal escape are limited [18], some studies have comprehensively investigated the molecular mechanisms underlying these processes [9,19,20]. For example, Gilleron et al. [9] used fluorescence imaging and electron microscopy to examine the cellular uptake and delivery characteristics of an ionizable lipid nanoparticle-based delivery system. This system mainly entered the cell through clathrin-mediated endocytosis (CME) and micropinocytosis (MP); however, only a small fraction of small interfering RNA (siRNA) (<2%) was released into the cytoplasm from endosomes/lysosomes. Similarly, another study reported that approximately 70% of siRNA was transported to the extracellular medium by circulating endosomes through a pathway mediated by Niemann–Pick C1 within 24 h of nanoparticle uptake [21]. Fichter et al. [20] indicated that Glycofect polymer nanoparticles were delivered via a non-sibling internal transport pathway, which induced their higher accumulation in the Golgi apparatus and endoplasmic reticulum (ER) owing to the presence of galactose in the nanoparticles. Therefore, the intracellular transport of drugs can be achieved by not only the endosome–lysosome pathway but also the different transport routes that may help to overcome endosomal/lysosomal barriers. However, the mechanisms underlying the internalization of extracellular materials and their intracellular transport vary greatly depending on the type of materials [22]. It is necessary to understand these mechanisms to design better delivery vectors and resolve the subsequent problem of endosomal/lysosomal degradation.
Cellular uptake mechanisms
Cellular uptake, intracellular transport, and localization of nanoparticles are primarily determined by the physical and chemical properties of nanocarriers, including their size, zeta potential, and surface modification [23]. On the basis of their size and surface modification, nanoparticles are internalized through phagocytosis or pinocytosis. Phagocytosis refers to the internalization of bacteria, fragments, or cargos of large sizes, whereas pinocytosis refers to the internalization of smaller components (Fig. 1). Endocytic pathways can be subdivided into CME, caveolae-mediated endocytosis (CvME), lipid raft-mediated endocytosis, MP, and membrane fusion [24,25].
Fig. 1.

Routes and mechanisms of cellular uptake and intracellular transport. ATPase, adenosine triphosphatase.
CME is regulated by clathrin and its adaptor proteins. Pentagonal or hexagonal grid-like structural concavities initially form capsule vesicles, and nanoparticles are endocytosed through several processes as follows: recruitment of an adaptor protein and clathrin, invagination and constriction of capsule vesicles, budding of vacuoles, and shelling of coated vacuoles [26]. CME is an important cellular uptake pathway for endogenous substances, including transferrin and low-density lipoprotein. Studies have demonstrated that a drug delivery system modified with endogenous substances is mainly internalized through CME [27]. For example, nanoparticles modified with peptide-22 targeting low-density lipoprotein enter cells mainly through reticulin-mediated endocytosis [28]. In addition, studies have demonstrated that most delivery systems designed based on lipids are internalized via CME [29].
CvME is coordinated by caveolae, which are cup-shaped/omega-shaped invaginations of the plasma membrane. Lipid density, cholesterol content, glycosphingolipid content, and sphingomyelin content are relatively high in the bilayer structure [30]. In addition to dynein, glycosylphosphatidylinositol-anchored proteins and tyrosine kinases, caveolin, and caveolae-related proteins are characteristic components of caveolae. Although the specific regulatory mechanisms remain unclear, many nanocarriers can enter the cell via CvME, such as magnetic nanoparticles containing siRNA of green fluorescent protein [31]. In addition, the uptake pathway of the polyethyleneimine (PEI)/DNA and poly(amidoamine) (PAMAM)/DNA complexes is associated with caveolin-mediated endocytosis [32]. Note that the diameter of caveolin vesicles is approximately 30 to 80 nm, and nanoparticles with a large particle size cannot be internalized through CvME. For example, silica nanoparticles with a 90-mm diameter modified with antibodies targeting HER2 can enter the cells through CvME; however, nanoparticles with a 200-nm diameter cannot be internalized through CvME [33]. In addition, surface properties can affect endocytosis. For example, a high-glucose environment can inhibit reticulin-mediated endocytosis and enhance CvME. Park et al. [34] prepared the polymannose PEI material (PMT) by cross-linking PEI with mannose diacrylate, and the results indicated that the PMT/DNA complex was internalized via caveolin-mediated endocytosis.
Similar to caveolae, lipid rafts are microregions on the cell membrane that are rich in protein receptors and sphingolipids and are highly ordered and more compact. Viral particles are internalized through lipid raft-induced endocytosis through glycoprotein binding [35], and low-density lipoprotein can promote the endocytosis of the amyloid precursor protein through this pathway [36].
MP is a method of nonselective endocytosis of water-soluble molecules [37]. It involves the formation of large and irregular endocytic vesicles derived from the cell membrane and mediates the endocytosis of nanoparticles with large particle sizes (250 nm) [38]. It depends on actin and is regulated by various proteins, such as phosphoinositide 3 kinase and Rab family members. Additionally, MP is sensitive to the pH of the cytoplasm. Cytoplasmic acidification can significantly reduce the movement of cell membrane folds [39]. Some lipid-based nucleic acid delivery systems are thought to enter cells through macrocytosis. For example, macrocytosis is considered the only pathway through which “core–shell” nanocarriers constructed on the basis of protamine/DNA/cationic liposomes are internalized by Chinese hamster ovary (CHO) cells [40].
Another mechanism of cell uptake is direct fusion with the cell membrane, which successively goes through lipid bilayers close to each other, external hydrophilic layer direct contact, protein-mediated fusion, and finally the fusion pore opens. This mechanism is mediated by different proteins, mainly including SNAREs, (soluble N -ethylmaleimide-sensitive factor attachment protein receptors), Rab proteins, and Sec1/Munc-18 related proteins [41,42]. Exosomes, biofilm carriers, and membrane-penetrating peptide-modified carriers are often internalized via membrane fusion because they have a corresponding affinity for target cell membranes, which renders membrane fusion more efficient. For example, Yang et al. [43] prepared virus-like exosomes that could directly deliver the encapsulated protein into target cells through membrane fusion.
Intracellular transport mechanisms
After nanoparticles are endocytosed through the abovementioned pathways, they are transported intracellularly through different pathways involving endosomes, lysosomes, the Golgi apparatus, and ER, owing to the differences in their cellular uptake pathways and particle characteristics. To date, 3 intracellular transport pathways have been widely reported, namely, the classical endosome–lysosome pathway, the endosome–Golgi pathway, and the Golgi–ER pathway.
The endosome–lysosome pathway is the most common intracellular transport mechanism, which is closely related to the maturation of endosomes. As shown in Fig. 1, primary endocytic vesicles deliver their contents to EEs around the cytoplasm. After approximately 8 to 15 min, the contents accumulate in EEs and are recycled to the plasma membrane (directly or through internal circulation of endosomes), resulting in the transformation of EEs to LEs. As LEs move along the microtubule (MT) to the periphery of the nucleus, they fuse with newly formed lysosomes and transform into the classic mature lysosomes with a low pH [44]. Consequently, when nanocarriers are transported through the endosome–lysosome pathway, they are bound to undergo degradation and destruction by acids and enzymes, which is detrimental to the subsequent effects of drugs. Therefore, the endosome–lysosome pathway is considered a degradative intracellular transport pathway. Studies have demonstrated that nanocarriers that are internalized by cells through CME or part of MP are transported intracellularly through the endosome–lysosome pathway. For example, the liposome/VEGF (vascular endothelial growth factor) siRNA system modified with angiopep and tLyP-1 peptides enters lysosomes after its internalization through CME [45]. Similarly, core–shell nanoparticles, which enter cells through macrocytosis, rapidly colocalize with lysosomes in the cells [40].
The Golgi apparatus acts as a “transit station” for intracellular transport (Fig. 1). After endocytosis, the internalized contents are initially transported to the Golgi apparatus, which generates new vesicles in the form of “budding.” These vesicles are transported to endosomes, lysosomes, or ER via regulation of various signals. The transport from endosomes to the Golgi apparatus is regulated by acid hydrolase receptors, transmembrane enzymes, and SNARE proteins, which can initiate retrograde transport and fusion from EEs/LEs to the trans-Golgi network (TGN) [46]. This transport pathway is considered an important mechanism to avoid lysosomal degradation after endocytosis [47]. SNAREs are membrane proteins that are widely distributed in the ER, Golgi apparatus, and endosomes and are sparsely distributed on the cell membrane. According to the SNARE hypothesis, v-SNARE on transport vesicles specifically interacts with homologous t-SNARE on the target membrane, and the formation of a very stable 4-helix bundle results in the fusion of the 2 diaphragms [47]. For example, shiga and cholera toxins are transported from endosomes to TGN and eventually to ER, where they exert their cytotoxic effects [48].
The 2 types of transport modes between the Golgi apparatus and ER are as follows: (a) COPI vesicles mediate the transport from the Golgi apparatus to ER, and (b) COPII vesicles mediate the transport from the ER to Golgi apparatus. Retrograde transport by COPI vesicles mainly involves various types of proteins [49], with the most important proteins being resident proteins with a representative KDEL signal (Lys-Asp-Glu-Leu tetrapeptide). The KDEL receptor interacts with soluble secreted proteins at the lower pH of the Golgi apparatus and is directed to ER through COPI vesicles. Subsequently, the receptor releases resident proteins into the cavity at the neutral pH of ER. COPI vesicles can induce transport from the Golgi apparatus to ER, thereby avoiding lysosomal degradation. Therefore, the Golgi–ER pathway is considered a non-degradative transport mode for overcoming lysosomal barriers.
Strategies for Overcoming Endosomal/Lysosomal Barriers
On the basis of the abovementioned mechanisms of cellular uptake and intracellular transport, researchers have developed several strategies for resolving the problem of lysosomal degradation and destruction (Fig. 2). These strategies are primarily divided into 3 categories as follows: (a) strategies for promoting endosomal/lysosomal escape, (b) strategies for directly crossing the cell membrane without entering endosomes or lysosomes, and (c) strategies in which different pathways are used to evade lysosomes and avoid degradation. Furthermore, these strategies for overcoming endo-/lysosomal barrier in nanodrug delivery have been summarized in Table.
Fig. 2.

A schematic overview of 3 main strategies used for overcoming lysosomal barriers. (A) Strategies to promote endosomal/lysosomal escape. (B) Strategies for directly crossing the cell membrane without entering endosomes or lysosomes. (C) Strategies to change the pathway to evade lysosomes and avoid degradation.
Table.
The summary of strategies for overcoming endo/lysosomal barrier in nanodrug delivery.
| Strategy | Mechanism | Materials | Drug/agent | Disease/cells | Ref. |
|---|---|---|---|---|---|
| Promote endo/lysosomal escape | Proton sponge effect | Poly-l-lysine (PLL), polyphenol (-)-epigallocatechin gallate (EGCG) | dsRNA | Sf9 cells | [50] |
| Poly(amidoamine) dendrimers (PAMAM) | Cisplatin | BxPC-3 cells | [51] | ||
| Poly(histidine-arginine)6-modified chitosan | siRNA | 4T1 cells and B16F10 cells | [52] | ||
| PEG-PCL-PEI | siRNA | SKOV3 cells | [53] | ||
| Pyridinium p-toluenesulfonate (PPTS) | Doxorubicin | Panc-1 cells | [54] | ||
| Osmotic lysis effect | PMPC-bPDPAEMA | Dyes | 22 different cells | [55] | |
| mPEG45-P(DPA50-co-DMAEMA56)-PT53 | siRNA | HepG2 cells | [56] | ||
| DOPA, DOTAP, CaP nanoparticles (NPs) | siRNA | H460 cells | [57,58] | ||
| Alendronate-hyaluronan, CaP NPs | siRNA | A549 cells | [59] | ||
| Swelling effect | PDEAEMA, PAEMA, PEGDMA | Ovalbumin | DC2.4 cells | [60] | |
| 2,4,6-Trimethoxy benzaldehyde, 5-methyl-2-(2,4,6-trimethoxy phenyl)-[1,3]-5-dioxanylmethanol | Paclitaxel | LLC cells | [61] | ||
| Poly(c-GA-co-c-GAOSu)-g-mPEG | Doxorubicin | HeLa cells | [62] | ||
| Pore formation | LPEI polyplexes | ODA/pDNA | HeLa cells | [63] | |
| PEI, HIV gp41 peptide | siRNA/pDNA | HeLa cells | [64] | ||
| PEGylated Au-NPs | LLO protein | MEF cells | [65] | ||
| Membrane disruption | Aptamer modified iron-deficient protein nanocages (Apn), HA-2 peptide | siRNA | MCF-7 cells | [66] | |
| DSPE-PCB lipoplexes | siRNA | HeLa cells | [67] | ||
| Ultrasound-responsive polymersome PEO-b-P(DEA-stat-MEMA) | Doxorubicin | L02 cells and HeLa cells | [68] | ||
| Temperature-sensitive “bubble liposomes”: PEG modified DOTAP/DOPE/cholesterol liposomes, ammonium bicarbonate | siRNA | H69AR cells | [69] | ||
| Membrane fusion | Core–shell liposomes: DSPE-PEG/DOTAP/DOPE/cholesterol | siRNA | U87 and MCF-7 cells | [70–73] | |
| R8/KALA-T-MEND | pDNA | JAWS II cells | [74] | ||
| PEgylated Tf-liposome incorporating Chol-GALA | pDNA | K562 cells | [75] | ||
| Photochemical internalization | CPP-cargo-linker-photosensitizer | shRNA | CHO cells | [76] | |
| TPPS2A, silica core–shell NPs | Photomorpholinos | B16-F0 cells | [77] | ||
| thiolated CPT (CPT-DP), thiolated PEG-b-P(Glu-DP) | Camptothecin (CPT) | HeLa cells | [78] | ||
| Non-endocytosis delivery by directly crossing the cell membrane | Membrane fusion | PEG/fusogenic porous silicon NPs | siRNA | CAOV-3 cells | [79] |
| F68, PCL, cancer cell membrane | Paclitaxel | 4T1 cells | [80] | ||
| Coiled-coil SNARE lipopeptides (CPK4, CPE4) | Doxorubicin | HeLa cells | [81] | ||
| Oligonucleotide (ON) template-assisted polymerization | Oligonucleotides | HeLa cells | [82] | ||
| CPP/single-wall carbon nanotubes | siRNA | HeLa cells | [83] | ||
| Carbon nanotube porins (CNTPs) | Doxorubicin | MDA-MB231 cells | [84] | ||
| Make a detour pathway to evade lysosomes | Cavosome-mediated transport | CPG-modified siRNA | siRNA | RAW264.7 and B16 cells | [85] |
| Histone/PEI polyplexes | pDNA | CHO cells | [86] | ||
| Transferrin-PEI/folate-PEI | siRNA | HeLa cells | [87] | ||
| Transported to Golgi/ER | KDEL modified Au NPs | pDNA | Sol8 cells | [88] | |
| ER membrane decorated DOTAP/DOPE/cholesterol liposome | siRNA | MCF-7 cells | [89] |
Endosomal/lysosomal escape
The endosome–lysosome pathway is considered a degradative intracellular transport pathway because nanoparticles are bound to undergo degradation and destruction by acids and enzymes, which is detrimental to the subsequent effects of encapsulated drugs. Therefore, the first goal is to escape from endosomes or lysosomes rapidly. The mechanisms underlying endosomal/lysosomal escape are highly controversial but involve trafficking across the endosomal/lysosomal membrane (Fig. 3).
Fig. 3.

(A to G) A schematic overview of mechanisms underlying endosomal escape.
This section describes 4 advanced strategies used for promoting endosomal/lysosomal escape of nanodrugs: the proton sponge effect of outstanding pH buffering; osmotic lysis resulting from pH-responsive disassembly of nanoparticles; as well as the swelling effect of pH-responsive nanoparticles and membrane destabilization induced by pore formation, membrane disruption, membrane fusion, and photochemical internalization.
Endosomal/lysosomal escape through the proton sponge effect
The proton sponge effect is a typical method of inducing endosomal/lysosomal escape based on the buffering action of materials in a physiologically relevant range (Fig. 3A) [90]. The protonated amino groups in cationic materials are chelated with protons provided using proton pumps (vacuolar-ATPase), resulting in the continuous opening of the pumps. Each proton can result in the entrapment of a chloride ion and water molecule in lysosomes, thereby leading to the swelling and rupture of lysosomes and the release of cationic nanocarriers into the cytoplasm. This strategy is usually applied to polycationic materials, including PEI [91], poly-l-lysine (PLL) [50,92,93], PAMAM dendrimers [94], chitosan [95], poly(silamine) [96], urocanic acid-modified chitosan [97], chloroquine [98], and others containing secondary or tertiary amines (Fig. 4).
Fig. 4.

Structure of representative materials with the capability to escape from endosomes or lysosomes via the proton sponge effect.
Owing to the inclusion of protonatable amino groups, the abovementioned materials are protonated to produce a “proton sponge” effect at the low pH (4 to 5.5) of endosomes or lysosomes. For example, Sun et al. [52] covalently linked poly(histidine arginine) (H6R6) with chitosan to encapsulate siRNAs, and the lysosomal escape ability of these modified chitosan nanoparticles was significantly stronger than that of unmodified chitosan nanoparticles. On the basis of the proton sponge effect, materials have been designed as a component of delivery systems for encapsulating drugs and facilitating their lysosomal escape, such as the triblock copolymers PEG-PCL-PEI for delivering siRNA [53] and PAMAM/carbon dot nanohybrids for chemotherapy [99]. Lee et al. [100] prepared rod-shaped nanoparticles formed by bundles of Janus base nanotubes (JBNTs) with RNA cargos incorporated inside via charge interactions. Similar to lipid nanoparticles, JBNTs/RNA nanoparticles efficiently entered cells via macrocytosis. Additionally, similar to cationic polymers, JBNTs/RNA nanoparticles had an enhanced endosomal escape ability because of their noncovalent structure and DNA-mimicking chemistry, which promoted the proton sponge effect. Chen et al. [12] developed metal-phenolic networks assembled with the polyphenol tannic acid and FeIII or AlIII as versatile and nontoxic modifications to promote endosomal/lysosomal escape through the proton sponge effect. The lysosomal escape ability was closely related to the amount of material used [101]. Effective transfection necessitates a significant excess of polymers, which frequently causes dose-limiting toxicity during in vivo application [14].
With the understanding and development of the proton sponge theory, researchers have developed various polymeric nanocarriers with increased buffering capacities. However, these nanocarriers have limited endosomal escape ability. Studies have reported that the buffering capacity of some polymer-based delivery systems was insufficient to achieve endosomal escape during their transport from endosomes/lysosomes to the cytoplsm [54,102]. Because only a few lysosomes per cell can fluctuate (a few hundred to several thousand lysosomes are found in each cell), only 1% of carriers can achieve escape [103,104]. Benjaminsen et al. [104] demonstrated that polyplexes prepared using PEI or derivatives did not alter the endosomal/lysosomal pH and suggested that the proton sponge effect did not play a vital role in escape. However, these results are based on the experimental data of a single study and have not been verified using a large number of reproducible statistics. Most importantly, escape is a very rapid and transient behavior, and the limitations of detection techniques necessitate the validation of study findings. Above all, as the earliest lysosomal escape mechanism, there is a large amount of literature supporting the existence of the proton sponge effect, and we mentioned it here in the hope that there will be more experimental data to give us a clearer direction in the future, just as truth arises in debate.
Endosomal/lysosomal escape through osmotic lysis
pH-responsive nanoparticles can disassemble at lower pH to mediate endosomal/lysosomal escape. The disassembly of nanoparticles into numerous polymer subunits results in osmotic shock that leads to the rupture of endosomes/lysosomes (Fig. 3B) [105–107]. This process is termed osmotic lysis. Massignani et al. [55] reported that the decrease of pH in endosomes mediated the rapid disassembly of nanoparticles prepared using the deblock copolymer PMPC-bPDPAEMA into abundant monomers, and the sharply increasing osmotic pressure promoted the release of cargo. Similarly, Li et al. [56] synthesized a range of quaternary ammonium-based amphiphilic triblock polymers to assemble pH-sensitive nanoparticles. The core was hydrophobic at physiological pH; however, the nanoparticles protonated to become hydrophilic and mutually exclusive in an acidic environment. When incubated with endosomes (pH 6.5 to 6.8), the siRNA-loaded mPEG45-P(DPA50-co-DMAEMA56)-PT53 nanoparticles rapidly disassembled, leading to the cytosolic release of siRNA and enhanced gene silencing activity.
Inorganic nanoparticles, such as those prepared using Ca2+ [108,109] and Zn2+ [110], may have an enhanced endosomal/lysosomal escape ability owing to their rapid dissolution in acidic environments. Drug-encapsulated inorganic nanocarriers undergo ionic reactions and dissolve in the acidic environment of lysosomes. Subsequently, they release a large number of ions, resulting in a dramatic increase in the internal osmotic pressure of lysosomes, leading to fluctuations in lysosomal water absorption and the release of drugs into the cytosol for better effects. The most representative inorganic nanocarriers are calcium phosphate (CAP) nanoparticles. Zhang et al. [57] and Li et al. [58] constructed cationic lipid membrane-coated CAP/siRNA nanoparticles through water-in-oil microemulsion using Ca2+, HPO42−, and DOPA (1,2-dioleoyl-sn-glycero-3-phosphate) as basic raw materials. Under the acidic conditions of lysosomes, the dissolution of CAP released a large number of ions, resulting in a rapid increase in lysosomal osmotic pressure, followed by water influx to disrupt lysosomal fluctuation and release siRNAs into the cytosol. These results strongly verified the lysosomal escape property of CAP nanoparticles.
CAP, a natural inorganic material with better biocompatibility and biodegradability, has high transfection efficiency and is considered a promising vehicle for gene delivery. However, its use as a nanocarrier is challenging owing to the lack of tissue specificity and the uncontrollable growth of size in a physiological solution. Therefore, various derivatives of chitosan, hyaluronic acid, and poly(ethylene glycol) (PEG) have been used to synthesize CAP nanoparticles with an enhanced lysosomal escape ability [111–117]. Qiu et al. [59] synthesized alendronate-hyaluronic acid (AHA) conjugates and prepared a novel core–shell CAP-AHA/siRNA delivery system by coating AHA around the inner core assembled through the chemical chelation of Ca2+ and phosphate ions (Fig. 5A). The internalized nanoparticles exhibited a pH-dependent siRNA release and contributed to rapid escape from endosomes/lysosomes. Together, nanocarriers that can promote osmotic lysis can convert the inferior environment of lysosomes into a superior environment using the acidic characteristics of lysosomes to facilitate the dissolution of nanocarriers and the release of drugs instead of only avoiding lysosomal degradation.
Fig. 5.

(A) Lysosomal escape induced by the pH-sensitive disassembling of CAP nanoparticles [59]. (B) Schematic diagram of DSPE-PCB lipoplexes for siRNA delivery with enhanced siRNA endosomal/lysosomal escape ability [67]. (C) Illustration of ultrasound-responsive polymersomes for facilely controlled drug delivery in tumor cells [68]. (D) GALA-mediated membrane fusion induced by the lowering of the pH in endosome and enhanced the endosomal escape of DNA core particles to the cytoplasmic space [75]. (E) The mechanism of photosensitizers enhancing endosomal escape [77]. UCNs, upconversion nanoparticles; DOX, doxorubicin; NIR, near infrared; UV, ultraviolet; Vis, visible.
Endosomal/lysosomal escape induced by the swelling effect
Various pH-responsive materials have been introduced to design nanoparticles that can swell at a lower pH in lysosomes after endocytosis. Tang et al. [118] reported that swelling polymers can enhance transfection. They used intact and fragmented polyacrylamide (PAM) dendrimers to regulate the flexibility and pH-sensitive enlargement of nanocarriers. Compared with nanocarriers synthesized using intact dendrimers with steric constraints, those synthesized using fragmented dendrimers with optimal flexibility had higher transfection efficiency after administration. On the basis of these findings, Szoka et al. [119] advocated using the term “umbrella hypothesis” to describe the volumetric expansion of polymers during protonation. A swelling nanoparticle is similar to an open umbrella, and the rapidly expanding volume of nanocarriers disrupts the membrane and promotes lysosomal escape (Fig. 3C). In addition, the rupture of endosomes into the cytosol is partly induced by mechanical destruction owing to the swelling of nanoparticles.
Hu et al. [60] developed core–shell nanoparticles using PEG dimethacrylate (PEGDMA) as a cross-linker. The particle size was approximately 200 nm at a pH of 7.4; however, the size of the nanoparticles increased to 550 nm at a lower pH of 4.9. Between pH values of 7.0 and 6.8, rapid swelling was observed owing to the electrostatic interactions and typical solvation of charged materials in the nanoparticle core. These nanoparticles successfully released ovalbumin and the small-molecule calcine into the cytoplasm of dendritic cells. Similarly, Griset et al. [61] synthesized expansile polymeric nanoparticles to deliver paclitaxel for treating lung cancer. These nanoparticles could expand several hundred-fold in volume (from 100 to 1,000 nm) in diameter in response to pH changes in lysosomes. Similar nanoparticles have been reported in other studies, such as nebulized anionic guanidinylated O-carboxymethyl chitosan/N-2-hydroxypropyltimehyl ammonium chloride chitosan nanoparticles for pulmonary delivery of siRNAs [120], dual-layered nanogel-coated hollow lipid/polypeptide for doxorubicin delivery [62], and pH-triggered polymeric nanogel for delivering proteins to treat lysosomal storage diseases [121].
Although these particles can contribute to endosomal escape, the factors affecting swelling remain unknown [15]. First, the cross-linking density can affect the swelling and transfection efficiency of nanoparticles. Nanoparticles with a lower cross-linking density have been demonstrated to have higher transfection efficiency [122]. This phenomenon suggests that a high cross-linking density makes the nanocarriers stronger and reduces the response to pH-sensitive swelling. Villani et al. [123] presented key evidence on the effects of structure and chemical composition on the swelling of nanocarriers. In their study, A(BC)n amphiphilic block copolymers with linear (n = 1) and branched (n = 2) architectures were synthesized to obtain pH-sensitive vesicles capable of releasing drugs in acidic conditions via controlled swelling instead of disaggregation. Kermaniyan et al. [124] developed a series of pH-sensitive nanoparticles (size ranging from 85 to 100 nm) that exhibited tunable pH-induced swelling (120% to 200%) and had good buffering capacity. However, the endosomal escape ability of these nanoparticles was weak. A possible reason underlying this result may be that the nanoparticles could not break endosomes after expansion, highlighting the requirement of designing nanocarriers with a larger expansion coefficient.
Endosomal/lysosomal escape through membrane destabilization
Escape from endosomes/lysosomes can be achieved through membrane destabilization. This section mainly discusses various mechanisms of membrane destabilization that help to prevent the enzymatic degradation of drugs. These mechanisms include pore formation, membrane disruption, membrane fusion, and photochemical internalization.
Therapeutic drugs can diffuse from endosomal or lysosomal compartments via membrane pores created owing to the direct interaction of polymers or peptides with membranes or the self-assembled defined pores by peptides (Fig. 3D) [125]. Bacterial toxins usually consist of a transmembrane domain for inducing endosomal/lysosomal escape [126]. After the proteins or peptides present in toxins accumulate in EEs, the transmembrane domain undergoes a conformational change as the pH of endosomes decreases and inserts into the membrane of endosomes/lysosomes to form different pores: barrel stave and toroidal pores [126,127]. For example, lipid nanoparticles can enter HeLa cells and liver cells through phagocytosis triggered by rabankyrin-5 [9]. After lipid nanoparticles are encapsulated in endosomes, a pore is formed temporarily and siRNAs are released into the cytoplasm through this pore. Similarly, the HIV envelope glycoprotein gp41 can enhance endosomal escape by adopting an amphipathic α-helical structure. Therefore, modification of the cationic polymer PEI with a lytic peptide derived from the endodomain of gp41 can significantly enhance PEI-mediated siRNA delivery [64]. In addition, melittin [128] and listeriolysin O [65] are potential peptides that can result in pore formation in endosomal membranes to enhance escape.
Together, bacterial toxins and/or cargos are translocated through endosomal membranes in a folded state or released into the cytoplasm without a complete nanostructure. ur Rehman et al. [63] investigated the interaction between lipo-/polyplexes and HeLa cells. Both the polymer and its genetic payload were separately released into the cytoplasm via local pores within the endosomal membrane. Similarly, Plaza-Ga et al. [65] demonstrated that endosomal acidification led to the release of listeriolysin O (LLO) protein from the nanoparticle surface and its self-assembly into a 300-Å pore that perforated the endosomal/lysosomal membrane, enabling the escape of gold nanoparticles. However, the size of the transmembrane pores is approximately 1 to 2 nm, which limits their use in inducing an efficient release of therapeutic cargoes [129]. Recent studies have indicated that nanodiamonds with sharp corners can escape from endosomes by piercing their lipid membrane [130,131].
Various materials interact with the endosomal/lysosomal membrane mainly through electrostatic interactions and increase the instability of the membrane to promote endosomal/lysosomal escape (Fig. 3E). Owing to their natural endosomal escape property, viruses may act as potential vectors to deliver drugs; however, their high toxicity and immunogenicity hinder their application in clinical settings [132]. Cell-penetrating peptides (CPPs) are short peptides with approximately 6 to 30 amino acid residues derived from viruses [133]. The TAT peptide (RKKRRQRRR) is a typical representative of cationic CPPs that can deliver cargos to the cytosol but cannot mediate endosomal escape [134]. By contrast, the pH-responsive peptides were mostly introduced for membrane-disrupting peptides specific toward endosomal membranes because of the low pH being a trigger for membrane insertion and leakage. For example, the influenza virus can use the N-terminal fusion peptide of hemagglutinin-2 (HA-2) to destroy the endosomal membrane [135]. Under acidic conditions, protonated peptides penetrate the lipid bilayer, resulting in disruption of the membrane, and allow viral nucleic acids to effectively escape from endosomes/lysosomes to the cytoplasm [66,136]. Similarly, pH (low) insertion peptides derived from the bacteriorhodopsin protein can change their conformation into a helix under acidic conditions and penetrate the membrane for enhancing the endosome/lysosome escape ability [137]. Zhang et al. [138] demonstrated that the incorporation of pH-sensitive triple-Glu-substituted peptides (AR-23) into PLL/DNA polyplexes enhanced the disruption of endosomal/lysosomal membranes, thereby promoting their entry into the cytoplasm and increasing the transfection efficiency.
The primary mechanism underlying membrane disruption is that the charge of the material changes to positive under an acidic environment and subsequently forms ion pairs with the negatively charged endosomal membrane, thereby damaging the stability of the membrane and releasing drugs. A similar method has been used by Peng et al. [139] to develop pH-sensitive nanoparticles (Fig. 5B). They developed DSPE-PCB (polycarboxybetaine) lipoplexes using a pH-sensitive zwitterionic PCB whose negative carboxyl acid groups could be protonated at low pH [140]. Neutral PCB lipids could be protonated in endosomes/lysosomes, which promoted the fusion of cationic liposomes with endosomal/lysosomal membranes and hence enhanced the endosomal/lysosomal escape of siRNA. The timing of pH-based membrane destabilization is affected by the components of materials, which may in turn affect endosomal/lysosomal escape [141]. Cupic et al. [5] synthesized 5 pH-based disassembly pHlexi particles by combining PEG-b-PDEAEMA with random copolymers of PDPAEMA. These particles were disassembled at various pH values ranging from 7.2 to 4.9, and membrane destabilization occurred at a pH of approximately 0.5 units above the disassembly point.
Furthermore, ionizable lipids (pKa; 6.2) used as delivery vehicles can mediate endosomal escape (Fig. 6A) [142,143]. They become protonated and positively charged as the endosome matures (pH 6), resulting in phase transformation from a cone-shaped structure into the hexagonal HII structure (Fig. 6B and 6C), followed by localized disruption of the endosomal lipid bilayer and endosomal escape of drugs into the cytoplasm [144,145]. Studies employing cryo-transmission electron microscopy have demonstrated that the structures of newly formed lipidic assemblies highly rely on the nature of ionizable lipids [9], which often possess different numbers of hydrocarbon chain and various arrangements (Fig. 6D). The ability to escape from the endosome was explored in various lipid nanoparticles based on 3 commercially available ionizable lipids, Dlin-MC3-DMA (MC3), Dlin-KC2-DMA (KC2), and SS-OP. The result indicated that lipid nanoparticles with 1.5 mol% of PEG and a hydrocarbon chain C14 resulted in optimal endosomal escape [146]. Dong et al. [147] developed the nucleoside-modified mRNA encoding VEGFA (vascular endothelial growth factor)-encapsulated ionizable lipid nanoparticles, which indicates an excellent lysosome escape capability to improve angiogenesis and increase wound healing rate. In addition, endosomal/lysosomal escape can be accelerated by destroying the components of the membrane via cationic agents. Joris et al. [148] showed that Food and Drug Administration-approved cationic amphiphilic drugs induced the phospholipidosis of lysosomes, resulting in transient lysosomal membrane permeabilization. Sequential incubation with nanogels/siRNAs significantly enhanced gene silencing in cancer cells. Similarly, Tamura et al. [149] designed acid-degradable cationic polyrotaxanes to destabilize endosomal/lysosomal membranes via rapid removal of phospholipids from the membranes, resulting in endosomal/lysosomal escape of siRNAs for gene silencing. Additionally, Donders et al. [150] established a tunable and generalizable strategy for endosomal disruption by manipulating the pKa of colloid-forming drugs.
Fig. 6.

(A to D) Structure and function of designed iPhos containing ionizable amines [142].
Furthermore, physical and mechanical characteristics can affect endosomal/lysosome escape, including ultrasound, temperature, and magnetism. Exogenous ultrasound is one of the classical membranes’ destabilizing escape methods due to the formation of transient pores under ultrasound exposure. For example, Liao et al. [151] used 2-methacryloyloxy ethyl trimethyl ammonium chloride (TMA) to prepare the TMA/plasmid DNA polyplexes, and the unique on–off phenomenon of TMA/pDNA polyplexes controlled by ultrasound exposure was found to be associated with endosomal escape. Additionally, the combination of ultrasound and nanobubbles is an important method of developing novel and noninvasive nanocarriers. Omata et al. [152,153] indicated that the transfection efficiency of TAT-PEG liposomes was enhanced by approximately 30-fold when bubble liposomes and ultrasound exposure were used to promote endosomal escape, and the mechanism was involved in endosomal acidification and vesicle fusion with Ca2+ and adenosine triphosphate. Similarly, Wei et al. [68] developed a new-generation ultrasound-responsive polymersome through self-assembly of PEO-b-P(DEA-stat-MEMA) block copolymers to evaluate its efficiency in delivering anticancer drugs. As shown in Fig. 5C, sonication accelerated the release of doxorubicin from the ultrasound-responsive polymersomes, resulting in rapid escape from LEs and significant inhibition of tumor growth (approximately 95%). However, these effects often depend on the amplitude of ultrasound waves used and are limited in deep tissues.
As a physical parameter, temperature can be used to enhance lysosomal escape. Alamoudi et al. [69] used PEG-modified DOTAP/DOPE/cholesterol liposomes to encapsulate both siRNA and ammonium bicarbonate to construct temperature-sensitive “bubble liposomes.” When the temperature of the tumor region was increased to 42 °C through external heating, ammonium bicarbonate was decomposed to produce carbon dioxide in the acidic environment of lysosomes, resulting in the disruption of lysosomes and effective release of Bcl2 or MRP1 siRNA into the cytoplasm.
Magnetic material-induced destabilization of lysosomes offers a novel strategy for the functionalization of nanocarriers. Superparamagnetic iron oxide nanoparticles can induce apoptosis through hyperthermia after stimulation with an alternating magnetic field [154]. Pucci et al. [155] and Domenech et al. [156] designed lipid-based nanocarriers encapsulating superparamagnetic iron oxide nanoparticles that resulted in membrane permeabilization with the consequent release of cargo from lysosomes.
Membrane fusion refers to the process through which 2 closely attached lipid membranes merge into a bilayer. It is a potential strategy for overcoming the poor release of nanodrugs from endosomes. The anionic lipids of endosomes interact with the cationic lipids of nanocarriers and rearrange to form a neutral ion pair, which sharply destabilizes the membrane and enhances endosomal escape (Fig. 3F).
Owing to its lamellar structure, liposomes can fuse more easily with lipid components present on the endosomal/lysosomal membranes to promote escape [157]. Dioleoyl-phosphatidyl ethanol-amine (DOPE) is the most commonly used membrane fusion agent (helper lipid) in delivery systems [158–160]. It can form hexagonal crystals under acidic conditions to induce membrane fusion between liposomes and endosomes or lysosomes [161,162]. The cationic lipid 1,2-dioleoyl-3-trimethylammoniumpropane (DOTAP), DOPE, and other helper lipids were used to prepare a series of siRNA-loaded core–shell nanoparticles for the treatment of breast cancer [70,71] and brain cancer [72,73]. After 2 h of incubation, the concentration of siRNAs was high in the cytoplasm than in lysosomes because DOPE as the helper lipid facilitated the fusion of liposomes with the lysosomal membrane through formation of a hexagonal inverted phase (HII) structure, thus allowing the efficient escape of nanocarriers into the cytosol.
Various pH-sensitive peptides can induce membrane fusion and enhance the endosomal/lysosomal escape of nanodrugs. Wyman et al. [163] designed the KALA peptide, whose amino acid sequence is WEAKLAKALAKALAKHLAKALAKALKACEA, and linked it with DNA to destroy the stability of the membrane. The conformation of KALA changes with pH, and KALA facilitated the escape of DNA from lysosomes under acidic conditions. Shaheen et al. [74] designed multilayer nanoparticles modified with octa arginine, in which KALA was decorated on the outermost layer of nanoparticles and had lysosomal escape ability. The transfection efficiency of nanoparticles modified with the functional KALA polypeptide was 20 times higher than that of unmodified nanoparticles.
Similarly, the GALA peptide, a 30-residue artificial amphiphilic peptide having a repeated sequence of Glu-Ala-Leu-Ala, can convert its structure to a helix (an amphipathic α-helical structure) when the pH is reduced from 7.0 to 5.0 [164]. The helical structure has a high affinity for the negatively charged endosomal/lysosomal membrane and forms aqueous pores consisting of 10 ± 2 peptides with a head-to-tail (N- to C-terminus) orientation [165,166]. Sasaki et al. [75] developed a novel method for encapsulating condensed plasmid DNA into PEGylated Tf-liposomes (Tf-PEG-L) to form core–shell-type nanoparticles (Fig. 5D). The transfection efficiency increased almost 100-fold because GALA interacted synergistically to induce the fusion of liposomes and endosomes/lysosomes.
Photochemical internalization is a light-triggered novel technology initially developed at the Norwegian Radium Hospital to promote endosomal/lysosomal escape of various nanocarriers encapsulated with therapeutic agents [11,76,167]. This unique mechanism relies on the activation of light to disturb an endocytic structure, usually exploits a small molecule that is susceptible to light and can help to produce a large amount of oxidizing active substances after illumination to disrupt the endosomal/lysosomal membrane for the release of encapsulated drugs (Fig. 3G).
Naturally, photosensitizers that form triplet states and can produce reactive oxygen species have tricyclic, heterocyclic, or porphyrin-like ring structures with conjugated double bonds (π-electron system), including disulfonated meso-tetraphenylporphine (TPPS2A), phthalocyanine dendrimers, disulfonated aluminum phthalocyanine, and tetra(4-sulfonatophenyl) porphine [168,169]. Jayakumar et al. [77] created silica core–shell nanoparticles that could emit both ultraviolet and visible light to active TPPS2A for enhanced gene knockdown (Fig. 5E). Cabral et al. [78] developed a novel nanocarrier using thiolated camptothecin and thiolated PEG-b-poly(glutamic acid) for photoactivated targeted chemotherapy. The results indicated that photochemical internalization-induced endosomal escape triggered drug release (approximately 90%) after 24 h.
Together, it is important to consider the influence of materials on membrane stability when designing nanocarriers. Compared with membrane disruption and pore formation, membrane fusion promotes drug delivery to the cytoplasm irrespective of the particle size [106]. Given that lysosomes play a key role in regulating apoptosis, the potentially toxic effects of endosomal escape should be considered to avoid the uncontrolled release of cathepsin, which significantly affects the viability of cells [170,171].
Directly crossing the cell membrane to bypass endosomes/lysosomes
Directly penetrating the membrane and entering the cytoplasm could bypass the degradation and destruction of endosomes/lysosomes, which is undoubtedly a good choice for the subsequent effects of drugs. Actually, for some nanocarriers, the cellular uptake mechanism is fusion with the cell membrane and entering into the cytoplasm directly without endocytosis (Fig. 2B).
Researchers have designed several delivery systems based on this strategy, mainly focusing on proteins, peptides, or other biomaterials. This endosomal/lysosomal evading mechanism is especially observed in virus-infected cells. Kim et al. [79] designed siRNA nanocarriers using porous silicon nanoparticles functionalized with tumor-targeting peptides and fusogenic lipids (Fig. 7A). The lipid coatings induced membrane fusion and promoted entry into cells through a distinctive mechanism that is independent of receptor-mediated endocytosis. Similarly, Yang et al. [43] prepared fusogenic exosomes by modifying exosomes with vascular stomatitis virus fusion protein. These modified exosomes could fuse with the plasma membranes through a process called “membrane editing,” which facilitated the transfer of biologically active membrane proteins into target cell membranes both in vitro and in vivo and significantly improved the transfection efficiency. This mechanism also applies to the binding and fusion of other extracellular vesicles [172].
Fig. 7.

(A) Schematic of the fusogenic porous silicon nanoparticles structure and hypothesized uptake for direct delivery to target cell [79]. (B) Schematic representation of the coiled-coil structure between peptides E and K, targeted liposome fusion mediated by the coiled-coil formation between CPE4-modified liposomes and CPK4-modified liposomes, and scheme of fusion between cells and liposomes [81]. (C and D) The membrane fusion induced by the carbon nanotube [83,84]. pSiNP, porous silicon nanoparticles;LUVs, large unilamellar 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) vesicles; SWCNT, single-wall carbon nanotube; CNTP, carbon nanotube porin.
The cell membrane has unique biological properties. Cell membrane-coated nanoparticles have the complex and unique surface physicochemical properties of protocells, especially the ability to fuse with nanocarriers [173]. Therefore, cell membrane-coated nanoparticles, primarily those coated with blood, bacterial, and tumor cells, have attracted extensive attention from researchers [174]. These nanoparticles can be prepared using the following method: Cells are lysed in a hypotonic solution, and the cell membrane is purified through differential centrifugation. The cell membrane is extruded from the polycarbonate membrane (aperture, 200 to 400 nm) several times to obtain cell membrane fragments with a uniform particle size. Subsequently, the cell membrane is coated or fused on the nanocore surface via repeated extrusion or ultrasonication. The nanocores are prepared using hard organic nanoparticles (such as PLGA (poly(lactic-co-glycolic acid) and PCL nanoparticles) or inorganic nanoparticles (gold nanoparticles, carbon nanorods, silica nanoparticles, and quantum dots) [175–177].
Red blood cells have several surface proteins that play a key role in recognizing homologous red blood cells. Guo et al. [178] proposed a biomimetic and controlled route to achieve the fusion of hydrophobic quantum dots with red blood cell membranes, and the resulting red blood cell-encapsulated quantum dots could fuse with the cell membrane. Similarly, Xuan et al. [179] coated leukocyte membranes on the surface of silica nanoparticles to synthesize a leucocyte-like carrier for targeted treatment of lesions. Wang et al. [180] used leukocyte membranes activated by inflammatory factors to construct “grapefruit-derived” nanocarriers. The experimental results revealed that doxorubicin-encapsulated nanoparticles effectively inhibited tumor growth, whereas curcumin-encapsulated nanoparticles effectively alleviated colitis. In addition, considering the hemostatic mechanism and function of platelets, researchers have developed nanocarriers modified with platelet membranes. For example, Anselmo et al. [181] coated the surface of nanoparticles with platelet membranes and prepared platelet-like nanocarriers, which specifically aggregated at the injured site and promoted coagulation. Surface antigens, such as galactoagglutinin-3 and carcinoembryonic antigen [182], specifically expressed by tumor cells have a structural domain that allows adhesion to homologous cells and, hence, endow nanoparticles with a unique tumor-targeting ability. Sun et al. [80] used PCL and F68 to encapsulate PTX to obtain core nanoparticles and coated them with the membrane of 4T1 tumor cells to obtain integrated CPPNs (cancer cell membrane-coated paclitaxel-loaded polymeric nanoparticles). These nanoparticles not only effectively inhibited the growth of tumors in situ but also significantly inhibited the metastasis of 4T1 tumors.
Cell-penetrating peptides (CPPs) have been used in the construction of vectors owing to their unique membrane-penetrating function. For example, polyarginine (R4 to R9) promotes membrane fusion not by direct penetration [17] but by “punching” the target membrane, resulting in the direct release of cargo into the cytoplasm. Jiang et al. [183] constructed nanoparticle-stabilized nanocapsules (NPSCs) to achieve direct delivery of siRNAs into the cytosol. siRNAs were allowed to adsorb on arginine-modified gold nanoparticles through electrostatic interactions, followed by the self-assembly of fatty acid nanodroplets on the surface to construct NPSC/siRNA nanocapsules, which could rapidly deliver siRNAs to the cytosol via membrane fusion. Similarly, Deng et al. [184] coated a tetrapeptide on the surface of “MEND” carriers delivering pDNA and found that it had no evident colocalization with lysosomes. The carrier entered the cell through direct membrane fusion, which was named “one-step into the cytoplasm”; however, the specific underlying mechanism was not discussed. Moreover, whether the nanocarrier was transported through mechanisms other than membrane fusion remained uncertain. To this end, Yao et al. [185] reported a comprehensive mechanism. They coated a tumor microenvironmental pH-sensitive polypeptide on the surface of PEG liposomes. In the tumor microenvironment, the polypeptide was exposed responsively and fused with lipid molecules on the cell membrane, as well as improved the uptake effect by directly fusing and releasing drugs to the cytoplasm or promoting lysosome escape after endocytosis. This membrane fusion method was very rapid and only consumed 8 ms. However, the carrier was not completely inserted into the cell directly; a large part of the carrier was endocytosed and transferred through lysosomes.
Lipid membrane fusion is the basic characteristic of cells to maintain efficient activity, especially during endocytosis, exocytosis, and intracellular transport [186–189]. However, it often requires a condition that the carrier contains ligand A, and the cell membrane surface has its receptor B. A and B interact to induce membrane fusion when they approach each other at a nanometer distance. For example, membrane fusion is mediated by SNAREs, which are membrane proteins whose C-terminal tail is anchored at the membrane. SNAREs are widely distributed in the ER, Golgi apparatus, and endosomes and sparsely distributed on the cell membrane [157,190]. According to the SNARE hypothesis, v-SNARE on transport vesicles (v represents the vesicle) specifically interacts with homologous t-SNARE on the target membrane (t represents the target). t-SNARE is usually complex composed of 3 SNARE motifs, which are composed of 3 Syn or Syn-like SNARE motifs, whereas v-SNARE usually contains one SNARE motif. During v-t-SNARE pairing, the formation of a stable 4-helix bundle induces the fusion of the 2 diaphragms. Ding et al. [191] demonstrated that stably assembled rHDL/Chol–siRNA complexes crossed the membrane and directly entered the cytoplasm via the scavenger receptor BI (SR-BI)-mediated non-endocytic mechanism, thereby evading endosomes/lysosomes. This phenomenon was observed in tumor cells with high expression of SR-BI, such as human breast cancer MCF-7 cells and human hepatocellular carcinoma HepG2 cells, but not in those with low expression of SR-BI, such as human fibrosarcoma HT1080 cells, indicating a selective behavior because of the interaction between A and B described previously.
Notably, not all cell surfaces contain corresponding receptors for membrane fusion. As shown in Fig. 7B, Yang et al. [81] designed a pair of complementary coiled-coil lipopeptides (CPK (cholesterol-PEG-peptides (KIAALKE)4) and CPE (cholesterol-PEG-peptides (EIAALEK)4)) and embedded them in the lipid bilayer of liposomes and cell membranes, respectively. When CPK-decorated liposomes were close to CPE-modified cell membranes, they interacted and induced membrane fusion with the concomitant release of liposome-encapsulated cargo for direct drug delivery without endocytosis. Similarly, Zhou et al. [82] developed an oligonucleotide (ON) template-assisted polymerization approach to synthesize ON nanospheres as gene vectors. In this approach, guanidinium-containing disulfide monomers were organized on templates, greatly increasing their local effective concentrations. Consequently, ring-opening disulfide-exchange polymerization between monomers was accelerated, further facilitating the self-assembly of ON nanospheres. These nanospheres were directly delivered into the cytosol via an endocytosis-independent pathway, followed by intracellular depolymerization in the reductive cytosolic environment to release the cargo, resulting in efficient gene silencing.
Introducing an intermediate material into nanocarriers for membrane fusion, such as carbon nanotubes [192], is a strategy for adjusting the mode of membrane penetration. Jiang et al. [83] designed the nanoplexes by encapsulating siRNAs with CPPs (NH2-VGalAvVvWlWlWlWbA-GSG-PKKRKVC-COOH). The siRNA–CPP complex was transported to lysosomes through endocytosis, whereas the siRNA–CPP complex modified with single-wall carbon nanotubes directly penetrated the cell membrane and had weak colocalization with lysosomes (Fig. 7C). Similarly, Ho et al. [84] reported that liposomes studded with 0.8-nm-wide carbon nanotube porins functioned as efficient vehicles for direct cytoplasmic drug delivery by facilitating the fusion of lipid membranes and complete mixing of the membrane material and vesicle content (Fig. 7D). Molecular dynamics simulations indicated that short fragments of carbon nanotubes inserted into lipid membranes potentially facilitated the fusion of lipid membranes [193,194]. In addition, Tai and Gao [195] reported the development of small, bifunctional chemical tags capable of transporting siRNAs directly into the cytosol. The bifunctional tags consisted of a siRNA-binding moiety that interacted with siRNAs noncovalently and a steroid domain that readily fused with the mammalian cell membrane. Compared with the conventional covalently conjugated siRNA–steroid complex that entered cells largely via endocytosis, which substantially limited siRNA bioavailability, the noncovalently tagged siRNAs directly penetrated the cell membrane, avoiding endocytosis.
As a strategy for overcoming lysosomal barriers, direct penetration into the cell membrane can prevent lysosomal degradation to some extent, but has strong limitations owing to the potential cytotoxicity of inorganic materials. Moreover, the transport mechanism remains elusive, thus limiting the application of membrane fusion. Direct penetration into the cell membrane is an interesting approach; however, it is challenging because delivery systems enter different cells nonspecifically.
Intracellular transport pathway bypassing endosomes/lysosomes
Unlike the endosome–lysosome pathway, the endosome–Golgi/ER pathway is considered a non-degradative pathway (Fig. 2C). Some studies have demonstrated that nanoparticles entering cells through macrocytosis or caveolin-mediated endocytosis are partly transported through the endosome–Golgi/ER pathway [46,196,197]. Small-molecule substances, toxins [198], or siRNAs [85] are internalized by cells through neutral vesicles called caveosomes, bypassing the endosomal/lysosomal pathway, and are directly transported to the Golgi apparatus or ER [86,87]. Reilly et al. [86] demonstrated that histone-targeted polyplexes were transported to the nucleus via the endosome–Golgi/ER retrograde pathway, evading endosomal/lysosomal trafficking routes. This transport mechanism was mainly induced by DPY-30, which was located in TGN [199].
However, some researchers have questioned the “caveosome-related pathway.” Owing to the identification of EEA-1, a maker protein of EEs, on caveosomes, researchers have indicated that the so-called caveosome is an EE, denying the existence of caveosome [200,201]. In addition, studies have reported that some nanoparticles are transported to lysosomes via caveolin-mediated endocytosis [202]. We speculate that the caveosome does not exist; however, the way in which nanoparticles enter cells should be closely related to subsequent intracellular localization. Different cell types, nanocarriers, and concentrations affect the underlying mechanism, and this relationship cannot be directly denied because of the cellular uptake mechanism and location on a certain cell.
Moreover, changing the formulation and synthesis process may regulate the intracellular transport pathway of nanoparticles, providing a new method for avoiding lysosomal degradation. We have previously investigated the lysosomal localization of nanocarriers with different assembly structures of gemini-like cationic lipid (CLD)/siRNA prepared using the AT (siRNA solution mixed with preformed CLD NPs), MT (ethanolic CLD solution dropped in a solutionof siRNA under sonication), and HT (a CLD thin film hydrated with siRNA solution) methods [203,204]. The siRNA nanoparticles prepared using the MT method mainly entered the cells through macrocytosis and caveolin-mediated endocytosis and did not colocalize with lysosomes.
Furthermore, the intracellular behavior of nanoparticles can be regulated through the functional modification of carriers by mimicking biochemical properties. Wang et al. [88] used the resident KDEL peptide of ER membrane proteins to modify gold nanoparticles containing siRNAs and found that the nanoparticles were internalized via CME and exhibited strong colocalization with the ER. KDEL can mediate retrograde transport from the Golgi apparatus to ER via COPI vesicles and helps co-transported drugs evade the lysosomal degradation pathway. The reverse transport of endosomes to Golgi or Golgi to ER may result in retrograde transport. The ER membrane derived from cancer cells has been used to prepare hybrid nanoplexes to enhance siRNA transfection (Fig. 8) [89]. Functional proteins on the ER membrane can alter the intracellular trafficking pathway of endosome–Golgi–ER to evade lysosomal degradation. However, it is noteworthy that different sources of the ER membrane and the combination of components can lead to uncertainty in formulation components and necessitate further simplification of formulation to ensure batch repeatability.
Fig. 8.
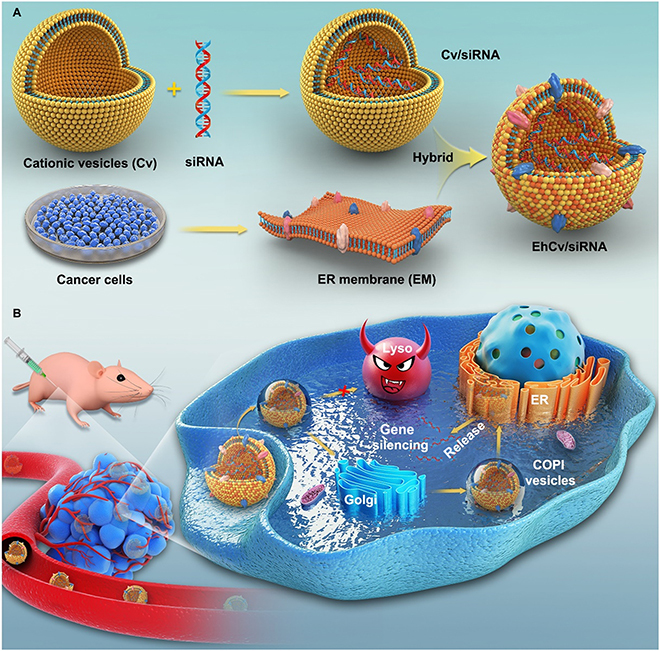
(A and B) Schematic of siRNA-loaded nanoplexes evading lysosomal degradation via an ER membrane-modified strategy [89].
Conclusion and Prospects
After decades of development and innovation, the use of nanotechnology-based drug therapies has increased in clinical practice in recent years as evidenced by the increasing number of clinically approved nanodrugs annually. Nanocarriers can penetrate biological barriers, improve biocompatibility, prolong the circulation time of drugs, and achieve targeted delivery of drugs. However, the lack of a safe and effective nanodrug delivery system limits the clinical application of nanodrugs. Moreover, the use of nanodrugs is limited owing to their immunogenicity, off-target effects, toxicity, and low stability. Low delivery efficiency owing to endosomal/lysosomal entrapment is a key problem that should be addressed.
Therefore, a comprehensive understanding of the mechanisms of escape from endosomes/lysosomes is important for enhancing the transport efficiency of nanocarriers. This review summarized recent advances in the development of different nanocarriers and described strategies for overcoming endosomal/lysosomal barriers based on the perspective of cellular uptake and intracellular transport mechanisms. The 3 main strategies are as follows: (a) strategies promoting the escape of endosomes/lysosomes, (b) strategies promoting non-endocytosis delivery through direct penetration into the cell membrane to evade endosomes/lysosomes, and (c) strategies involving a detour pathway to bypass lysosomes and evade degradation. Four advanced strategies for promoting escape from endosomes/lysosomes were discussed in this review, including the proton sponge effect of pH buffering; osmotic lysis resulting from pH-responsive disassembly of nanoparticles; as well as swelling of pH-responsive nanoparticles and membrane destabilization induced by pore formation, membrane disruption, membrane fusion, and photochemical internalization. Each nanocarrier has a unique intracellular fate owing to different physicochemical properties.
Traditional Chinese culture propagates a theory of yin and yang, which signifies that there is no absolute good or bad and everything is balanced. As acidic and enzyme-rich organelles, lysosomes play a crucial role in maintaining cell function. We speculate that strategies involving direct penetration into the cell membrane and evasion of lysosomes through a detour pathway are more promising for nanodrug delivery. However, some nanocarriers may benefit from lysosomal involvement to a certain degree, such as CAP nanoparticles that disassemble in response to the pH of lysosomes and liposomes that reverse surface charges under the acidic conditions of lysosomes to release drugs into the cytoplasm with better efficiency. The most important aspect of drug delivery is the reasonable design of carriers according to the requirements so that lysosomes can be used as a helper instead of a barrier.
Importantly, these advances may be more complex because the mechanism of trafficking, and escape depends on the characteristics of vehicles and cell types. Existing strategies for overcoming endosomal/lysosomal barriers may increase the delivery efficiency of nanocarriers; however, various challenges should be considered while designing nanocarriers in the future, including safety and biocompatibility, scalability and manufacturing, regulatory considerations, and so on. Liposomes are currently the main nanodrugs used in clinical practice or in clinical trials because of their good safety and biocompatibility. Importantly, the structure of liposome is stable, and it maintains high consistency of activity during the expansion from small to large batch preparation. Hence, various strategies based on liposomes to overcome endosomal/lysosomal barrier will be more potential, such as introducing cell membrane, ER membrane proteins, or DOPE into liposomes. Meanwhile, the translation of these strategies to clinical settings will require scalable and cost-effective manufacturing processes, and it is important to provide a standardized protocol to ensure reproducibility and quality control, as well as consider regulatory requirements based on their safety, efficacy, and quality.
Moreover, interdisciplinary collaboration is a crucial manner to unlock the smarter nanocarriers with innovative strategies for overcoming lysosomal barriers. Given the wide range of disciplines involved in nano-delivery systems, this includes collaborations between researchers from the fields of chemistry, materials science, biology, immunology, and medicine, as well as partnerships with industry and regulatory agencies. Finally, an in-depth understanding of nanocarrier trafficking may facilitate the rational design of smarter nanodrug delivery systems.
Acknowledgments
Funding: This work was supported by the Fundamental Research Funds for the National Natural Science Foundation (nos. 82204322 and 82104480), the Central Public Welfare Research Institutes (grant nos.: ZZ16-ND-10-05, ZZ16-ND-10-13, ZZ16-ND-10-17, ZZ16-ND-10-19, ZZ14-YQ-050, ZZ14-YQ-055, ZZ14-YQ-059, ZZ14-YQ-060, and ZZ16-YQ-046), and the Young Elite Scientists Sponsorship Program by CACM (2021QNRC2B29). Author contributions: Writing—original draft preparation: C.Q. Writing—review and editing: F.X., J.W., C.Q., Q.G., Q.S., J.Z., Y.M., C.W., H.P., L.G., and C.X. All authors have checked and agreed to the published version of the manuscript. Competing interes ts: The authors declare that they have no competing interests.
References
- 1.Sonksen M, Kerl K, Bunzen H. Current status and future prospects of nanomedicine for arsenic trioxide delivery to solid tumors. Med Res Rev. 2022;42(1):374–398. [DOI] [PubMed] [Google Scholar]
- 2.Tawfik M, Chen F, Goldberg JL, Sabel BA. Nanomedicine and drug delivery to the retina: Current status and implications for gene therapy. Naunyn Schmiedebergs Arch Pharmacol. 2022;395(12):1477–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun Q, Zhou Z, Qiu N, Shen Y. Rational design of cancer nanomedicine: Nanoproperty integration and synchronization. Adv Mater. 2017;29(14): Article 1606628. [DOI] [PubMed] [Google Scholar]
- 4.Cheng Z, Li Y, Zhao D, Zhao W, Wu M, Zhang W, Cui Y, Zhang P, Zhang Z. Nanocarriers for intracellular co-delivery of proteins and small-molecule drugs for cancer therapy. Front Bioeng Biotechnol. 2022;10: Article 994655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cupic KI, Rennick JJ, Johnston AP, Such GK. Controlling endosomal escape using nanoparticle composition: Current progress and future perspectives. Nanomedicine (Lond). 2019;14(2):215–223. [DOI] [PubMed] [Google Scholar]
- 6.Podinovskaia M, Spang A. The endosomal network: Mediators and regulators of endosome maturation. Prog Mol Subcell Biol. 2018;57:1–38. [DOI] [PubMed] [Google Scholar]
- 7.Such GK, Yan Y, Johnston AP, Gunawan ST, Caruso F. Interfacing materials science and biology for drug carrier design. Adv Mater. 2015;27(14):2278–2297. [DOI] [PubMed] [Google Scholar]
- 8.Lonn P, Kacsinta AD, Cui XS, Hamil AS, Kaulich M, Gogoi K, Dowdy SF. Enhancing endosomal escape for intracellular delivery of macromolecular biologic therapeutics. Sci Rep. 2016;6: Article 32301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilleron J, Querbes W, Zeigerer A, Borodovsky A, Marsico G, Schubert U, Manygoats K, Seifert S, Andree C, Stoter M, et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat Biotechnol. 2013;31(7):638–646. [DOI] [PubMed] [Google Scholar]
- 10.Upton DH, Ung C, George SM, Tsoli M, Kavallaris M, Ziegler DS. Challenges and opportunities to penetrate the blood-brain barrier for brain cancer therapy. Theranostics. 2022;12(10):4734–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad A, Khan JM, Haque S. Strategies in the design of endosomolytic agents for facilitating endosomal escape in nanoparticles. Biochimie. 2019;160:61–75. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Li J, Zhou J, Lin Z, Cavalieri F, Czuba-Wojnilowicz E, Hu Y, Glab A, Ju Y, Richardson JJ, et al. Metal-phenolic coatings as a platform to trigger endosomal escape of nanoparticles. ACS Nano. 2019;13(10):11653–11664. [DOI] [PubMed] [Google Scholar]
- 13.Degors IMS, Wang C, Rehman ZU, Zuhorn IS. Carriers break barriers in drug delivery: Endocytosis and endosomal escape of gene delivery vectors. Acc Chem Res. 2019;52(7):1750–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pei D, Buyanova M. Overcoming endosomal entrapment in drug delivery. Bioconjug Chem. 2019;30(2):273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith SA, Selby LI, Johnston APR, Such GK. The endosomal escape of nanoparticles: Toward more efficient cellular delivery. Bioconjug Chem. 2019;30(2):263–272. [DOI] [PubMed] [Google Scholar]
- 16.Xu E, Saltzman WM, Piotrowski-Daspit AS. Escaping the endosome: Assessing cellular trafficking mechanisms of non-viral vehicles. J Control Release. 2021;335:465–480. [DOI] [PubMed] [Google Scholar]
- 17.Allolio C, Magarkar A, Jurkiewicz P, Baxova K, Javanainen M, Mason PE, Sachl R, Cebecauer M, Hof M, Horinek D, et al. Arginine-rich cell-penetrating peptides induce membrane multilamellarity and subsequently enter via formation of a fusion pore. Proc Natl Acad Sci USA. 2018;115(47):11923–11928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrian T, Riera R, Pujals S, Albertazzi L. Nanoscopy for endosomal escape quantification. Nanoscale Adv. 2021;3(1):10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akinc A, Querbes W, De S, Qin J, Frank-Kamenetsky M, Jayaprakash KN, Jayaraman M, Rajeev KG, Cantley WL, Dorkin JR, et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther. 2010;18(7):1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fichter KM, Ingle NP, McLendon PM, Reineke TM. Polymeric nucleic acid vehicles exploit active interorganelle trafficking mechanisms. ACS Nano. 2013;7(1):347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahay G, Querbes W, Alabi C, Eltoukhy A, Sarkar S, Zurenko C, Karagiannis E, Love K, Chen D, Zoncu R, et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat Biotechnol. 2013;31(7):653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinde E, Thammasiraphop K, Duong HT, Yeow J, Karagoz B, Boyer C, Gooding JJ, Gaus K. Pair correlation microscopy reveals the role of nanoparticle shape in intracellular transport and site of drug release. Nat Nanotechnol. 2017;12(1):81–89. [DOI] [PubMed] [Google Scholar]
- 23.Mochida S. Mechanisms of synaptic vesicle exo- and endocytosis. Biomedicine. 2022;10(7): Article 1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3(1): Article 24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao F, Zhao Y, Liu Y, Chang X, Chen C, Zhao Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small. 2011;7(10):1322–1337. [DOI] [PubMed] [Google Scholar]
- 26.Smith SM, Smith CJ. Capturing the mechanics of clathrin-mediated endocytosis. Curr Opin Struct Biol. 2022;75: Article 102427. [DOI] [PubMed] [Google Scholar]
- 27.Arpino G, Somasundaram A, Shin W, Ge L, Villareal S, Chan CY, Ashery U, Shupliakov O, Taraska JW, Wu L-G. Clathrin-mediated endocytosis cooperates with bulk endocytosis to generate vesicles. iScience. 2022;25(2): Article 103809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang B, Sun X, Mei H, Wang Y, Liao Z, Chen J, Zhang Q, Hu Y, Pang Z, Jiang X. LDLR-mediated peptide-22-conjugated nanoparticles for dual-targeting therapy of brain glioma. Biomaterials. 2013;34(36):9171–9182. [DOI] [PubMed] [Google Scholar]
- 29.Rivolta I, Panariti A, Lettiero B, Sesana S, Gasco P, Gasco MR, Masserini M, Miserocchi G. Cellular uptake of coumarin-6 as a model drug loaded in solid lipid nanoparticles. J Physiol Pharmacol. 2011;62(1):45–53. [PubMed] [Google Scholar]
- 30.Sui ZH, Xu H, Wang H, Jiang S, Chi H, Sun L. Intracellular trafficking pathways of Edwardsiella tarda: From Clathrin- and Caveolin-mediated endocytosis to endosome and lysosome. Front Cell Infect Microbiol. 2017;7:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim J, Clements MA, Dobson J. Delivery of short interfering ribonucleic acid-complexed magnetic nanoparticles in an oscillating field occurs via caveolae-mediated endocytosis. PLOS ONE. 2012;7(12): Article e51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwang ME, Keswani RK, Pack DW. Dependence of PEI and PAMAM gene delivery on Clathrin- and Caveolin-dependent trafficking pathways. Pharm Res. 2015;32(6):2051–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang WC, Burnouf PA, Su YC, Chen BM, Chuang KH, Lee CW, Wei PK, Cheng TL, Roffler SR. Engineering chimeric receptors to investigate the size- and rigidity-dependent interaction of PEGylated nanoparticles with cells. ACS Nano. 2016;10(1):648–662. [DOI] [PubMed] [Google Scholar]
- 34.Park TE, Kang B, Kim YK, Zhang Q, Lee WS, Islam MA, Kang SK, Cho MH, Choi YJ, Cho CS. Selective stimulation of caveolae-mediated endocytosis by an osmotic polymannitol-based gene transporter. Biomaterials. 2012;33(29):7272–7281. [DOI] [PubMed] [Google Scholar]
- 35.Teissier E, Pecheur EI. Lipids as modulators of membrane fusion mediated by viral fusion proteins. Eur Biophys J. 2007;36(8):887–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lupien LE, Bloch K, Dehairs J, Traphagen NA, Feng WW, Davis WL, Dennis T, Swinnen JV, Wells WA, Smits NC, et al. Endocytosis of very low-density lipoproteins: An unexpected mechanism for lipid acquisition by breast cancer cells. J Lipid Res. 2020;61(2):205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kauffmann T, Evans DS. Macrocytosis. Treasure Island (FL): StatPearls; 2022.
- 38.Lim JP, Gleeson PA. Macropinocytosis: An endocytic pathway for internalising large gulps. Immunol Cell Biol. 2011;89(8):836–843. [DOI] [PubMed] [Google Scholar]
- 39.Egami Y. Molecular imaging analysis of Rab GTPases in the regulation of phagocytosis and macropinocytosis. Anat Sci Int. 2016;91(1):35–42. [DOI] [PubMed] [Google Scholar]
- 40.Pozzi D, Marchini C, Cardarelli F, Rossetta A, Colapicchioni V, Amici A, Montani M, Motta S, Brocca P, Cantu L, et al. Mechanistic understanding of gene delivery mediated by highly efficient multicomponent envelope-type nanoparticle systems. Mol Pharm. 2013;10(12):4654–4665. [DOI] [PubMed] [Google Scholar]
- 41.Chernomordik LV, Kozlov MM. Mechanics of membrane fusion. Nat Struct Mol Biol. 2008;15(7):675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuhrmans M, Marelli G, Smirnova YG, Muller M. Mechanics of membrane fusion/pore formation. Chem Phys Lipids. 2015;185:109–128. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y, Hong Y, Nam G-H, Chung JH, Koh E, Kim I-S. Virus-mimetic fusogenic exosomes for direct delivery of integral membrane proteins to target cell membranes. Adv Mater. 2017;29(13): Article 1605604. [DOI] [PubMed] [Google Scholar]
- 44.Scott CC, Vacca F, Gruenberg J. Endosome maturation, transport and functions. Semin Cell Dev Biol. 2014;31:2–10. [DOI] [PubMed] [Google Scholar]
- 45.Yang Z, Xiang B, Dong D, Wang Z, Li J, Qi X. Dual receptor-specific peptides modified liposomes as VEGF siRNA vector for tumor-targeting therapy. Curr Gene Ther. 2014;14(4):289–299. [DOI] [PubMed] [Google Scholar]
- 46.Tu Y, Zhao L, Billadeau DD, Jia D. Endosome-to-TGN trafficking: Organelle-vesicle and organelle-organelle interactions. Front Cell Dev Biol. 2020;8:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu L, Hong W. From endosomes to the trans-Golgi network. Semin Cell Dev Biol. 2014;31:30–39. [DOI] [PubMed] [Google Scholar]
- 48.Sandvig K, Skotland T, Deurs B, Klokk TI. Retrograde transport of protein toxins through the Golgi apparatus. Histochem Cell Biol. 2013;140(3):317–326. [DOI] [PubMed] [Google Scholar]
- 49.Gomez-Navarro N, Miller E. Protein sorting at the ER-Golgi interface. J Cell Biol. 2016;215(6):769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhandapani RK, Gurusamy D, Palli SR. Development of catechin, poly-l-lysine, and double-stranded RNA nanoparticles. ACS Appl Bio Mater. 2021;4(5):4310–4318. [DOI] [PubMed] [Google Scholar]
- 51.Li HJ, Du JZ, Du XJ, Xu CF, Sun CY, Wang HX, Cao ZT, Yang XZ, Zhu YH, Nie S, et al. Stimuli-responsive clustered nanoparticles for improved tumor penetration and therapeutic efficacy. Proc Natl Acad Sci USA. 2016;113(15):4164–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun P, Huang W, Kang L, Jin M, Fan B, Jin H, Wang Q-M, Gao Z. siRNA-loaded poly(histidine-arginine)6-modified chitosan nanoparticle with enhanced cell-penetrating and endosomal escape capacities for suppressing breast tumor metastasis. Int J Nanomedicine. 2017;12:3221–3234. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Endres T, Zheng M, Kilic A, Turowska A,Beck-Broichsitter M, Renz H, Merkel OM, Kissel T. Amphiphilic biodegradable PEG-PCL-PEI triblock copolymers for FRET-capable in vitro and in vivo delivery of siRNA and quantum dots. Mol Pharm. 2014;11(4):1273–1281. [DOI] [PubMed] [Google Scholar]
- 54.Wang P, Wang J, Tan H, Weng S, Cheng L, Zhou Z, Wen S. Acid- and reduction-sensitive micelles for improving the drug delivery efficacy for pancreatic cancer therapy. Biomater Sci. 2018;6(5):1262–1270. [DOI] [PubMed] [Google Scholar]
- 55.Massignani M, Canton I, Sun T, Hearnden V, Macneil S, Blanazs A, Armes SP, Lewis A, Battaglia G. Enhanced fluorescence imaging of live cells by effective cytosolic delivery of probes. PLOS ONE. 2010;5(5): Article e10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li C, Zhou J, Wu Y, Dong Y, Du L, Yang T, Wang Y, Guo S, Zhang M, Hussain A, et al. Core role of hydrophobic core of polymeric nanomicelle in endosomal escape of siRNA. Nano Lett. 2021;21(8):3680–3689. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Schwerbrock NM, Rogers AB, Kim WY, Huang L. Codelivery of VEGF siRNA and gemcitabine monophosphate in a single nanoparticle formulation for effective treatment of NSCLC. Mol Ther. 2013;21(8):1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J, Yang Y, Huang L. Calcium phosphate nanoparticles with an asymmetric lipid bilayer coating for siRNA delivery to the tumor. J Control Release. 2012;158(1):108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiu C, Wei W, Sun J, Zhang HT, Ding JS, Wang JC, Zhang Q. Systemic delivery of siRNA by hyaluronan-functionalized calcium phosphate nanoparticles for tumor-targeted therapy. Nanoscale. 2016;8(26):13033–13044. [DOI] [PubMed] [Google Scholar]
- 60.Hu Y, Litwin T, Nagaraja AR, Kwong B, Katz J, Watson N, Irvine DJ. Cytosolic delivery of membrane-impermeable molecules in dendritic cells using pH-responsive core-shell nanoparticles. Nano Lett. 2007;7(10):3056–3064. [DOI] [PubMed] [Google Scholar]
- 61.Griset AP, Walpole J, Liu R, Gaffey A, Colson YL,Grinstaff MW. Expansile nanoparticles: Synthesis, characterization, and in vivo efficacy of an acid-responsive polymeric drug delivery system. J Am Chem Soc. 2009;131(7):2469–2471. [DOI] [PubMed] [Google Scholar]
- 62.Chiang W-H, Huang W-C, Shen M-Y, Wang C-H, Huang Y-F, Lin S-C, Chern C-S, Chiu H-C. Dual-layered nanogel-coated hollow lipid/polypeptide conjugate assemblies for potential pH-triggered intracellular drug release. PLOS ONE. 2014;9(3): Article e92268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.ur Rehman Z, Hoekstra D, Zuhorn IS. Mechanism of polyplex- and lipoplex-mediated delivery of nucleic acids: Real-time visualization of transient membrane destabilization without endosomal lysis. ACS Nano. 2013;7(5):3767–3777. [DOI] [PubMed] [Google Scholar]
- 64.Kwon EJ, Bergen JM, Pun SH. Application of an HIV gp41-derived peptide for enhanced intracellular trafficking of synthetic gene and siRNA delivery vehicles. Bioconjug Chem. 2008;19(4):920–927. [DOI] [PubMed] [Google Scholar]
- 65.Plaza-Ga I, Manzaneda-Gonzalez V, Kisovec M, Almendro-Vedia V, Munoz-Ubeda M, Anderluh G, Guerrero-Martinez A, Natale P, Lopez Montero I. pH-triggered endosomal escape of pore-forming Listeriolysin O toxin-coated gold nanoparticles. J Nanobiotechnol. 2019;17(1): Article 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ranaweera A, Ratnayake PU, Weliky DP. The stabilities of the soluble ectodomain and fusion peptide hairpins of the influenza virus hemagglutinin subunit II protein are positively correlated with membrane fusion. Biochemistry. 2018;57(37):5480–5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Y, Cheng Q, Jiang Q, Huang Y, Liu H, Zhao Y, Cao W, Ma G, Dai F, Liang X, et al. Enhanced endosomal/lysosomal escape by distearoyl phosphoethanolamine-polycarboxybetaine lipid for systemic delivery of siRNA. J Control Release. 2014;176:104–114. [DOI] [PubMed] [Google Scholar]
- 68.Wei P, Sun M, Yang B, Xiao J, Du J. Ultrasound-responsive polymersomes capable of endosomal escape for efficient cancer therapy. J Control Release. 2020;322:81–94. [DOI] [PubMed] [Google Scholar]
- 69.Alamoudi K, Martins P, Croissant JG, Patil S, Omar H, Khashab NM. Thermoresponsive pegylated bubble liposome nanovectors for efficient siRNA delivery via endosomal escape. Nanomedicine (Lond). 2017;12(12):1421–1433. [DOI] [PubMed] [Google Scholar]
- 70.Feng Q, Yu MZ, Wang JC, Hou WJ, Gao LY, Ma XF, Pei XW, Niu YJ, Liu XY, Qiu C, et al. Synergistic inhibition of breast cancer by co-delivery of VEGF siRNA and paclitaxel via vapreotide-modified core-shell nanoparticles. Biomaterials. 2014;35(18):5028–5038. [DOI] [PubMed] [Google Scholar]
- 71.Gao LY, Liu XY, Chen CJ, Wang JC, Feng Q, Yu MZ, Ma XF, Pei XW, Niu YJ, Qiu C, et al. Core-shell type lipid/rPAA-Chol polymer hybrid nanoparticles for in vivo siRNA delivery. Biomaterials. 2014;35(6):2066–2078. [DOI] [PubMed] [Google Scholar]
- 72.Wei L, Guo XY, Yang T, Yu MZ, Chen DW, Wang JC. Brain tumor-targeted therapy by systemic delivery of siRNA with transferrin receptor-mediated core-shell nanoparticles. Int J Pharm. 2016;510(1):394–405. [DOI] [PubMed] [Google Scholar]
- 73.Yu MZ, Pang WH, Yang T, Wang JC, Wei L, Qiu C, Wu YF, Liu WZ, Wei W, Guo XY, et al. Systemic delivery of siRNA by T7 peptide modified core-shell nanoparticles for targeted therapy of breast cancer. Eur J Pharm Sci. 2016;92:39–48. [DOI] [PubMed] [Google Scholar]
- 74.Shaheen SM, Akita H, Nakamura T, Takayama S, Futaki S, Yamashita A, Katoono R, Yui N, Harashima H. KALA-modified multi-layered nanoparticles as gene carriers for MHC class-I mediated antigen presentation for a DNA vaccine. Biomaterials. 2011;32(26):6342–6350. [DOI] [PubMed] [Google Scholar]
- 75.Sasaki K, Kogure K, Chaki S, Nakamura Y, Moriguchi R, Hamada H, Danev R, Nagayama K, Futaki S, Harashima H. An artificial virus-like nano carrier system: Enhanced endosomal escape of nanoparticles via synergistic action of pH-sensitive fusogenic peptide derivatives. Anal Bioanal Chem. 2008;391(8):2717–2727. [DOI] [PubMed] [Google Scholar]
- 76.Miyoshi Y, Kadono M, Okazaki S, Nishimura A, Kitamatsu M, Watanabe K, Ohtsuki T. Endosomal escape of peptide-photosensitizer conjugates is affected by amino acid sequences near the photosensitizer. Bioconjug Chem. 2020;31(3):916–922. [DOI] [PubMed] [Google Scholar]
- 77.Jayakumar MK, Bansal A, Huang K, Yao R, Li BN, Zhang Y. Near-infrared-light-based nano-platform boosts endosomal escape and controls gene knockdown in vivo. ACS Nano. 2014;8(5):4848–4858. [DOI] [PubMed] [Google Scholar]
- 78.Cabral H, Nakanishi M, Kumagai M, Jang WD, Nishiyama N, Kataoka K. A photo-activated targeting chemotherapy using glutathione sensitive camptothecin-loaded polymeric micelles. Pharm Res. 2009;26(1):82–92. [DOI] [PubMed] [Google Scholar]
- 79.Kim B, Sun S, Varner JA, Howell SB, Ruoslahti E, Sailor MJ. Securing the payload, finding the cell, and avoiding the endosome: Peptide-targeted, fusogenic porous silicon nanoparticles for delivery of siRNA. Adv Mater. 2019;31(35): Article e1902952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun H, Su J, Meng Q, Yin Q, Chen L, Gu W, Zhang P, Zhang Z, Yu H, Wang S, et al. Cancer-cell-biomimetic nanoparticles for targeted therapy of homotypic tumors. Adv Mater. 2016;28(43):9581–9588. [DOI] [PubMed] [Google Scholar]
- 81.Yang J, Bahreman A, Daudey G, Bussmann J, Olsthoorn RC, Kros A. Drug delivery via cell membrane fusion using lipopeptide modified liposomes. ACS Cent Sci. 2016;2(9):621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou J, Sun L, Wang L, Liu Y, Li J, Li J, Li J, Yang H. Self-assembled and size-controllable oligonucleotide nanospheres for effective antisense gene delivery through an endocytosis-independent pathway. Angew Chem Int Ed Engl. 2019;58(16):5236–5240. [DOI] [PubMed] [Google Scholar]
- 83.Jiang X, Wang G, Liu R, Wang Y, Wang Y, Qiu X, Gao X. RNase non-sensitive and endocytosis independent siRNA delivery system: Delivery of siRNA into tumor cells and high efficiency induction of apoptosis. Nanoscale. 2013;5(16):7256–7264. [DOI] [PubMed] [Google Scholar]
- 84.Ho NT, Siggel M, Camacho KV, Bhaskara RM, Hicks JM, Yao YC, Zhang Y, Kofinger J, Hummer G, Noy A. Membrane fusion and drug delivery with carbon nanotube porins. Proc Natl Acad Sci USA. 2021;118(19): Article e2016974118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nechaev S, Gao C, Moreira D, Swiderski P, Jozwiak A, Kowolik CM, Zhou J, Armstrong B, Raubitschek A, Rossi JJ, et al. Intracellular processing of immunostimulatory CpG-siRNA: Toll-like receptor 9 facilitates siRNA dicing and endosomal escape. J Control Release. 2013;170(3):307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reilly MJ, Larsen JD, Sullivan MO. Polyplexes traffic through caveolae to the Golgi and endoplasmic reticulum en route to the nucleus. Mol Pharm. 2012;9(5):1280–1290. [DOI] [PubMed] [Google Scholar]
- 87.Gabrielson NP, Pack DW. Efficient polyethylenimine-mediated gene delivery proceeds via a caveolar pathway in HeLa cells. J Control Release. 2009;136(1):54–61. [DOI] [PubMed] [Google Scholar]
- 88.Wang G, Norton AS, Pokharel D, Song Y, Hill RA. KDEL peptide gold nanoconstructs: Promising nanoplatforms for drug delivery. Nanomedicine. 2013;9(3):366–374. [DOI] [PubMed] [Google Scholar]
- 89.Qiu C, Han HH, Sun J, Zhang HT, Wei W, Cui SH, Chen X, Wang JC, Zhang Q. Regulating intracellular fate of siRNA by endoplasmic reticulum membrane-decorated hybrid nanoplexes. Nat Commun. 2019;10(1):2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang H, Qin L, Zhang X, Guan J, Mao S. Mechanisms and challenges of nanocarriers as non-viral vectors of therapeutic genes for enhanced pulmonary delivery. J Control Release. 2022;352:970–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Neuberg P, Kichler A. Recent developments in nucleic acid delivery with polyethylenimines. Adv Genet. 2014;88:263–288. [DOI] [PubMed] [Google Scholar]
- 92.Chen J, Ding J, Wang Y, Cheng J, Ji S, Zhuang X, Chen X. Sequentially responsive shell-stacked nanoparticles for deep penetration into solid tumors. Adv Mater. 2017;29(32): Article 1701170. [DOI] [PubMed] [Google Scholar]
- 93.Shim G, Han SE, Yu YH, Lee S, Lee HY, Kim K, Kwon IC, Park TG, Kim YB, Choi YS, et al. Trilysinoyl oleylamide-based cationic liposomes for systemic co-delivery of siRNA and an anticancer drug. J Control Release. 2011;155(1):60–66. [DOI] [PubMed] [Google Scholar]
- 94.Bober Z, Bartusik-Aebisher D, Aebisher D. Application of dendrimers in anticancer diagnostics and therapy. Molecules. 2022;27(10): Article 3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ragelle H, Vandermeulen G, Préat V. Chitosan-based siRNA delivery systems. J Control Release. 2013;172(1):207–218. [DOI] [PubMed] [Google Scholar]
- 96.Christie RJ, Nishiyama N, Kataoka K. Delivering the code: Polyplex carriers for deoxyribonucleic acid and ribonucleic acid interference therapies. Endocrinology. 2010;151(2):466–473. [DOI] [PubMed] [Google Scholar]
- 97.Xiao B, Ma P, Viennois E, Merlin D. Urocanic acid-modified chitosan nanoparticles can confer anti-inflammatory effect by delivering CD98 siRNA to macrophages. Colloids Surf B Biointerfaces. 2016;143:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu J, Liu X, Han Y, Zhang J, Liu D, Ma G, Li C, Liu L, Kong D. Nanovaccine incorporated with hydroxychloroquine enhances antigen cross-presentation and promotes antitumor immune responses. ACS Appl Mater Interfaces. 2018;10(37):30983–30993. [DOI] [PubMed] [Google Scholar]
- 99.Guo Y, Shen M, Shi X. Construction of poly(amidoamine) dendrimer/carbon dot nanohybrids for biomedical applications. Macromol Biosci. 2021;21(4): Article e2100007. [DOI] [PubMed] [Google Scholar]
- 100.Lee J, Sands I, Zhang W, Zhou L, Chen Y. DNA-inspired nanomaterials for enhanced endosomal escape. Proc Natl Acad Sci USA. 2021;118(19): Article e2104511118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mahajan S, Tang T. Polyethylenimine-DNA nanoparticles under endosomal acidification and implication to gene delivery. Langmuir. 2022;38(27):8382–8397. [DOI] [PubMed] [Google Scholar]
- 102.Cabral H, Miyata K, Osada K, Kataoka K. Block copolymer micelles in nanomedicine applications. Chem Rev. 2018;118(14):6844–6892. [DOI] [PubMed] [Google Scholar]
- 103.Won Y-Y, Sharma R, Konieczny SF. Missing pieces in understanding the intracellular trafficking of polycation/DNA complexes. J Control Release. 2009;139(2):88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Benjaminsen RV, Mattebjerg MA, Henriksen JR, Moghimi SM, Andresen TL. The possible "proton sponge " effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol Ther. 2013;21(1):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lomas H, Massignani M, Abdullah KA, Canton I, Lo Presti C, MacNeil S, Du J, Blanazs A, Madsen J, Armes SP, et al. Non-cytotoxic polymer vesicles for rapid and efficient intracellular delivery. Faraday Discuss. 2008;139:143–159. [DOI] [PubMed] [Google Scholar]
- 106.Selby LI, Cortez-Jugo CM, Such GK, Johnston APR. Nanoescapology: Progress toward understanding the endosomal escape of polymeric nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(5): Article e1452. [DOI] [PubMed] [Google Scholar]
- 107.Lee WK, Braun M, Langeluddecke C, Thevenod F. Cyclosporin a, but not FK506, induces osmotic lysis of pancreas zymogen granules, intra-acinar enzyme release, and lysosome instability by activating K+ channel. Pancreas. 2012;41(4):596–604. [DOI] [PubMed] [Google Scholar]
- 108.Huang D, He B, Mi P. Calcium phosphate nanocarriers for drug delivery to tumors: Imaging, therapy and theranostics. Biomater Sci. 2019;7(10):3942–3960. [DOI] [PubMed] [Google Scholar]
- 109.Bassett DC, Robinson TE, Hill RJ, Grover LM, Barralet JE. Self-assembled calcium pyrophosphate nanostructures for targeted molecular delivery. Biomater Adv. 2022;140: Article 213086. [DOI] [PubMed] [Google Scholar]
- 110.Choi KY, Silvestre OF, Huang X, Min KH, Howard GP, Hida N, Jin AJ, Carvajal N, Lee SW, Hong JI, et al. Versatile RNA interference nanoplatform for systemic delivery of RNAs. ACS Nano. 2014;8(5):4559–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou Z, Li H, Wang K, Guo Q, Li C, Jiang H, Hu Y, Oupicky D, Sun M. Bioreducible cross-linked hyaluronic acid/calcium phosphate hybrid nanoparticles for specific delivery of siRNA in melanoma tumor therapy. ACS Appl Mater Interfaces. 2017;9(17):14576–14589. [DOI] [PubMed] [Google Scholar]
- 112.Yang Q, Liu DZ, Liu M, Ji QF, Mei QB, Cheng Y, Zhou SY. Bone-targeted calcium phosphate-polymer hybrid nanoparticle co-deliver zoledronate and docetaxel to treat bone metastasis of prostate cancer. J Pharm Sci. 2021;110(2):876–887. [DOI] [PubMed] [Google Scholar]
- 113.Lee MS, Lee JE, Byun E, Kim NW, Lee K, Lee H, Sim SJ, Lee DS, Jeong JH. Target-specific delivery of siRNA by stabilized calcium phosphate nanoparticles using dopa-hyaluronic acid conjugate. J Control Release. 2014;192:122–130. [DOI] [PubMed] [Google Scholar]
- 114.Lee JE, Yin Y, Lim SY, Kim ES, Jung J, Kim D, Park JW, Lee MS, Jeong JH. Enhanced transfection of human mesenchymal stem cells using a hyaluronic acid/calcium phosphate hybrid gene delivery system. Polymers (Basel). 2019(5):798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pittella F, Cabral H, Maeda Y, Mi P, Watanabe S, Takemoto H, Kim HJ, Nishiyama N, Miyata K, Kataoka K. Systemic siRNA delivery to a spontaneous pancreatic tumor model in transgenic mice by PEGylated calcium phosphate hybrid micelles. J Control Release. 2014;178:18–24. [DOI] [PubMed] [Google Scholar]
- 116.Pittella F, Zhang M, Lee Y, Kim HJ, Tockary T, Osada K, Ishii T, Miyata K, Nishiyama N, Kataoka K. Enhanced endosomal escape of siRNA-incorporating hybrid nanoparticles from calcium phosphate and PEG-block charge-conversional polymer for efficient gene knockdown with negligible cytotoxicity. Biomaterials. 2011;32(11):3106–3114. [DOI] [PubMed] [Google Scholar]
- 117.Giger EV, Puigmartí-Luis J, Schlatter R, Castagner B, Dittrich PS, Leroux J-C. Gene delivery with bisphosphonate-stabilized calcium phosphate nanoparticles. J Control Release. 2011;150(1):87–93. [DOI] [PubMed] [Google Scholar]
- 118.Tang MX, Redemann CT, Szoka FC Jr. In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjug Chem. 1996;7(6):703–714. [DOI] [PubMed] [Google Scholar]
- 119.Nguyen J, Szoka FC. Nucleic acid delivery: The missing pieces of the puzzle? Acc Chem Res. 2012;45(7):1153–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ni S, Xie Y, Tang Y, Liu Y, Chen J, Zhu S. Nebulized anionic guanidinylated O-carboxymethyl chitosan/N-2-hydroxypropyltimehyl ammonium chloride chitosan nanoparticles for siRNA pulmonary delivery: Preparation, characterization and in vitro evaluation. J Drug Target. 2017;25(5):451–462. [DOI] [PubMed] [Google Scholar]
- 121.Molla MR, Marcinko T, Prasad P, Deming D, Garman SC, Thayumanavan S. Unlocking a caged lysosomal protein from a polymeric nanogel with a pH trigger. Biomacromolecules. 2014;15(11):4046–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.You JO, Auguste DT. Nanocarrier cross-linking density and pH sensitivity regulate intracellular gene transfer. Nano Lett. 2009;9(12):4467–4473. [DOI] [PubMed] [Google Scholar]
- 123.Villani S, Adami R, Reverchon E, Ferretti AM, Ponti A, Lepretti M, Caputo I, Izzo L. pH-sensitive polymersomes: Controlling swelling via copolymer structure and chemical composition. J Drug Target. 2017;25(9–10):899–909. [DOI] [PubMed] [Google Scholar]
- 124.Kermaniyan SS, Chen M, Zhang C, Smith SA, Johnston APR, Such C, Such GK. Understanding the biological interactions of pH-swellable nanoparticles. Macromol Biosci. 2022;22(5): Article e2100445. [DOI] [PubMed] [Google Scholar]
- 125.Danial M, Perrier S, Jolliffe KA. Effect of the amino acid composition of cyclic peptides on their self-assembly in lipid bilayers. Org Biomol Chem. 2015;13(8):2464–2473. [DOI] [PubMed] [Google Scholar]
- 126.Mondal AK, Lata K, Singh M, Chatterjee S, Chauhan A, Puravankara S, Chattopadhyay K. Cryo-EM elucidates mechanism of action of bacterial pore-forming toxins. Biochim Biophys Acta Biomembr. 2022;1864(11): Article 184013. [DOI] [PubMed] [Google Scholar]
- 127.Ulhuq FR, Mariano G. Bacterial pore-forming toxins. Microbiology (Reading). 2022;168(3): Article 001154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee M-T, Sun T-L, Hung W-C, Huang HW. Process of inducing pores in membranes by melittin. Proc Natl Acad Sci USA. 2013;110(35):14243–14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qian S, Wang W, Yang L, Huang HW. Structure of the alamethicin pore reconstructed by x-ray diffraction analysis. Biophys J. 2008;94(9):3512–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chu Z, Zhang S, Zhang B, Zhang C, Fang CY, Rehor I, Cigler P, Chang HC, Lin G, Liu R, et al. Unambiguous observation of shape effects on cellular fate of nanoparticles. Sci Rep. 2014;4:4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pang YT, Ge Z, Zhang B, Xiu P, Li Q, Wang Y. Pore formation induced by nanoparticles binding to a lipid membrane. Nanoscale. 2020;12(14):7902–7913. [DOI] [PubMed] [Google Scholar]
- 132.Ren D, Fisson S, Dalkara D, Ail D. Immune responses to gene editing by viral and non-viral delivery vectors used in retinal gene therapy. Pharmaceutics. 2022;14(9): Article 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Endoh T, Ohtsuki T. Cellular siRNA delivery using cell-penetrating peptides modified for endosomal escape. Adv Drug Deliv Rev. 2009;61(9):704–709. [DOI] [PubMed] [Google Scholar]
- 134.Suzuki M, Iwaki K, Kikuchi M, Fujiwara K, Doi N. Characterization of the membrane penetration-enhancing peptide S19 derived from human syncytin-1 for the intracellular delivery of TAT-fused proteins. Biochem Biophys Res Commun. 2022;586:63–67. [DOI] [PubMed] [Google Scholar]
- 135.Cao X, Shang X, Guo Y, Zheng X, Li W, Wu D, Sun L, Mu S, Guo C. Lysosomal escaped protein nanocarriers for nuclear-targeted siRNA delivery. Anal Bioanal Chem. 2021;413(13):3493–3499. [DOI] [PubMed] [Google Scholar]
- 136.Ye S-f, Tian M-m, Wang T-x, Ren L, Wang D, Shen L-h, Shang T. Synergistic effects of cell-penetrating peptide tat and fusogenic peptide HA2-enhanced cellular internalization and gene transduction of organosilica nanoparticles. Nanomedicine. 2012;8(6):833–841. [DOI] [PubMed] [Google Scholar]
- 137.Wyatt LC, Lewis JS, Andreev OA, Reshetnyak YK, Engelman DM. Applications of pHLIP technology for cancer imaging and therapy. Trends Biotechnol. 2017;35(7):653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang SK, Gong L, Zhang X, Yun ZM, Li SB, Gao HW, Dai CJ, Yuan JJ, Chen JM, Gong F, et al. Antimicrobial peptide AR-23 derivatives with high endosomal disrupting ability enhance poly(l-lysine)-mediated gene transfer. J Gene Med. 2020;22(11): Article e3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Peng H, Ji W, Zhao R, Lu Z, Yang J, Li Y, Zhang X. pH-sensitive zwitterionic polycarboxybetaine as a potential non-viral vector for small interfering RNA delivery. RSC Adv. 2020;10(73):45059–45066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yameen B, Ali M, Neumann R, Ensinger W, Knoll W, Azzaroni O. Single conical nanopores displaying pH-tunable rectifying characteristics. Manipulating ionic transport with zwitterionic polymer brushes. J Am Chem Soc. 2009;131(6):2070–2071. [DOI] [PubMed] [Google Scholar]
- 141.Luo M, Wang H, Wang Z, Cai H, Lu Z, Li Y, Du M, Huang G, Wang C, Chen X, et al. A STING-activating nanovaccine for cancer immunotherapy. Nat Nanotechnol. 2017;12(7):648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Meng N, Grimm D. Membrane-destabilizing ionizable phospholipids: Novel components for organ-selective mRNA delivery and CRISPR-Cas gene editing. Signal Transduct Target Ther. 2021;6(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Habrant D, Peuziat P, Colombani T, Dallet L, Gehin J, Goudeau E, Evrard B, Lambert O, Haudebourg T, Pitard B. Design of ionizable lipids to overcome the limiting step of endosomal escape: Application in the intracellular delivery of mRNA, DNA, and siRNA. J Med Chem. 2016;59(7):3046–3062. [DOI] [PubMed] [Google Scholar]
- 144.Kulkarni JA, Cullis PR, Meel R. Lipid nanoparticles enabling gene therapies: From concepts to clinical utility. Nucleic Acid Ther. 2018;28(3):146–157. [DOI] [PubMed] [Google Scholar]
- 145.Jadhav SG, Dowdy SF. Overcoming delivery barriers with LNPs. Nat Mater. 2021;20(5):575–577. [DOI] [PubMed] [Google Scholar]
- 146.Bailey-Hytholt CM, Ulinski G, Dugas J, Haines M, Lazebnik M, Piepenhagen P, Zarraga IE, Bandekar A. Intracellular trafficking kinetics of nucleic acid escape from lipid nanoparticles via fluorescence imaging. Curr Pharm Biotechnol. 2023. [DOI] [PubMed] [Google Scholar]
- 147.Dong S, Wang J, Guo Z, Zhang Y, Zha W, Wang Y, Liu C, Xing H, Li X. Efficient delivery of VEGFA mRNA for promoting wound healing via ionizable lipid nanoparticles. Bioorg Med Chem. 2023;78: Article 117135. [DOI] [PubMed] [Google Scholar]
- 148.Joris F, De Backer L, Van de Vyver T, Bastiancich C, De Smedt SC, Raemdonck K. Repurposing cationic amphiphilic drugs as adjuvants to induce lysosomal siRNA escape in nanogel transfected cells. J Control Release. 2018;269:266–276. [DOI] [PubMed] [Google Scholar]
- 149.Tamura A, Yui N. A supramolecular endosomal escape approach for enhancing gene silencing of siRNA using acid-degradable cationic polyrotaxanes. J Mater Chem B. 2013;1(29):3535–3544. [DOI] [PubMed] [Google Scholar]
- 150.Donders EN, Slaughter KV, Dank C, Ganesh AN, Shoichet BK, Lautens M, Shoichet MS. Synthetic ionizable colloidal drug aggregates enable endosomal disruption. Adv Sci (Weinh). 2023; Article e2300311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liao WH, Hsiao MY, Lo CW, Yang HS, Sun MK, Lin FH, Chang Y, Chen WS. Intracellular triggered release of DNA-quaternary ammonium polyplex by ultrasound. Ultrason Sonochem. 2017;36:70–77. [DOI] [PubMed] [Google Scholar]
- 152.Omata D, Negishi Y, Hagiwara S, Yamamura S, Endo-Takahashi Y, Suzuki R, Maruyama K, Nomizu M, Aramaki Y. Bubble liposomes and ultrasound promoted endosomal escape of TAT-PEG liposomes as gene delivery carriers. Mol Pharm. 2011;8(6):2416–2423. [DOI] [PubMed] [Google Scholar]
- 153.Omata D, Negishi Y, Suzuki R, Oda Y, Endo-Takahashi Y, Maruyama K. Nonviral gene delivery systems by the combination of bubble liposomes and ultrasound. Adv Genet. 2015;89:25–48. [DOI] [PubMed] [Google Scholar]
- 154.Tapeinos C, Marino A, Battaglini M, Migliorin S, Brescia R, Scarpellini A, De Julian Fernandez C, Prato M, Drago F, Ciofani G. Stimuli-responsive lipid-based magnetic nanovectors increase apoptosis in glioblastoma cells through synergic intracellular hyperthermia and chemotherapy. Nanoscale. 2018;11(1):72–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pucci C, De Pasquale D, Marino A, Martinelli C, Lauciello S, Ciofani G. Hybrid magnetic nanovectors promote selective glioblastoma cell death through a combined effect of lysosomal membrane permeabilization and chemotherapy. ACS Appl Mater Interfaces. 2020;12(26):29037–29055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Domenech M, Marrero-Berrios I, Torres-Lugo M, Rinaldi C. Lysosomal membrane permeabilization by targeted magnetic nanoparticles in alternating magnetic fields. ACS Nano. 2013;7(6):5091–5101. [DOI] [PubMed] [Google Scholar]
- 157.Ramezanpour M, Tieleman DP. Computational insights into the role of cholesterol in inverted hexagonal phase stabilization and endosomal drug release. Langmuir. 2022;38(24):7462–7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chhabra R, Grabrucker AM, Veratti P, Belletti D,Boeckers TM, Vandelli MA, Forni F, Tosi G, Ruozi B. Characterization of lysosome-destabilizing DOPE/PLGA nanoparticles designed for cytoplasmic drug release. Int J Pharm. 2014;471(1–2):349–357. [DOI] [PubMed] [Google Scholar]
- 159.Shin J, Shum P, Grey J, Fujiwara S, Malhotra GS, Gonzalez-Bonet A, Hyun SH, Moase E, Allen TM, Thompson DH. Acid-labile mPEG-vinyl ether-1,2-dioleylglycerol lipids with tunable pH sensitivity: Synthesis and structural effects on hydrolysis rates, DOPE liposome release performance, and pharmacokinetics. Mol Pharm. 2012;9(11):3266–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Seraj S, Lee J, Ahn HJ. Systemic delivery of Eg5 shRNA-expressing plasmids using PEGylated DC-Chol/DOPE cationic liposome: Long-term silencing and anticancer effects in vivo. Biochem Pharmacol. 2019;166:192–202. [DOI] [PubMed] [Google Scholar]
- 161.Mochizuki S, Kanegae N, Nishina K, Kamikawa Y, Koiwai K, Masunaga H, Sakurai K. The role of the helper lipid dioleoylphosphatidylethanolamine (DOPE) for DNA transfection cooperating with a cationic lipid bearing ethylenediamine. Biochim Biophys Acta. 2013;1828(2):412–418. [DOI] [PubMed] [Google Scholar]
- 162.Koltover I, Salditt T, Radler JO, Safinya CR. An inverted hexagonal phase of cationic liposome-DNA complexes related to DNA release and delivery. Science. 1998;281(5373):78–81. [DOI] [PubMed] [Google Scholar]
- 163.Wyman TB, Nicol F, Zelphati O, Scaria PV, Plank C, Szoka FC Jr. Design, synthesis, and characterization of a cationic peptide that binds to nucleic acids and permeabilizes bilayers. Biochemistry. 1997;36(10):3008–3017. [DOI] [PubMed] [Google Scholar]
- 164.Li W, Nicol F, Szoka FC Jr. GALA: A designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Adv Drug Deliv Rev. 2004;56(7):967–985. [DOI] [PubMed] [Google Scholar]
- 165.Nishimura Y, Takeda K, Ezawa R, Ishii J, Ogino C, Kondo A. A display of pH-sensitive fusogenic GALA peptide facilitates endosomal escape from a bio-nanocapsule via an endocytic uptake pathway. J Nanobiotechnol. 2014;12: Article 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nakase I, Kogure K, Harashima H, Futaki S. Application of a fusiogenic peptide GALA for intracellular delivery. Methods Mol Biol. 2011;683:525–533. [DOI] [PubMed] [Google Scholar]
- 167.Berg K, Selbo PK, Weyergang A, Dietze A, Prasmickaite L, Bonsted A, Engesaeter BO, Angell-Petersen E, Warloe T, Frandsen N, et al. Porphyrin-related photosensitizers for cancer imaging and therapeutic applications. J Microsc. 2005;218(Pt 2):133–147. [DOI] [PubMed] [Google Scholar]
- 168.Rueda-Gensini L, Cifuentes J, Castellanos MC, Puentes PR, Serna JA, Munoz-Camargo C, Cruz JC. Tailoring iron oxide nanoparticles for efficient cellular internalization and endosomal escape. Nanomaterials (Basel). 2020;10(9): Article 1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Soe TH, Watanabe K, Ohtsuki T. Photoinduced endosomal escape mechanism: A view from photochemical internalization mediated by CPP-photosensitizer conjugates. Molecules. 2020;26(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Turk B, Turk V. Lysosomes as "suicide bags" in cell death: Myth or reality? J Biol Chem. 2009;284(33):21783–21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kavcic N, Pegan K, Turk B. Lysosomes in programmed cell death pathways: From initiators to amplifiers. Biol Chem. 2017;398(3):289–301. [DOI] [PubMed] [Google Scholar]
- 172.Prada I, Meldolesi J. Binding and fusion of extracellular vesicles to the plasma membrane of their cell targets. Int J Mol Sci. 2016;17(8):1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang M, Cheng S, Jin Y, Zhang N, Wang Y. Membrane engineering of cell membrane biomimetic nanoparticles for nanoscale therapeutics. Clin Transl Med. 2021;11(2): Article e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen HY, Deng J, Wang Y, Wu CQ, Li X, Dai HW. Hybrid cell membrane-coated nanoparticles: A multifunctional biomimetic platform for cancer diagnosis and therapy. Acta Biomater. 2020;112:1–13. [DOI] [PubMed] [Google Scholar]
- 175.Gao W, Hu CM, Fang RH, Luk BT, Su J, Zhang L. Surface functionalization of gold nanoparticles with red blood cell membranes. Adv Mater. 2013;25(26):3549–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xuan M, Shao J, Zhao J, Li Q, Dai L, Li J. Magnetic mesoporous silica nanoparticles cloaked by red blood cell membranes: Applications in cancer therapy. Angew Chem Int Ed Engl. 2018;57(21):6049–6053. [DOI] [PubMed] [Google Scholar]
- 177.Rao L, Bu LL, Xu JH, Cai B, Yu GT, Yu XL, He ZB, Huang QQ, Li A, Guo SS, et al. Red blood cell membrane as a biomimetic nanocoating for prolonged circulation time and reduced accelerated blood clearance. Small. 2015;11(46):6225–6236. [DOI] [PubMed] [Google Scholar]
- 178.Guo X, Zhang Y, Liu J, Yang X, Huang J, Li L, Wan L, Wang K. Red blood cell membrane-mediated fusion of hydrophobic quantum dots with living cell membranes for cell imaging. J Mater Chem B. 2016;4(23):4191–4197. [DOI] [PubMed] [Google Scholar]
- 179.Xuan M, Shao J, Dai L, He Q, Li J. Macrophage cell membrane camouflaged mesoporous silica nanocapsules for in vivo cancer therapy. Adv Healthc Mater. 2015;4(11):1645–1652. [DOI] [PubMed] [Google Scholar]
- 180.Wang Q, Ren Y, Mu J, Egilmez NK, Zhuang X, Deng Z, Zhang L, Yan J, Miller D, Zhang HG. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer Res. 2015;75(12):2520–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Anselmo AC, Modery-Pawlowski CL, Menegatti S, Kumar S, Vogus DR, Tian LL, Chen M, Squires TM, Sen Gupta A, Mitragotri S. Platelet-like nanoparticles: Mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano. 2014;8(11):11243–11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rao L, Bu LL, Cai B, Xu JH, Li A, Zhang WF, Sun ZJ, Guo SS, Liu W, Wang TH, et al. Cancer cell membrane-coated upconversion nanoprobes for highly specific tumor imaging. Adv Mater. 2016;28(18):3460–3466. [DOI] [PubMed] [Google Scholar]
- 183.Jiang Y, Tang R, Duncan B, Jiang Z, Yan B, Mout R, Rotello VM. Direct cytosolic delivery of siRNA using nanoparticle-stabilized nanocapsules. Angew Chem Int Ed Engl. 2015;54(2):506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Deng H, Zhao X, He D, Guo W, Yang K, Dong A, Liang XJ. One-step gene delivery into the cytoplasm in a fusion-dependent manner based on a new membrane fusogenic lipid. Chem Commun (Camb). 2016;52(46):7406–7408. [DOI] [PubMed] [Google Scholar]
- 185.Yao L, Daniels J, Wijesinghe D, Andreev OA, Reshetnyak YK. pHLIP(R)-mediated delivery of PEGylated liposomes to cancer cells. J Control Release. 2013;167(3):228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhou P, Bacaj T, Yang X, Pang ZP, Sudhof TC. Lipid-anchored SNAREs lacking transmembrane regions fully support membrane fusion during neurotransmitter release. Neuron. 2013;80(2):470–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lai Y, Zhao L, Bu B, Lou X, Li D, Ji B, Liu J, Diao J, Shin YK. Lipid molecules influence early stages of yeast SNARE-mediated membrane fusion. Phys Biol. 2015;12(2): Article 025003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kumar P, Guha S, Diederichsen U. SNARE protein analog-mediated membrane fusion. J Pept Sci. 2015;21(8):621–629. [DOI] [PubMed] [Google Scholar]
- 189.D'Agostino M, Risselada HJ, Endter LJ, Comte-Miserez V, Mayer A. SNARE-mediated membrane fusion arrests at pore expansion to regulate the volume of an organelle. EMBO J. 2018;37(19): Article e99193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Margiotta A. Membrane fusion and SNAREs: Interaction with Ras proteins. Int J Mol Sci. 2022;23(15):8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ding Y, Wang Y, Zhou J, Gu X, Wang W, Liu C, Bao X, Wang C, Li Y, Zhang Q. Direct cytosolic siRNA delivery by reconstituted high density lipoprotein for target-specific therapy of tumor angiogenesis. Biomaterials. 2014;35(25):7214–7227. [DOI] [PubMed] [Google Scholar]
- 192.Tunuguntla RH, Escalada A, Frolov VA, Noy A. Synthesis, lipid membrane incorporation, and ion permeability testing of carbon nanotube porins. Nat Protoc. 2016;11(10):2029–2047. [DOI] [PubMed] [Google Scholar]
- 193.Bhaskara RM, Linker SM, Vogele M, Kofinger J, Hummer G. Carbon nanotubes mediate fusion of lipid vesicles. ACS Nano. 2017;11(2):1273–1280. [DOI] [PubMed] [Google Scholar]
- 194.Geng J, Kim K, Zhang J, Escalada A, Tunuguntla R, Comolli LR, Allen FI, Shnyrova AV, Cho KR, Munoz D, et al. Stochastic transport through carbon nanotubes in lipid bilayers and live cell membranes. Nature. 2014;514(7524):612–615. [DOI] [PubMed] [Google Scholar]
- 195.Tai W, Gao X. Noncovalent tagging of siRNA with steroids for transmembrane delivery. Biomaterials. 2018;178:720–727. [DOI] [PubMed] [Google Scholar]
- 196.Chang CL, Chen YJ, Liou J. ER-plasma membrane junctions: Why and how do we study them? Biochim Biophys Acta, Mol Cell Res. 2017;1864(9):1494–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhou J, Shao Z, Liu J, Duan Q, Wang X, Li J, Yang H. From endocytosis to nonendocytosis: The emerging era of gene delivery. ACS Appl Bio Mater. 2020;3(5):2686–2701. [DOI] [PubMed] [Google Scholar]
- 198.Le PU, Nabi IR. Distinct caveolae-mediated endocytic pathways target the Golgi apparatus and the endoplasmic reticulum. J Cell Sci. 2003;116(Pt 6):1059–1071. [DOI] [PubMed] [Google Scholar]
- 199.Xu Z, Gong Q, Xia B, Groves B, Zimmermann M, Mugler C, Mu D, Matsumoto B, Seaman M, Ma D. A role of histone H3 lysine 4 methyltransferase components in endosomal trafficking. J Cell Biol. 2009;186(3):343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hayer A, Stoeber M, Ritz D, Engel S, Meyer HH, Helenius A. Caveolin-1 is ubiquitinated and targeted to intralumenal vesicles in endolysosomes for degradation. J Cell Biol. 2010;191(3):615–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Parton RG, Howes MT. Revisiting caveolin trafficking: The end of the caveosome. J Cell Biol. 2010;191(3):439–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nam HY, Kwon SM, Chung H, Lee SY, Kwon SH, Jeon H, Kim Y, Park JH, Kim J, Her S, et al. Cellular uptake mechanism and intracellular fate of hydrophobically modified glycol chitosan nanoparticles. J Control Release. 2009;135(3):259–267. [DOI] [PubMed] [Google Scholar]
- 203.Sun J, Qiu C, Diao Y, Wei W, Jin H, Zheng Y, Wang J, Zhang L, Yang Z. Delivery pathway regulation of 3',3''-Bis-peptide-siRNA conjugate via nanocarrier architecture engineering. Mol Ther Nucleic Acids. 2018;10:75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Qiu C, Cui S, Sun J, Wang J. In vitro comparative evaluation of three CLD/siRNA nanoplexes prepared by different processes. J Chin Pharm Sci. 2016;25(9):660–668. [Google Scholar]


