Abstract
Dysregulated neurite outgrowth and synapse formation underlie many psychiatric disorders, which are also manifested by wolfram syndrome (WS). Whether and how the causative gene WFS1 deficiency affects synapse formation remain elusive. By mirroring human brain development with cerebral organoids, WFS1-deficient cerebral organoids not only recapitulate the neuronal loss in WS patients, but also exhibit significantly impaired synapse formation and function associated with reduced astrocytes. WFS1 deficiency in neurons autonomously delays neuronal differentiation with altered expressions of genes associated with psychiatric disorders, and impairs neurite outgrowth and synapse formation with elevated cytosolic calcium. Intriguingly, WFS1 deficiency in astrocytes decreases the expression of glutamate transporter EAAT2 by NF-κB activation and induces excessive glutamate. When co-cultured with wildtype neurons, WFS1-deficient astrocytes lead to impaired neurite outgrowth and increased cytosolic calcium in neurons. Importantly, disrupted synapse formation and function in WFS1-deficient cerebral organoids and impaired neurite outgrowth affected by WFS1-deficient astrocytes are efficiently reversed with Riluzole treatment, by restoring EAAT2 expression in astrocytes. Furthermore, Riluzole rescues the depressive-like behavior in the forced swimming test and the impaired recognition and spatial memory in the novel object test and water maze test in Wfs1 conditional knockout mice. Altogether, our study provides novel insights into how WFS1 deficiency affects synapse formation and function, and offers a strategy to treat this disease.
Subject terms: Neuroscience, Stem cells
Introduction
Wolfram syndrome (WS) is a recessive genetic disease manifested by juvenile-onset diabetes mellitus, optic nerve atrophy, hearing loss and wide spectrum of neurological disorders [1–5]. WS patients are also characterized by severe psychiatric manifestations, such as schizophrenia, anxiety, depression, psychosis, panic attack and mood wings [6–10]. WFS1, highly expressed in brain and pancreas, is the major causative gene of WS, and its deficiency presents in 90% WS patients [11, 12]. WS is caused by homozygous or compound heterozygous (both alleles are mutated, but the mutations are not identical) loss of function (LOF) variants in the WFS1 gene, which are biallelic pathogenic variants and inherited in an autosomal recessive manner [12–14]. Patients with WFS1 homozygous or compound heterozygous mutations suffer from typical clinical features of WS [15]. WFS1 is of substantial importance for human mental health. However, due to ethical issues and scarcity of human samples, little is known of the underlying pathogenic mechanism.
During neurodevelopment to form neural connectivity, neurite outgrowth precedes and contributes to synapse formation by developing axon and dendrites [16–18]. Accumulative evidence suggests that dysregulated neurite outgrowth, synapse formation and synaptic function underlie psychiatric disorders, such as schizophrenia with reduced synapses and autism spectrum disorders with excess synapses [19–26]. Current studies on WS neuropathy mainly focus on investigating the role of WFS1 in dysfunction and degeneration of neurons in Drosophila, fly, and mouse model [27–29]. However, the mechanisms of how WFS1 deficiency impacts synapse formation underlying psychiatric disorders in WS remain elusive. Moreover, in Drosophila model of WS, knockdown of wfs1 in both neurons and glial cells resulted in more severe behavioral deficits than knockdown of wfs1 in neurons alone, indicating that interplay between neurons and astrocytes plays an essential role in WS neuropathogenesis [27]. Astrocytes constitute half of the cells in the brain, and have been considered as having an important pathogenic role in multiple neurodevelopmental and neurodegenerative disorders [30–33]. However, there is little evidence in whether and how WFS1 deficiency in astrocytes impacts neurons in WS.
Considering the significant divergences in structure, cell types and cognitive capacity between brains of human and experimental animals, lack of proper human disease models limits understanding how WFS1 deficiency contributes to psychiatric disorders in WS. Recently, various neural cells derived from isogenic human pluripotent stem cells are widely used to model human neurological or psychiatric disorders [23, 24], allowing investigation of pathogenesis in a fixed genetic background. Furthermore, technical breakthrough in generating human cerebral organoids from pluripotent stem cells holds promise to recapitulate human brain organogenesis and model neurodevelopmental disorders [34–38]. Taken together, these human pluripotent stem cell-based technological breakthroughs offer unprecedented opportunities to investigate whether and how WFS1 deficiency affects synapse formation in human disease models with various neural cell types.
Here, we apply a multi-dimensional strategy of combining 3D cerebral organoids and 2D neural differentiation derived from human embryonic stem cells (hESCs) harboring WFS1 deficiency. Our results reveal that WFS1 deficiency significantly impairs synapse formation and function in cerebral organoids associated with decreased astrocytes. WFS1 deficiency autonomously delays neuronal differentiation and affects synapse formation. Moreover, WFS1 deficiency decreases the expression of glutamate transporter EAAT2 by NF-κB activation and compromises glutamate clearance capacity of astrocytes, resulting in non-cell-autonomous reduced neurite outgrowth. Importantly, restoring EAAT2 expression by Riluzole efficiently reverses the impaired synapse formation and function induced by WFS1 deficiency in cerebral organoids and co-culture. Furthermore, Riluzole rescues the depressive-like behavior, the impaired recognition and spatial memory in Wfs1 conditional knockout mice. Thus, our study provides mechanistic insights into psychiatric disorders of WS, and highlights the pathogenic role of WFS1-deficient astrocytes and proposes a potential therapeutic approach with Riluzole.
Results
WFS1-deficient cerebral organoids recapitulate progressively neuronal loss in WS
Since WS is a recessive disease caused by LOF mutations in WFS1 gene, we applied CRISPR/Cas9 to introduce WFS1 knock-out (WFS1−/−) mutations in three hESCs lines, including H1, H9 and HuES8, respectively (Fig. S1A). There was no obvious difference in cell morphology, self-renewal capacity and pluripotency, and the growth rate between WFS1−/− and its wild-type counterpart (WT) hESCs (Fig. S1B–E). All three hESCs lines showed similar results (Fig. S1F).
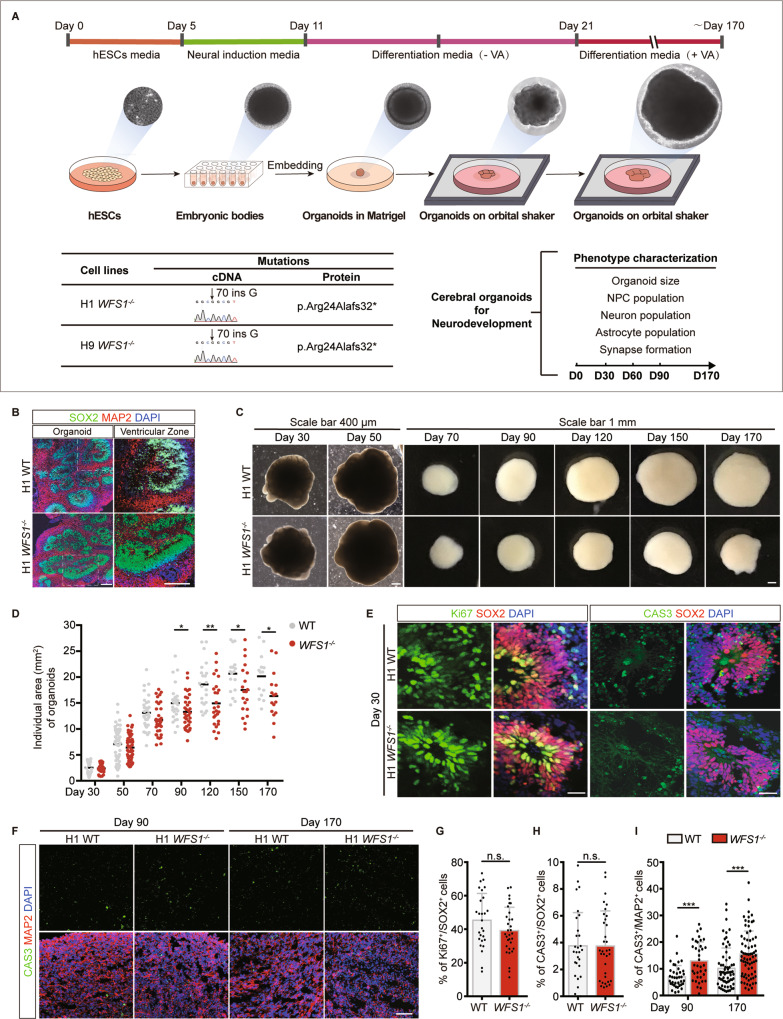
Next, we generated cerebral organoids from WT and WFS1−/− H1 and H9 hESCs (commonly used for cerebral organoids generation) in suspension culture containing cortical differentiation medium [36] (Fig. 1A). Indeed, both WT and WFS1−/− organoids formed 3D neural tissues with ventricular zone (VZ)-like structure surrounded by a thick layer of neural progenitor cells (NPCs) (SOX2-positive cells) on Day 50 (Fig. 1B). As the organoids kept on growing, in organoids from H1 cell line, a reduction in size of WFS1−/− organoids was observed from Day 90 to Day 170 (Fig. 1C, D). In organoids from H9, the WFS1−/− organoids showed decreased size since Day 60 (Fig. S2A, B). These results recapitulate the reduced brain volume observed in WS patients [39, 40]. To explore the mechanism underlying the size reduction, we assessed proliferative capacity and apoptosis of NPCs at Day 30 by immunostaining of Ki67 and cleaved caspase-3 (CAS3), and they were not affected by WFS1 deficiency in both H1 and H9 organoids (Figs. 1E, G and H; S2C–F). Next, we performed the immunostaining of SOX2 and neuronal marker MAP2 in cerebral organoids on Day 50, and quantified the layer thickness of the SOX2+ VZ-like zones and the number of rosettes in the whole cerebral organoid. We found that both of them were significantly decreased in WFS1-deficient cerebral organoids as compared to WT cerebral organoids (Fig. S3A–D). Further, reduction of neurons in WFS1−/− cerebral organoids was observed by MAP2 immunostaining on Day 90, Day 170 and Day 210 but not on Day 60 (Figs. 2A, C; S2I–M). Concurrently, we examined the apoptosis of neurons by immunostaining of MAP2 and CAS3. WFS1−/− organoids displayed a higher percentage of MAP2+CAS3+ cells at Day 90 and Day 170 (Fig. 1F, I). Together, these results suggest that neuronal loss may account for the reduction in organoid size at later stages of differentiation, and WFS1−/− cerebral organoids recapitulate aspects of reduced brain volume and increased neuronal loss observed in the patients of WS neuropathy in a progressive manner [41, 42].
Fig. 1. WFS1 deficiency reduces organoid size and increases neuronal loss in cerebral organoids.
A Schematic of the strategy of modeling WS neuropathy with cerebral organoids. B Representative immunostaining for SOX2 (green), MAP2 (red) and DAPI (blue) in WT and WFS1−/− cerebral organoids at Day 50. Scale bar, 200 μm. Right panels are magnified views of ventricular zone (VZ) in the hollow box region of left panels. Scale bar, 200 μm. C Representative bright-field images of WT and WFS1−/− cerebral organoids at Day 30, Day 50, Day 70, Day 90, Day 120, Day 150, Day 170. Scale bar, 400 μm (Day 30, 50). Scale bar, 1 mm (Day 70, 90, 120, 150, 170). D Quantification of the individual area (mm2) of WT and WFS1−/− cerebral organoids, n ≥ 17 individual organoids. E Immunostaining for SOX2 (red), the proliferation marker Ki67 and the apoptotic marker cleaved caspase-3 (CAS3) (green), and DAPI (blue) in WT and WFS1−/− cerebral organoids at Day 30. Scale bar, 20 μm. F Immunostaining for MAP2 (red), CAS3 (green) and DAPI (blue) in WT and WFS1−/− cerebral organoids at Day 90 and Day 170. Scale bar, 50 μm. G Quantification of the percentage of Ki67+ cells among the total number of SOX2+ NPCs in WT and WFS1−/− cerebral organoids at Day 30, n = 3 individual organoids. H Quantification of the percentage of CAS3+ cells among the total number of SOX2+ NPCs in WT and WFS1−/− cerebral organoids at Day 30, n = 3 individual organoids. I Quantification of the percentage of CAS3+ cells among the total number of MAP2+ neurons in WT and WFS1−/− cerebral organoids at Day 90 and Day 170, n = 3 individual organoids. Data are presented as mean ± SD. p values calculated by unpaired two-tailed Student’s t test were *p < 0.05, **p < 0.01, and ***p < 0.001.
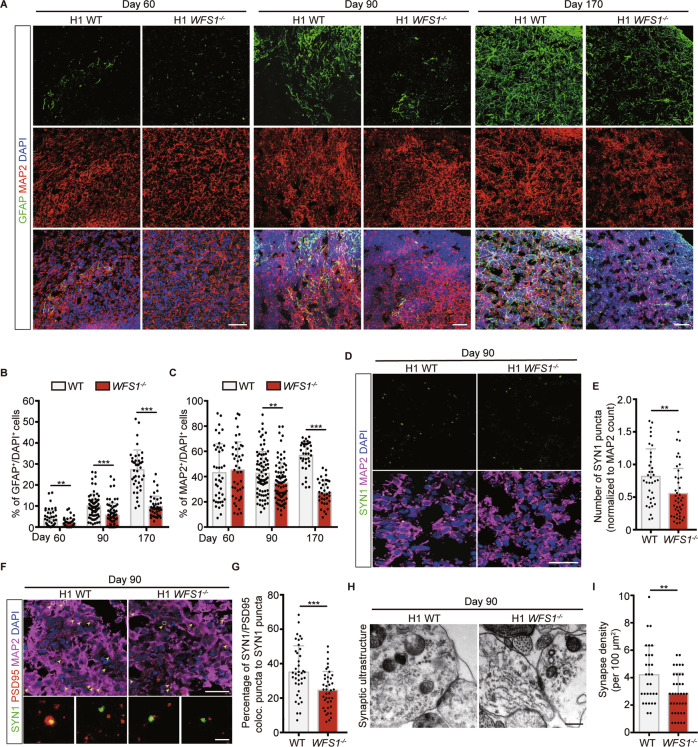
Fig. 2. WFS1 deficiency reduces astrocytes population and impairs synapse formation in cerebral organoids.
A Immunostaining for GFAP (green), MAP2 (red) and DAPI (blue) in WT and WFS1−/− cerebral organoids at Day 60, Day 90 and Day 170. Scale bar, 50 μm. B, C Quantification of the percentage of GFAP+ and MAP2+ cells among the total number of DAPI+ cells in WT and WFS1−/− cerebral organoids at Day 60, Day 90 and Day 170, n ≥ 3 individual organoids. D Immunostaining for the presynaptic marker Synapsin 1 (SYN1) (green) and MAP2 (magenta) in WT and WFS1−/− cerebral organoids at Day 90. Scale bar, 25 μm. E Quantification of the number of SYN1 puncta normalized to MAP2 count in WT and WFS1−/− cerebral organoids at Day 90, n = 3 individual organoids. F Immunostaining for the SYN1 (green), postsynaptic marker PSD95 (red) and MAP2 (magenta) in WT and WFS1−/− cerebral organoids at Day 90. Scale bar, 25 μm. Arrowheads indicate colocalization of SYN1 and PSD95. Lower panels are magnified views of the boxed region in the upper panel. Scale bar, 2 μm. G Quantification of the percentage of SYN1/PSD95 colocalized puncta to SYN1 puncta count in WT and WFS1−/− cerebral organoids at Day 90, n = 3 individual organoids. H Representative images of electron microscopy of synaptic ultrastructure in WT and WFS1−/− cerebral organoids at Day 90. Scale bar, 400 nm. I Quantification of the number of synapse structure normalized to area (100 μm2) in WT and WFS1−/− cerebral organoids at Day 90, n = 3 individual organoids. Data are presented as mean ± SD. p values calculated by unpaired two-tailed Student’s t test were *p < 0.05, **p < 0.01, and ***p < 0.001.
WFS1 deficiency reduces astrocytes population and impairs synapse formation in cerebral organoids
Astrocytes have been known as an essential cell type for neuronal survival and morphogenesis [30, 43]. During brain development, astrogenesis occurs following neurogenesis which could be recapitulated in the developing cerebral organoids (Fig. S3H). Astrocytes were observed as early as Day 60 and kept on increasing as cerebral organoids grew by immunostaining of astrocyte marker GFAP. The number of GFAP+ astrocytes was much lower in WFS1−/− cerebral organoids on Day 60, Day 90 and Day 170 compared to WT organoids (Figs. 2A, B; S2G, H). Astrocytes has been shown to induce synapse formation in neurons. We performed the immunostaining for the presynaptic marker Synapsin 1 (SYN1), and found that the number of SYN1 puncta colocalized with MAP2 was significantly decreased in WFS1−/− cerebral organoids (Fig. 2D, E). Concurrently, the percentage of SYN1/PSD95 colocalized puncta to SYN1 puncta was significantly decreased in WFS1−/− cerebral organoids as compared to WT organoids (Fig. 2F–G). Additionally, the synaptic ultrastructure was examined by electron microscopy and results showed a decrease of synapse density in WFS1−/− cerebral organoids (Fig. 2H, I). Further, to evaluate the effect of WFS1 deficiency on synapse function, we performed whole-cell patch-clamp recording on neurons within cerebral organoids. We found that the frequency but not amplitude of spontaneous excitatory postsynaptic current (sEPSC) of neurons within WFS1-deficient cerebral organoids was significantly decreased, compared to WT cerebral organoids (Fig. 5G, H). Altogether, these results demonstrate that WFS1 deficiency impairs synapse formation and function in cerebral organoids.
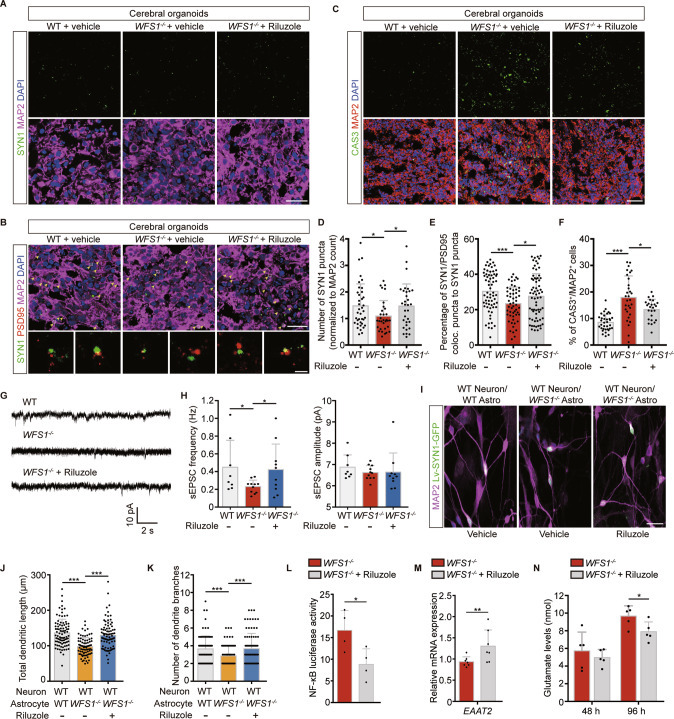
Fig. 5. Impaired synapse formation and function induced by WFS1 deficiency is reversed by Riluzole.
A Immunostaining for the SYN1 (green) and MAP2 (magenta) in WT and WFS1−/− cerebral organoids treated with 5 μM Riluzole or vehicle at Day 90. Scale bar, 25 μm. B Immunostaining for the SYN1 (green), PSD95 (red) and MAP2 (magenta) in WT and WFS1−/− cerebral organoids treated with 5 μM Riluzole or vehicle at Day 90. Scale bar, 25 μm. Arrowheads indicate colocalization of SYN1 and PSD95. Lower panels are magnified views of the boxed region in the upper panel. Scale bar, 2 μm. C Immunostaining for MAP2 (red), CAS3 (green) and DAPI (blue) in WT and WFS1−/− cerebral organoids treated with 5 μM Riluzole or vehicle at Day 90. Scale bar, 50 μm. D Quantification of the number of SYN1 puncta normalized to MAP2 count in WT and WFS1−/− cerebral organoids treated with 5 μM Riluzole or vehicle at Day 90, n = 3 individual organoids. E Quantification of the percentage of SYN1/PSD95 colocalized puncta normalized to SYN1 puncta count in WT and WFS1−/− cerebral organoids treated with 5 μM Riluzole or vehicle at Day 90, n = 3 individual organoids. F Quantification of the percentage of CAS3+ cells among the total number of MAP2+ neurons in WT and WFS1−/− cerebral organoids treated with 5 μM Riluzole or vehicle at Day 90, n = 3 individual organoids. G Representative traces of sEPSC of WT, WFS1−/− and Riluzole-treated WFS1−/− cerebral organoids. H Quantification of the frequency and amplitude of sEPSC for WT, WFS1−/− and Riluzole-treated WFS1−/− cerebral organoids, n = 3 individual organoids. I Representative images of co-culture of WT neurons and WT/WFS1−/− astrocytes treated with 5 μM Riluzole or vehicle, neurons labeled with lentivirus SYN1::GFP were stained with MAP2 (magenta). Scale bar, 25 μm. J, K Quantification of the total dendritic length (μm) and the number of dendrite branches, n = 3 independent experiments. L NF-κB luciferase activity in WFS1−/− astrocytes treated with 5 μM Riluzole or vehicle, n = 4 independent experiments. M Quantitative real-time PCR analysis of EAAT2 in WFS1−/− astrocytes treated with 5 μM Riluzole or vehicle, n = 7 independent experiments. N Glutamate levels (nmol) at 48 h and 96 h in WFS1−/− astrocytes culture treated with 5 μM Riluzole or vehicle, n = 5 independent experiments. Data are presented as mean ± SD. p values calculated by unpaired two-tailed Student’s t test were *p < 0.05, **p < 0.01, and ***p < 0.001.
WFS1 deficiency in neurons autonomously results in delayed neuronal differentiation and impaired synapse formation
Since recapitulated clinical features are from cerebral organoids, which contained diverse neural cell types, the results might be a reflection of changes in different cell populations. To evaluate WFS1’s role in neurons and astrocytes, we differentiated WT and WFS1−/− HuES8 into NPCs using previously reported 2D culture methods [18, 44], which could be maintained and subsequently differentiated into neurons and astrocytes separately.
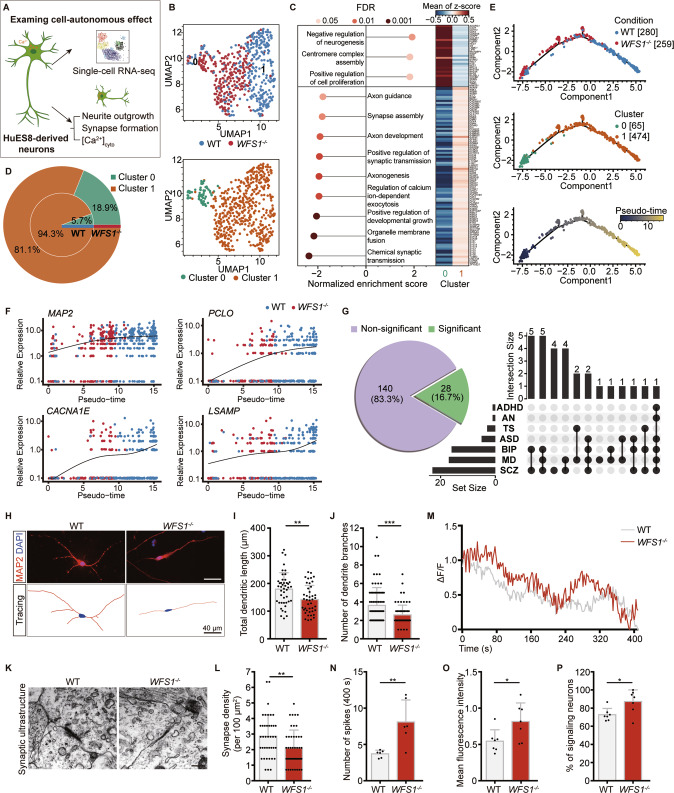
To explore the cell-autonomous effect of WFS1 deficiency on the transcriptome of neurons, we performed single-cell RNA sequencing (scRNA-seq) (Fig. 3A). Among total of 768 neurons, the scRNA-seq profiles of 539 neurons, including 280 neurons from WT NPCs and 259 neurons from WFS1−/− NPCs, passed quality control (Fig S4A). The scRNA-seq profiles of neurons derived from WFS1−/− NPCs looked generally different from those derived from WT NPCs, and all the 539 single neurons could be separated into two distinguishable clusters, cluster 0 and 1 (Fig. 3B). We carried out differential gene expression analysis using edgeR-zingeR [45, 46], and performed gene set enrichment analysis (GSEA). Among the GO terms enriched in cluster 0, we observed terms associated with negative regulation of differentiation and positive regulation of cell proliferation (Fig. 3C). By contrast, GO terms enriched in cluster 1 appeared to be associated with neuronal differentiation and synapse formation (Fig. 3C). So we speculated that the neurons in cluster 0 were less differentiated than those in cluster 1. Meanwhile, a significantly higher proportion of these less differentiated neurons were observed in the WFS1−/− group (18.9%) than in the WT group (5.7%) (Fig. 3D). To further analyze the effects of WFS1 deficiency on neuronal differentiation process, we constructed a trajectory for all WT and WFS1−/− neurons using Monocle [47–49] and ordered the single neurons by the pseudo-time inferred for each of them. Consistent with the results of GSEA, cells in cluster 0, the less differentiated cluster, were positioned on the early stage of the trajectory and the WFS1−/− neurons also intended to be on the left branch of the trajectory, suggesting WFS1 deficiency delays neuronal differentiation (Fig. 3E). Along the trajectory, we identified 1,931 genes whose expression was significantly associated with the pseudo-time using Monocle (FDR ≤ 0.05). We observed the expression of MAP2, PCLO, CACNA1E, LSAMP, ANKS1B, PPP2R2B, NEFM, GRIN2B, NRXN1 and KCNB1 increased over pseudo-time and were lower in the WFS1−/− group than in the WT group (Figs. 3F; S4B). These genes are known to be related with synapse formation and psychiatric disorders. Based on the above results, we carried out a systematic analysis to discover psychiatric disorders associated genes from the 1,931 genes. Specifically, we took the candidate genes of 146 linkage disequilibrium (LD)-independent SNPs predisposed to at least one psychiatric disorder reported by Cross-Disorder Group of the Psychiatric Genomics Consortium [50], and mapped them to 168 genes, which were considered as psychiatric disorders-associated genes. Among these genes, 28 showed significantly correlated expression with the pseudo-time, accounting for 16.7% (left panel of Fig. 3G), and most of these were reported to be associated with more than one mental disease (right panel of Fig. 3G). These results indicate that WFS1 deficiency leads to delayed differentiation of neurons and affects the expression of genes associated with synapse formation and common psychiatric disorders.
Fig. 3. WFS1 deficiency autonomously delays neuronal differentiation and impairs synapse formation.
A Schematic of the strategy of investigating the cell-autonomous effect of WFS1 deficiency in neurons. B UMAP plot of scRNA-seq data of 539 neurons that passed quality control. Each dot represents a single neuron. Cells are color coded for the corresponding conditions (WT or WFS1−/−, the top panel) and clusters (cluster 0 and 1, the bottom panel). C Dot plot showing significantly enriched GO terms of two clusters (left). GO terms with positive normalized enrichment scores (NES) mean they are enriched in cluster 0 and those with negative NESs mean they are enriched in cluster 1. And heatmap displaying the differentially expressed genes in the leading-edge genes of these terms (right). D Nested pie chart showing the difference in the proportion of cells in two clusters between WT and WFS1−/− neurons. E Principal graph of constructed trajectory of WT and WFS1−/−neurons. Each dot represents a single neuron. Cells are color coded for the corresponding conditions (WT or WFS1−/−, the top panel), clusters (cluster 0 and 1, the middle panel) and inferred pseudo-time (the bottom panel). F Scatter plot of expression of genes over pseudo-time. MAP2, a marker gene of neuron. PCLO, CACNA1E, LSAMP are genes associated with psychiatric disorder. The black curve is the fitted curve, representing the trend of expression changes over pseudo-time. G Pie chart showing that among the genes related to psychiatric disorders, genes significantly correlated with pseudo-time account for 16.7% (28 out of 168). UpSet plot depicting overlap of genes which are both seven psychiatric disorders-associated and significantly pseudo-time correlated. ADHD attention-deficit/hyperactivity disorder, AN anorexia nervosa, TS Tourette syndrome, ASD autism spectrum disorder, BIP bipolar disorder, MD major depression, SCZ schizophrenia. H Immunostaining for MAP2 (red) and DAPI (blue) in WT and WFS1−/− neurons. Scale bar, 40 μm. Lower panels are the corresponding tracings of WT and WFS1−/− neurons. Scale bar, 40 μm. I, J Quantification of the total dendritic length (μm) and the number of dendrite branches, n = 4 independent experiments. K Representative images of electron microscopy of synaptic ultrastructure in WT and WFS1−/− neurons. Scale bar, 400 nm. L Quantification of the number of synapse structure normalized to area (100 μm2) in WT and WFS1−/− neurons, n = 3 independent experiments. M Representative singlecell traces of intracellular spontaneous calcium activity of WT and WFS1−/− neurons. ΔF/F indicates the changes in fluorescence intensity reflecting cytosolic calcium activity in neurons. Intracellular spontaneous calcium activity analysis shown as calcium spike frequency (N), mean fluorescence intensity (O) and the percentage of signaling neurons (P) in WT and WFS1−/− neurons, n = 6 fields from 3 independent experiments. Data are presented as mean ± SD. p values calculated by unpaired two-tailed Student’s t test were *p < 0.05, **p < 0.01, and ***p < 0.001.
To validate the scRNA-seq analysis, neurons were stained with SOX2 and evaluated by intracellular flow cytometric analysis, to measure the population of NPCs in neuron cultures. Flow cytometry analysis showed the percentage of SOX2+ cells significantly increased in WFS1−/− neurons compared to WT neurons (Fig. S4E, F). Meanwhile, the percentage of differentiated neurons in the 3-week neuron cultures was quantified by magnetic-activated cell sorting for CD44-CD184- cells [51]. We found that the proportion of CD44-CD184- cells was significantly lower in WFS1−/− neurons compared to WT neurons (Fig. S4G). Further, we performed the immunostaining for SOX2, MAP2 and GFAP simultaneously in cerebral organoids and found that the number of SOX2+ cells was much higher in WFS1−/− cerebral organoids on Day 90 and Day 170 as compared to WT cerebral organoids (Fig. S3E, F). On Day 90 and Day 170, the majority of SOX2+ cells were SOX2-expressing astrocytes rather than NPCs [52]. To exclude this possibility, we then quantified the proportion of SOX2+GFAP-MAP2- cells more precisely to represent NPCs population. And we found that the percentage of SOX2+GFAP-MAP2- cells was significantly higher in WFS1−/− cerebral organoids on Day 90 and Day 170 as compared to WT cerebral organoids (Fig. S3G). Together, these results suggest that neuronal differentiation is delayed by WFS1 deficiency.
To validate the impaired synapse formation indicated by scRNA-seq data analysis, we examined neurite outgrowth measured by MAP2 staining in neurons. WFS1−/− neurons displayed significantly reduced total dendritic length and decreased number of dendrite branches compared with WT neurons (Fig. 3H–J). We also observed increased neuronal cell death in WFS1−/− neurons as compared with WT neurons (Fig. S4C, D). As expected, we observed decreased synapse density in WFS1−/− neurons as measured by electron microscopy compared to WT neurons (Fig. 3K, L). Moreover, it has been reported that WFS1 regulates calcium storage within ER, and its deficiency results in dysfunction of ER and consequent elevation of cytosolic Ca2+ ([Ca2+]cyto) [28, 53]. To further identify the role of disturbed cytosolic Ca2+ in regulation of neurite outgrowth resulted from WFS1 deficiency, we conducted a time-lapse recording of spontaneous cytosolic Ca2+ activity in neurons loaded with Fluo-4/AM. Spontaneous cytosolic Ca2+ activity as measured by changes in fluorescence intensity (ΔF/F) demonstrated that there was an increase in both frequency and amplitude of cytosolic Ca2+ activity in WFS1−/− neurons as compared to WT neurons (Fig. 3M–O). There was also an increase in the percentage of signaling neurons showing spontaneous Ca2+ transients in WFS1−/− neurons (Fig. 3P). To investigate whether the elevation of cytosolic Ca2+ contributes to impaired neurite outgrowth, we treated WT neurons with 0.125 μM Thapsigargin, an inhibitor for sarco/endoplasmic reticulum calcium ATPase (SERCA) pump, to increase cytosolic calcium levels. After 24 h treatment, increased spontaneous cytosolic Ca2+ activity resulted in reduced total dendritic length and the number of dendrite branches in WT neurons treated with Thapsigargin group as compared to control group (Fig. S5A–G). Previous studies have shown that Dantrolene is an inhibitor of the ryanodine receptors and suppresses calcium leakage from the ER to cytosol, lowering cytosolic calcium level [54, 55]. Thus, we treated WFS1−/− neurons with 8 μM Dantrolene for 48 h and found that the cytosolic Ca2+ activity was significantly decreased in WFS1−/− neurons (Fig. S5H–K); the total dendritic length and the number of dendrite branches in WFS1−/− neurons were also significantly rescued by Dantrolene treatment as compared to control (Fig. S5L–N). Altogether, these results suggest that cell-autonomous effect of WFS1 deficiency in neurons contributes to the impaired synapse formation, which is induced by elevation of cytosolic Ca2+.
WFS1 deficiency renders astrocytes toxic to neurite outgrowth through excessive glutamate in a non-cell-autonomous manner
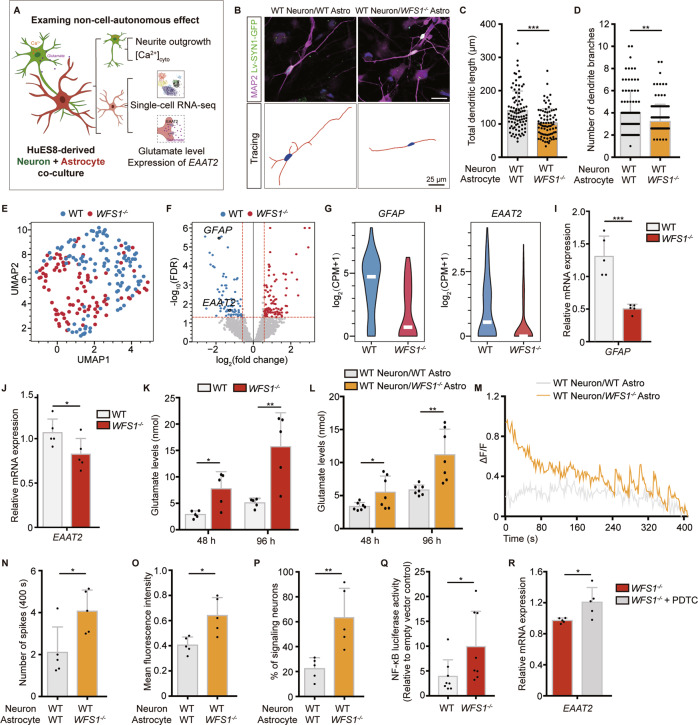
In many neurological disorders, disease-relevant astrocytes confer detrimental effects on neurons resulting in reduced neurite outgrowth and synapse formation [31, 33]. However, WFS1’s function in interplays between astrocytes and neurons remain elusive. To investigate the non-cell-autonomous effect of astrocytes with WFS1 deficiency on neurons, neuron-astrocyte co-culture was applied as a simplified model as previously reported [56] (Figs. 4A and S6B). We found that there was a significant decrease in neurite outgrowth including total dendritic length and the number of dendrite branches in WT neurons with WFS1−/− astrocytes group (WT neuron/WFS1−/− astro) compared with WT neurons with WT astrocytes group (WT neuron/WT astro) (Fig. 4B–D). And neuronal loss within neuron-astrocyte co-culture was also increased in WT neuron/WFS1−/− astro group (Fig. S6C, D). Accordingly, these results suggest that WFS1 deficiency in astrocytes plays an essential pathogenic role in neurite outgrowth with a non-cell-autonomous manner.
Fig. 4. Astrocytic WFS1 deficiency impairs neurite outgrowth in non-cell-autonomous manner.
A Schematic of the strategy of investigating the non-cell-autonomous effect of WFS1−/−astrocytes. B Representative images of co-culture of WT/WFS1−/− astrocytes and WT neurons, neurons labeled with lentivirus SYN1::GFP were stained with MAP2 (magenta). Scale bar, 25 μm. Lower panels are the corresponding tracings of neurons. Scale bar, 25 μm. C, D Quantification of the total dendritic length (μm) and the number of dendrite branches, n = 3 independent experiments. E UMAP plot of scRNA-seq data of 207 astrocytes that passed quality control. Each dot represents a single cell. Cells are color coded for the corresponding conditions (WT or WFS1−/−). F Volcano plot depicting differential gene analysis between WT and WFS1−/− astrocytes. Dots of color indicate differentially expressed genes, that is, those with |log2FC | > 0.58 and FDR ≤ 0.05 (red for up- and blue for down-regulated genes in WFS1−/− astrocytes). G, H Violin plots displaying the expression of GFAP and EAAT2 in WT and WFS1−/− astrocytes. The y-axis shows the log-transformed normalized read count. I, J Quantitative real-time PCR analysis of GFAP and EAAT2 in WT and WFS1−/− astrocytes, n = 5 independent experiments. K Glutamate levels (nmol) at 48 h and 96 h in WT and WFS1−/− astrocytes culture, n = 5 independent experiments. L Glutamate levels (nmol) at 48 h and 96 h in co-culture of WT neurons and WT/WFS1−/− astrocytes, n = 7 independent experiments. M Representative single cell traces of intracellular spontaneous calcium activity of WT neurons co-cultured with WT/WFS1−/− astrocytes. Intracellular spontaneous calcium activity analysis shown as calcium spike frequency (N), mean fluorescence intensity (O) and the percentage of signaling neurons (P) in WT neurons co-cultured with WT/WFS1−/− astrocytes, n = 5 fields from 3 independent experiments. Q NF-κB luciferase activity in WT and WFS1−/− astrocytes, relative to empty vector control group, n = 8 independent experiments. R Quantitative real-time PCR analysis of EAAT2 in WFS1−/− astrocytes and NF-κB inhibitor PDTC treated-WFS1−/− astrocytes, n = 5 independent experiments. Data are presented as mean ± SD. p values calculated by unpaired two-tailed Student’s t test were *p < 0.05, **p < 0.01, and ***p < 0.001.
To identify the possible causes underlying the detrimental effects of WFS1-deficient astrocytes on neurons, we performed scRNA-seq of WT and WFS1−/− astrocytes. The scRNA-seq profiles of 207 single astrocytes passed quality control and were retained for further analysis (Fig. S6A). At first glance, there was no obvious clusters among these cells (Fig. 4E). Genes with log2FC > 0.58 and FDR ≤ 0.05 were identified as differentially expressed genes (DEGs), including 100 up-regulated and 73 down-regulated genes in WFS1−/− group compared with WT group (Fig. 4F). Among them, GFAP was significantly down-regulated in WFS1−/− group (Fig. 4G). Moreover, EAAT2, which encodes glutamate transporter was also downregulated in WFS1−/− group (Fig. 4H). Altogether, these results indicate that the expression of GFAP and transportation of glutamate in WFS1-deficient astrocytes might be affected.
We performed q-PCR to empirically validate the decreased gene expression of GFAP in WFS1−/− astrocytes (Fig. 4I), which was in line with the reduced GFAP+ astrocytes population in cerebral organoids. EAAT2 is a transporter mainly expressed in astrocytes for glutamate transportation which is essential for maintaining glutamate homeostasis [57, 58]. The decreased EAAT2 level revealed by the scRNA-seq data was also validated by q-PCR (Fig. 4J). Next, glutamate assay was performed to assess extracellular glutamate level in astrocytes culture. There was a dramatic increase of the glutamate level in medium at 48 h and 96 h of WFS1−/− astrocytes as compared to WT astrocytes (Fig. 4K), suggesting excessive glutamate is induced by WFS1-deficient astrocytes with EAAT2 downregulation. To further confirm this, we also analyzed the glutamate level in neuron-astrocyte co-culture. Glutamate level was significantly higher in WT neuron/WFS1−/− astro group as compared to WT neuron/WT astro group (Fig. 4L). To examine the detrimental effect of excessive glutamate on neurite outgrowth, WT neurons were treated with 100 μM glutamate (close to the glutamate level monitored in medium of WFS1-deficient astrocytes) for 48 h. There was a decrease in total dendritic length, the number of dendrite branches and the percentage of MAP2+ neurons in glutamate-treated neurons as compared to control group (Fig. S7A–E). Accordingly, these results suggest that excessive glutamate elicited by WFS1−/− astrocytes with downregulation of EAAT2 expression leads to impaired neurite outgrowth in neurons.
Furthermore, it has been reported that excitotoxicity elicited by excessive glutamate induces calcium influx into neurons and elevates cytosolic calcium in these cells, which results in impaired neurite outgrowth and synapse formation [59–62]. Thus, we measured the spontaneous cytosolic Ca2+ activity in WT neurons co-cultured with WT/WFS1−/− astrocytes loaded with Rhod-4/AM. As expected, changes in fluorescence intensity (ΔF/F) showed that there was indeed an increase in the Ca2+ activity frequency, amplitude and percentage of signaling neurons in WT neuron/WFS1−/− astro group compared with WT neuron/WT astro group (Fig. 4M–P). Additionally, WT neurons treated with 100 μM glutamate also exhibited increased cytosolic Ca2+ activity (Fig. S7F–I). These results suggest that WFS1 deficiency renders astrocytes toxic to neurons by elevating neuronal cytosolic calcium via excessive glutamate. To note, our results demonstrate that WFS1 deficiency also autonomously elevates cytosolic calcium in neurons with impaired neurite outgrowth. Collectively, these results suggest that cell-autonomous and non-cell-autonomous detrimental effects induced by WFS1 deficiency in neurons and astrocytes converge on increased neuronal cytosolic calcium pathway (Fig. 6S).
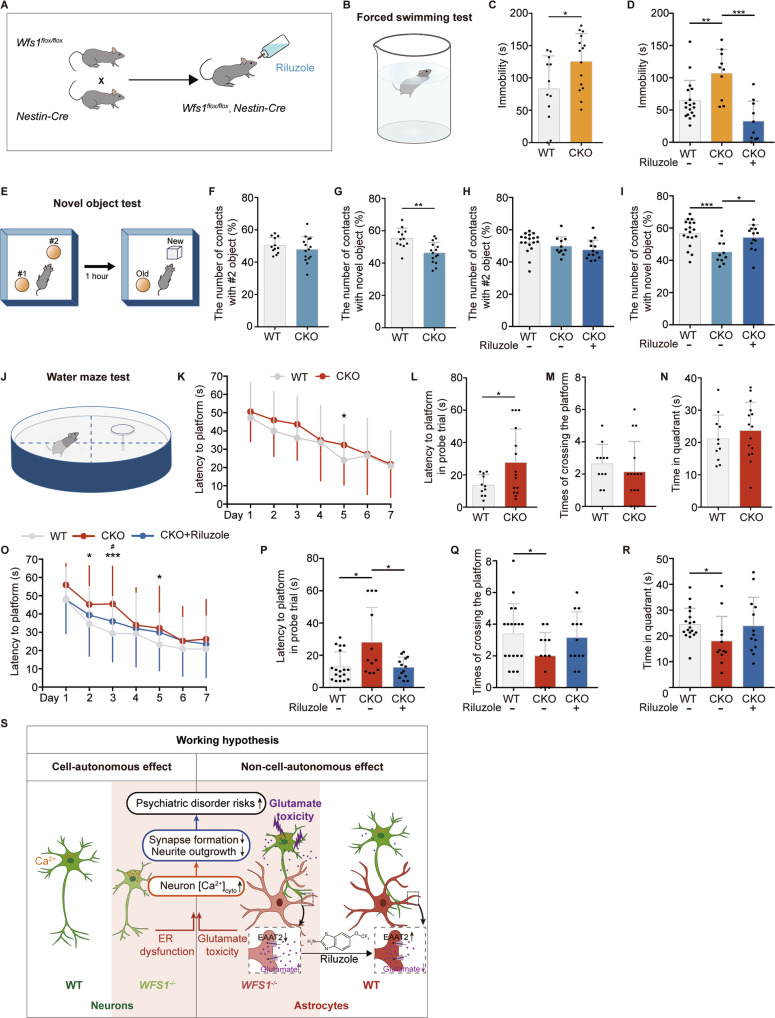
Fig. 6. Riluzole reverses behavioral defects in Wfs1 conditional knockout mice.
A Schematic of the strategy of generating Wfs1 conditional knockout mice for Riluzole treatment. B Schematic of the forced swimming test. C Analysis of the immobility time during the forced swimming test in WT and CKO mice, n ≥ 12. D Analysis of the immobility time during the forced swimming test after Riluzole treatment in WT and CKO mice, n ≥ 11. E Schematic of the novel object test. F, G Quantification of the percentage of the number of contacts with #2 or novel object in WT and CKO mice during acquisition and recognition session, n ≥ 12. H, I Quantification of the percentage of the number of contacts with #2 or novel object in WT and CKO mice during acquisition and recognition session after Riluzole treatment, n ≥ 11. J Schematic of the water maze test. K Latency to find the platform in WT and CKO mice during 7-day training sessions, n ≥ 12. On the probe trial day, quantification of the latency to the original position of the platform (L), times of crossing the platform area (M) and the time spent in quadrant of the platform (N) in WT and CKO mice, n ≥ 12. O Latency to find the platform in WT and CKO mice during 7-day training sessions after Riluzole treatment, n ≥ 11. On the probe trial day, quantification of the latency to the original position of the platform (P), times of crossing the platform area (Q) and the time spent in quadrant of the platform (R) in WT and CKO mice after Riluzole treatment, n ≥ 11. S The working hypothesis underlying the autonomous effect by WFS1 deficiency in neurons and non-autonomous effect by astrocytic WFS1 deficiency. Data are presented as mean ± SD. p values calculated by unpaired two-tailed Student’s t test were *p < 0.05, **p < 0.01, and ***p < 0.001. Two-way ANOVA was used for analysis of latency during 7-day training sessions after Riluzole treatment in the water maze test, *p < 0.05, **p < 0.01, and ***p < 0.001 for comparison of WT and CKO mice, #p < 0.05 for comparison of the effect of Riluzole treatment.
Several studies have shown that NF-κB pathway is critical for the transcriptional regulation of EAAT2, as EAAT2 has a binding site in its promotor for NF-κB [63–66]. To further investigate the mechanisms underlying downregulation of EAAT2 transcriptional level, we explored whether NF-κB pathway is involved in the regulation of EAAT2 mRNA levels under the situation of WFS1 deficiency. We examined the NF-κB activity by immunostaining of P50 and P65 nuclear translocation and luciferase reporter assay in differentiated astrocytes. Both the intensity of P50 and P65 in the nuclei area and the NF-κB luciferase activity were significantly increased in WFS1−/− astrocytes as compared to WT astrocytes (Figs. 4Q; S6E–G). To further identify the role of NF-κB activation in regulation of EAAT2 mRNA levels, we applied 100 μM PDTC, a pharmacological inhibitor of NF-κB, into WFS1−/− astrocytes. After 40 min treatment, the mRNA levels of EAAT2 were significantly reversed and NF-κB activation was suppressed in PDTC-treated WFS1−/− astrocytes, as compared to control group (Figs. 4R; S6H). These results suggest that the activation of NF-κB indeed results in the decreased mRNA level of EAAT2. WFS1 deficiency leads to high level of endoplasmic reticulum (ER) stress [42], and enhanced ER stress could activate NF-κB pathway [67]. To investigate whether WFS1 deficiency leads to NF-κB activation by upregulating ER stress, we examined the expression of three major components for sensing ER stress, PERK, ATF6 and IRE, by q-PCR. We found that the mRNA expression of PERK and ATF6 were significantly increased in WFS1−/− astrocytes as compared to WT astrocytes (Fig. S6I), suggesting that the ER stress is upregulated in WFS1−/− astrocytes. And then we treated WT astrocytes with 0.125 μM Thapsigargin, an ER stress inducer by increasing cytosolic calcium levels. After 24 h treatment, the NF-κB luciferase activity was significantly increased as compared to control group (Fig. S6J), indicating that upregulated ER stress induced by WFS1 deficiency leads to NF-κB activation. Altogether, these results suggest that activation of NF-κB contributes to downregulation of EAAT2 transcriptional level, which is induced by enhanced ER stress in WFS1-deficient astrocytes.
Riluzole reverses impaired synapse formation induced by WFS1 deficiency
It is known that Riluzole upregulates astrocytic EAAT2 expression to promote glutamate clearance [68, 69]. To investigate whether Riluzole could be used to treat impaired synapse formation in WS, we treated cerebral organoids on Day 90 with 5 μM Riluzole or vehicle for 7 days, respectively. We found that the number of SYN1 puncta significantly increased in Riluzole-treated cerebral organoids as compared to control group (Fig. 5A, D). Furthermore, Riluzole significantly increased the percentage of SYN1/PSD95 colocalized puncta to SYN1 puncta in WFS1−/− cerebral organoids compared with control group (Fig. 5B, E). Concurrently, the apoptosis of neurons in WFS1−/− cerebral organoids were significantly reduced by Riluzole as compared to control group (Fig. 5C, F). To examine the rescue effect of Riluzole on the electrophysiological properties of neurons, cerebral organoids were treated with 5 μM Riluzole since Day 50, then whole-cell patch-clamp recording was performed until Day 150. We found that the decreased frequency of sEPSCs was significantly rescued in Riluzole-treated cerebral organoids as compared to control in WFS1-deficient cerebral organoids (Fig. 5G, H). Next, we examined the effect of Riluzole on neurite outgrowth defects induced by WFS1-deficient astrocytes. We treated WT neuron co-cultured with WFS1−/− astrocytes with 5 μM Riluzole or vehicle for 48 h, respectively. As compared to WT neuron co-cultured with WFS1−/− astrocytes group, Riluzole almost fully reversed the defects in total dendritic length and the number of dendrite branches (Fig. 5I–K) and reduced the neuronal loss (Fig. S7J, K). Furthermore, Riluzole treatment decreased the NF-κB luciferase activity, restored astrocytic EAAT2 expression and decreased excessive glutamate level in WFS1−/− astrocytes (Fig. 5L–N). Taken together, these results suggest that Riluzole could rescue disrupted synapse formation and function, as well as neuronal loss induced by WFS1 deficiency.
Riluzole reverses behavioral defects in Wfs1 conditional knockout mice
Disrupted synapse formation and function underlies many psychiatric disorders, and patients with WS have been reported that suffering from a variety of psychiatric disorders including depression, impairments of recognition performance and memory [6, 70–73]. To recapitulate the psychiatric disturbances in patients, we conducted a series of behavioral studies including forced swimming test (depression), novel object test (recognition memory) and water maze test (spatial memory) in the Wfs1 conditional knockout mice (CKO mice). We generated the conditional Wfs1 knockout mice by crossing the Wfs1-flox mice (Wfs1flox/flox) with Nestin-Cre transgenic mice (Fig. 6A). The Nestin-Cre mice expressed Cre recombinase under the control of the Nestin promotor, thus Wfs1 would be knocked out in neuroepithelial cells as they convert to radial progenitors and initiate neurogenesis, which is in line with our in vitro system.
In the forced swimming test, CKO mice showed a significant increase in immobility time compared to WT mice, indicating that mice developed depressive-like behavior induced by Wfs1 deficiency (Fig. 6B, C). In the novel object test, both WT and CKO mice showed the similar number of contacts with the two objects in the session of acquisition (having two same objects) (Fig. 6E, F). However, in the session of recognition (having a familiar and a novel object), WT but not CKO mice showed significantly more contacts with the novel object than the familiar one (Fig. 6G), suggesting that CKO mice exhibit deficits in recognition memory. In the water maze test, CKO mice showed longer latency to find the platform during the 7-day training sessions, significant difference especially appeared on Day 5 (Fig. 6J, K). On the probe trial day, platform was removed and mice were allowed to swim for 60 s. Latency to the original platform position was significantly increased in CKO mice as compared to WT mice (Fig. 6L). There was no significant difference in the times of crossing the platform and time in the quadrant of platform in CKO mice compared to WT mice (Fig. 6M, N). These data suggest that Wfs1 deficiency could impair the spatial memory.
To further identify the rescue effect of Riluzole on these behavioral defects, we treated CKO mice with Riluzole at the concentration of 50 mg/kg/day in the drinking water from the age of 3 weeks for 2 months (this dose was previously tested in mice [74]). Riluzole treatment mice exhibited reversed increased immobility in the forced swimming test (Fig. 6D); more contacts with the novel object in the novel object test (Fig. 6H, I); shorter latency to the platform during the training sessions, which was significantly different on Day 3 in the water maze test (Fig. 6O); decreased latency to the platform on the probe trial (Fig. 6P) and an increased trend in the times of crossing the platform and time in the quadrant of platform compared to control in CKO mice (Fig. 6Q, R). These results demonstrate that Riluzole could reverse most of the behavioral defects in Wfs1-deficient mice. Overall, Riluzole could be potentially applied to treat both disrupted synapse formation and function, as well as neuronal loss in WS patients.
Discussion
The key to investigation of WS relies on proper human models, which is limited by ethic issue and scarcity of human samples. The effect of WFS1 loss of function on neuronal dysfunction has been modeled in rodents and Drosophila [27, 75, 76]. However, these models do not adequately recapitulate defects by WFS1 deficiency in human brain development, especially in investigating its role in mental illness. In this study, we applied a multi-dimensional strategy of combining 3D cerebral organoids and 2D neural differentiation derived from hESCs to illuminate the role of the causative gene WFS1 in psychiatric disorders underlying WS. We found that WFS1-deficient cerebral organoids not only phenocopied progressive neuronal loss in WS patients, but also showed impaired synapse formation which underlies common psychiatric disorders, providing a defective structural basis for WS.
Synapse is the basic structure element for neural circuit. Recent studies reveal that impaired synaptogenesis and synaptic dysfunction contribute to psychiatric disorders. Moreover, variants of numerous genes involved in synapse formation during brain neurodevelopment have been identified as genetic risk factors for psychiatric disorders by genome-wide association study (GWAS) [50, 77]. To further elucidate the pathogenesis, recent technical breakthroughs with pluripotent hESCs derived neural cells (2D) and cerebral organoids (3D) are applied to model psychiatric disorders possessing genetic predispositions [23, 24, 78, 79]. Here, from genotype to phenotype, by applying 3D cerebral organoids and 2D neural cells derived from hESCs, our results revealed that WFS1 deficiency resulted in reduced neurite outgrowth and consequent impaired synapse formation and function, which might explain the psychiatric symptoms observed in the WS patients. Nevertheless, further questions remain to be explored. Neurite outgrowth is a process of neurite extension. How WFS1 as an ER stress regulator controls this process and regulates the precise neuronal connectivity is unclear.
Astrocytes are essential for synapse formation and maintenance, and recent studies reveal that malfunction of astrocytes underlie psychiatric disorders, such as schizophrenia and autism [80, 81]. These facts lead us to further explore the interplay between neurons and astrocytes affected by WFS1 deficiency in neurite outgrowth and consequent synapse formation. The complexity was unraveled by 2D neural differentiation to induce neurons and astrocytes separately. On one hand, we found that WFS1 deficiency autonomously elevated cytosolic calcium and reduced neurite outgrowth, contributing to impaired synapse formation. On the other hand, WFS1-deficient astrocytes elicited reduced neurite outgrowth in non-cell-autonomous manner, highlighting the essentiality of astrocytes. Mechanistically, we found that WFS1 deficiency decreased the expression of the EAAT2 in astrocytes by NF-κB activation, resulting in excessive glutamate and consequent elevated cytosolic calcium and reduced neurite outgrowth in neurons. More specifically, how NF-κB regulates EAAT2 in astrocytes to control glutamate level warrants investigation. Further exploration should be focused on examining the underlying molecular mechanism.
Unfortunately, there is no effective therapy to treat WS which is hampered by limited understanding of the pathogenesis. It has been reported that Riluzole acts as a modulator to enhance EAAT2 expression level and activity in astrocytes for glutamate clearance [68, 82, 83]. Thus, we explored the therapeutic effect of Riluzole and found that Riluzole treatment reversed the phenotypes of reduced neurite outgrowth, impaired synapse formation and function, and neuronal loss. Furthermore, Riluzole rescued the depressive-like behavior, the impaired recognition and spatial memory in Wfs1 conditional knockout mice. Based on our discoveries, it will be highly valuable to test the therapeutic potential of Riluzole to treat WS neuropathy in future clinical studies.
Materials and methods
Generation of cerebral organoids
Cerebral organoids were generated as previously described [36]. Briefly, hESCs (H1 or H9) were dissociated to generate single cells. 9,000 cells were seeded in each well of an ultra-low-attachment 96-well plate (Corning) in mTeSR1 medium to form EBs. And then mTeSR1 was replaced with neural induction medium (NI medium) on Day 5 containing DMEM/F12 (Gibco™), 1× N2-supplement (Gibco™), 1× GlutaMAX (Gibco™), 1× MEM-NEAA (Sigma), 1× Penicillin/Streptomycin (Gibco™) and 1 μg/ml Heparin (Sigma). On Day 11, EBs were embedded in the center of the droplets of Matrigel (Corning), and then cultured in a 10-cm dish containing differentiation medium without vitamin A (1:1 mixture of DMEM/F12 and Neurobasal™ (Gibco™) medium containing 0.5× N2-supplement, 1× B27-supplement without vitamin A (Gibco™), 1× GlutaMAX, 0.5× MEM-NEAA, 1× Penicillin/Streptomycin, 1:4000 insulin (Sigma), 1 mg/mL NaHCO3). On Day 16, the EB droplets were transferred to an orbital shaker (57 r.p.m.). On Day 21, medium was replaced with differentiation medium with vitamin A same as above except B27-supplement without vitamin A replaced with 1× B27-Supplement (Gibco™), and 70 μg/mL vitamin C (Sigma) was added. On Day 40 onward, 1-2% Matrigel was added to differentiation medium with vitamin A. Medium was changed twice weekly until ready for further analysis.
NPC induction and neuronal differentiation
HuES8 were maintained in mTeSR1 medium for 3 days, after that medium was changed to N2 medium (DMEM/F12 supplemented with 0.5× N2-Supplement) with 1 μM dorsomorphin and 1 μM SB431542 for 2 days. hESCs colonies were lifted off and cultured in suspension as embryoid bodies for 1 week within N2 medium on the orbital shaker (95 r.p.m.). Embryoid bodies then were mechanically dissociated, and plated on 10 μg/mL poly-L-ornithine (Sigma) and laminin (Gibco™)-coated dishes, and cultured in N/B medium (DMEM/F12 supplemented with 0.5× N2-Supplement, 0.5× B27-Supplement) with 20 ng/mL bFGF (Stemcell Technologies). Formed neural rosettes were manually isolated. To differentiate NPCs into neurons, bFGF was withdrawn from medium. After that N/B medium was changed every other day.
Statistical analysis
The exact sample size for each experimental group were given in the figure legends. For cerebral organoids experiments, at least 3 individual organoids were collected for each experiment. For animal experiments, at least 11 animals were collected for each experiment. For other experiments, at least 3 independent experiments were performed for each experiment. The sample sizes were chosen based on our previous works and sufficient to confirm the conclusion, and no statistical methods were used to determine sample size. Investigator were blinded to the groups when assessing the outcomes. All data values were presented as mean ± SD. To compare the means of two normally distributed groups, unpaired two-tailed Student’s t-test was used. Two-way ANOVA followed by Tukey’s post hoc test was used with treatment and time as independent factors. Two-way ANOVA followed by Sidak’s post hoc test was used with the genotype and time as independent factors. The variance between the groups is similar. p values were *p < 0.05, **p < 0.01, and ***p < 0.001. All graphs were generated and analyzed using GraphPad Software Prism 7.
Supplementary information
Acknowledgements
This work was supported by the National Key Research and Development Program of China [Grant No. 2017YFA0106500, 2016YFA0102200, 2018YFA0107102, 2020YFA0112500]. WL is supported by the National Natural Science Foundation of China (Grant No. 31970751; Grant No. 32170740); the Key Project of the Science and Technology Commission of Shanghai Municipality (Grant No. 19JC1415300). Z-NZ is supported by the National Natural Science Foundation of China (Grant No. 81971078; Grant No. 82171166). ZS is supported by the National Key Research and Development Program of China (Grant No. 2018YFA0107602); the National Natural Science Foundation of China (Grant No. 31871280). The authors would like to thank Dr. Qin Shen from Tongji University for technical support and helpful suggestions. We also thank Dr. Qiurong Ding from Shanghai Institute of Nutrition and Health for human embryonic stem cells HuES8; Dr. Bing Luan from Tongji University for HEK293FT cells; Dr. Zhili Rong from Southern Medical University for P2U6 vectors; Dr. Shaorong Gao from Tongji University for lentivirus packaging plasmids PSPAX2 and PMD2.G.
Author contributions
FY, RH, Z-NZ and WL conceived, designed the experiments, and interpreted the results with YL and ZS. FY, MC and Z-NZ performed the experiments with assistance from MG, KJ, MX, QM, QS and CZ. RH performed single-cell RNA-seq library construction. YL, ML, and ZS performed single-cell RNA-seq profiling analyses. XM and KL performed the electrophysiology experiments of cerebral organoids. JC, PG and RZ helped to establish the platform for behavioral studies. SB helped to establish the cerebral organoid culture system. ZS, HW, YW, WY and LS provided critical suggestions to the overall study. FY, YL, RH, ZS, Z-NZ, and WL wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Fei Yuan, Yana Li, Rui Hu.
Contributor Information
Zhen Shao, Email: shaozhen@picb.ac.cn.
Zhen-Ning Zhang, Email: znzhang@tongji.edu.cn.
Weida Li, Email: liweida@tongji.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41380-023-01987-3.
References
- 1.Inoue H, Tanizawa Y, Wasson J, Behn P, Kalidas K, Bernal-Mizrachi E, et al. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome) Nat Genet. 1998;20:143–8. doi: 10.1038/2441. [DOI] [PubMed] [Google Scholar]
- 2.Pallotta MT, Tascini G, Crispoldi R, Orabona C, Mondanelli G, Grohmann U, et al. Wolfram syndrome, a rare neurodegenerative disease: from pathogenesis to future treatment perspectives. J Transl Med. 2019;17:238. doi: 10.1186/s12967-019-1993-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rando TA, Horton JC, Layzer RB. Wolfram syndrome: evidence of a diffuse neurodegenerative disease by magnetic resonance imaging. Neurology. 1992;42:1220–4. doi: 10.1212/WNL.42.6.1220. [DOI] [PubMed] [Google Scholar]
- 4.Swift M, Swift RG. Wolframin mutations and hospitalization for psychiatric illness. Mol Psychiatry. 2005;10:799–803. doi: 10.1038/sj.mp.4001681. [DOI] [PubMed] [Google Scholar]
- 5.Urano F. Wolfram Syndrome: Diagnosis, Management, and Treatment. Curr Diab Rep. 2016;16:6. doi: 10.1007/s11892-015-0702-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waschbisch A, Volbers B, Struffert T, Hoyer J, Schwab S, Bardutzky J. Primary diagnosis of Wolfram syndrome in an adult patient-case report and description of a novel pathogenic mutation. J Neurol Sci. 2011;300:191–3. doi: 10.1016/j.jns.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 7.Gowda GS, Rai D, Nadella RK, Tiwari S, Yadav R, Math SB. Schizophrenia in Wolfram Syndrome (DIDMOAD Syndrome): A case report in support of the mitochondrial dysfunction hypothesis. Schizophr Res. 2018;195:574–5. doi: 10.1016/j.schres.2017.09.044. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee SS, Mitra S, Pal SK. Mania in Wolfram’s Disease: From Bedside to Bench. Clin Psychopharmacol Neurosci. 2017;15:70–2. doi: 10.9758/cpn.2017.15.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torres R, Leroy E, Hu X, Katrivanou A, Gourzis P, Papachatzopoulou A, et al. Mutation screening of the Wolfram syndrome gene in psychiatric patients. Mol Psychiatry. 2001;6:39–43. doi: 10.1038/sj.mp.4000787. [DOI] [PubMed] [Google Scholar]
- 10.Matsunaga K, Tanabe K, Inoue H, Okuya S, Ohta Y, Akiyama M, et al. Wolfram syndrome in the Japanese population; molecular analysis of WFS1 gene and characterization of clinical features. PLoS One. 2014;9:e106906. doi: 10.1371/journal.pone.0106906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khanim F, Kirk J, Latif F, Barrett TG. WFS1/wolframin mutations, Wolfram syndrome, and associated diseases. Hum Mutat. 2001;17:357–67. doi: 10.1002/humu.1110. [DOI] [PubMed] [Google Scholar]
- 12.Rigoli L, Lombardo F, Di Bella C. Wolfram syndrome and WFS1 gene. Clin Genet. 2011;79:103–17. doi: 10.1111/j.1399-0004.2010.01522.x. [DOI] [PubMed] [Google Scholar]
- 13.Rigoli L, Di Bella C. Wolfram syndrome 1 and Wolfram syndrome 2. Curr Opin Pediatr. 2012;24:512–7. doi: 10.1097/MOP.0b013e328354ccdf. [DOI] [PubMed] [Google Scholar]
- 14.Barrett TTL, Gupta R, Rendtorff ND, WilliamsD, Wright B, Dias R. WFS1 Spectrum Disorder GeneReviews®. Seattle (WA): University of Washington, Seattle; 2009. https://www.ncbi.nlm.nih.gov/books/NBK4144/. [PubMed]
- 15.Gunn T, Bortolussi R, Little JM, Andermann F, Fraser FC, Belmonte MM. Juvenile diabetes mellitus, optic atrophy, sensory nerve deafness, and diabetes insipidus-a syndrome. J Pediatr. 1976;89:565–70. doi: 10.1016/S0022-3476(76)80387-3. [DOI] [PubMed] [Google Scholar]
- 16.Reese D, Drapeau P. Neurite growth patterns leading to functional synapses in an identified embryonic neuron. J Neurosci. 1998;18:5652–62. doi: 10.1523/JNEUROSCI.18-15-05652.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchetto MC, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143:527–39. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang ZN, Freitas BC, Qian H, Lux J, Acab A, Trujillo CA, et al. Layered hydrogels accelerate iPSC-derived neuronal maturation and reveal migration defects caused by MeCP2 dysfunction. Proc Natl Acad Sci USA. 2016;113:3185–90. doi: 10.1073/pnas.1521255113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srikanth P, Lagomarsino VN, Pearse RV, 2nd, Liao M, Ghosh S, Nehme R, et al. Convergence of independent DISC1 mutations on impaired neurite growth via decreased UNC5D expression. Transl Psychiatry. 2018;8:245. doi: 10.1038/s41398-018-0281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyoshi K, Honda A, Baba K, Taniguchi M, Oono K, Fujita T, et al. Disrupted-In-Schizophrenia 1, a candidate gene for schizophrenia, participates in neurite outgrowth. Mol Psychiatry. 2003;8:685–94. doi: 10.1038/sj.mp.4001352. [DOI] [PubMed] [Google Scholar]
- 21.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–9. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 22.Mirnics K, Middleton FA, Lewis DA, Levitt P. Analysis of complex brain disorders with gene expression microarrays: schizophrenia as a disease of the synapse. Trends Neurosci. 2001;24:479–86. doi: 10.1016/S0166-2236(00)01862-2. [DOI] [PubMed] [Google Scholar]
- 23.Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–5. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen Z, Nguyen HN, Guo Z, Lalli MA, Wang X, Su Y, et al. Synaptic dysregulation in a human iPS cell model of mental disorders. Nature. 2014;515:414–8. doi: 10.1038/nature13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guang S, Pang N, Deng X, Yang L, He F, Wu L, et al. Synaptopathology Involved in Autism Spectrum Disorder. Front Cell Neurosci. 2018;12:470. doi: 10.3389/fncel.2018.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sacai H, Sakoori K, Konno K, Nagahama K, Suzuki H, Watanabe T, et al. Autism spectrum disorder-like behavior caused by reduced excitatory synaptic transmission in pyramidal neurons of mouse prefrontal cortex. Nat Commun. 2020;11:5140. doi: 10.1038/s41467-020-18861-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakakibara Y, Sekiya M, Fujisaki N, Quan X, Iijima KM. Knockdown of wfs1, a fly homolog of Wolfram syndrome 1, in the nervous system increases susceptibility to age- and stress-induced neuronal dysfunction and degeneration in Drosophila. PLoS Genet. 2018;14:e1007196. doi: 10.1371/journal.pgen.1007196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu S, Kanekura K, Hara T, Mahadevan J, Spears LD, Oslowski CM, et al. A calcium-dependent protease as a potential therapeutic target for Wolfram syndrome. Proc Natl Acad Sci USA. 2014;111:E5292–301. doi: 10.1073/pnas.1421055111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Visnapuu T, Plaas M, Reimets R, Raud S, Terasmaa A, Koks S, et al. Evidence for impaired function of dopaminergic system in Wfs1-deficient mice. Behav Brain Res. 2013;244:90–9. doi: 10.1016/j.bbr.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 30.Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 2013;14:311–21. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobs S, Nathwani M, Doering LC. Fragile X astrocytes induce developmental delays in dendrite maturation and synaptic protein expression. BMC Neurosci. 2010;11:132. doi: 10.1186/1471-2202-11-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tong X, Ao Y, Faas GC, Nwaobi SE, Xu J, Haustein MD, et al. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat Neurosci. 2014;17:694–703. doi: 10.1038/nn.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams EC, Zhong X, Mohamed A, Li R, Liu Y, Dong Q, et al. Mutant astrocytes differentiated from Rett syndrome patients-specific iPSCs have adverse effects on wild-type neurons. Hum Mol Genet. 2014;23:2968–80. doi: 10.1093/hmg/ddu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–32. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez V, Llinares-Benadero C, Borrell V. Cerebral cortex expansion and folding: what have we learned? EMBO J. 2016;35:1021–44. doi: 10.15252/embj.201593701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–9. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Min Yang S, Berger DR, et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature. 2017;545:48–53. doi: 10.1038/nature22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Velasco S, Kedaigle AJ, Simmons SK, Nash A, Rocha M, Quadrato G, et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature. 2019;570:523–7. doi: 10.1038/s41586-019-1289-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghirardello S, Dusi E, Castiglione B, Fumagalli M, Mosca F. Congenital central diabetes insipidus and optic atrophy in a Wolfram newborn: is there a role for WFS1 gene in neurodevelopment? Ital J Pediatr. 2014;40:76. doi: 10.1186/s13052-014-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hershey T, Lugar HM, Shimony JS, Rutlin J, Koller JM, Perantie DC, et al. Early brain vulnerability in Wolfram syndrome. PLoS One. 2012;7:e40604. doi: 10.1371/journal.pone.0040604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samara A, Rahn R, Neyman O, Park KY, Samara A, Marshall B, et al. Developmental hypomyelination in Wolfram syndrome: new insights from neuroimaging and gene expression analyses. Orphanet J Rare Dis. 2019;14:279. doi: 10.1186/s13023-019-1260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L, Venkataraman L, Chen S, Fu H. Function of WFS1 and WFS2 in the Central Nervous System: Implications for Wolfram Syndrome and Alzheimer’s disease. Neurosci Biobehav Rev. 2020;118:775–83. doi: 10.1016/j.neubiorev.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allen NJ. Astrocyte regulation of synaptic behavior. Annu Rev Cell Dev Biol. 2014;30:439–63. doi: 10.1146/annurev-cellbio-100913-013053. [DOI] [PubMed] [Google Scholar]
- 44.Chailangkarn T, Trujillo CA, Freitas BC, Hrvoj-Mihic B, Herai RH, Yu DX, et al. A human neurodevelopmental model for Williams syndrome. Nature. 2016;536:338–43. doi: 10.1038/nature19067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vieth B, Parekh S, Ziegenhain C, Enard W, Hellmann I. A systematic evaluation of single cell RNA-seq analysis pipelines. Nat Commun. 2019;10:4667. doi: 10.1038/s41467-019-12266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trapnell C, Cacchiarelli D, Grimsby J, Pokharel P, Li S, Morse M, et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol. 2014;32:381–6. doi: 10.1038/nbt.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiu X, Hill A, Packer J, Lin D, Ma YA, Trapnell C. Single-cell mRNA quantification and differential analysis with Census. Nat Methods. 2017;14:309–15. doi: 10.1038/nmeth.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA, et al. Reversed graph embedding resolves complex single-cell trajectories. Nat Methods. 2017;14:979–82. doi: 10.1038/nmeth.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cross-Disorder Group of the Psychiatric Genomics Consortium. Electronic address pmhe, Cross-Disorder Group of the Psychiatric Genomics C. Genomic Relationships, Novel Loci, and Pleiotropic Mechanisms across Eight Psychiatric Disorders. Cell. 2019;179:1469–82.e11. doi: 10.1016/j.cell.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan SH, Martin J, Elia J, Flippin J, Paramban RI, Hefferan MP, et al. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS One. 2011;6:e17540. doi: 10.1371/journal.pone.0017540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–78. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fischer TT, Ehrlich BE. Wolfram Syndrome: a Monogenic Model to Study Diabetes Mellitus and Neurodegeneration. Curr Opin Physiol. 2020;17:115–23. doi: 10.1016/j.cophys.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei H, Perry DC. Dantrolene is cytoprotective in two models of neuronal cell death. J Neurochem. 1996;67:2390–8. doi: 10.1046/j.1471-4159.1996.67062390.x. [DOI] [PubMed] [Google Scholar]
- 55.Luciani DS, Gwiazda KS, Yang TL, Kalynyak TB, Bychkivska Y, Frey MH, et al. Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and beta-cell death. Diabetes. 2009;58:422–32. doi: 10.2337/db07-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Russo FB, Freitas BC, Pignatari GC, Fernandes IR, Sebat J, Muotri AR, et al. Modeling the Interplay Between Neurons and Astrocytes in Autism Using Human Induced Pluripotent Stem Cells. Biol Psychiatry. 2018;83:569–78. doi: 10.1016/j.biopsych.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 57.Pajarillo E, Rizor A, Lee J, Aschner M, Lee E. The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: Potential targets for neurotherapeutics. Neuropharmacology. 2019;161:107559. doi: 10.1016/j.neuropharm.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soni N, Reddy BV, Kumar P. GLT-1 transporter: an effective pharmacological target for various neurological disorders. Pharm Biochem Behav. 2014;127:70–81. doi: 10.1016/j.pbb.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–37. doi: 10.1016/S0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 60.Dubinsky JM. Intracellular calcium levels during the period of delayed excitotoxicity. J Neurosci. 1993;13:623–31. doi: 10.1523/JNEUROSCI.13-02-00623.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rueda CB, Llorente-Folch I, Traba J, Amigo I, Gonzalez-Sanchez P, Contreras L, et al. Glutamate excitotoxicity and Ca2+-regulation of respiration: Role of the Ca2+ activated mitochondrial transporters (CaMCs) Biochim Biophys Acta. 2016;1857:1158–66. doi: 10.1016/j.bbabio.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Lewerenz J, Maher P. Chronic Glutamate Toxicity in Neurodegenerative Diseases-What is the Evidence? Front Neurosci. 2015;9:469. doi: 10.3389/fnins.2015.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su ZZ, Leszczyniecka M, Kang DC, Sarkar D, Chao W, Volsky DJ, et al. Insights into glutamate transport regulation in human astrocytes: cloning of the promoter for excitatory amino acid transporter 2 (EAAT2) Proc Natl Acad Sci USA. 2003;100:1955–60. doi: 10.1073/pnas.0136555100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee SG, Su ZZ, Emdad L, Gupta P, Sarkar D, Borjabad A, et al. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J Biol Chem. 2008;283:13116–23. doi: 10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karki P, Webb A, Zerguine A, Choi J, Son DS, Lee E. Mechanism of raloxifene-induced upregulation of glutamate transporters in rat primary astrocytes. Glia. 2014;62:1270–83. doi: 10.1002/glia.22679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sitcheran R, Gupta P, Fisher PB, Baldwin AS. Positive and negative regulation of EAAT2 by NF-kappaB: a role for N-myc in TNFalpha-controlled repression. EMBO J. 2005;24:510–20. doi: 10.1038/sj.emboj.7600555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kitamura M. Biphasic, bidirectional regulation of NF-kappaB by endoplasmic reticulum stress. Antioxid Redox Signal. 2009;11:2353–64. doi: 10.1089/ars.2008.2391. [DOI] [PubMed] [Google Scholar]
- 68.Fumagalli E, Funicello M, Rauen T, Gobbi M, Mennini T. Riluzole enhances the activity of glutamate transporters GLAST, GLT1 and EAAC1. Eur J Pharm. 2008;578:171–6. doi: 10.1016/j.ejphar.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 69.Frizzo ME, Dall’Onder LP, Dalcin KB, Souza DO. Riluzole enhances glutamate uptake in rat astrocyte cultures. Cell Mol Neurobiol. 2004;24:123–8. doi: 10.1023/B:CEMN.0000012717.37839.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Medlej R, Wasson J, Baz P, Azar S, Salti I, Loiselet J, et al. Diabetes mellitus and optic atrophy: a study of Wolfram syndrome in the Lebanese population. J Clin Endocrinol Metab. 2004;89:1656–61. doi: 10.1210/jc.2002-030015. [DOI] [PubMed] [Google Scholar]
- 71.Nickl-Jockschat T, Kunert HJ, Herpertz-Dahlmann B, Grozinger M. Psychiatric symptoms in a patient with Wolfram syndrome caused by a combination of thalamic deficit and endocrinological pathologies. Neurocase. 2008;15:47–52. doi: 10.1080/13554790802613009. [DOI] [PubMed] [Google Scholar]
- 72.Chaussenot A, Bannwarth S, Rouzier C, Vialettes B, Mkadem SA, Chabrol B, et al. Neurologic features and genotype-phenotype correlation in Wolfram syndrome. Ann Neurol. 2011;69:501–8. doi: 10.1002/ana.22160. [DOI] [PubMed] [Google Scholar]
- 73.Swift RG, Sadler DB, Swift M. Psychiatric findings in Wolfram syndrome homozygotes. Lancet. 1990;336:667–9. doi: 10.1016/0140-6736(90)92157-D. [DOI] [PubMed] [Google Scholar]
- 74.Ishiyama T, Okada R, Nishibe H, Mitsumoto H, Nakayama C. Riluzole slows the progression of neuromuscular dysfunction in the wobbler mouse motor neuron disease. Brain Res. 2004;1019:226–36. doi: 10.1016/j.brainres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 75.Cagalinec M, Liiv M, Hodurova Z, Hickey MA, Vaarmann A, Mandel M, et al. Role of Mitochondrial Dynamics in Neuronal Development: Mechanism for Wolfram Syndrome. PLoS Biol. 2016;14:e1002511. doi: 10.1371/journal.pbio.1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seppa K, Toots M, Reimets R, Jagomae T, Koppel T, Pallase M, et al. GLP-1 receptor agonist liraglutide has a neuroprotective effect on an aged rat model of Wolfram syndrome. Sci Rep. 2019;9:15742. doi: 10.1038/s41598-019-52295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, Tomasini L, et al. FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders. Cell. 2015;162:375–90. doi: 10.1016/j.cell.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ye F, Kang E, Yu C, Qian X, Jacob F, Yu C, et al. DISC1 Regulates Neurogenesis via Modulating Kinetochore Attachment of Ndel1/Nde1 during Mitosis. Neuron. 2017;96:1041–54.e5. doi: 10.1016/j.neuron.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12:311–7. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sloan SA, Barres BA. Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr Opin Neurobiol. 2014;27:75–81. doi: 10.1016/j.conb.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu AY, Mathur R, Mei N, Langhammer CG, Babiarz B, Firestein BL. Neuroprotective drug riluzole amplifies the heat shock factor 1 (HSF1)- and glutamate transporter 1 (GLT1)-dependent cytoprotective mechanisms for neuronal survival. J Biol Chem. 2011;286:2785–94. doi: 10.1074/jbc.M110.158220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carbone M, Duty S, Rattray M. Riluzole elevates GLT-1 activity and levels in striatal astrocytes. Neurochem Int. 2012;60:31–8. doi: 10.1016/j.neuint.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.