Abstract
INTRODUCTION:
Continuous aerosolized β2 agonist, namely albuterol, is the most commonly used therapy for critical asthma. Benzalkonium chloride is a preservative present in some formulations of aerosolized albuterol solutions that can induce bronchospasm. Recent studies have shown that inhalation of albuterol containing benzalkonium chloride might induce unintended bronchoconstriction and poor outcomes. This study aimed to investigate whether using albuterol solutions containing benzalkonium chloride results in prolonged hospital length of stay (LOS).
METHODS:
This was a retrospective cohort study of pediatric subjects admitted to the pediatric ICU (PICU) and treated with continuous albuterol. Data were collected and compared before and after a change to benzalkonium chloride–containing solutions. Subjects who were treated with preservative-free solutions were used as control. The primary outcome was PICU and hospital LOS; secondary outcomes included the duration of continuous albuterol and use of adjunctive therapies.
RESULTS:
A total of 266 admissions were included in the study. One hundred forty subjects (52.6%) were exposed to benzalkonium chloride. Median age and severity of illness scoring were similar between groups. The initial dose of continuous albuterol was significantly higher in the benzalkonium chloride group (median 15 interquartile range [IQR] 10–20 mg/h) compared to the preservative-free group (median 10 IQR 10–20 mg/h) (P < .001). PICU LOS was longer for the preservative-free group, 2.5 (IQR 1.4–4.6) d vs 1.8 (IQR 1.1–2.9) d for benzalkonium chloride group (P = .002). There was no significant difference in duration of continuous albuterol therapy (P = .16) or need for adjunctive respiratory support (heliox [P = .32], noninvasive ventilation [P = .81], and invasive mechanical ventilation [P = .57]).
CONCLUSIONS:
In contrast to published literature showing that benzalkonium chloride may be associated with a longer duration of continuous albuterol nebulization and hospital LOS, our study demonstrated that benzalkonium chloride–containing albuterol is safe for continuous nebulization in critically ill children and not associated with worse outcomes.
Keywords: critical asthma, albuterol, aerosolized β2 agonist, benzalkonium chloride, PICU, pediatrics
Introduction
Nebulized β agonists are the first-line treatment for severe bronchospasm and critical asthma in the pediatric intensive care unit (PICU).1,2 Many studies have shown that continuous nebulized β agonists, namely albuterol, are equivalent or superior to intermittent treatment for critical asthma without significant added systemic side effects.3-6 Traditionally, sterile albuterol preservative-free vials have been used for single-dose nebulizer treatments; however, using these vials to prepare continuous albuterol solutions is time consuming and can delay timely therapy.7,8 As a result, many children’s hospitals in the United States shifted to using an albuterol multi-dose dropper bottle that contains the preservative benzalkonium chloride to prepare continuous aerosolized albuterol.7 This preservative is added for its bactericidal properties; however, recent literature suggests that inhalation of albuterol containing benzalkonium chloride may induce bronchoconstriction resulting in a slow response or worsening clinical condition leading to poor outcomes.7-12 As a result, some children’s hospitals switched back to preservative-free continuous albuterol. In October 2016, our hospital, UW Health American Family Children’s Hospital, switched to the use of sterile albuterol preservative-free vials to prepare continuous albuterol solutions.
Given the potential risks associated with the use of albuterol solutions containing benzalkonium chloride and previously mentioned safety concerns that might result from therapy delay associated with the use of preservative-free albuterol vials to prepare continuous albuterol, we sought to assess the outcomes of subjects treated with continuous albuterol in our PICU between January 2015–April 2019. Specifically, we sought to describe the characteristics of subjects who received benzalkonium chloride–containing albuterol and preservative-free continuous albuterol and assess both solutions’ effectiveness and safety. In addition, we hypothesized that in pediatric subjects treated for status asthmatics continuous albuterol solutions containing benzalkonium chloride would result in a poor therapeutic response with prolonged hospital length of stay (LOS).
QUICK LOOK.
Current knowledge
Benzalkonium chloride is a preservative present in some formulations of aerosolized albuterol solutions that can induce bronchospasm. Recent studies have shown that inhalation of albuterol containing benzalkonium chloride might induce unintended bronchoconstriction resulting in poor outcomes. As a result, some hospitals have switched to using preservative-free continuous albuterol.
What this paper contributes to our knowledge
In this retrospective cohort study, benzalkonium chloride–containing albuterol was found to be safe for continuous nebulization in critically ill children and not associated with worse outcomes. This study supports the use of multi-dose dropper bottles to prepare continuous albuterol solutions for critically ill children.
Methods
This was a single-center retrospective cohort study. The institutional review board at the University of Wisconsin-Madison approved the study protocol with a waiver of informed consent (ID: 2019–1047).
The medical records of all pediatric subjects (children > 37 weeks corrected gestational age and ≤ 18 y of age) admitted to a 21-bed mixed medical-surgical PICU and treated with continuous albuterol at our tertiary care children’s hospital between January 2015–April 2019 were identified and reviewed. Subjects with cystic fibrosis, congenital heart disease, tracheostomy, and long-term mechanical ventilation were excluded. Subjects were grouped based on exposure to benzalkonium chloride. Data collected included demographics; discharge diagnosis; Pediatric Risk of Mortality III (PRISM III); initial dose (first continuous albuterol dose recorded regardless of the location of initiation); the total time of continuous albuterol; use of adjunctive therapies (ie, heliox, terbutaline, aminophylline); and oxygen therapy, including invasive and noninvasive mechanical ventilation. During the study period, initiation, escalation, and de-escalation of continuous albuterol and other adjunctive therapies were per the intensivist discretions. Outcomes data included PICU and hospital LOS (d), need for intubation and invasive mechanical ventilation, and mortality within 90 d. The above data were obtained by abstraction from electronic medical records, Virtual PICU Systems database, and manual review of medical charts.
Our primary outcome was PICU and hospital LOS. Secondary outcomes included the duration of continuous albuterol, the use and duration of adjunctive therapies, and the need for mechanical ventilation.
Statistical Methodology
Continuous variables were summarized with the median and interquartile range (IQR) and categorical variables with frequencies and percentages. Characteristics of interest were compared between groups (albuterol solution either with or without benzalkonium chloride) using non-parametric tests (Wilcoxon rank sum), chi-square tests, or Fisher exact test as appropriate. LOS, for both the PICU and the hospital, was further explored using time-to-event analysis (ie, time to discharge), with subjects who died prior to discharge having their length of stay censored at the time of death. A sub-analysis, evaluating outcomes data only for children with a diagnosis of critical asthma and age > 2 y, was also completed (Critical asthma was identified using the International Classification of Diseases-9 or -10 codes for subjects > 2–17 y). Statistical significance was predefined as P ≤ .05 with no adjustment for multiple testing. Analyses were done using R v.4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).13
Subjects > 2 y of age with a discharge diagnosis of critical asthma were identified and included in a sub-analysis using the same methods just described.
Results
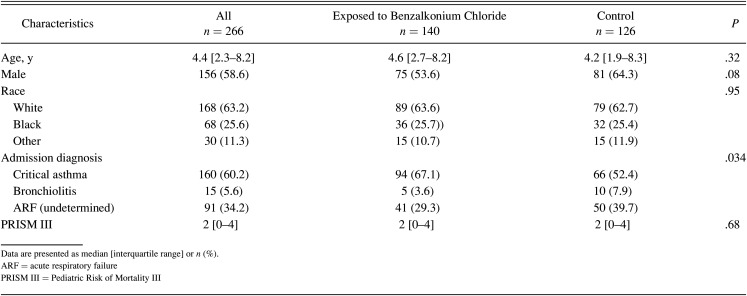
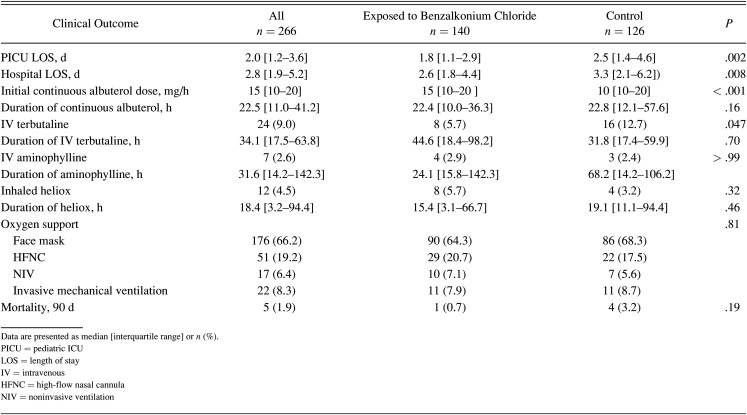
A total of 266 admissions involving 241 unique subjects was included in the study. One hundred forty (52.6%) encounters involved exposure to albuterol solution containing benzalkonium chloride. Table 1 lists the study sample’s characteristics separated into benzalkonium chloride–free control group and benzalkonium chloride group. More subjects were admitted with critical asthma in the benzalkonium chloride group (67.1%) when compared to the control group (52.4%) (P = .034). However, the severity of illness score (PRISM III) was not statistically different between groups (P = .68). The initial continuous albuterol median dose was higher in the benzalkonium chloride group (median 15 [IQR 10–20] mg/h) compared to the control group (median 10 [IQR 10–20] mg/h) (P < .001). However, duration of continuous albuterol was not statistically different between groups (22.4 h vs 22.8 h, P = .16). Similarly, a greater percentage of the control group was initiated on intravenous terbutaline (12.7%) when compared to the benzalkonium chloride group (5.7%) (P = .047). However, the duration of intravenous terbutaline was not statistically different between groups (P = .16). The use and duration of other adjunctive therapies, including intravenous aminophylline, heliox, intubation (within the first 72 h of admission), and noninvasive or invasive ventilation, were similar between groups (Table 2).
Table 1.
Demographics and Baseline Characteristics by Study Group
Table 2.
Clinical Outcomes by Study Group
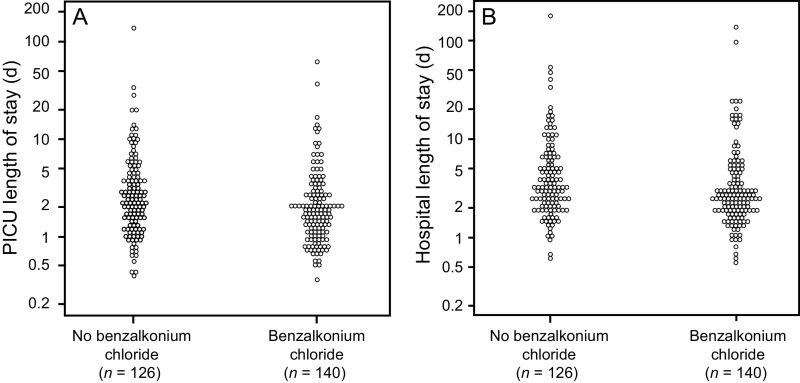
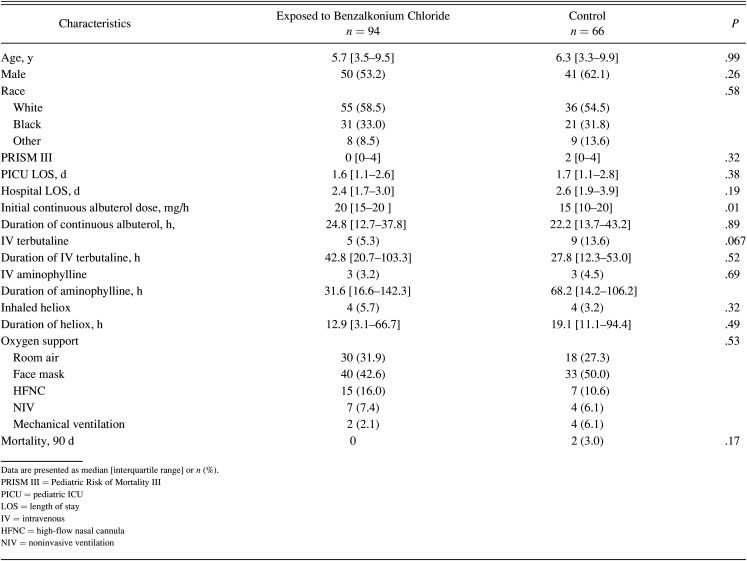
A total of 5 deaths within 90 d were observed during the study period was not statistically significant (P = .19). Both PICU and hospital LOS were longer in the control group when compared with benzalkonium chloride group (P = .002 and P = .008, respectively) (Fig. 1). However, when a sub-analysis was performed for critical asthma alone, none of these variables were statistically significant (Table 3).
Fig. 1.
Primary cohort outcomes: A: pediatric ICU stay; B: hospital length of stay. PICU = pediatric ICU.
Table 3.
Demographics, Baseline Characteristics, and Outcomes for Patients Admitted With Critical Asthma by Study Group
Discussion
In this retrospective cohort study of critically ill children admitted to the PICU and requiring treatment with continuous albuterol, we found that inhalation of albuterol containing benzalkonium chloride not associated with worse clinical outcomes (prolonged PICU or hospital LOS). This study adds to the currently limited data regarding the safe use of albuterol containing benzalkonium chloride and supports the convenient use of multi-dose dropper bottles to prepare continuous albuterol solutions.
We found that both PICU and hospital LOS were longer in the benzalkonium chloride–free group when compared with benzalkonium chloride group. This may be explained by the fact that approximately 40% of our cohort included subjects who were admitted with bronchiolitis or acute respiratory failure associated with wheezing and received continuous albuterol and that benzalkonium chloride–free group included a higher number of those subjects. In their large retrospective study describing the safety and effectiveness of continuous albuterol, Kenyon et al2 showed that previous hospitalizations and concomitant infections could be associated with prolonged therapy and potentially the LOS, which may explain our findings. Therefore, we performed a secondary analysis to have a more detailed picture regarding the safe use of albuterol containing benzalkonium chloride in subjects admitted only with critical asthma. We found that both PICU and hospital LOS were similar between both groups.
It is essential to highlight that the initial dose of continuous albuterol was higher in our subjects who were exposed to benzalkonium chloride–containing albuterol. This is consistent with a recent large retrospective analysis performed by Pertzborn et al12 who found that benzalkonium chloride was associated with a higher dose of continuous albuterol but without a significant difference in hospital LOS. However, in contrast to their finding, continuous albuterol or respiratory support duration did not differ between our groups, which is consistent with the finding of Orth et al8 who reported that for subjects who were exposed to benzalkonium chloride there was no significant difference in rates of poor response when compared to subjects who were not exposed. Moreover, our secondary outcomes, including the initial dose and duration of adjunct therapies, did not differ between groups. This inconsistency in results between different studies could be secondary to the variability in management approaches and strategies between pediatric centers, which could add a separate layer of confounders, highlighting the need for prospective randomized controlled studies to investigate both the short- and long-term effects of using benzalkonium chloride–containing albuterol solutions.
Based on the preliminary results of this study, the increased costs and more specifically turnaround time associated with preparing benzalkonium chloride–free albuterol, and the absence of randomized studies, our hospital reverted to using benzalkonium chloride–containing albuterol solutions in May 2019. This is not only due to the convenience benzalkonium chloride–containing albuterol multi-dose dropper bottle provides but, more importantly, the concerns regarding risks of contamination and the stability of preservative-free albuterol for subjects requiring prolonged nebulization of continuous albuterol. Gulley et al14 attempted to address the concerns related to the sterility and stability of benzalkonium chloride–free albuterol solutions. They conducted sterility testing on high- and low-concentration benzalkonium chloride–free albuterol stored at room temperature, found no bacterial growth detected on day 10 regardless of the concentration, and concluded that the benzalkonium chloride–free albuterol was stable up to 168 h. However, this study did not address sterility and stability during active nebulization and the risks of mold contamination.
This report has strengths and limitations. Strengths include the large sample size, the similarity of demographics between groups, and the severity of illness between groups. Our study limitations include the single-center and the retrospective nature of the study. In addition, study limitations include the lack of critical asthma clinical pathway during the study period; the use of PRISM III score, which focuses on multi-organ failure and mortality in a population that typically presents with a single-organ failure and a low mortality rate; and potential practice changes that could have occurred during the study. Finally, we included 5 subjects who died before discharge; although mortality was not statistically significant between groups, censoring their LOS at the time of death could potentially impact the interpretations of our results.
Conclusions
In this retrospective study of pediatric subjects admitted to the PICU and treated with continuous albuterol, we found that the use of benzalkonium chloride–containing albuterol solutions was safe for continuous nebulization in critically ill children and was not associated with a longer duration of continuous albuterol nebulization and PICU or hospital LOS.
Footnotes
Dr Al-Subu discloses a relationship with Edwards Lifesciences LLC. The remaining authors have disclosed no conflicts of interest.
REFERENCES
- 1.Peters SG. Continuous bronchodilator therapy. Chest 2007;131(1):286-289. [DOI] [PubMed] [Google Scholar]
- 2.Kenyon CC, Fieldston ES, Luan X, Keren R, Zorc JJ. Safety and effectiveness of continuous aerosolized albuterol in the non–intensive care setting. Pediatrics 2014;134(4):e976-e982. [DOI] [PubMed] [Google Scholar]
- 3.Lin AT, Moore-Clingenpeel M, Karsies TJ. Comparison of two continuous nebulized albuterol doses in critically ill children with status asthmaticus. J Asthma 2019:1-7. [DOI] [PubMed] [Google Scholar]
- 4.Nievas IF, Anand KJ. Severe acute asthma exacerbation in children: a stepwise approach for escalating therapy in a pediatric intensive care unit. J Pediatr Pharmacol Ther 2013;18(2):88-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig VL, Bigos D, Brilli RJ. Efficacy and safety of continuous albuterol nebulization in children with severe status asthmaticus. Pediatr Emerg Care 1996;12(1):1-5. [PubMed] [Google Scholar]
- 6.Papo MC, Frank J, Thompson AE. A prospective, randomized study of continuous versus intermittent nebulized albuterol for severe status asthmaticus in children. Crit Care Med 1993;21(10):1479-1486. [DOI] [PubMed] [Google Scholar]
- 7.Prabhakaran S, Abu-Hasan M, Hendeles L. Benzalkonium chloride: a bronchoconstricting preservative in continuous albuterol nebulizer solutions. Pharmacotherapy 2017;37(5):607-610. [DOI] [PubMed] [Google Scholar]
- 8.Orth LE, Kelly BJ, Lagasse CA, Collins SW, Ryan MF. Safety and effectiveness of albuterol solutions with and without benzalkonium chloride when administered by continuous nebulization. Am J Health Syst Pharm 2018;75(22):1791-1797. [DOI] [PubMed] [Google Scholar]
- 9.Lee BH, Kim SH. Benzalkonium chloride–induced bronchoconstriction in patients with stable bronchial asthma. Korean J Intern Med 2007;22(4):244-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asmus MJ, Barros MD, Liang J, Chesrown SE, Hendeles L. Pulmonary function response to EDTA, an additive in nebulized bronchodilators. J Allergy Clin Immunol 2001;107(1):68-72. [DOI] [PubMed] [Google Scholar]
- 11.Asmus MJ, Sherman J, Hendeles L. Bronchoconstrictor additives in bronchodilator solutions. J Allergy Clin Immunol 1999;104(2 Pt 2):S53-60. [DOI] [PubMed] [Google Scholar]
- 12.Pertzborn MC, Prabhakaran S, Abu-Hasan M, Baker D, Wu S, Wu Y, et al. Continuous albuterol with benzalkonium in children hospitalized with severe asthma. Pediatrics 2020;145(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.R Core Team, R Foundation for Statistical Computing. R: A language and environment for statistical computing, 2021. https://www.R-project.org.
- 14.Gulley SL, Baltzley SM, Junkins AD, Murray TD, Simms SF, Sullivan JE, et al. Sterility and stability testing of preservative-free albuterol. J Pediatr Pharmacol Ther 2019;24(1):53-57. [DOI] [PMC free article] [PubMed] [Google Scholar]