Abstract
In chickens, muscle development during embryonic growth is predominantly by myofiber hyperplasia. Following hatch, muscle growth primarily occurs via hypertrophy of the existing myofibers. Since myofiber number is set at hatch, production of more muscle fibers during embryonic growth would provide a greater myofiber number at hatch and potential for posthatch muscle growth by hypertrophy. Therefore, to improve performance in broilers, this study investigated the effect of in ovo spray application of probiotics on overall morphometry and muscle development in broiler embryos. For the study, fertile Ross 308 eggs were sprayed with different probiotics; Lactobacillus paracasei DUP 13076 (LP) and L. rhamnosus NRRL B 442 (LR) prior to and during incubation. The embryos were sacrificed on d 7, 10, 14, and 18 for embryo morphometry and pectoralis major muscle (PMM) sampling. Muscle sections were stained and imaged to quantify muscle fiber density (MFD), myofiber cross-sectional area (CSA), and nuclei density. Additionally, gene expression assays were performed to elucidate the effect of probiotics on myogenic genes. In ovo probiotic supplementation was found to significantly improve embryo weight, breast weight, and leg weight (P < 0.05). Further, histological analysis of PMM revealed a significant increase in MFD and nuclei number in the probiotic-treated embryos when compared to the control (P < 0.05). In 18-day-old broiler embryos, myofibers in the treatment group had a significantly smaller CSA (LP: 95.27 ± 3.28 μm2, LR: 178.84 ± 15.1 μm2) when compared to the control (211.41 ± 15.67 μm2). This decrease in CSA was found to be associated with a concomitant increase in MFD (fibers/mm2) in the LP (13,647 ± 482.15) and LR (13,957 ± 463.13) group when compared to the control (7,680 ± 406.78). Additionally, this increase in myofibrillar hyperplasia in the treatment groups was associated with upregulation in the expression of key genes regulating muscle growth including MYF5, MYOD, MYOG, and IGF-1. In summary, in ovo spray application of probiotics promoted overall embryo growth and muscle development in broilers.
Key words: broiler embryo, in ovo probiotic application, myogenesis, myogenic genes, embryonic growth
INTRODUCTION
In food animals, skeletal muscle is the largest contributor to mass and is therefore directly related to meat production (Gueller and Russell, 2010). In broiler chickens, breast muscle is the largest muscle by weight and sought out as a low-fat meat choice, it is the most valuable portion of the carcass (Harding et al., 2016). In effect, breast muscle weight represents around 18 to 25% of the birds’ live weight. As a consequence, even small differences in breast yield could have a significant economic impact (Scheuermann et al., 2003; Petracci et al., 2015). Consequently, the productivity of a broiler chicken is directly related to the cellular and molecular mechanisms regulating skeletal muscle myoblast growth and development, particularly in the pectoral muscle (Koohmaraie et al., 2002). In chicken, muscle hyperplasia mainly occurs during embryonic development which contributes to ∼30% of the lifespan of commercial broilers (Balaban et al., 2021; Liu et al., 2021; Wu et al., 2021). Hence, to improve posthatch muscle growth and meat yield it may be preferable to increase muscle fiber number and associated supportive vasculature during embryonic development (Wilkinson and Scott, 2006; Alcocer et al., 2021).
As in other species, muscle size and mass in chickens is determined by genetics, nutrition, health, and environmental factors (Wilkinson and Scott, 2006). Muscle tissue originates from the embryonic mesoderm, where the mesodermal cells proliferate and differentiate to somites and then myotome and myoblasts, which eventually form the skeletal muscle (Naya and Olson, 1999; Velleman, 2007; Berti et al., 2015). During primary myogenesis, the first wave of muscle development, myoblasts proliferate, and fuse with each other to form primary myofibers (Velleman and McFarland, 2015). This process is governed by the appropriate temporal expression of the myogenic regulatory factors (MRF), including myogenic factor 5 (MYF5), myogenic determination factor I (MYOD), myogenin (MYOG), and myogenic regulatory factor 4 (MRF4). A second wave of myogenesis leads to the formation of secondary fibers, using primary muscle fibers as a scaffold. During the late embryonic period, muscle growth occurs through hypertrophy, and muscle fiber number is fixed at hatch with no posthatch hyperplasia (Halevy et al., 2006b; Xu et al., 2021b). Subsequent posthatch muscle fiber growth occurs through hypertrophy (Gawel et al., 2022).
In ovo administration of nutrients and substances has been used to promote embryonic development and subsequent posthatch performance (Giviseiz et al., 2020). Specifically, administration of carbohydrates, vitamins, hormones, growth factors, prebiotics, and synbiotics were shown to promote intestinal development and embryonic metabolism (Uni and Ferket, 2003; Tako et al., 2004; Cheled-Shoval et al., 2011; Bhanja et al., 2014; Zhao et al., 2017; Moreiera Filho et al., 2018; Araujo et al., 2019; Dal Pont et al., 2019). Provision of these nutrients and substances likely enhanced the nutritional status of the embryo, thereby promoting growth including myogenesis (Xu et al., 2021b). A few studies have evaluated the effects of in ovo probiotic inoculation on posthatch performance and immune status of adult birds with varying results (Leao et al., 2021). However, their effects on embryonic growth and myogenesis have not been studied. Since myofiber number is set at hatch, production of more muscle fibers during embryonic growth would provide for a greater myofiber number at hatch and potential for posthatch muscle growth by hypertrophy (Halevy et al., 2006b; Willkinson and Scott, 2006; Velleman, 2007). Further, poultry researchers have now realized that future gains in production potential of these birds will come from advancements made on embryogenesis (Christensen et al., 2007; Collin et al., 2007; de Oliveira et al., 2008). Hence our study investigated the effects of in ovo probiotic spray application on embryo morphometry and muscle development in broilers. We hypothesized that in ovo probiotic application would promote embryonic muscle development in broilers. As opposed to inoculations, supplementation of probiotics by spray application provides for a noninvasive approach to promote growth. Further, egg spraying is a commonly employed method to disinfect hatching eggs (Brake and Sheldon, 1990; Buhr et al., 1994; Bourassa et al., 2002; Ernst, 2010; Copur et al., 2011). Hence, it is expected that spray application of probiotics on hatching eggs could be integrated with hatchery management practices to promote embryonic growth and muscle development.
MATERIALS AND METHODS
Probiotic Culture Conditions
Lactobacillus paracasei DUP-13076 (LP) and Lactobacillus rhamnosus NRRL-B-442 (LR) were obtained from Dr. Bhunia, Food Science Department, Purdue University, and the USDA NRRL culture collection, respectively. Each strain was cultured separately in de Mann, Rogosa, Sharpe broth (MRS) at 37°C overnight. The cultures were then centrifuged (3,200 × g, 12 min), and washed twice in phosphate buffered saline (PBS, pH 7.4). The pellet was resuspended in PBS and used as the inoculum. The bacterial population in the inoculum was determined by standard dilution and plating on MRS agar, followed by incubation at 37°C for 24 h. These cultures were selected based on our preliminary in ovo trials demonstrating absence of negative impact on embryonic growth and viability. Further, previous research from our lab also identified the ability of these strains to attenuate Salmonella virulence in vitro (Muyyarikkandy and Amalaradjou, 2017).
Experimental Design
All trials were conducted with the approval of the UConn Institutional Animal Care and Use Committee. Fertilized broiler (Ross 308) eggs from 42-wk-old birds were kindly provided by Aviagen, Inc. (Huntsville, AL). Upon receipt, any damaged eggs were discarded, and the rest stored at 12.8°C for no more than 24 h (Christensen et al., 2002). Prior to incubation, all settable eggs were weighed (starting egg weight) and randomly assigned to 1 of 3 groups namely, 1) Control [no probiotic, n = 100, eggs sprayed with solvent/carrier (PBS)], 2) LP [n = 100; eggs sprayed with LP (∼9 log CFU/egg)], and 3) LR [n = 100, eggs sprayed with LR (∼9 log CFU/egg)].
Spray Treatment and Egg Incubation
Eggs in each tray were individually sprayed on d 0, 3, 7, 10, 14, and 18 of incubation. On each treatment day, trays were removed one-at-time, eggs were individually sprayed, and the tray was put back into the incubator before moving to the next tray. The eggs were sprayed at room temperature and the time to spray each tray of eggs was observed to be less than 4 min. Eggs in the control group were individually sprayed with PBS while eggs in treatment groups were sprayed with LP or LR to ensure approximately 9 log CFU/egg using an atomizer (Amalaradjou, 2022). The treatment regimen was based on maintaining significant probiotic populations on the eggs (∼4 log CFU/egg) throughout incubation as determined in our preliminary trials. The sprayed eggs were incubated for 18 d in a thermostat incubator (2362N Hova-Bator, GQF Manufacturing Company, Inc., City, GA) with an automatic egg turner (1,611 egg turner with 6 universal racks, GQF Manufacturing Company, Inc.), at 37.8°C and 55% relative humidity.
Morphometric Measurements
On d 7, 10, 14, and 18 of incubation, 18 to 20 eggs/group were randomly sampled. The eggs were weighed, opened through the blunt end, and embryos euthanized by cervical dislocation. The embryos were then dissected to obtain embryo weight (yolk-free body mass; YFBM) and yolk sac weight. Additionally, on d 10, 14, and 18, the length of the curvature along the back from the top of the head to the tip of the tail was measured as the crown rump length (Henning et al., 2011). Relative embryo and yolk sac weight were calculated as percentage of starting egg weight (SEW) to account for variation in egg weights. Further, on d 18 additional tissue weights (breast, leg, heart, liver, and gizzard) and lengths (crown rump, tibial, radial, and the third digit) were measured (O'Dea et al., 2006; Li et al., 2013; de Oliveira et al., 2014). Relative organ, breast and leg weights were calculated as percentage of embryo weight (YFBM) to eliminate possible effects of variability in embryo weight. Additionally, on d 10, 14, and 18 of incubation, the left and right pectoralis major muscles (PMM) were collected from 8 to 10 embryos in each treatment group for immunohistochemistry and RT-qPCR, respectively.
Muscle Sample Collection
The left PMM was collected from the embryos and processed for muscle histology as previously described (Reed et al., 2014; Xu et al., 2021b). Briefly, samples were embedded in Tissue-Tek optimal cutting media (OCT; Fisher Scientific, Pittsburg, PA), frozen in dry ice cooled isopentane and stored at −80°C until further use.
Immunohistochemistry and Histology
Muscle samples were cryosectioned (10 µm) using a HM525 cryostat (Thermo Fisher, Waltham, MA) to determine muscle fiber cross-sectional area (CSA) and nuclei and muscle fiber density (MFD). Briefly, muscle sections were rehydrated in PBS with 0.1% triton X-100 for 5 min and fixed with 4% paraformaldehyde for 20 min. Fixed sections were then washed with PBS and blocked with 5% horse serum, 0.2% Triton X-100 in PBS for 20 min. To visualize the sarcolemma and nuclei, samples were incubated with Alexa Fluor 568 wheat germ agglutinin (WGA, 1:150, Invitrogen, Carlsbad, CA) and Hoechst 33342 (1:2,000, Invitrogen) overnight at 4°C in a humidified box. Sections were rinsed with PBS and cover-slipped with 9:1 glycerol/PBS solution. All sections were imaged at 400-fold magnification using an Axiovert Widefield microscope (Zeiss, Jena, Germany) mounted to an AxioCam Camera (Zeiss), false colored and merged using ImageJ (NIH, Bethesda, MD). Fiber CSA was measured as the region within the fiber boundary using the area measurement tool in ImageJ (Reed et al., 2014). At least 5 images were obtained from 5 different muscle sections resulting in the analysis of a minimum of 400 fibers per muscle sample. To determine muscle fiber and nuclei density, the number of fibers and nuclei within a 100 µm2 area were enumerated and represented as counts/mm2 (Reed et al., 2014; Xu et al., 2021a).
RT-qPCR of Genes Involved in Myogenesis
The right PMM was collected in RNAlater (Qiagen, Germantown, MD) and stored at −80°C until further processing (Zammit et al., 2006; Gu et al., 2022). RNA was extracted using RNeasy minikit (Qiagen) according to the manufacturer's protocol, and RNA quality was determined using the Nanodrop (Eppendorf, Enfield, CT). cDNA was synthesized using the iScript reverse transcriptase kit (Bio-Rad, Hercules, CA). Specific primers for candidate genes (MYF5, MRF4, FGF2, FGF4, IGF1, IGF1R, MYOD, and MYOG) were selected from published literature (Mitchell et al., 1999; Zammit et al., 2006; Al-Musawi et al., 2011; Davis et al., 2015; Harding et al., 2016). RT-qPCR was performed on the StepOnePlus platform using the SYBR green assay (Applied Biosystems, CA) under custom thermal cycling conditions. Duplicate samples were run from each biological sample and a total of 8 to 10 biological replicates from each treatment group were included in the assay. Data were normalized to the endogenous control (GAPDH), and comparative quantification (2−ΔΔCT) was carried out to detect changes in relative gene expression between the treatment and control samples (Bookout and Mangelsdorf, 2003).
Statistical Analysis
On each sampling day, 18 to 20 embryos per treatment were sampled for morphometric measurements, while muscle samples were collected from 8 to 10 embryos per group for histology and gene expression assays. All data were analyzed as a completely randomized design with embryo as the experimental unit. Data were sorted by embryonic day and analyzed using the Mixed procedure of SAS 9.4 (SAS Inst. Inc., Cary, NC) using treatment as the fixed effect. Treatment mean comparisons were performed using LSMEANS statement and PDIFF option. Differences were determined to be significant at P ≤ 0.05.
RESULTS AND DISCUSSION
Current commercial broiler chicken strains are a result of successful selection programs for rapid growth especially favoring the breast muscle (Scheuermann et al., 2003; Al-Musawi et al., 2011). As a result, the breast muscle is the largest muscle by weight and is the most valuable portion of the chicken carcass (Harding et al., 2016). Hence productivity in broilers is directly related to muscle growth and development (Koohmaraie et al., 2002). Since myofiber number is set at hatch, targeting embryonic myogenesis is crucial to improve breast muscle mass and yield (Halevy et al., 2006b; Willkinson and Scott, 2006; Velleman, 2007). Therefore, as a means to improve performance in broilers, this study investigated the effects of in ovo probiotic supplementation on overall embryo morphometry and muscle development in broiler embryos.
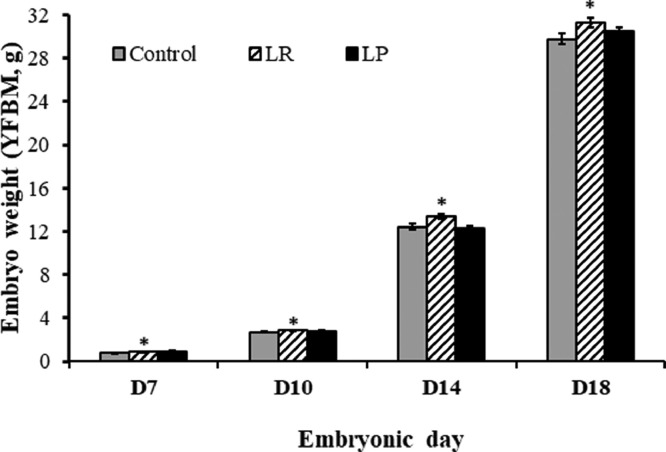
In the current study, in ovo supplementation with Lactobacillus rhamnosus NRRL-B-442 (LR) resulted in a significant (P < 0.05) increase in embryo weight (yolk-free body mass, Figure 1) and relative embryo weight (expressed as percentage of starting egg weight) when compared to the control (Figure 1 and Table 1). Specifically, on d 18, supplementation with LR increased relative embryo weight by 8% compared to the control. Additionally, as seen on d 18, we did not observe any significant differences in the yolk sac weight and crown rump length between treatments at all sampling times (data not shown). To the contrary, previous studies employing in ovo probiotic inoculation demonstrated increase in crown rump length with no observed improvement in embryo weights (de Oliveira et al., 2014). Further, we also observed a significant increase in relative breast and leg weight (expressed as percentage of embryo weight) when compared to the control (P < 0.05). For instance, LR supplementation increased breast and leg weight by 18 and 15%, respectively, when compared to the control (Table 1). Similarly, Lactobacillus paracasei DUP-13076 (LP) supplementation resulted in a 12% increase in breast weight and a 17% increase in leg weight when compared to the control. A similar increase in breast weight in d 19 embryos was reported by Xu et al. (2021b) following in ovo inoculation of nicotinamide riboside. Although probiotic supplementation led to significant improvement in embryo, breast and leg weight, in the current study, we did not observe any effects on the crown rump length or relative organ weights during embryogenesis (heart, liver, gizzard; P > 0.05; Table 1).
Figure 1.
Effect of in ovo probiotic supplementation on weight of growing embryos1. 1Weight was measured as yolk-free body mass. Data are represented as mean ± SEM. *Different superscripts indicate significant difference from control within each day at P < 0.05.
Table 1.
Effects of in ovo probiotic supplementation on embryo morphometry at d 18 of incubation.
| Morphometric parameters | Treatments |
||
|---|---|---|---|
| Control | LR | LP | |
| Embryo (YFBM; % SEW) relative percent increase | 44.83 ± 0.88a | 48.44 ± 0.87b +8.05% |
46.38 ± 0.85a +3.44% |
| Yolk sac (% SEW) | 27.02 ± 1.15 | 26.49 ± 1.33 | 28.03 ± 1.03 |
| Breast (% YFBM) relative percent increase | 4.72 ± 0.20a | 5.58 ± 0.34b +18.27% |
5.29 ± 0.18b +12.05% |
| Leg (% YFBM) Relative percent increase |
6.59 ± 0.26a | 7.59 ± 0.41b +15.13% |
7.75 ± 0.23b + 17.68% |
| Heart (% YFBM) | 1.20 ± 0.14 | 1.32 ± 0.29 | 1.01 ± 0.10 |
| Liver (% YFBM) | 3.62 ± 0.40 | 3.69 ± 0.52 | 3.85 ± 0.53 |
| Gizzard (% YFBM) | 7.82 ± 0.60 | 6.81 ± 0.77 | 7.77 ± 0.78 |
| Crown-rump length (cm) | 8.59 ± 0.07 | 8.74 ± 0.08 | 8.61 ± 0.10 |
| Tibial length (cm) | 2.61 ± 0.12 | 2.54 ± 0.14 | 2.50 ± 0.10 |
| Radial length (cm) | 1.34 ± 0.03 | 1.29 ± 0.04 | 1.36 ± 0.05 |
| Third digit length (cm) | 2.03 ± 0.03 | 2.08 ± 0.08 | 1.97 ± 0.04 |
Data are represented as mean ± SEM.
Treatments with different superscripts within a row are significantly different at P < 0.05.
Abbreviations: Embryo weight, YFBM; LP, Lactobacillus paracasei; LR, Lactobacillus rhamnosus; SEW, starting egg weight.
Breast weight includes the breast bone; Leg weight includes thigh and drumstick with femur and tibia; Tibial length: measured from knee cap to hock joint; Radial length: measured from elbow to carpal joint.
Probiotics have been widely studied as alternatives to growth promoters in poultry production. Specifically, probiotic supplementation was shown to promote weight gain, increase muscle mass, and improve feed efficiency (Maiorano et al., 2012; Park and Kim, 2014; Tavaniello et al., 2019). This increase in muscle mass can arise from a modulation in embryonic myogenesis and posthatch muscle development (Xu et al., 2021b). Toward this, Maiorano et al. (2012) and Bogucka et al. (2018) demonstrated an increase in MFD in adult birds following in ovo synbiotic supplementation (Maiorano et al., 2012; Bogucka et al., 2018; Bogucka et al., 2022). However, effects on embryo development and prenatal myogenesis have not been studied. Given the critical role that embryonic myogenesis has on posthatch muscle growth, we determined the effect of probiotics on PMM histology.
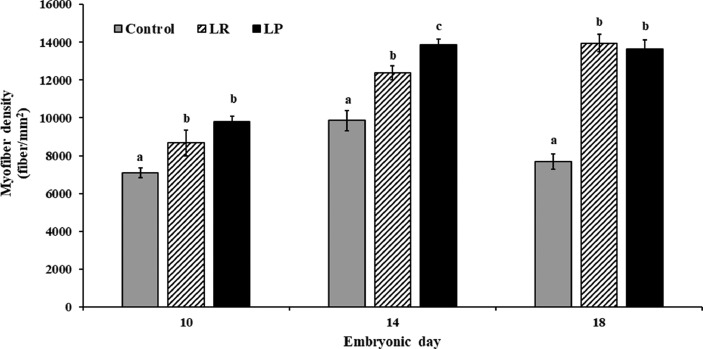
Since myofiber hyperplasia occurs prenatally, modulation in embryonic myogenesis could increase muscle fiber number (Halevy et al., 2006a; Xu et al., 2021a). Hence, to evaluate the effects of probiotics on embryonic myogenesis, MFD was determined in the pectoralis major muscles. Supplementation of probiotics to embryonated eggs significantly increased MFD when compared to the control (P < 0.05; Figure 2). For instance, on d 18 of incubation, PMM fiber density (fibers/mm2) in the LR and LP group was 13,957 ± 463.13 and 13,647 ± 482.15, respectively, when compared to 7,680 ± 406.78 in the control (Figure 3). Additionally, the increase in MFD was sustained throughout embryonic growth from d 10 to d 18 of incubation (Figures 2 and 3). More specifically, in ovo probiotic supplementation increased MFD by 22 to 38% on d 10, 25 to 40% on d 14, and 77 to 80% on d 18, when compared to the control (Figure 3).
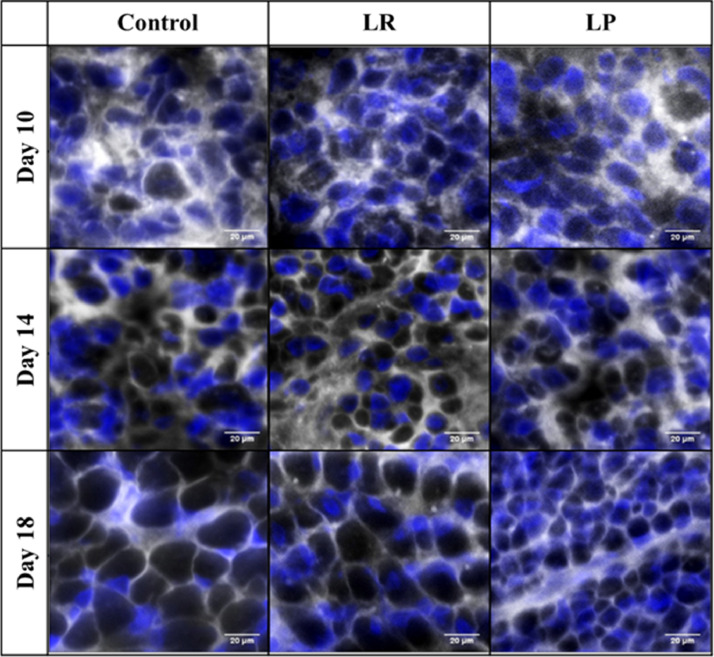
Figure 2.
Representative image of pectoralis major muscle sections of broiler embryos on embryonic d 10, 14, and 18. Cross sections (10 μm) of the PMM were stained with wheat germ agglutinin (WGA; gray) to delineate the muscle fiber membrane and Hoechst 33342 (blue) to identify nuclei.
Figure 3.
Effects of in ovo probiotic supplementation on myofiber density of the pectoralis major muscle in broiler embryos1. 1Data are represented as mean ± SEM. *Different superscripts indicate significant difference from control within each day at P < 0.05.
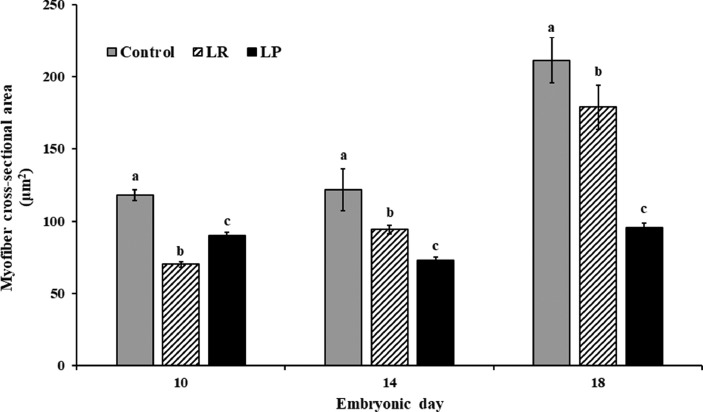
MFD is inversely related to muscle fiber CSA. Hence, an increase in MFD is often associated with a concomitant decrease in the myofiber CSA. In the current study, the control embryos consistently demonstrated significantly larger myofiber CSA when compared to the treatment groups (P < 0.001; Figures 2 and 4). At d 10 of incubation, CSA was 23 and 40% smaller in the LP and LR groups, respectively, when compared to the control (Control: 118.05 ± 3.94 µm2, LP: 89.91 ± 2.2 µm2, LR: 70.13 ± 1.97 µm2, P < 0.001, Figure 4). Similarly, on d 18, muscle fiber CSA in the LP and LR group was 54 and 15% smaller than in the control embryos (Control: 211.41 ± 15.67 µm2, LP: 95.27 ± 3.28 µm2, LR: 178.84 ± 15.31 µm2, P < 0.001). As previously described, smaller CSA was found to be associated with an accompanying increase in MFD (Figure 3). This smaller muscle fiber CSA coupled with increased MFD in probiotic supplemented groups suggests increased hyperplasia and not just the absence of hypertrophy. Since muscle growth during embryonic life predominantly occurs through hyperplasia, the increase in myofiber number observed in our study implies a positive effect of probiotic supplementation on embryonic muscle development.
Figure 4.
Effects of in ovo probiotic supplementation on myofiber cross-sectional area of the pectoralis major muscle in broiler embryos1. 1Data are represented as mean ± SEM. *Different superscripts indicate significant differences within each day at P < 0.05.
Although probiotics have not been previously evaluated for their effects on embryonic muscle development in chicken, in ovo supplementation of synbiotics was shown to have no effect on muscle fiber number or CSA in adult Ross 308 birds (Stasiak et al., 2021). However, supplementation of nutrients including L-glutamine, insulin-like growth factor I, silver nanoparticles, and nicotinamide riboside to embryos resulted in improved MFD (Liu et al., 2012; Grodzik et al., 2013; Gonzalez and Jackson, 2020; Husseiny et al., 2021; Xu et al., 2021a). Similarly, providing monochromatic green light during egg incubation was shown to promote myofiber hyperplasia in early posthatch chicks, with greater fiber density and reduced fiber diameter (Halevy et al., 2006a).
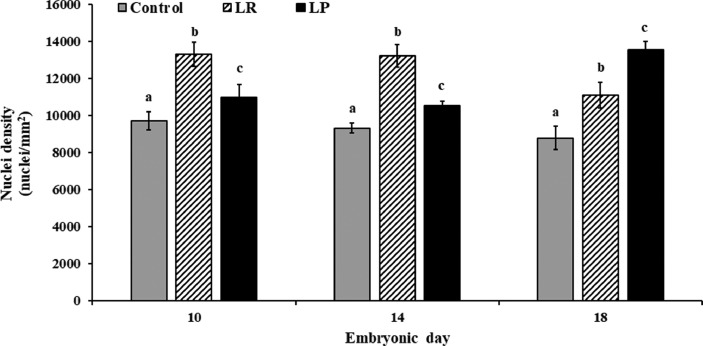
Embryonic myoblasts contribute nuclei and other organelles to both muscle fibers that are forming (hyperplasia) and fibers that are undergoing hypertrophy. Therefore, the effect of probiotic supplementation on nuclei density was determined. As seen with MFD, in ovo application of probiotics significantly improved nuclei density in PMM when compared to the control (P < 0.05; Figure 5). With the control samples, the nuclei density ranged from 8,780 to 9,706 nuclei per mm2 throughout the incubation period. On the other hand, with the LP group, the nuclei density was 10,986.67 ± 671.91, 10,530 ± 253.18 and 13,544 ± 441.72 nuclei per mm2 on d 10, 14, and 18, respectively. Similarly, with LR, the nuclei density was 13,300 ± 655.66, 13,223.33 ± 609.92, and 11,116.67 ± 692.22 per mm2 on d 10, 14, and 18, respectively. Overall, the nuclei density in the probiotic treatment groups was higher by 13 to 54% on d 10 and 18, respectively, when compared to the control. A similar increase in nuclei number was reported following in ovo administration of silver nanoparticles (Sawosz et al., 2012) and muscle extract and graphene oxide (Balaban et al., 2021) in chicken embryos.
Figure 5.
Effects of in ovo probiotic supplementation on nuclei density of the pectoralis major muscle in broiler embryos1. 1Data are represented as mean ± SEM. *Different superscripts indicate significant differences within each day at P < 0.05.
To determine the underlying mechanisms that may be responsible for the increase in breast weight with a greater number of muscle fibers, gene expression of key myogenic factors was investigated. Skeletal muscle development is tightly regulated by the myogenic regulatory factors including myogenic determination factor I (MYOD), myogenin (MYOG), including myogenic factor 5 (MYF5), and myogenic regulatory factor 4 (MRF4; Halevy et al., 2006b; Piestun et al., 2015; Harding et al., 2016; Wen et al., 2017). Of these factors, MYF5 and MYOD play early roles in the determination of muscle precursor cells to the myogenic lineage. Although these are first expressed in proliferating myoblasts, evidence suggests that MYF5 functions more toward proliferation while MYOD prepares the myoblast for efficient differentiation (Ishibashi et al., 2005; Al-Musawi et al., 2011). During differentiation, MYOD initiates cell cycle exit, which allows MYOG to stimulate fusion of myoblasts. Subsequently, MRF4 directs the maturation of the nascent muscle fiber (Hernandez-Hernandez et al., 2017). In our study, probiotic treatment was observed to significantly reduce the expression MYF5, MYOD, MYOG, and MRF4 initially (d 10 and 14) when compared to the control (P < 0.05, Table 2). Although both treatments reduced the expression of the above-mentioned myogenic factors, LR and LP significantly differed in the relative fold change observed with MYF5, MYOD, and MRF4 expression. For example, on d 14, LR resulted in ∼7-fold reduction in MYF5 expression when compared to ∼3-fold reduction by LP treatment. Overall, coupled with heavier breast muscle weights (12–18% heavier compared to control; Table 1) and smaller muscle fiber CSA (23–54% smaller compared to control, Figure 4), this could imply delayed maturation or increased secondary myogenesis in the treatment groups compared to the control.
Table 2.
Effects of in ovo probiotic supplementation on myogenic gene expression in the pectoralis major muscle of broiler embryos.
| D 10 |
D 14 |
D 18 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | Control | LR | LP | Control | LR | LP | Control | LR | LP |
| MYF5 | 1 ± 0.03a | −8.33 ± 0.41b | −6.66 ± 0.33c | 1.0 ± 0.28a | −7.14 ± 0.35b | −3.44± 0.17c | 1.0 ± .05a | 1.44 ± 0.07b | 1.56 ± 0.07c |
| MYOD | 1 ± 0.07a | −2.56 ± 0.12b | −2.12 ± 0.10c | 1.0 ± 0.14a | −2.5 ± 0.12b | −1.96 ± 0.09c | 1.0 ± 0.06a | 1.98 ± 0.09b | −1.16 ± 0.05a |
| MYOG | 1 ± 0.04a | −2.5 ± 0.125b | −1.58± 0.07b | 1.0 ± 0.11a | 1.00 ± 0.05a | −1.16± 0.05a | 1.0 ± 0.08a | 1.37 ± 0.06b | 4.37 ± 0.21c |
| MRF4 | 1 ± 0.15a | −2.43 ± 0.12b | −1.75 ± 0.08b | 1.0 ± 0.19a | −2.94 ± 0.14b | −2.04 ± 0.10c | 1.0 ± 0.16a | 1.47 ± 0.07b | −3.33 ± 0.16c |
| IGF1 | 1.0 ± 0.25a | 1.43 ± 0.07b | 3.04 ± 0.15c | 1.0 ± 0.21a | 1.35 ± 0.06b | 1.34 ± 0.06b | 1.0 ± 0.27a | 1.61 ± 0.08b | 1.14 ± 0.05a |
| IGF1R | 1.0 ± 0.13a | 2.73 ± 0.13b | 2.18 ± 0.10c | 1.0 ± 0.16a | −1.63 ± 0.08b | −1.13 ± 0.05a | 1.0 ± 0.15a | 3.60 ± 0.18b | −1.09 ± 0.05a |
| FGF2 | 1.0 ± 0.22a | −1.02 ± 0.05a | 1.02 ± 0.05a | 1.0 ± 0.34a | −1.29 ± 0.06a | 1.27 ± 0.12a | 1.0 ± 0.27a | 2.56 ± 0.10b | 2.17 ± 0.13c |
| FGF4 | 1.0 ± 0.28a | 1.15 ± 0.05a | 1.74 ± 0.08b | 1.0 ± 0.37a | −1.12 ± 0.05a | −1.63 ± 0.08b | 1.0 ± 0.32a | 3.53 ± 0.17b | 3.64 ± 0.18b |
Data are expressed as mean ± SEM.
Different superscripts indicate significant difference in relative gene expression between control (a) and treatments within each day at P < 0.05.
Abbreviations: LP, Lactobacillus paracasei; LR, Lactobacillus rhamnosus.
However, on d 18, all myogenic regulatory factors (MRF4, MYF5, MYOG, and MYOD) were significantly upregulated in LR relative to control (Table 2). In the LP-treated embryos, MRF4 was downregulated and MYF5 and MYOG were upregulated (Table 2; P < 0.05). The upregulation in MYOG at d 18 is in line with previous findings that report that transcription of MYOG is switched on at the end of embryogenesis, and consequently the process of proliferation decreases while the formation of myofibers becomes a highly active process (Brunetti and Goldfine, 1990; Husseiny et al., 2021). In the present study, probiotic treatments downregulated the expression of myogenic factors on d 10 and 14, suggesting prolonged secondary myogenesis in the pectoralis major muscles of these embryos. Further, the increased expression of MRFs in LR-treated eggs at d 18 suggests that probiotic treatment is priming muscle for additional postnatal growth.
Myogenesis is also under the influence of growth factors including fibroblast growth factor (FGF), platelet-derived growth factor, growth hormone, and insulin-like growth factor (IGF; Sklan et al., 2003; Al-Musawi et al., 2011). Of these, IGF has been implicated in the control of skeletal muscle growth and development during embryogenesis, posthatch hypertrophy, and regeneration (Florini et al., 1996; Duclos, 2005). In case of broilers, it has been shown that fast-growing birds may enhance myogenic determination and differentiation by upregulating the above-mentioned myogenic regulators both during embryonic (Al-Musawi et al., 2011) and postnatal muscle growth (Wen et al., 2017). In the present study, when compared to the control, IGF1 was significantly upregulated in both treatment groups at all the sampling time points, which could stimulate both muscle progenitor cell proliferation and differentiation (Table 2). Further, a significant increase in IGF1R (IGF1 receptor) expression was also observed on d 10 and 18. However, a slight but significant reduction in IGF1R expression was seen on d 14. With reference to FGF, acting through different pathways, FGF2 and FGF4 regulate myogenesis by stimulating myoblast proliferation and inhibiting their differentiation (Mitchell et al., 1999; Velleman, 2007). There was no significant change in FGF2 expression on d 10 or 14 in the treatment groups. However, FGF2 expression was significantly upregulated (∼2-fold increase) on d 18 in the LR- and LP-treated embryos. Taken together, the upregulation in FGF and MRF expression could imply that the muscle is primed for posthatch muscle growth. Similarly, FGF4 expression was increased 3.5-fold and 3.6-fold on d 18 in LR and LP groups, respectively. Increased expression of IGF1, FGF2, and FGF4 support the increased expression of MRFs and the apparent increase in total fiber number during embryonic development in probiotic-treated eggs (Figure 3; Grodzik et al., 2013). Further, our data demonstrate that although probiotic treatment improves overall muscle growth, the underlying mechanisms mediating these responses can differ with the individual strains.
Overall, in ovo spray application of probiotics was shown to promote embryonic growth and muscle development. Specifically, increased muscle growth was associated with an increase in muscle fiber and nuclei density. This is significant to broiler production since increased muscle fiber number at the time of hatch is expected to translate into increased muscle mass during posthatch development. Further, greater numbers of nuclei at the time of hatch are expected to support protein accretion during posthatch muscle hypertrophy. Moreover, the myogenic effects exerted by the probiotics were associated with the modulation of key genes that regulate muscle progenitor cell proliferation and differentiation. To further characterize the growth promoting effects of the candidate probiotics, additional studies on posthatch performance and muscle growth are ongoing. Additionally, experiments to modify and standardize treatment regimens that could be integrated with current industry practices are currently underway.
ACKNOWLEDGMENTS
This work was supported by the USDA-AFRI National Institute of Food and Agriculture Program [award number 2020-67016-30817] and the IDEA grant from the UConn Office for Undergraduate Research to Ms. Schlesinger.
DISCLOSURES
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Mary Anne Amalaradjou has patent #US11497197B2 issued to University of Connecticut. Corresponding author serves as an associate editor for Poultry Science.
REFERENCES
- Alcocer H.M., Xu X., Gravely M.E., Gonzalez J.M. In ovo feeding of commercial broiler eggs: an accurate and reproducible method to affect muscle development and growth. JoVE. 2021;175:e63006. doi: 10.3791/63006. [DOI] [PubMed] [Google Scholar]
- Al-Musawi S.L., Lock F., Simbi B.H., Bayol S.A., Stickland N.C. Muscle specific differences in the regulation of myogenic differentiation in chickens genetically selected for divergent growth rates. Differentiation. 2011;82:127–135. doi: 10.1016/j.diff.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalaradjou M.A. Methods for Enhancing Poultry Growth and Performance (U.S. Patent No. 11,497,197) 2022 https://patents.google.com/patent/US11497197B2 Accessed Jan. 2023. [Google Scholar]
- Araújo I.C., Café M.B., Noleto R.A., Martins J.M., Ulhoa C.J., Guareshi G.C., Reis M.M., Leandro N.S. Effect of vitamin E in ovo feeding to broiler embryos on hatchability, chick quality, oxidative state, and performance. Poult. Sci. 2019;98:3652–3661. doi: 10.3382/ps/pey439. [DOI] [PubMed] [Google Scholar]
- Bałaban J., Zielińska M., Wierzbicki M., Ostaszewska T., Fajkowska M., Rzepakowska M., Daniluk K., Sosnowska M., Chwalibog A., Sawosz E. Effect of muscle extract and graphene oxide on muscle structure of chicken embryos. Animals. 2021;11:3467. doi: 10.3390/ani11123467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti F., Nogueira J.M., Wöhrle S., Sobreira D.R., Hawrot K., Dietrich S. Time course and side-by-side analysis of mesodermal, pre-myogenic, myogenic and differentiated cell markers in the chicken model for skeletal muscle formation. J. Anat. 2015;227:361–382. doi: 10.1111/joa.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanja S.K., Sudhagar M., Goel A., Pandey N., Mehra M., Agarwal S.K., Mandal A. Differential expression of growth and immunity related genes influenced by in ovo supplementation of amino acids in broiler chickens. Czech. J. Anim. Sci. 2014;59:399–408. [Google Scholar]
- Bogucka J., Dankowiakowska A., Stanek M., Stadnicka K., Kirkiłło-Stacewicz K. Effect of synbiotics administered in ovo on microvascularization and histopathological changes in pectoral muscle and the biochemical profile of broiler chicken blood. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogucka J., Ribeiro D.M., Costa R., Bednarczyk M. Effect of synbiotic dietary supplementation on histological and histopathological parameters of Pectoralis major muscle of broiler chickens. Czech. J. Anim. Sci. 2018;63:263–271. [Google Scholar]
- Bookout A.L., Mangelsdorf D.J. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl. Recept. Signal. 2003;1 doi: 10.1621/nrs.01012. nrs.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourassa D.V., Buhr R.J., Wilson J.L. Hatchability of eggs sanitized with increasing concentrations of BioSentry 904 or Bio-Phene. J. Appl. Poult. Res. 2002;11:397–401. [Google Scholar]
- Brake J., Sheldon B.W. Effect of a quaternary ammonium sanitizer for hatching eggs on their contamination, permeability, water loss, and hatchability. Poult. Sci. 1990;69:517–525. doi: 10.3382/ps.0690517. [DOI] [PubMed] [Google Scholar]
- Brunetti A., Goldfine I.D. Role of myogenin in myoblast differentiation and its regulation by fibroblast growth factor. J. Biol. Chem. 1990;265:5960–5963. [PubMed] [Google Scholar]
- Buhr R.J., Mauldin J.M., Bailey J.S., Cox N.A. Automated spray sanitizing of broiler hatching eggs 2. Hatchability of nest clean and dirty eggs. J. Appl. Poult. Res. 1994;3:226–233. [Google Scholar]
- Cheled-Shoval S.L., Amit-Romach E., Barbakov M., Uni Z. The effect of in ovo administration of mannan oligosaccharide on small intestine development during the pre-and posthatch periods in chickens. Poult. Sci. 2011;90:2301–2310. doi: 10.3382/ps.2011-01488. [DOI] [PubMed] [Google Scholar]
- Christensen V.L., Ort D.T., Nestor K.E., Velleman S.G., Havenstein G.B. Genetic control of neonatal growth and intestinal maturation in turkeys. Poult. Sci. 2007;86:476–487. doi: 10.1093/ps/86.3.476. [DOI] [PubMed] [Google Scholar]
- Christensen V.L., Wineland M.J., Fasenko G.M., Donaldson W.E. Egg storage alters weight of supply and demand organs of broiler chicken embryos. Poult. Sci. 2002;81:1738–1743. doi: 10.1093/ps/81.11.1738. [DOI] [PubMed] [Google Scholar]
- Collin A., Berri C., Tesseraud S., Rodon F.E., Skiba-Cassy S., Crochet S., Duclos M.J., Rideau N., Tona K., Buyse J., Bruggeman V., Decuypere E., Picard M., Yahav S. Effects of thermal manipulation during early and late embryogenesis on thermotolerance and breast muscle characteristics in broiler chickens. Poult. Sci. 2007;86:795–800. doi: 10.1093/ps/86.5.795. [DOI] [PubMed] [Google Scholar]
- Copur G., Arslan M., Baylan M., Canogullari S. Use of allicin as an alternative hatching egg disinfectant versus formaldehyde fumigation in broiler hatching eggs. Biotechnol. Biotechnol. Equip. 2011;25:2494–2498. [Google Scholar]
- Dal Pont G.C., Goes E.C., da Silva K.F., de Oliveira S.G., da Rocha C., Miorka A. Glycerol in ovo feeding as an energy substrate improves performance of broilers from young breeders. J. Anim. Physiol. Anim. Nutr. 2019;103:1453–1461. doi: 10.1111/jpn.13153. [DOI] [PubMed] [Google Scholar]
- Davis R.V., Lamont S.J., Rothschild M.F., Persia M.E., Ashwell C.M., Schmidt C.J. Transcriptome analysis of post-hatch breast muscle in legacy and modern broiler chickens reveals enrichment of several regulators of myogenic growth. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira J.E., Uni Z., Ferket P.R. Important metabolic pathways in poultry embryos prior to hatch. World's Poult. Sci. J. 2008;64:488–499. [Google Scholar]
- de Oliveira J.E., Van der Hoeven-Hangoor E., Van de Linde I.B., Montijn R.C., Van der Vossen J. In ovo inoculation of chicken embryos with probiotic bacteria and its effect on post hatch Salmonella susceptibility. Poult. Sci. 2014;93:818–829. doi: 10.3382/ps.2013-03409. [DOI] [PubMed] [Google Scholar]
- Duclos M.J. Insulin-like growth factor-I (IGF-1) mRNA levels and chicken muscle growth. J. Physiol. Pharmacol. 2005;56:25–35. [PubMed] [Google Scholar]
- Ernst R.A. University of California; California: 2010. Pages 8120 in Hatching Egg Sanitation: The Key Step in Successful Storage and Production.https://anrcatalog.ucanr.edu/pdf/8120.pdf Accessed Jan. 2023. [Google Scholar]
- Florini J.R., Ewton D.Z., Coolican S.A. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr. Rev. 1996;17:481–517. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- Gaweł A., Madej J.P., Kozak B., Bobrek K. Early post-hatch nutrition influences performance and muscle growth in broiler chickens. Animals. 2022;12:3281. doi: 10.3390/ani12233281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givisiez P.E., Moreira Filho A.L., Santos M.R., Oliveira H.B., Ferket P.R., Oliveira C.J., Malheiros R.D. Chicken embryo development: metabolic and morphological basis for in ovo feeding technology. Poult. Sci. 2020;99:6774–6782. doi: 10.1016/j.psj.2020.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J.M., Jackson A.R. In ovo feeding of nicotinamide riboside affects broiler pectoralis major muscle development. Transl. Anim. Sci. 2020;4:txaa126. doi: 10.1093/tas/txaa126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzik M., Sawosz F., Sawosz E., Hotowy A., Wierzbicki M., Kutwin M., Jaworski S., Chwalibog A. Nano-nutrition of chicken embryos. The effect of in ovo administration of diamond nanoparticles and L-glutamine on molecular responses in chicken embryo pectoral muscles. Int. J. Mol. Sci. 2013;14:23033–23044. doi: 10.3390/ijms141123033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S., Wen C., Li J., Liu H., Huang Q., Zheng J., Sun C., Yang N. Temporal expression of myogenic regulatory genes in different chicken breeds during embryonic development. Int. J. Mol. Sci. 2022;23:10115. doi: 10.3390/ijms231710115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güller I., Russell A.P. MicroRNAs in skeletal muscle: their role and regulation in development, disease and function. J. Physiol. (Lond.) 2010;588:4075–4087. doi: 10.1113/jphysiol.2010.194175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O., Piestun Y., Rozenboim I., Yablonka-Reuveni Z. In ovo exposure to monochromatic green light promotes skeletal muscle cell proliferation and affects myofiber growth in post hatch chicks. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1062–R1070. doi: 10.1152/ajpregu.00378.2005. [DOI] [PubMed] [Google Scholar]
- Halevy O., Yahav S., Rozenboim I. Enhancement of meat production by environmental manipulations in embryo and young broilers. World's Poult. Sci. J. 2006;62:485–497. [Google Scholar]
- Harding R.L., Halevy O., Yahav S., Velleman S.G. The effect of temperature on proliferation and differentiation of chicken skeletal muscle satellite cells isolated from different muscle types. Physiol. Rep. 2016;4:e12770. doi: 10.14814/phy2.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning A.L., Jiang M.X., Yalcin H.C., Butcher J.T. Quantitative three-dimensional imaging of live avian embryonic morphogenesis via micro-computer tomography. Dev. Dyn. 2011;240:1949–1957. doi: 10.1002/dvdy.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Hernández J.M., García-González E.G., Brun C.E., Rudnicki M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017;72:10–18. doi: 10.1016/j.semcdb.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husseiny W.A., Hassanin A.A., El Nabtiti A.A., Khalil K., Elaswad A. Silver nanoparticles as modulators of myogenesis-related gene expression in chicken embryos. Genes. 2021;12:629. doi: 10.3390/genes12050629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi J., Perry R.L., Asakura A., Rudnicki M.A. MyoD induces myogenic differentiation through cooperation of its NH2- and COOH-terminal regions. J. Cell Biol. 2005;171:471–482. doi: 10.1083/jcb.200502101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koohmaraie M., Kent M.P., Shackelford S.D., Veiseth E., Wheeler T.L. Meat tenderness and muscle growth: is there any relationship? Meat Sci. 2002;62:345–352. [PubMed] [Google Scholar]
- Leão A.P.A., Alvarenga R.R., Zangeronimo M.G. In ovo inoculation of probiotics for broiler chickens: systematic review and meta-analysis. Anim. Feed Sci. Technol. 2021;280 [Google Scholar]
- Li X., Chen X., Wang X. Changes in relative organ weights and intestinal transporter gene expression in embryos from White Plymouth Rock and WENS Yellow feather chickens. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013;164:368–375. doi: 10.1016/j.cbpa.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Liu J., Li F., Hu X., Cao D., Liu W., Han H., Zhou Y., Lei Q. Deciphering the miRNA transcriptome of breast muscle from the embryonic to post-hatching periods in chickens. BMC Genom. 2021;22:1–14. doi: 10.1186/s12864-021-07374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Wang J., Zhang R., Chen X., Yu H., Jin H., Li L., Han C., Xu F., Kang B. In ovo feeding of IGF-1 to ducks influences neonatal skeletal muscle hypertrophy and muscle mass growth upon satellite cell activation. J. Cell. Physiol. 2012;227:1465–1475. doi: 10.1002/jcp.22862. [DOI] [PubMed] [Google Scholar]
- Maiorano G., Sobolewska A., Cianciullo D., Walasik K., Elminowska-Wenda G., Sławińska A., Tavaniello S., Żylińska J., Bardowski J., Bednarczyk M. Influence of in ovo prebiotic and synbiotic administration on meat quality of broiler chickens. Poult. Sci. 2012;91:2963–2969. doi: 10.3382/ps.2012-02208. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Steenstrup T., Hannon K. Expression of fibroblast growth factor family during postnatal skeletal muscle hypertrophy. J. Appl. Physiol. 1999;86:313–319. doi: 10.1152/jappl.1999.86.1.313. [DOI] [PubMed] [Google Scholar]
- Moreira Filho L.B.de Alexandre, Oliveira C.J., Freitas Neto O.C., de Leon C.M., Saraiva M., Andrade M.F., White B., Givisiez P.E. Intra-amnionic threonine administered to chicken embryos reduces Salmonella Enteritidis cecal counts and improves post hatch intestinal development. J. Immunol. Res. 2018;2018 doi: 10.1155/2018/9795829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyyarikkandy M.S., Amalaradjou M.A. Lactobacillus bulgaricus, Lactobacillus rhamnosus and Lactobacillus paracasei attenuate Salmonella Enteritidis, Salmonella Heidelberg and Salmonella Typhimurium colonization and virulence gene expression in vitro. Int. J. Mol. Sci. 2017;18:2381. doi: 10.3390/ijms18112381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya F.J., Olson E. MEF2: a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr. Opin. Cell Biol. 1999;11:683–688. doi: 10.1016/s0955-0674(99)00036-8. [DOI] [PubMed] [Google Scholar]
- O'Dea E.E., Fasenko G.M., Allison G.E., Korver D.R., Tannock G.W., Guan L.L. Investigating the effects of commercial probiotics on broiler chick quality and production efficiency. Poult. Sci. 2006;85:1855–1863. doi: 10.1093/ps/85.10.1855. [DOI] [PubMed] [Google Scholar]
- Park J.H., Kim I.H. Supplemental effect of probiotic Bacillus subtilis B2A on productivity, organ weight, intestinal Salmonella microflora, and breast meat quality of growing broiler chicks. Poult. Sci. 2014;93:2054–2059. doi: 10.3382/ps.2013-03818. [DOI] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Soglia F., Cavani C. Meat quality in fast-growing broiler chickens. World's Poult. Sci. J. 2015;71:363–374. [Google Scholar]
- Piestun Y., Yahav S., Halevy O. Thermal manipulation during embryogenesis affects myoblast proliferation and skeletal muscle growth in meat-type chickens. Poult. Sci. 2015;94:2528–2536. doi: 10.3382/ps/pev245. [DOI] [PubMed] [Google Scholar]
- Reed S.A., Raja J.S., Hoffman M.L., Zinn S.A., Govoni K.E. Poor maternal nutrition inhibits muscle development in ovine offspring. J. Anim. Sci. Biotechnol. 2014;5:1–11. doi: 10.1186/2049-1891-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawosz F., Pineda L., Hotowy A., Hyttel P., Sawosz E., Szmidt M., Niemiec T., Chwalibog A. Nano-nutrition of chicken embryos. The effect of silver nanoparticles and glutamine on molecular responses, and the morphology of pectoral muscle. Baltic J. Comp. Clin. Syst. Biol. 2012;2:29–45. [Google Scholar]
- Scheuermann G.N., Bilgili S.F., Hess J.B., Mulvaney D.R. Breast muscle development in commercial broiler chickens. Poult. Sci. 2003;82:1648–1658. doi: 10.1093/ps/82.10.1648. [DOI] [PubMed] [Google Scholar]
- Sklan D., Heifetz S., Halevy O. Heavier chicks at hatch improves marketing body weight by enhancing skeletal muscle growth. Poult. Sci. 2003;82:1778–1786. doi: 10.1093/ps/82.11.1778. [DOI] [PubMed] [Google Scholar]
- Stasiak K., Slawinska A., Bogucka J. Effects of probiotics, prebiotics and synbiotics injected in ovo on the microstructure of the breast muscle in different chicken genotypes. Animals. 2021;11:2944. doi: 10.3390/ani11102944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tako E., Ferket P.R., Uni Z. Effects of in ovo feeding of carbohydrates and beta-hydroxy-beta-methylbutyrate on the development of chicken intestine. Poult. Sci. 2004;83:2023–2028. doi: 10.1093/ps/83.12.2023. [DOI] [PubMed] [Google Scholar]
- Tavaniello S., Mucci R., Stadnicka K., Acaye O., Bednarczyk M., Maiorano G. Effect of in ovo administration of different synbiotics on carcass and meat quality traits in broiler chickens. Poult. Sci. 2019;98:464–472. doi: 10.3382/ps/pey330. [DOI] [PubMed] [Google Scholar]
- Uni Z., Ferket P.R. U.S. Patent and Trademark Office; 2003. Enhancement of Development of Oviparous Species by In Ovo Feeding (U.S. Patent No. 6,592,878)https://patents.google.com/patent/US6592878B2/en Accessed Jan. 2023. [Google Scholar]
- Velleman S.G. Muscle development in the embryo and hatchling. Poult. Sci. 2007;86:1050–1054. doi: 10.1093/ps/86.5.1050. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., McFarland D.C. In: Pages 379–402 in Sturkie's Avian Physiology. Scanes Colin G., editor. Elsevier; Boston, MA: 2015. Skeletal muscle. [Google Scholar]
- Wen C., Jiang X.Y., Ding L.R., Wang T., Zhou Y.M. Effects of dietary methionine on growth performance, meat quality and oxidative status of breast muscle in fast-and slow-growing broilers. Poult. Sci. 2017;96:1707–1714. doi: 10.3382/ps/pew432. [DOI] [PubMed] [Google Scholar]
- Wilkinson, S. J., and T. A. Scott. 2006. Preliminary results of in ovo strategies to increase breast meat yield in broiler chickens. Pages 206–209 in Proceedings of the 18th Australian Poultry Science Symposium, Sydney, New South Wales, Australia, 20–22 February 2006. Poultry Research Foundation.
- Wu H., Sun H., Ma C., Lian L., Lu L., Xu L., Xu L. Effects of maternal dietary energy restriction on breast muscle fibre development in the offspring of broiler breeders. Anim. Biosci. 2021;34:1829. doi: 10.5713/ab.20.0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Alcocer H.M., Gravely M.E., Turner K.K., Gonzalez J.M. 529 Late-breaking: effects of in ovo injection of high yield broilers with nicotinamide riboside on pectoralis major morphometrics, muscle fiber density, and mRNA expression. J. Anim. Sci. 2021;99:26. [Google Scholar]
- Xu X., Jackson A.R., Gonzalez J.M. The effects of in ovo nicotinamide riboside dose on broiler myogenesis. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit P.S., Partridge T.A., Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J. Histochem. Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- Zhao M.M., Gao T., Zhang L., Li J.L., Lv P.A., Yu L.L., Gao F., Zhou G.H. Effects of in ovo feeding of creatine pyruvate on the hatchability, growth performance and energy status in embryos and broiler chickens. Animals. 2017;11:1689–1697. doi: 10.1017/S1751731117000374. [DOI] [PubMed] [Google Scholar]