Abstract
Environmental exposures during early life are important for animals’ intestinal microbiota composition and their production performance. This experiment investigated the growth performance, hematology parameters, jejunal morphology, and cecal microbiota of broiler chicks as affected by exogenous factors from the aspects of drinking water quality and dietary manipulation. A total of 480-day-old broiler chicks (Arbor acre; 41.59 ± 0.88 g) were randomly assigned into 4 groups (CON, HWGM, CA, CAHWGM). Each group had 6 replicates with 20 birds per replicate. Broiler chicks in CON group were fed with basal diet and drank normal drinking water; in HWGM group were fed with basal diet supplemented with 1.5g/kg herbal extract blend (hops, grape seed, and wheat germ) and drank normal drinking water; in CA group were fed with basal diet and drank sodium dichlorocyanurate (50 mg/L) treated-drinking water; in CAHWGM group were fed with basal diet supplemented with 1.5 g/kg herbal extract blend and drank chlorinated drinking water. The experimental period was 42 d. We found that broiler chicks drank chlorinated drinking water led to an increase in body weight gain and feed efficiency during d 22 to 42 and 1 to 42, as well as a decrease in cecal Dysgonomonas and Providencia abundance. Dietary supplementation of herbal extract blend increased cecal Lactobacillus and Enterococcus abundance, whereas decreased Dysgonomonas abundance. Moreover, we observed that cecal Dysgonomonas abundance synergistically decreased by treating drinking water with sodium dichlorocyanurate and supplementing herbal extract blend to the diet. Therefore, results obtained in this study indicated that providing chlorinated drinking water is an effective strategy to improve the growth performance of broiler chicks by regulating intestinal microbiota. Additionally, dietary supplementation of herbal extract blend alone or combined with chlorinated drinking water is able to regulate cecal microbiota.
Key words: hop, wheat germ, grape seed, 16S rRNA, water quality
INTRODUCTION
Intestinal microbiota composition of animals is closely related to the environmental exposures during early life (Ballou et al., 2016). Any exogenous factor exposed in this crucial period will affect the performance of animals. Supplementing exogenous additives and/or improving drinking water quality are effective measures to optimize the production performance of broiler chicks (Guo et al., 2022; Dang et al., 2023a).
With the emergence of antibiotic-resistant bacteria, the use of antibiotics is gradually banned in animal husbandry. Herb-derived additives with antioxidant properties have been widely studied for developing a suitable alternative to antibiotics (Dang et al., 2022a). Supplementing herb-derived additives to the diet of animals has extensive positive effects on growth performance, intestinal health, immune response, and productive performance (Rafeeq et al., 2023).
Hops is a perennial herbaceous vine, which is famous for its application in beer brewing. When used in poultry husbandry, its supplementation can control the proliferation of pathogens in the intestine (Bortoluzzi et al., 2014), improve the growth performance (Vecchi et al., 2021), reduce the production of peroxide concentration in vivo (Tamizi Jooneghani et al., 2016), promote the colonization of beneficial bacteria in the intestine (Michalczuk et al., 2021), and change the intestinal morphology (Ząbek et al., 2020).
Wheat is an important cereal for human nutrition. Wheat germ is an important by-product in the cereal industry. Wheat germ extract has been demonstrated to improve the growth performance of broiler chicks (Arshad et al., 2013). Oil extracted from wheat germ can improve the damage of the liver and small intestine induced by carbon tetrachloride (Saleh, 2016). Wheat germ fermentation products have also been proved to improve growth performance, carcass traits, and antibody titer of broiler chicks (kany et al., 2017), and can effectively against the infection of Mycoplasma gallisepticum (Stipkovits et al., 2004).
Grape seed is a by-product in the juice and wine industry. Dietary supplementation of grape seed extract has been reported to regulate the composition of intestinal microbiota (Qamar et al., 2020), regulate the antioxidant status of the body (Qamar et al., 2020), improve immune response (Farahat et al., 2017), enhance production performance (Grandhaye et al., 2020), and increase nutrient digestibility (Brenes et al., 2010).
Therefore, all of the hops, wheat germ, and grape seed have the potential to improve the growth and production performance of broiler chicks. However, no study has investigated the effects of the blend of these herbal extracts on the performance of broiler chicks. It is reported that herbal extract blend can play more active effect than the use of herbs alone (Williamson, 2001).
Additionally, water is essential for organisms to maintain basic metabolic activities, and it is provided through the water pipe. Feed pipes are distributed to each cage to ensure the supply of drinking water. The quality of drinking water is undoubtedly related to the health and performance of animals (Maes et al., 2019). In routine production practice, some strategies are commonly adopted to improve water quality, of which acidified drinking water and magnetized drinking water are effective strategies to inhibit the growth of pathogens in drinking water (El-Katcha et al., 2018; Guo et al., 2022). Drinking animals with above treated-water can improve their growth and production performance, and decrease the spread of pathogens (Van Bunnik et al., 2012; Jassim and Aqeel, 2017; El-Katcha et al., 2018; Guo et al., 2022; Zhang et al., 2022a). Compared with the above methods, chlorinated drinking water is a common method in animal husbandry because it is easy to disperse, low in cost, and has a wide antimicrobial spectrum (Tsai et al., 1992; Maes et al., 2019). Its safety and effectiveness have been fully documented (Byrd et al., 2003; Barreiro et al., 2012). Sodium dichlorocyanurate has been approved by the United States Environmental Protection Agency and the WHO for the routine treatment of drinking water. Therefore, sodium dichlorocyanurate is a promising disinfectant for drinking water (Clasen and Edmondson, 2006).
To date, no study has investigated the effects of herbal extract blend (hop, wheat germ, and grape seed) supplementation plus chlorinated drinking water on performance of broiler chicks. We hypothesized that herbal extract blend supplementation and drinking water treated with sodium dichlorocyanurate synergistically improved intestinal morphology and intestinal microbiota of broiler chicks, thus enhanced growth performance. The objective of this study was to investigate the growth performance, hematology parameters, jejunal morphology, and cecal microbiota of broiler chicks as affected by exogenous factors from the aspects of drinking water quality and dietary manipulation.
MATERIALS AND METHODS
Experimental Design
A total of 480-day-old broiler chicks (Arbor acre; 41.59 ± 0.88 g) were randomly assigned into 4 groups (CON, HWGM, CA, CAHWGM). Each group had 6 replicates with 20 birds per replicate. Broiler chicks in CON group were fed with basal diet (nutrient requirements meet the recommendation of NRC, 1994) (Table 1), and drank normal drinking water; in HWGM group were fed with basal diet supplemented with 1.5 g/kg herbal extract blend (hop, grape seed, and wheat germ) and drank normal drinking water; in CA group were fed with basal diet and drank sodium dichlorocyanurate (50 mg/L) treated-drinking water; in CAHWGM group were fed with basal diet supplemented with 1.5 g/kg herbal extract blend and drank chlorinated drinking water. The dose of herbal extract blend was determined by our previous study (Zou et al., 2023). The experimental period was 42 d. Herbal extract blend composed in hop, wheat germ, and grape seed as well as sodium dichlorocyanurate were purchased from Liaoning Kaiwei Biotechnology Co., Ltd (Jinzhou, China). The Experimental protocol and the process were approved and supervised by the Animal Care and Use Committee of Jinzhou Medical University (Jinzhou, China).
Table 1.
Composition and nutrient levels of the experimental basal diet, (%, as-fed basis).
| Ingredients, % | d 1–12 | d 22–42 |
|---|---|---|
| Corn | 60.40 | 64.05 |
| Dicalcium phosphate | 1.40 | 1.30 |
| Soybean meal | 34.40 | 30.00 |
| Limestone | 1.21 | 1.12 |
| NaCl | 0.25 | 0.25 |
| Soy oil | 1.00 | 2.00 |
| Choline chloride | 0.05 | 0.05 |
| Lys | 0.08 | 0.10 |
| DL-Met | 0.21 | 0.13 |
| Mineral and vitamin mixture1 | 1.00 | 1.00 |
| Total | 100.00 | 100.00 |
| Analyzed composition, % | ||
| Metabolizable energy, MJ/kg | 12.14 | 12.51 |
| Crude protein | 21.17 | 19.24 |
| Calcium | 0.92 | 0.87 |
| Available phosphorus | 0.38 | 0.36 |
| Lysine | 1.29 | 1.15 |
| Methionine | 0.67 | 0.48 |
| Methionine + Cysteine | 1.00 | 0.72 |
Provided per kg of complete diet: vitamin A 5,000 IU; vitamin D3 1,000 IU; vitamin E 75 IU; vitamin K3 18.80 mg; vitamin B1, 9.80 mg; vitamin B2 28.80 mg; vitamin B6 19.60 mg; vitamin B12 0.10 mg; biotin 2.50 mg; folic acid 4.90 mg; pantothenic acid 58.80 mg; niacin 196 mg; Zn 37.60 mg; Mn 50 mg; Fe 40 mg; Cu 4 mg; I 0.2 mg; Se 0.2 mg.
All birds were housed in the same environment. They were raised in 3-floor battery cages (1.25 × 0.98 × 0.47 m/cage) and placed on a mesh grate dotted with hexagonal grids. The stocking density of birds not exceed 33 kg/m2 at the slaughtering time (d 42) (Regulation of the Ministry of Agriculture and Rural Development, Dz. U. 2010.56.344; MRiRW, 2010). Each cage was equipped with nipple drinkers and feed hoppers. The lighting program was 24 h a d for the first week (d 1–7) and then reduced to 16 h of light and 8 h of dark during d 7 to 42. The temperature of the room was started at 33°C and reduced by 3°C every week up to 22°C. Relative humidity was 65%. According to the mean kg of body weight of broiler chicks, during the experimental period, the volume of air exchange increase from 0 m3 per hour to 4.5 m3 to ensure the concentration of carbon dioxide and ammonia lower than 3,000 ppm and 20 ppm, respectively. Birds were monitored twice a day and excreta located on excreta collection tray was cleared daily. Birds had free access to get feed and water during the experiment.
Growth Performance
Replicate cage-based body weight was measured on d 1, 21, and 42 to calculate the body weight gain (BWG). Feed intake was measured weekly based on the replicate cage to calculate the feed intake (FI). Feed efficiency was calculated as the ratio of gain to feed.
Hematology Parameters
On d 21 and 42, 24 birds (4 birds per replicate cage) were randomly selected from each treatment according to the mean body weight of broiler chick flock for blooding. Blood samples were collected from the wing vein using a sterile syringe and stored at 4°C. Blood samples were centrifuged (3000 × g) for 15 min at 4 °C to obtain serum samples and then stored at -20 °C until analysis. The Biuret method of Armstrong and Carr (1964) was used to determine the concentration of total protein. The concentrations of albumin were assayed using colorimetric methods (Hill, 1985). Globulin concentration was obtained by subtracting albumin concentration from total protein concentration. Serum alanine aminotransferase and creatinine concentrations were measured using a fully automated biochemical analyzer (SMT-120VP, Chengdu Seamaty Technology Co., Ltd, Chengdu, P. R. China) with specific kits (Seamaty AW00668, Chengdu Polytech Biological Technology Co., Ltd, Chengdu, P. R. China). Company-provided calibrators and quality controls were run before each measurement in samples.
Jejunal Morphology
On d 42, blood sampling birds (1 bird was selected from each replicate cage according to the mean body weight of broiler chicks’ flock, in total of 6 birds per group) were then slaughtered for measuring the jejunal morphology according to the study of Dang et al. (2023b). Samples of jejunum were cut into small pieces and fixed with 10% neutral buffered formalin for 12 h, followed by dehydration in increasing concentrations of alcohol (70, 80, 90, 95, and 100%) and xylene. Therefore, samples were embedded in paraffin and stored in an oven at 60°C. Twelve hours later, samples were removed from the oven and histological cassettes. Fragments were placed in “paper boxes” and covered with paraffin. After the paraffin solidified into blocks, the “papers” were removed and the blocks were kept under refrigeration until the cuts were realized.
Serial tissue sections (5 specimens with 3μm thickness) were excised using a cryostat. After sectioning, put the paraffin section ribbon on the coating slide glass. Dried slides were kept in oven at 60°C for 2 h to eliminate any excess paraffin. The next step consisted of paraffin removal and slide hydration, using xylene, different concentrations of ethanol. Samples were then stained following the hematoxylin and eosin staining protocol (Felício et al., 2013). Samples were then dehydrated again and mounted. An optical microscope (Olympus, BX53F, Tokyo, Japan) was used to measure the values of villus height and crypt depth at 10X magnification. A minimum of 10 measurements per slide were made for each parameter and averaged into one value.
Cecal Microbiota
Cecal contents were collected from the above jejunal morphology-sampling birds (1 bird per replicate cage, in total of 6 birds per group) and were placed in a sterile bag, and stored at -20°C until DNA extraction. A magnetic Soil and Stool DNA kit (cat# DP712, TIANGEN Biotech Co., Ltd., Beijing, China) was used for extracting total DNA from the cecal content (0.5 g). The concentration and purity of the extracted DNA were determined using a Qubit 2.0 spectrophotometer (Invitrogen, Carlsbad, CA) and 1% (w/v) agarose gel electrophoresis. The DNA samples were diluted with sterile water to a concentration of 1 ng/μL and stored at −20°C before analysis. Then, quantitative PCR was used to amplify the V3–V4 hypervariable regions of extracted DNA with specific full length universal forward (5′-ACTCCTACGGGAGGCAGCA-3′) and reverse primers (5′-GGACTACGVGGGTVVTCTAAT-3′). Subsequently, a Qiagen Gel Extraction Kit (cat# 28706, Qiagen, Germany) was used to further purify the PCR products. Simultaneously, the purity of the PCR mixture was evaluated using a Qubit 2.0 dsDNA HS Assay Kit (cat# Q32854, Invitrogen). The 16S rRNA gene sequencing was performed to analyze the cecal microbial community structures using the NovaSeq 6000 platform (Illumina, San Diego, CA) in Novogene Bioinformatics Co., Ltd. (Tianjin, China). Raw data were obtained by cutting low-quality reads using Cutadapt software version 1.9.1. Chimeric sequences were trimmed by alignment and detection. High-quality reads were clustered into operational taxonomic units (OTUs) at 97% sequence identity using Uparse v7.0.1001. The taxonomic assignment of the representative sequences was performed using QIIME v1.9.1. The alpha-diversity was estimated by Chao1, Faith_pd, Observed_species, Shannon, and Simpson indices. The beta-diversity was estimated by the P-value of Anosim, Permanova, and Permdisp analyses.
Statistical Analysis
The PROC UNIVARIATE procedure of SAS software (version 9.4) was used to test the normality of data. Data of growth performance, hematology parameters, jejunal morphology, alpha-diversity of cecal microbiota, differential bacteria in cecal microbiota were analyzed using the PROC MIXED procedure of SAS software (version 9.4), and the beta-diversity of cecal microbiota was analyzed using the Anosim, Permanova, and Permdisp analyses. The experimental birds were assigned to 4 treatments and 6 replicates in a completely randomized design, where each replicate cage was considered an individual experimental unit. Tukey's post hoc test was used to separate means among treatments. Variability in the data was expressed as the standard error of means (SEM). Results were considered significant at P < 0.05.
RESULTS
Counting bacterial load of drinking water samples by plate method indicated that drinking water treated with sodium dichlorocyanurate had lower colony number (68,000 vs. 2.5 colony-forming unit [CFU]), coliform bacteria (1042.25 vs. 4 CFU), mould colonies (30 vs. 0 CFU) than normal drinking water in the quantity. However, the pH of drinking water did not differ.
Effects of broiler chicks’ growth performance as affected by drinking water disinfection and/or herbal extract blend supplementation were presented in Table 2. Broiler chicks drank chlorinated drinking water increased BWG during d 22 to 42 (P = 0.03) and 1 to 42 (P = 0.02) as well as feed efficiency during d 22 to 42 (P = 0.04) and 1 to 42 (P = 0.03), but did not affect FI and mortality rate. Result of Tukey's post hoc test indicated that the chlorination of the drinking water led to an increase in BWG during d 22 to 42 (P < 0.05) and 1 to 42 (P < 0.05) as well as feed efficiency during d 22 to 42 (P < 0.05) and 1 to 42 (P < 0.05) in comparison to the control group. However, herbal extract blend supplementation did not affect growth performance parameters, and no synergistic effects between drinking water disinfection and herbal extract blend supplementation have been observed.
Table 2.
Growth performance of broiler chicks as affected by drinking water disinfection and/or herbal extract blend supplementation.
| W1 | - |
+ |
SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|---|
| H2 | - | + | - | + | W | H | W × H | |
| BWG, g/d | ||||||||
| d 1–21 | 692.32 | 735.57 | 725.91 | 724.18 | 11.061 | 0.35 | 0.07 | 0.06 |
| d 22–42 | 1497.87b | 1596.07a | 1602.69a | 1595.54ab | 28.573 | 0.03 | 0.18 | 0.12 |
| d 1–42 | 2190.18b | 2331.64a | 2328.59a | 2319.72ab | 32.846 | 0.02 | 0.10 | 0.07 |
| FI, g/d | ||||||||
| d 1–21 | 1044.47 | 1046.79 | 1047.92 | 1050.07 | 2.448 | 0.24 | 0.31 | 0.97 |
| d 22–42 | 2768.47 | 2770.87 | 2772.14 | 2775.07 | 1.631 | 0.10 | 0.10 | 0.87 |
| d 1–42 | 3812.94 | 3817.66 | 3820.06 | 3825.14 | 3.274 | 0.13 | 0.09 | 0.95 |
| Feed efficiency | ||||||||
| d 1–21 | 0.663 | 0.703 | 0.693 | 0.690 | 0.010 | 0.44 | 0.10 | 0.06 |
| d 22–42 | 0.541b | 0.576a | 0.578a | 0.575a,b | 0.011 | 0.04 | 0.19 | 0.13 |
| d 1–42 | 0.574b | 0.611a | 0.610a | 0.607a,b | 0.009 | 0.03 | 0.12 | 0.07 |
| Mortality rate, % | ||||||||
| d 1–21 | 0.026 | 0.040 | 0.023 | 0.029 | 0.008 | 0.43 | 0.26 | 0.62 |
| d 22–42 | 0.064 | 0.082 | 0.070 | 0.044 | 0.021 | 0.44 | 0.85 | 0.37 |
| d 1–42 | 0.045 | 0.061 | 0.047 | 0.036 | 0.013 | 0.38 | 0.87 | 0.41 |
Abbreviations: BWG, body weight gain; FI, feed intake.
Different superscripts within a row indicate a significant difference (P < 0.05).
W was defined as drinking water treated with 50 mg/L sodium dichlorocyanurate.
H was defined as dietary supplementation of 1.5 g/kg herbal extract blend (hops, grape seed, and wheat germ).
The contents of serum albumin, total protein, globulin, alanine aminotransferase, and creatinine did not differ among groups (Table 3).
Table 3.
Hematology parameters of broiler chicks as affected by drinking water disinfection and/or herbal extract blend supplementation.
| W1 | - |
+ |
SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|---|
| H2 | - | + | - | + | W | H | W × H | |
| Albumin, g/L | ||||||||
| d 21 | 10.87 | 10.80 | 10.38 | 10.53 | 0.290 | 0.26 | 0.88 | 0.69 |
| d 42 | 12.33 | 12.00 | 12.32 | 11.67 | 0.743 | 0.81 | 0.54 | 0.84 |
| Total protein, g/L | ||||||||
| d 21 | 24.08 | 24.43 | 23.10 | 22.92 | 0.934 | 0.25 | 0.92 | 0.76 |
| D 42 | 32.80 | 30.27 | 32.80 | 28.50 | 2.613 | 0.75 | 0.20 | 0.73 |
| Globulin, g/L | ||||||||
| d 21 | 13.18 | 13.63 | 12.73 | 12.38 | 0.806 | 0.35 | 0.95 | 0.61 |
| d 42 | 20.45 | 18.27 | 20.50 | 16.52 | 2.115 | 0.72 | 0.14 | 0.65 |
| Alanine aminotransferase, U/L | ||||||||
| d 21 | 7.00 | 5.67 | 8.00 | 5.67 | 1.193 | 0.64 | 0.20 | 0.71 |
| d 42 | 7.67 | 8.00 | 9.50 | 7.33 | 0.967 | 0.57 | 0.35 | 0.21 |
| Creatinine, umol/L | ||||||||
| d 21 | 13.10 | 13.10 | 15.83 | 12.42 | 0.942 | 0.17 | 0.16 | 0.16 |
| d 42 | 11.20 | 10.62 | 10.78 | 10.98 | 0.586 | 0.97 | 0.71 | 0.45 |
W was defined as drinking water treated with 50 mg/L sodium dichlorocyanurate.
H was defined as dietary supplementation of 1.5 g/kg herbal extract blend (hops, grape seed, and wheat germ).
Parameters of jejunal morphology including villus height, crypt depth, and their ratio were not affected by treating water with chlorate and supplementing herbal extract blend to the diet (Table 4).
Table 4.
Jejunal morphology of broiler chicks as affected by drinking water disinfection and/or herbal extract blend supplementation.
| W1 | - |
+ |
SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|---|
| H2 | - | + | - | + | W | H | W × H | |
| Villus height, μm | 1363.39 | 1471.33 | 1564.69 | 1590.45 | 85.243 | 0.14 | 0.35 | 0.56 |
| Crypt depth, μm | 169.54 | 198.05 | 186.06 | 208.77 | 11.920 | 0.21 | 0.09 | 0.83 |
| Villus to crypt ratio | 7.99 | 7.54 | 8.49 | 7.78 | 0.467 | 0.52 | 0.12 | 0.72 |
W was defined as drinking water treated with 50 mg/L sodium dichlorocyanurate.
H was defined as dietary supplementation of 1.5 g/kg herbal extract blend (hops, grape seed, and wheat germ).
Chlorinated drinking water and herbal extract blend supplementation had no significant effects on alpha-diversity indexes (Table 5).
Table 5.
Alpha-diversity in cecal microbiota of broiler chicks as affected by drinking water disinfection and/or herbal extract blend supplementation.
| W1 | - |
+ |
SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|---|
| H2 | - | + | - | + | W | H | W × H | |
| Chao1 | 979.39 | 1215.10 | 1070.39 | 1355.19 | 105.314 | 0.17 | 0.07 | 0.85 |
| Faith_pd | 48.58 | 51.33 | 45.89 | 53.46 | 3.002 | 0.90 | 0.18 | 0.52 |
| Observed_species | 906.47 | 1051.48 | 909.13 | 1093.13 | 79.920 | 0.70 | 0.13 | 0.85 |
| Shannon | 7.65 | 7.73 | 7.38 | 7.57 | 0.142 | 0.11 | 0.41 | 0.74 |
| Simpson | 0.99 | 0.99 | 0.98 | 0.99 | 0.002 | 0.22 | 0.68 | 0.53 |
W was defined as drinking water treated with 50 mg/L sodium dichlorocyanurate.
H was defined as dietary supplementation of 1.5 g/kg herbal extract blend (hops, grape seed, and wheat germ).
Moreover, the results of Anosim, Permanova, and Permdisp analyses indicated that drinking water disinfection and/or herbal extract blend supplementation did not affect the beta-diversity of cecal microbiota (Table 6).
Table 6.
The P-value of beta-diversity in cecal microbiota of broiler chicks as affected by drinking water disinfection and/or herbal extract blend supplementation.
| Anosim | Permanova | Permdisp |
|---|---|---|
| 0.30 | 0.22 | 0.20 |
Cecal microbiota analyzed by 16S rRNA revealed that chlorinated drinking water decreased cecal Dysgonomonas (P < 0.01) and Providencia (P = 0.02) abundance. Result of Tukey's post hoc test indicated that the chlorination of the drinking water led to a decrease in cecal Dysgonomonas (P < 0.05) and Providencia (P < 0.05) abundance in comparison to the control group. Herbal extract blend supplementation increased cecal Lactobacillus (P = 0.01) and Enterococcus (P = 0.03) abundance, whereas decreased cecal Dysgonomonas (P < 0.01) abundance. Result of Tukey's post hoc test indicated that the chlorination of the drinking water led to an increase in cecal Lactobacillus (P < 0.05) and Enterococcus (P < 0.05) abundance, as well as a decrease in cecal Dysgonomonas (P < 0.05) abundance in comparison to the control group. Additionally, drinking water disinfection and herbal extract blend supplementation synergistically reduced the abundance of Dysgonomonas in cecum (P < 0.01) (Table 7).
Table 7.
Differential bacteria in cecal microbiota of broiler chicks as affected by drinking water disinfection and/or herbal extract blend supplementation.
| W1 | - |
+ |
SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|---|
| H2 | - | + | - | + | W | H | W × H | |
| Lactobacillus | 0.016b | 0.038a | 0.022b | 0.062a | 0.011 | 0.24 | 0.01 | 0.33 |
| Enterococcus | 0.002b | 0.003a | 0.001a,b | 0.004a | 0.001 | 0.96 | 0.03 | 0.40 |
| Dysgonomonas | <.001a | 0b | 0b | 0b | <.001 | <.01 | <.01 | <.01 |
| Providencia | <.001a | <.001a,b | 0b | 0b | <.001 | 0.02 | 0.10 | 0.10 |
Different superscripts within a row indicate a significant difference (P < 0.05).
W was defined as drinking water treated with 50 mg/L sodium dichlorocyanurate.
H was defined as dietary supplementation of 1.5 g/kg herbal extract blend (hops, grape seed, and wheat germ).
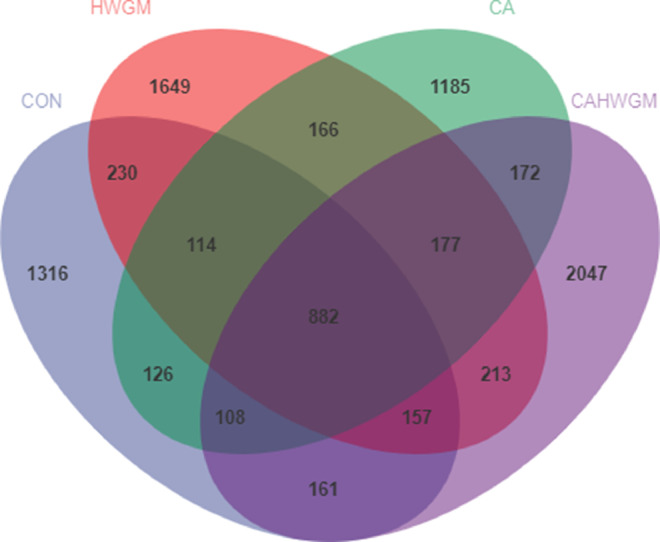
Venn diagram of cecal microbiota revealed that a total of 882 bacterial OTUs were shared among groups, 1,316 unique OTUs were presented in cecal content from broiler chicks fed with basal diet and drank normal drinking water, 1,649 unique OTUs were presented in cecal content from broiler chicks fed with basal diet supplemented with herbal extract blend and drank normal drinking water, 1,185 unique OTUs were presented in cecal content from broiler chicks fed with basal diet and drank chlorinated drinking water, and 2,047 unique OTUs were presented in cecal content from broiler chicks fed with basal diet supplemented with herbal extract blend and drank chlorinated drinking water (Figure 1).
Figure 1.
Venn diagram for cecal microbiota of broiler chicks.
Group CON was defined as the cecal content sample from broiler chicks fed with basal diet and drank normal drinking water.
Group HWGM was defined as the cecal content sample from broiler chicks fed with basal diet supplemented with 1.5g/kg herbal extract blend (hops, grape seed, and wheat germ) and drank normal drinking water.
Group CA was defined as the cecal content sample from broiler chicks fed with basal diet and drank chlorinated drinking water.
Group CAHWGM was defined as the cecal content sample from broiler chicks fed with basal diet supplemented with 1.5 g/kg herbal extract blend and drank chlorinated drinking water.
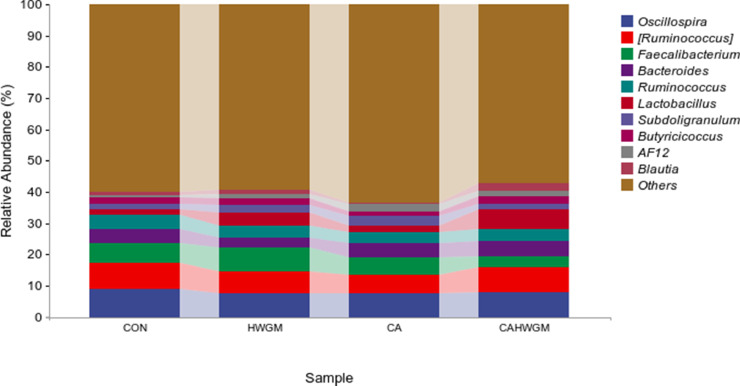
The 10 most abundant bacteria in cecal content samples at genus level were mainly involved in Oscillospira, [Ruminococcus], Faecalibacterium, Bacteroides, Ruminococcus, Lactobacillus, Subdoligranulum, Butyricicoccus, AF12, and Blautia (Figure 2).
Figure 2.
The 10 most abundant bacteria from cecal microbiota of broiler chicks.
Group CON was defined as the cecal content sample from broiler chicks fed with basal diet and drank normal drinking water.
Group HWGM was defined as the cecal content sample from broiler chicks fed with basal diet supplemented with 1.5g/kg herbal extract blend (hops, grape seed, and wheat germ) and drank normal drinking water.
Group CA was defined as the cecal content sample from broiler chicks fed with basal diet and drank chlorinated drinking water.
Group CAHWGM was defined as the cecal content sample from broiler chicks fed with basal diet supplemented with 1.5 g/kg herbal extract blend and drank chlorinated drinking water.
DISCUSSION
Dietary manipulation or water quality improvement are effective strategies to improve the growth performance of broiler chicks (Alipour et al., 2015; Guo et al., 2022). However, in this study, dietary supplementation of herbal extract blend had no significant effects on growth performance parameters, but chlorinated drinking water increased broiler chicks’ BWG and feed efficiency. As an important component constituting the additive used in this study, hops extract (Vecchi et al., 2021), wheat germ (Ellakany et al., 2017), and grape seed extract (Ao and Kim, 2020) have been demonstrated to be able to improve the growth performance of birds when added to the diet alone. Although it is commonly believed that herbal extract blend can play more active effect than the use of herbs alone (Williamson, 2001). However, results are not always good, Ahmed et al. (2016) noted that dietary supplementation of 30% pomegranate peel extract, 4.5% ginkgo biloba leaf power, and 0.5% licorice root powder had negative effects on growth performance of animals. Therefore, supplementing different herbs together to the diet cannot be considered as an effective measure to improve the growth performance. The results observed in this study suggested that dietary supplementation of the blend of hops, grape seed, and wheat germ did not improve the growth performance of broiler chicks, but also did not generate any adverse effects. Water quality is also an important factor affecting the health and performance of animals (Maes et al., 2019). The objective of drinking water disinfection is to reduce bacterial load (El-Katcha et al., 2018; Guo et al., 2022). Oral intake of bacteria will directly affect the growth performance of animals (Dang et al., 2023a). El-Katcha et al. (2018) provided broiler chicks with acidified drinking water and/or magnetized drinking water and found that this measure was able to ameliorate the growth performance impairment induced by Salmonella Enteritidis challenge. Guo et al. (2022) reported that supplementing acidifier to the drinking water of broiler chicks tend to increase their BWG and feed efficiency. Our results also demonstrated that chlorinated drinking water is an effective measure to improve the BWG and feed efficiency of broiler chicks.
The physiology of the intestinal tract is the cornerstone for ensuring high growth performance (Khan and Islam, 2012; Pluske, 2012), which is reflected in villus height and crypt depth. Increasing crypt depth and villus height will cause the absorption of more nutrients into the bloodstream, thus improving growth and feed efficiency (Lisnahan and Nahak, 2020). However, no morphological changes in the intestinal tract caused by treating drinking water with chlorate and supplementing herbal extract blend to the diet have been observed in this study. The results obtained in this study against the results of Sadati et al. (2022) and Saki et al. (2012). They reported that broiler chicks received acidified drinking water or herbal extract-contained diet had positive effects on intestinal morphology. However, studies of Guo et al. (2022) and Dang et al. (2020b) supported the results observed in this study, they noted that drinking water disinfection or herbal extract supplementation had no significant effects on intestinal morphology. Different results may be due to different types of water and dietary additives used in the experiments (Eftekhari et al., 2015). As the results observed in this study, we considered that herbal extract blend and chlorate used in this study are not able to improve the intestinal morphology of broiler chicks.
The microbiota present in the intestine plays important role in regulating growth performance. Any factors that regulate the composition and abundance of intestinal microbiota will inevitably affect growth performance. Alpha- and beta- indexes are parameters reflecting the richness and diversity of intestinal microbiota. In this study, we did not observe that dietary supplementation of herbal extract blend and chlorinated drinking water had significant effects on alpha- and beta- indexes, indicating that herbal extract blend supplementation and chlorinated drinking water had no significant effects on the richness and diversity of broiler chicks’ cecal microbiota. Further bacteria composition analysis revealed that drinking water treated with chlorate decreased cecal Dysgonomonas and Providencia abundance. The abundance of Dysgonomonas is positively correlated to the levels of intestinal inflammation/prethrombotic markers (Nakajima et al., 2022). Providencia is also a pathogen, and has been demonstrated to play an etiologic role in acute intestinal diseases as early as 1977 (Kholodkova et al., 1977). Recently, Providencia has been demonstrated to be able to adhere to intestinal epithelial cells and invade cells, which are related to its pathogenicity for intestinal dysfunction (Zhang et al., 2022b). Therefore, we considered that providing broiler chicks with chlorinated drinking water was beneficial to decrease the abundance of harmful bacteria in cecum, and therefore benefited to improve the growth performance. Similarly, Barreiro et al. (2012) reported that drinking water treated with chlorate reduced the pathogens counts in the intestine. Byrd et al. (2003) noted that drinking water administrated with chlorate was able to reduce the contamination of Salmonella in the flock of broiler chick. On the other hand, herbal extract blend supplementation increased the abundance of Lactobacillus and Enterococcus in cecum, whereas decreased the abundance of Dysgonomonas. Lactobacillus and Enterococcus are producers of short-chain fatty acids, which can induce significant anti-inflammatory effects and contribute to the integrity of the intestinal epithelium (De Vrese and Schrezenmeir, 2008; Hati et al., 2019). Lactobacillus and Enterococcus are included as food supplements in several probiotic preparations, that have been proven to be effective and safe (De Vrese and Schrezenmeir, 2008; Zhang et al., 2018). Moreover, Dysgonomonas is a pathogen as described above. Therefore, herbal extract blend supplementation increased the abundance of beneficial bacteria whereas decreased the abundance of harmful bacteria in the cecum, which was confirmed by the studies of Rahimi et al. (2011) and Viveros et al. (2011). The regulatory effects of components constituting the additive used in this study on intestinal microbiota have been reported. Tamizi Jooneghani et al. (2016) found that hops extract supplementation increased ileal Lactobacillus counts of broiler chicks. Michalczuk et al. (2021) observed an increase in the relative abundance of Bifidobacterium in cecum of broiler chicks caused by supplementing hops extract to the diet. Additionally, Viveros et al. (2011) noted that broiler chicks fed the diet supplemented with grape seed extract increased ileal Lactobacillus abundance of broiler chicks. Grandhaye et al. (2020) found an increase in the relative abundance of Bifidobacteriaceae, Lactobacilliaceae, and Lachnospiraceae in the intestine of layers caused by supplementing grape seed extract to the diet. Therefore, herbal extract blend used in this study had a regulatory effect on the cecal microbiota of broiler chicks. Moreover, a synergistic decrease in cecal Dysgonomonas abundance was observed, which indicated that chlorinated drinking water plus herbal extract blend supplementation had regulatory effects on cecal microbiota.
Serum biochemical parameters including albumin, globulin, and total protein can be used as an indicator to indicate nutritional status in vivo. In the present study, chlorinated drinking water and/or herbal extract blend supplementation had no significant effects on the above hematological parameters. However, we observed an improvement in feed efficiency caused by treating drinking water with chlorate. Therefore, it is necessary to understand more about the effect of chlorinated drinking water on nutrient digestibility. Additionally, alanine aminotransferase and creatinine are clinical and regulatory tools for detecting the liver and kidney injury, respectively (Waikar and Bonventre, 2009; Senior, 2012). Chlorate and herbal extract blend used in this study did not affect the concentration of alanine aminotransferase and creatinine in serum, indicating their supplementation have no toxic effects on the liver and kidney.
CONCLUSIONS
This study demonstrated that chlorinated drinking water reduced the abundance of Dysgonomonas and Providencia in the cecum, and therefore beneficial to improve broiler chicks’ BWG and feed efficiency. Herbal extract blend supplementation was able to regulate the composition of cecal microbiota, manifesting in an increase in cecal Lactobacillus and Enterococcus abundance and a decrease in cecal Dysgonomonas abundance. Additionally, we observed that herbal extract blend supplementation and chlorinated drinking water synergistically decreased the abundance of Dysgonomonas in cecum. Therefore, results obtained in this study indicated that providing chlorinated drinking water is an effective strategy to improve the growth performance of broiler chicks by regulating intestinal microbiota. Moreover, dietary supplementation of herbal extract blend alone or combined with chlorinated drinking water is able to regulate the cecal microbiota.
ACKNOWLEDGMENTS
This study is supported by National College Students' innovation and entrepreneurship training projects (S202210160027).
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- Ahmed S.T., Mun H.S., Islam M.M., Ko S.Y., Yang C.J. Effects of dietary natural and fermented herb combination on growth performance, carcass traits and meat quality in grower-finisher pigs. Meat Sci. 2016;122:7–15. doi: 10.1016/j.meatsci.2016.07.016. [DOI] [PubMed] [Google Scholar]
- Alipour F., Hassanabadi A., Golian A., Nassiri-Moghaddam H. Effect of plant extracts derived from thyme on male broiler performance. Poult. Sci. 2015;94:2630–2634. doi: 10.3382/ps/pev220. [DOI] [PubMed] [Google Scholar]
- Ao X., Kim I.H. Effects of grape seed extract on performance, immunity, antioxidant capacity, and meat quality in Pekin ducks. Poult. Sci. 2020;99:2078–2086. doi: 10.1016/j.psj.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong W.D., Carr C.W. E. Burses Publishing Co; Minneapollis, MN: 1964. Physiological Chemistry Laboratory Direction (3rd.) [Google Scholar]
- Arshad M.S., Anjum F.M., Khan M.I., Shahid M., Akhtar S., Sohaib M. Wheat germ oil enrichment in broiler feed with α-lipoic acid to enhance the antioxidant potential and lipid stability of meat. Lipids Health Dis. 2013;12:1–14. doi: 10.1186/1476-511X-12-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou A.L., Ali R.A., Mendoza M.A., Ellis J.C., Hassan H.M., Croom W.J., Koci M.D. Development of the chick microbiome: how early exposure influences future microbial diversity. Front. Vet. Sci. 2016;3:2. doi: 10.3389/fvets.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro F.R., Baraldi-Artoni S.M., Pinto F.R., Barbosa M.M.C., Barbosa J.C., Amaral L.A. Influence of chlorine added to drinking water during the preslaughter feed withdrawal on microbiology and morphology of the broiler gastrointestinal tract. Poult. Sci. 2012;91:2778–2784. doi: 10.3382/ps.2012-02455. [DOI] [PubMed] [Google Scholar]
- Bortoluzzi C., Menten J.F.M., Romano G.G., Pereira R., Napty G.S. Effect of hops β-acids (Humulus lupulus) on performance and intestinal health of broiler chickens. J. Appl. Poult. Res. 2014;23:437–443. [Google Scholar]
- Brenes A., Montoro A.V., Cambrodón I.G., Centeno C., Calixto F.S., Arija I. Effect grape seed extract on growth performance, protein and polyphenol digestibilities, and antioxidant activity in chickens. Spanish J. Agric. Res. 2010;2:326–333. [Google Scholar]
- Byrd J.A., Anderson R.C., Callaway T.R., Moore R.W., Knape K.D., Kubena L.F., Ziprin R.L., Nisbet D.J. Effect of experimental chlorate product administration in the drinking water on Salmonella Typhimurium contamination of broilers. Poult. Sci. 2003;82:1403–1406. doi: 10.1093/ps/82.9.1403. [DOI] [PubMed] [Google Scholar]
- Clasen T., Edmondson P. Sodium dichloroisocyanurate (NaDCC) tablets as an alternative to sodium hypochlorite for the routine treatment of drinking water at the household level. Int. J. Hyg. Environ. Health. 2006;209:173–181. doi: 10.1016/j.ijheh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Dang D.X., Cho S., Wang H., Seok W.J., Ha J.H., Kim I.H. Quercetin extracted from Sophora japonica flower improves growth performance, nutrient digestibility, cecal microbiota, organ indexes, and breast quality in broiler chicks. Anim Biosci. 2022;35:577. doi: 10.5713/ab.21.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang D.X., Choi S.Y., Choi Y.J., Lee J.H., Castex M., Chevaux E., Saronil D., de Laguna F.B., Jimenez G., Kim I.H. Probiotic, araprobiotic, and hydrolyzed yeast mixture supplementation has comparable effects to zinc oxide in improving growth performance and ameliorating post-weaning Diarrhea in weaned piglets. Probiotics Antimicro. Prot. 2023;2023:1–10. doi: 10.1007/s12602-022-10008-8. [DOI] [PubMed] [Google Scholar]
- Dang D.X., Li C.J., Zhou H., Lou Y., Liu X., Li D. Development of small intestine and sugar absorptive capacity in goslings during pre-and post-hatching periods. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang D.X., Yun K.S., Kim I.H. Achyranthes Japonica Nakai root extract supplementation improves apparent nutrient digestibility, caecum microbiota, and excreta gas emission in broiler chicks. Canadian J. Anim. Sci. 2022;102:266–273. [Google Scholar]
- De Vrese M., Schrezenmeir A.J. In: Page 1-66 in Food Biotechnology. Stahl U., Donalies U.E. B., Nevoigt E., editors. Springer Berlin; Heidelberg, Germany: 2008. Probiotics, prebiotics, and synbiotics. [Google Scholar]
- Eftekhari A., Rezaeipour V., Abdullahpour R. Effects of acidified drinking water on performance, carcass, immune response, jejunum morphology, and microbiota activity of broiler chickens fed diets containing graded levels of threonine. Livestock Sci. 2015;180:158–163. [Google Scholar]
- El-Katcha M., Soltan M.A., El-Shobokshy S.A., Kasser M. Impact of water acidification or magnetic treatment on growth performance, health and oxidative status of broiler chicks challenged by Salmonella Enteritidis. Alex. J. Vet. Sci. 2018;59:154–168. [Google Scholar]
- Farahat M.H., Abdallah F.M., Ali H.A., Hernandez-Santana A. Effect of dietary supplementation of grape seed extract on the growth performance, lipid profile, antioxidant status and immune response of broiler chickens. Animal. 2017;11:771–777. doi: 10.1017/S1751731116002251. [DOI] [PubMed] [Google Scholar]
- Felício A.M., Gaya L.G., Ferraz J.B., Moncau C.T., Mattos E.C., Santos N.P., Michelan Filho T., Balieiro J.C., Eler J.P. Heritability and genetic correlation estimates for performance, meat quality and quantitative skeletal muscle fiber traits in broiler. Livestock Sci. 2013;157:81–87. [Google Scholar]
- Grandhaye J., Douard V., Rodriguez-Mateos A., Xu Y., Cheok A., Riva A., Guabiraba R., Zemb O., Philippe C., Monnoye M., Staub C., Venturi E., Barbe A., Rame C., Dupont J., Froment P. Microbiota changes due to grape seed extract diet improved intestinal homeostasis and decreased fatness in parental broiler hens. Microorganisms. 2020;8:1141. doi: 10.3390/microorganisms8081141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.J., Wang Z.Y., Wang Y.S., Chen B., Huang Y.Q., Li P., Tan Q., Zhang H.Y., Chen W. Impact of drinking water supplemented 2-hydroxy-4-methylthiobutyric acid in combination with acidifier on performance, intestinal development, and microflora in broilers. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hati S., Patel M., Mishra B.K., Das S. Short-chain fatty acid and vitamin production potentials of Lactobacillus isolated from fermented foods of Khasi Tribes, Meghalaya, India. Ann. Microbiol. 2019;69:1191–1199. [Google Scholar]
- Hill P.G. The measurement of albumin in serum and plasma. Ann. Clin. Biochem. 1985;6:565–578. doi: 10.1177/000456328502200604. [DOI] [PubMed] [Google Scholar]
- Jassim E.Q., Aqeel C.H. Effect of alkaline water and/or magnetic water on some physiological characteristic in broiler chicken. J. Entomol. Zool. Stud. 2017;5:1643–1647. [Google Scholar]
- Kany H., El-Sayed A.E.H., Soliman F., Elbestawy A. The effect of fermented wheat germ extract on biochemical, physiological and performance parameters of broiler chickens. Alex. J. Vet. Sci. 2017;55:91–97. [Google Scholar]
- Khan J., N.slam M. In: Pages 133–152 in Histopathology-Reviews and Recent Advances. Martinez E.P., editor. Intech Publishers; Rijeka, Croatia: 2012. Morphology of the intestinal barrier in different physiological and pathological conditions. [Google Scholar]
- Kholodkova E.V., IuM K., Baturo A.P., Lifshits M.B., Glebovskaia M.A. Etiologic role of bacteria of the genus Providencia in acute intestinal diseases. Zh. Mikrobiol. Epidemiol. Immunobiol. 1977;12:20–23. [PubMed] [Google Scholar]
- Lisnahan C.V., Nahak O.R. Growth performance and small intestinal morphology of native chickens after feed supplementation with tryptophan and threonine during the starter phase. Vet. World. 2020;13:2765. doi: 10.14202/vetworld.2020.2765-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes S., Vackier T., Nguyen Huu S., Heyndrickx M., Steenackers H., Sampers I., Raes K., Verplaetse A., De Reu K. Occurrence and characterisation of biofilms in drinking water systems of broiler houses. BMC Microbiol. 2019;19:1–15. doi: 10.1186/s12866-019-1451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalczuk M., Holl E., Möddel A., Jóźwik A., Slósarz J., Bień D., Ząbek K., Konieczka P. Phytogenic ingredients from hops and organic acids improve selected indices of welfare, health status markers, and bacteria composition in the caeca of broiler chickens. Animals. 2021;11:3249. doi: 10.3390/ani11113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MRiRW (2010). Rozporządzenie Ministra Rolnictwa i Rozwoju Wsi z dnia 15 lutego 2010 r. w sprawie wymagań i sposobu postępowania przy utrzymywaniu gatunków zwierząt gospodarskich, dla których normy ochrony zostały określone w przepisach Unii Europejskiej. Dz.U. 2010 nr 56 poz. 344. Accessed Sept. 2022 https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20100560344/O/D20100344.pdf (in Polish)
- Nakajima A., Mitomo S., Yuki H., Araki M., Seegers L.M., McNulty I., Lee H., Kuter D., Ishibashi M., Kobayashi K., Dijkstra J., Onishi H., Yabushita H., Matsuoka S., Kawamoto H., Watanabe Y., Tanaka K., Chou S., Naganuma T., Okutsu M., Tahara S., Kurita N., Nakamura S., Das S., Nakamura S., Jang I. Gut microbiota and coronary plaque characteristics. J. Am. Heart Assoc. 2022;11 doi: 10.1161/JAHA.122.026036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . 9th rev. ed. National Academies Press; Washington, D.C: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Pluske J.R. In: Page 239–257 in Feed Efficiency in Swine. Patience J.F., editor. Wageningen Academic Publishers; Wageningen, Netherlands: 2012. Physiology of feed efficiency in the pig: emphasis on the gastrointestinal tract and specific dietary examples. [Google Scholar]
- Qamar H., Li A., Zeng Z., Wang Y., Mehmood K., Waqas M., Iqbal M., Zhang J., Li J. Effect of grape seed extract on tibial dyschondroplasia incidence, liver weight, and tibial angiogenesis in chickens. Pak. Vet. J. 2020;40:187–194. [Google Scholar]
- Rafeeq M., Bilal R.M., Batool F., Yameen K., Farag M.R., Madkour M., Elnesr S.S., El-Shall N.A., Dhama K., Alagawany M. Application of herbs and their derivatives in broiler chickens: a review. World's Poult. Sci. J. 2023;2023:1–23. [Google Scholar]
- Rahimi S., Teymori Zadeh Z., Torshizi K., Omidbaigi R., Rokni H. Effect of the three herbal extracts on growth performance, immune system, blood factors and intestinal selected bacterial population in broiler chickens. J. Agric. Sci. Technol. 2011;13:527–539. [Google Scholar]
- Sadati M.M., Rezaeipour V., Abdullahpour R. Efficacy of whole wheat grain in combination with acidified drinking water on growth performance, gizzard development, intestinal morphology, and microbial population of broiler chickens. Livestock Sci. 2022;259 [Google Scholar]
- Saki A.A., Harcini R.N., Rahmatnejad E., Salary J. Herbal additives and organic acids as antibiotic alternatives in broiler chickens diet for organic production. African J. Biotechnol. 2012;11:2139–2145. [Google Scholar]
- Saleh H. Modulatory effect of wheat germ oil on intestinal oxidative stress and DNA damage induced by carbon tetrachloride in Mice. J. Appl. Pharm. Sci. 2016;6:67–74. [Google Scholar]
- Senior J.R. Alanine aminotransferase: a clinical and regulatory tool for detecting liver injury–past, present, and future. Clin. Pharmacol. Ther. 2012;92:332–339. doi: 10.1038/clpt.2012.108. [DOI] [PubMed] [Google Scholar]
- Stipkovits L., Lapis K., Hidvégi M., Kósa E., Glávits R., Resetár A. Testing the efficacy of fermented wheat germ extract against Mycoplasma gallisepticum infection of chickens. Poult. Sci. 2004;83:1844–1848. doi: 10.1093/ps/83.11.1844. [DOI] [PubMed] [Google Scholar]
- Tamizi Jooneghani M., Ghazanfari S., Aghashahi A., Sharifi S.D., Hosseini S.A. Effects of Humulus lupulus essential oils on productive performance, meat oxidative stability and ileum microbial population in broiler chickens. Anim. Production. 2016;18:501–512. [Google Scholar]
- Tsai L.S., Schade J.E., Molyneux B.T. Chlorination of poultry chiller water: chlorine demand and disinfection efficiency. Poult. Sci. 1992;71:188–196. [Google Scholar]
- Van Bunnik B.A.D., Katsma W.E.A., Wagenaar J.A., Jacobs-Reitsma W.F., De Jong M.C.M. Acidification of drinking water inhibits indirect transmission, but not direct transmission of Campylobacter between broilers. Prev. Vet. Med. 2012;105:315–319. doi: 10.1016/j.prevetmed.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Vecchi B., Gumina E., Matte F., Bata A., Bata S., Molnar-Nagy V., Hall J.W., Hernandez-Velasco X., Layton S. Effect of Herbanoplex CP on broiler chicken's performance following a nondefined challenge or intestinal lesion score using a necrotic enteritis challenge model. J. Appl. Poult. Res. 2021;30 [Google Scholar]
- Viveros A., Chamorro S., Pizarro M., Arija I., Centeno C., Brenes A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011;90:566–578. doi: 10.3382/ps.2010-00889. [DOI] [PubMed] [Google Scholar]
- Waikar S.S., Bonventre J.V. Creatinine kinetics and the definition of acute kidney injury. J. Am. Soc. Nephrol. 2009;20:672–679. doi: 10.1681/ASN.2008070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson E.M. Synergy and other interactions in phytomedicines. Phytomedicine. 2001;8:401–409. doi: 10.1078/0944-7113-00060. [DOI] [PubMed] [Google Scholar]
- Ząbek K., Szkopek D., Michalczuk M., Konieczka P. Dietary phytogenic combination with hops and a mixture of a free butyrate acidifier and gluconic acid maintaining the health status of the gut and performance in chickens. Animals. 2020;10:1335. doi: 10.3390/ani10081335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Guo Y., Wang Z., Wang Y., Chen B., Du P., Zhang X., Huang Y., Li P., Michiels J., Chen W. Acidification of drinking water improved tibia mass of broilers through the alterations of intestinal barrier and microbiota. Anim. Biosci. 2022;35:902–915. doi: 10.5713/ab.21.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Lv J., Pan L., Zhang Y. Roles and applications of probiotic Lactobacillus strains. Appl. Microbiol. Biotechnol. 2018;102:8135–8143. doi: 10.1007/s00253-018-9217-9. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Zhao L., Song M., Luo J., Liu H., Xue K., Huang C., Chen H., Ge J. Providencia heimbachae associated with Post-weaning Diarrhea in Piglets: identification, phenotype, and pathogenesis. Curr. Microbiol. 2022;79:1–12. doi: 10.1007/s00284-021-02697-1. [DOI] [PubMed] [Google Scholar]
- Zou Q., Meng W., Li C., Wang T., Li D. Feeding broilers with wheat germ, hops and grape seed extract mixture improves growth performance. Front. Physiol. 2023;14 doi: 10.3389/fphys.2023.1144997. [DOI] [PMC free article] [PubMed] [Google Scholar]