Abstract
Active microrheology was conducted in living cells by applying an optical-trapping force to vigorously fluctuating tracer beads with feedback-tracking technology. The complex shear modulus was measured in HeLa cells in an epithelial-like confluent monolayer. We found that over a wide range of frequencies (1 Hz < < 10 kHz). Actin disruption and cell-cycle progression from G1 to S and G2 phases only had a limited effect on in living cells. On the other hand, was found to be dependent on cell metabolism; ATP-depleted cells showed an increased elastic modulus at low frequencies, giving rise to a constant plateau such that . Both the plateau and the additional frequency dependency of ATP-depleted cells are consistent with a rheological response typical of colloidal jamming. On the other hand, the plateau disappeared in ordinary metabolically active cells, implying that living cells fluidize their internal states such that they approach the critical jamming point.
Significance
Intracellular mechanical properties were measured using optical-trap-based microrheology. Despite expectations to the contrary, shear viscoelasticity was hardly affected by reorganization of cytoskeletal structures during cell-cycle progression (G1 to S and G2 phases) nor by artificial disruption of the actin cytoskeleton induced by chemical inhibitors. Rather, the mechanics of cell interiors is governed by the glassy cytoplasm. Cells depleted of ATP solidified, whereas living cells that maintained metabolic activities were more fluid-like. Instead of a completely fluid response, however, we observed a characteristic power-law viscoelasticity over the whole range of frequencies measured. Based on our current understanding of jamming rheology, we discuss how cells fluidize their internal state in a way that pushes the system toward the critical jamming transition.
Introduction
The mechanics of cells is a fundamental aspect of biology; it governs the dynamics of functional biomacromolecules that perform various physiological processes (1,2,3,4). For instance, motor proteins and other mechanoenzymes change their shapes to catalyze biochemical reactions (5,6). They also need to be present at the right place at the right time (2); thus their performance depends on the mechanical properties (i.e., fluidity) of the surrounding medium. At the same time, mechanical stability is also necessary for cells in order for them to maintain their complex internal organization. Living cells thus meet these apparently conflicting requirements: dynamic fluidity and mechanical stability. However, the physical machinery underlying the regulation of intracellular mechanics remains elusive (7,8).
It has been believed that the mechanics of cells is determined by the properties of the cytoskeleton, a network of interlinking protein filaments (9,10). After decades of intense research, a consensus has been reached that the mechanical properties of the cytoskeleton are described using a theory based on the semiflexible polymer network model (11). However, in living cells, the interstices of the sparse network of the cytoskeleton are densely filled with other colloidal constituents such as proteins, nucleic acids, polysaccharides, and their assemblies such as ribosomes (3,12,13,14). In contrast to the cytoskeleton, it remains to be elucidated whether and/or how the cytoplasm excluding the cytoskeleton (hereafter referred to as just the cytoplasm) contributes to intracellular mechanics. As it was recently pointed out, cytoplasmic rheology may be similar to that of dense colloidal suspensions close to jamming (4,8).
As is typical in soft materials, both the cytoskeleton and cytoplasm respond in a highly nonlinear manner to mechanical perturbations. For instance, when subjected to mechanical loads such as shear stress or a locally applied force, semiflexible polymer networks typically stiffen (15,16,17,18,19,20,21), whereas dense colloidal suspensions are fluidized (22,23,24,25). Active mechanoenzymes such as molecular motors also apply forces to the cytoskeleton (20), which will eventually be transmitted to the surrounding cytoplasm. Conformational changes of enzymes that occur during enzymatic reactions (5,6) may also mechanically perturb a crowded environment. It is then reasonable to expect that driving the cell interior out of equilibrium will lead to the emergence of a complex intracellular mechanics.
Living cells dynamically change their internal organization during their cell-cycle progression. They replicate chromosomes, double cytoplasmic components, and reconstruct cytoskeleton structures (26). Accordingly, it has been reported that the elastic and viscous moduli of cells depend on the cell-cycle phase (27,28,29). All the factors listed above, i.e., the cytoskeleton, cytoplasm (in the interstices of the cytoskeleton), their reorganization during cell-cycle progression, and their metabolic activity, seem to be integrated into intracellular mechanics. Prior studies of intracellular mechanics mostly focused on the cytoskeleton, but attention has rarely been paid to the cytoplasmic contribution. In this study, we aim to investigate how the mechanics of the cytoplasm is regulated by metabolic activities, using interphase cells that make up a majority of our body and also cell cultures.
Microrheology (MR) is a method to investigate the viscoelastic properties of a medium at microscopic (∼μm) length scales (30), by tracking the motion of micron-sized tracer beads imbedded in a specimen (31,32). In active microrheology (AMR) (33,34), an external force is applied to a tracer bead, and its displacement response is measured. When a sinusoidal force is applied to the bead, we obtain the complex shear modulus of the surrounding medium as a function of the angular frequency . Here, G′ and G″ are the real and imaginary part of the complex modulus . AMR can be performed simultaneously by applying an optical-trapping force to the probe particle and measuring the probe displacements using the back-focal-plane laser interferometry technique (BFPI) (35). Because of the vigorous nonthermal fluctuations, a probe particle in living cells moves out of the laser focus within the experimental time scale. Although a strong laser could trap the probe in the laser focus, it also hinders bead motion, causing large errors in estimating the intrinsic response and fluctuations that should have occurred in the absence of optical trapping. In a prior study, we therefore developed an optical-trap-based MR implemented with a three-dimensional feedback-controlled sample stage (feedback-tracking MR, see Supporting material S1) (7,36). This technique allowed us to stably track a vigorously fluctuating probe particle in living cells with a laser power as small as 0.4–1.8 mW, significantly lower than ordinary optical-trapping measurements (34,37). As seen in Fig. S2, this noninvasive technology is crucial for conducting optical-trap-based MR in living cells.
Using this MR technique, we measured the intracellular mechanics of living HeLa cells. To focus on the cytoplasmic mechanics, the contribution of the cytoskeleton was reduced by 1) using probe particles that do not bind to cytoskeletal filaments and 2) measuring the location where the actin cytoskeleton is supposed to be sparse, i.e., the middle of cells cultured in an epithelial-like monolayer sheet. In this study, rheological property measured by our experimental setup is referred to as intracellular mechanics or cell mechanics. We found that the cell-cycle progression during interphase and cytoskeletal organization merely have a marginal effect on intracellular mechanics measured in this way. The complex modulus did not show large variation from cell to cell; they consistently showed a frequency dependence predicted for disordered colloidal jamming (7,8).
On the other hand, the intracellular was highly dependent on metabolic activities. The viscoelasticity was seen to increase in ATP-depleted HeLa cells. A plateau elasticity appeared that is real and constant over the frequency range, i.e., . We discuss how these observations are consistent with what is expected for a glassy cytoplasm (4,8,38). Although it is inferred that the metabolic activity fluidized the cytoplasm (4,8), a typical fluid-like response was not observed in the range of frequencies measured (0.1–104 Hz). Instead, our results imply that the rheological properties typical for critical jamming (39,40) are achieved during the active fluidization of the glassy cytoplasm in living cells (41).
Materials and methods
Cell preparation
HeLa cells and HeLa/Fucci2 cells (Riken, Cell Bank) were seeded on fibronectin-coated glass-bottom petri dishes in Dulbecco’s modified Eagle’s medium (Wako, D-Mem, high glucose) with glucose (1 mg/mL), penicillin (100 U/mL), streptomycin (0.1 mg/mL), amphotericin B (250 mg/mL), and 10% fetal bovine serum (FBS) at 37°C. Cells were cultured in a CO2 incubator until they formed a confluent epithelial-like monolayer sheet. The surface of the probe particles (melamine particles, diameter, micro Particles GmbH) were coated with polyethylene glycol (PEG) strands (NANOCS, mPEG-NH2, 1000 Da, PG1-AM-1k) (36). PEG coating generally passivates probe surfaces; in aqueous environments, hydrophilic PEG acts as a polymer brush and prevents sticking to other objects or to other molecules. Probe particles were introduced into cells using a gene gun (Bio-Rad, PDS-1000/He). Excess beads that did not enter the cells were removed by washing the dishes with phosphate-buffered saline. After replenishing the dishes with fresh culture medium, they were placed in an incubator at least overnight to allow for the recovery from cell damage. Since supplying CO2 causes additional noise, MR experiments were performed in a -independent culture medium (Gibco, L-15 medium) with 10% FBS serum. All measurements were performed at 37°C. For MR experiments under ATP depletion and actin disruption, FBS was not included in the media as stated below.
Actin disruption
To disrupt the actin network, polymerization of actin filaments was inhibited by adding chemical agents (cytochalasin D 50 μg/mL or latrunculin B 10 μM). After MR experiments were performed at 37°C in L-15 medium with 10% FBS (w/o chemical agents), the culture medium was replaced with the actin disruption medium (cytochalasin D or Latrunculin B, without FBS). Cells were then incubated at 37°C on the MR setup for ∼30 min before the next MR measurement so that the actin network would be disrupted in cells.
ATP depletion
The high-energy molecule ATP is mainly produced in cells via two metabolic pathways: glycolytic in the cytoplasm and oxidative phosphorylation in the mitochondria. Therefore, in order to deplete intracellular ATP, cells were cultured in a nutrient-free media with 50 mM 2-deoxy-D-glucose and 10 mM sodium azide (42). 2-Deoxy-D-glucose and sodium azide inhibit glycolysis and oxidative ATP production, respectively.
HeLa cells were incubated in the medium for ATP depletion (L-15 medium with 50 mM 2-deoxy-D-glucose, 10 mM sodium azide, without FBS) at 37°C for ∼10 h before the MR measurements so that ATP stored in cells would be consumed.
Luminescent ATP detection assay
Intracellular ATP was measured using a CellTiter-Glo Luminescent Cell Viability Assay kit (Promega, G7570) according to the manufacturer’s instructions. Briefly, 5000 cells were plated on a 96-well plate and incubated for 24 h at 37°C in 5% CO2. After incubation, the medium was replaced with 50 μL of either 1) control medium (phenol-red free DMEM (Thermo Fisher, 31,053,028) supplemented with 4 mM glutamine (Thermo Fisher, 25,030,081) and 1% Penicillin-Streptomycin Solution (Sigma-Aldrich, P4333)), or 2) control medium supplemented with 50 mM 2-deoxyglucose (Nacalai, 10,722-11) and 10 mM NaN3 (WAKO, 199-11095), and then it was incubated for 7 h at 37°C in 5% CO2. Subsequently, 50 μL of reagent was directly added to the well, and the contents were mixed for 2 min on an orbital shaker at 25°C. Luminescence was recorded after 30 min of incubation at 25°C in the dark.
Immunocytochemistry
HeLa cells plated on a poly-lysine-coated glass-bottom dish were fixed with 4% paraformaldehyde phosphate buffer solution (Wako, 163–20145) for 10 min at 25°C, permeabilized with PBS containing 0.5% Triton-100 for 15 min at 25°C, and stained with ActinRed 555 ReadyProbes Reagent (ThermoFisher, R37112) for 30 min at 25°C. Subsequently, the cells were incubated with Hoechst 33,342 (ThermoFisher, H1399) for 10 min at 25°C for nuclear staining. The cells were washed three times with PBS after each step. Imaging was performed using an Eclipse Ti2-E microscope equipped with NIS-Elements AR imaging software. The representative images were processed by Clarify.ai and Denoise.ai using NIS-Elements AR 5.30, as previously described (43).
AMR in living cells
The technical details of conventional optical-trapping-based AMR (without feedback operation) are given elsewhere (34). We will repeat the essence here briefly for the reader’s convenience. As shown in Fig. 1 A and B, a small sinusoidal force was applied to a probe particle, by optically trapping it with the drive laser (λ = 1064 nm Nd:YVO4, Coherent, Tokyo, Japan) whose focus position was controlled by an acousto-optic deflector (AOD; DTSX-400-1064, AA Opto-Electronic, Orsay, France). The probe displacement synchronous to the applied force was measured using BFPI conducted with a fixed probe laser (λ = 830 nm IQ1C140, Power Technology, Alexander, AR, USA) (35). Typical values of and are given in Supporting material S6. The frequency response function is then obtained as a complex quantity. The complex shear modulus of the surrounding media is also obtained as a complex quantity via the Stokes relation extended to the sinusoidal response,
| (1) |
where a is the radius of the spherical probe particle.
Figure 1.
(A) Schematic of the feedback-tracking microrheology setup. A fixed probe laser is used to measure using a quadrant photodiode (QPD). A drive laser and an acousto-optic deflector (AOD) are used to apply an oscillatory external force. The PEG-coated probe particle surrounded by dense colloidal constituents does not bind to the sparse cytoskeletal network. (B) Typical time series of the probe displacement (black line) and applied force (red line). The sinusoidal signal was hidden in the experimental noise (e.g., thermal fluctuation) but can be detected by using a lock-in amplifier. (C) Schematic illustration of stage feedback. (D) The displacements of a probe are obtained by the sum of the displacement of the piezo stage and the displacement from the focus of the probe laser. The focus of the drive laser is moved sinusoidally around the focus of the probe laser, following . To see this figure in color, go online.
A tracer bead fluctuates vigorously in a living cell because the cytoplasm is driven far from equilibrium by the energy derived from metabolism. As explained in detail in Refs. (7,36), such a fluctuating particle was smoothly tracked by analog PID-feedback control of the piezo-mechanical sample stage on which the specimen was placed. The optical-trapping force was then stably applied to the probe particle by the oscillating drive laser while maintaining the probe particle in the focus of the fixed probe laser. As shown in Fig. 1 C, the displacement of the probe particle from the probe laser , which is measured by BFPI of the probe laser, is used to control the position of the piezo stage by finding
| (2) |
Here, τ (typically set at 10–100 ms) is the delay time for the feedback response of our experimental setup. The displacement of the probe in the coordinate system traveling with the feedback-controlled piezo stage is given as (Fig. 1 D). From Eq. 2, the frequency-response relation is obtained as
| (3) |
where ˆ denotes the amplitude of the sinusoidal signal, e.g., . From Eq. 3, it can be seen that and are high- and low-pass filtered from the total probe displacement . Thus, the slow/large displacements of the probe are tracked by the piezo stage as , whereas the fast/small displacements are measured with the QPD as . By measuring either or as a function of frequencies, we obtain the total probe response under feedback tracking. By estimating the sinusoidal force applied to the probe under the feedback (as given in Supporting material S1), we obtain the probe particle’s response function as . We then obtain via Eq. 1.
Statistical analysis
Since the distributions of G′ and G″ were non-Gaussian, statistical analysis was performed by using the Mann-Whitney U test, unless otherwise stated in the figure caption. p values <0.05 were considered a significant difference. All the statistical tests were performed using Igor and MATLAB software. A list of all p values from the MR experiments is given in Supporting material S7.
Results
Power-law intracellular rheology of untreated HeLa cells
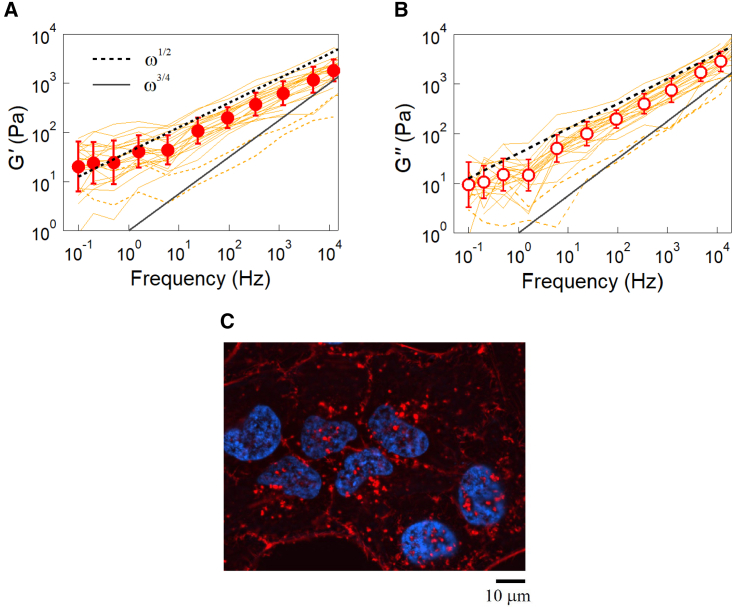
Using feedback-tracking AMR, we measured the complex modulus of HeLa cells derived from a cervical cancer. Melamine particles (2a = 1 μm) were incorporated into cells forming an epithelial-like confluent monolayer on the surface of a glass-bottom dish. Particles incorporated at the center of cells between the cell membrane and the nuclear membrane were used as probes. The average of the complex shear modulus of the untreated HeLa cells is shown in Fig. 2 together with the standard deviation (n = 21). Filled circles in (A) and open circles in (B) show the real (G′) and imaginary (G″) parts of the complex shear modulus, , respectively. Curves show raw data before averaging. Markers are averaged values, and bars indicate the log-normal SD. Since the logarithms of mechanical properties are approximately distributed with Gaussian (42), averages and SDs were calculated for the logarithm of experimental results. Our results showed a characteristic frequency dependence of the form . G′(ω) slightly deviates from the power-law dependency at low frequencies (), but this may be an artifact. As shown in Supporting material S2, low-frequency displacement fluctuations of probe particles in Madin-Darby canine kidney (MDCK) cells measured by passive MR are decreased by prolonged irradiation with a 1.7-mW laser, implying an increased elasticity at low frequencies. Accordingly, our recent preliminary experiments performed more carefully with a reduced laser power (<0.5 mW) in MDCK cells seem to remove this deviation. This will be reported in more detail elsewhere.
Figure 2.
Intracellular complex modulus measured with AMR using 2a = 1 μm diameter melamine particles. (A) G′(ω) and (B) G”(ω) of HeLa cells in a confluent monolayer formed on a glass substrate. Curves are the raw data obtained in 23 different cells. Data shown as dotted lines were excluded from the average (n = 21). Since cell cycles were not monitored, cells in different cell-cycle phases (G1, S, and G2) are included. Circles are the statistical average, and bars indicate the log-normal SD. Broken and solid lines show the power-law dependency typical of the glassy cytoplasm () and cytoskeletal networks (), respectively. The result follows over a wide range of frequencies, indicating that the intracellular mechanics are predominantly governed by the glassy cytoplasm. (C) Immunofluorescent images of confluent HeLa cells. The cells were stained with rhodamine phalloidin and Hoechst33342 to visualize F-actin (red) and the nucleus (blue). To see this figure in color, go online.
It has been reported that cytoskeletal networks prepared in vitro exhibit an elastic plateau at low frequencies and a power-law dependency at higher frequencies (17,20,44,45). The frequency dependency, which is now accepted as the hallmark of the cytoskeleton (21,44), is explained by the theory for semiflexible polymer networks (46,47,48,49). In biological cells, a consistent with this understanding was found when MR was conducted using probe particles coupled to the actin cytoskeleton (7,42,50). On the other hand, the power-law dependence observed in this study is not consistent with that expected for the cytoskeleton. It is to be noted that the probe particles used in this study were coated with PEG polymers that generally inhibit adherence to objects in cells, including the cytoskeleton. The actin cytoskeleton is usually less expressed in confluent monolayers of cells compared with isolated cells (51). In addition, the probes were incorporated deep inside of living cells where the actin cytoskeleton is only sparsely expressed (Fig. 2 C), as reported in confluent HeLa cells (52,53) and also in confluent epithelial-like monolayers of MCF10A and MDCK (51,54).
Marginal contribution of the actin cytoskeleton to intracellular mechanics
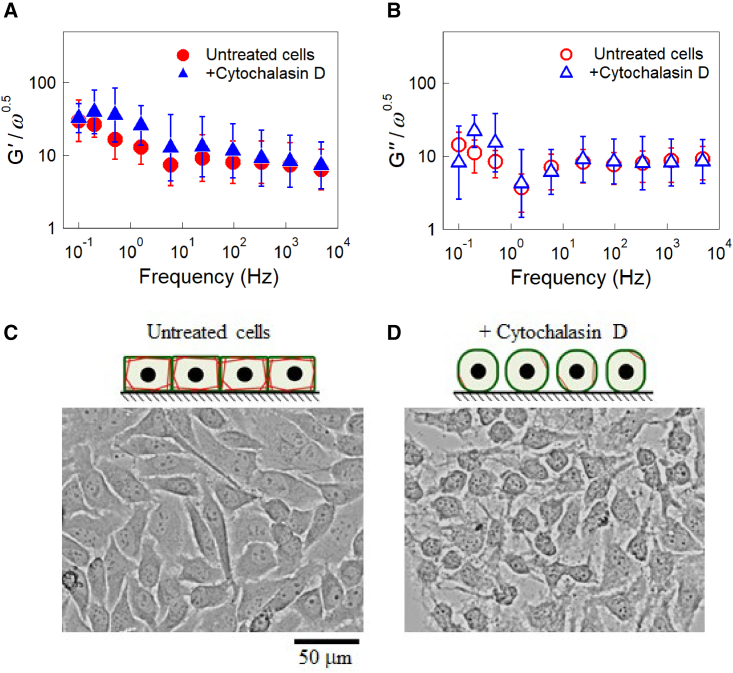
In order to clarify the contribution of the cytoskeleton to intracellular , we conducted AMR in HeLa cells in which F-actin was disrupted. After conducting the first AMR experiments in untreated HeLa cells, actin polymerization was inhibited by adding cytochalasin D or latrunculin B to the culture medium (55,56). A second round of AMR measurements was then performed using the same particles as those used in the first AMR experiments. Since shows a general trend proportional to , results normalized as are shown in Fig. 3 in order to clarify the effect of the chemical dose at each frequency. Cytochalasin D at 50 μg/mL did not alter in any frequency range (n = 9, Fig. 3 A and B). Statistical tests at each frequency were conducted for and before and after the addition of cytochalasin D. The p values obtained by the Mann-Whitney U test were larger than 0.05 for all frequencies measured, which indicates no significant difference. Similar results were obtained by treating cells with another inhibitor for actin polymerization, latrunculin B at 10 μM (Fig. S3). Cells changed their morphology after the inhibitors were administrated (Fig. 3 C). They became more rounded and lost cell-cell contact while leaving branched protrusions; some cells were even removed from the glass substrate. These observations indicate that the inhibitors worked effectively and disrupted cytoskeletal organization, since the actin cytoskeleton is responsible for maintaining the shape of cells and their adhesion to the substrate (57). We thus conclude that the actin cytoskeleton does not contribute to the cytoplasmic of HeLa cells cultured in a confluent monolayer in any remarkable way.
Figure 3.
(A) and (B) of HeLa cells, untreated (circles) and treated (triangles) with 50 μg/mL cytochalasin D. The same probe particles in the same cells were measured before and after adding cytochalasin D to the culture media (n = 9). Bars indicate the log-normal SD. Similar experiments performed before and after treatment with 10 μM latrunculin B are given in Fig. S3. (C, D) A microscopy image of HeLa cells. When the actin cytoskeleton was disrupted with 50 μg/mL cytochalasin D, cells strongly contracted, leaving branched protrusions. (A, B) The Mann-Whitney U test was used to calculate the p value between untreated cells and cells treated with 50 μg/mL cytochalasin D. No significant difference was observed for any of the frequencies. To see this figure in color, go online.
HeLa cells in confluent monolayers are laterally confined and polarized under normal conditions. They are roughly as tall as they are wide and tightly connected to each other. In such epithelial-like tissue, actin stress fibers are densely expressed at the cell-cell and cell-substrate interfaces, and they contribute to the organization of the tissue. The disruption of actin thus remarkably affects cell morphology. In prior studies, mechanical properties of cells have been measured by using other techniques, e.g. AFM (atomic force microscopy) (10,58,59,60), magnetic twisting cytometry (38,42,61), and others (42,62,63). These techniques have shown that the mechanical properties of cells indeed depend on actin expression (60). It is therefore commonly believed that the mechanical properties of cells are directly correlated with their shape and cytoskeletal organization (62,63), in contrast to our experimental results. It is to be noted, however, that the techniques used in prior studies measure the properties of cell surfaces at which the actin cytoskeleton is densely expressed. Considering that F-actin is less expressed inside of the epithelial cells in a confluent monolayer, results obtained in this study are reasonable and do not contradict any of the prior measurements conducted on cell surfaces.
Cell surface elasticities measured using conventional techniques are usually widely distributed, over orders of magnitude (42,59,64). The organization of the actin cytoskeleton at the cell cortex is heterogeneous and changes with time. Depending on the situation, actin filaments bundle to form thick fibers, couple to cell membranes, and create tension due to actomyosin contractile activity, leading to remarkable stiffening as demonstrated in vivo (65,66,67) and in vitro (17,20,68). All these and many other factors contribute to variations in the mechanical properties of cell surfaces. On the other hand, in living cytoplasm is found to be narrowly distributed, even though they were measured with different probes in different cells. This observation also supports the claim that the cytoplasmic mechanics of the epithelium are not strongly associated with the actin cytoskeleton.
Cell mechanics during cell-cycle progression: G1 to S and G2 phases
Next, we performed feedback-tracking AMR in HeLa cells while the cell cycle progressed during interphase. HeLa cells used here (HeLa/Fucci2) were labeled using the Fucci system that enables us to identify G1 and S/G2 phases by the fluorescence color in the cell nucleus (69). In Fig. 4, we show the normalized complex shear modulus (A: and B: ) of HeLa cells as a function of frequency. Circles and squares indicate cells at G1 and S/G2 phases respectively. The intracellular in the S/G2 phase slightly increased compared with cells at G1 phase; G′ and G″ of S/G2 phase were 40% and 30% larger than those of the G1 phase on average, respectively. Note that these differences were only marginal but observed because the cell cycle phases could be resolved.
Figure 4.
(A) of HeLa/Fucci2 cells in G1 (circles, n = 12) and S/G2 phases (squares, n = 9). (B) of HeLa/Fuuci2 cells in G1 (circles) and S/G2 phases (squares). Bars indicate the estimate of the log-normal SD. Remarkable differences were not observed. (C) Schematic illustration of the Fucci system used for identifying cell-cycle phases. The Mann-Whitney U test was used to calculate the p value for differences between the G1 and S/G2 phases. ∗p < 0.05. No symbol: p > 0.05. To see this figure in color, go online.
In the literature, the cell-cycle dependency of cell mechanics has been investigated using AFM (27) and micropipette aspiration (28), both of which showed a 50%–100% increase of surface elasticity of S-phase cells compared with that of G1-phase cells. Here, we emphasize that our results and prior studies do not contradict. Our AMR methodology is indifferent to F-actin and likely measures the interstices of the sparse network of the cytoskeleton. On the other hand, prior studies measured the response of the cytoskeleton at cell surfaces whose expression is likely strongly affected by cell-cycle progression.
As explained in the introduction, various processes that occur during cell-cycle progression are inherently mechanical. Especially, cells in the M phase experience drastic variations in cytoskeletal organization (63,70), which would affect cell mechanics derived from the cytoskeleton (29,71,72). When the probe particle was coupled to the cytoskeleton, MR in the M phase could exhibit mechanical properties more evidently different from other phases; the cell interior underwent softening, and the viscoelastic fluid contributions increased (29). However, AMR experiments were not conducted in M phase cells in this study. Because mitosis and cytokinesis are highly dynamic processes and complete in a relatively short period of time, it was not possible to stably track the probe particle in M phase cells with the feedback technique.
ATP-dependent intracellular mechanical properties
After finding that the actin cytoskeleton and cell-cycle progression during interphase have marginal effects on the intracellular mechanics, we next investigated the effect of metabolism on active mechanics in HeLa cells. In order to regulate the metabolic activity, cells were incubated with a culture media that causes ATP depletion (42). Firstly, intracellular ATP was measured using the luminescent ATP detection assay. Fig. 5 A shows that the ATP level significantly decreased by more than an order of magnitude in ATP-depleted cells. On applying AMR, it was found that the ATP-depleted cells showed an elastic plateau such that in contrast to for untreated cells, as shown in Fig. 5 B. To quantitatively evaluate the effect of ATP depletion on , we fitted of untreated and ATP-depleted cells with (black dashed lines in Fig. 5 B). The coefficient hardly changed, whereas the elastic plateau of ATP-depleted cells became ∼10 times larger than that of untreated cells. This result clearly indicated that only at low frequency significantly increased due to ATP depletion. In a similar manner to previous sections, and of untreated HeLa cells (n = 21, red circles) and ATP-depleted cells (n = 9, black squares) are shown in Fig. 5 C and D, respectively. Error bars indicate log-normal SDs except for in ATP-depleted cells. Instead, we show the median, 25%, and 75% quantiles for in ATP-depleted cells because at small sometimes showed negative values due to and measurement noise. The Mann-Whitney U test for G′ in the low frequency range (0.1–94 Hz) showed a significant increase of G′ upon ATP depletion (p < 0.05) compared with untreated cells.
Figure 5.
(A) Intracellular ATP level in HeLa cells treated with 50 mM 2-deoxyglucose and 10 mM NaN3 (ATP-depleted) for 7 h was measured. Quantification was performed on five independent experiments. All data are presented as mean ± standard deviation. ∗∗∗p < 0.001 (Student's t-test). (B) of untreated and ATP-depleted HeLa cells. of untreated and ATP-depleted HeLa cells are fitted by . Untreated: G0 = 136 Pa. A = 11.1 Pa s−0.5. ATP-depleted: G0 = 13.1 Pa. A = 11.2 Pa s−0.5. (C) and (D) of untreated (red circles, n = 21) and ATP-depleted HeLa cells (black squares, n = 9). (C) Bars indicate the log-normal SD. (D) Symbols and bars of for untreated cells indicate the log-normal mean and SD, respectively. of ATP-depleted cells at low frequency sometimes became negative due to and measurement noise. Thus, symbols of for ATP-depleted cells represent median values, and the bars connect 25% and 75% quantiles. At low frequencies (), showed a significant increase after ATP depletion (p < 0.05). (C, D) The Mann-Whitney U test was used to calculate the p value for differences between untreated and ATP-depleted cells. ∗∗∗p < 0.001. ∗∗p < 0.01. ∗p < 0.05. No symbol: p > 0.05. (E) Microscope image of ATP-depleted HeLa cells. ATP-depleted HeLa cells became round and lost cell-cell contact. (F) Immunofluorescent images of ATP-depleted HeLa cells. The cells were stained with rhodamine phalloidin and Hoechst33342 to visualize F-actin (red) and nuclei (blue). To see this figure in color, go online.
We note that feedback-tracking MR in cells is most easily conducted when the probe particle is situated in the homogeneous part of the cell. Experimental errors increase when the probe particle is in a region close to the nucleus, cell membranes, or another large organelle, because the inhomogeneity of optical properties in such a region disturbs the propagation of the probe and the drive laser. It frequently occurred that a probe particle that was used in the first AMR measurement moved to regions where measurement is no longer possible. In Fig. S4, we compare the limited number of data points (n = 3) successfully measured using the same probe particle in the same cells. All the results showed a remarkable increase in viscoelasticity when ATP was depleted.
Fig. 5E shows microscope images of HeLa cells taken after ATP depletion. ATP-depleted cells lose tight adherence to the substrate and to neighboring cells. Cells change their morphology and take on more rounded shapes due to decreased cytoskeletal tension. To clarify the effect of ATP depletion on actin cytoskeletal structures, F-actin was stained using rhodamine phalloidin (Fig. 5 F). Similar to the untreated cells (Fig. 2 C), the actin cytoskeleton of ATP-depleted cells was also sparse. Thus, we concluded that the actin cytoskeleton was not responsible for the increase in the elastic plateau during ATP depletion. Note that the in vitro cytoskeleton is remarkably stiffened under active tension or prestress (15,16,17). Consistently, the cell surface elasticity measured with AFM decreases when ATP is depleted (73,74) likely because the active stiffening of the cytoskeleton is eased owing to the relax of actomyosin contraction at the cell cortex (62,75,76,77). On the contrary, intracellular elasticity measured in this study increased significantly when the intracellular ATP was depleted. This observation again indicates that the cell cortex cytoskeleton is not relevant to intracellular mechanics. When cells are subjected to the ATP depletion medium for a prolonged period, they could enter the path toward cell death (78,79). The fluorescence indicator (fluorescence caspase 3) for apoptosis indeed showed positive in HeLa cells prepared using the similar protocol (Fig. S5). Except for the emergence of the elastic plateau, however, the mechanical properties typical for intracellular rheology were still observed for ATP-depleted cells. Therefore, our results indicate that the cytoplasm with less expression of the cytoskeleton stiffens in a metabolism-dependent manner.
Discussion
It has been believed that mechanical properties of cells are mostly determined by the cytoskeleton (9,10). This study showed, however, that intracellular mechanics in a confluent epithelium are scarcely affected by the actin cytoskeleton. Note that there are multitudes of other biomacromolecules that are crowded together in living cells. Although their effects on cell mechanics have not been fully appreciated until recently, it was shown that in vitro cytoplasm that lacks the cytoskeleton has glass-forming capabilities; the cytoplasm becomes glassy (i.e., practically solidified owing to the slowdown of dynamics) when the concentration of polymeric components is increased and/or metabolic activities are inhibited (4,8,38,80). Vigorous fluctuations were observed in cells maintaining ordinary metabolism, but these were remarkably decreased in ATP-depleted cells (4,37,42,81). It was then inferred that the glassy cytoplasm is fluidized by metabolic activities. However, the decrease/increase of fluctuations does not necessarily mean vitrification/fluidization. Even if glass-forming materials are deeply quenched and solidified, they could still flow if they are actively stirred (82). Besides, only observing the spontaneous fluctuations does not tell us how strongly the medium is stirred by metabolic activities. In this study, we directly measured intracellular viscoelasticity with AMR by observing a probe particle’s response to a well-determined force. Our direct measurement showed that the cytoplasm in ATP-depleted cells was glassy, and cells with ordinary metabolic activities were fluidized.
Cells are constructed of soft materials that respond nonlinearly to applied forces. For instance, semiflexible polymer networks (a model for the cytoskeleton) typically stiffen under tension (15,16,17,18,19,20,21), whereas glassy colloidal suspensions (model for the cytoplasm without the cytoskeleton) are fluidized by mechanical loads (22,23,24,25). In living cells, various mechanoenzymes such as motor proteins perform physiological functions by generating forces utilizing energy supplied by metabolism. The motor-generated forces then create nonthermal fluctuations that surpass thermal fluctuations as demonstrated by the violation of the fluctuation-dissipation theorem, both in vivo (7,37,83,84,85,86) and in vitro (17,20,34,87,88). Owing to the nonlinear response of constituent materials, mechanical properties of cells are profoundly altered by the active fluctuations. In the living cytoplasm, vigorous active fluctuations presumably induce structural relaxations that could not occur with thermal activation (7,8), leading to active fluidization. Here, the fluidization of the cytoplasm under metabolic activities is consistent with the concept of a glassy cytoplasm, but not with the cytoskeleton that stiffens with active metabolism (17,20,68).
The complex modulus in ordinary HeLa cells showed a characteristic frequency dependence . This dependency may seem to coincide with the prediction of the Rouse model (89) that describes the high-frequency dynamics of flexible polymers. In accordance with the theory, cross-linked poly-acrylamide gels show , but only at high frequencies (7,90). Networks of cross-linked semiflexible polymers usually show , but this changes to when the network is placed under tension (17,49). Therefore, polymer networks could also show at high frequencies when they are cross-linked. However, the polymer network models predict that the prefactor A is proportional to monomer concentration (48,89). On the other hand, A for extracted cytoplasm and for cytoplasm in living cells grows much more rapidly, super-exponentially and exponentially, as a function of macromolecule concentration, respectively (8). Such rapid growth of viscoelasticity has been typically observed in glass-forming materials close to the glass transition (91) but not in polymer networks. Note that the static resilience of cross-linked polymer networks should not increase at all when the metabolic activity is inhibited: in fact, the opposite should happen for the cross-linked cytoskeleton (17). Also, the mechanical properties of flexible polymer networks are hardly affected by the kind of mechanical perturbations expected under physiological conditions (16,18).
We believe that the universal frequency dependency of living cytoplasm also conforms to the concept of a glassy cytoplasm. Recent theoretical studies on jamming rheology have shown that this frequency dependency may be derived from the anomalous relaxation modes in the disordered medium close to the jamming transition, crowded with colloidal objects with slippery interfaces (39,40). In a critical point scenario, the system is marginally stable; it displays excess relaxation modes at low frequencies and becomes highly susceptible to external perturbations (92). When metabolic activity was decreased in ATP-depleted cells, the elastic plateau arose as . Similar viscoelastic behavior with an elastic plateau has been commonly observed in glassy or jammed suspensions that were quenched beyond jamming (40,93,94). However, the plateau disappeared when they are driven out of equilibrium under the application of external flow (41), and only remains, much like in the living cytoplasm. The glassy suspensions exhibited the rheological properties typical for critical jamming (i.e., ) when they were mechanically driven out of equilibrium. Our results in cells may indicate that living cytoplasm also reflects such a critical-point-like situation.
The cytoskeleton also shows glassiness when it is driven out of equilibrium, e.g., when the network of semiflexible polymers is forced to flow beyond the linear response regime (95,96). A glassy worm-like chain model explains such behavior by assuming that the height of the free-energy barrier for the structural modification of the network is widely spread (97). Even if thermal activation is not sufficient to overcome the barrier within the experiment duration, the broad spectrum of the energy landscape becomes apparent in the nonlinear flow behavior. At equilibrium, the linear response of in vitro cytoskeleton is understood as a network of semiflexible polymers (34,47,98). However, the cytoskeleton is driven out of equilibrium in living cells by active mechanoenzymes (82). With nonthermal perturbation, structural relaxations are induced in the cytoskeleton of living cells, leading to glassy cytoskeletal rheology. Such glassy behavior may be described by an effective noise temperature as determined in the theory of “soft glassy rheology” (23). Indeed, glassy rheology was observed in the cytoskeleton at cell surfaces in vivo (61,64) but in a way distinct from colloidal crowding. of the cell cortex cytoskeleton showed widely spread power-law exponents ( for ), depending on the situations and the types of cells.
On the other hand, seems to be universally observed in the intracellular cytoplasm of MDCK cells, mouse embryonic stem cells, etc., as we will report elsewhere. Not only the power-law exponent (), but the prefactor A also does not largely differ for different kind of cells, as long as cells and the probe particles are prepared such that the intracellular cytoplasm (but not the cytoskeleton) is measured, as done in this study. Again, we emphasize that these results are consistent with glassy materials close to jamming. One way to disprove theoretical models listed above is to seek the reason 1) why this universal behavior appears specifically for cytoplasmic mechanics in living cells and 2) why normal cells do not fluidize beyond the critical jamming point, but stay there. These questions may be related to the marginal stability of active cytoplasm; the activity of mechanoenzymes is regulated in a specific manner to achieve a situation similar to critical jamming, as we will discuss in a forthcoming paper (Fig. 6) (86).
Figure 6.
Jamming phase diagram of the cell cytoplasm, which is inferred from this study and (8). The dependence of phases on the inverse density of solid components and mechanical force is schematically shown. Since mechanoenzymes apply micro-mechanical force on the cytoplasm, the magnitude of metabolic activity should positively relate to the magnitude of mechanical force (inset). Compared with the untreated cells, violation of fluctuation-dissipation theorem in the ATP-depleted cell is weak (86). Thus, under ATP depletion, active force generated by mechanoenzymes should be weakened. Then, energy-depleted cells are in a glassy state due to molecular crowding (black circle, , (39)). On the other hand, as mechanoenzymes of the untreated cell can generate active mechanical force on their surroundings, the cytoplasm reaches a marginal glass phase (red circle, , (39)), but it does not enter a liquid phase (blue circle). To see this figure in color, go online.
The interior of a biological cell is an enormously complex system, made up of an innumerous number of molecule types and is maintained under continuous metabolic turnover. It is therefore challenging to unequivocally prove a physical model of cells by solely investigating cells in vivo. Even if one inhibits or enhances a specific biochemical pathway to achieve more detailed control of metabolism, cells try to maintain homeostasis by bypassing the path in the complex network of metabolism. Thus, it would be necessary to establish a simplified model system that includes only the essences of living cytoplasm: activity and crowding. During the last decade, a model system collectively referred to as “active matter” has been intensively investigated. Active matter is composed of objects that convert the internal or ambient source of energy into mechanical form. During the dissipation of the input energy, novel collective behavior of the objects was seen to emerge (99,100). On the other hand, the physics of densely packed disordered materials (glass and jamming), especially the mechanism for dynamic arrest and melting, has been a key topic of nonequilibrium statistical mechanics over 100 years (91,101). Recently, the active matter and glass research communities have begun to share a common interest in investigating dense active matter or active glasses (102), which we believe is a good theoretical model of living cytoplasm. Whereas fluctuations and dynamics have been investigated so far (103,104,105,106,107,108,109), physical and mechanical properties behind readily observable phenomena have been rarely measured. In this study, we found that mechanical properties predicted for critical jamming somehow emerge in the living cytoplasm even though some of the fundamental premises of jamming theory do not apply. Therefore, to reveal the nature of jamming rheology in the cytoplasm, simplified dense active matter that allows for control over density, active force, and other conditions is desirable for future work.
Conclusions
In this study, we investigated the mechanical properties of HeLa cells in a confluent epithelial monolayer. The probe particle’s displacement in response to a sinusoidal optical-trapping force was observed using a feedback-tracking microrheology technique (7,21,36). The complex shear modulus of cytoplasm surrounding the probe was then obtained. In contrast to prior measurements of the cell’s cortex, the intracellular complex modulus showed a frequency dependency , which was seldom affected by the cell-cycle progression during interphase and the disruption of the actin cytoskeleton.
On the other hand, ATP depletion clearly affected the mechanical properties of the cell interior; an elastic plateau at low frequencies emerged in ATP-depleted cells. These observations are consistent with the recently emerging idea that the cell interior is glassy (4,8). Mechanoenzymes such as molecular motors enhance structural relaxations (yielding) by actively stirring the cytoplasm, as is typical in glassy media under mechanical loads. Our results imply that the intracellular cytoplasm is fluidized such that it approaches the critical jamming state, characterized by (39,92). Instead of complete fluidization, the slow relaxation typical of a marginally stabilized system was observed in the range of frequencies measured. We note that metabolism-dependent glassy mechanics has various implications for biology. For example, a positive feedback between mechanics and metabolism may occur in cells, as a cytoplasm fluidized by nonthermal activity may facilitate metabolic processes (6,81,110,111,112,113).
Author contributions
H.E., K.U., K.N., W.N., and S.I. collected/analyzed MR data. T.M. performed biochemical assays and immunocytochemistry. H.E., Y.S., and D.M. analyzed/discussed the results. D.M. designed/supervised the project and wrote the manuscript with the support of H.E. and Y.S.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number JP22H04848, JP21H01048, JP20H05536, JP20H00128. We thank Christoph Schmidt in Duke University, and Atsushi Ikeda in the University of Tokyo for helpful discussions and technical support.
Declaration of interests
The authors declare no competing interests.
Editor: Timo Betz.
Footnotes
Supporting material can be found online at https://doi.org/10.1016/j.bpj.2023.04.011.
Supporting material
References
- 1.Heilbrunn L.V. Academic Press; 1956. The Dynamics of Living Protoplasm. [Google Scholar]
- 2.Dix J.A., Verkman A.S. Crowding effects on diffusion in solutions and cells. Annu. Rev. Biophys. 2008;37:247–263. doi: 10.1146/annurev.biophys.37.032807.125824. [DOI] [PubMed] [Google Scholar]
- 3.Minton A.P. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J. Biol. Chem. 2001;276:10577–10580. doi: 10.1074/jbc.R100005200. [DOI] [PubMed] [Google Scholar]
- 4.Parry B.R., Surovtsev I.V., et al. Jacobs-Wagner C. The bacterial cytoplasm has glass-like properties and is fluidized by metabolic activity. Cell. 2014;156:183–194. doi: 10.1016/j.cell.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riedel C., Gabizon R., et al. Bustamante C. The heat released during catalytic turnover enhances the diffusion of an enzyme. Nature. 2015;517:227–230. doi: 10.1038/nature14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jee A.Y., Cho Y.K., et al. Tlusty T. Catalytic enzymes are active matter. Proc. Natl. Acad. Sci. USA. 2018;115:E10812–E10821. doi: 10.1073/pnas.1814180115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishizawa K., Bremerich M., et al. Mizuno D. Feedback-tracking microrheology in living cells. Sci. Adv. 2017;3:e1700318. doi: 10.1126/sciadv.1700318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishizawa K., Fujiwara K., et al. Mizuno D. Universal glass-forming behavior of in vitro and living cytoplasm. Sci. Rep. 2017;7:15143. doi: 10.1038/s41598-017-14883-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fletcher D.A., Mullins R.D. Cell mechanics and the cytoskeleton. Nature. 2010;463:485–492. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rigato A., Miyagi A., et al. Rico F. High-frequency microrheology reveals cytoskeleton dynamics in living cells. Nat. Phys. 2017;13:771–775. doi: 10.1038/nphys4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broedersz C., MacKintosh F. Modeling semiflexible polymer networks. Rev. Mod. Phys. 2014;86:995–1036. [Google Scholar]
- 12.Zimmerman S.B., Trach S.O. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J. Mol. Biol. 1991;222:599–620. doi: 10.1016/0022-2836(91)90499-v. [DOI] [PubMed] [Google Scholar]
- 13.Zimmerman S.B., Minton A.P. Macromolecular crowding - biochemical, biophysical, and physiological consequences. Annu. Rev. Biophys. Biomol. Struct. 1993;22:27–65. doi: 10.1146/annurev.bb.22.060193.000331. [DOI] [PubMed] [Google Scholar]
- 14.Delarue M., Brittingham G.P., et al. Holt L.J. mTORC1 controls phase separation and the biophysical properties of the cytoplasm by tuning crowding. Cell. 2018;174:338–349.e20. doi: 10.1016/j.cell.2018.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardel M.L., Shin J.H., et al. Weitz D.A. Elastic behavior of cross-linked and bundled actin networks. Science. 2004;304:1301–1305. doi: 10.1126/science.1095087. [DOI] [PubMed] [Google Scholar]
- 16.Storm C., Pastore J.J., et al. Janmey P.A. Nonlinear elasticity in biological gels. Nature. 2005;435:191–194. doi: 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- 17.Mizuno D., Tardin C., et al. Mackintosh F.C. Nonequilibrium mechanics of active cytoskeletal networks. Science. 2007;315:370–373. doi: 10.1126/science.1134404. [DOI] [PubMed] [Google Scholar]
- 18.Head D.A., Ikebe E., et al. Mizuno D. High-frequency affine mechanics and nonaffine relaxation in a model cytoskeleton. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2014;89:042711. doi: 10.1103/PhysRevE.89.042711. [DOI] [PubMed] [Google Scholar]
- 19.Head D.A., Mizuno D. Local mechanical response in semiflexible polymer networks subjected to an axisymmetric prestress. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2013;88:022717. doi: 10.1103/PhysRevE.88.022717. [DOI] [PubMed] [Google Scholar]
- 20.Mizuno D., Tardin C., Schmidt C.F. Rapid local compression in active gels is caused by nonlinear network response. Soft Matter. 2020;16:9369–9382. doi: 10.1039/c9sm02362c. [DOI] [PubMed] [Google Scholar]
- 21.Honda N., Shiraki K., et al. Mizuno D. Nonlinear master relation in microscopic mechanical response of semiflexible biopolymer networks. New J. Phys. 2022;24:053031. [Google Scholar]
- 22.Liu A.J., Nagel S.R. Jamming is not just cool any more. Nature. 1998;396:21–22. [Google Scholar]
- 23.Sollich P., Lequeux F., et al. Cates M.E. Rheology of soft glassy materials. Phys. Rev. Lett. 1997;78:2020–2023. [Google Scholar]
- 24.Petekidis G., Vlassopoulos D., Pusey P.N. Yielding and flow of sheared colloidal glasses. J Phys. Condens. Mat. 2004;16:S3955–S3963. [Google Scholar]
- 25.Wilson L.G., Harrison A.W., et al. Puertas A.M. Microrheology and the fluctuation theorem in dense colloids. Epl Europhys Lett. 2011;93:58007. [Google Scholar]
- 26.Alberts B. Garland Science, Taylor and Francis Group; 2015. Molecular Biology of the Cell. [Google Scholar]
- 27.Kelly G.M., Kilpatrick J.I., et al. Jarvis S.P. Bone cell elasticity and morphology changes during the cell cycle. J. Biomech. 2011;44:1484–1490. doi: 10.1016/j.jbiomech.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Tsai M.A., Waugh R.E., Keng P.C. Cell cycle-dependence of HL-60 cell deformability. Biophys. J. 1996;70:2023–2029. doi: 10.1016/S0006-3495(96)79768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurst S., Vos B.E., et al. Betz T. Intracellular softening and increased viscoelastic fluidity during division. Nat. Phys. 2021;17:1270–1276. [Google Scholar]
- 30.Nijenhuis N., Mizuno D., et al. Schmidt C.F. High-resolution microrheology in the pericellular matrix of prostate cancer cells. J. R. Soc. Interface. 2012;9:1733–1744. doi: 10.1098/rsif.2011.0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacKintosh F.C., Schmidt C.F. Microrheology. Curr Opin Colloid In. 1999;4:300–307. [Google Scholar]
- 32.Mason T.G., Ganesan K., et al. Kuo S.C. Particle tracking microrheology of complex fluids. Phys. Rev. Lett. 1997;79:3282–3285. [Google Scholar]
- 33.Hough L.A., Ou-Yang H.D. Correlated motions of two hydrodynamically coupled particles confined in separate quadratic potential wells. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2002;65(2 Pt 1):021906. doi: 10.1103/PhysRevE.65.021906. [DOI] [PubMed] [Google Scholar]
- 34.Mizuno D., Head D.A., et al. Schmidt C.F. Active and passive microrheology in equilibrium and nonequilibrium systems. Macromolecules. 2008;41:7194–7202. [Google Scholar]
- 35.Gittes F., Schmidt C.F. Interference model for back-focal-plane displacement detection in optical tweezers. Opt. Lett. 1998;23(1):7–9. doi: 10.1364/ol.23.000007. [DOI] [PubMed] [Google Scholar]
- 36.Sugino Y., Ikenaga M., Mizuno D. Optimization of optical trapping and laser interferometry in biological cells. Appl Sci-Basel. 2020;10:4970. [Google Scholar]
- 37.Guo M., Ehrlicher A.J., et al. Weitz D.A. Probing the stochastic, motor-driven properties of the cytoplasm using force spectrum microscopy. Cell. 2014;158:822–832. doi: 10.1016/j.cell.2014.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou E.H., Trepat X., et al. Fredberg J.J. Universal behavior of the osmotically compressed cell and its analogy to the colloidal glass transition. Proc. Natl. Acad. Sci. USA. 2009;106:10632–10637. doi: 10.1073/pnas.0901462106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tighe B.P. Relaxations and rheology near jamming. Phys. Rev. Lett. 2011;107:158303. doi: 10.1103/PhysRevLett.107.158303. [DOI] [PubMed] [Google Scholar]
- 40.Liu A.J., Ramaswamy S., et al. Weitz D.A. Anomalous viscous loss in emulsions. Phys. Rev. Lett. 1996;76:3017–3020. doi: 10.1103/PhysRevLett.76.3017. [DOI] [PubMed] [Google Scholar]
- 41.Jacob A.R., Poulos A.S., et al. Petekidis G. Flow dynamics of concentrated starlike micelles: a superposition rheometry investigation into relaxation mechanisms. J. Rheol. 2019;63:641–653. [Google Scholar]
- 42.Hoffman B.D., Massiera G., et al. Crocker J.C. The consensus mechanics of cultured mammalian cells. Proc. Natl. Acad. Sci. USA. 2006;103:10259–10264. doi: 10.1073/pnas.0510348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyamoto T., Uosaki H., et al. Shimano H. Rapid manipulation of mitochondrial morphology in a living cell with iCMM. Cell Rep. Methods. 2021;1:100052. doi: 10.1016/j.crmeth.2021.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koenderink G.H., Atakhorrami M., et al. Schmidt C.F. High-frequency stress relaxation in semiflexible polymer solutions and networks. Phys. Rev. Lett. 2006;96:138307. doi: 10.1103/PhysRevLett.96.138307. [DOI] [PubMed] [Google Scholar]
- 45.Mason T.G., Gisler T., et al. Weitz D.A. Rheology of F-actin solutions determined from thermally driven tracer motion. J. Rheol. 2000;44:917–928. [Google Scholar]
- 46.Morse D.C. Viscoelasticity of concentrated isotropic solutions of semiflexible polymers. 1. Model and stress tensor. Macromolecules. 1998;31:7030–7043. [Google Scholar]
- 47.Morse D.C. Viscoelasticity of concentrated isotropic solutions of semiflexible polymers. 2. Linear response. Macromolecules. 1998;31:7044–7067. [Google Scholar]
- 48.Gittes F., MacKintosh F.C. Dynamic shear modulus of a semiflexible polymer network. Phys. Rev. E. 1998;58:R1241–R1244. [Google Scholar]
- 49.Caspi A., Elbaum M., et al. Zbaida D. Semiflexible polymer network: a view from inside. Phys. Rev. Lett. 1998;80:1106–1109. [Google Scholar]
- 50.Caspi A., Granek R., Elbaum M. Diffusion and directed motion in cellular transport. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2002;66(1 Pt 1):011916. doi: 10.1103/PhysRevE.66.011916. [DOI] [PubMed] [Google Scholar]
- 51.Pavel M., Renna M., et al. Rubinsztein D.C. Contact inhibition controls cell survival and proliferation via YAP/TAZ-autophagy axis. Nat. Commun. 2018;9:2961. doi: 10.1038/s41467-018-05388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haase K., Pelling A.E. Resiliency of the plasma membrane and actin cortex to large-scale deformation. Cytoskeleton. 2013;70:494–514. doi: 10.1002/cm.21129. [DOI] [PubMed] [Google Scholar]
- 53.Simone L.C., Caplan S., Naslavsky N. Role of phosphatidylinositol 4,5-bisphosphate in regulating EHD2 plasma membrane localization. PLoS One. 2013;8:e74519. doi: 10.1371/journal.pone.0074519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stevenson B.R., Begg D.A. Concentration-dependent effects of cytochalasin D on tight junctions and actin filaments in MDCK epithelial cells. J. Cell Sci. 1994;107:367–375. doi: 10.1242/jcs.107.3.367. [DOI] [PubMed] [Google Scholar]
- 55.Casella J.F., Flanagan M.D., Lin S. Cytochalasin D inhibits actin polymerization and induces depolymerization of actin filaments formed during platelet shape change. Nature. 1981;293:302–305. doi: 10.1038/293302a0. [DOI] [PubMed] [Google Scholar]
- 56.Wakatsuki T., Schwab B., et al. Elson E.L. Effects of cytochalasin D and latrunculin B on mechanical properties of cells. J. Cell Sci. 2001;114(Pt 5):1025–1036. doi: 10.1242/jcs.114.5.1025. [DOI] [PubMed] [Google Scholar]
- 57.Godman G.C., Miranda A.F., et al. Tanenbaum S.W. Action of CYTOCHALASIN D on cells of established lines. J. Cell Biol. 1975;64:644–667. doi: 10.1083/jcb.64.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haga H., Sasaki S., et al. Sambongi T. Elasticity mapping of living fibroblasts by AFM and immunofluorescence observation of the cytoskeleton. Ultramicroscopy. 2000;82:253–258. doi: 10.1016/s0304-3991(99)00157-6. [DOI] [PubMed] [Google Scholar]
- 59.Wang A., Vijayraghavan K., et al. Butte M.J. Fast stiffness mapping of cells using high-bandwidth atomic force microscopy. ACS Nano. 2016;10:257–264. doi: 10.1021/acsnano.5b03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rotsch C., Radmacher M. Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: an atomic force microscopy study. Biophys. J. 2000;78:520–535. doi: 10.1016/S0006-3495(00)76614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fabry B., Maksym G.N., et al. Fredberg J.J. Scaling the microrheology of living cells. Phys. Rev. Lett. 2001;87:148102. doi: 10.1103/PhysRevLett.87.148102. [DOI] [PubMed] [Google Scholar]
- 62.Bacabac R.G., Mizuno D., et al. Smit T.H. Round versus flat: bone cell morphology, elasticity, and mechanosensing. J. Biomech. 2008;41:1590–1598. doi: 10.1016/j.jbiomech.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 63.Stewart M.P., Helenius J., et al. Hyman A.A. Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding. Nature. 2011;469:226–230. doi: 10.1038/nature09642. [DOI] [PubMed] [Google Scholar]
- 64.Fabry B., Maksym G.N., et al. Fredberg J.J. Time scale and other invariants of integrative mechanical behavior in living cells. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2003;68(4 Pt 1):041914. doi: 10.1103/PhysRevE.68.041914. [DOI] [PubMed] [Google Scholar]
- 65.Tee S.Y., Fu J., et al. Janmey P.A. Cell shape and substrate rigidity both regulate cell stiffness. Biophys. J. 2011;100:L25–L27. doi: 10.1016/j.bpj.2010.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grimm K.B., Oberleithner H., Fels J. Fixed endothelial cells exhibit physiologically relevant nanomechanics of the cortical actin web. Nanotechnology. 2014;25:215101. doi: 10.1088/0957-4484/25/21/215101. [DOI] [PubMed] [Google Scholar]
- 67.Ayala Y.A., Pontes B., et al. Nussenzveig H.M. Effects of cytoskeletal drugs on actin cortex elasticity. Exp. Cell Res. 2017;351:173–181. doi: 10.1016/j.yexcr.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 68.Koenderink G.H., Dogic Z., et al. Weitz D.A. An active biopolymer network controlled by molecular motors. Proc. Natl. Acad. Sci. USA. 2009;106:15192–15197. doi: 10.1073/pnas.0903974106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sakaue-Sawano A., Kurokawa H., et al. Miyawaki A. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 2008;132:487–498. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 70.Taubenberger A.V., Baum B., Matthews H.K. The mechanics of mitotic cell rounding. Front. Cell Dev. Biol. 2020;8:687. doi: 10.3389/fcell.2020.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fischer-Friedrich E., Toyoda Y., et al. Jülicher F. Rheology of the active cell cortex in mitosis. Biophys. J. 2016;111:589–600. doi: 10.1016/j.bpj.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garzon-Coral C., Fantana H.A., Howard J. A force-generating machinery maintains the spindle at the cell center during mitosis. Science. 2016;352:1124–1127. doi: 10.1126/science.aad9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Streppa L., Ratti F., et al. Argoul F. Prestressed cells are prone to cytoskeleton failures under localized shear strain: an experimental demonstration on muscle precursor cells. Sci. Rep. 2018;8:8602. doi: 10.1038/s41598-018-26797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Embry A.E., Mohammadi H., et al. Miller R.T. Biochemical and cellular determinants of renal glomerular elasticity. PLoS One. 2016;11:e0167924. doi: 10.1371/journal.pone.0167924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sutton T.A., Mang H.E., Atkinson S.J. Rho-kinase regulates myosin II activation in MDCK cells during recovery after ATP depletion. Am. J. Physiol. Renal. 2001;281:F810–F818. doi: 10.1152/ajprenal.2001.281.5.F810. [DOI] [PubMed] [Google Scholar]
- 76.Yubero M.L., Kosaka P.M., et al. Tamayo J. Effects of energy metabolism on the mechanical properties of breast cancer cells. Commun. Biol. 2020;3:590. doi: 10.1038/s42003-020-01330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gupta S.K., Guo M. Equilibrium and out-of-equilibrium mechanics of living mammalian cytoplasm. J. Mech. Phys. Solids. 2017;107:284–293. [Google Scholar]
- 78.Eguchi Y., Shimizu S., Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- 79.Lieberthal W., Menza S.A., Levine J.S. Graded ATP depletion can cause necrosis or apoptosis of cultured mouse proximal tubular cells. Am. J. Physiol. 1998;274:F315–F327. doi: 10.1152/ajprenal.1998.274.2.F315. [DOI] [PubMed] [Google Scholar]
- 80.Fernández-Marín B., Kranner I., et al. García-Plazaola J.I. Evidence for the absence of enzymatic reactions in the glassy state. A case study of xanthophyll cycle pigments in the desiccation-tolerant moss Syntrichia ruralis. J. Exp. Bot. 2013;64:3033–3043. doi: 10.1093/jxb/ert145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weber S.C., Spakowitz A.J., Theriot J.A. Nonthermal ATP-dependent fluctuations contribute to the in vivo motion of chromosomal loci. Proc. Natl. Acad. Sci. USA. 2012;109:7338–7343. doi: 10.1073/pnas.1119505109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fakhri N., Wessel A.D., et al. Schmidt C.F. High-resolution mapping of intracellular fluctuations using carbon nanotubes. Science. 2014;344:1031–1035. doi: 10.1126/science.1250170. [DOI] [PubMed] [Google Scholar]
- 83.Ahmed W.W., Fodor É., et al. Betz T. Active mechanics reveal molecular-scale force kinetics in living oocytes. Biophys. J. 2018;114:1667–1679. doi: 10.1016/j.bpj.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Turlier H., Fedosov D.A., et al. Betz T. Equilibrium physics breakdown reveals the active nature of red blood cell flickering. Nat. Phys. 2016;12:513–519. [Google Scholar]
- 85.Mizuno D., Bacabac R., et al. Schmidt C.F. High-resolution probing of cellular force transmission. Phys. Rev. Lett. 2009;102:168102. doi: 10.1103/PhysRevLett.102.168102. [DOI] [PubMed] [Google Scholar]
- 86.Umeda K., Nishizawa K., et al. Mizuno D. Activity-dependent glassy cell mechanics II : non-thermal fluctuations under metabolic activity. arXiv. 2023 doi: 10.48550/arXiv.2302.13323. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ariga T., Tomishige M., Mizuno D. Nonequilibrium energetics of molecular motor kinesin. Phys. Rev. Lett. 2018;121:218101. doi: 10.1103/PhysRevLett.121.218101. [DOI] [PubMed] [Google Scholar]
- 88.Bertrand O.J.N., Fygenson D.K., Saleh O.A. Active, motor-driven mechanics in a DNA gel. Proc. Natl. Acad. Sci. USA. 2012;109:17342–17347. doi: 10.1073/pnas.1208732109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Doi M., Edwards S.F. Clarendon Press; Oxford University Press; 1986. The Theory of Polymer Dynamics. [Google Scholar]
- 90.Schnurr B., Gittes F., et al. Schmidt C.F. Determining microscopic viscoelasticity in flexible and semiflexible polymer networks from thermal fluctuations. Macromolecules. 1997;30:7781–7792. [Google Scholar]
- 91.Angell C.A., Ngai K.L., et al. Martin S.W. Relaxation in glassforming liquids and amorphous solids. J. Appl. Phys. 2000;88:3113–3157. [Google Scholar]
- 92.Berthier L., Biroli G. In: Encyclopedia of Complexity and Systems Science. Meyers R.A., editor. Springer New York; 2009. Glasses and aging, A statistical mechanics perspective on; pp. 4209–4240. [Google Scholar]
- 93.Wyss H.M., Miyazaki K., et al. Weitz D.A. Strain-rate frequency superposition: a rheological probe of structural relaxation in soft materials. Phys. Rev. Lett. 2007;98:238303. doi: 10.1103/PhysRevLett.98.238303. [DOI] [PubMed] [Google Scholar]
- 94.Krishan K., Helal A., et al. Cohen-Addad S. Fast relaxations in foam. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2010;82(1 Pt 1):011405. doi: 10.1103/PhysRevE.82.011405. [DOI] [PubMed] [Google Scholar]
- 95.Semmrich C., Storz T., et al. Kroy K. Glass transition and rheological redundancy in F-actin solutions. Proc. Natl. Acad. Sci. USA. 2007;104:20199–20203. doi: 10.1073/pnas.0705513104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chapman C.D., Robertson-Anderson R.M. Nonlinear microrheology reveals entanglement-driven molecular-level viscoelasticity of concentrated DNA. Phys. Rev. Lett. 2014;113:098303. doi: 10.1103/PhysRevLett.113.098303. [DOI] [PubMed] [Google Scholar]
- 97.Kroy K., Glaser J. The glassy wormlike chain. New J. Phys. 2007;9:416. [Google Scholar]
- 98.Atakhorrami M., Koenderink G., et al. Schmidt C. Scale-dependent nonaffine elasticity of semiflexible polymer networks. Phys. Rev. Lett. 2014;112:088101. [Google Scholar]
- 99.Marchetti M.C., Joanny J.F., et al. Simha R.A. Hydrodynamics of soft active matter. Rev. Mod. Phys. 2013;85:1143–1189. [Google Scholar]
- 100.Bechinger C., Di Leonardo R., et al. Volpe G. Active particles in complex and crowded environments. Rev. Mod. Phys. 2016;88:45006. [Google Scholar]
- 101.Hunter G.L., Weeks E.R. The physics of the colloidal glass transition. Rep. Prog. Phys. 2012;75:066501. doi: 10.1088/0034-4885/75/6/066501. [DOI] [PubMed] [Google Scholar]
- 102.Janssen L.M.C. Active glasses. J. Phys. Condens. Matter. 2019;31:503002. doi: 10.1088/1361-648X/ab3e90. [DOI] [PubMed] [Google Scholar]
- 103.Mongera A., Rowghanian P., et al. Campàs O. A fluid-to-solid jamming transition underlies vertebrate body axis elongation. Nature. 2018;561:401–405. doi: 10.1038/s41586-018-0479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Berthier L., Kurchan J. Non-equilibrium glass transitions in driven and active matter. Nat. Phys. 2013;9:310–314. [Google Scholar]
- 105.Nandi S.K., Mandal R., et al. Gov N.S. A random first-order transition theory for an active glass. Proc. Natl. Acad. Sci. USA. 2018;115:7688–7693. doi: 10.1073/pnas.1721324115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oyama N., Kawasaki T., et al. Ikeda A. Glassy dynamics of a model of bacterial cytoplasm with metabolic activities. Phys. Rev. Res. 2019;1:032038. [Google Scholar]
- 107.Mandal R., Bhuyan P.J., et al. Rao M. Extreme active matter at high densities. Nat. Commun. 2020;11:2581. doi: 10.1038/s41467-020-16130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mandal R., Bhuyan P.J., et al. Dasgupta C. Active fluidization in dense glassy systems. Soft Matter. 2016;12:6268–6276. doi: 10.1039/c5sm02950c. [DOI] [PubMed] [Google Scholar]
- 109.Koyano Y., Kitahata H., Mikhailov A.S. Diffusion in crowded colloids of particles cyclically changing their shapes. Epl-Europhys Lett. 2019;128:40003. [Google Scholar]
- 110.Ross J.L. The dark matter of biology. Biophys. J. 2016;111:909–916. doi: 10.1016/j.bpj.2016.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ariga T., Tateishi K., et al. Mizuno D. Noise-induced acceleration of single molecule kinesin-1. Phys. Rev. Lett. 2021;127:178101. doi: 10.1103/PhysRevLett.127.178101. [DOI] [PubMed] [Google Scholar]
- 112.Nishizawa K., Honda N., et al. Mizuno D. Feedback microrheology in soft matter. arXiv. 2021 doi: 10.48550/arXiv.2106.05119. Preprint at. [DOI] [Google Scholar]
- 113.Ezber Y., Belyy V., et al. Yildiz A. Dynein harnesses active fluctuations of microtubules for faster movement. Nat. Phys. 2020;16:312–316. doi: 10.1038/s41567-019-0757-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.