Summary
Background
China has approximately 300 million current smokers, and smoking cessation services are limited. This study aimed to assess the efficacy of a Cognitive Behavioral Theory-based smoking cessation intervention (‘WeChat WeQuit’) via the most popular social media platform in China, WeChat.
Methods
A parallel, single-blind, two-arm randomised controlled trial was conducted via WeChat between March 19, 2020 and November 16, 2022. Chinese-speaking adult smokers (n = 2000) willing to quit within one month were recruited and randomised in a 1:1 ratio. The intervention group (n = 1005) received the ‘WeChat WeQuit’ program and the control group (n = 955) received control messages for 14 weeks (2-week prequit and 12-week postquit). Participants were followed up to 26 weeks after the quit date. The primary outcome was self-reported continuous smoking abstinence rate, biochemically validated at 26 weeks. The secondary outcomes were self-reported 7-day and continuous abstinence rates at 6 months. All analyses were by intention to treat. The trial is registered at ClinicalTrials.gov (NCT03169686).
Findings
By intention-to-treat analysis, the biochemically verified 26-week continuous abstinence rate was 11.94% in the intervention group and 2.81% in the control group (OR = 4.68, 95% CI: 3.07–7.13, p < 0.0001). The self-reported 7-day abstinence rates ranged from 39.70% at week 1–32.04% at week 26 for the intervention group and 14.17%–11.86% in the control group for weeks 1 and 26, respectively; the self-reported continuous abstinence rates at weeks 1 and 26 ranged from 34.33% to 24.28% and 9.65% to 6.13% in the intervention group and the control group respectively (all p < 0.0001). Participants with low nicotine dependence or previous quit attempts were more likely to successfully quit smoking.
Interpretation
The ‘WeChat WeQuit’ intervention significantly increased smoking abstinence rates at 6 months and should be considered for treatment-seeking smokers in China.
Funding
The research is supported by the Natural Science Foundation of Hunan Province (2020JJ4794, YLiao), the K.C. Wong Postdoctoral Fellowship for YLiao to study at King’s College London, and China Medical Board (CMB) Open Competition Program (grant no. 15-226, 22-485, YLiao).
Keywords: Online smoking cessation, Quit smoking, ‘WeChat WeQuit’, WeChat-based intervention, Randomised controlled trial
Research in context.
Evidence before this study
We conducted a literature search on PubMed, Google Scholar, Cochrane database, the China National Knowledge Infrastructure, Wanfang data resource for randomised controlled trials (RCT) of the most popular social media platform of WeChat-based interventions for smoking cessation published in Chinese and English, from database inception to March 1, 2020 using “WeChat” and “smoking” or “tobacco” or “smoking cessation”. The strategy identified 4 relevant articles, of which one was a real world two-arm study to examine bupropion hydrochloride sustained-release tablets with psychological intervention, this trial used the WeChat platform to follow up the rate of smoking cessation, reporting that the 1-month smoking cessation rate in 3 months follow-up was 57.8% in the bupropion group (n = 199) and 10.7% in the only psychological intervention group (n = 84); one was a survey study examining the acceptability of WeChat and text messaging-based tobacco control intervention among parents who smoked from rural China; another was a qualitative study exploring the perception of using Facebook messenger, WeChat, and other instant messaging (IM) apps to deliver chat-based smoking cessation intervention for Chinese community smokers in Hong Kong; we only identified one study protocol for a randomised controlled trial of WhatsApp/WeChat-based brief motivational interviewing (i-BMI) for smokers with chronic diseases. We failed to find any results reported by the RCT of the efficacy of WeChat-based smoking cessation.
Added value of this study
This trial shows that WeChat-based smoking cessation support (‘WeChat WeQuit’ program) is effective in increasing abstinence among cigarette smokers from the general population of China. The biochemically verified 26-week continuous abstinence rate was significantly higher for participants who received the ‘WeChat WeQuit’ program than those allocated to the control group. We also noted over twice as many participants who received the ‘WeChat WeQuit’ cessation intervention had self-reported 7-day and continuous abstinence rates than those who did not receive it.
Implications of all the available evidence
To the best of our knowledge, we have provided the first robust evidence to support the use of the ‘WeChat WeQuit’ program as a novel method for smoking cessation in China. Based on the results of our trial, health care providers and policy makers should recommend WeChat-based cessation support to every smoker, to address the high levels of smoking and the levels of unmet need for the vast number of treatment-seeking smokers in China.
Introduction
Tobacco use has enormous health and economic consequences. The Global Burden of Disease Study 2019 revealed that cigarette smoking is the leading risk factor for risk-attributable cancer deaths and disability-adjusted life-years (DALYs).1,2 In China, the situation is particularly severe, with approximately 300 million smokers and more than 1 million annual deaths from tobacco-caused diseases.1,3,4 Chinese people consumed 44% of the world’s cigarettes in 2014, at an estimated total cost of $57 billion per year.5 According to the Global Adult Tobacco Surveys (GATS) in 2018, the smoking rate among smokers aged 15 and older in China was 26.6%, with 50.5% of adult men and 2.1% of women being smokers.6 According to GATS China 2010 and 2018, despite a slight decrease in smoking prevalence over the past decade, the actual number of adult smokers has increased from 301 million in 2010 to 307.6 million in 2018 due to population growth.
In China, smoking cessation services and medications are not yet covered by basic health insurance, and access to them is limited.7 More than 90% of smokers who tried to quit smoking in the past 12 months did not receive any professional help,6 due to a lack of trained health care providers and a lack of access to effective medications. According to a national survey, 366 health care institutions have established smoking cessation clinics around China, but only 43% of them provided smoking cessation medications (also difficult to obtain in pharmacies), and only 1–2 patients per week visit cessation clinics on average.8,9 Furthermore, cultural and social barriers to seeking help for tobacco dependence may also play a role. According to GATS China 2018, although up to 86% of people believe that smoking causes disease, and 19.8% of smokers (nearly 60 million Chinese) had tried to quit smoking in the past 12 months, the 6-month continuous abstinence rate remains very low.6,10 Most ex-smokers quit smoking only by their own willpower,11 further highlighting the need for support. Given the current situation, exploring evidence-based, effective, low-cost, and widely accessible smoking cessation services is of great practical significance.
Billions of people worldwide use social media12; thus, using social media for smoking cessation seems feasible.13,14 Given the popularity of social media, there may be more opportunities to reach people who are challenging to engage through traditional smoking cessation forms. Besides, social media platforms are highly interactive, and allow users to connect with others, to get support from others, and share their experiences with others. Social media-based smoking cessation programs usually help smokers quit by delivering information related to smoking cessation and providing opportunities to directly interact with others. Studies have shown that Facebook and other social media is effective for smoking cessation.14,15 In China, WeChat, similar to Facebook, hasover 1 billion monthly active users.
Recently, WeChat has become the premier mobile social media app in China, offering a wide array of features including instant messaging for text, images, and files, video calling, and content sharing for web pages, images and articles. Its robust social network capabilities also allow it to merge features and functionalities commonly found on Facebook, Instagram, and WhatsApp, making it an all-in-one social media platform. WeChat Public Platform accounts and mini-programs (https://mp.weixin.qq.com/) provide various health, business, and other services for individuals and organizations. With the features of simplicity, affordability, immediacy, widespread availability, and ease of access, the WeChat Users by Country 202216 reported that there are more than 1 billion people in China who use WeChat regularly. In recent years, a growing number of researchers or organizations use WeChat to carry out scientific research or medical and health services, such as health education, behavior promotion, follow-up care interventions.17 Its advantages of improving the communication efficiency, increasing follow-up rates, wide coverage, convenience for the elderly and inexperienced smartphone users, make it the most promising platform for conducting online interventions for smokers to quit.17, 18, 19, 20, 21 Several studies indicate that WeChat-based smoking cessation is feasible and effective for Chinese smokers, but the results of abstinence rates are inconsistent across studies.22, 23, 24, 25, 26 Thus, there is a need for evidence from large sample size, high-quality research on smoking cessation interventions through WeChat to provide more accurate and reliable results.
Cognitive Behavioral Therapy (CBT) has been considered as a gold standard of psychotherapy,27 and is widely used in smoking cessation and other substance use disorders treatment.28 Comprehensive intervention measures for quitting smoking include strengthening smoking cessation motivation, correcting misperceptions that lead to smoking, training in skills to cope with stress and cravings, preventing relapse, and maintenance of long-term smoking abstinence.29 Our previous research demonstrated the efficacy of a smoking cessation intervention via text messages (CBT-based ‘Happy Quit’) in China.30 This ‘WeChat WeQuit’ program focuses on changing participants’ thoughts and behaviors that lead to smoking, such as changing thinking patterns, identifying social or environmental cues, seeking social support, relaxation training, problem-solving strategies, managing weight via healthy eating and exercise (the curriculums in details see Supplementary File S1). The WeChat-based intervention includes all features of text message-based intervention, and it has some unique features, such as hold-to-talk voice messaging, broadcast (one-to-many) messaging in WeChat group with as many as 500 users, video calls, sharing for photograph, audio, video, and web links. However, our assessment of the existing literature indicated there was no research exploring the efficacy of CBT-based smoking cessation intervention through WeChat. In response to this, we designed a CBT-based mobile smoking cessation intervention (‘WeChat WeQuit’ program) for treatment-seeking smokers in China. The primary aim of this study is to evaluate the efficacy (preliminary by evaluating biochemically verified 26-week continuous quit rate) of the ‘WeChat WeQuit’ program in China. We hypothesized that this program could significantly improve quit rates among current smokers interested in cessation.
Methods
Study design, participants and recruitment
This was a WeChat-based, single-blind, two-arm RCT (randomised controlled trial) undertaken in China without regional restrictions between March 19, 2020 and November 16, 2022. Research staff, including clinical trial monitors, research assistants, and statisticians, were blinded to the treatment group assignments. Current smokers who wanted to quit smoking within a month were invited to join the program through online or offline advertisements, and participants who met eligibility criteria were randomly assigned to the intervention group or the control group in a 1:1 ratio. The intervention group received the ‘WeChat WeQuit’ program for 14 weeks (2 weeks during prequit stage and 12 weeks during the postquit stage), while the control group received control messages (e.g. thanks for their participation, reminders of selected quit date, and reminders of the last follow-up date). Both groups were followed up at 26 weeks after the quit date.
The eligibility criteria for participants were: cigarette smokers (smoked more than 100 cigarettes, and currently smoked five cigarettes or more per day), aged 18 and above, could read and write in Chinese, had a smartphone and owned a WeChat personal account, wanted to quit smoking within one month (by responding YES to the question of “I really want to stop smoking and intend to within one month”), and were willing to provide informed consent to participate in the study. Participants who had begun to quit smoking within the past three months or were receiving other smoking cessation treatment at the time of information checking were excluded from this study.
Participants were recruited from March 19, 2020 to May 2, 2022 via online (WeChat, web news, and Weibo) and offline (communities and hospitals) advertisements. Potential participants were encouraged to contact us by sending messages or making a call. More than 90% of participants were recruited from online advertisements. Research assistants (Dr. Yi Liu, Dr. Ling Li, PN. (Nurse Practitioner) Zitang Zhou, Dr. Yunfei Wang) responded to evaluate eligibility and explain the purpose of the study to each participant. All participants were told that they would be randomly assigned to the intervention group or the control group, and they were free to withdraw from the research at any point in time. Then, they were asked to provide their telephone number (strictly confidential and used only for this study) for use in an emergency and subscribe to the WeChat subscription account named “科学戒烟日志” (“Evidence-based Journey to Quit Smoking”, “Ke-Xue-Jie-Yan-Ri-Zhi” in Chinese pinyin) in Chinese. Finally, a link containing electronically informed consent and another link containing baseline information were sent to each participant separately.
Intervention
The design of this WeChat-based ‘WeChat WeQuit’ intervention was primarily based on Cognitive Behavioral Theory (CBT) and our previous text message-based ‘Happy Quit’ intervention.30 The ‘WeChat WeQuit’ program contained two different sections: 1) “preparing to quit” focuses on motivation to quit and helps participants develop a quit plan, identifies smoking patterns, triggers, reasons for quit, and sources of support, teaches relaxation techniques, etc. 2) “quitting and relapse prevention” teaches tips to cope with cravings or difficult situations, strategies to deal with weight gain, skills for preventing relapse, resources for how to maintain a healthy lifestyle, identifying peer support, and so on. The intervention has been developed by Dr. Liao (Supplementary File S1 for the intervention curriculums) and evaluated by a pilot study (Supplementary File S2 for pilot results). Three to five messages were sent to participants who were allocated to the intervention group during the first four weeks, then one to three messages were sent until 12 weeks. Relaxation audios and pictures were presented in the WeChat subscription account for them to use at any time. About 80% of information was read by participants. This CBT based program focuses on how participants think about quitting (‘cognitive’ principles) and what they do when quitting (‘behavior’ principles). More details about the development of the ‘WeChat WeQuit’ program is described in the study protocol.31
Ethics statement
Each participant was signed an electronically informed consent before enrolling in this study. Ethical approval was granted by the ethics committee of the National Clinical Research Center, the Second Xiangya Hospital (2018 Ethics Approval File no. 010), and the Sir Run Run Shaw Hospital (2020 Ethics Approval File no. 34).
Baseline data collection, randomisation, and group allocation
Demographic information (such as gender, age, and educational level) and smoking characteristics (including cigarettes smoked per day, motivation to quit, self-efficacy to quit, nicotine dependence, and craving) were collected from all participants at baseline. Motivation to quit and self-efficacy were measured by the Visual Analogue Scale (VAS = response options were integers from 0 (not at all) to 10 (very much), used to assess the levels of motivation, self-efficacy, and craving, i.e. higher scores indicate greater motivation or self-efficacy, stronger craving); nicotine dependence was measured by the Chinese version of the Fagerström Test for Cigarette Dependence (FTCD)32 and severe or high dependence was defined as FTCD ≥ 5; smoking craving was measured by the VAS and the modified Penn Alcohol Craving Scale with replacement of “alcohol” to “smoking” (PACS). Adverse events were measured by an open-ended question 2 weeks after quit date and at the last follow-up. Participants were equally assigned to the intervention group or the control group based on the stratified random method after completing the baseline information and being checked for eligibility by the research assistants. The process was fully automated and computerized by Microsoft SQL Server 2008. The balanced factors included gender (male or female), age (≤34 or >34 years), educational level (≤ or >12 years), cigarettes smoked per day (≤20 or >20 cigarettes), and FTCD (≤5 or >5 scores).
Procedures
We used “Quit Date” to divide the process of the program into two stages: preparation/prequit stage (2 weeks) and postquit stage (12 weeks, earlier postquit stage focused on dealing with withdrawal symptoms and later on preventing relapse). After randomisation, all participants from both groups were mailed a physical copy of a book with smoking cessation guidance. Participants from the intervention group received the ‘WeChat WeQuit’ program from the WeChat subscription account. The period for intervention was 14 weeks, including 2-week quitting preparation intervention and 12-week postquit intervention. The number of messages/pictures/audios intensively sent to them during the 2-week prequit and 4 weeks postquit, then less intensively from week 5 to week 12 postquit. All interventions were automatically delivered by the WeChat subscription account. A WeChat group was created for answering questions from participants, and sharing the latest resources related to quitting smoking, and promoting communication between participants (peer support), as well as providing interactive platform between participants and health care providers. In addition, intervention participants could contact us at any time through WeChat one-to-one private chat, or phone calls for help (no additional support for participants from the control group). Participants from the control group received no smoking cessation messages by the study team; they only received follow-up questionnaires at check-up points, reminders of their quit date and the end of the trial to receive the WeChat-based intervention of “Evidence-based Journey to Quit Smoking”.
Online-based follow-up questionnaires were automatically sent to all participants at weeks 1, 4, 8, 12, 16, 20, and 26 after the quit date. Participants completed information at each time-point, including baseline and follow-up questionnaires, via a link that automatically sent by the WeChat subscription account (“Ke-Xue-Jie-Yan-Ri-Zhi”). No participants withdrew from the study, thus all participants received all links automatically for completing follow-up questionnaires. Automatic reminders were sent to participants who did not submit any follow-up questionnaires on the next day. All data were collected and stored on a cloud server. The groups were unblinded after completing data analysis.
Outcomes
The primary outcome measure was self-reported continuous smoking abstinence rate (abstinence over the whole follow-up period), biochemically validated at 26 weeks after quit day. The researcher contacted participants who had self-reported 26-week continuous smoking abstinence to further confirm their smoking status, then mailed cotinine urine dipsticks with urine cotinine cut-off point of 200 ng/ml to verify their smoking status. All test results were confirmed by sending photographs33 and then further confirmed by themselves and their family members via online communication. The secondary outcome measures included: acceptability and feasibility of the program (assessed at 4 weeks after the quit date), and the rate of completion of the program/rate of follow-up; self-reported 7-day point prevalence of abstinence at weeks 1, 4, 8, 12, 16, 20, and 26 after the quit date, and self-reported continuous smoking abstinence rate at weeks 4, 8, 12, 16, 20, and 26 after the quit date; and the reduction of cigarette consumption (compared to baseline, the reduction of cigarettes smoked per day at 1, 4, 8, 12, 16, 20, and 26 weeks among participants who were still smoking after the quit date).
Statistical analyses
The sample size estimation of recruiting 2000 participants was primarily based on our previous study of ‘Happy Quit’30 as well as our pilot results (see Supplementary File S2) (for more details, see the study protocol31). On the basis of the results of Happy Quit with a total of 1369 participants, biochemically verified continuous smoking abstinence at 24 weeks was 6.5%, 6.0% and 1.9% in the high-frequency messaging (HFM) group, the low-frequency messaging (LFM) group, and the control group, respectively. It is estimated that, for assessing biologically verified continuous smoking abstinence, 1162 participants (518 participants in each group) are required to achieve 95% power (1-beta = 0.95), as significant at the 5% level (alpha = 0.05), of an increase in the primary outcome measure from 1.9% in the control group to 6.0% in the intervention group. On the basis of the pilot results (biochemically verified continuous smoking abstinence at 26 weeks was 8.1% in the intervention group and 1.8% in the control group), we only needed to recruit a total of 488 participants. In this study, a final target sample size of 2000 participants (1000 in each arm) could have more than a 95% chance of detecting a significant difference.
Data were analyzed by R software (version 4.2.1) between November 20 and December 12, 2022. Smoking cessation/abstinence rates were calculated as the number of abstinent participants divided by the number of total participants at baseline. Baseline characteristics between the study groups, between completers and incompleters, and incompleters’ baseline characteristics between the two study groups were compared by the chi-square test for categorical variables and the Student’s t-test or Wilcoxon rank-sum test for continuous variables, as appropriate. The Shapiro–Wilk test was used to test the normality.
Data analyses were based on an intention-to-treat (ITT),34 all randomised participants were included in the analysis. For the primary analyses, we used multiple imputation (MI) to impute missing outcome data. Participants with missing abstinence outcomes (self-reported 7-day point prevalence of abstinence at 1, 4, 8, 12, 16, 20, and 26 weeks) were imputed by multiple imputation models, using study group, age, gender, education, urban-rural, regions, cigarettes smoked per day at baseline, FTCD score, BMI, years of smoking, previous quit history, PACS score, and VAS score for motivation to quit, self-efficacy, and craving. Rubin’s rule has been used to pool the estimates from 10 imputed datasets. We also did two sensitivity analyses for the abstinence outcomes. First, we applied the last observation carried forward (LOCF) analyses: participants with missing outcome measures were considered to have no change in smoking behavior from the last observation. Second, we did per-protocol (PP) analyses by excluding participants with missing outcomes.
Logistic regression models have been applied to examine the intervention effect on outcomes, including biochemically verified continuous abstinence at 26 weeks; self-reported 7-day point prevalence of abstinence at 1, 4, 8, 12, 16, 20, and 26 weeks; and self-reported continuous abstinence at 4, 8, 12,16, 20, and 26 weeks. Odds ratios (OR) were used to measure the outcomes for the intervention group compared with the control group. Each model was further adjusted for the following eight baseline categorical variables: gender, age, education, urban-rural, regions, cigarettes smoked per day at baseline, FTCD score, and previous quit attempt. We also examined the intervention effect of the primary outcome in subgroups that were defined by eight categorical baseline variables: gender, age, education, urban-rural, regions, cigarettes smoked per day at baseline, FTCD score, and previous quit attempt. The condition × subgroup interactions and cessation rates in the intervention and control groups, were assessed with the use of the logistic regression models. We used Kaplan–Meier curves and the logrank test for analyses of time to relapse. All tests were two-sided, with p values of less than 0.005 judged as statistically significant.
This study has been registered at ClinicalTrials.gov, Identifier: NCT03169686, website: https://clinicaltrials.gov/ct2/show/NCT03169686.
Role of the funding source
The research is supported by the Natural Science Foundation of Hunan Province (2020JJ4794, YLiao), the K.C. Wong Postdoctoral Fellowship for YLiao to study at King’s College London, and China Medical Board (CMB) Open Competition Program (grant no. 15-226, 22-485, YLiao). The funders had no role in study design, data collection and analysis, decision to write the report or to submit the paper for publication. The corresponding author Dr. Yanhui Liao had access to dataset and decision to submit for publication.
Results
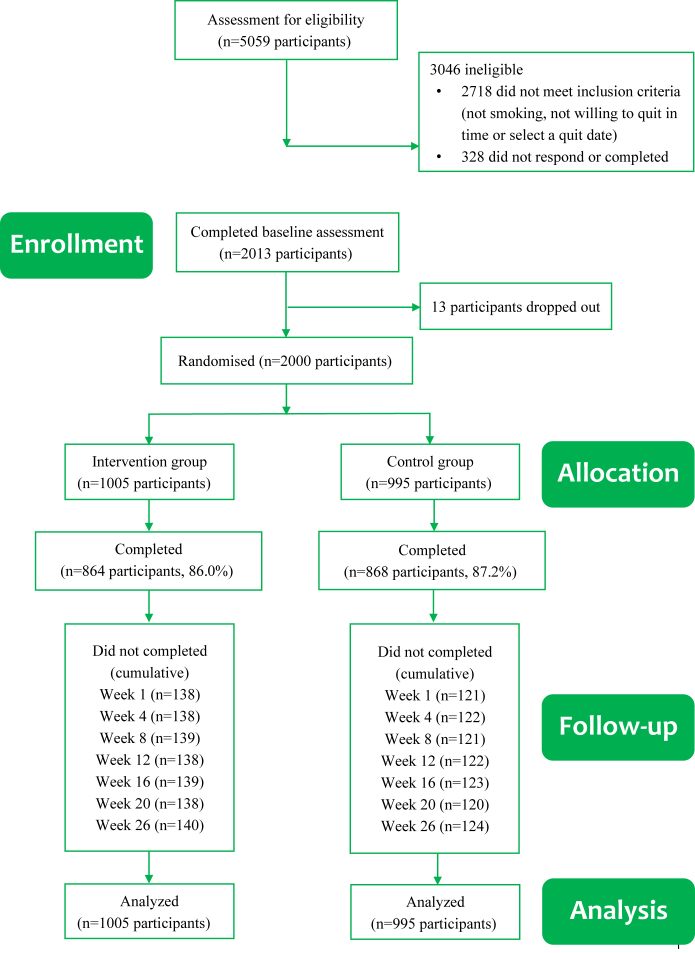
Between March 19, 2020 and November 16, 2022, 5059 potential participants were screened for eligibility; 2341 were found eligible; 2013 provided informed consent; and 2000 completed baseline assessment, were included in the trial, and were randomly assigned to either the intervention or the control group (Fig. 1). The acceptability of the ‘WeChat WeQuit’ program (see the study protocol31) was assessed between March 19 and June 28, 2020 (the first 100 participants from the intervention group, response rate was 96%), and displayed in Supplementary Table S1. The overall program satisfaction was 89.6%. During the trial, no treatment-related serious adverse events were reported from the participants, except for some common withdrawal symptoms (such as irritability, anxiety, depression, and insomnia) during the first one or two weeks after quit date. A few participants reported using smoking cessation medication (n = 8) or electronic cigarettes (n = 3). None of these participants reported any safety issues, and none of them succeeded in quitting smoking.
Fig. 1.
CONSORT flow diagram.
By ITT, the primary analyses included all participants randomly assigned to the intervention group (n = 1005) or control group (n = 995). There were no significant differences in overall follow-up rates between the two study groups (86.0% in the intervention group and 87.2% in the control group, χ2 = 0.691, p = 0.406). The baseline characteristics were well balanced between groups (Table 1). Baseline characteristics between participants who completed all follow-ups and loss to follow-up participants is shown in Supplementary Table S2; participants who received no more than 12 years of education were more likely to be lost to follow-up. Baseline characteristics of those lost to follow-up shows no statistical significance between the two study groups (Supplementary Table S3). The geographical distribution across China’s 31 provinces/regions of participants is provided in Supplementary Table S4.
Table 1.
Demographic characteristics at baseline.
| Characteristic | Intervention group (n = 1005) | Control group (n = 995) | p-values |
|---|---|---|---|
| Gender | 0.8094 | ||
| Male | 942 (93.73%) | 930 (93.47%) | |
| Female | 63 (6.27%) | 65 (6.53%) | |
| Age (years) | 0.9524 | ||
| ≤34 | 326 (32.44%) | 324 (32.56%) | |
| >34 | 679 (67.66%) | 671 (67.44%) | |
| Education (years) | 0.2566 | ||
| ≤12 | 205 (20.40%) | 183 (18.39%) | |
| >12 | 800 (79.60%) | 812 (81.61%) | |
| Urban-Rural | 0.3765 | ||
| Urban | 881 (87.66%) | 859 (86.33%) | |
| Rural | 124 (12.34%) | 136 (13.67%) | |
| Regions | 0.6241 | ||
| East | 511 (50.85%) | 507 (50.95%) | |
| Middle | 300 (29.85%) | 311 (31.26%) | |
| West | 194 (19.30%) | 177 (17.79%) | |
| Cigarettes smoked per day | 0.2141 | ||
| ≤10 | 216 (21.49%) | 222 (22.31%) | |
| 11–20 | 596 (59.30%) | 564 (56.68%) | |
| 21–30 | 146 (14.53%) | 142 (14.27%) | |
| >30 | 47 (4.68%) | 67 (6.73%) | |
| FTCD score | 0.2665 | ||
| ≤5 | 693 (68.96%) | 663 (66.63%) | |
| >5 | 312 (31.04%) | 332 (33.37%) | |
| BMI | 24.82 ± 3.41 | 24.76 ± 3.57 | 0.4129 |
| Years of smoking | 18.9 ± 9.6 | 19.3 ± 9.7 | 0.9975 |
| Previous quit attempts | 0.3698 | ||
| Yes | 741 (73.73%) | 751 (75.48%) | |
| No | 264 (26.27%) | 244 (24.52%) | |
| Motivation to quit | 8.3 ± 1.9 | 8.2 ± 2.1 | 0.9869 |
| Self-efficacy to quit | 7.4 ± 2.3 | 7.2 ± 2.4 | 0.3958 |
| PACS score | 17.3 ± 4.9 | 17.6 ± 4.8 | 0.7518 |
| VAS score | 6.5 ± 2.1 | 6.7 ± 2.0 | 0.2506 |
Data are n (%) or Mean ± SD.
FTCD: Fagerstrom Test for Nicotine Dependence; BMI: Body Mass Index (kg/m2); PACS: Penn Alcohol Craving Scale; VAS: Visual Analogue Scale (0–10 scores); p-values: chi-square test or t-test/Mann Whitney Wilcoxon test.
The biochemically verified 26-week continuous smoking abstinence rate was higher in the intervention group (11.94% vs 2.81%, OR = 4.68, 95% CI: 3.07–7.13, p < 0.0001) compared with the control group (Table 2). Self-reported 7-day point prevalence abstinence rate at 1, 4, 8, 12, 16, 20, and 26 weeks after the quit date, and self-reported continuous smoking abstinence rate at weeks 4, 8, 12, 16, 20, and 26 after the quit date are shown in Table 2, Supplementary Figs. S1 and S2. The secondary outcomes of self-reported 7-day point and continuous abstinence rates at all follow-up points were higher in the intervention groups than those in the control group (all p < 0.0001). The probability of continuous abstinence between the two study groups is shown in Supplementary Fig. S3. Quit/abstinence rates were gradually decreased at each assessment point in both groups. The self-reported 7-day point abstinence rates ranged from 39.70% at week 1%–32.04% at week 26 for the intervention group and 14.17%–11.86% in the control group. The continuous abstinence rates ranged from 34.33% at week 1%–24.28% at week 26 for the intervention group and 9.65%–6.13% in the control group (Table 2). The results of significantly higher quit rates in the intervention group than the control group were confirmed by the last observation carried forward (Supplementary Table S5) and per-protocol analyses (Supplementary Table S6). In addition, smokers (those who reported smoking more than 5 cigarettes after the quit date) in both groups consumed about 4 fewer cigarettes per day over the 26 weeks follow-up after the quit date (Supplementary Table S7).
Table 2.
Verified continuous smoking abstinence and 7-day point prevalence of abstinence between two groups by intention-to-treat analysis (multiple imputation).
| Outcome | Intervention group |
Control group |
OR (95%CI) | AOR (95%CI)a | p-value |
|---|---|---|---|---|---|
| N = 1005 | N = 995 | ||||
| Primary outcome | |||||
| Verified abstinence | 120 (11.94%) | 28 (2.81%) | 4.68 (3.07–7.13) | 4.65 (3.09–7.23) | <0.0001 |
| Secondary outcomes | |||||
| Self-reported 7-day point prevalence of abstinence | |||||
| 1 week | 399 (39.70%) | 141 (14.17%) | 3.99 (3.21–4.96) | 4.08 (3.28–5.10) | <0.0001 |
| 4 weeks | 394 (39.20%) | 139 (13.97%) | 3.97 (3.19–4.94) | 4.09 (3.28–5.12) | <0.0001 |
| 8 weeks | 398 (39.60%) | 143 (14.37%) | 3.91 (3.15–4.86) | 4.05 (3.25–5.07) | <0.0001 |
| 12 weeks | 371 (36.92%) | 135 (13.57%) | 3.73 (2.99–4.66) | 3.84 (3.07–4.83) | <0.0001 |
| 16 weeks | 359 (35.72%) | 121 (12.16%) | 4.01 (3.19–5.05) | 4.11 (3.26–5.21) | <0.0001 |
| 20 weeks | 339 (33.73%) | 131 (13.17%) | 3.36 (2.68–4.21) | 3.43 (2.73–4.32) | <0.0001 |
| 26 weeks | 322 (32.04%) | 118 (11.86%) | 3.50 (2.77–4.42) | 3.54 (2.81–4.50) | <0.0001 |
| Self-reported continuous abstinence | |||||
| 4 weeks | 345 (34.33%) | 96 (9.65%) | 4.90 (3.83–6.28) | 5.01 (3.91–6.46) | <0.0001 |
| 8 weeks | 311 (30.95%) | 81 (8.14%) | 5.06 (3.89–6.59) | 5.18 (3.98–6.80) | <0.0001 |
| 12 weeks | 293 (29.15%) | 70 (7.04%) | 5.44 (4.12–7.19) | 5.59 (4.24–7.46) | <0.0001 |
| 16 weeks | 277 (27.56%) | 67 (6.73%) | 5.27 (3.97–7.00) | 5.38 (4.06–7.21) | <0.0001 |
| 20 weeks | 261 (25.97%) | 64 (6.43%) | 5.10 (3.82–6.81) | 5.18 (3.89–6.99) | <0.0001 |
| 26 weeks | 244 (24.28%) | 61 (6.13%) | 4.91 (3.65–6.60) | 4.95 (3.70–6.73) | <0.0001 |
OR: odds ratio; 95% CI: 95% confidence interval; AOR: adjusted odds ratio.
The analysis was adjusted for gender, age, education, urban-rural, regions, cigarettes smoked per day, FTCD score and previous quit attempts at baseline.
The effect of the ‘WeChat WeQuit’ intervention on the primary outcome of the ITT-based biochemically verified 26-week continuous smoking abstinence rate by subgroup is shown in Fig. 2. Compared with high nicotine dependence participants (FTCD > 5), those who had low nicotine dependence (FTCD ≤ 5) were suggestively more likely to quit smoking (χ2 = 7.77, p = 0.0053). Moreover, participants who had previous quit attempt(s) were suggestively more likely to quit smoking (χ2 = 7.66, p = 0.0056).
Fig. 2.
Rates of biochemically validated continuous smoking abstinence in various subgroups at 6 months by intention-to-treat analysis (multiple imputation). Data are n (%); the intervention group had higher cessation rate than the control group across all variables (all p < 0.0001); OR: odds ratio; 95% CI: 95% confidence interval.
Discussion
Compared with participants from the control group, participants who received the ‘WeChat WeQuit’ intervention were significantly more likely to quit smoking at 6 months. The intervention was more effective than the control at all assessment points during the 6 months of follow up. The results were consistent across several different analyses. Therefore, we conclude that the ‘WeChat WeQuit’ program is effective for achieving cessation at six months among treatment-seeking smokers in China. We observed no significant adverse events with this intervention. The observed effect on validated abstinence was similar to or better than those of other mobile phone-based interventions for smoking cessation.30,35
This large sample size RCT assessed the efficacy of a CBT-based smoking cessation intervention delivered via WeChat. Different from text messaging-based services which utilized only text message as an intervention, this WeChat-based intervention, developed based on CBT principles, was integrated into a multicomponent, multimedia, proactive, interactive, and treatment model for treatment-seeking smokers. This suggests that WeChat-based smoking cessation support can provide more intuitive online content to guide users than text messaging-based services, and may be a crucial component for improving smoking cessation in China, benefiting from the unique characteristics of WeChat such as ease of deployment, low cost, and ability to reach vulnerable populations. Considering 1) the extremely high rates of smoking in China, especially among men, 2) smoking cessation services are not widespread and may be difficult to access by some populations in China, and 3) WeChat is extremely popular in China, then the current study findings indicate that smoking cessation via WeChat could help a substantive number of Chinese smokers to stop in the future.
To be specific, this study shows that biochemically verified 26-week continuous smoking abstinence rate (often regarded as the ‘gold standard’ in verification of quitting smoking) in the intervention group (almost 12%) was more than 4 times higher than that in the control group, which is better than our previous study of a text messaging-based intervention (6.5%),30 indicating that WeChat-based smoking cessation services could be more effective than text messaging-based services (also used predominantly online recruitment), as the WeChat platform can provide more intuitive interventions (e.g. text, image, video). However, a text message-based smoking cessation intervention via WeChat with 722 participants conducted in 5 cities in China found that biochemically verified continuous abstinence at 6 months was only 6.9% in the intervention group and 3.0% in the control group.25 The quit rate for the intervention group was slightly higher than the result of 6.5% in our text messaging-based study,30 and the quit rate of 3.0% for the control group was consistent with 2.8% in the current trial. A pilot three-arm RCT with 403 participants found that the 7-day point quit rate at 4-week follow-up was 15.6% and 20.6% in the Standard Group and Enhanced Group respectively, and 10.6% in the Waitlist Group,26 which was much lower than the current study (39.2% in the intervention group and 14% in the control group).
The results from the current trial along with other studies indicate that the long-term quitting rate is still low. In China, it is well known that cigarettes are closely connected with social and cultural activities.36 Our previous study revealed that more than 90% of current smokers have shared and accepted cigarettes and even more than 60% of non-smokers have given cigarettes as gifts to others,37 which greatly prevents people from quitting smoking. Nevertheless, short-term (especially during the first week) quit rates are quite promising with the ‘WeChat WeQuit’ intervention. Over a third of participants reported 7-day point abstinence. Although a large number of participants didn’t successfully quit for 26 weeks, participants from the two study groups consumed approximately three fewer cigarettes per day during the follow-up period. It is worth noting that, participants who smoked fewer cigarettes per day or had previous quit attempt(s) were more likely to quit smoking. Our previous text-messaging study also found light smokers or smokers who had many previous quit attempts were more likely to maintain long-term abstinence.38 Another WeChat-based study also found that smokers with low nicotine dependence were more likely to quit.25
The study sample consisted mainly of men (>90% in both intervention and control group) and two thirds were older than 34 years. This is reflective of the population of smokers in China. More than 50% of adult men and only 2.1% of women are current smokers in China.6 Findings from the nationwide China Health Literacy Survey during 2018–2019 revealed that older smokers are more likely to develop tobacco dependence than younger ones, and smokers with tobacco dependence experience more difficulty quitting smoking.39 Thus, older smokers would be more likely to seek help to quit. Furthermore, the sample consisted of 260 rural participants. Our results showed a trend of lower quit rates among rural compared to urban participants. However, a study with 233 rural, 107 suburban, 63 urban participants found that rural smokers reported better smoking cessation outcomes than urban smokers.40 This study has several strengths. A WeChat-based intervention has unique advantages in China, such as wide coverage, low cost, real time, flexibility, multimedia; the sample size is large, and participants came from 31 provinces and autonomous regions of China; blind randomisation was used; baseline prognostic factors were well balanced between the two study groups; researchers who gathered data and conducted analyses were masked to treatment allocation; the primary outcome of 26-week continuous abstinence was biochemically verified; all analyses were on an ITT basis, and we also conducted sensitivity analyses with the traditional approaches to deal with missing data in smoking cessation studies and per-protocol analyses, with consistent findings. This study was conducted during COVID-19 pandemic (from March 19, 2020 to November 16, 2022), the effectiveness of our RCT demonstrates that the WeChat-based online intervention is a useful method for smoking cessation under the circumstances of restricted activities. However, our related survey study indicates that the COVID-19 pandemic failed to improve Chinese smokers’ motivation to quit and to reduce cigarettes smoked per day.38
Nonetheless, our trial has several limitations. Firstly, similar to other mobile-based interventions, this trial failed to achieve perfect follow-up, and some potential for bias may exist. However, there were less than 15% participants lost over time in the current study. We applied different methods to deal with missing data, and each analysis consistently showed that both self-reported and biologically verified abstinences achieved statistically significant differences between the two study groups. The potential for self-reporting and social desirability bias in the study outcomes should also be considered. Secondly, a very small group of participants used medications or e-cigarettes to quit during the trial. However, none of them quit smoking during the trial, which may indicate that these participants did not impact the cessation outcomes. Thirdly, to ensure the accuracy of biologically verified abstinence, we sent two cotinine urine dipsticks to each participant who reported successfully quitting smoking, and confirmed the test results by sending pictures and making calls, and successful outcomes were further confirmed by their family members. However, the cotinine urine test can only detect smoking status within the past few days, rather than over the past 26 weeks. Fourth, this trial only assessed the efficacy of the ‘WeChat WeQuit’ program on its own, and we don’t know its effectiveness when used alongside other smoking cessation interventions. Fifth, this WeChat-based intervention was delivered in mainland China where more than 300 million adult smokers live, the intervention might require adaptation, translation into other languages with local evaluation if delivered to other populations. Sixth, we recruited participants from each province/region in Mainland China. Our team members are from Zhejiang and Hunan provinces, which may have contributed to the high proportion of recruited participants. We can’t be certain that this is a clinically representative sample, but the demographical and clinical characteristics of this sample are similar to our previous of text-messaging study and reach widely in provinces across China.30 Finally, participants assigned to the control group could have reduced motivation to quit. To minimize this effect, we mailed all participants a self-guided smoking cessation book. However, research found that providing smoking cessation manuals for participants can increase quit rates.41 Compared with controls from our previous ‘Happy Quit’ who received no smoking cessation intervention,30 providing a smoking cessation book slightly increased quit rates at 6 months.
Future research in the area of online interventions for smoking cessation could focus on exploring the use of more personalized and tailored interventions based on online feedback. Through a powerful online platform such as WeChat, individuals’ instant reactions towards the intervention can be traced, and advanced algorithms such as artificial intelligence can be used to analyze such large-scale user feedback for constructing or recommending more attractive and persuasive intervention materials. By tailoring the intervention to the individual’s unique characteristics and needs, as well as incorporating online feedback, researchers may be able to improve the effectiveness of online interventions for smoking cessation. Additionally, live streaming in China is a thriving and fast-growing industry, and the inauguration of a WeChat live stream communication platform quickly gained popularity. The potential of using WeChat-based live streaming communication as an intervention for smoking cessation in real-time should be investigated in future studies.
This trial found that the WeChat-based online smoking cessation intervention quadrupled rates of abstinence among treatment-seeking Chinese smokers. It demonstrates the efficacy of CBT-based smoking cessation intervention via WeChat (‘WeChat WeQuit’ program) in China. Considering China’s high smoking rate, the extreme lack of smoking cessation services, and the widespread use of WeChat, we believe that the ‘WeChat WeQuit’ program may have broad application prospects for reducing the prevalence of smoking. This WeChat-based intervention has a low cost and likely to be highly cost-effective, to scale up the ‘WeChat WeQuit’ program for delivery at a national level would potentially improve population health in China.
Contributors
YLiao conceived the study and developed ‘WeChat WeQuit’ program. YLiao and JT designed the trial. WC, AM, BK, and JC advised on the trial conduct plan and the statistical analysis plan. Yueheng Liu coordinated the randomisation. Yi Liu, LL, JJ, YF, ZZ, and YW undertook the trial. JT cleaned the data. JY, XL and YS did the statistical analysis. YLiao, JT and JY wrote the first draft of the manuscript and WC, AM, BK, and JC commented on the drafts of the manuscript. All authors provided final approval for publication submission.
Data sharing statement
The de-identified participant data generated during this study are attached as a supporting information and shared with other investigators. The contents (in Chinese) of WeChat-based smoking cessation interventions are available from Dr. Yanhui Liao on reasonable request. Requests should be directed to the corresponding author by email. The study protocol has been published and shared.
Declaration of interests
YLiao reports grant from CMB, grant from J&J; JC reports grant from Bloomberg Philanthropie, grants from NIH. All other authors declare no competing interests.
Acknowledgements
The research is supported by the Natural Science Foundation of Hunan Province (2020JJ4794, YLiao), the K.C. Wong Postdoctoral Fellowship for YLiao to study at King’s College London, and China Medical Board (CMB) Open Competition Program (15-226, 22-485, YLiao). The funders had no role in study design, data collection and analysis, decision to write the report or to submit the paper for publication. We thank all the participants in the trial, thank all the volunteers who helped promote the study recruitment.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102009.
Appendix A. Supplementary data
References
- 1.Reitsma M.B., Kendrick P.J., Ababneh E., et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet. 2021;397(10292):2337–2360. doi: 10.1016/S0140-6736(21)01169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2019 Cancer Risk Factors Collaborators The global burden of cancer attributable to risk factors, 2010-19: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2022;400(10352):563–591. doi: 10.1016/S0140-6736(22)01438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang M., Yang L., Wang L., et al. Trends in smoking prevalence in urban and rural China, 2007 to 2018: findings from 5 consecutive nationally representative cross-sectional surveys. PLoS Med. 2022;19(8) doi: 10.1371/journal.pmed.1004064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan K.H., Wright N., Xiao D., et al. Tobacco smoking and risks of more than 470 diseases in China: a prospective cohort study. Lancet Public Health. 2022;7(12):e1014–e1026. doi: 10.1016/S2468-2667(22)00227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Regional Office for the Western Pacific . WHO Regional Office for the Western Pacific; Manila: 2017. The bill China cannot afford : health, economic and social costs of China’s tobacco epidemic. [Google Scholar]
- 6.World Health Organization . Global adult tobacco survey (GATS) China factsheet. 2018. https://extranet.who.int/ncdsmicrodata/index.php/catalog/803 [Online resource]. Available from: [Google Scholar]
- 7.Sun D., Pang Y., Lyu J., Li L. Current progress and challenges to tobacco control in China. China CDC Wkly. 2022;4(6):101–105. doi: 10.46234/ccdcw2022.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang G., Wang Y., Wu Y., Yang J., Wan X. The road to effective tobacco control in China. Lancet. 2015;385(9972):1019–1028. doi: 10.1016/S0140-6736(15)60174-X. [DOI] [PubMed] [Google Scholar]
- 9.Lin H., Xiao D., Liu Z., Shi Q., Hajek P., Wang C. National survey of smoking cessation provision in China. Tob Induc Dis. 2019;17:25. doi: 10.18332/tid/104726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S., Zhang M., Yang L., et al. Prevalence and patterns of tobacco smoking among Chinese adult men and women: findings of the 2010 national smoking survey. J Epidemiol Commun Health. 2017;71(2):154–161. doi: 10.1136/jech-2016-207805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y.Y., Tang W.T., Zhang H., et al. Barriers and facilitators for smoking cessation in Chinese smokers with chronic obstructive pulmonary disease: a qualitative study. Int J Chronic Obstr Pulm Dis. 2022;17:1107–1120. doi: 10.2147/COPD.S356935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortiz-Ospin E. The rise of social media. 2019. https://ourworldindata.org/rise-of-social-media Published online at OurWorldInData.org [Online Resource]. Available from:
- 13.Taylor G.M., Dalili M.N., Semwal M., Civljak M., Sheikh A., Car J. Internet-based interventions for smoking cessation. Cochrane Database Syst Rev. 2017;9 doi: 10.1002/14651858.CD007078.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naslund J.A., Kim S.J., Aschbrenner K.A., et al. Systematic review of social media interventions for smoking cessation. Addict Behav. 2017;73:81–93. doi: 10.1016/j.addbeh.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desrichard O., Moussaoui L.S., Blondé J., et al. Cessation rates from a national collective social network smoking cessation programme: results from the 'I quit smoking with Facebook on March 21' Swiss programme. Tob Control. 2022;31(6):762–764. doi: 10.1136/tobaccocontrol-2020-056182. [DOI] [PubMed] [Google Scholar]
- 16.Review WP. 2022. https://worldpopulationreview.com/country-rankings/wechat-users-by-country Available from:
- 17.Chen X., Zhou X., Li H., Li J., Jiang H. The value of WeChat application in chronic diseases management in China. Comput Methods Progr Biomed. 2020;196 doi: 10.1016/j.cmpb.2020.105710. [DOI] [PubMed] [Google Scholar]
- 18.Rong L., Shilin X., Li Z., Xiaonan W., Jian K., Xuejun H. A study on the real world effect of the combination of bupropion hydrochloride sustained release tablets with behavior intervention on smoking cessation. Chinese J Pract Intern Med. 2019;39(10):873–878. [Google Scholar]
- 19.Chen X., Zhao D., Wen T., et al. To text or not to text? Acceptability of WeChat and text messaging intervention to promote tobacco control assistance among parents who smoke in rural China. Tob Induc Dis. 2019;17:88. doi: 10.18332/tid/114089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luk T.T., Wong S.W., Lee J.J., Chan S.S., Lam T.H., Wang M.P. Exploring community smokers' perspectives for developing a chat-based smoking cessation intervention delivered through mobile instant messaging: qualitative study. JMIR mHealth and uHealth. 2019;7(1) doi: 10.2196/11954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W.H.C., Ho K.Y., Lam K.K.W., et al. A study protocol for a randomised controlled trial evaluating the use of information communication technology (WhatsApp/WeChat) to deliver brief motivational interviewing (i-BMI) in promoting smoking cessation among smokers with chronic diseases. BMC Publ Health. 2019;19(1):1083. doi: 10.1186/s12889-019-7417-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo T., Li M.S., Williams D., et al. A WeChat-based smoking cessation intervention for Chinese smokers: a feasibility study. Transl Behav Med. 2022;12(10):1018–1027. doi: 10.1093/tbm/ibac067. [DOI] [PubMed] [Google Scholar]
- 23.Chen J., Ho E., Jiang Y., Whittaker R., Yang T., Bullen C. Mobile social network-based smoking cessation intervention for Chinese male smokers: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2020;8(10) doi: 10.2196/17522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo T., Li M.S., Williams D., et al. Implementation of a WeChat-based smoking cessation program for Chinese smokers. Int J Environ Res Publ Health. 2021;18(21):11189. doi: 10.3390/ijerph182111189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin H., Liu Y., Zhang H., Zhu Z., Zhang X., Chang C. Assessment of a text message-based smoking cessation intervention for adult smokers in China: a randomized clinical trial. JAMA Netw Open. 2023;6(3) doi: 10.1001/jamanetworkopen.2023.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo T., Li M.S., Williams D., et al. A WeChat-based smoking cessation intervention for Chinese smokers: a pilot study. Internet Interv. 2022;28 doi: 10.1016/j.invent.2022.100511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.David D., Cristea I., Hofmann S.G. Why cognitive behavioral therapy is the current gold standard of psychotherapy. Front Psychiatr. 2018;9:4. doi: 10.3389/fpsyt.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dutra L., Stathopoulou G., Basden S.L., Leyro T.M., Powers M.B., Otto M.W. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatr. 2008;165(2):179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- 29.Perkins K.A., Conklin C.A., Levine M. Cognitive-behavioral therapy for smoking cessation : a practical guidebook to the most effective treatments. J Addict Dis. 2008;17(7):729–730. [Google Scholar]
- 30.Liao Y., Wu Q., Kelly B.C., et al. Effectiveness of a text-messaging-based smoking cessation intervention (“Happy Quit”) for smoking cessation in China: a randomized controlled trial. PLoS Med. 2018;15(12) doi: 10.1371/journal.pmed.1002713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao Y., Wang Y., Liu Y., McNeill A., Tang J. Effectiveness of the WeChat-based smoking cessation intervention ('WeChat WeQuit' program) in China: study protocol for a randomized controlled trial. Addiction. 2020;116(5):1279–1290. doi: 10.1111/add.15166. [DOI] [PubMed] [Google Scholar]
- 32.Huang C.L., Lin H.H., Wang H.H. The psychometric properties of the Chinese version of the Fagerstrom test for nicotine dependence. Addict Behav. 2006;31(12):2324–2327. doi: 10.1016/j.addbeh.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 33.Ramo D.E., Thrul J., Chavez K., Delucchi K.L., Prochaska J.J. Feasibility and quit rates of the tobacco status project: a Facebook smoking cessation intervention for young adults. J Med Internet Res. 2015;17(12):e291. doi: 10.2196/jmir.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montori V.M., Guyatt G.H. Intention-to-treat principle. CMAJ. 2001;165(10):1339–1341. [PMC free article] [PubMed] [Google Scholar]
- 35.Whittaker R., McRobbie H., Bullen C., Rodgers A., Gu Y., Dobson R. Mobile phone text messaging and app-based interventions for smoking cessation. Cochrane Database Syst Rev. 2019;10 doi: 10.1002/14651858.CD006611.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mao A., Yang T., Bottorff J.L., Sarbit G. Personal and social determinants sustaining smoking practices in rural China: a qualitative study. Int J Equity Health. 2014;13:12. doi: 10.1186/1475-9276-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao Y., Tang J., McNeill A., Kelly B.C., Cohen J.E. Impact of cigarette package warnings on attitudes towards sharing and gifting cigarettes in China: a nationwide study of smokers and non-smokers. Tob Control. 2021;31(6):750–753. doi: 10.1136/tobaccocontrol-2020-056160. [DOI] [PubMed] [Google Scholar]
- 38.Liao Y., Wang Y., Tang J., Wu Q., Wu Z., McNeill A. Predictors of long-term abstinence in a randomized controlled trial of smoking cessation by mobile phone text messaging ('Happy Quit') in China. Tob Prev Cessat. 2022;8:31. doi: 10.18332/tpc/152255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Z., Li Y.H., Cui Z.Y., et al. Prevalence of tobacco dependence and associated factors in China: findings from nationwide China Health Literacy Survey during 2018-19. Lancet Reg Health West Pac. 2022;24 doi: 10.1016/j.lanwpc.2022.100464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo T., Li M., Williams D., et al. Urban and rural disparities in a WeChat-based smoking cessation intervention among Chinese smokers. Int J Environ Res Publ Health. 2021;18(13):6731. doi: 10.3390/ijerph18136731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walters J., Ferguson S. The effect of mobile text messages on short and long term quitting in motivated smokers: a randomised controlled trial. Annual Meeting of the Society for Research on Nicotine & Tobacco; Philadelphia Pennsylvania: 25-28 February, 2015. p. 252. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.