Abstract
Gastric signet ring cell carcinoma (GSRC) is a special subtype of gastric cancer (GC) associated with poor prognosis, but an in-depth and systematic study of GSRC is lacking. Here, we perform single-cell RNA sequencing to assess GC samples. We identify signet ring cell carcinoma (SRCC) cells. Microseminoprotein-beta (MSMB) can be used as a marker gene to guide the identification of moderately/poorly differentiated adenocarcinoma and signet ring cell carcinoma (SRCC). The upregulated differentially expressed genes in SRCC cells are mainly enriched in abnormally activated cancer-related signalling pathways and immune response signalling pathways. SRCC cells are also significantly enriched in mitogen-activated protein kinase and oestrogen signalling pathways, which can interact and promote each other in a positive feedback loop. SRCC cells are shown to have lower cell adhesion and higher immune evasion capabilities as well as an immunosuppressive microenvironment, which may be closely associated with the relatively poor prognosis of GSRC. In summary, GSRC exhibits unique cytological characteristics and a unique immune microenvironment, which may be advantageous for accurate diagnosis and treatment.
Subject terms: Cancer microenvironment, Gastric cancer, Gastric cancer
The tumour microenvironment in signet ring cell carcinoma (SRCC) remains to be characterised. Here, the authors perform single-cell RNA sequencing and reveal distinct features compared to moderately/poorly differentiated adenocarcinoma.
Introduction
Gastric cancer (GC) is a common cancer worldwide that ranks fifth in incidence and fourth in mortality1. Fewer early-stage GC patients are diagnosed in China than in other countries, and late-stage GC patients account for the highest proportion of patients with large tumours at the time of diagnosis2. Despite advances in early-stage diagnosis and treatment, the current understanding of the biology of GC is still preliminary, and the 5-year survival rate of patients with advanced GC remains below 5%3.
Gastric adenocarcinoma (GA) is the most common subtype of GC, accounting for 95% of all GC cases. GA is a highly heterogeneous disease4,5 and is classified into different histological subtypes based on Lauren’s classification system, including intestinal-type, diffuse-type and mixed-type6. Gastric signet ring cell carcinoma (GSRC) is classified as diffuse-type GA, which develops from poorly cohesive cells without gland formation7. GSRC is histologically diagnosed based on microscopic characteristics, specifically, the presence of signet ring cells in over 50% of cancer cells, according to the guidelines of the World Health Organization (WHO). Cancer cells secrete a large amount of mucus, but most of it is not discharged from the cell. Rather, the large amount of mucus in the cytoplasm squeezes the nucleus to one side, making the cancer cell look like a signet ring, hence the name signet ring cell carcinoma (SRCC). GSRC has the characteristics of low differentiation, high malignancy, strong invasiveness, higher risk of metastasis, poor response to radiotherapy and chemotherapy, and its incidence is increasing yearly, especially in women8–11.
The molecular mechanisms involved in the occurrence and heterogeneity of GSRC have been described using exome and transcriptome sequencing, and changes in several driving factors have been recognized12–14, However, these sequencing data reflect the average expression level of the gene in bulk cells.They mask the molecular characteristics of different cell subgroups within the tissue and cannot truly reflect the specificity and heterogeneity of the gastric printed cells. In recent years, single-cell RNA sequencing (scRNA-seq) has been used to directly analyse gene expression and intracellular population heterogeneity at the single-cell level, define dynamic transformations in cell type and cell state, and recognize new cell subtypes, thereby improving the understanding of transcription kinetics and gene regulation in rare cells15–17. The method can also be used to analyse biological tissues and has been widely applied in tumour microenvironment analysis, including tumorigenesis and immune tolerance contexts18. Furthermore, several studies have profiled the single-cell landscape of immune cell heterogeneity and tumour cell heterogeneity in GC19,20. However, a detailed understanding of the cytological and immune microenvironment in GSRC at the single-cell resolution level remains elusive.
In this work, we analyse para-cancerous and GA tissues by scRNA-seq. We compare the differences between moderately/poorly differentiated adenocarcinoma(M/PDA) and GSRC in cytology and immune microenvironment. Our data provide an in-depth understanding of GSRC and may provide a resource for the accurate diagnosis and treatment of GSRC.
Results
scRNA-seq overview and identification of major cell types in para-cancerous and GA tissues
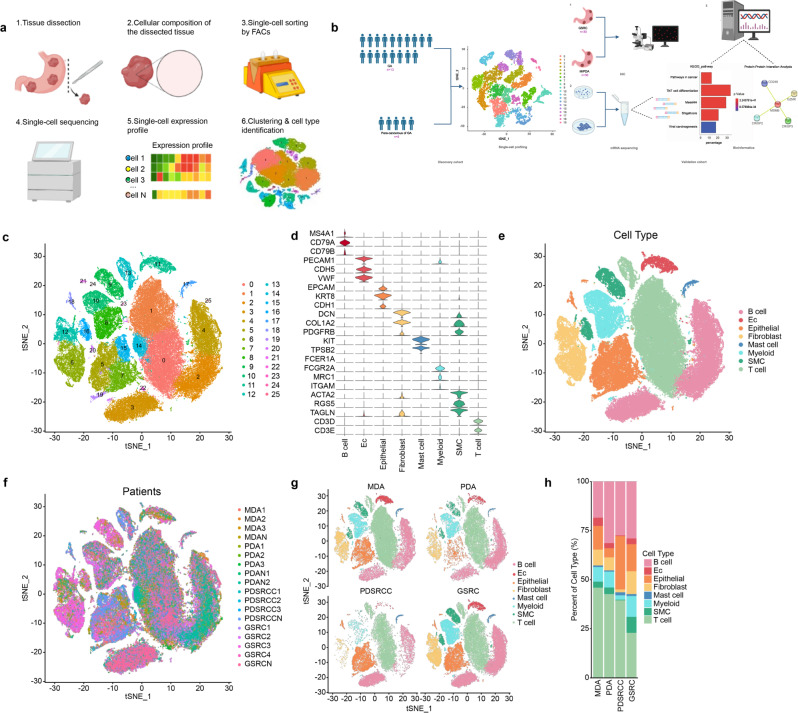
From December 2019 to December 2021, 13 patients with GA who underwent radical gastrectomy at Shandong Cancer Institute and Jinan Central Hospital (Jinan, China) were enrolled. No enrolled patient received any other therapies before surgery, such as radiotherapy, chemotherapy, and immunotherapy, or had other tumours. All patients were negative for a family history of tumours. The patients were divided into 4 groups based mainly on the degree of differentiation and the signet ring cell content, and no gender-based analysis was performed. Additional patient data are presented in Supplementary Table 1. scRNA-seq was used to analyse the samples of 13 GA patients. After filtering out low-quality cells, removing doublet reads, and correcting for batch effects, the transcriptomes of a total of 149,782 single cells were analysed (Fig. 1a). In the discovery cohort, 32,456 single cells were obtained from para-cancerous tissues from 5 patients, and 117,326 single cells were obtained from cancer tissues from 13 patients. The validation cohort was used to confirm the differences found in the discovery cohort (Fig. 1b). Using CellRanger, the official analysis software from 10× Genomics, the cells were divided into 26 clusters based on principal component analysis (PCA) and cluster analysis (Fig. 1c). Seurat software was utilized to analyse the gene expression differences among the cell clusters and to screen the genes that were upregulated in different cells (Fig. 1d). The distribution of 8 different cell clusters was finally determined based on unbiased cell type recognition (Fig. 1e). The cell clusters were named according to specific marker genes: B cells (expressing CD79B, CD79A, and MS4A1), endothelial cells (expressing VWF, CDH5, and PECAM), epithelial cells (expressing CDH1, KRT8, and EPCAM), fibroblast cells (expressing PDGFRB, COL1O2, and DCN), mast cells (expressing SLC18A2, FCER1A, TPSB2, and KIT), myoid cells (expressing FCGR2A, CD163, and MRC1), smooth muscle cells (expressing TAGLN, RGS5, and ACTA2) and T cells (expressing TRBC2, CD2, and CD3E)19. To further understand the cell clustering, in-depth analysis was carried out from the aspects of cell source, grouping and cell proportion (Fig. 1f–h).
Fig. 1. scRNA-seq overview and identification of major cell types in para-cancerous and GA tissues.
a Schematic diagram of the scRNA-seq process. Designed with BioRender©. b Experimental design for scRNA-seq and the corresponding validation. In the discovery cohort, cancer tissues (n = 13) and para-cancerous tissues (n = 5) of 13 patients with GA who underwent radical gastrectomy were analysed. In the validation cohort, GSRC (n = 30) and M/PDA (n = 30) samples were used for IHC verification. The mRNA transcriptome was sequenced from cultured cells. Relevant databases were used for bioinformatics validation. Designed with BioRender©. c The t-distributed stochastic neighbour embedding (t-SNE) plot of the 26 main cell types identified from cancer tissues (n = 13) and para-cancerous tissues (n = 5); 32,456 single cells were obtained from para-cancerous tissues, and 117,326 single cells were obtained from cancer tissues. d Violin plot showing the expression levels of cell type marker genes in 8 cell types. e t-SNE plot of cells from cancer and para-cancerous tissues (coloured by cell type). f t-SNE plot of cells from cancer and para-cancerous tissues (coloured by sample origin). g t-SNE plots of cells from cancer tissues, which were grouped by the degree of differentiation and signet ring cell content. h Scale plot of cells from cancer tissues (n = 13), which were grouped by the degree of differentiation and signet ring cell content. Source data are provided as a Source Data file.
Identification of malignant and nonmalignant epithelial cells
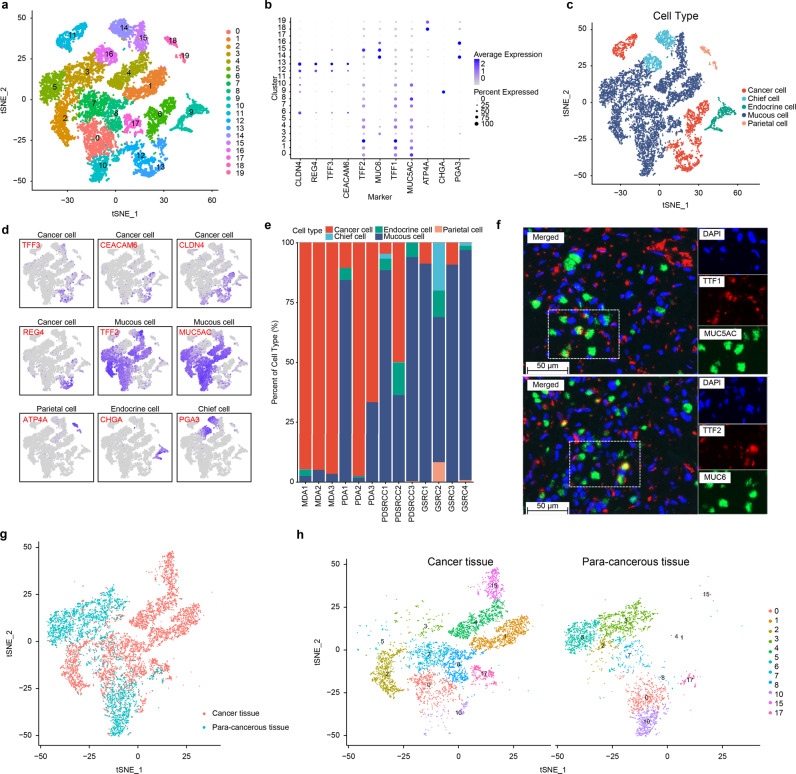
Cell clusters 5, 7, 15, and 19 were defined as epithelial cell clusters. Through cluster analysis of the epithelial cells, 20 subclusters (Fig. 2a) were obtained. According to marker genes cancer cells (expressing CLDN4, REG4, TTF3, and CEACAM6), mucous cells (expressing TFF1, MUC5AC, TFF2, and MUC6), chief cells (expressing PGA3 and PGA4), parietal cells (expressing ATP4A and ATP4B), and endocrine cells (expressing CHGA and CHGB)19 (Fig. 2b), the subclusters were redivided into 5 subclusters. Subclusters 6, 11, 12, and 13 were identified as M/PDA cells, subclusters 14 and 16 were identified as chief cells, subclusters 18 and 19 were identified as parietal cells, subcluster 9 was identified as endocrine cells, and other subclusters were identified as mucous cells (Fig. 2c, d).
Fig. 2. Single-cell transcriptome plots of epithelial cells.
a t-SNE plot of 20 epithelial cells from cancer tissues (n = 13) and para-cancerous tissues (n = 5). b Dot plot of the expression levels of cell type marker genes in 5 cell types. c t-SNE plot of epithelial cells from cancer tissues (n = 13) and para-cancerous tissues (n = 5) (coloured by cell type). Subclusters 6, 11, 12, and 13 were identified as M/PDA cells, subclusters 14 and 16 were identified as chief cells, subclusters 18 and 19 were identified as parietal cells, subcluster 9 was identified as endocrine cells, and other subclusters were identified as mucous cells. d t-SNE plot of expression levels of marker genes for 5 cell types. e Scale plot of epithelial cells from cancer tissues (n = 13). f Immunofluorescence plot of characteristic genes of selected cell types (n = 26). The gastric antrum mucus cells were stained with DAPI (blue), TTF1 (red) and MUC5AC (green). The gastric antrum basal gland mucus cells were stained with MUC6 (green), TTF2 (red), and DAPI (blue). Scale bar: 50 µm. g t-SNE plot of mucous cells from cancer tissues (n = 13) and para-cancerous tissues (n = 5) (coloured by tissue origin). h t-SNE plots of mucous cells of subclusters from cancer tissues (n = 13) and para-cancerous tissues (n = 5) (categorized by tissue origin and coloured by cell type). Source data are provided as a Source Data file.
The proportion of mucous cells increased significantly in poorly differentiated adenocarcinoma with signet ring cell carcinoma (PDSRCC) and GSRC (Fig. 2e). According to the abovementioned findings, immunofluorescence analysis of mucous cell markers was conducted, and it was revealed that these markers were also expressed in SRCC cells (Fig. 2f). SRCC cells contained a great deal of mucous, thus, these cells may be identified as mucous cells. To further distinguish mucous and SRCC cells, tissue origin was considered. Subclusters 1, 4, 8, and 15 were independently derived from cancer tissues and were defined as SRCC cells, and subclusters 0, 2, 3, 5, 7, 10, and 17 were defined as mucous cells (Fig. 2g, h).
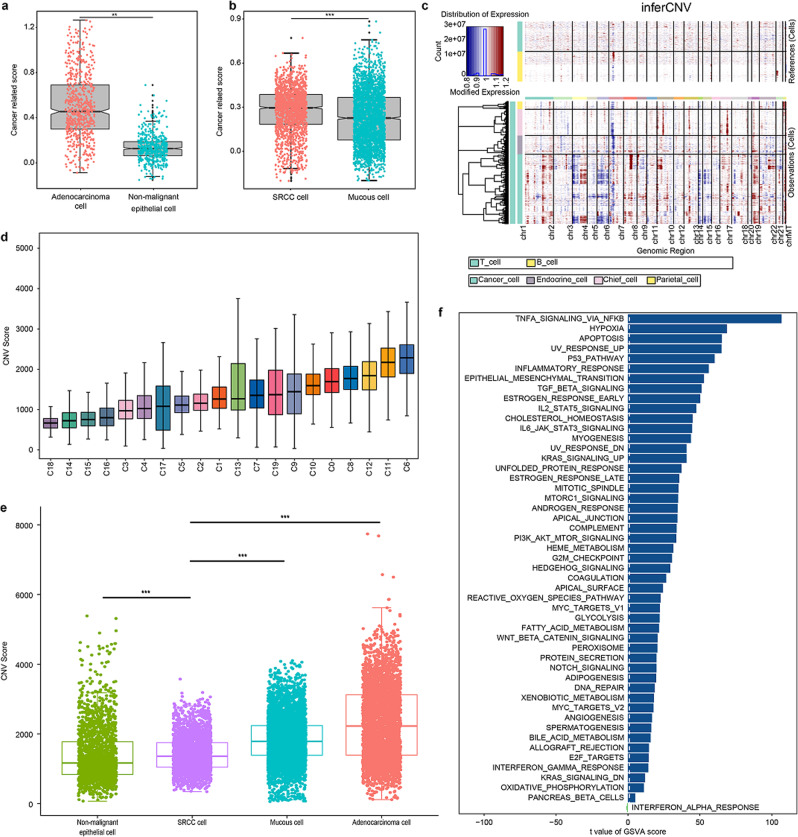
To verify the accuracy of clustering, the following steps were performed. First, to facilitate analysis, the chief cells, parietal cells, and endothelial cells were classified as nonmalignant epithelium, and their identification was relatively clear. The average expression levels of 80 cancer cell-elevated genes obtained from The Cancer Genome Atlas (TCGA) were used; the cancer-related score was compared between nonmalignant epithelium and M/PDA cells, and there was a significant difference between the two groups (Fig. 3a). The same results were also found between SRCC and mucous cells (Fig. 3b). Second, InferCNV is a tool used to analyse copy number variations (CNV) in tumour cells using scRNA-seq data. Based on the expression matrix of InferCNV, the CNV of M/PDA cells was significantly higher than that of nonmalignant epithelium and mucous cells, while the CNV of SRCC cells was lower than that of mucous cells (Fig. 3c, Supplementary Fig. 2). Moreover, to further verify the accuracy of the identification, the CNV scores of all subclusters were calculated based on InferCNV. The CNV scores of subclusters 6, 11, 12, and 13 were relatively high; thus, these subclusters were identified as M/PDA cells with characteristics typical of malignant tumours (Fig. 3d). The CNV score of SRCC cells was lower than that of M/PDA and mucous cells but higher than that of nonmalignant epithelial cells (t-test, p < 0.001) (Fig. 3e). Finally, volcano plot analysis revealed that REG4, S100A10, TSPAN8, OLMF4, and PHGR1 were upregulated in M/PDA cells compared with nonmalignant epithelial cells (Supplementary Fig. 3a). According to the results of the GSVA, M/PDA cells were mainly enriched in cancer-related signalling pathways contributing to tumour growth, proliferation, and metastasis, such as the epithelial-mesenchymal transition, E2F targets, PI3K/AKT/mTOR and KRAS signalling pathways (Supplementary Fig. 3b, Supplementary Data 1). GSVA was also performed to characterize different sources of mucous and SRCC cells.PDAucous cells, SRCC cells were enriched for signalling pathways such as the tumour necrosis factor-α (TNF-α), nuclear factor-κB (NF-κB), and transforming growth factor-β (TGF-β) signalling pathways, which are crucial for cancer development (Fig. 3f).
Fig. 3. Verification plots of nonmalignant epithelium and malignant epithelium after identification.
a Box plot showing distribution of cancer-related scores (average expression levels of cancer-related epithelial marker genes) for cells categorized as nonmalignant epithelial cells and M/PDA cells (t-test, p < 2.2 × 10−16). b Box plot showing distributions of cancer-related scores (average expression levels of cancer-related epithelial marker genes) for cells categorized as SRCC and mucous cells (t-test, p < 2.2 × 10−16). c Heatmap showing inferCNV for all subclusters of epithelial cells. Red: amplifications; blue: deletions. d Histogram showing CNV score plot of all subclusters of epithelial cells. e Box plot showing CNV score plot of epithelial cells by cell type. The CNV score of SRCC cells was lower than that of M/PDA and mucous cells, while higher than that of nonmalignant epithelial cells (t-test, p < 2.2 × 10−16). f GSVA of SRCC and mucous cells. SRCC cells were mainly enriched in cancer-related signalling pathways such as the TNF-α signalling pathway, NF-κB signalling pathway, and TGF-β signalling pathway. GSVA data were plotted according to the t value of limma, and at value > 5 was considered significant. The statistical strategy were two-sided Student’s t-test. In a, b, d and e, All specimens were participated in the analysis (n = 18), Data are presented as mean values ± SEM. In the box plot, the black dots represent outliers, the error bars represent SEM, the box midpoints represent means and the boxes represent inter-quartile positions. p values were calculated using the two-sided unpaired Student’s t-test, P values < 0.05 were considered to indicate significance: **p < 0.01; ***p < 0.001. Source data are provided as a Source Data file.
Collectively, the subclusters of epithelial cells were reidentified, and corresponding verification was carried out.
scRNA-seq revealed the characteristics of SRCC
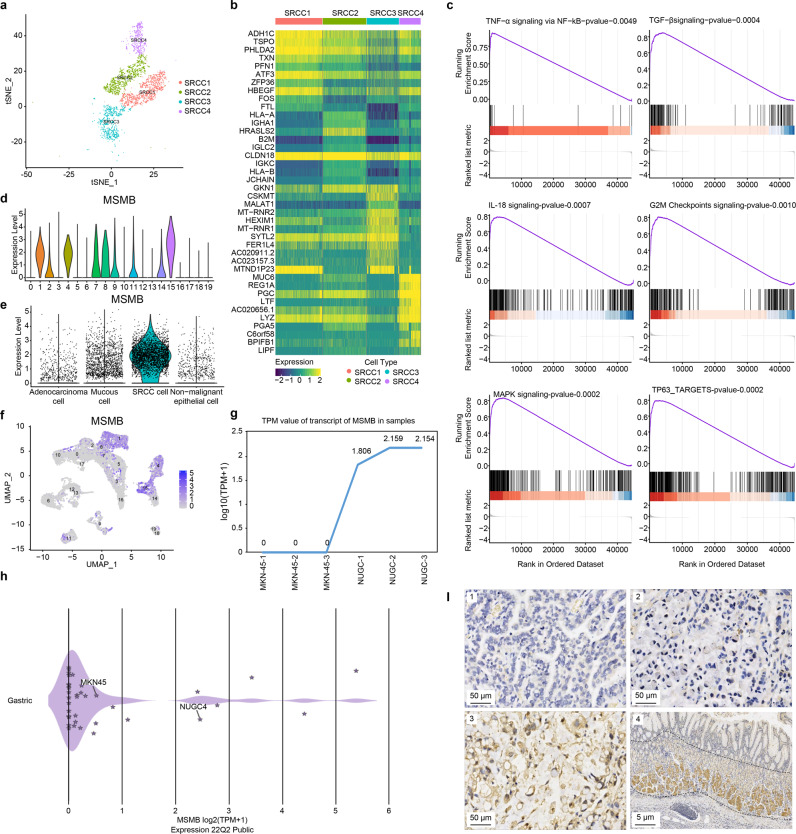
SRCC cells were divided into four subclusters (Fig. 4a). There was significant heterogeneity among SRCC cells according to heatmap analysis (Fig. 4b). However, in the four subclusters, some common highly expressed genes were identified, such as CLDN18, PHLDA2, ATF3, HBEGF, SYTL2, PGC, and LYZ, which might also be involved in the occurrence and development of GSRC.
Fig. 4. Basic features of SRCC cells.
a t-SNE plot of 4 subclusters of SRCC cells. b Heatmap of 4 subclusters of SRCC cells. Some common highly expressed genes, such as CLDN18, PHLDA2, ATF3, HBEGF, SYTL2, PGC, and LYZ were identified and might also be involved in the occurrence and development of GSRC. c The results of GSEA showed that cancer-related signalling pathways were enriched in SRCC cells. The genes upregulated in SRCC cells were mainly enriched in signalling pathways associated with immune response, such as the TNF-α signalling pathway, NF-kB signalling pathway, and TGF-β signalling pathway, as well as cell proliferation-related pathways, including the G2/M checkpoint signalling pathway, MAPK signalling pathway, and TP63 signalling pathway. The statistical strategy were one-sided Student’s t-test. d Violin plot of expression level of MSMB in subclusters. The expression level of MSMB was generally consistent with that in subclusters of 1, 4, 8, and 15 of SRCC cells. MSMB is weakly or not at all expressed in M/PDA cells (subclusters of 0, 2, 3, 5, 7,10, and 17). e Violin plot of expression level of MSMB in epithelial cells(categorized by cell type). The expression level of MSMB was relatively higher in SRCC cells than mucous and M/PDA cells. f UMAP plot of the expression level of MSMB in subclusters. g TPM value of transcript of MSMB in MKN-45 and NUGC4 cells by cell culture. h TPM value of transcript of MSMB in cell lines by DepMap database. The transcriptional expression data of gastric cancer cell lines were downloaded from DepMap database (https://depmap.org/portal/). i IHC plots of the expression level of MSMB in cancer tissues and para-cancerous tissues (n = 69). 1: PDA; 2: PDSRCC; 3: GSRC; 4: para-cancerous tissues. 1, 2 and 3, Scale bar: 50 μm; 4, Scale bar: 5 μm. Source data are provided as a Source Data file.
According to the gene set enrichment analysis (GSEA) results, the genes upregulated in SRCC cells were mainly enriched in the immune response and included TNF-α signalling via the NF-kB signalling pathway, the TGF-β signalling pathway, and the IL-18 signalling pathway. In addition, SRCC cells were enriched in cell proliferation-related signalling pathways, including the G2/M checkpoint, mitogen-activated protein kinase (MAPK), and TP63-targets signalling pathways (Fig. 4c). In addition, oestrogen response signalling and cholesterol homoeostasis signalling could be specifically enriched in SRCC cells, indicating that oestrogen receptor levels and cholesterol metabolism could be involved in cancer occurrence and development.
Importantly, MSMB was found to be a potential marker gene of GSRC. The expression level of MSMB was basically consistent with that in the subclusters of SRCC cells. MSMB exhibited essentially no/low expression in M/PDA cells (Fig. 4d, e). The scRNA-seq results showed that the expression level of MSMB was higher in SRCC cells than in mucous cells and M/PDA cells (Fig. 4f). Verification was carried out in the corresponding cell lines. The SRCC cell line NUGC4 exhibited a higher expression level of MSMB than the poorly differentiated MKN-45 adenocarcinoma cell line (Fig. 4g, h). Verification was conducted using IHC, and the results confirmed that the MSMB expression level in SRCC cells was higher than that in M/PDA cells but lower than that in the gastric foveal proliferation area in para-cancerous tissues (Fig. 4I). Taken together, these data supported the high MSMB expression level in SRCC cells.
Similarities and differences between M/PDA and SRCC cells
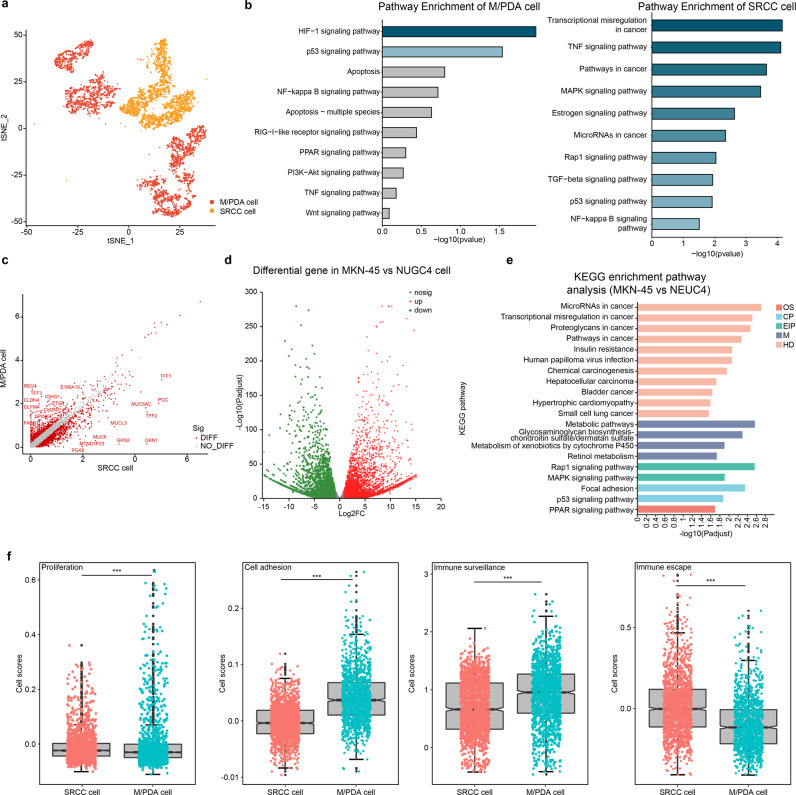
Clinically, there were significant differences in biological behaviours between M/PDA and GSRC, and these differences were mainly manifested in the tumour parenchyma. To clarify these differences, DEGs were analysed in M/PDA and SRCC cells (Fig. 5a). Through the enrichment analysis of upregulated DEGs and signalling pathways, it was revealed thatPDA/PDA cells, the upregulated genes in SRCC cells were mainly enriched with abnormally active cancer-related signalling pathways (e.g., transcriptional misregulation in cancer, pathways in cancer and microRNAs in cancer) and were closely correlated with signalling pathways of immune escape (e.g., the TNF-α signalling pathway, TGF-β signalling pathway, and NF-kB signalling pathway) (Fig. 5b, c). The corresponding verification was carried out in cell culture, and the same results were obtained (Fig. 5d, e). M/PDA cells had high proliferation and immune surveillance capabilities, while SRCC cells had low cell adhesion and high immune escape capabilities (t-test, p < 0.001). (Fig. 5f).
Fig. 5. Similarities and differences between M/PDA and SRCC cells.
a t-SNE plot of M/PDA and SRCC cells (coloured by cell type). b Pathway enrichment analysis plots of DEGs upregulated in M/PDA and SRCC cells. Compared with M/PDA cells, the DEGs upregulated in SRCC cells were mainly enriched in abnormally activated cancer-related signalling pathways (e.g., transcriptional misregulation in cancer, pathways in cancer and microRNAs in cancer) and were closely associated with signalling pathways of immune escape (e.g., TNF signalling pathway, TGF-β signalling pathway and NF-kB signalling pathway). c Heatmap plot of DEGs in M/PDA and SRCC cells. d Heatmap plot of DEGs in MKN-45 and NUGC4 cells. e KEGG pathway enrichment analysis of MKN-45 and NUGC4 cells among DEGs. f Differences between M/PDA and SRCC cells in terms of cell proliferation, cell adhesion, immune surveillance, and immune escape. M/PDA cells had high proliferation and immune surveillance capabilities, while SRCC cells had low cell adhesion and high immune escape capabilities (t-test, p < 0.001). n = 10 (include 3 samples in MDA and PDA groups, respectively; and 4 samples in GSRC group). Data are presented as mean values ± SEM. In the box plot, the black dots represent outliers, the error bars represent SEM, the box midpoints represent means and the boxes represent inter-quartile positions. p values were calculated using the two-sided unpaired Student’s t-test, p values < 0.05 were considered to indicate significance: ***p < 0.001. Source data are provided as a Source Data file.
Infiltration characteristics of B cells in M/PDA and GSRC
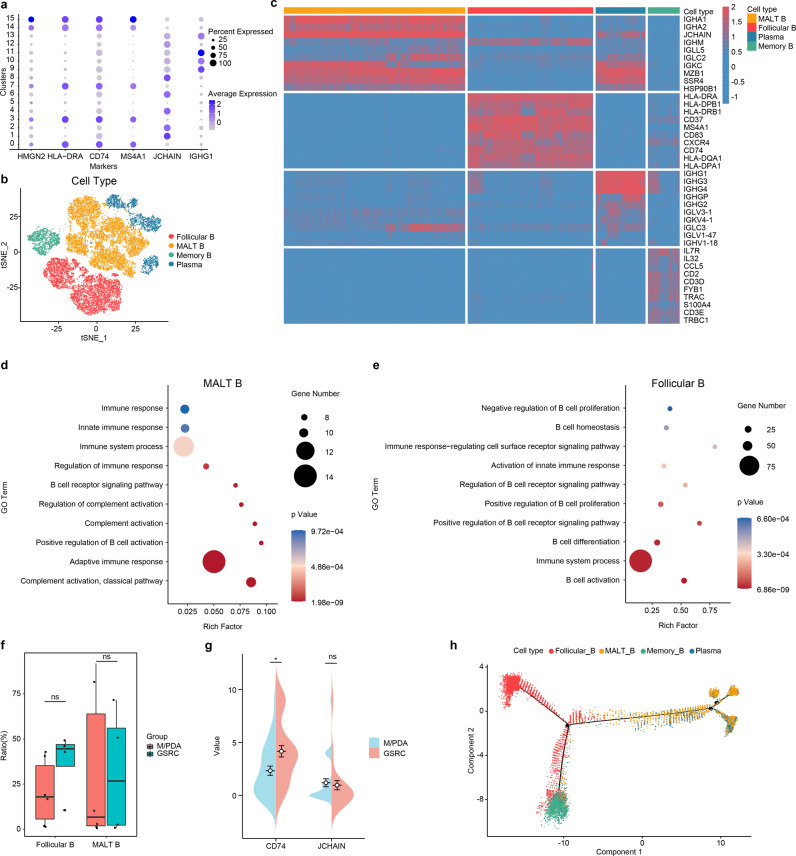
B cells from clusters 3 and 4 were reclustered into 16 subclusters (Fig. 6b, Supplementary Fig. 4a–c) using the marker gene (Fig. 6a). Of these, subclusters 1, 2, 4, 6, 8 and 12 were defined as mucosa-associated lymphoid tissue-derived B (MALT-B) cells (expressing immunoglobulin (Ig) and JCHAIN); subclusters 0, 3, 7, 14 and 15 were defined as follicular B cells (expressing MS4A1, CD74 and HLA-DRA); subclusters 9, 10, 11 and 13 were defined as plasma cells (expressing Ig-related genes and IGHG1); and subcluster 5 was defined as memory B cells (expressing HMGN2 and H2AFZ)21,22.
Fig. 6. Similarities and differences of tumour-infiltrating B cells between M/PDA and GSRC.
a Dot plot showing the expression levels of cell type marker genes in 4 cell types. b t-SNE plot showing subclusters of B cells (coloured by cell type). c Heatmap plot showing subclusters of B cells. d Functional signalling pathway enrichment plot of MALT-B cells. MALT-B cells were mainly related to immune response, B-cell activation, and participation in complement activation. e Functional signalling pathway enrichment plot of follicular B cells. Follicular B cells were related to immune system and regulation of B cells. f Box plot of tumour-infiltrating follicular B and MALT-B cells in M/PDA and GSRC. Compared with M/PDA, tumour-infiltrating follicular B cells in GSRC were higher, the difference was not statistically significant (t-test, p = 0.19); Similarly, there is no difference in tumour-infiltrating MALT-B cells between M/PDA and GSRC (t-test, p = 1). n = 10 (include 3 samples in MDA and PDA groups, respectively; and 4 samples in GSRC group). Data are presented as mean values ± SEM. In the box plot, the black dots represent outliers, the error bars represent SEM, the box midpoints represent means and the boxes represent inter-quartile positions. g Pod plot of tumour-infiltrating follicular B and MALT-B cells in M/PDA and GSRC by IHC score (n = 30). Compared with that of M/PDA, the IHC score of tumour-infiltrating follicular B cells (expressing CD74) in GSRC was higher, and the difference was statistically significant (Wilcoxon rank-sum test, p = 0.012); The IHC score of tumour-infiltrating MALT-B cells (expressing JCHAIN) in GSRC was higher, but the difference was not statistically significant (Wilcoxon rank-sum test, p = 0.154). p values were calculated using Wilcoxon rank-sum test. h Pseudochronological analysis of subclusters of B cells. Starting from marker point 1, B cells were divided into two branches: one consisting of follicular B cells and memory B cells; the other consisting of MALT-B cells and plasma cells. In d, e and f, the statistical strategy were two-sided Student’s t-test. p values < 0.05 were considered to indicate significance: *p < 0.05; ns no significance. Source data are provided as a Source Data file.
After extracting the top 10 genes from each subcluster of B cells to form a heatmap, it was found that MALT-B cells mainly expressed IgA-related genes, while plasma cells mainly expressed IgG-related genes (Fig. 6c). MALT-B cells are similar to plasma cells in their functions and play an important role in the humoral immunity of B cells. Afterwards, enrichment analysis was performed on each subcluster, and it was found that MALT-B and plasma cells had similar functions, which were mainly related to the immune response, B-cell activation, and participation in complement activation. However, follicular B cells had relatively weak immune responses (immune system process and regulation of B cells) (Fig. 6d).
Analysis of the samples revealed that the infiltration of MALT-B cells was the mainstay in MDA, PDA, and PDSRCC in para-cancerous tissues, while GSRC was mainly infiltrated by follicular B cells. The analysis of cancer tissue showed that MALT-B and memory B cells were the main infiltrating cells in MDA and PDA, while follicular B and memory B cells were the main infiltrating cells in PDSRCC and GSRC. The increased proportion of SRCC was accompanied by a decreased proportion of MALT-B cells and a significant increase in the number of follicular B cells, which was highly consistent with para-cancerous tissues in GSRC (Supplementary Fig. 4d). Compared with M/PDA, tumour-infiltrating follicular B cells in GSRC were higher, the difference was not statistically significant (t-test, p = 0.19); Similarly, there was no difference in tumour-infiltrating MALT-B cells between M/PDA and GSRC (t-test, p = 1) (Fig. 6e). Compared with that of M/PDA, the IHC score of tumour-infiltrating follicular B cells (expressing CD74) in GSRC was higher, and the difference was statistically significant (Wilcoxon rank-sum test, p = 0.012); The IHC score of tumour-infiltrating MALT-B cells (expressing JCHAIN) in GSRC was higher, but the difference was not statistically significant (Wilcoxon rank-sum test, p = 0.154) (Fig. 6f, Supplementary Fig. 5).
To gain insight into the evolutionary relationship among B-cell subclusters, Monocle algorithm analysis was performed based on the definition of subclusters. The majority of cells from different subclusters were clustered according to the similarity of their gene expression, while different subclusters, which were clustered in pseudotime, illustrated a stepwise process, starting from marker point 1. B cells were divided into two branches: one consisting of follicular B cells and memory B cells; the other consisting of MALT-B cells and plasma cells (Fig. 6g).
In summary, compared with M/PDA, subclusters of B cells exhibited unique infiltration characteristics in GSRC, mainly follicular B cells. Moreover, follicular B cells had relatively weak immune responses; this could also be one of the reasons for the poor prognosis of GSRC.
Infiltration characteristics of T cells in M/PDA and GSRC
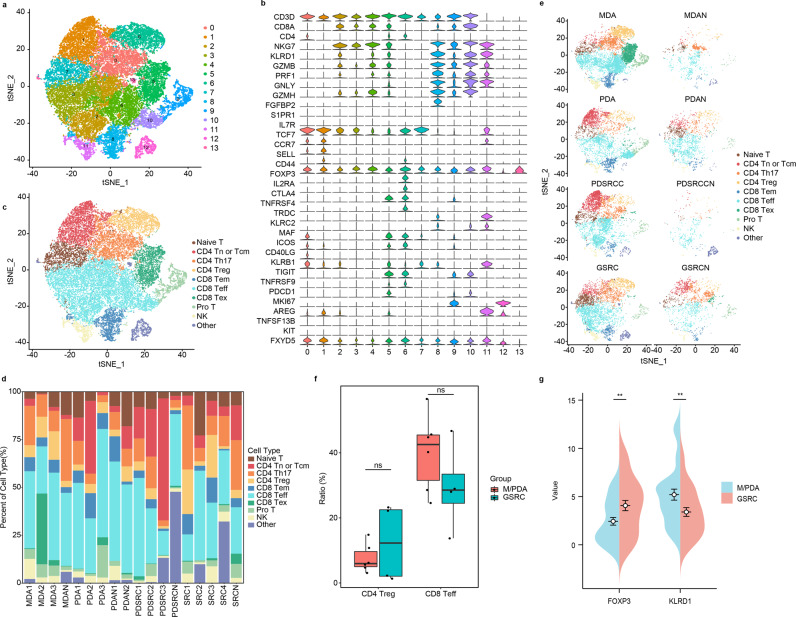
T cells from clusters of 0, 1, 13, and 14 were reclustered into 14 independent subclusters (Fig. 7a). The subclusters were subdivided into 10 cell subclusters (Fig. 7c) using marker genes (Fig. 7b). Among them, the subcluster from cluster 0 was defined as CD4-Th17 cells (expressing CD4, MAF, ICOS, CD40LG, and KLRB1); the subcluster from cluster 1 was defined as CD4-Tn or central memory T (Tcm) cells (expressing CD4, IL7R, TCF7, CCR7, SELL, and CD44); subclusters from clusters 2, 3, 4, and 10 were defined as CD8-Teff cells (expressing CD8A, NKG7, KLRD1, and GZMB); the subcluster from cluster 5 was defined as CD8-Tex cells (expressing CD8A, CTLA4, TIGIT, TNFRSF9, and PDCD1); the subcluster from cluster 6 was defined as CD4-Treg cells (expressing CD4 and FOXP3); the subcluster from cluster 7 was defined as naive T cells (expressing CD3); the subcluster from cluster 8 was defined as CD8-Tem cells (expressing PRF1, GNLY, GZMH, FGFBP2, S1PR1, and S1PR5); the subcluster from cluster 9 was defined as pro-T cells (expressing MKI67, CD3D, CD8A, and CD4); the subcluster from cluster 11 was defined as natural killer (NK) cells (expressing CD3D, XCL1, TRDC, and XCL2), and the subcluster from cluster 12 was defined as other cells21,22.
Fig. 7. Similarities and differences of tumour-infiltrating T cells between M/PDA and GSRC.
a t-SNE plot showing subclusters of T cells. b Dot plot showing the expression levels of cell type marker genes in 10 cell types. c t-SNE plot showing subclusters of T cells (coloured by cell type). d Scale plot of subclusters of T cells (n = 13). e t-SNE plots showing subclusters of T cells (coloured by group). f BOX plot of tumour-infiltrating CD4-Treg and CD8-Teff cells in M/PDA and GSRC. Compared with that in M/PDA, the proportion of tumour-infiltrating CD4-Treg cells in GSRC was higher, and the difference was not statistically significant (t-test, p = 0.25). The proportion of tumour-infiltrating CD8-Teff cells in GSRC was lower, the difference was not statistically significant (t-test, p = 0.25). n = 10 (include 3 samples in MDA and PDA groups, respectively; and 4 samples in GSRC group). Data are presented as mean values ± SEM. In the box plot, the black dots represent outliers, the error bars represent SEM, the box midpoints represent means and the boxes represent inter-quartile positions. P values were calculated using the two-side unpaired Student’s t-test. g Differential plot of tumour-infiltrating CD4-Treg and CD8-Teff cells in M/PDA and GSRC by IHC score (n = 30). Compared with that of M/PDA, the IHC score of tumour-infiltrating CD4-Treg cells (expressing FOXP3) in GSRC was higher, and the difference was statistically significant (Wilcoxon rank-sum test, p = 0.0032); The IHC score of tumour-infiltrating CD8-Teff cells (expressing KLRD1) was lower in GSRC, and the difference was statistically significant (Wilcoxon rank-sum test, p = 0.0021). p values were calculated using the Wilcoxon rank-sum test. p values were calculated using the Wilcoxon rank-sum test. p values < 0.05 were considered to indicate significance: **p < 0.01; ns: no significance. Source data are provided as a Source Data file.
Through the analysis of the samples, it was found that CD4-Th17, CD4-Tn or Tcm and CD8-Teff cells mainly infiltrated para-cancerous tissues and mainly exhibited immune functions. The infiltration rate of CD4-Treg cells was significantly higher in cancer tissues than in para-cancerous tissues, especially in GSRC. Meanwhile, GSRC had fewer tumour-infiltrating CD8-Teff cells than M/PDA (Fig. 7d). Compared with that in M/PDA, the proportion of tumour-infiltrating CD4-Treg cells in GSRC was higher, and the difference was not statistically significant (t-test, p = 0.25). The proportion of tumour-infiltrating CD8-Teff cells in GSRC was lower, the difference was not statistically significant (t-test, p = 0.25) (Fig. 6e). Compared with that in M/PDA, the IHC score of tumour-infiltrating CD4-Treg cells (expressing FOXP3) in GSRC was higher, and the difference was statistically significant (Wilcoxon rank-sum test, p = 0.0032); The IHC score of tumour-infiltrating CD8-Teff cells (expressing KLRD1) was lower in GSRC, and the difference was statistically significant (Wilcoxon rank-sum test, p = 0.0021) (Fig. 6f, Supplementary Fig. 5).
Collectively, the subclusters of T cells in GSRC exhibited unique infiltration characteristics, with increased infiltration of CD4-Treg cells and decreased infiltration of CD8-Teff cells, mainly manifesting as an immunosuppressive microenvironment.
Discussion
GC is a common gastrointestinal malignancy and remains the fourth leading cause of cancer-related deaths worldwide1. Although recent epidemiological surveys have shown that the overall incidence of GC has decreased significantly, the incidence of diffuse GC is continuously increasing. This is especially true for GSRC due to its high heterogeneity, and it accounts for 3.4–45% of new GA cases23–26. Patients frequently exhibit advanced GSRC (stage III or IV) at the time of diagnosis, which is associated with poor prognosis. Therefore, this type of GC has particularly attracted oncologists’ attention27–30. However, there is still a lack of in-depth understanding of the biological characteristics of GSRC, and further research is required to formulate targeted treatment strategies for GSRC.
In this work, relying on scRNA-seq, it is very important to accurately identify the SRCC cells. Starting from the unique characteristics of SRCC, that is, the large amount of mucus contained in the cells. The marker genes of gastric antrum basal gland mucus cells (MUC6 and TFF2) and of gastric antrum mucus cells (MUC5AC and TFF1) were used for immunofluorescence examination19, and it was confirmed that SRCC cells were identified in mucous cells. Further characterization, mainly depending on tissue origin, combined with comprehensive verification, such as cancer-related score, InferCNV, CNV score, and GSVA, identified SRCC cells. The degree of variation and CNV score of SRCC cells were lower than those of mucous and M/PDA cells. In general, tumour cells are prone to CNV mutations, and genes in the regions where CNV changes occur are always overexpressed or downregulated compared with normal cells. InferCNV estimated the single-cell CNV spectrum to distinguish between tumour cells and normal epithelial cells, which is irrelevant to the malignancy of tumours. The CNV score of cluster 15 was lower than that of the mucous cell clusters, which may have consequences on the total CNV score of clusters of SRCC cells. The role of tumour heterogeneity is noteworthy. According to the abovementioned results, the level of CNV does not discriminate benign and malignant GA cells, which is consistent with the results of another single-cell analysis, in which only 25.0% of static malignant cells exhibited high levels of CNV in GC31. In summary, InferCNV and CNV score provide favourable support for the regrouping of epithelial cells.
Furthermore, heatmap analysis revealed that there was also significant heterogeneity among SRCC subclusters. Similar to the case in other malignant tumours, tumour heterogeneity mainly has a noticeable influence on patient survival and prognosis32, which may justify the poor prognosis of GSRC patients. Among the 4 subclusters, further analysis indicated that there were also some highly expressed genes among the subclusters, such as CLDN18, PHLDA2, ATF3, HBEGF, SYTL2, PGC, and LYZ. For instance, the importance of CLDN18-ARHGAP26 was confirmed because of its frequent fusion in response to chemotherapy in GSRC13. High expression of CLDN18.2 is considered to have potential value for targeted therapy of patients with advanced GSRC; the analysis of CLDN18.2 expression and genetic abnormalities provides a new treatment option for advanced GSRC33. However, other genes have not been reported in studies on GSRC, and this area deserves further in-depth research.
In the process of studying GSRC, it was found that MSMB was highly expressed in SRCC cells but had no/low expression in M/PDA cells, consistent with previous studies34,35. In combination with data from relevant studies, our findings suggest that SRCC may originate from MUC5AC-/low MUC6- pre-pit cells in the proliferative zone of gastric glands2; SRCC of the gastric foveolar epithelium is positive for MUC1, MUC5AC, and MUC6, and SRCC, which is derived from the gastric foveolar epithelium, may originate from the proliferative region of the bottom of the gastric pit and gland neck. The IHC results confirmed the MSMB expression level in the gastric foveal proliferation area. The UMAP plot of MSMB also demonstrated that subclusters 7 and 8 were adjacent and were closely associated. Thus, a high MSMB expression level was detected in subcluster 7, while subclusters 0, 2, 3, 5, 10, and 17 were derived from mucous cells in other parts. SRCC may originate from subcluster 7. Therefore, MSMB was found to be a potential marker of GSRC and could be related to the differentiation and development of GSRC, and its specific biological function deserves further research.
The heterogeneity of GC is determined mainly by the tumour parenchyma and is closely correlated with its tumour microenvironment (TME). To clarify the significant differences in the biological behaviours between M/PDA and GSRC, the tumour parenchyma and TME were analysed.
Through the enrichment analysis of upregulated differentially expressed genes (DEGs), it was found that compared with M/PDA cells, SRCC cells were enriched for abnormally active cancer-related signalling pathways (transcriptional misregulation in cancer, pathways in cancer and microRNAs in cancer), which might be closely associated with the relatively poor prognosis and insensitivity to chemotherapy of GSRC36–38. The DEGs were also closely correlated with the signalling pathways of the immune response (TNF-α signalling pathway, TGF-β signalling pathway, and NF-kB signalling pathway), and the activity of the signalling pathways of the immune response in malignant tumours mainly indicates that the tumour has a high immune escape function18. The abovementioned pathway enrichment analysis results were highly consistent with the pathway enrichment analysis results of SRCC cell lines and adenocarcinoma cell lines in this study. Further analysis indicated that M/PDA cells had stronger proliferation and immune surveillance capabilities, while SRCC cells had weaker cell adhesion and stronger immune evasion capabilities. GSRC cells appeared less sensitive to chemotherapy than M/PDA cells due to their low proliferation. Studies have demonstrated that changes in cell adhesion play an important role in the occurrence and development of GSRC. E-cadherin, encoded by the CDH1 gene, is a cell‒cell adhesion molecule that participates in the early formation of GSRC and plays a crucial role39,40. The frequency of cell adhesion-related gene mutations increased in GSRC3. PPI network analysis revealed 107 mutated genes related to cell adhesion, indicating that the cell adhesion pathway plays an important role in GSRC tumorigenesis13. However, it was revealed that the CDH1 expression level was essentially the same between M/PDA and SRCC cells, which could be related to the fact that GSRC was not detected in the early stage of formation. Compared with that in M/PDA cells, the intercellular adhesion function was decreased in SRCC cells; however, the intercellular adhesion function was decreased or destroyed, which is the key in causing cancer cells to break away from the parent tumour to initiate metastasis and may also be associated with higher risks of invasive growth and metastasis, especially for implanted metastases of GSRC.
The cell signalling pathways of M/PDA were enriched in the hypoxia-inducible factor 1 (HIF-1), P53, and PI3K-AKT signalling pathways, which is consistent with previously reported findings22. SRCC cells could also be significantly enriched in the MAPK signalling pathway, which is involved in tumour growth, proliferation, and metastasis41,42. This may be one of the intrinsic reasons for the high levels of invasion and metastasis of GSRC. In addition, it was found that the oestrogen signalling pathway could also be abnormally activated in SRCC cells, confirming that oestrogen could play an important role in the occurrence and development of GSRC43,44. Previous studies demonstrated that young female GA patients are prone to GSRC, and more than 80% of GSRC cells can secrete mucin and express oestrogen receptors, which are more likely to metastasize to the ovary, indicating that GSRC has a higher affinity for oestrogen and can promote tumour growth and invasion45,46. The oestrogen and MAPK signalling pathways can interact and promote each other through membrane-initiated steroid signalling47–49. Oestrogen signalling could also be activated by nuclear-initiated steroid signalling and can be regulated by HPS70, HPS90, and FKBP550–52. In this study, heat shock protein family members were significantly enriched in the oestrogen signalling pathway. It was indicated that the nuclear-initiated signalling of oestrogen was one of the important pathways for its function in GSRC. Collectively, these findings show that blocking the MAPK and oestrogen signalling pathways is a potential method for the treatment of GSRC, and the associated specific internal mechanisms should be explored further.
Tumour tissues are composed of not only tumour cells themselves but also the cells in the TME, such as immune cells, fibroblasts, vascular endothelial cells, and stromal cells. Among these cells, tumour-infiltrating immune cells (TICs) play a vital role in tumorigenesis, development, and therapy53,54. Therefore, quantitative analysis of TICs in GC, especially comparative analysis of the immune microenvironment of M/PDA and GSRC, may clarify the characteristics of the mechanism of the immune response in the occurrence and development of GC, thereby laying a theoretical foundation for immunotherapy55.
The subclusters of B cells in GSRC have a unique infiltrating feature, dominated by the infiltration of follicular B cells, whose main function is to activate immune system processes and regulate B cells56. Their performance in the immune response is relatively weak, indicating a relatively suppressed immune microenvironment, and the same results were obtained from functional cluster analysis and quasi-sequential analysis40,57,58. Follicular B cells account for approximately 25% of the total number of cells, which is indicative of the importance of B cells in antitumour immunity in GA and suggests one of the reasons for the poor prognosis of GSRC.
The infiltration rate of CD4-Treg cells in cancer tissues was significantly higher than that in para-cancerous tissues, especially in GSRC. Moreover, there were more infiltrating FOXP3+ CD4-Treg cells in GSRC than in M/PDA, and the infiltrating proportion of CD8-Teff cells in cancer tissues was lower than that in para-cancerous tissues, especially in GSRC. The current study revealed that Treg cells could inhibit the functions of effector T lymphocytes, and the positive expression of FOXP3 was its main feature. The higher the positive rate of FOXP3+ CD4-Treg cells is, the faster the progression of GC and the lower the survival rate59,60. This may also be one of the reasons for the poor prognosis of GSRC.
Taken together, the findings here show that the subclusters of B and T cells in GSRC have unique infiltration characteristics. The infiltration of follicular B and CD4-Treg cells increased, and that of CD8-Teff cells decreased, in GSRC. Therefore, GSRC has an immunosuppressive microenvironment, which could be closely associated with the relatively poor prognosis and poor efficacy of immunotherapy. Regrettably, the underlying mechanisms need further study.
In conclusion, 32,456 single cells from para-cancerous tissues of 5 patients and 117,326 single cells from cancer tissues of 13 patients were analysed by scRNA-seq. We have identified SRCC cells. MSMB could be used as a marker to guide the identification of SRCC and M/PDA cells. The DEGs upregulated in SRCC were mainly enriched in abnormally activated cancer-related signalling pathways and signalling pathways of the immune response. SRCC could also be significantly enriched in the MAPK and oestrogen signalling pathways, which could interact and promote each other and continue to amplify each other’s effects. SRCC cells exhibited lower cell adhesion and higher immune evasion capabilities, as well as an immunosuppressive microenvironment, which could be closely associated with the relatively poor prognosis of GSRC. One limitation of the present study is that it is difficult to collect sufficient samples and lack of analytical methods for multi-omics. Overall, compared with M/PDA cells, GSRC cells have unique cytological characteristics and a unique immune microenvironment, which may be advantageous for the accurate diagnosis and treatment of GSRC.
Methods
Ethics
The present study was approved by the Institutional Review Boards of Shandong Cancer Institute (Approval No. SDTHEC2020011006) and Jinan Central Hospital (Approval No. GZR2019-041-01). All specimens were obtained with written informed consent and were fully anonymized. Consent to publish relevant clinical information potentially identifying individuals (e.g., age, gender, histological grade, etc.) was obtained. We have consent to publish the information that identifies individuals. Participants were not granted any corresponding compensation, as our study exclusively utilized residual tissue samples (secondary use of surplus materials), effectively obviating the necessity for supplementary patient follow-up or communication. This research was conducted according to the principles of the Declaration of Helsinki.
Human specimens
The gastroscopic biopsy pathology of enrolled patients at first diagnosis was GA; they had not received antitumour treatments, such as radiotherapy, chemotherapy, and immunotherapy before surgery, and did not have tumours in other organs. In the discovery cohort, 13 patients were prospectively enrolled for scRNA-seq, including 7 males and 6 females with median ages of 63 and 51 years old. The patients were grouped mainly based on the degree of differentiation and signet ring cell content, and no gender-based analysis was performed. In the validation cohort, 60 patients were retrospectively enrolled for immunohistochemistry (IHC).
Tissue dissociation and preparation of single-cell suspensions
Human gastric tissue was obtained from patients and placed into a sterile RNase-free culture dish containing an appropriate amount of calcium-free and magnesium-free 1× phosphate-buffered saline (PBS) on ice. The tissue was then transferred into the culture dish, cut into 0.5 mm2 pieces, and washed with 1× PBS, and blood residue and fatty layers were removed. Tissues were dissociated into single cells in dissociation solution. The overall cell viability was more than 85%, as confirmed by trypan blue exclusion. The single-cell suspensions were counted using the Countess II Automated Cell Counter, and the concentration was adjusted to 700–1200 cells/μl before single-cell analysis.
10X Genomics Chromium library and sequencing
Single-cell suspensions were loaded onto a 10X Genomics Chromium instrument to capture 8000 single cells according to the manufacturer’s instructions for the 10X Genomics Chromium Single-Cell 3′ kit (V3). The following cDNA amplification and library construction steps were performed according to the standard protocol. Libraries were sequenced on an Illumina NovaSeq 6000 sequencing system (paired-end multiplexing run, 150 bp) by LC-Bio Technology Co., Ltd, (Hangzhou, China) at a minimum depth of 20,000 reads per cell.
CellRanger software (v7.0.0, 10X Genomics) was then used to analyse the sequencing data, and gene expression information was obtained for each cell. Cell Ranger (http://support.10xgenomics.com/single-cell/software/overview/welcome) used the STAR aligner (https://github.com/alexdobin/STAR) to perform splicing-aware alignment of reads to the genome. The Cell Ranger output was loaded onto Seurat (v4.1.1) software for dimensionality reduction, clustering, and analysis of scRNA-seq data. Overall, 149,782 cells passed the quality control threshold; all genes expressed in less than three cells were removed, the number of genes expressed per cell was between 500 and 5000, with a unique molecular identifier (UMI) count less than 500, and the rate of mitochondrial gene expression was <25%.
DoubletFinder (v2.0.3) was used to remove multiplet cells from sequencing data. To visualize data, the LogNormalize method of the “Normalization” function of Seurat software was utilized to calculate the expression levels of the genes. PCA was performed using the normalized expression levels, and the top 10 PCs were used to carry out clustering and t-distributed stochastic neighbour embedding (t-SNE) analysis. Due to the obvious batch effect among samples, Harmony (v0.1.0) was used for batch effect correction.
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses of differentially expressed genes (DEGs)
GO enrichment analysis provides all GO terms that are significantly enriched in DEGs compared with the genome background and filters the DEGs that correspond to biological functions. First, all related genes were mapped to GO terms in the Gene Ontology database (http://www.geneontology.org/), gene numbers were calculated for every term, and GO terms significantly enriched in DEGs compared with the genome background were defined by hypergeometric test.
Pathway-based analysis helps to further improve the understanding of the biological functions of genes. KEGG is the major public pathway database. Pathway enrichment analysis identified significantly enriched metabolic pathways or signal transduction pathways in DEGs compared with the whole genome background.
Gene set enrichment analysis (GSEA)
Using the “clusterProfiler” R package, GSEA was performed on the GO and KEGG databases for RNA-seq data. GO and KEGG pathway enrichment analyses were carried out, followed by visualization using the “clusterProfiler” R package.
Gene set variation analysis (GSVA)
GSVA is a nonparametric unsupervised analysis method that is mainly used to evaluate gene set enrichment results of microarray nuclear transcriptomes. It is typically utilized to indicate whether different metabolic pathways are enriched between different samples by converting the expression matrix of genes, mainly from the perspective of bioinformatics, to explain the causes of phenotypic differences.
DepMap database
DepMap is a cancer cell line database that integrates and provides the existing cell line database. The transcriptional expression data of gastric cancer cell lines were downloaded from the DepMap database (https://depmap.org/portal/).
InferCNV
InferCNV is a relatively efficient analytical tool for determining intracellular chromosomal copy number variation (CNV) changes. Tumour RNA-seq data were explored using InferCNV to analyse somatic large-scale CNVs, such as gains or losses of whole chromosomes and large segments of chromosomes. In general, tumour cells are prone to CNV mutations, and genes in the regions where CNV changes occur are always overexpressed or downregulated compared with normal cells.
Cell culture
MKN-45 (CBP60488) and NUGC4(CBP60493) cells were purchased from Nanjing Kebai Biotechnology Co., Ltd (Nanjing, China). MKN-45 and NUGC4 cells were authenticated using short tandem repeat analysis. No mycoplasma contamination was detected. MKN-45 and NUGC4 cells cultured in Roswell Park Memorial Institute (RPMI)−1640 medium (HyClone, Logan, UT, USA) containing 10% foetal bovine serum (FBS; Hangzhou Sijiqing Co., Ltd., Hangzhou, China) at 37 °C in the presence of 5% CO2.
RNA extraction
Total RNA was extracted from the cells using TRIzol® reagent (RNA isolation from plant tissue) according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA), and genomic DNA was removed using DNase I (TaKaRa, Shiga, Japan). Then, RNA quality was determined by the 2100 Bioanalyzer system (Agilent Technologies, Inc., Santa Clara, CA, USA) and quantified using the ND-2000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Only high-quality RNA samples (optical density (OD) 260/280 = 1.8 ~ 2.2, OD260/230 ≥ 2.0, RNA integrity number (RIN) ≥ 6.5, 28S:18S ≥ 1.0, >1 μg) were used to construct the sequencing library.
Library preparation and Illumina HiSeq X Ten/NovaSeq 6000 sequencing
An RNA-seq transcriptome library was constructed following TruSeqTM RNA sample preparation using a kit (Illumina Inc., San Diego, CA, USA) with 1 μg of total RNA. Briefly, messenger RNA (mRNA) was isolated according to the poly(A) selection method by oligo(dT) beads and then initially fragmented by fragmentation buffer. Second, double-stranded cDNA was synthesized using a SuperScript double-stranded cDNA synthesis kit (Invitrogen) with random hexamer primers (Illumina). Afterwards, the synthesized cDNA was subjected to end repair, phosphorylation, and ‘A’ base addition according to the Illumina library construction protocol (Illumina Inc.). Libraries were size-selected for cDNA target fragments of 300 bp on 2% Low Range Ultra Agarose, followed by polymerase chain reaction (PCR) amplification via Phusion DNA polymerase (NEB) for 15 PCR cycles. After quantification by TBS380, a paired-end RNA sequencing library was sequenced using the Illumina HiSeq X Ten/NovaSeq 6000 sequencer (2 × 150-bp read length). Data were analysed by the Majorbio cloud platform (www.majorbio.com).
Immunohistochemistry (IHC)
IHC was performed with a HistoMouse SP Broad Spectrum DAB kit (Invitrogen) following the manufacturer’s instructions. Briefly, samples were routinely deparaffinized, and antigen retrieval was conducted at high temperature and high pressure. Subsequently, the samples were incubated with primary antibody at 4 °C overnight. Then, 3,3′-diaminobenzidine (DAB) colour was developed, and each section was counterstained with haematoxylin for 30 s. Antibody was replaced with PBS as a blank control. The following antibodies were used to detect the proteins: anti-EpCAM (rabbit, 1:400, bs-0593R, Bioss, China), anti-TTF1 (rabbit, 1:1000, 66034-1-IG, 2C8F3, PROTEINTECH GROUP, USA), anti-TTF2 (rabbit, 1:100, 13722-1-AP, PROTEINTECH GROUP, USA), anti-MUC5AC (mouse,1:200, GB14112, l107, Servicebio, China), anti-MUC6 (mouse, 1:50, GB14113, l108, Servicebio, China), anti-JCHAIN (rabbit, 1:1000, bsm-60277R, J2H8, Bioss, China), anti-MSMB (rabbit, 1:200, bs-19185R, Bioss, China), anti-CD74 (mouse, 1:1000, GB121179, 3H10A4, Servicebio, China), anti-FOXP3 (rabbit, 1:400, GB11093, Servicebio, China), antiKLRD1 (rabbit, 1:100, DF6773, Affinity Biosciences, USA).The histopathological images of the cases were examined by two experienced pathologists. The results were judged by the semiquantitative integral method, and the score was comprehensively assessed according to the percentage of positive cells and the positive intensity. (1) Percentage of positive cells: ≤5% positive cells, 0 points; 6–25%, 1 point; 26–50%, 2 points; 51–75%, 3 points; and >75%, 4 points. (2) Positive intensity: colourless, 0 points; pale yellow, 1 point; yellow, 2 points; and brownish yellow; 3 points. The final score was calculated as follows: final score = positive ratio score × positive intensity score.
Statistical analysis
Marker genes for transcriptional subpopulations in scRNA-seq profiles were identified using the FindAllMarkers function of Seurat software with a minimum log-fold change threshold of 0.25 and with p values calculated by the Wilcoxon rank-sum test. p values were computed using a hypergeometric test and adjusted for multiple hypothesis testing with the Benjamini‒Hochberg procedure. The experimental data were statistically analysed using SPSS 24.0 software (IBM, Armonk, NY, USA). The t-test and Wilcoxon rank-sum test were utilized to compare mean values between two groups. The Bonferroni correction method was used to make pairwise comparisons when the difference was statistically significant; correlations were explored using Pearson’s correlation analysis. p < 0.05 was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This study was supported by the Major Innovation Project of Science and Technology of Shandong Province (No. 2019ZZZY011008, by J.C.), the Natural Science Foundation of Shandong Province (No. ZR2021MH108, by J.C.), the Academic Promotion Program of Shandong First Medical University (No. 2019QL024, by Y.J.), and the Hospital-level project of Binzhou People’s Hospital (No. XJ202202505, by W.Z.). We thank LC-Bio Technology Co., Ltd (Hangzhou, China) and Majorbio for their assistance in sequencing.
Source data
Author contributions
L.X. and J.C. were the principal investigators who conceived and designed the study, obtained financial supports and approved the final version of the manuscript; W.Z. and Y.J. performed data acquisition and analysis and wrote and revised the manuscript.; G.S., L.L., X.Q. and J.D. collected clinical samples and performed immunohistochemistry; H.Y. and H.Yu performed data compilation and statistics; B.X. and S.Z. were responsible for cell culture. All the authors read and approved the final version of the manuscript.
Peer review
Peer review information
Nature Communications thanks Matthew Benesch and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The raw scRNA-seq data used in this study are available in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in the National Genomics Data Center (Nucleic Acids Res 2022), China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences database under accession code GSA-Human: HRA003647. The raw RNA-seq data generated in this study have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in the National Genomics Data Center (Nucleic Acids Res 2022), China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences database under accession code GSA-Human: HRA003650. The published data used for validation of the expression of MSMB in cell lines in this manuscript were retrieved from DepMap databases (https://depmap.org/portal/Interactive). The data are provided in the form of a single Excel file and placed in multiple label files in a compressed folder. This file or folder was also named “Source Data”. The remaining data are also available in this manuscript. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Weizhu Zhao, Yanfei Jia.
Contributor Information
Ligang Xing, Email: xinglg@medmail.com.cn.
Jie Chai, Email: jchai@sdfmu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-38426-4.
References
- 1.Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Feng R, Zong Y, Cao S, Xu R. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics. Cancer Commun. 2019;39:22. doi: 10.1186/s40880-019-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galletti G, et al. Microtubule engagement with taxane is altered in taxane-resistant gastric cancer. Clin. Cancer Res. 2020;26:3771–3783. doi: 10.1158/1078-0432.CCR-19-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lordick F, Janjigian YY. Clinical impact of tumour biology in the management of gastroesophageal cancer. Nat. Rev. Clin. Oncol. 2016;13:348–360. doi: 10.1038/nrclinonc.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudler P. Challenges of deciphering gastric cancer heterogeneity. World J. Gastroenterol. 2015;21:10510–10527. doi: 10.3748/wjg.v21.i37.10510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LAUREN P, et al. The two histological main types of gastric carcinoma: diffuse and so called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 7.Lee JJ, et al. Advanced gastric carcinoma with signet ring cell carcinoma versus non-signet ring cell carcinoma: differentiation with multidetector CT. J. Comput. Assist. Tomogr. 2006;30:880–884. doi: 10.1097/01.rct.0000220800.76276.46. [DOI] [PubMed] [Google Scholar]
- 8.Xie Y, et al. Anatomic subsites and prognosis of gastric signet ring cell carcinoma: a SEER population-based 1: 1 propensity-matched study. BioMed. Res. Int. 2022;2022:1565207. doi: 10.1155/2022/1565207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torre LA, Siegel RL, Ward EM, Jemal M. Global cancer incidence and mortality rates and trends-an update. Cancer Epidemiol. Biomark. Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 10.Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann. Surg. 2009;250:878–887. doi: 10.1097/SLA.0b013e3181b21c7b. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Zhong Y, Xu Q, Zhu Z, Tian Y. Prognostic significance of signet ring cells in gastric cancer: the higher proportion, the better survival. Front. Oncol. 2021;11:713587. doi: 10.3389/fonc.2021.713587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, et al. Estrogen receptor beta prevents signet ring cell gastric carcinoma progression in young patients by inhibiting pseudopodia formation via the mTOR-Arpc1b/EVL signaling pathway. Front. Cell Dev. Biol. 2020;8:592919. doi: 10.3389/fcell.2020.592919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shu Y, et al. Prognostic significance of frequent CLDN18-ARHGAP26/6 fusion in gastric signet-ring cell cancer. Nat. Commun. 2018;9:2447. doi: 10.1038/s41467-018-04907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang KH, et al. The clinicopathological characteristics and genetic alterations of signet-ring cell carcinoma in gastric cancer. Cancers. 2020;12:2318. doi: 10.3390/cancers12082318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zilionis R. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity. 2019;50:1317–1334. doi: 10.1016/j.immuni.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng J, et al. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res. 2019;29:725–738. doi: 10.1038/s41422-019-0195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao S, et al. Tracing the temporal-spatial transcriptome landscapes of the human fetal digestive tract using single-cell RNA-sequencing. Nat. Cell Biol. 2018;20:721–734. doi: 10.1038/s41556-018-0105-4. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, et al. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell. 2021;184:404–421. doi: 10.1016/j.cell.2020.11.041. [DOI] [PubMed] [Google Scholar]
- 19.Zhang P, et al. Dissecting the single-cell transcriptome network underlying gastric premalignant lesions and early gastric cancer. Cell Rep. 2019;27:1934–1947. doi: 10.1016/j.celrep.2019.04.052. [DOI] [PubMed] [Google Scholar]
- 20.Wang R, et al. Single-cell dissection of intratumoral heterogeneity and lineage diversity in metastatic gastric adenocarcinoma. Nat. Med. 2021;27:141–151. doi: 10.1038/s41591-020-1125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei X, et al. Identification of novel tumor microenvironment-associated genes in gastric cancer based on single-cell RNA-sequencing datasets. Front. Genetis. 2022;13:896064. doi: 10.3389/fgene.2022.896064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia L, et al. Single-cell profiling of infiltrating B cells and tertiary lymphoid structures in the TME of gastric adenocarcinomas. Oncoimmunology. 2021;10:1969767. doi: 10.1080/2162402X.2021.1969767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou ZH, Zhang JD, Zhao HB, Zhao LN, Shan BZ. Cell origin and premalignant lesions of gastric signetring cell carcinoma: A histopathologic study. World Chin. J. Digestol. 2010;18:2001–2006. doi: 10.11569/wcjd.v18.i19.2001. [DOI] [Google Scholar]
- 24.Xie Y, Shi L, He X, Luo Y. Gastrointestinal cancers in China, the USA, and Europe. Gastroenterol. Rep. 2021;9:91–104. doi: 10.1093/gastro/goab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pernot S, et al. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J. Gastroenterol. 2015;21:11428–11438. doi: 10.3748/wjg.v21.i40.11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mariette C, et al. Consensus on the pathological definition and classification of poorly cohesive gastric carcinoma. Gastric Cancer. 2019;22:1–9. doi: 10.1007/s10120-018-0868-0. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C, et al. Difference between signet ring cell gastric cancers and non-signet ring cell gastric cancers: a systematic review and meta-analysis. Front. Oncol. 2021;11:618477. doi: 10.3389/fonc.2021.618477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, et al. Clinicopathological characteristics and survival outcomes of primary signet ring cell carcinoma in the stomach: retrospective analysis of single center database. PLoS ONE. 2015;10:e0144420. doi: 10.1371/journal.pone.0144420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kao YC, et al. Clinicopathological differences in signet ring cell adenocarcinoma between early and advanced gastric cancer. Gastric Cancer. 2019;22:255–263. doi: 10.1007/s10120-018-0860-8. [DOI] [PubMed] [Google Scholar]
- 30.Bamboat ZM, et al. Stage-stratified prognosis of signet ring cell histology in patients undergoing curative resection for gastric adenocarcinoma. Ann. Surg. Oncol. 2014;21:1678–1685. doi: 10.1245/s10434-013-3466-8. [DOI] [PubMed] [Google Scholar]
- 31.Zhang M, et al. Dissecting transcriptional heterogeneity in primary gastric adenocarcinoma by single cell RNA sequencing. Gut. 2021;70:464–475. doi: 10.1136/gutjnl-2019-320368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma L, et al. Tumor cell biodiversity drives microenvironmental reprogramming in liver cancer. Cancer Cell. 2019;36:418–430. doi: 10.1016/j.ccell.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu B, et al. Highly expressed Claudin18.2 as a potential therapeutic target in advanced gastric signet-ring cell carcinoma (SRCC) J. Gastrointest. Oncol. 2020;11:1431–1439. doi: 10.21037/jgo-20-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng L, et al. Identification of gastric cancer-related genes by multiple high throughput analysis and data mining. Chin. J. Gastrointest. Surg. 2007;10:169–172. [PubMed] [Google Scholar]
- 35.Ohnuma S, et al. Cancer-associated splicing variants of the CDCA1 and MSMB genes expressed in cancer cell lines and surgically resected gastric cancer tissues. Surgery. 2009;145:57–68. doi: 10.1016/j.surg.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Zu H, Wang H, Li C, Xue Y. Clinicopathologic characteristics and prognostic value of various histological types in advanced gastric cancer. Int. J. Clin. Exp. Pathol. 2014;7:5692–5700. [PMC free article] [PubMed] [Google Scholar]
- 37.Ma J, Meng Y, Zhou X, Guo L, Fu W. The prognostic significance and gene expression characteristics of gastric signet-ring cell carcinoma: a study based on the SEER and TCGA databases. Front. Surg. 2022;9:819018. doi: 10.3389/fsurg.2022.819018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altay SB, Akkurt G, Yılmaz N, Özdemir N. Clinicopathological evaluation of gastric signet ring cell carcinoma: our experience. Gastroenterol. Rep. 2020;10:76–84. doi: 10.5005/jp-journals-10018-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muta H, et al. E-cadherin gene mutations in signet ring cell carcinoma of the stomach. Jpn. J. Cancer Res. 1996;87:843–848. doi: 10.1111/j.1349-7006.1996.tb02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen J, et al. Screening of differential microRNA expression in gastric signet ring cell carcinoma and gastric adenocarcinoma and target gene prediction. Oncol. Rep. 2015;33:2963–2971. doi: 10.3892/or.2015.3935. [DOI] [PubMed] [Google Scholar]
- 41.Zhu Q, et al. Irinotecan induces autophagy-dependent apoptosis and positively regulates ROS-related JNK- and P38-MAPK pathways in gastric. Cancer Cells OncoTargets Ther. 2020;13:2807–2817. doi: 10.2147/OTT.S240803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang M, et al. NAIF1 inhibits gastric cancer cells migration and invasion via the MAPK pathways. J. Cancer Res. Clin. Oncol. 2015;141:1037–1047. doi: 10.1007/s00432-014-1865-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang W, et al. Expression of estrogen receptors and androgen receptor and their clinical significance in gastric cancer. Oncotarget. 2017;8:40765–40777. doi: 10.18632/oncotarget.16582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taghavi S, Jayarajan S, Davey A, Willis AI. Prognostic significance of signet ring gastric cancer. J. Clin. Oncol. 2012;30:3493–3498. doi: 10.1200/JCO.2012.42.6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang XF, et al. Pathobiological behavior and molecular mechanism of signet ring cell carcinoma and mucinous adenocarcinoma of the stomach: a comparative study. World J Gastroenterol. 2004;10:750–754. doi: 10.3748/wjg.v10.i5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin X, et al. Clinical features and risk factors for lymph node metastasis in early signet ring cell gastric cancer. Front. Oncol. 2021;11:630675. doi: 10.3389/fonc.2021.630675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tong JS, et al. ER-α36, a novel variant of ER-α, mediates estrogen-stimulated proliferation of endometrial carcinoma cells via the PKCδ/ERK pathway. PLoS ONE. 2010;5:e15408. doi: 10.1371/journal.pone.0015408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun Q, Liang Y, Zhang T, Wang K, Yang X. ER-α36 mediates estrogen-stimulated MAPK/ERK activation and regulates migration, invasion, proliferation in cervical cancer cells. Biochemical. 2017;487:625–632. doi: 10.1016/j.bbrc.2017.04.105. [DOI] [PubMed] [Google Scholar]
- 49.Caiazza F, Galluzzo P, Lorenzetti S, Marino M. 17Beta-estradiol induces ERbeta up-regulation via p38/MAPK activation in colon cancer cells. Biochemical. 2007;359:102–107. doi: 10.1016/j.bbrc.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 50.Powell E, Wang Y, Shapiro DJ, Xu W. Differential requirements of Hsp90 and DNA for the formation of estrogen receptor homodimers and heterodimers. J. Biol. Chem. 2010;285:16125–16134. doi: 10.1074/jbc.M110.104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fliss A, Benzeno S, Rao J, Caplan AJ. Control of estrogen receptor ligand binding by Hsp90. J. Steroid Biochem Mol. Biol. 2000;72:223–230. doi: 10.1016/S0960-0760(00)00037-6. [DOI] [PubMed] [Google Scholar]
- 52.Dhamad AE, Zhou Z, Zhou J, Du Y. Systematic proteomic identification of the heat shock proteins (Hsp) that interact with estrogen receptor alpha (ERα) and biochemical characterization of the ERα-Hsp70 interaction. PLoS ONE. 2016;11:e0160312. doi: 10.1371/journal.pone.0160312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parker TM, et al. Cell competition in intratumoral and tumor microenvironment interactions. EMBO J. 2021;40:e107271. doi: 10.15252/embj.2020107271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao S, Hsu TW, Li M. Immunity beyond cancer cells: perspective from tumor tissue. Trends Cancer. 2021;7:1010–1019. doi: 10.1016/j.trecan.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Havel JJ, Chowell D, Chan T. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer. 2019;19:133–150. doi: 10.1038/s41568-019-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hennequin A, et al. Tumor infiltration by Tbet+ effector T cells and CD20+B cells is associated with survival in gastric cancer patients. Oncoimmunology. 2016;5:e1054598. doi: 10.1080/2162402X.2015.1054598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tortajada A, Gutierrez E, Pickering MC, Terente MP, Medjeral-Thomas N. The role of complement in IgA nephropathy. Mol. Immunol. 2019;114:123–132. doi: 10.1016/j.molimm.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 58.Tezuka H, et al. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–933. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- 59.Ma K, et al. Correlations between CD4 FoxP3 Treg and expression of FoxM1 and Ki-67 in gastric cancer patients. Asia Pac. J. Clin. Oncol. 2021;17:e63–e69. doi: 10.1111/ajco.13302. [DOI] [PubMed] [Google Scholar]
- 60.Pernot S, et al. Infiltrating and peripheral immune cell analysis in advanced gastric cancer according to the Lauren classification and its prognostic significance. Gastric Cancer. 2020;23:73–81. doi: 10.1007/s10120-019-00983-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The raw scRNA-seq data used in this study are available in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in the National Genomics Data Center (Nucleic Acids Res 2022), China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences database under accession code GSA-Human: HRA003647. The raw RNA-seq data generated in this study have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in the National Genomics Data Center (Nucleic Acids Res 2022), China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences database under accession code GSA-Human: HRA003650. The published data used for validation of the expression of MSMB in cell lines in this manuscript were retrieved from DepMap databases (https://depmap.org/portal/Interactive). The data are provided in the form of a single Excel file and placed in multiple label files in a compressed folder. This file or folder was also named “Source Data”. The remaining data are also available in this manuscript. Source data are provided with this paper.