Abstract
Rotavirus is the major cause of diarrhea among young infants in both humans and animals. Immune protection of newborns by vaccination is difficult to achieve since there is not enough time to mount an immune response before exposure to the virus. We have designed a vaccination strategy mediating transfer of neutralizing antibodies from the mother to the offspring during pregnancy and/or lactation. Adult female mice were nasally immunized with virus-like particles (VLPs) made of viral proteins VP2 and 6 (VLP2/6) or VP 2, 6, and 7 (VLP2/6/7) derived from the RF rotavirus strain in the presence or absence of cholera toxin. Both vaccines elicited serum and milk antibodies against the respective VPs. Four days after parturition, suckling pups were challenged orally with RF rotavirus. Pups from mothers immunized with VLP2/6/7 but not VLP2/6 were protected against rotavirus diarrhea, indicating that VP7 plays a key role in protection. Protection was mediated by milk rather than serum antibodies, and mucosal adjuvants were not required. In conclusion, VLPs containing VP7 administered nasally to mothers represent a promising vaccine candidate for the protection of suckling newborns against rotavirus-induced diarrhea, even in the absence of a mucosal adjuvant.
Rotavirus, a member of the Reoviridae family, is the leading cause of severe diarrhea in newborns worldwide (16). The infection is disseminated by feco-oral transmission. The virus targets the small intestine mucosa and replicates strictly in the epithelial cells. Villi are reduced in size and destroyed. Sodium adsorption is reduced and water accumulates in the lumen (27, 30). These processes cause diarrhea. Since the disease results in a high rate of mortality in the developing countries and high morbidity in the industrialized countries, and due to the absence of antirotavirus drugs, efforts have been made to design vaccine strategies to prevent the disease.
Different strategies of vaccination have been based on the use of live rotavirus or subunit vaccines. Usually, protection against rotavirus infection in adult mice was measured by reduction of fecal virus shedding after oral challenge, but in all species, including human and mice, rotavirus infection does not cause diarrhea in adults. In contrast to adults, newborn mice during the first 2 weeks of life develop diarrhea when infected. Protection of pups through vaccination, however, is difficult to achieve since there is not time to develop an immune response able to protect the pups during the short susceptibility period, and their immune system is not fully mature (22, 31). Thus, immunization of mothers with live heterologous viruses that results in the transfer of their antibody repertoire to the offspring represents an alternative that has already been explored by others (19, 25, 28, 32). Since some side effects have been observed with live viruses, subunit vaccines in the form of virus-like particles (VLPs) have been developed and tested in cows by intramammary gland injection. Calves receiving milk from immunized mothers were protected (12).
Rotaviruses are composed of three protein layers surrounding 11 segments of single-stranded RNA (11). The inner layer is composed of three viral proteins, VP1, VP2, and VP3; the middle layer contains VP6, and the outer layer contains VP4 and VP7. Rotavirus genes encoding VPs have been expressed in insect cells using baculovirus vectors. In this expression system, VP2 alone drives the formation of stable VLPs (21), and coexpression of other VPs results in the assembly of multilayered VLPs (9, 21), i.e., double layered with VP2 and 6 and triple layered when VP7 is added. Since VPs retain their native structure in VLPs, one can expect them to elicit conformational antibodies similar to those triggered by live viruses. Thus VLPs represent a promising alternative to live-virus vaccines. Systemic or mucosal administration of VP7-containing VLPs induces immune responses (8, 26), but protection against diarrhea was not assessed, the experiments being restricted to adults. In addition, nasal immunization appeared to be the best route of vaccination (26).
In this study, we observe that immunization of dams with VLPs containing VP7 protects their pups against rotavirus-induced diarrhea. We demonstrate for the first time that nasal immunization of mothers with VLPs in the absence of mucosal adjuvants triggers high milk and serum antibody titers that protect suckling newborns. Finally, milk but not serum antibodies are required for protection.
MATERIALS AND METHODS
VLPs and viruses.
VLP2/6 and VLP2/6/7 were assembled in insect cells using the VP2, VP6, and VP7 genes of the bovine RF strain (G6 serotype) and were purified as previously described (21). The virulent bovine RF strain viruses (G6 serotype) and the heterologous virulent simian RRV strain (G3 serotype) were produced as previously described (9).
Immunization and breeding of mice.
Ten-week-old BALB/c mice (Harlan, Horst, The Netherlands) were used in this study. Each group contained eight animals. Female mice were anesthetized by intraperitoneal injection of 200 μl of anesthetic containing 1 mg of Ketasol-100 (GraeuB, Bern, Switzerland) and 0.4 mg of Rompun (Bayer, Leverkusen, Germany) per 20 g of body weight in phosphate-buffered saline (PBS). Anesthetized mice were immunized nasally with 20 μl of solution containing 5 μg of VLPs or 5 μg of VLPs and 5 μg of cholera toxin (CT) (Vibrio cholerae, type INABA 569B [Calbiochem, La Jolla, Calif.]). Ten microliters of vaccine was instilled in each nostril with a micropipette. Mice were boosted twice nasally, on days 7 and 14. One week after the last immunization, mice were mated. One male was added for two females in each cage.
Sampling of milk.
A total of 100 μl of oxytocin (5 IU/ml) (Syntocinon; Sandoz) was injected intraperitoneally into lactating female mice, and 5 min later the animals were anesthetized as described above. Milk was harvested using a vacuum pump adapted to the mouse breast and was collected in a tube kept on ice. Milk was diluted 1:3 with PBS, centrifuged 5 min at 180 × g to remove debris and fat, and stored at −70°C. Rotavirus-specific antibodies were measured by enzyme-linked immunosorbent assay (ELISA).
ELISA.
ELISA was performed in Maxisorp 96-well immunoplates (Nunc, Life Technologies, Basel, Switzerland). For immunoglobulin G (IgG) measurements, plates were coated with 100 μl of VLP2/6 (1 μg/ml) per well. After a blocking step of 30 min at 37°C with PBS–1% milk, plates were washed five times, and serial twofold dilutions, starting at 1:100, of milk or serum samples in PBS–1% milk–0.1% Tween 20 were added to each well and incubated for 2 h at 37°C. After washing, plates were incubated with a goat anti-mouse IgG linked to horseradish peroxidase (diluted 1:20,000) (Bio-Rad, Hercules, Calif.) for 1.5 h at 37°C. For IgA, plates were treated as described above but with initial serum samples diluted 1:5 and milk samples diluted 1:3. Plates were further incubated with a 1:500 dilution of a biotinylated goat immunoglobulin directed against mouse IgA (Sigma, Buchs, Switzerland) for 1 h at 37°C. After washing, plates were incubated with a 1:1,000 dilution of streptavidin conjugated to horseradish peroxidase (Amersham, Arlington Heights, Ill.) for 30 min at 37°C. In both cases, plates were developed with 0.1% O-phenylenediamine (Sigma) and 0.03% H2O2 in citrate buffer (44.4 mM citric acid, 103 mM Na2HPO4). After 15 min, absorbency was measured in a Packard Spectracount at 495 nm. Preimmune sera and milk from nonimmunized mice served as controls. The specific IgA or IgG titers were expressed as the reciprocal of the highest dilution that yielded an absorbency equivalent to four times that of the preimmune samples. Samples from control mice (PBS or CT) were negatives for VLP2/6-specific antibodies (titer < 100).
SDS-PAGE and immunoblot analysis.
Proteins separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were electroblotted onto nitrocellulose membrane in a transfer buffer containing methanol. Membranes were blocked for 30 min with PBS–0.1% Tween 20–4% dry milk at room temperature, washed three times for 5 min in PBS–0.1% Tween 20, and incubated overnight at 4°C with mouse serum (1:100) or rabbit anti-bovine RF serum (8184 serum) (1:1,000) diluted in PBS–0.1% Tween 20–1% dry milk buffer. After three washes of 10 min, horseradish peroxidase-conjugated anti-mouse (Bio-Rad) or anti-rabbit (Sigma) antibody, diluted 1:5,000, was added and incubated for 60 min at room temperature. Blots were washed again and developed with an ECL kit (Amersham) following the manufacturer's instructions. An X-Omat Kodak film (Sigma) was exposed to the membrane.
Protection studies of newborns.
Rotavirus challenge of 4-day-old suckling mice from both control and immunized dams was performed by oral inoculation with a micropipette of 10 μl of virus-containing medium. Each pup received 2 × 106 PFU of RF virus, a virus dose that induces diarrhea in 90% of challenged animals, or 2 × 106 PFU of RRV virus (10 times the virus dose that induces diarrhea in 50% of challenged animals). Pups were monitored daily for onset of diarrhea during 5 days. They were considered sick when yellow and liquid stools appeared upon gentle abdominal palpation.
Statistical analysis.
The χ2 test was performed to determine the significance of protection compared to the PBS- or CT-treated group.
RESULTS
Nasal administration of VLPs elicits a rotavirus-specific antibody response in milk.
Maternal antibodies are known to protect babies from rotavirus infection (17, 25, 32). Since in mice the maternal antibody repertoire is transferred before birth via the placenta, and after birth via milk, we measured by ELISA antibody titers in both serum and milk. Following nasal administration of VLP2/6 or VLP2/6/7, both with CT, sera were collected each week and milk was obtained twice, at 4 and 8 days after delivery (Fig. 1). Three weeks after priming, serum VLP2/6-specific IgG antibody titers reached a plateau (reciprocal log 10 titer, 4.7 ± 0.4) that was maintained until parturition (Fig. 2a). IgG1 represented the major immunoglobulin isotype, suggesting a TH2 response (data not shown). Under our experimental conditions, serum VLP2/6-specific IgA antibodies were not detected. In milk, 4 days after parturition VLP2/6-specific IgG titers (3.5 ± 0.3) (Fig. 2b) were sustained for at least 8 days postpartum. Four days after birth, no VLP2/6-specific IgA antibodies were detected, while at day 8 low titers (1.7 ± 0.2), similar to intestinal concentrations reported by O'Neal et al. (26), were measured for both VLP-immunized groups. In conclusion, nasal VLP immunization was efficient in eliciting a serum antibody response, as expected, but also a milk IgG and, to a lesser extent, an IgA antibody response.
FIG. 1.
Protocol of immunization. Female mice were immunized three times nasally with 5 μg of VLP2/6 or VLP2/6/7 and 5 μg of CT (arrows at top). Control mice were immunized with 10 μl of PBS or 5 μg of CT. Breeding was initiated at week 4 by adding males. Before delivery, females were separated in order to analyze independently each litter. Sera (●) were sampled every week. Milk samples (■) were collected on days 4 and 8 postpartum. Pups were challenged orally 4 days after the delivery with rotavirus RF, and diarrhea was monitored during 5 days.
FIG. 2.
Antibody response elicited by nasal immunization with VLPs. Female mice were nasally immunized with 5 μg of VLP2/6 or VLP2/6/7 and 5 μg of CT at weeks 0, 1, and 2. VLP2/6-specific IgGs were titrated by ELISA. (a) Antibody response in serum. (b) Antibody response in milk.
Attempts to absorb VP2/6-specific antibodies with VLP2/6 and to measure VP7-specific antibodies by ELISA using VLP2/6/7 as the coating antigen were unsuccessful, possibly because VP7 antibody titers were low or due to alteration of VP7 conformation upon coating. In addition, since VP7 in its native configuration is not yet available, we were unable to measure VP7-specific conformational antibodies. Therefore, the presence of serum VP7-specific antibodies in mice immunized with VLP2/6/7 and CT was assessed by immunoblot (Fig. 3). A rabbit polyclonal serum against RF virus served as a positive control. The anti-RF serum revealed a band for VP2 (94 kDa) and known degradation products VP6 (44 kDa) and VP7 (38 kDa) (Fig. 3). In VLP2/6-immunized animals, VP2- and VP6-specific antibodies were detected, and sera from VLP2/6/7-immunized mice contained VP2-, VP6-, and VP7-specific antibodies. These experiments indicate that VLP2/6/7 is able to induce a VP7-specific antibody response in serum but not in milk.
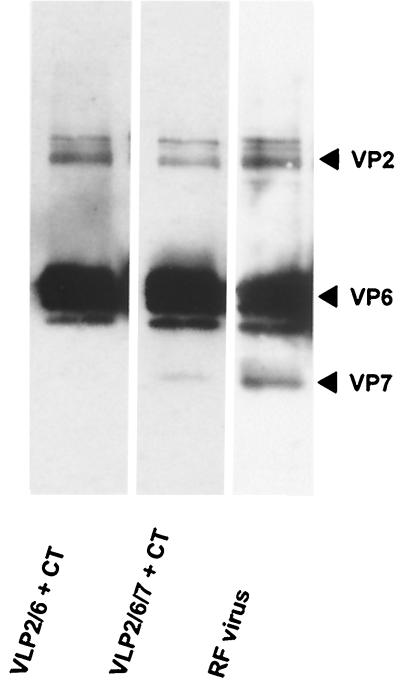
FIG. 3.
Detection of VP7-specific antibodies in serum of immunized animals. Two micrograms of VLP2/6/7 per lane were separated by SDS-PAGE. After transfer onto nitrocellulose membrane, the specificity of serum antibodies from mice immunized with VLP2/6 and CT (lane 1), mice immunized with VLP2/6/7 and CT (lane 2), or a rabbit polyclonal serum against RF virus (lane 3) as positive control were tested by immunoblot.
Newborns from mice nasally immunized with VLP2/6/7 are protected against rotavirus-induced diarrhea.
To test whether mothers immunized with VLPs and CT as an adjuvant can transfer antibodies to their newborns and thus protect them against viral challenge, 4-day-old newborns from nasally immunized mice were challenged with an oral dose of 2.5 × 106 PFU of RF virus. The RF strain shares the same VP2, VP6, and VP7 proteins that the VLPs used for immunization. As a readout of infection, we scored diarrhea during the 4-day period following viral challenge (Fig. 4). The incidence of diarrhea in pups from PBS or CT sham-immunized mice was 93.7 and 81%, and the difference between the two groups was not significant (P = 0.13). In the group of pups from VLP2/6-immunized mice, the diarrhea incidence was 71.4% (Fig. 4). This value was not significantly different from that of the sham-immunized group (P = 0.31). In contrast, pups from VLP2/6/7-immunized mothers were clearly protected, with only 27% diarrheic animals. Our data indicate that VLPs and CT given nasally are immunogenic and induce maternal immune effector antibodies that are transmitted to the pups and protect them against rotavirus-induced diarrhea. Moreover, we showed that passive protection requires the presence of VP7.
FIG. 4.
Passive protection of newborns against rotavirus. Female mice were immunized three times nasally with 5 μg of VLP2/6 or VLP2/6/7 and 5 μg of CT. Control mice were immunized with 10 μl of PBS or 5 μg of CT. Breeding was initiated at week 4 by adding males. Four-day-old newborns were orally infected with 2.5 × 106 PFU of RF virus. Diarrhea was checked daily. Pups presenting diarrhea were not protected. ∗, P < 0.05.
Milk antibodies play a key role in passive protection against rotavirus-induced diarrhea.
To determine the respective contribution of antibodies acquired by pups through the placenta or via milk, foster nursing experiments were performed. To assess the importance of maternally transferred serum antibodies, pups from VLP2/6/7 plus CT-immunized mice were foster nursed on nonimmunized mice. The role of milk antibodies was determined by an inverse setup, with pups from nonimmunized mothers fed by immunized lactating mice. The results illustrated in Fig. 5 indicate that pups born from immunized mice were not protected, while 60% of the pups nursed by immune mothers did not develop diarrhea. The difference in protection (Fig. 5) observed between pups receiving only the milk of an immunized female (milk group) and pups born of and nursed by immunized animals (milk + placenta group) was not significant (P = 0.42). This result indicates that only milk antibodies play a role in passive protection.
FIG. 5.
Contribution of milk antibodies to protection. Female mice were nasally immunized with 5 μg of VLP2/6/7 and 5 μg of CT at weeks 0, 1, and 2. Females of the control group were not immunized. Females of the immunized and control groups delivering the same days were inverted. At day 4 postpartum, pups were orally infected with 2.5 × 106 PFU of RF virus. Diarrhea was checked daily. Pups presenting diarrhea were not protected. The χ2 test was performed to determine the significance of protection compared to the negative, nonexchanged group. ∗, P < 0.05. Pups were born of and nursed by (placenta + milk), only born of (placenta), or only nursed by (milk) VLP2/6/7- plus CT-immunized mice; i.e., they received antibodies against VLP from both placenta and milk, only from placenta, or only from milk, respectively.
Mucosal adjuvants are not required for antibody-mediated protection against rotavirus-induced diarrhea in newborns.
Usually mucosal adjuvants are necessary to mount efficient immune responses against mucosally delivered antigens (14). Due to their toxicity, CT and Escherichia coli labile toxin cannot be used in humans, and toxoids have been generated (14). Since viral particles trigger strong antibody responses in the absence of adjuvants due to the repetitive structure of their surfaces (3), we tested whether rotavirus VLPs, which share the same surface structure as native viruses, were able to stimulate an antibody response sufficient to confer protection upon transfer of the antibodies from the mother to the offspring. Mice were immunized and challenged following the schedule outlined in Fig. 1. The protection was not significantly different from the groups with CT (P = 0.16) (Fig. 6).
FIG. 6.
Immunity induced by VLP2/6/7 nasal immunization without CT. Female mice were nasally immunized with 5 μg of VLP2/6/7 with or without 5 μg of CT and control mice were immunized with 10 μl of PBS or 5 μg of CT at weeks 0, 1, and 2. Levels of VLP2/6-specific antibodies were analyzed by ELISA. (a) Serum antibody response. Control mice immunized with PBS or CT developed no detectable response. (b) Milk antibody response. Mice were milked twice, at 4 and 8 days postpartum. (c) Protection of newborns. Breeding was initiated at week 4 by adding males. Four-day-old newborns were orally infected with 2.5 × 106 PFU of RF virus. Diarrhea was checked daily. Pups presenting diarrhea were not protected. ∗, P < 0.05.
Finally, to determine whether VP7-mediated protection was also efficient against heterotypic virus strains, we challenged pups of mice immunized with VLP2/6/7 derived from RF strain (G6 serotype) with an RRV virus (G3 serotype). Since no protection was observed (91% diarrheic animals), we concluded that VLPs derived from G6 serotype strains were unable to protect against a G3 serotype strain.
DISCUSSION
Rotavirus VLPs made of the viral proteins VP2, 6, and 7 are immunogenic in the absence of mucosal adjuvant and elicit a strong rotavirus-specific antibody response in serum and milk when administered nasally to female mice. Pups suckling immunized mothers are protected against infection with a virus strain homotypic to the challenge and do not develop diarrhea. We provide evidence that protection is mainly mediated by milk and not placentally transferred antibodies.
Protection against rotavirus infection by passive maternal transfer of antibodies has already been reported for mice immunized with live-rotavirus vaccines (25) or recombinant adenovirus expressing rotavirus VP7 (5), administered orally or intraperitoneally. Since a licensed tetravalent live-rotavirus vaccine has recently been linked to intussusception, a bowel occlusion syndrome (1, 4, 7, 15, 20), safer vaccines probably will have to be constructed and tested. Subunit vaccines may represent an alternative to produce safer, although less immunogenic, vaccines. Thus, we decided to examine whether subunit vaccines in the form of VLPs assembled in insect cells with recombinant baculovirus vectors would induce strong antibody responses that could be transferred by immunized dams to their pups and protect them against rotavirus diarrhea. In humans, passive transfer of the maternal antibody repertoire occurs exclusively before birth through the placenta, while in mice both the placental and the oral pathways are involved (18). Thus, in mice it is possible to analyze the contribution of each pathway. We clearly demonstrate that milk antibodies but not maternally acquired serum antibodies are essential for the protection.
The role of the various rotavirus capsid proteins as protective immunogens remains controversial (6, 12, 26, 29). To analyze the role of VP7 in passive protection against diarrhea and not fecal virus shedding, we compared the immunogenicity of VLP2/6 and VLP2/6/7 in the rotavirus newborn mouse model (29). Although both VLPs were highly immunogenic, only VLP2/6/7 conferred protection against diarrhea. We were unable to correlate protection with the presence of neutralizing VP7-specific antibodies, although VP7-specific antibodies were detected by immunoblots in serum and, to a lesser extent, in milk of the vaccinees. It is likely that VP7 in the VLPs generates conformational antibodies unable to interact efficiently with partially denatured VP7. In addition, the G6 serotype-specific VP7 antibodies were unable to recognize VP7 from another serotype, G3, further supporting the crucial role of VP7 as a protective antigen.
Our study confirms previous experiments showing that passive transfer of VP6-specific antibodies passively transferred to newborns was not sufficient to protect against diarrhea (2, 29). In contrast to the VP6 protein produced by adenovirus (2), the VP6 displayed by the VLP2/6 is in its native conformation, therefore eliciting conformational antibodies. This suggests that VP6-specific conformational antibodies are not sufficient to confer passive protection. In adults, VP6 was shown to be protective against virus shedding. Differences in the protection mechanisms in adults and newborns could explain the discrepancy in the results. It has been proposed (6) that neutralization of rotavirus by VP6-specific antibodies occurs in the cell rather than outside in the intestinal lumen, as described for influenza and Sendaï virus (23, 24). This implies that the virus or subviral particle and the antibodies meet in an endosomal compartment. In the adult, polymeric IgA antibodies taken up by the polymeric Ig receptor were proposed to mediate intracellular protection (6). In our study, IgG but probably not IgA antibodies are involved. Uptake and internalization of IgG antibodies also occur in the newborn via the neonatal Fc receptor (18). Therefore, if intracellular neutralization is involved in protection against infection, VP2/6-specific IgG antibodies should also protect against, or at least reduce the duration of, the diarrheic episode. Lack of protection suggests that intracellular neutralization of the virus does not correlate with protection against diarrhea. Moreover, the passive transfer of milk antibodies excludes any role of VP6-specific cytotoxic T lymphocyte in protection (10, 13). In our system, VP7 is essential for protection against diarrhea, as described previously (2).
In conclusion, nasal administration of VLP2/6/7 in the absence of a mucosal adjuvant to female mice is sufficient to passively protect suckling newborns against diarrhea during the breast-feeding period. Such a vaccinal strategy could be assessed to prevent rotaviral infection in human newborns during the breast-feeding period of life. However, this period does not overlap the time of susceptibility to rotavirus in infants. It remains to be established whether infants can also be actively protected by nasal administration of VLP2/6/7, as suggested by the work of O'Neal et al. (26). Such studies will require an animal model in which the period of susceptibility to infection exceeds the weaning period that is typical of mice.
ACKNOWLEDGMENTS
We are grateful to Annie Charpilienne for technical support in VLP purification and virus production. We thank Pierre-André Christinet and his staff for help with animal handling. We also thank Lucy Hathaway for critically reading and revising the manuscript.
This work was supported by a grant from the Swiss National Science Foundation, grant 31-56936-99 to J.-P.K.
REFERENCES
- 1.Abramson J S, Baker C J, Fisher M C, Gerber M A, Meissner H C, Murray D L, Overturf G D, Prober C G, Rennels M B, Saari T N, Weiner L B, Whitley R J. Possible association of intussusception with rotavirus vaccination. American Academy of Pediatrics. Committee on Infectious Diseases. Pediatrics. 1999;104:575. [PubMed] [Google Scholar]
- 2.Andrew M E, Boyle D B, Coupar B E, Reddy D, Bellamy A R, Both G W. Vaccinia-rotavirus VP7 recombinants protect mice against rotavirus-induced diarrhoea. Vaccine. 1992;10:185–191. doi: 10.1016/0264-410x(92)90010-h. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann M F, Rohrer U H, Kundig T M, Burki K, Hengartner H, Zinkernagel R M. The influence of antigen organization on B cell responsiveness. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 4.Barnes G. Intussusception and rotavirus vaccine. J Pediatr Gastroenterol Nutr. 1999;29:375. doi: 10.1097/00005176-199910000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Both G W, Lockett L J, Janardhana V, Edwards S J, Bellamy A R, Graham F L, Prevec L, Andrew M E. Protective immunity to rotavirus-induced diarrhoea is passively transferred to newborn mice from naive dams vaccinated with a single dose of a recombinant adenovirus expressing rotavirus VP7sc. Virology. 1993;193:940–950. doi: 10.1006/viro.1993.1203. [DOI] [PubMed] [Google Scholar]
- 6.Burns J W, Siadat-Pajouh M, Krishnaney A A, Greenberg H B. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science. 1996;272:104–107. doi: 10.1126/science.272.5258.104. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Intussusception among recipients of rotavirus vaccine—United States, 1998–1999. Morbid Mortal Weekly Rep. 1999;48:577–581. [PubMed] [Google Scholar]
- 8.Conner M E, Crawford S E, Barone C, Estes M K. Rotavirus vaccine administered parenterally induces protective immunity. J Virol. 1993;67:6633–6641. doi: 10.1128/jvi.67.11.6633-6641.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crawford S E, Labbé M, Cohen J, Burroughs M H, Zhou Y-J, Estes M K. Characterization of virus-like particles produced by the expression of rotavirus capsid proteins in insect cells. J Virol. 1994;68:5945–5952. doi: 10.1128/jvi.68.9.5945-5952.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dharakul T, Labbe M, Cohen J, Bellamy A R, Street J E, Mackow E R, Fiore L, Rott L, Greenberg H B. Immunization with baculovirus-expressed recombinant rotavirus proteins VP1, VP4, VP6, and VP7 induces CD8+ T lymphocytes that mediate clearance of chronic rotavirus infection in SCID mice. J Virol. 1991;65:5928–5932. doi: 10.1128/jvi.65.11.5928-5932.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estes M K. Rotavirus and their replication. In: Fields B N, Knipe D M, editors. Virology. New York, N.Y: Raven Press; 1996. pp. 1329–1352. [Google Scholar]
- 12.Fernandez F M, Conner M E, Hodgins D C, Parwani A V, Nielsen P R, Crawford S E, Estes M K, Saif L J. Passive immunity to bovine rotavirus in newborn calves fed colostrum supplements from cows immunized with recombinant SA11 rotavirus core-like particle (CLP) or virus-like particle (VLP) vaccines. Vaccine. 1998;16:507–516. doi: 10.1016/S0264-410X(97)80004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franco M A, Greenberg H B. Immunity to rotavirus infection in mice. J Infect Dis. 1999;179:S466–S469. doi: 10.1086/314805. [DOI] [PubMed] [Google Scholar]
- 14.Freytag L C, Clements J D. Bacterial toxins as mucosal adjuvants. Curr Top Microbiol Immunol. 1999;236:215–236. doi: 10.1007/978-3-642-59951-4_11. [DOI] [PubMed] [Google Scholar]
- 15.Gay N, Ramsay M, Waight P. Rotavirus vaccination and intussusception. Lancet. 1999;354:956. doi: 10.1016/S0140-6736(05)75710-X. [DOI] [PubMed] [Google Scholar]
- 16.Glass R I, Kilgore P E, Holman R C, Jin S, Smith J C, Woods P A, Clarke M J, Ho M S, Gentsch J R. The epidemiology of rotavirus diarrhea in the United States: surveillance and estimates of disease burden. J Infect Dis. 1996;174:S5–S11. doi: 10.1093/infdis/174.supplement_1.s5. [DOI] [PubMed] [Google Scholar]
- 17.Heiman H S, Weisman L E. Transplacental or enteral transfer of maternal immunization-induced antibody protects suckling rats from type III group B streptococcal infection. Pediatr Res. 1989;26:629–632. doi: 10.1203/00006450-198912000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Hunziker W, Kraehenbuhl J P. Epithelial transcytosis of immunoglobulins. J Mammary Gland Biol Neoplasia. 1998;3:287–302. doi: 10.1023/a:1018715511178. [DOI] [PubMed] [Google Scholar]
- 19.Ijaz M K, Sabara M I, Frenchick P J, Babiuk L A. Effect of different routes of immunization with bovine rotavirus on lactogenic antibody response in mice. Antivir Res. 1987;8:283–297. doi: 10.1016/S0166-3542(87)80006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.JAMA. From the Centers for Disease Control and Prevention. Withdrawal of rotavirus vaccine recommendation. JAMA. 1999;282:2113–2114. [PubMed] [Google Scholar]
- 21.Labbé M, Charpilienne A, Crawford S E, Estes M K, Cohen J. Expression of rotavirus VP2 produces empty core-like particles. J Virol. 1991;65:2946–2952. doi: 10.1128/jvi.65.6.2946-2952.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu C Y, Beller D I, Unanue E R. During ontogeny, Ia-bearing accessory cells are found early in the thymus but late in the spleen. Proc Natl Acad Sci USA. 1980;77:1597–1601. doi: 10.1073/pnas.77.3.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazanec M B, Coudret C L, Fletcher D R. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J Virol. 1995;69:1339–1343. doi: 10.1128/jvi.69.2.1339-1343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazanec M B, Kaetzel C S, Lamm M E, Fletcher D, Peterra J, Nedrud J G. Intracellular neutralization of Sendai and influenza viruses by IgA monoclonal antibodies. Adv Exp Med Biol. 1995;371A:651–654. doi: 10.1007/978-1-4615-1941-6_137. [DOI] [PubMed] [Google Scholar]
- 25.Offit P A, Clark H F. Maternal antibody-mediated protection against gastroenteritis due to rotavirus in newborn mice is dependent on both serotype and titer of antibody. J Infect Dis. 1985;152:1152–1158. doi: 10.1093/infdis/152.6.1152. [DOI] [PubMed] [Google Scholar]
- 26.O'Neal C M, Crawford S E, Estes M K, Conner M E. Rotavirus virus-like particles administered mucosally induce protective immunity. J Virol. 1997;71:8707–8717. doi: 10.1128/jvi.71.11.8707-8717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osborne M P, Haddon S J, Spencer A J, Collins J, Starkey W G, Wallis T S, Clarke G J, Worton K J, Candy D C, Stephen J. An electron microscopic investigation of time-related changes in the intestine of neonatal mice infected with murine rotavirus. J Pediatr Gastroenterol Nutr. 1988;7:236–248. doi: 10.1097/00005176-198803000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Pickering L K, Morrow A L, Herrera I, O'Ryan M, Estes M K, Guilliams S E, Jackson L, Carter-Campbell S, Matson D O. Effect of maternal rotavirus immunization on milk and serum antibody titers. J Infect Dis. 1995;172:723–728. doi: 10.1093/infdis/172.3.723. [DOI] [PubMed] [Google Scholar]
- 29.Ruggeri F M, Johansen K, Basile G, Kraehenbuhl J P, Svensson L. Antirotavirus immunoglobulin A neutralizes virus in vitro after transcytosis through epithelial cells and protects infant mice from diarrhea. J Virol. 1998;72:2708–2714. doi: 10.1128/jvi.72.4.2708-2714.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salim A F, Phillips A D, Walker-Smith J A, Farthing M J. Sequential changes in small intestinal structure and function during rotavirus infection in neonatal rats. Gut. 1995;36:231–238. doi: 10.1136/gut.36.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spear P G, Edelman G M. Maturation of the humoral immune response in mice. J Exp Med. 1974;139:249–263. doi: 10.1084/jem.139.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ward L A, Rich E D, Besser T E. Role of maternally derived circulating antibodies in protection of neonatal swine against porcine group A rotavirus. J Infect Dis. 1996;174:276–282. doi: 10.1093/infdis/174.2.276. [DOI] [PubMed] [Google Scholar]