Abstract
A growing body of work suggests that individuals with aggressive behavior and/or aggressive tendencies have evidence of chronic, low level, inflammation as manifested by elevated circulating levels of acute phase reactant proteins and pro-inflammatory cytokines. While animal studies report that direct application of pro-inflammatory proteins in brain increase aggressive behavior, there is no data on the relationship of central levels of these proteins and aggression in human subjects. We simultaneously measured levels of both plasma and lumbar cerebrospinal fluid (CSF) C-Reactive Protein (CRP) and IL-6, IL-8, and TNF-α in 77 medically healthy, drug-free, individuals with varying degrees of aggression including 22 individuals with DSM-5 Intermittent Explosive Disorder (IED). Aggression was assessed using the Life History of Aggression (LHA) and the Buss-Perry Aggression Questionnaire (BPAQ). Plasma and CSF levels of CRP, IL-8, and TNF-α, but not IL-6, correlated significantly with each other. Aggressive individuals with IED displayed elevated plasma, but not CSF, levels of proinflammatory markers and this relationship was specific to IED. Similarly, composite aggression scores correlated significantly with plasma, but not CSF, pro-inflammatory markers. Aggressive behavior in humans is correlated with Plasma, but not CSF, proinflammatory markers despite the observation that these two sets of markers are significantly correlated. Since the direct application of proinflammatory proteins in brains of animals increase aggressive behavior, proinflammatory proteins likely influence brain-based behavior in a manner not reflected in lumbar CSF.
Subject terms: Biomarkers, Neuroscience
Introduction
A growing body of work suggests that individuals with aggressive behavior and/or aggressive tendencies have evidence of chronic, low level, inflammation as manifest by elevated circulating levels of acute phase reactant proteins and pro-inflammatory cytokines. Specifically, circulating levels of C-Reactive Protein (CRP) displays a positive correlation with hostile [1, 2], angry [3], and aggressive dispositions [4, 5]; elevations of IL-6 levels are associated with the same variables [5–9]. Animal studies report similar observations. For example, defensive rage in cats is associated with elevated levels of IL-1β and IL-6 and blocking IL-1β activity reduces these effects [10–12]. In addition, mice deficient in inflammatory cytokine receptors fail to exhibit aggressive and defensive behavior even when threatened [13].
This led us to study inflammatory markers in those with Intermittent Explosive Disorder (IED), a disorder of impulsive aggression not due to the presence of other disorders or conditions [14]. IED is present in up to 4% of individuals in the U.S. lifetime and in up to 2.6% in any one year [15] and it is associated with a variety of features suggestive of neurochemical, neuroanatomical, and neurophysiological aberrations such as reduced central serotonergic function [16], reduced gray matter in cortico-limbic regions [17], and imbalance in frontal-amygdala function [18, 19].
Our initial work reported elevations in plasma levels of CRP and IL-6 in individuals with IED compared with both healthy and psychiatric controls, who did not differ in this regard [20]. In addition, these pro-inflammatory markers correlated directly with dimensional measures of aggression. Subsequently, we conducted another study to compare the role of pro-inflammatory markers obtained from Plasma and from Cerebrospinal Fluid (CSF) in the same individuals in order to: (a) replicate and extend these findings in the periphery and, (b) test the hypothesis that pro-inflammatory markers in the CSF would be correlated with those in Plasma and, in turn, elevated in aggressive individuals and display direct correlations with measures of aggression. The latter hypothesis was based on evidence suggesting that peripheral inflammatory processes influence central neuronal processes and on evidence of pro-inflammatory activity in the post-mortem brains of individuals who died by suicide, many, but certainly not all, of whom were aggressive in life.
Methods
Subjects
Seventy-seven physically healthy study participants were recruited from public service announcements, seeking individuals with history of impulsive aggressive behavior and seeking individuals without this history. All participants gave signed informed consent as approved by the Institutional Review Board (IRB) of the University of Chicago to undergo assessment of both circulating (plasma) and cerebrospinal fluid (CSF) pro-inflammatory markers. Subjects with bipolar disorder, schizophrenia, current substance use disorder, intellectual disability, or episodes of loss of consciousness greater than 30 min, were excluded. Medical health was documented by comprehensive medical history and physical exam which included a screen for drugs of abuse (all subjects tested negative).
Diagnostic assessment
Major syndromal disorder diagnoses were made by DSM-5 criteria [14]. Research assessments were performed by individuals with masters/doctoral degrees in clinical psychology with inter-rater (kappa) reliability ranging from 0.79 to 0.93 (mean ± sd: 0.84 ± 0.05) across mood, anxiety, substance use, and other disorders. Final diagnoses were assigned by previously described best-estimate consensus procedures [21], utilizing information from: (a) Structured Clinical Interview for DSM Diagnoses (SCID) [22], (b) clinical interview by a research psychiatrist and, (c) review of all available clinical data. Twenty-two study participants were identified as “aggressive”, as documented by the presence of a current DSM-5 diagnosis of Intermittent Explosive Disorder (IED); fifty-five participants were identified as “non-aggressive”. Of the latter group, 28 had no history of any DSM-5 disorder and 27 met DSM-5 criteria for a current and/or past DSM-5 syndromal disorder. Specific DSM-5 diagnoses for participants with a DSM-5 disorder are listed in Table 1.
Table 1.
DSM-5 diagnoses among study participants with a psychiatric disorder.
| Psychiatric controls (N = 27) | Intermittent explosive disorder (N = 22) | p | Group differences* | |
|---|---|---|---|---|
| Current syndromal disorders: | ||||
| Any depressive disorder | 0 (0.0%) | 5 (22.7%) | = 0.014 | PC = IED |
| Any anxiety disorder | 4 (14.8%) | 5 (22.7%) | = 0.713 | PC = IED |
| Post-traumatic stress disorder | 1 (3.7%) | 6 (27.3%) | = 0.036 | PC = IED |
| Lifetime syndromal disorders: | ||||
| Any depressive disorder | 14 (51.9%) | 12 (54.5%) | = 0.999 | PC = IED |
| Any anxiety disorder | 9 (33.3%) | 7 (31.8%) | = 0.999 | PC = IED |
| Post-traumatic stress disorder | 4 (14.8%) | 12 (54.5%) | = 0.005 | PC = IED* |
| Any substance use disorder | 11 (40.7%) | 10 (45.5%) | = 0.779 | PC = IED |
*p < 0.05 after correction for multiple comparisons (uncorrected p < 0.006).
Psychometric measures relevant aggression, anger, impulsivity, and related behavioral dimensions
Aggression was assessed using the Aggression Scale from Life History of Aggression (LHA; e.g., history of “temper tantrums”, “verbal” and “physical” fights, “breaking things/objects”) [23] and the sum of the Verbal (e.g., “I can’t help getting into arguments when people disagree with me”) and Physical (e.g., “If somebody hits me I hit them back”) Aggression sub-scales from the Buss-Perry Aggression Questionnaire (BPAQ) [24]. Anger and Hostility were assessed with the Anger (e.g., “Sometimes I fly off the handle for no good reason”) and Hostility (e.g., “I wonder why sometimes I fell so bitter about things”) subscales from the BPAQ. Impulsivity was assessed with the total scores from the Life History of Impulsive Behavior (LHIB) [25] and the Barratt Impulsivity Scale (BIS-11) [26]. LHA and LHIB assess the number of times a person has engaged in aggressive, or impulsive, behavior in their life and the BPA and BIS-11 assess a person’s disposition to act aggressively, or impulsively, as a personality trait. Life history of suicidal behavior was assessed during the SCID interviews. Other relevant behavioral assessments included Beck Depression Scale (BDI-II); [27] for state depression, Pittsburgh Sleep Quality Inventory (PSQI) [28] for overall sleep quality, and Perceived Stress Scale (PSS) [29] for perceived stressful events in the past month. Ethnicity data, collected by diagnostic assessors, reflected self-identified racial characteristics of the study participants. Socioeconomic status (SES) was assessed using the method of Hollingshead [30].
Assessment of pro-inflammatory levels
Study participants were free of all medications for at least four weeks, typically much longer. At time of study, participants had no signs of physical trauma, or skin rash, and reported received no vaccinations in the previous six months. Participants were admitted to the University of Chicago’s Clinical Research Center (CRC) by 10 pm the evening before the lumbar puncture. Participants went to bed and lay in a supine position by 11 pm. Participants remained at rest, and fasting, for a lumbar puncture the next morning between 8 am and 9 am. Whole blood, anticoagulated with EDTA, was obtained by venipuncture through a forearm vein no more than 30 min prior to the lumbar puncture. Plasma was processed after centrifugation and stored at −80 °C until assay at UCLA. The lumbar puncture was performed at L3-L4 under local anesthesia by a Board-Certified Anesthesiologist (AK or RF). After the lumbar puncture, participants drank a liter of water over the next four hours while they spent two hours on their abdomen followed by two hours on their back. Participants were discharged home after examined by a research psychiatrist (RL or EFC). The first 1.5 cc cerebrospinal fluid (CSF) fraction was set aside with the next 7.5 cc fraction collected in a single tube. This tube was vortexed to yield 15 homogenized 0.5 cc CSF fractions. All CSF fractions were frozen at the bedside using a dry ice bath and kept at −80 °C until assay at UCLA. One Plasma sample and one CSF sample was used to assay C-Reactive Protein using a high sensitivity assay. Another sample of Plasma and CSF was used to assay IL-6, IL-8, and TNF-α. We did not assay for IL-1 related cytokines (e.g., IL-1β) because we found these to largely be undetectable in similar study participants in our pilot work. Samples were run together in the same assay and levels reported represent the mean of the duplicates. Sensitivity and coefficient of variation were: 0.2 mg/L / < 6.1% for CRP and 0.21 pg/ml / < 8.0% for the other inflammatory markers, respectively.
Statistical analysis and data reduction
Comparisons between groups were performed by t test, analysis of covariance, and by X2 tests as appropriate. Correlational analyses were conducted by parametric methods including multiple regression. Alpha = 0.05 denoted statistical significance. All inflammatory markers were log-transformed for analysis as all followed a non-normal distribution. Controls with and without any DSM-5 psychiatric disorder did not differ in Plasma or CSF pro-inflammatory levels and were combined into a single control group. Data was analyzed both categorically (Controls vs. IED) and dimensionally as a function of aggression, anger-hostility, and impulsivity. Analyses of demographic and other relevant variables revealed that only age correlated with pro-inflammatory levels, thus, all analyses used age as a covariate. Body Mass Index, known to influence pro-inflammatory marker levels was also used as a covariate. Follow-up analyses including potentially relevant variables were also performed and reported below. A composite pro-inflammatory variable was created from each participant’s z-score for log CRP, IL-6, IL-8, and TNF-α levels. In addition, composite variables for aggression (LHA/BPAQ), anger-hostility (BPAQ), and impulsivity (LHIB / BIS-11) were created from each participant’s z-scores for these measures. While highly correlated, none of the three composite behavioral variables fully overlapped (r2 from 0.56 to 0.71) and, thus, we also created an omnibus composite behavioral variable to use in our primary analyses.
Results
Demographic, behavioral, and other characteristics of study participants
Table 2 aggressive and non-aggressive study participants did not significantly differ in demographic variables. Rates of current regular, and heavy (>14 drinks/week for males and >7 drink/week for females), alcohol consumption did not differ between the two groups. In contrast, current history of smoking was more common in the IED group (23 vs. 6%). As expected, the two groups differed in aggression (LHA, BPA), anger/hostility (BPAQ), impulsivity (LHIB, BIS-11), depression (BDI-II) scores, in scores for perceived stress (PSS), and in overall quality of sleep (PSQI).
Table 2.
Demographic, behavioral, and other relevant variables.
| Controls (N = 55) | Intermittent explosive disorder (N = 22) | Group differences | |
|---|---|---|---|
| Demographic variables: | |||
| Age (Years ± SD) | 34.8 ± 9.2 | 38.4 ± 10.4 | CONT = IEDa |
| Sex (% Male) | 45.5% | 50.0% | CONT = IEDb |
| Race (% White / AA / Other) | 42% / 33% / 25% | 27% / 59% / 14% | CONT = IEDb |
| SES Score | 40.6 ± 3.6 | 36.1 ± 15.1 | CONT = IEDa |
| Behavioral variables: | |||
| Aggression | |||
| LHA Aggression | 4.4 ± 3.7 | 18.5 ± 3.2 | CONT < IEDa,c |
| BPAQ Aggression | 27.4 ± 8.0 | 45.9 ± 12.5 | CONT < IEDa,c |
| Anger / Hostility | |||
| BPAQ Anger | 12.4 ± 5.8 | 23.7 ± 4.7 | CONT < IEDa,c |
| BPAQ Hostility | 16.4 ± 7.0 | 22.4 ± 5.6 | CONT < IEDa,c |
| Impulsivity | |||
| LHIB Impulsivity | 26.8 ± 16.6 | 51.8 ± 23.6 | CONT < IEDa,c |
| BIS-11 Impulsivity | 54.8 ± 9.6 | 70.6 ± 10.9 | CONT < IEDa,c |
| Other relevant variables: | |||
| Body Mass Index (BMI) | 25.6 ± 3.8 | 25.7 ± 4.2 | CONT = IED |
| State Depression Score (BDI-II) | 4.2 ± 7.2 | 11.6 ± 9.8 | CONT < IEDa,c |
| PSQI Sleep Score | 5.7 ± 2.3 | 7.5 ± 3.1 | CONT < IEDa,c |
| Perceived Stress Score | 21.2 ± 3.1 | 23.9 ± 3.9 | CONT < IEDa,c |
| Other relevant lifestyle variables: | |||
| Current Alcohol Consumption (%) | 66% | 68% | CONT = IED |
| Heavy Alcohol Consumption (%) | 13% | 10% | CONT = IED |
| Log Mean Drinks/Week | 1.04 ± 0.06 | 1.04 ± 0.05 | CONT = IEDa |
| Current Smoking (%) | 6% | 23% | CONT < IEDb,c |
aBy t test.
bBy X2 test.
cp < 0.05.
Correlations among plasma and CSF levels of inflammatory markers (Fig. 1)
Fig. 1. Plasma/CSF Correlation of CRP and Cytokines.
a Correlation between Log Plasma and Log CSF C-Reactive Protein (CRP); (b) correlation between Log Plasma and Log CSF TNF-a; (c) correlation between Log Plasma and Log CSF IL-8; (d) correlation between Log Plasma and Log CSF IL- 6.
Plasma log CRP and CSF log CRP levels were highly correlated (r = 0.85, p < 0.001, Fig. 1a) as were Plasma/CSF log TNF-α (r = 0.46, p < 0.001; Fig. 1b) and Plasma/CSF log IL-8 (r = 0.28, p = 0.014, Fig. 1c) levels. In contrast, log IL-6 levels displayed no correlation (r = −0.08, p = 0.502, Fig. 1d).
Correlations among inflammatory markers in plasma and CSF (Supplementary Table 1)
CRP correlated with IL-6 in plasma, as expected, but not in CSF, while IL-8 correlated with TNF-α in both plasma and CSF. All other correlations were statistically non-significant.
Plasma pro-inflammatory levels in aggressive vs. control participants (Fig. 2a)
Fig. 2. Plasma and CSF Pro-Inflammatory Levels as a Function Group.
a Marginal mean (±SEM) for log composite, CRP, IL-6, IL-8, and TNF-a plasma levels for IED compared with controls. b Marginal mean (±SEM) for log CSF CRP, IL-6, IL-8, and TNF-a levels for IED compared with controls.
Composite log Plasma Pro-Inflammatory levels were significantly higher in IED compared with Controls as were each of the four plasma pro-inflammatory markers (Fig. 2a). Examining the presence or absence of IED, and for lifetime disorders commonly comorbid with IED (Coccaro et al., 2019; i.e., Depressive Disorder, Anxiety Disorder, Post-Traumatic Stress Disorder, and Substance Use Disorder) in the same ANCOVA model revealed that differences between groups were statistically significant only for IED (Supplementary Figure). The same was true for those with current comorbidities, though only about a third of participants had current (21 of 49), compared with lifetime (58 of 49), comorbidities.
CSF pro-inflammatory levels in aggressive vs. control participants (Fig. 2b)
In contrast, to plasma, the IED-Control difference in Composite log CSF Pro-Inflammatory level only approached statistical significance. This was due to a trend for statistical significance for the IED-Control difference in log CSF CRP levels only.
Correlation with composite aggression, anger/hostility, and impulsivity scores
Composite log Plasma (β = 0.35, p < 0.001), but not Composite log CSF Pro-Inflammatory (β = 0.14, p = 0.183), levels correlated significantly with the composite of behavioral variables for Composite Aggression, Composite Anger/Hostility, and Composite Impulsivity (Supplemental Table 2). When placed in the same statistical model, the beta value for Plasma log Pro-Inflammatory level increased by 29% (β = 0.45, p = 0.002) while the beta value for CSF log Pro-Inflammatory level decreased by 57% (β = −0.08, p = 0.586). Among the four plasma pro-inflammatory levels, CRP (β = 0.30, p = 0.013) and IL-8 (β = 0.33, p = 0.006) correlated significantly with this composite behavioral variable. Unlike with the IED-Control analysis, above, in which all pro-inflammatory levels were higher in IED compared with Controls, the relationship with IL-6 only approached statistical significance (β = 0.21, p = 0.089) while the relationship with TNF-α (β = 0.19, p = 0.103) was just beyond the criteria for a trend toward statistical significance.
Examining the individual composite behavioral variables (Supplementary Table 3), Composite Aggression (β = 0.35, p < 0.001), Composite Anger/Hostility (β = 0.33, p = 0.002), and Composite Impulsivity (β = 0.29, p = 0.006) each correlated significantly with Composite Plasma log Pro-Inflammatory levels. Placing each of these individual composite behavioral variables in the same statistical model, however, revealed that none were uniquely related to Composite Plasma log Pro-Inflammatory levels. In the final model (F[5,71] = 5.92, p < 0.001), beta values for Composite Aggression dropped from 0.35 (p = 0.001), examined alone, to 0.22 (p = 0.288) while beta values for Composite Anger dropped from 0.33 (p = 0.002) to 0.13 (p = 0.531), and beta values for Composite Impulsivity dropped from 0.29 (p = 0.006) to 0.03 (p = 0.861).
Correlation with physical vs. verbal aggression
Of the two source aggression measures, only the BPAQ divides “physical” aggression and “verbal” aggression into separate valid subscales. Composite log Plasma Pro-Inflammatory levels correlated significantly with both BPAQ Physical Aggression (β = 0.33, p = 0.002) and Verbal Aggression (β = 0.23, p = 0.036). When placed in the same statistical model, BPAQ Physical Aggression (β = 0.30, p = 0.027), but not Verbal Aggression (β = 0.04, p = 0.789), scores correlated significantly with Composite log Plasma Pro-Inflammatory levels.
Correlation with anger vs. hostility
The BPAQ also assesses “anger” and “hostility” as separate valid subscales. Examined separately, Composite log Plasma Pro-Inflammatory levels correlated significantly with both BPA Anger (β = 0.41, p < 0.001) and Hostility (β = 0.32, p = 0.011) scores. When placed in the same statistical model, BPAQ Anger (β = 0.35, p = 0.029), but not Hostility (β = −0.00, p = 0.997), scores correlated significantly with Composite log Plasma Pro-Inflammatory levels.
Influence of other potentially relevant variables
Relevant variables related to demographics (i.e., sex, ethnicity, ses), lifestyle (number of cigarettes per week, number of standard alcohol drinks per week), depressive symptoms (BDI-II), perceived stress (PSS), and overall sleep quality (PSQI) did not impact these results. Adding all covariates did not meaningfully affect the IED-Control difference in Composite log Plasma Pro-Inflammatory levels (F[1,65] = 4.61, p = 0.035) or in the latter’s correlation with Composite Aggression, Anger-Hostility, Impulsivity scores (β = 0.36, p = 0.014; Fig. 3).
Fig. 3. Correlation Between COMP Plasama Pro-Inflammatory Level and COMP Aggression/Anger-Hostility/Impulsivity Score.
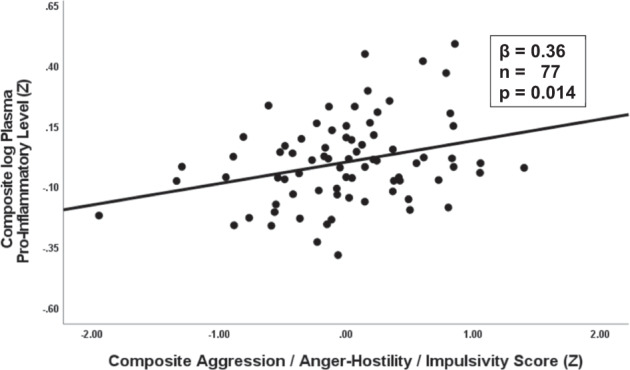
Correlation between composite aggression, anger/hostility, impulsivity and log composite plasma pro-inflammatory levels (all co-variates).
Discussion
The results of this study replicate positive relationships between aggression and plasma pro-inflammatory markers both from an aggression vs. non-aggressive (control), and from a dimensional, standpoint. Previously, we reported that, compared with controls, individuals with Intermittent Explosive Disorder (IED) have elevations of plasma levels of CRP and IL-6 [20]. Here, we replicate that observation and extend it by noting that this may also be true for IL-8 and TNF-α.
This study replicates IED-Control differences with CRP and IL-6 and extends this observation to include IL-8 and TNF-α. In addition, we show that the presence of relevant co-morbid diagnoses do not impact these IED-Control findings when placed in the same statistical model as IED. This further supports the hypothesis of a pro-inflammatory role in IED is not due to other relevant psychiatric comorbidities. Finally, we report correlations for measures of aggression, anger/hostility, and impulsivity with composite plasma pro-inflammatory levels and replicate previous findings in this regard. For dimensional analyses, while plasma CRP and IL-8 levels were of moderate size and significantly related to our behavioral measures, the relationships with IL-6 and TNF-α were smaller in magnitude and of marginal statistical significance, likely due to modest sample size. Overall, the dimensional relationships seem to be accounted for by aggression and anger rather than impulsivity since the latter accounts for little of the variance in plasma pro-inflammatory levels when the three composite behavioral variables are examined simultaneously.
These data are consistent with other human studies. First, self-reported anger and aggressive disposition is increased in patients treated with cytokine immunotherapy [31, 32]. Second, plasma CRP and IL-6 correlate with measures of hostility [1–3, 7–9], anger [3], and aggressive disposition [4, 5, 20], in adults. Third, greater levels of anger and/or hostility have been associated with greater production of inflammatory cytokines from blood monocytes in healthy adults after stimulation by bacterial lipopolysaccharide [33].
Given evidence of pro-inflammatory activity in the post-mortem brains of those who completed suicide, many of whom are aggressive, we hypothesized the presence of elevated levels of pro-inflammatory markers in CSF as a function of aggression and possibly anger. In addition, we hypothesized that plasma levels of these markers would correlate with those in the CSF and that, in turn, findings from plasma studies would correlate with those from the CSF. This study provides only partial support for these hypotheses.
While three of the four pro-inflammatory markers correlate significantly in Plasma and CSF, we did not see a clear signal of elevated pro-inflammatory cytokines in the CSF in those with IED, or a correlation with aggression, in samples from the CSF. In fact, when examined in the same statistical model, only plasma pro-inflammatory levels displayed a medium-to-large relationship with composite aggression, anger/hostility, and impulsivity. We did see a trend for an elevation of Composite CSF log Pro-Inflammatory level in IED study participants, but this was due to a similar trend for log CSF CRP level; none of the other CSF markers were elevated in the IED group though each was elevated in the Plasma.
It is possible that the relationship between aggression and CSF CRP levels represent evidence of a brain-based relationship. CRP is produced in the brain at perivascular interfaces in macrophages, and possibly in brain microglia, which are known to produce pro-inflammatory cytokines in the brain [34]. However, the observation of a near unity correlation between CRP In plasma and CSF which replicates the observation of Felger et al. [35], the observation that none of the CSF pro-inflammatory cytokines were elevated in IED study participants (or were correlated with measures of aggression), as they were in plasma, and that CRP correlated in plasma but not CSF, suggests that the relationship between CSF CRP and aggression represents in this study, reflects what is observed in the periphery.
The lack of evidence of a positive finding in CSF, however, is not evidence of absence. CSF in living human subjects is collected from the lumbar sack below the terminus of the spinal cord, an anatomic location far from brain regions and circuits of interest. It is likely that much, if not the vast majority, of inflammatory proteins measured in lumbar CSF is produced in the distal spinal cord. While levels of inflammatory proteins may correlate with those in the brain, we know of no published studies comparing inflammatory protein levels in lumbar CSF and brain in post-mortem humans. Even so, a relationship between the two would not provide evidence of pro-inflammatory processes in brain regions and circuits of interest. The answer to this question will have to wait for neuroimaging studies that putatively image processes related to neuroinflammation such as PET TSPO binding [36] and/or micro-structural MRI using magnetization transfer Harrison [37].
Currently, the best evidence that peripheral inflammation influences central neuronal processes comes from structural and functional MRI studies as well as fMRI studies during pro-inflammatory challenge with endotoxin which produces a robust increase of pro-inflammatory mediators. Several reports note that circulating levels of IL-6 are correlated with reduced gray matter volume in the hippocampus of non-patient volunteers [38] and that CRP is inversely correlated with the functional connectivity of both the striatum and amygdala to mPFC [39, 40]. In addition, endotoxin challenge has been shown to enhance fMRI BOLD responses to social threat in the amygdala [41] and in the orbitofrontal cortex [42]. It is thought that peripheral inflammatory processes influence central neuronal processes by any one, or more, mechanisms including passage through leaky regions in the blood-brain-barrier, active transport through saturable transporters, activation of cells lining the cerebral vasculature which produce cytokines and, binding to receptors on peripheral afferent nerve fibers that relay cytokine signals to relevant brain regions such as the hypothalamus, and other brain structures [43, 44]. Finally, pro-inflammatory cytokines administered into medial hypothalamus or periaquiductal gray in animal models of aggression, for example, increase threat-related defensive aggression [12, 45–48] suggesting that the brain’s response to pro-inflammatory proteins is aggressive responding.
This study also sheds light on what aspects of aggression and anger/hostility are best related to elevated levels of plasma pro-inflammatory levels. Analysis of the data reveal that physical, but not verbal, aggression likely accounts for elevations of plasma pro-inflammatory levels and that anger, but not hostility, likely accounts for elevations of plasma pro-inflammatory levels, in our study participants. Since physical and verbal aggression, anger, hostility, and impulsivity are each correlated to a high degree, the variance associated with verbal aggression and hostility is subsumed in the variance related to physical aggression and hostility and that related to plasma pro-inflammatory levels.
These results are not likely due to other, potentially, relevant factors. Study participants were physically healthy, free of systemic illness or recent physical injury, skin rash, recent history of vaccination, free of psychotropic or anti-inflammatory medication, and without current history of alcohol, or drug, use disorder. Study participants were studied at rest in a controlled environment, and had been fasting for at least eight hours, at time of study procedures and results were unchanged when relevant variables (e.g., body mass index, state depression, recent perceived stress, etc.) were included in the statistical models. In addition, the primacy of aggression (and anger) is supported by the observation that elevations of pro-inflammatory markers were only found for IED when examined, simultaneously with other relevant comorbidities.
Next steps may include studies with endotoxin challenge to determine if acute inflammation increases aggressive responding in laboratory models of aggression as well as brain imaging studies targeting neuroinflammation in relevant cortico-limbic circuits.
The strengths of this study include a well characterized sample, validated measures of aggression, anger/hostility, and impulsivity, in the context of a multi-measure study, and a standardized approach to minimize the effect of extraneous factors on Plasma or CSF inflammatory levels. The fact that study participants were medication free is another strength, given that many studies involving the relationship of inflammatory markers with aggression [49, 50] or anger [51, 52] do not exclude concomitant medications unless they are anti-inflammatory in action. Limitations include the cross-sectional nature of the study, the modest number of study participants, and the fact that correlational analysis cannot establish causality.
In conclusion, we report a direct relationship between Plasma, but not CSF, pro-inflammatory markers and aggression in human subjects, particularly in those with IED. This relationship was not accounted for by various potential confounds. Given that IED, a disorder of impulsive aggression, displays a 2–3% one year prevalence rate in the U.S. [15], and that currently available treatments bring less than 50% of those into remission [53], additional strategies for the examination and intervention of human impulsive aggression are needed.
Supplementary information
Acknowledgements
Alan Klock, M.D. and Robert Fong, M.D. (Department of Anesthesiology and Critical Care at the University of Chicago, Division of Biological Sciences) performed the lumbar punctures.
Author contributions
EFC: Conceptualization, Funding Acquisition, Project Management, Data Collection, Statistical Analysis, Writing. RL: Data collection, Writing—review and editing. ECB: Laboratory analysis (assays) and Interpretation of Data. MRI: Conceptualization, Interpretation of Data, and Revising Manuscript Critically for Important Intellectual Content.
Funding
This work was supported by a grant from the National Institute of Mental Health: RO1 MH104673 (Dr. Coccaro).
Competing interests
EFC reports being a consultant to and being on the Scientific Advisory Boards of Azevan Pharmaceuticals, Inc., Avanir Pharmaceuticals, Inc., Boerhinger Ingelheim, Pharma, Inc., and being a current recipient of a grant award from the NIMH and NIAAA. RL, ECB, and MRI report no biomedical financial interests or competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-023-01541-3.
References
- 1.Graham JE, Robles TF, Kiecolt-Glaser JK, Malarkey WB, Bissell MG, Glaser R. Hostility and pain are related to inflammation. in older adults. Brain Behav Immun. 2006;20:389–400. doi: 10.1016/j.bbi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Ranjit N, Diez-Roux AV, Shea S, Cushman M, Seeman T, Jackson SA, et al. Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Arch Intern Med. 2007;2007:174–81. doi: 10.1001/archinte.167.2.174. [DOI] [PubMed] [Google Scholar]
- 3.Suarez EC. C-reactive protein is associated with psychological risk factors of cardiovascular disease in apparently healthy adults. Psychosom Med. 2004;66:684–91. doi: 10.1097/01.psy.0000138281.73634.67. [DOI] [PubMed] [Google Scholar]
- 4.Coccaro EF. Association of C-reactive protein elevation with trait aggression and hostility in personality disordered subjects: a pilot study. J Psychiatr Res. 2006;40:460–5. doi: 10.1016/j.jpsychires.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Marsland AL, Prather AA, Petersen KL, Cohen S, Manuck SB. Antagonistic characteristics are positively associated with inflammatory markers independently of trait negative emotionality. Brain Behav Immun. 2008;22:753–61. doi: 10.1016/j.bbi.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, et al. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry. 2005;62:1377–84. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- 7.Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA. Cynical hostility, depressive symptoms, and the expression of inflammatory risk markers for coronary heart disease. J Behav Med. 2003;26:501–15. doi: 10.1023/A:1026273817984. [DOI] [PubMed] [Google Scholar]
- 8.Sjogren E, Leanderson P, Kristenson M, Ernerudh J. Interleukin-6 levels in relation to psychosocial factors: Studies on serum, saliva, and in vitro production by blood mononuclear cells. Brain Behav Immun. 2006;20:270–8. doi: 10.1016/j.bbi.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Suarez EC. Joint effect of hostility and severity of depressive symptoms on plasma interleukin-6 concentration. Psychosom Med. 2003;65:523–7. doi: 10.1097/01.PSY.0000062530.94551.EA. [DOI] [PubMed] [Google Scholar]
- 10.Pesce M, Speranza L, Franceschelli S, Ialenti V, Patruno A, Febo MA, et al. Biological role of interleukin-1beta in defensive-aggressive behaviour. J Biol Regul Homeost Agents. 2011;25:323–9. [PubMed] [Google Scholar]
- 11.Bhatt S, Bhatt R, Zalcman SS, Siegel A. Role of IL-1 beta and 5-HT2 receptors in midbrain periaqueductal gray (PAG) in potentiating defensive rage behavior in cat. Brain Behav Immunity. 2008;22:224–33. doi: 10.1016/j.bbi.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zalcman SS, Siegel A. The neurobiology of aggression and rage: role of cytokines. Brain Behav Immunity. 2006;20:507–14. doi: 10.1016/j.bbi.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Patel A, Siegel A, Zalcman SS. Lack of aggression and anxiolytic-like behavior in TNF receptor (TNF-R1 and TNF-R2) deficient mice. Brain Behav Immun. 2010;24:1276–80. doi: 10.1016/j.bbi.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Psychiatric Association, American Psychiatric Association. Task Force on DSM-5: Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 15.Coccaro EF, Lee RJ. Disordered Aggression and Violence in the United States. J Clin Psychiatry. 2020;81:e1–e8. [DOI] [PubMed]
- 16.Coccaro EF, Lee R, Kavoussi RJ. Aggression, suicidality, and intermittent explosive disorder: serotonergic correlates in personality disorder and healthy control subjects. Neuropsychopharmacology. 2010;35:435–44. doi: 10.1038/npp.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coccaro EF, Fitzgerald DA, Lee R, McCloskey M, Phan KL. Frontolimbic morphometric abnormalities in intermittent explosive disorder and aggression. Biol Psychiatry. 2016;1:32–8. doi: 10.1016/j.bpsc.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol Psychiatry. 2007;62:168–78. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 19.McCloskey MS, Phan KL, Angstadt M, Fettich KC, Keedy S, Coccaro EF. Amygdala hyperactivation to angry faces in intermittent explosive disorder. J Psychiatr Res. 2016;79:34–41. doi: 10.1016/j.jpsychires.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Coccaro EF, Lee R, Coussons-Read M. Elevated plasma inflammatory markers in individuals with intermittent explosive disorder and correlation with aggression in humans. JAMA Psychiatry. 2014;71:158–65. doi: 10.1001/jamapsychiatry.2013.3297. [DOI] [PubMed] [Google Scholar]
- 21.Coccaro EF, Nayyer H, McCloskey MS. Personality disorder-not otherwise specified evidence of validity and consideration for DSM-5. Compr Psychiatry. 2012;53:907–14. doi: 10.1016/j.comppsych.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 22.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) New York: Psychiatric Institute, Biometrics Research; 1997. [Google Scholar]
- 23.Coccaro EF, Berman ME, Kavoussi RJ. Assessment of life history of aggression: development and psychometric characteristics. Psychiatry Res. 1997;73:147–57. doi: 10.1016/S0165-1781(97)00119-4. [DOI] [PubMed] [Google Scholar]
- 24.Buss AH, Perry M. The aggression questionnaire. J Pers Soc Psychol. 1992;63:452–9. doi: 10.1037/0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- 25.Coccaro EF, Schmidt-Kaplan CA. Life history of impulsive behavior: development and validation of a new questionnaire. J Psychiatr Res. 2012;46:346–52. doi: 10.1016/j.jpsychires.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Patton J, Stanford M, Barratt E. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Brown GK. Beck Depression Inventory-II (BDI-II) San Antonio, TX.: The Psychological Corporation; 1996. [Google Scholar]
- 28.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 29.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:386–96. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- 30.Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]
- 31.Kraus MR, Schafer A, Faller H, Csef H, Scheurlen M. Psychiatric symptoms in patients with chronic hepatitis C receiving interferon alfa-2b therapy. J Clin Psychiatry. 2003;64:708–14. doi: 10.4088/JCP.v64n0614. [DOI] [PubMed] [Google Scholar]
- 32.McHuthison J, Gordon S, Schiff E. Interferon alpha-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. NEJM. 1998;339:1485–92. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 33.Suarez EC, Lewis JG, Krishnan RR, Young KH. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology. 2004;29:1119–28. doi: 10.1016/j.psyneuen.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Felger JC, Haroon E, Patel TA, Goldsmith DR, Wommack EC, Woolwine BJ, et al. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry. 2020;25:1301–11. doi: 10.1038/s41380-018-0096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer JH, Cervenka S, Kim MJ, Kreisl WC, Henter ID, Innis RB. Neuroinflammation in psychiatric disorders: PET imaging and promising new targets. Lancet Psychiatry. 2020;7:1064–74. doi: 10.1016/S2215-0366(20)30255-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrison NA, Cooper E, Dowell NG, Keramida G, Voon V, Critchley HD, et al. Quantitative magnetization transfer imaging as a biomarker for effects of systemic inflammation on the brain. Biol Psychiatry. 2015;78:4957. doi: 10.1016/j.biopsych.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 2008;64:484–90. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2016;21:1358–65. doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehta ND, Haroon E, Xu X, Woolwine BJ, Li L, Felger JC. Inflammation negatively correlates with amygdala-ventromedial prefrontal functional connectivity in association with anxiety in patients with depression: preliminary results. Brain Behav Immun. 2018;73:725–30. doi: 10.1016/j.bbi.2018.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI. Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage. 2012;59:3222–6. doi: 10.1016/j.neuroimage.2011.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kullmann JS, Grigoleit JS, Lichte P, Kobbe P, Rosenberger C, Banner C, et al. Neural response to emotional stimuli during experimental human endotoxemia. Hum Brain Mapp. 2013;34:2217–27. doi: 10.1002/hbm.22063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immunol. 2007;21:727–35. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Hassanain M, Zalcman S, Bhatt S, Siegel A. Interleukin-1 beta in the hypothalamus potentiates feline defensive rage: role of serotonin-2 receptors. Neuroscience. 2003;120:227–33. doi: 10.1016/S0306-4522(03)00264-1. [DOI] [PubMed] [Google Scholar]
- 46.Hassanain M, Bhatt S, Zalcman S, Siegel A. Potentiating role of interleukin-1beta (IL-1beta) and IL-1beta type 1 receptors in the medial hypothalamus in defensive rage behavior in the cat. Brain Res. 2005;1048:1–11. doi: 10.1016/j.brainres.2005.04.086. [DOI] [PubMed] [Google Scholar]
- 47.Bhatt S, Siegel A. Potentiating role of interleukin 2 (IL-2) receptors in the midbrain periaqueductal gray (PAG) upon defensive rage behavior in the cat: role of neurokinin NK(1) receptors. Behav Brain Res. 2006;167:251–60. doi: 10.1016/j.bbr.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 48.Bhatt S, Zalcman S, Hassanain M, Siegel A. Cytokine modulation of defensive rage behavior in the cat: role of GABAA and interleukin-2 receptors in the medial hypothalamus. Neuroscience. 2005;133:17–28. doi: 10.1016/j.neuroscience.2005.01.065. [DOI] [PubMed] [Google Scholar]
- 49.Chang HB, Munroe S, Gray K, Porta G, Douaihy A, Marsland A, et al. The role of substance use, smoking, and inflammation in risk for suicidal behavior. J Affect Disord. 2019;243:33–41. doi: 10.1016/j.jad.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coryell W, Wilcox H, Evans SJ, Pandey GN, Jones-Brando L, Dickerson F, et al. Aggression, impulsivity and inflammatory markers as risk factors for suicidal behavior. Psychiatry Res. 2018;106:38–42. doi: 10.1016/j.jpsychires.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Barlow MA, Wrosch C, Gouin J-P, Kunzmann U. Is anger, but not sadness, associated with chronic inflammation and illness in older adulthood? Psychol Aging. 2019;34:330–40. doi: 10.1037/pag0000348. [DOI] [PubMed] [Google Scholar]
- 52.Yong JC, Hartanto A, Tan JJX. Subjective social status and inflammation: The role of culture and anger control. Health Psychol. 2021;40:62–70. doi: 10.1037/hea0001029. [DOI] [PubMed] [Google Scholar]
- 53.McCloskey MS, Fahlgren MK, Coccaro EF. Assessment and treatment of intermittent explosive disorder. In: Coccaro EF, McCloskey MS, editors. Aggression: Clinical Features and Treatment Across the Diagnostic Spectrum. Washington, D.C.: American Psychiatric Association Publishing; 2019. pp. 31–49. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




