Abstract
Wnt signaling comprises a group of complex signal transduction pathways that play critical roles in cell proliferation, differentiation, and apoptosis during development, as well as in stem cell maintenance and adult tissue homeostasis. Wnt pathways are classified into two major groups, canonical (β-catenin-dependent) or non-canonical (β-catenin-independent). Most previous studies in the eye have focused on canonical Wnt signaling, and the role of non-canonical signaling remains poorly understood. Additionally, the crosstalk between canonical and non-canonical Wnt signaling in the eye has hardly been explored. In this review, we present an overview of available data on ocular non-canonical Wnt signaling, including developmental and functional aspects in different eye compartments. We also discuss important changes of this signaling in various ocular conditions, such as keratoconus, aniridia-related keratopathy, diabetes, age-related macular degeneration, optic nerve damage, pathological angiogenesis, and abnormalities in the trabecular meshwork and conjunctival cells, and limbal stem cell deficiency.
Keywords: Planar cell polarity, calcium signaling, Wnt signaling, eye development, eye diseases, cornea, retina, lens, conjunctiva, stem cells
1. Introduction
Since the discovery of wingless (Wg) in Drosophila (Sharma 1973; Baker 1987) and Integration-1 (Int-1) in mouse (Rijsewijk et al. 1987) over four decades ago, the Wnt family of proteins (Nusse et al. 1991) have been extensively studied in disease and development (Yang 2012; Duchartre et al. 2016; Nusse and Clevers 2017; Taciak et al. 2018; Foulquier et al. 2018; Steinhart and Angers 2018; Wang et al. 2019). Acting as intracellular signaling molecules, these highly conserved family of proteins are encoded by 19 WNT genes in humans (Miller 2002) and play a critical role in early development and stem cell control, as well as in later cell growth and maintenance (Nusse and Clevers 2017). Wnt signal transduction comprises pathways including the canonical Wnt/β-catenin dependent pathway and the non-canonical planar cell polarity (PCP) and calcium (Ca+2) signaling pathways (Komiya and Habas 2008). Whereas the canonical pathway is well-characterized and widely studied, the molecular mechanisms involved in non-canonical Wnt signaling remain unclear. In this review, we will focus on and present a broad overview of non-canonical Wnt signaling in the eye.
2. Wnt Family, Signaling Pathways and Wnt Receptors
Wingless-related integration site genes encode evolutionarily conserved, secreted proteins that are essential for multiple biological processes. The Wnt signaling pathways play various roles during embryonic development and adult homeostasis, and their disruption may be linked to several diseases such as cancer, cardiometabolic diseases, and degenerative disorders (Logan and Nusse 2004; Sedgwick and D’Souza-Schorey 2016; Gay and Towler 2016) as well as genetic diseases due to mutations in various Wnt components (Human genetic diseases and Wnt signaling components 2022 https://web.stanford.edu/group/nusselab/cgi-bin/wnt/human_genetic_diseases, last accessed October 28, 2022).
The human Wnt family contains 19 WNT genes that encode proteins about 40 kDa in size and contain highly conserved cysteine and serine residues (Miller 2002; Clevers and Nusse 2012). Conserved serines of Wnt proteins are palmitoylated by a special palmitoyl transferase, porcupine (PORCN), in the endoplasmic reticulum (Galli et al 2007; Rios-Esteves et al 2014), which is essential for the secretion of Wnt ligands. Eventually, mature hydrophobic Wnt proteins are escorted by the transmembrane protein Wntless (Wls) to be secreted at the plasma membrane or released in exosomes, leading to both autocrine and paracrine effects (Bänziger et al. 2006; Routledge and Scholpp 2019). Vertebrate animals such as mouse and frog have the same number of 19 Wnt genes, whereas others such as chicken have 11 and zebrafish have 12 known Wnt genes (Vertebrate Wnt genes, the Wnt homepage 2022. https://web.stanford.edu/group/nusselab/cgi-bin/wnt/vertebrate; accessed October 27, 2022). Ocular cell expression of various Wnt ligands is summarized in Figure 1.
Figure 1.
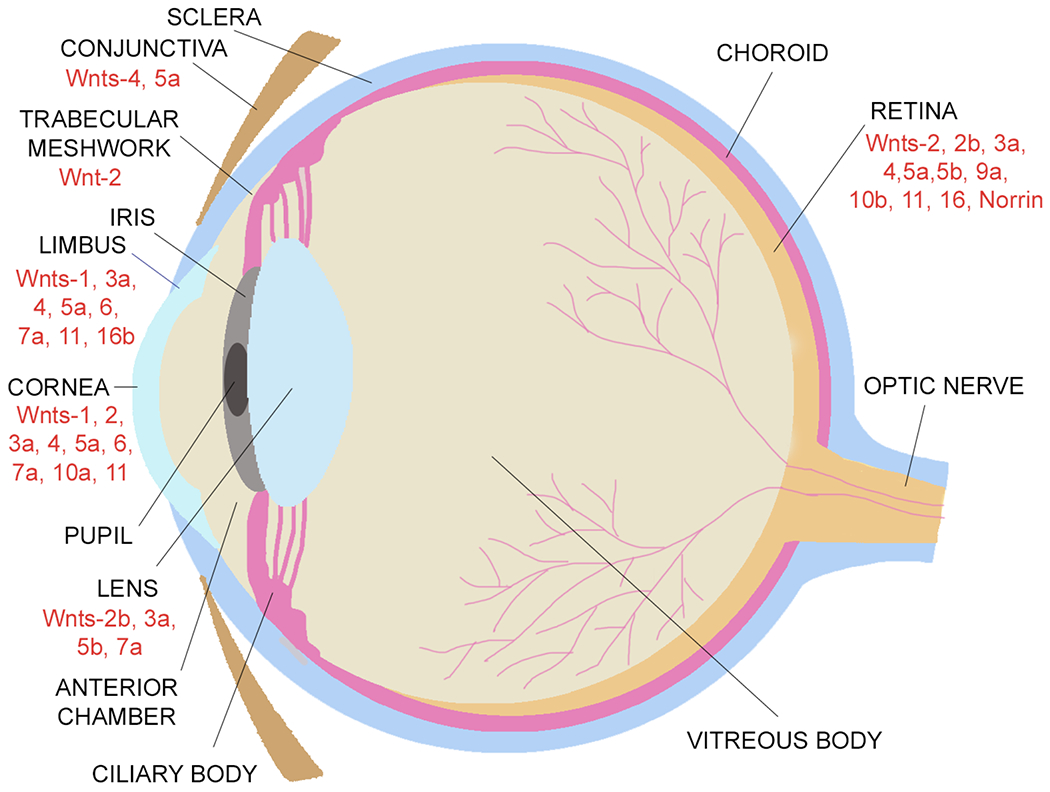
A schematic illustration of the expression of Wnt ligands in the eye.
The Wnt signaling pathways are divided into two main branches: the canonical (β-catenin-dependent) signaling pathway and the non-canonical (β-catenin-independent) signaling pathways (Figure 2, Table 1). Wnt ligands bind to various receptors and co-receptors. Eleven members of the Frizzled protein family serve as receptors for both the canonical and non-canonical Wnts. The coupling between certain Frizzled proteins and another receptor, such as the low-density lipoprotein receptor-related protein 5 or 6 (LRP5/6), protein tyrosine kinase 7 (PTK7), the receptor like tyrosine kinase (RYK) or the receptor Tyr kinase-like orphan receptor 1 or 2 (ROR1/2), launches the downstream signaling (Endo et al. 2015; Azbazdar et al. 2021). In contrast, Wnt antagonists regulate the fine-tuning of Wnt signaling by inhibiting its activation and thus control various cellular processes. To date, six families of secreted Wnt antagonists and four families of transmembrane Wnt antagonists have been described. The secreted antagonists include the Dickkopf proteins (Dkks), secreted Frizzled-related proteins (sFRPs), Wnt-inhibitory factor 1 (WIF-1), Wise/SOST, Cerberus, and insulin-like growth-factor binding protein 4 (IGFBP-4), whereas the transmembrane antagonists are Shisa, Wnt-activated inhibitory factor 1 (Waif1/5T4), adenomatosis polyposis coli down-regulated 1 (APCDD1), and Tiki1. These Wnt antagonists have been described in detail by Cruciat and Niehrs (2013).
Figure 2.
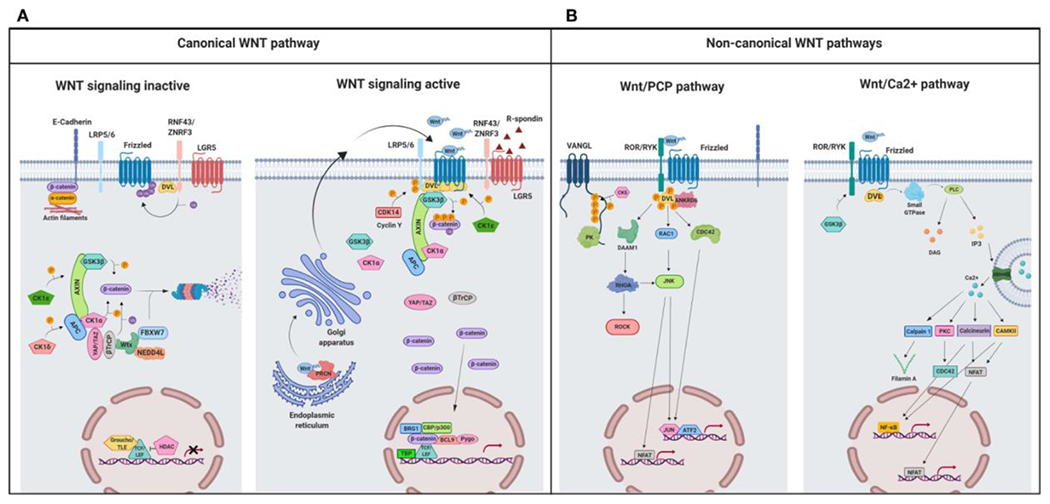
A schematic illustration representing different Wnt signaling pathways. (A) Canonical Wnt signaling. Left panel shows inactive pathway. In the absence of Wnt ligands, β-catenin is phosphorylated by the destruction complex, constituted by the scaffolding proteins APC and AXIN and the kinases GSK3β and CK1α. Then, β-catenin is ubiquitinated and targeted for proteasomal degradation by the complex containing β-TrCP, FBXW7, NEDDL4, and WTX proteins. Thus, β-catenin degradation prevents its presence in the nucleus where a complex formed by TCF/LEF and TLE/Groucho binds HDACs to inhibit transcription of target genes. Right panel shows canonical Wnt signaling active. The binding of Wnt ligands to FZD receptors and LRP co-receptors activates Wnt signaling. LRP receptors are phosphorylated by CK1α and GSK3β. Then, DVL proteins polymerize and are activated at the plasma membrane inhibiting the destruction complex. This results in stabilization and accumulation of β-catenin in the cytosol and its subsequent translocation into the nucleus where it displaces TLE/Groucho repressors forming an active complex with TCF/LEF proteins that bind co-activators such as CBP/p300, BRG1, BCL9, and PYGO. An alternative way of β-catenin signaling includes the disruption of epithelial E-cadherin interactions, which breaks the binding of β-catenin to the cytoplasmic domain of cadherin and leads to the accumulation of β-catenin first in the cytosol, and later in the nucleus. (B) Schematic illustration representing the main non-canonical Wnt pathways. Left panel shows the Wnt/PCP pathway. Wnt ligands bind to the FZD receptor and the co-receptors ROR 1/2 (or RYK). Then, DVL is recruited and activated followed by VANGL activation. Then DVL binds to the small GTPase RHO A with the collaboration of the cytoplasmic protein DAAM1. The small GTPases RAC1 and RHO activate ROCK and JNK. This leads to rearrangements of the cytoskeleton and/or transcriptional responses via for example, ATF2 and/or NFAT. Right panel shows the Wnt/Ca2+ pathway. The signaling is initiated when Wnt ligands bind to the FZD receptor and the co-receptor ROR 1/2 (or RYK). Then, DVL is recruited and activated and binds to the small GTPase which activates phospholipase C leading to intracellular calcium fluxes and downstream calcium dependent cytoskeletal and/or transcriptional responses. APC, adenomatous polyposis coli; BCL9, B-cell CLL/lymphoma 9 protein; β-TrCP, β-Transducin repeat-containing protein; BRG1, Brahma related gene 1; CAMKII, calmodulin-dependent protein kinase II; CBP, CREB-binding protein; CDC42, cell division control protein 42; CELSR, cadherin EGF LAG seven-pass G-type receptor; CK1α,ε,δ, casein kinase 1α,ε,δ; DAAM1, DVL associated activator of morphogenesis; DAG, diacylglycerol; DVL, disheveled; FBXW7, F box/WD repeat-containing protein 7; FZD, Frizzled; GSK3b, glycogen synthase kinase 3β; IP3, inositol 1,4,5 triphosphate; JNK, JUN kinase; LGR5, Leucine-rich repeat-containing G-protein-coupled receptor 5; LRP5/6, low-density lipoprotein receptor-related protein 5/6; NEDD4L, neural precursor cell expressed, developmentally downregulated 4-like; NFAT, nuclear factor of activated T cells; NF-κB, nuclear factor kappa B; PK, Prickle; PKC, protein kinase C; PLC, Phospholipase C; p300, E1A Binding Protein p300; RAC, Ras-related C3 botulinum toxin substrate; RHOA, Ras homolog gene family member A; ROCK, Rho kinase; ROR 1/2, bind tyrosine kinase-like orphan receptor 1 or 2; RYK, receptor-like tyrosine kinase; TBP, TATA-binding protein; PRCN, Porcupine; PYGO, Pygopus; RNF43, Ring finger protein 43; RSPO, R-spondin; TCF/LEF, T-cell factor/lymphoid enhancer factor; TLE, Transducin-Like Enhancer of Split proteins; VANGL, Van Gogh-like; WTX, Wilms tumor suppressor protein complex; YAP/TAZ, Yes-associated protein/Transcriptional co-activator with a PDZ-binding domain; ZNRF3, Zinc and Ring Finger 3. Created with BioRender.com.
From: Martin-Orozco E, Sanchez-Fernandez A, Ortiz-Parra I, Ayala-San Nicolas M. WNT signaling in tumors: the way to evade drugs and immunity. Front Immunol. 2019; 10:2854. doi:10.3389/fimmu.2019.02854.
Table 1.
Wnt ligands, their possible receptors and ocular cell expression
| Wnt ligand | Canonical signaling | Non-canonical signaling | Possible Receptors | Ocular cell expression | References |
|---|---|---|---|---|---|
| Wnt-1 | + | RYK, LRP5/6, FZD4, FZD5, FZD8 | Corneal endothelial cells, limbal epithelial cells, trabecular meshwork cells | Lu et al. 2004; Mao et al. 2001; Voloshanenko et al. 2017; McGowan et al. 2007 | |
| Wnt-2 | + | + | FZD1, FZD8, FZD9, LRP5/6 | Müller cells, corneal epithelial cells | Gazit et al. 1999; Bravo et al. 2013; Karasawa et al. 2002; Ren et al. 2021; Musada et al. 2020; Liu et al. 2003. |
| Wnt-2b/Wnt-13 | + | FZD4, LRP5/6 | Lens epithelial cells, retinal pigment epithelial cells, retinal progenitor cells | Ohta et al. 2011; Katoh and Katoh 2009; Ren et al. 2021; Jasoni, Hendrickson and Roelink 1999; Liu et al. 2003; Nakagawa et al. 2003; Kubo, Takeichi and Nakagawa 2003; | |
| Wnt-3/3a | + | FZD1-10, LRP5/6 | Corneal and limbal epithelial cells, lens epithelial cells, retinal pigment epithelial cells | Voloshanenko et al. 2017; Kozielewicz et al. 2021; Sebastian et al. 2017; Liu et al. 2003; Kim et al. 2015 | |
| Wnt-4 | + | + | FZD1, FZD2, FZD6, LRP5/6 | Müller cells, corneal and limbal epithelial cells, conjunctival epithelial cells | Zhang et al. 2021; Musada et al. 2020; Liu et al. 2003; Yuan et al. 2013; Watson et al. 2013; Figueria et al. 2007; Mantelli et al. 2009 |
| Wnt-5a | + | FZD2, FZD3, FZD4, FZD5, FZD6, FZD7, FZD8, RYK, ROR2, PTK7, CD146 | Corneal and limbal epithelial cells, conjunctival epithelial cells, Müller cells, rods, bipolar cells, retinal myeloid cells, retinal pigment epithelial cells, stromal cells | Kumawat and Gosens 2016; Martinez et al. 2015; Musada et al. 2020; Sarin et al. 2018; Stefater et al. 2011; Kim et al. 2015; Yuan et al. 2013; Che et al. 2018; Mantelli et al. 2009 | |
| Wnt-5b | + | + | FZD1, FZD2, FZD4, FZD5, FZD6, FZD7, FZD8 | Müller cells, rods bipolar cells, lens epithelial cells | Suthon et al. 2021; Musada et al. 2020; Sarin et al. 2018; Liu et al. 2003 |
| Wnt-6 | + | + | FZD4, LRP6 | Corneal and limbal epithelial cells, | Wei et al. 2020; He et al. 2020; MacDonald and He 2012; Liu et al. 2003; Bonnet et al. 2021. |
| Wnt-7a | + | + | FZD5, LRP5/6 | Corneal and limbal epithelial cells, lens fiber cells | Voloshanenko et al. 2017; Ren et al. 2021; Ouyang et al. 2014; Liu et al. 2003 |
| Wnt-7b | + | + | FZD1, FZD5, FZD7, FZD8, FZD10, LRP5 | Voloshanenko et al. 2017; Wang et al. 2005; Ferrari et al. 2018 | |
| Wnt-8 | + | FZD54, FZD5, FZD8, FZD10, LRP6 | Voloshanenko et al. 2017; Dawson et al. 2013 | ||
| Wnt-9a/Wnt-14 | + | + | FZD4, FZD10, LRP5/6 | Müller cells | Voloshanenko et al. 2017; Ren et al. 2021; Musada et al. 2020 |
| Wnt-9b | + | + | FZD4, FZD5, FZD8, FZD10, LRP5/6 | Voloshanenko et al. 2017; Ren et al. 2021 | |
| Wnt-10a | + | + | FZD5, FZD8, LRP5/6 | Corneal epithelial cells | Voloshanenko et al. 2017; Ren et al. 2021; Foster et al. 2021 |
| Wnt-10b/Wnt-12 | + | + | FZD6, LRP5/6 | Müller cells | Neuhaus et al. 2021; Ren et al. 2021; Musada et al. 2020 |
| Wnt-11 | + | ROR2, FZD7, FZD8, RYK | Corneal and limbal epithelial cells, retinal myeloid cells | Bai et al. 2014; Kim et al. 2008; Murillo-Garzón et al. 2018; Stefater et al. 2011 | |
| Wnt-16 | + | FZD1, FZD2, FZD7 | Müller cells, limbal epithelial cells | Martínez-Bartolomé and Range 2019; Musada et al. 2020; Zhao et al. 2022. | |
| Norrin | + | + | FZD4 | Müller cells, retinal endothelial cells | Wang et al. 2012; Madaan et al. 2019; Yang et al. 2021; Ye et al. 2009; Chidiac et al. 2021 |
Canonical Wnt signaling includes binding of Wnt ligands (e.g., Wnt-1 and Wnt-3) to a receptor complex formed by a Frizzled and LRP5/6. The output of the canonical Wnt pathway signaling is determined by the level of cytosolic β-catenin, as receptor recruitment leads to the accumulation of cytoplasmic β-catenin. Upon translocation into the nucleus, β-catenin binds to the T cell factor/lymphoid enhancer factor (TCF/LEF) transcription factor to initiate transcription of Wnt/β-catenin target genes such as c-Myc, c-Jun, cyclin D1, Axin regulating a number of cellular processes (Dijksterhuis et al. 2014; Lecarpentier et al. 2019) (Figure 2, Table1).
Other Wnt ligands (e.g., Wnt-4, Wnt-5a, Wnt-5b and Wnt-11) can activate non-canonical Wnt pathways independent of β-catenin accumulation (Xiao et al. 2017). Non-canonical Wnt signaling can be subdivided into two main pathways: the planar cell polarity (PCP) signaling pathway and the Ca2+ signaling pathway (Figure 2, Table 1). In different cell types, non-canonical Wnt may use various Frizzled co-receptors with ROR, RYK, MuSK, or PTK7, but act on different downstream effectors (Chen et al. 2021). In PCP signaling pathway, disheveled (DVL) forms a complex with DVL-associated activator of morphogenesis 1 (DAAM1) to induce the small GTPase Ras homology family member (Rho) activation (Habas et al. 2001), which activates downstream kinases such as Rho-associated protein kinase (ROCK) (Winter et al. 2001) or JUN-N-terminal kinase (JNK) (Strutt et al. 1997). Additionally, DVL activates RAC to trigger JNK activity, which controls gene expression via JNK-dependent transcription factors (Fanto et al. 2000). The activation of PCP signaling results in cell polarity establishment, cell migration and convergent extension during embryogenesis (Niehrs 2012).
The Ca2+ signaling pathway involves the activation of phospholipase C (PLC) by heterotrimeric G proteins. This in turn, upregulates diacylglycerol (DAG) and type 3 inositol-1,4,5-triphosphate (IP3) production leading to Ca2+ mobilization (Slusarski et al. 1997; Anastas and Moon 2013) and activation of Ca2+ sensing enzymes calmodulin-dependent kinase II (CaMKII) and protein kinase C (PKC) (Kuhl et al. 2000; Wang et al. 2010) regulating cell fate and migration. Through multiple effectors, non-canonical pathways are involved in regulating cell polarity, promoting cell movement and invasion, maintenance of stem cells and inhibiting the canonical Wnt/β-catenin signaling pathway (Katoh 2017; Kukichi et al. 2012). In addition, the transcriptional regulator nuclear factor associated with T cells (NFAT) is stimulated through calcineurin and is translocated to the nucleus to control transcription of genes involved in morphogenesis and mesenchymal to epithelial transition (Dejmek et al. 2006; Burn et al. 2011). Noteworthy, the loss of normal adhesion and cell polarity, as well as the acquisition of motility and invasiveness, are important steps during tumor progression and metastasis, which therefore, can be all mediated by non-canonical Wnt signaling. Unlike canonical signaling activity, which can be measured by various luciferase or GFP based transcriptional reporters, reporter assays to measure non-canonical signaling activity are limited. Loss-of-function and gain-of-function approaches are required to establish the involvement of specific effector pathways. Ohkawara and Niehrs (2011) have described an ATF2 response element-based luciferase assay to monitor non-canonical PCP signaling activity in Xenopus embryos. Similarly, Karuna, Susman, and Ho (2018) have designed a non-transcriptional Wnt-5a-Ror-Kif26b (WRK) reporter assay that measures Wnt-5a-Ror2 signaling activity in real time.
3. Non-canonical Wnt Signaling in Eye Development
Formation of the eye begins with the neural tube ectoderm, which is the progenitor of the retina, iris and its smooth muscle, ciliary body, optic nerve and vitreous humor, and the surface ectoderm that gives rise to the lens, conjunctiva, corneal epithelium, eyelid and lacrimal system (Graw 2010). Whereas canonical β-catenin dependent pathway is crucial for other aspects of eye development, several studies indicate that non-canonical signaling is essential for the formation of eye field in the anterior neural plate, lens, and retinal ganglion cell (RGC) axon outgrowth (Fuhrmann 2008).
3.1. Eye Field Development
In frog, ephrinB1 via its extracellular domain interacts with DVL through PCP pathway and regulates retinal progenitor movement in the eye field (Lee et al. 2006). In zebrafish, Wnt-11 and Frizzled-5 activate non-canonical signaling to direct the morphogenesis of the eye field and antagonizes β-catenin signaling to suppress retinal identity and promote the coherence of eye field cells (Cavodeassi et al. 2005). Similarly, Wnt-5 also negatively regulates canonical pathway through Ca2+ signaling in vertebrate axis formation in zebrafish. Loss-of-function experiments with Wnt-5 revealed defects in cell movement and hyperdorsalization and axis-duplication phenotypes due to accumulation of β-catenin and activation of its downstream genes (Westfall et al. 2003). Moreover, in medaka fish, Esteve et al. (2004) postulated that sFRP1 is essential for proper eye field formation within the forebrain by enhancing Wnt-11 signaling, possibly via the activation of PCP pathway. Another non-canonical Wnt-4 is also involved in eye development in frogs through EAF2, a component of the ELL-mediated RNA polymerase II elongation factor complex. Loss-of-function studies showed that Wnt-4 was critical for eye field formation, as indicated by the loss of eye field markers, Rx and Pax6 (Maurus et al. 2005).
3.2. Lens Development
In the lens, non-canonical Wnt signaling is involved in the regulation of such processes as embryonic differentiation, cytoskeleton organization, and the formation of three-dimensional architecture that occurs during embryogenesis (Chen et al. 2008; Han et al. 2018; Dawes et al. 2013; Chen et al. 2006). Human lens development begins with the differentiation of embryonic stem cells (hESCs) into lens progenitor cells and lentoid bodies (LBs), with regulation mediated by non-canonical Wnt-5a (Figure 3) (Han et al. 2018; Kohn and Moon 2005). In an in vitro model paralleling early lens development in vivo, the addition of exogenous Wnt-5a to hESCs produced activation of the non-canonical Wnt/JNK PCP pathway (Chen et al. 2008; Han et al. 2018). The upregulation of this pathway activated downstream JNK signaling cascade (Chen et al. 2008; Han et al. 2018). This in turn promoted the differentiation of ESCs into LBs, evidenced by increased quantity and size of LBs as well as increased expression of the lens-specific genes (Han et al. 2018).
Figure 3.
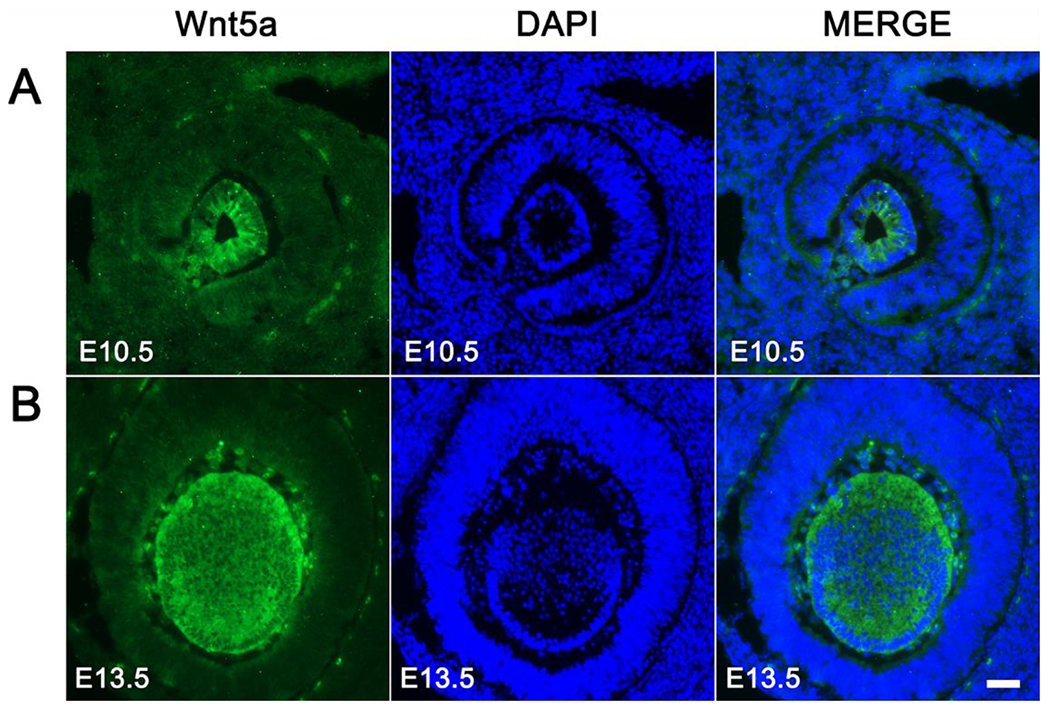
Spatiotemporal expression pattern of Wnt-5a during lens development in vivo. Immunofluorescent staining of sagittal sections of mouse embryonic eyes at E10.5 (A) and E13.5 (B) for Wnt-5a (green) and DAPI (blue; nuclei) is shown. Scale bar. 100 μm.
From: Han C, Li J, Wang C, Ouyang H, Ding X, Liu Y, Chen S, Luo L. Wnt5a contributes to the differentiation of human embryonic stem cells into lentoid bodies through the noncanonical Wnt/JNK signaling pathway. Invest. Ophthalmol. Vis. Sci. 2018;59:3449-3460. doi:10.1167/iovs.18-23902
Subsequently, the lens is being formed from the lens placode - an ectodermally derived precursor associated with the optic vesicle that expresses Wnt-5a, with regulation by PAX6 (Sugiyama et al. 2011; McAvoy et al. 1999; Xie et al. 2013). The lens placode and optic vesicle invaginate together developing, respectively, into the lens pit and optic cup, with roughly equivalent levels of expression for several non-canonical Wnt genes (Wnt5a, Wnt5b, Wnt7a, and Wnt7b) throughout the lens placode, pit, and vesicle (Ang et al. 2004). Similar expression patterns were also induced by the Wnt signaling regulators Dickkopfs (DKK1, 2, and 3) (Ang et al. 2004). Anterior lens vesicle cells differentiate into the lens epithelium, and the posterior cells withdraw from the cell cycle, elongate and differentiate into primary lens fiber cells (Sugiyama et al. 2011; McAvoy et al. 1999). At this point that mammalian lens displays increased expression of Wnt-5a, Wnt-5b, Wnt-7a, and Wnt-7b in presumptive epithelial cells and elongating primary fibers (Ang et al. 2004). Human SIPA1L3 gene is necessary for proper development of lens fibers and maturation, and its missense mutations will result in congenital cataracts and in some cases in microphthalmia (Greenlees et al. 2015, Rothe et al. 2017). The data obtained in Xenopus suggested that in the normal ocular development, the interaction between Epha4 and its downstream Sipa1l3 inhibits Wnt/β-catenin signaling and activates non-canonical signaling, whereas Sipa1l3 knockdown results in the activation of β-catenin signaling and its nuclear translocation leading to disturbed ocular development including microphthalmia and cataract formation (Rothe et al. 2017). Thus, inhibiting canonical Wnt signaling with the activation of non-canonical signaling may be an important therapeutic approach to treat congenital cataracts with SIPA1L3 mutations. Expression of the aforementioned non-canonical Wnts and their Dkk regulators gradually declines in the primary lens fiber cells and primarily begins to localize near the lens equator region, though Wnt-7b remains strongly expressed in central primary fibers (Ang et al. 2004).
Several fibroblast growth factors (FGFs) and their receptors (FGFRs) play an essential role in initiating and promoting both the initial differentiation of lens vesicle cells into primary lens fibers and the subsequent proliferation and differentiation of lens epithelial cells into secondary lens fibers (Robinson 2006; Zhao et al. 2008). β-catenin independent Wnt signaling is involved in enhancing the morphological aspects of this FGF2-induced lens fiber differentiation (Lyu and Joo 2004). In line with this finding, rat lens epithelial explants cultured with FGF-2 displayed upregulation of non-canonical Wnt-Frizzled/PCP signaling, confirming the involvement of non-canonical Wnts in the FGF-induced cascade crucial to initiating and regulating epithelial to lens fiber differentiation (Dawes et al. 2013; Chen et al. 2006).
Upon the induction of lens cell differentiation by FGF-2, Wnt-5a was found to influence the behavior of lens cells by acting as a directional cue for lens cell migration (Dawes et al. 2014). This mechanism guided the formation of complex organization and architecture needed to develop and maintain lens polarity. Potential interactions of Notch signaling with Wnt-5a in this mechanism have been suggested based on other cases of Notch promoting Wnt-5a in epithelial progenitor cells, though they have not been confirmed in the lens specifically (Dawes et al. 2014; Koyanagi et al. 2007). In the epithelial to fiber differentiation and elongation, PCP signaling in the lens of transgenic mice was reported to be essential to multiple facets of lens structural development (Chen et al. 2008). Blocking of PCP signaling by overexpression of Wnt signaling antagonist, sFRP2, led to significant adverse effects in lens development that ultimately led to the formation of severe cataracts. These effects were the result of hindered secondary lens fiber elongation that interfered with proper fiber cell cytoskeletal organization and prevented the convex curvature required for proper three-dimensional lens architecture (Chen et al. 2008). A recent study provided further evidence for the role of PCP signaling in this cytoskeletal organization with in vitro treatment with Wnt-5a resulting in elevated levels of activated (phosphorylated) Dvl and Rac1 proteins implicated in providing scaffolding for components of the cytoskeleton and regulation of lens placodal cell elongation, respectively (Han et al. 2018; Malbon and Wang 2006).
3.3. Retinal Development
In the developing retina, in situ hybridization analysis revealed that Wnt-5a and Wnt-7b were expressed in the neural retina at E12.5 and promoted RGC axon outgrowth (Liu et al. 2003). Moreover, in determining the functional and homeostasis processes of cone development and establishment in mouse retina, significant activation of the Wnt/Ca2+ pathway to modulate calcium concentrations and expression of Bmp/Smad genes was observed (Yu et al. 2004). Additionally, Wnt-5a becomes expressed and controlled by Zic2-switch at the optic chiasm midline, promoting crossing of retinal axons without relying on canonical pathway activation (Morenilla-Palao et al. 2020). These data support an alternative Zic2-switch-dependent Wnt signaling pathway in ipsilateral neurons, that includes the expression of a group of specific Wnt proteins and receptors.
More recently, a study utilizing RNA-seq analysis of chick periocular neural crest (pNC) showed differential expression of Wnt signaling intermediates with significant expression in several ligands involved in the PCP pathway (i.e DAMM2, MAPK10, ROCK2, PRICKLE2, RHOA, RAC1, CDC42) and the Wnt Ca2+ pathway (i.e NFACTC1, PRKCA, CAMK2D, RYK, CAMK2B) in pNC and neural crest-derived corneal cell development into highly specialized corneal endothelial cells and keratocytes. The expression of the genes was validated by in situ hybridization, establishing their involvement in the corneal endothelium and keratocyte identity by inducing cell migration and polarity during development (Bi and Lwigale 2019).
Sarin et al. (2018) support non-canonical signaling activity during the development of the retinal neuropil, specifically the outer plexiform layer (OPL), which is the integrative system of neurons and glia where rods and cones photoreceptors synapse with bipolar cells. Through RNA-sequencing, specific genes that encode for cell surface and secreted proteins, Wnt-5a and Wnt-5b were identified. Furthermore, CRISPR-Cas9 electroporation was used to assess their part in OPL development, revealing that Wnt-5a and Wnt-5b affect the placement of synapse, but not the localization of synaptic markers. Wnt-5a and Wnt-5b were found to interact through a Ryk-dependent pathway requiring Dvl; expressed primarily in rods.
4. Non-Canonical Wnt Signaling in Eye Diseases
4.1. Cornea
The cornea is the most anterior transparent part of the eye and its most powerful lens. It functions not only to protect the inner eye, but also to focus light onto the retina and ensure visual acuity. The corneal epithelium is constantly renewed and sustained by corneal epithelial stem cells located at the limbus, the junction between the conjunctiva and cornea (Sridhar 2018). Limbal epithelial stem cells (LESC) are maintained and differentiated into corneal epithelium by key molecular events aided by Wnt proteins that allow communication between these cells to help regulate their growth, functions, differentiation, and death (Nakatsu et al. 2011). In this section, we discuss the involvement of non-canonical signaling in LESC maintenance and its implication in pathological corneal conditions including limbal stem cell deficiency (LSCD), diabetic keratopathy and corneal wound healing, as well as in aniridia related keratopathy and keratoconus.
4.1.1. Limbal Stem Cell Maintenance and Corneal Wound Healing in Normal and Disease Conditions
The role of non-canonical Wnt signaling in LESC maintenance has been explored only by a handful of studies, and hence remains poorly understood. Mei et al. (2014) reported the contribution of Frizzled-7 in the maintenance of LESC in their undifferentiated state through a β-catenin independent non-canonical pathway. Frizzled-7 is preferentially expressed in the basal layer of the limbal epithelium, housing LESC. shRNA knockdown of FZD7 gene in primary LESC resulted in a significant decrease in the expression of putative LESC markers, ABCG2, ΔNp63α, and K14. The authors further revealed that Frizzled-7 interacted and colocalized with syndecan-4 and fibronectin in the human basal limbal epithelium (Mei et al. 2014). This complex also promoted LESC proliferation and sternness via Wnt-11 stimulation and the activation of PCP signaling through ROCK upregulation in rabbit LESC (Zheng et al. 2019).
The importance of Wnt-6 and Wnt-16b in regulating LESC proliferation and self-renewal via the activation of non-canonical signaling through the phosphorylation of c-Jun in cultured LESC was also reported (Bonnet et al. 2021; Robertson et al. 2022; Zhao et al. 2022). LESC grown on 3T3 cells that overexpress Wnt-16b at low levels using a Wnt-16b-IRES-GFP lentivirus vector showed increased expression of p63-positive cells, indicating putative LESC expression. They also increased the canonical pathway markers, Axin2 and Lrp5, and non-canonical Wnt co-receptor, Ror2 (Robertson et al. 2020) and showed increased phosphorylation of cJun as compared to the control vector. This study suggests the role of Wnt-16b in maintaining a balance of canonical and non-canonical pathways to regulate LESC proliferation, differentiation, and self-renewal (Robertson et al. 2022; Zhao et al. 2022). Similarly, LESC grown on 3T3 cells overexpressing Wnt-6 at low, medium, and high levels showed highest increase in phosphorylation of CamKII at low levels of Wnt-6, indicating the activation of Ca2+ signaling, while RhoA was phosphorylated (activation of PCP signaling by Wnt-6) in a dose-dependent manner. In addition, Wnt-6 also induced the activation of β-catenin signaling immediately after exposure as compared to the slightly later activation of non-canonical pathways, when β-catenin was beginning to decline, suggesting time-dependent modulation of the activation of these divergent pathways (Bonnet et al. 2021).
Similarly, Wnt-7a promoted human corneal epithelial cell proliferation during wound healing via upregulation of metalloproteinase-12 (MMP-12) in a synergistic β-catenin dependent and independent manner through the activation of Rac and PCP signaling (Lyu and Joo 2005).
Our recent work has shown that Wnt-5a stimulates diabetic corneal wound healing via Ca+2 signaling pathway (Shah et al. 2021; Figure 4). Global DNA methylation study from our lab using 850,000-sites Illumina EPIC arrays found hypermethylation of WNT5A gene in human primary diabetic LESC-enriched cultured cells and subsequent suppression of its protein expression in the human limbal epithelium. Addition of exogenous recombinant Wnt-5a as well as its modulation via demethylating agents, zebularine and decitabine, and through nanobioconjugate-based gene therapy targeting miRNA-203a that regulates Wnt-5a, stimulated diabetic wound healing in both limbal epithelial cell cultures enriched in LESC and in human organ-cultured corneas via the phosphorylation of PLC and PKC and the activation of Ca+2 signaling (Shah et al. 2021). In human corneal endothelial cells, Wnt-5a significantly increased cell migration induced by brief stimulation of IL-1β through NF-κB (Lee and Heur 2014). Subsequently, Wnt-5a binding to Frizzled-5 and ROR2 resulted in activation of DVL and later binding between DAAM1 and Cdc42. Activated Cdc42 inhibited RhoA to allow parallel dephosphorylation and activation of slingshot 1, a protein phosphatase. This ultimately led to dephosphorylation and activation of actin-binding protein cofilin, a regulator of actin filament dynamics, to result in enhanced cell migration.
Figure 4.
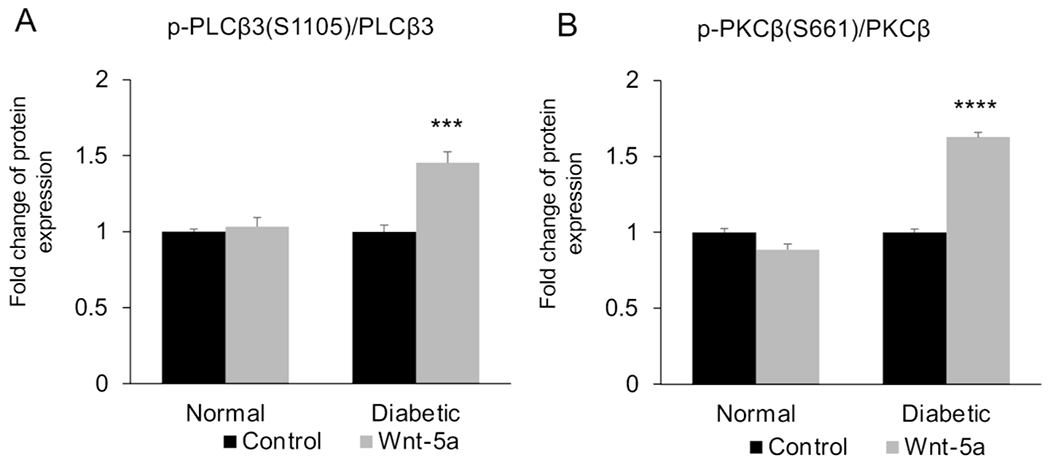
Wnt phospho array analysis of (A) p-PLCβ3 at Ser 1105 and (B) p-PKCβ at Ser 661 in normal and diabetic human limbal epithelial cells with or without Wnt-5a treatment (200 μg/ml). Values are mean ± SEM. ***, p<0.001; ****, p<0.0001 vs. untreated.
From: Shah R, Spektor TM, Punj V, Turjman S, Ghiam S, Kim J, Tolstoff S, Amador; C, Chun ST, Weisenberger DJ, Saghizadeh M, Kramerov AA, Ljubimov AV. Wnt5a promotes diabetic corneal epithelial wound healing and limbal stem cell expression Invest. Ophthalmol. Vis. Sci. 2021;62:847.
Transforming growth factor-α (TGF-a) also plays a role in the corneal wound healing process (Schultz et al. 1992; McClintock and Ceresa 2010). However, when TGF-α is overexpressed in human corneal epithelial cells, it may lead to anterior segment abnormalities and disruption in ocular surface homeostasis (Yuan et al. 2013a). In a study using double-transgenic mice overexpressing TGF-α, peripheral anterior synechiae, a condition in which the iris adheres to the angle was associated with canonical Wnt signaling suppression (Lef1 and Axin2 downregulation) with activation of non-canonical Wnt signaling including Wnt-4 and Wnt-5a upregulation. An increase in the phosphorylation of myosin light chain through RhoA activation suggested the involvement of PCP signaling (Yuan et al. 2013a).
Thus, manipulation and application of non-canonical signaling Wnt ligands at the transcriptional and translational levels may provide an alternate therapeutic approach to the challenges faced in corneal wound healing in certain conditions including diabetes. However, it is important to note that changes in Wnt ligands alone may not contribute to these disease conditions. A single cell RNA sequencing analysis from our laboratory demonstrated an increase in FZD6 gene expression in human diabetic ex vivo corneolimbal cells that was confirmed by quantitative RT-PCR analysis (Figure 5), suggesting the role of Wnt receptors as targets for therapeutic interventions.
Figure 5.
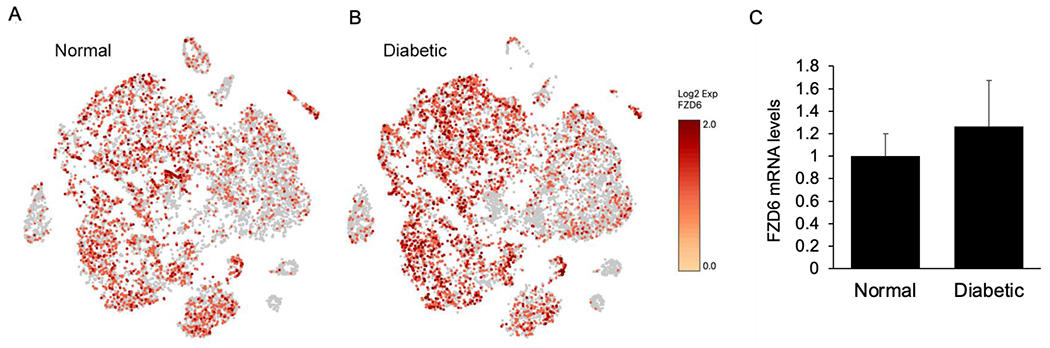
Single cell RNA sequencing analysis of (A) normal and (B) diabetic human limbal epithelial ex vivo cells showing the gene expression of Wnt receptor FZD6 in various cell clusters. (C) Quantitative RT-PCR analysis also shows increased mRNA levels of FZD6 in human diabetic ex vivo limbal tissue.
4.1.2. Aniridia-Associated Keratopathy
Aniridia-associated keratopathy (AAK) is thought to be related with LSCD. This deficiency hinders the ability to maintain the cornea clear and recover from irritation and injury, thus predisposing the cornea after childhood to become thickened, opaque, and excessively vascularized (Ihnatko et al. 2016; Bausili et al. 2016; Auw-Haedrich et al. 2011). This rare disorder is reported to be caused by mutations in PAX6, an essential gene that plays a role in the eye development (Lim et al. 2017).
Davis and Piatigorsky (2011) reported that the overexpression of Pax6 (Pax6-Tg) in mouse cornea altered corneal epithelial cell morphology, neovascularization, and immune function via the transcriptional upregulation of Wnt inhibitory factor 1 (Wif1). This change in expression may have contributed to the observed abnormal phenotype in the Pax6-Tg corneas. At the same time, non-canonical Wnt ligands, Wnt-5a and Wnt-11, were increased, suggesting the role of Pax6 in maintaining normal corneal function through a fine balance between the suppression of canonical and activation of non-canonical Wnt pathways. Ouyang et al. (2014) created an in vitro limbal epithelial cell expansion and three-dimensional corneal differentiation system to investigate the molecular events in maintenance and differentiation of LESC into corneal epithelium. It was found that Wnt-7a facilitates corneal epithelial differentiation through Pax6. In the absence of Wnt-7a or Pax6, the LESC were differentiated into a squamous skin-like epithelium, the process linked to some human corneal diseases such as AAK or Stevens-Johnson syndrome. The attempt to convert skin epithelium stem cells into LESC by PAX6 transduction was unsuccessful in vitro. However, when the transduced skin epithelial stem cells were transplanted onto rabbit eyes with corneal injuries, the cells were able to differentiate into corneal epithelium and aid in healing the damaged corneal surface. These data suggested a critical interaction between Wnt-7a and Pax6 in the maintenance and differentiation of corneal epithelial cell fate that may aid future strategies in treating corneal diseases such as AAK.
4.1.3. Keratoconus
Keratoconus is a progressive non-inflammatory disease presenting with thinning cornea that progressively bulges outward acquiring a cone shape. This may result in irregular astigmatism, blurred vision, glare and sensitivity to light (Asimellis et al. 2021). Keratoconus is the most common cause of corneal transplant in developing countries with a prevalence of 1.38 per 1000 worldwide (Hashemi et al. 2020). This disease appears to be multifactorial due to its influence by environmental and genetic factors as well as biomechanics and sex hormones (Sharif et al. 2018; Asimellis et al. 2021; Karamichos et al. 2022). Although the frequency of eye rubbing (McMonnies 2007), atopy (Sharma et al. 2013), excessive sunlight exposure, industrial toxins (Gordon-Shaag et al. 2015), and contact lens use (Macsai et al. 1990) are important environmental risk factors, there is evidence of the role of Wnt proteins and their signaling pathways in the pathogenesis of keratoconus, highlighting the effect of genetic factors (Gao et al. 2016; Cuellar-Partida et al. 2015; Foster et al. 2021; Khaled et al. 2018; Kabza et al. 2019).
Genome-wide association studies (GWAS) identified single nucleotide polymorphisms (SNPs) in the RNAs of non-canonical Wnts, WNT7B and WNT10A in keratoconus affected humans (Gao et al. 2016; Cuellar-Partida et al. 2015). Variants in WNT7B and WNT10A appear to be associated with an increased risk of developing keratoconus and play an important role in regulating the central corneal thickness (Gao et al. 2016; Cuellar-Partida et al. 2015). It may be hypothesized that the missense mutations in these proteins may lower their receptor affinity and interfere with their downstream signaling. Additionally, transcriptomic and immunohistochemical analysis of keratoconus-affected human corneas found WNT10A to be underexpressed at the mRNA and protein levels compared to healthy human corneas, particularly in the Bowman’s layer (Foster et al. 2021). Wang et al. (2018a) reported that in WNT10A deficient mice, the expression and synthesis of type I collagen (COL1A1) is impaired. Since COL1A1 is a major component of the corneal stromal extracellular matrix, providing the tensile strength, it has been hypothesized that underexpression of WNT10A in keratoconus may result in decreased production of COL1A1 and reduced biomechanical strength of the cornea (Foster et al. 2021). RNA-seq study by Khaled et al. (2018) identified differentially expressed coding and long non-coding RNAs in keratoconus-affected corneas and showed increased expression of Lnc-WNT4-2:1, a sense transcript overlapping with exon 5 of WNT4 gene. Flowever, there was no significant difference in WNT4 mRNA in these corneas. Another study reported DNA hypermethylation in genes encoding Wnt-3 and Wnt-5a proteins in keratoconus corneas (Kabza et al. 2019). Although WNT5A was transcriptionally downregulated, WNT3 was remarkably upregulated. This irregularity was explained by the fact that DNA methylation can affect alternative splicing and gene expression indirectly by different mechanisms (Kabza et al. 2019).
4.2. Conjunctiva
The conjunctiva functions as a protective barrier between the external environment and the ocular surface and contributes to the maintenance of proper lubrication of the eye by secreting mucus and tear film. It harbors conjunctival stem cells located predominantly at the medial canthal and inferior fornix, which give rise to mucin-producing goblet cells and epithelial cells (Shumway et al. 2021; Stewart et al. 2015). Currently, there is very little evidence on the role of Wnt signaling in the conjunctiva. Schrader et al. (2014) suggested that the downregulation of WNT4 and WNT7B gene expression may be necessary for proper maintenance and differentiation in human conjunctival progenitor cells, as determined by qRT-PCR. In patients with dry eye, Wnt-4 and Wnt-5a, along with their cell surface receptors Frizzled-6 and Frizzled-7, were found to be downregulated, which could have an effect on abnormal differentiation of conjunctival epithelium in relation to Wnt/Notch signaling (Mantelli et al. 2009).
4.3. Trabecular Meshwork
The trabecular meshwork of the eye is found at the iridocorneal angle and functions to maintain intraocular pressure, which is imperative to resisting evacuation of aqueous humor from the eye (Llobet et al. 2003). Active canonical and non-canonical Wnt signaling systems were described in human trabecular meshwork cells, where 25 WNT-related genes were detected by qRT-PCR, including genes encoding non-canonical Wnt ligands, WNT5A and WNT5B, genes encoding transduction proteins, DVL1, DVL2, and DVL3, and receptors FZD2 and FZD4 of the β-catenin independent signaling systems (Shyam et al. 2010). Dexamethasone (DEX) may induce ocular hypertension reminiscent of primary angle glaucoma. Treatment of trabecular meshwork cells with DEX was found to upregulate non-canonical Wnt-5a through ROR2/RhoA/ROCK PCP signaling axis, which led to the stress-induced formation of cross-linked actin networks, ultimately compromising the function of the trabecular meshwork (Yuan et al. 2013b). ROR2 receptor knock-down eliminated the effects of dexamethasone on the cells. These results provide a mechanism of DEX action and suggest that applying ROCK inhibitor may successfully treat primary open-angle glaucoma. In agreement with these data, treatment of primary human trabecular meshwork cells with small molecule broad spectrum Wnt signaling inhibitor 3235-0367 suppressed DEX-induced accumulation of collagen and fibronectin extracellular matrix common to steroid-induced glaucoma (Ahadome et al. 2017). As cross-linked actin networks appear to be regulated by non-canonical Wnt signaling, the 3235-0367 inhibitor effects could be also primarily due to the inhibition of non-canonical Wnts. Counteracting Wnt effects may constitute a novel approach to primary open-angle glaucoma treatment.
4.4. Retina
Multiple stages of retinal development are dependent on the canonical Wnt/β-catenin signaling pathway, such as field establishment, retinal and hyaloid vasculogenesis, and stem cell maintenance (Fujimura et al. 2016). However, research supports that vertebrate retinal development and degeneration may require an interplay between or parallel expression of the canonical and non-canonical Wnt/PCP and Wnt/Ca2+ ligands and signaling pathways (Liu et al 2003; Van Raay and Vetter 2004; Hackam 2005).
4.4.1. Maintenance of Blood-Brain Barrier and Blood-Retina Barrier
Binding to and signaling of norrin through Wnt receptor Frizzled-4 activates canonical Wnt/p-catenin signaling to develop and maintain retinal vascularization, blood-brain barrier (BBB) and blood-retina barrier (BRB) plasticity (Drenser 2016, Wang et al. 2012). A loss-of-function experiments with regards to norrin and Wnt-7a/Wnt-7b of the alternative ligand-receptor systems suggest their importance in the development of BBB and BRB in mouse models (Wang et al 2018b). The combined knockout of Wnt7a and Tspan12 (a Norrin-signaling coactivator) or Wnt7a and Fz4 resulted in a defective BBB anatomical structure, as compared to the knockout of each one individually that resulted in little to no severity. The partial impairment of the norrin system suggests that Wnt-7a and Wnt-7b have a minor role in BRB development, as there is a redundancy among the signaling systems (Wang et al. 2018b, Díaz-Coránguez et al. 2020). Another study found that norrin-induced signaling activating β-catenin restored BRB function compromised by exogenous VEGF in bovine retinal epithelial cells and by injected VEGF in rat retinas or upon diabetes induction (Díaz-Coránguez et al. 2020). However, despite norrin binding to a Wnt receptor Frizzled-4, neither a canonical (Wnt-3a) nor a non-canonical (Wnt-5a) Wnt could duplicate beneficial effect of norrin on BRB function in vitro. These data suggest that Wnt receptors may be utilized by other ligands for functional signaling that is independent from both canonical and non-canonical Wnt pathways.
4.4.2. Angiogenesis
Ocular angiogenesis in the retina and choroid is regulated by a fine balance between pro-angiogenic and anti-angiogenic factors, the failure of which may lead to significant visual impairment either by inadequate vascularization or by excessive pathological vascularization. Diabetic retinopathy and AMD are two of the main causes of vision loss related to abnormal angiogenesis (Cabral et al. 2017).
Carvalho et al. (2019) identified the role of non-canonical Wnt-5a signaling in the regulation of murine vascular morphogenesis, a process led by pro-angiogenic factors to cause the endothelial tip cells to trigger the formation of a vascular sprout that migrates into and invades avascular tissues (Figure 6). Wnt-5a activated PCP pathway (Figure 2) through the ROR2/Cdc42 signaling axis that strengthened the coupling between adherens junctions and the actin cytoskeleton, an essential event for stable binding of vinculin to α-catenin, and efficient mechanocoupling between endothelial cells. Additionally, in a conditional knockout of the Wnt secretion factor, Evi, in mouse endothelial cells, Wnt-5a rescued the reduced vascularization and regulated endothelial cell survival, proliferation and subsequent vascular pruning (Korn et al. 2014).
Figure 6.
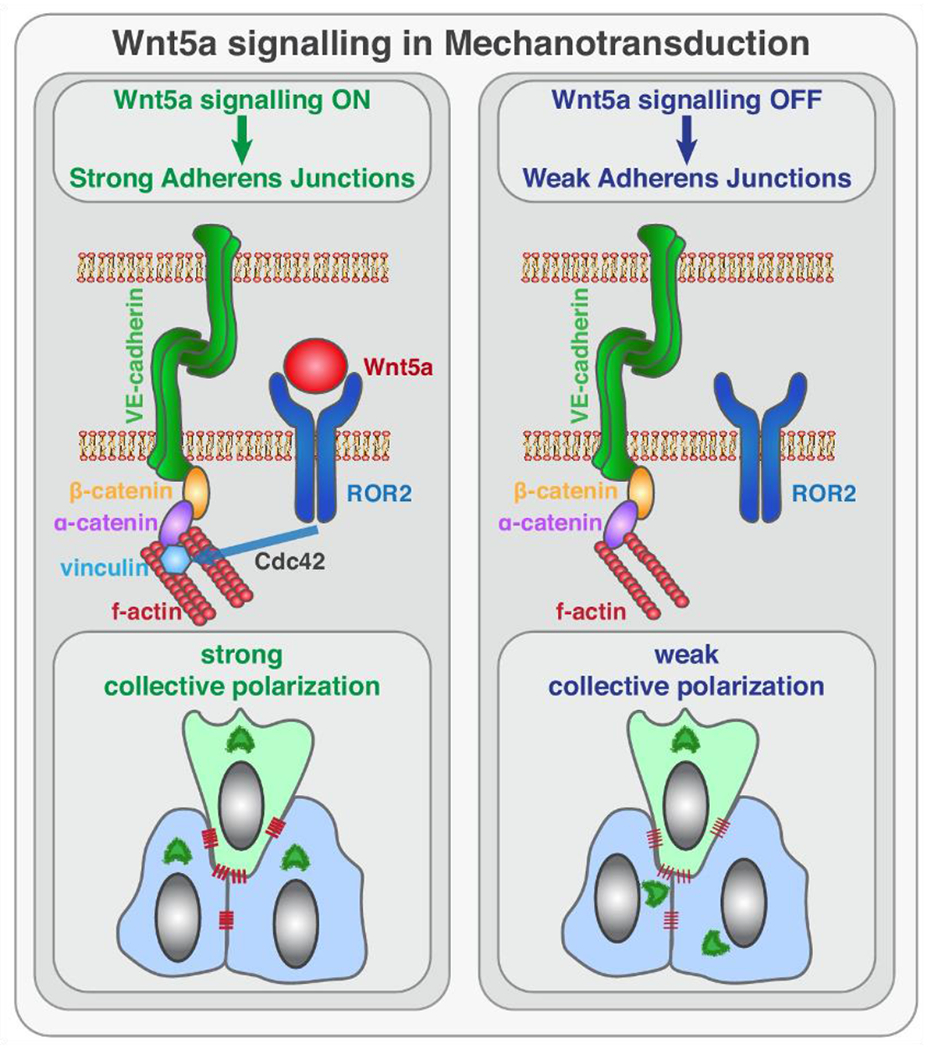
Working model for the role of non-canonical Wnt ligand Wnt-5a in mechanotransduction. Wnt-5a, through ROR2, activates Cdc42 at adherens junctions, which is necessary for stable binding of vinculin to α-catenin, and efficient mechanocoupling between endothelial cells. Low non-canonical Wnt signaling weakens adherens junctions, impairs force propagation, and disrupts collective cell migration of endothelial cells.
From: Carvalho JR, Fortunato IC, Fonseca CG, Pezzarossa A, Barbacena P, Dominguez-Cejudo MA, Vasconcelos FA, Santos NC, Carvalho FA, Franco CA. Non-canonical Wnt signaling regulates junctional mechanocoupling during angiogenic collective cell migration. eLife. 2019;8:e45853. doi: 10.7554/eLife.45853.
In mouse retinal myeloid cells, mutations in Wnt5a and Wnt11 increased angiogenesis through the activation of non-canonical Wnt signaling and can directly control angiogenic branching by producing Flt1, an inhibitory VEGF receptor (Stefater et al. 2011). During vascular remodeling, non-canonical Wnt-5a and Wnt-11 also modulated endothelial shear stress flow sensor. Loss of Wnt-5a or Wnt-11 in mice increased sensitivity of endothelial cells to shear stress and at lower levels polarized against the blood flow direction thus resulting in premature and excessive vessel regression (Franco et al. 2016). Moreover, Lee et al. reported a regulatory role of Wnt-5a and an ECM protein CCN1 in pathological angiogenesis (Lee et al. 2017). CCN1 is secreted mainly by angiogenic endothelial cells and controls the bidirectional flow of information between the cells and the matrix. However, in a mouse model of oxygen-induced retinopathy, pericytes become the major source of CCN1, which induced the expression of Wnt-5a in these cells, but not in endothelial cells. Treatment with Wnt-5a inhibited CCN1 gene expression and also stimulated endothelial cell proliferation and hypersprouting. In contrast, treatment with a Wnt-5a inhibitor (TNP470) restored the expression of CCN1 in endothelial cells and reduced neovascular growth. Thus, a tight balance between CCN1 and Wnt-5a in endothelial cells and pericytes appears to govern pathological angiogenesis during ischemic retinopathy (Lee et al. 2017).
4.4.3. Age-related Macular Degeneration
Age-related macular degeneration (AMD) is a progressive disease affecting both retina and choroid and causing loss of central vision. Dry/early AMD presents with degeneration of retinal pigment epithelium (RPE) with formation of hard exudates (drusen). Wet/late AMD is characterized by leaky choroidal neovascularization traversing the Bruch’s membrane and growing into the retina (Mitchell et al. 2018). Whereas the activation of Wnt/β-catenin signaling is one of the key drivers of the degenerative process during wet AMD (Hu et al. 2013; Zhou et al. 2010), many studies have shown its protective role in dry AMD and inherited retinal degenerations by reducing apoptosis (Kassumeh et al. 2021).
Retinal progenitor cells (RPC) have been widely used in ocular cell therapy as a source for generating new RPE and photoreceptor cells (Lamba et al. 2006; Tucker et al. 2011). Application of small molecule Wnt activator CHIR99021 in combination with basic fibroblast growth factor (FGF-2) stabilized adult mouse neural retinal progenitor cell (mNRPC) renewal in vitro by activating the non-canonical Wnt-5a/Ca2+ pathway (Jin et al. 2018). Both FGF-2 and CHIR99021 were found to enhance cell proliferation individually, but together they significantly enhanced cell cycle growth in G1/S and G2/M transition phases. CHIR99021 contributed to Ca2+ homeostasis, which may be essential to mNRPCs self-renewal and differentiation into mature retinal cells. Moreover, the study showed that the in vitro induction of the self-renewing mNRPC could make them differentiate into rod photoreceptor-like cells and RPE-like cells. At the same time, non-canonical Wnt-5a was shown to attenuate the degeneration of human RPE cells caused by Wnt/β-catenin signaling (Kim et al. 2015). In this study, Wnt-5a inhibited the β-catenin dependent pathway by antagonizing the effects of Wnt-3a, and significantly decreasing VEGF, TNF-α, and NF-κB. Subsequently, Wnt-5a upregulated the expression of E-cadherin and attenuated cell migration by down regulating Snail expression, ultimately blocking epithelial-mesenchymal transition induced by Wnt-3a (Kim et al. 2015).
Overall, the mitigation of canonical Wnt signaling by the activation of antagonistic non-canonical Wnts may be a potential therapeutic approach for the treatment of AMD and retinal degenerations, as well as for the maintenance of retinal progenitor cells suitable for transplantation in these conditions.
4.5. Optic Nerve
The optic nerve is an extension of the central nervous system that directly relays sensory information as electrical impulses from the eyes to the brain (Smith and Czyz 2021). Damage to the optic nerve in zebrafish caused an upregulation of non-canonical wnt5a mRNA (Matsukawa et al 2018). As a result, small G-proteins Cdc42 and Rac1, involved in cytoskeletal arrangement and cell proliferation and differentiation (Mott et al. 1999), were activated and Wnt/β-catenin signaling was downregulated. In an adult optic nerve crush injury mouse model, Wnt-5a promoted axonal growth and protected RGCs in the retina. Treatment with exogenous Wnt-5a induced pro-survival and regenerative pathways by stimulating both forms of non-canonical signaling, JNK/STAT3 (PCP pathway) and CamKII/CREB (Ca2+ pathway) in murine RGC, resulting in significantly increased RGC survival, RGC neurite growth, and optic nerve regeneration (Musada et al. 2022; Figure 7). These studies highlight the importance of Wnt-5a in the regeneration of optic nerve after damage. As RGC cell death is a major cause of glaucoma, the activation of non-canonical Wnts to counteract this process might be a useful therapeutic approach.
Figure 7.
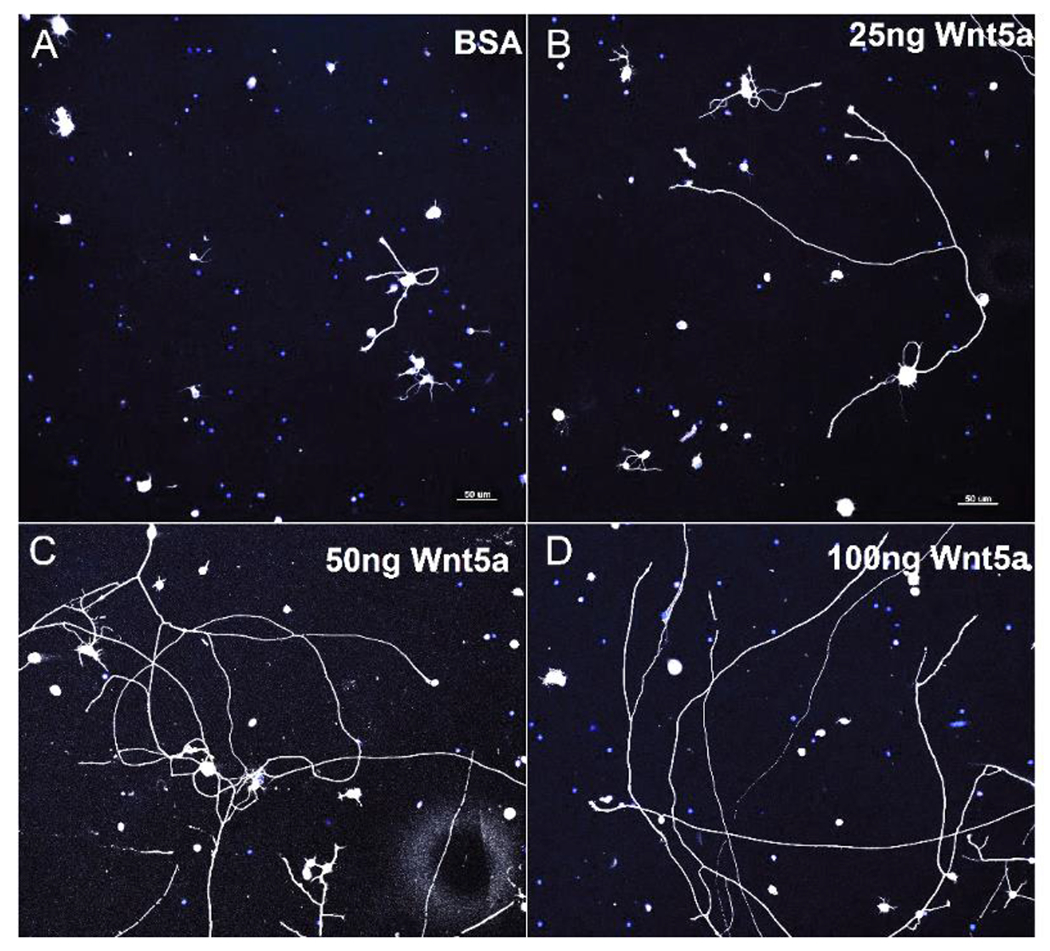
Wnt-5a treatment induced neurite growth in RGC primary cultures. Representative images of RGC cultures treated with BSA (control), 25 ng, 50 ng and 100 ng recombinant Wnt-5a. Neurites are shown in white, DAPI-stained nuclei are blue. Scale bar: 50 μm.
From: Musada GR, Carmy-Bennun T, Hackam AS. Identification of a novel axon regeneration role for non-canonical Wnt signaling in the adult retina after injury. eNeuro. 2022;9:ENEURO.0182-22.2022. doi: 10.1523/ENEURO.0182-22.2022.
5. Conclusions and Future Directions
The complex Wnt signaling system is a key regulator of processes involved in the development of the eye as well as in various ocular diseases. Major efforts have been made to describe and understand the canonical β-catenin dependent Wnt signaling. At the same time, non-canonical pathways in the eye are severely understudied. In recent years, the importance of non-canonical Wnts as usual antagonists of canonical pathways has been recognized for ocular stem cell maintenance and for potential use in diseases such as different keratopathies, primary open angle glaucoma and AMD. In non-ocular systems, canonical and non-canonical Wnt pathways can be co-activated, but the regulation of their interplay in the eye tissues is poorly understood. Therefore, future studies should be focused on addressing this limitation by pinpointing regulators of crosstalk between canonical and non-canonical signaling pathways, as well as the crosstalk between non-canonical signaling and other cellular signaling pathways, such as NF-κB, Akt, and Notch pathways among others. There is also a need for identifying the role of various non-canonical Wnt receptors in the development of disease. A single cell RNA sequencing approach may prove to be beneficial in elucidating cellular expression and function of various Wnt ligands and their receptors not only in maintaining homeostasis but also in the development of disease.
Future studies should also address translational aspects of non-canonical signaling in common diseases for therapeutic purposes. Currently, several potentially useful approaches have been developed to regulate non-canonical Wnt expression using different drugs. It is anticipated that specific combinations of such drugs could provide maximum beneficial effect to the patients affected by e.g., diabetes. The role of non-canonical Wnts in maintenance and differentiation of ocular stem cells should also be studied in more detail given an increasing use of such stem cells for transplantation in corneal and retinal diseases.
Acknowledgments
Supported by: NIH grants R01 EY013431, EY031377 (AVL), and EY025377 (MS), and grants from the Board of Governors Regenerative Medicine Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none.
Bibliography
- Ahadome SD, Zhang C, Tannous E, Shen J, Zheng JJ. Small-molecule inhibition of Wnt signaling abrogates dexamethasone-induced phenotype of primary human trabecular meshwork cells. Exp Cell Res. 2017;357:116–123. doi: 10.1016/j.yexcr.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- Ang SJ, Stump RJ, Lovicu FJ, McAvoy JW. Spatial and temporal expression of Wnt and Dickkopf genes during murine lens development. Gene Expr Patterns. 2004;4:289–295. doi: 10.1016/j.modgep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Asimellis G, Kaufman EJ. Keratoconus. [Updated 2022 May 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470435/ [Google Scholar]
- Auw-Haedrich C, Agrawal M, Gabbert HE, Meyer P, Arnold N, Reinhard T. Immunohistochemical expression of epithelial cell markers in corneas with congenital aniridia and ocular cicatrizing pemphigoid. Acta Ophthalmol. 2011;89:47–53. doi: 10.1111/j.1755-3768.2009.01603.x. [DOI] [PubMed] [Google Scholar]
- Azbazdar Y, Karabicici M, Erdal E, Ozhan G. Regulation of Wnt signaling pathways at the plasma membrane and their misregulation in cancer. Front Cell Dev Biol. 2021;9:631623. doi: 10.3389/fcell.2021.631623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NE. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J. 1987;6:1765–1773. doi: 10.1002/j.1460-2075.1987.tb02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Tan X, Zhang H, Liu C, Zhao B, Li Y, Lu L, Liu Y, Zhou J. Ror2 receptor mediates Wnt11 ligand signaling and affects convergence and extension movements in zebrafish. J Biol Chem. 2014;289:20664–76. doi: 10.1074/jbc.M114.586099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bänziger C, Soldini D, Schütt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Bausili MM, Alvarez de Toledo J, Barraquer RI, Michael R, Tresserra F, de la Paz MF. Histopathology findings of corneal buttons in congenital aniridia patients. Ophthalmic Res. 2016;56:202–206. doi: 10.1159/000444930. [DOI] [PubMed] [Google Scholar]
- Bi L, Lwigale P. Transcriptomic analysis of differential gene expression during chick periocular neural crest differentiation into corneal cells. Dev Dyn. 2019;248:583–602. doi: 10.1002/dvdy.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet C, Oh D, Mei H, Robertson S, Chang D, Bourges JL, Behar-Cohen F, Zheng JJ, Deng SX. Wnt6 plays a complex role in maintaining human limbal stem/progenitor cells. Sci Rep. 2021;11:20948. doi: 10.1038/s41598-021-00273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo DT, Yang YL, Kuchenbecker K, Hung MS, Xu Z, Jablons DM, You L. Frizzled-8 receptor is activated by the Wnt-2 ligand in non-small cell lung cancer. BMC Cancer. 2013;13:316. doi: 10.1186/1471-2407-13-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral T, Mello LGM, Lima LH, Polido J, Regatieri CV, Belfort R Jr, Mahajan VB. Retinal and choroidal angiogenesis: a review of new targets. Int J Retina Vitreous. 2017;3:31. doi: 10.1186/s40942-017-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho JR, Fortunato IC, Fonseca CG, Pezzarossa A, Barbacena P, Dominguez-Cejudo MA, Vasconcelos FF, Santos NC, Carvalho FA, Franco CA. Non-canonical Wnt signaling regulates junctional mechanocoupling during angiogenic collective cell migration. eLife. 2019;8:e45853. doi: 10.7554/eLife.45853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavodeassi F, Carreira-Barbosa F, Young RM, Concha ML, Allende ML, Houart C, Tada M, Wilson SW. Early stages of zebrafish eye formation require the coordinated activity of Wnt11, Fz5, and the Wnt/β-catenin pathway. Neuron. 2005;47:43–56. doi: 10.1016/j.neuron.2005.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che C, Li C, Lin J, Zhang J, Jiang N, Yuan K, Zhao G. Wnt5a contributes to dectin-1 and LOX-1 induced host inflammatory response signature in Aspergillus fumigatus keratitis. Cell Signal. 2018;52:103–111. doi: 10.1016/j.cellsig.2018.08.020. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen Z, Tang Y, Xiao Q. The involvement of noncanonical Wnt signaling in cancers. Biomed Pharmacother. 2021; 133:110946. doi: 10.1016/j.biopha.2020.110946. [DOI] [PubMed] [Google Scholar]
- Chen Y, Stump RJ, Lovicu FJ, McAvoy JW. A role for Wnt/planar cell polarity signaling during lens fiber cell differentiation? Semin Cell Dev Biol. 2006;17:712–725. doi: 10.1016/j.semcdb.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Stump RJ, Lovicu FJ, Shimono A, McAvoy JW. Wnt signaling is required for organization of the lens fiber cell cytoskeleton and development of lens three-dimensional architecture. Dev Biol. 2008;324:161–176. doi: 10.1016/j.ydbio.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidiac R, Abedin M, Macleod G, Yang A, Thibeault PE, Blazer LL, Adams JJ, Zhang L, Roehrich H, Jo HN, Seshagiri S, Sidhu SS, Junge HJ, Angers S. A Norrin/Wnt surrogate antibody stimulates endothelial cell barrier function and rescues retinopathy. EMBO Mol Med. 2021. ;13:e13977. doi: 10.15252/emmm.202113977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Cruciat CM, Niehrs C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb Perspect Biol. 2013;5:a015081. doi: 10.1101/cshperspect.a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuellar-Partida G, Springelkamp H, Lucas SE, Yazar S, Hewitt AW, Iglesias Al, Montgomery GW, Martin NG, Pennell CE, van Leeuwen EM, Verhoeven VJ, Hofman A, Uitterlinden AG, Ramdas WD, Wolfs RC, Vingerling JR, Brown MA, Mills RA, Craig JE, Klaver CC, van Duijn CM, Burdon KP, MacGregor S, Mackey DA. WNT10A exonic variant increases the risk of keratoconus by decreasing corneal thickness. Hum Mol Genet. 2015;24:5060–5068. doi: 10.1093/hmg/ddv211. [DOI] [PubMed] [Google Scholar]
- Davis J, Piatigorsky J. Overexpression of Pax6 in mouse cornea directly alters corneal epithelial cells: changes in immune function, vascularization, and differentiation. Invest Ophthalmol Vis Sci. 2011;52:4158–4168. doi: 10.1167/iovs.10-6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes LJ, Sugiyama Y, Lovicu FJ, Harris CG, Shelley EJ, McAvoy JW. Interactions between lens epithelial and fiber cells reveal an intrinsic self-assembly mechanism. Dev Biol. 2014;385:291–303. doi: 10.1016/j.ydbio.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes LJ, Sugiyama Y, Tanedo AS, Lovicu FJ, McAvoy JW. Wnt-frizzled signaling is part of an FGF-induced cascade that promotes lens fiber differentiation. Invest Ophthalmol Vis Sci. 2013;54:1582–1590. doi: 10.1167/iovs.12-11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson K, Aflaki M, Nattel S. Role of the Wnt-Frizzled system in cardiac pathophysiology: a rapidly developing, poorly understood area with enormous potential. J Physiol. 2013;591:1409–1432. doi: 10.1113/jphysiol.2012.235382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejmek J, Säfholm A, Kamp Nielsen C, Andersson T, Leandersson K. Wnt-5a/Ca2+-induced NFAT activity is counteracted by Wnt-5a/Yes-Cdc42-casein kinase 1α signaling in human mammary epithelial cells. Mol Cell Biol. 2006;26:6024–6036. doi: 10.1128/MCB.02354-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Coránguez M, Lin CM, Liebner S, Antonetti DA. Norrin restores blood-retinal barrier properties after vascular endothelial growth factor-induced permeability. J Biol Chem. 2020;295:4647–4660. doi: 10.1074/jbc.RA119.011273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijksterhuis JP, Petersen J, Schulte G. WNT/Frizzled signalling: receptor-ligand selectivity with focus on FZD-G protein signalling and its physiological relevance: IUPHAR Review 3. Br J Pharmacol. 2014;171:1195–1209. doi: 10.1111/bph.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenser KA. Wnt signaling pathway in retinal vascularization. Eye Brain. 2016;8:141–146. doi: 10.2147/EB.S94452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchartre Y, Kim YM, Kahn M. The Wnt signaling pathway in cancer. Crit Rev Oncol Hematol. 2016;99:141–149. doi: 10.1016/j.critrevonc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Nishita M, Fujii M, Minami Y. Insight into the role of Wnt5a-induced signaling in normal and cancer cells. Int Rev Cell Mol Biol. 2015;314:117–148. doi: 10.1016/bs.ircmb.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Esteve P, Lopez-Rios J, Bovolenta P. SFRP1 is required for the proper establishment of the eye field in the medaka fish. Mech Dev. 2004;121:687–701. doi: 10.1016/j.mod.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Fanto M, Weber U, Strutt DI, Mlodzik M. Nuclear signaling by Rac and Rho GTPases is required in the establishment of epithelial planar polarity in the Drosophila eye. Curr Biol. 2000;10:979–988. doi: 10.1016/s0960-9822(00)00645-x. [DOI] [PubMed] [Google Scholar]
- Ferrari ME, Bernis ME, McLeod F, Podpolny M, Coullery RP, Casadei IM, Salinas PC, Rosso SB. Wnt7b signalling through Frizzled-7 receptor promotes dendrite development by coactivating CaMKII and JNK. J Cell Sci. 2018;131:jcs216101. doi: 10.1242/jcs.216101. [DOI] [PubMed] [Google Scholar]
- Figueira EC, Di Girolamo N, Coroneo MT, Wakefield D. The phenotype of limbal epithelial stem cells. Invest Ophthalmol Vis Sci. 2007;48:144–156. doi: 10.1167/iovs.06-0346. [DOI] [PubMed] [Google Scholar]
- Foster JW, Parikh RN, Wang J, Bower KS, Matthaei M, Chakravarti S, Jun AS, Eberhart CG, Soiberman US. transcriptomic and immunohistochemical analysis of progressive keratoconus reveal altered WNT10A in epithelium and Bowman’s layer. Invest Ophthalmol Vis Sci. 2021;62:16. doi: 10.1167/iovs.62.6.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulquier S, Daskalopoulos EP, Lluri G, Hermans KCM, Deb A, Blankesteijn WM. WNT signaling in cardiac and vascular disease. Pharmacol Rev. 2018;70:68–141. doi: 10.1124/pr.117.013896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco CA, Jones ML, Bernabeu MO, Vion AC, Barbacena P, Fan J, Mathivet T, Fonseca CG, Ragab A, Yamaguchi TP, Coveney PV, Lang RA, Gerhardt H. Non-canonical Wnt signalling modulates the endothelial shear stress flow sensor in vascular remodelling. Elife. 2016;5:e07727. doi: 10.7554/eLife.07727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann S Wnt signaling in eye organogenesis. Organogenesis. 2008;4:60–67. doi: 10.4161/org.4.2.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura N WNT/β-catenin signaling in vertebrate eye development. Front Cell Dev Biol. 2016;4:138. doi: 10.3389/fcell.2016.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli LM, Barnes TL, Secrest SS, Kadowaki T, Burrus LW. Porcupine-mediated lipid-modification regulates the activity and distribution of Wnt proteins in the chick neural tube. Development. 2007;134:3339–3348. doi: 10.1242/dev.02881. [DOI] [PubMed] [Google Scholar]
- Gao X, Nannini DR, Corrao K, Torres M, Chen YI, Fan BJ, Wiggs JL; International Glaucoma Genetics Consortium, Taylor KD, Gauderman WJ, Rotter Jl, Varma R. Genome-wide association study identifies WNT7B as a novel locus for central corneal thickness in Latinos. Hum Mol Genet. 2016;25:5035–5045. doi: 10.1093/hmg/ddw319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay A, Towler DA. Wnt signaling in cardiovascular disease: opportunities and challenges. Curr Opin Lipidol. 2017;28:387–396. doi: 10.1097/MOL.0000000000000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit A, Yaniv A, Bafico A, Pramila T, Igarashi M, Kitajewski J, Aaronson SA. Human frizzled 1 interacts with transforming Wnts to transduce a TCF dependent transcriptional response. Oncogene. 1999;18:5959–5966. doi: 10.1038/sj.onc.1202985. [DOI] [PubMed] [Google Scholar]
- Gordon-Shaag A, Millodot M, Shneor E, Liu Y. The genetic and environmental factors for keratoconus. Biomed Res Int. 2015;2015:795738. doi: 10.1155/2015/795738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graw J Eye development. Curr Top Dev Biol. 2010;90:343–386. doi: 10.1016/S0070-2153(10)90010-0. [DOI] [PubMed] [Google Scholar]
- Greenlees R, Mihelec M, Yousoof S, Speidel D, Wu SK, Rinkwitz S, Prokudin I, Perveen R, Cheng A, Ma A, Nash B, Gillespie R, Loebel DA, Clayton-Smith J, Lloyd IC, Grigg JR, Tam PP, Yap AS, Becker TS, Black GC, Semina E, Jamieson RV. Mutations in SIPA1L3 cause eye defects through disruption of cell polarity and cytoskeleton organization. Hum Mol Genet. 2015;24:5789–5804. doi: 10.1093/hmg/ddv298. [DOI] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Hackam AS. The Wnt signaling pathway in retinal degenerations. IUBMB Life. 2005;57:381–388. doi: 10.1080/15216540500137586. [DOI] [PubMed] [Google Scholar]
- Han C, Li J, Wang C, Ouyang H, Ding X, Liu Y, Chen S, Luo L. Wnt5a contributes to the differentiation of human embryonic stem cells into lentoid bodies through the noncanonical Wnt/JNK signaling pathway. Invest Ophthalmol Vis Sci. 2018;59:3449–3460. doi: 10.1167/iovs.18-23902. [DOI] [PubMed] [Google Scholar]
- Hashemi H, Heydarian S, Hooshmand E, Saatchi M, Yekta A, Aghamirsalim M, Valadkhan M, Mortazavi M, Hashemi A, Khabazkhoob M. The prevalence and risk factors for keratoconus: a systematic review and meta-analysis. Cornea. 2020;39:263–270. doi: 10.1097/ICO.0000000000002150. [DOI] [PubMed] [Google Scholar]
- He QJ, Wang P, Liu QQ, Wu QG, Li YF, Wang J, Lee SC. Secreted Wnt6 mediates diabetes-associated centrosome amplification via its receptor FZD4. Am J Physiol Cell Physiol. 2020;318:C48–C62. doi: 10.1152/ajpcell.00091.2019. [DOI] [PubMed] [Google Scholar]
- Hu Y, Chen Y, Lin M, Lee K, Mott RA, Ma JX. Pathogenic role of the Wnt signaling pathway activation in laser-induced choroidal neovascularization. Invest Ophthalmol Vis Sci. 2013;54:141–154. doi: 10.1167/iovs.12-10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human genetic diseases and Wnt signaling components 2022. https://web.stanford.edu/group/nusselab/cgi-bin/wnt/human_genetic_diseases, last accessed October 28, 2022.
- Ihnatko R, Eden U, Fagerholm P, Lagali N. Congenital aniridia and the ocular surface. Ocul Surf. 2016;14:196–206. doi: 10.1016/j.jtos.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Jasoni C, Hendrickson A, Roelink H. Analysis of chicken Wnt-13 expression demonstrates coincidence with cell division in the developing eye and is consistent with a role in induction. Dev Dyn. 1999;215:215–224. doi: . [DOI] [PubMed] [Google Scholar]
- Jin C, Ou Q, Li Z, Wang J, Zhang J, Tian H, Xu JY, Gao F, Lu L, Xu GT. The combination of bFGF and CHIR99021 maintains stable self-renewal of mouse adult retinal progenitor cells. Stem Cell Res Ther. 2018;9:346. doi: 10.1186/s13287-018-1091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabza M, Karolak JA, Rydzanicz M, Udziela M, Gasperowicz P, Ploski R, Szaflik JP, Gajecka M. Multiple Differentially Methylated Regions Specific to Keratoconus Explain Known Keratoconus Linkage Loci. Invest Ophthalmol Vis Sci. 2019;60:1501–1509. doi: 10.1167/iovs.18-25916. [DOI] [PubMed] [Google Scholar]
- Karamichos D, Escandon P, Vasini B, Nicholas SE, Van L, Dang DH, Cunningham RL, Riaz KM. Anterior pituitary, sex hormones, and keratoconus: Beyond traditional targets. Prog Retin Eye Res. 2022;88:101016. doi: 10.1016/j.preteyeres.2021.101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa T, Yokokura H, Kitajewski J, Lombroso PJ. Frizzled-9 is activated by Wnt-2 and functions in Wnt/β-catenin signaling. J Biol Chem. 2002;277:37479–37486. doi: 10.1074/jbc.M205658200. [DOI] [PubMed] [Google Scholar]
- Karuna EP, Susman MW, Ho HH. Quantitative live-cell reporter assay for noncanonical Wnt activity. Bio Protoc. 2018;8:e2762. doi: 10.21769/BioProtoc.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassumeh S, Weber GR, Nobl M, Priglinger SG, Ohlmann A. The neuroprotective role of Wnt signaling in the retina. Neural Regen Res. 2021;16:1524–1528. doi: 10.4103/1673-5374.303010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M, Katoh M. Transcriptional regulation of WNT2B based on the balance of Hedgehog, Notch, BMP and WNT signals. Int J Oncol. 2009;34:1411–1415. [PubMed] [Google Scholar]
- Katoh M Canonical and non-canonical WNT signaling in cancer stem cells and their niches: Cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review). Int J Oncol. 2017;51:1357–1369. doi: 10.3892/ijo.2017.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaled ML, Bykhovskaya Y, Yablonski SER, Li H, Drewry MD, Aboobakar IF, Estes A, Gao XR, Stamer WD, Xu H, Allingham RR, Hauser MA, Rabinowitz YS, Liu Y. Differential expression of coding and long noncoding RNAs in keratoconus-affected corneas. Invest Ophthalmol Vis Sci. 2018;59:2717–2728. doi: 10.1167/iovs.18-24267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto H, Sato A, Matsumoto S. Wnt5a: its signalling, functions and implication in diseases. Acta Physiol (Oxf). 2012;204:17–33. doi: 10.1111/j.1748-1716.2011.02294.x. [DOI] [PubMed] [Google Scholar]
- Kim GH, Her JH, Han JK. Ryk cooperates with Frizzled 7 to promote Wnt11-mediated endocytosis and is essential for Xenopus laevis convergent extension movements. J Cell Biol. 2008;182:1073–1082. doi: 10.1083/jcb.200710188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Park S, Chung H, Oh S. Wnt5a attenuates the pathogenic effects of the Wnt/β-catenin pathway in human retinal pigment epithelial cells via down-regulating β-catenin and Snail. BMB Rep. 2015;48:525–530. doi: 10.5483/bmbrep.2015.48.9.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn AD, Moon RT. Wnt and calcium signaling: β-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn C, Scholz B, Hu J, Srivastava K, Wojtarowicz J, Arnsperger T, Adams RH, Boutros M, Augustin HG, Augustin I. Endothelial cell-derived non-canonical Wnt ligands control vascular pruning in angiogenesis. Development. 2014;141:1757–1766. doi: 10.1242/dev.104422. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Bushoven P, Iwasaki M, Urbich C, Zeiher AM, Dimmeler S. Notch signaling contributes to the expression of cardiac markers in human circulating progenitor cells. Circ Res. 2007;101:1139–1145. doi: 10.1161/CIRCRESAHA.107.151381. [DOI] [PubMed] [Google Scholar]
- Kozielewicz P, Shekhani R, Moser S, Bowin CF, Wesslowski J, Davidson G, Schulte G. Quantitative profiling of WNT-3A binding to all human Frizzled paralogues in HEK293 cells by NanoBiT/BRET assessments. ACS Pharmacol Transl Sci. 2021;4:1235–1245. doi: 10.1021/acsptsci.1c00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo F, Takeichi M, Nakagawa S. Wnt2b controls retinal cell differentiation at the ciliary marginal zone. Development. 2003;130:587–598. doi: 10.1242/dev.00244. [DOI] [PubMed] [Google Scholar]
- Kühl M, Sheldahl LC, Malbon CC, Moon RT. Ca2+/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J Biol Chem. 2000;275:12701–12711. doi: 10.1074/jbc.275.17.12701. [DOI] [PubMed] [Google Scholar]
- Kumawat K, Gosens R. WNT-5A: signaling and functions in health and disease. Cell Mol LifeSci. 2016;73:567–587. doi: 10.1007/s00018-015-2076-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:12769–12774. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecarpentier Y, Schussler O, Hébert JL, Vallée A. Multiple Targets of the Canonical WNT/β-Catenin Signaling in Cancers. Front Oncol. 2019. Nov 18;9:1248. doi: 10.3389/fonc.2019.01248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Bong YS, Moore KB, Soria K, Moody SA, Daar IO. Dishevelled mediates ephrinB1 signalling in the eye field through the planar cell polarity pathway. Nat Cell Biol. 2006;8:55–63. doi: 10.1038/ncb1344. [DOI] [PubMed] [Google Scholar]
- Lee JG, Heur M. Interleukin-1β-induced Wnt5a enhances human corneal endothelial cell migration through regulation of Cdc42 and RhoA. Mol Cell Biol. 2014;34:3535–3545. doi: 10.1128/MCB.01572-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Elaskandrany M, Lau LF, Lazzaro D, Grant MB, Chaqour B. Interplay between CCN1 and Wnt5a in endothelial cells and pericytes determines the angiogenic outcome in a model of ischemic retinopathy. Sci Rep. 2017;7:1405. doi: 10.1038/s41598-017-01585-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HT, Kim DH, Kim H. PAX6 aniridia syndrome: clinics, genetics, and therapeutics. Curr Opin Ophthalmol. 2017;28:436–447. doi: 10.1097/ICU.0000000000000405. [DOI] [PubMed] [Google Scholar]
- Liu H, Mohamed O, Dufort D, Wallace VA. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn. 2003;227:323–334. doi: 10.1002/dvdy.10315. [DOI] [PubMed] [Google Scholar]
- Llobet A, Gasull X, Gual A. Understanding trabecular meshwork physiology: a key to the control of intraocular pressure? News Physiol Sci. 2003;18:205–209. doi: 10.1152/nips.01443.2003. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Lyu J, Joo CK. Expression of Wnt and MMP in epithelial cells during corneal wound healing. Cornea. 2006;25:S24–S28. doi: 10.1097/01.ico.0000247209.01262.4e. [DOI] [PubMed] [Google Scholar]
- Lyu J, Joo CK. Wnt signaling enhances FGF2-triggered lens fiber cell differentiation. Development. 2004;131:1813–1824. doi: 10.1242/dev.01060. [DOI] [PubMed] [Google Scholar]
- Lyu J, Joo CK. Wnt-7a up-regulates matrix metalloproteinase-12 expression and promotes cell proliferation in corneal epithelial cells during wound healing. J Biol Chem. 2005;280:21653–21660. doi: 10.1074/jbc.M500374200. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, He X. Frizzled and LRP5/6 receptors for Wnt/β-catenin signaling. Cold Spring Harb Perspect Biol. 2012;4:a007880. doi: 10.1101/cshperspect.a007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macsai MS, Varley GA, Krachmer JH. Development of keratoconus after contact lens wear. Patient characteristics. Arch Ophthalmol. 1990;108:534–538. doi: 10.1001/archopht.1990.01070060082054. [DOI] [PubMed] [Google Scholar]
- Madaan A, Chaudhari P, Nadeau-Vallée M, Hamel D, Zhu T, Mitchell G, Samuels M, Pundir S, Dabouz R, Howe Cheng CW, Mohammad Nezhady MA, Joyal JS, Rivera JC, Chemtob S. Müller cell-localized G-protein-coupled receptor 81 (hydroxycarboxylic acid receptor 1) regulates inner retinal vasculature via Norrin/Wnt pathways. Am J Pathol. 2019;189:1878–1896. doi: 10.1016/j.ajpath.2019.05.016. [DOI] [PubMed] [Google Scholar]
- Malbon CC, Wang HY. Dishevelled: a mobile scaffold catalyzing development. Curr Top Dev Biol. 2006;72:153–66. doi: 10.1016/S0070-2153(05)72002-0. [DOI] [PubMed] [Google Scholar]
- Mantelli F, Schaffer L, Dana R, Head SR, Argüeso P. Glycogene expression in conjunctiva of patients with dry eye: downregulation of Notch signaling. Invest Ophthalmol Vis Sci. 2009;50:2666–2672. doi: 10.1167/iovs.08-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Wang J, Liu B, Pan W, Farr GH 3rd, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7:801–809. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Martínez-Bartolomé M, Range RC. A biphasic role of non-canonical Wnt16 signaling during early anterior-posterior patterning and morphogenesis of the sea urchin embryo. Development. 2019;146:dev168799. doi: 10.1242/dev.168799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez S, Scerbo P, Giordano M, Daulat AM, Lhoumeau AC, Thomé V, Kodjabachian L, Borg JP. The PTK7 and ROR2 protein receptors interact in the vertebrate WNT/Planar Cell Polarity (PCP) Pathway. J Biol Chem. 2015;290:30562–30572. doi: 10.1074/jbc.M115.697615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Orozco E, Sanchez-Fernandez A, Ortiz-Parra I, Ayala-San Nicolas M. WNT signaling in tumors: the way to evade drugs and immunity. Front Immunol. 2019;10:2854. doi: 10.3389/fimmu.2019.02854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa T, Morita K, Omizu S, Kato S, Koriyama Y. Mechanisms of RhoA inactivation and CDC42 and Rac1 activation during zebrafish optic nerve regeneration. Neurochem Int. 2018;112:71–80. doi: 10.1016/j.neuint.2017.11.004. [DOI] [PubMed] [Google Scholar]
- Maurus D, Héligon C, Bürger-Schwärzler A, Brändli AW, Kühl M. Noncanonical Wnt-4 signaling and EAF2 are required for eye development in Xenopus laevis. EMBO J. 2005;24:1181–1191. doi: 10.1038/sj.emboj.7600603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy JW, Chamberlain CG, de longh RU, Hales AM, Lovicu FJ. Lens development. Eye (Lond). 1999;13:425–437. doi: 10.1038/eye.1999.117. [DOI] [PubMed] [Google Scholar]
- McClintock JL, Ceresa BP. Transforming growth factor-α enhances corneal epithelial cell migration by promoting EGFR recycling. Invest Ophthalmol Vis Sci. 2010;51:3455–3461. doi: 10.1167/iovs.09-4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan SL, Edelhauser HF, Pfister RR, Whikehart DR. Stem cell markers in the human posterior limbus and corneal endothelium of unwounded and wounded corneas. Mol Vis. 2007;13:1984–2000. [PubMed] [Google Scholar]
- McMonnies CW. Abnormal rubbing and keratectasia. Eye Contact Lens. 2007;33:265–271. [DOI] [PubMed] [Google Scholar]
- Mei H, Nakatsu MN, Baclagon ER, Deng SX. Frizzled 7 maintains the undifferentiated state of human limbal stem/progenitor cells. Stem Cells. 2014;32:938–945. doi: 10.1002/stem.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JR. The Wnts. Genome Biol. 2001;3:reviews3001.1. doi: 10.1186/gb-2001-3-1-reviews3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet. 2018;392:1147–1159. doi: 10.1016/S0140-6736(18)31550-2. [DOI] [PubMed] [Google Scholar]
- Morenilla-Palao C, López-Cascales MT, López-Atalaya JP, Baeza D, Calvo-Diíaz L, Barco A, Herrera E. A Zic2-regulated switch in a noncanonical Wnt/βcatenin pathway is essential for the formation of bilateral circuits. Sci Adv. 2020;6:eaaz8797. doi: 10.1126/sciadv.aaz8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott HR, Owen D, Nietlispach D, Lowe PN, Manser E, Lim L, Laue ED. Structure of the small G protein Cdc42 bound to the GTPase-binding domain of ACK. Nature. 1999;399:384–388. doi: 10.1038/20732. [DOI] [PubMed] [Google Scholar]
- Murillo-Garzón V, Gorroño-Etxebarria I, Åkerfelt M, Puustinen MC, Sistonen L, Nees M, Carton J, Waxman J, Kypta RM. Frizzled-8 integrates Wnt-11 and transforming growth factor-β signaling in prostate cancer. Nat Commun. 2018;9:1747. doi: 10.1038/s41467-018-04042-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musada GR, Carmy-Bennun T, Hackam AS. Identification of a novel axon regeneration role for non-canonical Wnt signaling in the adult retina after injury. eNeuro. 2022;9:ENEURO.0182-22.2022. doi: 10.1523/ENEUR0.0182-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musada GR, Dvoriantchikova G, Myer C, Ivanov D, Bhattacharya SK, Hackam AS. The effect of extrinsic Wnt/β-catenin signaling in Muller glia on retinal ganglion cell neurite growth. Dev Neurobiol. 2020;80:98–110. doi: 10.1002/dneu.22741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Takada S, Takada R, Takeichi M. Identification of the laminar-inducing factor: Wnt-signal from the anterior rim induces correct laminar formation of the neural retina in vitro. Dev Biol. 2003;260:414–425. doi: 10.1016/s0012-1606(03)00320-8. [DOI] [PubMed] [Google Scholar]
- Nakatsu MN, Ding Z, Ng MY, Truong TT, Yu F, Deng SX. Wnt/β-catenin signaling regulates proliferation of human cornea epithelial stem/progenitor cells. Invest Ophthalmol Vis Sci. 2011;52:4734–4741. doi: 10.1167/iovs.10-6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus J, Weimann A, Berndt-Paetz M. Immunocytochemical analysis of endogenous Frizzled-(Co-)receptor interactions and rapid Wnt pathway activation in mammalian cells. Int J Mol Sci. 2021;22:12057. doi: 10.3390/ijms222112057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- Nusse R, Brown A, Papkoff J, Scambler P, Shackleford G, McMahon A, Moon R, Varmus H. A new nomenclature for int-1 and related genes: the Wnt gene family. Cell. 1991. Jan 25;64(2):231. [DOI] [PubMed] [Google Scholar]
- Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Ohkawara B, Niehrs C. An ATF2-based luciferase reporter to monitor non-canonical Wnt signaling in Xenopus embryos. Dev Dyn. 2011;240:188–194. doi: 10.1002/dvdy.22500. [DOI] [PubMed] [Google Scholar]
- Ohta K, Ito A, Kuriyama S, Lupo G, Kosaka M, Ohnuma S, Nakagawa S, Tanaka H. Tsukushi functions as a Wnt signaling inhibitor by competing with Wnt2b for binding to transmembrane protein Frizzled4. Proc Natl Acad Sci U S A. 2011;108:14962–14967. doi: 10.1073/pnas.1100513108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang H, Xue Y, Lin Y, Zhang X, Xi L, Patel S, Cai H, Luo J, Zhang M, Zhang M, Yang Y, Li G, Li H, Jiang W, Yeh E, Lin J, Pei M, Zhu J, Cao G, Zhang L, Yu B, Chen S, Fu XD, Liu Y, Zhang K. WNT7A and PAX6 define corneal epithelium homeostasis and pathogenesis. Nature. 2014;511:358–361. doi: 10.1038/nature13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Q, Chen J, Liu Y. LRP5 and LRP6 in Wnt signaling: similarity and divergence. Front Cell Dev Biol. 2021;9:670960. doi: 10.3389/fcell.2021.670960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijsewijk F, Schuermann M, Wagenaar E, Parren P, Weigel D, Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987;50:649–657. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- Rios-Esteves J, Haugen B, Resh MD. Identification of key residues and regions important for porcupine-mediated Wnt acylation. J Biol Chem. 2014;289:17009–17019. doi: 10.1074/jbc.M114.561209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S, Bonnet C, Islam J, Deng SX. Wnt signaling crosstalk via Wnt16b improves human limbal epithelial stem/progenitor cell maintenance. Invest. Ophthalmol. Vis. Sci 2020;61:3283. [Google Scholar]
- Robertson S, Bonnet C, Islam J, Deng SX. Non-canonical Wnt signaling via Wnt16b improves human limbal epithelial stem/progenitor cell maintenance. Invest. Ophthalmol. Vis. Sci 2022;63:3631–A0196. [Google Scholar]
- Robinson ML. An essential role for FGF receptor signaling in lens development. Semin Cell Dev Biol. 2006;17:726–740. doi: 10.1016/j.semcdb.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe M, Kanwal N, Dietmann P, Seigfried FA, Hempel A, Schütz D, Reim D, Engels R, Linnemann A, Schmeisser MJ, Bockmann J, Kühl M, Boeckers TM, Kühl SJ. An Epha4/Sipa1l3/Wnt pathway regulates eye development and lens maturation. Development. 2017;144:321–333. doi: 10.1242/dev.147462. [DOI] [PubMed] [Google Scholar]
- Routledge D, Scholpp S. Mechanisms of intercellular Wnt transport. Development. 2019;146:dev176073. doi: 10.1242/dev.176073. [DOI] [PubMed] [Google Scholar]
- Sarin S, Zuniga-Sanchez E, Kurmangaliyev YZ, Cousins H, Patel M, Hernandez J, Zhang KX, Samuel MA, Morey M, Sanes JR, Zipursky SL. Role for Wnt Signaling in retinal neuropil development: analysis via RNA-seq and in vivo somatic CRISPR mutagenesis. Neuron. 2018;98:109–126.e8. doi: 10.1016/j.neuron.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader S, O’Callaghan AR, Tuft SJ, Beaconsfield M, Geerling G, Daniels JT. Wnt signalling in an in vitro niche model for conjunctival progenitor cells. J Tissue Eng Regen Med. 2014;8:969–977. doi: 10.1002/term.1599. [DOI] [PubMed] [Google Scholar]
- Schultz G, Chegini N, Grant M, Khaw P, MacKay S. Effects of growth factors on corneal wound healing. Acta Ophthalmol Suppl. 1992;202:60–66. doi: 10.1111/j.1755-3768.1992.tb02170.x. [DOI] [PubMed] [Google Scholar]
- Sebastian A, Hum NR, Murugesh DK, Hatsell S, Economides AN, Loots GG. Wnt co-receptors Lrp5 and Lrp6 differentially mediate Wnt3a signaling in osteoblasts. PLoS One. 2017;12:e0188264. doi: 10.1371/journal.pone.0188264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick AE, D’Souza-Schorey C. Wnt signaling in cell motility and invasion: drawing parallels between development and cancer. Cancers (Basel). 2016;8:80. doi: 10.3390/cancers8090080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R, Spektor TM, Punj V, Turjman S, Ghiam S, Kim J, Tolstoff S, Amador C, Chun ST, Weisenberger DJ, Saghizadeh M, Kramerov AA, Ljubimov AV. Wnt5a promotes diabetic corneal epithelial wound healing and limbal stem cell expression Invest. Ophthalmol. Vis. Sci 2021;62:847. [Google Scholar]
- Sharif R, Bak-Nielsen S, Hjortdal J, Karamichos D. Pathogenesis of keratoconus: The intriguing therapeutic potential of prolactin-inducible protein. Prog Retin Eye Res. 2018;67:150–167. doi: 10.1016/j.preteyeres.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Rao K, Maharana PK, Vajpayee RB. Ocular allergy and keratoconus. Indian J Ophthalmol. 2013;61:407–409. doi: 10.4103/0301-4738.116063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R. Wingless, a new mutant in D. melanogaster. Drosoph. Inf. Serv 1973;50:134. [Google Scholar]
- Shumway CL, Motlagh M, Wade M. Anatomy, Head and Neck, Eye Conjunctiva. 2021. Jul 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. [PubMed] [Google Scholar]
- Shyam R, Shen X, Yue BY, Wentz-Hunter KK. Wnt gene expression in human trabecular meshwork cells. Mol Vis. 2010;16:122–129. [PMC free article] [PubMed] [Google Scholar]
- Slusarski DC, Yang-Snyder J, Busa WB, Moon RT. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev Biol. 1997;182:114–120. doi: 10.1006/dbio.1996.8463. [DOI] [PubMed] [Google Scholar]
- Smith AM, Czyz CN. Neuroanatomy, Cranial Nerve 2 (Optic). 2021. Nov 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. [PubMed] [Google Scholar]
- Sridhar MS. Anatomy of cornea and ocular surface. Indian J Ophthalmol. 2018;66:190–194. doi: 10.4103/ijo.IJO_646_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefater JA 3rd, Lewkowich I, Rao S, Mariggi G, Carpenter AC, Burr AR, Fan J, Ajima R, Molkentin JD, Williams BO, Wills-Karp M, Pollard JW, Yamaguchi T, Ferrara N, Gerhardt H, Lang RA. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature. 2011;474:511–5. doi: 10.1038/nature10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart Z, Angers S. Wnt signaling in development and tissue homeostasis. Development. 2018;145:dev146589. doi: 10.1242/dev.146589. [DOI] [PubMed] [Google Scholar]
- Stewart RM, Sheridan CM, Hiscott PS, Czanner G, Kaye SB. Human conjunctival stem cells are predominantly located in the medial canthal and inferior forniceal areas. Invest Ophthalmol Vis Sci. 2015;56:2021–2030. doi: 10.1167/iovs.14-16266. [DOI] [PubMed] [Google Scholar]
- Strutt DI, Weber U, Mlodzik M. The role of RhoA in tissue polarity and Frizzled signalling. Nature. 1997;387:292–295. doi: 10.1038/387292a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Lovicu FJ, McAvoy JW. Planar cell polarity in the mammalian eye lens. Organogenesis. 2011;7:191–201. doi: 10.4161/org.7.3.18421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthon S, Perkins RS, Bryja V, Miranda-Carboni GA, Krum SA. WNT5B in physiology and disease. Front Cell Dev Biol. 2021;9:667581. doi: 10.3389/fcell.2021.667581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taciak B, Pruszynska I, Kiraga L, Bialasek M, Krol M. Wnt signaling pathway in development and cancer. J Physiol Pharmacol. 2018;69:185–196. doi: 10.26402/jpp.2018.2.07. [DOI] [PubMed] [Google Scholar]
- Tokuda Y, Okumura N, Komori Y, Hanada N, Tashiro K, Koizumi N, Nakano M. Transcriptome dataset of human corneal endothelium based on ribosomal RNA-depleted RNA-Seq data. Sci Data. 2020;7:407. doi: 10.1038/s41597-020-00754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Park IH, Qi SD, Klassen HJ, Jiang C, Yao J, Redenti S, Daley GQ, Young MJ. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS One. 2011;6:e18992. doi: 10.1371/journal.pone.0018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raay TJ, Vetter ML. Wnt/frizzled signaling during vertebrate retinal development. Dev Neurosci. 2004;26:352–358. doi: 10.1159/000082277. [DOI] [PubMed] [Google Scholar]
- Vertebrate Wnt genes, the Wnt homepage 2022. https://web.stanford.edu/group/nusselab/cgi-bin/wnt/vertebrate; accessed October 27, 2022).
- Voloshanenko O, Gmach P, Winter J, Kranz D, Boutros M. Mapping of Wnt-Frizzled interactions by multiplex CRISPR targeting of receptor gene families. FASEB J. 2017;31:4832–4844. doi: 10.1096/fj.201700144R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KY, Yamada S, Izumi H, Tsukamoto M, Nakashima T, Tasaki T, Guo X, Uramoto H, Sasaguri Y, Kohno K. Critical in vivo roles of WNT10A in wound healing by regulating collagen expression/synthesis in WNT10A-deficient mice. PLoS One. 2018a;13:e0195156. doi: 10.1371/journal.pone.0195156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Symes AJ, Kane CA, Freeman A, Nariculam J, Munson P, Thrasivoulou C, Masters JR, Ahmed A. A novel role for Wnt/Ca2+ signaling in actin cytoskeleton remodeling and cell motility in prostate cancer. PLoS One. 2010;5:e10456. doi: 10.1371/journal.pone.0010456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cho C, Williams J, Smallwood PM, Zhang C, Junge HJ, Nathans J. Interplay of the Norrin and Wnt7a/Wnt7b signaling systems in blood-brain barrier and blood-retina barrier development and maintenance. Proc Natl Acad Sci U S A. 2018b;115:E11827–E11836. doi: 10.1073/pnas.1813217115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rattner A, Zhou Y, Williams J, Smallwood PM, Nathans J. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell. 2012;151:1332–1344. doi: 10.1016/j.cell.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu CH, Huang S, Chen J. Wnt signaling in vascular eye diseases. Prog Retin Eye Res. 2019;70:110–133. doi: 10.1016/j.preteyeres.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Shu W, Lu MM, Morrisey EE. Wnt7b activates canonical signaling in epithelial and vascular smooth muscle cells through interactions with Fzd1, Fzd10, and LRP5. Mol Cell Biol. 2005;25:5022–5030. doi: 10.1128/MCB.25.12.5022-5030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S, Sarris M, Kuishek M, McKelvie P, Figueria E, McCluskey P, Coroneo M, Wakefield D. Limbal dermoid epithelium shares phenotypic characteristics common to both hair epidermal and limbal epithelial stem cells. Curr Eye Res. 2013;38:835–842. doi: 10.3109/02713683.2013.780625. [DOI] [PubMed] [Google Scholar]
- Wei M, Zhang C, Tian Y, Du X, Wang Q, Zhao H. Expression and function of WNT6: From development to disease. Front Cell Dev Biol. 2020;8:558155. doi: 10.3389/fcell.2020.558155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall TA, Brimeyer R, Twedt J, Gladon J, Olberding A, Furutani-Seiki M, Slusarski DC. Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/β-catenin activity. J Cell Biol. 2003;162:889–898. doi: 10.1083/jcb.200303107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter CG, Wang B, Ballew A, Royou A, Karess R, Axelrod JD, Luo L. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Chen Z, Jin X, Mao R, Chen Z. The many postures of noncanonical Wnt signaling in development and diseases. Biomed Pharmacother. 2017;93:359–369. doi: 10.1016/j.biopha.2017.06.061. [DOI] [PubMed] [Google Scholar]
- Xie Q, Yang Y, Huang J, Ninkovic J, Walcher T, Wolf L, Vitenzon A, Zheng D, Götz M, Beebe DC, Zavadil J, Cvekl A. Pax6 interactions with chromatin and identification of its novel direct target genes in lens and forebrain. PLoS One. 2013;8:e54507. doi: 10.1371/journal.pone.0054507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Li S, Liu W, Li X, He Y, Yang Y, Sun K, Zhang L, Tian W, Duan L, Chen H, Yao D, Yang Z, Zhu X. The ER membrane protein complex subunit Emc3 controls angiogenesis via the FZD4/WNT signaling axis. Sci China Life Sci. 2021;64:1868–1883. doi: 10.1007/s11427-021-1941-7. [DOI] [PubMed] [Google Scholar]
- Yang Y. Wnt signaling in development and disease. Cell Biosci. 2012;2:14. doi: 10.1186/2045-3701-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, Peachey NS, Nathans J. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139:285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, He S, Friedman JS, Akimoto M, Ghosh D, Mears AJ, Hicks D, Swaroop A. Altered expression of genes of the Bmp/Smad and Wnt/calcium signaling pathways in the cone-only Nrl−/− mouse retina, revealed by gene profiling using custom cDNA microarrays. J Biol Chem. 2004;279:42211–42220. doi: 10.1074/jbc.M408223200. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Call MK, Yuan Y, Zhang Y, Fischesser K, Liu CY, Kao WW. Dexamethasone induces cross-linked actin networks in trabecular meshwork cells through noncanonical Wnt signaling. Invest Ophthalmol Vis Sci. 2013b;54:6502–6509. doi: 10.1167/iovs.13-12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Yeh LK, Liu H, Yamanaka O, Hardie WD, Kao WW, Liu CY. Targeted overexpression of TGF-α in the corneal epithelium of adult transgenic mice induces changes in anterior segment morphology and activates noncanonical Wnt signaling. Invest Ophthalmol Vis Sci. 2013a;54:1829–1837. doi: 10.1167/iovs.12-11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Pan Y, Ji J, Xu Y, Zhang Q, Qin L. Roles and action mechanisms of WNT4 in cell differentiation and human diseases: a review. Cell Death Discov. 2021;7:287. doi: 10.1038/s41420-021-00668-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yang T, Madakashira BP, Thiels CA, Bechtle CA, Garcia CM, Zhang H, Yu K, Ornitz DM, Beebe DC, Robinson ML. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol. 2008;318:276–288. doi: 10.1016/j.ydbio.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Wan X, Dai Y, Gong L, Le Q. WNT16B enhances the proliferation and self-renewal of limbal epithelial cells via CXCR4/MEK/ERK signaling. Stem Cell Reports. 2022;17:864–878. doi: 10.1016/j.stemcr.2022.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Tian C, Fan T, Xu B. Fibronectin regulates the self-renewal of rabbit limbal epithelial stem cells by stimulating the Wnt11/Fzd7/ROCK non-canonical Wnt pathway. Exp Eye Res. 2019;185:107681. doi: 10.1016/j.exer.2019.05.021. [DOI] [PubMed] [Google Scholar]
- Zhou T, Hu Y, Chen Y, Zhou KK, Zhang B, Gao G, Ma JX. The pathogenic role of the canonical Wnt pathway in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51:4371–4379. doi: 10.1167/iovs.09-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]


