Abstract
Vinca alkaloids including vincristine, vinblastine, vindesine, and vinflunine are chemotherapeutic compounds commonly used to treat various cancers. Vinca alkaloids are one of the first microtubule-targeting agents to be produced and certified for the treatment of hematological and lymphatic neoplasms. Microtubule targeting agents like vincristine and vinblastine work by disrupting microtubule dynamics, causing mitotic arrest and cell death. The key issues facing vinca alkaloids applications include establishing an environment-friendly production technique based on microorganisms, as well as increasing bioavailability without causing harm to patient’s health. The low yield of these vinca alkaloids from the plant and the difficulty of meeting their huge colossal demand around the globe prompted researchers to create a variety of approaches. Endophytes could thus be selected to produce beneficial secondary metabolites required for the biosynthesis of vinca alkaloids. This review covers the significant aspects of these vital drugs, from their discovery to the present day, in a concise manner. In addition, we emphasize the major hurdles that must be overcome in the coming years to improve vinca alkaloid’s effectiveness.
Keywords: Cancer, Cell death, Endophytes, Microtubules, Vinca alkaloids
Introduction
Vinca alkaloids are a class of organic compounds often derived from plants and usually made up of hydrogen, oxygen, carbon, and nitrogen. Even though, the name of alkaloids represents alkali, some of the alkaloids do not exhibit alkaline properties. Some of the alkaloids have poisonous characteristics and show physiological effects too, making them helpful in being used as medicines (Sahelian 2011). Vinca alkaloids seem to be the most primitive group of plant alkaloids used to treat cancer (Brogan 2010). They are nitrogenous bases obtained from the periwinkle plant Catharanthus roseus G. Don that are either naturally occurring or semisynthetic (Mckay and Cidlowski 2003). Canadian scientists Robert Noble and Charles Beer discovered Vinca alkaloids for the first time in the 1950s. Medicinal applications of this plant necessitate the examination of these chemicals for hypoglycemic action, which is minor compared to their cytotoxic effects (Gidding et al. 1999). Nonetheless, the vinca alkaloids are critical cancer fighters. Vinblastine (VBL), vinorelbine (VRL), vincristine (VCR), and vindesine (VDS) are the four primary vinca alkaloids in clinical usage; however, only VCR, VBL, and VRL are approved for use in the USA (Mckay and Cidlowski 2003). There are first-generation (vincristine, vinblastine), second-generation semisynthetic derivatives (vinorelbine, vindesine), and third-generation (vinflunine) vinca alkaloids developed from Catharanthus roseus (Arora and Menzes 2021). There is also a novel synthetic vinca alkaloid, vinflunine, licensed for therapeutic use in Europe since 2008 (Bennouna et al. 2008; Schutz et al. 2011). Vinca alkaloids (vincristine and vinblastine), as well as semisynthetic derivatives (vinorelbine, vindesine, and vinflunine), are potent antimitotic chemotherapeutics used to treat hematological and lymphatic neoplasms (Galindo-Solís and Fernández 2022). Vinblastine is a chemotherapeutic medication used to treat several human malignancies, including leukemia, lymphoma, breast cancer, and lung cancer (Van Tellingen et al. 1993). When vinblastine was crushed into tea, its utility as a chemotherapeutic agent was recognized. Because tea consumption reduced the number of white blood cells, it was thought that vinblastine would be beneficial against malignancies of the white blood cells, such as lymphoma (Mollaamin et al. 2014). Vincristine, sometimes known as leurocristine, a chemotherapy medication used to treat various cancer problems. Acute myeloid leukemia, neuroblastoma, small cell lung cancer, Hodgkin's disease, and acute lymphocytic leukemia are some conditions for which the medication is injected intravenously (Ashoka et al. 2017). Vincristine, an oxidized derivative of vinblastine, was first synthesized in 1963 (Kumar et al. 2019).
Vinorelbine is a semisynthetic vinca alkaloid having antitumor efficacy across the board. The vinca alkaloids are classified as spindle poisons, and their method of action is to interfere with the polymerization of tubulin. This protein is vital for the formation of the microtubule system during cell division (Gregory and Smith 2000). Vindesine (VDS) or deacetyl vinblastine amide sulfate (DVAS) is a vinca alkaloid homolog. Vinblastine (VLB) and vincristine (VCR), the two similar medicines, differ in only one functional group, R3. This slight change, however, accounts for significant differences in the oncolytic range, potency, and toxicity of the two drugs. VDS is a semisynthetic derivative of VLB that differs on its side chain by one hydroxyl (R2) and one carboxy-amino group (R1) (Barnett et al. 1978). Although those compounds are available from the leaves, the plant does not produce them in large amounts while alive (Kumar et al. 2019). Vinblastine and vincristine, the miracle drugs, employed for the shoot and tissue culturing which includes semi- and complete techniques for synthesis, are isolated from Catharanthus roseus plant leaves. These synthesis methods allow medications to be retrieved but their sources are insufficient and cannot meet current needs. Because plant extract has a meager yield, new strategies to improve its production are needed as global demand for these compounds rises (Liu et al. 2014). Microbial endophytes, which live in plant symbionts, are widely distributed and therefore are generally perceived as producers of novel secondary metabolites with prospective applications in industry, medicine, and agriculture (Zaferanloo et al. 2013). A variety of endophytic microorganisms from Catharanthus roseus have been isolated to fulfill the demand of pioneer drugs, i.e., vincristine and vinblastine, sufficiently and at inexpensive rates, and nature conservation responsibilities are taken into consideration (Kumar et al. 2013).
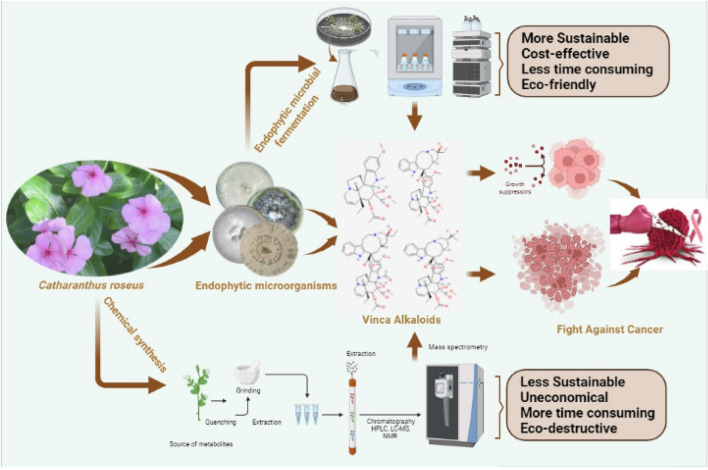
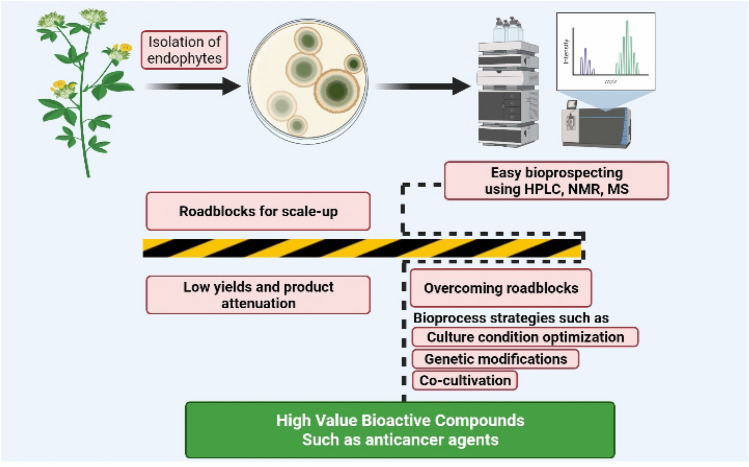
The importance of VAs will be explored further in the following sections, focusing on their modes of action and the pharmacological characteristics of each vinca alkaloid in clinical application. Furthermore, recent developments and the most potential vinca alkaloid analogs will be explored to provide a comprehensive perspective of the VAs world. As a result, the emphasis of this study is on the exploitation of endophytic microbial diversity, which can lead to the discovery of several promising vinca drugs in a more cost-effective and accessible manner. The endophyte-based production of high-value bioactive compounds is summarized schematically in Fig. 1.
Fig. 1.
Schematic summary of endophyte-based production of high-value bioactive compounds
Types of vinca alkaloids
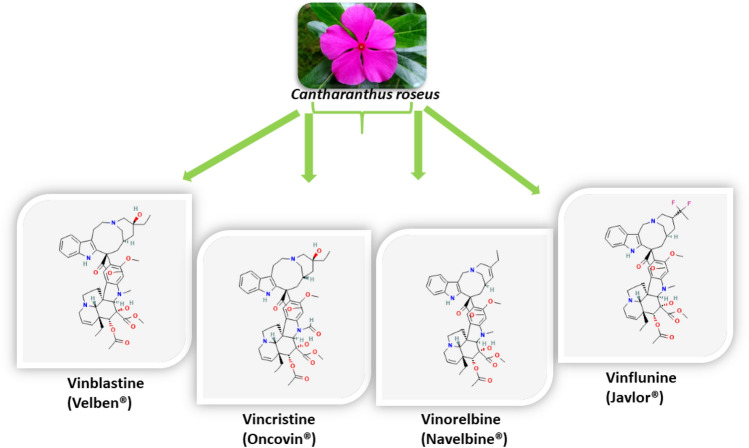
Vinca alkaloids block cell division at mitotic metaphase/anaphase and inhibit microtubule dynamics (Dumontet and Sikic 1999). These compounds have become increasingly important in the battle against cancer, with evidence that they are particularly effective during the treatment of lymphatic and hematological neoplasms, as well as against solid tumors (i.e., non-small cell lung cancer, breast cancer) (Johnson 1968; Johnson et al. 1963; Rowinsky and Donehower 1991). VAs are commonly used as anticancer medications, either alone or in amalgamation with some other drugs, for treating breast cancer, osteosarcoma, and acute lymphocytic leukemia. They were first used to treat hematologic malignancies in childhood and then expanded to embrace adult and solid hematologic malignancies (Johnson et al. 1960; Silvestri 2013). The list of essential elements of WHO (World Health Organization) includes vinca alkaloids (VBL, VCR, and VRL) that are described alongside their toxicological and clinical applications (https://www.who.int/publications/i/item/eml-20). Figure 2 presents the types of the major vinca alkaloids and their structures (Da Silva and Meijer 2012).
Fig. 2.
Types of major vinca alkaloids and their structures
Vinblastine
Vinblastine is a natural drug that can be used for the treatment of cancer and Hodgkin’s disease. It can be isolated from Catharanthus roseus leaves using plant tissue culture techniques such as expression via shoot culture, semisynthesis, total synthesis, cell culture, and tissue culture (Pandey et al. 2016). Vinblastine can be synthesized using enzymatic tryptophan decarboxylation, a crucial step in the biosynthesis of indole alkaloids. This process converts tryptophan to tryptamine, which is then transformed into bisindole alkaloid such as vinblastine. Plant tissue culture, metabolic engineering (Moreno et al. 1995; Datta and Srivastava 1997), and semisynthetic bioprocesses (Ataei-Azimi et al. 2008; Yokoshima et al. 2002) reported for the production of vinblastine are expensive and have low yields which resulting in insufficient supplies to meet rising demand (Chandra 2012). Vinblastine, an autophagy maturation inhibitor (Craig and Jensen 2017), increased LS174T (human colon) cell lines and apoptotic cell death in HepG2 (human hepatocarcinoma) by inhibiting autophagy maturation and increasing autophagic vacuole accumulation. Vinblastine is excreted mainly, with negligible renal excretion in bile and feces. The vinblastine dose is typically 6 mg/m2 for children and adults, with hepatic and hematological tolerance modifications (Owellen et al. 1977a, b). Vinblastine is the most researched of the vinca alkaloids family, and it is used in a variety of chemotherapy regimens for melanoma, non-small cell lung cancer, testicular cancer, brain cancer, and Hodgkin's lymphoma (Moudi et al. 2013; Maswadeh et al. 2002; Dandamudi and Campbell 2007).
Vincristine
Vincristine acts as an inhibitor during the cell cycle's metaphase, preventing the mitotic spindle from developing by attaching to microtubules (Zhou et al. 2018). In plants, vincristine and vinblastine production is deficient (ranging from 0.001 to 0.0003%), which results in their exorbitant price (Pernot et al. 2018). Vincristine is often used off-label for adult patients with Ewing sarcoma, advanced progressive thymoma, multiple myeloma, CNS primary lymphoma, gestational trophoblastic tumors, ovarian tumors cancer, and central nervous system malignancies (Martino et al. 2018). The terpenoid indole alkaloid vincristine holds great medicinal importance, despite its relatively low abundance in plants (approximately 0.0005% of dry weight). In order to enhance the production of Catharanthus roseus alkaloids, particularly vincristine, various in vitro culture investigations have been conducted using biotic or abiotic elicitation strategies. These studies aim to stimulate the biosynthesis pathways of terpenoid indole alkaloids, which are still not fully understood. (Sheard et al. 2007; O'Donnell et al. 2002). Vincristine is an integral component of the treatment regimen that has successfully treated juvenile leukemia. In combination therapy, vincristine is used to treat lymphomas and acute leukemia (Fahy et al. 1997). Vincristine has been proven to be effective in treating Hodgkin’s disease, reticulum cell sarcoma, lymphosarcoma, choriocarcinoma, and to a lesser extent, some breast tumors. It is usually unsuccessful in leukemia and most carcinomas (Byrd et al. 1981; Ripps et al. 1984).
Vindesine
Vindesine is a semisynthetic vinca alkaloid derivative that was first used in clinical oncology. Desacetyl vinblastine amide is another name for vindesine. Vindesine inhibits the net tubulin addition of the microtubules near respective assembly endpoints in circumstances such as blast crisis of chronic myeloid leukemia, malignant melanoma, pediatric solid tumors, acute lymphocytic leukemia, esophageal carcinomas, and breast, and renal cancer (Pandrangi et al. 2022). Because of its side effects, it was only approved in a few countries (Dyke et al. 1979).
Vinorelbine
Vinorelbine is a semisynthetic derivative that is an antimitotic medication commonly utilized for cancer treatment. Vinorelbine, the most current vinca alkaloid to receive clinical approval, has enhanced efficacy while also lowering toxicity and found to be successful in ovarian cancer, metastatic breast cancer, and non-small cell lung cancer as well as promising in prostatic carcinoma, esophageal cancer, and lymphoma (Dyke et al. 1979). Second-generation vinca alkaloids, including vinorelbine show a wide variety of antiproliferative activity, particularly in metastatic/advanced non-small lung cancer and advanced breast cancer. Vinorelbine is available in an injectable form that is commonly used in clinical practice (Rowinsky and Donehower 1991). In Europe (1991) and the USA (1995), vinorelbine was licensed for non-small cell lung cancer therapy and vindesine was licensed for the treatment of melanoma (Fahy et al. 1997).
Vinflunine
Vinflunine is a third-generation compound, i.e., a fluorinated alkaloid derivative and the current member of the vinca alkaloids family. It is important to note that it is the least neurotoxic in Vinca alkaloids, and as compared to others, it has greater anticancerous activity (Bonfil et al. 2002). Vinflunine is a novel semisynthetic bifluorinated chemical in phase II clinical trials after completing phase I (Armand et al. 2001). In various murine malignancies and human tumor xenografts, Vinflunine is more effective than vincristine, vinblastine, and vinorelbine. For example, in 64% of tumor models, vinflunine shows high or moderate antitumor efficacy, i.e., in 7 of 11, while vinorelbine in 27% shows moderate activity (Kruczynski et al. 1998a, b, c). Currently, vinflunine is being tested in several clinical trials (Schutz et al. 2011). Vinflunine is also being licensed in Europe for treating advanced or metastatic transitional cell carcinoma of the urothelial tract after a prior platinum-containing regimen failed (Bellmunt et al. 2009). The molecular mechanism of vinflunine-killing cells in murine P388 is identified in a study that demonstrated that vinflunine has led to apoptotic cell death via a succession of processes. After treatment with vinflunine, these tumor cells showed apoptosis-related cellular morphological alterations and DNA fragmentation (Kruczynski et al. 1998a, b, c). Vinflunine has shown antitumor effectiveness against various experimental tumors with varying biological characteristics and chemo sensibilities (Hill et al. 1999). According to various schedules, vinflunine was given intraperitoneally as a single or repeated dose or intravenously or peroral dose, with marked activity against the murine P388 leukemia implanted intravenously. This activity was not linked to significant toxicity, since the lack of substantial body weight loss and early deaths were judged. According to the National Cancer Institute (NCI) USA standards, a single intraperitoneal injection of vinflunine at the highest non-toxic dose of 40 mg/kg resulted in a considerable increase in life span (ILS) of 100 percent (T/C of 200 percent), which was considered to be of a high level of activity (T/C175%) (Kruczynski et al. 1998a, b, c).
Mechanism of action
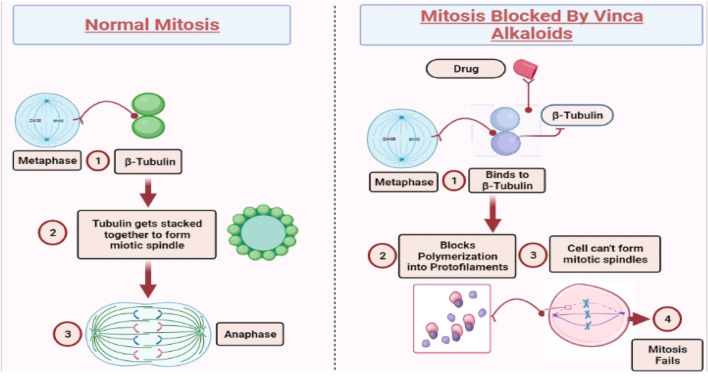
The significance of vinca alkaloid cytotoxicity include tubulin interactions and microtubule function disruption, especially of microtubules including the mitotic spindle apparatus, resulting in metaphase arrest (Himes 1991). The exact vinca alkaloid’s mode of action is undetermined; nevertheless, it has been demonstrated that their antitumor efficacy is linked to their strong binding affinity for tubulin, the basic protein subunit of microtubules. These drugs are considered to halt cell mitosis during metaphase by blocking tubulin polymerization to generate microtubules and causing microtubule depolymerization, making them cell cycle-dependent antiproliferative agents (spindle poisons). Vincristine, vinblastine, and vindesine have binding constants for tubulin of 8.0 × 106, 6.0 × 106, and 3.3 × 106 mol/L, respectively (Owellen et al. 1972; Owellen et al. 1977a, b). Vinca alkaloids were examined at the assembly ends of bovine brain microtubules to prevent net tubulin addition. The inhibition constants (Kj) for vincristine, vinblastine, and vindesine (0.085, 0.178, and 0.110 mol/ L, respectively) were similar; however, the K; values did not correspond well with the drugs’ capacity to block intact cell cycle progression (Jordan et al. 1985). As a result, the main difference between these agents appeared to be their tumor tissue retention (Ferguson and Cass 1985; Ferguson et al. 1984; Singer and Himes 1992). The establishment and stability of vinca alkaloid tubulin complexes are thought to be a determinant of this. Vincristine inhibits the production of microtubules via binding to tubulin as a result mitosis is arrested at metaphase. Due to this inhibition mitotic spindle formation is disrupted notably during the M and S phases. Vincristine another action mechanism involves preventing the use of glutamic acid to disturb nucleic acid and protein synthesis. Figure 3 illustrates the mechanism of action of vinca alkaloids, shedding light on the underlying processes by which these compounds exert their therapeutic effects. Vincristine is usually given as a bolus intravenous infusion to adults at 1.4 mg/m2 (maximum dose-2 mg) and for children vary from 1.5 to 2.0 mg/m2 (maximum dose ranges from 2.0 to 2.5 mg). While its therapeutic properties have been extensively used in clinical use for the treatment of various cancer disorders, vincristine is constrained by its extreme neurotoxicity (Moreno et al. 1995; Munier et al. 1992). Guanosine 5′-triphosphate (GTP) was required to develop and stabilize vincristine tubulin complexes in the cytosols of mice carrying human rhabdomyosarcoma xenografts with varying vincristine stability. The initial rate of vincristine binding and the maximal amount of bound drug were two- to threefold higher in the presence of 0.1 mmol/L GTP than in the absence, suggesting that GTP may have had a role in vinca alkaloid therapeutic selectivity (Bowman et al. 1986; Houghton et al. 1987). The pharmacological mechanism, trade names, and applications of the most important vinca alkaloids are listed in Table 1.
Fig. 3.
Mechanism of action of vinca alkaloids
Table 1.
Applications, trade names and pharmacological mechanism of major vinca alkaloids
| Indole alkaloid | Trade (market name) | Applications | Pharmacological mechanisms | References |
|---|---|---|---|---|
| Vinblastine | Velban | Carcinoma of breast, Lymphosarcoma, Neuroblastoma, and Hodgkin’s disease | Inhibits microtubules from undergoing mitosis by adhering to tubulin | Aslam et al. (2010) |
| Vincristine | Oncovin | Wilkins’s tumor, Hodgkin’s disease and Neuroblastoma | Suppresses the formation of mitosis in microtubule structures by binding to tubulin dimer | Aslam et al. (2010) |
| Vindesine | Eldisine, Fildesin | Lung cancers, Uterine malignancies and Melanoma | Antimitotic | Dhyani et al. (2022) |
| Vinflunine | Javlor | Breast cancer, transitional cell carcinoma | Anaphase transition, preventing cancer cells from entering mitosis | Bennouna et al. (2008) |
| Vinorelbine | Navelbine | Non-small cell lung cancer, Breast cancer | Antimitotic | Goa and Faulds (1994) |
Clinical indications
The vinca alkaloids have been extensively incorporated for curative and palliative therapies into combination chemotherapy regimens. They are based not only on the alkylation of DNA due to lack of cross-resistance with medications but also on their discrete mode of action. An additional advantage is the lack of myelosuppression and VCR which enables the use of vincristine in combination with myelosuppression mediators in full doses in several chemotherapy regimens. A wide range of antitumor effects of VCR has been reported. It has shown a high curative response in combination with other chemotherapeutic agents for Wilms tumor, neuroblastoma, Hodgkin and non-Hodgkin lymphomas, childhood and adult acute lymphocytic leukemia, Ewing sarcoma, and rhabdomyosarcoma (Gidding et al. 1999; Armand et al. 2001; Schutz et al. 2011). VCR and other antineoplastic compounds have shown efficient results for chronic lymphocytic leukemia, sarcomas, multiple myeloma, and lymphoblastic crises of chronic myelogenous and small cell lung cancer. In addition, VCR has shown its curative properties in treating some non-malignant hematological disorders like hemolytic uremic syndrome, refractory autoimmune thrombocytopenia, and thrombotic thrombocytopenic purpura.
The therapeutic approach against menacing diseases such as Hodgkin and non-Hodgkin lymphomas and testicular carcinoma is vinblastine (Schutz et al. 2011). The conventional treatment against the advanced carcinomas of the testes was termed PVB, consisting of bleomycin, cisplatin, and vinblastine. The replacement of VBL was done by etoposide because of the more favorable toxicity profile of the cisplatin–etoposide regimen. A combination of vinblastine with bleomycin, doxorubicin, and dacarbazine (ABVD) is mainly used in Hodgkin lymphoma. The non-cross-resistance properties are shown by MOPP (nitrogen mustard, VCR, procarbazine, and prednisone) against ABVD. The studies suggest the mixture sees the incorporation of both the VCR and VBL of MOPP/ABV. In various carcinomas such as Kaposi sarcoma, choriocarcinomas, bladder, terminal phase of chronic myelogenous, breast, lung, mycosis fungoides, leukemia, and Letterer–Siwe disease (histiocytosis X). As a single agent or in combination with other antineoplastic compounds, vinblastine is responsible for the antineoplastic activity. In the case of refractory autoimmune thrombocytopenia, the effective insertion of VBL or VBL-loaded platelets is seen. This is mainly due to the overall binding of one antibody molecule to an antigen in platelets (Datta and Srivastava 1997; Schutz et al. 2011).
VDS is limited to investigational purposes in the USA. It has been reported that coupling of VDS with cisplatin or mitomycins shows good therapeutic responses in non-small cell lung cancer, better than responses of frequently used standard combinations or with either single compound. Furthermore, VDS has shown antineoplastic activity in acute lymphocytic leukemia, malignant melanoma, metastatic renal carcinoma, blast crisis of chronic myeloid leukemia, breast, pediatric solid tumors, colorectal and esophageal carcinomas; but the specific role of VDS in oncology is still undefined (Joel 1995). The USA has accepted VRL for treating patients at the initial stages of non-small cell lung cancer in combination with cisplatin or as a single therapeutic compound (Budman 1997; Gregory and Smith 2000). It has shown efficient anticancerous activity in initial treatment and as a part of first-line chemotherapeutics comprising other non-cross-resistant chemotherapeutic compounds in patients suffering from advanced or metastatic breast cancer compounds (Budman 1997; Gregory and Smith 2000; Johnson et al. 1996; Domenech and Vogel 2001). VRL can be seen as an endurable compound in elderly growing individuals. Still, it is under invigilation as a part of first-line chemotherapeutic compound in non-small cell lung and breast carcinoma in elderly patients. Furthermore, VRL has shown efficient therapeutic results in patients with ovarian cancer and advanced Hodgkin and non-Hodgkin lymphomas. Still, the role in the therapy of these and different malignancies is currently being accessed.
Preliminary studies on vinca alkaloids production
In pursuit of semisynthetic derivatives, substantial pharmacological research was carried out in the 1970s to develop more potent and less harmful medications. Many different academic or industrial groups have synthesized vinca alkaloid derivatives, however only two natural substances, vinblastine and vincristine, and two semisynthetic derivatives, vinorelbine and vindesine, have been registered and are now utilized as anticancer medicines (Duflos et al. 2002). The plant produces terpenoids as a secondary metabolite in a minimal amount approximately less than 2% of the dry weight (Roberts 2007). For example, 500 kg of dried leaves of Catharanthus roseus is required to produce 1 g of vinblastine (Noble 1990). Chromatographic techniques like mixed bed vacuum liquid chromatography (VLC), vacuum liquid chromatographic column on silica gel: Aluminum oxide (1:1), charcoal column, and centrifugally accelerated radial chromatography are widely used to quantify the pure form of vinblastine and vincristine from Catharanthus roseus (Shams et al. 2009). Some other methods, such as supercritical fluid extraction (Song et al. 1992), and high-performance liquid chromatography (Hisiger and Jolicoeur 2007; Siddiqui et al. 2011) are also used for the extraction of vinblastine and vincristine from plants. Combining mono indole alkaloids like vindoline and catharanthine, present excessively in the aerial parts of the plant leads to the formation of dimeric compounds like vinblastine and vincristine (Renault et al. 1999). Accumulation of monomeric compounds, vindoline, and catharanthine is very low in quantity in aerial regions of Catharanthus roseus resulting in a meager yield of vinca alkaloids leading to very high market pricing which can exceed millions of dollars per kilogram (Courdavault et al. 2020). The biogenetic pathway of these alkaloids has been established under the harsh developmental regulation in the plant (Chandra and Chandra 2011). Biosynthesis of vinca alkaloids can proceed by three methods: total synthesis, chemical synthesis, and semisynthesis. These anticancerous monoterpene indole alkaloids (MIA) follow semisynthesis method for their manufacturing which partially incorporates chemical synthesis by using chemicals as starting components, obtained from natural sources, since FDA approval in 1963 (Courdavault et al. 2020). Many variations have been found in the chemical synthesis of these therapeutically active components depending on the origin of the plant, harvesting, and geographical conditions (Sharma et al. 2020). But these methods are significantly costlier and quantifying the amount of therapeutic compound is very low. In these cultures, the quantity of dimeric compounds is deficient, but at least they are formed. Culturing Catharanthus roseus in the field is more economizing since it is hard to grow shoot cultures in a large-scale bioreactor (Wink et al. 2005). Table 2 presents the plant tissue culture/cell culture and chemical synthesis-based production of vinca alkaloids from Catharanthus roseus.
Table 2.
Plant tissue culture/cell culture and chemical synthesis-based production of vinca alkaloids from Catharanthus roseus
| Culture type | Bioactive compounds | Yield obtained | References |
|---|---|---|---|
| Cell suspension | Vinblastine | 4090 µg/L | Pliankong et al. (2018) |
| Protoplast derived | Vinblastine | 15.5 µg/g | Maqsood and Abdul (2017) |
| Callus culture | Vincrsitine | 0.22 µg/g | Tonk et al. (2016) |
| Hairy root culture | Vinblastine | 700 µg/g | Vu et al. (2022) |
| Cell suspension | Vincrsitine | 5470 µg/L | Pliankong et al. (2018) |
| Callus culture | Vinblastine | 0.90 µg/g | Tonk et al. (2016) |
| Hairy root culture | Vincristine | 52.0 µg/g | Vu et al. (2022) |
| Protoplast derived | Vincrsitine | 4.14 µg/g | Maqsood and Abdul (2017) |
| Total synthesis | Vincrsitine | –a | Ishikawa et al. (2009) |
aNot available
Hence, endosymbiotic fungi attracted researchers as a source of secondary metabolite production and presented as a substitute for microbial fermentation or chemical synthesis (Yan et al. 2018). Although microbial fermentation has various advantages over the use of plant raw materials for the production of therapeutic products and compounds, the study of cytotoxic substances produced by endophytic fungi of vinca plants may provide an alternative method of production (Banyal et al. 2021). Vinblastine and vincristine hold very high costs because of plants' low production (0.001–0.0003). In recent years fungal endophytes have opted as an alternative method for producing these drugs. This alternative method helps conserve plants in some regions and reduces the effective price of these therapeutic compounds (Kumar 2016). It has been reported for the first time that vinblastine can be extracted from the Alternaria sp., an endophytic fungus from the phloem of Catharanthus roseus (Bo et al. 1998). Later on, another endophytic fungus Fusarium oxysporum from the phloem of Catharanthus roseus has been reported to produce vincristine (Lingqi et al. 2000). The previously unknown vincristine-producing endophytic fungus was discovered (Yang et al. 1994). Curvularia verruculosa has been reported to produce vinblastine in high quantity, i.e., 182 µg/L for the first time (Parthasarathy et al. 2020). Then, compared to other fungi, El-Sayed (2021) isolated the endophytic fungus Alternaria alternata, which has been reported to produce vinblastine in large quantities, i.e., 1553 µg/L. The endophytic bacteria Microbacterium sp. which has been reported to produce vindoline in significant quantity (82 µg/L), was isolated by Anjum and Chandra (2019). Even though Nigrospora sphaerica, an endophytic fungus, only produces 0.868 µg/mL vinblastine, studies have shown that it has a significant level of cytotoxicity against the breast cancer cell line (MDA-MB 231). The endophytic microorganisms that produce vinca alkaloids are listed in Table 3.
Table 3.
Endophytic microorganisms as a source of vinca alkaloids
| Endophytic microorganism | Plant source | Bioactive compounds | Yield obtained (µg/L) | References |
|---|---|---|---|---|
| Alternaria alternata | Melissa officinalis | Vinblastine | 1553 | El-Sayed (2021) |
| Botryosphaeria laricina | Catharanthus roseus | Vincristine | 2800 | Bandara et al. (2021) |
| Talaromyces radicus | C. roseus | Vinblastine | 70 | Palem et al. (2016) |
| Fusarium oxysporum | C. roseus | Vincristine | 67 | Kumar et al. (2013) |
| Curvularia verruculosa | C. roseus | Vinblastine | 182 | Parthasarathy et al. (2020) |
| B. laricina | C. roseus | Vincristine | 2400 | Bandara et al. (2021) |
| F. oxysporum | C. roseus | Vinblastine | 76 | Kumar et al. (2013) |
| T. radicus | C. roseus | Vincristine | 670 | Palem et al. (2016) |
Biosynthesis of vinca alkaloids
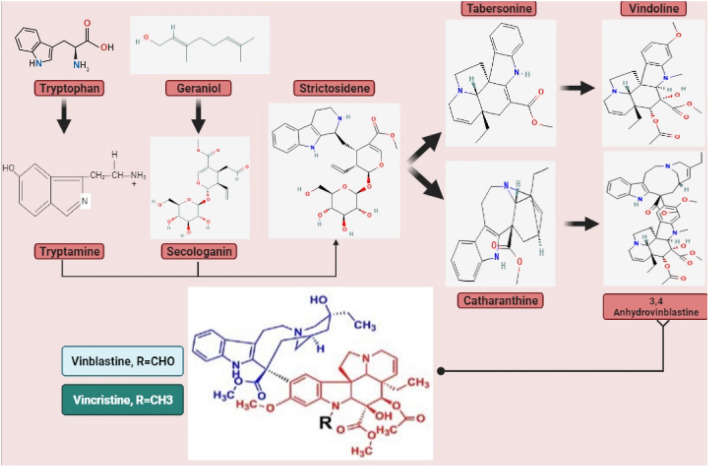
Catharanthus roseus is a remedial plant having great pharmaceutical potential. It has the potency to biosynthesize secondary metabolites of more than 130 types, collectively known as terpenoid indole alkaloids (TIAs) and includes anticancerous compounds such as vinblastine and vincristine (El-Sayed and Verpoorte 2007). TIAs comprise a huge family of secondary metabolites showing several biological activities (Facchini 2001; Groger 1985) and are restricted to four families such as Nyssaceae, Loganiaceae, Apocynaceae, and Rubiaceae (Memelink et al. 2001). The biosynthesis of indole alkaloids might be fragmented into three phases (Zhu et al. 2015). The biosynthetic pathway of vinca alkaloids is illustrated in Fig. 4 (Sottomayor and Barcelo 2006).
Fig. 4.
Biosynthetic pathway of vinca alkaloids
Formation of strictosidine
Vinca alkaloids are terpenoid indole alkaloids (MIAs) and they are all derived from tryptamine, amino acid, and iridoid terpene secologanin. TIAs biosynthesis is a multi-step enzyme-based network extensively regulated by cell-specific, developmental, and organo-environmental controls and completed through 30 enzymatic steps and 35 intermediates. In the biosynthetic pathway, the production of vincristine and vinblastine requires five intercellular compartments which include vacuole, cytosol, endoplasmic reticulum, chloroplast, and nucleus, and four types of cells are idioblasts, epidermis, and internal phloem associated with laticifers and parenchyma, for the completion of the biosynthetic pathway (St-Pierre et al. 1999; Jacobs et al. 2004; Rischer et al. 2006; Verma et al. 2012). Tryptamine is synthesized from an amino acid, i.e., tryptophan (Leete 1961) with the help of the enzyme tryptophan decarboxylase (a pyridoxal-dependent enzyme) (De Luca et al. 1989; Facchini et al. 2000; Hong et al. 2006) while other is synthesized with the help of enzyme geraniol-8-hydroxylase (G8H) from geraniol. The central intermediate for all TIAs is strictosidine or it acts as a precursor which is then converted into the number of monoterpenoids indole alkaloids (MIAs) (Oudin et al. 2007). The essential intermediate molecule for the biosynthesis of monoterpenoid indole alkaloids (MIAs), strictosidine, is introduced by combining the aromatic amino acid tryptamine and the secoiridoid monoterpene molecule secologenin (Sharma et al. 2022).
Formation of vindoline and catharanthine
The formation of vinblastine is by two different types of monoterpenoid indole alkaloids (MIAs): MIA catharanthine and MIA vindoline. These two alkaloids are extracted in parallel branches from 3-α(s) strictosidine. In the biosynthetic pathway, removing a glucose molecule from strictosidine occurs with the help of 3α(S)-strictosidine β-glucosidase and converts it into strictosidine aglycones, i.e., an aldehyde (Tatsis et al. 2017). Moreover, it is then followed by various enzyme activity that includes enzymes such as two dehydrogenases and a cytochrome P-450 oxidase which produces stemmadenine and several unstable intercedes (Qu et al. 2018). Furthermore, by undergoing various enzymatic reactions stemmadenine generates two monoterpenoid indole alkaloids (MIAs), catharanthine and taberosine. These two MIAs are generated when the taberosine synthetase hydrolases and catharanthine synthetase act on dihydroprecondylocarpine acetate (enamine form) (Qu et al. 2018; Caputi et al. 2018).
Furthermore, monoterpenoid indole alkaloid taberosine is converted into one of vinca alkaloids 'vindoline' in different seven-step pathways. The steps included in this pathway are O-methylation, aromatic hydroxylation, hydroxylation at position 4, hydration of the 2, 3-double bond, 4-O-acetylation, and N (1)-methylation (Liscombe and O’Connor 2011) In the first step, tabersonine is converted into 16-hydroxytabersonine by enzyme tabersonine 16-hydroxylase which is cytochrome P450 and this step is known as aromatic hydroxylation in which NADPH is oxidized into NADH + (St-Pierre and De Luca 1995). After hydroxylation, 16-hydroxytabersonine, with the help of the 16-hydroxytabersonine 16-O-methyltransferase is O-methylated into 16-methoxytabersonine (Levac et al. 2008). Further, through hydration, 16-methoxytabersonine is converted into 3-hydroxy-16-methoxy-2, 3-dihydroxytabersonine by an enzyme tabersonine, 3-oxygenase. In the next step, desacetoxyvindoline is formed through an uncharacterized mechanism of hydration, and N-methylated at the indolic nitrogen by 16-methoxy-2, 3-dihydro-3-hydroxytabersonine N-methyltransferase (Liscombe et al. 2010). The last two steps of this biosynthetic pathway involve the additional hydroxylation catalyzed by an enzyme desacetoxyvindoline-4-hydroxylase and forms the deactylevindoline (Vazquez-Flota et al. 1997), and finally, vindoline is formed through O-acetylation which is performed by an enzyme deacetylvindoline-4-O-acetyltransferase (St‐Pierre et al. 1998). Catharanthine and vindoline are finally coupled by secondary metabolic pathways to produce the monoterpenoid indole alkaloids (MIAs) vinblastine and vincristine (Sharma et al. 2018; Mistry et al. 2022).
Formation of bisindole alkaloids
Vinblastine and vincristine are two highly interesting bisindole alkaloids (Zhu et al. 2015). Furthermore, vincristine and vinblastine are produced from monomeric alkaloids. This product 3′,4′-anhydrovinblastine (AVLB) is converted into vinblastine, and vinblastine is converted into vincristine. So even though Catharanthus roseus is the only source of antineoplastic drugs, researchers have spent considerable time investigating the accumulation to increase their productive capacity in the in vitro organ, cell, and tissue cultures using metabolic engineering techniques, genetic and developmental regulation of MIAs synthesis (Verma et al. 2012; Liu et al. 2017).
Molecular docking studies of vinca alkaloids
Tubulin, the protein monomer of microtubules, is the intracellular target of dimeric vinca alkaloids, a class of potent antitumor drugs. Additionally, the vinca drugs bind to microtubules through a small number of sites at the ends of microtubules that appear to have a high affinity and are involved in the inhibition of tubulin dimer addition to the microtubule ends, as well as through sites along the microtubule wall that appear to have low affinity (Himes 1991). Computational techniques provide efficient and inexpensive working tools to accomplish the docking process in a stepwise manner. The three-dimensional structure of the protein molecules can be obtained from the PDB database (https://pubmed.ncbi.nlm.nih.gov), which is a repository of all obtained protein molecules and nucleic acids with the help of X-ray crystallography and NMR techniques. A three-dimensional structure of alpha and beta protein has been obtained from the PDB database. The downloaded structures were refined by removing previously incorporated ligands, ions, and extra chains. This can be done with the help of the chimera application (https://www.cgl.ucsf.edu/chimera/). Chimera is an online tool that helps molecular structure visualization, protein and ligand structure refining, and editing. The chemical structure of different available vinca alkaloids (used as ligands) was obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). It is a platform for various available chemical molecules. It shows molecular weight, molecular formula, applications, surface features, and even the synonyms of that particular drug. PubChem contains the ID of a chemical in different formats like canonical smiles, Inchl, and PubChem id. We can search for the desired chemical by entering the query in the form of chemical formula, molecular weight, or targets for which chemical is used. Three-dimensional or two-dimensional files can be downloaded in various formats like sdf, mol, etc., protein molecules have various binding pockets over which a ligand can bind easily. CastP (http://sts.bioe.uic.edu/castp/) help us find the desired protein molecule's binding pockets for better docking results.
The ligand molecules were prepared by adding hydrogen atoms and charges and removing water molecules with the help of chimera tools. Before proceeding toward the docking process, bioactivity score, toxicity and ADME properties of ligands have been studied with the help of computational tools like Molinspiration (https://www.molinspiration.com/) and PreADMET (https://preadmet.bmdrc.kr/), respectively. Molinspiration organization develops cheminformatics tools where bioactivity and physical properties can be calculated of any molecule. Molinspiration SMILES as input to generate molecular structure or you can draw your molecule in the provided area. User-defined interface makes it very easy for users. Molinspiration supports the virtual screening of molecules. PreADMET is a web-based tool used for predicting ADME data. It indicates various properties (drug likeness, ADME, and toxicity) based on the designed structure of the chemical compound. Drug-likeness prediction calculates Lipinski’s rule, lead-like rule, and drug DB-like rule. Ames test and different rodent carcinogenicity can be predicted in toxicity calculation. Here we use this tool to predict the toxicity of different ligands.
Docking of vinca alkaloids with tubulin proteins has been done using two tools, PyRx (https://pyrx.sourceforge.io/) and Swiss Dock (http://www.swissdock.ch/). PyRx is used for docking large no. of ligands with targets. It guides you on every step to make the process easy-going and learnable. It has easy to uses auto dock wizard which includes screening molecule preparation, and result visualization. It consists of an open babel, which can be used to remove salts and minimize energy. It uses wxPython as a scripting language. It can be downloaded free of cost from the server according to the system configuration. SWISS Dock is an open web server that is used for the target ligand docking process. It is based on EADock DSS Engine. The HTML interface of the swiss dock makes it convenient for users to easily submit docking molecules and visualize them in the result file. Input can be given by uploading the prepared target file (in PDB format) and ligand (in MoL2 format) or the user can directly search for ligands and targets by using its search option for docking. Visualization and analysis of the obtained docking result can be done with the help of a discovery studio (https://discover.3ds.com/discovery-studio-visualizer), an online and offline available computational tool.
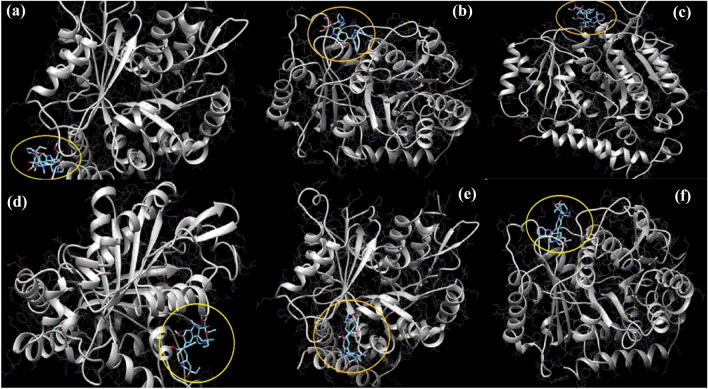
Docking results of vinca alkaloids with alpha and beta-tubulin proteins have been obtained and analyzed with the help of the chimera tool. The yellow highlighted region in the figures shows the binding places of different vinca alkaloids with the alpha and beta-tubulin proteins. Swiss dock result binding analysis of vinorelbine with chain A (alpha-tubulin) of tubulin protein forms two conventional hydrogen bonds with Arginine (215) and Asparagine (216). Four alkyl bonding can be seen with alanine (273), valine (275), isoleucine (276), and alanine (294). Vinorelbine also forms one pi–anion and two carbon–hydrogen bonds with glutamic acid (297) and one carbon–hydrogen bind and two pi–anion bonds with glutamic acid (290) can be seen in the highlighted region of Fig. 5a. Swiss dock result binding analysis of vinorelbine with chain B (beta-tubulin) of tubulin protein. Vinorelbine binds with beta-tubulin with two conventional hydrogen bonds at threonine (276) and arginine (369) also it makes three carbon–hydrogen bonds with leucine (275), glutamic acid (27), and histidine (229). One pi-alkyl bonding with phenylalanine (272) and four different alkyl bonding with leucine (219), alanine (233), leucine 230, and arginine (278) are shown in Fig. 5b. Swiss dock result binding analysis of vinblastine with chain A (alpha-tubulin) of tubulin protein is shown in Fig. 5c. Swiss dock result binding analysis of vinblastine with chain B (beta-tubulin) of tubulin protein is shown in Fig. 5d. Swiss dock result binding analysis of vindesine with chain A (alpha-tubulin) of tubulin protein forms one conventional hydrogen bond with alanine (389) and pi–sigma bond with histidine (393). A carbon–hydrogen bond with aspartic acid (306) is also observed in Fig. 5e. Swiss dock result binding analysis of vindesine with chain B (beta-tubulin) of tubulin protein shows two conventional hydrogen bonding with glycine (370) and a pi–pi T-shaped bonding with Histidine (229). Alkyl and pi-alkyl bonding were also observed. Alkyl bonding was observed with leucine (219), arginine (278), proline (360), arginine (9369), and a pi-alkyl with leucine (230) and alanine (233) are shown in in Fig. 5f (Coderch et al. 2012). Understanding the binding mechanisms and predicting the prospective effectiveness of vinca alkaloids as cancer therapies can be done with the aid of molecular docking studies. Researchers can develop more potent Vinca alkaloid derivatives for the treatment of cancer in the near future by understanding the molecular interactions through analysis of the docking results.
Fig. 5.
a Swiss dock result binding analysis of vinorelbine with chain A (alpha-tubulin) of tubulin protein. b Swiss dock result binding analysis of vinorelbine with chain B (beta-tubulin) of tubulin protein. c Swiss dock result binding analysis of vinblastine with chain A (alpha-tubulin) of tubulin protein. d Swiss dock result binding analysis of vinblastine with chain B (beta-tubulin) of tubulin protein. e Swiss dock result binding analysis of vindesine with chain A (alpha-tubulin) of tubulin protein. f Swiss dock result binding analysis of vindesine with chain B (beta-tubulin) of tubulin protein
Current bottlenecks and solutions
Microbes produce complex biological molecules known as microbial secondary metabolites after the completion of their development phase. Secondary metabolites are essential for other secondary needs, but they play little part in the growth and reproduction of microorganisms themselves. These compounds have been used in cosmetics, food, medications, etc. (Barrios-Gonzalez et al. 2005). Attempts to use endophytic microorganisms in the scale-up synthesis of metabolites have met with low yields and performance, even though production and isolation of secondary metabolites can occasionally be successful in small-scale laboratory circumstances (Stadler and Schulz 2009). One of the main obstacles to obtaining their commercial production is the significant decrease in secondary metabolite production with repeated subculturing under axenic monoculture conditions. Unfortunately, practically all of the attempts to extract natural compounds from endophytic microbes relevant to biotechnology have been made through the fermentation of axenic monocultures (Scherlach and Hertweck 2009). Numerous methods for increasing yield and productivity have been suggested to produce critical secondary metabolites on an industrial scale. The current techniques are genetic engineering, co-culture fermentation, and process optimization (Yan et al. 2018). Mutagenesis can be performed on endophytes to change an organism’s genetic makeup and increase metabolite outputs (Venugopalan and Srivastava 2015). Co-culture fermentation includes growing two or more microorganisms together in a small habitat, potentially more closely resembling natural microbial communities. This technique is based on the general hypothesis that some microbial secondary metabolite gene clusters may be induced or triggered in the presence of other microbes (Bertrand et al. 2014). Numerous other factors, many of which are poorly understood, affect the development of secondary metabolites produced from endophytic microbes. Composition of cultivation medium, pH, temperature, nutrient ratios, duration of incubation, and aeration are some known parameters (Elmoslamy et al. 2017). More incredible applications must be made possible by overcoming current difficulties in scale-up development, potential product toxicity, and synthesis (Yan et al. 2018). Furthermore, developing industrial-scale bioprocesses for endophyte co-cultures for the sustainable manufacture of biopharmaceuticals is a topic that has little been studied and is entirely open to further research and development (Kusari et al. 2014).
Conclusion and future directions
Cancer will likely be one of the most essential human challenges for decades. Vincristine and vinblastine, as well as the other Catharanthus roseus-derived agents, is highly beneficial in the fight against cancer. However, the primary disadvantage of their use persists today: their production is both exorbitant and inefficient. Endophytes constitute a potential alternate source for developing secondary metabolites derived from plants. The use of bioprocess optimization has also increased the yield of bioactive compounds produced during the endophytic fermentation process. To compete with chemical synthesis and plant extraction, the most feasible strategy is microbial fermentation of vinca alkaloids. However, a better knowledge of microbial metabolism and the development of more advanced genetic engineering techniques are still required. We must continue to research this area to expand our understanding to improve the efficacy and yield of vinca alkaloids and other anticancer drugs. Figure 6 presents the schematic summary of overcoming roadblocks in scale-up for bioprocess development of high value bioactive compounds.
Fig. 6.
Schematic summary of overcoming roadblocks in scale-up for bioprocess development of high value bioactive compounds
Acknowledgements
The authors would like to thank the Prof. Prem Kumar Khosla, Chancellor, Shoolini University of Biotechnology and Management Sciences, Solan, Himachal Pradesh, India, and the School of Biotechnology here for providing the laboratory and technical facilities in the field of secondary metabolite production. All the structures have been adopted from PubChem, https://pubchem.ncbi.nlm.nih.gov/, and modified for the creation of the images.
Author contributions
AB and PK were involved in conceptualization, data curation, formal analysis, writing—original draft, writing—reviewing and editing, and validation; ST was responsible for data curation, formal analysis, writing—original draft, writing—reviewing and editing, and validation; AS and SKSP contributed to data curation, formal analysis, and validation; and IC took part in data curation and formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical statement
This article does not have any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
All the authors mutually agreed that the work should be published in the 3 Biotech.
References
- Anjum N, Chandra R. Endophytic bacteria of Catharanthus roseus as an alternative source of vindoline and application of response surface methodology to enhance its production. Arch Biol Sci. 2019;71(1):27–38. doi: 10.2298/ABS180802044A. [DOI] [Google Scholar]
- Armand JP, Fumoleau P, Marty M, Variol P, Pinel MC, Picard M, Puozzo C. Pharmacokinetics of vinflunine, a novel Vinca alkaloid, during the Phase I dose-escalation study. Proc Am Assoc Cancer Res. 2001;42:381. [Google Scholar]
- Arora RD, Menezes RG. Vinca alkaloid toxicity. Treasure Island: StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- Ashoka H, Hegde P, Madihalli C, Kumar A, Shettihalli A. Isolation and detection of vinca alkaloids from endophytes isolated from Catharanthus roseus. Eur J Biomed Pharm Sci. 2017;4(10):675–683. [Google Scholar]
- Aslam J, Khan SH, Siddiqui ZH, Fatima Z, Maqsood M, Bhat MA, Sharma MP. Catharanthus roseus (L.) G. Don. An important drug: its applications and production. Pharm Glob (IJCP) 2010;4(12):1–16. [Google Scholar]
- Ataei-Azimi A, Hashemloian BD, Ebrahimzadeh H, Majd A (2008) High in vitro production of anti-cancer indole alkaloids from periwinkle (Catharanthusroseus) tissue culture. Afr J Biotechnol 7(16)
- Ayob FW, Simarani K, Zainal Abidin N, Mohamad J. First report on a novel Nigrospora sphaerica isolated from Catharanthus roseus plant with anticarcinogenic properties. Microb Biotechnol. 2017;10(4):926–932. doi: 10.1111/1751-7915.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandara CJ, Siriwardhana A, Karunaratne DN, Ratnayake Bandara BM, Wickramasinghe A, Krishnarajah SA, Karunaratne V. Production of vincristine and vinblastine by the endophytic fungus Botryosphaeria laricina strain (CRS1) is dependent on stimulating factors present in Catharanthus roseus. J Nat Prod. 2021;11(2):221–230. doi: 10.2174/2210315510666200108102735. [DOI] [Google Scholar]
- Banyal A, Thakur V, Thakur R, Kumar P. Endophytic microbial diversity: a new hope for the production of novel anti-tumor and anti-HIV agents as future therapeutics. Curr Microbiol. 2021;78(5):1699–1717. doi: 10.1007/s00284-021-02359-2. [DOI] [PubMed] [Google Scholar]
- Barnett CJ, Cullinan GJ, Gerzon K, Hoying RC, Jones WE, Newlon WM, Sweeney MJ. Structure-activity relationships of dimeric Catharanthus roseus alkaloids. 1. Deacetyl vinblastine amide (vindesine) sulfate. J Med Chem. 1978;21(1):88–96. doi: 10.1021/jm00199a016. [DOI] [PubMed] [Google Scholar]
- Barrios-Gonzalez J, Fernandez FJ, Tomasini A, Mejia A. Secondary metabolites production by solid-state fermentation. Malays J Microbiol. 2005;1(1):1–6. [Google Scholar]
- Bellmunt J, Théodore C, Demkov T, Komyakov B, Sengelov L, Daugaard G, von der Maase H. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27(27):4454–4461. doi: 10.1200/JCO.2008.20.5534. [DOI] [PubMed] [Google Scholar]
- Bennouna J, Delord JP, Campone M, Nguyen L. Vinflunine: a new microtubule inhibitor agent. Clin Cancer Res. 2008;14(6):1625–1632. doi: 10.1158/1078-0432. [DOI] [PubMed] [Google Scholar]
- Bertrand S, Bohni N, Schnee S, Schumpp O, Gindro K, Wolfender JL. Metabolite induction via microorganism co-culture: a potential way to enhance chemical diversity for drug discovery. Biotechnol Adv. 2014;32(6):1180–1204. doi: 10.1016/j.biotechadv.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Bo G, Haiyan L, Lingqi Z. Isolation of a fungus producing Vinblastine. J Yunnan Univ Nat Sci Ed. 1998;20(3):214–215. [Google Scholar]
- Bonfil RD, Russo DM, Binda MM, Delgado FM, Vincenti M. Higher antitumor activity of vinflunine than vinorelbine against an orthotropic murine model of transitional cell carcinoma of the bladder. Urol Oncol. 2002;7(4):159–166. doi: 10.1016/s1078-1439(02)00184-9. [DOI] [PubMed] [Google Scholar]
- Bowman LC, Houghton JA, Houghton PJ. GTP influences the binding of vincristine in human tumor cytosols. Biochem Biophys Res Commun. 1986;135(3):695–700. doi: 10.1016/0006-291x(86)90984-8. [DOI] [PubMed] [Google Scholar]
- Brogan C (2010) Alkaloids cancer treatments
- Budman DR. Vinorelbine (Navelbiner): a third-generation Vinca alkaloid. Cancer Invest. 1997;15(5):475–490. doi: 10.3109/07357909709047587. [DOI] [PubMed] [Google Scholar]
- Byrd RL, Rohrbaugh TM, Beverly Raney Jr R, Norris DG. Transient cortical blindness secondary to vincristine therapy in childhood malignancies. Cancer. 1981;47(1):37–40. doi: 10.1002/1097-0142(19810101). [DOI] [PubMed] [Google Scholar]
- Caputi L, Franke J, Farrow SC, Chung K, Payne RM, Nguyen TD, O’Connor SE. Missing enzymes in the biosynthesis of the anticancer drug vinblastine in Madagascar periwinkle. Science. 2018;360(6394):1235–1239. doi: 10.1126/science.aat4100. [DOI] [PubMed] [Google Scholar]
- Chandra S. Endophytic fungi: novel sources of anticancer lead molecules. Appl Microbiol Biotechnol. 2012;95(1):47–59. doi: 10.1007/s00253-012-4128-7. [DOI] [PubMed] [Google Scholar]
- Chandra S, Chandra R. Engineering secondary metabolite production in hairy roots. Phytochem Rev. 2011;10(3):371. doi: 10.1007/s11101-011-9210-8. [DOI] [Google Scholar]
- Coderch C, Morreale A, Gago F. Tubulin-based structure-affinity relationships for antimitotic Vinca alkaloids. Anticancer Agents Med Chem. 2012;12(3):219–225. doi: 10.1002/vjch.201900087. [DOI] [PubMed] [Google Scholar]
- Courdavault V, O’Connor SE, Oudin A, Besseau S, Papon N. Towards the microbial production of plant-derived anticancer drugs. Trends Cancer. 2020;6(6):444–448. doi: 10.1016/j.trecan.2020.02.004. [DOI] [PubMed] [Google Scholar]
- Craig SL, Jensen VB. Cancer nanotechnology. Amsterdam: Elsevier; 2017. Animal models in cancer nanotechnology; pp. 45–69. [Google Scholar]
- Da Silva PP, Meijer L. Search for natural substances with therapeutic activity: Pierre Potier (1934–2006) Med Sci. 2012;28(5):534–542. doi: 10.1051/medsci/2012285020. [DOI] [PubMed] [Google Scholar]
- Dandamudi S, Campbell RB. The drug loading, cytotoxicity and tumor vascular targeting characteristics of magnetite in magnetic drug targeting. Biomaterials. 2007;28(31):4673–4683. doi: 10.1016/j.biomaterials.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Datta A, Srivastava PS. Variation in vinblastine production by Catharanthus roseus, during in vivo and in vitro differentiation. Phytochemistry. 1997;46(1):135–137. doi: 10.1016/S0031-9422(97)00165-9. [DOI] [Google Scholar]
- De Luca V, Marineau C, Brisson N. Molecular cloning and analysis of cDNA encoding a plant tryptophan decarboxylase: comparison with animal dopa decarboxylases. Proc Natl Acad Sci. 1989;86(8):2582–2586. doi: 10.1073/pnas.86.8.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhyani P, Quispe C, Sharma E, Bahukhandi A, Sati P, Attri DC, Cho WC. Anticancer potential of alkaloids: a key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022;22(1):1–20. doi: 10.1186/s12935-022-02624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenech GH, Vogel CL. A review of vinorelbine in the treatment of breast cancer. Clin Breast Cancer. 2001;2(2):113–128. doi: 10.3816/CBC.2001.n.016. [DOI] [PubMed] [Google Scholar]
- Duflos A, Kruczynski A, Barret JM. Novel aspects of natural and modified vinca alkaloids. Curr Med Chem Anticancer Agents. 2002;2(1):55–70. doi: 10.2174/1568011023354452. [DOI] [PubMed] [Google Scholar]
- Dumontet C, Sikic BI. Mechanisms of action of and resistance to anti-tubulin agents: microtubule dynamics, drug transport, and cell death. J Clin Oncol. 1999;17(3):1061–1061. doi: 10.1200/JCO.1999.17.3.1061. [DOI] [PubMed] [Google Scholar]
- Dyke RW, Nelson RL, Brade WP. Vindesine a short review of preclinical and first clinical data. Cancer Chemother Pharmacol. 1979;2(4):229–232. doi: 10.1007/BF00257185. [DOI] [PubMed] [Google Scholar]
- Elmoslamy SH, Elkady MF, Rezk AH, Abdelfattah YR. Applying taguchi design and large-scale strategy for mycosynthesis of nanosilver from endophytic Trichoderma harzianum SYA.F4 and its application against phytopathogens. Sci Rep. 2017;7:45297. doi: 10.1038/srep45297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed ER. Discovery of the anticancer drug vinblastine from the endophytic Alternaria alternata and yield improvement by gamma irradiation mutagenesis. J Appl Microbiol. 2021;131(6):2886–2898. doi: 10.1111/jam.15169. [DOI] [PubMed] [Google Scholar]
- El-Sayed M, Verpoorte R. Catharanthus terpenoid indole alkaloids: biosynthesis and regulation. Phytochem Rev. 2007;6(2):277–305. doi: 10.1007/s11101-006-9047-8. [DOI] [Google Scholar]
- Facchini PJ. Alkaloid biosynthesis in plants: biochemistry, cell biology, molecular regulation, and metabolic engineering applications. Annu Rev Plant Biol. 2001;52(1):29–66. doi: 10.1146/annurev.arplant.52.1.29. [DOI] [PubMed] [Google Scholar]
- Facchini PJ, Huber-Allanach KL, Tari LW. Plant aromatic L-amino acid decarboxylases: evolution, biochemistry, regulation, and metabolic engineering applications. Phytochemistry. 2000;54(2):121–138. doi: 10.1016/s0031-9422(00)00050-9. [DOI] [PubMed] [Google Scholar]
- Fahy J, Duflos A, Ribet JP, Jaequesy JC, Berrier C, Jouannetaud MP, Zunino F (1997) Vinca alkaloids in superacidic media: a method for creating a new family of antitumor derivatives. J Am Chem Soc 119(36):8576–8577
- Ferguson PJ, Cass CE. Differential cellular retention of vincristine and vinblastine by cultured human promyelocytic leukemia HL-60/Cl cells: the basis of differential toxicity. Cancer Res. 1985;45(11 Pt 1):5480–5488. [PubMed] [Google Scholar]
- Ferguson PJ, Phillips JR, Selner M, Cass CE. Differential activity of vincristine and vinblastine against cultured cells. Cancer Res. 1984;44(8):3307–3312. [PubMed] [Google Scholar]
- Galindo-Solís JM, Fernández FJ. Endophytic fungal terpenoids: natural role and bioactivities. Microorganisms. 2022;10(2):339. doi: 10.3390/microorganisms10020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidding CM, Kellie SJ, Kamps WA, De Graaf SN. Vincristine revisited. Crit Rev Oncol Hematol. 1999;29(3):267–287. doi: 10.1016/S1040-8428(98)00023-7. [DOI] [PubMed] [Google Scholar]
- Goa KL, Faulds D. Vinorelbine. Drugs Aging. 1994;5(3):200–234. doi: 10.2165/00002512-199405030-00006. [DOI] [PubMed] [Google Scholar]
- Gregory RK, Smith IE. Vinorelbine—a clinical review. Br J Cancer. 2000;82(12):1907–1913. doi: 10.1054/bjoc.2000.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groger D (1985) Alkaloids derived from tryptophan. In: Mothes K, Schutte HR, Luckner M (ed) Biochemistry of Alkaloids. VEB Deutscher Verlag, Berlin, pp 272–313
- Hill BT, Fiebig HH, Waud WR, Poupon MF, Colpaert F, Kruczynski A. Superior in vivo experimental antitumor activity of vinflunine, relative to vinorelbine, in a panel of human tumor xenografts. Eur J Cancer. 1999;35(3):512–520. doi: 10.1016/s0959-8049(98)00416-x. [DOI] [PubMed] [Google Scholar]
- Himes RH. Interactions of the Catharanthus (Vinca) alkaloids with tubulin and microtubules. Pharmacol Ther. 1991;51(2):257–267. doi: 10.1016/0163-7258(91)90081-v. [DOI] [PubMed] [Google Scholar]
- Hisiger S, Jolicoeur M. Analysis of Catharanthus roseus alkaloids by HPLC. Phytochem Rev. 2007;6(2–3):207–234. doi: 10.1007/s11101-006-9036-y. [DOI] [Google Scholar]
- Hong SB, Peebles CA, Shanks JV, San KY, Gibson SI. Expression of the Arabidopsis feedback-insensitive anthranilate synthase holoenzyme and tryptophan decarboxylase genes in Catharanthus roseus hairy roots. J Biotechnol. 2006;122(1):28–38. doi: 10.1016/j.jbiotec.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Houghton PJ, Houghton JA, Bowman LC, Hazelton BJ. Therapeutic selectivity of vinca alkaloids: a role for guanosine 5'-triphosphate. Anticancer Drug Des. 1987;2(2):165–179. [PubMed] [Google Scholar]
- Ishikawa H, Colby DA, Seto S, Va P, Tam A, Kakei H, Boger DL. Total synthesis of vinblastine, vincristine, related natural products, and key structural analogues. J Am Chem Soc. 2009;131(13):4904–4916. doi: 10.1021/ja809842b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs DI, Snoeijer W, Hallard D, Verpoorte R. The Catharanthus alkaloids: pharmacognosy and biotechnology. Curr Med Chem. 2004;11(5):607–628. doi: 10.2174/0929867043455846. [DOI] [PubMed] [Google Scholar]
- Joel S. The comparative clinical pharmacology of vincristine and vindesine: does vindesine offer any advantage in clinical use. Cancer Treat Rev. 1995;21(6):513–525. doi: 10.1016/0305-7372(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Johnson IS. Historical background of Vinca alkaloid research and areas of future interest. Cancer Treat Rep. 1968;52(4):455–461. [PubMed] [Google Scholar]
- Johnson IS, Wright HF, Svoboda GH, Vlantis J. Anti-tumor principles derived from Vinca rosea Linn I. Vinca leukoblastine and leurosine. Cancer Res. 1960;20(7):1016–1022. [PubMed] [Google Scholar]
- Johnson IS, Armstrong JG, Gorman M, Burnett JP. The vinca alkaloids: a new class of oncolytic agents. Cancer Res. 1963;23(8 Part 1):1390–1427. [PubMed] [Google Scholar]
- Johnson SA, Harper P, Hortobagyi GN, Pouillart P. Vinorelbine: an overview. Cancer Treat Rev. 1996;22(2):127–142. doi: 10.1016/s0305-7372(96)90032-8. [DOI] [PubMed] [Google Scholar]
- Jordan MA, Himes RH, Wilson L. Comparison of the effects of vinblastine, vincristine, vindesine, and vinepidine on microtubule dynamics and cell proliferation in vitro. Cancer Res. 1985;45(6):2741–2747. [PubMed] [Google Scholar]
- Kruczynski A, Barret JM, Etiévant C, Colpaert F, Fahy J, Hill BT. Antimitotic and tubulin-interacting properties of vinflunine, a novel fluorinated Vinca alkaloid. Biochem Pharmacol. 1998;55(5):35–648. doi: 10.1016/s0006-2952(97)00505-4. [DOI] [PubMed] [Google Scholar]
- Kruczynski A, Colpaert F, Tarayre JP, Mouillard P, Fahy J, Hill BT. Preclinical in vivo antitumor activity of vinflunine, a novel fluorinated Vinca alkaloid. Cancer Chemother Pharmacol. 1998;41(6):437–447. doi: 10.1007/s002800050764. [DOI] [PubMed] [Google Scholar]
- Kruczynski A, Etievant C, Chansard N, Cabrol N, Astruc J, Chazottes E, Hill BT. Induction of apoptosis by vinflunine, a novel fluorinated Vinca alkaloid. Ann Oncol. 1998;9:102–102. [Google Scholar]
- Kumar A. Vincristine and vinblastine: a review. IJMPS. 2016;6:23–30. [Google Scholar]
- Kumar A, Patil D, Rajamohanan PR, Ahmad A. Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PLoS ONE. 2013 doi: 10.1371/journal.pone.0071805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Singh B, Thakur V, Thakur A, Thakur N, Pandey D, Chand D. Hyper-production of taxol from Aspergillus fumigatus, an endophytic fungus isolated from Taxus sp. of the Northern Himalayan region. Biotechnol Rep. 2019;24:e00395. doi: 10.1016/j.btre.2019.e00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusari S, Singh S, Jayabaskaran C. Biotechnological potential of plant-associated endophytic fungi: hope versus hype. Trends Biotechnol. 2014;32(6):297–303. doi: 10.1016/j.tibtech.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Leete E. Biogenesis of the Rauwolfia alkaloids—II: the incorporation of tryptophan into serpentine and reserpine. Tetrahedron. 1961;14(1–2):35–41. doi: 10.1016/0040-4020(61)80084-7. [DOI] [Google Scholar]
- Levac D, Murata J, Kim WS, De Luca V. Application of carborundum abrasion for investigating the leaf epidermis: molecular cloning of Catharanthus roseus 16-hydroxytabersonine-16-O-methyltransferase. Plant J. 2008;53(2):225–236. doi: 10.1111/j.1365-313X.2007.03337.x. [DOI] [PubMed] [Google Scholar]
- Lingqi Z, Bo G, Haiyan L, Songrong Z, Hua S, Su G, Rongcheng W. Preliminary study on the isolation of endophytic fungus of Catharanthus roseus and its fermentation to produce products of therapeutic value. Chin Tradit Herb Drugs. 2000;31(11):805–807. [Google Scholar]
- Liscombe DK, O’Connor SE. A virus-induced gene silencing approach to understanding alkaloid metabolism in Catharanthus roseus. Phytochemistry. 2011;72(16):1969–1977. doi: 10.1016/j.phytochem.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscombe DK, Usera AR, O'Connor SE. Homolog of tocopherol C methyltransferases catalyze N methylation in anticancer alkaloid biosynthesis. Proc Natl Acad Sci. 2010;107(44):18793–18798. doi: 10.1073/pnas.1009003107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhu J, Tang L, Wen W, Lv S, Yu R. Enhancement of vindoline and vinblastine production in suspension-cultured cells of Catharanthus roseus by artemisinic acid elicitation. World J Microbiol Biotechnol. 2014;30:175–180. doi: 10.1007/s11274-013-1432-z. [DOI] [PubMed] [Google Scholar]
- Liu J, Cai J, Wang R, Yang S. Transcriptional regulation and transport of terpenoid indole alkaloid in Catharanthus roseus: exploration of new research directions. Int J Mol Sci. 2017;18(1):53. doi: 10.3390/ijms18010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqsood M, Abdul M. Yeast extract elicitation increases vinblastine and vincristine yield in protoplast derived tissues and plantlets in Catharanthus roseus. Rev Bras Farmacogn. 2017;27:549–556. doi: 10.1016/j.bjp.2017.05.008. [DOI] [Google Scholar]
- Martino E, Casamassima G, Castiglione S, Cellupica E, Pantalone S, Papagni F, Collina S. Vinca alkaloids and analogues as anti-cancer agents: looking back, peering ahead. Bioorganic Med Chem Lett. 2018;28(17):2816–2826. doi: 10.1016/j.bmcl.2018.06.044. [DOI] [PubMed] [Google Scholar]
- Maswadeh H, Demetzos C, Daliani I, Kyrikou I, Mavromoustakos T, Tsortos A, Nounesis G. A molecular basis explanation of the dynamic and thermal effects of vinblastine sulfate upon di palmitoyl phosphatidylcholine bilayer membranes. Biochim Biophys Acta Biomembr. 2002;1567:49–55. doi: 10.1016/s0005-2736(02)00564-3. [DOI] [PubMed] [Google Scholar]
- Mckay LI, Cidlowski JA (2003) Corticosteroids in the treatment of neoplasms. In: Kufe DW, Pollock RE, Weichselbaum RR, Bast RC, Gansler TS, Holland JF and Frei E (ed) Cancer Medicine, 6th edn. BC Decker, Hamilton ON, Canada. https://www.ncbi.nlm.nih.gov/books/NBK13383/
- Memelink J, Verpoorte R, Kijne JW. Orcanization of jasmonate-responsive gene expression in alkaloid metabolism. Trends Plant Sci. 2001;6(5):212–219. doi: 10.1016/s1360-1385(01)01924-0. [DOI] [PubMed] [Google Scholar]
- Mistry V, Darji S, Tiwari P, Sharma A. Engineering Catharanthus roseus monoterpenoid indole alkaloid pathway in yeast. J Appl Microbiol. 2022;106(7):2337–2347. doi: 10.1007/s00253-022-11883-5. [DOI] [PubMed] [Google Scholar]
- Mollaamin F, Monajjemi M, Mehrzad J. Molecular modelling investigation of an anti-cancer agent joint to SWCNT using theoretical methods. Fuller Nanotub Carbon Nanostruct. 2014;22(8):738–751. doi: 10.1080/1536383X.2012.731582. [DOI] [Google Scholar]
- Moreno PR, van der Heijden R, Verpoorte R. Cell and tissue cultures of Catharanthus roseus: a literature survey. Plant Cell Tissue Organ Cult. 1995;42(1):1–25. doi: 10.1007/BF00037677. [DOI] [Google Scholar]
- Moudi M, Go R, Yien CYS, Nazre M. Vinca alkaloids. Int J Prev Med. 2013;4(11):1231. [PMC free article] [PubMed] [Google Scholar]
- Munier F, Perentes E, Herbort CP, Uffer S, Biollaz J. Selective loss of optic nerve β-tubulin in vincristine-induced blindness. Am J Med. 1992;93(2):232–234. doi: 10.1016/0002-9343(92)90058-j. [DOI] [PubMed] [Google Scholar]
- Ngan VK, Bellman K, Hill BT, Wilson L, Jordan MA. Mechanism of mitotic block and inhibition of cell proliferation by the semisynthetic Vinca alkaloids vinorelbine and its newer derivative vinflunine. Mol Pharmacol. 2001;60(1):225–232. doi: 10.1124/mol.60.1.225. [DOI] [PubMed] [Google Scholar]
- Noble RL. The discovery of the vinca alkaloids—chemotherapeutic agents against cancer. Biochem Cell Biol. 1990;68(12):1344–1351. doi: 10.1139/o90-197. [DOI] [PubMed] [Google Scholar]
- O'Donnell PH, Guo WX, Reynolds CP, Maurer BJ. N-(4-hydroxyphenyl) retinamide increases ceramide and is cytotoxic to acute lymphoblastic leukemia cell lines, but not to non-malignant lymphocytes. Leukemia. 2002;16(5):902–910. doi: 10.1038/sj.leu.2402485. [DOI] [PubMed] [Google Scholar]
- Oudin A, Mahroug S, Courdavault V, Hervouet N, Zelwer C, Rodríguez-Concepción M, Burlat V. Spatial distribution and hormonal regulation of gene products from methyl erythritol phosphate and monoterpene-secoiridoid pathways in Catharanthus roseus. Plant Mol Biol. 2007;65(1):13–30. doi: 10.1007/s11103-007-9190-7. [DOI] [PubMed] [Google Scholar]
- Owellen RJ, Owens AH, Jr, Donigian DW. The binding of vincristine, vinblastine and colchicine to tubulin. Biochem Biophys Res Commun. 1972;47(4):685–691. doi: 10.1016/0006-291x(72)90546-3. [DOI] [PubMed] [Google Scholar]
- Owellen RJ, Donigian DW, Hartke CA, Hains FO. Correlation of biologic data with physico-chemical properties among the vinca alkaloids and their congeners. Biochem Pharmacol. 1977;26(13):1213–1219. doi: 10.1016/0006-2952(77)90108-3. [DOI] [PubMed] [Google Scholar]
- Owellen RJ, Hartke CA, Hains FO. Pharmacokinetics and metabolism of vinblastine in humans. Cancer Res. 1977;37(8 Part 1):2597–2602. [PubMed] [Google Scholar]
- Palem PPC, Kuriakose GC, Jayabaskaran C. An endophytic fungus, Talaromyces radicus, isolated from Catharanthus roseus, produces vincristine and vinblastine, which induce apoptotic cell death. PLoS ONE. 2016;11(4):e0153111. doi: 10.1371/journal.pone.0153111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SS, Singh S, Babu CV, Shanker K, Srivastava NK, Shukla AK, Kalra A. Fungal endophytes of Catharanthus roseus enhance vindoline content by modulating structural and regulatory genes related to terpenoid indole alkaloid biosynthesis. Sci Rep. 2016;6(1):1–14. doi: 10.1038/srep26583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrangi SL, Chalumuri SS, Garimella S. Emerging therapeutic efficacy of alkaloids as anticancer agents. Ann Romanian Soc Cell Biol. 2022;26(01):64–74. [Google Scholar]
- Parthasarathy R, Shanmuganathan R, Pugazhendhi A. Vinblastine production by the endophytic fungus Curvularia verruculosa from the leaves of Catharanthus roseus and its in vitro cytotoxicity against HeLa cell line. Anal Biochem. 2020;593:113530. doi: 10.1016/j.ab.2019.113530. [DOI] [PubMed] [Google Scholar]
- Pernot B, Gyan E, Maillot F, Hodges P, Ertault M, Ferreira-Maldent N. Lymphomas diagnosed in an internal medicine department compared to lymphomas diagnosed in other departments: clinical and outcome differences. Medicine. 2018 doi: 10.1097/MD.0000000000013228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliankong P, Suksa-Ard P, Wannakrairoj S. Chitosan elicitation for enhancing of vincristine and vinblastine accumulation in cell culture of Catharanthus roseus (L.) G. Don. J Agric Sci. 2018;10(12):287–293. doi: 10.5539/jas.v10n12p287. [DOI] [Google Scholar]
- Qu Y, Easson ME, Simionescu R, Hajicek J, Thamm AM, Salim V, De Luca V. Solution of the multistep pathway for assembly of corynanthean, strychnos, iboga, and aspidosperma monoterpenoid indole alkaloids from 19E-geissoschizine. Proc Natl Acad Sci. 2018;115(12):3180–3185. doi: 10.1073/pnas.1719979115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault JH, Nuzillard JM, Le Crouérour G, Thépenier P, Zèches-Hanrot M, Le Men-Olivier L. Isolation of indole alkaloids from Catharanthus roseus by centrifugal partition chromatography in the pH-zone refining mode. J Chromatogr A. 1999;849(2):421–431. doi: 10.1016/s0021-9673(99)00495-1. [DOI] [PubMed] [Google Scholar]
- Ripps H, Carr RE, Siegel IM, Greenstein VC. Functional abnormalities in vincristine-induced night blindness. Investig Ophthalmol vis Sci. 1984;25(7):787–794. [PubMed] [Google Scholar]
- Rischer H, Orešič M, Seppänen-Laakso T, Katajamaa M, Lammertyn F, Ardiles-Diaz W, Goossens A. Gene-to-metabolite networks for terpenoid indole alkaloid biosynthesis in Catharanthus roseus cells. Proc Natl Acad Sci. 2006;103(14):5614–5619. doi: 10.1073/pnas.0601027103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SC. Production and engineering of terpenoids in plant cell culture. Nat Chem Biol. 2007;3(7):387–395. doi: 10.1038/nchembio.2007.8. [DOI] [PubMed] [Google Scholar]
- Rowinsky EK, Donehower RC. The clinical pharmacology and use of anti-microtubule agents in cancer chemotherapeutics. Pharmacol Ther. 1991;52(1):35–84. doi: 10.1016/0163-7258(91)90086-2. [DOI] [PubMed] [Google Scholar]
- Sahelian R (2011) Alkaloid substances in plants, information on vinca, ergot and ephedra alkaloid compounds
- Scherlach K, Hertweck C. Triggering cryptic natural product biosynthesis in microorganisms. Org Biomol Chem. 2009;7(9):1753–1760. doi: 10.1039/b821578b. [DOI] [PubMed] [Google Scholar]
- Schutz FA, Bellmunt J, Rosenberg JE, Choueiri TK. Vinflunine: drug safety evaluation of this novel synthetic vinca alkaloid. Expert Opin Drug Saf. 2011;10(4):645–653. doi: 10.1517/14740338.2011.581660. [DOI] [PubMed] [Google Scholar]
- Shams K, Nazif N, Azim N, Shafeek K, Missiry M, Ismail S, Nasr M. Isolation and characterization of antineoplastic alkaloids from Catharanthus roseus L. Don. cultivated in Egypt. Afr J Tradit Complement Altern Med. 2009 doi: 10.4314/ajtcam.v6i2.57082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Verma P, Mathur A, Mathur AK. Genetic engineering approach using early Vinca alkaloid biosynthesis genes led to increased tryptamine and terpenoid indole alkaloids biosynthesis in differentiating cultures of Catharanthus roseus. Protoplasma. 2018;255:425–435. doi: 10.1007/s00709-017-1151-7. [DOI] [PubMed] [Google Scholar]
- Sharma A, Amin D, Sankaranarayanan A, Arora R, Mathur AK. Present status of Catharanthus roseus monoterpenoid indole alkaloids engineering in homo-and heterologous systems. Biotechnol Lett. 2020;42(1):11–23. doi: 10.1007/s10529-019-02757-4. [DOI] [PubMed] [Google Scholar]
- Sharma A, Tiwari P, Arora R, Sankaranarayanan A. Madagascar periwinkle alkaloids: biosynthesis, ethnobotanical attributes, and pharmacological functions. S Afr J Bot. 2022;151:108–115. doi: 10.1016/j.sajb.2022.09.039. [DOI] [Google Scholar]
- Sheard M, Kang M, Cabral D, Lee J, Castro L, Khankaldyyan V, Reynolds C. Bone marrow-level oxygen tension enables enhanced and sustained growth of new pediatric acute lymphoblastic leukemia cell lines. Cancer Res. 2007 doi: 10.1038/sj.leu.2402485. [DOI] [Google Scholar]
- Siddiqui MJA, Ismail Z, Saidan NH. Simultaneous determination of secondary metabolites from Vinca rosea plant extractives by reverse phase high performance liquid chromatography. Pharmacogn Mag. 2011;7(26):92. doi: 10.4103/0973-1296.80662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri R. New prospects for vinblastine analogues as anticancer agents. J Med Chem. 2013;56(3):625–627. doi: 10.1021/jm400002j. [DOI] [PubMed] [Google Scholar]
- Singer WD, Himes RH. Cellular uptake and tubulin binding properties of four Vinca alkaloids. Biochem Pharmacol. 1992;43(3):545–551. doi: 10.1016/0006-2952(92)90577-6. [DOI] [PubMed] [Google Scholar]
- Song KM, Park SW, Hong WH, Lee H, Kwak SS, Liu JR. Isolation of vindoline from Catharanthus roseus by supercritical fluid extraction. Biotechnol Prog. 1992;8(6):583–586. doi: 10.1021/bp00018a018. [DOI] [PubMed] [Google Scholar]
- Sottomayor M, Barceló AR. The Vinca alkaloids: from biosynthesis and accumulation in plant cells, to uptake, activity and metabolism in animal cells. Stud Nat Prod Chem. 2006;33:813–857. doi: 10.1016/S1572-5995(06)80041-4. [DOI] [Google Scholar]
- Stadler M, Schulz B. High energy biofuel from endophytic fungi? Trends Plant Sci. 2009;14(7):353–355. doi: 10.1016/j.tplants.2009.05.001. [DOI] [PubMed] [Google Scholar]
- St-Pierre B, De Luca V. A cytochrome P-450 monooxygenase catalyzes the first step in the conversion of tabersonine to vindoline in Catharanthus roseus. Plant Physiol. 1995;109(1):131–139. doi: 10.1104/pp.109.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre B, Laflamme P, Alarco AM, Luca E. The terminal O-acetyltransferase involved in vindoline biosynthesis defines a new class of proteins responsible for coenzyme A-dependent acyl transfer. Plant J. 1998;14(6):703–713. doi: 10.1046/j.1365-313x.1998.00174.x. [DOI] [PubMed] [Google Scholar]
- St-Pierre B, Vazquez-Flota FA, De Luca V. Multicellular compartmentation of Catharanthus roseus alkaloid biosynthesis predicts intercellular translocation of a pathway intermediate. Plant Cell. 1999;11(5):887–900. doi: 10.1105/tpc.11.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsis EC, Carqueijeiro I, de Bernonville TD, Franke J, Dang TT, Oudin A, O’Connor SE. A three enzyme system to generate the Strychnos alkaloid scaffold from a central biosynthetic intermediate. Nat Commun. 2017;8(1):1–10. doi: 10.1038/s41467-017-00154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmaiah KN, Sethi VS. Chemical characterization of the degradation products of vinblastine dihydrogen sulfate. Cancer Res. 1985;45(11 Part 1):5382–5385. [PubMed] [Google Scholar]
- Tonk D, Mujib A, Maqsood M, Ali M, Zafar N. Aspergillus flavus fungus elicitation improves vincristine and vinblastine yield by augmenting callus biomass growth in Catharanthus roseus. Plant Cell Tissue Organ Cult. 2016;126(2):291–303. doi: 10.1007/s11240-016-0998-1. [DOI] [Google Scholar]
- Van Tellingen O, Beijnen JH, Nooijen WJ, Bult A. Plasma pharmacokinetics of vinblastine and the investigational Vinca alkaloid N-(deacetyl-O-4-vinblastoyl-23)-L-ethyl isoleucinate in mice as determined by high-performance liquid chromatography. Cancer Res. 1993;53(9):2061–2065. [PubMed] [Google Scholar]
- Vazquez-Flota F, De Carolis E, Alarco AM, De Luca V. Molecular cloning and characterization of desacetoxyvindoline-4-hydroxylase, a 2-oxoglutarate dependent-dioxygenase involved in the biosynthesis of vindoline in Catharanthus roseus (L.) G. Don. Plant Mol Biol. 1997;34(6):935–948. doi: 10.1023/a:1005894001516. [DOI] [PubMed] [Google Scholar]
- Venugopalan A, Srivastava S. Endophytes as in vitro production platforms of high value plant secondary metabolites. Biotechnol Adv. 2015;33(6):873–887. doi: 10.1016/j.biotechadv.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Verma P, Mathur AK, Srivastava A, Mathur A. Emerging trends in research on spatial and temporal organization of terpenoid indole alkaloid pathway in Catharanthus roseus: a literature update. Protoplasma. 2012;249(2):255–268. doi: 10.1007/s00709-011-0291-4. [DOI] [PubMed] [Google Scholar]
- Vu PTB, Bui AL, Nguyen NN, Quach PND. In vitro growth and content of vincristine and vinblastine of Catharanthus roseus L. hairy roots in response to precursors and elicitors. Plant Sci Today. 2022;9(1):21–28. doi: 10.14719/pst.1337. [DOI] [Google Scholar]
- WHO Model List of Essential Medicines, 20th List (2017)
- Wink M, Alfermann AW, Franke R, Wetterauer B, Distl M, Windhövel J, Ripplinger P. Sustainable bio production of phytochemicals by plant in vitro cultures: anticancer agents. Plant Genet Resour. 2005;3(2):90–100. doi: 10.1079/PGR200575. [DOI] [Google Scholar]
- Yan L, Zhao H, Zhao X, Xu X, Di Y, Jiang C, Jin M. Production of bio products by endophytic fungi: chemical ecology, biotechnological applications, bottlenecks, and solutions. Appl Microbiol Biotechnol. 2018;102(15):6279–6298. doi: 10.1007/s00253-018-9101-7. [DOI] [PubMed] [Google Scholar]
- Yang X, Zhang L, Guo B, Guo S (1994) Preliminary study of a vincristine-producing endophytic fungus isolated from leaves of Catharanthusroseus. Chin Tradit Herb Drugs
- Yokoshima S, Ueda T, Kobayashi S, Sato A, Kuboyama T, Tokuyama H, Fukuyama T. Stereo controlled total synthesis of (+)-vinblastine. J Am Chem Soc. 2002;124(10):2137–2139. doi: 10.1021/ja0177049. [DOI] [PubMed] [Google Scholar]
- Zafari D, Leylaiee S, Tajick MA. Isolation and identification of vinblastine from the fungus of Chaetomium globosum Cr95 isolated from Catharanthus roseus plant. BJM. 2019;8(32):1–14. doi: 10.22108/BJM.2018.107927.1095. [DOI] [Google Scholar]
- Zaferanloo B, Virkar A, Mahon PJ, Palombo EA. Endophytes from an Australian native plant are a promising source of industrially useful enzymes. World J Microbiol Biotechnol. 2013;29(2):335–345. doi: 10.1007/s11274-012-1187-y. [DOI] [PubMed] [Google Scholar]
- Zhou X, Xu Z, Li A, Zhang Z, Xu S. Double-sides sticking mechanism of vinblastine interacting with α, β-tubulin to get activity against cancer cells. J Biomol Struct Dyn. 2018 doi: 10.1080/07391102.2018.1539412. [DOI] [PubMed] [Google Scholar]
- Zhu J, Wang M, Wen W, Yu R. Biosynthesis and regulation of terpenoid indole alkaloids in Catharanthus roseus. Pharmacogn Rev. 2015;9(17):24–28. doi: 10.4103/0973-7847.156323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.