Abstract
Andaliman (Z. acanthopodium DC) is a kind of flowering plant that belongs to the Rutaceae family. The habitats are found in southwestern China (Guangxi, Guizhou, Sichuan, Tibet, and Yunnan), Bangladesh, Bhutan, northern India, and northeastern India (Arunachal Pradesh, Assam, Manipur, Meghalaya, Nagaland, Sikkim, Uttar Pradesh, and West Bengal), Nepal, Laos, Burma, Vietnam, North Sumatra highlands, Peninsular Malaysia, and northern Thailand. Andaliman is indigenous to North Sumatra, more specifically the Toba Samosir District, North Tapanuli, and the Dairi region. The phytochemical investigation showed some terpenoids as well as other compounds such as alkaloids, flavonoids, glycosides, tannins, and saponins, but they have not been entirely identified. In Indonesia, the plant is employed both in the culinary industry as an additive for flavoring food and in the traditional medical system for treating various diseases. It was reported to possess antibacterial, antifungal, anti-inflammatory, anticancer, cardioprotective, hepatoprotective, nephroprotective, and wound healing properties, alongside other activities related to pregnancy that were tested in vitro and in vivo. The results of the investigation were based on previously published studies. This review serves as information and a summary, thereby making further exploration of Andaliman to be easier.
Keywords: Andaliman, Zanthoxylum acanthopodium DC, Phytochemistry, Pharmacology
1. Introduction
Indonesia is a country rich in biodiversity with 30,000–40,000 plant species, out of which 2500–7500 are medicinal, whether native, introduced, wild, or cultivated species [1,2]. Moreover, their value has been recognized around the globe for centuries, as drugs and cosmetics, and used in both traditional and modern ways. The large demand for different types of plants produced as raw materials for modern and traditional medicine (herbs) leads to tremendous trade from local to international scales [3,4]. Indonesia ranked fourth as the world's main producer of medicinal plants. Despite possessing many herbal plants, their medicinal potential is not yet used fully [5,6].
One of the spices with an application limited to primary commodities is Z. acanthopodium DC. In the plant family Rutaceae, locally known as Andaliman [7]. Andaliman is a common wild plant species in North Sumatera Utara, Indonesia. Andaliman has many names in Indonesia such as Toba, Inti-intir, Sinyar-sinyar, and Syarnyar [8]. Andaliman is classified as rare and this plant has not been widely used even among Indonesian people because Andaliman is only found in highland areas [9]. The Andaliman can only be found in North Sumatra, particularly in North Tapanuli, Tobasa, and Dairi. These plants are difficult to cultivate because needed a good location. Most local farmers rely on naturally-grown seedlings from prescribed burning lands rather than sowing. Many natural populations exist in high-slope habitats, while some are in low and mid-altitude rainforests and thickets [10].
Andaliman is a shrub or small tree that grows to a height of 6 m and in a persistent manner [7], with low prickly branches, trunks, and twigs. Its compound leaves containing oil glands are dispersed, stemmed, odd-pinnate, 5–20 cm long, and 3–15 cm wide. Leaflets attached to the winged rachis are 3–11, thorny, shaped oblong, with tapering ends, finely serrated edges, 1–7 cm in length, and 0.5–2.0 cm in breadth. The young or light leaves possess a sparkling green top surface and a green bottom, while some have a green top surface and a reddish-green underside [5,7]. Moreover, there is limited interest in compounds found in the leaves, with axillary flowers that are flat or conical in shape. The petals are 5–7, 1–2 cm long, pale yellow, androgynous. About 5–6 stamens sit at the flower's base, with reddish anthers, 3–4 pistils, and an apocarpous ovule. Andaliman produces true box fruits or capsules, which are round, 2–3 mm in diameter, light green (at a young age), dark red (at maturity), and each has one seed, hard skin, and shiny black color. The seeds are black and will emerge from the old fruit after 10 days at room temperature. The fruits, as well as seeds, are often consumed, and 5–10% are sold in the form of fresh mixed green and red fruit, as presented in Fig. 1 [5,8,10].
Fig. 1.
(A) Leaves and branches of Andaliman (Z acanthopodium), (B) Andaliman (Z acanthopodium) fruit [5].
Andaliman is widely used by the Batak ethnic group in processing, mainly fish and meat. Due to the spicy flavor and distinct aroma, it is called “Batak pepper” [7]. Traditional cuisine seasoned with harvested fruit has a distinct flavor and is empirically more durable. The Batak tribe traditionally uses the plant as a blended seasoning for a variety of dishes, including Arsik carp (carp curry without coconut milk), Natinombur (grilled fish with Andaliman chili sauce), and Sangsang (meat cooked with Andaliman spices) [9]. Apart from its use in food processing, the Batak community also uses Andaliman as a traditional medicine. Andaliman's medicinal potential is due to the flavonoids, terpenes, pyrroloquinoline, quaternary isoquinoline, and apophyrine alkaloids [11,12]. The components provide antimicrobial activity against insects, bronchitis, and dyspepsia, besides functioning as antiviral, anticonvulsant, antifungal, analgesic, antibiotic, hepatoprotective, anti-cancer, and anti-preeclampsia agents [8,12]. Andaliman also reported that it is rich in essential oil content. The high essential oil content was reported to have naturally applicable antimicrobial properties and could serve as an antioxidant [13]. Therefore, this study aims to explore the secondary metabolite content and pharmacological activity of Andaliman which has been proven in vitro and in vivo.
2. Method
2.1. Design
This study is a literature review, namely a survey that combines information from scientific papers, books, and other relevant sources to create an informative, critical, and beneficial synthesis for a certain issue [14].
2.2. Article criteria
The inclusion criteria established to narrow down the search for this review paper were 1) Focus on Andaliman (Z. acanthopodium), 2) In the subject of medicine, 3) Full text, 4) Original study article, and 5) English language articles. The exclusion criteria were 1) Just abstract, 2) Incomplete text, and 3) Double publication. The question of this study is how often scientifically sound is the testing of Andaliman concerning phytochemical analysis and pharmacological activity.
2.3. Article search
For this mini-review and viewpoint, data on Andaliman and herbal medicine were searched and gathered. Google Scholar, PubMed, Science Direct, SciFinder, e.t.c., were employed to search for the articles using the combination of specific words like “Zanthoxylum acanthopodium”, “Andaliman”, “Ethnoecology”, “Phytochemical”, and “Pharmacological”.
2.4. Study selection
The prism technique was applied to select articles, beginning with identification, screening, and eligibility, then ending with the included item, as shown in Fig. 2.
Fig. 2.
Decision trail of included studies.
3. Phytochemical properties of Andaliman (Z. acanthopodium DC)
Phytochemical screening is a preliminary test for determining the group of compounds and providing an overview of secondary metabolites that possess a plant's biological activity. This is used as preliminary information in knowing the group of chemical compounds contained in a plant. Phytochemical screening was performed in this study using certain reagents to identify the chemical compounds found in Andaliman. Secondary metabolite compounds are produced for protection against unfavorable environmental conditions e.g. temperature, climate, as well as pest disorders and diseases. Furthermore, they are grouped based on chemical structure, namely phenolics, saponins, flavonoids, tannins, triterpenoids and alkaloids, and steroids [15,16].
The results showed that extracting Andaliman fruit with methanol generated the highest yield, followed by ethyl acetate, water, and n-hexane (4.15%, 3.97%, 3.23%, and 3.02%, respectively). In this study, single-stage extractions were employed, and the yield is presented in Table 1 [17]. The mentioned solvents were effective for a variety of phytochemicals. Methanol, ethyl acetate, and n-hexane can be used to extract alkaloids. Flavonoids, glycosides, and tannins are extracted with water, methanol, and ethyl acetate. Methanol and ethyl acetate are capable of extracting saponins. Triterpenes/steroids can be extracted using both methanol and n-hexane, but glycoside anthraquinone is only be extracted with methanol. These phytochemical compositions are presented in Table 2 [17,18].
Table 1.
The yield of Andaliman (Z acanthopodium DC) fruit extract.
| Solvent | Yield (%) |
|---|---|
| Water | 3.23 |
| Methanol | 4.15 |
| Ethyl acetate | 3.97 |
| n-Hexane | 3.02 |
Table 2.
The results of the phytochemical composition test of Andaliman (Z acanthopodium DC).
| Phytochemical | Solvent extract [(+) means present, (−) means absent] |
|||
|---|---|---|---|---|
| Water | Methanol | Ethyl acetate | n-Hexane | |
| Alkaloids | – | + | + | + |
| Flavonoids | + | + | + | – |
| Glycosides | + | + | + | – |
| Saponins | – | + | + | – |
| Tannins | + | + | + | – |
| Triterpenes/steroids | – | + | – | + |
| Glycoside anthraquinones | – | + | – | – |
Based on previous articles, there are terpenoids in Andaliman extract. The biological activity of the main components found in the leaves extracted with petroleum ether is exhibited by β-sitosterol, palmitic acid, phytol, paulownin, and eudesmin as presented in Table 3 [19]. Terpenoids constitute a class of compounds that include essential oils and are built by sub-units C5. Most essential oils are referred to as volatile oils. They produce distinct aromas and many have a monetary value. The majority comes from the monoterpenoid (C10) and sesquiterpenoid (C15) groups [[20], [21], [22]]. Further examination showed the main content of Andaliman was primarily in the form of essential oils. After more investigation using GC/MS, 20 different chemicals were detected in the fruit's methanolic extract. Up to 16 chemical compounds were terpenes and terpenoids derivatives, namely citronellol, geraniol, geranyl acetate, E-β-caryophyllene, 2-hexadecen-1-ol, myrtenol, (E,E)-farnesylacetone, farnesol, citronellyl propionate, geranyl hexanoate, citronellyl acetate, 1,5,9-decatriene, 2,3,5,8-tetramethyl, and myrtenyl acetate (Table 3) [23]. These were also confirmed in the n-hexane fraction [24].
Table 3.
Identified phytochemical compounds of Andaliman (Z acanthopodium).
| Active Compounds | IUPAC Name | Chemical Structure | Source Parts | Biological activity | References |
|---|---|---|---|---|---|
| Geranyl acetat | (E)-3,7-dimethylocta-2,6-dien-1-yl acetate | Andaliman fruit | Anticancer | [24] | |
| Limonene | 1-methyl-4-(prop-1-en-2-yl)cyclohex-1-ene | 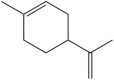 |
Andaliman fruit | Anticancer | [24] |
| Myrcene | 7-methyl-3-methyleneocta-1,6-diene | 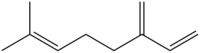 |
Andaliman fruit | Anticancer | [24] |
| Ocimene | (E)-3,7-dimethylocta-1,3,6-triene | 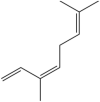 |
Andaliman fruit | Anticancer | [24] |
| Linalool | 3,7-dimethylocta-1,6-dien-3-ol | 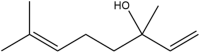 |
Andaliman fruit | Anticancer | [24] |
| Citronellol | 3,7-dimethyloct-6-en-1-ol |  |
Andaliman fruit | Anticancer and Antibacteria | [23,24] |
| Neral | (Z)-3,7-dimethylocta-2,6-dienal | 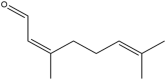 |
Andaliman fruit | Anticancer | [24] |
| Geraniol | (E)-3,7-dimethylocta-2,6-dien-1-ol | 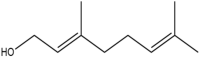 |
Andaliman fruit | Anticancer and Antibacteria | [23,24] |
| E-β-caryophyllene | (4E)-4,11,11-trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene |  |
Andaliman fruit | Anticancer and Antibacteria | [23,24] |
| 2-hexadecen-1-ol | (E)-hexadec-2-en-1-ol | Andaliman fruit | Anticancer and Antibacteria | [23,24] | |
| Ethyl linoleate | (9Z,12Z)-ethyl octadeca-9,12-dienoate | Andaliman fruit | Anticancer and Antibacteria | [23,24] | |
| Myrtenyl acetate | (6,6-dimethylbicyclo[3.1.1]hept-2-en-2-yl)methyl acetate | 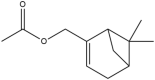 |
Andaliman fruit | Anticancer and Antibacteria | [23,24] |
| 5- (propenyl-2)-1,3,7-nonatriene | (3E,7E)-5-(prop-1-en-2-yl)nona-1,3,7-triene |  |
Andaliman fruit | Anticancer and Antibacteria | [23,24] |
| (E,E)-farnesylacetone | (5E,9E)-6,10,14-trimethylpentadeca-5,9,13-trien-2-one | Andaliman fruit | Anticancer and Antibacteria | [23,24] | |
| Farnesol | (2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-ol | Andaliman fruit | Anticancer and Antibacteria | [23,24] | |
| Citronellyl propionate | 3,7-dimethyloct-6-en-1-yl propionate |  |
Andaliman fruit | Anticancer and Antibacteria | [23,24] |
| Geranyl hexanoate | (E)-3,7-dimethylocta-2,6-dien-1-yl hexanoate | Andaliman fruit | Anticancer and Antibacteria | [23,24] | |
| Citronellyl acetate | 3,7-dimethyloct-6-en-1-yl acetate |  |
Andaliman fruit | Anticancer and Antibacteria | [23,24] |
| 1,5,9-decatriene,2,3,5,8-tetramethyl | (E)-2,3,5,8-tetramethyldeca-1,5,9-triene | 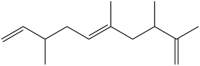 |
Andaliman fruit | Anticancer and Antibacteria | [23,24] |
| Neryl butanoate | (Z)-3,7-dimethylocta-2,6-dien-1-yl butyrate |  |
Andaliman fruit | Anticancer and Antibacteria | [23,24] |
| β-sitosterol | (3S,8S,9S,10R,13R,14S,17R)-17-[(2R,5R)-5-ethyl-6-methylheptan-2-yl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol |  |
Andaliman leaf | Antifungal | [19] |
| Phytol | (E)-3,7,11,15-tetramethylhexadec-2-en-1-ol | Andaliman leaf | Antifungal | [19] | |
| Paulownin | (3R,3aS,6S,6aR)-3,6-bis(1,3-benzodioxol-5-yl)-3,4,6,6a-tetrahydro-1H-furo [3,4-c]furan-3a-ol | 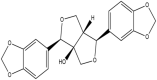 |
Andaliman leaf | Antifungal | [19] |
| Eudesmin | (3S,3aR,6S,6aR)-3,6-bis(3,4-dimethoxyphenyl)-1,3,3a,4,6,6a-hexahydrofuro [3,4-c]furan | 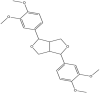 |
Andaliman leaf | Antifungal | [19] |
4. Pharmacological activity of Andaliman (Z. acanthopodium DC)
The study conducted showed several reports concerning the activity of Andaliman in vitro and in vivo. Beneficial effects of the plant include antimicrobial, antifungal, anti-inflammatory, cardioprotective, anticancer, wound healing activity, and other qualities as described in Table 4.
Table 4.
Pharmacological activities of Andaliman (Z. acanthopodium).
| Sample | Sample Doses | Effect | Study Design | Mechanism/Parameter | References |
|---|---|---|---|---|---|
| Andaliman fruit extracts | Extract concentration is 75%, 50%, and 25% | Antibacterial | In vitro | Inhibition zone and Minimum Inhibitory Concentration | [17] |
| Andaliman fruit extracts | Not identified | Antifungal | In vitro | Inhibition zone and Minimum Inhibitory Concentration | [19] |
| Methanolic extract of Andaliman fruit | Dose series of 500 μg/mL, 250 μg/mL, 125 μg/mL, 62,5 μg/mL, and 31,25 μg/mL | Anticancer | In vitro using murine P388 leukemia cells | Cytotoxic activity | [23] |
| n-hexane extract of Andaliman fruit | The range of extract concentration is 1 μg/mL - 256 μg/mL | Antibacterial | In vitro | Minimum Inhibitory Concentration | [25] |
| The active fraction of Andaliman fruit | A dose of 10–25 μg/mL | Anti-inflammatory | In vitro | Decrease of TNF-α, IL-6, and MMP-9 Reduce the mRNA expression of TNF-α, IL-6, COX-2, iNOS, and MMP-9 |
[26] |
| Aqueous and Methanolic extract of Andaliman fruit | Doses of 200 mg/kg BW and 400 mg/kg BW | Anticancer | In vivo using Dalton's Lymphoma Ascites (DLA) cell lines induced cancer in Swiss albino mice | Reduce the tumor volume, packed cell volume, cell viability, and improvement in the hematological parameters | [27] |
| Chloroform fraction (pH 3, 7, and 9) of Andaliman fruit | Doses series of 500 μg/mL, 250 μg/mL, 125 μg/mL, 62,5 μg/mL, and 31,25 μg/mL | Anticancer | In vitro using T47D, 4T1, MCF-7, HeLa, and Raji Cells line | Inhibitory concentration 50 on T47D, 4T1, MCF-7, HeLa, and Raji Cells line | [28] |
| Ethanol extract of Andaliman fruit | Doses series of 500 μg/mL, 250 μg/mL, 125 μg/mL, 62,5 μg/mL, and 31,25 μg/mL | Anticancer | In vitro using MCF-7 cell line | Inhibition of MCF-7 proliferation with IC50 is 222.31 μg/mL | [29] |
| Ethyl acetate fraction of Andaliman fruit | Doses series of 500 μg/mL, 250 μg/mL, 125 μg/mL, 62,5 μg/mL, and 31,25 μg/mL | Anticancer | In vitro using 4T1 cell line | Anti-proliferation activity, cell cycle arrest activity, decrease the COX-2 and VEGFR expressions | [30] |
| Ethyl acetate fraction of Andaliman fruit | Dose series of 500 μg/mL, 250 μg/mL, 125 μg/mL, 62,5 μg/mL, and 31,25 μg/mL | Anticancer | In vitro using T47D cell line | Cytotoxic activity, cell cycle arrest activity, decrease the cyclin D1 and increase p53 | [31] |
| Methanolic extract of Andaliman fruit | Doses of 100, mg/kg BW, 200 mg/kg BW, and 400 mg/kg BW | Anticancer | In vivo using cervical cancer rat models | Histological changes in cervical rats, decrease some proteins like IL-1β, TGFβ1, and VEGFR-1 | [32] |
| Methanolic extract of Andaliman fruit | Doses of 100, mg/kg BW, 200 mg/kg BW, and 400 mg/kg BW | Anticancer | In vivo using cervical cancer rat models | Increase cytochrome c expression | [33] |
| Methanolic extract of a Andaliman fruit |
Doses of 100, mg/kg BW, 200 mg/kg BW, and 400 mg/kg BW | Anticancer | In vivo using cervical cancer rat models | Increase PI3K expression and decrease Wnt expression | [34] |
| Ethyl acetate extract of Andaliman fruit | A dose of 300 mg/kg BW | Cardioprotective | In vivo using doxorubicin-induced rats | Decrease CtnT and CK-MB (markers of cardiotoxicity), histopathological change | [35] |
| Methanolic extract of Andaliman fruit | Doses of 100, mg/kg BW, 200 mg/kg BW, and 400 mg/kg BW | Nephroprotective | In vivo using benzopyrene-induced rats | Decrease cell necrosis, and histopathological change | [36] |
| Methanolic extract of Andaliman fruit | Doses of 100, mg/kg BW, 200 mg/kg BW, and 400 mg/kg BW | Hepatoprotective | In vivo using benzopyrene-induced rats | Decrease cell necrosis, and histopathological change | [36] |
| Andaliman extract-bacterial cellulose | A dose of 100 mg/kg BW | Wound healing | In vivo using diabetic rat models | Histopathological change | [37] |
| Nano herbal formulation of Andaliman fruit | A dose of 100 mg/kg BW | Wound healing | In vivo using diabetic rat models | Histopathological change, fibroblast cell increase | [38] |
| Nano herbal formulation of Andaliman fruit | Not identified | Placental trophoblast protective | In vitro using trophoblast cells of the human placenta | Decrease NOTCH1 and Hes1 expressions | [39] |
| Nano herbal formulation of Andaliman fruit | A 100 mg/200g BW dose and an addition of 1 mL virgin olive oil extract of 1 mL/200g BW for doses combination | Antipreeclampsia | In vivo using hypertension rat models | Reduce the cytochrome c and FasL, increase apoptosis | [40] |
4.1. Antibacterial and antifungal activity
According to Muzafri et al. (2018) [17], the ethyl acetate extract of Andaliman fruit had higher antibacterial activity against S. aureus and S. typhimurium than E. Coli with inhibition zones of 20.06 and 20.19 mm, respectively. This was followed by methanolic extract which had greater antibacterial activity against S. aureus and S. typhimurium with inhibition zones of 17.78 and 17.76 mm, respectively, compared to inhibition against E. Coli. Both extracts were observed at a concentration of 100%. The MIC value of the ethyl acetate extract was determined using the direct method on nutrient broth media against E. coli, S. aureus, and S.typhimurium. MIC values of the fruit extracts ranged from 0.1 to 0.25%, where 0.25% was found in E. coli and S. typhimurium, while S. aureus had 0.1%. Several studies reported the antibacterial activity of Andaliman against a variety of human pathogenic bacteria. The n-hexane extract has also been stated to exhibit antibacterial activity against Mycobacterium smegmatis with a MIC of 64 μg/mL [25].
It was reported that the petroleum ether extract showed moderate activity against two fungi namely Candida albicans and C. Krusei compared to standard Amphotericin-B. C. Krusei had a larger zone of inhibition (18 mm) than C. albicans (10 mm). In comparison to the other solvent extracts, such as chloroform and ethyl acetate, the petroleum ether extract possessed high antifungal activity (18 mm). The extracts' microbial susceptibility was also determined by calculating their minimum inhibitory concentration (MIC) against fungi, where the petroleum ether extract produced the lowest MIC (0.9375 mg/mL). GC-MS analysis of the petroleum ether extract revealed the presence of many bioactive compounds. Among the identified compounds, paulownin, eudesmin, β-sitosterol, dodecanoic acid, tetradecanoic acid, n-hexadecanoic acid, phytol, and octacosanol had antifungal activity [19].
4.2. Anti-inflammatory activity
Polysaccharides, proteins, polyphenols, and essential oil fractions in Andaliman have been reported as anti-inflammatory agents in cells activated with Escherichia lipopolysaccharide (LPS). This augments the production of inflammatory cytokines and proteins e.g. TNF-, IL-6, COX-2, and iNOS. Based on the results, active fractions of ethanolic extract from Andaliman fruits decreased TNF-α and COX-2 expression and MMP-9 activity in macrophages treated with LPS. At the gene level, the extract can effectively block mRNA expression from TNF-α, IL-6, iNOS, COX-2, and MMP-9 [26].
4.3. Anticancer activity
Based on the literature search concerning the pharmacological activity of Andaliman, there were more discussions about cytotoxic activity than other activity tests. According to the reported study, aqueous extract of the fruits has cytotoxic activity in vitro while considering several cancer parameters such as tumor volume, packed cell volume (PCV), cell viability, and improvement in hematological indices. The extract can reduce tumor volume by 5.13 ± 0.24 mL once compared to the Dalton's Lymphoma Ascites (DLA) group, which induced cancer in Swiss albino mice at a rate of 26.12 ± 0.47 mL. These results were supported by a decrease in PCV of the extract group, namely 3.150 ± 0.12 mL compared to the DLA group, which was 11.750 ± 0.55 mL, and a decrease in cell viability, namely 33% compared to 93% in the DLA group alone. In terms of hematological indices, the total white blood cell (WBC) concentration was found to be 12.9 ± 0.06 (x 106/mL) lower in the extract group compared to the DLA group (19.8 ± 0.17 (x 106/mL). This suggests that the extract has a protective effect on the hemopoietic system, albeit with varying degrees of protection [27].
The Andaliman fruit at pH 7 and 9 had cytotoxic or anti-cancer activity against T47D, 4T1, MCF-7, HeLa, and Raji cell lines with IC50 values at pH 7 being 92.67 ± 1.37, 71.87 ± 1.04, 159.87 ± 0.63, 123.39 ± 0.81, and 103.09 ± 0.58 μg/mL, respectively. The IC50 values at pH 9 were, 451.29 ± 25.48, 247.18 ± 2.82, 318.46 ± 5.40, 303.96 ± 8.75, and 181.45 ± 1.35 μg/mL, respectively. These results showed the fruits' alkaloid fractions are effective anticancer agents against various cell lines [28]. This study was supported by the results that the ethanol extract had a fairly active potential as an anti-cancer against the inhibition of MCF-7 cell proliferation with an IC50 value of 221.31 μg/mL [29]. This value indicated the potency of Andaliman seeds' ethanol extract as an anticancer because the IC50 was below 1000 μg/mL [[41], [42], [43]]. Another study showed the antimigration activity of the ethyl acetate fraction of Andaliman fruit against 4T1 and T47D cell lines. The IC50 value for the fraction was calculated to be 48.1 ± 1.06 μg/mL, which became 62.3 ± 0.28% after 72 h of incubation at a dose of 10 μg/mL with live cells. This was accompanied by accumulation in the G2-M phase, and suppression of cell migration by decreasing COX-2 (0.62 ± 0.01) and VEGFR-2 (0.39 ± 0.003) expressions in the conducted experiment [30]. On T47D, the ethyl acetate fraction had a high activity with an IC50 value of 48.94 ± 0.32 μg/mL. A fraction of 25 μg/mL caused cell accumulation at G0/G1 (60.48%) and in a control cell (51.69%) as well as decreased expression of cyclin D1 and increased expression of p53 [31].
Changes were reported in the expression of cytokines such as IL-10, IL1β, and VEGF, which were previously linked to cervical cancer etiology. In serum and cervical cancer histology, there was a significant decrease in the expression of IL1β, TGFβ1, and VEGFR1. Furthermore, it was discovered that Andaliman administration aided IL-10 in inhibiting the proliferation of differentiated abnormal cells. IL-10's primary biological role is to limit and terminate the inflammatory response, as well as to suppress tumor development. Therefore, Andaliman may be a promising molecular cytokine therapy for cervical cancer treatment [32]. Other evidence of the activity of this plant as an anticancer has been stated. The methanolic extract was found to trigger apoptosis in vivo in cervical cancer rats through induction of cytochrome c and suppression of Wnt expression by Increasing PI3K. There was a statistically significant difference between the body weight and the cervical region's weight (p < 0.01). Histology also demonstrated a statistically significant difference between each therapy relating to cytochrome c (p < 0.01). Cytochrome c expression was highest in the dose of 200 mg/kg BW/day [33]. In another pathway, there was a significant difference between all groups (p < 0.001) concerning Wnt and PI3K expression. The real role of Andaliman methanolic extract in cervical cancer tissue was seen at the highest dose of 400 mg/b.wt./day. Irregular mucosal folds and stretched interstitial connective tissue in the K+ group can return to regularity and improve at the 400 mg/kg Bw/day dose. The extract administration showed a significant difference in cervical tissue after benzopyrene injection [34].
In the leukemia case, another study reported that the methanolic extract had a potent anticancer activity with an IC50 value of 19.14 μg/mL. This means a 19.14 μg/mL concentration can inhibit 50% (IC50) of murine P388 leukemia cell proliferation, while doxorubicin has an IC50 value of 0.02 μg/mL. In preliminary testing, the American National Cancer Institute (NCI) considered IC50 30 μg/mL as a potential cytotoxic agent, hence Andaliman crude extract was suggested as a potential source of anticancer agents for treating leukemia [23].
4.4. Cardioprotective, nephroprotective, and hepatoprotective activity
This study showed that Andaliman extract exhibited cardioprotective activity against levels of biomarkers such as cTnT and CK-MB in rats. CTnT values obtained for the group administered with the extract were 0.15 μg/L and CK-MB of 132 U/L compared to the Dox treatment group as presented in Table 5. Similarly, CMC Na 0.5% given to the control group did not cause damage to cardiomyocytes (normal form), and the boundary between heart muscle fiber cells was clear and regular. Bleeding, irregular heart muscle fibers, muscle fiber fragmentation, and pyknosis were observed in the Dox group. Free radicals have a high affinity for cardiomyocytes. Dox-generated free radicals react with unsaturated fatty acids to form lipid peroxides. Cons, changing the structure of the lipid bilayer membrane causes cell damage and death. Based on the results, Andaliman extract has the potential to be cardioprotective by lowering cTnT and CK-MB levels while protecting cardiomyocytes. These are supported by the results of a histopathological examination of the heart using HE (Hematoxylin-Eosin) staining. It was discovered that the Dox-inducing group had blood vessel congestion, pyknosis, myocytolysis, fragmentation, and karyolysis in heart cells, while the extract group had normal myofibrils and no abnormalities [35].
Table 5.
cTnT and CK-MB levels.
| Treatments | cTnT (μg/L) | CK-MB (U/L) |
|---|---|---|
| Control (CMC Na) | 0.31 | 126 |
| Dox | 1.89 | 321 |
| Extract | 0.15 | 132 |
Moreover, the administration of Andaliman methanolic extract has also been observed to be nephroprotective and hepatoprotective in benzopyrene-induced rats. The tubulosus renal constriction of the positive and negative control groups had a statistically significant difference (p < 0.01) once compared. However, there was no statistically significant difference found between the group given the extract treatment dose of 200 mg/kg BW and K+ groups (p > 0.05). In contrast, the value of 300 mg/kg BW and 400 mg/kg BW doses was statistically significant (p < 0.05). The kidney tissue showed the benzopyrene-injected group experienced 96 ± 8.94 necrosis, while 300 mg/kg BW and 400 mg/kg BW groups had decreased necrosis, namely 86 ± 15,57 and 85 ± 20.61 (p < 0.05). The liver histology demonstrated necrosis at values that were significantly different (p < 0.001) from those observed in the K+ groups. Once compared to K+ groups, there was a statistically significant difference in the amount of parenchymal degeneration that occurred in hepatocyte cells (p < 0.01). A statistically significant difference was also found in the liver histology of groups treated with 200 mg/kg BW and 400 mg/kg BW doses (p < 0.001), but no significant change was obtained from the 300 mg/kg BW dose (p > 0.05) [36]. This study showed the methanolic extract dose of 300 mg/kg BW produced nephroprotective and hepatoprotective activity in benzopyrene-induced rats.
4.5. Wound healing activity
Andaliman was stated to have good anti-inflammatory and antibacterial activity. This led to commencement of the plant development in wound treatment. Based on reports, the extract is used as an active ingredient in wound dressing-bacterial cellulose preparations (BC-MZA). An in-vivo test was conducted on 28 male and female rats (Rattus norvegicus) aged 3 months, with body weights ranging from 150 to 180 g, to investigate the efficacy of BC-MZA composites in expediting the burn healing process. The in-vivo test indicated the 15 g/L MZA composite as the most effective for wound dressing. The histopathological examination results showed the burned tissues that were treated with the BC-15 g/L MZA composite had a fully formed epithelial layer and softer scars in the granulation tissues. These are in accordance with the study performed by Pasaribu et al., 2020 [37].
The wound-healing activity of nano herbal Andaliman fruit was also tested in diabetic rats. A significant difference was observed between all groups (p < 0.001). The wound healing results showed that the epithelialization had covered the epidermis, the thick basal membrane had been neatly structured, the dermis was filled with dense collagen connective tissue, and the fibroblast cells increased in number. The proliferation in the wounded skin layer demonstrated that the flavonoids, steroids, saponins, and tannins included in the nano herbal formulation of Haramonting and Andaliman play roles in cell division and growth to build wound tissue. These compounds are found in both herbs [38] and based on the reports, Andaliman has the potential to accelerate the wound healing process. Although, further tests must be carried out to determine the mechanism of action.
4.6. Other activity
There is a possibility that issues with the placental trophoblast could cause pregnancy complications. The Notch gene functionality is essential for the processes of cell death, proliferation, organogenesis, and placental development. In this study, the HTR-8 trophoblast cell was used to determine NOTCH1 and Hes1 expressions. The nano herbal Andaliman has an activity to reduce the NOTCH1 gene expression 30 min after treatment in the human placental trophoblast and Hes1 in less than 16 h [39].
In another study, the nano herbal Andaliman effect was tested to increase apoptosis in preeclampsia rats. Injecting rats with 3 mL of 6% NaCl led to the development of hypertension. Between the 13th and 19th day of pregnancy, the nano herbal (100 mg/kg BW) and EVOO (1 mL) were administered to the pregnant rats. On the 20th day, pregnant rats were dissected using a procedure called cervical dislocation. The results showed the levels of cytochrome c and FasL protein exposure were elevated during hypertensive pregnancy in rats (P < 0.0001) in the labyrinth, basal, and yolk sac zones. The nano herbal administration reduced the expression of cytochrome c as well as FasL. The combination of nano herbal and EVOO was proven to provide a statistically significant difference [40].
5. Toxicological study of Andaliman (Z. acanthopodium DC)
Toxicity studies are important for the development of drugs derived from natural ingredients. Based on our studies, we found several reports regarding the toxicity of Andaliman. The methanolic extract of Andaliman fruit was poisonous in both acute and subacute doses. A 5000 mg/kg BW dosage was used to investigate acute toxicity. In contrast, mice were given 200, 500, and 1000 mg of the extract/kg BW for subacute toxicity. The acute test showed that the mean body weight growth in treated mice was dramatically reduced, although there were no symptoms of morbidity or fatality. Meanwhile, the sub-acute toxicity test demonstrated that the Andaliman extract had a detrimental effect on blood and biochemical markers. Light and electron microscopy of the liver and kidney revealed that the extract harmed the tissues and cellular structures. Furthermore, the extract caused sperm abnormalities by significantly lowering sperm count and viability and increasing the percentage of improperly shaped sperm [44]. Another study looked at the toxicity of the nano herbal Andaliman. According to the published Lethal Concentration 50 (LC50) value of 1737.80 ppm and Lethal Doses 50 (LD50) value of 9.807 g/kg BW 0.075 after providing nano herbal andaliman, this plant in nanosize is categorized as mildly poisonous. A substantial difference (p < 0.05) at each dose level resulted in histological abnormalities in animal tests' liver, lungs, heart, and brain [45].
6. Conclusions
This article provides an overview of the pharmacological activities of Andaliman (Z. acanthopodium DC) that have been investigated in vitro and in vivo, as well as the active components. Terpene groups comprised most of the identified compounds, including geranyl acetate, linalool, and citronellol. On the other hand, the plant contained alkaloids, flavonoids, glycosides, tannins, and saponins. The pharmacological activity can be attributed to the presence of active components. Antibacterial, antifungal, anti-inflammatory, anticancer, cardioprotective, hepatoprotective, nephroprotective, and wound healing are some of the reported pharmacological activities. Investigations on the pharmacological effects are currently in the first stage of laboratory testing. Of course, further tests are needed in the future using Andaliman, including identifying pure active compounds from this plant. Studies, such as those examining the potentially harmful effects of Andaliman, are still lacking. However, the acute and sub-acute toxicity of the methanolic extract and nano herbal of Andaliman fruit was performed. Therefore, further investigation into the benefits and usefulness of clinical testing is needed to create the herbal product containing Andaliman.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
Data included in article/supplementary material/referenced in article.
Funding
No external funding was received.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
References
- 1.Elfahmi, Woerdenbag H.J., Kayser O. Jamu: Indonesian traditional herbal medicine towards rational phytopharmacological use. J. Herb. Med. 2014;4(2):51–73. doi: 10.1016/j.hermed.2014.01.002. [DOI] [Google Scholar]
- 2.Lubis M.F., Hasibuan P.A., Syahputra H., Astyka R. A review on phytochemicals and pharmacological activities as ethnomedicinal uses of duku (lansium domesticum corr.) Open Access Maced J Med Sci. 2022;10(F):57–65. doi: 10.3889/oamjms.2022.8394. [DOI] [Google Scholar]
- 3.Sumarni W., Sudarmin S., Sumarti S.S. The scientification of jamu: a study of Indonesian's traditional medicine. J Phys Conf Ser. 2019;1321(3) doi: 10.1088/1742-6596/1321/3/032057. [DOI] [Google Scholar]
- 4.Lubis M.F., Syahputra H., Illian D.N., Kaban V.E. Antioxidant activity and nephroprotective effect of Lansium parasiticum leaves in doxorubicin-induced rats. J Res Pharm. 2022;26(3):565–573. doi: 10.29228/jrp.154. [DOI] [Google Scholar]
- 5.Rahmawaty. Samosir J.B., Batubara R., Rauf A. Diversity and distribution of medicinal plants in the Universitas Sumatera Utara arboretum of deli serdang, North Sumatra, Indonesia. Biodiversitas. 2019;20(5):1457–1465. doi: 10.13057/biodiv/d200539. [DOI] [Google Scholar]
- 6.Lubis M.F., Hasibuan P.A.Z., Harahap U., Satria D., Syahputra H., Muhammad M., Astyka R. The molecular approach of natural products as pancreatic cancer treatment. Review. 2022;15(2):1362–1377. doi: 10.31788/RJC.2022.1526765. [DOI] [Google Scholar]
- 7.Sonangda M., Sinaga H., Limbong L.N. Effect of ratio of andaliman (Zanthoxylum acanthopodium) with garlic and aging time on the quality of sambal tuk tuk. IOP Conf. Ser. Earth Environ. Sci. 2019;260(1):0–8. doi: 10.1088/1755-1315/260/1/012084. [DOI] [Google Scholar]
- 8.Ompusunggu N.P., Irawati W. Andaliman (zanthoxylum acanthopodium DC.), a rare endemic plant from North Sumatra that rich in essential oils and potentially as antioxidant and antibacterial. J. Biol. Trop. 2021;21(3):1063–1072. doi: 10.29303/jbt.v21i3.2961. [DOI] [Google Scholar]
- 9.Li H.L., Zhou N., Guo D.Q. The complete chloroplast genome of Zanthoxylum acanthopodium DC. (Rutaceae) and its phylogenetic analysis. Mitochondrial DNA Part B Resour. 2020;5(3):3636–3637. doi: 10.1080/23802359.2020.1823268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seal T. HPLC determination of phenolic acids, flavonoids and ascorbic acid in four different solvent extracts of Zanthoxylum acanthopodium, a wild edible plant of Meghalaya state of India. Int. J. Pharm. Pharmaceut. Sci. 2016;8(3):103–109. https://innovareacademics.in/journals/index.php/ijpps/article/view/10062 [Google Scholar]
- 11.Xuliang X., Florenly Johannes Bastira Ginting, Fioni Analysis of wound healing from andaliman fruit essential oil ointment (zanthoxylum canthopodium dc.) on wistar rats (Rattus norvegicus) Brit. Int. Exact Sci. J. 2021;4(1):31–42. doi: 10.33258/bioex.v4i1.542. [DOI] [Google Scholar]
- 12.Saragih D.E., Arsita E.V. The phytochemical content of Zanthoxylum acanthopodium and its potential as a medicinal plant in the regions of Toba Samosir and North Tapanuli, North Sumatra. Pros Semin Nas Masy Biodiversitas Indones. 2019;5(1):71–76. doi: 10.13057/psnmbi/m050114. [DOI] [Google Scholar]
- 13.Syaputri Ira, Girsang Ermi, Chiuman Linda. Test of antioxidant and antibacterial activity of ethanol extract of andaliman fruit (zanthoxylum acanthopodium dc.) with dpph (1.1-diphenyl-2-picrylhydrazil) trapping method and minimum inhibitory concentration. Int. J. Heal. Pharm. 2022;2(2):215–224. doi: 10.51601/ijhp.v2i2.36. [DOI] [Google Scholar]
- 14.Lubis M.F., Zaitun Hasibuan P.A., Syahputra H., Surbakti C., Astyka R. Saurauia vulcani (Korth.) as herbal medicine potential from North Sumatera, Indonesia: a literature review. Heliyon. 2022;8(4) doi: 10.1016/j.heliyon.2022.e09249. 4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasibuan P.A.Z., Harahap U., Sitorus P., Lubis M.F., Satria D. In-silico analysis of vernonioside d and vernonioside e from Vernonia amygdalina delile. Leaves as inhibitor of epidermal growth factor receptor (egfr) and mammalian target of rapamycin (mtor) Rasayan J. Chem. 2021;14(3):1539–1543. doi: 10.31788/RJC.2021.1436092. [DOI] [Google Scholar]
- 16.Lubis M.F., Kaban V.E., Aritonang J.O., Satria D., Mulina A.A., Febriani H. Acute toxicity and antifungal activity of the ointment Murraya koenigii ethanol extract. Rasayan J. Chem. 2022;15(1):256–261. doi: 10.31788/RJC.2022.1516401. [DOI] [Google Scholar]
- 17.Muzafri A., Julianti E., Rusmarilin H. The extraction of antimicrobials component of andaliman (Zanthoxylum acanthopodium DC.) and its application on catfish (Pangasius sutchi) fillet. IOP Conf. Ser. Earth Environ. Sci. 2018;122(1) doi: 10.1088/1755-1315/122/1/012089. [DOI] [Google Scholar]
- 18.Lelyana S., Widyarman A.S., Amtha R. Indonesian andaliman fruit (Zanthoxylum acanthopodium DC.) extract supresses the expression of inflammatory mediators on fibroblasts cells in vitro. Pharm. Sci. Asia. 2021;48(5):491–497. doi: 10.29090/psa.2021.05.20.064. [DOI] [Google Scholar]
- 19.Devi O., Rao K., Bidalia A., Wangkheirakpam R., Singh O. GC-MS analysis of phytocomponents and antifungal activities of zanthoxylum acanthopodium DC. Collected from Manipur, India. Eur. J. Med. Plants. 2015;10(1):1–9. doi: 10.9734/EJMP/2015/19353. [DOI] [Google Scholar]
- 20.Yao J.L., Fang S.M., Liu R., Oppong M.B., Liu E.W., Fan G.W., Zhang H. A review on the terpenes from genus vitex. Molecules. 2016;21(9):1–20. doi: 10.3390/molecules21091179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sommano S.R., Chittasupho C., Ruksiriwanich W., Jantrawut P. The cannabis terpenes. Molecules. 2020;25(24):1–16. doi: 10.3390/molecules25245792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ben Salha G., Abderrabba M., Labidi J. A status review of terpenes and their separation methods. Rev. Chem. Eng. 2019 doi: 10.1515/revce-2018-0066. [DOI] [Google Scholar]
- 23.Sibero M.T., Siswanto A.P., Murwani R., Frederick E.H., Wijaya A.P., Syafitri E., Farabi K., Saito S., Igarashi Y. Antibacterial, cytotoxicity and metabolite profiling of crude methanolic extract from andaliman (Zanthoxylum acanthopodium) fruit. Biodiversitas. 2020;21(9):4147–4154. doi: 10.13057/biodiv/d210928. [DOI] [Google Scholar]
- 24.Satria D., Silalahi J., Haro G., Ilyas S., Hasibuan P.A.Z. Chemical analysis and cytotoxic activity of N-hexane fraction of Zanthoxylum acanthopodium DC. fruits. Rasayan J. Chem. 2019;12(2):803–808. doi: 10.31788/RJC.2019.1225180. [DOI] [Google Scholar]
- 25.Julistiono H., Lestari F.G., Iryanto R., Lotulung P.D. Antimycobacterial activity of fruit of Zanthoxylum acanthopodium DC against Mycobacterium smegmatis. Avicenna J. Phytomed. 2018;8(5):432–438. PMID: 30345230. [PMC free article] [PubMed] [Google Scholar]
- 26.Yanti Y. Active fractions from Zanthoxylum acanthopodium fruit modulate inflammatory biomarkers in lipopolysaccharide-induced macrophages in vitro. Int. J. Infect. Dis. [Internet] 2016;45:289. doi: 10.1016/j.ijid.2016.02.639. Available from. [DOI] [Google Scholar]
- 27.Bhattacharya J., aparna Lakshmi, Ata S., sen Sarma oly. Evaluation of cytotoxic activity of Zanthoxylum acanthopodium in dalton ’ s lymphoma ascites cells induced. Eur. J. Biomed. Pharmaceut. Sci. 2020;4(10):541–548. [Google Scholar]
- 28.Syari D.M., Rosidah R., Hasibuan P.A.Z., Haro G., Satria D. Evaluation of cytotoxic activity alkaloid fractions of Zanthoxylum acanthopodium DC. Fruits. Open Access Maced J. Med. Sci. 2019;7(22):3745–3747. doi: 10.3889/oamjms.2019.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arsita E.V., Saragih D.E., Aldrin K. Anticancer potential from ethanol extract of Zanthoxylum acanthopodium DC. seed to against MCF-7 cell line. IOP Conf. Ser. Earth Environ. Sci. 2019;293(1) doi: 10.1088/1755-1315/293/1/012016. [DOI] [Google Scholar]
- 30.Harahap U., Hasibuan P.A.Z., Sitorus P., Arfian N., Satria D. Antimigration activity of an ethylacetate fraction of Zanthoxylum acanthopodium DC. fruits in 4T1 breast cancer cells. Asian Pac. J. Cancer Prev. APJCP. 2018;19(2):565–569. doi: 10.22034/APJCP.2018.19.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satria D., Silalahi J., Haro G., Ilyas S., Hasibuan P.A.Z. Cell cycle inhibition of ethylacetate fraction of zanthoxylum acanthopodium DC. Fruit against T47D cells. Open Access Maced J Med Sci. 2019 Mar;7(5):726–729. doi: 10.3889/oamjms.2019.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simanullang R.H., Situmorang P.C., Herlina M., Noradina Silalahi B., Manurung S.S. Histological changes of cervical tumours following Zanthoxylum acanthopodium DC treatment, and its impact on cytokine expression. Saudi J. Biol. Sci. 2022;29(4):2706–2718. doi: 10.1016/j.sjbs.2021.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simanullang R.H., Situmorang P.C., Herlina M., Silalahi B. Cytochrome c expression by andaliman (zanthoxylum acanthopodium) on cervical cancer histology. Pakistan J. Biol. Sci. 2022;25(1):49–55. doi: 10.3923/pjbs.2022.49.55. [DOI] [PubMed] [Google Scholar]
- 34.Ilyas S., Simanullang R.H., Hutahaean S., Rosidah R., Situmorang P.C. Suppression of Wnt expression by increasing PI3K in rats cervical carcinoma by andaliman (zanthoxylum acanthopodium) Pakistan J. Biol. Sci. 2022;25(1):29–36. doi: 10.3923/pjbs.2022.29.36. [DOI] [PubMed] [Google Scholar]
- 35.Sihotang Y., Silalahi J., Anjelisa P. Cardioprotective effect of ethylacetate extract of Zanthoxylum acanthopodium DC. against doxorubicin-induced cardiotoxicity in rats. Int. J. Pharm. Tech. Res. 2016;9(4):249–253. [Google Scholar]
- 36.Simanullang R.H., Ilyas S., Hutahaean S., Rosidah Effect of andaliman (Zanthoxylum acanthopodium DC) methanol extract on rat's kidney and liver histology induced by benzopyrene. Pakistan J. Biol. Sci. 2021;24(2):274–281. doi: 10.3923/pjbs.2021.274.281. [DOI] [PubMed] [Google Scholar]
- 37.Pasaribu K.M., Gea S., Ilyas S., Tamrin T., Sarumaha A.A., Sembiring A., Radecka I. Fabrication and in-vivo study of micro-colloidal Zanthoxylum acanthopodium-loaded bacterial cellulose as a burn wound dressing. Polymers. 2020;12(7) doi: 10.3390/polym12071436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manurung R.D., Ilyas S., Hutahaean S., Rosidah R., Situmorang P.C. Diabetic wound healing in fgf expression by nano herbal of Rhodomyrtus tomentosa L. And Zanthoxylum acanthopodium fruits. Pakistan J. Biol. Sci. 2021;24(3):401–408. doi: 10.3923/pjbs.2021.401.408. [DOI] [PubMed] [Google Scholar]
- 39.Situmorang P.C., Ilyas S., Hutahaean S., Rosidah Effect of nano herbal andaliman (Zanthoxylum acanthopodium) fruits in notch1 and hes1 expressions to human placental trophoblasts. Pakistan J. Biol. Sci. 2021;24(1):165–171. doi: 10.3923/pjbs.2021.165.171. [DOI] [PubMed] [Google Scholar]
- 40.Situmorang P.C., Ilyas S., Hutahaean S., Rosidah R. Histological changes in placental rat apoptosis via FasL and cytochrome c by the nano-herbal Zanthoxylum acanthopodium. Saudi J. Biol. Sci. [Internet] 2021;28(5):3060–3068. doi: 10.1016/j.sjbs.2021.02.047. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravi L., Krishnan K. Cytotoxic potential of N-hexadecanoic acid extracted from kigelia pinnata leaves. Asian J. Cell Biol. 2016;12(1):20–27. doi: 10.3923/ajcb.2017.20.27. [DOI] [Google Scholar]
- 42.Dalimunthe A., Hasibuan P.A.Z., Satria D. Cell cycle arrest activity of Litsea cubeba Lour: heartwood and fruit extracts against T47D breast cancer cells. Asian J. Pharmaceut. Clin. Res. 2017;10(11):404–406. doi: 10.22159/ajpcr.2017.v10i11.20204. [DOI] [Google Scholar]
- 43.Dalimunthe A., Hasibuan P.A.Z., Fujiko M., Masfria Satria D. Phytochemicals analysis and cell cycle arrest activity of ethanol extract of Litsea cubeba Lour. Fruits towards mcf-7/her-2 cell line. Rasayan J. Chem. 2021;14(1):16–18. doi: 10.31788/RJC.2021.1415734. [DOI] [Google Scholar]
- 44.Shylla A., Pathaw D., Roy B. Toxicological assessment of methanolic fruit extract of Zanthoxylum acanthopodium DC. In swiss albino mice: acute and sub-acute toxicity study. Toxicol. Int. 2022;29(1):75–93. doi: 10.18311/ti/2022/v29il/28051. [DOI] [Google Scholar]
- 45.Situmorang P.C., Ilyas S., Hutahaean S., Rosidah, Manurung R.D. Acute toxicity test and histological description of organs after giving nano herbal andaliman (Zanthoxylum acanthopodium) Rasayan J. Chem. 2020;13(2):780–788. doi: 10.31788/RJC.2020.1325621. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.




