Abstract
Older individuals fatigue more rapidly during, and recover more slowly from, dynamic exercise. Women are particularly vulnerable to these deleterious effects of aging, which increases their risk of falling. We have shown that dietary nitrate (NO3 −), a source of nitric oxide (NO) via the NO3 − → nitrite (NO2 −) → NO pathway, enhances muscle speed and power in older individuals in the non‐fatigued state; however, it is unclear if it reduces fatigability and/or improves recoverability in this population. Using a double‐blind, placebo‐controlled, crossover design, we studied 18 older (age 70 ± 4 years) women who were administered an acute dose of beetroot juice (BRJ) containing either 15.6 ± 3.6 or <0.05 mmol of NO3 −. Blood samples were drawn throughout each ~3 h visit for plasma NO3 − and NO2 − analysis. Peak torque was measured during, and periodically for 10 min after, 50 maximal knee extensions performed at 3.14 rad/s on an isokinetic dynamometer. Ingestion of NO3 −‐containing BRJ increased plasma NO3 − and NO2 − concentrations by 21 ± 8 and 4 ± 4 fold, respectively. However, there were no differences in muscle fatigue or recovery. Dietary NO3 − increases plasma NO3 − and NO2 − concentrations but does not reduce fatigability during or enhance recoverability after high intensity exercise in older women.
Keywords: aging, beetroot juice, isokinetic dynamometry, nitric oxide
The effect of acute NO3 − intake on maximal isokinetic knee extensor torque during and after high intensity dynamic exercise was determined in older women. Despite resulting in significant elevations in plasma NO3 − and NO2 − levels, and hence presumably in NO levels, NO3 − supplementation had no effect on muscle fatigability or recoverability under these conditions.
1. INTRODUCTION
Physical function is diminished in older individuals, due in large part to changes in the properties of skeletal muscle. The latter include decreases in the maximal force, speed, and power of muscle contraction (Larsson et al., 2019), as well as reductions in mitochondrial respiratory capacity (Coggan et al., 1992, 1993; Ferri et al., 2020) and in blood flow during exercise (Casey et al., 2015; Ridout et al., 2010). The latter lead to a greater disturbance of cellular energetics during activity (Coggan et al., 1993; Lewsey et al., 2020), which along with the overall slowing of contractile properties result in increased fatigability during dynamic exercise (Christie et al., 2012), especially at higher movement velocities (Callahan & Kent‐Braun, 2011; Dalton et al., 2010). The rate of recovery from fatiguing exercise, i.e., recoverability, is also diminished in older individuals (Schwendner et al., 1997; Yoon et al., 2012). Since most activities of daily living are intermittent, not continuous, in nature, the latter may be of particular practical and clinical relevance. Women are especially susceptible to the deleterious effects of aging on physical function (Freedman et al., 2016; Murtagh & Hubert, 2004), with increased fatigability and reduced recoverability of muscle predisposing them to falls (Schwendner et al., 1997) and an increased risk of hospitalization and even institutionalization.
Dietary nitrate (NO3 −) supplementation may provide a viable means of offsetting some of these detrimental age‐related changes in skeletal muscle function. Upon ingestion and concentration/secretion by the salivary glands, NO3 − can be reduced by oral bacteria to form nitrite (NO2 −), which once absorbed can in turn be reduced by, e.g., deoxyhemoglobin to generate nitric oxide (NO) (Lundberg & Weitzberg, 2009). Given the importance of NO in modulating muscle function, including contractility, mitochondrial respiration, blood flow, etc. (Stamler & Meissner, 2001), numerous studies in recent years have therefore assessed the effects of dietary NO3 − (usually in the form of beetroot juice [BRJ]) on exercise capacity. As reviewed elsewhere, such studies have demonstrated that acute or short‐term dietary NO3 − supplementation can improve the inherent contractile properties of muscle (Coggan et al., 2021; Esen et al., 2023) as well as enhance performance during high intensity dynamic exercise (Silva et al., 2022), at least/especially during open‐ended tests performed by non‐athletes. Increasing NO bioavailability via NO3 − intake could therefore potentially ameliorate at least some of the negative effects of aging described above. Indeed, we recently found that acute ingestion of BRJ containing 13.4 mmol of NO3 − significantly increased maximal knee extensor speed and power in twelve 71 year old men and women (Coggan, Hoffman, et al., 2020). However, there were no changes in fatigability during an “all‐out”, 50 contraction (~1 min) fatigue test conducted at 3.14 rad/s on an isokinetic dynamometer. The latter finding could be due to the numerous factors likely contributing to muscle fatigue under such conditions (e.g., changes in high energy phosphate and/or H+ concentrations, altered Ca2+ handling kinetics, impaired excitation‐contraction coupling, etc. [Kent‐Braun et al., 2012]), only some of which might be influenced by increased NO bioavailability. However, they could also be due to the relatively small sample size, or to the fact that we studied older men as well as women when we have found that women appear to be more likely to benefit from NO3 − supplementation (Coggan, Broadstreet, Mikhalkova, et al., 2018).
Although dietary NO3 − may not (or may) enhance muscle fatigue resistance in older persons, it might still improve the rate of recovery from fatiguing activities. As a potent vasodilator, NO could increase muscle blood flow post‐exercise, when intramuscular pressure is no longer elevated by contractions. This might speed the “washout” of fatigue‐related metabolites such as H+ from muscle (Kemp et al., 1997). Furthermore, by delivering more O2, higher post‐exercise blood flow could increase the rate of mitochondrial ATP synthesis, thus resulting in more rapid restoration of high energy phosphate levels (Layec et al., 2013) and hence muscle function (Jansson et al., 1990). In fact, two studies of healthy young men and women found that under hypoxic conditions dietary NO3 − accelerated phosphocreatine (PCr) recovery kinetics following high intensity knee extensor exercise (Vanhatalo et al., 2011, 2014), indicative of increased O2 delivery via enhanced blood flow and/or improved mitochondrial coupling. However, NO3 − ingestion had no effect on PCr recovery in healthy older men and women studied under normoxic conditions (Kelly et al., 2013). Thus, whether dietary NO3 − supplementation might augment recovery of muscle function following fatiguing exercise in older persons is unclear.
The purpose of the present study was to test the hypothesis that acute dietary NO3 − supplementation would reduce fatigability and/or improve recoverability of muscle specifically in older women. If so, this could have broad implications for this population of individuals, who are particularly vulnerable to the deleterious effects of reductions in muscle function with aging.
2. MATERIALS AND METHODS
2.1. Participants
A total of 18 community‐dwelling women with a mean age, height, weight, and body mass index of 70 ± 4 years, 1.65 ± 0.06 m, 67.1 ± 9.5 kg, and 24.6 ± 3.2 kg/m2, respectively, completed this study, which represents a secondary analysis of data collected during two registered clinical trial (NCT03513302 and NCT03595774). These women were studied after screening a total of 143 potential participants, of whom n = 26 were enrolled and then n = 8 were excluded after an initial visit to the University Hospital Clinical Research Center (CRC) (Figure 1). During this visit, potential participants underwent a health history, physical examination, resting ECG, and phlebotomy (CBC, CMP, fasting insulin, and lipids), and also practiced the isokinetic dynamometry testing protocol (see below).
FIGURE 1.

CONSORT diagram illustrating flow of subjects through the study.
Individuals were excluded if they were not between the ages of 65 and 79 years, were unable to provide informed consent, were currently smokers, pregnant, or lactating, were taking proton pump inhibitors, antacids, or xanthine oxidoreductase inhibitors, or had a history of major metabolic (thyroid disorders, type I or II diabetes), cardiovascular (e.g., moderate or severe valvular disease, myocardial/pericardial disease, stage II or greater hypertension, heart failure, myocardial infarction/ischemia), renal (eGFR <60 mL/min/1.73 m2, or 61–90 mL/min/1.73 m2 and albumin: creatine ratio > 30), neuromuscular (e.g., cervical spondylotic radiculomyelopathy, lumbar spondylosis, amyotrophic lateral sclerosis, Guillain‐Barre syndrome, acquired demyelinating polyneuropathies), or liver (e.g., SGOT/SGPT >2× normal) diseases, were anemic (hematocrit <30%), or had any other contraindications to vigorous exercise. Other exclusion criteria included whether the participant was on hormone replacement therapy or used phosphodiesterase inhibitors, since these can respectively diminish (Obach et al. 2004) or potentiate (Webb et al., 1999) the effects of dietary NO3 −. All other participants were included. The Human Subjects Office at Indiana University approved this study and written informed consent was obtained from each individual.
2.2. Experimental design and protocol
Starting approximately 1 week after completion of the initial screening visit, eligible participants were studied using a randomized, double‐blind, placebo‐controlled crossover design. Participants were asked to avoid foods high in NO3 − (e.g., spinach, arugula, beets) for 24 h and alcohol, caffeine, or food for 12 h before testing. Otherwise, no dietary restrictions were imposed. Upon arrival at the CRC, an intravenous catheter was placed and a baseline blood sample taken for future analysis of plasma NO3 − and NO2 − concentrations as described below. The participant then ingested 140 mL of a concentrated beetroot juice (BRJ) supplement (Beet it Sport, James White Drinks) containing (based on direct measurement) either 15.6 ± 3.6 or <0.05 mmol of NO3 −. The latter placebo, which is produced by the manufacturer by passing BRJ over a highly selective anion exchange resin, is indistinguishable from the NO3 −‐containing BRJ in terms of color, texture, taste, smell, and packaging. A randomization scheme assigning participants to treatment order ‘A’ or ‘B’ was generated, after which an individual not involved with the study chose which was active and which was placebo and labeled the bottles accordingly. This blinding was broken only after data collection was complete. After 1 and 2 h of quiet rest, additional blood samples were collected, after which muscle function testing was performed as described below. Ten minutes later, a final blood sample was obtained, after which participants were fed lunch then released. They then returned to the CRC after a 1 week washout period and repeated the experiment, this time ingesting the opposite BRJ supplement than before. Participants therefore made a total of three study visits, i.e., initial screening, placebo BRJ, NO3 −‐containing BRJ, with the latter two in random order.
2.3. Measurement of muscle function
Fatigability and recoverability of the knee extensor muscles were assessed using isokinetic dynamometry (Biodex System 4 Pro, Biodex Medical Systems). This method of assessing muscle function was chosen due to its excellent reliability (Pincivero et al., 2001; Saenz et al., 2010; Sinacore et al., 1974) and because it results in less cardiovascular strain and hence risk compared to intense exercise with a large muscle mass (e.g., sprint cycling). The femoral condyle of the participant's dominant leg was aligned with the dynamometer's axis of rotation while the lower leg, thigh, waist, and torso were securely restrained with straps to prevent any extraneous movement. The participant first performed three maximal knee extensions at each of five velocities (i.e., 0, 1.57, 3.14, 4.71, and 6.28 rad/s), with 120 s of rest between sets, to determine their peak torque‐velocity relationship (data reported separately). After an additional 120 s of rest, they then performed 50 maximal knee extensions at a velocity of 3.14 rad/s. The velocity of knee flexion was set to 6.28 rad/s, allowing the participant to freely reposition their leg against minimal resistance between repetitions. Participants were instructed to go “all‐out” during this fatigue test, i.e., to extend their leg as quickly and as forcefully as possible during each repetition and to not pace themselves in an attempt to maintain torque. Strong verbal encouragement was provided throughout each test. After completion of the fatigue test, participants performed sets of three additional maximal knee extensions at 3.14 rad/s after 30, 60, 150, 300, and 600 s (nominally) of recovery.
Data from the isokinetic dynamometer were processed by windowing, filtering, and smoothing as previously described (Coggan et al., 2015). Fatigability was assessed based on the average torque, total work, work during the first and last 1/3rd of the fatigue test, and the ratio of the latter as reported by the manufacturer's software. The torque data were also exported and analyzed to determine the peak torque during each of the 50 contractions. Recoverability was assessed based on the degree of restoration of peak torque at the different time points following the fatigue test. Because it was not possible to measure peak torque at identical times in every participant, a monoexponential function was also fit to the peak torque‐recovery duration data (including the final value from the fatigue test) for each individual to determine their time constant (τ) and half‐life (=ln(2)/τ) of torque recovery:
where t = the exact timeafter the 50 contraction fatigue test and torque is expressed as a percentage of the maximum torque observed at the start of the fatigue test (which almost always occurred during the 2nd or 3rd of the 50 knee extensions).
2.4. Measurement of plasma NO3 − and NO2 −
A dedicated high‐performance liquid chromatography system (ENO‐30, Eicom USA) was used to measure plasma NO3 − and NO2 − concentrations as previously described in detail (Coggan, Broadstreet, Mahmood, et al., 2018). Briefly, 25 μL of thawed plasma was mixed 1:1 with methanol, centrifuged, and then a 10 μL aliquot of the protein‐poor supernatant was injected into the HPLC. Plasma NO3 − and NO2 − concentrations were determined from calibration curves generated using NIST‐traceable standard solutions.
2.5. Statistical analysis
Data were analyzed using GraphPad Prism version 9.3.1 (GraphPad Software). The normality of data distribution was tested using the D'Agostino‐Pearson omnibus test. Plasma NO3 − and NO2 − and peak torque data were compared between trials using two‐way (treatment × time or treatment × contraction number) repeated measures ANOVA. Post‐hoc testing was performed using the Holm‐Šidák multiple comparison procedure. Fatigue test summary statistics (e.g., average torque) and monoexponential recovery curve fit parameters were compared between trials using paired t‐tests. Multiplicity‐corrected p values less than 0.05 were considered significant. Deidentified data are available from the authors upon request.
3. RESULTS
3.1. Plasma NO3 − and NO2 − levels
There were no significant changes in plasma NO3 − or NO2 − levels following ingestion of the NO3 −‐depleted BRJ placebo (Table 1). In contrast, plasma NO3 − and NO2 − concentrations were significantly elevated 1 and 2 h post‐ingestion of NO3 −‐containing BRJ, as well as 10 min after completion of the exercise testing (approximately 2.5 h post‐ingestion).
TABLE 1.
Effects of NO3 − supplementation on plasma NO3 − and NO2 − concentrations.
| Trial | Time of measurement | ||||
|---|---|---|---|---|---|
| Pre ingestion | 1 h post ingestion | 2 h post ingestion | 10 min post exercise | ||
| Plasma [NO3 −] (μmol/L) | Placebo | 47.7 ± 30.3 | 47.7 ± 23.5 | 51.9 ± 25.9 | 51.1 ± 24.4 |
| Nitrate | 42.6 ± 17.7 | 622.9 ± 227.6 | 751.3 ± 156.9 | 735.2 ± 158.2 | |
| p value | 0.312 | 1.20 x 10−7 | 3.35 x 10−10 | 5.60 x 10−10 | |
| Plasma [NO2 −] (μmol/L) | Placebo | 0.343 ± 0.230 | 0.363 ± 0.286 | 0.387 ± 0.358 | 0.411 ± 0.330 |
| Nitrate | 0.376 ± 0.286 | 0.786 ± 0.783 | 0.968 ± 0.803 | 1.209 ± 0.883 | |
| p value | 0.404 | 2.28 x 10−2 | 4.50 x 10−3 | 2.45 x 10−3 | |
Note: Values are mean ± S.D. for n = 18.
3.2. Fatigability
There were no significant differences between placebo and NO3 − trials in the time required to complete the 50 contraction fatigue test (i.e., 62 ± 6 vs. 64 ± 9 s; p = 0.153) or in the total time spent extending (33 ± 2 vs. 34 ± 2 s; p = 0.078) or flexing (29 ± 4 vs. 30 ± 7 s; p = 0.384) the leg. There were also no significant differences between trials in the average torque, total work, work during the first 1/3rd or last 1/3rd of the test, or their ratio (Table 2). Finally, analysis of the raw data to determine the peak torque during each of the 50 contractions also revealed no significant treatment (p = 0.851) or interaction (p = 0.576) effects (Figure 2). However, there was a significant effect of repetition number (p = 1.52 x 10−20), with peak torque being reduced below initial from the 10th repetition onward (p = 7.30 × 10−4 to 1.84 × 10−101).
TABLE 2.
Fatigue test summary results.
| Trial | Average torque (nm/kg) | Total work (J/kg) | Work first 1/3rd (J/kg) | Work last 1/3rd (J/kg) | Fatigue index (%) |
|---|---|---|---|---|---|
| Placebo | 0.57 ± 0.10 | 34.6 ± 8.0 | 15.7 ± 8.0 | 8.0 ± 2.1 | 50.8 ± 4.8 |
| Nitrate | 0.57 ± 0.10 | 35.3 ± 8.2 | 16.0 ± 3.7 | 8.2 ± 2.1 | 51.4 ± 5.8 |
| p value | 0.969 | 0.260 | 0.204 | 0.229 | 0.593 |
Note: Values are mean ± S.D. for n = 18.
FIGURE 2.

Knee extensor peak torque during the fatigue test. Note that although 5 repetition averages are presented for clarity, statistical analysis was performed using the peak torque from each of the 50 repetitions. Median values are shown by a line, with boxes and whiskers representing 25th/75th percentile and minimum/maximum values, respectively. ***Peak torque during contractions 6–50 significantly lower than during contractions 1–5 (p < 0.001). Exact p values are provided in the text.
3.3. Recoverability
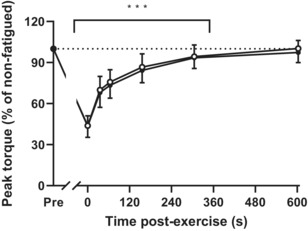
Peak torque was measured after 35 ± 3, 64 ± 5, 159 ± 19, 309 ± 20, and 603 ± 4 s of recovery in the placebo trial and after 34 ± 3, 65 ± 2, 154 ± 3, 302 ± 4, and 603 ± 7 s of recovery in the NO3 − trial. The treatment and interaction effects were not significant (p = 0.161 and p = 0.255, respectively), although by design there was a significant time effect (p = 3.14 × 10−35). NO3 − ingestion had no significant effect on peak torque measured at these time points, regardless of whether torque was expressed relative to body mass (treatment effect, p = 0.326; interaction effect, p = 0.281) or as a percentage of the non‐fatigued baseline (treatment effect, p = 0.275; interaction effect, p = 0.500) (Figure 3). In both cases, however, there was a significant effect of time (p = 8.09 × 10−22 for peak torque relative to body mass and p = 1.94 × 10−31 for peak torque as a percentage of the non‐fatigued baseline), with peak torque being reduced below initial at all but the last time point (p = 5.10 × 10−3 to 4.74 × 10−63). There were also no differences in the predicted minimum or maximum torque or time course of recovery of torque determined via exponential curve fitting (Table 3).
FIGURE 3.

Recovery of knee extensor peak torque following the fatigue test. Data are expressed either relative to body mass (top panel) or as a percentage of the maximum non‐fatigued value (bottom panel). Values are mean ± S.D. for n = 18. ***Peak torque during recovery significantly lower than before the fatigue test (Pre) (p < 0.001). Exact p values are provided in the text.
TABLE 3.
Curve fit parameters for recovery of isokinetic torque at 3.14 rad/s.
| Trial | Minimum torque (% of non‐fatigued) | Maximum torque (% of non‐fatigued) | Time constant (s−1) | Half‐life (s) | S.E.E. (%) |
|---|---|---|---|---|---|
| Placebo | 45.6 ± 7.9 | 97.8 ± 8.0 | 0.01515 ± 0.00898 | 72.4 ± 82.0 | 4.3 ± 1.2 |
| Nitrate | 45.8 ± 6.2 | 97.4 ± 5.5 | 0.01457 ± 0.00671 | 58.1 ± 26.7 | 4.7 ± 1.6 |
| P value | 0.882 | 0.856 | 0.785 | 0.506 | 0.336 |
Note: Values are mean ± S.D. for n = 18.
4. DISCUSSION
Aging results in more rapid fatigue during, and slower recovery from, dynamic exercise. Older women in particular are prone to these negative effects of aging, which increases their risk of falls. The purpose of the present study was to determine whether acute dietary NO3 − intake might ameliorate these age‐related changes in muscle function. However, despite producing large changes in plasma NO3 − and NO2 − levels, and hence presumably in NO bioavailability, we found no effect of such supplementation on either the fatigability or recoverability of muscle in this population.
In the present study, the ingestion of BRJ containing 15.6 ± 3.6 mmol of NO3 − increased the plasma concentration 21 ± 8‐fold, or to an individual peak of 783 ± 139 μmol/L above baseline. Assuming essentially complete absorption, this corresponds to a volume of distribution of 20.1 ± 3.3 L, which agrees exactly with our recent novel pharmacokinetic modeling study of the combined NO3 −/NO2 − system (Coggan, Racette, et al., 2020). Plasma NO2 − concentrations also rose, albeit to a lesser extent, i.e., 4 ± 4‐fold, due to the larger volume of distribution and almost 70‐fold more rapid clearance of the latter and the fact that only 3% of ingested NO3 − is converted to NO2 − (Coggan, Racette, et al., 2020). These changes in plasma NO3 − and NO2 − are consistent with numerous previous studies of dietary NO3 − supplementation that have employed a similar dose (e.g., Jonvik et al., 2018). We did not measure breath NO levels as we have in our previous studies of NO3 − supplementation in older individuals (Coggan, Hoffman, et al., 2020; Gallardo et al., 2021), but based on such data the latter likely would have increased by at least 100%. It therefore seems reasonable to conclude that the ingestion of NO3 −‐containing BRJ was effective in increasing whole‐body NO bioavailability.
Despite the above, acute NO3 − supplementation has no impact on muscle fatigability during a 50 contraction (~60 s) isokinetic knee extensor exercise test. The present results corroborate and extend our prior study of a smaller group of older women and men combined (Coggan, Hoffman, et al., 2020). Although direct comparisons are challenging due to potential differences between small muscle mass and whole‐body exercise, they are also consistent with those of prior studies examining the effects of NO3 − ingestion on, e.g., 30 s Wingate test performance in younger, healthier individuals (Jonvik et al., 2018; Rimer et al., 2016). Indeed, a recent meta‐analysis by Silva et al. (2022) concluded that dietary NO3 − is effective at improving performance only during exercise tasks lasting 2–10 min, but not during shorter or longer efforts. In contrast, Kadach et al. (2023) recently found that NO3 − supplementation increased muscle torque throughout the first 90 s of a 300 s bout of repeated maximal isometric knee extensions (3 s contraction, 2 s rest). Notably, however, numerous other investigations have failed to demonstrate any effect of dietary NO3 − on maximal isometric torque (Esen et al., 2023), making the study by Kadach et al. (2023) an exception. Regardless, based on the present as well as our previous results, it seems clear that although acute ingestion of NO3 − enhances maximal muscle speed and hence power in older individuals (Coggan, Hoffman, et al., 2020; Gallardo et al., 2021), it does not alter fatigability during intense contractile activity in older persons.
A novel aspect of the present investigation was determination of the effects of dietary NO3 − on recovery of muscle contractile function following fatiguing exercise. Specifically, we hypothesized that NO3 − supplementation might enhance recovery of muscle torque by magnifying post‐exercise hyperemia. Reductions in intramuscular pO2 and/or pH during exercise should accelerate conversion of NO3 −/NO2 − to NO (Lundberg & Weitzberg, 2009), which in turn could lead to greater vasodilation and higher blood flow following exercise, once intramuscular pressure decreases. If so, this could result in more rapid restoration of muscle pH and/or high energy phosphate levels, and hence muscle function. Indeed, NO3 − intake has been previously reported to increase PCr recovery kinetics in healthy young men and women studied in hypoxia (Vanhatalo et al., 2011, 2014), but not in younger (Vanhatalo et al., 2014) or older (Kelly et al., 2013) individuals studied in normoxia. Although we did not measure changes in muscle pH and/or high energy phosphate levels in the older women in the present study, acute NO3 − ingestion had no effect on the recovery of muscle torque following fatiguing exercise. Thus, either any increase in post‐exercise blood flow was insufficient to result in more rapid restoration of function, or increased flow simply did not improve recovery, reflecting the likely multifactorial nature of fatigue under the present conditions (Kent‐Braun et al., 2012).
There are a number of strengths to the present study, including a relatively large sample size, careful screening of participants to avoid confounding the effects of aging with those due to chronic disease, and the rigorous evaluation of muscle fatigability/recoverability during/after high intensity dynamic exercise. There are, however, a number of limitations as well. Specifically, as indicated above we did not collect any mechanistic measurements (e.g., changes in blood flow, PCr kinetics, etc.) that could have provided further insight into the effects of acute NO3 − intake on skeletal muscle physiology in this population, even if function was unchanged. It is also possible that we might have obtained different results had we utilized a different dose of NO3 −, had studied the participants after multiple days of supplementation, and/or had studied men instead of women. Since the effects of aging on muscle fatigability are most evident at higher speeds of movement (Callahan & Kent‐Braun, 2011; Dalton et al., 2010), we might also have observed an effect of dietary NO3 − on fatigability had we tested at a higher velocity of knee extension. Similarly, it is possible that NO3 − supplementation might enhance recovery from fatigue induced by other types and/or durations/intensities of exercise.
5. CONCLUSION
In summary, we have assessed the effects of acute NO3 − intake on maximal isokinetic knee extensor torque during and after high intensity dynamic exercise in older women. Despite resulting in significant elevations in plasma NO3 − and NO2 − levels, and hence presumably in NO levels, NO3 − supplementation had no effect on muscle fatigability or recoverability under these conditions.
AUTHOR CONTRIBUTIONS
Conception and design of research: Andrew R. Coggan. Data collection and analysis: William S. Zoughaib, Richard L. Hoffman, Brandon A. Yates, Ranjani N. Moorthi, Kenneth Lim, and Andrew R. Coggan. Drafting of manuscript: William S. Zoughaib and Andrew R. Coggan. Edited and revised manuscript: Andrew R. Coggan. Approved final version of manuscript: William S. Zoughaib, Richard L. Hoffman, Brandon A. Yates, Ranjani N. Moorthi, Kenneth Lim, and Andrew R. Coggan.
FUNDING INFORMATION
This work was supported by the Office of the Vice Provost for Research at Indiana University Purdue University Indianapolis and by the National Institutes of Health (grant numbers R21 AG053606 to ARC, R34 HL 138253 to ARC and Linda Peterson, R61 HL155858 to ARC, Linda Peterson, and Ken Schechtman, K23 DK102824 to RNM, P30 AR072581 to Sharon Moe, and UL1 TR002529 to Anantha Shekhar). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health.
Zoughaib, W. S. , Hoffman, R. L. , Yates, B. A. , Moorthi, R. N. , Lim, K. , & Coggan, A. R. (2023). The influence of acute dietary nitrate supplementation on skeletal muscle fatigue and recovery in older women. Physiological Reports, 11, e15694. 10.14814/phy2.15694
REFERENCES
- Callahan, D. M. , & Kent‐Braun, J. A. (2011). Effect of old age on human skeletal muscle force‐velocity and fatigue properties. Journal of Applied Physiology, 111, 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey, D. P. , Ranadive, S. M. , & Joyner, M. J. (2015). Aging is associated with altered vasodilator kinetics in dynamically contracting muscle: Role of nitric oxide. Journal of Applied Physiology, 119, 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie, A. , Snook, E. M. , & Kent‐Braun, J. A. (2012). Systematic review and meta‐analysis of skeletal muscle fatigue in old age. Medicine and Science in Sports and Exercise, 43, 568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggan, A. R. , Abduljalil, A. M. , Swanson, S. C. , Earle, M. S. , Farris, J. W. , Mendenhall, L. A. , & Robitaille, P.‐M. (1993). Muscle metabolism during exercise in young and older untrained and endurance‐trained men. Journal of Applied Physiology, 75, 2125–2133. [DOI] [PubMed] [Google Scholar]
- Coggan, A. R. , Baranauskas, M. N. , Hinrichs, R. L. , Liu, Z. , & Carter, S. J. (2021). Effect of dietary nitrate on human muscle power: A systematic review and individual subject data meta‐analysis. Journal of the International Society of Sports Nutrition, 18, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggan, A. R. , Broadstreet, S. R. , Mahmood, K. , Mikhalkova, D. , Madigan, M. , Bole, I. , Leibowitz, J. L. , Kadkhodayan, A. , Thomas, D. P. , Thies, D. , & Peterson, L. R. (2018). Dietary nitrate increases VO2peak and performance but does not alter ventilation or efficiency in patients with heart failure with reduced ejection fraction. Journal of Cardiac Failure, 24, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggan, A. R. , Broadstreet, S. R. , Mikhalkova, D. , Bole, I. , Leibowitz, J. L. , Kadkhodayan, A. , Park, S. , Thomas, D. P. , Thies, D. , & Peterson, L. R. (2018). Dietary nitrate‐induced increases in human muscle power: High versus low responders. Physiological Reports, 6, e13575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggan, A. R. , Hoffman, R. L. , Gray, D. A. , Moorthi, R. N. , Thomas, D. P. , Leibowitz, J. L. , Thies, D. , & Peterson, L. R. (2020). A single dose dietary of nitrate increases maximal muscle speed and power in healthy older men and women. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 75, 1154–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggan, A. R. , Leibowitz, J. L. , Kadkhodayan, A. , Thomas, D. T. , Ramamurthy, S. , Anderson Spearie, C. , Waller, S. , Farmer, M. , & Peterson, L. R. (2015). Effect of acute dietary nitrate intake on knee extensor speed and power in healthy men and women. Nitric Oxide, 48, 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggan, A. R. , Racette, S. B. , Thies, D. , Peterson, L. R. , & Stratford, R. E., Jr. (2020). Simultaneous pharmacokinetic analysis of nitrate and its reduced metabolite, nitrite, following ingestion of inorganic nitrate in a mixed patient population. Pharmaceutical Research, 37, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggan, A. R. , Spina, R. J. , Rogers, M. A. , King, D. S. , Brown, M. , Nemeth, P. M. , & Holloszy, J. O. (1992). Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Geront, 47, B71–B76. [DOI] [PubMed] [Google Scholar]
- Dalton, B. H. , Power, G. A. , Vandervoort, A. A. , & Rice, C. L. (2010). Power loss is greater in old men than young men during fast plantar flexion contractions. Journal of Applied Physiology, 109, 1441–1447. [DOI] [PubMed] [Google Scholar]
- Esen, O. , Dobbin, N. , & Callaghan, M. J. (2023). The effect of dietary nitrate on the contractile properties of human skeletal muscle: A systematic review and meta‐analysis. Journal of the American Nutrition Association, 42, 327–338. 10.1080/07315724.2022.2037475 [DOI] [PubMed] [Google Scholar]
- Ferri, E. , Marzetti, E. , Calvani, R. , Picca, A. , Cesari, M. , & Arosio, B. (2020). Role of age‐related mitochondrial dysfunction in sarcopenia. International Journal of Molecular Sciences, 21, 5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman, V. A. , Wolf, D. A. , & Spillman, B. C. (2016). Disability‐free life expectancy over 30 years: A growing female disadvantage in the US population. American Journal of Public Health, 106, 1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo, E. J. , Gray, D. A. , Hoffman, R. L. , Yates, B. A. , Moorthi, R. N. , & Coggan, A. R. (2021). Dose‐response effect of dietary nitrate on muscle contractility and blood pressure in older subjects: A pilot study. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 76, 591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson, E. , Dudley, G. A. , Norman, B. , & Tesch, P. A. (1990). Relationship of recovery from intense exercise to the oxidative potential of skeletal muscle. Acta Physiologica Scandinavica, 139, 147–152. [DOI] [PubMed] [Google Scholar]
- Jonvik, K. L. , Nyakayiru, J. , van Dijk, J. W. , Masse, K. , Ballak, S. B. , Senden, J. M. G. , van Loon, L. J. C. , & Verdijk, L. B. (2018). Repeated‐sprint performance and plasma responses following beetroot juice supplementation do not differ between recreational, competitive, and elite sprint athletes. European Journal of Sport Science, 7, 1–10. [DOI] [PubMed] [Google Scholar]
- Kadach, S. , Park, J. W. , Stoyanov, Z. , Black, M. I. , Vanhatalo, A. , Burnley, M. , Walter, P. J. , Cai, H. , Schechter, A. N. , Piknova, B. , & Jones, A. M. (2023). 15N‐labeled dietary nitrate supplementation increases human skeletal muscle nitrate concentration and improves muscle torque production. Acta Physiologica (Oxf.), 237, e13924. [DOI] [PubMed] [Google Scholar]
- Kelly, J. , Fulford, J. , Vanhatalo, A. , Blackwell, J. R. , French, O. , Bailey, S. J. , Gilchrist, M. , Winyard, P. G. , & Jones, A. M. (2013). Effects of short‐term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. The American Journal of Physiology, 304, R73–R83. [DOI] [PubMed] [Google Scholar]
- Kemp, G. J. , Thompson, C. H. , Taylor, D. J. , & Radda, G. K. (1997). Proton efflux in human skeletal muscle during recovery from exercise. European Journal of Applied Physiology, 76, 462–471. [DOI] [PubMed] [Google Scholar]
- Kent‐Braun, J. A. , Fitts, R. H. , & Christie, A. (2012). Skeletal muscle fatigue. Comprehensive Physiology, 2, 997–1044. [DOI] [PubMed] [Google Scholar]
- Larsson, L. , Degens, H. , Li, M. , Salviati, L. , Lee, Y. I. , Thompson, W. , Kirkland, J. L. , & Sandri, M. (2019). Sarcopenia: Aging‐related loss of muscle mass and function. Physiological Reviews, 99, 427–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layec, G. , Haseler, L. J. , Trinity, J. D. , Hart, C. R. , Liu, X. , Le Fur, Y. , Jeong, E.‐K. , & Richardson, R. S. (2013). Mitochondrial function and increased convective O2 transport: Implications for the assessment of mitochondrial respiration in vivo. Journal of Applied Physiology, 115, 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewsey, S. C. , Weiss, K. , Schär, M. , Zhang, Y. , Bottomley, P. A. , Samuel, T. J. , Xue, Q.‐L. , Steinberg, A. , Walston, J. D. , Gerstenblith, G. , & Weiss, R. G. (2020). Exercise intolerance and rapid skeletal muscle energetic decline in human age‐associated frailty. JCI Insight, 5(20), e141246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg, J. O. , & Weitzberg, E. (2009). NO generation from inorganic nitrate and nitrite: Role in physiology, nutrition, and therapeutics. Archives of Pharmacal Research, 32, 1119–1126. [DOI] [PubMed] [Google Scholar]
- Murtagh, K. N. , & Hubert, H. B. (2004). Gender differences in physical disability among an elderly cohort. American Journal of Public Health, 94, 1406–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obach, R. S. , Huynh, P. , Allen, M. C. , & Beedham, C. (2004). Human liver aldehyde oxidase: inhibition by 239 drugs. Journal of Clinical Pharmacology, 44, 7–19. [DOI] [PubMed] [Google Scholar]
- Pincivero, D. M. , Gear, W. S. , & Sterner, R. L. (2001). Assessment of the reliability of high‐intensity quadriceps muscle fatigue. Medicine and Science in Sports and Exercise, 33, 334–338. [DOI] [PubMed] [Google Scholar]
- Ridout, S. J. , Parker, B. A. , Smithmyer, S. L. , Gonzales, J. U. , Beck, K. C. , & Proctor, D. N. (2010). Age and sex influence the balance between maximal cardiac output and peripheral vascular reserve. Journal of Applied Physiology, 108, 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimer, E. G. , Peterson, L. R. , Coggan, A. R. , & Martin, J. C. (2016). Acute dietary nitrate supplementation increases maximal cycling power in athletes. International Journal of Sports Physiology and Performance, 11, 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz, A. , Avellanet, M. , Hijos, E. , Chaler, J. , Garreta, R. , Pujol, E. , Sandoval, B. , Buen, C. , & Farreny, A. (2010). Knee isokinetic test‐retest: A multicentre knee isokinetic test‐retest study of a fatigue protocol. European Journal of Physical and Rehabilitation Medicine, 46, 81–88. [PubMed] [Google Scholar]
- Schwendner, K. I. , Mikesky, A. E. , Holt, W. S., Jr. , Peacock, M. , & Burr, D. B. (1997). Differences in muscle endurance and recovery between fallers and nonfallers, and between young and older women. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 52, MI55–M160. [DOI] [PubMed] [Google Scholar]
- Silva, K. V. C. , Costa, B. D. , Gomes, A. C. , Saunders, B. , & Mota, J. F. (2022). Factors that moderate the effect of nitrate ingestion on exercise performance in adults: A systematic review with meta‐analyses and meta‐regressions. Advances in Nutrition, 13, 1866–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinacore, D. R. , Bander, B. L. , & Delitto, A. (1974). Recovery from a 1‐minute bout of fatiguing exercise: Characteristics, reliability, and responsiveness. Physical Therapy, 74, 234–244. [DOI] [PubMed] [Google Scholar]
- Stamler, J. S. , & Meissner, G. (2001). Physiology of nitric oxide in skeletal muscle. Physiological Reviews, 81, 209–237. [DOI] [PubMed] [Google Scholar]
- Vanhatalo, A. , Fulford, J. , Bailey, S. J. , Blackwell, J. R. , Winyard, P. G. , & Jones, A. M. (2011). Dietary nitrate reduces muscle metabolic perturbation and improves exercise tolerance in hypoxia. The Journal of Physiology, 589, 5517–5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhatalo, A. , Jones, A. M. , Blackwell, J. R. , Winyard, P. G. , & Fulford, J. (2014). Dietary nitrate accelerates postexercise muscle metabolic recovery and O2 delivery in hypoxia. Journal of Applied Physiology, 117, 1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb, D. J. , Freestone, S. , Allen, M. J. , & Muirhead, G. J. (1999). Sildenafil citrate and blood‐pressure–lowering drugs: Results of drug interaction studies with an organic nitrate and a calcium antagonist. The American Journal of Cardiology, 83, 21C–28C. [DOI] [PubMed] [Google Scholar]
- Yoon, T. , Schlinder‐Delap, B. , Keller, M. L. , & Hunter, S. K. (2012). Supraspinal fatigue impedes recovery from a low‐intensity sustained contraction in old adults. Journal of Applied Physiology, 112, 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]


