Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in solid organ transplant (SOT) recipients is associated with poorer antibody response (AbR) compared with non-SOT recipients. However, its impact on the risk of breakthrough infection (BI) has yet to be assessed.
Methods
Single-center prospective longitudinal cohort study enrolling adult SOT recipients who received SARS-CoV-2 vaccination during a 1-year period (February 2021 – January 2022), end of follow-up April 2022. Patients were tested for AbR at multiple time points. The primary end-point was BI (laboratory-confirmed SARS-CoV-2 infection ≥14 days after the second dose). Immunization (positive AbR) was considered an intermediate state between vaccination and BI. Probabilities of being in vaccination, immunization, and BI states were obtained for each type of graft and vaccination sequence using multistate survival analysis. Then, multivariable logistic regression was performed to analyze the risk of BI related to AbR levels.
Results
614 SOT (275 kidney, 163 liver, 137 heart, 39 lung) recipients were included. Most patients (84.7%) received 3 vaccine doses. The first 2 consisted of BNT162b2 and mRNA-1273 in 73.5% and 26.5% of cases, respectively. For the third dose, mRNA-1273 was administered in 59.8% of patients. Overall, 75.4% of patients reached immunization and 18.4% developed BI. Heart transplant recipients showed the lowest probability of immunization (0.418) and the highest of BI (0.323); all mRNA-1273 vaccine sequences showed the highest probability of immunization (0.732) and the lowest of BI (0.098). Risk of BI was higher for non–high-level AbR, younger age, and shorter time from transplant.
Conclusions
SOT patients with non–high-level AbR and shorter time from transplantation and heart recipients are at highest risk of BI.
Keywords: SARS-CoV-2 infection, COVID-19, vaccination, antibody response, solid organ transplantation
Relationship between antibody response (AbR) and breakthrough infection (BI) after SARS-Cov-2 vaccination in 614 SOT recipients was analysed. Heart recipients showed lowest probability of immunization and highest of infection. Non-high-level AbR and shorter time from transplantation were associated with BI.
Immune response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines as well as their clinical effectiveness have been shown to be lower in solid organ transplant (SOT) recipients compared with the general population [1–4]. Indeed, immune monitoring in this setting has been proposed by some experts [5] in order to identify patients at higher risk of infection in which other preventive strategies (ie, preexposure monoclonal antibodies, booster doses associated with temporary reduction of immunosuppressive treatment) could be implemented.
However, several controversies surround the practice of antibody testing in SOT recipients, including the lack of an established antibody threshold associated with protection in patients on long-term immunosuppression and the potential role of cellular response [5]. Indeed, studies investigating the relationship between the presence and level of antibody response with the rate and severity of SARS-CoV-2 infection in SOT recipients are limited.
To fill this gap, in the current prospective longitudinal study, we aimed to assess the rate and severity of SARS-CoV-2 breakthrough infections (BIs) and their relationship with antibody response (AbR) in fully vaccinated SOT recipients.
METHODS
Study Design
CONTRAST (The impact of COvid-19 pandemic on the saNt’orsola TRAnSplant and cancer cohorT: an observational study) is a single-center prospective longitudinal cohort study of SOT recipients who underwent SARS-CoV-2 vaccination within the Horizon 2020 ORCHESTRA project (https://orchestra-cohort.eu/).
The recruitment period was 1 year starting 1 February 2021 and corresponding to the onset of a vaccination campaign in fragile patients in the Emilia–Romagna (Italy) region. All patients were followed up until 30 April 2022, and the minimum follow-up period per patient was 1 month after the last vaccination dose.
Clinical charts and hospital electronic records were used as data sources. Data were recorded anonymously and managed using REDCap electronic data capture tools hosted at the University of Bologna [6]. The study database was locked on 15 May 2022 after a careful revision of missing and/or incongruent data.
The study was approved by the local institutional review board. Informed consent was obtained before patients were enrolled.
Setting
The IRCCS Azienda Ospedaliero-Universitaria di Bologna is a 1400-bed tertiary teaching hospital with 4 active transplant programs: kidney, liver, heart, and lung with an average volume of transplantation of 120, 90, 25 and 10 per year, respectively.
Participants
All adult (aged ≥18 years) SOT recipients who received ≥2 doses of a SARS-CoV-2 vaccine or 1 dose in patients with a prior history of infection during the recruitment period and provided consent to participate in the study were included.
Variables
The primary end point was diagnosis of BI defined as the detection of SARS-CoV-2 RNA or antigen in a respiratory specimen collected ≥14 days after the administration of the second vaccine dose (https://www.cdc.gov/coronavirus/2019-ncov/vaccines/effectiveness/why-measure-effectiveness/breakthrough-cases.html). Diagnostic testing for SARS-CoV-2 infection was performed according to local policy (generally active infection was ruled out in all patients before being visited at daycare or outpatient facilities and twice weekly in hospitalized patients) and clinical judgment and was not dictated by study protocol. Information about BI was retrieved during routine hospital visits and in periodic phone and/or email interviews when patients were asked about self-administered tests, symptoms, or exposure to positive persons that occurred since the last contact. Data on clinical severity according to World Health Organization (WHO) criteria (https://www.COVID19treatmentguidelines.nih.gov/overview/clinical-spectrum), hospitalization, and death were collected for all patients diagnosed with BI.
AbR was assessed at multiple time points scheduled from, and including, the administration of the first dose (t0), second dose (t1), 3 ± 1 months after the first dose (t2), and 6 ± 2 months after the first dose (t3). During the study period, administration of booster doses in SOT recipients was recommended, and most patients received a third dose within 4–5 months after the first dose. Consequently, t3 included patients assessed at 1 month after the third dose. AbR was determined using the Elecsys Anti-SARS-CoV-2 Electrochemiluminescence Immunoassay (ECLIA) assay (Roche Diagnostics AG, Rotkreuz, Switzerland). Minimum and maximum thresholds for detection of anti–receptor binding domain (RBD) antibody levels were 0.4 and 2500 UI/mL, respectively. Positive AbR was defined as an anti-RBD titer of ≥5 UI/mL, as previously described [7]. The positive AbR was stratified into very low (5.58–<45 UI/mL), low (45–204 UI/mL), medium (205–<817 UI/mL), and high level (>817 UI/mL) following the WHO reference panel for anti–SARS-CoV-2 immunoglobulin (WHO/BS/2020.2403) [8]. The last available determination of AbR before the diagnosis of BI or at the end of follow-up was considered.
Data on sex, age, comorbidities according to the Charlson score (active underlying diseases other than those related to end-stage organ failure requiring transplantation were considered active comorbidities), type of transplant and time from transplant to first vaccine dose administration, basal (t0 or t1) immune parameters including lymphocyte subpopulations and immunoglobulin G (IgG) levels, type of each vaccine dose administration, exposure to an induction regimen within 6 months before the administration of the last vaccine dose, immunosuppressive drugs (calcineurin inhibitors, antimetabolites, mammalian target of rapamycin inhibitors, steroids) at the time of the last vaccine dose administration, and graft function were also collected.
Statistical Analyses
Patients’ characteristics represented by categorical variables were described as absolute numbers and percentages. Continuous variables were presented as mean ± standard deviation (SD) if normally distributed and as median and interquartile range (IQR) if nonnormally distributed.
Multistate survival analysis was conducted considering AbR as an intermediate state between vaccination and BI, thus building separate models for each 1 of the 3 transitions: vaccination–immunization, vaccination–infection, and immunization–infection. For immunization, the outcome was the time from administration of the first vaccine dose to the first time that a positive antibody response was found. For BI, the outcome was the time from administration of the first vaccine dose or from the first positive antibody response to the time of the first BI. In the case that no event occurred, patients remained in the vaccination state and were censored at the date of their last follow-up. The best-fitting survival model was chosen among Cox, exponential, Gompertz, gamma, log logistic, log normal, Weibull, and Royston–Parmar models based on the lowest values of Akaike Information Criteria (AIC) and Bayesian Information Criteria (BIC). Multivariable models with type of graft or vaccination pattern as exposure were built, including as covariates the main predictors of immunization and BI. Covariates that did not satisfy the proportionality of hazards assumption were modeled as time-varying variables. The probabilities of being in each state during 1 year of follow-up since vaccination were then obtained for each type of graft and vaccination sequence. Finally, a multivariable logistic regression was carried out to identify the prediction role of AbR, type of graft, and vaccination sequence on BI, adjusting for clinical covariates. Multistate and logistic regression analyses were conducted on the same subset of patients who were administered 3 vaccine doses and with complete data on all covariates. Stata v.17 was used for all analyses; specifically, the merlin package was used for multistate modeling [9].
RESULTS
Characteristics of the Study Cohort
Overall, 614 patients were analyzed (Strobe flow chart; Supplementary Figure 1); of them, 213 (34.7%) were female and the mean (±SD) age was 57.3 ± 13.6 years (Table 1). The distribution of SOT type was kidney (n = 275, 44.8%), liver (n = 163, 26.5%), heart (n = 137, 22.3%), and lung (n = 39, 6.4%). All patients received messenger RNA (mRNA)-based vaccines. The median time from transplantation to the first dose was 6.82 years (IQR, 3.12–12.89). The primary vaccine series consisted of BNT162b2 and mRNA-1273 in 73.5% and 26.5% of patients, respectively. The median duration of follow-up was 260 days (IQR, 226–281), and the fifth percentile was 82 days. At the end of follow-up, the majority of patients (n = 520, 84.7%) had received 3 vaccine doses. In those patients, mRNA-1273 was frequently used for the third dose (n = 307, 59.8%). History of SARS-CoV-2 infection prior to vaccination or between the first 2 vaccine doses was present in 19 patients, additionally 12 had positive serology at t0.
Table 1.
Characteristics of the Study Population and Comparison of Patients With Positive and Negative Antibody Responses
| Characteristic, N = 614 (Except Where Otherwise Stated) | Overall | Positive (n = 463, 75.4%) |
Negative (n = 151, 24.6%) |
Test; P Value |
|---|---|---|---|---|
| Age at first vaccine dose, mean ± standard deviation, y | 57.3 ± 13.6 | 57.0 ± 13.6 | 58.1 ± 11.9 | 0.88; 0.380a |
| Female | 213 (34.7) | 153 (33.1) | 60 (39.7) | 2.25; 0.134 |
| Time from transplant to vaccine first dose, median (interquartile range), y | 6.82 (3.12–12.89) | 7.51 (3.78–13.77) | 4.82 (1.68–9.14) | −4.8; < 0.001b |
| Type of graft | 46.0; < 0.001 | |||
| Kidney | 275 (44.8) | 205 (44.3) | 70 (46.4) | |
| Liver | 163 (26.5) | 150 (32.4) | 13 (8.6) | |
| Heart | 137 (22.3) | 80 (17.3) | 57 (37.8) | |
| Lung | 39 (6.4) | 28 (6.1) | 11 (7.3) | |
| Comorbidity | 215 (35.0) | 152 (32.8) | 63 (41.7) | 3.96; 0.047 |
| Booster | 520 (84.7) | 401 (86.6) | 119 (78.8) | 5.34; 0.021 |
| Vaccine sequence (n = 513) | 4.53; 0.104 | |||
| BNT162b2–BNT162b2–BNT162b2 | 70 (13.7) | 47 (11.9) | 23 (19.5) | |
| mRNA-1273–mRNA-1273–mRNA-1273 | 136 (26.5) | 108 (27.3) | 28 (23.7) | |
| BNT162b2–BNT162b2–mRNA-1273 | 307 (59.8) | 240 (60.8) | 67 (56.8) | |
| Graft function (n = 561) | 1.68; 0.195 | |||
| Good | 500 (89.1) | 382 (90.1) | 118 (86.1) | |
| Impaired and/or failure | 61 (10.9) | 42 (9.9) | 19 (13.9) | |
| Induction regimen in the last 6 m | 9 (1.5) | 5 (1.1) | 4 (2.7) | 0.234c |
| Immunosuppressive drug | ||||
| Calcineurin inhibitors | 592 (96.4) | 443 (95.7) | 149 (98.7) | 2.96; 0.086 |
| Mycophenolate | 326 (53.1) | 213 (46.0) | 113 (74.8) | 38.0; < 0.001 |
| Mammalian target of rapamycin inhibitors | 81 (13.2) | 64 (13.8) | 17 (11.3) | 0.65; 0.419 |
| Steroids | 404 (65.8) | 277 (59.8) | 127 (84.1) | 29.8; < 0.001 |
| Baseline immune parameters | ||||
| CD4 (n = 199) | 584 ± 401 | 630 ± 405 | 420 ± 343 | −3.13; 0.002a |
| CD4 >500 (n = 199) | 101 (50.7) | 89 (57.4) | 12 (27.3) | 12.5; < 0.001 |
| CD8 (n = 209) | 556 ± 339 | 581 ± 331 | 475 ± 357 | −1.90; 0.058a |
| IgG (n = 179) | 1004 ± 283 | 1040 ± 265 | 881 ± 314 | −3.21; 0.002a |
| IgG >600 (n = 179) | 171 (95.5) | 137 (98.6) | 34 (85.0) | 0.002c |
The statistical test was χ2 test except when noted.
Abbreviations: IgG, immunoglobulin G; mRNA, messenger RNA.
t test.
Mann–Whitney test.
Fisher exact test.
Antibody Response Assessment
In 94 patients (15.3%), the last available AbR assessment was after 2 doses and in 520 (84.7%) it was after 3 doses. Mean titers of antibody levels at each time point are shown in Supplementary Figure 2. Overall, positive AbR was observed in 463 (75.4%) patients. In patients with positive AbR, anti-RBD levels were classified as very low, low, medium, and high level in 47 (10.1%), 50 (10.6%), 51 (10.8%), and 317 (68.5%) patients, respectively. The comparison of patients with positive and negative AbR showed that positive AbR rates were significantly higher in patients with longer time from transplantation, in liver transplant recipients, and in those who received a third dose. Conversely, positive AbR rates were significantly lower in heart transplant recipients, in patients with comorbidities, and in those who received mycophenolate and/or steroids (Table 1). In patients with available baseline immune parameters, mean CD4 count and mean IgG level were significantly higher among patients with positive AbR (Table 1).
Description of Breakthrough Infections
Overall, 113 (18.4%) patients were diagnosed with BI within a median of 294 days (IQR, 273–325) after t0. Of them, 92 (81.4%) had received 3 vaccine doses and 80 (70.8%) had positive AbR.
Infection was classified as asymptomatic/mild, moderate, and severe/critical in 80.2%, 14.3%, and 5.5% of cases, respectively. Hospitalization occurred in 15 of 113 (16.5%) patients, and death occurred in 3 of 113 (1.9%). Hospitalized patients were older (63.7 ± 9.3 years vs 50.9 ± 14.1 years, P = .001), reported a higher rate of comorbidities (60.0% vs 32.9%, P = .047), had received a third dose less frequently (66.7% vs 86.8%, P = .054), and showed a lower rate of positive AbR (33.3% vs 77.6%, P = .001) compared with nonhospitalized patients (Supplementary Table 1).
Patients who developed BI compared with those who did not (Table 2) were younger (52.4 ± 14.1 years vs 58.4 ± 12.7 years, P < .001) and showed a shorter median time from transplantation (4.69 years vs 7.22 years, P < .001). They were also administered mycophenolate (63.7% vs 50.7%, P = .012) and steroids (74.3% vs 63.9%, P = .034) at higher rates. A nonsignificant lower proportion of positive AbR (70.8% vs 76.4%, P = .208) was observed among patients with BI. In patients with available immune parameters, mean CD4 count was not significantly lower in patients with BI (434 ± 255 vs 604 ± 413, P = .051), while mean IgG levels were higher in BI patients compared with non-BI patients (1123 ± 366 vs 989 ± 268, P = .040).
Table 2.
Comparison of Patients With and Without Diagnosis of Breakthrough Infection
| Characteristic, N = 614 (Except Where Otherwise Specified) |
Yes (n = 113, 18.4%) |
No (n = 501, 81.6%) |
Test; P Value |
|---|---|---|---|
| Age at first vaccine dose, mean ± standard deviation, y | 52.4 ± 14.1 | 58.4 ± 12.7 | 4.44; < 0.001a |
| Female | 37 (17.4) | 176 (82.6) | 0.23; 0.630 |
| Time from transplant to vaccine first dose, median (interquartile range), y | 4.69 (2.11–9.24) | 7.22 (3.45–13.57) | 3.50; < 0.001b |
| Type of graft | 5.49; 0.139 | ||
| Kidney | 60 (21.8) | 215 (78.2) | |
| Heart | 20 (14.6) | 117 (85.4) | |
| Liver | 24 (14.7) | 139 (85.3) | |
| Lung | 9 (23.1) | 30 (76.9) | |
| Comorbidities | 40 (18.6) | 175 (81.4) | 0.01; 0.925 |
| Booster | 92 (17.7) | 428 (82.3) | 1.15; 0.285 |
| Vaccine sequence (n = 513) | 0.37; 0.830 | ||
| BNT162b2–BNT162b2–BNT162b2 | 13 (18.6) | 57 (81.4) | |
| mRNA-1273–mRNA-1273–mRNA-1273 | 21 (15.4) | 115 (84.6) | |
| BNT162b2–BNT162b2–mRNA-1273 | 53 (17.3) | 254 (82.7) | |
| Severe acute respiratory syndrome coronavirus 2 infection preceding vaccination | 2 (10.5) | 17 (89.5) | 0.550c |
| Positive serum test at first vaccine | 1 (8.3) | 11 (91.7) | 0.705c |
| Positive serum test at last serology test | 80 (17.3) | 383 (82.7) | 1.59; 0.208 |
| Graft function (n = 561) | 0.04; 0.840 | ||
| Good | 85 (17.0) | 415 (83.0) | |
| Impaired and/or failure | 11 (18.0) | 50 (82.0) | |
| Induction regimen in the last 6 m | 2 (22.2) | 7 (77.8) | 0.674c |
| Immunosuppressive drug | |||
| Calcineurin inhibitors | 107 (18.1) | 485 (81.9) | 0.267c |
| Mycophenolate | 72 (22.1) | 254 (77.9) | 6.27; 0.012 |
| Mammalian target of rapamycin | 14 (17.3) | 67 (82.7) | 0.08; 0.780 |
| Steroids | 84 (20.8) | 320 (79.2) | 4.49; 0.034 |
| Baseline immune parameters | |||
| CD4 (n = 199) | 434 ± 255 | 604 ± 413 | 1.97; 0.051a |
| CD4 >500 (n = 199) | 9(37.5) | 92(52.6) | 1.92; 0.166 |
| CD8 (n = 209) | 466 ± 221 | 568 ± 351 | 1.43; 0.154a |
| IgG (n = 179) | 1123 ± 366 | 989 ± 268 | −2.07; 0.040a |
| IgG >600 (n = 179) | 21(100.0) | 150(94.9) | 0.599c |
The statistical test was χ2 test except when noted.
Abbreviations: IgG, immunoglobulin G; mRNA, messenger RNA.
t test.
Mann–Whitney test.
Fisher exact test.
Multistate Analysis
The multistate analysis showed that, starting from the 614 vaccinated patients, 463 were found to have a positive AbR, 33 recorded BI without being previously AbR-positive, and 118 did not experience any event; 80 AbR-positive patients developed a subsequent BI. Thus, at the end of follow-up, immunization was the most frequent state, including 383 of 614 patients (Supplementary Figure 3).
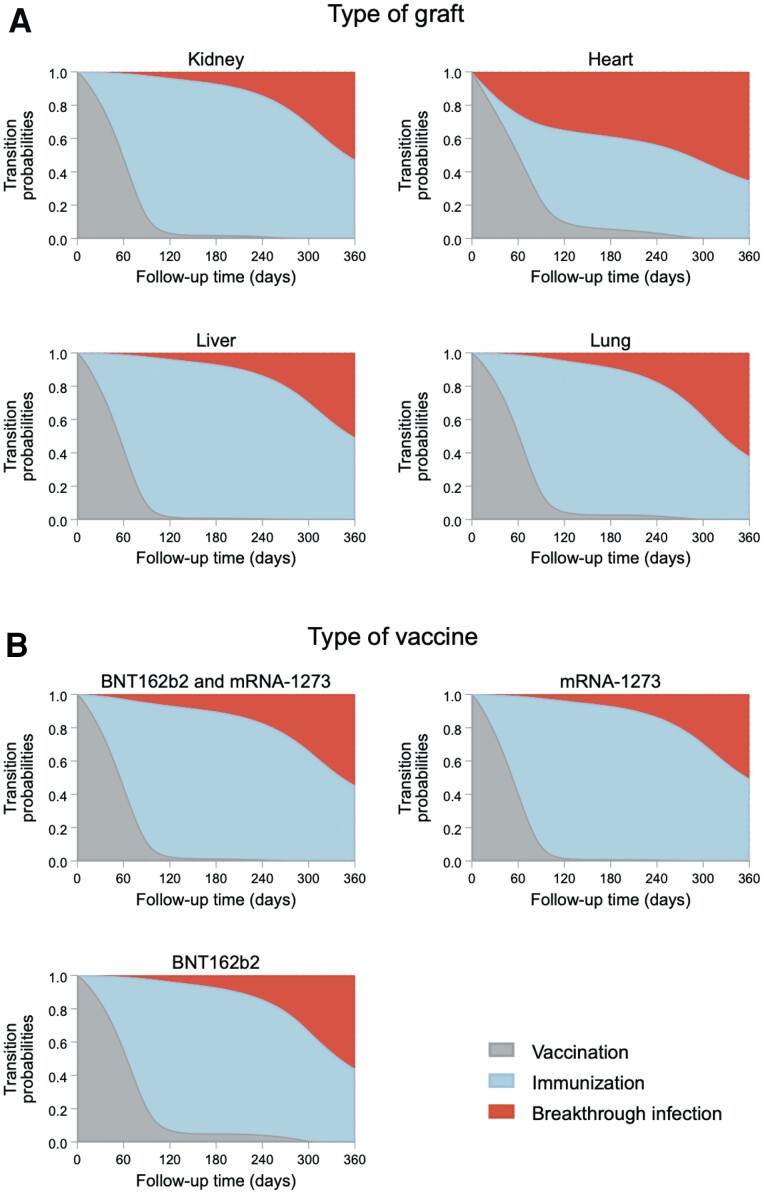
The multistate survival models were carried out on 505 patients after excluding 94 patients who did not receive the third vaccine dose, 13 patients who were AbR-positive at or before the date of the first vaccination, and 2 who had missing data on the type of vaccine administered. In addition to the exposure (type of graft or type of vaccine), the following covariates were included: age at first vaccination, gender, time from transplant to first vaccination, and the indicators of use of mycophenolate, steroids, and calcineurin inhibitors. With regard to the type of graft, heart transplant patients were estimated to have the lowest probability of being in the immunized state (0.418) and, conversely, to have the highest probability of being in the vaccination (0.259) and BI (0.323) states. The lowest probability of BI was estimated for liver transplant patients (0.093), who had the highest probability of being in the immunization status (0.729; Figure 1A, Supplementary Table 2). The probabilities for being in a specific state were less differentiated among vaccine sequence groups: patients who received mRNA-1273 for all 3 doses showed the highest probability of being in the immunization state (0.732) and the lowest probability of being in the BI state (0.098; Figure 1B, Supplementary Table 2).
Figure 1.
Probability of breakthrough infection according to type of graft (panel A) and type of vaccine (panel B). Abbreviation: mRNA, messenger RNA.
Multivariable Logistic Regression Analysis
From multivariable logistic regression, patients who achieved medium-level antibody response had a higher risk of BI than patients with high response (odds ratio [OR] = 2.447, P = .040; Table 3). High risk of BI was also observed in patients classified as having very low (OR = 1.971, P = .164) and low (OR = 1.849, P = .176) AbR. Possibly, the OR for these groups did not reach statistical significance because of the small number of patients (further reduced to n = 37 and n = 38, respectively, in this multivariable model because of missing data in the covariates). Other factors associated with BI were younger age (OR = 0.970, P = .004) and shorter time elapsed from transplant to vaccination (OR = 0.946, P = .014).
Table 3.
Multivariable Logistic Regression Analysis of Breakthrough Infection for n = 505 Patients
| Covariates | Odds Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| Antibody response | ||
| Negative (<5 lU/mL) | 1.053 (0.523–2.118) | 0.884 |
| Very low (5–44 lU/mL) | 1.971 (0.757–5.132) | 0.164 |
| Low (45–204 IU/mL) | 1.849 (0.75–4.504) | 0.176 |
| Medium (205–817 IU/mL) | 2.447 (1.039–5.763) | 0.040 |
| High (>817 lU/mL) | ref. | |
| Type of graft | ||
| Kidney | ref. | |
| Liver | 0.505 (0.218–1.172) | 0.112 |
| Heart | 0.666 (0.277–1.601) | 0.364 |
| Lung | 0.78 (0.305–2.017) | 0.616 |
| Vaccine sequence | ||
| BNT162b2–BNT162b2–mRNA-1273 | ref. | |
| mRNA-1273–mRNA-1273–mRNA-1273 | 0.608 (0.330–1.11) | 0.110 |
| BNT162b2–BNT162b2–BNT162b2 | 1.456 (0.643–3.300) | 0.367 |
| Age at first vaccine dose | 0.969 (0.950–0.98) | 0.003 |
| Time from transplant to vaccine first dose | 0.945 (0.904–0.988) | 0.013 |
| Female | 1.083 (0.634–1.849) | 0.770 |
| Calcineurin inhibitors | 0.777 (0.165–3.647) | 0.750 |
| Mycophenolate | 1.335 (0.704–2.532) | 0.375 |
| Mammalian target of rapamycin inhibitors | 0.755 (0.294–1.933) | 0.558 |
| Steroids | 1.186 (0.599–2.350) | 0.624 |
| Comorbidities | 0.969 (0.560–1.677) | 0.913 |
| Severe acute respiratory syndrome coronavirus 2 infection preceding vaccination | 0.404 (0.037–4.388) | 0.457 |
| Constant | 2.055 (0.249–16.959) | 0.503 |
Abbreviation: mRNA, messenger RNA.
DISCUSSION
We are the first to assess the relationship between antibody response and BI after 3 doses of mRNA SARS-CoV-2 vaccines in a large prospective longitudinal cohort of SOT recipients using multistate model analysis. The rates of positive AbR and BI were 75.4% and 18.4%, respectively. Heart transplant patients were estimated to have the lowest probability of reaching positive AbR and the highest of developing BI. For the type of vaccine manufacturer, patients who received mRNA-1273 for all 3 doses showed a slightly higher probability of reaching immunization and the lowest probability of developing BI. Surprisingly, younger patients had an increased risk of BI. As expected, compared with high levels, very low, low, and medium levels of AbR were associated with increased risk of BI as well as shorter time from transplant to vaccination onset.
In a multicenter longitudinal cohort study of 1467 SOT recipients from United States, 150 (10.2%) reported a BI during the Omicron wave, with 11 (7.3%) hospitalizations and 2 (1.3%) deaths [10]. Among SOT recipients with serological available data, 96 of 666 (14.4%) were seronegative, 24% had medium-level AbR (anti-RBD, 250–2500 U/mL), and 47% had high-level AbR (anti-RBD, >2500 U/mL). Rates of seronegative status were higher, although not at a significant level, among patients with BI (21.6% vs 14%), and high-level AbR response was lower in BI patients (29.7% vs 48%) compared with patients without BI. In addition, there was a statistically significant relationship between increasing titer categories and proportion with confirmed infection (Wilcoxon rank sum, P = .006) [10]. It is also worth mentioning that in other cohorts of SOT recipients with BI, patients with negative or non–high-level AbR had a higher risk of unfavorable outcomes in terms of mortality and hospitalization compared with patients with high-level AbR [11–13].
In our analysis, in the assessed exposures (type of graft and type of vaccine manufacturer), as expected, the higher the probability of achieving immunization, the lower the probability of developing BI and vice versa. As reported in the literature, liver recipients were confirmed as being more likely to have positive AbR and the lowest probability of BI, while heart transplant patients had the lowest probability of achieving immunization and the highest probability of BI. The highest incidence of BI in heart recipients compared with other types of transplantation was reported in a study that included 4776 SOT recipients with BI from the United States [14]. These findings could be explained by the different distribution of age, gender, comorbidities, and immunosuppressive regimens administered in heart transplant patients compared with other SOT recipients (Supplementary Table 3).
The independent risk factors for BI were younger age, shorter time from transplant to vaccination, and medium level of antibody response. The younger age underlines an issue rarely explored in clinical studies on the risk of BI in fragile patients, that is, the role of social distancing and occupational activities as well as compliance with mask-wearing and hand hygiene [15]. However, it should be emphasized that older age was associated with an increased risk of severe BI that required hospitalization. Surprisingly, on multivariate analysis, immunosuppressive drugs were not associated with an increased risk of BI, as reported by others mainly for the use of mycophenolate and steroids [14]. Finally, confirming the increased risk of BI among patients with non-high-level AbR in SOT recipients, we argue in favor of the controversial role of serology monitoring in this setting [5].
Some limitations of our study should be considered. First, the single-center design may limit generalizability of our results. This is primarily true for lung transplantation due to the small number of patients with this type of transplant included in the study. Second, due to the censored structure of the detection thresholds of antibody levels, we refrained from investigating antibody level as a quantitative variable. Third, we did not assess cellular immune response, which may play a role in this framework. Furthermore, genotype analysis of the viral variant was not performed in all enrolled patients during the study period. Thus, we could not determine if it had a role in the transition from vaccination to immunization and/or BI. However, as the majority of BIs occurred between December 2021 and April 2022 when the Omicron variant started to be dominant in our region, our findings may be considered primarily applicable to SOT recipients exposed to Omicron variants. Since BIs in patient subgroups were limited to a few cases, some estimates may lack robustness. Thus, additional studies on larger numbers of patients are needed to corroborate our findings. Finally, we were able to collect baseline immune parameters for a limited number of patients. Our finding that low CD4 count was associated with poorer AbR and increased susceptibility to BI, while lower IgG levels were associated with poorer AbR but not with an increased rate of BI, should be corroborated by additional larger studies.
To conclude, our results support the practice of immune monitoring in SOT recipients in order to identify patients at highest risk of BI and confirm the need for adjunctive strategies in heart transplant recipients, patients who recently underwent solid organ transplantation, and in those with evidence of low- to medium-level AbR. Additional studies are needed to confirm our results and, in particular, to link the role of social behaviors in the transition from vaccination to immunization and/or BI.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Cecilia Bonazzetti, Infectious Diseases Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy.
Beatrice Tazza, Infectious Diseases Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy.
Dino Gibertoni, Research and Innovation Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Zeno Pasquini, Infectious Diseases Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Natascia Caroccia, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy.
Francesca Fanì, Infectious Diseases Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Giacomo Fornaro, Infectious Diseases Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Renato Pascale, Infectious Diseases Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Matteo Rinaldi, Infectious Diseases Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Beatrice Miani, Infectious Diseases Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Chiara Gamberini, Microbiology Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Maria Cristina Morelli, Internal Medicine Unit for the Treatment of Severe Organ Failure, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Mariarosa Tamé, Gastroenterology Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Marco Busutti, Nephrology, Dialysis and Transplantation Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Giorgia Comai, Nephrology, Dialysis and Transplantation Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Luciano Potena, Unit of Heart Failure and Transplantation, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Laura Borgese, Unit of Heart Failure and Transplantation, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Elena Salvaterra, Division of Interventional Pulmonology Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Tiziana Lazzarotto, Microbiology Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; Department of Experimental, Diagnostic and Specialty Medicine, University of Bologna, Bologna, Italy.
Luigia Scudeller, Research and Innovation Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Pierluigi Viale, Infectious Diseases Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy.
Maddalena Giannella, Infectious Diseases Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy.
the CONTRAST Study Group:
Michela Di Chiara, Maria Eugenia Giacomini, Oana Vatamanu, Lorenzo Marconi, Clara Solera Horna, Caterina Campoli, Michele Bartoletti, Linda Bussini, Fabio Trapani, Luciano Attard, Milo Gatti, Antonio Gramegna, Gaetano La Manna, Valeria Grandinetti, Marcello Demetri, Simona Barbuto, Chiara Abenavoli, Giovanni Vitale, Laura Turco, Matteo Ravaioli, Matteo Cescon, Valentina Bertuzzo, Paola Messina, Alessandra Trombi, Marco Masetti, Paola Prestinenzi, Mario Sabatino, Laura Giovannini, Aloisio Alessio, Antonio Russo, Maria Francesca Scuppa, Giampiero Dolci, Gianmaria Paganelli, Liliana Gabrielli, Matteo Pavoni, Marta Leone, and Federica Lanna
Notes
Acknowledgments. The following are members of the CONTRAST (The impact of COvid-19 pandemic on the saNt’orsola TRAnSplant and cancer cohorT: an observational study) Study Group: Michela Di Chiara, Maria Eugenia Giacomini, Oana Vatamanu, Lorenzo Marconi, Clara Solera Horna, Caterina Campoli, Michele Bartoletti, Linda Bussini, Fabio Trapani, Luciano Attard, Milo Gatti, Antonio Gramegna, Gaetano La Manna, Valeria Grandinetti, Marcello Demetri, Simona Barbuto, Chiara Abenavoli, Giovanni Vitale, Laura Turco, Matteo Ravaioli, Matteo Cescon, Valentina Bertuzzo, Paola Messina, Alessandra Trombi, Marco Masetti, Paola Prestinenzi, Mario Sabatino, Laura Giovannini, Aloisio Alessio, Antonio Russo, Maria Francesca Scuppa, Giampiero Dolci, Gianmaria Paganelli, Liliana Gabrielli, Matteo Pavoni, Marta Leone, and Federica Lanna.
Financial support. This work was supported by the European Union’s Horizon 2020 Research and Innovation Program, grant agreement 101016167, within the ORCHESTRA project (EU H2020 ORCHESTRA), and from the Ministero della Salute within Ricerca Corrente 2022 Funding Program (N. RC-2022-2774290). N. C. reports support for this work in the form of a research grant within the EU H2020 ORCHESTRA project for management and coordination of project activities such as scheduling patient visits and blood withdrawals for the study-specific biosampling, coordinating personnel (clinicians, nurses and biologists) involved in the study, and managing data (data collection, data entry, data query resolution, data export). C. G. reports support for this work in the form of a research grant within the EU H2020 ORCHESTRA project for performing of serological tests.
REFERENCES
- 1. Manothummetha K, Chuleerarux N, Sanguankeo A, et al. . Immunogenicity and risk factors associated with poor humoral immune response of SARS-CoV-2 vaccines in recipients of solid organ transplant: a systematic review and meta-analysis. JAMA Netw Open 2022; 5:e226822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kwon JH, Tenforde MW, Gaglani M, et al. . mRNA vaccine effectiveness against COVID-19 hospitalization among solid organ transplant recipients. J Infect Dis 2022; 226:797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Giannella M, Pierrotti LC, Helanterä I, Manuel O. SARS-CoV-2 vaccination in solid-organ transplant recipients: what the clinician needs to know. Transpl Int 2021; 34:1776–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Galmiche S, Luong Nguyen LB, Tartour E, et al. . Immunological and clinical efficacy of COVID-19 vaccines in immunocompromised populations: a systematic review. Clin Microbiol Infect 2022; 28:163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Werbel WA, Segev DL. SARS-CoV-2 antibody testing for transplant recipients: a tool to personalize protection versus COVID-19. Am J Transplant 2022; 22:1316–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harris PA, Taylor R, Minor BL, et al. . The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giannella M, Righi E, Pascale R, et al. . Evaluation of the kinetics of antibody response to COVID-19 vaccine in solid organ transplant recipients: the prospective multicenter ORCHESTRA cohort. Microorganisms 2022; 10:1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mattiuzzo G, Bentley EM, Hassall M, et al . Establishment of the WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody. WHO/BS/2020.2403. 2020:1–60.
- 9. Crowther MJ. Merlin—a unified modeling framework for data analysis and methods development in Stata. Stata Journal 2020; 20:763–84. [Google Scholar]
- 10. Alejo JL, Chiang TPY, Bowles Zeiser L, et al. . Incidence and severity of COVID-19 among vaccinated solid organ transplant recipients during the Omicron wave. Transplantation 2022; 106:e413–5. [DOI] [PubMed] [Google Scholar]
- 11. Biagio P, Rosa C, Nicola SM, et al. . Serological response and clinical protection of anti-SARS-CoV-2 vaccination and the role of immunosuppressive drugs in a cohort of kidney transplant patients. Viruses 2022; 14:1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malahe SRK, Hoek RAS, Dalm VASH, et al. . Clinical characteristics and outcome of immunocompromised patients with COVID-19 caused by the Omicron variant: a prospective observational study [manuscript published online ahead of print 23 July 2022]. Clin Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamm SR, Rezahosseini O, Møller DL, et al. . Incidence and severity of SARS-CoV-2 infections in liver and kidney transplant recipients in the post-vaccination era: real-life data from Denmark. Am J Transplant 2022; 22:2637–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vinson AJ, Anzalone AJ, Sun J, et al. . The risk and consequences of breakthrough SARS-CoV-2 infection in solid organ transplant recipients relative to non-immunosuppressed controls. Am J Transplant 2022; 22:2418–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Lucas Ramos P, García-Botella A, García-Lledó A, et al. . Actions and attitudes on the immunized patients against SARS-CoV-2. Rev Esp Quimioter 2022; 35:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.