Abstract
Using intraerythrocytic tenofovir-diphosphate data from the emtricitabine/tenofovir disoproxil fumarate arms of HIV Prevention Trials Network (HPTN) 083 (men) and HPTN 084 (women), approximately 99% efficacy was achieved at a lower adherence threshold in HPTN 083 (≥2 doses/week) compared with HPTN 084 (daily), suggesting higher adherence is necessary for women vs men.
Keywords: PrEP, sex differences, adherence, tenofovir, pharmacology
HPTN 084 was a ground-breaking trial for human immunodeficiency virus (HIV) prevention among persons assigned female at birth, demonstrating a 91% (95% confidence interval [CI], 73%–96%) lower risk of HIV acquisition in those randomized to blinded cabotegravir (CAB; n = 1614) compared with blinded oral emtricitabine/tenofovir disoproxil fumarate (F-TDF) (n = 1610) [1]. HPTN 083 was an analogous trial in cisgender men and transgender women who have sex with men, showing a 66% (38%–82%) reduced risk of HIV in those randomized to CAB (n = 2280) vs F-TDF (n = 2281) [2]. Both trials observed significantly fewer incident infections for CAB compared with F-TDF, with 12 vs 39 infections and 3 vs 36 infections during the blinded phases of HPTN 083 and HPTN 084, respectively [3, 4].
In both studies, all HIV infections in the F-TDF arms were characterized with drug concentrations around the time of the first HIV-positive visit [3, 4], which is of interest here. Understanding the adherence–response relationship for F-TDF in women is critical because significant debate and uncertainty surround whether oral F-TDF is less effective and less pharmacologically forgiving for vaginal vs anal intercourse. This stems from the lack of F-TDF efficacy in women from the Vaginal and Oral Interventions to Control the Epidemic (VOICE) and Preexposure Prophylaxis Trial for HIV Prevention among African Women (FEM-PrEP) trials and from the parallel finding that tenofovir diphosphate (TFV-DP) concentrations were lower in vaginal compared with rectal tissue homogenate (as much as 100-fold lower) [5]. A comprehensive pharmacokinetic–pharmacodynamic model, based on tissue concentrations of TFV-DP and emtricitabine-triphosphate (FTC-TP) in a ratio with their competitor deoxynucleotides, deoxyadenosine-triphosphate (dATP) and deoxycytidine-triphosphate (dCTP), predicted that 6 F-TDF doses per week were needed for maximal protection in the vaginal compartment vs only 2 F-TDF doses per week for rectal tissue [6]. Most of the effect in vaginal tissue was driven by high FTC-TP/dCTP; TFV-DP/dATP contributed little. However, these data were not consistent with findings from the Partners preexposure prophylaxis (PrEP) trial, which showed equal efficacy for TDF alone compared with F-TDF in women; hazard ratio vs placebo of 0.29 (95% CI, .13–.63) for TDF alone and 0.34 for F-TDF (95% CI, .16–.72). Additionally, efficacy was approximately 90% in women and men with quantifiable plasma TFV concentrations [7, 8]. These data underscore uncertainty around the pharmacologic requirements for F-TDF protection in women.
HPTN 084 is the first large, blinded trial in women that used intraerythrocytic TFV-DP collected as dried blood spots (DBS) to assess gradients of adherence, providing the first opportunity to examine the TFV-DP–response relationship in women in a large trial setting. DBS were collected at the end of the oral lead-in phase, at study week 33, and every third injection visit thereafter. DBS were also collected at the visit following the first visit where the site obtained a reactive or positive HIV test result. Intraerythrocytic TFV-DP concentrations were determined at 2 visits, when available: the first HIV-positive visit or the next visit with a DBS sample and the closest visit prior to the first HIV-positive visit. TFV-DP was also evaluated in a randomly selected adherence cohort of 405 participants.
Intraerythrocytic TFV-DP in DBS is used to estimate gradients of adherence averaged over the preceding 4–8 weeks as follows: <350 fmol/punch, 2 doses/week; 350–699 fmol/punch, 2–3 doses/week; 700–1249 fmol/punch, 4–6 doses/week; and ≥1250 fmol/punch daily dosing. The adherence–response relationship using intraerythrocytic TFV-DP in DBS was previously defined in men who have sex with men, where it predicted near complete F-TDF efficacy for ≥4 doses/week (99% risk reduction) and substantial efficacy for 2–3 doses per week (84% risk reduction), consistent with the IPERGAY trial results using episodic dosing [9, 10].
In both HPTN 083 and HPTN 084, a breakthrough infection was defined as HIV acquisition in the setting of protective drug exposure, >700 fmol/punch for TFV-DP in DBS or >500 fmol/punch if collected prior to week 8 (ie, pre-steady state) [3, 4]. No breakthrough infections associated with daily adherence were observed during the blinded phase of HPTN 084. However, 1 participant had intraerythrocytic TFV-DP concentrations consistent with 4–6 doses/week, based on DBS collected 23 days after enrollment and 11 days after the first HIV-positive visit (HIV was not detected at the enrollment visit).
There were additional post-injection study visits in HPTN 083; however, a similar testing schema was used to interrogate F-TDF use in study participants who acquired HIV. This study also included a randomly selected adherence cohort (n = 390). Two breakthrough infections were observed in participants randomized to the F-TDF arm. For 1 case, a sample was not available for TFV-DP measurements the first HIV-positive visit, and adherence was based on a plasma TFV concentrations >40 ng/mL at the first HIV-positive visit and previous TFV-DP concentrations >700 fmol/punch [3, 4]. In the other breakthrough infection, the participant had nucleoside reverse-transcriptase inhibitor/nonnucleoside reverse-transcriptase inhibitor resistance, including K65R, at the first HIV-positive visit, which may have been responsible for viral acquisition in the presence of target TFV and TFV-DP concentrations.
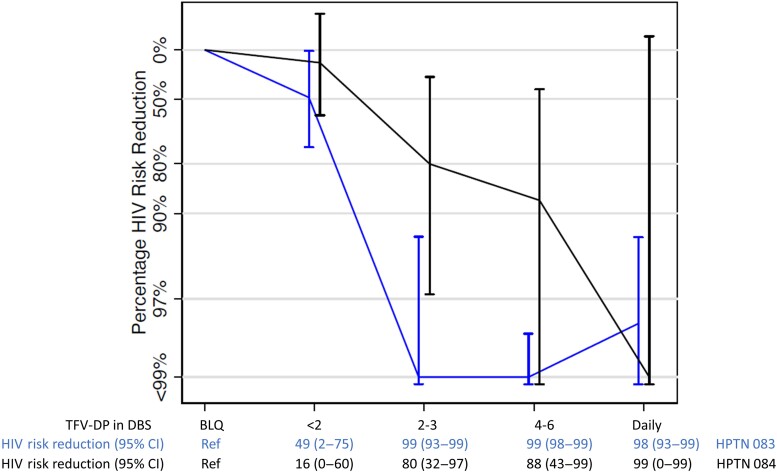
Despite similar breakthrough infection rates in men and women (2 and 1, respectively), a different pattern of pharmacologic forgiveness emerged when the full adherence–efficacy relationship was evaluated (Figure 1) [3, 4, 10]. In both studies, high efficacy (approximately 99%) is achieved for the highest adherers, but approximately 99% efficacy is reached at a lower adherence threshold in HPTN 083 (≥2 doses per week on average in the preceding 4–8 weeks) compared with HPTN 084 (women), where 99% efficacy is reached only at the highest adherence level. Confidence intervals are wide for HPTN 084 estimates, owing to low person-years for the higher adherence categories; person-years were 738, 505, 350, 291, and 58 for Below the Limit of Quantification (BLQ), <2, 2–3, 4–6, and daily F-TDF use, respectively [1]. Confidence intervals were tighter for HPTN 083, with corresponding person-years of 274, 290, 316, 1235, and 1072, respectively [2]. The low follow-up in the high-adherence categories for cisgender women indicates poor acceptance of daily F-TDF, which is a limitation to this analysis.
Figure 1.
Percentage HIV risk-reduction estimates (y-axis) for HPTN 083 and HPTN 084 according to adherence as measured with TFV-DP at or near the seroconversion visit (x-axis). Point estimates and 95% CIs are included on the x-axis. For HPTN 083, person-years (number of infections), from left to right, were 274 (24), 290 (13), 316 (0), 1235 (0), and 1072 (2). For HPTN 084, person-years (number of infections), from left to right, were 738 (21), 505 (12), 350 (2), 291 (1), 58 (0). Abbreviations: BLQ, Below the Limit of Quantification; CI, confidence interval; DBS, dried blood spot; HIV, human immunodeficiency virus; HPTN, HIV Prevention Trials Network; TFV-DP, tenofovir diphosphate.
An advantage of these studies was the collection of paired plasma TFV and intraerythrocytic TFV-DP, allowing for a more granular assessment of adherence. For instance, high plasma TFV concentrations (>40 ng/mL) in the setting of low TFV-DP (BLQ-350 fmol/punch) suggest significant, persistent nonadherence (low TFV-DP), except dosing just before the clinic visit (ie, “white-coat” dosing). Eight of 36 cases (25%) in HPTN 084 had plasma TFV concentrations >40 ng/mL at the first HIV-positive visit despite low TFV-DP measurements at or near this visit, consistent with dosing close to sampling. This pattern was only observed in 3 of 39 cases (8%) in HPTN 083 [3, 4]. A recent dose in the setting of long-term nonadherence could influence the TFV-DP collected at that visit, resulting in low but measurable TFV-DP that, without a recent dose, would otherwise be BLQ. Given that BLQ was the comparator for this analysis (Figure 1), this could bias HIV risk-reduction estimates at the lower adherence thresholds toward less forgiveness, as was observed for women (HPTN 084) in this analysis.
In the CAB arms for HPTN 083 and HPTN 084, there are currently no established pharmacologic correlates of protection. Based on rectal and vaginal Simian-Human Immunodeficiency Virus (SHIV) challenge studies, CAB plasma concentrations 3 and 4 times greater than the protein-adjusted 90% inhibition concentration (PA-IC90; 0.166 µg/mL) were associated with 100% protection, respectively [11, 12]. In order to probe the biological efficacy of CAB, both studies estimated the number of breakthrough infections, which was defined as HIV acquisition in the setting of expected CAB plasma exposures maintained over the injection interval (≥4× PA-IC90 or 0.664 µg/mL). In HPTN 084, 1 participant randomized to the CAB arm acquired HIV during the injection phase of the trial. However, there were several injection visit delays, including a 16.1-week gap between injections. The CAB plasma concentration at the first HIV-positive visit was <4× PA-IC90, which occurred after this delay [3]. In HPTN 083, 4 CAB breakthrough infections were identified [4]. The frequency of infections despite on-time injections was higher in men than in women. This may be attributed to the higher total number of person-years for CAB in HPTN 083 (3205) vs HPTN 084 (1956) [1, 2]. These findings are also consistent with more efficient per-act HIV transmission via rectal vs vaginal exposures [13]. Another consideration is that CAB exposure in the rectal compartment is modestly lower than in cervical and vaginal compartments. In a single-dose multicompartment pharmacokinetic study, rectal tissue was 9% of plasma concentrations, while cervical and vaginal tissue concentrations were 14% and 16% of plasma concentrations, respectively (statistical comparison not reported) [14]. Further analyses will be needed to understand if these findings illustrate different biological efficacies. These considerations aside, CAB efficacy was excellent.
In summary, the observations from HPTN 084 and HPTN 083 suggest different pharmacologic forgiveness profiles for oral F-TDF in women vs men consistent with previously described findings of decreased TFV-DP concentrations in vaginal compared with rectal tissues. More data are needed in the higher adherence categories for cisgender women to tighten the confidence intervals around the risk-reduction estimates. Also, additional directly observed dosing studies of F-TDF in African women are needed to refine the adherence benchmarks in this population [15]. We look forward to gaining more information from the open-label extensions of these trials and other PrEP trials that are underway to further guide the optimal use of F-TDF as PrEP for women and men.
Contributor Information
Peter L Anderson, Department of Pharmaceutical Sciences, University of Colorado Anschutz Medical Campus, Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora, Colorado, USA.
Mark A Marzinke, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
David V Glidden, Department of Epidemiology and Biostatistics, University of California–San Francisco, San Francisco, California, USA.
Notes
Acknowledgments . The authors acknowledge the HIV Prevention Trials Network (HPTN) 083 and HPTN 084 study participants and the research teams led by Drs Raphael Landovitz and Beatriz Grinsztejn (HPTN 083 protocol co-chairs) and Sinead Delany-Moretlwe and Mina Hosseinipour (HPTN 084 protocol co-chairs).
Financial support . This work was supported by UM1 AI068613 (M. A. M., P. L. A.), R01 AI122298 (P. L. A.), and R01 AI143357 (D. V. G.). D. V. G. reports support for this work from the National Institutes of Health (NIH) paid to institution. P. L. A. and M. A. M. report support for this work from the NIH/National Institute of Allergy and Infectious Diseases paid to institution.
References
- 1. Delany-Moretlwe S, Hughes JP, Bock P, et al. . Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. Lancet 2022; 399:1779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Landovitz RJ, Donnell D, Clement ME, et al. . Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med 2021; 385:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eshleman SH, Fogel JM, Piwowar-Manning E, et al. . Characterization of human immunodeficiency virus (HIV) infections in women who received injectable cabotegravir or tenofovir disoproxil fumarate/emtricitabine for HIV prevention: HPTN 084. J Infect Dis 2022; 225:1741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marzinke MA, Grinsztejn B, Fogel JM, et al. . Characterization of HIV infection in cisgender men and transgender women who have sex with men receiving injectable cabotegravir for HIV prevention: HPTN 083. J Infect Dis 2021; 224:1581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patterson KB, Prince HA, Kraft E, et al. . Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med 2011; 3:112re4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cottrell ML, Yang KH, Prince HM, et al. . A translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis 2016; 214:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baeten JM, Donnell D, Mugo NR, et al. . Single-agent tenofovir vs combination emtricitabine plus tenofovir for pre-exposure prophylaxis for HIV-1 acquisition: an update of data from a randomised, double-blind, phase 3 trial. Lancet Infect Dis 2014; 14:1055–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baeten JM, Donnell D, Ndase P, et al. . Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Molina JM, Capitant C, Spire B, et al. . On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015; 373:2237–46. [DOI] [PubMed] [Google Scholar]
- 10. Grant RM, Anderson PL, McMahan V, et al. . Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andrews CD, Spreen WR, Mohri H, et al. . Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science 2014; 343:1151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andrews CD, Yueh YL, Spreen WR, et al. . A long-acting integrase inhibitor protects female macaques from repeated high-dose intravaginal SHIV challenge. Sci Transl Med 2015; 7:270ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. Aids 2014; 28:1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shaik JS, Weld ED, Edick S, et al. . Multicompartmental pharmacokinetic evaluation of long-acting cabotegravir in healthy adults for HIV preexposure prophylaxis. Br J Clin Pharmacol 2022; 88:1667–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stranix-Chibanda L, Anderson PL, Kacanek D, et al. . Tenofovir diphosphate concentrations in dried blood spots from pregnant and postpartum adolescent and young women receiving daily observed pre-exposure prophylaxis in sub-Saharan Africa. Clin Infect Dis 2020; 73:e1893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]