Abstract
Background
As people with human immunodeficiency virus (HIV) (PWH) age, it remains unclear whether they are at higher risk for age-related neurodegenerative disorders—for example, Alzheimer disease (AD)—and, if so, how to differentiate HIV-associated neurocognitive impairment from AD. We examined a clinically available blood biomarker test for AD (plasma amyloid-β [Aβ] 42/Aβ40 ratio) in PWH who were cognitively normal (PWH_CN) or cognitively impaired (PWH_CI) and people without HIV (PWoH) who were cognitively normal (PWoH_CN) or had symptomatic AD (PWoH_AD).
Methods
A total of 66 PWH (age >40 years) (HIV RNA <50 copies/mL) and 195 PWoH provided blood samples, underwent magnetic resonance imaging, and completed a neuropsychological battery or clinical dementia rating scale. Participants were categorized by impairment (PWH_CN, n = 43; PWH_CI, n = 23; PWoH_CN, n = 138; PWoH_AD, n = 57). Plasma Aβ42 and Aβ40 concentrations were obtained using a liquid chromatography–tandem mass spectrometry method to calculate the PrecivityAD amyloid probability score (APS). The APS incorporates age and apolipoprotein E proteotype into a risk score for brain amyloidosis. Plasma Aβ42/Aβ40 ratios and APSs were compared between groups and assessed for relationships with hippocampal volumes or cognition and HIV clinical characteristics (PWH only).
Results
The plasma Aβ42/Aβ40 ratio was significantly lower, and the APS higher, in PWoH_AD than in other groups. A lower Aβ42/Aβ40 ratio and higher APS was associated with smaller hippocampal volumes for PWoH_AD. The Aβ42/Aβ40 ratio and APS were not associated with cognition or HIV clinical measures for PWH.
Conclusions
The plasma Aβ42/Aβ40 ratio can serve as a screening tool for AD and may help differentiate effects of HIV from AD within PWH, but larger studies with older PWH are needed.
Keywords: HIV, Alzheimer disease, biomarkers
The plasma amyloid-β (Aβ) 42/Aβ40 ratio, a blood-based biomarker for brain amyloid in Alzheimer disease, is not abnormal in older cognitively normal or cognitively impaired people with human immunodeficiency virus (HIV), compared with similarly aged, cognitively normal people without HIV.
With the advent of effective antiretroviral therapy (ART) for human immunodeficiency virus (HIV), people with HIV (PWH) are now living longer, reaching ages similar to people without HIV (PWoH). The Centers for Disease Control estimates that more than half of PWH in the United States are >50 years old, and an increasing proportion of PWH have reached or will reach the age of 65 years [1]. Consequently, differentiating between HIV-related neurocognitive disorders and age-related neurodegenerative diseases, primarily Alzheimer disease (AD), has become a critical focus for clinicians caring for PWH.
Previous research has suggested that brain aging may be accelerated or accentuated in PWH [2–5]. Changes to brain integrity have been reported to occur at younger ages in PWH than in PWoH [2–5], even in individuals on a stable ART regimen who are virologically well controlled [4]. In addition, these changes have also been linked to poorer cognitive functioning in PWH [2–4]. Although research is limited, several studies also indicate a potentially higher prevalence of AD among PWH compared with PWoH [6–8]. However, current research is divided as to whether these changes also imply greater risk for the accumulation of amyloid at younger ages, as reflected by AD-related biomarkers.
Amyloid-β (Aβ) deposition, a hallmark of AD, has been a primary focus for neuropathological studies. Research suggests that abnormal Aβ deposition may occur several decades before cognitive symptoms become apparent [9, 10]. Currently, the most common methods to evaluate cerebral Aβ pathology include positron emission tomography (PET) and cerebrospinal fluid (CSF) analysis. Inconsistent results have been seen in studies that have previously used these 2 methods to evaluate for potential Aβ changes in PWH. Several PET imaging studies have not observed increased Aβ deposition in PWH compared with PWoH [11–13], while another study suggests some evidence of elevated brain Aβ levels in an older person with HIV [14]. Similarly, results vary when CSF Aβ42 is considered. Reduced CSF Aβ42/Aβ40, indicative of greater cerebral Aβ pathology, has been reported in some studies of PWH with cognitive impairment compared with PWoH [15, 16] while others observed no relationship between HIV serostatus, cognitive impairment, and CSF Aβ42/Aβ40 [13, 17–19].
In addition to the inclusion of PWH both with and without virological suppression, small group sizes resulting from difficulty in obtaining PET and CSF measures may influence these discrepant results. PET and CSF analyses are invasive, costly procedures, limiting their utility in certain clinical settings. Consequently, the need for more easily obtainable markers has led to the advent of potential blood-based biomarkers (BBMs) for Aβ pathology. BBMs are less expensive and more convenient for patients and can be obtained quickly and easily, allowing collection to be completed at routine clinic or study visits [20]. BBMs may have particular utility as screening measures for Aβ pathology that can be easily and widely implemented in many clinics and used as screeners or prescreeners for Aβ pathology, for entry into clinical trials [20].
One promising BBM to screen for Aβ pathology is the ratio of plasma Aβ42 to Aβ40. This ratio has been evaluated in relation to PET amyloid in both cognitively impaired and unimpaired older adults [21–23]. The first commercially available blood test uses immunoprecipitation mass spectrometry (PrecivityAD; C2N Diagnostics). In HIV, the serum Aβ42/Aβ40 ratio was previously found to be reduced in PWH compared with a small sample of PWoH but not individuals with AD [24]. Notably, that study used an electrochemiluminescence assay (Meso Scale Discovery; Meso Scale Diagnostics) to assess Aβ42/Aβ40. However, more recent work suggests that immunoprecipitation mass spectrometry may be more accurate in determining cerebral amyloid status than methods using immunoassays [25].
In addition, the amyloid probability score (APS) has been developed, which incorporates plasma Aβ42/Aβ40, age, and apolipoprotein proteotype into a risk score for brain amyloidosis. The APS has a stronger association with brain amyloidosis than the plasma Aβ42/Aβ40 ratio [26]. The current study compares the plasma Aβ42/Aβ40 ratio and APS between older, cognitively healthy PWoH, patients with symptomatic AD, and PWH with or without cognitive impairment. The plasma Aβ42/Aβ40 ratio and APS were also correlated with hippocampal volumes within each group, and with HIV clinical characteristics (nadir and current CD4 T-cell count, CD8 T-cell count, CD4/CD8 ratio, and duration of HIV infection) within the PWH groups.
METHODS
Participants
Between 2017 and 2020, a total of 66 PWH (age, >40 years; range, 49–71 years) who were receiving ART and had HIV RNA levels <50 copies/mL were recruited from the Washington University School of Medicine Infectious Disease Clinic and the Washington University School of Medicine AIDS Clinical Trial Unit. Between 2004 and 2019, a total of 195 PWoH (age range, 43–88 years; cognitively normal, n = 138; symptomatic AD, n = 57) were recruited from studies performed at the Knight Alzheimer Disease Research Center. Exclusion criteria for all participants included current or past confounding neurological disorders (eg, stroke, untreated severe depression, head injury with loss of consciousness >30 minutes); a positive result on a urine drug screen for a substance other than tobacco, alcohol, or marijuana; and <8 years of education. All participants provided written informed consent that was approved by the Institutional Review Board at Washington University in St Louis.
Plasma Aβ42 and Aβ40 Measurement
Blood samples were collected at study visits for participants and frozen using methods described elsewhere [21]. Plasma samples were prepared and analyzed for Aβ42 and Aβ40 concentrations, using the analytically and clinically validated liquid chromatography–tandem mass spectrometry platform developed at C2N Diagnostics [26–28]. In short, plasma Aβ isoforms (Aβ42 and Aβ40) were immunoprecipitated from 450 µL of ethylenediaminetetraacetic acid plasma via a monoclonal antibody (HJ5.1) conjugated to magnetic beads (M-270 Epoxy Dynabeads; Thermo Fisher Scientific). Extracted proteins were digested (Lys-N; Thermo Fisher Scientific), C-terminal peptides (Aβ28–40 and Aβ28–42) were further purified using solid-phase extraction (Waters), separated, and quantified using liquid chromatography–mass spectrometry (Thermo Fisher Scientific Fusion Lumos Tribrid Mass Spectrometer). Lower plasma Aβ42/Aβ40 ratios are concordant with a high likelihood of brain amyloidosis [21, 27, 28].
APOE Genotyping
For PWH, apolipoprotein E4 (APOE ε4) allele status was extracted from 1 × 106 peripheral blood mononuclear cells using the Quick-DNA Miniprep kit (Zymo Research).
APOE ε4 allele status was obtained from the Knight Alzheimer Disease Research Center Genetics Core using methods described elsewhere for PWoH [29]. APOE ε4 status was coded as a binary variable, with participants classified as having no APOE ε4 alleles or ≥1 APOE ε4 allele.
Calculation of APS
The APS was also calculated for all participants using a logistic regression formula that incorporates plasma Aβ42/Aβ40, age, and apolipoprotein E proteotype (1 of 6 possible genotypes). The output from this algorithm is scaled to a range from 0 (low likelihood of amyloid positivity) to 100 (high likelihood of amyloid positivity) [26].
Neuropsychological Evaluation
PWH completed a comprehensive neuropsychological test battery that assessed the learning, delayed recall, recognition memory, executive function, processing speed, attention, language, and fine motor performance domains [30]. Raw test scores were standardized into demographically adjusted (age, sex, race, education) z scores [30]. Test z scores within each cognitive domain were averaged to created domain z scores, and domain z scores were averaged to create a global z score. Domain z scores were used to categorize PWH as cognitively normal (PWH_CN) or cognitively impaired (PWH_CI), with cognitive impairment defined as ≥2 cognitive domain z scores ≤ −1 or ≥1 domain z score ≤ −2 [31]. All PWoH were evaluated by experienced clinicians using a semistructured interview based on the clinical dementia rating scale [32]. Individuals with a clinical dementia rating scale score of 0 were classified as cognitively normal (PWoH_CN), while those with a score >0 were classified as presenting with symptomatic AD (PWoH_AD).
HIV Clinical Characteristics
Current HIV RNA level, CD4 T-cell count, CD8 T-cell count, and CD4/CD8 ratio were obtained via blood sampling at the study visit for all PWH. The duration of known HIV infection and nadir T-cell count were obtained through self-report or medical records, when available.
Neuroimaging
Magnetic resonance imaging was collected on Siemens 3 T scanners at Washington University School of Medicine in St Louis. The imaging protocol included a high-resolution 3D T1-weighted magnetization-prepared rapid acquisition gradient-echo (MPRAGE) sequence (repetition time, 2400 ms; echo time, 3.16 ms; 256 × 256 acquisition matrix with 1-mm isotropic resolution; flip angle, 8˚). Freesurfer software, version 5.3, was used to segment and calculate volume of the hippocampus. The intracranial volume was regressed out of the hippocampus, and the standardized residual was used as the outcome variables in applicable analyses.
Statistical Analyses
Demographic variables (age, sex, race, education level) were first compared between all groups using χ2 tests for categorical variables or Mann-Whitney U or Kruskal-Wallis tests for continuous variables. Similarly, HIV clinical characteristics were compared between PWH_CN (n = 43) and PWH_CI (n = 23). Plasma Aβ42/Aβ40 ratios were log-transformed. Group differences in plasma Aβ42/Aβ40 ratios were assessed using general linear models, using age, sex, race, and APOE ε4 status as covariates. In addition, group differences in the APS were assessed using general linear models, with sex and race included as covariates. Relationships between plasma Aβ42/Aβ40 ratios or APSs and cognitive domain z scores were also assessed using linear regression models within PWH. Domain z scores were not available for PWoH.
To assess relationships between the plasma Aβ42/Aβ40 ratio and hippocampus volumes, linear regression models using age, sex, race, APOE ε4 status, and plasma Aβ42/Aβ40 as predictors were conducted within each group. Similarly, regression analyses for PWH assessed relationships between plasma Aβ42/Aβ40 and HIV disease characteristics (current and nadir CD4 T-cell count, current CD8 T-cell count, CD4/CD8 ratio, and duration of infection). Relationships with the APS were assessed with similar analyses, using only sex and race as covariates when appropriate. The false discovery rate method was used to correct for multiple comparisons. In addition, plasma Aβ42/Aβ40 ratios for PWH in the oldest quartile of age were compared with PWoH_AD participants in the youngest age quartile. Further details are provided in the Supplementary Material, section I.
RESULTS
Participant demographic, clinical, and cognitive characteristics are provided in Table 1. The PWoH_AD group was significantly older and included more white participants than the other 3 groups (P < .001). In general, the 2 PWoH groups were more highly educated and had more female participants than the 2 PWH groups (P < .01). On average, among PWH, HIV had been diagnosed >20 years earlier (mean duration of known infection, 247.2 months; standard deviation [SD], 109.6); ART duration, 212 months [104.7 months]) and had a mean CD4 T-cell count of 667.4/µL (280.5/µL). There were no differences in demographic or clinical characteristics between PWH_CN and PWH_CI (P >.05).
Table 1.
Demographic, Clinical, and Cognitive Characteristics of Participants by Group
| Characteristic | PWoH_CN (n = 138) | PWoH_AD (n = 57) | PWH_CN (n = 43) | PWH_CI (n = 23) | P Value | Effect Size |
|---|---|---|---|---|---|---|
| Age, mean (SD), y | 57.0 (6.9)a | 66.2 (5.7) | 57.3 (5.6)a | 55.5 (4.8)a | <.001b | 0.28c |
| Male sex, % | 46 | 61 | 77d | 83d | <.001b | 0.28e |
| Educational level, mean (SD), y | 16.0 (4.8) | 15.2 (2.7) | 13.4 (2.1)a,d | 13.0 (2.9)a,d | <.001b | 0.16c |
| African American race, % | 43a | 7 | 63a | 70a | <.001b | 0.41e |
| Hispanic ethnicity, % | 0 | 0 | 2.3 | 0 | .17 | 0.14e |
| ≥1 APOE ε4 allele, % | 43a | 71 | 33a | 39a | <.001b | 0.27e |
| T-cell count, median (IQR), cells/µL | ||||||
| Recent CD4 T-cell count, | NA | NA | 639 (444–898) | 580 (362–771) | .1 | 0.04c |
| Nadir CD4 T-cell count | NA | NA | 190 (17–275) | 54 (24–300) | .89 | 0.002c |
| Recent CD8 T-cell count | NA | NA | 867 (606–1116) | 1011 (559–1085) | .97 | 0.00c |
| CD4/CD8 ratio, median (IQR) | NA | NA | 0.7 (0.5–0.9) | 0.7 (0.3–1.2) | .19 | 0.02c |
| Duration of HIV infection, median (IQR), mo | NA | NA | 240 (126–288) | 300 (204–372) | .08 | 0.04c |
| Current cART medication, % | ||||||
| NRTI | NA | NA | 95 | 96 | .96 | 0.01e |
| NNRTI | NA | NA | 40 | 30 | .46 | 0.10e |
| PI | NA | NA | 19 | 30 | .27 | 0.14e |
| INSTI | NA | NA | 65 | 74 | .47 | 0.10e |
| Cognitive characterization | ||||||
| CDR >0.5, % | 0% | 37% | NA | NA | <.001b | 1.0e |
| z Score, mean (SD) | ||||||
| Learning domain | NA | NA | 0.1 (0.7) | −0.6 (0.7) | <.001b | 1.0f |
| Delayed recall domain | NA | NA | 0.3 (0.8) | −0.6 (0.7) | <.001b | 1.2f |
| Recognition domain | NA | NA | −0. (0.5) | −0.6 (0.8) | .01b | 0.4f |
| Executive function domain | NA | NA | 0.05 (0.6) | −0.5 (0.7) | .003b | 0.8f |
| Attention domain | NA | NA | 0.2 (0.6) | −1.0 (1.0) | <.001b | 1.5f |
| Fine motor domain | NA | NA | −0.3 (0.9) | −0.9 (0.8) | .008b | 0.7f |
| Psychomotor speed domain | NA | NA | 0.3 (0.7) | −0.3(0.5) | .001b | 1.0f |
| Language domain | NA | NA | 0.1 (0.7) | −0.6 (0.7) | .002b | 1.0f |
| Global cognition | NA | NA | 0.1 (0.3) | −0.6 (0.3) | <.001b | 2.3f |
| Hippocampus volume | 0.36 (0.70)a | −1.34 (0.94) | 0.19 (0.82)a | −0.09 (0.83)a | <.001b | 0.3c |
| Log Aβ42/Aβ40 ratio, median (IQR) | −0.97 (−0.99 to −0.95)a | −1.03 (−1.04 to −1.01) | −0.98 (−1.00 to −0.94)a | −0.98 (−1.0 to −0.95)a | <.001b | 0.1c |
| APS, median (IQR) | 1.0 (0–3.3)a | 17.0 (8.2–70.5) | 1.5 (0–7.5)a | 2.5 (0–15.5)a | <.001b | 0.4c |
Abbreviations: Aβ, amyloid-β; APS, amyloid probability score; cART, combination antiretroviral therapy; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; NA, not available; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitors; PWH_CI, people with HIV (PWH) with cognitive impairment; PWH_CN, cognitively normal PWH; PWoH_AD, people without HIV (PWoH) who had symptomatic AD; PWoH_CN, cognitively normal PWoH; SD, standard deviation.
Significant difference from PWoH_AD group (P < .05).
Significant at P < .05.
Effect size given as partial η2.
Significant difference from PWoH_CN group (P < .05).
Effect size given as Cramer V.
Effect size given as Cohen d.
For PWH, all cognitive domain z scores and the global cognition z score were significantly lower in the PWH_CI than in the PWH_CN group (P <.01). The attention, psychomotor speed, and delayed recall domains demonstrated the largest differences in scores between the PWH_CN and PWH_CI groups (average group difference in z scores, 1.2, 1.0, and 1.0, respectively).
Effects of HIV and Cognitive Impairment on the Plasma Aβ42/Aβ40 Ratio and APS
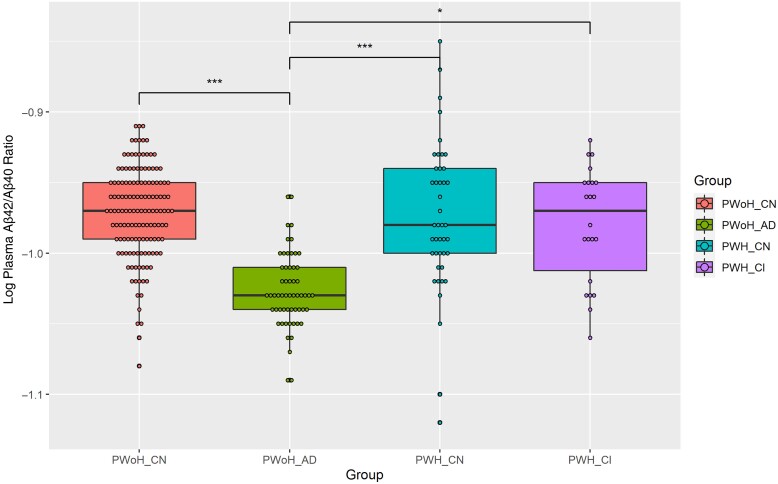
Overall, analyses demonstrated a significant group difference in log Aβ42/Aβ40 ratios (P < .001) (Figure 1). Pairwise comparisons revealed a significantly lower Aβ42/Aβ40 ratio for the PWoH_AD group (mean ratio [SD], −1.03 [0.03]) compared with the 3 other groups ( −0.97 [0.03], −0.98 [0.05], and −0.99 [0.06] for PWoH_CN, PWoH_CN, and PWH_CI, respectively; P < .01). There were no other significant differences between these groups, including between PWH who did and those who did not have cognitive impairment (P > .05). Results did not change when comparing the oldest PWH with the youngest PWoH_AD participants (Supplementary Material, section II).
Figure 1.
Log-transformed plasma amyloid-β (Aβ) 42/Aβ40 ratios by group. People without human immunodeficiency virus (HIV) (PWoH) who had symptomatic Alzheimer disease (PWoH_AD) had significantly lower plasma Aβ42/Aβ40 ratios than the other 3 groups. People with HIV (PWH), regardless of cognitive impairment status, did not have lower ratios than cognitively normal PWoH (PWoH_CN). *P < .05; ***P < .001. Abbreviations: PWH_CI, cognitively impaired PWH; PWH_CN, cognitively normal PWH.
Similarly, the mean APS was significantly higher for the PWoH_AD group (mean [SD], 41.2 [28.9]) than for the other 3 groups (4.73 [11.3], 6.63 [13.6], and 10.2 [17.7] for PWoH_CN, PWH_CN, and PWH_CI, respectively; P < .001). There were no other significant group differences in APS between the PWoH_CN, PWH_CN, and PWH_CI groups (P > .05). For PWH, there were no significant correlations between the plasma Aβ42/Aβ40 ratio or APS and the global cognition z score or any of the cognitive domain z scores (all P > .05).
Relationships Between Aβ42/Aβ40 or APS and Hippocampus Volume
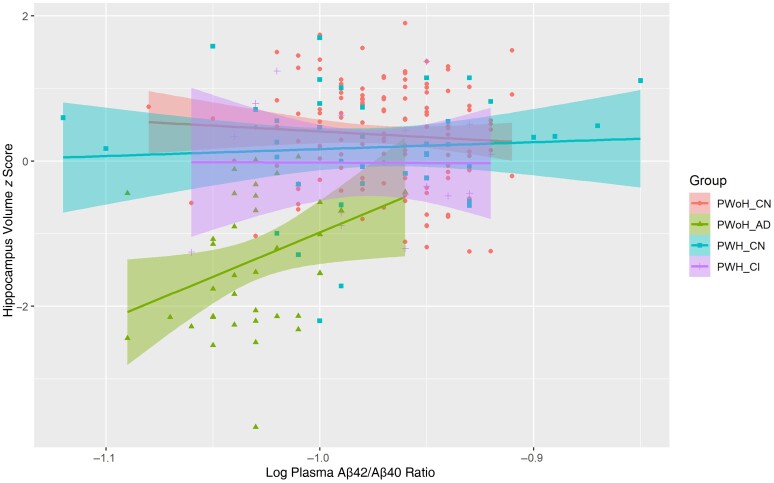
Regression analyses revealed lower plasma Aβ42/Aβ40 ratios (r = 0.46; P = .003) (Figure 2) and higher APSs (r = −0.40; P = .009) were significantly associated with smaller hippocampal volumes for the PWoH_AD group. These relationships did not reach significance for any other group (P > .05).
Figure 2.
Lower plasma amyloid-β (Aβ) 42/Aβ40 ratios were significantly correlated with smaller hippocampus volumes only in the people without human immunodeficiency virus (HIV) (PWoH) who had symptomatic Alzheimer disease (PWoH_AD). Abbreviations: PWH_CI, people with HIV (PWH) with cognitive impairment; PWH_CN, cognitively normal PWH; PWoH_CN, cognitively normal PWoH.
Relationships Between Aβ42/Aβ40 or APS and HIV Clinical Characteristics
Among PWH, there were no significant relationships between the plasma Aβ42/Aβ40 ratio or APS and the nadir CD4 T-cell count, current CD4 and CD8 T-cell counts, CD4/CD8 ratio, or duration of known HIV infection (P > .05).
DISCUSSION
As PWH continue to age, one of the most significant questions clinicians are facing is whether HIV results in increased risk for age-related neurodegenerative disorders, primarily AD, as assessed by increased AD biomarker accumulation compared with PWoH. The current study was among the first to evaluate a clinically available blood biomarker test for AD in a group of virologically well-controlled PWH, with or without cognitive impairment, compared with cognitively normal PWoH and PWoH with symptomatic AD.
Results indicated that the plasma Aβ42/Aβ40 ratio and APS were sensitive in discriminating the PWoH_AD group from PWoH_CN and the 2 PWH groups. Within PWH, the plasma Aβ42/Aβ40 ratio and APS did not significantly differ between cognitively normal and cognitively impaired PWH. In addition, neither the Aβ42/Aβ40 ratio nor the APS was associated with any of the examined HIV disease characteristics (eg, CD4 and CD8 T-cell counts) in this sample. Finally, we observed the expected relationship between lower plasma Aβ42/Aβ40 ratio and smaller hippocampal volumes within the symptomatic AD group, but this relationship was not significant for the cognitively normal PWoH or the 2 PWH groups. These results suggest that, within this sample, HIV-related cognitive changes were likely not attributable to brain amyloid accumulation and lower hippocampal volume, and the plasma Aβ42/Aβ40 ratio and APS may be useful in distinguishing AD-related cognitive changes from HIV-related cognitive changes.
Our results are consistent with those of previous studies of Aβ in HIV that demonstrated no significant differences in amyloid between PWH and cognitively unimpaired PWoH, using either CSF biomarkers or PET imaging [11–13, 17, 19]. Taken together, these results suggest that while there may be some evidence of accelerated brain aging in PWH [2–5], it is unlikely that these changes can be attributed to an accelerated AD phenotype, as we observed no significant reduction in Aβ42/Aβ40 ratio or increase in APS in PWH compared with age-matched PWoH_CN. Instead, changes to brain integrity in older PWH may be more attributable to other factors, such as legacy effects of early untreated HIV infection, comorbid conditions (eg, cardiovascular disease and diabetes), environmental factors, and ongoing neuroinflammation [33–37].
Importantly, we also did not observe any significant differences in the plasma Aβ42/Aβ40 ratio or APS in our sample of cognitively impaired PWH compared with PWH_CN, with both PWH groups demonstrating similarly normal plasma Aβ42/Aβ40 ratios and lower APSs compared with the PWoH_AD group. Notably, the pattern of cognitive deficits within our PWH_CI sample did not reflect those typically observed within individuals with AD. Specifically, the neuropsychological profile of PWH within the current study was largely heterogeneous, with significantly poorer performance recorded for PWH_CI compared with PWH_CN across all tested cognitive domains. The greatest differences in cognitive performance between the PWH groups were identified within the attention and psychomotor speed domains. These deficits more closely correspond to a pattern of HIV-related neurocognitive impairment compared with the episodic and recognition memory impairments that are characteristic of AD [38].
In addition, there were no significant associations between any of the cognitive domain z scores and the plasma Aβ42/Aβ40 ratio or APS in PWH, even within AD-typical domains (eg, recognition memory). However, it should be noted that even within PWH_CI, cognitive deficits were relatively mild (mean global cognition z score [SD], −0.6 [0.3]). Although longitudinal studies are needed to examine relationships between further cognitive decline and changes in the plasma Aβ42/Aβ40 ratio, the observed differences in plasma Aβ42/Aβ40 ratios between cognitively impaired PWH and individuals with symptomatic AD suggest a potential utility of this ratio in infectious disease clinics as a screening method to differentiate between HIV-related cognitive impairment and brain amyloid accumulation typical of AD in older PWH who present with cognitive complaints.
Several limitations of the current study must be considered. First, this study was cross-sectional, limiting conclusions regarding the progression of changes in plasma Aβ42/Aβ40 ratios over time in PWH. Importantly, PWH included in the study were relatively young (mean age [SD], 56.6 [5.4] years) and slightly younger than the cohort used to clinically validate the plasma Aβ42/Aβ40 ratios and APSs with amyloid PET status (age ≥60 years). While the younger and similar age ranges of our PWH and PWoH_CN groups further suggest a lack of evidence for an accelerated AD phenotype in PWH compared with the general population, longitudinal studies examining patterns of change in plasma Aβ42/Aβ40 ratios in PWH as they reach ages associated with increased AD risk (>65 years) are needed to elucidate any potential links between amyloidosis and aging with HIV. In addition, the PWoH and PWH groups were not well matched on other demographic variables (sex and race) that may affect results. Future studies need to focus on recruiting PWoH and PWH samples that are demographically more similar. Finally, cognitive impairment was assessed differently in PWH and PWoH. Future studies using similar methods across all participants are needed to establish patterns of associations between cognitive domains and plasma Aβ42/Aβ40 ratios.
Virologically well-controlled PWH with or without cognitive impairment did not exhibit reduced plasma Aβ42/Aβ40 ratios or increased APSs compared with cognitively unimpaired PWoH. Only PWoH with symptomatic AD demonstrated significantly lower plasma Aβ42/Aβ40 ratios and higher APSs, which were also correlated with smaller hippocampus volumes. Clinical markers of HIV disease were not associated with the plasma Aβ42/Aβ40 ratio or APS. Overall, these results demonstrate that the plasma Aβ42/Aβ40 ratio, a marker that can be quickly and easily obtained during a clinical visit, may help differentiate the effects of HIV from AD within PWH, but larger studies with older PWH are needed. The ability to distinguish causes will help inform the best treatments to help relieve or potentially slow cognitive decline.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Sarah A Cooley, Department of Neurology, Washington University in St Louis, St Louis, Missouri, USA.
Brittany Nelson, Department of Neurology, Washington University in St Louis, St Louis, Missouri, USA.
Anna Boerwinkle, Department of Neurology, Washington University in St Louis, St Louis, Missouri, USA.
Kevin E Yarasheski, C2N Diagnostics, St Louis, Missouri, USA.
Kris M Kirmess, C2N Diagnostics, St Louis, Missouri, USA.
Matthew R Meyer, C2N Diagnostics, St Louis, Missouri, USA.
Suzanne E Schindler, Department of Neurology, Washington University in St Louis, St Louis, Missouri, USA; Knight Alzheimer Disease Research Center, Washington University School of Medicine, St Louis, Missouri, USA.
John C Morris, Department of Neurology, Washington University in St Louis, St Louis, Missouri, USA; Knight Alzheimer Disease Research Center, Washington University School of Medicine, St Louis, Missouri, USA; Department of Radiology, Washington University in St Louis, St Louis, Missouri, USA.
Anne Fagan, Knight Alzheimer Disease Research Center, Washington University School of Medicine, St Louis, Missouri, USA.
Beau M Ances, Department of Neurology, Washington University in St Louis, St Louis, Missouri, USA.
Jane A O’Halloran, Division of Infectious Diseases, Department of Medicine, Washington University School of Medicine, St Louis, Missouri, USA.
Notes
Financial support. This work was supported by the National Institute on Aging (grants R03AG067995-02, R01AG070941, P30AG066444, P01AG003991, and P01AG026276), the National Institute of Nursing Research (grant R01NR015738), the National Institute of Mental Health (grant R01MH118031), and the National Institute of Drug Abuse (grant R01DA054009). K. E. Y., K. M. K., and M. R. M. report support for this work from C2N Diagnostics as full-time employees.
References
- 1. Dailey A, Gant Z, Johnson S, et al. HIV surveillance report 2018 (updated). 2018. Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html.http://www.cdc.gov/hiv/library/reports/hiv-surveillance.htmlhttp://wwwn.cdc.gov/dcs/ContactUs/Form. Accessed 8 August 2022.
- 2. Cole JH, Underwood J, Caan MWA, et al. Increased brain-predicted aging in treated HIV disease. Neurology 2017; 88:1349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuhn T, Kaufmann T, Doan NT, et al. An augmented aging process in brain white matter in HIV. Hum Brain Mapp 2018; 39:2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petersen KJ, Metcalf N, Cooley S, et al. Accelerated brain aging and cerebral blood flow reduction in persons with human immunodeficiency virus. Clin Infect Dis An Off Publ Infect Dis Soc Am 2021; 73:1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Petersen KJ, Strain J, Cooley S, Vaida F, Ances BM. Machine learning quantifies accelerated white-matter aging in persons with HIV. J Infect Dis 2022; 226:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lam JO, Lee C, Gilsanz P, et al. Comparison of dementia incidence and prevalence between individuals with and without HIV infection in primary care from 2000 to 2016. AIDS 2022; 36:437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang CC, Chien WC, Chung CH, et al. No association between human immunodeficiency virus infections and dementia: a nationwide cohort study in Taiwan. Neuropsychiatr Dis Treat 2019; 15:3155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu X, Raji MA, Giordano TP, Berenson AB, Baillargeon J, Kuo YF. Prevalence of Alzheimer's disease and related dementias among older US Medicare beneficiaries with and without HIV: a successive cross-sectional study. Lancet Heal Longev 2022; 3:S6. [Google Scholar]
- 9. Bateman RJ, Xiong C, Benzinger TLS, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med 2012; 367:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jack CR, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013; 12:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ances BM, Christensen JJ, Teshome M, et al. Cognitively unimpaired HIV-positive subjects do not have increased 11C-PiB: a case-control study. Neurology 2010; 75:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Howdle GC, Quidé Y, Kassem MS, et al. Brain amyloid in virally suppressed HIV-associated neurocognitive disorder. Neurol Neuroimmunol Neuroinflammation 2020; 7:e739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ortega M, Ances BM. Role of HIV in amyloid metabolism. J Neuroimmune Pharmacol 2014; 9:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mohamed M, Skolasky RL, Zhou Y, et al. Beta-amyloid (Aβ) uptake by PET imaging in older HIV+ and HIV- individuals. J Neurovirol 2020; 26:382–90. [DOI] [PubMed] [Google Scholar]
- 15. Clifford DB, Fagan AM, Holtzman DM, et al. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology 2009; 73:1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krut JJ, Zetterberg H, Blennow K, et al. Cerebrospinal fluid Alzheimer's biomarker profiles in CNS infections. J Neurol 2013; 260:620–6. [DOI] [PubMed] [Google Scholar]
- 17. Ances BM, Benzinger TL, Christensen JJ, et al. 11C-PiB imaging of human immunodeficiency virus–associated neurocognitive disorder. Arch Neurol 2012; 69:72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ellis RJ, Chenna A, Petropoulos CJ, et al. Higher cerebrospinal fluid biomarkers of neuronal injury in HIV-associated neurocognitive impairment. J NeuroVirology 2022; 1:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Steinbrink F, Evers S, Buerke B, et al. Cognitive impairment in HIV infection is associated with MRI and CSF pattern of neurodegeneration. Eur J Neurol 2013; 20:420–8. [DOI] [PubMed] [Google Scholar]
- 20. Hansson O, Edelmayer RM, Boxer AL, et al. The Alzheimer's Association appropriate use recommendations for blood biomarkers in Alzheimer's disease. Alzheimers Dement 2022; 18:2669–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schindler SE, Bollinger JG, Ovod V, et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 2019; 93:E1647–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Y, Schindler SE, Bollinger JG, et al. Validation of plasma amyloid-β 42/40 for detecting Alzheimer disease amyloid plaques. Neurology 2022; 98:E688–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burnham SC, Fandos N, Fowler C, et al. Longitudinal evaluation of the natural history of amyloid-β in plasma and brain. Brain Commun 2020; 2:fcaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Almeida SM, Ribeiro CE, Rotta I, et al. Blood amyloid-β protein isoforms are affected by HIV-1 in a subtype-dependent pattern. J Neurovirol 2020; 26:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Janelidze S, Teunissen CE, Zetterberg H, et al. Head-to-head comparison of 8 plasma amyloid-β 42/40 assays in Alzheimer disease. JAMA Neurol 2021; 78:1375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu Y, Kirmess KM, Meyer MR, et al. Assessment of a plasma amyloid probability score to estimate amyloid positron emission tomography findings among adults with cognitive impairment. JAMA Netw Open 2022; 5:E228392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. West T, Kirmess KM, Meyer MR, et al. A blood-based diagnostic test incorporating plasma Aβ42/40 ratio, ApoE proteotype, and age accurately identifies brain amyloid status: findings from a multi cohort validity analysis. Mol Neurodegener 2021; 16:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kirmess KM, Meyer MR, Holubasch MS, et al. The PrecivityADTM test: accurate and reliable LC-MS/MS assays for quantifying plasma amyloid beta 40 and 42 and apolipoprotein E proteotype for the assessment of brain amyloidosis. Clin Chim Acta 2021; 519:267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pastor P, Roe CM, Villegas A, et al. Apolipoprotein epsilon4 modifies Alzheimer's disease onset in an E280A PS1 kindred. Ann Neurol 2003; 54:163–9. [DOI] [PubMed] [Google Scholar]
- 30. Paul RH, Cooley SA, Garcia-Egan PM, Ances BM. Cognitive performance and frailty in older HIV-positive adults. J Acquir Immune Defic Syndr 2018; 79:375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paul R, Rhee G, Baker LM, Vaida F, Cooley SA, Ances BM. Effort and neuropsychological performance in HIV-infected individuals on stable combination antiretroviral therapy. J Neurovirol 2017; 23:725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology 1993; 43:2412–4. [DOI] [PubMed] [Google Scholar]
- 33. Burdo TH, Lackner A, Williams KC. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev 2013; 254:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ellis RJ, Badiee J, Vaida F, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS 2011; 25:1747–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qu Y, Weinstein A, Wang Z, et al. Legacy effect on neuropsychological function in HIV-infected men on combination antiretroviral therapy. AIDS 2022; 36:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rubin LH, Xu Y, Norris PJ, et al. Early inflammatory signatures predict subsequent cognition in long-term virally suppressed women with HIV. Front Integr Neurosci 2020; 14:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Winston A, Spudich S. Cognitive disorders in people living with HIV. Lancet HIV 2020; 7:e504–13. [DOI] [PubMed] [Google Scholar]
- 38. Rubin LH, Sundermann EE, Moore DJ. The current understanding of overlap between characteristics of HIV-associated neurocognitive disorders and Alzheimer's disease. J Neurovirol 2019; 25:661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.