Highlights
-
•
Signs and symptoms of COVID-19 at hospital admission are similar between patients infected with HIV and controls.
-
•
Mortality from COVID-19 in patients infected with HIV was higher compared to the controls in 2020, but no difference in 2021.
-
•
Similar rates of ICU and invasive mechanical ventilation were observed in the different waves.
Keywords: COVID-19, HIV, Intensive care unit, Mechanical ventilation, Mortality
Abstract
Objective
To evaluate clinical characteristics and outcomes of COVID-19 patients infected with HIV, and to compare with a paired sample without HIV infection.
Methods
This is a substudy of a Brazilian multicentric cohort that comprised two periods (2020 and 2021). Data was obtained through the retrospective review of medical records. Primary outcomes were admission to the intensive care unit, invasive mechanical ventilation, and death. Patients with HIV and controls were matched for age, sex, number of comorbidities, and hospital of origin using the technique of propensity score matching (up to 4:1). They were compared using the Chi-Square or Fisher's Exact tests for categorical variables and the Wilcoxon for numerical variables.
Results
Throughout the study, 17,101 COVID-19 patients were hospitalized, and 130 (0.76%) of those were infected with HIV. The median age was 54 (IQR: 43.0;64.0) years in 2020 and 53 (IQR: 46.0;63.5) years in 2021, with a predominance of females in both periods. People Living with HIV (PLHIV) and their controls showed similar prevalence for admission to the ICU and invasive mechanical ventilation requirement in the two periods, with no significant differences. In 2020, in-hospital mortality was higher in the PLHIV compared to the controls (27.9% vs. 17.7%; p = 0.049), but there was no difference in mortality between groups in 2021 (25.0% vs. 25.1%; p > 0.999).
Conclusions
Our results reiterate that PLHIV were at higher risk of COVID-19 mortality in the early stages of the pandemic, however, this finding did not sustain in 2021, when the mortality rate is similar to the control group.
Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic has significantly impacted the assistance services offered to People Living with Human Immunodeficiency Virus (PLHIV). The introduction of restrictive measures aimed at slowing COVID-19 dissemination has resulted in reduced access to PLHIV to specialized care.1 This is an alarming scenario, due to the important contribution of regular follow-up towards declining the morbidity and mortality of PLHIV.2
Evidence demonstrates higher susceptibility to severe COVID-19 in PLHIV with uncontrolled infection due to the lower CD4+ T cells count in these patients.3, 4, 5, 6 There is evidence that the SARS-CoV-2 binds to CD4+ T-cells via the Angiotensin-Converting Enzyme 2 (ACE2) receptor, and it replicates in a wide range of T-helper cells. This process of viral replication induces the death of defense cells and compromises the immune system. Findings also indicate the expression of a large amount of cytokine by SARS-CoV-2 infected CD4+ T-cells, which is markedly associated with viral persistence and disease severity.7 Furthermore, it is known that pre-existing underlying diseases, commonly observed due to increased survival in PLHIV, are considered potential risk factors for the severity of COVID-19 in this population.8
A recent systematic review with data from over 20 million COVID-19 patients from Africa, Asia, Europe, and North America showed an increased mortality risk in PLHIV and a higher risk of hospitalization for COVID-19 among those without viral suppression and in a more advanced stage of HIV infection.5 Data from other meta-analyses that grouped studies developed in Africa, European, China, India, and the United States corroborate these findings.9,10 However, another systematic review involving seven studies found no increased risk of worse COVID-19 outcomes among PLHIV.11 On the other hand, some studies indicate that PLHIV are subject to lower infection rates and lower risk of developing severe forms of the disease, as a consequence of the activity attributed to the use of antiretroviral drugs.12, 13, 14 Divergences about the severity of COVID-19 in PLHIV remain prominent in the current scenario.
So far, no robust studies have been developed to investigate the influence of HIV infection on the outcomes of COVID-19 in Latin America in different waves of the pandemic. This continent was severely hit by the pandemic,15 with higher mortality when compared to countries in other regions.16 It is believed that the higher COVID-19 mortality in Latin America is a result of different factors, including high population density, precarious sanitary conditions, and low socioeconomic and educational levels, which are usually associated with a higher prevalence of chronic comorbidities and delays in seeking care.16
Given the divergences in the clinical course of COVID-19 in PLHIV and the limited knowledge in this regard in different waves, further evidence from large cohorts is still required to investigate the profile of the disease in this specific population, especially in Latin America. Therefore, the present study aimed to evaluate clinical characteristics and outcomes in patients with SARS-CoV-2 and HIV coinfection, as well as to compare their clinical outcomes to COVID-19 patients without HIV infection in different periods.
Methods
Study design and setting
The present study design followed the Strengthening the Report of Observational Studies in Epidemiology (STROBE) recommendations, to cover the essential items for the description of observational studies. It is a substudy of two large Brazilian cohorts. The Brazilian COVID-19 Registry is a multicenter retrospective cohort involving 37 public and private hospitals, which comprised two periods: March to September 2020 and March to December 2021. This cohort included consecutive adult patients (≥ 18 years old) with laboratory-confirmed COVID-19 admitted to the participating hospitals. The CO-FRAIL (COVID-19 and Frailty) Study was a cohort developed at the University Hospital of the University of Sao Paulo Medical School (HCFMUSP), which included consecutive patients ≥ 50 years old with laboratory-confirmed COVID-19 admitted to the hospital in March to July 2020. Details about the research setting of both registries are described in previous publications.17,18
Patients who manifested COVID-19 while admitted for other reasons or those transferred to other hospitals who had no outcome (discharged or death) were excluded from the present analysis.
Data collection
In the Brazilian COVID-19 Registry, data collection was performed from the medical records by healthcare professionals properly trained to obtain the variables of interest in the study. Data were managed using the Research Electronic Data Capture (REDCap). The REDCap was hosted at the Telehealth Center, University Hospital, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil.17 A coding manual guiding data collection was provided to the researchers involved and remained accessible for questions throughout the collection period. The outliers and missing information were verified and corrected by local references from each hospital, to ensure data reliability. At the HCFMUSP cohort, data were extracted after a detailed review of electronic medical records, nursing records, and consulting notes. These records included information documented by frontline health professionals in standardized electronic forms, specially designed for the pandemic.18
In both cohorts, information on sociodemographic characteristics, comorbidities, clinical assessment, laboratory findings, treatment, and outcomes was obtained. Specific variables such as CD4+ T-lymphocyte count, viral load, and antiretroviral therapy were also obtained for all PLHIV through the Brazilian official records: Laboratory Tests Control System (SISCEL) and Logistic Control System of Medication (SICLOM).
Outcomes
Primary outcomes were admission to the Intensive Care Unit (ICU), invasive mechanical ventilation, and death. The secondary outcomes were other clinical complications such as renal replacement therapy requirement, adult respiratory distress syndrome, septic shock, nosocomial infection, acute myocardial infarction, deep vein thrombosis, and pulmonary thromboembolism.
Statistical analysis
Initially, a descriptive analysis of the population was performed, in which sociodemographic and clinical characteristics of the patients under study were represented by frequency distribution, measures of central tendency, and variability. The Kolmogorov-Smirnov test was applied to verify data normality.
COVID-19 patients infected with HIV and COVID-19 patients without concomitant diagnosis of HIV infection were matched for age, sex, number of comorbidities, and hospital of admission by propensity score matching using the nearest neighbor algorithm (0.25 standard deviations of the logit of the propensity score, on a scale from 0‒1.00), up to 4:1. Groups were compared to using the Chi-Square Test and Fisher's exact test for categorical variables, and the Wilcoxon test for continuous variables. Statistical analysis was performed with R software (version 4.0.2). Results were considered statistically significant at a level of p<0.05.
Ethics statement
The Brazilian National Commission for Research Ethics (CAAE 30350820.5.1001.0008) and the Research Ethics Committee of participating institutions approved the study protocol. Individual informed consent was waived due to the pandemic situation and the use of data from unidentified medical records. Additionally, administrative permissions to access and use the medical records were obtained from each institution. This study was performed in line with the principles of the Declaration of Helsinki.
Results
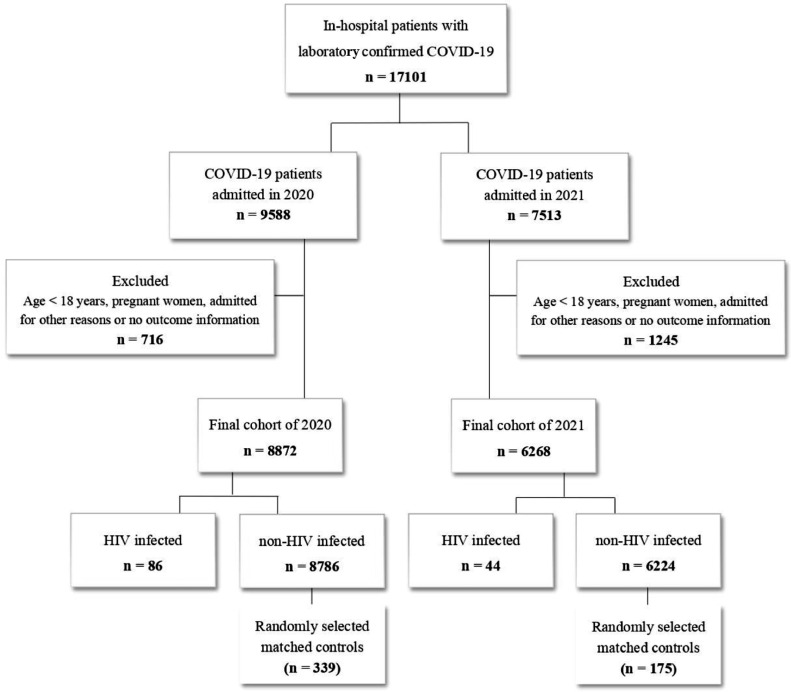
The study included 17,101 COVID-19 patients, 130 (0.76%) were PLHIV. Of these, 86 were admitted in 2020 (0.50% of the total; median age 55 years; 62.8% women), and 44 were admitted in 2021 (0.26% of the total; median age 53 years; 59.1% women) (Fig. 1).
Fig. 1.
Flowchart representative of the study population.
Table 1 shows the sociodemographic and clinical characteristics of study patients. PLHIV and matched controls had a similar frequency of comorbidities in both periods, except for a lower frequency of obesity (5.8% vs. 16.8%; p = 0.016), and a higher frequency of chronic obstructive pulmonary disease (9.3% vs. 3.8%; p = 0.049), and cancer (15.1% vs. 7.1%; p = 0.032) among those with HIV, in 2020. Regarding lifestyle habits, smoking was more frequent among PLHIV in both periods (12.8% vs. 4.7%, p = 0.013 in 2020; 13.6% vs. 4.0%, p=0.027 in 2021). Specific data related to HIV infection is shown in Supplementary Table 1.
Table 1.
Sociodemographic characteristics and previous clinical history of patient's study.
| 2020 |
2021 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Total (n = 425) | HIV infected (n = 86) | non-HIV infected (n = 339) | p-value | Total (n = 219) | HIV infected (n = 44) | non-HIV infected (n = 175) | p-value |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||
| Demographic data | ||||||||
| Age (years)a | 54.0 (43.0, 64.0) | 55.0 (47.0, 64.0) | 54.0 (43.0, 63.5) | 0.584 | 53.0 (46.0, 63.5) | 53.0 (44.0, 59.5) | 53.0 (46.0, 64.5) | 0.789 |
| Female | 252 (59.3%) | 54 (62.8%) | 198 (58.4%) | 0.538 | 119 (54.3%) | 26 (59.1%) | 93 (53.1%) | 0.590 |
| Lifestyle habits | ||||||||
| Current smoker | 27 (6.4%) | 11 (12.8%) | 16 (4.7%) | 0.013 | 13 (5.9%) | 6 (13.6%) | 7 (4.0%) | 0.027 |
| Previous smoker | 61 (14.4%) | 17 (19.8%) | 44 (13.0%) | 0.152 | 20 (9.1%) | 5 (11.4%) | 15 (8.6%) | 0.562 |
| Comorbidities | ||||||||
| Hypertension | 162 (38.1%) | 30 (34.9%) | 132 (38.9%) | 0.571 | 97 (44.3%) | 18 (40.9%) | 79 (45.1%) | 0.737 |
| Coronary artery disease | 19 (4.5%) | 7 (8.1%) | 12 (3.5%) | 0.079 | 7 (3.2%) | 1 (2.3%) | 6 (3.4%) | >0.999 |
| Heart failure | 21 (4.9%) | 5 (5.8%) | 16 (4.7%) | 0.780 | 7 (3.2%) | 2 (4.5%) | 5 (2.9%) | 0.630 |
| Atrial fibrillation/flutter | 9 (2.1%) | 2 (2.3%) | 7 (2.1%) | >0.999 | 2 (0.9%) | 0 (0.0%) | 2 (1.1%) | >0.999 |
| Stroke | 9 (2.1%) | 3 (3.5%) | 6 (1.8%) | 0.395 | 6 (2.7%) | 1 (2.3%) | 5 (2.9%) | >0.999 |
| Diabetes mellitus | 88 (20.7%) | 21 (24.4%) | 67 (19.8%) | 0.422 | 45 (20.5%) | 8 (18.2%) | 37 (21.1%) | 0.821 |
| Obesity | 62 (14.6%) | 5 (5.8%) | 57 (16.8%) | 0.016 | 44 (20.1%) | 8 (18.2%) | 36 (20.6%) | 0.886 |
| Asthma | 38 (8.9%) | 5 (5.8%) | 33 (9.7%) | 0.354 | 16 (7.3%) | 3 (6.8%) | 13 (7.4%) | >0.999 |
| Chronic obstructive pulmonary disease | 21 (4.9%) | 8 (9.3%) | 13 (3.8%) | 0.049 | 7 (3.2%) | 2 (4.5%) | 5 (2.9%) | 0.630 |
| Cancer | 37 (8.7%) | 13 (15.1%) | 24 (7.1%) | 0.032 | 6 (2.7%) | 3 (6.8%) | 3 (1.7%) | 0.097 |
| Cirrhosis | 3 (0.7) | 0 (0.0%) | 3 (0.9%) | >0.999 | 2 (0.9%) | 1 (2.3%) | 1 (0.6%) | 0.362 |
| Chronic kidney disease | 33 (7.8%) | 10 (11.6%) | 23 (6.8%) | 0.203 | 5 (2.3%) | 1 (2.3%) | 4 (2.3%) | >0.999 |
| Rheumatological disease | 7 (1.6%) | 1 (1.2%) | 6 (1.8%) | >0.999 | 4 (1.8%) | 1 (2.3%) | 3 (1.7%) | >0.999 |
| Psychiatric illness | 21 (4.9%) | 9 (10.5%) | 12 (3.5%) | 0.021 | 17 (7.8%) | 5 (11.4%) | 12 (6.9%) | 0.345 |
| Number of comorbidities | ||||||||
| 0 | 171 (40.2%) | 34 (39.5%) | 137 (40.4%) | 78 (35.6%) | 17 (38.6%) | 61 (34.9%) | ||
| 1 | 132 (31.1%) | 24 (27.9%) | 108 (31.9%) | 80 (36.5%) | 15 (34.1%) | 65 (37.1%) | ||
| 2 | 85 (20.0%) | 19 (22.1%) | 66 (19.5%) | 0.810 | 43 (19.6%) | 8 (18.2%) | 35 (20.0%) | 0.910 |
| 3 | 27 (6.4%) | 6 (7.0%) | 21 (6.2%) | 15 (6.8%) | 3 (6.8%) | 12 (6.9%) | ||
| 4 | 10 (2.3%) | 3 (0.7%) | 7 (1.6%) | 3 (1.4%) | 1 (2.3%) | 2 (1.1%) | ||
Median (Interquartile Range ‒ IQR). Statistical tests: Wilcoxon rank-sum test; Chi-Square test of independence; Fisher's exact test.
Dyspnea (65.6% in 2020; 69.4% in 2021) and fever (60.5% in 2020; 43.8% in 2021) were the most frequent symptoms in both groups and both periods, with no significant differences (Supplementary Table 2). Concerning the laboratory exams, HIV-infected patients admitted in 2020 had lower counts of leukocytes (5,950.0 [4,595.0, 8,220.0] vs. 7,370.5 [5,415.0, 10,257.5]; p < 0.001) and neutrophils (4,303.5 [2,715.0, 6,088.0] vs 5,435.5 [3,658.8, 8,015.0]; p = 0.001), in which HIV infected patients had lower counts compared to controls. Alanine aminotransferase level (30.0 [22.7, 42.0] vs. 37.0 [23.0, 63.8]; p = 0.021) was also lower among HIV-infected patients. These differences were observed among patients admitted in 2021 (Table 2).
Table 2.
Clinical and laboratory assessment at hospital admission.
| 2020 |
2021 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Total (n = 425) | HIV infected (n = 86) | non-HIV infected (n = 339) | p-value | Total (n = 219) | HIV infected (n = 44) | non-HIV infected (n = 175) | p-value |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||
| Clinical assessmenta | ||||||||
| Glasgow <15 | 79 (18.6%) | 16 (18.6%) | 63 (18.6%) | >0.999 | 11 (5.0%) | 3 (6.8%) | 8 (4.6%) | 0.465 |
| Heart rate (bpm) | 90.0 (79.0, 101.2) | 88.5 (77.8, 101.2) | 90.0 (79.8, 101.2) | 0.410 | 86.0 (77.8, 95.2) | 86.0 (80.0, 95.0) | 86.0 (76.0, 95.0) | 0.666 |
| Respiratory rate (irpm) | 20.0 (18.0, 25.0) | 20.0 (19.0, 24.0) | 20.5 (18.0, 25.2) | 0.859 | 20.0 (18.0, 24.0) | 22.0 (18.0, 26.0) | 20.0 (18.8, 24.0) | 0.608 |
| SF ratio | 411.0 (247.9, 456.0) | 431.0 (254.6, 458.3) | 407.5 (247.9, 452.4) | 0.316 | 342.9 (275.3, 419.0) | 344.6 (276.7, 422.6) | 339.3 (275.3, 419.0) | 0.741 |
| Mechanical ventilation | 54 (12.7%) | 11 (12.8%) | 43 (12.7%) | >0.999 | 2 (1.1%) | 2 (5.6%) | 0 (0.0%) | 0.041 |
| Systolic blood pressure | ||||||||
| ≥90 (mm Hg) | 369 (91.8%) | 73 (91.2%) | 296 (91.9%) | 166 (97.1%) | 33 (97.1%) | 133 (97.1%) | ||
| <90 (mm Hg) | 5 (1.2%) | 1 (1.2%) | 4 (1.2%) | 0.921 | 2 (1.2%) | 0 (0.0%) | 2 (1.5%) | 0.675 |
| Inotrope requirement | 28 (7.0%) | 6 (7.5%) | 22 (6.8%) | 3 (1.8%) | 1 (2.9%) | 2 (1.5%) | ||
| Diastolic blood pressure | ||||||||
| >60 (mm Hg) | 319 (79.4%) | 62 (77.5%) | 257 (79.8%) | 143 (83.6%) | 27 (79.4%) | 116 (84.7%) | ||
| ≤60 (mm Hg) | 55 (13.7%) | 12 (15.0%) | 43 (13.4%) | 0.900 | 25 (14.6%) | 6 (17.6%) | 19 (13.9%) | 0.542 |
| Inotrope requirement | 28 (7.0%) | 6 (7.5%) | 22 (6.8%) | 3 (1.8%) | 1 (2.9%) | 2 (1.5%) | ||
| Laboratory assessment 1 | ||||||||
| Hemogram parameters | ||||||||
| Hemoglobin (g/dL) | 13.1 (11.4, 14.4) | 12.7 (10.6, 14.2) | 13.2 (11.6, 14.4) | 0.149 | 13.4 (12.3, 14.6) | 13.4 (12.1, 14.8) | 13.3 (12.4, 14.5) | 0.919 |
| Leukocytes (cels/mm3) | 7,100.0 (5,100.0, 9,850.0) | 5,950.0 (4,595.0, 8,220.0) | 7,370.5 (5,415.0, 10,257.5) | <0.001 | 6,890.0 (5,765.0, 9,310.0) | 6,770.0 (5,840.0, 8,430.0) | 6,941.0 (5,597.0, 9,310.0) | 0.551 |
| Neutrophiles (cels/mm3) | 5,219.0 (3,420.5, 7,666.5) | 4,303.5 (2,715.0, 6,088.0) | 5,435.5 (3,658.8, 8,015.0) | 0.001 | 5,301.2 (3,966.8, 7,331.2) | 4,989.0 (4,194.4, 6,718.0) | 5,410.0 (3,849.8, 7,392.0) | 0.692 |
| Lymphocytes (cels/mm3) | 1,000.0 (710.0, 1,382.0) | 1,013.0 (593.2, 1,398.2) | 1,000.0 (737.0, 1,380.0) | 0.500 | 978.5 (673.8, 1,492.5) | 965.0 (508.0, 1,434.0) | 994.0 (684.0, 1,499.0) | 0.374 |
| Platelet count (109/L) | 199,000.0 (158,000.0, 266,750.0) | 182,000.0 (147,500.0, 251,500.0) | 205,000.0 (162,000.0, 268,000.0) | 0.089 | 214,500.0 (159,500.0, 273,250.0) | 219,000.0 (178,500.0, 262,500.0) | 210,000.0 (158,000.0, 274,000.0) | 0.700 |
| NLR (cels/mm3) | 5.1 (3.0, 8.7) | 4.4 (2.5, 7.5) | 5.3 (3.0, 8.9) | 0.142 | 5.6 (3.5, 9.1) | 6.2 (3.9, 10.6) | 5.3 (3.4, 8.8) | 0.466 |
| Kidney parameters | ||||||||
| Creatinine (mg/dL) | 0.9 (0.7, 1.2) | 0.9 (0.7, s1.4) | 0.9 (0.7, 1.2) | 0.530 | 0.8 (0.7, 1.1) | 0.9 (0.7, 1.2) | 0.8 (0.7, 1.0) | 0.340 |
| Urea (mg/dL) | 35.0 (25.0, 52.3) | 35.5 (24.0, 55.6) | 35.0 (26.0, 50.8) | 0.856 | 32.6 (26.0, 45.8) | 37.0 (26.2, 54.7) | 31.7 (26.0, 44.2) | 0.249 |
| Liver parameters | ||||||||
| AST (U/L) | 41.0 (30.0, 61.0) | 37.0 (27.8, 53.0) | 42.0 (30.0, 63.0) | 0.090 | 47.2 (36.2, 69.7) | 41.0 (32.5, 58.7) | 51.8 (39.2, 69.8) | 0.066 |
| ALT (U/L) | 34.0 (23.0, 58.0) | 30.0 (22.7, 42.0) | 37.0 (23.0, 63.8) | 0.021 | 44.0 (28.0, 72.0) | 42.0 (24.0, 54.5) | 47.5 (32.0, 72.8) | 0.139 |
| Ions | ||||||||
| Sodium (mmoL/L) | 138.0 (135.0, 141.0) | 138.0 (135.0, 140.0) | 138.0 (136.0, 141.0) | 0.291 | 137.0 (134.0, 139.0) | 137.0 (134.8, 139.0) | 137.0 (134.0, 139.0) | 0.868 |
| Others parameters | ||||||||
| INR | 1.1 (1.0, 1.2) | 1.1 (1.0, 1.2) | 1.1 (1.0, 1.2) | 0.652 | 1.1 (1.0, 1.2) | 1.1 (1.0, 1.2) | 1.1 (1.0, 1.1) | 0.181 |
| Lactate (mmoL/L) | 1.3 (0.9, 1.8) | 1.5 (1.1, 1.9) | 1.2 (0.9, 1.6) | 0.006 | 1.5 (1.1, 2.2) | 1.8 (1.3, 2.3) | 1.5 (1.1, 2.1) | 0.219 |
| Arterial pH | 7.4 (7.4, 7.5) | 7.4 (7.4, 7.4) | 7.4 (7.4, 7.5) | 0.013 | 7.4 (7.4, 7.5) | 7.4 (7.4, 7.5) | 7.4 (7.4, 7.5) | 0.759 |
| Arterial pO2 | 77.0 (63.2, 96.0) | 79.0 (60.8, 104.8) | 76.1 (63.8, 94.1) | 0.381 | 71.4 (61.5, 95.0) | 77.0 (65.1, 99.7) | 70.5 (61.0, 95.0) | 0.192 |
| Arterial pCO2 | 35.5 (32.0, 40.0) | 35.2 (30.0, 42.0) | 35.8 (32.1, 39.2) | 0.659 | 35.0 (31.6, 38.2) | 33.6 (30.0, 35.6) | 35.9 (32.0, 39.0) | 0.046 |
| Bicarbonate | 23.6 (21.0, 25.9) | 23.0 (19.0, 25.2) | 23.8 (21.3, 26.0) | 0.073 | 23.2 (21.1, 25.1) | 22.0 (19.6, 23.8) | 24.0 (22.0, 25.3) | 0.002 |
Median (Interquartile Range ‒ IQR). Statistical tests: Wilcoxon rank-sum test; Chi-Square test of independence; Fisher's exact test.
INR, International Normalized Ratio; NLR, Neutrophil to Lymphocyte Ratio; SF, Ratio – Peripheral; SpO2/FiO2 ratio, Capillary Oxygen Saturation/Fraction of Inspired Oxygen.
In both periods, no significant differences were found between HIV-infected patients and controls for any of the medications or supportive care during the hospital stay (Supplementary Table 3). There was higher mortality among patients with HIV in 2020 when compared to matched controls (27.9% vs. 60 17.7%; p = 0.049), with no difference in other clinical outcomes. In 2021, there was no difference in outcomes between groups (Table 3).
Table 3.
Clinical outcomes during hospitalization.
| 2020 |
2021 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Total (n = 425) | HIV infected (n = 86) | non-HIV infected (n = 339) | p-value | Total (n = 219) | HIV infected (n = 44) | non-HIV infected (n = 175) | p-value |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||
| Outcomes | ||||||||
| Septic shock | 81 (19.1%) | 18 (20.9%) | 63 (18.6%) | 0.733 | 31 (14.2%) | 6 (13.6%) | 25 (14.3%) | >0.999 |
| Adult respiratory distress syndrome | 88 (20.7%) | 16 (18.6%) | 72 (21.2%) | 0.697 | 71 (32.4%) | 15 (34.1%) | 56 (32.0%) | 0.932 |
| Acute myocardial infarction | 6 (1.4%) | 2 (2.3%) | 4 (1.2%) | 0.350 | 2 (0.9%) | 0 (0.0%) | 2 (1.1%) | >0.999 |
| Nosocomial infection | 73 (17.2%) | 14 (16.3%) | 59 (17.4%) | 0.931 | 41 (18.7%) | 9 (20.5%) | 32 (18.3%) | 0.910 |
| Deep vein thrombosis | 12 (2.8%) | 2 (2.3%) | 10 (2.9%) | >0.999 | 6 (2.7%) | 1 (2.3%) | 5 (2.9%) | >0.999 |
| Pulmonary thromboembolism | 24 (5.6%) | 4 (4.7%) | 20 (5.9%) | 0.798 | 15 (6.8%) | 6 (13.6%) | 9 (5.1%) | 0.086 |
| Renal replacement therapy requirement | 53 (12.5%) | 13 (15.1%) | 40 (11.8%) | 0.516 | 26 (11.9%) | 6 (13.6%) | 20 (11.4%) | 0.885 |
| Admission to the ICU | 178 (42.0%) | 37 (43.5%) | 141 (41.6%) | 0.841 | 94 (43.1%) | 17 (38.6%) | 77 (44.3%) | 0.616 |
| Length of stay (days)a | 9.0 (4.0, 21.0) | 9.0 (4.0, 18.0) | 9.0 (4.0, 23.0) | 0.512 | 10.5 (5.0, 16.8) | 11.0 (5.0, 18.0) | 10.0 (5.0, 16.0) | 0.906 |
| Mechanical ventilation support | 136 (32.5%) | 30 (35.7%) | 106 (31.7%) | 0.572 | 71 (32.4%) | 15 (34.1%) | 56 (32.0%) | 0.932 |
| Death | 84 (19.8%) | 24 (27.9%) | 60 (17.7%) | 0.049 | 55 (25.1%) | 11 (25.0%) | 44 (25.1%) | >0.999 |
Median (Interquartile Range ‒ IQR). Statistical tests: Wilcoxon rank-sum test; Chi-Square test of independence; Fisher's exact test.
Discussion
This study presents the clinical characteristics and outcomes of COVID-19 in PLHIV and matched controls, admitted in participating hospitals from 14 Brazilian cities in 2020 and 2021. Clinical characteristics were similar between groups, except for comorbidities such as obesity, chronic obstructive pulmonary disease, and cancer and laboratory results of total leukocytes and neutrophils, which showed differences in the first period under analysis. Although patients with HIV admitted in 2020 had higher mortality than matched controls, this finding has not been observed among those admitted in 2021.
Despite the conflicting evidence regarding the influence of HIV infection on the clinical course and outcome of COVID-19, most studies – including systematic reviews and meta-analyses – agree that PLHIV has a higher risk of death from COVID-19.5,9,10,19, 20, 21, 22 However, a thorough analysis of the literature demonstrates that most of the available studies comprise samples from 2020 when there were a large number of publications due to the urgency of updates on COVID-19. These studies have not included the vaccination period, as the World Health Organization issued the first document supporting the approval of a COVID-19 vaccine on December 31, 2020. Additionally, the most recent studies evaluating COVID-19 outcomes in PLHIV include data collected no later than July 2021 and therefore do not cover a more advanced stage of COVID-19 vaccination.6,19,20,22, 23, 24
Although the vaccination program in Brazil began in January 2021, the immunization rates advanced slowly in the first half of that year due to the limited availability of vaccines. By the end of 2021, with a significant advance in immunization, the country had reached the coverage of 67.9% of the population fully vaccinated and 78.2% with at least one vaccine dose.25 Regarding the definition of target groups for immunization by the Ministry of Health, the PLHIV were considered a priority if they had a CD4+ count < 350 mm3, otherwise, the vaccination followed the age criteria, with older people being vaccinated first.26 In our cohort, which includes patients admitted up to December 2021, the mortality rate in PLHIV was stable in both periods (before and after the vaccination program started in Brazil).
Regarding specific information related to HIV infection, the authors obtained data from 99 (76.1%) patients in the study. Most patients with available data were on antiretroviral therapy (100.0%) and had suppressed viral loads (2020: 78.1% and 2021: 71.4%). Similarly, in a meta-analysis that analyzed the outcomes of COVID-19 in HIV-infected individuals from seven countries, 96.0% were on antiretroviral therapy, and about 80.0% were suppressed.5 Brazil is a country that stands out in Latin America for a strong HIV response, with an established national public program designated for the identification, monitoring, surveillance, and access to free antiretrovirals since the 1990s.27 Currently, 650,000 PLHIV are on antiretroviral therapy and 66.0% have suppressed viral load in the country, observed constant efforts to reach the global goal of viral suppression to 90.0%.28
The present results also showed that only 54.7% of PLHIV admitted in 2020, with available data, had CD4+ T-lymphocyte counts above 500 cells/µL, a condition that may have contributed to the finding of greater severity of COVID-19 among PLHIV compared to controls. Notably, the immune dysregulation resulting from HIV infection is a contributing factor to the severity of COVID-19.6 According to Davanzo et al.,7 the binding of SARS-CoV-2 to the defense cells of patients with HIV coinfection is capable of inducing a pronounced cell death process. Liu et al.29 and Hoffman et al.4 also have described an exacerbated expression of cytokines and chemokines in patients with SARS-CoV-2 and HIV coinfection, which might trigger a hyperinflammatory response and potentiate the negative outcomes of COVID-19. Furthermore, it is known that immunocompromise may be established before the initiation of antiretroviral therapy and, therefore, even with treatment adherence, HIV-infected patients may find themselves in a state of persistent immune dysregulation and, consequently, present a higher risk of severity during the COVID-19 course.3
In 2021, 70.0% of PLHIV, with available data, had a CD4+ T-lymphocyte count greater than or equal to 500 cells/μL. The clinical trial developed by Frater et al.,30 provides clear evidence of the efficacy and immunogenicity of vaccination against COVID-19 for PLHIV with CD4+ T-lymphocyte counts greater than 350 cells/μL. Despite high CD4+ T-lymphocyte counts in 2021, the study design does not allow concluding that vaccination is responsible for the absence of difference in mortality between patients living with HIV and the control group in 2021.
Information regarding the clinical history obtained for patients admitted for COVID-19 in 2020 indicates that PLHIV had a higher frequency of comorbidities, such as chronic obstructive pulmonary disease (9.3% vs. 3.8%; p = 0.049) and cancer (15.1% vs. 7.1%; p = 0.032) than patients not infected with HIV. According to Maciel et al.,31 PLHIV are susceptible to premature aging as a result of the inflammatory process associated with HIV infection and, even at younger ages, have a higher prevalence of chronic conditions common in older populations not infected with the virus. Therefore, it is believed that advanced age and the presence of a greater number of comorbidities are capable of influencing the severity of COVID-19 in PLHIV, which may explain the higher mortality observed in this specific population in 2020.5,32 According to this standard, the authors observe that in 2021, both the comorbidities and mortality rates were similar in both groups.
During the assessment of aspects related to lifestyle habits, PLHIV showed a higher frequency of smoking compared to controls in both periods (12.8% vs. 4.7%, p = 0.013 in 2020; 13.6% vs. 4.0%, p = 0.027 in 2021). There is concrete evidence that PLHIV is two to three times more exposed to smoking than the general population.33 Studies involving patients with COVID-19 demonstrate greater disease severity among smokers.34 Thus, the higher frequency of comorbidities such as chronic obstructive pulmonary disease and cancer, as well as the high number of smokers among PLHIV in this study, may have an influence on findings of higher mortality in this population compared to patients not infected with HIV.
As for clinical signs and symptoms upon hospital presentation, the findings were similar between both groups, with dyspnea and fever being the most prevalent symptoms. These findings are in line with previous studies and reinforce that the screening and clinical suspicion of COVID-19 for the HIV-infected population should be similar to the general population.35 Clinical assessment characteristics upon hospital presentation were similar for HIV-infected and non-HIV-infected patients in both study periods. Laboratory findings also were mostly similar to non-HIV-infected patients. Despite the lower leukocytes and neutrophil counts compared to the non-infected population, that difference did not maintain in 2021. Although chronic HIV infection might lead to lower neutrophil counts,36 it is unclear the clinical significance of this finding in the context of an acute SARS-CoV-2 infection.
Most patients included in both study periods were on antiretroviral therapy, with lamivudine (96.9%), tenofovir (61.5%) and dolutegravir (57.3%) being the most used antiretrovirals. There is evidence of the benefit of antiretrovirals such as tenofovir against COVID-19.13,14 In vitro and molecular docking studies suggest that tenofovir inhibits the Ribonucleic Acid (RNA)-dependent RNA polymerase of SARS-CoV-2.37 This antiretroviral also has immunomodulatory effects, including decreased production of Interleukin (IL)-8 and IL-10,38 cytokines that are associated with COVID-19 severity and mortality.29
Concerning the study limitations, the sample of PLHIV was small, which indicates the need for a careful interpretation of the results. Even so, the point estimate for mortality in 2021 was approximately the same for patients with HIV and matched controls. Despite the restricted sample size, this study presents great relevance since to the best of our knowledge there are no publications directed to the investigation of patients with coinfection by SARS-COV-2 and HIV in Latin America in different waves of the pandemic. In this context, it is noticeable that knowledge about the clinical course of COVID-19 in this specific population is crucial to ensure the appropriate management of the disease, as well as to improve the management of health care costs, which is relevant for many countries of the continent that had great restriction of resources during the COVID-19 pandemic.39 Another limitation is due to the retrospective study design. Information such as the vaccination status of patients and specific data related to HIV infection could not be fully recovered in the hospital records and other records under analysis. The resources used to obtain the variables of interest in the study are secondary sources of information, and since they were not specifically designed for data collection, the absence of indispensable information for the study is inevitable. Additionally, the work overload of health professionals can make it difficult to assiduously record information about the assistance provided to the patient, directly affecting the quality of the data source.
The major advantage of this study focuses on the involvement of large Brazilian cohorts, including patients from 27 hospitals in 14 cities and 4 different states (Minas Gerais, São Paulo, Rio Grande do Sul, and Pernambuco), ensuring the diversity of the population studied. Another relevant factor is the comparison of clinical characteristics and outcomes in patients with SARS-CoV-2 and HIV co-infection in two consecutive years (2020 and 2021), which has not been observed in other cohorts published until the moment.
Conclusions
The present results reiterate that PLHIV were at higher risk of COVID-19 mortality in the early stages of the pandemic, indicating the importance of prioritizing the clinical management of the disease in this specific population. However, this finding did not sustain in 2021, when the mortality rate is similar to the control group. The study has limitations and, therefore, further investigations are needed to elucidate the impact of HIV infection on COVID-19.
Authors’ contributions
MSM, TLSS, and MCP had substantial contributions to the conception or design of the work. TLSS, MVRSS, PDP, JVBN, MFS, VCMA, HD, NRO, NCSS, LEFR, AVS, AOJ, ALBAS, BMC, CTCAS, CMR, FA, FAB, GMSG, GFN, KBR, LSMM, LCC, LAN, MC, MFG, MCAN, MHGJ, PKZ, RCA, SCF, STSN, SFA, TJAS, MJRA, MCP, ESS, and MSM had substantial contributions to the acquisition, analysis, or interpretation of data for the work. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgments
Funding source
This study was supported in part by the Minas Gerais State Agency for Research and Development (Fundação de Amparo à Pesquisa do Estado de Minas Gerais - FAPEMIG) [grant number APQ-01154-21], National Institute of Science and Technology for Health Technology Assessment (Instituto de Avaliação de Tecnologias em Saúde – IATS)/ National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico ‒ CNPq) [grant number 465518/2014-1] and Graduate Program in Infectious Diseases and Tropical Medicine at the Federal University of Minas Gerais (Programa de Pós-Graduação em Infectologia e Medicina Tropical – PPGIMT / UFMG). Funders were not involved in the study design; in the collection, analysis, and interpretation of data; in writing the report; and in the decision to submit the article for publication.
Acknowledgments
The authors would like to thank the hospitals which are part of this collaboration, for supporting this project: Hospitais da Rede Mater Dei, Hospital das Clínicas da UFMG, Hospital das Clínicas de Porto Alegre, Hospital Santo Antônio, Hospital Eduardo de Menezes, Hospital Tacchini, Hospital Márcio Cunha, Hospital Metropolitano Dr. Célio de Castro, Hospital Metropolitano Odilon Behrens, Hospital Risoleta Tolentino Neves, Hospital Santa Cruz, Hospital São João de Deus, Hospital Semper, Hospital Unimed, Hospital Universitário Canoas, Hospital Universitário de Santa Maria, Hospital Moinhos de Vento, Hospital Nossa Senhora da Conceição, Hospital Julia Kubitschek, Hospital Regional Antônio Dias, Hospital Mãe de Deus, Hospital das Clínicas da Universidade Federal de Pernambuco, Hospital Bruno Born, Hospital São Lucas da PUCRS, Orizonti- Instituto de Saúde e Longevidade Ltda, Santa Casa de Misericórdia de Belo Horizonte and Universidade de São Paulo. The authors also thank all the clinical staff at those hospitals, who cared for the patients, and all undergraduate students who helped with data collection.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.clinsp.2023.100223.
Appendix. Supplementary materials
References
- 1.Jiang H, Zhou Y, Tang W. Maintaining HIV care during the COVID-19 pandemic. Lancet HIV. 2020;7(5) doi: 10.1016/S2352-3018(20)30105-3. e308-e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO - World Health Organization Consolidated guidelines on HIV prevention, testing, treatment, service delivery, and monitoring: recommendations for a public health approach. Geneva: World Health Organization; 2021 a. [PubMed] [Google Scholar]
- 3.Geretti AM, Stockdale AJ, Kelly SH, Cevik M, Collins S, Waters L, et al. Outcomes of coronavirus disease 2019 (COVID-19) related hospitalization among people with human immunodeficiency virus (HIV) in the ISARIC World Health Organization (WHO) Clinical Characterization Protocol (UK): a prospective observational study. Clin Infect Dis. 2021;73(7):e2095–e2106. doi: 10.1093/cid/ciaa1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann C, Casado JL, Härter G, Vizcarra P, Moreno A, Cattaneo D, et al. Immune deficiency is a risk factor for severe COVID-19 in people living with HIV. HIV Med. 2021;22(5):372–378. doi: 10.1111/hiv.13037. [DOI] [PubMed] [Google Scholar]
- 5.Ssentongo P, Heilbrunn ES, Ssentongo AE, Advani S, Chinchilli VM, Nunez JJ, et al. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: a systematic review and meta-analysis. Sci Rep. 2021;11(6283):1–12. doi: 10.1038/s41598-021-85359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun J, Patel RC, Zheng Q, Madhira V, Olex AL, Islam JY, et al. COVID-19 disease severity among people with HIV infection or solid organ transplant in the United States: a nationally-representative, multicenter, observational cohort study. medRxiv. Preprint. 2021 2021.07.26.21261028. [Google Scholar]
- 7.Davanzo GG, Codo AC, Brunetti NS, Boldrini V, Knittel TL, Monterio LB, et al. SARS-CoV-2 Uses CD4 to Infect T Helper Lymphocytes. medRxiv. Preprint. 2020 doi: 10.7554/eLife.84790. 2020.09.25.20200329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etienne N, Karmochkine M, Slama L, Pavie J, Batisse D, Usubillaga R, et al. HIV infection and COVID-19: risk factors for severe disease. AIDS. 2020;34(12):1771–1774. doi: 10.1097/QAD.0000000000002651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mellor MM, Bast AC, Jones NR, Roberts NW, Ordóñez-Mena JM, Reith AJM, et al. Risk of adverse coronavirus disease 2019 outcomes for people living with HIV. AIDS Lond Engl. 2021;35(4):F1–F10. doi: 10.1097/QAD.0000000000002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oyelade T, Alqahtani JS, Hjazi AM, Li A, Kamila A, Raya RP. Global and regional prevalence and outcomes of COVID-19 in people living with HIV: a systematic review and meta-analysis. Trop Med Infect Dis. 2022;7(2):22. doi: 10.3390/tropicalmed7020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee KW, Yap SF, Ngeow YF, Lye MS. Covid-19 in people living with HIV: A systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(7):1–25. doi: 10.3390/ijerph18073554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berenguer J, Díez C, Martín-Vicente M, Micán R, Pérez-Elías MJ, García-Fraile LJ, et al. Prevalence and factors associated with SARS-CoV-2 seropositivity in the Spanish HIV Research Network Cohort. Clin Microbiol Infect. 2021;27(11):1678–1684. doi: 10.1016/j.cmi.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chien M, Anderson TK, Jockusch S, Tao C, Li X, Kumar S, et al. Nucleotide analogues as inhibitors of SARS-CoV-2 polymerase, a key drug target for COVID-19. J Proteome Res. 2020;19(11):4690–4697. doi: 10.1021/acs.jproteome.0c00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO - World Health Organization. Cumulative confirmed and probable COVID-19 cases reported by countries and territories in the region of the Americas [Internet]. Geneva: World Health Organization; 2022. [cited 2020 May 20]; Available from: https://ais.paho.org/phip/viz/COVID19Table.asp.
- 16.Zahid MN, Perna S. Continent-wide analysis of COVID-19: total cases, deaths, tests, socio-economic, and morbidity factors associated to the mortality rate, and forecasting analysis in 2020–2021. Int J Environ Res Public Health. 2021;18(10):5350. doi: 10.3390/ijerph18105350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcolino MS, Ziegelmann PK, Souza-Silva MVR, Nascimento IJB, Oliveira LM, Monteiro LS, et al. Clinical characteristics and outcomes of patients hospitalized with COVID-19 in Brazil: Results from the Brazilian COVID-19 registry. Int J Infect Dis. 2021;107:300–310. doi: 10.1016/j.ijid.2021.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aliberti MJR, Covinsky KE, Garcez FB, Smith AK, Curiati PK, Lee SJ, et al. A fuller picture of COVID-19 prognosis: the added value of vulnerability measures to predict mortality in hospitalized older adults. Age Ageing. 2021;50(1):32–39. doi: 10.1093/ageing/afaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertagnolio S, Thwin SS, Silva R, Nagarajan S, Jassat W, Fowler R, et al. Clinical features of, and risk factors for, severe or fatal COVID-19 among people living with HIV admitted to hospital: analysis of data from the WHO Global Clinical Platform of COVID-19. Lancet HIV. 2022 doi: 10.1016/S2352-3018(22)00097-2. S2352-3018(22)00097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danwang C, Noubiap JJ, Robert A, Yombi JC. Outcomes of patients with HIV and COVID-19 co-infection: a systematic review and meta-analysis. AIDS Res Ther. 2022;19(3):1–12. doi: 10.1186/s12981-021-00427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong Y, Li Z, Ding S, Liu S, Tang Z, Jia L, et al. HIV infection and risk of COVID-19 mortality. Medicine (Baltimore) 2021;100(26):e26573. doi: 10.1097/MD.0000000000026573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kouhpayeh H, Ansari H. HIV infection and increased risk of COVID-19 mortality: a meta-analysis. Eur J Transl Myol. 2021;31(4):10107. doi: 10.4081/ejtm.2021.10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jassat W, Cohen C, Tempia S, Masha M, Goldstein S, Kufa T, et al. Risk factors for COVID-19-related in-hospital mortality in a high HIV and tuberculosis prevalence setting in South Africa: a cohort study. Lancet HIV. 2021;8(9):e554–e567. doi: 10.1016/S2352-3018(21)00151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Sun J, Patel RC, Zhang J, Guo S, Zheng Q, et al. Associations between HIV infection and clinical spectrum of COVID-19: a population level analysis based on US National COVID Cohort Collaborative (N3C) data. Lancet HIV. 2021;8(11):e690–e700. doi: 10.1016/S2352-3018(21)00239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO - World Health Organization. WHO Coronavirus (COVID-19) Dashboard: situation by region, country, territory, and area [Internet]. Geneva: World Health Organization; 2021b. [cited 2020 May 20]; Available from: https://covid19.who.int/table.
- 26.BRASIL. Ministério da Saúde Secretaria de Vigilância em Saúde. Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis. Nota Informativa n° 03 de 2021. Dispõe sobre a vacinação de COVID-19 em pessoas vivendo com HIV. 2021 [Google Scholar]
- 27.BRASIL . Vol. 1996. Diário Oficial da União; Brasília: 1996. (Dispõe sobre a distribuição gratuita de medicamentos aos portadores do HIV e doentes de AsIDS). [Google Scholar]
- 28.UNAIDS - Joint United Nations Programme on HIV/AIDS. Country factsheets - Brazil 2020: HIV and AIDS estimates [Internet]. Geneva: Joint United Nations Programme on HIV/AIDS; 2022. [cited 2020 May 18]; Available from: https://aidsinfo.unaids.org/.
- 29.Liu N, Jiang C, Cai P, Shen Z, Sun W, Xu H, et al. Single-cell analysis of COVID-19, sepsis, and HIV infection reveals hyperinflammatory and immunosuppressive signatures in monocytes. Cell Rep. 2021;37(109793):1–17. doi: 10.1016/j.celrep.2021.109793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frater J, Ewer KJ, Ogbe A, Pace M, Adele S, Adland E, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV. 2021;8(8) doi: 10.1016/S2352-3018(21)00103-X. e474-e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maciel RA, Klück HM, Durand M, Sprinz E. Comorbidity is more common and occurs earlier in persons living with HIV than in HIV-uninfected matched controls, aged 50 years and older: a cross-sectional study. Int J Infect Dis. 2018;70:30–35. doi: 10.1016/j.ijid.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Nomah DK, Reyes-Urueña J, Díaz Y, Moreno S, Aceiton J, Bruguera A, et al. Sociodemographic, clinical, and immunological factors associated with SARS-CoV-2 diagnosis and severe COVID-19 outcomes in people living with HIV: a retrospective cohort study. Lancet HIV. 2021;8(11):e701–e710. doi: 10.1016/S2352-3018(21)00240-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mdege ND, Shah S, Ayo-Yusuf OA, Hakim J, Siddiqi K. Tobacco use among people living with HIV: analysis of data from demographic and health surveys from 28 low-income and middle-income countries. Lancet Glob Health. 2017;5(6):e578–e592. doi: 10.1016/S2214-109X(17)30170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Q, Meng M, Kumar R, Wu Y, Huang J, Lian N, et al. The impact of COPD and smoking history on the severity of COVID-19: A systemic review and meta-analysis. J Med Virol. 2020;92(10):1915–1921. doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inciarte A, Gonzalez-Cordon A, Rojas J, Torres B, de Lazzari E, de la Mora L, et al. Clinical characteristics, risk factors, and incidence of symptomatic coronavirus disease 2019 in a large cohort of adults living with HIV: a single-center, prospective observational study. AIDS Lond Engl. 2020;34(12):1775–1780. doi: 10.1097/QAD.0000000000002643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madzime M, Rossouw TM, Theron AJ, Anderson R, Steel HC. Interactions of HIV and antiretroviral therapy with neutrophils and platelets. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.634386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elfiky AA. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253 doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melchjorsen J, Risør MW, Søgaard OS, O'Loughlin KL, Chow S, Paludan SR, et al. Tenofovir selectively regulates production of inflammatory cytokines and shifts the IL-12/IL-10 balance in human primary cells. JAIDS J Acquir Immune Defic Syndr. 2011;57(4):265–275. doi: 10.1097/QAI.0b013e3182185276. [DOI] [PubMed] [Google Scholar]
- 39.Pablos-Méndez A, Vega J, Aranguren FP, Tabish H, Raviglione MC. Covid-19 in Latin America. BMJ. 2020;370:m2939. doi: 10.1136/bmj.m2939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.