Abstract
A 6-year-old castrated male Cavalier King Charles Spaniel was referred to the Animal Medical Center, Tokyo University of Agriculture and Technology, for examination and treatment of recurrent pneumothorax. Chest radiography and computed tomography showed multiple cavitary lesions in the caudal right posterior lobe. These lesions were surgically excised via thoracotomy. Subsequent histopathological examination revealed paragonimiasis. In the postoperative review, we found that the owner had fed raw deer meat to the dog four months earlier. Deer meat has attracted attention as a source of Paragonimus in humans. To our knowledge, this is the first report of Paragonimus infection in a dog due to deer meat consumption.
Keywords: deer, dog, game dish, Paragonimus, pneumothorax
Paragonimus infects the lungs of mammals including humans, dogs, and cats, causing chronic respiratory diseases and paragonimiasis. In humans, paragonimiasis is typically foodborne, and the main route of infection is by oral ingestion of raw or undercooked freshwater crustaceans, a second intermediate host [1, 11, 24]. Currently, 1 million individuals are infected every year worldwide, thus Paragonimus remains an important parasite in public health [24]. In contrast, in veterinary medicine, paragonimiasis in dogs and cats has been reported in various parts of the world, albeit sporadically. Freshwater crustaceans are thought to be the infection source [5,6,7, 17, 18, 20]. However, in recent years, paragonimiasis has been frequently observed in wild boar-hunting dogs, indicating that wild boar meat could be a source of Paragonimus in dogs [8, 9, 15]. To the best of our knowledge, no deer meat-derived Paragonimus infection has been reported in dogs. In this report, we described a domestic dog with recurrent spontaneous pneumothorax after Paragonimus infection caused by eating raw deer meat purchased by the owner.
A 6-year-old castrated male Cavalier King Charles Spaniel, weighing 10.4 kg, with a body condition score of 3/5, was diagnosed with recurrent pneumothorax by a family veterinarian. The dog was referred to the Animal Medical Center of the Tokyo University of Agriculture and Technology in May 2022 for further examination and treatment. The dog was kept completely indoors and had no trauma history. The owner reported progressive coughing for approximately two months. An accurate evaluation of the respiratory rate and respiratory pattern was difficult owing to panting. A complete blood count showed mild increases in the total white blood cell count (16,090/μL) and the lobulated neutrophil count (13,460/μL). The left lateral chest radiograph showed multiple ring-shaped opacities, with an outer diameter of 6–14 mm and an inner diameter of 5–13 mm, near the right crus of the diaphragm in the right posterior lobe (Fig. 1). The lung parenchyma in the right posterior lobe showed a diffuse nonstructural interstitial pattern centered on a ring-shaped opacity, and enhanced radiopacity was observed (Fig. 1).
Fig. 1.

Chest radiograph. The left lateral image shows multiple ring-shaped opacities near the right crus of the diaphragm in the right posterior lobe.
Computed tomography (CT) revealed irregular nodular soft-tissue opacities in the caudal right posterior lobe. Multiple single cavitary lesions of varying sizes were found inside the opacities, and a small amount of gas remained in the thoracic cavity (Fig. 2). Based on these findings, we speculated that the recurrent pneumothorax was caused by cavitary lesions in the lung parenchyma. We judged that medical treatment would not be effective for this lesion and attempted surgical excision of the lesion under thoracotomy.
Fig. 2.

Computed tomography (CT). CT examination shows multiple single cavitary lesions of varying sizes, each with an irregular nodular soft-tissue opacity around them, in the caudal of the right posterior lobe.
The primary lesion in the right posterior lobe was confirmed to be approximately 3 cm × 2 cm × 1.5 cm, convex, and reddish-brown in color, during thoracotomy (Fig. 3). No gross abnormalities were observed in the non-lesional lung tissues. Therefore, the right posterior lobe including the lesions, was resected.
Fig. 3.
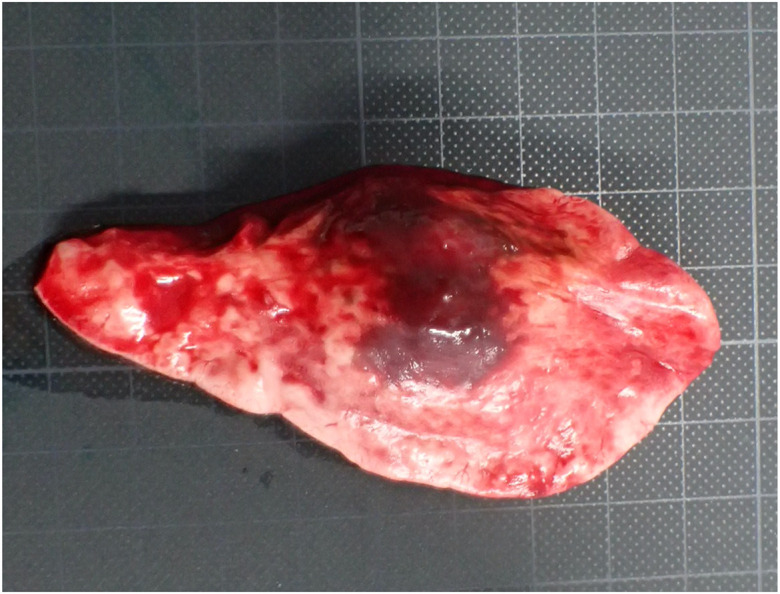
Gross findings of the surgically excised lesion. The lesion measuring approximately 3 cm × 2 cm × 1.5 cm, appears swollen, convex, and reddish-brown in color.
Histopathological examination of the resected lung revealed thickening of the alveolar walls and diffuse alveolar collapse in the lung parenchyma. Hyperplastic fibrous tissues, hemorrhage, and infiltration of inflammatory cells including lymphocytes, plasma cells, neutrophils, and macrophages, were observed in the stroma. A cyst containing adult flukes was found and many ova were observed around it. Granulation tissue formation in response to these parasites was also observed (Fig. 4). Based on these findings, our dog was diagnosed with parasitic granulomatous pneumonia. The parasite was diagnosed as Paragonimus based on its morphology and histological features. The parasite was assumed to be P. ohirai, because four to five cuticular spines clustered onto the body surface, the eggshell had uniform thickness, and a small protrusion was observed at the posterior end of the eggshell [13] (Fig. 5). However, it was difficult to obtain genetic information from formalin-fixed samples, and thus, complete identification of the species was not possible.
Fig. 4.
Adult Paragonimus with surrounding ova. (A) A cyst containing adult flukes is found in the resected lung. The parasite was diagnosed as Paragonimus based on its morphology and histological features. (B) Many ova are observed around the adult Paragonimus. Granulomatous inflammation, in response to the parasites, is evident. Bars=1,000 μm.
Fig. 5.
Morphological features of the body and eggshell of the fluke. Based on the characteristics shown in (A) and (B), the Paragonimus was assumed to be P. Ohirai. (A) Clusters of 4–5 cuticular spines are observed on the body surface (arrowheads). (B) An almost constant thickness and a small protrusion at the posterior end are observed in the eggshell (arrowheads). Bars=100 μm.
In the postoperative review, we found that the owner had fed the dog raw deer meat from Hokkaido, which he had purchased to be served as game dishes, four months earlier. We found that the dog had never been in an environment where it could eat wild animals or freshwater crustaceans. In Paragonimus infection, eggs are expelled from the lungs through coughing [17]. The prepatent period for Paragonimus in dogs is approximately 5–7 weeks [17]. This was consistent with the timing of the dog’s asymptomatic period and the onset of progressive coughing. Therefore, we concluded that severe granulomatous pneumonia and cavitary lesions were caused by Paragonimus infection from raw deer meat, resulting in recurrent pneumothorax. We administered praziquantel orally at a dose of 25 mg/kg, three times a day for two consecutive days, as an additional postoperative treatment, since Paragonimus may be present outside the resected area [10]. At 390 days postoperatively, the dog was doing well with no signs of recurrence.
The life cycle of Paragonimus is complex and requires two intermediate hosts for development, such as a freshwater snail as the first intermediate host and a freshwater crustacean as the second intermediate host [1, 11, 24]. Definitive hosts, including humans and dogs, are infected via oral ingestion of metacercaria-parasitizing freshwater crustaceans. Metacercariae excyst in the stomach or small intestine and migrate to the peritoneal cavity, perforate the diaphragm, and finally reach the lungs [18]. Subsequently, cysts are formed in the lung parenchyma, where they mature and lay ova [18].
The main clinical signs of paragonimiasis in dogs include a chronic productive cough and dyspnea, which correlate with the behavioral sequence of paragonimiasis [6, 7, 17, 18, 20]. Dyspnea, which is usually caused by pneumothorax [6, 7, 17, 18, 20], is acute, and can often be fatal [6, 10]. Similar to previous reports, the dog presented with chronic coughing followed by acute dyspnea due to recurrent spontaneous pneumothorax, consistent with the typical clinical signs of paragonimiasis [10]. Chest radiography and CT showed multiple cavitary lesions in the right posterior lobe of the lung. Therefore, we concluded that lesion rupture caused recurrent pneumothorax. Diseases that form cavitary lesions in the lungs include emphysematous pulmonary cysts and pneumonia caused by fungal or bacterial infections [3, 4, 16]. Notably, paragonimiasis is known to cause lesions in the posterior lobe, especially in the right posterior lobe of the lung, in line with the migratory route of the fluke [17, 18, 20]. Based on the imaging results, we may have been able to diagnose paragonimiasis preoperatively if we had tested for parasite ova in the feces or conducted a cytological examination of the tracheal lavage fluid [20]. East Asia is an endemic area for paragonimiasis in humans [24]. Therefore, in Japan, veterinarians need to consider paragonimiasis in the differential diagnosis of chronic respiratory diseases. In our case, no significant blood results were observed. In humans, eosinophilia is known to aid in the diagnosis of paragonimiasis [21, 24]. In contrast, in dogs, eosinophilia is absent at certain stages [18, 19], and the eosinophil count is often within the normal range by the time clinical symptoms are observed [18]. Consistent with previous reports, our dog also showed no increase in the eosinophil count. This suggests that blood tests are not useful in diagnosing paragonimiasis in dogs.
In Japan, paragonimiasis is caused by P. westermani, P. miyazakii, and P. ohirai. Of these, deer meat has attracted attention as a new source of P. westermani infection in humans [2, 25]. To date, it has not been thought that deer, being herbivorous, can serve as paratenic hosts for Paragonimus. However, Banzai et al. detected triploid larvae of P. westermani in deer meat samples [2]. Matsuo et al. found freshwater crab exoskeletons in deer stomachs [12]. These findings indicate that deer may be a potential paratenic host for Paragonimus, possibly by accidentally preying on freshwater crabs [12, 25]. Based on the morphology of the parasite body and the eggs in the histopathological examination, our dog was assumed to have been infected with P. ohirai. However, unlike P. westermani, the relationship between P. ohirai and deer has not yet been clarified. Therefore, further research is required to determine whether deer can serve as a paratenic host in the life cycle of P. ohirai.
With the increasing popularity of game dishes in Japan recently, raw deer meat has been made available through online sales [14]. Therefore, it is speculated that the risk of Paragonimus infection from consuming deer meat will likely increase [14]. In our dog, it was strongly suspected that the source of infection was raw deer meat from Hokkaido, which was purchased online by the owner, based on the breeding environment and history. According to an established theory, domestic Paragonimus, including this species, does not inhabit Hokkaido [23]. However, since the 1960s, the habitat of domestic Paragonimus species in Hokkaido has not been surveyed, and the recent pollution status cannot be clarified [23]. In humans, Paragonimus infection has been reported in Hokkaido [22]. Therefore, the new colonization of P. ohirai in Hokkaido may have been overlooked. Furthermore, production information including the origin of the deer meat is generally sent by the seller. Consumers have no way of verifying the authenticity, and origin information alone is insufficient to ensure the quality and safety of the meat. Therefore, veterinarians must educate dog owners regarding the dangers of feeding raw deer meat to dogs, and thoroughly instruct them on cooking the meat sufficiently before consumption by their dogs.
Systemic therapy with anthelmintic drugs, such as praziquantel and fenbendazole, is effective as the first-line treatment for paragonimiasis [9, 17, 19, 20]. Therefore, it is possible that treatment of our dog may only require systemic therapy. However, even if the Paragonimus is gone, it is possible that the cavitary lesions can persist, leading to pneumothorax. The need for surgical intervention in these cases warrants future investigations.
As the distribution of wild game dishes increases, there is a possibility that the incidence of paragonimiasis will increase in the future. Veterinarians need to be careful not to overlook paragonimiasis when examining animals with chronic respiratory symptoms. For this purpose, it is essential to conduct a thorough medical interview regarding the clinical course and diet, and to accurately interpret the images.
CONFLICT OF INTEREST
There are no conflicts of interest.
Acknowledgments
The authors would like to thank all the hospital staff and the owner involved.
REFERENCES
- 1.Aka NA, Adoubryn K, Rondelaud D, Dreyfuss G. 2008. Human paragonimiasis in Africa. Ann Afr Med 7: 153–162. doi: 10.4103/1596-3519.55660 [DOI] [PubMed] [Google Scholar]
- 2.Banzai A, Sugiyama H, Hasegawa M, Morishima Y, Kawakami Y. 2021. Paragonimus westermani metacercariae in two freshwater crab species in Kagoshima Prefecture, Japan, as a possible source of infection in wild boars and sika deer. J Vet Med Sci 83: 412–418. doi: 10.1292/jvms.20-0576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brady CA. 2004. Bacterial pneumonia in dogs and cats. pp. 412–421. In: Text Book of Respiratory Disease in Dog and Cat (King LG ed.), W. B. Saunders Co., Philadelphia. [Google Scholar]
- 4.Brockman DJ, Puerto DA. 2004. Pneumomediastinum and Pneumothorax. pp. 616–624. In: Text Book of Respiratory Disease in Dog and Cat (King LG ed.), W. B. Saunders Co., Philadelphia. [Google Scholar]
- 5.Dubey JP, Stromberg PC, Toussant MJ, Hoover EA, Pechman RD. 1978. Induced paragonimiasis in cats: clinical signs and diagnosis. J Am Vet Med Assoc 173: 734–742. [PubMed] [Google Scholar]
- 6.Gillick A. 1972. Paragonimiasis in a dog. Can Vet J 13: 175–179. [PMC free article] [PubMed] [Google Scholar]
- 7.Harrus S, Nyska A, Colorni A, Markovics A. 1997. Sudden death due to Paragonimus kellicotti infection in a dog. Vet Parasitol 71: 59–63. doi: 10.1016/S0304-4017(97)00007-1 [DOI] [PubMed] [Google Scholar]
- 8.Irie T, Yamaguchi Y, Doanh PN, Guo ZH, Habe S, Horii Y, Nonaka N. 2017. Infection with Paragonimus westermani of boar-hunting dogs in Western Japan maintained via artificial feeding with wild boar meat by hunters. J Vet Med Sci 79: 1419–1425. doi: 10.1292/jvms.17-0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirino Y, Nakano N, Hagio M, Hidaka Y, Nakamura-Uchiyama F, Nawa Y, Horii Y. 2008. Infection of a group of boar-hunting dogs with Paragonimus westermani in Miyazaki Prefecture, Japan. Vet Parasitol 158: 376–379. doi: 10.1016/j.vetpar.2008.09.017 [DOI] [PubMed] [Google Scholar]
- 10.Kirkpatrick CE, Shelly EA. 1985. Paragonimiasis in a dog: treatment with praziquantel. J Am Vet Med Assoc 187: 75–76. [PubMed] [Google Scholar]
- 11.Lane MA, Barsanti MC, Santos CA, Yeung M, Lubner SJ, Weil GJ. 2009. Human paragonimiasis in North America following ingestion of raw crayfish. Clin Infect Dis 49: e55–e61. doi: 10.1086/605534 [DOI] [PubMed] [Google Scholar]
- 12.Matsuo K, Moribe J, Takashima Y, Kasuya S, Yoshida A, Abe N, Saijuntha W, Agatsuma T. 2018. Possibility of paragonimiasis due to consumption of raw deer meat. Nippon Juishikai Zasshi 71: 449–453 (in Japanese with English abstract). [Google Scholar]
- 13.Miyazaki I, To Y. 1988. Paragonimiasis. pp. 277–366. In: Parasitic Zoonoses (Miyazaki I, To Y eds.), Kyushu University Press, Fukuoka (in Japanese). [Google Scholar]
- 14.Nagayasu E, Yoshida A, Hombu A, Horii Y, Maruyama H. 2015. Paragonimiasis in Japan: a twelve-year retrospective case review (2001–2012). Intern Med 54: 179–186. doi: 10.2169/internalmedicine.54.1733 [DOI] [PubMed] [Google Scholar]
- 15.Nakano N, Kirino Y, Uchida K, Nakamura-Uchiyama F, Nawa Y, Horii Y. 2009. Large-group infection of boar-hunting dogs with Paragonimus westermani in Miyazaki Prefecture, Japan, with special reference to a case of sudden death due to bilateral pneumothorax. J Vet Med Sci 71: 657–660. doi: 10.1292/jvms.71.657 [DOI] [PubMed] [Google Scholar]
- 16.Norris CR. 2004. Fungal Pneumonia. pp. 446–456. In: Text Book of Respiratory Disease in Dog and Cat (King LG ed.), W. B. Saunders Co., Philadelphia. [Google Scholar]
- 17.Pechman RD. 1976. The radiographic features of pulmonary paragonimiasis in the dog and cat. J Am Vet Radiol Soc 17: 182–190. doi: 10.1111/j.1740-8261.1976.tb00573.x [DOI] [Google Scholar]
- 18.Pechman RD, Jr. 1980. Pulmonary paragonimiasis in dogs and cats: a review. J Small Anim Pract 21: 87–95. doi: 10.1111/j.1748-5827.1980.tb01218.x [DOI] [PubMed] [Google Scholar]
- 19.Saini N, Ranjan R, Singla LD, Anand A, Randhawa CS. 2012. Successful treatment of pulmonary paragonimiasis in a German shepherd dog with fenbendazole. J Parasit Dis 36: 171–174. doi: 10.1007/s12639-012-0098-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherding RG. 2004. Parasites of the lung. pp. 548–558. In: Text Book of Respiratory Disease in Dog and Cat (King LG ed.), W. B. Saunders Co., Philadelphia. [Google Scholar]
- 21.Singh TS, Mutum SS, Razaque MA. 1986. Pulmonary paragonimiasis: clinical features, diagnosis and treatment of 39 cases in Manipur. Trans R Soc Trop Med Hyg 80: 967–971. doi: 10.1016/0035-9203(86)90275-0 [DOI] [PubMed] [Google Scholar]
- 22.Yasumura S, Ito N, Tanaka C, Hiraki M, Kanazawa T. 1989. A case of chronic cerebral paragonimiasis infested in Hokkaido. Journal of Hokkaido Brain Research Foundation 2: 41–45 (in Japanese with English abstract). [Google Scholar]
- 23.Yokogawa M, Tsuji M, Okura T, Yoshimura H, Ichikawa K. 1961. Epidemiological survey using the intradermal test for paragonimiasis in Hokkaido, Japan. Kiseichugaku Zasshi 10: 578–581 (in Japanese with English abstract). [Google Scholar]
- 24.Yoshida A, Doanh PN, Maruyama H. 2019. Paragonimus and paragonimiasis in Asia: An update. Acta Trop 199: 105074. doi: 10.1016/j.actatropica.2019.105074 [DOI] [PubMed] [Google Scholar]
- 25.Yoshida A, Matsuo K, Moribe J, Tanaka R, Kikuchi T, Nagayasu E, Misawa N, Maruyama H. 2016. Venison, another source of Paragonimus westermani infection. Parasitol Int 65 6 Pt A: 607–612. doi: 10.1016/j.parint.2016.09.009 [DOI] [PubMed] [Google Scholar]




