Summary
Dysregulations in cholesterol metabolism are associated with neurodegenerative and vascular pathologies, and dementia. Diet-derived plant sterols (phytosterols) have cholesterol-lowering, anti-inflammatory, and antioxidant properties and may interfere with neurodegeneration and cognitive decline. Here we performed multivariate analysis in 720 individuals enrolled in a population-based prospective study to determine whether circulating cholesterol precursors and metabolites, triglycerides, and phytosterols, are associated with cognitive impairment and decline in the older population. We report specific dysregulations of endogenous cholesterol synthesis and metabolism, and diet-derived phytosterols, and their changes over time associated with cognitive impairment, and decline in the general population. These findings suggest circulating sterols levels could be considered in risk evaluation and are relevant for the development of strategies to prevent cognitive decline in older people.
Subject areas: Age, Human metabolism, Behavioral neuroscience
Graphical abstract
Highlights
-
•
Endogenous cholesterol metabolism is associated with cognitive impairment
-
•
Specific sterols level changes over time are associated with cognitive decline
-
•
Plasma sterol levels might be useful in risk assessment in the general population
Age; Human metabolism; Behavioral neuroscience
Introduction
Cholesterol metabolism dysregulation in middle age is associated with increased risk of cognitive impairment in later life. Multiple cholesterol metabolic alterations are linked to the development of cerebrovascular and Alzheimer’s disease (AD) pathologies, which together represent the most common causes of cognitive impairment and dementia in old age.1,2,3,4,5 Previous evidence has associated high cholesterol levels with changes in rate of cognitive decline in the general population, although this depends on the age of hypercholesterolemia onset.6,7 In addition, this relationship changes over time. Indeed, cerebral pathology itself reduces cholesterol absorption, which is in turn associated with decreased cognitive performance and increased all-cause and vascular mortality.8,9 This suggests that changes in cholesterol metabolite levels could be implicated in the development of cognitive decline. In addition, altered cholesterol synthesis and metabolism may contribute to AD pathogenesis by modifying amyloid accumulation and affecting neurodegeneration.10,11
Cerebral cholesterol is unable to pass the blood-brain barrier (BBB)12,13 and is synthesized in majority in situ by neurons and astrocytes.14 Cholesterol synthesis is a complex process, including many intermediates, such as lanosterol, lathosterol and desmosterol.15 In the event of excessive cholesterol accumulation in the brain, resulting from metabolic dysregulations or myelin breakdown, cholesterol is enzymatically converted into the oxysterol 24-hydroxycholesterol (24S-OHC) by the cytochrome P450 enzyme CYP46A1 (cholesterol 24S-hydroxylase,16,17). Polymorphism of this enzyme has been associated with cognitive decline18 and is considered an AD risk factor.19 Altered levels of circulating 24S-OHC are associated with mild cognitive impairment8,20 and with dementia and cognitive decline21,22 in clinical settings. In the context of AD, 24S-OHC is related to neurodegeneration and tau pathology.10,23 Contrary to cholesterol, 24S-OHC can diffuse into circulating blood,24 where it can be considered a marker of cerebral cholesterol turnover. Furthermore, increased levels of the neurotoxic 27-hydroxycholesterol (27-OHC), a cholesterol derivative like 24S-OHC, are associated with cognitive decline.20 Both these oxysterols (24S-OHC and 27-OHC) are also associated with elevated amyloid levels in the brain.11
Plant sterols (or phytosterols) which are entirely diet-derived25 can cross the BBB and enter the brain where they regulate cerebral cholesterol metabolism and cerebral pathological processes by their cholesterol-lowering, anti-inflammatory, and antioxidant properties.25,26 Amongst phytosterols, cerebrospinal fluid (CSF) levels of both campesterol and sitosterol are associated with amyloid aggregation and neurodegeneration 23 whereas CSF levels of stigmasterol are associated with reduced amyloid generation.27 The plant sterol brassicasterol could be a biomarker candidate for cerebrovascular pathology.26
Although there is evidence that cholesterol synthesis and metabolism, and the diet-derived phytosterols, contribute to brain pathologies leading to cognitive impairment, their relationships with cognitive decline in the general elder population are unknown. In this study, we hypothesize that specific cholesterols metabolism intermediates, and plant sterols present in circulating blood are associated with cognitive performance at baseline and with cognitive decline at five-year follow up in the general population. As the progression of specific sterol-related processes may parallel or contribute to cognitive decline over a longer period, we will further assess if longitudinal changes in endogenous sterols and phytosterols are associated with cognitive decline at follow-up visits.
Results
Study population
Clinical and demographic characteristics of the cohort are shown in Table 1. Age, depression and former smoking status did not differ between groups (CDR = 0 and CDR = 0.5) at TP0 in the 720 participants considered at baseline (TP0). Of note, current treatment with statins at baseline was significantly higher in participants with CDR = 0.5. Longitudinal cognitive data (TP1) was available for 720 participants with MMSE data (the same participants as at TP0), sterol concentrations were available for 691 participants and CDR data for 510 participants with a similar male/female distribution. Both MMSE and CDR-SoB scores differed in both groups at TP1.
Table 1.
Characteristics of the study population
| Total n = 720 | CDR = 0 n = 343 | CDR = 0.5 n = 377 | p-value | ||
|---|---|---|---|---|---|
| TP0 | N (n, % male) | 720 (445, 61.81) | 343 (253, 73.76) | 377 (192, 50.93) | <0.0001 |
| Age, mean (S.E.) | 70.04 (0.17) | 70.03 (0.24) | 70.05 (0.24) | 0.9415 | |
| BMI, mean (S.E.) | 26.49 (0.17) | 26.1 (0.24) | 26.84 (0.23) | 0.0256 | |
| MMSE, mean (S.E.) | 29.26 (0.05) | 29.62 (0.04) | 28.94 (0.09) | <0.0001 | |
| CDR-SoB, mean (S.E.) | 0.91 (0.02) | 0.44 (0.02) | 1.34 (0.03) | <0.0001 | |
| MDD, % (n) | 4.58 (33) | 5.25 (18) | 3.98 (15) | 0.5255 | |
| Hypertension, % (n) | 62.26 (447) | 56.14 (192) | 67.82 (255) | 0.0016 | |
| Diabetes, % (n) | 15.86 (114) | 12.83 (44) | 18.62 (70) | 0.0433 | |
| Dyslipidemia, % (n) | 54.03 (389) | 48.69 (167) | 58.89 (222) | 0.0076 | |
| Education level, % low (n) | 61.53 (443) | 56.27 (193) | 66.31 (250) | 0.0071 | |
| Never smoker, % (n) | 41.82 (299) | 46.76 (159) | 37.33 (140) | 0.0122 | |
| Former smoker, % (n) | 44.76 (320) | 41.18 (140) | 48 (180) | 0.0617 | |
| Current smoker, % (n) | 13.43 (96) | 12.06 (41) | 14.67 (55) | 0.0378 | |
| Statins treatment, % (n) | 14.7 (106) | 11.6 (40) | 17.5 (66) | 0.0292 | |
| TP1 | MMSE, mean (S.E.) | 28.98 (0.05) | 29.24 (0.06) | 28.75 (0.09) | <0.0001 |
| CDR SoB, mean (S.E.) | 0.86 (0.03) | 0.65 (0.04) | 1.06 (0.05) | <0.0001 | |
| CDR = 0, % (n) | 48.04 (245) | 65.99 (163) | 31.18 (82) | <0.0001 | |
| CDR = 0.5, % (n) | 51.37 (262) | 33.2 (82) | 68.44 (180) | <0.0001 | |
| CDR =1, % (n) | 0.59 (3) | 0.81 (2) | 0.38 (1) | <0.0001 |
Group comparisons between CDR = 0 and CDR = 0.5 groups were performed using Mann–Whitney U-Test for continuous variables and Chi-square tests for categorical variables. Significant p-values are shown in bold. The number of study participants within each groups is denoted n.
Associations with baseline cognitive performance
We first investigated the association of sterol clusters with impairment in global cognition (MMSE) and cognitive and functional impairment (CDR-SoB) at baseline (Table 2). Baseline plasma levels of all sterols and phytosterols are shown in Table S1. We found that in the endogenous sterol cluster, triglyceride levels were positively associated with baseline MMSE (transformed) score in both models, indicating an association of higher triglyceride levels with more severe impairment in global cognition. Triglyceride levels were not associated with baseline CDR-SoB score. 7α-hydroxy-cholesterol was positively associated with baseline CDR-SoB score, suggesting an association of higher levels with higher cognitive and functional impairment. HDL-cholesterol was negatively associated with baseline CDR-SoB score and lathosterol was negatively associated with both (transformed) MMSE and CDR-SoB scores in all models, indicating that lower levels of these compounds are associated with worse cognitive impairment at baseline. Similarly, 24S-OHC was negatively associated with CDR-SoB baseline scores. In the phytosterol cluster, higher sitosterol levels was associated with lower baseline scores of both CDR-SoB and (transformed) MMSE, indicating an association of higher levels of this phytosterol with less impairment in global cognition and related cognitive decline. Associations with the presence of cognitive impairment at baseline (CDR >0) are shown in Table S2 and revealed an association of higher stigmasterol with CDR >0.
Table 2.
Results of linear regression between baseline cognitive scores and the two sterol clusters considered in this study
| Parsimonious model | Complete Model | |
|---|---|---|
| Endogenous sterols | ||
| MMSE | Lathosterol∗∗ (−0.060) Desmosterol∗ (0.052) Triglycerides∗∗ (0.092) |
Lathosterol∗ (−0.067) Triglycerides∗ (0.069) |
| CDR-SoB | Lathosterol∗∗ (−0.099) 24S-OHC∗∗ (−0.070) 7α-hydroxycholesterol∗∗ (0.054) HDL-cholesterol∗ (−0.081) |
Lathosterol∗ (−0.119) Lanosterol ∗(0.073) 24S-OHC∗∗ (−0.065) 7α-hydroxycholesterol∗ (0.055) HDL-cholesterol∗ (−0.068) |
| Phytosterols | ||
| MMSE | Sitosterol∗ (−0.138) | |
| CDR-SoB | Sitosterol∗ (−0.223) | Sitosterol∗ (−0.181) |
Only two-tailed t-test significant associations are shown. Individual β coefficients are shown in parentheses. The parsimonious model considers age and sex as covariates, whereas the complete model considers age, sex, smoking status, hypertension, diabetes, BMI, the presence of MDD, and years of education. Note that MMSE = ln(31-MMSE), therefore a positive coefficient indicates an association with more severe impairment in global cognition. ∗ = p < 0.05; ∗∗ = p < 0.01.
Associations with cognitive decline at five-year follow-up
We next assessed the associations of sterol clusters with cognitive decline over five years. In the endogenous cholesterol cluster, higher desmosterol levels were associated with decline in global cognition over time. In addition, higher 24S-OHC and cholesterol levels were associated with more marked cognitive and functional decline over time (Table 3). Furthermore, higher dihydrolanosterol was associated with greater cognitive and functional decline and sitosterol levels were negatively associated global cognition change (Table S3). In all models, statins intake at baseline was not associated with cognitive decline over five years. In addition, the presence of diabetes was positively associated with more marked cognitive and functional decline over time in both the endogenous sterol cluster and the phytosterol cluster (data not shown).
Table 3.
Results of logistic regression between cognitive decline at five years and the endogenous sterols considered in this study
| Parsimonious model | Complete Model | |
|---|---|---|
| Endogenous sterols | ||
| ΔMMSE ≥2 | Desmosterol∗∗ (1.368) | Desmosterol∗ (1.342) |
| ΔCDR-SoB >0 | 24S-OHC∗ (1.390) | 24S-OHC∗∗ (1.081) Cholesterol∗ (1.074) |
| ΔCDR >0 | 24S-OHC∗ (1.438) | Cholesterol∗ (1.056) |
Only two-tailed t-test significant associations are shown. Individual β coefficients are shown in parentheses. The parsimonious model considers age and sex as covariates, while the complete model considers age, sex, smoking status, hypertension, diabetes, BMI, the presence of MDD, and years of education. Note that MMSE = ln(31-MMSE), therefore a positive coefficient indicates an association with more severe impairment in global cognition. ∗ = p < 0.05; ∗∗ = p < 0.01.
Changes in sterols levels associated with cognitive decline
We finally investigated how changes in the circulating levels of individual sterols between TP0 and TP1 were associated with cognitive decline. Follow-up plasma levels of all sterols and phytosterols are shown in Table S1. Increase in 24S-OHC, 27-OHC and cholesterol levels over time was associated with an increase in cognitive and related functional impairment (Table 4). In the phytosterol cluster, stigmasterol increase was associated with a decrease in global cognitive performance, and both campestanol and sitostanol increase were associated with cognitive decline. In participants treated with statins at TP0, all endogenous cholesterol metabolites, except desmosterol, were significantly decreased at TP1, compared to participants without treatment (Table S4). Phytosterol levels did not differ between participants with statins intake at baseline and those without. In all models, statins intake at baseline did not modify associations between sterols changes and cognitive decline. In all models, the presence of diabetes at baseline was also associated with an increase in cognitive and related functional impairment (data not shown).
Table 4.
Results of logistic regression between cognitive decline at five years and concentration change (TP1- TP0) of individual sterols within both clusters considered in this study
| Parsimonious model | Complete Model | |
|---|---|---|
| Endogenous sterols | ||
| ΔMMSE ≥ 2 | ||
| ΔCDR-SoB>0 | Cholesterol∗ (1.498) | 24S-OHC∗ (1.087) 27-OHC∗ (0.928) Cholesterol∗ (1.073) |
| ΔCDR>0 | ||
| Phytosterols | ||
| ΔMMSE ≥ 2 | Stigmasterol∗ (1.501) | Stigmasterol∗ (1.558) |
| ΔCDR-SoB>0 | ||
| ΔCDR>0 | Campestanol∗ (1.708) Sitostanol∗ (0.637) |
Campestanol∗ (1.094) Sitostanol∗ (0.930) |
Only two-tailed t-test significant associations are shown. Individual β coefficients are shown in parentheses. The parsimonious model considers age and sex as covariates, while the complete model considers age, sex, smoking status, hypertension, diabetes, BMI, the presence of MDD, and years of education. Note that MMSE = ln(31-MMSE), therefore a positive coefficient indicates an association with more severe impairment in global cognition. ∗ = p < 0.05; ∗∗ = p < 0.01.
Discussion
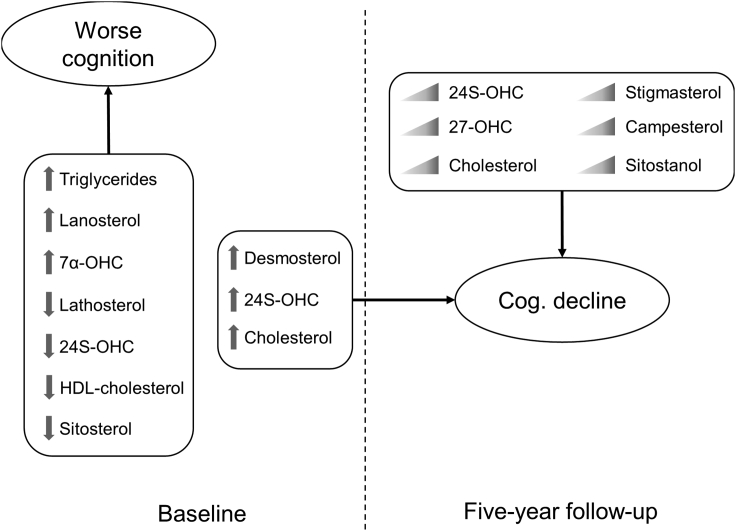
Metabolites from endogenous cholesterol metabolism (lathosterol, HDL-cholesterol, triglyceride, and 24S-OHC) were associated with impairment in global cognition and cognitive and related functional impairment at baseline. Furthermore, the phytosterol sitosterol was associated with cognitive impairment. Baseline desmosterol and cholesterol were associated with a decrease in global cognition whereas 24S-OHC was associated with cognitive and related functional decline at follow-up. Changes in plasma levels of 24S-OHC and 27-OHC over time were associated with cognitive and related functional decline. This was also observed for the phytosterols stigmasterol, campestanol and sitostanol whose changes were associated with cognitive decline. (Figure 1). Results from our parsimonious model might be useful for identifying risk profiles for cognitive decline, whereas our complete models provide a more comprehensive view of the relationship between cholesterol metabolism and phytosterols, with cognitive impairment and decline in the general population.
Figure 1.
Graphical summary of the present study
Arrows next to individual sterols indicate high or low levels at baseline. Triangular gradients indicate an increase in concentration of the designated metabolite at five-year follow up. Cognitive performance at baseline was derived from either MMSE or CDR-SoB scores. Cognitive decline (Cog. Decline) was determined using MMSE, CDR-SoB or CDR score difference at five-year follow up.
Higher baseline total cholesterol was associated with cognitive impairment at baseline. In addition, increasing total cholesterol over the 5-year follow-up period was associated with more marked cognitive and related functional decline. These findings are in line with several previous reports relating high circulating cholesterol levels with higher risk of cognitive decline in the general population.27,28 Moreover, lower concentrations of HDL-cholesterol were associated with cognitive impairment at baseline. This finding is in line with previous reports where plasma HDL-cholesterol levels are negatively associated with cognitive impairment7,29,30 and with AD and vascular dementias.30,31,32 This association could be explained by 1) the association between cardiovascular risk and HDL-cholesterol levels33 and 2) the interactions between HDL-cholesterol and apolipoproteins,34 which promote the degradation of the amyloid peptide,35 reducing the risk of amyloid aggregation and subsequent AD pathology and related cognitive decline.
We observed that lower concentrations of the cholesterol precursor lathosterol36 were associated with more pronounced impairment in global cognition. This is contrary to previous results where increased lathosterol levels were related to impaired cognitive performance.37 However, lower plasma levels of lathosterol have also been observed in AD dementia patients compared to non-demented controls.38 This suggests cholesterol synthesis may be differentially affected in AD dementia compared to other causes of cognitive impairment. Plasma levels of desmosterol, another cholesterol precursor,36 may also be reduced in AD dementia and MCI,23,39 further suggesting a dysregulation of cholesterol biosynthesis in association with neurodegeneration. In our general population cohort, we observed that increasing levels of desmosterol are associated with a decline in global cognition over time. This suggests that the alterations of cholesterol biosynthesis related to cognitive decline are distinct but concomitant with those happening in the context of developing neurodegenerative pathologies. This hypothesis is supported by the observation that cholesterol absorption into the brain is dysregulated in the context of AD.40 Further investigating these differential alterations would require the consideration of the specific pathologies underlying cognitive impairment and decline.
In line with previous reports,41 we observed an association between plasma triglyceride levels and cognitive impairment. Triglycerides are implicated in the pathogenesis of cardiovascular disease.42 In addition, they are associated with inflammation, a risk factor for cognitive impairment43 and regulate BBB function.44 Triglycerides are also associated with altered amyloid levels independently of total and HDL-cholesterol levels.45 Therefore, we hypothesize that the observed association between triglyceride levels and cognition is mediated by an alternative pathway unrelated to cholesterol metabolism.
We observed that baseline concentration of oxysterol 24S-OHC was negatively associated with cognitive and functional impairment, whilst higher concentrations were associated with more pronounced cognitive decline at follow-up visits. This apparently dichotomic effect of 24S-OHC is supported by previous findings suggesting 24S-OHC can have both neuroprotective effects by reducing neuronal loss46 and neurotoxic inflammatory effects47 which may contribute to neurodegeneration and cognitive decline. Further, we found an increase in circulating levels of 24S-OHC over time was associated with cognitive and functional decline. Because circulating 24S-OHC derives mainly from brain efflux and can be regarded as a biomarker of cholesterol metabolism in the brain, this suggests an increase in cerebral cholesterol levels in parallel to cognitive decline, possibly resulting from neurodegeneration and myelin degradation. This increase could also result from disrupted brain cholesterol clearance associated with cognitive decline.48 This is supported by previous work suggesting 24S-OHC is a direct biomarker of cholesterol metabolism in the brain.24 In addition, 24S-OHC is enzymatically produced by CYP46A1 whose levels have been reported to both be increased49 and decreased in the context of AD.50 Here, we have also observed apparently contradictory associations of 24S-OHC with cognitive decline in the general population. This suggests the relationships between cholesterol metabolism and cognitive decline are complex and may depend on multiple factors, including underlying pathologies or demographics. We hypothesize that 24S-OHC levels in plasma change over the pathological course of AD as brain cholesterol metabolism is altered by the disease. In this context, our results suggest 24S-OHC is a key component of cerebral cholesterol metabolism and a potential blood plasma marker indicating increased risk of cognitive decline in the elder general population.
Increasing cholesterol levels occurring during the progression of cognitive decline will also result in an increase in low density lipoprotein-cholesterol levels. This will drive entry of 27-OHC into the brain from blood51 resulting in increased cerebral levels of 27-OHC which have previously been associated with cognitive decline in clinical cohorts.20 Here, we observed that plasma levels of 27-OHC increasing over time are associated with worse cognitive and functional decline. This is in accordance with a recent study investigating a comparable general population cohort, where increased 27-OHC at baseline was associated with poorer cognitive performance.52 Whether the circulating 27-OHC increase is caused by disrupted cholesterol metabolism or rate-limiting brain influx is unknown, although it has been reported that circulating 27-OHC function in the brain is independent of cholesterol.53
The increasing levels of both 24S-OHC and 27-OHC over time we observed in association with cognitive decline may also be directly linked to cerebral pathophysiological mechanisms, especially in the context of AD, where altered levels of these oxysterols have been associated with amyloid aggregation and tau pathology.10,11 Another possibility is that separate mechanisms underlie the emergence of cognitive impairment and further cognitive decline. In the context of AD, for example, impaired enzymatic cholesterol catabolism and efflux result in reduced de novo reduced cholesterol synthesis, supporting this hypothesis.54 Further considering that a single serum measurement of 24S-OHC or 27-OHC can reliably estimate their respective average levels over a one-year period48 these oxysterols may represent interesting biomarker candidates of cognitive decline in the general population. This is further supported by studies in animal models showing that both 24S-OHC and 27-OHC act as suppressors of cholesterol biosynthesis in the brain55 and that their interplay is the main driving force of cholesterol homeostasis and normal neuronal function in the brain.15
Among the phytosterols, low baseline levels of sitosterol were associated with more pronounced cognitive and functional impairment. Increasing concentrations of campesterol and sitostanol were associated with worse cognitive and functional decline whereas an increase in stigmasterol was associated with more marked decline in global cognition. These findings indicate relevance of diet for cognitive decline prevention. This is supported by animal studies, where sitosterol supplementation reduced cognitive deficits.56 Therefore, increase over time of circulating levels of campesterol, stigmasterol and sitostanol could accelerate cognitive decline. For campesterol, this is in line with previous reports.23 Concerning stigmasterol, it has been reported in mouse models that increased levels of this sterol do not interfere with memory and results in decreased levels of desmosterol and 24S-OHC in the hippocampus.57 Both these sterols (desmosterol and 24S-OHC) were associated with cognitive decline in our study. These conflicting results could be caused by region specific effects of stigmasterol in the brain because these changes were observed in the hippocampus specifically. This animal study57 also reports an increase in lanosterol in the cortex with no apparent effect on cognition, whereas in the present study lanosterol was associated with worse cognition. We therefore hypothesize that the effects of phytosterols in the brain depend on pathological background and the etiologies of dementia. This is supported by previous studies suggesting stigmasterol can reduce amyloid burden.58 Lastly, it is also possible that the changes we have observed in plasma stigmasterol levels are related to a change in dietary habits in people with poorer cognitive performance. Our results also conflict with the cholesterol reducing effects of sitostanol59 observed in hyperlipidemic men. These results suggest a positive effect on cognition of these phytosterols in specific cases, which may consequently contribute to slower cognitive decline. Future investigations of dietary interventions modulating these phytosterols are required to better understand their potential positive or negative effects on cognition in the general population. It would be particularly interesting to investigate diets with a focus on the intake of vegetable oils and nuts as these are the principal sources of campesterol, stigmasterol and sitostanol.
Statins treatment has been associated with lower risks of all-cause dementia and mild cognitive impairment.60 Statins also have been shown to lower sterol levels, in both the periphery and in the central nervous system in AD patients,61 which suggests statins may have neuroprotective effects. However, randomized controlled trials failed to find beneficial effects of statins on cognitive decline and there is evidence that statins could even contribute to (reversible) cognitive impairment.62,63 In the present study in older people from the general population we did not find an association between treatment with statins at baseline and cognitive decline at five-years follow-up. Considering the fact that statins intake was only present in a small proportion of the participants and the heterogeneity of the cohort with significantly higher statin treatment rate in participants with CDR = 0.5 at baseline compared to cognitively healthy participants, no clear conclusion can be made on whether statins may slow down cognitive decline, especially in people with specific risk profiles.
Our results highlight the role of plasma circulating levels of cholesterol, non-cholesterol-sterols, and phytosterols as risk factors for all cause cognitive impairment and decline in the general population. Although the results presented here do not account for different pathological origins of cognitive impairment and decline, they support the further investigation and development of lipid modulating therapies to lower circulating concentrations of phytosterols and cholesterol metabolites to reduce cerebral cholesterol levels and prevent cognitive decline, although the efficacy of such approaches to prevent cognitive decline is yet to be proven.
Limitations of the study
There are some limitations to this study. First, there is no data available on the brain pathologies underlying cognitive decline in the study participants. Accordingly, we cannot differentiate between cognitive decline resulting from AD, vascular, mixed, and other brain pathologies. This lack of patient stratification does limit our ability to associate circulating sterol concentrations with specific pathologies. It does, however, demonstrate that the associations observed here are robust and could be useful in real-world applications. Further studies considering both circulating sterols as well as plasma biomarkers of AD and other neurodegenerative processes, such as Neurofilament light (NfL) protein, tau phosphorylated at threonine 181 (p-tau181), and Glial fibrillary acidic protein (GFAP), would help better understand which circulating sterols are associated with specific diseases and brain pathologies. In addition, we have not directly measured low-density lipoprotein (LDL) cholesterol in this study, affecting data interpretation. Future studies could still, however, benefit from the results provided here and could use the Friedewald formula to estimate circulating LDL-cholesterol levels.64 Furthermore, the follow-up time was limited to five years and some alterations may be associated with cognitive decline only after a longer period. Finally, this study does not include dietary habits data. Although food intake of individual participants may affect circulating sterol levels, the cohort considered in this study is large enough that inter-participants differences should be smoothed out between groups. Therefore, differences in diet across the cohort should not affect the results presented here. Although some of the associations of sterols with cognitive decline have been previously reported in clinical settings, our study is the first to provide a comprehensive analysis of cholesterol and non-cholesterol sterols, and diet-derived plant sterols, in relation to global cognitive performance and cognitive decline in the general population. In addition, we measured these metabolites at both baseline and five-years follow up visit, which allowed us to explore how their change over time relates to the evolution of the cognitive performance. Our study furthermore considers a large array of confounders that could influence the identified associations, further strengthening the utility and relevance of circulating sterol levels for the development of prevention and early interventions in the elderly population.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| R script (version 4.2.1) | R Core Team (2022) | RRID:SCR_001905; |
Resource availability
Lead contact
Further information and requests for resources should be directed to the lead contact, Pr Julius Popp, e-mail: julius.popp@uzh.ch.
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request. Requests must be justified and will be subject to data access agreement between the parties.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and subject details
CoLaus/PsyCoLaus study design
Data for the present study stem from CoLaus/PsyCoLaus, a prospective population-based cohort-study designed to determine cardiovascular risk factors and mental disorders and their associations in the community.65,66 A total of 6734 individuals aged 35–75 years were randomly selected from the residents of the city of Lausanne, Switzerland, between 2003 and 2006 according to the civil register. The first combined physical and psychiatric follow-up evaluations of the cohort were completed between 2009-2013 and 2014–2018. At the first follow-up, in addition to the semi-structured Diagnostic Interview for Genetic Studies (DIGS;67) already conducted at baseline, comprehensive cognitive assessments were introduced for all participants aged 65 years and older.65 Accordingly, this evaluation was used as baseline assessment for the present study (Time-point 0 (TP0)) and the subsequent investigation as five-year follow-up (Time-point 1 (TP1)).
Study sample
The present analyses were based on 720 community-dwelling participants aged over 65 years at TP0 who completed the full cognitive test battery (see below) and for whom plasma samples were available. Of these participants, 343 had a Clinical Dementia Rating (CDR,68) score of 0 (considered as cognitively healthy) and 377 a CDR score of 0.5 (considered as mildly cognitively impaired). Participants with a CDR score ≥ 1 were excluded. At TP1, Mini Mental State Examination (MMSE,69) and full cognitive test data were available from 691 to 510 participants, respectively, together with plasma samples.
Method details
Cognitive measurements
A detailed neuropsychological assessment of cognitive performance at baseline (TP0) and follow up visits (TP1) including: assessment of global cognitive performance using Mini Mental State Examination (MMSE), assessment of memory using the Grober and Buschke Double Memory Test (DMT), assessment of verbal fluency using the DO40 picture-naming test, assessment of executive functions including cognitive flexibility, selective attention, cognitive inhibition, and information processing speed using the Stroop Test, and assessment of visuo-spatial construction using the figures from the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropsychological test battery was performed.70 Baseline global cognitive performance score (MMSE) was derived from the original CoLaus survey, one year before other cognitive assessments. Overall cognitive and functional status was assessed using CDR and CDR Sum of Boxes (CDR-SoB).
Biological data
Plasma concentrations of phytosterols (campesterol, campestanol, stigmasterol, sitosterol, sitostanol, brassicasterol), cholesterol, cholesterol precursors (desmosterol, lanosterol, dihydrolanosterol, lathosterol), the gut metabolite 5α-saturated cholesterol cholestanol as well as cholesterol enzymatic metabolites (24S-hydroxycholesterol, 7α-hydroxycholesterol, and 27-hydroxycholesterol) were measured at baseline (TP0) and at five-year follow up visits (TP1). Sterols and oxysterols were extracted from serum by cyclohexane after saponification. Gas chromatography flame ionization was performed with an HP6890 gas-chromatograph (Hewlett-Packard, Böblingen, Germany) using hydrogen as a carrier gas. Quantification of lathosterol, desmosterol, lanosterol, campesterol, sitosterol, 24S-OH-Chol, and 27-OH-Chol was performed using an HP5890 Series II plus gas-chromatograph combined with an HP5972 mass selective detector.37,71 Notably, this approach includes a saponification step using by cyclohexane in order to measure both free and esterified oxysterols. Plasma concentrations of triglyceride, and HDL-cholesterol was measured at TP0 at the CHUV Clinical Laboratory on fresh blood samples.65 All other measurements were obtained from fasted venous blood samples spun down at 4 °C, immediately aliquoted, and snap frozen at −80 °C until assayed.
Ethics
The Institutional Ethic’s Committee of the University of Lausanne approved the CoLaus and subsequently the PsyCoLaus study. All participants signed a written informed consent after having received a detailed description of the goal and funding of the study.
Role of funders
Funding sources had no role in the conduct or reporting of the research.
Quantification and statistical analysis
Before analysis, baseline MMSE score was transformed using ln(31-MMSE) to approach normal distribution. All values of continuous variables are shown as means and standard error (SE, the approximate standard deviation of the sample population). Means and percentages within the CDR = 0 and CDR = 0.5 groups were then compared using Mann–Whitney U-Test for continuous variables and Chi-square tests for categorical variables. The number of participants within each group is denoted n.
Concentrations of plant and endogenous sterols at baseline (TP0) and their changes over five years of follow up (TP1) were associated with cognitive performance at TP0 and decline over time (TP0 - TP1) considering possible confounders including age, sex, tobacco use, hypertension, BMI, major depressive disorder (MDD) according to DSM-IV diagnostic criteria,72 current lipid-lowering medication (statins) intake, and years of education using regression models. These approaches were performed considering two different sterol clusters separately: 1) the endogenous cholesterol cluster including total cholesterol, HDL-cholesterol, triglyceride, cholestanol, lathosterol, lanosterol, desmosterol, dihydrolanosterol, 24S-hydroxy-cholesterol, 7-α-hydroxy-cholesterol, 27-hydroxycholesterol and 2) the plant sterol cluster, including campesterol, campestanol, stigmasterol, sitosterol, sitostanol and brassicasterol. For each of these clusters, we performed linear and logistic regression to evaluate the association of the cluster at baseline with A) cognition at baseline defined by MMSE (impairment in global cognition), CDR-SoB (cognitive and related functional impairment) or CDR (cognitive-functional status), and B) cognitive decline at TP1. Decline measures were ΔMMSE, ΔCDR-SoB for linear models and ΔMMSE≥2, ΔCDR>0, and ΔCDR-SoB>0 for logistic models. For each dependent variable (i.e. cognitive impairment or cognitive decline) we constructed two different models: a parsimonious model considering age and sex as covariates and a complete model adjusted for age, sex, baseline cognitive status (CDR, only for decline models), smoking status, hypertension, diabetes, BMI, MDD, current lipid-lowering medication (statins) intake, and years of education. This approach was repeated considering changes in individual cholesterol metabolites concentration levels between TP1 and TP0 within the endogenous and phytosterol clusters as independent variables and cognitive decline at TP1 as dependent variable. All regression models were performed with R software (R Foundation for Statistical Computing, Vienna, Austria).
To determine significant association in these models we used the T-test statistic for linear regression or the Wald statistic for logistic regression models to determine whether or not there was a correlation between the response and predictor variables. The resulting pvalues were subsequently corrected for multiple comparison using the Benjamini-Hochberg method with a false discovery rate of 0 · 1. Only pvalues statistically significant (<0.05) are shown in the results Tables.
Acknowledgments
The authors wish to thank Vera van der Velpen, Dr Pedro Marques-Vidal and Dr Peter Vollenweider for their contribution. This work was funded by the Swiss National Research Foundation (320030_141179 and 320030_204886) and Synapsis Foundation—Dementia Research Switzerland (2017-PI01). The CoLaus/PsyCoLaus study was supported by research grants from GlaxoSmithKline, the Faculty of Biology and Medicine of Lausanne, the Swiss National Research Foundation [grant numbers 3200B0–105993, 3200B0-118308, 33CSCO-122661, 33CS30-139468,33CS30-148401, 33CS30_177535 and 3247730_204523]; and the Swiss Personalized Health Network [project: Swiss Aging Citizen Reference].
Author contributions
J.P. and D.L. designed the study. A.v.G. and M.P. contributed to the CoLaus/PsyCoLaus study design. E.C. contributed to the data management. C.C. and M.G. performed the data analysis. C.C., D.L., and J.P. wrote the manuscript. L.Z., A.K., E.C., A.v.G., M.P., D.L., and J.P. contributed to the data collection and critically revised the manuscript. All authors had reviewed and approved the final manuscript. J.P. is the guarantor responsible for the contents of the manuscript. The authors read and approved the final manuscript.
Declaration of interests
J.P. received consultation and speaker honoraria from Nestle Institute of Health Sciences, Innovation Campus, EPFL, Lausanne, Switzerland, Ono Pharma, Schwabe Pharma Switzerland, OM Pharma Switzerland, Roche Pharma, and from Fujirebio Europe. C.C. received consultation and speaker honoraria from OM Pharma Suisse. The other authors declare no conflicts of interest.
Published: April 24, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.106740.
Supplemental information
References
- 1.Sáiz-Vazquez O., Puente-Martínez A., Ubillos-Landa S., Pacheco-Bonrostro J., Santabárbara J. Cholesterol and Alzheimer’s disease risk: a meta-meta-analysis. Brain Sci. 2020;10:386. doi: 10.3390/brainsci10060386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rundek T., Tolea M., Ariko T., Fagerli E.A., Camargo C.J. Vascular cognitive impairment (VCI) Neurotherapeutics. 2022;19:68–88. doi: 10.1007/s13311-021-01170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Launer L.J., White L.R., Petrovitch H., Ross G.W., Curb J.D. Cholesterol and neuropathologic markers of AD: a population-based autopsy study. Neurology. 2001;57:1447–1452. doi: 10.1212/wnl.57.8.1447. [DOI] [PubMed] [Google Scholar]
- 4.Pappolla M.A., Bryant-Thomas T.K., Herbert D., Pacheco J., Fabra Garcia M., Manjon M., Girones X., Henry T.L., Matsubara E., Zambon D., et al. Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Neurology. 2003;61:199–205. doi: 10.1212/01.wnl.0000070182.02537.84. [DOI] [PubMed] [Google Scholar]
- 5.Martin M., Dotti C.G., Ledesma M.D. Brain cholesterol in normal and pathological aging. Biochim. Biophys. Acta. 2010;1801:934–944. doi: 10.1016/j.bbalip.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Power M.C., Rawlings A., Sharrett A.R., Bandeen-Roche K., Coresh J., Ballantyne C.M., Pokharel Y., Michos E.D., Penman A., Alonso A., et al. Association of midlife lipids with 20-year cognitive change: a cohort study. Alzheimers Dement. 2018;14:167–177. doi: 10.1016/j.jalz.2017.07.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benito-León J., Vega-Quiroga S., Villarejo-Galende A., Bermejo-Pareja F. Hypercholesterolemia in elders is associated with slower cognitive decline: a prospective, population-based study (NEDICES) J. Neurol. Sci. 2015;350:69–74. doi: 10.1016/j.jns.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Hughes T.M., Rosano C., Evans R.W., Kuller L.H. Brain cholesterol metabolism, oxysterols, and dementia. J. Alzheimers Dis. 2013;33:891–911. doi: 10.3233/JAD-2012-121585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tilvis R.S., Valvanne J.N., Strandberg T.E., Miettinen T.A. Prognostic significance of serum cholesterol, lathosterol, and sitosterol in old age; a 17-year population study. Ann. Med. 2011;43:292–301. doi: 10.3109/07853890.2010.546363. [DOI] [PubMed] [Google Scholar]
- 10.Popp J., Meichsner S., Kölsch H., Lewczuk P., Maier W., Kornhuber J., Jessen F., Lütjohann D. Cerebral and extracerebral cholesterol metabolism and CSF markers of Alzheimer’s disease. Biochem. Pharmacol. 2013;86:37–42. doi: 10.1016/j.bcp.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Popp J., Lewczuk P., Kölsch H., Meichsner S., Maier W., Kornhuber J., Jessen F., Lütjohann D. Cholesterol metabolism is associated with soluble amyloid precursor protein production in Alzheimer’s disease. J. Neurochem. 2012;123:310–316. doi: 10.1111/j.1471-4159.2012.07893.x. [DOI] [PubMed] [Google Scholar]
- 12.Loera-Valencia R., Goikolea J., Parrado-Fernandez C., Merino-Serrais P., Maioli S. Alterations in cholesterol metabolism as a risk factor for developing Alzheimer’s disease: potential novel targets for treatment. J. Steroid Biochem. Mol. Biol. 2019;190:104–114. doi: 10.1016/j.jsbmb.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Björkhem I., Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 2004;24:806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- 14.Kulig W., Cwiklik L., Jurkiewicz P., Rog T., Vattulainen I. Cholesterol oxidation products and their biological importance. Chem. Phys. Lipids. 2016;199:144–160. doi: 10.1016/j.chemphyslip.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J., Liu Q. Cholesterol metabolism and homeostasis in the brain. Protein Cell. 2015;6:254–264. doi: 10.1007/s13238-014-0131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lund E.G., Guileyardo J.M., Russell D.W. cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc. Natl. Acad. Sci. USA. 1999;96:7238–7243. doi: 10.1073/pnas.96.13.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lütjohann D., Breuer O., Ahlborg G., Nennesmo I., Sidén A., Diczfalusy U., Björkhem I. Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc. Natl. Acad. Sci. USA. 1996;93:9799–9804. doi: 10.1073/pnas.93.18.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu B.Y., Ma S.L., Tang N.L.S., Tam C.W.C., Lui V.W.C., Chiu H.F.K., Lam L.C.W. Cholesterol 24-hydroxylase (CYP46A1) polymorphisms are associated with faster cognitive deterioration in Chinese older persons: a two-year follow up study. Int. J. Geriatr. Psychiatry. 2009;24:921–926. doi: 10.1002/gps.2196. [DOI] [PubMed] [Google Scholar]
- 19.Li L., Zeng F., Liu Y.-H., Li H.-Y., Dong S.-Y., Peng Z.-Y., Wang Y.-J., Zhou H.-D. CYP46A1 and the APOEε4 allele polymorphisms correlate with the risk of Alzheimer’s disease. Mol. Neurobiol. 2018;55:8179–8187. doi: 10.1007/s12035-018-0952-9. [DOI] [PubMed] [Google Scholar]
- 20.Wang H.-L., Wang Y.-Y., Liu X.-G., Kuo S.-H., Liu N., Song Q.-Y., Wang M.-W. Cholesterol, 24-hydroxycholesterol, and 27-hydroxycholesterol as surrogate biomarkers in cerebrospinal fluid in mild cognitive impairment and Alzheimer’s disease: a meta-analysis. J. Alzheimers Dis. 2016;51:45–55. doi: 10.3233/JAD-150734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lütjohann D., Papassotiropoulos A., Björkhem I., Locatelli S., Bagli M., Oehring R.D., Schlegel U., Jessen F., Rao M.L., von Bergmann K., et al. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J. Lipid Res. 2000;41:195–198. doi: 10.1016/S0022-2275(20)32052-6. [DOI] [PubMed] [Google Scholar]
- 22.van den Kommer T.N., Dik M.G., Comijs H.C., Fassbender K., Lütjohann D., Jonker C. Total cholesterol and oxysterols: early markers for cognitive decline in elderly? Neurobiol. Aging. 2009;30:534–545. doi: 10.1016/j.neurobiolaging.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Jahn T., Clark C., Kerksiek A., Lewczuk P., Lütjohann D., Popp J. Cholesterol metabolites and plant sterols in cerebrospinal fluid are associated with Alzheimer’s cerebral pathology and clinical disease progression. J. Steroid Biochem. Mol. Biol. 2021;205:105785. doi: 10.1016/j.jsbmb.2020.105785. [DOI] [PubMed] [Google Scholar]
- 24.Sun M.-Y., Linsenbardt A.J., Emnett C.M., Eisenman L.N., Izumi Y., Zorumski C.F., Mennerick S. 24(S)-Hydroxycholesterol as a modulator of neuronal signaling and survival. Neuroscientist. 2016;22:132–144. doi: 10.1177/1073858414568122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanmierlo T., Bogie J.F.J., Mailleux J., Vanmol J., Lütjohann D., Mulder M., Hendriks J.J.A. Plant sterols: friend or foe in CNS disorders? Prog. Lipid Res. 2015;58:26–39. doi: 10.1016/j.plipres.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Vanmierlo T., Popp J., Kölsch H., Friedrichs S., Jessen F., Stoffel-Wagner B., Bertsch T., Hartmann T., Maier W., von Bergmann K., et al. The plant sterol brassicasterol as additional CSF biomarker in Alzheimer’s disease. Acta Psychiatr. Scand. 2011;124:184–192. doi: 10.1111/j.1600-0447.2011.01713.x. [DOI] [PubMed] [Google Scholar]
- 27.Solomon A., Kåreholt I., Ngandu T., Winblad B., Nissinen A., Tuomilehto J., Soininen H., Kivipelto M. Serum cholesterol changes after midlife and late-life cognition: twenty-one-year follow-up study. Neurology. 2007;68:751–756. doi: 10.1212/01.wnl.0000256368.57375.b7. [DOI] [PubMed] [Google Scholar]
- 28.Solomon A., Kivipelto M., Wolozin B., Zhou J., Whitmer R.A. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement. Geriatr. Cogn. Disord. 2009;28:75–80. doi: 10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He Q., Li Q., Zhao J., Wu T., Ji L., Huang G., Ma F. Relationship between plasma lipids and mild cognitive impairment in the elderly Chinese: a case-control study. Lipids Health Dis. 2016;15:146. doi: 10.1186/s12944-016-0320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svensson T., Sawada N., Mimura M., Nozaki S., Shikimoto R., Tsugane S. The association between midlife serum high-density lipoprotein and mild cognitive impairment and dementia after 19 years of follow-up. Transl. Psychiatry. 2019;9:26. doi: 10.1038/s41398-018-0336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reitz C., Tang M.-X., Schupf N., Manly J.J., Mayeux R., Luchsinger J.A. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch. Neurol. 2010;67:1491–1497. doi: 10.1001/archneurol.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reitz C., Tang M.-X., Luchsinger J., Mayeux R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch. Neurol. 2004;61:705–714. doi: 10.1001/archneur.61.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saito I., Yamagishi K., Kokubo Y., Yatsuya H., Iso H., Sawada N., Inoue M., Tsugane S. Association of high-density lipoprotein cholesterol concentration with different types of stroke and coronary heart disease: the Japan Public Health Center-based prospective (JPHC) study. Atherosclerosis. 2017;265:147–154. doi: 10.1016/j.atherosclerosis.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 34.de Chaves E.P., Narayanaswami V. Apolipoprotein E and cholesterol in aging and disease in the brain. Future Lipidol. 2008;3:505–530. doi: 10.2217/17460875.3.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee C.Y.D., Tse W., Smith J.D., Landreth G.E. Apolipoprotein E promotes β-amyloid trafficking and degradation by modulating microglial cholesterol levels. J. Biol. Chem. 2012;287:2032–2044. doi: 10.1074/jbc.M111.295451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitsche M.A., McDonald J.G., Hobbs H.H., Cohen J.C. Flux analysis of cholesterol biosynthesis in vivo reveals multiple tissue and cell-type specific pathways. Elife. 2015;4:e07999. doi: 10.7554/eLife.07999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teunissen C.E., De Vente J., von Bergmann K., Bosma H., van Boxtel M.P.J., De Bruijn C., Jolles J., Steinbusch H.W.M., Lütjohann D. Serum cholesterol, precursors and metabolites and cognitive performance in an aging population. Neurobiol. Aging. 2003;24:147–155. doi: 10.1016/s0197-4580(02)00061-1. [DOI] [PubMed] [Google Scholar]
- 38.Solomon A., Leoni V., Kivipelto M., Besga A., Oksengård A.R., Julin P., Svensson L., Wahlund L.-O., Andreasen N., Winblad B., et al. Plasma levels of 24S-hydroxycholesterol reflect brain volumes in patients without objective cognitive impairment but not in those with Alzheimer’s disease. Neurosci. Lett. 2009;462:89–93. doi: 10.1016/j.neulet.2009.06.073. [DOI] [PubMed] [Google Scholar]
- 39.Sato Y., Suzuki I., Nakamura T., Bernier F., Aoshima K., Oda Y. Identification of a new plasma biomarker of Alzheimer’s disease using metabolomics technology. J. Lipid Res. 2012;53:567–576. doi: 10.1194/jlr.M022376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feringa F.M., van der Kant R. Cholesterol and Alzheimer’s disease; from risk genes to pathological effects. Front. Aging Neurosci. 2021;13:690372. doi: 10.3389/fnagi.2021.690372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parthasarathy V., Frazier D.T., Bettcher B.M., Jastrzab L., Chao L., Reed B., Mungas D., Weiner M., DeCarli C., Chui H., et al. Triglycerides are negatively correlated with cognitive function in nondemented aging adults. Neuropsychology. 2017;31:682–688. doi: 10.1037/neu0000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harchaoui K.E.L., Visser M.E., Kastelein J.J.P., Stroes E.S., Dallinga-Thie G.M. Triglycerides and cardiovascular risk. Curr. Cardiol. Rev. 2009;5:216–222. doi: 10.2174/157340309788970315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welty F.K. How do elevated triglycerides and low HDL-cholesterol affect inflammation and atherothrombosis? Curr. Cardiol. Rep. 2013;15:400. doi: 10.1007/s11886-013-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urayama A., Banks W.A. Starvation and triglycerides reverse the obesity-induced impairment of insulin transport at the blood-brain barrier. Endocrinology. 2008;149:3592–3597. doi: 10.1210/en.2008-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi H.J., Byun M.S., Yi D., Choe Y.M., Sohn B.K., Baek H.W., Lee J.H., Kim H.J., Han J.Y., Yoon E.J., et al. Association between serum triglycerides and cerebral amyloidosis in cognitively normal elderly. Am. J. Geriatr. Psychiatry. 2016;24:604–612. doi: 10.1016/j.jagp.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Ullrich C., Pirchl M., Humpel C. Effects of cholesterol and its 24S-OH and 25-OH oxysterols on choline acetyltransferase-positive neurons in brain slices. Pharmacology. 2010;86:15–21. doi: 10.1159/000314333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staurenghi E., Cerrato V., Gamba P., Testa G., Giannelli S., Leoni V., Caccia C., Buffo A., Noble W., Perez-Nievas B.G., et al. Oxysterols present in Alzheimer’s disease brain induce synaptotoxicity by activating astrocytes: a major role for lipocalin-2. Redox Biol. 2021;39:101837. doi: 10.1016/j.redox.2020.101837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vitali C., Wellington C.L., Calabresi L. HDL and cholesterol handling in the brain. Cardiovasc. Res. 2014;103:405–413. doi: 10.1093/cvr/cvu148. [DOI] [PubMed] [Google Scholar]
- 49.Bogdanovic N., Bretillon L., Lund E.G., Diczfalusy U., Lannfelt L., Winblad B., Russell D.W., Björkhem I. On the turnover of brain cholesterol in patients with Alzheimer’s disease. Abnormal induction of the cholesterol-catabolic enzyme CYP46 in glial cells. Neurosci. Lett. 2001;314:45–48. doi: 10.1016/S0304-3940(01)02277-7. [DOI] [PubMed] [Google Scholar]
- 50.Brown J., Theisler C., Silberman S., Magnuson D., Gottardi-Littell N., Lee J.M., Yager D., Crowley J., Sambamurti K., Rahman M.M., et al. Differential expression of cholesterol hydroxylases in Alzheimer’s disease. J. Biol. Chem. 2004;279:34674–34681. doi: 10.1074/jbc.M402324200. [DOI] [PubMed] [Google Scholar]
- 51.Heverin M., Meaney S., Lütjohann D., Diczfalusy U., Wahren J., Björkhem I. Crossing the barrier: net flux of 27-hydroxycholesterol into the human brain. J. Lipid Res. 2005;46:1047–1052. doi: 10.1194/jlr.M500024-JLR200. [DOI] [PubMed] [Google Scholar]
- 52.Sandebring-Matton A., Goikolea J., Björkhem I., Paternain L., Kemppainen N., Laatikainen T., Ngandu T., Rinne J., Soininen H., Cedazo-Minguez A., et al. 27-Hydroxycholesterol, cognition, and brain imaging markers in the FINGER randomized controlled trial. Alz. Res. Therapy. 2021;13:56. doi: 10.1186/s13195-021-00790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ismail M.-A.-M., Mateos L., Maioli S., Merino-Serrais P., Ali Z., Lodeiro M., Westman E., Leitersdorf E., Gulyás B., Olof-Wahlund L., et al. 27-Hydroxycholesterol impairs neuronal glucose uptake through an IRAP/GLUT4 system dysregulation. J. Exp. Med. 2017;214:699–717. doi: 10.1084/jem.20160534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varma V.R., Büşra Lüleci H., Oommen A.M., Varma S., Blackshear C.T., Griswold M.E., An Y., Roberts J.A., O’Brien R., Pletnikova O., et al. Abnormal brain cholesterol homeostasis in Alzheimer’s disease-a targeted metabolomic and transcriptomic study. NPJ Aging Mech. Dis. 2021;7:11. doi: 10.1038/s41514-021-00064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ali Z., Heverin M., Olin M., Acimovic J., Lövgren-Sandblom A., Shafaati M., Båvner A., Meiner V., Leitersdorf E., Björkhem I. On the regulatory role of side-chain hydroxylated oxysterols in the brain. Lessons from CYP27A1 transgenic and Cyp27a1(-/-) mice. J. Lipid Res. 2013;54:1033–1043. doi: 10.1194/jlr.M034124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ye J.-Y., Li L., Hao Q.-M., Qin Y., Ma C.-S. β-Sitosterol treatment attenuates cognitive deficits and prevents amyloid plaque deposition in amyloid protein precursor/presenilin 1 mice. Korean J. Physiol. Pharmacol. 2020;24:39–46. doi: 10.4196/kjpp.2020.24.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vanmierlo T., Rutten K., van Vark-van der Zee L.C., Friedrichs S., Bloks V.W., Blokland A., Ramaekers F.C., Sijbrands E., Steinbusch H., Prickaerts J., et al. Cerebral accumulation of dietary derivable plant sterols does not interfere with memory and anxiety related behavior in Abcg5-/- mice. Plant Foods Hum. Nutr. 2011;66:149–156. doi: 10.1007/s11130-011-0219-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burg V.K., Grimm H.S., Rothhaar T.L., Grösgen S., Hundsdörfer B., Haupenthal V.J., Zimmer V.C., Mett J., Weingärtner O., Laufs U., et al. Plant sterols the better cholesterol in Alzheimer’s disease? A mechanistical study. J. Neurosci. 2013;33:16072–16087. doi: 10.1523/JNEUROSCI.1506-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones P.J., Ntanios F.Y., Raeini-Sarjaz M., Vanstone C.A. Cholesterol-lowering efficacy of a sitostanol-containing phytosterol mixture with a prudent diet in hyperlipidemic men. Am. J. Clin. Nutr. 1999;69:1144–1150. doi: 10.1093/ajcn/69.6.1144. [DOI] [PubMed] [Google Scholar]
- 60.Chu C.-S., Tseng P.-T., Stubbs B., Chen T.-Y., Tang C.-H., Li D.-J., Yang W.-C., Chen Y.-W., Wu C.-K., Veronese N., et al. Use of statins and the risk of dementia and mild cognitive impairment: a systematic review and meta-analysis. Sci. Rep. 2018;8:5804. doi: 10.1038/s41598-018-24248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoglund K., Thelen K.M., Syversen S., Sjogren M., von Bergmann K., Wallin A., Vanmechelen E., Vanderstichele H., Lutjohann D., Blennow K. The effect of simvastatin treatment on the amyloid precursor protein and brain cholesterol metabolism in patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2005;19:256–265. doi: 10.1159/000084550. [DOI] [PubMed] [Google Scholar]
- 62.Schultz B.G., Patten D.K., Berlau D.J. The role of statins in both cognitive impairment and protection against dementia: a tale of two mechanisms. Transl. Neurodegener. 2018;7:5. doi: 10.1186/s40035-018-0110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Evans M.A., Golomb B.A. Statin-associated adverse cognitive effects: survey results from 171 patients. Pharmacotherapy. 2009;29:800–811. doi: 10.1592/phco.29.7.800. [DOI] [PubMed] [Google Scholar]
- 64.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 65.Firmann M., Mayor V., Vidal P.M., Bochud M., Pécoud A., Hayoz D., Paccaud F., Preisig M., Song K.S., Yuan X., et al. The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc. Disord. 2008;8:6. doi: 10.1186/1471-2261-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Preisig M., Waeber G., Vollenweider P., Bovet P., Rothen S., Vandeleur C., Guex P., Middleton L., Waterworth D., Mooser V., et al. The PsyCoLaus study: methodology and characteristics of the sample of a population-based survey on psychiatric disorders and their association with genetic and cardiovascular risk factors. BMC Psychiatr. 2009;9:9. doi: 10.1186/1471-244X-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Preisig M., Fenton B.T., Matthey M.L., Berney A., Ferrero F. Diagnostic interview for genetic studies (DIGS): inter-rater and test-retest reliability of the French version. Eur. Arch. Psychiatry Clin. Neurosci. 1999;249:174–179. doi: 10.1007/s004060050084. [DOI] [PubMed] [Google Scholar]
- 68.Morris J.C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 69.Folstein M.F., Folstein S.E., McHugh P.R. Mini-mental state. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 70.Ouanes S., Castelao E., von Gunten A., Vidal P.M., Preisig M., Popp J. Personality, cortisol, and cognition in non-demented elderly subjects: results from a population-based study. Front. Aging Neurosci. 2017;9:63. doi: 10.3389/fnagi.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kölsch H., Heun R., Jessen F., Popp J., Hentschel F., Maier W., Lütjohann D. Alterations of cholesterol precursor levels in Alzheimer’s disease. Biochim. Biophys. Acta. 2010;1801:945–950. doi: 10.1016/j.bbalip.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 72.Bell C.C. DSM-IV: diagnostic and statistical manual of mental disorders. JAMA. 1994;272:828. doi: 10.1001/jama.1994.03520100096046. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request. Requests must be justified and will be subject to data access agreement between the parties.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.