Abstract
Patients with cerebrovascular disease (CeVD) have been shown to benefit from lipid-lowering therapies, but guideline-recommended levels of low-density lipoprotein cholesterol (LDL-C) are often not attained with statin treatment alone. The ORION-9, ORION-10, and ORION-11 trials evaluated the efficacy and safety of inclisiran in 3660 primary and secondary prevention patients with hyperlipidemia despite maximum tolerated statin treatment. This pooled post hoc analysis comprised 202 randomized patients from those trials with established CeVD who had received either 284 mg inclisiran (equivalent to 300 mg inclisiran sodium, n = 110) or placebo (n = 92) on Days 1, 90, and 6-monthly thereafter up to Day 540. At baseline, mean (SD) LDL-C was 108.4 (34.3) mg/dL and 110.5 (35.3) mg/dL in inclisiran and placebo arms, respectively. Inclisiran produced a mean (95% CI) placebo-corrected percentage change in LDL-C from baseline to Day 510 of –55.2 (–64.5 to –45.9; p < 0.0001); the corresponding time-adjusted percentage change from baseline after Day 90 and up to Day 540 was –55.2 (–62.4 to –47.9; p < 0.0001). Treatment-emergent adverse events (TEAEs) and TEAEs at the injection site, mostly mild, were more frequent with inclisiran versus placebo (82.7% vs 70.7% and 3.6% vs 0%, respectively). In patients with CeVD, twice-yearly dosing with inclisiran (after the initial and 3-month doses) in combination with maximally tolerated statins provided effective and consistent LDL-C reductions and was well tolerated.
Keywords: Inclisiran, siRNA, Cerebrovascular disease, LDL-cholesterol, Safety
1. Introduction
Hyperlipidemia is a modifiable risk factor for atherosclerotic cardiovascular disease (ASCVD) including stroke [1]. In ischemic stroke survivors, recurrence of stroke and other vascular events remains high, with the reported 5-year risk of recurrence of stroke varying between 9.5%–26% across studies [1], [2], [3], [4], [5], [6]. Statins, are the first line treatment for the management of elevated low-density lipoprotein cholesterol (LDL-C) levels, and significantly reduce the risk of cardiovascular events by 22% and stroke by 16% for every 38.7 mg/dL (1.0 mmol/L) reduction in LDL-C [7,8]. However, suboptimal long-term adherence to statins or other oral lipid-lowering therapy remains a challenge, and ∼70% patients on statins fail to achieve guideline-recommended LDL-C goals [9]. With guidelines increasingly advocating for lower LDL-C levels in secondary prevention, combination therapeutic strategies are needed when patients do not attain the specified LDL-C goals [8,9]. Monoclonal antibodies (mAbs) that inhibit proprotein convertase subtilisin/kexin type 9 (PCSK9) have been shown to provide further reduction in LDL-C levels when added to statin therapy, and further reduce the risk of future cardiovascular events including stroke but require subcutaneous injection once or twice per month [10], [11], [12], [13].
Inclisiran is a first-in-class small interfering ribonucleic acid (siRNA) therapy that specifically targets PCSK9 mRNA in the liver and reduces PCSK9 protein production. This leads to increased recycling and availability of LDL receptors on the hepatocyte cell surface, thereby decreasing plasma LDL-C [14]. The pivotal Phase 3 studies, ORION-9 (patients with heterozygous familial hypercholesterolemia [HeFH]), ORION-10 (patients with ASCVD), and ORION-11 (patients with ASCVD or ASCVD risk equivalent), comprised a total of 3660 patients, demonstrated that twice-yearly subcutaneous dosing of inclisiran (after initial doses at baseline and 3 months) resulted in significant and consistent reductions in levels of LDL-C and other atherogenic lipoproteins including lipoprotein(a) [Lp(a)], and was well tolerated [15], [16], [17].
This pooled post hoc analysis evaluated the efficacy and safety of inclisiran in a subgroup of patients with established cerebrovascular disease (CeVD) from the ORION-9, ORION-10, and ORION-11 studies.
2. Methods
2.1. Study design
The ORION-9 (NCT03397121), ORION-10 (NCT03399370), and ORION-11 (NCT03400800) trials were Phase 3, double-blind, randomized, placebo-controlled trials that evaluated the efficacy and safety of inclisiran in patients with elevated LDL-C (≥70 mg/dL for patients with ASCVD, ≥100 mg/dL for patients with ASCVD risk equivalent and those with HeFH) despite receiving maximum tolerated statin therapy. Patients were randomized 1:1 to receive 284 mg inclisiran (300 mg inclisiran sodium) or placebo on Days 1 and 90, and 6-monthly thereafter; they were followed up for 540 days (18 months). Details of the ORION-9, ORION-10, and ORION-11 studies have been published previously [15], [16], [17].
2.2. Participants
Patients who had a history of established CeVD (defined as ischemic stroke, carotid artery stenosis by angiography or ultrasound of >70%, or prior percutaneous or surgical carotid artery revascularization) were included in this post hoc analysis. History of prior hemorrhagic stroke was not a criterion for inclusion in this subgroup analysis, nor was it an exclusion criterion.
2.3. Endpoints
The co-primary efficacy endpoints included percentage change in LDL-C from baseline to Day 510 and time-adjusted percentage change in LDL-C from baseline after Day 90 and up to Day 540. Key secondary endpoints included absolute change in LDL-C from baseline to Day 510; time-adjusted absolute change in LDL-C from baseline after Day 90 and up to Day 540; percentage change from baseline in total cholesterol, non–high-density lipoprotein cholesterol (non–HDL-C), and apolipoprotein B (apoB) to Day 510 and in Lp(a) from baseline to Day 540; proportion of patients achieving prespecified LDL-C thresholds, and overall treatment-emergent adverse events (TEAEs).
2.4. Statistical analysis
The percentage change and absolute change in LDL-C from baseline to Day 510 were analyzed using an analysis of covariance model with a multiple imputation washout model for missing data. The time-adjusted percentage change in LDL-C from baseline after Day 90 and up to Day 540 was analyzed by a mixed-effects model for repeated measures (MMRM) with a control-based pattern mixture model for data imputation. Other efficacy outcomes were analyzed using an MMRM without imputation, assuming missing data are missing at random. Statistical tests were performed at a 2-sided 5% alpha level without multiplicity adjustment.
3. Results
The analysis included 202 patients (n = 110 inclisiran arm; n = 92 placebo arm) with established CeVD. Baseline demographic and clinical characteristics were generally balanced across both treatment arms (Table 1). The mean ± standard deviation (SD) age of patients was 65.1 ± 8.5 years and 64.2 ± 8.6 years, 48.2% and 45.7% of patients were female in the inclisiran and placebo arms, respectively. At baseline, 90.0% (n = 99/110) of patients in the inclisiran arm and 84.8% (n = 78/92) in the placebo arm had a history of ischemic stroke(s), 10.9% and 18.5% of patients had carotid artery stenosis, and 13.6% and 17.4% of patients had carotid revascularization, respectively (these categories were not mutually exclusive). Hypertension and diabetes were present in 83.6% and 35.5% of patients in the inclisiran arm, and 90.2% and 32.6% of patients in the placebo arm, respectively. In all, 90.9% and 88.0% of patients in the inclisiran and placebo arms were receiving statins, of whom 69.1% and 69.6% were receiving a high-intensity statin regimen. Ezetimibe was used in 5.5% and 5.4% of patients, respectively. The mean ± SD baseline LDL-C was 108.4 ± 34.3 mg/dL for patients in the inclisiran arm and 110.5 ± 35.3 mg/dL in the placebo arm (Table 1).
Table 1.
Demographics and clinical characteristics of patients with CeVD at baseline.
| Inclisiran (n = 110) | Placebo (n = 92) | |
|---|---|---|
| Age (years), mean ± SD | 65.1 ± 8.5 | 64.2 ± 8.6 |
| Female, n (%) | 53 (48.2) | 42 (45.7) |
| White race, n (%) | 94 (85.5) | 81 (88.0) |
| BMI (kg/ m2), mean ± SD | 31.0 ± 6.1 | 30.6 ± 4.8 |
| Lipid modifying therapy, n (%) | ||
| Statin | 100 (90.9) | 81 (88.0) |
| High-intensity statin | 76 (69.1) | 64 (69.6) |
| Ezetimibe | 6 (5.5) | 5 (5.4) |
| ASCVD status, n (%) | ||
| ASCVD | 110 (100.0) | 92 (100.0) |
| Medical History, n (%) | ||
| PAD | 0 (0.0) | 0 (0.0) |
| CHD | 0 (0.0) | 0 (0.0) |
| MI | 0 (0.0) | 0 (0.0) |
| CeVD | 110 (100.0) | 92 (100.0) |
| CeVD patients: Carotid artery stenosis by angiography or Ultrasound >70% | 12 (10.9) | 17 (18.5) |
| CeVD patients: Prior percutaneous or surgical carotid artery revascularization | 15 (13.6) | 16 (17.4) |
| Hypertension | 92 (83.6) | 83 (90.2) |
| CHF | 4 (3.6) | 7 (7.6) |
| Hyperlipidemia | 107 (97.3) | 85 (92.4) |
| FH | 9 (8.2) | 7 (7.6) |
| Ischemic stroke | 99 (90.0) | 78 (84.8) |
| Diabetes | 39 (35.5) | 30 (32.6) |
| Smoking | 21 (19.1) | 14 (15.2) |
| Lipid measures (mg/dL), mean ± SD | ||
| LDL-C | 108.4 ± 34.3 | 110.5 ± 35.3 |
| Total cholesterol | 191.6 ± 42.2 | 190.3 ± 41.7 |
| Non-HDL-C | 138.8 ± 40.3 | 139.1 ± 37.4 |
| HDL | 52.8 ± 17.7 | 51.3 ± 16.6 |
| ApoB | 97.0 ± 23.6 | 96.5 ± 21.8 |
| Triglycerides | 151.5 ± 80.1 | 142.4 ± 56.1 |
| Lp(a) (nmol/L), median (Q1–Q3) | 52.0 (17–157) | 31.5 (16–187) |
| PCSK9 (ng/mL) | 382.8 ± 108.8 | 369.7 ± 123.9 |
Intention-to-treat population – CeVD only subset.
ApoB, apolipoprotein B; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CeVD, cerebrovascular disease; CHF, congestive heart failure; CHD, coronary heart disease; FH, familial hypercholesterolemia; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein(a); PAD, peripheral artery disease; PCSK9, proprotein convertase subtilisin/kexin type 9; SD, standard deviation.
3.1. Efficacy outcomes
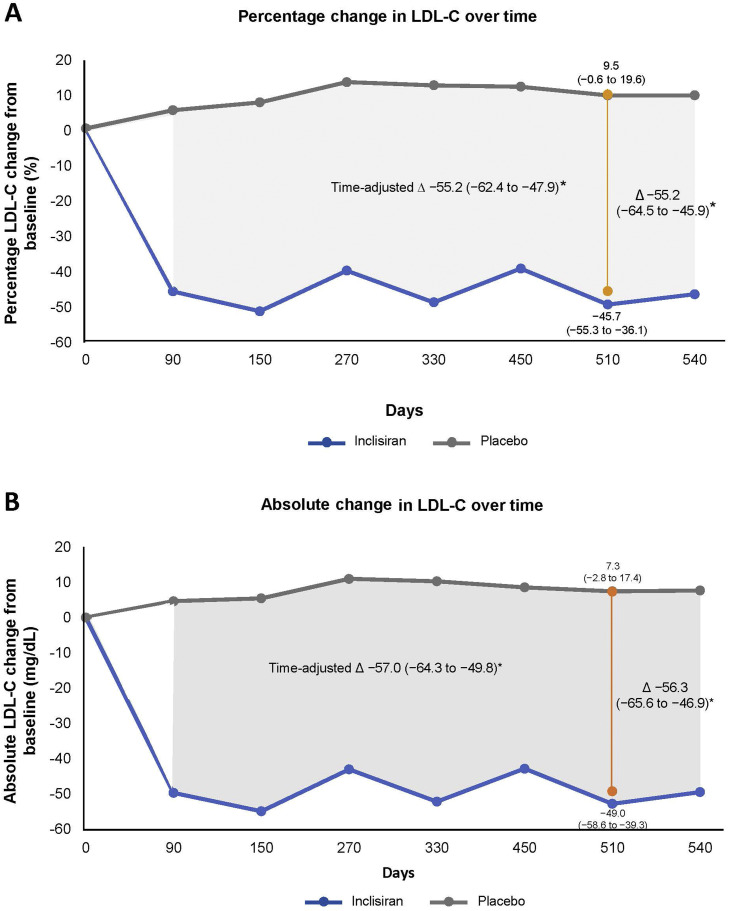
The mean percentage change in LDL-C from baseline to Day 510 in patients with CeVD was −45.7% in the inclisiran arm and 9.5% in the placebo arm, resulting in a placebo-corrected difference of –55.2% (95% confidence interval [CI]: –64.5 to –45.9; p < 0.0001; Fig. 1A). The time-adjusted percentage change in LDL-C after Day 90 and up to Day 540 was –45.6% with inclisiran and 9.6% with placebo compared with baseline (placebo-corrected difference –55.2%; 95% CI: –62.4 to –47.9; p < 0.0001; Fig. 1A).
Fig. 1.
Change in LDL-C over time. A) Percentage change in LDL-C from baseline to Day 510 (vertical line) was analyzed using ANCOVA model with a multiple imputation washout model for missing data,the time-adjusted percentage change from baseline after Day 90 and up to Day 540 was analyzed by MMRM with a controlbased pattern mixture model for data imputation (shaded gray area) Percentage change in LDL-C for post-baseline visits from Day 90 to Day 540 (gray line for placebo group and blue line for inclisiran group) is based on observed data analyzed using MMRM without imputation. (B) Absolute change in LDL-C from baseline to Day 510 (yellow line) was analyzed using ANCOVA model with a multiple imputation washout model for missing data. Absolute time-adjusted change from baseline after Day 90 and up to Day 540 (shaded gray area) and absolute change in LDL-C for post-baseline visits from Day 90 up to Day 540 (gray line for placebo group and blue line for inclisiran group) is based on observed data analyzed using MMRM without imputation assuming missing data MAR. Efficacy analyses were performed on the intention-to-treat population – CeVD only subset that included all randomized patients with established CeVD in the 3 trials. ANCOVA, analysis of covariance; CeVD, cerebrovascular disease; CI, confidence interval; LDL-C, low-density lipoprotein cholesterol; LS, least square; MMRM, mixed-effects model for repeated measures; MAR, missing at random.
*Inclisiran vs placebo, p < 0.0001.
The corresponding placebo-adjusted absolute change in LDL-C with inclisiran at Day 510 was −56.3 mg/dL (95% CI: –65.6 to –46.9 mg/dL; p < 0.0001), and the time-adjusted absolute change from baseline after Day 90 and up to Day 540 was –57.0 mg/dL (95% CI: –64.3 to −49.8 mg/dL; p < 0.0001; Fig. 1B).
Decreased levels of total cholesterol, apoB, and non–HDL-C from baseline to Day 510 and Lp(a) to Day 540 were observed in patients in the inclisiran arm compared with placebo (p < 0.0001 between-group difference for all parameters; Table 2). In the inclisiran arm, 59.4% of patients with CeVD had ≥50% LDL-C reductions from baseline at Day 510 visit (vs 3.6% in placebo); 59.1%, 70.0%, and 79.1% of patients achieved LDL-C threshold levels <50 mg/dL, <70 mg/dL, and <100 mg/dL at Day 510, respectively.
Table 2.
Percentage change in other atherogenic lipids and lipoproteins from baseline to Day 510.
| Parameter | Inclisiran (n = 110) | Placebo (n = 92) | Between-group difference* |
|---|---|---|---|
| Total cholesterol | −29.5 (−34.8, −24.1) | 3.9 (−1.8, 9.6) | −33.4 (−39.2, −27.5) |
| Non–HDL-C | −44.0 (−51.0, −37.1) | 4.7 (−2.7, 12.0) | −48.7 (−56.4, −41.0) |
| ApoB | −41.1 (−47.3, −34.9) | 3.0 (−3.6, 9.5) | −44.0 (−50.7, −37.3) |
| Triglycerides | –9.1 (–17.4, –0.9) | –7.1 (–15.9, 1.7) | –2.0 (–11.4, 7.4) |
| Lp(a)† | –16.6 (–27.0, –6.2) | 1.0 (–9.3, 11.4) | –17.6 (–25.8, –9.4) |
Inclisiran vs placebo, p < 0.0001 for all atherogenic lipids and lipoproteins except triglycerides where p = 0.6714; †Lp(a) assessed at Day 540.
Efficacy analyses were performed on the intention-to-treat population – CeVD only subset that included all randomized patients with established CeVD in the 3 trials. Data shown are LS mean (95% CI), except for Lp(a) which is median (95% CI).
ApoB, apolipoprotein B; CeVD, cerebrovascular disease; CI, confidence interval; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein(a); LS, least squares; HDL-C, high-density lipoprotein cholesterol.
3.2. Safety outcomes
A higher proportion of TEAEs were reported in patients in the inclisiran arm (82.7%; n = 91) versus placebo (70.7%; n = 65; risk ratio: 1.17; 95% CI: 1.00 to 1.37). Treatment-emergent serious adverse events (TESAE) were reported in 21 patients (19.1%) with inclisiran and 12 (13.0%) patients in placebo (risk ratio: 1.46; 95% CI: 0.76 to 2.81). None of the TESAEs were considered to be drug related in the inclisiran arm; 1 TESAE in the placebo arm was considered drug related. Overall, when assessed by system organ class or preferred term, no increase in any specific safety event was noted between the treatment arms. TEAEs leading to drug discontinuation were reported in 1 (0.9%) patient in the inclisiran arm and by none in the placebo arm; 4 (3.6%) patients in the inclisiran arm reported ≥1 clinically relevant TEAE at the injection site, all mild (n = 3) to moderate (n = 1). No TEAE of hemorrhagic stroke was reported in this subgroup of patients with CeVD. There was only one TESAE of ischemic stroke in one patient reported in the inclisiran arm and two TESAEs of lacunar stroke reported in two patients in the placebo arm.
4. Discussion
The results of this post hoc analysis highlight the beneficial effect of inclisiran in combination with statins on LDL-C levels in patients with established CeVD who require further LDL-C lowering in addition to statin. Consistent with the findings from the overall ORION-9, ORION-10, and ORION-11 trials, the subgroup of patients with established CeVD had substantial and consistent reductions in LDL-C levels with twice-yearly dosing of inclisiran (after the initial and 3-month doses) [15,16]. By Day 510, patients in the CeVD subgroup treated with inclisiran had a mean 55.2% reduction in LDL-C from baseline, compared with 50.7% for the overall pooled ORION-9, ORION-10, ORION-11 cohort [17]. The efficacy of inclisiran has also been evaluated in patients with and without polyvascular disease (PVD), and the results were similar to that observed in this study. The mean percentage reduction in LDL-C in patients with PVD and those without was 48.9% and 51.5% respectively [18]. The results of the current analysis in addition to prior published data, support the consistent effect of inclisiran in different ASCVD sub-populations. In agreement with previous findings, notable improvements in other atherogenic lipids were also observed with inclisiran in patients with CeVD.
Anti-PCSK9 mAbs bind to extracellular PCSK9 protein inhibiting its interaction with LDL receptors, while inclisiran prevents PCSK9 mRNA translation in hepatocytes and thus reduces the production of PCSK9 by the liver. Both approaches result in greater hepatic uptake of circulating LDL-C by LDL receptors [14,19]. The magnitude of LDL-C lowering achieved with inclisiran is similar to that reported with anti-PCSK9 mAbs, but with a less frequent, twice-yearly dosing regimen (after initial and 3-month doses) compared with self-injection of anti-PCSK9 mAb every 2 or 4 weeks [10,11,14,16,17,19]. Moreover, data from the Phase 3 trials and ORION-3 study show that treatment with inclisiran provides a consistent LDL-C−lowering effect sustained for 6 months per dose [16,20]. In this subset of patients with CeVD who had a mean (SD) baseline LDL-C of 108.4 (34.3) mg/dL, 59.1%, 70.0%, and 79.1% of patients achieved LDL-C thresholds of <50 mg/dl, <70 mg/dL, and <100 mg/dL, respectively, with the addition of inclisiran to maximally tolerated statin therapy.
Overall, the safety profile of inclisiran in patients with established CeVD was generally consistent with that reported for the pooled population from the Phase 3 studies, although rates of TEAEs and TSEAEs were numerically higher with inclisiran than with placebo. This finding is likely due to the lower rate of TEAEs and TSEAEs among placebo-treated individuals with CeVD (70.7%) compared to the aggregate placebo groups from the 3 trials (77.3%) [17], which in turn may be related to the relatively small size of the CeVD subgroup. TEAEs at the injection site were few and mostly mild; none were severe with inclisiran.
Limitations include the post hoc nature of this analysis, the small sample size of the subgroup of patients with established CeVD, and the lack of longer-term efficacy and safety data. Patients were not randomized according to a history of CeVD, resulting in a modest imbalance in the numbers of patients in the inclisiran versus placebo groups. Furthermore, the effect of inclisiran in lowering the risk of stroke and other cardiovascular events in these patients needs to be determined. Although the incidence of major adverse cardiovascular events was less frequent with inclisiran compared with placebo in the ORION-10 and ORION-11 trials, the analysis was exploratory [16,21,22]. The ongoing cardiovascular outcomes trials, ORION-4 NCT03705234) and VICTORION-2 Prevent (NCT05030428), will provide further insights into the long-term efficacy of inclisiran in improving cardiovascular outcomes in patients with ASCVD, including CeVD [23,24]. There is also a need for real-world data to understand the effectiveness of inclisiran in routine clinical settings.
In conclusion, in addition to maximally tolerated statin therapy, twice-yearly dosing with inclisiran (after the initial and 3-month doses) is an effective option for LDL-C lowering and is well tolerated in adults with established CeVD.
Author contributions
The lead author (WK) directed the analysis and manuscript development, and all authors contributed to its revision and concurred with the decision to submit the final manuscript for publication. Jackie Han was responsible for the statistical analysis.
Funding
This study was funded by Novartis Pharmaceuticals AG, Basel, Switzerland.
Declaration of Competing Interest
Wolfgang Koenig reports receiving consulting fees and lecture fees from AstraZeneca, Novartis, and Amgen; consulting fees from Pfizer, The Medicines Company, DalCor Pharmaceuticals, Kowa, Corvidia Therapeutics, Esperion, Genentech, OMEICOS, Novo Nordisk, LIB Therapeutics, TenSixteen Bio, New Amsterdam Pharma and Daiichi Sankyo; lecture fees from Berlin-Chemie, Bristol-Myers Squibb, and Sanofi; and grant support and provision of reagents from Singulex, Abbott, Roche Diagnostics, and Dr Beckmann Pharma. Kausik K. Ray reports grants from Amgen, Sanofi, Daiichi Sankyo, Regeneron, and Pfizer; consulting fees from Daiichi Sankyo, Silence Therapeutics, Novartis, SCRIBE, CRISPR, VAXXINITY, Cargene, Novo Nordisk, Sanofi, Astra Zeneca, Eli Lilly, Silence Therapeutics, Resverlogix, New Amsterdam, Kowa, Bayer, Amarin, and Esperion; and personal fees from Novartis, Amgen, Viatris, Novo Nordisk, Boehringer Ingelheim, Daiichi Sankyo, Kyrka, and Astra Zeneca. Ulf Landmesser reports grants from Novartis; consulting fees from Novartis, Amgen, Sanofi, and Bayer; and personal fees from Novartis, Amgen, Sanofi, Bayer, and Daiichi Sankyo Lawrence A. Leiter has received research funding from, has provided CME on behalf of, and/or has acted as an advisor to Amarin, Amgen, AstraZeneca, Esperion, HLS, Kowa, Merck, Novartis, Pfizer, and Sanofi. Gregory G. Schwartz reports receiving research support paid to his institution from AstraZeneca, Resverlogix, Sanofi, Silence Therapeutics and The Medicines Company and a pending US patent (62/806,313, Methods for Reducing Cardiovascular Risks) assigned in full to the University of Colorado R. Scott Wright report consulting fees from Boehringer Ingelheim. Lorena Garcia Conde and Jackie Han are employees of Novartis. Frederick J. Raal reports receiving advisory board fees and lecture fees from Amgen, Sanofi-Aventis, Regeneron Pharmaceuticals, Novartis and LIB Therapeutics.
Acknowledgments
Scientific writing and editorial assistance in the development of this manuscript was provided by Shivani Vadapalli (Novartis Healthcare Pvt. Ltd., Hyderabad, India) and Aisling Towell (Novartis Ireland Ltd., Dublin, Ireland), in accordance with Good Publication Practice (GPP4) 2022 guidelines. The authors also thank Klaus Molle of Novartis Pharma AG, Basel, Switzerland for his scientific review of the manuscript.
References
- 1.Virani S.S., Alonso A., Aparicio H.J., Benjamin E.J., Bittencourt M.S., Callaway C.W., et al. Heart disease and stroke statistics-2021 Update: a Report from the American heart association. Circulation. 2021;143(8):e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.Amarenco P., Lavallee P.C., Monteiro Tavares L., Labreuche J., Albers G.W., Abboud H., et al. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378(23):2182–2190. doi: 10.1056/NEJMoa1802712. [DOI] [PubMed] [Google Scholar]
- 3.Flach C., Muruet W., Wolfe C.D.A., Bhalla A., Douiri A. Risk and secondary prevention of stroke recurrence: a population-base cohort study. Stroke. 2020;51(8):2435–2444. doi: 10.1161/STROKEAHA.120.028992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohan K.M., Wolfe C.D., Rudd A.G., Heuschmann P.U., Kolominsky-Rabas P.L., Grieve A.P. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke. 2011;42(5):1489–1494. doi: 10.1161/STROKEAHA.110.602615. [DOI] [PubMed] [Google Scholar]
- 5.Rucker V., Heuschmann P.U., O'Flaherty M., Weingartner M., Hess M., Sedlak C., et al. Twenty-year time trends in long-term case-fatality and recurrence rates After ischemic stroke stratified by etiology. Stroke. 2020;51(9):2778–2785. doi: 10.1161/STROKEAHA.120.029972. [DOI] [PubMed] [Google Scholar]
- 6.Zhao W., Wu J., Liu J., Wu Y., Ni J., Gu H., et al. Trends in the incidence of recurrent stroke at 5 years after the first-ever stroke in rural China: a population-based stroke surveillance from 1992 to 2017. Aging. 2019;11(6):1686–1694. doi: 10.18632/aging.101862. (Albany NY) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cholesterol Treatment Trialists C., Baigent C., Blackwell L., Emberson J., Holland L.E., Reith C., et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 9.Ray K.K., Molemans B., Schoonen W.M., Giovas P., Bray S., Kiru G., et al. EU-Wide cross-sectional observational study of lipid-modifying therapy use in secondary and primary care: the DA VINCI study. Eur J Prev Cardiol. 2021;28(11):1279–1289. doi: 10.1093/eurjpc/zwaa047. [DOI] [PubMed] [Google Scholar]
- 10.Giugliano R.P., Pedersen T.R., Saver J.L., Sever P.S., Keech A.C., Bohula E.A., et al. Stroke prevention with the PCSK9 (Proprotein Convertase Subtilisin-Kexin Type 9) inhibitor evolocumab added to statin in high-risk patients with stable atherosclerosis. Stroke. 2020;51(5):1546–1554. doi: 10.1161/STROKEAHA.119.027759. [DOI] [PubMed] [Google Scholar]
- 11.Guedeney P., Giustino G., Sorrentino S., Claessen B.E., Camaj A., Kalkman D.N., et al. Efficacy and safety of alirocumab and evolocumab: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J. 2019 doi: 10.1093/eurheartj/ehz430. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz G.G., Bessac L., Berdan L.G., Bhatt D.L., Bittner V., Diaz R., et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J. 2014;168(5):682–689. doi: 10.1016/j.ahj.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz G.G., Steg P.G., Szarek M., Bhatt D.L., Bittner V.A., Diaz R., et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 14.Kosmas C.E., Munoz Estrella A., Skavdis A., Pena Genao E., Martinez I., Guzman E. Inclisiran for the treatment of cardiovascular disease: a short review on the emerging data and therapeutic potential. Ther Clin Risk Manag. 2020;16:1031–1037. doi: 10.2147/TCRM.S230592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raal F.J., Kallend D., Ray K.K., Turner T., Koenig W., Wright R.S., et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;382(16):1520–1530. doi: 10.1056/NEJMoa1913805. [DOI] [PubMed] [Google Scholar]
- 16.Ray K.K., Wright R.S., Kallend D., Koenig W., Leiter L.A., Raal F.J., et al. Two Phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382(16):1507–1519. doi: 10.1056/NEJMoa1912387. [DOI] [PubMed] [Google Scholar]
- 17.Wright R.S., Ray K.K., Raal F.J., Kallend D.G., Jaros M., Koenig W., et al. Pooled patient-level analysis of inclisiran trials in patients with familial hypercholesterolemia or atherosclerosis. J Am Coll Cardiol. 2021;77(9):1182–1193. doi: 10.1016/j.jacc.2020.12.058. [DOI] [PubMed] [Google Scholar]
- 18.Koenig, W., L.G. Conde, U. Landmesser, L.A. Leiter, K.K. Ray, G.G. Schwartz, et al., Efficacy and safety of inclisiran in patients with polyvascular disease: pooled, post hoc analysis of the ORION-9, ORION-10, and ORION-11 Phase 3 randomized controlled trials. Cardiovasc Drugs Ther 2022. DOI: 10.1007/s10557-022-07413-0. [DOI] [PMC free article] [PubMed]
- 19.Nishikido T., Ray K.K. Inclisiran for the treatment of dyslipidemia. Expert Opin Investig Drugs. 2018;27(3):287–294. doi: 10.1080/13543784.2018.1442435. [DOI] [PubMed] [Google Scholar]
- 20.Ray K.K., Troquay R.P.T.V., LA Leiter F.L.J., Wright S.V., Talloczy S., Zang Z.X., Maheux P., et al. Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated low-density lipoprotein cholesterol (ORION-3): results from the 4-Year open-label extension of the ORION-1 Trial. Lancet. 2022 doi: 10.1016/S2213-8587(22)00353-9. https://ssrn.com/abstract=4252675 DOI: Available at SSRN: [DOI] [PubMed] [Google Scholar]
- 21.Ray K.K., Kallend D., Leiter L.A., Raal F.J., Koenig W., Jaros M.J., et al. Effect of inclisiran on lipids in primary prevention: the ORION-11 trial. Eur Heart J. 2022 doi: 10.1093/eurheartj/ehac615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ray K.K., Raal F.J., Kallend D.G., Jaros M.J., Koenig W., Leiter L.A., et al. Inclisiran and cardiovascular events: a patient-level analysis of phase III trials. Eur Heart J. 2023;44(2):129–138. doi: 10.1093/eurheartj/ehac594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ClinicalTrial.gov. A randomized trial assessing the effects of inclisiran on clinical outcomes among people with cardiovascular disease (ORION-4). [Accessed September.2022]; Available from: https://clinicaltrials.gov/ct2/show/NCT03705234.
- 24.ClinicalTrial.gov. Study of inclisiran to prevent cardiovascular (CV) events in participants with established cardiovascular disease (VICTORION-2P). [Accessed September.2022]; Available from: https://clinicaltrials.gov/ct2/show/NCT05030428?term=VICTORION-2+Prevent&draw=2&rank=1.