Abstract
We report a case of a patient with Li Fraumeni Syndrome (LFS) who developed synchronous EGFR exon deletion 19 and EGFR exon 20 insertion NSCLC and characterize the diagnostic and therapeutic challenges in managing her care. Osimertinib was effective in the EGFR deletion 19 population but did not garner a response in the EGFR exon 20 insertion population, which was treated definitively with surgical resection. At the time of oligoprogression, she underwent surgical resection, and radiation therapy was minimized. The biologic link between LFS and EGFR mutation remains unclear, and using larger, real-world cohorts could help to clarify the relationship between LFS and EGFR-mutant NSCLC.
Keywords: Li Fraumeni Syndrome, EGFR mutations, NSCLC, Case report
Introduction
Germline mutations in the TP53 TSG are the defining feature of the cancer predisposition condition known as Li Fraumeni syndrome (LFS).1 Lung cancer is a component of the LFS tumor spectrum with a risk of 2% to 7%, though the clinical phenotype of these lung cancers is not well described.2 Though data are limited, most patients with LFS who develop lung cancer are younger and of the female sex, and reports describe a high incidence of somatic EGFR mutations, with an occurrence rate of up to 40% in one report.1
EGFR mutations are among the most frequent actionable driver mutations seen in NSCLC, with variations in prevalence by ethnicity.3 EGFR exon 19 deletions (del19) are the most common sensitizing mutations, followed by EGFR exon 21 mutations; less common are activating EGFR exon 20 insertions.3 Although EGFR del19 and L858R mutations predict sensitivity to EGFR tyrosine kinase inhibitors (TKIs) such as osimertinib, EGFR exon 20 insertions are relatively insensitive to osimertinib and other standard EGFR TKIs.4 Here, we report a case of a patient with LFS who developed synchronous EGFR del 19 and EGFR exon 20 insertion NSCLC.
Case Presentation
A 54-year-old woman with a history of hormone receptor–positive/HER2-negative bilateral breast cancer treated with bilateral mastectomy in 2016 and a diagnosis of LFS by means of a de novo germline TP53 mutation presented to the clinic after a screening computed tomography (CT) of the chest in March 2019 revealed asymptomatic, right upper lobe (RUL) and left upper lobe (LUL) lung masses (Fig. 1). A magnetic resonance imaging of the brain was performed, which did not reveal any metastases; a positron emission tomography - CT was also done, which revealed a 2.5 cm RUL mass with an enlarged right hilar lymph node, a contralateral 1.9 cm LUL mass, and a left seventh rib metastasis—all of which were hypermetabolic (Fig. 2). Both the RUL and LUL lesions were confirmed to be lung adenocarcinoma by biopsy. However, through next-generation sequencing by means of FoundationOne (Foundation Medicine, Inc., Cambridge, MA), it was found that the RUL lesion had an EGFR del19 mutation, whereas the LUL lesion was noted to have had an EGFR exon 20 insertion (p.P772_H773 duplication) detected by NeoGenomics (NeoGenomics Laboratories, Inc., Fort Myers, FL). FoundationOne testing of the RUL biopsy also detected co-occurring mutations including MAP2K4 S5I, MYC S326F, TP53 G245S, EGFR amp, and RB1 loss. Circulating tumor DNA (ctDNA) with Guardant360 (Guardant, Redwood City, CA) revealed the known germline TP53 G245S with nearly a 50% allelic fraction. In May 2019, she began osimertinib 80 mg and tolerated therapy well. There was a considerable reduction in the size of the RUL mass and adenopathy but no change in the LUL lung mass (Fig. 3A–D). She then underwent an LUL lobectomy in July 2019, and pathological findings identified the EGFR exon 20 insertion by means of Caris MiSeq (Caris Life Sciences, Irving, TX). She continued osimertinib to treat the right-sided lesion and her nodal and osseous metastases. She had an ongoing response for more than 2 years until she developed oligoprogression in the RUL. ctDNA analysis with Guardant360 again revealed only the germline TP53 G245S. With concern about radiation risks in the context of LFS, she underwent an RUL lobectomy in December 2021. Caris MiSeq from the resected specimen confirmed EGFR del19 and the known TP53 mutation with no acquired alterations and no histologic shift. She remains on osimertinib with ongoing disease control.
Figure 1.
Timeline of patient’s clinical course. The figure displays the patient’s clinical course incorporating imaging, treatment, and pathologic and molecular data. CT, computed tomography; ctDNA, circulating tumor DNA; del 19, exon 19 deletion; LUL, left upper lobe; PET, positron emission tomography; PR, partial response; RUL, right upper lobe; VAF, variant allele frequency.
Figure 2.
FDG-Whole Body PET/CT. PET/CT revealed a 2.5 cm RUL mass (SUV max 11.5) with an enlarged right hilar lymph node (SUV max 9.5), a contralateral 1.9 cm LUL mass (SUV max 4.2), and a left seventh rib metastasis (SUV max 4.1). CT, computed tomography; FDG, fluorodeoxyglucose; LUL, left upper lobe; PET, positron emission tomography; RUL, right upper lobe; SUV max, maximum standardized uptake value.
Figure 3.
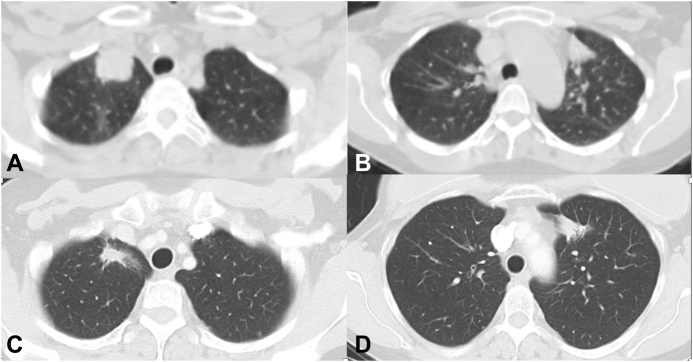
CT chest with contrast. (A) Pretreatment scan before osimertinib revealing mass in the right upper lobe of the lung. (B) Pretreatment scan before osimertinib revealing mass in the left upper lobe of the lung. (C) Posttreatment scan after osimertinib revealing a good response in the right upper lobe of the lung. (D) Posttreatment scan after osimertinib revealing no change in the left upper lobe of the lung. CT, computed tomography.
Discussion
Previous reports revealed that patients with LFS and advanced lung cancer have a higher frequency of associated somatic mutations compared with the general NSCLC population, with EGFR mutation rates of 40% to 45%.1,4,5 In one study, 90% of LFS-associated lung tumors (19 of 21) had somatic oncogenic drivers, in which 18 cases were EGFR-mutated with one patient having a ROS1 fusion.5 In contrast to those with concurrent somatic TP53 and EGFR mutations, patients with germline TP53 and somatic EGFR mutations still benefit from EGFR TKIs, such as osimertinib.5 Observational studies suggest TP53 genetic carriers act permissively, requiring somatic driver alterations such as EGFR to induce oncogenesis.4,5
Whereas ctDNA is not validated yet for germline analysis, studies have found that variant allele frequency (VAF) can be useful in determining germline versus somatic status.6 Heterozygous germline variants often have a VAF of approximately 50% or 100% when homozygous or owing to loss of heterozygosity. After birth, somatic changes are acquired, and their VAF is typically less than 50%. However, such high VAF values prompt a need for confirmational germline testing.
In the case presented, an EGFR mutation was found in the context of NSCLC and LFS. Two different EGFR mutations in two synchronous tumors were identified. Whereas the underlying biology of this association is not known, patients with co-occurring somatic EGFR and germline TP53 mutations present unique challenges in disease management, playing a role in surveillance and treatment. The expected tumor harboring the EGFR exon 20 insertion mutation did not respond to osimertinib. We elected for surgical lobectomy to minimize radiation exposure with a relatively high risk of secondary malignancies in patients with LFS. Though localized radiation was an option, the evidence of forgoing such exposure in LFS remains controversial, especially in later stages in which it is a standard of care and would be warranted.5 Moreover, for similar reasons, we used serial CT monitoring judiciously to minimize radiation exposure. Our study has limitations inherent to case reports, including sample size and retrospective design, among several others. There remains an unmet need regarding the optimal management of patients with LFS and somatic EGFR mutations, and additional studies using larger, real-world cohorts would be of benefit.
Conclusions
There is a clear but poorly described connection between LFS and NSCLC harboring a somatic EGFR mutation. The precise pathway is not clear but may be incredibly important in establishing the origin of these non–smoking-related lung cancers. Here, we describe a case of two synchronous EGFR lung cancers in a patient with LFS and provide insight into the unique challenges of managing this population, including exposure to ionizing radiation. A more devoted study of this link may be of benefit to understanding the relationship between LFS and the development of EGFR-mutant NSCLC.
CRediT Authorship Contribution Statement
Jennifer A. Marks: Conceptualization, Writing - original draft, Visualization, Writing - review & editing.
Stephen V. Liu: Conceptualization, Writing - review & editing, Visualization, Supervision.
Informed Consent
Informed consent was obtained from the patient before the submission of this article.
Footnotes
Disclosure: Dr. Marks has received honoraria from Targeted Healthcare Communications and Curio Science and travel awards from IDEOlogy Health, IASLC, and Creative Educational Concepts. Dr. Liu serves on the advisory board as a consultant for the following organizations: Amgen, AbbVie, AstraZeneca, Bristol-Myers Squibb, Catalyst, Daiichi Sankyo, Eisai, Elevation Oncology, Genentech/Roche, Gilead, Guardant Health, Janssen, Jazz Pharmaceuticals, Merck, Merus, Novartis, Regeneron, Sanofi, Takeda, and Turning Point Therapeutics; receives research grant funding from the following institutions: Alkermes, Bristol-Myers Squibb, Elevation Oncology, Genentech, Gilead, Merck, Merus, Nuvalent, Pfizer, RAPT, and Turning Point Therapeutics; and serves on the Data Safety Monitoring Board for Candel Therapeutics.
Cite this article as: Marks JA, Liu SV. NSCLC with synchronous EGFR mutations in Li Fraumeni syndrome: a case report. JTO Clin Res Rep. 2023;4:100521.
References
- 1.Kerrigan K., Chan J., Vagher J., et al. Lung cancer in Li-Fraumeni syndrome. JCO Precis Oncol. 2021;5:552–556. doi: 10.1200/PO.20.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McBride K.A., Ballinger M.L., Killick E., et al. Li-Fraumeni syndrome: cancer risk assessment and clinical management. Nat Rev Clin Oncol. 2014;11:260–271. doi: 10.1038/nrclinonc.2014.41. [DOI] [PubMed] [Google Scholar]
- 3.Melosky B., Kambartel K., Häntschel M., et al. Worldwide prevalence of epidermal growth factor receptor mutations in non-small cell lung cancer: a meta-analysis. Mol Diagn Ther. 2022;26:7–18. doi: 10.1007/s40291-021-00563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang W., Huang Y., Hong S., et al. EGFR exon 20 insertion mutations and response to osimertinib in non-small-cell lung cancer. BMC Cancer. 2019;19:1–9. doi: 10.1186/s12885-019-5820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mezquita L., Jové M., Nadal E., et al. High prevalence of somatic oncogenic driver alterations in patients with NSCLC and Li-Fraumeni syndrome. J Thorac Oncol. 2020;15:1232–1239. doi: 10.1016/j.jtho.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Veyseh M., Ricker C., Espenschied C., Raymond V., D’Souza A., Barzi A. Secondary germline finding in liquid biopsy of a deceased patient; case report and review of the literature. Front Oncol. 2018;8:259. doi: 10.3389/fonc.2018.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]