Abstract
Latent tuberculosis infection (LTBI) constitutes an important public health problem because of risk of progression to TB disease. Effective treatment of multi-drug resistant (MDR) LTBI would prevent progression to MDR TB disease, which would improve patient and public health outcomes. The majority of MDR LTBI treatment studies have focused on the use of fluoroquinolone-based antibiotic regimens. Options for and experience in the treatment of fluoroquinolone-resistant MDR LTBI are limited in the published literature and not comprehensively addressed in current guidelines. In this review, we share our experience with the treatment of fluoroquinolone-resistant MDR LTBI with linezolid. We discuss treatment options for MDR TB that provide context for predicting effective MDR LTBI treatment, with a focus on the microbiologic and pharmacokinetic properties of linezolid that support its use. We then summarize the evidence for treatment of MDR LTBI. Finally, we present our experiences treating fluoroquinolone-resistant MDR LTBI with linezolid with an emphasis on dosing considerations to optimize efficacy and minimize potential toxicities.
Keywords: Latent tuberculosis infection, Multidrug-resistant tuberculosis, Linezolid
1. Introduction
Mycobacterium tuberculosis is a major cause of morbidity and mortality worldwide and is the leading cause of death from a single infectious agent. In 2020, an estimated 1.3 million persons not infected with HIV died of tuberculosis (TB) worldwide, and an additional 214,000 persons died among those co-infected with HIV [1]. With one-fourth of the world’s population estimated to have latent TB infection (LTBI) and 5%–10% of infected persons expected to become ill with TB disease, treatment of LTBI to prevent TB disease is a critical component of the World Health Organization (WHO) End TB Strategy and is increasingly recognized as an important strategy to accelerate the progress towards TB elimination [2], [3], [4], [5]. Approximately 13 million people in the United States have LTBI, and untreated LTBI accounts for approximately 80% of U.S. incident TB cases, highlighting the importance of treatment of LTBI in the United States [6], [7]. Multidrug resistant LTBI (MDR LTBI) also represents a growing public health concern, with recent modelling estimating more than 19 million people with MDR LTBI worldwide and children <15 years old twice as likely to have MDR LTBI [8].
The decision to treat LTBI must consider both treatment efficacy and medication toxicity, balancing the benefits of avoiding disease progression with the risk of adverse effects stemming from treatment. For drug-susceptible LTBI, the balance has long favored treatment, with the Centers for Disease Control and Prevention (CDC), American Thoracic Society (ATS), and National TB Controllers’ Association recommending treatment to reduce the risk of reactivation and subsequent TB transmission since the CDC’s published guideline in 2000 [9], [10]. More recently, studies have also demonstrated the benefits of treating MDR LTBI, both in terms of reduction of reactivation rates as well as cost effectiveness [11], [12], [13]. Relying on the growing evidence and expert opinion, CDC, ATS, Infectious Diseases Society of America, and European Respiratory Society in 2019 updated guidelines with the recommendation to treat MDR LTBI with a fluoroquinolone-based regimen [14]. Additionally, the 2020 WHO guidelines provide a conditional recommendation for fluoroquinolone-based regimens for household contacts and people living with HIV [15]. While these updated guidelines provide further clarity for the treatment of MDR LTBI, little evidence is available to guide the treatment of fluoroquinolone-resistant MDR LTBI. To address this gap in guidance, a careful consideration of drugs with high efficacy against MDR TB can help in proposing regimens for treatment of MDR LTBI.
2. Regimens for treating multidrug-resistant TB
MDR TB is caused by infection with a Mycobacterium tuberculosis strain that is resistant to at least isoniazid and rifampicin [16]. In addition to the complexity introduced by potentially resistant organisms, TB treatment is challenging because of the drug tolerance of nonreplicating M. tuberculosis, responsible for the lengthened treatment course needed for effective cure. The addition of antimicrobials with bactericidal activity against sporadically dividing mycobacteria (i.e., sterilizing effect) such as pyrazinamide or the rifamycins has led to shortened duration of treatment for drug-susceptible TB disease [17], [18]. For MDR TB, second-line therapies that provide this sterilizing effect are attractive options to improve the likelihood of therapeutic success. Consistent with this distinction, WHO re-categorized the second-line drugs based on efficacy and safety in 2016 and 2018 (Group A, B, and C) [19]. These recommendations placed bedaquiline, levofloxacin, moxifloxacin, and linezolid in Group A, drugs with the highest level of efficacy and safety in MDR TB treatment.
Multiple studies have demonstrated the importance of the Group A drugs in shortening MDR TB treatment. With the NixTB and subsequent ZeNix trials, patients were given 6–9 months of an oral treatment regimen with bedaquiline, pretomanid, and linezolid (BPaL) with improved efficacy and tolerability compared to previous regimens [20], [21]. The TB Practecal trial, which combined BPaL plus moxifloxacin, was terminated early because superiority to the control regimen was evident early in the trial [22]. Additionally, a 6-month regimen containing bedaquiline, levofloxacin, and linezolid plus 2 other second line drugs was shown to be as effective as other regimens used for 18–24 months [23]. More recently, the results of the STREAM 2 trial demonstrated that bedaquiline-containing regimens had superior efficacy compared to the 9-month injectable-containing (STREAM 1) regimen [24]. Delamanid-containing regimens for MDR TB are also associated with high treatment-success rates [25]. Together, these results highlight that drugs with sterilizing effect can shorten TB regimens while maintaining efficacy and minimizing toxicity.
3. Linezolid in MDR TB treatment
Linezolid, an oxazolidinone antimicrobial developed for its activity against drug-resistant isolates of staphylococci, streptococci, and enterococci, inhibits protein synthesis of bacteria by interfering with translation. Linezolid binds to a site on the bacterial 23S ribosomal RNA of the 50S subunit, which prevents the formation of a functional 70S initiation complex [26]. Linezolid also has in vitro activity against M. tuberculosis including MDR TB isolates resistant to fluoroquinolones and rifabutin [27] as well as activity in murine models of treatment [28]. Consistent with this experimental susceptibility, clinical efficacy with approximately 80% cure rate has been achieved in regimens including linezolid for MDR TB management [29], [30], [31], [32]. Linezolid also inhibits mitochondrial protein synthesis, which is the proposed etiology of its toxicity [33].
During infection, M. tuberculosis shifts between a variety of metabolic states. Three of the most well studied are the log-growth phase, the acid-growth phase, and the nonreplicative-persister phenotype. Log-phase M. tuberculosis has the most rapid replication rate, while acid-phase organisms have a prolonged doubling time. Nonreplicative-persister (NRP)-phenotype M. tuberculosis doubles very slowly or not at all [34]. These phases have varying degrees of susceptibility to antibiotics, and the ability of linezolid to achieve effective antimicrobial activity in these metabolic states has been assessed experimentally. First, activity against rapidly dividing M. tuberculosis can be estimated by measurement of early bactericidal activity (EBA) or by the fall in M. tuberculosis counts per mL sputum per day [35]. In a study monitoring quantitative sputum cultures of 30 patients treated with linezolid monotherapy, linezolid was shown to have moderate EBA from days 0 to 2, supporting bactericidal activity against rapidly growing TB bacilli with good permeation into TB cavities (34). In this same study, linezolid was shown to have minimal extended EBA from days 2 to 7, a property suggested to predict sterilizing capacity in infected tissues. However, the ability to predict sterilizing activity from EBA at days 2–7 has limitations as demonstrated by the low extended EBA of pyrazinamide, a drug with demonstrable sterilizing activity [36]. The bactericidal activity of linezolid against acid growth phase and the non-replicative persister phenotype has been demonstrated using an in vitro hollow fiber infection model. During in vitro acid growth phase, linezolid demonstrated bactericidal activity over a range of tested doses (33). Furthermore, in a streptomycin auxotroph model of nonreplicative persistence, linezolid was still active, demonstrating bactericidal activity at a variety of concentrations [34].
Linezolid has low protein binding and is highly bioavailable in the bronchial mucosa and alveolar lining [37], [38]. The minimum inhibitory concentrations (MICs) against clinical M. tuberculosis isolates are persistently low and an MIC of 1 mg/L is considered to represent the clinical susceptibility breakpoint. [39], [40], [41]. For other pathogens, linezolid activity is sensitive to time and accumulation, and favorable pharmacodynamics of linezolid can be predicted by measuring the time above the MIC (T > MIC) as well as the area under the curve divided by MIC (AUC/MIC). In a murine infection model, both AUC/MIC and T > MIC predicted linezolid efficacy [42]. Prior modeling of optimal linezolid dosing for treatment of TB has used an AUC/MIC of >119 as the pharmacokinetic/pharmacodynamic target [43]. However, separate experiments using an in vitro hollow fiber model have demonstrated sterilizing effect at lower AUC/MIC levels [44]. While the optimal dosing of linezolid for treatment of LTBI and TB disease has not been validated clinically, these in vitro and in silico modeling studies indicate an attainable therapeutic window for linezolid in treating M. tuberculosis infections.
Linezolid is a promising candidate for treating MDR LTBI because of its microbiological and pharmacokinetic properties and its clinical efficacy as a component of combination therapy for MDR TB. However, long term use results in toxicities such as peripheral and optic neuropathy and myelosuppression [32], [45], and can affect up to a third of patients [45] in a dose-dependent manner [43]. Measurement of linezolid trough concentrations has been proposed as a strategy for lessening these adverse events, with target trough serum drug concentration less than 2 mg/L [46]. However, recent retrospective analysis from a cohort of patients with MDR TB treated with linezolid 600 mg daily did not show a significant association between trough concentration and neurotoxicity [45], although the authors of this study did note that the sample size was not adequately powered to identify differences. Furthermore, a notable proportion of these patients had relevant comorbidities including Hepatitis C, alcohol dependency, and ethambutol use which may have contributed to neurotoxicity.
Reducing the dose of linezolid can also be used to prevent toxicity; however, sufficient concentrations are required for antitubercular activity. The dosing of linezolid 600 mg BID or 1200 mg daily is based on the registered indication for treatment of gram-positive bacterial infections. However, measurement of EBA at 0–2 days is similar when comparing linezolid 600 mg given once and twice daily, which suggests similar microbiological effect in this context [47]. For the treatment of MDR TB, once-daily dosing of linezolid as a part of combination chemotherapy for MDR TB was used with good results in limiting toxicity [21]. Further efforts to optimize the dose of linezolid to promote efficacy and limit toxicity have been employed in in vitro hollow fiber infection models and pharmacokinetic/pharmacodynamic modeling. In general, these studies have predicted dose dependent toxicity that is more likely to develop with dosing above 900 mg daily as well as decreasing efficacy as dosing decreases below 600 mg daily [29], [43], [44]. Data on this topic are sparse, but some evidence suggests that even lower doses of linezolid may still be effective. Modeling from Srivastava et al. predicted that doses of 300 mg daily had high probability of target attainment to a MIC of 0.5 mg/L [44]. Furthermore, Drusano et al. demonstrated enhanced killing of M. tuberculosis within the hollow fiber infection model system with 600 mg of linezolid every other day compared to 300 mg daily, both at acid growth phase and in a model of nonreplicative persistence [34]. Finally, pharmacodynamic studies in a murine mouse model did demonstrate preserved activity with intermittent dosing as infrequent as three times weekly during the chronic phase of infection, which indicated that intermittent dosing of linezolid may be yet another strategy to limit toxicity while maintaining efficacy [42]. Consistent with this PD/PD modelling of low and intermittent linezolid dosing, a case series of patients with MDR TB treated with regimens containing linezolid demonstrated superior efficacy and decreased toxicity at low (300 mg daily) or intermittent (600 mg less than 5 times per week) dosing compared to 600 mg daily dosing [48].
4. Current MDR LTBI treatment regimens
Treatment of MDR LTBI is recommended to prevent progression to TB disease and subsequent TB transmission. Treatment is supported by studies demonstrating the benefits of treatment of MDR LTBI including reduced MDR TB rates, reduced TB transmission and cost effectiveness [11], [14]. Treatment guidelines for MDR LTBI have focused on the use of fluoroquinolone alone or with a second drug for 6–12 months based on susceptibility testing of the M. tuberculosis from the source-case [14]. For contacts with fluoroquinolone-resistant MDR LTBI, pyrazinamide plus ethambutol has been used if the source case M. tuberculosis isolate is susceptible, although this regimen is associated with increased risk of hepatotoxicity [49]. If none of these options are available, some have advocated for the use of two drugs with demonstrated susceptibility of the source case [50]. Unfortunately, measuring LTBI treatment efficacy is challenging without reliable biomarkers of therapeutic response, and further trials with long-term clinical outcomes are needed to validate MDR LTBI treatment options. Two such trials, VQUIN MDR and TB-CHAMP are testing the efficacy of levofloxacin for 24 weeks [51], [52]. Additionally, PHOENix MDR TB is investigating the use of isoniazid and delamanid for 26 weeks [53]. Clinical experience beyond the use of fluoroquinolones or pyrazinamide plus ethambutol for treatment of MDR LTBI is limited, and further study is needed to expand the arsenal of available treatments.
5. Linezolid in fluoroquinolone-resistant MDR LTBI management
While linezolid has been shown to have promising microbiological and pharmacokinetic properties as well as clinical efficacy as part of multidrug regimens for MDR TB, its use as monotherapy for MDR LTBI has not been reported. We describe three separate instances when patients were diagnosed with fluoroquinolone-resistant MDR LTBI and treated with linezolid alone, where individualized dosing regimens were used to create an improved adverse events profile.
5.1. Case report #1
A 65-year-old woman travelled from Mexico to the Midwestern United States. Three weeks later, she was found dead in her bedroom with evidence of massive hemoptysis. Autopsy showed cavitary TB which on susceptibility testing was determined to be MDR TB. Contact tracing led to testing 16 persons, with three grandchildren found to have positive results from an interferon-gamma release assay (IGRA). The three contacts who tested positive were a 13-year-old girl, 18-year-old woman, and a 20-year-old man, all previously healthy. Notably, each of these grandchildren was born in the United States with no known prior TB exposure risk factors: as such, these positive IGRA results were concerning for LTBI with MDR M. tuberculosis. Because of the significant drug resistance of the index TB case strain (Table 1), including broad fluoroquinolone resistance, a compassionate use request for delamanid was submitted but denied based on the parent company (Otsuka) compassionate use program protocols, focused only on TB disease treatment and excluding patients with LTBI. A regimen of ethambutol and cycloserine was considered, but given the bacteriostatic nature of that regimen, the decision was made to instead use linezolid. The three persons were treated with linezolid 600 mg daily for 2 weeks followed by 600 mg every other day for a total duration of 9 months. During the treatment course, dosing modifications and therapeutic drug monitoring to help guide dosing were discussed, but all three patients declined.
Table 1.
Antibiotic susceptibility testing of index M. tuberculosis strain from case series #1. Numbers in parentheses indicate concentration(s) of antibiotic tested in mcg/mL.
| First-Line Agents | |
| Isoniazid | Resistant (0.1, 0.2, 1) |
| Rifampin | Resistant (1) |
| Rifabutin | Resistant (2) |
| Pyrazinamide | Resistant (100) |
| Second Line Agents | |
| Group A | |
| Moxifloxacin* | Resistant (>4) |
| Linezolid* | Susceptible (0.5) |
| Bedaquiline* | Susceptible (0.12) |
| Group B | |
| Clofazimine* | Susceptible (0.06) |
| Cycloserine | Susceptible (30) |
| Group C | |
| Ethambutol | Susceptible (5, 10) |
| Amikacin | Susceptible (4, 6) |
| Streptomycin | Resistant (2, 10) |
| p-aminosalicylic acid | Resistant (2) |
| Ethionamide | Susceptible (5, 10) |
*Indicates drug testing performed by broth microdilution.
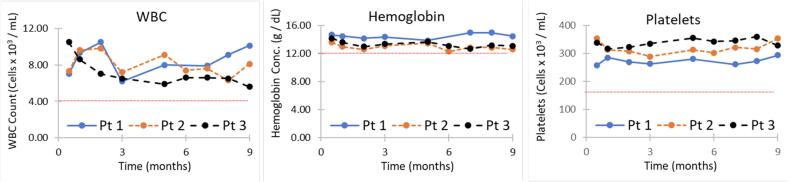
Blood counts were monitored at two weeks after treatment initiation and then monthly throughout the course of treatment without cytopenia (Fig. 1). The 13-year-old girl developed mild diarrhea and subjective shortness of breath and the 18-year-old woman developed nausea initially while on treatment, but both were able to remain on therapy and the symptoms resolved within 1 to 2 months (Table 2). All three patients completed the full 9 months of therapy with at most 8 doses missed over the 9-month course (Table 2). In the 4 years since beginning treatment, none has had symptoms of TB disease.
Fig. 1.
Complete blood count monitoring during therapy in case series #1. White blood cell count (WBC), Hemoglobin, and platelet count remained normal throughout therapy. Blood counts were monitored at two weeks and then monthly throughout the course of treatment without any evidence of cytopenia. A comprehensive metabolic panel was performed 4 months into therapy without evidence of impaired renal function or liver inflammation.
Table 2.
Patient outcomes during therapy from case series #1.
| Patient | Side effects reported during therapy (duration) | Total Doses Missed | Weight Change During Therapy |
|---|---|---|---|
| 13-year-old girl | Diarrhea (1-2 months)Shortness of Breath (1 week) |
8 | 81.5 to 88 lbs. (48th to 46th percentile) |
| 18-year-old woman | Nausea (1 month), improved with taking pills with meal | 6 | 138 to 136 lbs. |
| 20-year-old man | None | 6 | 252 to 243 lbs. |
5.2. Case report #2
A 36-year-old woman from Columbia was diagnosed with infectious cavitary pulmonary TB. The strain was resistant to all first line agents, isoniazid, rifampin, pyrazinamide, ethambutol, and fluoroquinolones. She was treated using BPaL. She had two household contacts, her 9-year-old daughter was negative on serial TB testing. Her 35-year-old fiancé had a positive IGRA result and was assessed to have fluoroquinolone-resistant MDR LTBI. He was treated with linezolid 600 mg daily for 24 weeks. After the first 3 weeks, linezolid serum trough levels were measured to be less than 2 mcg/ml. No dose adjustments were made. A complete blood count and comprehensive metabolic panel were also obtained and normal. No further laboratory monitoring was performed during treatment. The treatment was well tolerated and no adverse drug reactions developed during the 24-week period. He remains asymptomatic 2 years after treatment completion.
5.3. Case report #3
A 36-year-old woman from China was diagnosed with cavitary pulmonary TB. She had a history of pulmonary TB treated 10 years earlier in China. Her AFB smear was 4 + and the strain was found to have rpoB, katG, embB, pncA, gyrA, and eis mutation indicating resistance to all first line agents as well as fluoroquinolones and potentially, aminoglycosides. Two household contacts were identified, her husband and 8-year-old son. The only contact with a positive IGRA result was her son. The son weighed 27 kg, and was started on Linezolid 300 mg daily (11 mg/kg) for 24 weeks. No therapeutic drug monitoring was performed during the course of treatment. A complete blood count and comprehensive metabolic panel were obtained after initiation of linezolid and normal. No further laboratory monitoring was performed during treatment. The treatment was well tolerated and completed. He has remained asymptomatic 4 years after treatment completion.
6. Discussion
In cases of fluoroquinolone-resistant MDR LTBI, linezolid offers a therapeutic option with attractive microbiological and pharmacokinetic properties. However, a key limitation to its use is the potential of significant toxicity. In this series of five patients treated with three different dosing strategies, linezolid was successfully used without serious adverse events. The tolerability of linezolid within this case series supports the further study of its efficacy in treatment of MDR LTB. The dosing of linezolid must balance both tolerability and efficacy of treatment. The dosing strategies used in this case series all fall within the range of doses used in PK/PD modeling studies as discussed previously, with the lower dosing in case report #1 predicted to have higher tolerability while the regimens in case reports #2 and #3 predicted to have higher efficacy. In case report #2, despite the higher dose of 600 mg daily, trough serum concentrations were measured below the target of 2 mg/L at 3 weeks, and in both case report #2 and #3 the regimens were well tolerated despite using a relatively higher dose. Whether lower doses of linezolid lead to increased risk of development of linezolid-resistant subpopulations of M. tuberculosis is an important area of further study. Notably, the PK/PD studies that have informed the dosing strategies used in this case series were designed to mimic treatment conditions of pulmonary TB, a potential limitation to their application to the treatment of LTBI. Further studies using therapeutic drug monitoring of linezolid during LTBI treatment would help validate optimal linezolid dosing. Based on currently available data, using linezolid at 600 mg daily if tolerated seems most prudent.
New drug trials evaluating several combinations of drugs and regimens are underway to find the shortest, most effective and tolerated MDR TB treatments. Next generation oxazolidinones like sutezolid and delpazolid may maintain the efficacy of linezolid with fewer toxic effects, and their effectiveness in M. tuberculosis management is being reviewed [54]. A randomized trial to evaluate the efficacy, duration, dosing, and safety of linezolid and newer oxazolidinones in latent MDR TB infections should also be considered to contribute to a standardized practice guideline for MDR LTBI treatment.
7. Conclusions
Linezolid has been shown to have treatment success in MDR TB treatment and has favorable microbiologic and pharmacokinetic properties that support its utility as an agent for MDR LTBI with close monitoring for adverse events. While the clinical data are limited at present, here we describe the use of linezolid to treat fluoroquinolone-resistant MDR LTBI in 5 persons with good tolerability. Further study of the efficacy and tolerability of linezolid for the treatment MDR LTBI could validate its use and provide a much-needed tool to work toward WHO’s end-TB strategy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Global Tuberculosis Report 202World Health Organization. 2021.
- 2.Cohen A., Mathiasen V.D., Schön T., Wejse C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2019;54(3):1900655. doi: 10.1183/13993003.00655-2019. [DOI] [PubMed] [Google Scholar]
- 3.Kahwati L.C., Feltner C., Halpern M., Woodell C.L., Boland E., Amick H.R., et al. Primary care screening and treatment for latent tuberculosis infection in adults: evidence report and systematic review for the US preventive services task force. JAMA. 2016;316(9):970. doi: 10.1001/jama.2016.10357. [DOI] [PubMed] [Google Scholar]
- 4.Uplekar M., Weil D., Lonnroth K., Jaramillo E., Lienhardt C., Dias H.M., et al. WHO's new end TB strategy. Lancet. 2015;385(9979):1799–1801. doi: 10.1016/S0140-6736(15)60570-0. [DOI] [PubMed] [Google Scholar]
- 5.Houben R.M., Dodd P.J. The global burden of latent tuberculosis infection: A re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. doi: 10.1371/journal.pmed.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miramontes R., et al. Tuberculosis infection in the United States: prevalence estimates from the national health and nutrition examination survey, 2011-2012. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0140881. e0140881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shea K.M., Kammerer J.S., Winston C.A., Navin T.R., Horsburgh, C.R. Estimated rate of reactivation of latent tuberculosis infection in the United States, overall and by population subgroup. Am J Epidemiol. 2014;179(2):216–225. doi: 10.1093/aje/kwt246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight G.M., McQuaid C.F., Dodd P.J., Houben R.M.G.J. Global burden of latent multidrug-resistant tuberculosis: trends and estimates based on mathematical modelling. Lancet Infect Dis. 2019;19(8):903–912. doi: 10.1016/S1473-3099(19)30307-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep, 2000. 49(Rr-6): p. 1-51. [PubMed]
- 10.Sterling T.R., Njie G., Zenner D., Cohn D.L., Reves R., Ahmed A., et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the national tuberculosis controllers association and CDC, 2020. MMWR Recomm Rep. 2020;69(1):1–11. doi: 10.15585/mmwr.rr6901a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marks S.M., Mase S.R., Morris S.B. Systematic review, meta-analysis, and cost-effectiveness of treatment of latent tuberculosis to reduce progression to multidrug-resistant tuberculosis. Clin Infect Dis. 2017;64(12):1670–1677. doi: 10.1093/cid/cix208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denholm J.T., Leslie D.E., Jenkin G.A., Darby J., Johnson P.D.R., Graham S.M., et al. Long-term follow-up of contacts exposed to multidrug-resistant tuberculosis in Victoria, Australia, 1995–2010. Int J Tuberc Lung Dis. 2012;16(10):1320–1325. doi: 10.5588/ijtld.12.0092. [DOI] [PubMed] [Google Scholar]
- 13.Bamrah S., Brostrom R., Dorina F., Setik L., Song R., Kawamura L.M., et al. Treatment for LTBI in contacts of MDR-TB patients, Federated States of Micronesia, 2009–2012. Int J Tuberc Lung Dis. 2014;18(8):912–918. doi: 10.5588/ijtld.13.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nahid P., Mase S.R., Migliori G.B., Sotgiu G., Bothamley G.H., Brozek J.L., et al. Treatment of drug-resistant tuberculosis. An official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med. 2019;200(10):e93–e142. doi: 10.1164/rccm.201909-1874ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO Guidelines Approved by the Guidelines Review Committee, in WHO consolidated guidelines on tuberculosis: tuberculosis preventive treatment: Module 1: prevention. 2020, World Health Organization © World Health Organization 2020.: Geneva. [PubMed]
- 16.Langer A.J.S., Angela M. Centers for Disease Control and Prevention; 2022. Surveillance definitions for extensively drug resistant (XDR) and pre-XDR tuberculosis, in Dear Colleague Letters. [Google Scholar]
- 17.McCune R.M., Feldmann F.M., McDermott W. Microbial persistence. II. Characteristics of the sterile state of tubercle bacilli. J Exp Med. 1966;123(3):469–486. doi: 10.1084/jem.123.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grosset J. The sterilizing value of rifampicin and pyrazinamide in experimental short-course chemotherapy. Bull Int Union Tuberc. 1978;53(1):5–12. [PubMed] [Google Scholar]
- 19.WHO Guidelines Approved by the Guidelines Review Committee, in WHO consolidated guidelines on tuberculosis: Module 4: Treatment - Drug-resistant tuberculosis treatment. 2020, World Health Organization © World Health Organization 2020.: Geneva.
- 20.Conradie F., Diacon A.H., Ngubane N., Howell P., Everitt D., Crook A.M., et al. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med. 2020;382(10):893–902. doi: 10.1056/NEJMoa1901814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conradie F., Bagdasaryan T.R., Borisov S., Howell P., Mikiashvili L., Ngubane N., et al. Bedaquiline-pretomanid-linezolid regimens for drug-resistant tuberculosis. N Engl J Med. 2022;387(9):810–823. doi: 10.1056/NEJMoa2119430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berry C., du Cros P., Fielding K., Gajewski S., Kazounis E., McHugh T.D., et al. TB-PRACTECAL: study protocol for a randomised, controlled, open-label, phase II-III trial to evaluate the safety and efficacy of regimens containing bedaquiline and pretomanid for the treatment of adult patients with pulmonary multidrug-resistant tuberculosis. Trials. 2022;23(1) doi: 10.1186/s13063-022-06331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esmail A., Oelofse S., Lombard C., Perumal R., Mbuthini L., Goolam Mahomed A., et al. An All-Oral 6-Month Regimen for Multidrug-Resistant Tuberculosis: A Multicenter, Randomized Controlled Clinical Trial (the NExT Study) Am J Respir Crit Care Med. 2022;205(10):1214–1227. doi: 10.1164/rccm.202107-1779OC. [DOI] [PubMed] [Google Scholar]
- 24.Goodall R.L., Meredith S.K., Nunn A.J., Bayissa A., Bhatnagar A.K., Bronson G., et al. Evaluation of two short standardised regimens for the treatment of rifampicin-resistant tuberculosis (STREAM stage 2): an open-label, multicentre, randomised, non-inferiority trial. Lancet. 2022;400(10366):1858–1868. doi: 10.1016/S0140-6736(22)02078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasiri M.J., Zangiabadian M., Arabpour E., Amini S., Khalili F., Centis R., et al. Delamanid-containing regimens and multidrug-resistant tuberculosis: A systematic review and meta-analysis. Int J Infect Dis. 2022;124:S90–S103. doi: 10.1016/j.ijid.2022.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livermore D.M. Linezolid in vitro: mechanism and antibacterial spectrum. J Antimicrob Chemother. 2003;51 Suppl 2:p. ii9-16. doi: 10.1093/jac/dkg249. [DOI] [PubMed] [Google Scholar]
- 27.Huang T.-S., Liu Y.-C., Sy C.-L., Chen Y.-S., Tu H.-Z., Chen B.-C. In vitro activities of linezolid against clinical isolates of Mycobacterium tuberculosis complex isolated in Taiwan over 10 years. Antimicrob Agents Chemother. 2008;52(6):2226–2227. doi: 10.1128/AAC.00414-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cynamon M.H., Klemens S.P., Sharpe C.A., Chase S. Activities of several novel oxazolidinones against Mycobacterium tuberculosis in a murine model. Antimicrob Agents Chemother. 1999;43(5):1189–1191. doi: 10.1128/aac.43.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee M., Lee J., Carroll M.W., Choi H., Min S., Song T., et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med. 2012;367(16):1508–1518. doi: 10.1056/NEJMoa1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L., Pang Y., Yu X., Wang Y., Gao M., Huang H., et al. Linezolid in the treatment of extensively drug-resistant tuberculosis. Infection. 2014;42(4):705–711. doi: 10.1007/s15010-014-0632-2. [DOI] [PubMed] [Google Scholar]
- 31.Tang S., Yao L., Hao X., Zhang X., Liu G., Liu X., et al. Efficacy, safety and tolerability of linezolid for the treatment of XDR-TB: a study in China. Eur Respir J. 2015;45(1):161–170. doi: 10.1183/09031936.00035114. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X., et al. Systematic review and meta-analysis of the efficacy and safety of therapy with linezolid containing regimens in the treatment of multidrug-resistant and extensively drug-resistant tuberculosis. J Thorac Dis. 2015;7(4):603–615. doi: 10.3978/j.issn.2072-1439.2015.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soriano A., Miró O., Mensa J. Mitochondrial toxicity associated with linezolid. N Engl J Med. 2005;353(21):2305–2306. doi: 10.1056/NEJM200511243532123. [DOI] [PubMed] [Google Scholar]
- 34.Drusano G.L., Myrick J., Maynard M., Nole J., Duncanson B., Brown D., et al. Linezolid kills acid-phase and nonreplicative-persister-phase mycobacterium tuberculosis in a hollow-fiber infection model. Antimicrob Agents Chemother. 2018;62(8) doi: 10.1128/AAC.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donald P.R., Diacon A.H. The early bactericidal activity of anti-tuberculosis drugs: a literature review. Tuberculosis (Edinb) 2008;88(Suppl 1):S75–S83. doi: 10.1016/S1472-9792(08)70038-6. [DOI] [PubMed] [Google Scholar]
- 36.Dietze R., Hadad D.J., McGee B., Molino L.P.D., Maciel E.L.N., Peloquin C.A., et al. Early and extended early bactericidal activity of linezolid in pulmonary tuberculosis. Am J Respir Crit Care Med. 2008;178(11):1180–1185. doi: 10.1164/rccm.200806-892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honeybourne D., et al. Intrapulmonary penetration of linezolid. J Antimicrob Chemother. 2003;51(6):1431–1434. doi: 10.1093/jac/dkg262. [DOI] [PubMed] [Google Scholar]
- 38.Conte J.E., Golden J.A., Kipps J., Zurlinden E. Intrapulmonary pharmacokinetics of linezolid. Antimicrob Agents Chemother. 2002;46(5):1475–1480. doi: 10.1128/AAC.46.5.1475-1480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.EUCAST Technical Note on linezolid. Clin Microbiol Infect, 2006. 12(12): p. 1243-5. [DOI] [PubMed]
- 40.Schön T., Juréen P., Chryssanthou E., Giske C.G., Sturegård E., Kahlmeter G., et al. Wild-type distributions of seven oral second-line drugs against Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2011;15(4):502–509. doi: 10.5588/ijtld.10.0238. [DOI] [PubMed] [Google Scholar]
- 41.Lopez B., et al. Bedaquiline and linezolid MIC distributions and epidemiological cut-off values for Mycobacterium tuberculosis in the Latin American region. J Antimicrob Chemother. 2019;74(2):373–379. doi: 10.1093/jac/dky414. [DOI] [PubMed] [Google Scholar]
- 42.Bigelow K.M., et al. Pharmacodynamic correlates of linezolid activity and toxicity in murine models of tuberculosis. J Infect Dis. 2021;223(11):1855–1864. doi: 10.1093/infdis/jiaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alghamdi W.A., Al-Shaer M.H., An G., Alsultan A., Kipiani M., Barbakadze K., et al. Population pharmacokinetics of linezolid in tuberculosis patients: dosing regimen simulation and target attainment analysis. Antimicrob Agents Chemother. 2020;64(10) doi: 10.1128/AAC.01174-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srivastava S., Magombedze G., Koeuth T., Sherman C., Pasipanodya J.G., Raj P., et al. Linezolid dose that maximizes sterilizing effect while minimizing toxicity and resistance emergence for tuberculosis. Antimicrob Agents Chemother. 2017;61(8) doi: 10.1128/AAC.00751-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaspard M., Butel N., El Helali N., Marigot-Outtandy D., Guillot H., Peytavin G., et al. Linezolid-associated neurologic adverse events in patients with multidrug-resistant tuberculosis, France. Emerg Infect Dis. 2020;26(8):1792–1800. doi: 10.3201/eid2608.191499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song T., Lee M., Jeon H.-S., Park Y., Dodd L.E., Dartois V., et al. Linezolid trough concentrations correlate with mitochondrial toxicity-related adverse events in the treatment of chronic extensively drug-resistant tuberculosis. EBioMedicine. 2015;2(11):1627–1633. doi: 10.1016/j.ebiom.2015.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park I.N., et al. Efficacy and tolerability of daily-half dose linezolid in patients with intractable multidrug-resistant tuberculosis. J Antimicrob Chemother. 2006;58(3):701–704. doi: 10.1093/jac/dkl298. [DOI] [PubMed] [Google Scholar]
- 48.Mase A., et al. Low-dose linezolid for treatment of patients with multidrug-resistant tuberculosis. Open Forum Infect Dis. 2022;9(12):ofac500. doi: 10.1093/ofid/ofac500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Younossian A.B., et al. High hepatotoxicity of pyrazinamide and ethambutol for treatment of latent tuberculosis. Eur Respir J. 2005;26(3):462–464. doi: 10.1183/09031936.05.00006205. [DOI] [PubMed] [Google Scholar]
- 50.Clinical programs and policy manual: New York City Department of Health and Mental Hygiene Bureau of Tuberculosis Control 2022. 2022.
- 51.Fox G.J., et al. Levofloxacin versus placebo for the treatment of latent tuberculosis among contacts of patients with multidrug-resistant tuberculosis (the VQUIN MDR trial): a protocol for a randomised controlled trial. BMJ Open. 2020;10(1) doi: 10.1136/bmjopen-2019-033945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seddon J.A., Garcia-Prats A.J., Purchase S.E., Osman M., Demers A.-M., Hoddinott G., et al. Levofloxacin versus placebo for the prevention of tuberculosis disease in child contacts of multidrug-resistant tuberculosis: study protocol for a phase III cluster randomised controlled trial (TB-CHAMP) Trials. 2018;19(1) doi: 10.1186/s13063-018-3070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee A., et al. Current and future treatments for tuberculosis. BMJ. 2020;368:m216. doi: 10.1136/bmj.m216. [DOI] [PubMed] [Google Scholar]
- 54.Black T.A., Buchwald U.K. The pipeline of new molecules and regimens against drug-resistant tuberculosis. J Clin Tubercul Other Mycobact Diseases. 2021;25 doi: 10.1016/j.jctube.2021.100285. [DOI] [PMC free article] [PubMed] [Google Scholar]